Novel and Highly Efficient Carboxylative Cyclization of CO2 to 2-Oxazolidinones Using Nano-SiO2-Supported Ionic Liquid Sustainable Catalysts
Abstract
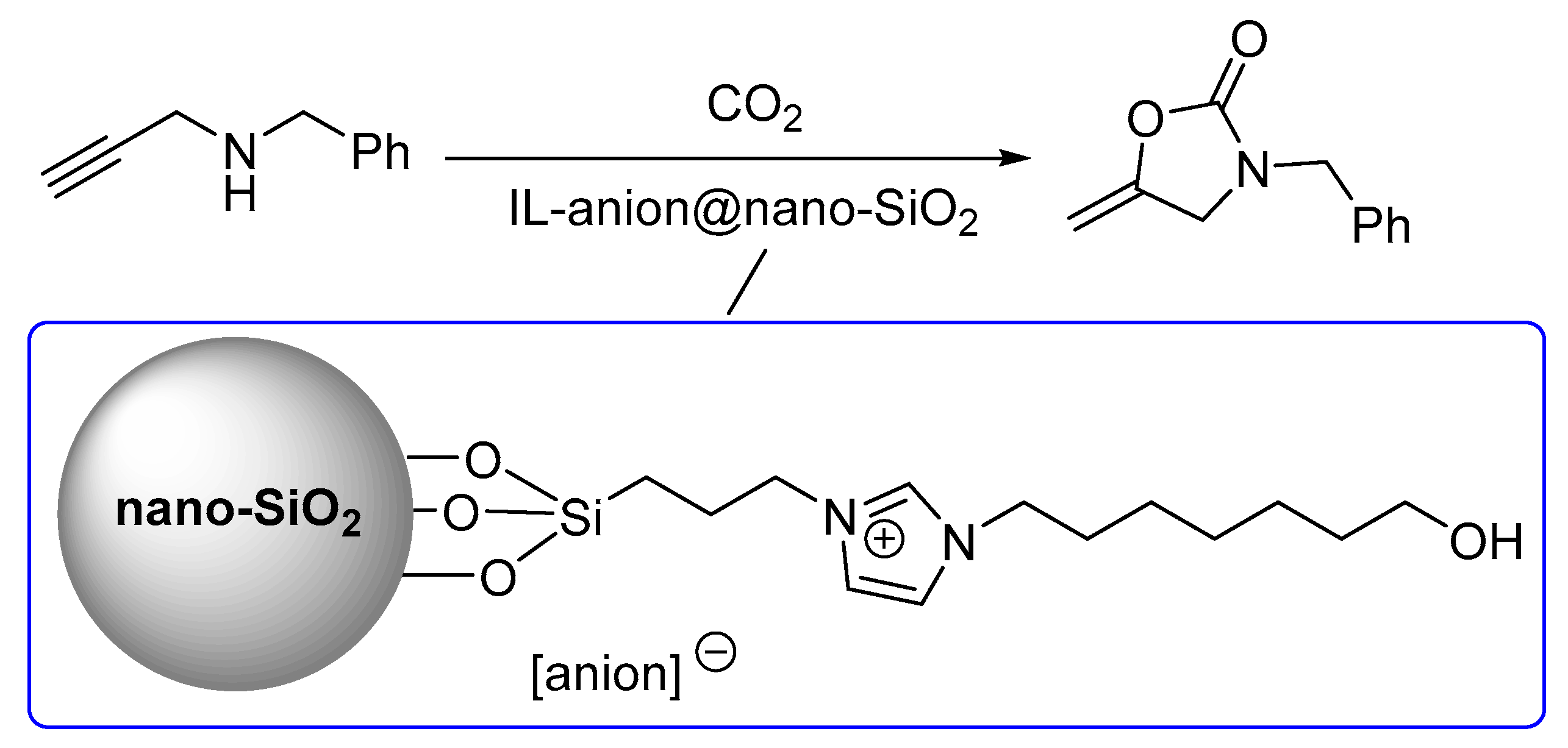
1. Introduction
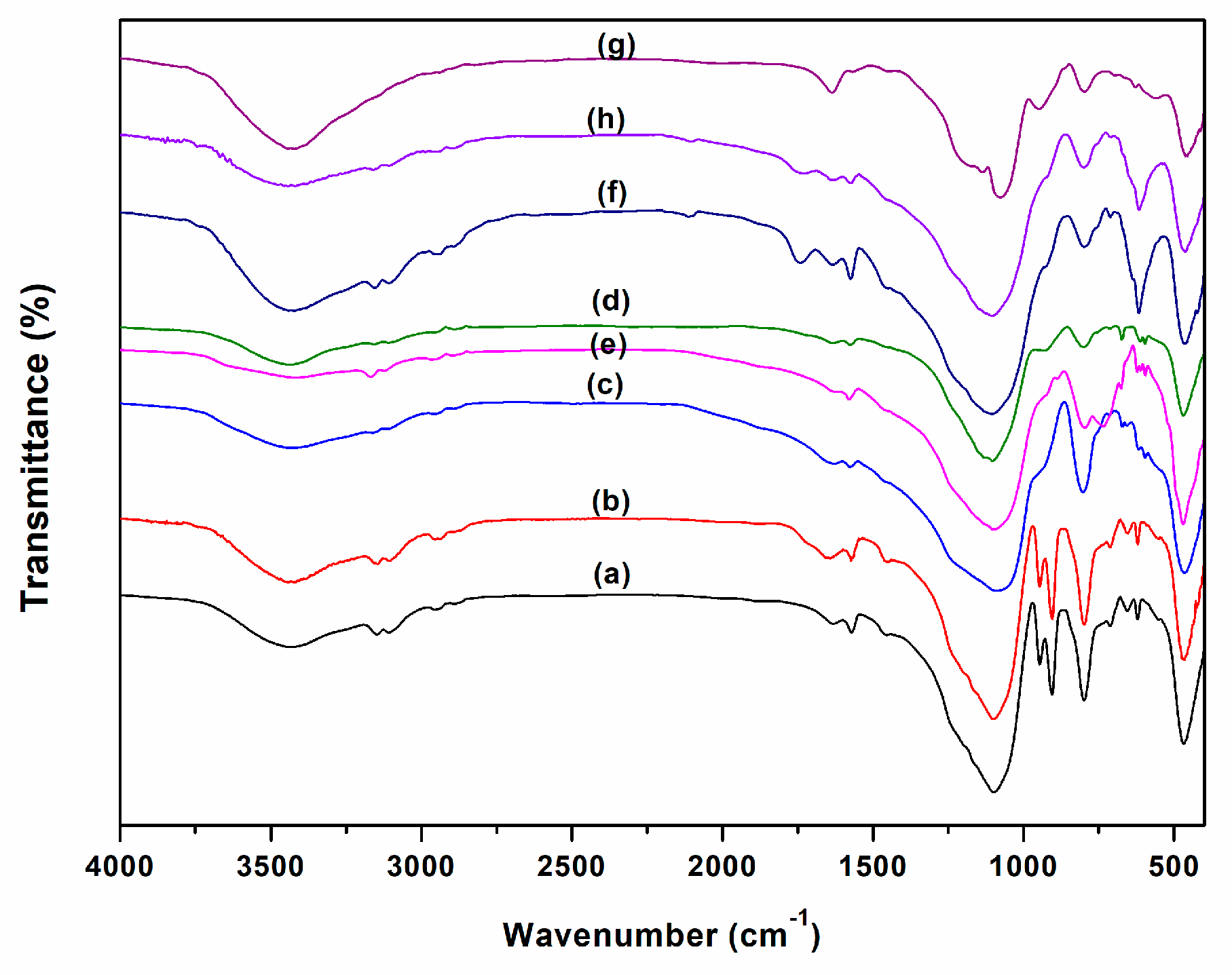
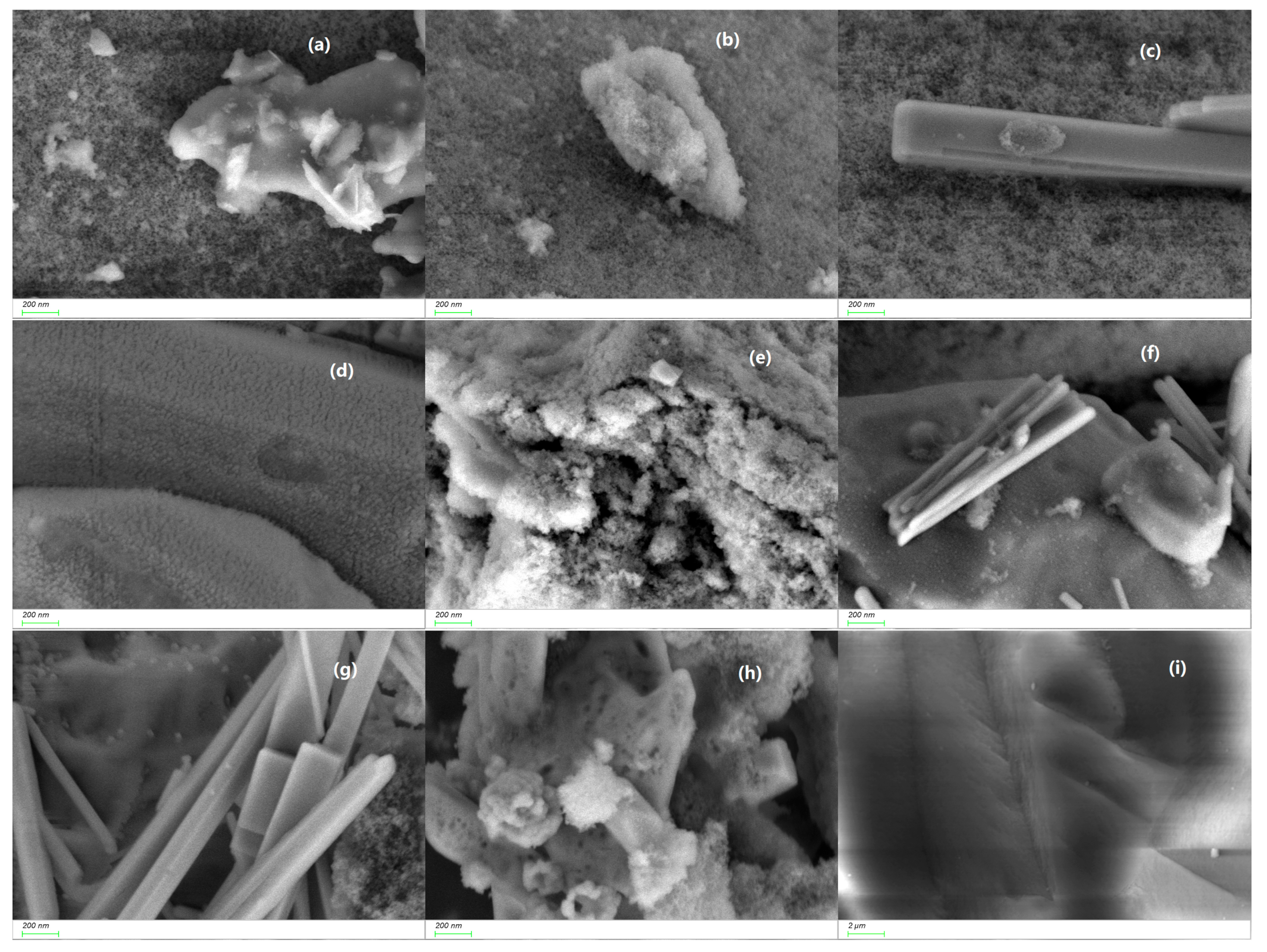
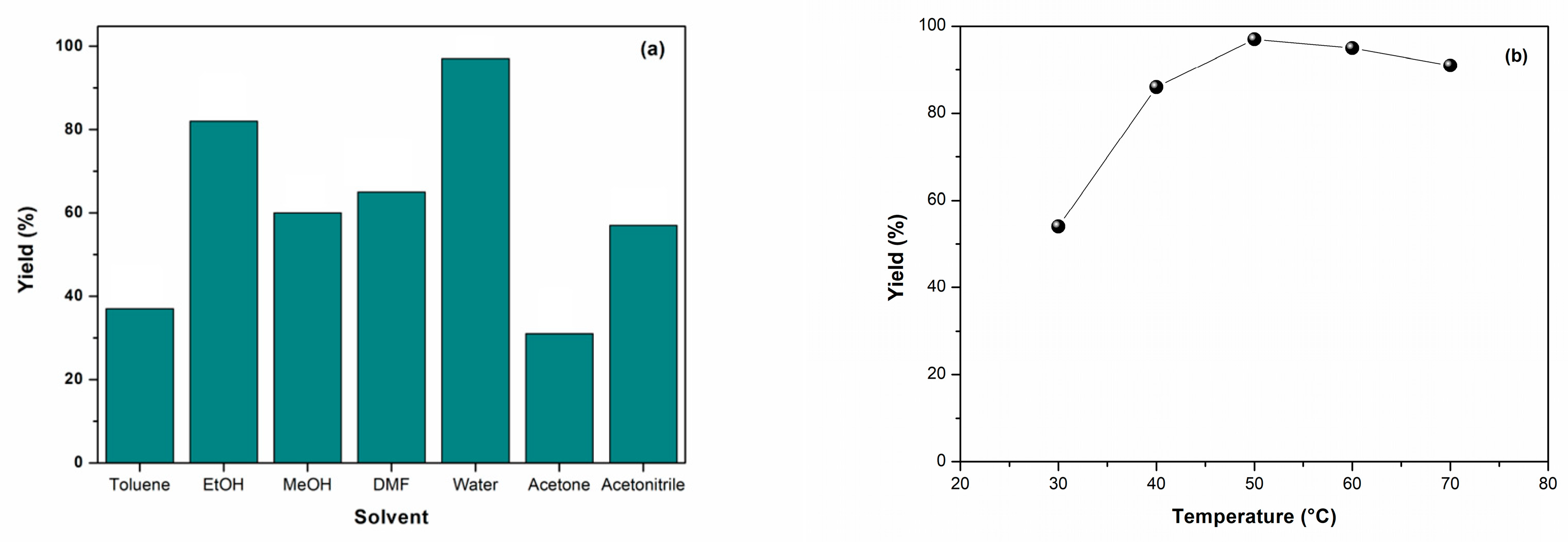
2. Results and Discussion
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sun, Z.; Liao, Y.; Zhao, S.; Zhang, X.; Liu, Q.; Shi, X. Research progress in metal–organic frameworks (MOFs) in CO2 capture from post-combustion coal-fired flue gas: Characteristics, preparation, modification and applications. J. Mater. Chem. A 2022, 10, 5174–5211. [Google Scholar] [CrossRef]
- Chen, T.W.; Chen, S.M.; Anushya, G.; Kannan, R.; Al-Sehemi, A.G.; Alargarsamy, S.; Gajendran, P.; Ramachandran, R. Development of different kinds of electrocatalyst for the electrochemical reduction of carbon dioxide reactions: An overview. Molecules 2023, 28, 7016. [Google Scholar] [CrossRef] [PubMed]
- Jelmy, E.J.; Thomas, N.; Mathew, D.T.; Louis, J.; Padmanabhan, N.T.; Kumaravel, V.; John, H.; Pillai, S.C. Impact of structure, doping and defect-engineering in 2D materials on CO2 capture and conversion. React. Chem. Eng. 2021, 6, 1701–1738. [Google Scholar] [CrossRef]
- Velty, A.; Corma, A. Advanced zeolite and ordered mesoporous silica-based catalysts for the conversion of CO2 to chemicals and fuels. Chem. Soc. Rev. 2023, 52, 1773–1946. [Google Scholar] [CrossRef]
- Khan, M.N.; Ingen, Y.; Boruah, T.; McLauchlan, A.; Wirth, T.; Melen, R.L. Advances in CO2 activation by frustrated Lewis pairs: From stoichiometric to catalytic reactions. Chem. Sci. 2023, 14, 13661–13695. [Google Scholar] [CrossRef]
- Baran, T.; Caringella, D.; Dibenedetto, A.; Aresta, M. Pitfalls in photochemical and photoelectrochemical reduction of CO2 to energy products. Molecules 2024, 29, 4758. [Google Scholar] [CrossRef]
- Arshadi, S.; Vessally, E.; Sobati, M.; Hosseinian, A.; Bekhradnia, A. Chemical fixation of CO2 to N-propargylamines: A straightforward route to 2-oxazolidinones. J. CO2 Util. 2017, 19, 120–129. [Google Scholar] [CrossRef]
- Wang, B.; Guo, Z.; Wei, X. Recent advances on oxazolidinones synthesize from carbon dioxide. J. Fuel Chem. Technol. 2023, 51, 85–99. [Google Scholar] [CrossRef]
- Yoshida, M.; Mizuguchi, T.; Shishido, K. Synthesis of oxazolidinones by efficient fixation of atmospheric CO2 with propargylic amines by using a silver/1,8-diazabicyclo [5.4.0]undec-7-ene (DBU) dual-catalyst system. Chem. Eur. J. 2012, 18, 15578–15581. [Google Scholar] [CrossRef]
- Liu, X.; Wang, M.Y.; Wang, S.Y.; Wang, Q.; He, L.N. In situ generated zinc(II) catalyst for incorporation of CO2 into 2-oxazolidinones with propargylic amines at atmospheric pressure. ChemSusChem 2017, 10, 1210–1216. [Google Scholar] [CrossRef]
- Zhao, Y.; Qiu, J.; Tian, L.; Li, Z.; Fan, M.; Wang, J. New copper(I)/DBU catalyst system for the carboxylative cyclization of propargylic amines with atmospheric CO2: An experimental and theoretical study. ACS Sustain. Chem. Eng. 2016, 4, 5553–5560. [Google Scholar] [CrossRef]
- Wu, Z.L.; Zhai, Y.T.; Bian, G.G.; Guo, L.J.; Zhang, Y.X.; Wei, H.Y. Conversion of propargylic amines with CO2 catalyzed by a highly stable copper(I) iodide thorium-based heterometal–organic framework. Inorg. Chem. 2024, 63, 13450–13458. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.H.; Hu, T.D.; Zhai, Y.T.; Zhang, Y.X.; Wu, Z.L. Stepwise engineering of the pore environment within metal–organic frameworks for green conversion of CO2 and propargylic amines. Green Chem. 2023, 25, 1938–1947. [Google Scholar] [CrossRef]
- Ding, M.; Flaig, R.W.; Jiang, H.L.; Yaghi, O.M. Carbon capture and conversion using metal–organic frameworks and MOF-based materials. Chem. Soc. Rev. 2019, 48, 2783–2828. [Google Scholar] [CrossRef] [PubMed]
- Giri, P.K.; Parihar, V.; Kumar, S.; Nagaraja, C.M. Copper nanoparticles anchored on the metal–organic framework as recyclable catalyst for CO2 fixation to high-value compounds. ACS Appl. Nano Mater. 2024, 7, 15488–15497. [Google Scholar] [CrossRef]
- Ghosh, S.; Molla, R.A.; Kayal, U.; Bhaumik, A.; Islam, S.M. Ag NPs decorated on a COF in the presence of DBU as an efficient catalytic system for the synthesis of tetramic acids via CO2 fixation into propargylic amines at atmospheric pressure. Dalton Trans. 2019, 48, 4657–4666. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, H.; He, X.; Wang, A.; Bai, G.; Lan, X. Covalent organic frameworks embedding single cadmium sites for efficient carboxylative cyclization of CO2 with propargylic amines. Green Chem. 2023, 25, 5557–5565. [Google Scholar] [CrossRef]
- Ghosh, S.; Khan, T.S.; Ghosh, A.; Chowdhury, A.H.; Haider, M.A.; Khan, A.; Islam, S.M. Utility of silver nanoparticles embedded covalent organic frameworks as recyclable catalysts for the sustainable synthesis of cyclic carbamates and 2-oxazolidinones via atmospheric cyclizative CO2 capture. ACS Sustain. Chem. Eng. 2020, 8, 5495–5513. [Google Scholar] [CrossRef]
- Qiu, J.; Qi, X.; Zhu, K.; Zhao, Y.; Wang, H.; Li, Z.; Wang, H.; Zhao, Y.; Wang, J. CuI-anchored porous covalent organic frameworks for highly efficient conversion of propargylic amines with CO2 from flue gas. Green Chem. 2024, 26, 6172–6179. [Google Scholar] [CrossRef]
- Kishan, R.; Rani, P.; Singh, G.; Nagaraja, C.M. Functionalized Covalent Triazine Framework (CTF) for catalytic CO2 fixation and synthesis of value-added chemicals. Cryst. Growth Des. 2024, 24, 7878–7887. [Google Scholar] [CrossRef]
- Matsuo, H.; Choi, J.C.; Fujitani, T.; Fujita, K. Silica-catalyzed carboxylative cyclization of propargylic amines with CO2. Tetrahedron Lett. 2020, 61, 152557. [Google Scholar] [CrossRef]
- Fujita, K.; Inoue, K.; Sato, J.; Tsuchimoto, T.; Yasuda, H. Carboxylative cyclization of propargylic amines with CO2 catalyzed by dendritic N-heterocyclic carbene–gold(I) complexes. Tetrahedron 2016, 72, 1205–1212. [Google Scholar] [CrossRef]
- Tariq, M.; Freire, M.G.; Saramago, B.; Coutinho, J.A.P.; Lopes, J.N.C.; Rebelo, L.P.N. Surface tension of ionic liquids and ionic liquid solutions. Chem. Soc. Rev. 2012, 41, 829–868. [Google Scholar] [CrossRef]
- Ab Rahim, A.H.; Yunus, N.M.; Bustam, M.A. Ionic liquids hybridization for carbon dioxide capture: A review. Molecules 2023, 28, 7091. [Google Scholar] [CrossRef]
- Więcławik, J.; Chrobok, A. Gallium(III)- and indium(III)-containing ionic liquids as highly active catalysts in organic synthesis. Molecules 2023, 28, 1955. [Google Scholar] [CrossRef]
- McNeice, P.; Marr, P.C.; Marr, A.C. Basic ionic liquids for catalysis: The road to greater stability. Catal. Sci. Technol. 2021, 11, 726–741. [Google Scholar] [CrossRef]
- Zhang, S.J.; Lu, X.M. Ionic Liquids: From Fundamental Research to Industrial Applications; Science Press: Beijing, China, 2006. [Google Scholar]
- Wang, M.Y.; Song, Q.W.; Ma, R.; Xie, J.N.; He, L.N. Efficient conversion of carbon dioxide at atmospheric pressure to 2-oxazolidinones promoted by bifunctional Cu(II)-substituted polyoxometalate-based ionic liquids. Green Chem. 2016, 18, 282–287. [Google Scholar] [CrossRef]
- Wu, J.; Niu, J.; Liu, H.; Xie, R.; Zhu, N. Conversion of atmospheric CO2 catalyzed by thiolate-based ionic liquids under mild conditions: Efficient synthesis of 2-oxazolidinones. Org. Biomol. Chem. 2024, 22, 8138–8143. [Google Scholar] [CrossRef]
- Vishwakarma, N.K.; Singh, A.K.; Hwang, Y.H.; Ko, D.H.; Kim, J.O.; Babu, A.G.; Kim, D.P. Integrated CO2 capture-fixation chemistry via interfacial ionic liquid catalyst in laminar gas/liquid flow. Nat. Commun. 2017, 8, 14676. [Google Scholar] [CrossRef]
- Pappuru, S.; Shpasser, D.; Gazit, O. Synthesis of polycarbonates from CO2 promoted by immobilized ionic liquid functionalized di-Mg complex catalyst. ChemCatChem 2023, 15, e202201359. [Google Scholar] [CrossRef]
- Nale, D.B.; Saigaonkar, S.D.; Bhanage, B.M. An efficient synthesis of quinazoline-2,4(1H,3H)-dione from CO2 and 2-aminobenzonitrile using [Hmim]OH/SiO2 as a base functionalized supported ionic liquid phase catalyst. J. CO2 Util. 2014, 8, 67–73. [Google Scholar] [CrossRef]
- Akbari, Z.; Ghiaci, M. Heterogenization of a green homogeneous catalyst: Synthesis and characterization of imidazolium ionene/Br–Cl–@SiO2 as an efficient catalyst for the cycloaddition of CO2 with epoxides. Ind. Eng. Chem. Res. 2017, 56, 9045–9053. [Google Scholar] [CrossRef]
- Martinez, A.S.; Hauzenberger, C.; Sahoo, A.R.; Csendes, Z.; Hoffmann, H.; Bica, K. Continuous conversion of carbon dioxide to propylene carbonate with supported ionic liquids. ACS Sustain. Chem. Eng. 2018, 6, 13131–13139. [Google Scholar] [CrossRef]
- Weiss, E.; Dutta, B.; Kirschning, A.; Abu-Reziq, R. BMIm-PF6@SiO2 microcapsules: Particulated ionic liquid as a new material for the heterogenization of catalysts. Chem. Mater. 2014, 26, 4781–4787. [Google Scholar] [CrossRef]
- Garcia, I.M.; Souza, V.S.; Balhaddad, A.A.; Mokeem, L.; Melo, M.A.S.; Scholten, J.D.; Collares, F.M. Ionic liquid-based silane for SiO2 nanoparticles: A versatile coupling agent for dental resins. ACS Appl. Mater. Interfaces 2024, 16, 34057–34068. [Google Scholar] [CrossRef]
- Li, Z.; Luo, A.; Zhou, R.; Li, X.; Li, H. Preparation of ionic liquid@SiO2 nanocapsules for improving self-lubricating performance of PA6 composites. Polymer 2024, 290, 126537. [Google Scholar] [CrossRef]
- Hu, F.; Qi, F.; Xiang, Z.; Zhang, B.; Qi, F.; Zhao, N.; Ouyang, X. Synergistic enhancement effect of nano-SiO2 and ionic liquids on mechanical properties and impact resistance of polyurethane elastomer. Compos. Commun. 2021, 27, 100876. [Google Scholar] [CrossRef]
- Kumar, H.; Katal, A. Temperature dependent physicochemical and spectroscopic (FT-IR) studies of citrate salts (trilithium citrate and triammonium citrate) in aqueous ionic liquid [C4mim][BF4] (1-butyl-3-methyl imidazolium tetrafluroborate) solutions. J. Mol. Liq. 2018, 256, 148–162. [Google Scholar] [CrossRef]
- Cascales, C.; Blas, A.M.; Rico, M.; Volkov, V.; Zaldo, C. The optical spectroscopy of lanthanides R3+ in ABi(XO4)2 (A = Li, Na; X = Mo, W) and LiYb(MoO4)2 multifunctional single crystals: Relationship with the structural local disorder. Opt. Mater. 2005, 27, 1672–1680. [Google Scholar] [CrossRef]
- Świetlik, R.; Jankowski, D.; Fourmigué, M.; Yakushi, K. Infrared and Raman studies of the anion ordering transitions in paramagnetic organometallic radical cation salts [Cp2Mo(dmit)]X (X = PF6, SbF6). Vib. Spectrosc. 2011, 55, 195–200. [Google Scholar] [CrossRef]
- Lutz, W.; Rüscher, C.H.; Heidemann, D. Determination of the framework and non-framework [SiO2] and [AlO2] species of steamed and leached faujasite type zeolites: Calibration of IR, NMR, and XRD data by chemical methods. Micropor. Mesopor. Mater. 2002, 55, 193–202. [Google Scholar] [CrossRef]
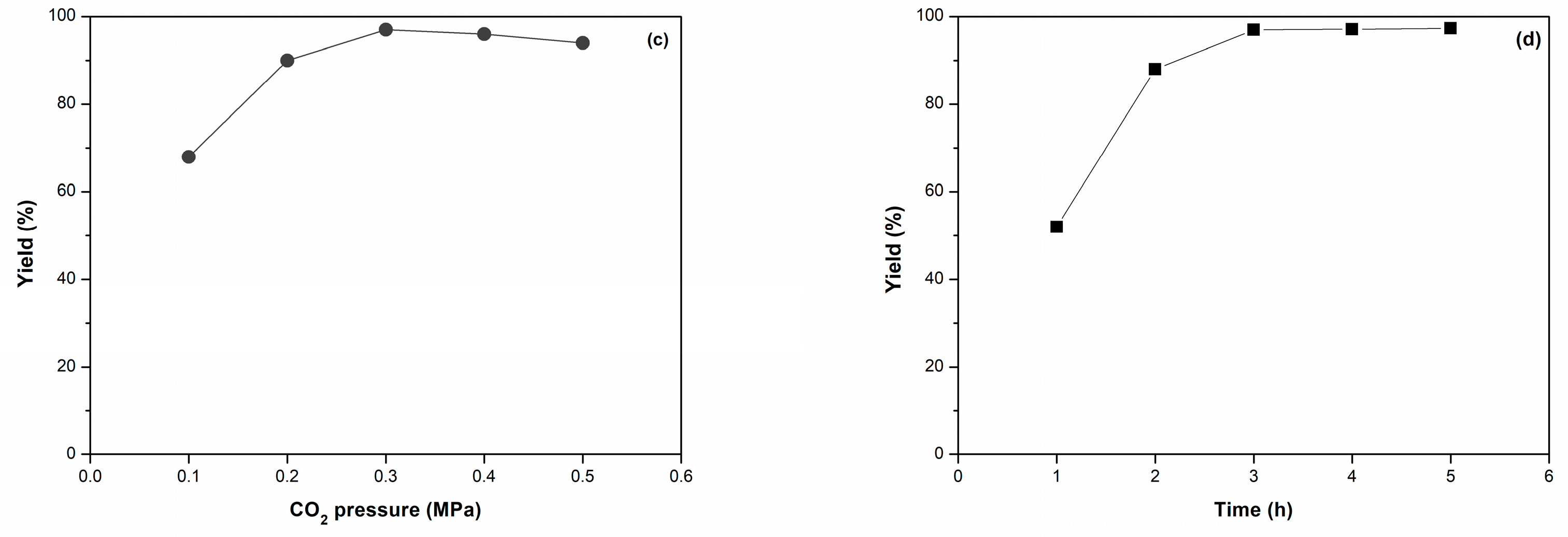
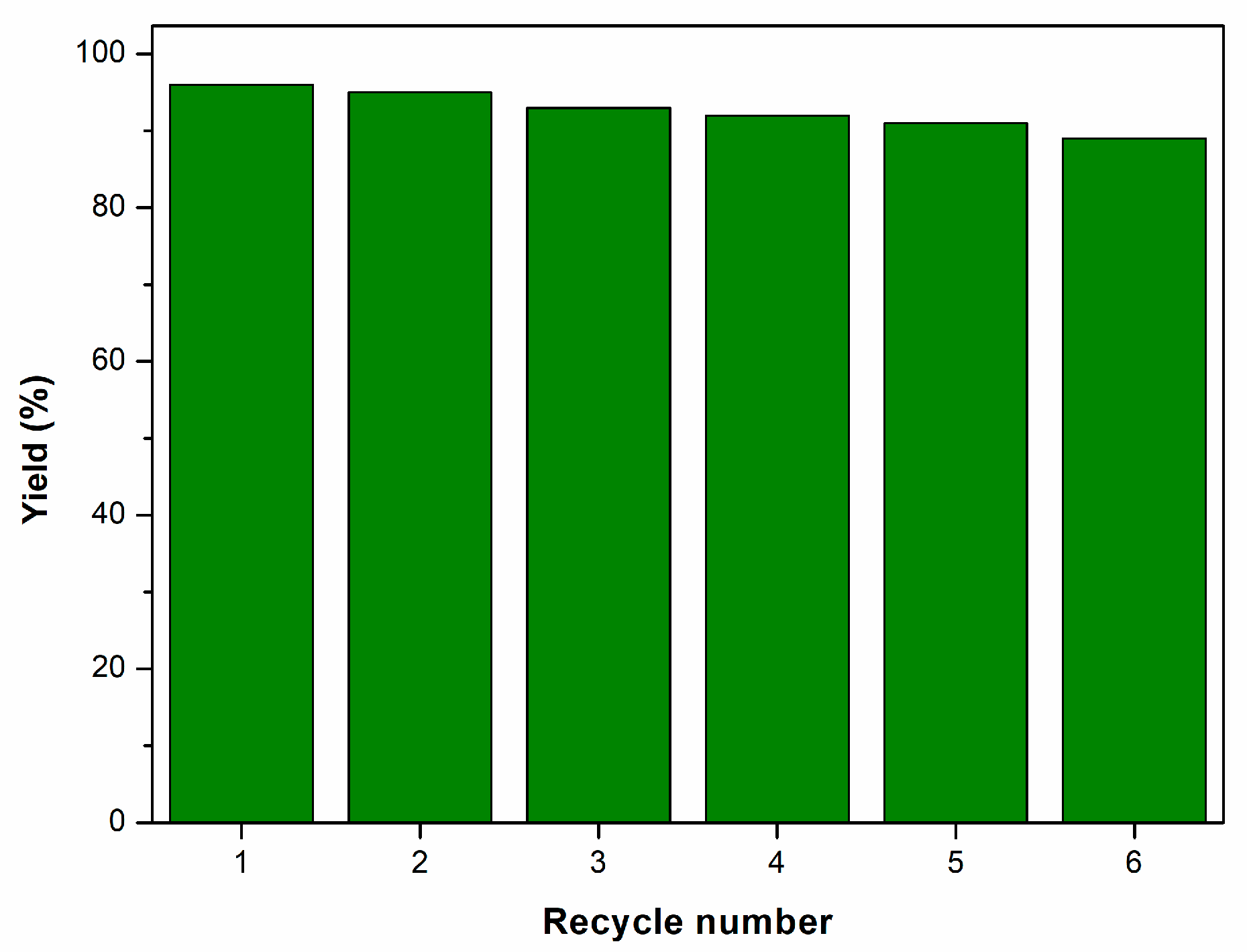
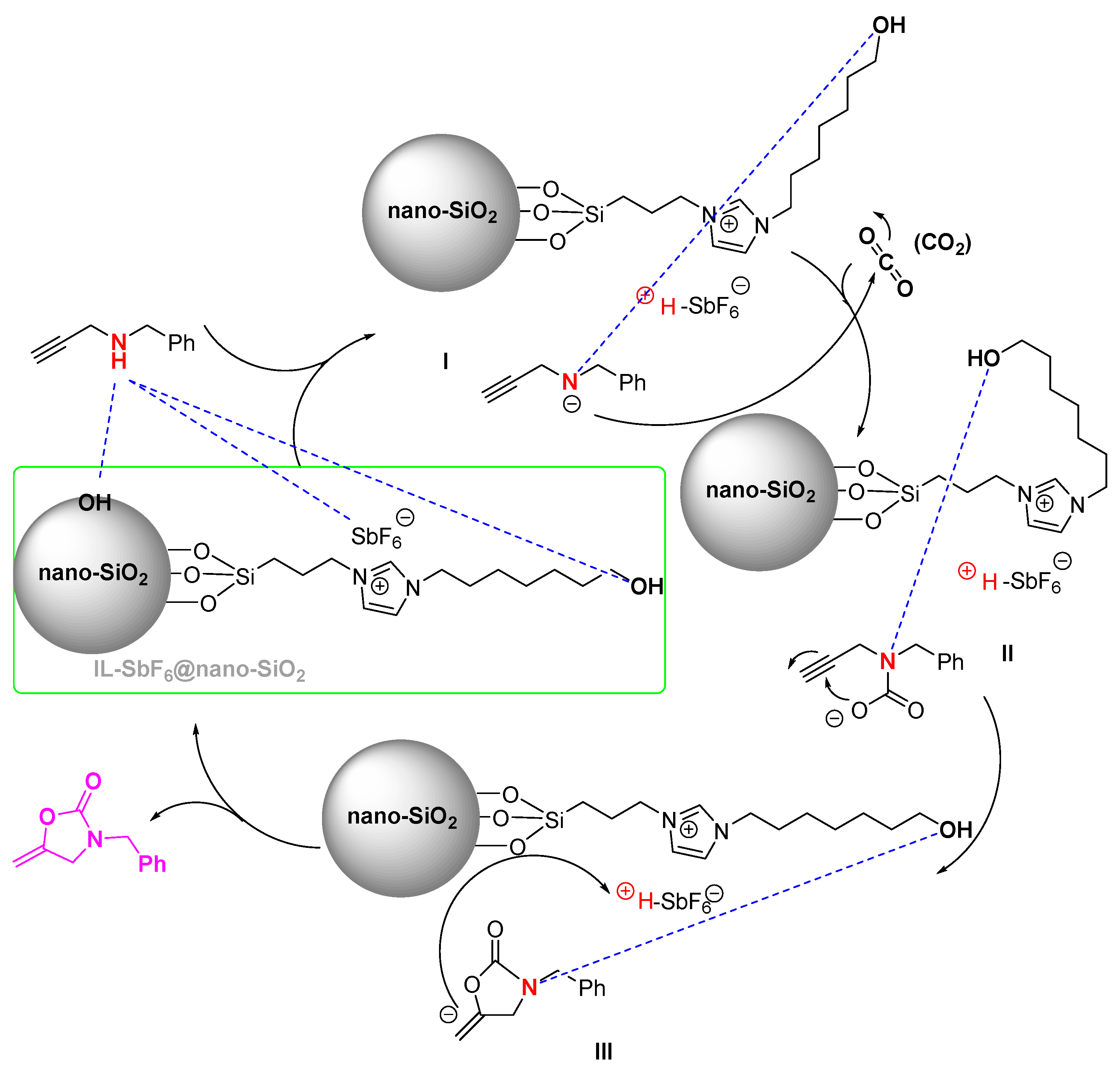
| Entry | Catalyst | Catalyst (g) | Time (h) | Yield (%) b |
|---|---|---|---|---|
| 1 | IL−MoO4@nano−SiO2 | 0.4 | 3 | 84 |
| 2 | IL−OH@nano−SiO2 | 0.4 | 5 | 65 |
| 3 | IL−HOCH2COO@nano−SiO2 | 0.4 | 5 | 60 |
| 4 | IL−BF4@nano−SiO2 | 0.4 | 5 | 70 |
| 5 | IL−SbF6@nano−SiO2 | 0.4 | 3 | 97 |
| 6 | IL−CH3COO@nano−SiO2 | 0.4 | 6 | 56 |
| 7 | IL−AlO2@nano−SiO2 | 0.4 | 3 | 80 |
| 8 | IL−HCO3@nano−SiO2 | 0.4 | 3 | 51 |
| 9 | IL−MoO4 | 0.4 | 3 | 82 |
| 10 | IL−SbF6 | 0.4 | 3 | 87 |
| 11 | IL−AlO2 | 0.4 | 3 | 79 |
| 12 | nano−SiO2 | 0.5 | 24 | trace |
| 13 | none | 0 | 24 | 0 |
| 14 | IL−SbF6@nano−SiO2 | 0.1 | 5 | 49 |
| 15 | IL−SbF6@nano−SiO2 | 0.2 | 5 | 67 |
| 16 | IL−SbF6@nano−SiO2 | 0.3 | 3 | 88 |
| 17 | IL−SbF6@nano−SiO2 | 0.5 | 3 | 97 |
| Entry | Substrate | Product | Time (h) | Yield (%) b |
|---|---|---|---|---|
| 1 | 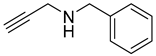 | 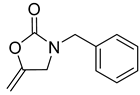 | 3 | 97 |
| 2 | 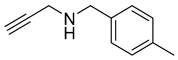 | 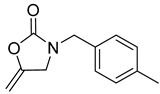 | 3 | 96 |
| 3 | 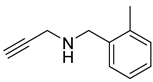 | 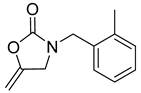 | 3 | 96 |
| 4 | 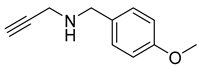 | 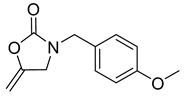 | 3 | 98 |
| 5 | 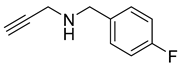 | 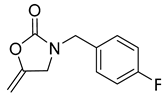 | 3 | 98 |
| 6 | 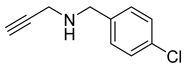 | 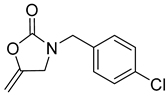 | 3 | 96 |
| 7 | 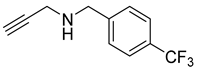 | 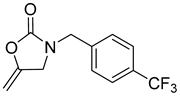 | 3 | 94 |
| 8 | 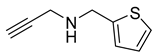 | 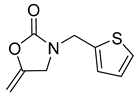 | 3 | 97 |
| 9 | 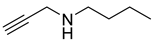 | 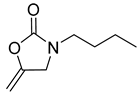 | 4 | 92 |
| 10 | 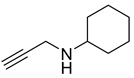 | 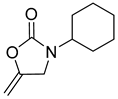 | 4 | 90 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, Y.; Tang, Z.; Liu, X. Novel and Highly Efficient Carboxylative Cyclization of CO2 to 2-Oxazolidinones Using Nano-SiO2-Supported Ionic Liquid Sustainable Catalysts. Molecules 2025, 30, 633. https://doi.org/10.3390/molecules30030633
Hu Y, Tang Z, Liu X. Novel and Highly Efficient Carboxylative Cyclization of CO2 to 2-Oxazolidinones Using Nano-SiO2-Supported Ionic Liquid Sustainable Catalysts. Molecules. 2025; 30(3):633. https://doi.org/10.3390/molecules30030633
Chicago/Turabian StyleHu, Yulin, Zongyan Tang, and Xiaobing Liu. 2025. "Novel and Highly Efficient Carboxylative Cyclization of CO2 to 2-Oxazolidinones Using Nano-SiO2-Supported Ionic Liquid Sustainable Catalysts" Molecules 30, no. 3: 633. https://doi.org/10.3390/molecules30030633
APA StyleHu, Y., Tang, Z., & Liu, X. (2025). Novel and Highly Efficient Carboxylative Cyclization of CO2 to 2-Oxazolidinones Using Nano-SiO2-Supported Ionic Liquid Sustainable Catalysts. Molecules, 30(3), 633. https://doi.org/10.3390/molecules30030633






