New Benzothiazole–Monoterpenoid Hybrids as Multifunctional Molecules with Potential Applications in Cosmetics
Abstract
1. Introduction
2. Results and Discussion
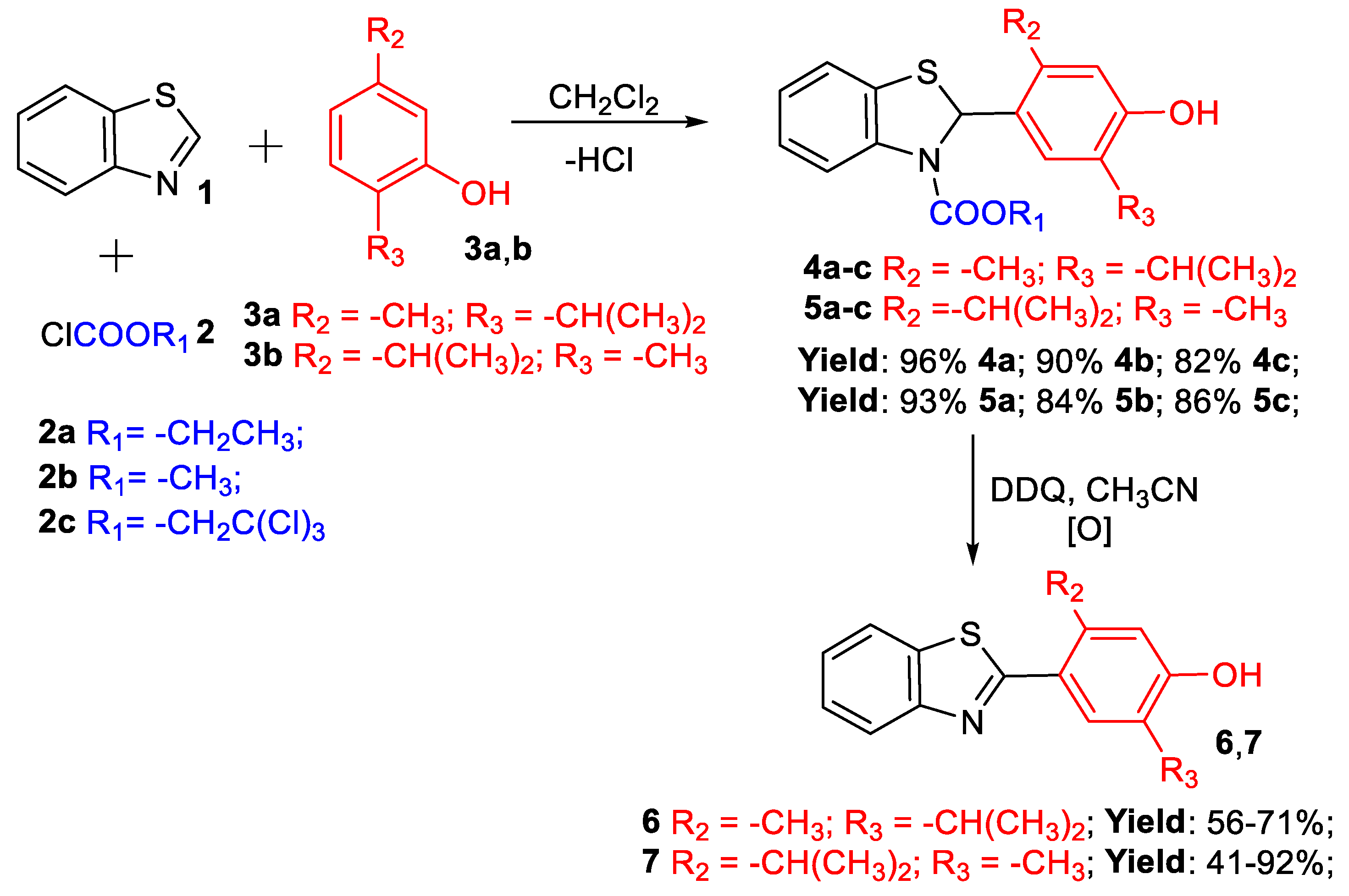
2.1. Synthesis of Benzothiazole–Monoterpenoid Hybrids
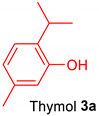
2.1.1. Amidoalkylation of Natural Monoterpenoids—Thymol and Carvacrol
- (a)
- Effect of Solvent Polarity
- (b)
- Effect of Alkyl Chloroformates
- (c)
- Effect of the Alkyl Substituent in Phenolics to Formation of C-C Bond in BT Ring
2.1.2. Experimental Results of Oxidative Rearomatization
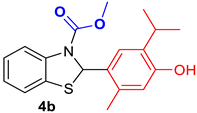
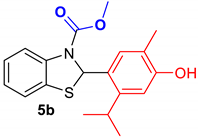
2.1.3. Synthesis of Compounds 4a,b and 5a,b from Essential Oils of Thyme (Thymus vulgaris) and Oregano (Origanum vulgare)
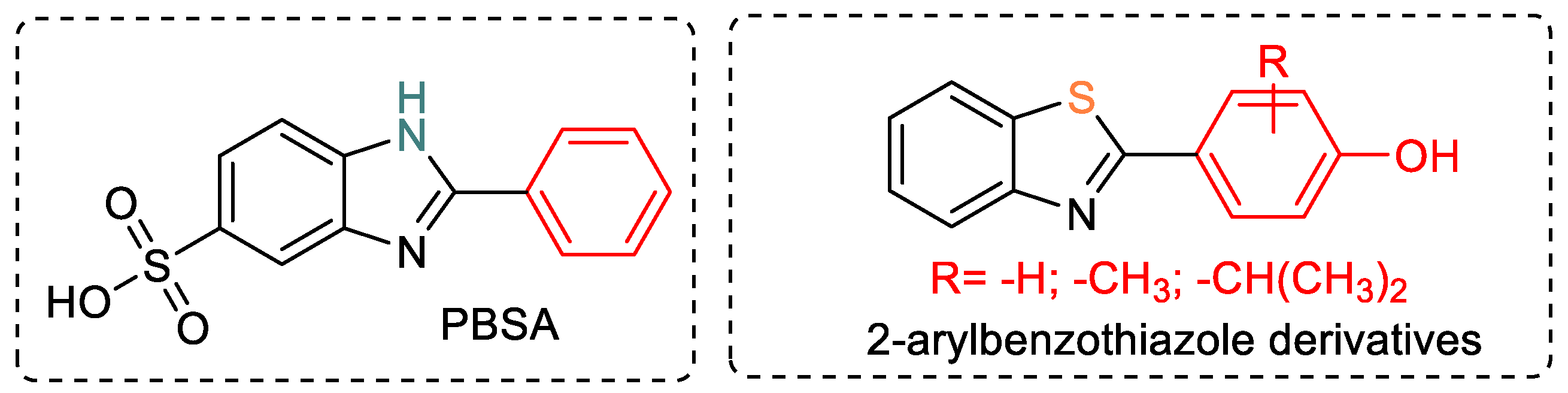
2.2. Biological Activity
2.2.1. In Silico Predictions of Benzothiazole-Based Hybrids Toxicity
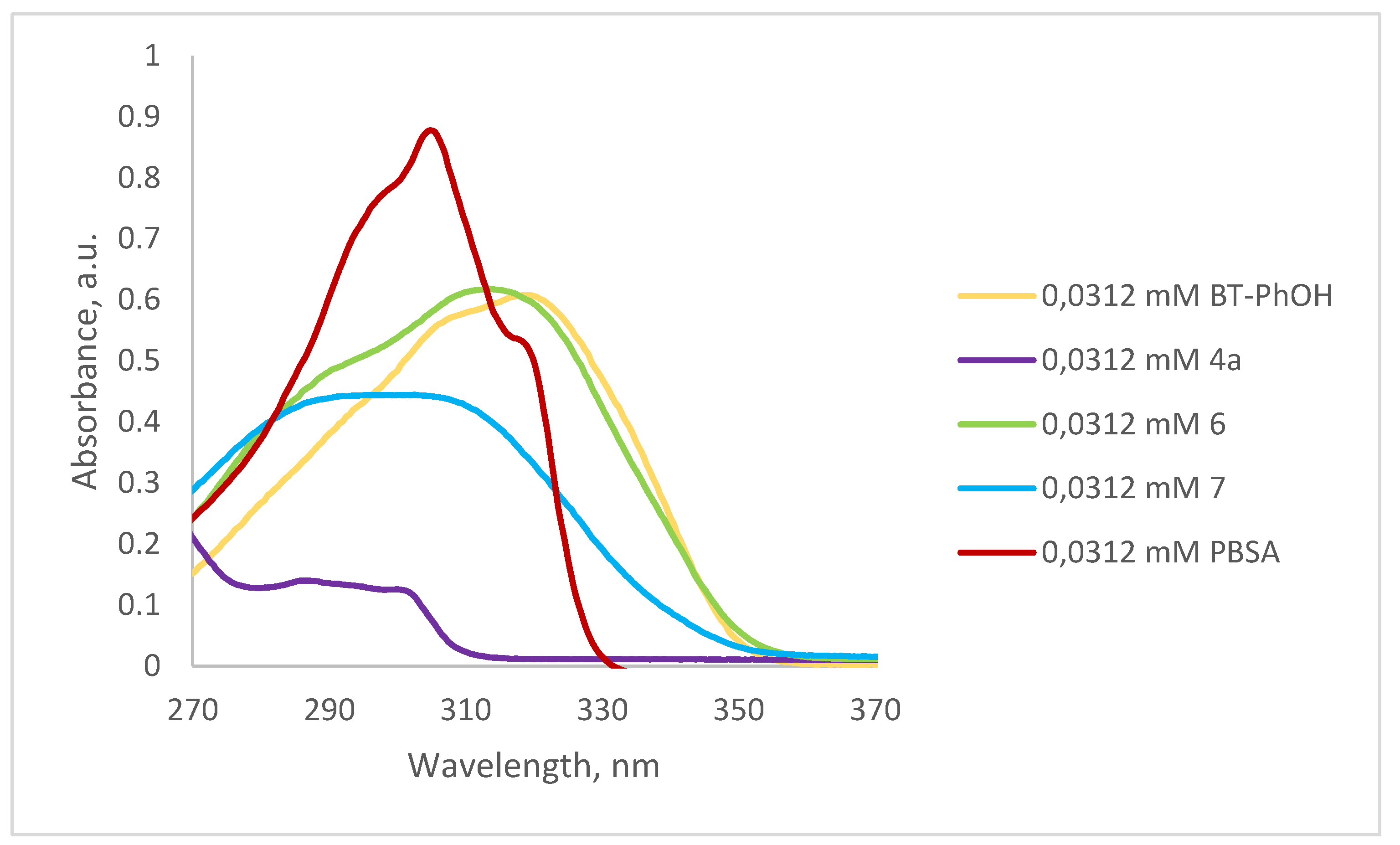
2.2.2. In Vitro Determination of the Sun-Protection Factor (SPF)
2.2.3. Antimicrobial Activity Assay
2.2.4. Radical Scavenging Activity Assay
3. Materials and Methods
3.1. Chemistry
3.1.1. General Information
3.1.2. General Procedure for the Synthesis of Benzothiazole–Monoterpenoid Hybrids 4a–c and 5a–c
3.1.3. General Procedure for the Oxidation of Thymol and Carvacrol Containing Benzothiazolines 4a–c and 5a–c Toward Aromatized Products 6 and 7
3.1.4. General Procedure for the Synthesis of Compounds 4a,b and 5a,b from Essential Oils of Thyme (Thymus vulgaris) and Oregano (Origanum vulgare)
3.1.5. GC-MS/MS Analysis of Thyme and Oregano Essential Oils
3.1.6. In Silico QSAR Analysis
3.1.7. Method for Determining the SPF Factor
3.1.8. Method for Determination of Antimicrobial Activity
3.1.9. Radical Scavenging Activity Assay
DPPH Free Radical Scavenging Assay
ABTS Free Radical Scavenging Assay
3.1.10. STATISTICS
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gümüş, M.; Yakan, M.; Koca, I. Recent advances of thiazole hybrids in biological applications. Future Med. Chem. 2019, 11, 1979–1998. [Google Scholar] [CrossRef] [PubMed]
- Moodley, R.; Mashaba, C.; Rakodi, G.H.; Ncube, N.B.; Maphoru, M.V.; Balogun, M.O.; Jordan, A.; Warner, D.F.; Khan, R.; Tukulula, M. New Quinoline–Urea–Benzothiazole Hybrids as Promising Antitubercular Agents: Synthesis, In Vitro Antitubercular Activity, Cytotoxicity Studies, and In Silico ADME Profiling. Pharmaceuticals 2022, 15, 576. [Google Scholar] [CrossRef] [PubMed]
- Reddy, V.G.; Reddy, T.S.; Jadala, C.; Reddy, M.S.; Sultana, F.; Akunuri, R.; Bhargava, S.K.; Wlodkowic, D.; Srihari, P.; Kamal, A. Pyrazolo-benzothiazole hybrids: Synthesis, anticancer properties and evaluation of antiangiogenic activity using in vitro VEGFR-2 kinase and in vivo transgenic zebrafish model. Eur. J. Med. Chem. 2019, 182, 111609. [Google Scholar] [CrossRef] [PubMed]
- Valverde Sancho, J.; Carreño Amate, C.; Caparrós Pérez, M.d.M.; Santana Méridas, O.; Julio, L.F. Biological activity of hybrid molecules based on major constituents of Cinnammomun verum and Thymus vulgaris essential oils. Life 2023, 13, 499. [Google Scholar] [CrossRef]
- Jovanović, A.; Balanč, B.; Petrović, P.; Pravilović, R.; Djordjević, V. Pharmacological potential of Thymus serpyllum L. (wild thyme) extracts and essential oil: A review. J. Eng. Process. Manag. 2021, 13, 32–41. [Google Scholar] [CrossRef]
- Salehi, B.; Mishra, A.P.; Shukla, I.; Sharifi-Rad, M.; Contreras, M.M.; Segura-Carretero, A.; Fathi, H.; Nasrabadi, N.N.; Kobarfard, F.; Sharifi-Rad, J. Thymol, thyme, and other plant sources: Health and potential uses. J. Phytother. Res. 2018, 32, 1688–1706. [Google Scholar] [CrossRef]
- Asha, D.; Mathew, L. Chemical profiling of Thymus vulgaris L. using HPTLC. J. Pharmacogn. Phytochem. 2017, 6, 1017–1023. Available online: https://www.phytojournal.com/archives/2017/vol6issue4/PartO/6-4-12-368.pdf (accessed on 1 July 2017).
- Kosakowska, O.; Bączek, K.; Przybył, J.L.; Pawełczak, A.; Rolewska, K.; Węglarz, Z. Morphological and chemical traits as quality determinants of common thyme (Thymus vulgaris L.), on the example of ‘Standard Winter’ cultivar. Agronomy 2020, 10, 909. [Google Scholar] [CrossRef]
- Alihosseini, F. Plant-based compounds for antimicrobial textiles. In Antimicrobial Textiles; Woodhead Publishing: Sawston, UK; Elsevier Ltd.: Amsterdam, The Netherlands, 2016; pp. 155–195. [Google Scholar] [CrossRef]
- Brotzman, N.; Xu, Y.; Graybill, A.; Cocolas, A.; Ressler, A.; Seeram, N.P.; Ma, H.; Henry, G.E. Synthesis and tyrosinase inhibitory activities of 4-oxobutanoate derivatives of carvacrol and thymol. Bioorg. Med. Chem. Lett. 2019, 29, 56–58. [Google Scholar] [CrossRef]
- Kowalczyk, A.; Przychodna, M.; Sopata, S.; Bodalska, A.; Fecka, I. Thymol and thyme essential oil-new insights into selected therapeutic applications. Molecules 2020, 25, 4125. [Google Scholar] [CrossRef]
- Aazza, S.; Lyoussi, B.; Miguel, M.G. Antioxidant and ntiacetylcholinesterase activities of some commercial essential oils and their major compounds. Molecules 2011, 16, 7672–7690. [Google Scholar] [CrossRef] [PubMed]
- Miladi, H.; Zmantar, T.; Chaabouni, Y.; Fedhila, K.; Bakhrouf, A.; Mahdouani, K.; Chaieb, K. Antibacterial and efflux pump inhibitors of thymol and carvacrol against food-borne pathogens. Microb. Pathog. 2016, 99, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Bayir, A.G.; Kiziltan, H.S.; Kocyigit, A.; Güler, E.M.; Karataş, E.; Toprak, A. Effects of natural phenolic compound carvacrol on the human gastric adenocarcinoma (AGS) cells in vitro. Anticancer Drugs 2017, 28, 522–530. [Google Scholar] [CrossRef] [PubMed]
- Mari, A.; Mani, G.; Nagabhishek, S.N.; Balaraman, G.; Subramanian, N.; Mirza, F.B.; Sundaram, J.; Thiruvengadam, D. Carvacrol promotes cell cycle arrest and apoptosis through PI3K/AKT signaling pathway in MCF-7 breast cancer cells. Chin. J. Integr. Med. 2021, 27, 680–687. [Google Scholar] [CrossRef]
- Elbe, H.; Yigitturk, G.; Cavusoglu, T.; Baygar, T.; Onal, M.O.; Ozturk, F. Comparison of ultrastructural changes and the anticarcinogenic effects of thymol and carvacrol on ovarian cancer cells: Which is more effective? Ultrastruct. Pathol. 2020, 44, 193–202. [Google Scholar] [CrossRef]
- Yu, Y.; Chao, T.; Chang, W.; Chang, M.; Lee, M. Thymol reduces oxidative stress, aortic intimal thickening, and inflammation-related gene expression in hyperlipidemic rabbits. J. Food Drug Anal. 2016, 24, 556–563. [Google Scholar] [CrossRef]
- Braga, P.C.; Sasso, M.D.; Culici, M.; Bianchi, T.; Bordoni, L.; Marabini, L. Anti-inflammatory activity of thymol: Inhibitory effect on the release of human neutrophil Elastase. Pharmacology 2006, 77, 130–136. [Google Scholar] [CrossRef]
- Marchese, A.; Orhan, I.E.; Daglia, M.; Barbieri, R.; Di Lorenzo, A.; Nabavi, S.F.; Gortzi, O.; Izadi, M.; Nabavi, S.M. Antibacterial and antifungal activities of thymol: A brief review of the literature. Food Chem. 2016, 210, 402–414. [Google Scholar] [CrossRef]
- Djuidje, E.N.; Barbari, R.; Baldisserotto, A.; Durini, E.; Sciabica, S.; Balzarini, J.; Liekens, S.; Vertuani, S.; Manfredini, S. Benzothiazole Derivatives as Multifunctional Antioxidant Agents for Skin Damage: Structure–Activity Relationship of a Scaffold Bearing a Five-Membered Ring System. Antioxidants 2022, 11, 407. [Google Scholar] [CrossRef]
- Barbari, R.; Tupini, C.; Durini, E.; Gallerani, E.; Nicoli, F.; Lampronti, I.; Baldisserotto, A.; Manfredini, S. Design, Synthesis and Evaluation of New Multifunctional Benzothiazoles as Photoprotective, Antioxidant and Antiproliferative Agents. Molecules 2023, 28, 287. [Google Scholar] [CrossRef]
- Nichols, J.A.; Katiyar, S.K. Skin photoprotection by natural polyphenols: Anti-inflammatory, antioxidant and DNA repair mechanisms. Arch. Dermatol. Res. 2010, 302, 71–83. [Google Scholar] [CrossRef] [PubMed]
- Sies, H.; Stahl, W. Nutritional Protection against Skin Damage from Sunlight. Annu. Rev. Nutr. 2004, 24, 173–200. [Google Scholar] [CrossRef] [PubMed]
- Bastien, N.; Millau, J.F.; Rouabhia, M.; Davies, R.J.H.; Drouin, R. The Sunscreen Agent 2-Phenylbenzimidazole-5 Sulfonic Acid photosensitizes the formation of oxidized guanines in cellulo after UV-A or UV-B exposure. J. Investig. Dermatol. 2010, 130, 2463–2471. [Google Scholar] [CrossRef] [PubMed]
- Baldisserotto, A.; Demurtas, M.; Lampronti, I.; Tacchini, M.; Moi, D.; Balboni, G.; Pacifico, S.; Vertuani, S.; Manfredini, S.; Onnis, V. In vitro evaluation of antioxidant, antiproliferative and photo-protective activities of benzimidazolehydrazone derivatives. Pharmaceuticals 2020, 13, 68. [Google Scholar] [CrossRef] [PubMed]
- Stremski, Y.; Kirkova, D.; Statkova-Abeghe, S.; Angelov, P.; Ivanov, I.; Georgiev, D. Synthesis and antibacterial activity of hydroxylated 2-arylbenzothiazole derivatives. Synth. Commun. 2020, 50, 3007–3015. [Google Scholar] [CrossRef]
- Djuidje, E.N.; Sciabica, S.; Buzzi, R.; Dissette, V.; Balzarini, J.; Liekens, S.; Serra, E.; Andreotti, E.; Manfredini, S.; Vertuani, S.; et al. Design, synthesis and evaluation of benzothiazole derivatives as multifunctional agents. Bioorg. Chem. 2020, 101, 103960. [Google Scholar] [CrossRef]
- Khan, K.M.; Rahim, F.; Halim, S.A.; Taha, M.; Khan, M.; Perveen, S.; Zaheer-ul-Haq; Mesaik, M.A.; Iqbal Choudhary, M. Synthesis of novel inhibitors of β-glucuronidase based on benzothiazole skeleton and study of their binding affinity by molecular docking. Bioorg. Med. Chem. 2011, 19, 4286–4294. [Google Scholar] [CrossRef]
- Tang, L.; Zou, Y.; Zhong, K.; Bian, Y. A novel benzothiazole-based enaminone as a fluorescent probe for highly selective and sensitive detection of CN. RSC Adv. 2016, 6, 48351–48356. [Google Scholar] [CrossRef]
- Yang, P.; Zhao, J.; Wu, W.; Yu, X.; Liu, Y. Accessing the long-lived triplet excited states in bodipy-conjugated 2-(2-hydroxyphenyl) benzothiazole/benzoxazoles and applications as organic triplet photosensitizers for photooxidations. J. Org. Chem. 2012, 77, 6166–6178. [Google Scholar] [CrossRef]
- Kurt, B.Z.; Gazioglu, I.; Dag, A.; Salmas, R.E.; Kayık, G.; Durdagi, S.; Sonmez, F. Synthesis, anticholinesterase activity and molecular modeling study of novel carbamate-substituted thymol/carvacrol derivatives. Bioorganic Med. Chem. 2017, 25, 1352–1363. [Google Scholar] [CrossRef]
- Rahim, N.; Asari, A.; Ismail, N.; Abdullah, F.; Osman, H.; Tahier, S.M. Synthesis and antibacterial study of thymol derivatives. Asian J. Chem. 2018, 30, 126–128. [Google Scholar] [CrossRef]
- Kirkova, D.; Stremski, Y.; Statkova-Abeghe, S.; Docheva, M. Quercetin hybrids-synthesis, spectral characterization and radical scavenging potential. Molbank 2022, 1, M1329. [Google Scholar] [CrossRef]
- Stremski, Y.; Bachvarova, M.; Kirkova, D.; Statkova-Abeghe, S. New 2-(2,4-Dihydroxyphenyl)benzimidazolines. MolBank 2023, 1, M1602. [Google Scholar] [CrossRef]
- ISO 19817:2017; Essential Oil of Thyme [Thymus vulgaris L. and Thymus zygis L.], Thymol Type. International Organization for Standardization: Geneva, Switzerland, 2017. Available online: https://www.iso.org/standard/66267.html (accessed on 11 November 2017).
- ISO 13171:2016; Essential Oil of Oregano [Origanum vulgare L. subsp. hirtum (Link) letsw]. International Organization for Standardization: Geneva, Switzerland, 2016. Available online: https://www.iso.org/standard/66202.html (accessed on 1 August 2016).
- Hristozova, A.; Batmazyan, M.; Simitchiev, K.; Tsoneva, S.; Kmetov, V.; Rosenberg, E. Headspace-Solid phase microextraction vs liquid injection GC-MS analysis of essential oils: Prediction of linear retention indices by multiple linear regression. Acta Chromatogr. 2024, 1–11. [Google Scholar] [CrossRef]
- Raies, A.B.; Bajic, V.B. In silico toxicology: Computational methods for the prediction of chemical toxicity. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2016, 6, 147–172. [Google Scholar] [CrossRef]
- Ames, B.N.; Durston, W.E.; Yamasaki, E.; Lee, F.D. Carcinogens are mutagens: A simple test system combining liver homogenates for activation and bacteria for detection. Proc. Natl. Acad. Sci. USA 1973, 70, 2281–2285. [Google Scholar] [CrossRef]
- Honma, M.; Kitazawa, A.; Cayley, A.; Williams, R.V.; Barber, C.; Hanser, T.; Saiakhov, R.; Chakravarti, S.; Myatt, G.J.; Cross, K.P.; et al. Improvement of quantitative structure–activity relationship (QSAR) tools for predicting Ames mutagenicity: Outcomes of the Ames/QSAR International Challenge Project. Mutagenesis 2019, 34, 3–16. [Google Scholar] [CrossRef]
- Feeney, S.V.; Lui, R.; Guan, D.; Matthews, S. Multiple instance learning improves Ames Mutagenicity prediction for problematic molecular species. Chem. Res. Toxicol. 2023, 36, 1227–1237. [Google Scholar] [CrossRef]
- Uesawa, Y. Progress in predicting Ames Test Outcomes from Chemical Structures: An In-Depth Re-Evaluation of models from the 1st and 2nd Ames/QSAR International Challenge Projects. Int. J. Mol. Sci. 2024, 25, 1373. [Google Scholar] [CrossRef]
- Kirk, R.G.W. Recovering the principles of humane experimental technique: The 3Rs and the human essence of animal Research. Sci. Technol. Hum. Values 2018, 43, 622–648. [Google Scholar] [CrossRef]
- Russell, W.M.S.; Burch, R.L. The principles of humane experimental technique. Med. J. Aust. 1959, 1, 500. [Google Scholar] [CrossRef]
- Commission EU Regulation (EC) No 1907/2006 of the European Parliament and of the Council of 18 December 2006, European Commission, Brussels. Available online: https://eur-lex.europa.eu/eli/reg/2006/1907/oj (accessed on 30 December 2006).
- Raitano, G.; Roncaglioni, A.; Manganaro, A.; Honma, M.; Sousselier, L.; Do, Q.T.; Paya, E.; Benfenati, E. Integrating in silico models for the prediction of mutagenicity (Ames Test) of botanical ingredients of cosmetics. Comput. Toxicol. 2019, 12, 100–108. [Google Scholar] [CrossRef]
- Martin, T.M. User’s Guide for T.E.S.T. (Toxicity Estimation Software Tool), & Todd. User’s Guide for T.E.S.T. Version 5.1 A Java Application to Estimate Toxicities and Physical Properties from Molecular Structure. 2020. Available online: https://www.epa.gov/chemical-research/toxicity-estimation-software-tool-test (accessed on 21 February 2024).
- Pedretti, A.; Mazzolari, A.; Gervasoni, S.; Fumagalli, L.; Vistoli, G. The VEGA Suite of Programs: An Versatile Platform for Cheminformatics and Drug Design Projects. Bioinformatics 2021, 37, 1174–1175. [Google Scholar] [CrossRef] [PubMed]
- Mswahili, M.E.; Jeong, Y. Transformer-based models for chemical SMILES representation: A comprehensive literature review. Heliyon 2024, 10, e39038. [Google Scholar] [CrossRef]
- Weininger, D. SMILES, a chemical language and information system. 1. introduction to methodology and encoding rules. J. Chem. Inf. Comput. Sci. 1988, 28, 31–36. [Google Scholar] [CrossRef]
- Weininger, D.; Weininger, A.; Weininger, J.L. SMILES. 2. Algorithm for generation of unique SMILES notation. J. Chem. Inf. Comput. Sci. 1989, 29, 97–101. [Google Scholar] [CrossRef]
- Mansur, J.S.; Breder, M.N.R.; Mansur, M.C.A.; Azulay, R.D. Determination of sun protection factor by spectrophotometry. An. Bras. Dermatol. 1986, 61, 121–124. [Google Scholar]
- Sayre, R.M.; Agin, P.P.; Levee, G.J.; Marlowe, E. Comparison of in vitro and in vivo testing of sun screening formulas. Photochem. Photobiol. 1979, 29, 559–566. [Google Scholar] [CrossRef]
- Skowron, K.; Budzyńska, A.; Wiktorczyk-Kapischke, N.; Warzonkoska, W.; Gospodarek-Komkowska, E.; Grudlewska-Buda, K. Assessment of antimicrobial efficacy in selected antibacterial cosmetics. Pomeranian J. Life Sci. 2024, 70, 74–82. [Google Scholar]
- Kirkova, D.; Statkova-Abeghe, S.; Docheva, M.; Stremski, Y.; Minkova, S. Structure-activity relationship of in vitro radical-scavenging activity of 2-(hydroxyphenyl) benzothiazole derivatives. Bulg. Chem. Commun. 2020, 52, 196–200. [Google Scholar]
- Docheva, M.; Dagnon, S.; Statkova-Abeghe, S. Flavonoid content and radical scavenging potential of extracts prepared from tobacco cultivars and waste. Nat. Prod. Res. 2014, 28, 1328–1334. [Google Scholar] [CrossRef] [PubMed]
- ISO 24443:2021; Determination of Sunscreen UVA Photoprotection In Vitro. International Organization for Standardization: Geneva, Switzerland. Available online: https://www.iso.org/standard/75059.html (accessed on 12 December 2021).
- Diffey, B.; Robson, J. A New Substrate to Measure Sunscreen Protection Factors Throughout the Ultraviolet Spectrum. J. Soc. Cosmet. Chem. 1989, 40, 127–133. [Google Scholar]
- Goranov, B.; Gaytanska, Y.; Denkova-Kostova, R.; Ivanova, P.; Denkova, Z.; Kostov, G. Examination of the Bactericidal and Fungicidal Activity of Bacillus Amyloliquefaciens M Isolated from Spring Waters in Bulgaria. Appl. Sci. 2024, 14, 3612. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]

| Product | R1 | R2 | R3 | *Ratio BT:3a,b | Reaction Time, h | Yield, % |
|---|---|---|---|---|---|---|
| 4a 4b 4c | CH2CH3 | CH3 | CH(CH3)2 | 2:1 | 6 | 96 |
| CH3 | CH3 | CH(CH3)2 | 2:1 | 3 | 90 | |
| CH2C(Cl)3 | CH3 | CH(CH3)2 | 1.3:1 | 1 | 82 | |
| 5a 5b 5c | CH2CH3 | CH(CH3)2 | CH3 | 2:1 | 24 | 93 |
| CH3 | CH(CH3)2 | CH3 | 2:1 | 6 | 84 | |
| CH2C(Cl)3 | CH(CH3)2 | CH3 | 1.3:1 | 1.5 | 86 |
| No. | Solvent | *Ratio BT:3a | Reaction Time, h | Yield 4a, % |
|---|---|---|---|---|
| 1 | CH3CN | 2:1 | 24 | 84 |
| 2 | CH2Cl2 | 2:1 | 6 | 96 |
| 3 | CH2Cl2 | 1.3:1 | 24 | 95 |
| Product | Oxidation of | R2 | R3 | *Ratio DDQ:4,5 | Reaction Time, h | Yield, % |
|---|---|---|---|---|---|---|
| 6 | 4a | CH3 | CH(CH3)2 | 2:1 | 3 | 70 |
| 4b | CH3 | CH(CH3)2 | 2:1 | 2 | 71 | |
| 4c | CH3 | CH(CH3)2 | 2:1 | 2 | 56 | |
| 7 | 5a | CH(CH3)2 | CH3 | 2:1 | 3 | 92 |
| 5b | CH(CH3)2 | CH3 | 2:1 | 2 | 82 | |
| 5c | CH(CH3)2 | CH3 | 2:1 | 2 | 41 |
| Reference Compounds | Benzothiazole Hybrids |
|---|---|
 |  |
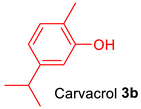 | 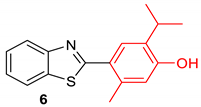 |
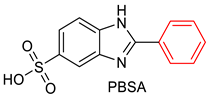 |  |
| Benzothiazoline-hybrid | |
 | 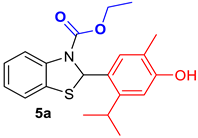 |
 |  |
| Compound | Mutagenicity Model (CAESAR) 2.1.14 | Mutagenicity Model (ISS) 1.0.3 | Mutagenicity Model (SarPy-IRFMN) 1.0.8 | Acute Toxicity (LD50), mg/kg | Skin Irritation | Thyroid Receptor Alpha Effect (TRα) | Reproductive Toxicity |
|---|---|---|---|---|---|---|---|
| BT-PhOH | Mutagenic | NON | NON | 2706.41 | NON | Inactive | NON |
| 3a | NON | NON | NON | 970.11 | Irritating | Inactive | NON |
| 3b | NON | NON | NON | 806.9 | Irritating | Inactive | NON |
| 4a | Mutagenic | NON | Mutagenic | 40.76 | NON | Inactive | NON |
| 4b | Mutagenic | NON | Mutagenic | 38.35 | NON | Inactive | NON |
| 5a | Mutagenic | NON | Mutagenic | 155.46 | NON | Inactive | NON |
| 5b | Mutagenic | NON | Mutagenic | 38.30 | NON | Inactive | NON |
| 6 | NON | NON | NON | 3192.7 | NON | Inactive | NON |
| 7 | NON | NON | NON | 3191.84 | NON | Inactive | NON |
| PBSA | NON | NON | NON | 1465.52 | NON | Inactive | NON |
| Compound | MW, g/mol | Oral rat LD50 Nearest Neighbour mg/kg | T. pyriformis IGC50 (48 h) Nearest Neighbour mg/L | Daphnia magna LC50 (48 h) Nearest Neighbour mg/L |
|---|---|---|---|---|
| BT-PhOH | 227.28 | 1363.33 | 4.78 | 1.89 |
| 3a | 150.22 | * 981.26/507.13 | 12.69 | 20.72 |
| 3b | 150.22 | * 810.56/1072.64 | 12.69 | 10.13 |
| 4a | 357.47 | 68.44 | 1.76 | 0.13 |
| 4b | 343.44 | 65.75 | 1.69 | 0.13 |
| 5a | 357.47 | 68.44 | 1.76 | 0.18 |
| 5b | 343.44 | 65.75 | 1.69 | 0.38 |
| 6 | 283.39 | 1164.39 | 1.65 | 2.35 |
| 7 | 283.39 | 1164.39 | 1.65 | 2.35 |
| PBSA | 274.29 | 319.83 | 7.54 | 8.12 |
| nm | Absorption (A) for 0.0312 mM Solution in 96% Ethanol | EE × I | Compound 7—A × EE × I | ||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 1 | 2 | 3 | ||
| 290 | 0.4101 | 0.4067 | 0.4458 | 0.0150 | 0.00615150 | 0.00610050 | 0.00668700 |
| 295 | 0.4145 | 0.4176 | 0.4494 | 0.0817 | 0.03386465 | 0.03411792 | 0.03671598 |
| 300 | 0.4122 | 0.4112 | 0.4494 | 0.2874 | 0.11846628 | 0.11817888 | 0.12915756 |
| 305 | 0.4095 | 0.4105 | 0.4478 | 0.3278 | 0.13423410 | 0.13456190 | 0.14678884 |
| 310 | 0.3976 | 0.3958 | 0.4336 | 0.1864 | 0.07411264 | 0.07377712 | 0.08082304 |
| 315 | 0.3608 | 0.3633 | 0.3954 | 0.0839 | 0.03027112 | 0.03048087 | 0.03317406 |
| 320 | 0.3081 | 0.3069 | 0.3336 | 0.0180 | 0.00554580 | 0.00552420 | 0.00600480 |
| Sum × CF | 0.40264609 | 0.40274139 | 0.43935128 | ||||
| SPF value | 4.03 | 4.03 | 4.39 | ||||
| SPF ± SD values | 4.15 ± 0.21 | ||||||
| Concentration, mM | SPF ± SD Values of Synthetic 2-arylbenzazole Compounds | |||
|---|---|---|---|---|
| BT-PhOH | 6 | 7 | PBSA | |
| 1 | 33.42 ± 0.76 | 34.32 ± 0.10 | 36.19 ± 0.69 | 30.45 ± 0.05 |
| 0.5 | 33.67 ± 0.10 | 34.18 ± 0.03 | 35.99 ± 0.60 | 30.28 ± 0.07 |
| 0.25 | 32.65 ± 0.03 | 33.74 ± 0.08 | 31.55 ± 0.80 | 30.08 ± 0.08 |
| 0.125 | 20.28 ± 0.52 | 21.86 ± 0.02 | 14.50 ± 2.73 | 26.72 ± 0.04 |
| 0.0625 | 10.29 ± 0.20 | 11.29 ± 0.14 | 8.26 ± 0.22 | 15.18 ± 0.06 |
| 0.0312 | 5.27 ± 0.06 | 5.68 ± 0.06 | 4.15 ± 0.21 | 7.66 ± 0.16 |
| 0.0156 | 2.84 ± 0.07 | 3.22 ± 0.22 | 2.23 ± 0.19 | 3.89 ± 0.02 |
| 0.01 | 1.69 ± 0.06 | 1.77 ± 0.09 | 1.38 ± 0.02 | 2.10 ± 0.08 |
| 0.005 | 1.27 ± 0.14 | 1.11 ± 0.10 | 0.67 ± 0.12 | 1.63 ± 0.01 |
| Compound | Content µmol in Wells 6 mm | Content µg in Wells 6 mm | Diameter zone of Inhibition ± SD (mm) | |||||
|---|---|---|---|---|---|---|---|---|
| E. coli ATCC 25922 | E. coli ATCC 8739 | S. aureus ATCC 25923 | S. aureus ATCC 6538 | C. albicans NBIMCC 74 | P. aeruginosa NBIMCC 1390 | |||
| Essential oil of Thyme | - | 60 30 15 | NA NA NA | NA NA NA | NA NA NA | NA NA NA | 12 ± 0.00 10 ± 0.00 9 ± 0.00 | NA NA NA |
| Thymol | 0.399 0.200 0.100 | 60 30 15 | NA NA NA | NA NA NA | NA NA NA | NA NA NA | 12 ± 0.00 11 ± 0.71 10 ± 0.71 | NA NA NA |
| Carvacrol | 0.399 0.200 0.100 | 60 30 15 | NA NA NA | NA NA NA | NA NA NA | NA NA NA | 11 ± 0.00 10 ± 0.71 9 ± 0.00 | NA NA NA |
| 4a | 0.168 0.084 0.042 | 60 30 15 | 15 ± 0.71 13 ± 0.00 13 ± 0.00 | 15 ± 0.71 13 ± 0.00 13 ± 0.00 | 11 ± 0.71 10 ± 0.71 9 ± 0.71 | 16 ± 0.71 13 ± 0.71 13 ± 0.71 | 12 ± 0.00 12 ± 0.00 12 ± 0.71 | 14 ± 0.71 13 ± 0.00 13 ± 0.00 |
| 4b | 0.175 0.087 0.044 | 60 30 15 | 16 ± 0.71 14 ± 0.71 12 ± 0.00 | 15 ± 0.71 14 ± 0.71 13 ± 0.00 | 15 ± 0.71 14 ± 0.00 14 ± 0.00 | 15 ± 0.71 14 ± 0.00 14 ± 0.00 | 12 ± 0.00 12 ± 0.71 12± 0.71 | 14 ± 0.71 12 ± 0.00 12 ± 0.71 |
| 5a | 0.168 0.084 0.042 | 60 30 15 | NA NA NA | NA NA NA | 9 ± 0.71 NA NA | NA NA NA | 13 ± 0.71 13 ± 0.71 13 ± 0.71 | NA NA NA |
| 5b | 0.175 0.087 0.044 | 60 30 15 | 13 ± 0.00 12 ± 0.00 10 ± 0.00 | 15 ± 0.00 14 ± 0.71 11 ± 0.71 | 24 ± 2.12 22 ± 0.00 20 ± 0.00 | 14 ± 0.71 12 ± 0.00 10 ± 0.00 | 13 ± 0.71 10 ± 0.00 10 ± 0.00 | 13 ± 0.71 12 ± 0.71 10 ± 0.00 |
| 6 | 0.212 0.106 0.053 | 60 30 15 | NA NA NA | NA NA NA | NA NA NA | NA NA NA | 10 ± 0.00 NA NA | NA NA NA |
| 7 | 0.212 0.106 0.053 | 60 30 15 | NA NA NA | NA NA NA | NA NA NA | NA NA NA | 9 ± 0.00 9 ± 0.00 8 ± 0.71 | NA NA NA |
| Compound | DPPH | ABTS |
|---|---|---|
| Gallic acid | NA* | 37.70 ± 1.25 |
| Quercetin [33,56] | 4.60 ± 0.30 | 48.00 ± 4.40 |
| Rutin [33,56] | 5.02 ± 0.40 | 95.30 ± 4.50 |
| Thymol | 506 ± 15 | 92.50 ± 10 |
| Carvacrol | 456 ± 15 | 52.60 ± 5.00 |
| 4a | >1000 | 133.70 ± 10 |
| 4b | >1000 | 157.50 ± 10 |
| 5a | >1000 | 300 ± 25 |
| 5b | >1000 | 213 ± 20 |
| 6 | NA* | 421.30 ± 20 |
| 7 | NA* | >2000 |
| Wavelength (λ, nm) | EE × I (Normalized) |
|---|---|
| 290 | 0.0150 |
| 295 | 0.0817 |
| 300 | 0.2874 |
| 305 | 0.3278 |
| 310 | 0.1864 |
| 315 | 0.0839 |
| 320 | 0.0180 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kirkova, D.; Stremski, Y.; Bachvarova, M.; Todorova, M.; Goranov, B.; Statkova-Abeghe, S.; Docheva, M. New Benzothiazole–Monoterpenoid Hybrids as Multifunctional Molecules with Potential Applications in Cosmetics. Molecules 2025, 30, 636. https://doi.org/10.3390/molecules30030636
Kirkova D, Stremski Y, Bachvarova M, Todorova M, Goranov B, Statkova-Abeghe S, Docheva M. New Benzothiazole–Monoterpenoid Hybrids as Multifunctional Molecules with Potential Applications in Cosmetics. Molecules. 2025; 30(3):636. https://doi.org/10.3390/molecules30030636
Chicago/Turabian StyleKirkova, Desislava, Yordan Stremski, Maria Bachvarova, Mina Todorova, Bogdan Goranov, Stela Statkova-Abeghe, and Margarita Docheva. 2025. "New Benzothiazole–Monoterpenoid Hybrids as Multifunctional Molecules with Potential Applications in Cosmetics" Molecules 30, no. 3: 636. https://doi.org/10.3390/molecules30030636
APA StyleKirkova, D., Stremski, Y., Bachvarova, M., Todorova, M., Goranov, B., Statkova-Abeghe, S., & Docheva, M. (2025). New Benzothiazole–Monoterpenoid Hybrids as Multifunctional Molecules with Potential Applications in Cosmetics. Molecules, 30(3), 636. https://doi.org/10.3390/molecules30030636









