Abstract
The polyphenol oxidase (PPO) enzyme leads to undesirable consequences by causing enzymatic browning during the processing of vegetables and fruits. As these browning reactions occur, many phenolic compounds of PPO can lead to significant changes in active metabolites due to substrate utilization. This may cause a loss of appearance and nutritional and commercial value of food. The sickleweed (Falcaria vulgaris Bernh.) plant studied in the current research is considered an edible and medicinal food. In the present research, polyphenol oxidase was purified 15.65-fold with a yield of 23.61% by affinity chromatography. The optimum pH and temperature for catechol, 4-methylcatechol, and 3,4-dihydroxyphenylpropionic acid substrates were determined in separate experiments. For all three substrates, the optimum pH was 7.0, while the optimum temperature was 20 °C. The catalytic efficiency ratio (Vmax/Km) was employed to assess the substrate specificity. Since the highest Vmax/Km ratio reflects the greatest substrate affinity, 4-methylcatechol was identified as the substrate with the highest affinity for sickleweed PPO based on these values. pH stability and thermal stability were examined in the presence of 4-methylcatechol. The inhibitory effects of widely used antibrowning agents, sodium metabisulphite, citric acid, and ascorbic acid, on PPO activity were investigated. The results show that ascorbic acid was the most efficient inhibitor.
1. Introduction
Falcaria vulgaris is a fast-growing edible and medicinal plant from the Apiaceae (Umbelliferae) family, with an average height of 30 cm. In Iğdır province, it is cooked with yogurt or milk and bulgur, roasted with eggs and pickled. The plant’s leaves are called sickleweed and gazeyağı because they resemble a goose’s foot. Geographically, it is spread in America, Europe, Türkiye, Iran, the Caucasus, Central Asia, and Northwest Africa [1,2,3]. In Persian medicine, Falcaria vulgaris Bernh. is widely used to prevent, control, and treat many diseases [4]. Research has demonstrated that sickleweed has anti-inflammatory, antiviral, antibacterial, antifungal, and antioxidant effects [5,6]. The literature reports that sickleweed is used for medical purposes in the Iranian region to treat gastrointestinal [7] and skin diseases [5], fertility regulation [8], heart diseases [9], and as an anti-cancer agent [10]. Falcaria vulgaris Bernh. contains various phenolic constituents and flavonoids, which contribute significantly to the plant’s specific biological activities. Some environmental factors can easily affect the contents of these chemical components. These compounds found in plants may cause a browning reaction due to the activity of polyphenol oxidase during processing, impact, and storage [11].
Polyphenol oxidase (E.C.1.14.18.1) represents a copper-dependent metalloenzyme responsible for enzymatic browning during harvesting, storing, processing, and handling various plant materials. PPO is ubiquitously present in plants and other organisms [12,13]. PPO catalyzes two main reactions: (1) the hydroxylation of monophenols to form o-diphenols, and (2) the oxidation of o-diphenols to produce o-quinones. The products acquired at the end of the oxidation reactions are converted into brown, red, or black pigments by polymerization [14,15]. While these browning reactions occur, many phenolic compounds of PPO may cause significant changes in active metabolites due to substrate use. This may cause a loss of appearance and food’s nutritional and commercial value [13,16].
PPO, one of the enzymes involved in enzymatic browning, has been inactivated by inhibitors, and various studies have been carried out to control this process [17]. Diverse methods for the inhibition of polyphenol oxidase can be applied to prevent enzymatic browning. These methods include removing O2 or substrates from the medium, reducing the pH to two or more units less than the optimum pH, temperature deactivation of the enzyme, adding compounds that inhibit PPO, and using substances that prevent melanin production [18]. The most effective control method is inhibiting enzymatic browning reactions with chemical and natural antibrowning agents [19]. Many chemicals have been used to inhibit the polyphenol oxidase enzyme. A few of these inhibitors can be safely used in the food industry because the chemicals used to inhibit PPO activity in foods should be non-toxic and allergen-free and not alter the food’s structural properties, taste, and aroma. L-cysteine, ascorbic, colic, and citric acids, which prevent enzymatic browning, are inhibitors that can be safely used in the food industry [20]. Although sulfites and their derivatives can prevent enzymatic browning quite effectively, they adversely affect human health [17].
Several studies have researched the impacts of PPO, the causative agent of enzymatic browning, on postharvest and processed food quality in many fruits and vegetables such as Agaricus bisporus [16], purslane [21], potato (Solanum tuberosum) tubers [22], Camellia sinensis [23], rape flower [24], water yam (Dioscorea alata) [25], Ocimum basilicum L. [26] and edible yam (Dioscorea opposita Thunb.) [27]. The literature review shows that PPO from sickleweed has not been purified and characterized. This research aimed to purify, characterize, and inhibit PPO from sickleweed, which has both food and medicinal uses.
2. Results and Discussion
2.1. Purification of PPO from Sickleweed
PPO was purified from sickleweed (Falcaria vulgaris Bernh.) using an affinity column. A qualitative protein assay was performed on the eluates using the Walburg–Christian method [28] and activity determination was conducted using a 4-methylcatechol substrate (Figure 1). Quantitative protein determination in the crude enzyme extract and pure enzyme was performed following the Bradford method [29]. Table 1 contains purification data for PPO.
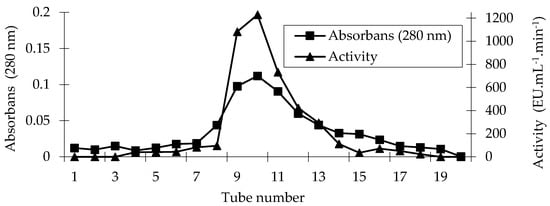
Figure 1.
Affinity chromatography profile of the PPO purified from sickleweed (Falcaria vulgaris Bernh.).

Table 1.
Table for the purification of sickleweed PPO.
PPO from sickleweed was purified 15.65-fold with a 23.61% yield using the affinity column (Table 1). By utilizing the same affinity column, PPO was purified 13.9-fold from Lactarius piperatus [30], 11.2-fold from Cimin grape (Vitis vinifera spp., Cimin) [31], 43-fold from artichoke (Cynara scolymus L.) [32], 5.21-fold from unripe Japanese pear (Pyrus pyrifolia (Burm.) Nakai) fruit, and 9.77-fold from ripe ones [33].
2.2. Native Polyacrylamide Gel Electrophoresis and Sodium Dodecyl Sulfate Polyacrylamide Gel Electrophoresis
The purity of the PPO purified from sickleweed was determined by performing native polyacrylamide gel electrophoresis. The observation of a single band in the Coomassie Brilliant Blue-R250 staining of the resulting gel shows that the enzyme was purified (Figure 2A). Additionally, another gel prepared with the same properties was stained with Levodopa (L-DOPA) as a substrate staining solution, and a single band was obtained (Figure 2B). These results show that the enzyme was purified and that the purified enzyme was PPO (Figure 2). To demonstrate the purity of the PPO purified from different sources, native PAGE was carried out, the gel was stained with Coomassie Brilliant Blue-R250, and a single band was found. L-DOPA was also used for substrate staining, and a single band was found [15,34,35,36].

Figure 2.
Native PAGE staining of the PPO purified from sickleweed (Falcaria vulgaris Bernh.) (A) Coomassie Brilliant Blue-R250 staining (B) 24 mM L-DOPA staining.
The molecular weight of the PPO purified from sickleweed was found to be approximately 67.60 kDa using the Log Mw-Rf graph by SDS-PAGE (Figure 3). The literature review showed that the PPO purified from different sources had different molecular weights. The molecular weight of PPO was calculated as 65 kDa for mulberry (Morus alba L.) [37], 60 kDa for oil lettuce (Lactuca sativa var. capitata L.) [38], 72.44 kDa for plum (Prunus domestica) [39], 50 kDa for tea leaf (Camellia sinensis) [40] and 40 kDa for Boletus erythropus [14].
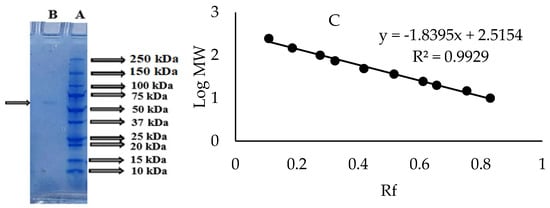
Figure 3.
SDS-PAGE image (A) Protein standard (B) Coomassie Brilliant Blue-R250 staining of the PPO purified from sickleweed (Falcaria vulgaris Bernh.) (C) Log Mw-Rf graph.
2.3. The Optimum pH
Enzymes affect the ionization of amino acids in the active site depending on the pH level of their medium. This changes the enzyme’s conformation and affects the substrate’s binding strength and affinity. Generally, the optimum pH of PPO varies depending on the substrates used and the enzyme source and extraction method [41]. The optimum pH of sickleweed PPO was 7.0 for catechol, 4-methylcatechol, and DHPPA substrates (Figure 4). For PPO from corn stover, the optimum pH was 8.0 for catechol and 6.0 for 4-methylcatechol [42], For LacPPO, the optimum pH was 5.0 for 4-methylcatechol and 7.0 for DHPPA [43]. For PPO from Kirmizi Kismis grape (Vitis vinifera L.), the optimum pH was 5.0 for the 4-methylcatechol substrate [44]. For PPO from Cistanche deserticola, the optimum pH was 7.0 for the catechol substrate [45].
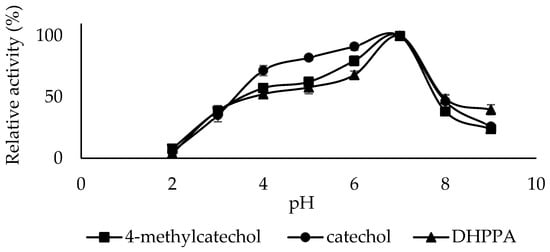
Figure 4.
Optimum pH plots of PPO purified from sickleweed (Falcaria vulgaris Bernh.) using three substrates: catechol, 4-methylcatechol, and DHPPA. Experiments were performed in triplicate, and error bars represent standard deviations.
2.4. Determination of the Optimum Temperature
The optimum temperature for PPO activity can vary based on the enzyme’s source, the environmental conditions under which the source is cultivated, and the specific substrate employed [46]. It is, therefore, essential to research the optimum temperature for polyphenol oxidase enzymes isolated from new sources. The optimum temperature for PPO in the presence of 4-methylcatechol, catechol, and DHPPA substrates was found to be 20 °C (Figure 5). For LacPPO, the optimum temperature was 20 °C for 4-methylcatechol and 30 °C for DHPPA [43]. For PPO from Sarali plum, the optimum temperature was 20 °C for catechol and -4-methylcatechol [39]. For PPO from the persimmon fruit, the optimum temperature was 20 °C for catechol, 40 °C for 4-methylcatechol, and 60 °C for DHPPA [47].
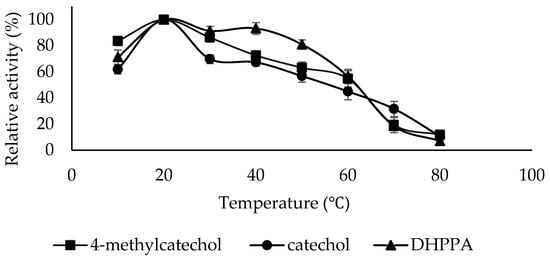
Figure 5.
Optimum Temperature plots of PPO purified from sickleweed (Falcaria vulgaris Bernh.) using three substrates: catechol, 4-methylcatechol, and DHPPA. Experiments were performed in triplicate, and error bars represent standard deviations.
2.5. Km and Vmax
The Michaelis-Menten constant (Km) and the maximum velocity (Vmax) values of PPO in the presence of 4-methylcatechol, catechol, and DHPPA substrates were calculated by utilizing the Lineweaver–Burk plots. Table 2 lists the Km, Vmax, and Vmax/Km values, and the results are compared with those of other studies in the literature. The Km and Vmax values of sickleweed PPO in the presence of 4-methylcatechol, catechol, and DHPPA were computed to be 2 mM and 500 EU·mL−1·min−1 (Figure 6), 5 mM and 10,000 EU·mL−1·min−1 (Figure 6), and 11.43 mM and 14,285.71 EU·mL−1·min−1 (Figure 6), respectively. This study used the catalytic efficiency ratio (Vmax/Km) to determine the substrates’ specificity [48]. The (Vmax/Km) ratios for catechol, 4-methylcatechol, and DHPPA substrates were calculated as 2000 (EU·mL−1·min−1), 2500 (EU·mL−1·min−1), and 1249.84 (EU·mL−1·min−1), respectively. The highest (Vmax/Km) value indicates the best substrate affinity. Among the substrates studied, sickleweed PPO had the highest (Vmax/Km) value for 4-methylcatechol (Table 2). The literature has reported that the wide range of Km values of PPO originates from different source types, the use of different purification methods, substrates, and buffers [49].

Table 2.
Comparison of Km and Vmax values from the current work with other studies in the literature.
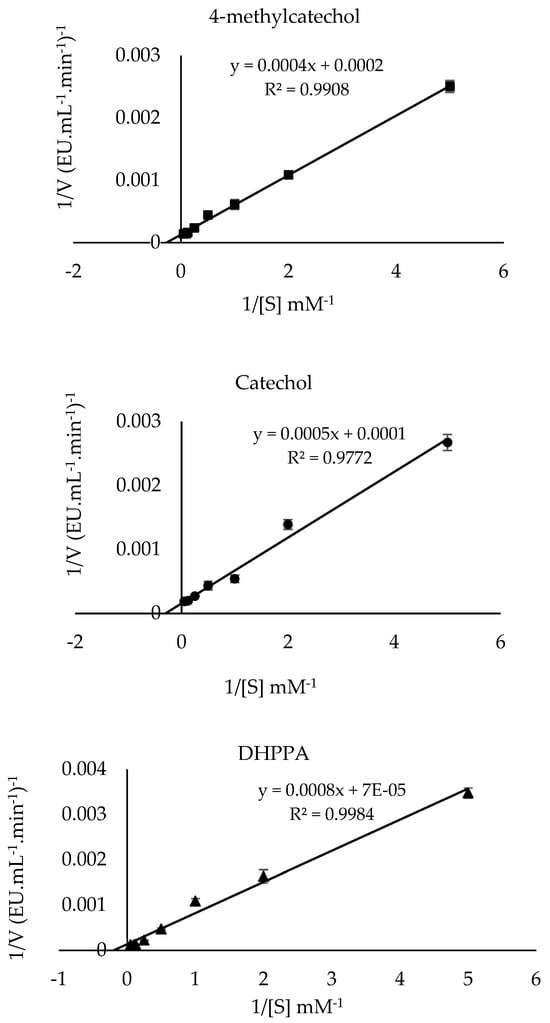
Figure 6.
Lineweaver–Burk plots of PPO purified from sickleweed (Falcaria vulgaris Bernh.) using three substrates: 4-methylcatechol, catechol, and DHPPA. Experiments were performed in triplicate, and error bars represent standard deviations.
2.6. pH Stability
Temperature and pH are two parameters that influence the enzyme’s catalytic activity. pH and temperature can vary in stability depending on the source of enzymes, the buffers, and the substrates used. These two parameters should work to control the enzyme’s activity [20,48]
The enzyme activity was maintained at different rates at pH 6.0, 7.0, and 8.0 for 24, 48, 72, and 96 h. After 24 h of incubation at 4 °C using 4-methylcatechol as a substrate, PPO activity was preserved over 90% at pH 6.0, 7.0, and 8.0. PPO activity was maintained at 53.56%, 58.51%, and 37.78% at pH 6.0, 7.0, and 8.0 for 96 h of incubation at 4 °C (Figure 7). PPO from the snake fruit was found to retain more than 80% of its initial activity in the pH range of 6.0–6.5 after 24 h of incubation at 4 °C and lost almost 50% of its initial activity above pH 7.5 [52]. Upon investigating the pH stability of PPO purified from Macrolepiota gracilenta, it was reported that after 5 days of incubation at 4 °C, PPO retained its activity by 62% at pH 5.0 and 42% at pH 7.0 and lost its activity at pH 8.0 [15]. The pH stability of Ispir sugar bean was examined in the range of 4.0–8.5 for 10 days, and it was found that the enzyme activity was highest at pH 6.0 and was maintained at different rates at other pH values [53].
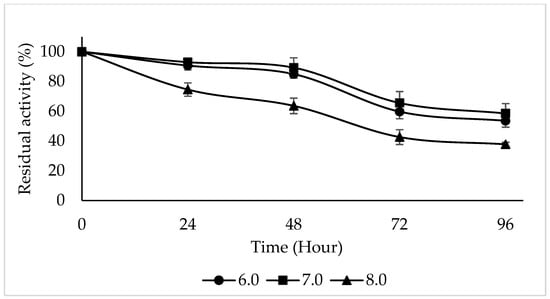
Figure 7.
pH stability of PPO purified from sickleweed (Falcaria vulgaris Bernh.) was evaluated in the pH range 6.0–7.0–8.0 after incubation at 4 °C for 24, 48, 72, and 96 h using 4-methylcatechol as the substrate. Experiments were performed in triplicate, and error bars represent standard deviations.
2.7. Thermal Stability
To determine the thermal stability of PPO, the enzyme solution was studied in the range of 10–70 °C in 10 °C increments for 20, 40, and 60 min using the 4-methylcatechol substrate (Figure 8A,B). The thermal stability results showed that the enzyme maintained its activity best at 20 °C after 60 min of incubation. At higher temperatures, the enzyme activity gradually decreased with increasing temperature and incubation time (Figure 8B). After 60 min of incubation, the enzyme activity was retained by 28.37% at 60 °C and 15.08% at 70 °C, respectively (Figure 8B). The thermal stability of Sarali plum (Prunus domestica) PPO was investigated in the presence of catechol and 4-methylcatechol, and it was found that the enzyme activity was maintained by 92% and 88% at 4 °C for 90 min, respectively. At 60 °C, the enzyme activity was preserved by 22% and 30.5% in the presence of catechol and 4-methylcatechol, respectively. At 70 °C, the activity was preserved by 6.6% and 5.1% at the end of 90 min, respectively [39]. The PPO obtained from the banana was thermally stable at 30 °C. Even at temperatures above 60 °C, PPO could retain some of its activity [54]. The PPO from green bean (Phaseolus vulgaris L.) was thermally stable between 0 and 40 °C for 30 min [55]. Medlar fruit PPO was moderately stable at the optimum temperature and up to 60 °C for 30 min. It was reported that enzyme activity decreased after 10 min of incubation at high temperatures, and this loss of activity could be due to deterioration in the enzyme’s secondary and tertiary structures [51].
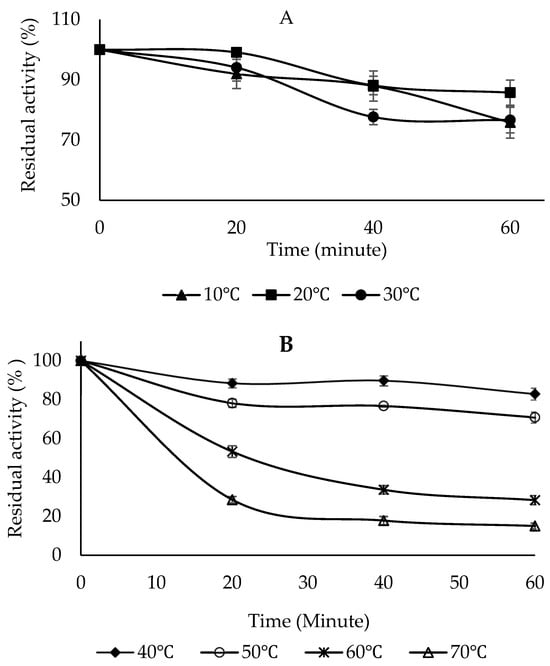
Figure 8.
Temperature stability of PPO purified from sickleweed (Falcaria vulgaris Bernh.). (A) Enzyme activity after incubation at 10, 20, and 30 °C for 20, 40, and 60 min. (B) Enzyme activity after incubation at 40, 50, 60, and 70 °C for 20, 40, and 60 min. 4-Methylcatechol was used as the substrate. Experiments were conducted in triplicate, and error bars indicate standard deviations.
2.8. Inhibition
The mechanism of L-ascorbic acid inhibition is that PPO oxidizes phenolic substrates to o-quinones, whereas L-ascorbic acid converts the formed o-quinones back into phenolic compounds [56]. Additionally, ascorbic acid can cause PPO inhibition by reducing the pH value of the medium to which it is added or by capturing the oxygen in the medium necessary for the PPO reaction [57,58]. The literature has described the inhibitory mechanism of citric acid as follows. It causes PPO inhibition by lowering the pH value or chelating copper in the enzyme’s active site [59]. It has been suggested that SH groups display a strong affinity to copper, potentially displacing histidine residues coordinated to the copper in PPO’s active site or even causing the copper to be removed from the enzyme entirely. Furthermore, PPO inhibitors are categorized into two groups: those interacting with the copper binding site and those affecting the phenolic site. The category interacting with the copper site displays competitive inhibition. The other category exhibits non-competitive inhibition [40]. The present research found the IC50 values for ascorbic acid, sodium metabisulphite, and citric acid to be 0.026 mM, 0.027 mM, and 31.98 mM, respectively. The Ki constants and modes of inhibition were determined for the three inhibitors from the Lineweaver-Burk plots (Figure 9). The lowest Ki constant was observed for ascorbic acid (0.0101 mM), followed by sodium metabisulphite (0.0237 mM) and citric acid (8.73 mM) (Figure 9). The present research findings demonstrated that ascorbic and citric acids exhibited competitive inhibition, while sodium metabisulphite demonstrated non-competitive inhibition. Ascorbic acid showed the most potent inhibition among the inhibitors tested. Table 3 presents a comparison of this research with other studies in the literature. The literature has reported that the type of inhibition varies depending on the source of PPO, the substrate, and the inhibitor studied [60].
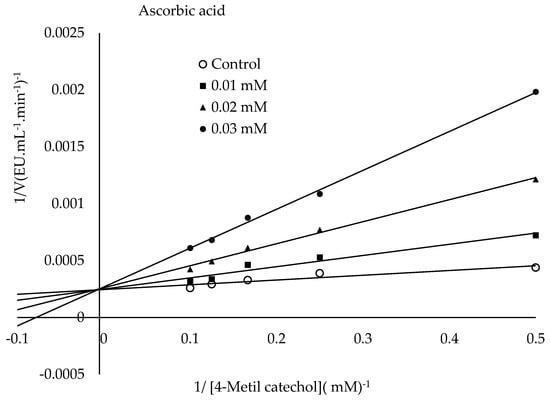
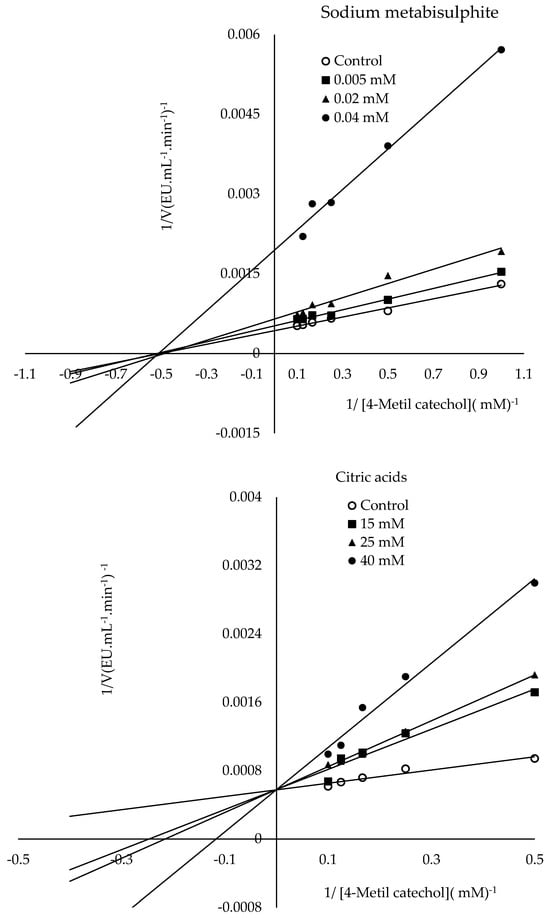
Figure 9.
Lineweaver–Burk plots were drawn to determine the Ki constants of ascorbic acid, sodium metabisulfite, and citric acid in the presence of 4-methylcatechol as the substrate.

Table 3.
Comparison of IC50 and Ki values from this study with those from other studies in the literature.
3. Materials and Methods
3.1. Materials and Chemicals
The plant used in this study, sickleweed (Falcaria vulgaris Bernh.), was obtained from a local market in Iğdır province, Turkey, in June 2024, and stored at −20 °C until use. The plant was identified by Prof. Dr. Ahmet Zafer TEL and deposited at the Iğdır University National Wildlife Museum (INWM) Herbarium under the accession number INWM00000236.
All chemicals and reagents employed in this study were of analytical grade. Sodium acetate (CH3COONa), potassium phosphate monobasic (KH2PO4), potassium phosphate dibasic (K2HPO4), tris base, glycine, 3-methyl-2-benzothiazolinone hydrazone (MBTH), HCl, cyanogen bromide-activated-Sepharose™ 4B, dimethylformamide (DMF), L-3,4-dihydroxyphenylalanine (L-DOPA), 4-methylcatechol, ascorbic acid, sodium metabisulfite, citric acid, catechol, Coomassie Brilliant Blue R-250 and DHPPA were purchased from Sigma Chem. Co. (St. Louis, MO, USA) and Merck A.G. (Darmstadt, Germany). Spectrophotometric measurements were carried out using a UV–Vis Spectrophotometer (Agilent Cary 60, Santa Clara, CA, USA). Native and SDS–PAGE electrophoresis experiments were performed using a Bio-Rad electrophoresis system (Hercules, CA, USA).
3.2. Methods
3.2.1. Preparation of the Crude Enzyme Extract
Five grams were taken from the sickleweed plant, put into the mortar, and crushed. After several freeze–thaw cycles, 10 mL of 50 mM pH 5.0 acetate buffer containing 1% PEG was added. The mixture was thoroughly mixed, filtered, and centrifuged at 10,000 rpm for a period of 30 min, and a supernatant was utilized as crude enzyme extract [62].
3.2.2. Determination of Polyphenol Oxidase Activity
PPO activity was determined by measuring the increase in absorbance at 496 nm for 4-methylcatechol and 500 nm for catechol and DHPPA with a UV–Vis spectrophotometer [63]. PPO activity was detected by the increment in absorbance in 1 min by adding 100 μL of the substrate (100 mM), 100 μL of 3-Methyl-2-benzothiazolinone hydrazone (MBTH, 10 mM), 20 μL of Dimethylformamide (DMF), 680 μL of 50 mM pH 5.0 acetate buffer, and finally 100 μL of the enzyme. The blank contains all solutions, excluding the enzyme. The enzyme unit is defined as the amount of the enzyme producing an absorbance increase of 0.001 per minute in 1 mL of the reaction mixture. The specific activity of PPO was defined as units per mg of protein [64].
3.2.3. Purification of Polyphenol Oxidase from Sickleweed
The was synthesized following Arslan et al. (2004) [37]. The column was balanced with sodium acetate buffer (50 mM pH 5.0). The enzyme was applied to an affinity column. The column was washed with 50 mM pH 5.0 sodium acetate buffer with the objective of removing impurities. The PPO enzyme retained on the affinity column was collected in affinity column (Sepharose-4B-l-Tyr-p-amino benzoic acid) tubes of 2 mL each using 50 mM pH 8.0 phosphate buffer containing 1 M NaCl. Protein and activity determination was performed separately in the collected tubes.
3.2.4. Native-PAGE and SDS-PAGE
A 5% loading gel and a 12% separating gel were used for native PAGE [65]. After the gels were ready, they were placed in the tank and loaded with proteins. The tank was placed in a container filled with ice to prevent protein denaturation. After the gel was run, it was removed from the tank and stained with a freshly prepared 24 mM L-DOPA substrate solution.
A 5% loading gel and a 12% separating gel were used for SDS-PAGE [65]. The proteins were subjected to the steps required for SDS-PAGE. At the end of the run, the gel was costained with Coomassie Brilliant Blue R-250. The bands were visualized when they became visible.
3.2.5. Optimum pH
Glycine-HCI (pH 2.0–3.0), sodium acetate (pH 4.0–5.0), phosphate (pH 6.0–7.0), and tris-HCl (pH 8.0) buffers at a concentration of 50 mM were utilized to investigate the impact of pH on polyphenol oxidase activity [66]. The pH value at which polyphenol oxidase activity was highest was considered 100%. Other values were calculated as % relative activity over this value, and the pH-% relative activity graph was plotted.
3.2.6. Optimum Temperature
To assess the impact of temperature on PPO activity, the enzyme was measured in a water bath at temperatures between 10 and 70 °C. The reaction medium containing the buffer (at optimum pH) and substrate were kept in the water bath at the specified temperatures for 10 min, following which enzyme activity was measured. The obtained data were analyzed and a plot of percent relative activity versus temperature was constructed [15].
3.2.7. Kinetic Studies
To determine substrate specificity, catechol (0.2–20 mM), 4-methylcatechol (0.2–20 mM), and DHPPA (0.2–20 mM) concentrations were studied. Enzyme activity was carried out at optimum temperature and pH. The kinetic data were analyzed in terms of 1/activity (1/V) and 1/substrate concentration ([S]). The Michaelis-Menten constant (Km) and the maximum velocity (Vmax) were computed by plotting the Lineweaver-Burk graph. The catalytic efficiency ratio (Vmax/Km) was used as a criterion to evaluate substrate specificity [67].
3.2.8. Thermal Stability
The thermal stability of the purified PPO was studied by keeping the enzyme for 20, 40, and 60 min in the temperature range of 10–70 °C using 4-methylcatechol as a substrate. At the end of the period, the enzyme was put in an ice container for 5 min and brought to room temperature. The activity of the enzyme was carried out under determined optimum conditions. The activity of the unincubated PPO was taken as 100%, the required calculations were performed, and the graph was plotted [43].
3.2.9. pH Stability
Phosphate (pH 6.0–7.0, 50 mM) and 50 mM tris-HCl (pH 8.0) buffers were used to investigate the pH stability of PPO. The enzyme was mixed with the specified buffers at a 1:1 ratio and incubated at a temperature of 4 °C for 24, 48, 72, and 96 h. The activity of the enzyme was carried out under determined optimum conditions. The activity of the unincubated PPO was taken as 100%, the required calculations were performed, and the graph was plotted [51].
3.2.10. Inhibition of PPO
The common inhibitors of PPO were preferred to study the inhibitory effects on PPO activity. For the 4-methylcatechol substrate, sodium metabisulphite (0.005–0.1 mM), citric acid (5–60 mM), and ascorbic acid (0.005–0.04 mM) inhibitors were utilized to determine enzyme activities under optimum conditions. The half maximal inhibitory concentration (IC50), which halves the enzyme activity, was calculated using the graph of percentage residual activity (%) versus inhibitor concentration (enzyme activity in the absence of inhibitor was accepted as one hundred percent). Then, enzyme activities were studied at five substrate concentrations and three inhibitor concentrations under optimum conditions. The Lineweaver-Burk graphs were drawn by calculating 1/V and 1/[S] values with the obtained data. From these graphs, inhibition constant (Ki) values were calculated for each inhibitor, and the inhibition type was determined [67].
4. Conclusions
In the present research, PPO was purified from sickleweed (Falcaria vulgaris Bernh.) using affinity chromatography. Native and SDS-PAGE showed the enzyme’s purity. The optimum pH and temperature values for PPO were determined to be similar to those found in the literature. Substrate specificity was studied in the presence of catechol, 4-methylcatechol, and DHPPA substrates. By calculating the catalytic activity (Vmax/Km) values, it was determined that the enzyme had the highest affinity for 4-methylcatechol. IC50 and Ki values and types of inhibition were found for ascorbic acid, citric acid, and sodium metabisulfite. The best inhibitor was ascorbic acid. Sickleweed is an edible and medicinal plant. Hence it is important to control PPO activity to prevent browning during sickleweed processing and storage. In addition, the purification and inhibition of PPO from the sickleweed plant may contribute to preserving and increasing the effectiveness of the plant’s biological and medicinal activities by preventing the oxidation of phenolic compounds.
Author Contributions
Conceptualization, A.T.; methodology, A.T.; formal analysis, A.T. and C.B.A.O.; investigation, C.B.A.O.; data curation, A.T.; writing—original draft, A.T.; writing—review and editing, A.T. and C.B.A.O.; visualization, A.T.; supervision, A.T.; project administration, A.T.; funding acquisition, A.T. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by Iğdır University Scientific Research Projects Coordinatorship (Project No. TBY0624Y22).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Acknowledgments
The authors would like to thank Iğdır University (BAP, Project No: TBY0624Y22) for their financial support. The authors would like to thank Ahmet Zafer Tel, a member of the Department of Agricultural Biotechnology, Faculty of Agriculture, Iğdır University, for identifying the plant that was used in this master’s thesis.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
| PPO | Polyphenol oxidase |
| Vmax/Km | Catalytic efficiency ratio |
| L-DOPA | Levodopa |
| DHPPA | 3,4-Dihydroxyphenylpropionic acid |
| Km | Michaelis-Menten constant |
| Vmax | Maximum velocity |
| IC50 | Half maximal inhibitory concentration |
| Ki | Inhibition constant |
| Native-PAGE | Native Polyacrylamide Gel Electrophoresis |
| SDS-PAGE | Sodium Dodecyl Sulfate Polyacrylamide Gel Electrophoresis |
| MBTH | 3-Methyl-2-benzothiazolinone hydrazone |
| DMF | Dimethylformamide |
| Affinity column | Sepharose-4B-l-Tyr-p-amino benzoic acid |
References
- Cakir, E.A. Traditional knowledge of wild edible plants of Igdir Province (East Anatolia, Turkey). Acta Soc. Bot. Pol. 2017, 86, 3568. [Google Scholar] [CrossRef]
- Ozturk, H.I.; Nas, H.; Ekinci, M.; Turan, M.; Ercisli, S.; Narmanlioglu, H.K.; Yildirim, E.; Assouguem, A.; Almeer, R.; Sayed, A.A.; et al. Antioxidant Activity, Phenolic Composition, and Hormone Content of Wild Edible Vegetables. Horticulturae 2022, 8, 427. [Google Scholar] [CrossRef]
- Rahimi, M.; AhmadiAfzadi, M.; Kordrostami, M. Genetic diversity in Sickleweed (Falcaria vulgaris) and using stepwise regression to identify markers associated with traits. Sci. Rep. 2023, 13, 12142. [Google Scholar] [CrossRef] [PubMed]
- Jafari, Z.; Farzaei, M.H.; Morovati, M.R.; Foroughini, A. Potential therapeutic effects of Falcaria vulgaris Bernh: A systematic review. J. Rep. Pharm. Sci. 2022, 11, 18–27. [Google Scholar] [CrossRef]
- Khazaei, M.; Salehi, H. Protective effect of Falcaria vulgaris extract on ethanol induced gastric ulcer in rat. Iran. J. Pharmacol. Ther. 2006, 5, 43–46. [Google Scholar]
- Shafaghat, A. Free radical scavenging and antibacterial activities, and GC/MS analysis of essential oils from different parts of Falcaria vulgaris from two regions. Nat. Prod. Commun. 2010, 5, 981–984. [Google Scholar] [CrossRef] [PubMed]
- Yadegari, M.; Khazaei, M.; Ghorbani, R.; Rezae, M.; Izadi, B.; Sheikholeslam, A. Wound healing effect of Falcaria vulgaris leaves on aspirin-induced gastric ulcer in rats. J. Kermanshah Univ. Med. Sci. 2006, 10, 195–203. [Google Scholar]
- Yadegari, M.; Khazaei, M.; Hamzavi, Y.; Toloei, A.R. Antifertility effects of Falcaria vulgaris in female rat. J. Arak Univ. Med. Sci. 2011, 14, 94–99. [Google Scholar]
- Goudini, A.; Shakibaei, D. Effect of Falcaria vulgaris extract on isolated rat heart. In Proceedings of the Iranıan Congress of Physıology and Pharmacology, Kashan, Iran, 26–30 August 2007; Available online: https://sid.ir/paper/904934/en (accessed on 4 November 2025).
- Hosseini, K.; Jasori, S.; Delazar, A.; Asgharian, P.; Tarhriz, V. Phytochemical analysis and anticancer activity of Falcaria vulgaris Bernh growing in Moghan plain, northwest of Iran. BMC Complement. Med. Ther. 2021, 21, 294. [Google Scholar] [CrossRef]
- Çınar, F.; Aksay, S. Purification and characterization of polyphenol oxidase from myrtle berries (Myrtus communis L.). J. Food Meas. Charact. 2022, 16, 2282–2291. [Google Scholar] [CrossRef]
- Lee, C.Y.; Whitaker, J.R. (Eds.) Enzymatic Browning and Its Prevention; ACS Symposium Series 600; American Chemical Society: Washington, DC, USA, 1995. [Google Scholar]
- Mayer, A.M. Polyphenol oxidases in plants and fungi: Going places? A review. Phytochemistry 2006, 67, 2318–2331. [Google Scholar] [CrossRef]
- Özel, A.; Colak, A.; Arslan, O.; Yildirim, M. Purification and characterisation of a polyphenol oxidase from Boletus erythropus and investigation of its catalytic efficiency in selected organic solvents. Food Chem. 2010, 119, 1044–1049. [Google Scholar] [CrossRef]
- Kolcuoğlu, Y. Purification and comparative characterization of monophenolase and diphenolase activities from a wild edible mushroom (Macrolepiota gracilenta). Process Biochem. 2012, 47, 2449–2454. [Google Scholar] [CrossRef]
- Wu, J.; Gao, J.; Chen, H.; Liu, X.; Cheng, W.; Ma, X.; Tong, P. Purification and characterization of polyphenol oxidase from Agaricus bisporus. Int. J. Food Prop. 2013, 16, 1483–1493. [Google Scholar] [CrossRef]
- McEvily, A.J.; Iyengar, R.; Otwell, W.S. Inhibition of enzymatic browning in foods and beverages. Crit. Rev. Food Sci. Nutr. 1992, 32, 253–273. [Google Scholar] [CrossRef]
- Whitaker, J.R.; Lee, C.Y. Recent Advances in Chemistry of Enzymatic Browning: An Overview; American Chemical Society: Washington, DC, USA, 1995; pp. 2–7. [Google Scholar] [CrossRef]
- Zhou, L.; Liao, T.; Liu, W.; Zou, L.; Liu, C.; Terefe, N.S. Inhibitory effects of organic acids on polyphenol oxidase: From model systems to food systems. Crit. Rev. Food Sci. Nutr. 2020, 60, 3594–3621. [Google Scholar] [CrossRef]
- Zhou, X.; Xiao, Y.; Meng, X.; Liu, B. Full inhibition of Whangkeumbae pear polyphenol oxidase enzymatic browning reaction by L-cysteine. Food Chem. 2018, 266, 1–8. [Google Scholar] [CrossRef]
- Gul Guven, R.; Guven, K.; Matpan Bekler, F.; Acer, O.; Alkan, H.; Dogru, M. Purification and characterization of polyphenol oxidase from purslane. Food Sci. Technol. 2017, 37, 356–362. [Google Scholar] [CrossRef]
- Marri, C.; Frazzoli, A.; Hochkoeppler, A.; Poggi, V. Purification of a polyphenol oxidase isoform from potato (Solanum tuberosum) tubers. Phytochemistry 2003, 63, 745–752. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.W.; Huang, Y.Y.; Ding, J.; Liu, C.; Xiao, X.D.; Ni, D.J. Prokaryotic expression and purification of Camellia sinensis polyphenol oxidase. J. Sci. Food Agric. 2010, 90, 2490–2494. [Google Scholar] [CrossRef]
- Sun, H.J.; Wang, J.; Tao, X.M.; Shi, J.; Huang, M.Y.; Chen, Z. Purification and characterization of polyphenol oxidase from rape flower. J. Agric. Food Chem. 2012, 60, 823–829. [Google Scholar] [CrossRef]
- Peng, X.; Du, C.; Yu, H.; Zhao, X.; Zhang, X.; Wang, X. Purification and characterization of polyphenol oxidase (PPO) from water yam (Dioscorea alata). CyTA-J. Food 2019, 17, 676–684. [Google Scholar] [CrossRef]
- Doğan, S.; Turan, P.; Doğan, M.; Arslan, O.; Alkan, M. Purification and characterization of Ocimum basilicum L. polyphenol oxidase. J. Agric. Food Chem. 2005, 53, 10224–10230. [Google Scholar] [CrossRef] [PubMed]
- Fujita, S.; Han, Y.Z.; Kouno, C.; Matsuo, T.; Yamashita, M.; Haraguchi, Y.; Yang, C.P. Purification and characterization of polyphenol oxidase from edible yam (Dioscorea opposita Thunb.). Food Sci. Technol. Res. 2006, 12, 235–239. [Google Scholar] [CrossRef]
- Warburg, O.; Christian, W. Isolierung und Kristallisation des Gärungsferments Enolase. Naturwissenschaften 1941, 29, 589–590. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Öz, F.; Colak, A.; Özel, A.; Sağlam Ertunga, N.; Sesli, E. Purification and characterization of a mushroom polyphenol oxidase and its activity in organic solvents. J. Food Biochem. 2013, 37, 36–44. [Google Scholar] [CrossRef]
- Faiz, O. Purification and characterization of a polyphenol oxidase from Cimin grape (Vitis vinifera spp., Cimin). Res. J. Biotechnol. 2016, 11, 87–94. [Google Scholar]
- Doğan, S.; Turan, Y.; Ertürk, H.; Arslan, O. Characterization and purification of polyphenol oxidase from artichoke (Cynara scolymus L.). J. Agric. Food Chem. 2005, 53, 776–785. [Google Scholar] [CrossRef]
- Demir, D.; Çağlayan, K.; Eken, C. Polyphenol oxidase activities in Japanese pear (Pyrus pyrifolia (Burm.) Nakai) fruit at different development stages. Biol. Bull. 2023, 50, 1115–1124. [Google Scholar] [CrossRef]
- Kihara, T.; Murata, M.; Homma, S.; Kaneko, S.; Komae, K. Purification and characterization of wheat (Triticum aestivum) polyphenol oxidase. Food Sci. Technol. Res. 2005, 11, 87–94. [Google Scholar] [CrossRef][Green Version]
- Liu, N.N.; Liu, W.; Wang, D.J.; Zhou, Y.B.; Lin, X.J.; Wang, X.; Li, S.B. Purification and partial characterization of polyphenol oxidase from the flower buds of Lonicera japonica Thunb. Food Chem. 2013, 138, 478–483. [Google Scholar] [CrossRef]
- Aksoy, M. A new insight into purification of polyphenol oxidase and inhibition effect of curcumin and quercetin on potato polyphenol oxidase. Protein Expr. Purif. 2020, 171, 105612. [Google Scholar] [CrossRef]
- Arslan, O.; Erzengin, M.; Sinan, S.; Ozensoy, O. Purification of mulberry (Morus alba L.) polyphenol oxidase by affinity chromatography and investigation of its kinetic and electrophoretic properties. Food Chem. 2004, 88, 479–484. [Google Scholar] [CrossRef]
- Gawlik-Dziki, U.; Złotek, U.; Świeca, M. Characterization of polyphenol oxidase from butter lettuce (Lactuca sativa var. capitata L.). Food Chem. 2008, 107, 129–135. [Google Scholar] [CrossRef]
- Kaya, E.D. Biochemical properties of polyphenol oxidase purified from Sarali plum (Prunus domestica). J. Food Meas. Charact. 2024, 18, 6473–6484. [Google Scholar] [CrossRef]
- Öztürk, C.; Aksoy, M.; Küfrevioğlu, Ö.İ. Purification of tea leaf (Camellia sinensis) polyphenol oxidase by using affinity chromatography and investigation of its kinetic properties. J. Food Meas. Charact. 2020, 14, 31–38. [Google Scholar] [CrossRef]
- Benaceur, F.; Chaibi, R.; Berrabah, F.; Neifar, A.; Leboukh, M.; Benaceur, K.; Gargouri, A. Purification and characterization of latent polyphenol oxidase from truffles (Terfezia arenaria). Int. J. Biol. Macromol. 2020, 145, 885–893. [Google Scholar] [CrossRef]
- Gul Guven, R.; Aslan, N.; Guven, K.; Matpan Bekler, F.; Acer, O. Purification and characterization of polyphenol oxidase from corn tassel. Cell. Mol. Biol. 2016, 62, 6–11. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kolcuoğlu, Y.; Kuyumcu, I.; Colak, A. A catecholase from Laccaria laccata, a wild edible mushroom, and its catalytic efficiency in organic media. J. Food Biochem. 2018, 42, e12605. [Google Scholar] [CrossRef]
- Kaya, E.D.; Bağci, O. Purification and biochemical characterization of polyphenol oxidase extracted from Kirmizi Kismis grape (Vitis vinifera L.). J. Food Biochem. 2021, 45, e13627. [Google Scholar] [CrossRef]
- Huang, J.; Gao, X.; Su, L.; Liu, X.; Guo, L.; Zhang, Z.; Hao, J. Purification, characterization and inactivation kinetics of polyphenol oxidase extracted from Cistanche deserticola. Planta 2023, 257, 85. [Google Scholar] [CrossRef]
- Derardja, A.E.; Pretzler, M.; Kampatsikas, I.; Barkat, M.; Rompel, A. Purification and characterization of latent polyphenol oxidase from apricot (Prunus armeniaca L.). J. Agric. Food Chem. 2017, 65, 8203–8212. [Google Scholar] [CrossRef]
- Özen, A.; Colak, A.; Dincer, B.; Güner, S. A diphenolase from persimmon fruits (Diospyros kaki L., Ebenaceae). Food Chem. 2004, 85, 431–437. [Google Scholar] [CrossRef]
- Doğan, M.; Arslan, O.; Doğan, S. Substrate specificity, heat inactivation and inhibition of polyphenol oxidase from different aubergine cultivars. Int. J. Food Sci. Technol. 2002, 37, 415–423. [Google Scholar] [CrossRef]
- Sarsenova, A.; Demir, D.; Çağlayan, K.; Abiyev, S.; Darbayeva, T.; Eken, C. Purification and properties of polyphenol oxidase of dried Volvariella bombycina. Biology 2022, 12, 53. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Ng, A.W.R.; Wong, C.W. Partial purification and characterization of polyphenol oxidase from Chinese parsley (Coriandrum sativum). Food Sci. Biotechnol. 2016, 25, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Dincer, B.; Colak, A.; Aydin, N.; Kadioglu, A.; Güner, S. Characterization of polyphenoloxidase from medlar fruits (Mespilus germanica L., Rosaceae). Food Chem. 2002, 77, 1–7. [Google Scholar] [CrossRef]
- Zaini, N.A.M.; Osman, A.; Hamid, A.A.; Ebrahimpour, A.; Saari, N. Purification and characterization of membrane-bound polyphenoloxidase (mPPO) from Snake fruit [Salacca zalacca (Gaertn.) Voss]. Food Chem. 2013, 136, 407–414. [Google Scholar] [CrossRef]
- Sakıroglu, H.; Yılmaz, E.; Erat, M.; Öztürk, A.E. Selected properties of polyphenol oxidase obtained from Ispir sugar bean. Int. J. Food Prop. 2013, 16, 1314–1321. [Google Scholar] [CrossRef]
- Yang, C.P.; Fujita, S.; Ashrafuzzaman, M.D.; Nakamura, N.; Hayashi, N. Purification and characterization of polyphenol oxidase from banana (Musa sapientum L.) pulp. J. Agric. Food Chem. 2000, 48, 2732–2735. [Google Scholar] [CrossRef]
- Guo, L.; Ma, Y.; Shi, J.; Xue, S. The purification and characterization of polyphenol oxidase from green bean (Phaseolus vulgaris L.). Food Chem. 2009, 117, 143–151. [Google Scholar] [CrossRef]
- Duangmal, K.; Apenten, R.K.O. A comparative study of polyphenoloxidases from taro (Colocasia esculenta) and potato (Solanum tuberosum var. Romano). Food Chem. 1999, 64, 351–359. [Google Scholar] [CrossRef]
- Zhou, L.; Liu, W.; Stockmann, R.; Terefe, N.S. Effect of citric acid and high pressure thermal processing on enzyme activity and related quality attributes of pear puree. Innov. Food Sci. Emerg. Technol. 2018, 45, 196–207. [Google Scholar] [CrossRef]
- Fan, X. Chemical inhibition of polyphenol oxidase and cut surface browning of fresh-cut apples. Crit. Rev. Food Sci. Nutr. 2023, 63, 8737–8751. [Google Scholar] [CrossRef]
- Ali, H.M.; El-Gizawy, A.M.; El-Bassiouny, R.E.; Saleh, M.A. Browning inhibition mechanisms by cysteine, ascorbic acid and citric acid, and identifying PPO-catechol-cysteine reaction products. J. Food Sci. Technol. 2015, 52, 3651–3659. [Google Scholar] [CrossRef]
- Alici, E.H.; Arabaci, G. Purification of polyphenol oxidase from borage (Trachystemon orientalis L.) by using three-phase partitioning and investigation of kinetic properties. Int. J. Biol. Macromol. 2016, 93, 1051–1056. [Google Scholar] [CrossRef]
- Gawlik-Dziki, U.; Szymanowska, U.; Baraniak, B. Characterization of polyphenol oxidase from broccoli (Brassica oleracea var. botrytis italica) florets. Food Chem. 2007, 105, 1047–1053. [Google Scholar] [CrossRef]
- Aydemir, T. Partial purification and characterization of polyphenol oxidase from artichoke (Cynara scolymus L.) heads. Food Chem. 2004, 87, 59–67. [Google Scholar] [CrossRef]
- Espín, J.C.; Morales, M.; Varón, R.; Tudela, J.; Garciacanovas, F. A continuous spectrophotometric method for determining the monophenolase and diphenolase activities of apple polyphenol oxidase. Anal. Biochem. 1995, 231, 237–246. [Google Scholar] [CrossRef] [PubMed]
- Galeazzi, M.A.M.; Sgarbieri, V.C. Substrate specificity and inhibition of polyphenoloxidase (PPO) from a dwarf variety of banana (Musa cavendishii L.). J. Food Sci. 1981, 46, 1404–1406. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Colak, A.; Özen, A.; Dincer, B.; Güner, S.; Ayaz, A.F. Diphenolases from two cultivars of cherry laurel (Laurocerasus officinalis Roem.) fruits at early stage of maturation. Food Chem. 2005, 90, 801–807. [Google Scholar] [CrossRef]
- Lineweaver, H.; Burk, D. The determination of enzyme dissociation constants. J. Am. Chem. Soc. 1934, 56, 658–666. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).