Chemical Analysis and Antioxidant Activities of Resin Fractions from Pistacia lentiscus L. var. Chia in Neuroblastoma SH-SY5Y Cells
Abstract
1. Introduction
2. Results
2.1. Chemical Characterization of the Three Different Polarity Fractions of P. lenticonus/Chios Mastiha
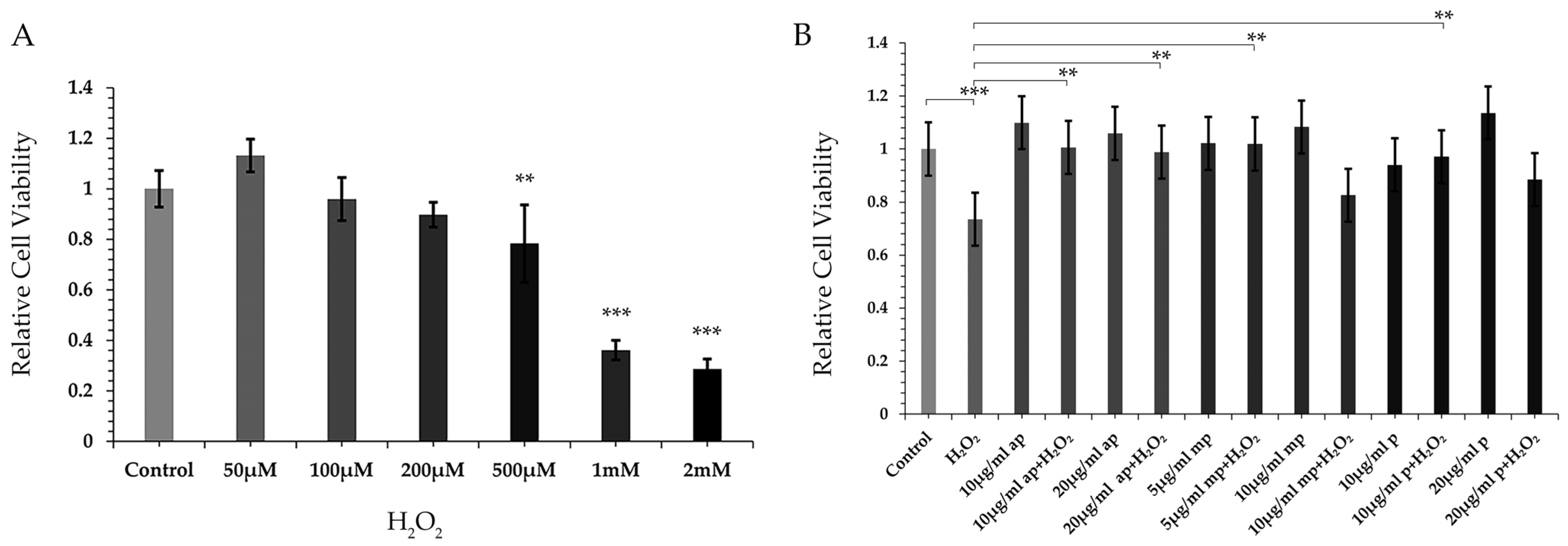
2.2. Different Polarity Fractions from P. lenticonus/Chios Mastiha Induced Resistance to H2O2-Triggered Reduction in Cell Viability of SH-SY5Y Cells
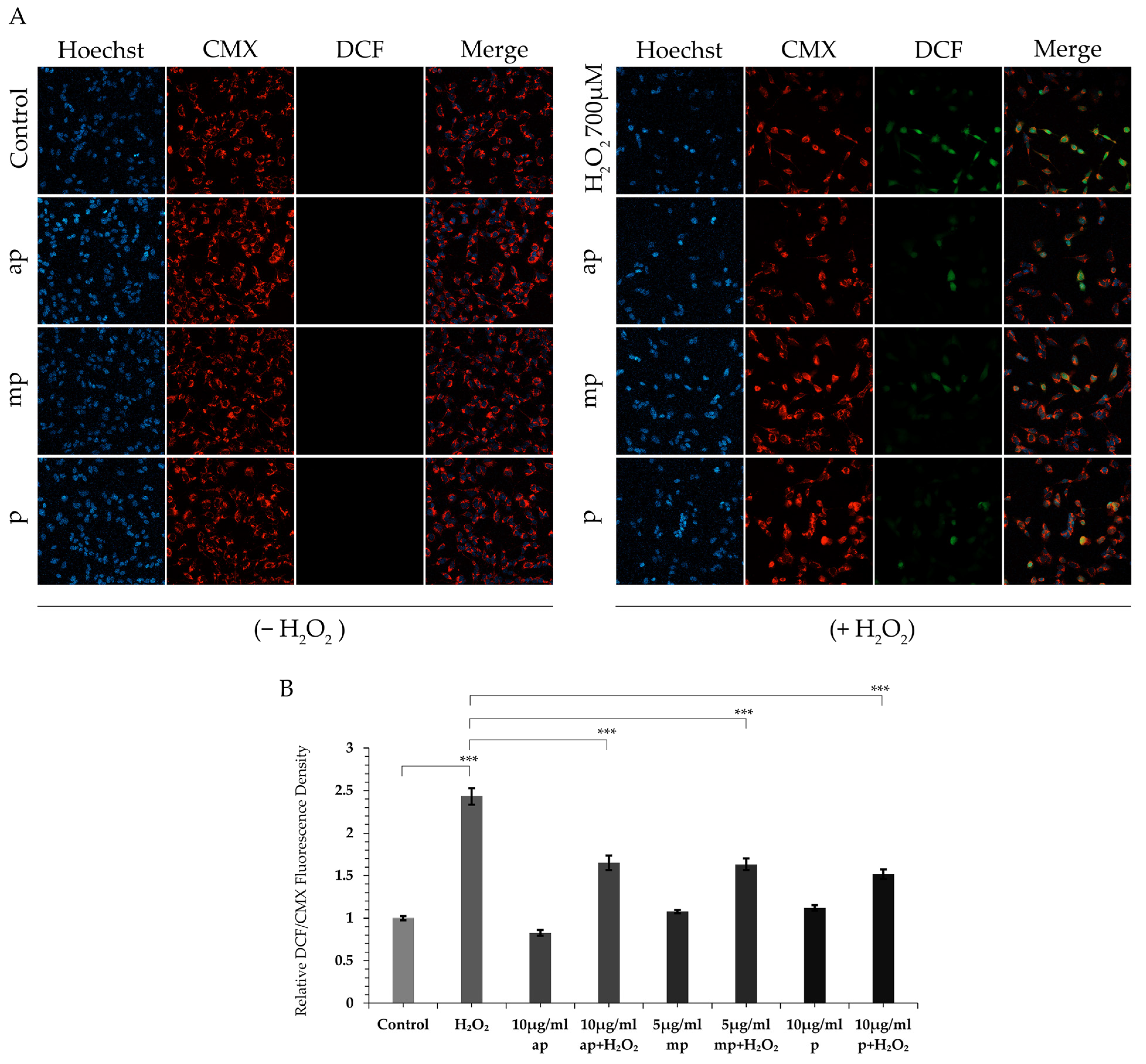
2.3. Protective Effect of the Different Polarity P. lenticonus/Chios Mastiha Fractions on H2O2-Induced ROS Production in SH-SY5Y Cells
2.4. Maintenance of the Mitochondrial Functionality by the Different Polarity P. lenticonus/Chios Mastiha Fractions upon Conditions of H2O2-Induced Oxidative Stress
2.5. Regulation of GR, PPARα και Bcl-2 Protein Levels by the Apolar Fraction
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Chemicals
5.2. Chios Mastiha Fractionation and Chemical Characterization
5.3. Cell Culture
5.4. Antibodies
5.5. Cell Viability Assay-MTT
5.6. Electrophoresis and Western Blot Analysis
5.7. Intracellular ROS Measurement
5.8. Analysis of the Mitochondrial Membrane Potential
5.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pachi, V.K.; Mikropoulou, E.V.; Gkiouvetidis, P.; Siafakas, K.; Argyropoulou, A.; Angelis, A.; Mitakou, S.; Halabalaki, M. Traditional uses, phytochemistry and pharmacology of Chios mastic gum (Pistacia lentiscus var. Chia, Anacardiaceae): A review. J. Ethnopharmacol. 2020, 254, 112485. [Google Scholar] [CrossRef] [PubMed]
- Soulaidopoulos, S.; Tsiogka, A.; Chrysohoou, C.; Lazarou, E.; Aznaouridis, K.; Doundoulakis, I.; Tyrovola, D.; Tousoulis, D.; Tsioufis, K.; Vlachopoulos, C.; et al. Overview of Chios Mastic Gum (Pistacia lentiscus) Effects on Human Health. Nutrients 2022, 14, 590. [Google Scholar] [CrossRef]
- Balan, K.V.; Demetzos, C.; Prince, J.; Dimas, K.; Cladaras, M.; Han, Z.; Wyche, J.H.; Pantazis, P. Induction of apoptosis in human colon cancer HCT116 cells treated with an extract of the plant product, Chios mastic gum. In Vivo 2005, 19, 93–102. [Google Scholar] [PubMed]
- Dimas, K.S.; Pantazis, P.; Ramanujam, R. Review: Chios mastic gum: A plant-produced resin exhibiting numerous diverse pharmaceutical and biomedical properties. In Vivo 2012, 26, 777–785. [Google Scholar]
- He, M.L.; Yuan, H.Q.; Jiang, A.L.; Gong, A.Y.; Chen, W.W.; Zhang, P.J.; Young, C.Y.; Zhang, J.Y. Gum mastic inhibits the expression and function of the androgen receptor in prostate cancer cells. Cancer 2006, 106, 2547–2555. [Google Scholar] [CrossRef] [PubMed]
- Dedoussis, G.V.; Kaliora, A.C.; Psarras, S.; Chiou, A.; Mylona, A.; Papadopoulos, N.G.; Andrikopoulos, N.K. Antiatherogenic effect of Pistacia lentiscus via GSH restoration and downregulation of CD36 mRNA expression. Atherosclerosis 2004, 174, 293–303. [Google Scholar] [CrossRef]
- Triantafyllou, A.; Chaviaras, N.; Sergentanis, T.N.; Protopapa, E.; Tsaknis, J. Chios mastic gum modulates serum biochemical parameters in a human population. J. Ethnopharmacol. 2007, 111, 43–49. [Google Scholar] [CrossRef]
- Paraschos, S.; Mitakou, S.; Skaltsounis, A.L. Chios gum mastic: A review of its biological activities. Curr. Med. Chem. 2012, 19, 2292–2302. [Google Scholar] [CrossRef]
- Zhou, L.; Satoh, K.; Takahashi, K.; Watanabe, S.; Nakamura, W.; Maki, J.; Hatano, H.; Takekawa, F.; Shimada, C.; Sakagami, H. Re-evaluation of anti-inflammatory activity of mastic using activated macrophages. In Vivo 2009, 23, 583–589. [Google Scholar]
- Loizou, S.; Paraschos, S.; Mitakou, S.; Chrousos, G.P.; Lekakis, I.; Moutsatsou, P. Chios mastic gum extract and isolated phytosterol tirucallol exhibit anti-inflammatory activity in human aortic endothelial cells. Exp. Biol. Med. 2009, 234, 553–561. [Google Scholar] [CrossRef]
- Papada, E.; Forbes, A.; Amerikanou, C.; Torovic, L.; Kalogeropoulos, N.; Tzavara, C.; Triantafillidis, J.K.; Kaliora, A.C. Antioxidative Efficacy of a Pistacia Lentiscus Supplement and Its Effect on the Plasma Amino Acid Profile in Inflammatory Bowel Disease: A Randomised, Double-Blind, Placebo-Controlled Trial. Nutrients 2018, 10, 1779. [Google Scholar] [CrossRef]
- Kalousi, F.D.; Pollastro, F.; Karra, A.G.; Tsialtas, I.; Georgantopoulos, A.; Salamone, S.; Psarra, A.G. Regulation of Energy Metabolism and Anti-Inflammatory Activities of Mastiha Fractions from Pistacia lentiscus L. var. chia. Foods 2023, 12, 1390. [Google Scholar] [CrossRef] [PubMed]
- Andrikopoulos, N.K.; Kaliora, A.C.; Assimopoulou, A.N.; Papapeorgiou, V.P. Biological activity of some naturally occurring resins, gums and pigments against in vitro LDL oxidation. Phytother. Res. 2003, 17, 501–507. [Google Scholar] [CrossRef]
- Kalousi, F.D.; Pollastro, F.; Christodoulou, E.C.; Karra, A.G.; Tsialtas, I.; Georgantopoulos, A.; Salamone, S.; Psarra, A.G. Apoptotic, Anti-Inflammatory Activities and Interference with the Glucocorticoid Receptor Signaling of Fractions from Pistacia lentiscus L. var. chia Leaves. Plants 2022, 11, 934. [Google Scholar] [CrossRef] [PubMed]
- Papada, E.; Gioxari, A.; Amerikanou, C.; Forbes, A.; Tzavara, C.; Smyrnioudis, I.; Kaliora, A.C. Regulation of faecal biomarkers in inflammatory bowel disease patients treated with oral mastiha (Pistacia lentiscus) supplement: A double-blind and placebo-controlled randomised trial. Phytother. Res. 2019, 33, 360–369. [Google Scholar] [CrossRef]
- Kaliora, A.C.; Stathopoulou, M.G.; Triantafillidis, J.K.; Dedoussis, G.V.; Andrikopoulos, N.K. Chios mastic treatment of patients with active Crohn’s disease. World J. Gastroenterol. 2007, 13, 748–753. [Google Scholar] [CrossRef] [PubMed]
- Kanoni, S.; Kumar, S.; Amerikanou, C.; Kurth, M.J.; Stathopoulou, M.G.; Bourgeois, S.; Masson, C.; Kannt, A.; Cesarini, L.; Kontoe, M.S.; et al. Nutrigenetic Interactions Might Modulate the Antioxidant and Anti-Inflammatory Status in Mastiha-Supplemented Patients with NAFLD. Front. Immunol. 2021, 12, 683028. [Google Scholar] [CrossRef]
- Checa, J.; Aran, J.M. Reactive Oxygen Species: Drivers of Physiological and Pathological Processes. J. Inflamm. Res. 2020, 13, 1057–1073. [Google Scholar] [CrossRef] [PubMed]
- Piccolella, S.; Nocera, P.; Carillo, P.; Woodrow, P.; Greco, V.; Manti, L.; Fiorentino, A.; Pacifico, S. An apolar Pistacia lentiscus L. leaf extract: GC-MS metabolic profiling and evaluation of cytotoxicity and apoptosis inducing effects on SH-SY5Y and SK-N-BE(2)C cell lines. Food Chem. Toxicol. 2016, 95, 64–74. [Google Scholar] [CrossRef] [PubMed]
- Galluzzi, L.; Kepp, O.; Trojel-Hansen, C.; Kroemer, G. Mitochondrial control of cellular life, stress, and death. Circ. Res. 2012, 111, 1198–1207. [Google Scholar] [CrossRef]
- Assimopoulou, A.N.; Papageorgiou, V.P. GC-MS analysis of penta- and tetra-cyclic triterpenes from resins of Pistacia species. Part I. Pistacia lentiscus var. Chia. Biomed. Chromatogr. 2005, 19, 285–311. [Google Scholar] [CrossRef] [PubMed]
- Paraschos, S.; Magiatis, P.; Mitakou, S.; Petraki, K.; Kalliaropoulos, A.; Maragkoudakis, P.; Mentis, A.; Sgouras, D.; Skaltsounis, A.L. In vitro and in vivo activities of Chios mastic gum extracts and constituents against Helicobacter pylori. Antimicrob. Agents Chemother. 2007, 51, 551–559. [Google Scholar] [CrossRef] [PubMed]
- Ottria, R.; Xynomilakis, O.; Casati, S.; Abbiati, E.; Maconi, G.; Ciuffreda, P. Chios Mastic Gum: Chemical Profile and Pharmacological Properties in Inflammatory Bowel Disease: From the Past to the Future. Int. J. Mol. Sci. 2023, 24, 12038. [Google Scholar] [CrossRef]
- Vallianou, I.; Peroulis, N.; Pantazis, P.; Hadzopoulou-Cladaras, M. Camphene, a plant-derived monoterpene, reduces plasma cholesterol and triglycerides in hyperlipidemic rats independently of HMG-CoA reductase activity. PLoS ONE 2011, 6, e20516. [Google Scholar] [CrossRef] [PubMed]
- Magiatis, P.; Melliou, E.; Skaltsounis, A.L.; Chinou, I.B.; Mitaku, S. Chemical composition and antimicrobial activity of the essential oils of Pistacia lentiscus var. chia. Planta Med. 1999, 65, 749–752. [Google Scholar] [CrossRef]
- Timmermans, S.; Souffriau, J.; Libert, C. A General Introduction to Glucocorticoid Biology. Front. Immunol. 2019, 10, 1545. [Google Scholar] [CrossRef]
- Georgatza, D.; Gorgogietas, V.A.; Kylindri, P.; Charalambous, M.C.; Papadopoulou, K.K.; Hayes, J.M.; Psarra, A.G. The triterpene echinocystic acid and its 3-O-glucoside derivative are revealed as potent and selective glucocorticoid receptor agonists. Int. J. Biochem. Cell Biol. 2016, 79, 277–287. [Google Scholar] [CrossRef]
- Karra, A.G.; Konstantinou, M.; Tzortziou, M.; Tsialtas, I.; Kalousi, F.D.; Garagounis, C.; Hayes, J.M.; Psarra, A.G. Potential Dissociative Glucocorticoid Receptor Activity for Protopanaxadiol and Protopanaxatriol. Int. J. Mol. Sci. 2018, 20, 94. [Google Scholar] [CrossRef]
- Georgantopoulos, A.; Vougioukas, A.; Kalousi, F.D.; Tsialtas, I.; Psarra, A.G. Comparative Studies on the Anti-Inflammatory and Apoptotic Activities of Four Greek Essential Oils: Involvement in the Regulation of NF-kappaBeta and Steroid Receptor Signaling. Life 2023, 13, 1534. [Google Scholar] [CrossRef]
- Boutemine, I.M.; Amri, M.; Dorgham, K.; Amir, Z.C.; Benazzouz, S.; Ameur, F.; Layaida, K.; Yssel, H.; Touil-Boukoffa, C. Beneficial role of Pistacia lentiscus aqueous extract in experimental colitis: Anti-inflammatory and potential therapeutic effects. Inflammopharmacology 2021, 29, 1225–1239. [Google Scholar] [CrossRef]
- Cui, H.; Li, X.; An, X.R.; Liu, W.; Yuan, T. Masticadienonic acid from Chios mastic gum mitigates colitis in mice via modulating inflammatory response, gut barrier integrity and microbiota. Phytomedicine 2023, 108, 154518. [Google Scholar] [CrossRef]
- Lee, W.; Yang, E.J.; Ku, S.K.; Song, K.S.; Bae, J.S. Anti-inflammatory effects of oleanolic acid on LPS-induced inflammation in vitro and in vivo. Inflammation 2013, 36, 94–102. [Google Scholar] [CrossRef]
- Dharmappa, K.K.; Kumar, R.V.; Nataraju, A.; Mohamed, R.; Shivaprasad, H.V.; Vishwanath, B.S. Anti-inflammatory activity of oleanolic acid by inhibition of secretory phospholipase A2. Planta Med. 2009, 75, 211–215. [Google Scholar] [CrossRef]
- Park, J.S.; Rehman, I.U.; Choe, K.; Ahmad, R.; Lee, H.J.; Kim, M.O. A Triterpenoid Lupeol as an Antioxidant and Anti-Neuroinflammatory Agent: Impacts on Oxidative Stress in Alzheimer’s Disease. Nutrients 2023, 15, 3059. [Google Scholar] [CrossRef] [PubMed]
- Dai, D.; Zeldin, D.C.; Blaisdell, J.A.; Chanas, B.; Coulter, S.J.; Ghanayem, B.I.; Goldstein, J.A. Polymorphisms in human CYP2C8 decrease metabolism of the anticancer drug paclitaxel and arachidonic acid. Pharmacogenetics 2001, 11, 597–607. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Bai, L.; Chen, L.; Tong, R.; Feng, Y.; Shi, J. Terpenoid natural products exert neuroprotection via the PI3K/Akt pathway. Front. Pharmacol. 2022, 13, 1036506. [Google Scholar] [CrossRef] [PubMed]
- Stamou, P.; Gianniou, D.D.; Trougakos, I.P.; Mitakou, S.; Halabalaki, M.; Kostakis, I.K.; Skaltsounis, A.L. Anti-Inflammatory Activity of the Major Triterpenic Acids of Chios Mastic Gum and Their Semi-Synthetic Analogues. Biomolecules 2024, 14, 1618. [Google Scholar] [CrossRef]
- Said, S.A.; Fernandez, C.; Greff, S.; Torre, F.; Derridj, A.; Gauquelin, T.; Mevy, J.P. Inter-population variability of terpenoid composition in leaves of Pistacia lentiscus L. from Algeria: A chemoecological approach. Molecules 2011, 16, 2646–2657. [Google Scholar] [CrossRef]
- Floris, S.; Di Petrillo, A.; Pintus, F.; Delogu, G.L. Pistacia lentiscus: Phytochemistry and Antidiabetic Properties. Nutrients 2024, 16, 1638. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.H.; Feng, Y.P.; Liu, W.; Yuan, T. Diverse Triterpenoids from Mastic Produced by Pistacia lentiscus and Their Anti-Inflammatory Activities. Chem. Biodivers 2022, 19, e202101012. [Google Scholar] [CrossRef] [PubMed]
- Kahaer, G.; Abdulla, R.; Wu, T.; Aisa, H.A. Systematic qualitative analysis of terpenes in mastic (Pistacia lentiscus L.) extract and their fragmentations by UHPLC-Q-Orbitrap-HRMS. Phytochem. Anal. 2024, 35, 1072–1087. [Google Scholar] [CrossRef]
- Karadimou, C.; Petsa, E.; Ouroumi, N.A.; Papadakis, E.N.; Kontoudakis, N.; Theocharis, S.; Mourtzinos, I.; Menkissoglu-Spiroudi, U.; Kalogiouri, N.P.; Koundouras, S. Exploration of the anthocyanin and proanthocyanidin profile of Greek red grape skins belonging to Vradiano, Limnio, and Kotsifali cultivars, analyzed by a novel LC-QTOF-MS/MS method. Phytochem. Anal. 2024, 35, 1781–1793. [Google Scholar] [CrossRef] [PubMed]
- Mitsikaris, P.D.; Kostas, S.; Mourtzinos, I.; Menkissoglu-Spiroudi, U.; Papadopoulos, A.; Kalogiouri, N.P. Investigation of Rosa species by an optimized LC-QTOF-MS/MS method using targeted and non-targeted screening strategies combined with multivariate chemometrics. Phytochem. Anal. 2024, 35, 1100–1111. [Google Scholar] [CrossRef] [PubMed]
- Lemberger, T.; Staels, B.; Saladin, R.; Desvergne, B.; Auwerx, J.; Wahli, W. Regulation of the peroxisome proliferator-activated receptor alpha gene by glucocorticoids. J. Biol. Chem. 1994, 269, 24527–24530. [Google Scholar] [CrossRef]
- Wang, L.; Cai, Y.; Jian, L.; Cheung, C.W.; Zhang, L.; Xia, Z. Impact of peroxisome proliferator-activated receptor-alpha on diabetic cardiomyopathy. Cardiovasc. Diabetol. 2021, 20, 2. [Google Scholar] [CrossRef] [PubMed]
- Kamata, S.; Oyama, T.; Saito, K.; Honda, A.; Yamamoto, Y.; Suda, K.; Ishikawa, R.; Itoh, T.; Watanabe, Y.; Shibata, T.; et al. PPARalpha Ligand-Binding Domain Structures with Endogenous Fatty Acids and Fibrates. iScience 2020, 23, 101727. [Google Scholar] [CrossRef]
- Hostetler, H.A.; Petrescu, A.D.; Kier, A.B.; Schroeder, F. Peroxisome proliferator-activated receptor alpha interacts with high affinity and is conformationally responsive to endogenous ligands. J. Biol. Chem. 2005, 280, 18667–18682. [Google Scholar] [CrossRef] [PubMed]
- Varga, T.; Czimmerer, Z.; Nagy, L. PPARs are a unique set of fatty acid regulated transcription factors controlling both lipid metabolism and inflammation. Biochim. Biophys. Acta 2011, 1812, 1007–1022. [Google Scholar] [CrossRef] [PubMed]
- Iversen, L.; Kragballe, K. Arachidonic acid metabolism in skin health and disease. Prostaglandins Other Lipid Mediat. 2000, 63, 25–42. [Google Scholar] [CrossRef]
- Venn-Watson, S.; Schork, N.J. Pentadecanoic Acid (C15:0), an Essential Fatty Acid, Shares Clinically Relevant Cell-Based Activities with Leading Longevity-Enhancing Compounds. Nutrients 2023, 15, 4607. [Google Scholar] [CrossRef] [PubMed]
- Fulda, S. Betulinic acid: A natural product with anticancer activity. Mol. Nutr. Food Res. 2009, 53, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Costa, J.F.; Barbosa-Filho, J.M.; Maia, G.L.; Guimaraes, E.T.; Meira, C.S.; Ribeiro-dos-Santos, R.; de Carvalho, L.C.; Soares, M.B. Potent anti-inflammatory activity of betulinic acid treatment in a model of lethal endotoxemia. Int. Immunopharmacol. 2014, 23, 469–474. [Google Scholar] [CrossRef] [PubMed]
- Dai, W.; Dong, P.; Liu, J.; Gao, Y.; Hu, Y.; Lin, H.; Song, Y.; Mei, Q. Euscaphic acid inhibits proliferation and promotes apoptosis of nasopharyngeal carcinoma cells by silencing the PI3K/AKT/mTOR signaling pathway. Am. J. Transl. Res. 2019, 11, 2090–2098. [Google Scholar] [PubMed]
- Shi, C.; Li, Z.; Wu, Y.; Li, X.; Li, Y.; Wei, J.; Li, J.; Zhang, Y.; Li, L. Euscaphic acid and Tormentic acid protect vascular endothelial cells against hypoxia-induced apoptosis via PI3K/AKT or ERK 1/2 signaling pathway. Life Sci. 2020, 252, 117666. [Google Scholar] [CrossRef]
- Kim, S.Y.; Lee, H.J.; Nam, J.W.; Seo, E.K.; Lee, Y.S. Coniferyl aldehyde reduces radiation damage through increased protein stability of heat shock transcriptional factor 1 by phosphorylation. Int. J. Radiat. Oncol. Biol. Phys. 2015, 91, 807–816. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.M.; Heo, D.R.; Kim, Y.A.; Lee, J.; Kim, N.S.; Bang, O.S. Coniferaldehyde inhibits LPS-induced apoptosis through the PKC alpha/beta II/Nrf-2/HO-1 dependent pathway in RAW264.7 macrophage cells. Environ. Toxicol. Pharmacol. 2016, 48, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Karamac, M.; Koleva, L.; Kancheva, V.D.; Amarowicz, R. The Structure-Antioxidant Activity Relationship of Ferulates. Molecules 2017, 22, 527. [Google Scholar] [CrossRef] [PubMed]
- Yi, B.; Hu, L.; Mei, W.; Zhou, K.; Wang, H.; Luo, Y.; Wei, X.; Dai, H. Antioxidant phenolic compounds of cassava (Manihot esculenta) from Hainan. Molecules 2011, 16, 10157–10167. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, Y.; Li, X.; Sun, X.; Wang, Z.; Wang, H.; Nie, R.; Yu, W.; Zhou, Y. Coniferyl Aldehyde Inhibits the Inflammatory Effects of Leptomeningeal Cells by Suppressing the JAK2 Signaling. BioMed Res. Int. 2020, 2020, 4616308. [Google Scholar] [CrossRef] [PubMed]
- Park, J.M.; Park, J.E.; Park, J.S.; Leem, Y.H.; Kim, D.Y.; Hyun, J.W.; Kim, H.S. Anti-inflammatory and antioxidant mechanisms of coniferaldehyde in lipopolysaccharide-induced neuroinflammation: Involvement of AMPK/Nrf2 and TAK1/MAPK/NF-kappaB signaling pathways. Eur. J. Pharmacol. 2024, 979, 176850. [Google Scholar] [CrossRef]
- Dong, Y.; Stewart, T.; Bai, L.; Li, X.; Xu, T.; Iliff, J.; Shi, M.; Zheng, D.; Yuan, L.; Wei, T.; et al. Coniferaldehyde attenuates Alzheimer’s pathology via activation of Nrf2 and its targets. Theranostics 2020, 10, 179–200. [Google Scholar] [CrossRef] [PubMed]
- Al-Khayri, J.M.; Sahana, G.R.; Nagella, P.; Joseph, B.V.; Alessa, F.M.; Al-Mssallem, M.Q. Flavonoids as Potential Anti-Inflammatory Molecules: A Review. Molecules 2022, 27, 2901. [Google Scholar] [CrossRef]
- Avalos, Y.; Hernandez-Caceres, M.P.; Lagos, P.; Pinto-Nunez, D.; Rivera, P.; Burgos, P.; Diaz-Castro, F.; Joy-Immediato, M.; Venegas-Zamora, L.; Lopez-Gallardo, E.; et al. Palmitic acid control of ciliogenesis modulates insulin signaling in hypothalamic neurons through an autophagy-dependent mechanism. Cell Death Dis. 2022, 13, 659. [Google Scholar] [CrossRef]
- To, N.B.; Nguyen, Y.T.; Moon, J.Y.; Ediriweera, M.K.; Cho, S.K. Pentadecanoic Acid, an Odd-Chain Fatty Acid, Suppresses the Stemness of MCF-7/SC Human Breast Cancer Stem-Like Cells through JAK2/STAT3 Signaling. Nutrients 2020, 12, 1663. [Google Scholar] [CrossRef] [PubMed]
- Ysrafil, Y.; Sapiun, Z.; Slamet, N.S.; Mohamad, F.; Hartati, H.; Damiti, S.A.; Alexandra, F.D.; Rahman, S.; Masyeni, S.; Harapan, H.; et al. Anti-inflammatory activities of flavonoid derivates. ADMET DMPK 2023, 11, 331–359. [Google Scholar] [CrossRef]
- Venn-Watson, S.K.; Butterworth, C.N. Broader and safer clinically-relevant activities of pentadecanoic acid compared to omega-3: Evaluation of an emerging essential fatty acid across twelve primary human cell-based disease systems. PLoS ONE 2022, 17, e0268778. [Google Scholar] [CrossRef]
- Wang, Z.J.; Li, G.M.; Tang, W.L.; Yin, M. Neuroprotective effects of stearic acid against toxicity of oxygen/glucose deprivation or glutamate on rat cortical or hippocampal slices. Acta Pharmacol. Sin. 2006, 27, 145–150. [Google Scholar] [CrossRef]
- Charlot, A.; Morel, L.; Bringolf, A.; Georg, I.; Charles, A.L.; Goupilleau, F.; Geny, B.; Zoll, J. Octanoic Acid-Enrichment Diet Improves Endurance Capacity and Reprograms Mitochondrial Biogenesis in Skeletal Muscle of Mice. Nutrients 2022, 14, 2721. [Google Scholar] [CrossRef]
- Gupta, R.; Sharma, A.K.; Sharma, M.C.; Dobhal, M.P.; Gupta, R.S. Evaluation of antidiabetic and antioxidant potential of lupeol in experimental hyperglycaemia. Nat. Prod. Res. 2012, 26, 1125–1129. [Google Scholar] [CrossRef] [PubMed]
- Rychlicka, M.; Rot, A.; Gliszczynska, A. Biological Properties, Health Benefits and Enzymatic Modifications of Dietary Methoxylated Derivatives of Cinnamic Acid. Foods 2021, 10, 1417. [Google Scholar] [CrossRef]
- Georgiadis, I.; Karatzas, T.; Korou, L.M.; Katsilambros, N.; Perrea, D. Beneficial health effects of Chios Gum Mastic and peroxisome proliferator-activated receptors: Indications of common mechanisms. J. Med. Food 2015, 18, 1–10. [Google Scholar] [CrossRef]
- Toyama, T.; Nakamura, H.; Harano, Y.; Yamauchi, N.; Morita, A.; Kirishima, T.; Minami, M.; Itoh, Y.; Okanoue, T. PPARalpha ligands activate antioxidant enzymes and suppress hepatic fibrosis in rats. Biochem. Biophys. Res. Commun. 2004, 324, 697–704. [Google Scholar] [CrossRef] [PubMed]
- Shin, M.H.; Lee, S.R.; Kim, M.K.; Shin, C.Y.; Lee, D.H.; Chung, J.H. Activation of Peroxisome Proliferator-Activated Receptor Alpha Improves Aged and UV-Irradiated Skin by Catalase Induction. PLoS ONE 2016, 11, e0162628. [Google Scholar] [CrossRef] [PubMed]
- Muzio, G.; Barrera, G.; Pizzimenti, S. Peroxisome Proliferator-Activated Receptors (PPARs) and Oxidative Stress in Physiological Conditions and in Cancer. Antioxidants 2021, 10, 1734. [Google Scholar] [CrossRef]
- Popeijus, H.E.; van Otterdijk, S.D.; van der Krieken, S.E.; Konings, M.; Serbonij, K.; Plat, J.; Mensink, R.P. Fatty acid chain length and saturation influences PPARalpha transcriptional activation and repression in HepG2 cells. Mol. Nutr. Food Res. 2014, 58, 2342–2349. [Google Scholar] [CrossRef]
- Lo Verme, J.; Fu, J.; Astarita, G.; La Rana, G.; Russo, R.; Calignano, A.; Piomelli, D. The nuclear receptor peroxisome proliferator-activated receptor-alpha mediates the anti-inflammatory actions of palmitoylethanolamide. Mol. Pharmacol. 2005, 67, 15–19. [Google Scholar] [CrossRef]
- Malcher-Lopes, R.; Franco, A.; Tasker, J.G. Glucocorticoids shift arachidonic acid metabolism toward endocannabinoid synthesis: A non-genomic anti-inflammatory switch. Eur. J. Pharmacol. 2008, 583, 322–339. [Google Scholar] [CrossRef] [PubMed]
- Zeb, A. Concept, mechanism, and applications of phenolic antioxidants in foods. J. Food Biochem. 2020, 44, e13394. [Google Scholar] [CrossRef] [PubMed]
- Benadiba, M.; Miyake, J.A.; Colquhoun, A. Gamma-linolenic acid alters Ku80, E2F1, and bax expression and induces micronucleus formation in C6 glioma cells in vitro. IUBMB Life 2009, 61, 244–251. [Google Scholar] [CrossRef]
- Kou, J.J.; Shi, J.Z.; He, Y.Y.; Hao, J.J.; Zhang, H.Y.; Luo, D.M.; Song, J.K.; Yan, Y.; Xie, X.M.; Du, G.H.; et al. Luteolin alleviates cognitive impairment in Alzheimer’s disease mouse model via inhibiting endoplasmic reticulum stress-dependent neuroinflammation. Acta Pharmacol. Sin. 2022, 43, 840–849. [Google Scholar] [CrossRef] [PubMed]
- Cui, D.; Chen, Y.; Ye, B.; Guo, W.; Wang, D.; He, J. Natural products for the treatment of neurodegenerative diseases. Phytomedicine 2023, 121, 155101. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Suarez, L.; Awabdh, S.A.; Coumoul, X.; Chauvet, C. The SH-SY5Y human neuroblastoma cell line, a relevant in vitro cell model for investigating neurotoxicology in human: Focus on organic pollutants. Neurotoxicology 2022, 92, 131–155. [Google Scholar] [CrossRef] [PubMed]
- Tsialtas, I.; Georgantopoulos, A.; Karipidou, M.E.; Kalousi, F.D.; Karra, A.G.; Leonidas, D.D.; Psarra, A.M.G. Anti-Apoptotic and Antioxidant Activities of the Mitochondrial Estrogen Receptor Beta in N2A Neuroblastoma Cells. Int. J. Mol. Sci. 2021, 22, 7620. [Google Scholar] [CrossRef]
- Psarra, A.M.G.; Sekeris, C.E. Glucocorticoids induce mitochondrial gene transcription in HepG2 cells Role of the mitochondrial glucocorticoid receptor. BBA-Mol. Cell Res. 2011, 1813, 1814–1821. [Google Scholar] [CrossRef]
- Tsialtas, I.; Gorgogietas, V.A.; Michalopoulou, M.; Komninou, A.; Liakou, E.; Georgantopoulos, A.; Kalousi, F.D.; Karra, A.G.; Protopapa, E.; Psarra, A.G. Neurotoxic effects of aluminum are associated with its interference with estrogen receptors signaling. Neurotoxicology 2020, 77, 114–126. [Google Scholar] [CrossRef] [PubMed]
- Sivandzade, F.; Bhalerao, A.; Cucullo, L. Analysis of the Mitochondrial Membrane Potential Using the Cationic JC-1 Dye as a Sensitive Fluorescent Probe. Bio-Protocol 2019, 9, e3128. [Google Scholar] [CrossRef]
- Pisha, E.; Chai, H.; Lee, I.-S.; Chagwedera, T.E.; Farnsworth, N.R.; Cordell, G.A.; Beecher, C.W.; Fong, H.H.; Kinghorn, A.D.; Brown, D.M.; et al. Discovery of betulinic acid as a selective inhibitor of human melanoma that functions by induction of apoptosis. Nat. Med. 1995, 1, 1046–1051. [Google Scholar] [CrossRef] [PubMed]
- Lou, H.; Li, H.; Zhang, S.; Lu, H.; Chen, Q. A Review on Preparation of Betulinic Acid and Its Biological Activities. Molecules 2021, 26, 5583. [Google Scholar] [CrossRef] [PubMed]
- Armah, F.A.; Annan, K.; Mensah, A.Y.; Amponsah, I.K.; Tocher, D.A.; Habtemariam, S. Erythroivorensin: A novel anti-inflammatory diterpene from the root-bark of Erythrophleum ivorense (A Chev.). Fitoterapia 2015, 105, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Quan, H.Y.; Jeong, K.J.; Kim, D.Y.; Kim, G.W.; Jo, H.K.; Chung, S.H. Beneficial Effect of Betulinic Acid on Hyperglycemia via Suppression of Hepatic Glucose Production. J. Agric. Food Chem. 2013, 62, 434–442. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.J.; Lee, Y.J.; Han, B.H.; Choi, E.S.; Kho, M.C.; Park, J.H.; Ahn, Y.M.; Kim, H.Y.; Kang, D.G.; Lee, H.S. Protective effect of betulinic acid on early atherosclerosis in diabetic apolipoprotein-E gene knockout mice. Eur. J. Pharmacol. 2017, 796, 224–232. [Google Scholar] [CrossRef]
- Gai, H.; Zhou, F.; Zhang, Y.; Ai, J.; Zhan, J.; You, Y.; Huang, W. Coniferaldehyde ameliorates the lipid and glucose metabolism in palmitic acid-induced HepG2 cells via the LKB1/AMPK signaling pathway. J. Food Sci. 2020, 85, 4050–4060. [Google Scholar] [CrossRef] [PubMed]
- Jeong, N.-H.; Lee, S.; Choi, Y.-A.; Song, K.-S.; Kim, S.-H. Inhibitory Effects of Euscaphic Acid in the Atopic Dermatitis Model by Reducing Skin Inflammation and Intense Pruritus. Inflammation 2022, 45, 1680–1691. [Google Scholar] [CrossRef] [PubMed]
- Jayaprakasha, G.K.; Rao, L.J.; Sakariah, K.K. Antioxidant activities of flavidin in different in vitro model systems. Bioorganic Med. Chem. 2004, 12, 5141–5146. [Google Scholar] [CrossRef]
- Wang, K.-L.; Yu, Y.-C.; Hsia, S.-M. Perspectives on the Role of Isoliquiritigenin in Cancer. Cancers 2021, 13, 115. [Google Scholar] [CrossRef]
- Hirchaud, F.; Hermetet, F.; Ablise, M.; Fauconnet, S.; Vuitton, D.A.; Prétet, J.-L.; Mougin, C. Isoliquiritigenin Induces Caspase-Dependent Apoptosis via Downregulation of HPV16 E6 Expression in Cervical Cancer Ca Ski Cells. Planta Medica 2013, 79, 1628–1635. [Google Scholar] [CrossRef]
- Caporali, S.; De Stefano, A.; Calabrese, C.; Giovannelli, A.; Pieri, M.; Savini, I.; Tesauro, M.; Bernardini, S.; Minieri, M.; Terrinoni, A. Anti-Inflammatory and Active Biological Properties of the Plant-Derived Bioactive Compounds Luteolin and Luteolin 7-Glucoside. Nutrients 2022, 14, 1155. [Google Scholar] [CrossRef] [PubMed]
- Samec, M.; Liskova, A.; Koklesova, L.; Samuel, S.M.; Zhai, K.; Buhrmann, C.; Varghese, E.; Abotaleb, M.; Qaradakhi, T.; Zulli, A.; et al. Flavonoids against the Warburg phenotype—Concepts of predictive, preventive and personalised medicine to cut the Gordian knot of cancer cell metabolism. EPMA J. 2020, 11, 377–398. [Google Scholar] [CrossRef] [PubMed]
- Kang, K.A.; Piao, M.J.; Ryu, Y.S.; Hyun, Y.J.; Park, J.E.; Shilnikova, K.; Zhen, A.X.; Kang, H.K.; Koh, Y.S.; Jeong, Y.J.; et al. Luteolin induces apoptotic cell death via antioxidant activity in human colon cancer cells. Int. J. Oncol. 2017, 51, 1169–1178. [Google Scholar] [CrossRef] [PubMed]
- Junior, R.F.d.A.; Eich, C.; Jorquera, C.; Schomann, T.; Baldazzi, F.; Chan, A.B.; Cruz, L.J. Ceramide and palmitic acid inhibit macrophage-mediated epithelial–mesenchymal transition in colorectal cancer. Mol. Cell. Biochem. 2020, 468, 153–168. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Luo, H.; Zhang, N.; Wang, Y.; Li, Y.; Huang, H.; Liu, Y.; Hu, Y.; Liu, H.; Zhang, J.; et al. Loss of p53 Sensitizes Cells to Palmitic Acid-Induced Apoptosis by Reactive Oxygen Species Accumulation. Int. J. Mol. Sci. 2019, 20, 6268. [Google Scholar] [CrossRef] [PubMed]
- Mancini, A.; Imperlini, E.; Nigro, E.; Montagnese, C.; Daniele, A.; Orrù, S.; Buono, P. Biological and Nutritional Properties of Palm Oil and Palmitic Acid: Effects on Health. Molecules 2015, 20, 17339–17361. [Google Scholar] [CrossRef]
- Innis, S.M. Palmitic Acid in Early Human Development. Crit. Rev. Food Sci. Nutr. 2015, 56, 1952–1959. [Google Scholar] [CrossRef]
- Acosta-Montaño, P.; Rodríguez-Velázquez, E.; Ibarra-López, E.; Frayde-Gómez, H.; Mas-Oliva, J.; Delgado-Coello, B.; Rivero, I.A.; Alatorre-Meda, M.; Aguilera, J.; Guevara-Olaya, L.; et al. Fatty Acid and Lipopolysaccharide Effect on Beta Cells Proteostasis and its Impact on Insulin Se-cretion. Cells 2019, 8, 884. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Xiao, M.; Mo, Y.; Wang, H.; Han, Y.; Zhao, X.; Yang, X.; Liu, Z.; Xu, B. Emerging roles of protein palmitoylation and its modifying enzymes in cancer cell signal transduction and cancer therapy. Int. J. Biol. Sci. 2022, 18, 3447–3457. [Google Scholar] [CrossRef] [PubMed]
- Librán-Pérez, M.; Pereiro, P.; Figueras, A.; Novoa, B. Antiviral activity of palmitic acid via autophagic flux inhibition in zebrafish (Danio rerio). Fish Shellfish. Immunol. 2019, 95, 595–605. [Google Scholar] [CrossRef]
- Syed, I.; Lee, J.; Moraes-Vieira, P.M.; Donaldson, C.J.; Sontheimer, A.; Aryal, P.; Wellenstein, K.; Kolar, M.J.; Nelson, A.T.; Siegel, D.; et al. Palmitic Acid Hydroxystearic Acids Activate GPR40, Which Is Involved in Their Beneficial Effects on Glucose Homeostasis. Cell Metab. 2018, 27, 419–427. [Google Scholar] [CrossRef]
- Hammarstedt, A.; Syed, I.; Vijayakumar, A.; Eliasson, B.; Gogg, S.; Kahn, B.B.; Smith, U. Adipose tissue dysfunction is associated with low levels of the novel Palmitic Acid Hydroxystearic Acids. Sci. Rep. 2018, 8, 15757. [Google Scholar] [CrossRef] [PubMed]
- Yore, M.M.; Syed, I.; Moraes-Vieira, P.M.; Zhang, T.; Herman, M.A.; Homan, E.A.; Patel, R.T.; Lee, J.; Chen, S.; Peroni, O.D.; et al. Discovery of a Class of Endogenous Mammalian Lipids with Anti-Diabetic and Anti-inflammatory Effects. Cell 2014, 159, 318–332. [Google Scholar] [CrossRef]
- Khan, A.A.; Alanazi, A.M.; Jabeen, M.; Chauhan, A.; Abdelhameed, A.S. Design, synthesis and in vitro anticancer evaluation of a stearic acid-based ester conjugate. Anticancer Res. 2013, 33, 2517–2524. [Google Scholar]
- Fekete, K.; Györei, E.; Lohner, S.; Verduci, E.; Agostoni, C.; Decsi, T. Long-chain polyunsaturated fatty acid status in obesity: A systematic review and meta-analysis. Obes. Rev. 2015, 16, 488–497. [Google Scholar] [CrossRef]
- Hodson, L.; Skeaff, C.M.; Fielding, B.A. Fatty acid composition of adipose tissue and blood in humans and its use as a biomarker of dietary intake. Prog. Lipid Res. 2008, 47, 348–380. [Google Scholar] [CrossRef]
- Ren, J.; Chung, S.H. Anti-inflammatory effect of alpha-linolenic acid and its mode of action through the inhibition of nitric oxide production and inducible nitric oxide synthase gene expression via NF-kappaB and mitogen-activated protein kinase pathways. J. Agric. Food Chem. 2007, 55, 5073–5080. [Google Scholar] [CrossRef]
- Fan, N.; Fusco, J.L.; Rosenberg, D.W. Antioxidant and Anti-Inflammatory Properties of Walnut Constituents: Focus on Personalized Cancer Prevention and the Microbiome. Antioxidants 2023, 12, 982. [Google Scholar] [CrossRef]
- Yan, H.; Zhang, S.; Yang, L.; Jiang, M.; Xin, Y.; Liao, X.; Li, Y.; Lu, J. The Antitumor Effects of α-Linolenic Acid. J. Pers. Med. 2024, 14, 260. [Google Scholar] [CrossRef] [PubMed]
- Azrad, M.; Turgeon, C.; Demark-Wahnefried, W. Current Evidence Linking Polyunsaturated Fatty Acids with Cancer Risk and Progression. Front. Oncol. 2013, 3, 224. [Google Scholar] [CrossRef] [PubMed]
- Vieira, C.; Evangelista, S.; Cirillo, R.; Lippi, A.; Maggi, C.A.; Manzini, S. Effect of ricinoleic acid in acute and subchronic experimental models of inflammation. Mediat. Inflamm. 2000, 9, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Pabiś, S.; Kula, J. Synthesis and Bioactivity of (R)-Ricinoleic Acid Derivatives: A Review. Curr. Med. Chem. 2016, 23, 4037–4056. [Google Scholar] [CrossRef]
- Minto, R.E.; Blacklock, B.J. Biosynthesis and function of polyacetylenes and allied natural products. Prog. Lipid Res. 2008, 47, 233–306. [Google Scholar] [CrossRef]
- Xu, T.; Tripathi, S.K.; Feng, Q.; Lorenz, M.C.; Wright, M.A.; Jacob, M.R.; Mask, M.M.; Baerson, S.R.; Li, X.-C.; Clark, A.M.; et al. A Potent Plant-Derived Antifungal Acetylenic Acid Mediates Its Activity by Interfering with Fatty Acid Homeostasis. Antimicrob. Agents Chemother. 2012, 56, 2894–2907. [Google Scholar] [CrossRef]
- Rengachar, P.; Bhatt, A.N.; Polavarapu, S.; Veeramani, S.; Krishnan, A.; Sadananda, M.; Das, U.N. Gamma-Linolenic Acid (GLA) Protects against Ionizing Radiation-Induced Damage: An In Vitro and In Vivo Study. Biomolecules 2022, 12, 797. [Google Scholar] [CrossRef]
- Das, U.N.; Rao, K.P. Effect of γ-linolenic acid and prostaglandins E1 on gamma-radiation and chemical-induced genetic damage to the bone marrow cells of mice. Prostaglandins Leukot. Essent. Fat. Acids 2006, 74, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Leaver, H.A.; Wharton, S.B.; Bell, H.S.; Leaver-Yap, I.M.M.; Whittle, I. Highly unsaturated fatty acid induced tumour regression in glioma pharmacodynamics and bioavaila-bility of gamma linolenic acid in an implantation glioma model: Effects on tumour biomass, apoptosis and neuronal tissue histology. Prostaglandins Leukot Essent Fatty Acids 2002, 67, 283–292. [Google Scholar] [CrossRef]
- Das, U.N. Gamma-linolenic acid therapy of human glioma-a review of in vitro, in vivo, and clinical studies. Med. Sci. Monit. 2007, 13, RA119–RA131. [Google Scholar]
- Miyake, J.A.; Gomes, R.N.; Colquhoun, A. Gamma-Linolenic acid alters migration, proliferation and apoptosis in human and rat glioblastoma cells. Prostaglandins Other Lipid Mediat. 2020, 150, 106452. [Google Scholar] [CrossRef]
- Miyake, J.A.; Benadiba, M.; Colquhoun, A. Gamma-linolenic acid inhibits both tumour cell cycle progression and angi-ogenesis in the orthotopic C6 glioma model through changes in VEGF, Flt1, ERK1/2, MMP2, cyclin D1, pRb, p53 and p27 protein expression. Lipids Health Dis. 2009, 8. [Google Scholar] [CrossRef]
- Oepen, K.; Özbek, H.; Schüffler, A.; Liermann, J.C.; Thines, E.; Schneider, D. Myristic Acid Inhibits the Activity of the Bacterial ABC Transporter BmrA. Int. J. Mol. Sci. 2021, 22, 13565. [Google Scholar] [CrossRef] [PubMed]
- Contreras, C.M.; Rodríguez-Landa, J.F.; García-Ríos, R.I.; Cueto-Escobedo, J.; Guillen-Ruiz, G.; Bernal-Morales, B. Myristic Acid Produces Anxiolytic-Like Effects in Wistar Rats in the Elevated Plus Maze. Bio. Med. Res. Int. 2014, 2014, 1–8. [Google Scholar] [CrossRef]
- Javid, S.; Ather, H.; Hani, U.; Siddiqua, A.; Ansari, S.M.A.; Shanmugarajan, D.; Kumar, H.Y.; Arivuselvam, R.; Purohit, M.N.; Kumar, B.R.P. Discovery of Novel Myristic Acid Derivatives as N-Myristoyltransferase Inhibitors: Design, Synthesis, Analysis, Computational Studies and Antifungal Activity. Antibiotics 2023, 12, 1167. [Google Scholar] [CrossRef]
- Prasath, K.G.; Alexpandi, R.; Parasuraman, R.; Pavithra, M.; Ravi, A.V.; Pandian, S.K. Anti-inflammatory potential of myristic acid and palmitic acid synergism against systemic candidiasis in Danio rerio (Zebrafish). Biomed. Pharmacother. 2020, 133, 111043. [Google Scholar] [CrossRef]
- Alonso-Castro, A.J.; Serrano-Vega, R.; Gutiérrez, S.P.; Isiordia-Espinoza, M.A.; Solorio-Alvarado, C.R. Myristic acid reduces skin inflammation and nociception. J. Food Biochem. 2021, 46, e14013. [Google Scholar] [CrossRef]
- Pompéia, C.; Lopes, L.; Miyasaka, C.; Procópio, J.; Sannomiya, P.; Curi, R. Effect of fatty acids on leukocyte function. Braz. J. Med Biol. Res. 2000, 33, 1255–1268. [Google Scholar] [CrossRef]
- Brash, A.R. Arachidonic acid as a bioactive molecule. J. Clin. Investig. 2001, 107, 1339–1345. [Google Scholar] [CrossRef] [PubMed]
- Tallima, H.; El Ridi, R. Arachidonic acid: Physiological roles and potential health benefits—A review. J. Adv. Res. 2018, 11, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Tokuda, H.; Kontani, M.; Kawashima, H.; Kiso, Y.; Shibata, H.; Osumi, N. Differential effect of arachidonic acid and docosahexaenoic acid on age-related decreases in hippocampal neurogenesis. Neurosci. Res. 2014, 88, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Fukaya, T.; Gondaira, T.; Kashiyae, Y.; Kotani, S.; Ishikura, Y.; Fujikawa, S.; Kiso, Y.; Sakakibara, M. Arachidonic acid preserves hippocampal neuron membrane fluidity in senescent rats. Neurobiol. Aging 2007, 28, 1179–1186. [Google Scholar] [CrossRef] [PubMed]
- Antollini, S.S.; Barrantes, F.J. Fatty Acid Regulation of Voltage- and Ligand-Gated Ion Channel Function. Front. Physiol. 2016, 7, 573. [Google Scholar] [CrossRef]
- Wang, Z.-J.; Liang, C.-L.; Li, G.-M.; Yu, C.-Y.; Yin, M. Neuroprotective effects of arachidonic acid against oxidative stress on rat hippocampal slices. Chem. Interactions 2006, 163, 207–217. [Google Scholar] [CrossRef]
- Pérez, R.; Matabosch, X.; Llebaria, A.; Balboa, M.A.; Balsinde, J. Blockade of arachidonic acid incorporation into phospholipids induces apoptosis in U937 promonocytic cells. J. Lipid Res. 2006, 47, 484–491. [Google Scholar] [CrossRef]
- Trostchansky, A.; Wood, I.; Rubbo, H. Regulation of arachidonic acid oxidation and metabolism by lipid electrophiles. Prostaglandins Other Lipid Mediat. 2020, 152, 106482. [Google Scholar] [CrossRef]
- Trostchansky, A.; Rubbo, H. Anti-inflammatory signaling actions of electrophilic nitro-arachidonic acid in vascular cells and astrocytes. Arch. Biochem. Biophys. 2017, 617, 155–161. [Google Scholar] [CrossRef]
- Sala, A.; Proschak, E.; Steinhilber, D.; Rovati, G.E. Two-pronged approach to anti-inflammatory therapy through the modulation of the arachidonic acid cascade. Biochem. Pharmacol. 2018, 158, 161–173. [Google Scholar] [CrossRef]
- Wang, T.; Fu, X.; Chen, Q.; Patra, J.K.; Wang, D.; Wang, Z.; Gai, Z. Arachidonic Acid Metabolism and Kidney Inflammation. Int. J. Mol. Sci. 2019, 20, 3683. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wei, X.; Xiao, X.; Hui, R.; Card, J.W.; Carey, M.A.; Wang, D.W.; Zeldin, D.C. Arachidonic acid epoxygenase metabolites stimulate endothelial cell growth and angiogenesis via mito-gen-activated protein kinase and phosphatidylinositol 3-kinase/Akt signaling pathways. J. Pharmacol. Exp. Ther. 2005, 314, 522–532. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Liu, J.-Y.; Qiu, H.; Harris, T.R.; Sirish, P.; Hammock, B.D.; Chiamvimonvat, N. Use of Metabolomic Profiling in the Study of Arachidonic Acid Metabolism in Cardiovascular Disease. Congest. Hear. Fail. 2011, 17, 42–46. [Google Scholar] [CrossRef] [PubMed]
- Sonnweber, T.; Pizzini, A.; Nairz, M.; Weiss, G.; Tancevski, I. Arachidonic Acid Metabolites in Cardiovascular and Metabolic Diseases. Int. J. Mol. Sci. 2018, 19, 3285. [Google Scholar] [CrossRef] [PubMed]
- Tavolari, S.; Bonafe, M.; Marini, M.; Ferreri, C.; Bartolini, G.; Brighenti, E.; Manara, S.; Tomasi, V.; Laufer, S.; Guarnieri, T. Licofelone, a dual COX/5-LOX inhibitor, induces apoptosis in HCA-7 colon cancer cells through the mi-tochondrial pathway independently from its ability to affect the arachidonic acid cascade. Carcinogenesis 2008, 29, 371–380. [Google Scholar] [CrossRef] [PubMed]
- Colombero, C.; Cárdenas, S.; Venara, M.; Martin, A.; Pennisi, P.; Barontini, M.; Nowicki, S. Cytochrome 450 metabolites of arachidonic acid (20-HETE, 11,12-EET and 14,15-EET) promote pheo-chromocytoma cell growth and tumor associated angiogenesis. Biochimie 2020, 171, 147–157. [Google Scholar] [CrossRef]
- Shao, J.; Wang, H.; Yuan, G.; Chen, Z.; Li, Q. Involvement of the arachidonic acid cytochrome P450 epoxygenase pathway in the proliferation and invasion of human multiple myeloma cells. Peer.J. 2016, 4, e1925. [Google Scholar] [CrossRef][Green Version]
- Pozzi, A.; Popescu, V.; Yang, S.; Mei, S.; Shi, M.; Puolitaival, S.M.; Caprioli, R.M.; Capdevila, J.H. The Anti-tumorigenic Properties of Peroxisomal Proliferator-activated Receptor α Are Arachidonic Acid Epoxygenase-mediated. J. Biol. Chem. 2010, 285, 12840–12850. [Google Scholar] [CrossRef]
- Venn-Watson, S.; Lumpkin, R.; Dennis, E.A. Efficacy of dietary odd-chain saturated fatty acid pentadecanoic acid parallels broad associated health benefits in humans: Could it be essential? Sci. Rep. 2020, 10, 8161. [Google Scholar] [CrossRef]
- Shafaghat, A. Antioxidant, Antimicrobial Activities and Fatty Acid Components of Flower, Leaf, Stem and Seed of Hypericum scabrum. Nat. Prod. Commun. 2011, 6, 1739–1742. [Google Scholar] [CrossRef]
- Singh, D.; Mehghini, P.; Rodriguez-Palacios, A.; Di Martino, L.; Cominelli, F.; Basson, A.R. Anti-Inflammatory Effect of Dietary Pentadecanoic Fatty Acid Supplementation on Inflammatory Bowel Disease in SAMP1/YitFc Mice. Nutrients 2024, 16, 3031. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ye, X.-L.; Liu, R.; Chen, H.-L.; Bai, H.; Liang, X.; Zhang, X.-D.; Wang, Z.; Li, W.-L.; Hai, C.-X. Antioxidant activities of oleanolic acid in vitro: Possible role of Nrf2 and MAP kinases. Chem. Biol. Interact. 2010, 184, 328–337. [Google Scholar] [CrossRef]
- Wang, X.; Liu, R.; Zhang, W.; Zhang, X.; Liao, N.; Wang, Z.; Li, W.; Qin, X.; Hai, C. Oleanolic acid improves hepatic insulin resistance via antioxidant, hypolipidemic and anti-inflammatory effects. Mol. Cell. Endocrinol. 2013, 376, 70–80. [Google Scholar] [CrossRef]
- Dzubak, P.; Hajduch, M.; Vydra, D.; Hustova, A.; Kvasnica, M.; Biedermann, D.; Markova, L.; Urban, M.; Sarek, J. Pharmacological activities of natural triterpenoids and their therapeutic implications. Nat. Prod. Rep. 2006, 23, 394–411. [Google Scholar] [CrossRef]
- Allouche, Y.; Warleta, F.; Campos, M.; Sánchez-Quesada, C.; Uceda, M.; Beltrán, G.; Gaforio, J.J. Antioxidant, Antiproliferative, and Pro-apoptotic Capacities of Pentacyclic Triterpenes Found in the Skin of Olives on MCF-7 Human Breast Cancer Cells and Their Effects on DNA Damage. J. Agric. Food Chem. 2010, 59, 121–130. [Google Scholar] [CrossRef]
- Du, Y.; Ko, K.M. Oleanolic Acid Protects against Myocardial Ischemia-Reperfusion Injury by Enhancing Mitochondrial Antioxidant Mechanism Mediated by Glutathione and α-Tocopherol in Rats. Planta Medica 2005, 72, 222–227. [Google Scholar] [CrossRef]
- Tang, Z.Y.; Li, Y.; Tang, Y.T.; Ma, X.D.; Tang, Z.Y. Anticancer activity of oleanolic acid and its derivatives: Recent advances in evidence, target profiling and mechanisms of action. Biomed. Pharmacother. 2022, 145, 112397. [Google Scholar] [CrossRef]
- Gupta, S.; Kalani, K.; Saxena, M.; Srivastava, S.K.; Agrawal, S.K.; Suri, N.; Saxena, A.K. Cytotoxic Evaluation of Semisynthetic Ester and Amide Derivatives of Oleanolic Acid. Nat. Prod. Commun. 2010, 5, 1567–1570. [Google Scholar] [CrossRef]
- Roohbakhsh, A.; Parhiz, H.; Soltani, F.; Rezaee, R.; Iranshahi, M. Molecular mechanisms behind the biological effects of hesperidin and hesperetin for the prevention of cancer and cardiovascular diseases. Life Sci. 2015, 124, 64–74. [Google Scholar] [CrossRef]
- Parhiz, H.; Roohbakhsh, A.; Soltani, F.; Rezaee, R.; Iranshahi, M. Antioxidant and Anti-Inflammatory Properties of the Citrus Flavonoids Hesperidin and Hesperetin: An Updated Review of their Molecular Mechanisms and Experimental Models. Phytotherapy Res. 2014, 29, 323–331. [Google Scholar] [CrossRef]
- de Oliveira, J.M.P.F.; Santos, C.; Fernandes, E. Therapeutic potential of hesperidin and its aglycone hesperetin: Cell cycle regulation and apoptosis induction in cancer models. Phytomedicine. 2019, 73, 152887. [Google Scholar] [CrossRef]
- Fontana, A.; Spolaore, B.; de Laureto, P.P. The biological activities of protein/oleic acid complexes reside in the fatty acid. Biochim. Biophys. Acta (BBA)-Proteins Proteom. 2013, 1834, 1125–1143. [Google Scholar] [CrossRef] [PubMed]
- Carrillo, C.; Cavia Camarero, M.D.M.; Alonso-Torre, S.R. Antitumor effect of oleic acid; mechanisms of action: A review. Nutr. Hosp. 2012, 27, 1860–1865. [Google Scholar] [CrossRef]
- Carrillo, C.; Cavia Mdel, M.; Alonso-Torre, S. Role of oleic acid in immune system; mechanism of action; a review. Nutr. Hosp. 2012, 27, 978–990. [Google Scholar] [CrossRef]
- Masner, M.; Lujea, N.; Bisbal, M.; Acosta, C.; Kunda, P. Linoleic and oleic acids enhance cell migration by altering the dynamics of microtubules and the remodeling of the actin cytoskeleton at the leading edge. Sci. Rep. 2021, 11, 14984. [Google Scholar] [CrossRef]
- Benjamin, S.; Spener, F. Conjugated linoleic acids as functional food: An insight into their health benefits. Nutr. Metab. 2009, 6, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Kolar, M.J.; Konduri, S.; Chang, T.; Wang, H.; McNerlin, C.; Ohlsson, L.; Härröd, M.; Siegel, D.; Saghatelian, A. Linoleic acid esters of hydroxy linoleic acids are anti-inflammatory lipids found in plants and mammals. J. Biol. Chem. 2019, 294, 10698–10707. [Google Scholar] [CrossRef]
- Cahoon, E.B.; Li-Beisson, Y. Plant unusual fatty acids: Learning from the less common. Curr. Opin. Plant Biol. 2020, 55, 66–73. [Google Scholar] [CrossRef]
- Scott, S.; Cahoon, E.B.; Busta, L. Variation on a theme: The structures and biosynthesis of specialized fatty acid natural products in plants. Plant J. 2022, 111, 954–965. [Google Scholar] [CrossRef]
- Lin, W.-C.; Hsu, K.-C.; You, M.-F.; Lee, K.-H.; Chi, C.-H.; Chen, J.-Y. Octanoic acid promotes clearance of antibiotic-tolerant cells and eradicates biofilms of Staphylococcus aureus isolated from recurrent bovine mastitis. Biofilm 2023, 6, 100149. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Dolan, H.L.; Ding, Q.; Wang, S.; Tikekar, R.V. Antimicrobial action of octanoic acid against Escherichia coli O157:H7 during washing of baby spinach and grape tomatoes. Food Res. Int. 2019, 125, 108523. [Google Scholar] [CrossRef]
- Mączka, W.; Duda-Madej, A.; Grabarczyk, M.; Wińska, K. Natural Compounds in the Battle against Microorganisms—Linalool. Molecules 2022, 27, 6928. [Google Scholar] [CrossRef] [PubMed]
- Pereira, I.; Severino, P.; Santos, A.C.; Silva, A.M.; Souto, E.B. Linalool Bioactive Properties and Potential Applicability in Drug Delivery Systems. Colloid Surface Biointerface 2018, 171, 566–578. [Google Scholar] [CrossRef]
- Liu, X.; Cai, J.; Chen, H.; Zhong, Q.; Hou, Y.; Chen, W.; Chen, W. Antibacterial activity and mechanism of linalool against Pseudomonas aeruginosa. Microb. Pathog. 2020, 141, 103980. [Google Scholar] [CrossRef]
- Elbe, H.; Ozturk, F.; Yigitturk, G.; Baygar, T.; Cavusoglu, T. Anticancer activity of linalool: Comparative investigation of ultrastructural changes and apoptosis in breast cancer cells. Ultrastruct. Pathol. 2022, 46, 348–358. [Google Scholar] [CrossRef]
- Zielińska-Błajet, M.; Feder-Kubis, J. Monoterpenes and Their Derivatives—Recent Development in Biological and Medical Applications. Int. J. Mol. Sci. 2020, 21, 7078. [Google Scholar] [CrossRef]
- Koziol, A.; Stryjewska, A.; Librowski, T.; Salat, K.; Gawel, M.; Moniczewski, A.; Lochynski, S. An Overview of the Pharmacological Properties and Potential Applications of Natural Monoterpenes. Mini-Reviews Med. Chem. 2015, 14, 1156–1168. [Google Scholar] [CrossRef]
- Khusnutdinova, E.F.; Sinou, V.; Babkov, D.A.; Kazakova, O.; Brunel, J.M. Development of New Antimicrobial Oleanonic Acid Polyamine Conjugates. Antibiotics 2022, 11, 94. [Google Scholar] [CrossRef]
- Castellano, J.M.; Ramos-Romero, S.; Perona, J.S. Oleanolic Acid: Extraction, Characterization and Biological Activity. Nutrients 2022, 14, 623. [Google Scholar] [CrossRef]
- Vuorinen, A.; Seibert, J.; Papageorgiou, V.P.; Rollinger, J.M.; Odermatt, A.; Schuster, D.; Assimopoulou, A.N. Pistacia lentiscus Oleoresin: Virtual Screening and Identification of Masticadienonic and Isomasticadi-enonic Acids as Inhibitors of 11beta-Hydroxysteroid Dehydrogenase 1. Planta Med. 2015, 81, 525–532. [Google Scholar] [CrossRef]
- Meng, Q.; Li, J.; Wang, C.; Shan, A. Biological function of resveratrol and its application in animal production: A review. J. Anim. Sci. Biotechnol. 2023, 14, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Bhat, K.P.L.; Kosmeder, J.W., 2nd; Pezzuto, J.M. Biological effects of resveratrol. Antioxid. Redox Signal 2001, 3, 1041–1064. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Jia, Y.; Ren, F. Multidimensional biological activities of resveratrol and its prospects and challenges in the health field. Front. Nutr. 2024, 11, 1408651. [Google Scholar] [CrossRef]
- Santos, J.A.; de Carvaho, G.S.; Oliveira, V.; Raposo, N.R.; da Silva, A.D. Resveratrol and Analogues: A Review of Antioxidant Activity and Applications to Human Health. Recent Patents Food Nutr. Agric. 2013, 5, 144–153. [Google Scholar] [CrossRef] [PubMed]
- Płowuszyńska, A.; Gliszczyńska, A. Recent Developments in Therapeutic and Nutraceutical Applications of p-Methoxycinnamic Acid from Plant Origin. Molecules 2021, 26, 3827. [Google Scholar] [CrossRef]
- Adisakwattana, S. Cinnamic Acid and Its Derivatives: Mechanisms for Prevention and Management of Diabetes and Its Complications. Nutrients 2017, 9, 163. [Google Scholar] [CrossRef] [PubMed]
- Yoon, B.K.; Jackman, J.A.; Valle-González, E.R.; Cho, N.-J. Antibacterial Free Fatty Acids and Monoglycerides: Biological Activities, Experimental Testing, and Therapeutic Applications. Int. J. Mol. Sci. 2018, 19, 1114. [Google Scholar] [CrossRef]
- Kim, J.J.; Kim, H.K. Antioxidant and Antibacterial Activity of Caprylic Acid Vanillyl Ester Produced by Lipase-Mediated Transesterification. J. Microbiol. Biotechnol. 2020, 31, 317–326. [Google Scholar] [CrossRef]
- Altinoz, M.A.; Ozpinar, A.; Seyfried, T.N. Seyfried, Caprylic (Octanoic) Acid as a Potential Fatty Acid Chemotherapeutic for Glio-blastoma. Prostaglandins Leukot Essent Fatty Acids 2020, 159, 102142. [Google Scholar] [CrossRef]
- Okere, I.C.; McElfresh, T.A.; Brunengraber, D.Z.; Martini, W.; Sterk, J.P.; Huang, H.; Chandler, M.P.; Brunengraber, H.; Stanley, W.C. Differential effects of heptanoate and hexanoate on myocardial citric acid cycle intermediates following ischemia-reperfusion. J. Appl. Physiol. 2006, 100, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.B.; Alimova, Y.; Myers, T.M.; Ebersole, J.L. Short- and medium-chain fatty acids exhibit antimicrobial activity for oral microorganisms. Arch. Oral Biol. 2011, 56, 650–654. [Google Scholar] [CrossRef]
- Wisetsai, A.; Lekphrom, R.; Bua-art, S.; Suebrasri, T.; Boonlue, S.; Tontapha, S.; Amornkitbamrung, V.; Senawong, T.; Schevenels, F.T. Scalarane Sesterterpenoids with Antibacterial and Anti-Proliferative Activities from the Mushroom Ne-onothopanus nambi. Molecules 2021, 26, 7667. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, Y. Targeting cancer with sesterterpenoids: The new potential antitumor drugs. J. Nat. Med. 2015, 69, 255–266. [Google Scholar] [CrossRef]
- Jarboe, L.R.; Royce, L.A.; Liu, P. Understanding biocatalyst inhibition by carboxylic acids. Front. Microbiol. 2013, 4, 272. [Google Scholar] [CrossRef] [PubMed]
- Kallscheuer, N.; Polen, T.; Bott, M.; Marienhagen, J. Reversal of beta-oxidative pathways for the microbial production of chemicals and polymer building blocks. Metab. Eng. 2017, 42, 33–42. [Google Scholar] [CrossRef]
- Zhang, B.; Du, H.; Sun, M.; Wu, X.; Li, Y.; Wang, Z.; Xiao, Y.; Peng, F. Comparison of lauric acid and 12-hydroxylauric acid in the alleviation of drought stress in peach (Prunus persica (L.) Batsch). Front. Plant Sci. 2022, 13, 1025569. [Google Scholar] [CrossRef] [PubMed]
- Deen, A.; Visvanathan, R.; Wickramarachchi, D.; Marikkar, N.; Nammi, S.; Jayawardana, B.C.; Liyanage, R. Chemical composition and health benefits of coconut oil: An overview. J. Sci. Food Agric. 2020, 101, 2182–2193. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.; Gao, W.; Ge, T.; Tan, X.; Wang, J.; Liu, H.; Wang, Y.; Han, C.; Xu, Q.; Wang, Q. Lauric Acid Is a Potent Biological Control Agent That Damages the Cell Membrane of Phytophthora sojae. Front. Microbiol. 2021, 12. [Google Scholar] [CrossRef] [PubMed]
- Takagi, T.; Fujiwara-Tani, R.; Mori, S.; Kishi, S.; Nishiguchi, Y.; Sasaki, T.; Ogata, R.; Ikemoto, A.; Sasaki, R.; Ohmori, H.; et al. Lauric Acid Overcomes Hypoxia-Induced Gemcitabine Chemoresistance in Pancreatic Ductal Adenocarci-noma. Int. J. Mol. Sci. 2023, 24, 7506. [Google Scholar] [CrossRef]
- Aihara, K.-I.; Azuma, H.; Akaike, M.; Ikeda, Y.; Yamashita, M.; Sudo, T.; Hayashi, H.; Yamada, Y.; Endoh, F.; Fujimura, M.; et al. Disruption of Nuclear Vitamin D Receptor Gene Causes Enhanced Thrombogenicity in Mice. J. Biol. Chem. 2004, 279, 35798–35802. [Google Scholar] [CrossRef] [PubMed]
- Pilz, S.; Tomaschitz, A.; Drechsler, C.; Zittermann, A.; Dekker, J.M.; Marz, W. Vitamin D Supplementation: A Promising Approach for the Prevention and Treatment of Strokes. Curr. Drug Targets 2011, 12, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.J.; Pencina, M.J.; Booth, S.L.; Jacques, P.F.; Ingelsson, E.; Lanier, K.; Benjamin, E.J.; D’Agostino, R.B.; Wolf, M.; Vasan, R.S. Vitamin D Deficiency and Risk of Cardiovascular Disease. Circulation 2008, 117, 503–511. [Google Scholar] [CrossRef]
- Pilkey, N.G.; Novosel, O.; Roy, A.; Wilson, T.E.; Sharma, J.; Khan, S.; Kapuria, S.; Adams, M.A.; Holden, R.M. Does Native Vitamin D Supplementation Have Pleiotropic Effects in Patients with End-Stage Kidney Disease? A Systematic Review of Randomized Trials. Nutrients 2023, 15, 3072. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.D.; Prykhodko, O.; Hållenius, F.F.; Nyman, M. Monovalerin and trivalerin increase brain acetic acid, decrease liver succinic acid, and alter gut microbiota in rats fed high-fat diets. Eur. J. Nutr. 2018, 58, 1545–1560. [Google Scholar] [CrossRef]
- Nguyen, T.D.; Hållenius, F.F.; Lin, X.; Nyman, M.; Prykhodko, O. Monobutyrin and Monovalerin Affect Brain Short-Chain Fatty Acid Profiles and Tight-Junction Protein Expression in ApoE-Knockout Rats Fed High-Fat Diets. Nutrients 2020, 12, 1202. [Google Scholar] [CrossRef] [PubMed]
- Onrust, L.; Van Driessche, K.; Ducatelle, R.; Schwarzer, K.; Haesebrouck, F.; Van Immerseel, F. Valeric acid glyceride esters in feed promote broiler performance and reduce the incidence of necrotic en-teritis. Poult. Sci. 2018, 97, 2303–2311. [Google Scholar] [CrossRef]
- Zhao, L.; Zheng, L. A Review on Bioactive Anthraquinone and Derivatives as the Regulators for ROS. Molecules 2023, 28, 8139. [Google Scholar] [CrossRef]
- Berillo, D.; Kozhahmetova, M.; Lebedeva, L. Overview of the Biological Activity of Anthraquinons and Flavanoids of the Plant Rumex Species. Molecules 2022, 27, 1204. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Wang, W. Bioactivities and Structure–Activity Relationships of Natural Tetrahydroanthraquinone Compounds: A Review. Front. Pharmacol. 2020, 11, 799. [Google Scholar] [CrossRef]
- Wang, X.; Fu, H.Y.; He, W.; Xiang, Y.T.; Yang, Z.C.; Kuang, Y.; Yang, S.X. Synthesis and Antibacterial Activity Evaluation of Biphenyl and Dibenzofuran Derivatives as Potential An-timicrobial Agents against Antibiotic-Resistant Bacteria. Curr. Issues Mol. Biol. 2022, 44, 4087–4099. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, Q.; Zhang, L.; Zheng, C.; Xu, H. Biphenyls in Clusiaceae: Isolation, structure diversity, synthesis and bioactivity. Front. Chem. 2022, 10, 987009. [Google Scholar] [CrossRef] [PubMed]


| Compounds | Quantitative Measurement (μg/L) | Relative Enrichment | ||||
|---|---|---|---|---|---|---|
| Apolar, ap | Medium Polar, mp | Polar, p | Apolar, ap | Medium Polar, mp | Polar, p | |
| Coniferaldehyde | 103.9 | - | - | - | - | - |
| Palmitic acid | 9174.3 | 1768.3 | 1102.9 | 8.3 | 1.6 | 1.0 |
| Stearic acid | 13,951.4 | 2437.4 | 1620.9 | 8.6 | 1.5 | 1.0 |
| Myristic acid | 395.7 | 69.3 | 79.8 | 5.7 | 1.0 | 1.2 |
| Arachidonic acid | 25.9 | 7.0 | 0.6 | 43.2 | 11.7 | 1.0 |
| Pentadecanoic acid | 246.2 | 48.9 | 38.8 | 6.3 | 1.3 | 1.0 |
| Betulinic acid | 2119.7 | 11,462.9 | 11,725.7 | 1.0 | 5.4 | 5.5 |
| Euscaphic Acid | 40.4 | 199.6 | 482.0 | 1.0 | 4.9 | 11.9 |
| Flavidin | 30.7 | 265.9 | 148.1 | 1.0 | 8.7 | 4.8 |
| Luteolin glucoside | 0.4 | 2.2 | 2.3 | 1.0 | 5.5 | 5.8 |
| α-Linolenic acid | 23.0 | 55.7 | 103.6 | 1.0 | 2.4 | 4.5 |
| Hesperidin | 4.9 | 6.0 | 5.3 | 1.0 | 1.2 | 1.1 |
| Oleic acid | 2502.3 | 1443.1 | 1131.4 | 2.2 | 1.3 | 1.0 |
| Linoleic acid | 267.3 | 248.3 | 320 | 1.1 | 1.0 | 1.3 |
| Caprylic acid | 54.1 | 35.4 | 31.7 | 1.7 | 1.1 | 1.0 |
| Nebraskanic acid | 47.5 | 50.0 | 65.8 | 1.0 | 1.1 | 1.4 |
| Octyl formate | 122.2 | 54.7 | 40.6 | 3.0 | 1.3 | 1.0 |
| 6,7-dihydro-7-hydroxylinalool | 64.1 | 51.3 | 53.7 | 1.2 | 1.0 | 1.04 |
| α-irone | 10.3 | 18.5 | 13.5 | 1.0 | 1.8 | 1.3 |
| Oleanonic acid | 29,571.4 | 32,200.0 | 25,114.3 | 1.2 | 1.3 | 1.0 |
| Resveratrol | 5.7 | 6.4 | 6.7 | 1.0 | 1.1 | 1.2 |
| Isoliquiritigenin di-glucoside | 3.1 | 0.1 | 3.1 | 31.0 | 1.0 | 31.0 |
| Methoxycinnamic acid | 10.3 | 18.5 | 13.5 | 1.0 | 1.8 | 1.3 |
| Masticadecanoic acid | 29,571.4 | 32,200.0 | 25,114.3 | 1.2 | 1.3 | 1.0 |
| Gamma linolenic acid | 23.0 | 55.7 | 103.6 | 1.0 | 2.4 | 4.5 |
| Oleanolic acid | 601.1 | 2920.0 | 2885.7 | 1.0 | 4.9 | 4.8 |
| Ricinoleic acid | 23.0 | 55.7 | 103.6 | 1.0 | 2.4 | 4.5 |
| Crepenynic acid | 23.0 | 55.7 | 103.6 | 1.0 | 2.4 | 4.5 |
| Non-Target Compounds | Non-Target Compounds Chemical Classification |
|---|---|
| Ethyl 2-acetyl heptanoate | straight-chain fatty acid |
| Sesterstatin | sesterterpenoid |
| Cyclohexanecarboxylic acid | organic compound |
| 1,2-Hydroxylauric acid | medium-chain fatty acid |
| Dodecanedioic acid | saturated aliphatic dicarboxylic acid |
| Tisocalcitate | organic compound (vitamin D derivative) |
| Trivalerin | (ester of valeric acid) straight-chain saturated fatty acid |
| Frangulin B | anthraquinone |
| 4,4′,6,6′-Tetra-tert-butyl-2,2′-biphenol | aromatic hydrocarbon |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Georgantopoulos, A.; Kalousi, F.D.; Pollastro, F.; Tsialtas, I.; Kalogiouri, N.P.; Psarra, A.-M.G. Chemical Analysis and Antioxidant Activities of Resin Fractions from Pistacia lentiscus L. var. Chia in Neuroblastoma SH-SY5Y Cells. Molecules 2025, 30, 997. https://doi.org/10.3390/molecules30050997
Georgantopoulos A, Kalousi FD, Pollastro F, Tsialtas I, Kalogiouri NP, Psarra A-MG. Chemical Analysis and Antioxidant Activities of Resin Fractions from Pistacia lentiscus L. var. Chia in Neuroblastoma SH-SY5Y Cells. Molecules. 2025; 30(5):997. https://doi.org/10.3390/molecules30050997
Chicago/Turabian StyleGeorgantopoulos, Achilleas, Foteini D. Kalousi, Federica Pollastro, Ioannis Tsialtas, Natasa P. Kalogiouri, and Anna-Maria G. Psarra. 2025. "Chemical Analysis and Antioxidant Activities of Resin Fractions from Pistacia lentiscus L. var. Chia in Neuroblastoma SH-SY5Y Cells" Molecules 30, no. 5: 997. https://doi.org/10.3390/molecules30050997
APA StyleGeorgantopoulos, A., Kalousi, F. D., Pollastro, F., Tsialtas, I., Kalogiouri, N. P., & Psarra, A.-M. G. (2025). Chemical Analysis and Antioxidant Activities of Resin Fractions from Pistacia lentiscus L. var. Chia in Neuroblastoma SH-SY5Y Cells. Molecules, 30(5), 997. https://doi.org/10.3390/molecules30050997








