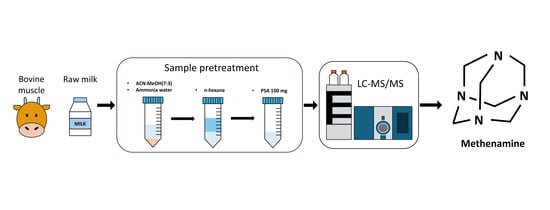
Development and Validation of an LC-MS/MS Method for the Quantification of Methenamine in Raw Milk and Bovine Muscle and Its Application to Incurred Samples
Abstract
1. Introduction
2. Results and Discussion
2.1. Optimization of Sample Pretreatment
2.1.1. Comparative Evaluation with MFDS Method
2.1.2. Optimization of Purification
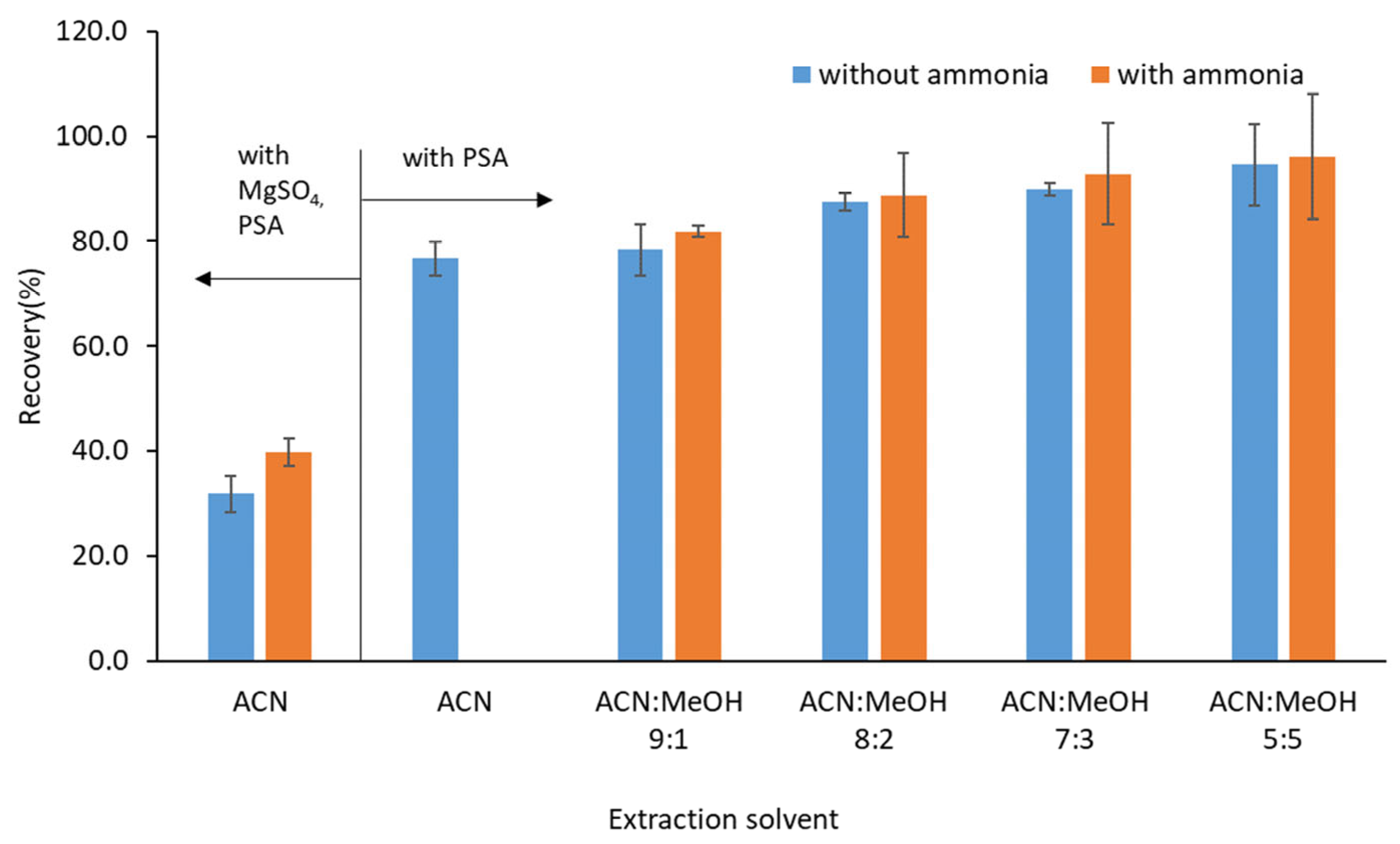
2.1.3. Optimization of Extraction
2.2. LC Method Development
2.3. Validation of Method
2.3.1. Matrix Effect
2.3.2. Linearity, Limits of Detection (LOD) and Limits of Quantification (LOQ)
2.3.3. Accuracy and Precision
2.3.4. Stability Tests
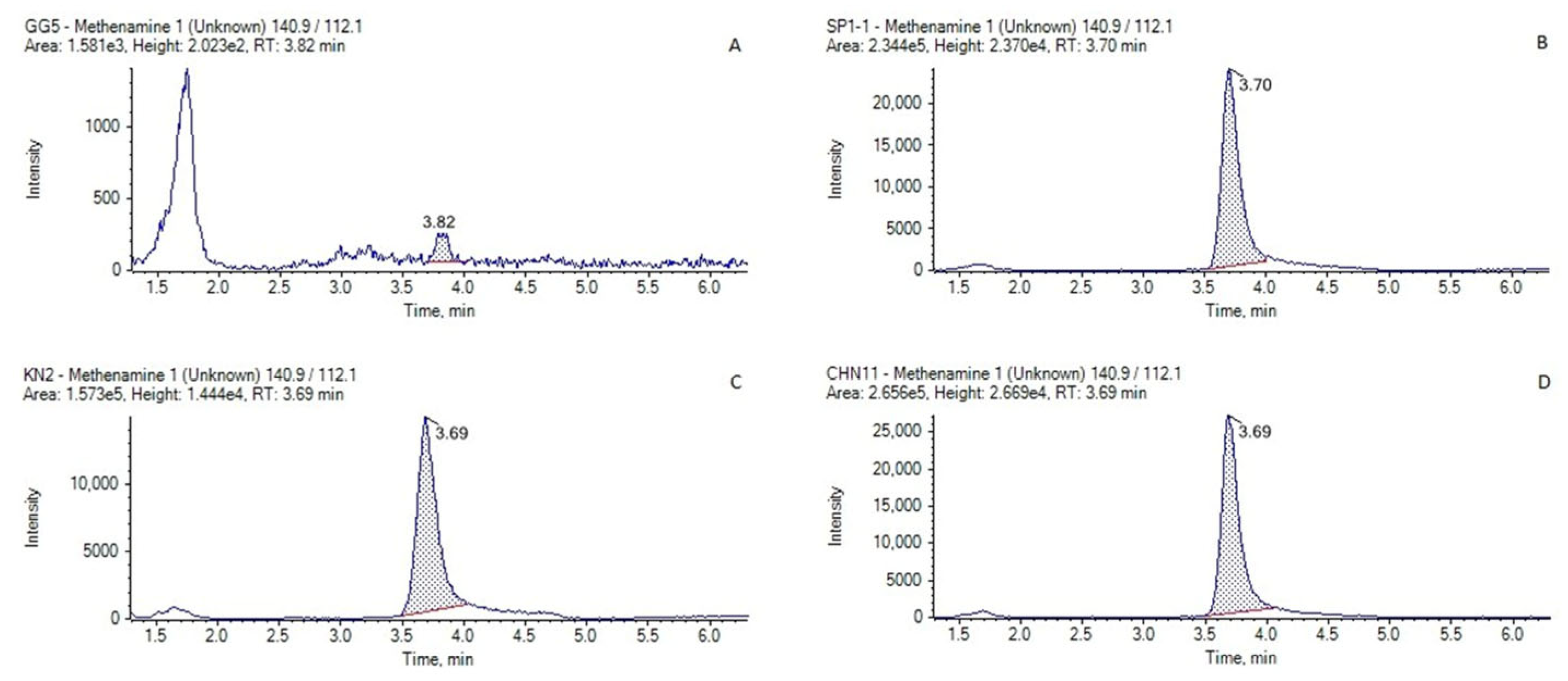
2.4. Application to Incurred Samples
2.5. Convenience and Economics of Proposed Method
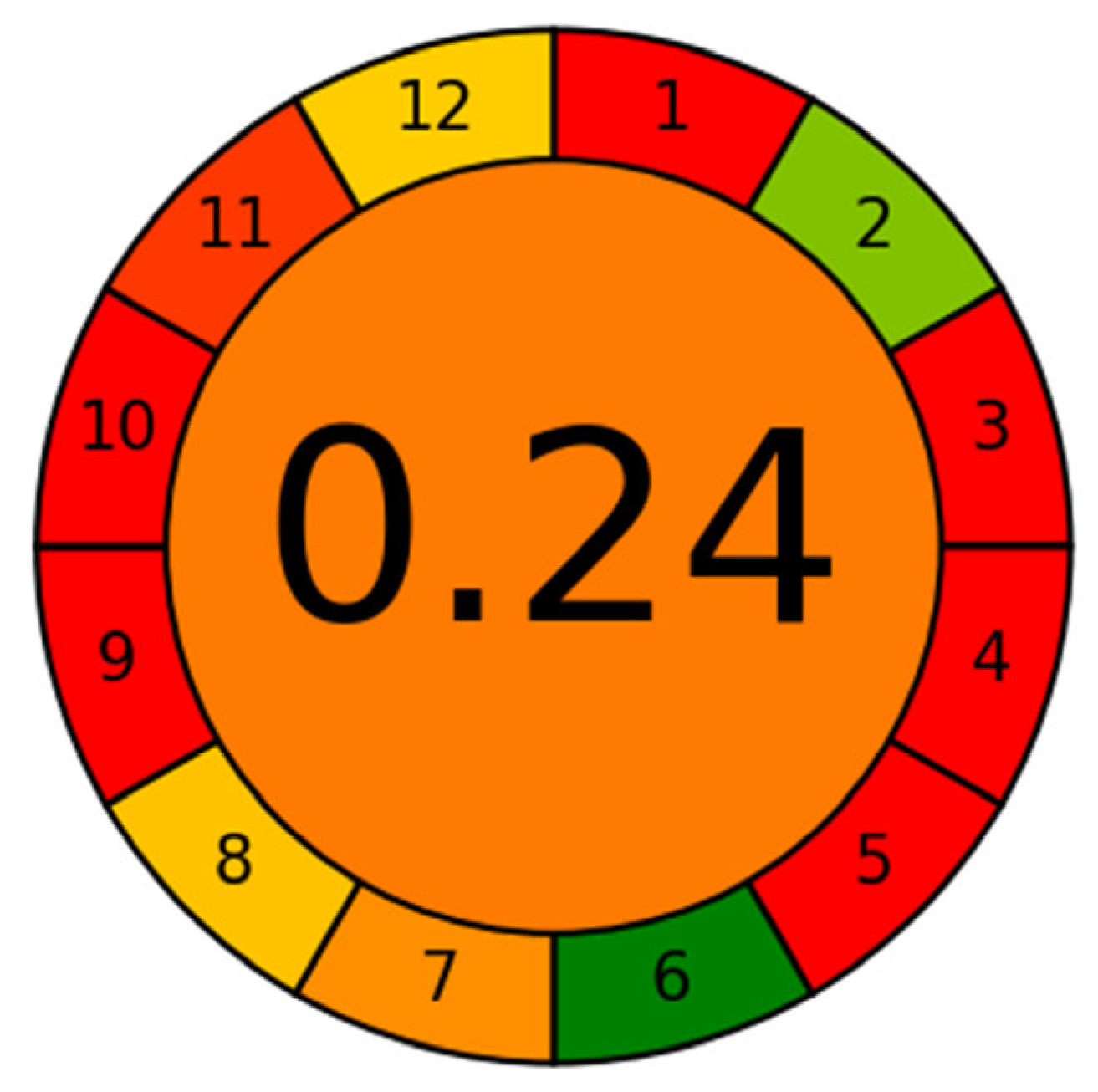
2.6. Greenness Assessment
2.7. Limitations and Future Perspectives
3. Materials and Methods
3.1. Reagents and Materials
3.2. Analytical Procedure
3.2.1. Sample Collection
3.2.2. Sample Pretreatment
3.2.3. HPLC-MS/MS Analysis
3.3. Validation of the Method
3.3.1. Assessments of Linearity, LOD, LOQ, Accuracy and Precision
3.3.2. Stability Tests
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Musher, D.M.; Griffith, D.P. Generation of formaldehyde from methenamine; effect of pH and concentration and antibacterial effect. Antimicrob. Agents Chemother. 1974, 6, 708–711. [Google Scholar] [CrossRef] [PubMed]
- Ariëns, E.J.; Hanselaar, A.G.; Henderson, P.T.; Simonis, A.M. Beware of formaldehyde in disguise. Eur. J. Clin. Pharmacol. 1982, 23, 373–375. [Google Scholar] [CrossRef] [PubMed]
- Josiane, K.N.G.; Raphaël, K.J.; Ruben, N.T.; Divine, Y.M.D.; Agwa, E.D.; Gilchrist, T.D.; Valdes, N.T.B.; Issa, I.B. Effects of methenamine feeding regime on growth performances, gut microbiota, organs histology and haemato-biochemical profile of broiler chickens. Open J. Anim. Sci. 2021, 11, 238–254. [Google Scholar] [CrossRef]
- EFSA. Scientific Opinion on the Safety and Efficacy of Hexamethylene Tetramine as a Silage Additive for Pigs, Poultry, Bovines, Sheep, Goats, Rabbits and Horses. EFSA J. 2015, 13, 4014. [Google Scholar] [CrossRef][Green Version]
- EFSA. Scientific Opinion on the Re-Evaluation of Hexamethylene Tetramine (E239) as a Food Additive. EFSA J. 2014, 12, 3696. [Google Scholar] [CrossRef]
- Xu, X.; Duhoranimana, E.; Zhang, X. Selective extraction of methenamine from chicken eggs using molecularly imprinted polymers and LC-MS/MS confirmation. Food Control 2017, 73, 265–272. [Google Scholar] [CrossRef]
- Fuselli, F.; Guarino, C.; La Mantia, A.; Longo, L.; Faberi, A.; Marianellea, R.M. Multi-detection of preservatives in cheeses by liquid chromatography-tandem mass spectrometry. J. Chromatogr. B 2012, 906, 9–18. [Google Scholar] [CrossRef]
- Jia, W.; Ling, Y.; Lin, Y.; Chang, J.; Chu, X. Analysis of additives in dairy products by liquid chromatography coupled to quadrupole-orbitrap mass spectrometry. J. Chromatogr. A 2014, 1336, 67–75. [Google Scholar] [CrossRef]
- Molognoni, L.; Daguer, H.; de Sá Ploêncio, L.A.; De Dea Lindner, J. A multi-purpose tool for food inspection: Simultaneous determination of various classes of preservatives and biogenic amines in meat and fish products by LC-MS. Talanta 2018, 178, 1053–1066. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, X.; Abbas, S.; Karangwa, E.; Duhoranimana, E.; Shu, F.P.P. Methenamine in dairy products by isotope dilution gas chromatography coupled with triple quadrupole mass spectrometry: Method validation and occurrence. Food Control 2015, 57, 89–95. [Google Scholar] [CrossRef]
- Liu, M.; Zhu, C.; Li, X.; Wang, F. 3DG functionalized magnetic solid phase extraction materials for the efficient enrichment of hexamethylenetetramine in Vermicelli. Molecules 2022, 27, 1548. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.S.; Kim, J.I.; Ko, K.Y.; Kim, M. Determination of hexamethylenetetramine in foods by high-performance liquid chromatography (HPLC). Food Addit. Contam. 2014, 31, 1489–1495. [Google Scholar] [CrossRef]
- Kim, W.; Kim, E.; Lee, J.; Song, C.H.; Jung, W.; Shin, S.; Kim, K.B.; Shin, B.S.; Kim, T.H. Development of an LC-MS/MS Assay and Toxicokinetic Characterization of Hexamethylenetetramine in Rats. Toxics 2023, 11, 337. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Zhang, X.; Duhoranimana, E.; Zhang, Y.; Shu, P. Determination of methenamine residues in edible animal tissues by HPLC-MS/MS using a modified QuEChERS method: Validation and pilot survey in actual samples. Food Control 2016, 61, 99–104. [Google Scholar] [CrossRef]
- Xu, X.; Duhoranimana, E.; Zhang, X. Preparation and characterization of magnetic molecularly imprinted polymers for the extraction of hexamethylenetetramine in milk samples. Talanta 2017, 163, 31–38. [Google Scholar] [CrossRef]
- Ministry of Food and Drug Safety (MFDS). Korean Food Code; Ministry of Food and Drug Safety: Cheongju, Republic of Korea, 2023; Available online: https://www.Foodsafetykorea.go.kr/foodcode/01_01.jsp (accessed on 7 August 2023).
- Wang, P.C.; Lee, R.J.; Chen, C.Y.; Chou, C.C.; Lee, M.R. Determination of cyromazine and melamine in chicken eggs using quick, easy, cheap, effective rugged and safe (QuEChERS) extraction coupled with liquid chromatography-tandem mass spectrometry. Anal. Chim. Acta 2012, 752, 78–86. [Google Scholar] [CrossRef]
- Lee, Y.J.; Rahman, M.M.; Abd El-Ary, A.M.; Choi, J.H.; Chung, H.S.; Kim, S.W.; Abdel-Aty, A.M.; Shin, H.C.; Shim, J.H. Detection of three herbicide, and one metabolite, residues in brown rice and rice straw using various versions of the QuEChERS method and liquid chromatography-tandem mass spectrometry. Food Chem. 2016, 210, 442–450. [Google Scholar] [CrossRef]
- Ferreira, J.A.; Ferreira, J.M.S.; Talamini, V.; Facco, J.F.; Rizzetti, T.M.; Prestes, O.D.; Adaime, M.B.; Zanella, R.; Bottoli, C.B.G. Determination of pesticides in coconut (Cocos nucifera Linn.) water and pulp using modified QuEChERS and LC-MS/MS. Food Chem. 2016, 213, 616–624. [Google Scholar] [CrossRef]
- Yoon, S.H.; Kim, M.S.; Kim, S.H.; Park, H.M.; Pyo, H.; Lee, Y.M.; Lee, K.T.; Hong, J. Effective application of freezing lipid precipitation and SCX-SPE for determination of pyrrolizidine alkaloids in high lipid foodstuffs by LC-ESI-MS/MS. J. Chromatogra. B 2015, 992, 56–66. [Google Scholar] [CrossRef]
- Musarurwa, H.; Chimuka, L.; Pakade, V.E.; Tavengwa, N.T. Recent developments and applications of QuEChERS based techniques on food samples during pesticide analysis. J. Food Compos. Anal. 2019, 84, 103314. [Google Scholar] [CrossRef]
- MacGibbon, A.K.H.; Taylor, M.W. Composition and Structure of Bovine Milk Lipids. In Advanced Dairy Chemistry Volume 2: Lipids, 3rd ed.; Fox, P.F., McSweeney, P.L.H., Eds.; Springer: New York, NY, USA, 2006; pp. 1–42. [Google Scholar] [CrossRef]
- Dittrich, B.; Harrowfield, J.M.; Koutsantonis, G.A.; Nealon, G.L.; Skelton, B.W. Long tailed cage amines: Synthesis, metal complexation, and structure. Dalton Trans. 2010, 39, 3433–3448. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Park, H.; Kang, H.S.; Cho, B.H.; Oh, J.H. Comparison of sample preparation and determination of 60 veterinary drug residues in flatfish using liquid chromatography-tandem mass spectrometry. Molecules 2020, 25, 1206–1221. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.; Kim, S.; Shin, J.Y.; Kim, M.K.; Kim, J.H. Development and verification for analysis of pesticides in eggs and egg products using QuEChERS and LC-MS/MS. Food Chem. 2015, 173, 1236–1242. [Google Scholar] [CrossRef] [PubMed]
- Patel, D. Matrix effect in a view of LC-MS/MS: An overview. Int. J. Pharma Bio Sci. 2011, 2, 559–564. [Google Scholar]
- Xu, X.; Xu, X.; Han, M.; Qiu, S.; Hou, X. Development of a modified QuEChERS method based on magnetic multiwalled carbon nanotubes for the simultaneous determination of veterinary drugs, pesticides and mycotoxins in eggs by UPLS-MS/MS. Food Chem. 2019, 276, 419–426. [Google Scholar] [CrossRef]
- Stahnke, H.; Kittlaus, S.; Kempe, G.; Alder, L. Reduction of matrix effects in liquid chromatography-electospray ionization-mass spectrometry by dilution of the sample extracts: How much dilution is needed? Anal. Chem. 2012, 84, 1474–1482. [Google Scholar] [CrossRef]
- Lehotay, S.J.; Mastovska, K.; Lightfield, A.R.; Gates, R.A. Multi-analyst, Multi-matrix performance of the QuEChERS approach for pesticide residues in foods and feeds using HPLC/MS/MS analysis with different calibration techniques. J. AOAC Int. 2010, 93, 355–367. [Google Scholar] [CrossRef]
- CAC/GL 71-2009; Guidelines for the Design and Implementation of National Regulatory Food Safety Assurance Programme Associated with the Use of Veterinary Drugs in Food Producing Animals. Food and Agriculture Organization: Rome, Italy, 2009. Available online: https://www.fao.org/fao-who-codexalimentarius/codex-texts/guidelines/en/ (accessed on 7 August 2023).
- FDA. Bioanalytical Method Validation Guidance for Industry. 2018. Available online: https://www.fda.gov/files/drugs/published/Bioanalytical-Method-Validation-Guidance-for-Industry.pdf (accessed on 26 November 2025).
- EMA. Guideline on Bioanalytical Method Validation. 2011. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-bioanalytical-method-validation_en.pdf (accessed on 26 November 2025).
- Pena-Pereira, F.; Wojnowski, W.; Tobiszewski, M. AGREE-Analytical GREEnness Metric Approach and Software. Anal. Chem. 2020, 92, 10076–10082. [Google Scholar] [CrossRef]
| Method | 1 (Proposed Method) | 2 (MFDS Method) |
|---|---|---|
| Sample | 2 g of samples | 5 g of samples |
| Extraction | mixture of ACN and MeOH (ACN:MeOH = 7:3, v/v) (10 mL) ammonia water (100 µL) | 10 mM Ammonium formate in ACN (20 mL) |
| Purification | Hexane (10 mL) PSA (100 mg) | Hexane (20 mL) MgSO4 (900 mg) PSA (50 mg) |
| Evaporation | Under N2(g), 40 °C until 0.5 mL of extracts | |
| Reconstitution | ACN 0.5 mL | ACN 1.5 mL |
| Mobile phase A | 0.1% formic acid and 5 mM ammonium acetate in water | 10 mM ammonium formate in water |
| Spiked Concentration (µg/kg) | Intra-Day (n = 6) | Inter-Day (n = 12) | |||
|---|---|---|---|---|---|
| Recovery (%) | CV (%) | Recovery (%) | CV (%) | ||
| raw milk | 1 | 95.4 | 5.94 | 96.0 | 7.10 |
| 10 | 102.8 | 7.64 | 101.6 | 5.97 | |
| 40 | 95.8 | 3.13 | 95.9 | 4.59 | |
| bovine muscle | 5 | 91.5 | 3.82 | 91.4 | 7.74 |
| 10 | 78.1 | 3.57 | 83.5 | 8.75 | |
| 40 | 94.3 | 4.30 | 91.5 | 7.03 | |
| Matrix | QC | Room Temp (Time-Zero) | Short Term Freezer −80 °C (2 d) | Freeze–Thaw (3 Cycles) | Autosampler 24 h |
|---|---|---|---|---|---|
| Raw milk | Low QC | 95.34 ± 4.44 | 93.31 ± 3.22 (−2.13) | 97.41 ± 3.78 (2.17) | 91.58 ± 4.98 (−3.94) |
| High QC | 89.90 ± 3.69 | 88.37 ± 5.21 (−1.70) | 95.92 ± 3.11 (6.70) | 87.66 ± 10.52 (−2.49) | |
| Bovine muscle | Low QC | 98.56 ± 3.36 | 102.20 ± 2.28 (3.69) | 98.07 ± 0.99 (−0.50) | 95.40 ± 3.28 (−3.21) |
| High QC | 91.51 ± 4.70 | 94.75 ± 4.05 (3.54) | 85.67 ± 4.77 (−6.38) | 94.26 ± 5.22 (3.01) |
| Precursor Ion (m/z) | Product Ion (m/z) | Dwell Time (ms) | Retention Time (min) | DP (Volts) | EP (Volts) | CE (Volts) | CXP (Volts) |
|---|---|---|---|---|---|---|---|
| 140.9 | 112.1 | 100 | 3.71 | 136 | 10 | 19 | 12 |
| 85.1 | 25 | 14 | |||||
| 98 | 19 | 18 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, S.; Hong, C.-O.; Kim, S.-H.; Lee, S.-Y.; Jeon, I.; Kim, D.H.; Ku, H.-O.; Park, M.-Y. Development and Validation of an LC-MS/MS Method for the Quantification of Methenamine in Raw Milk and Bovine Muscle and Its Application to Incurred Samples. Molecules 2025, 30, 4807. https://doi.org/10.3390/molecules30244807
Park S, Hong C-O, Kim S-H, Lee S-Y, Jeon I, Kim DH, Ku H-O, Park M-Y. Development and Validation of an LC-MS/MS Method for the Quantification of Methenamine in Raw Milk and Bovine Muscle and Its Application to Incurred Samples. Molecules. 2025; 30(24):4807. https://doi.org/10.3390/molecules30244807
Chicago/Turabian StylePark, Sunjin, Chung-Oui Hong, Se-Hyung Kim, Seon-Young Lee, Inhae Jeon, Do Hui Kim, Hyun-Ok Ku, and Mi-Young Park. 2025. "Development and Validation of an LC-MS/MS Method for the Quantification of Methenamine in Raw Milk and Bovine Muscle and Its Application to Incurred Samples" Molecules 30, no. 24: 4807. https://doi.org/10.3390/molecules30244807
APA StylePark, S., Hong, C.-O., Kim, S.-H., Lee, S.-Y., Jeon, I., Kim, D. H., Ku, H.-O., & Park, M.-Y. (2025). Development and Validation of an LC-MS/MS Method for the Quantification of Methenamine in Raw Milk and Bovine Muscle and Its Application to Incurred Samples. Molecules, 30(24), 4807. https://doi.org/10.3390/molecules30244807