Synthesis of 1,2,4-Triazole-3-Thiol Derivatives from Thiosemicarbazides and Carboxylic Acids Using Polyphosphate Ester
Abstract
1. Introduction
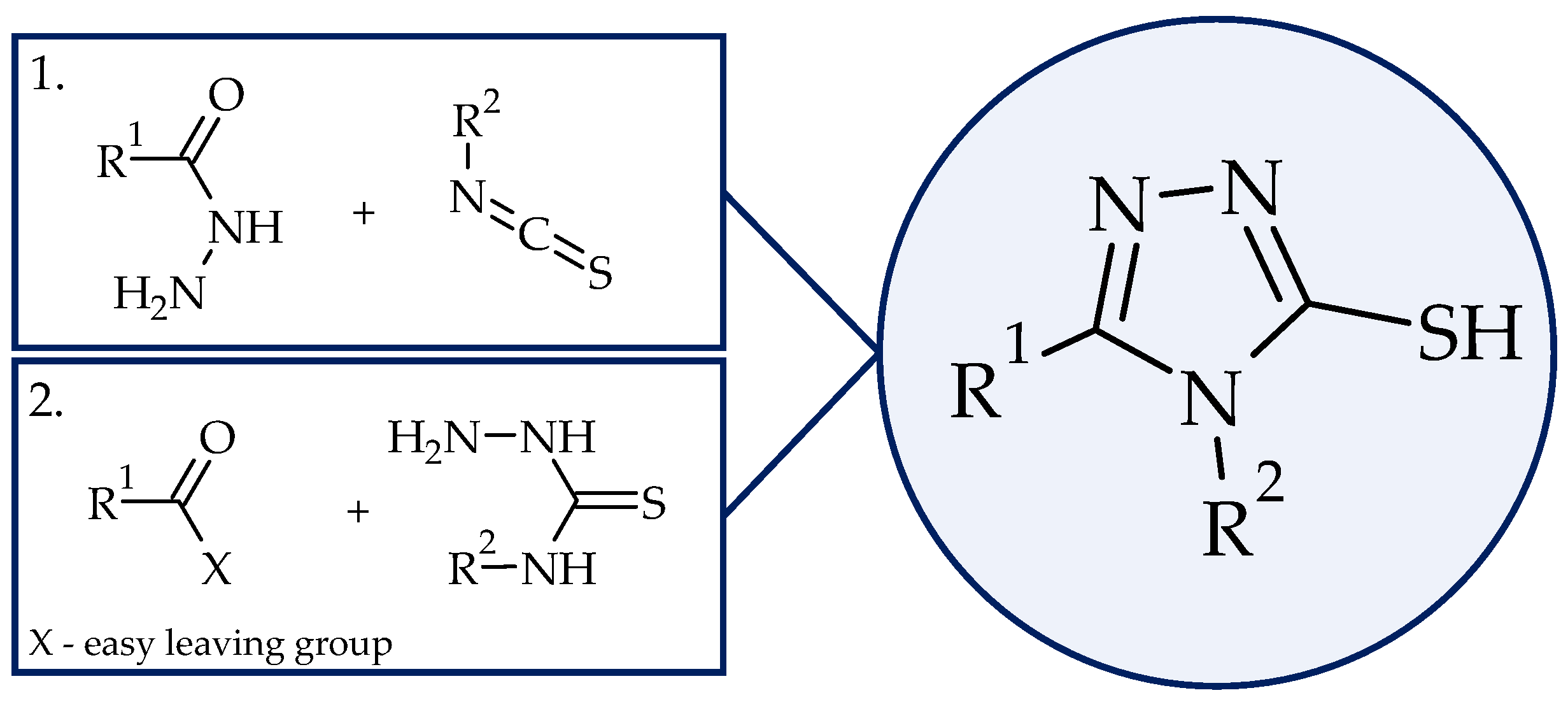
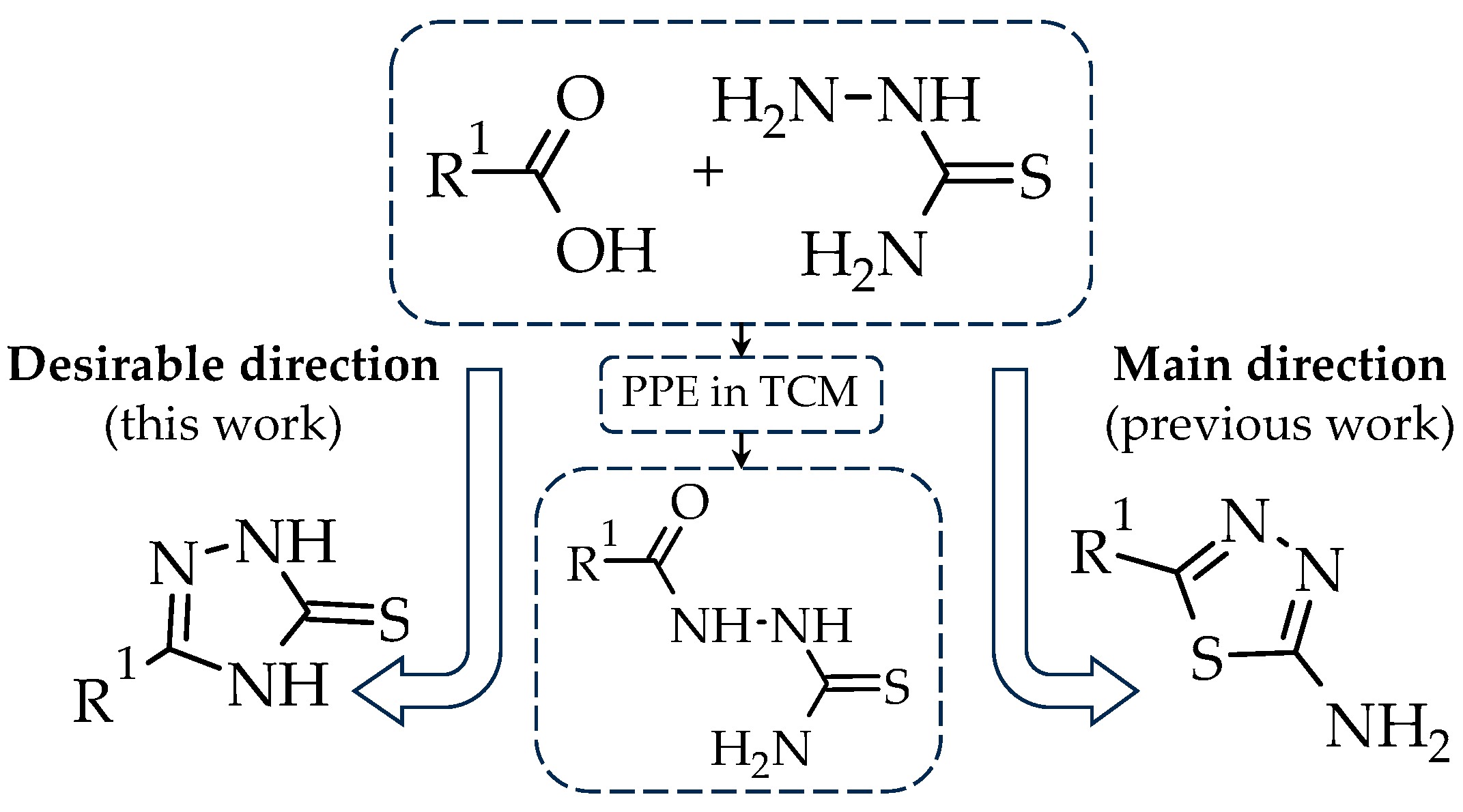
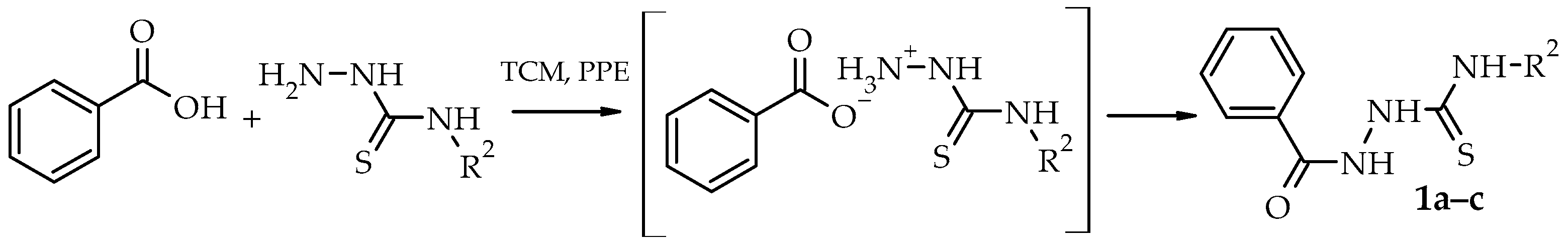
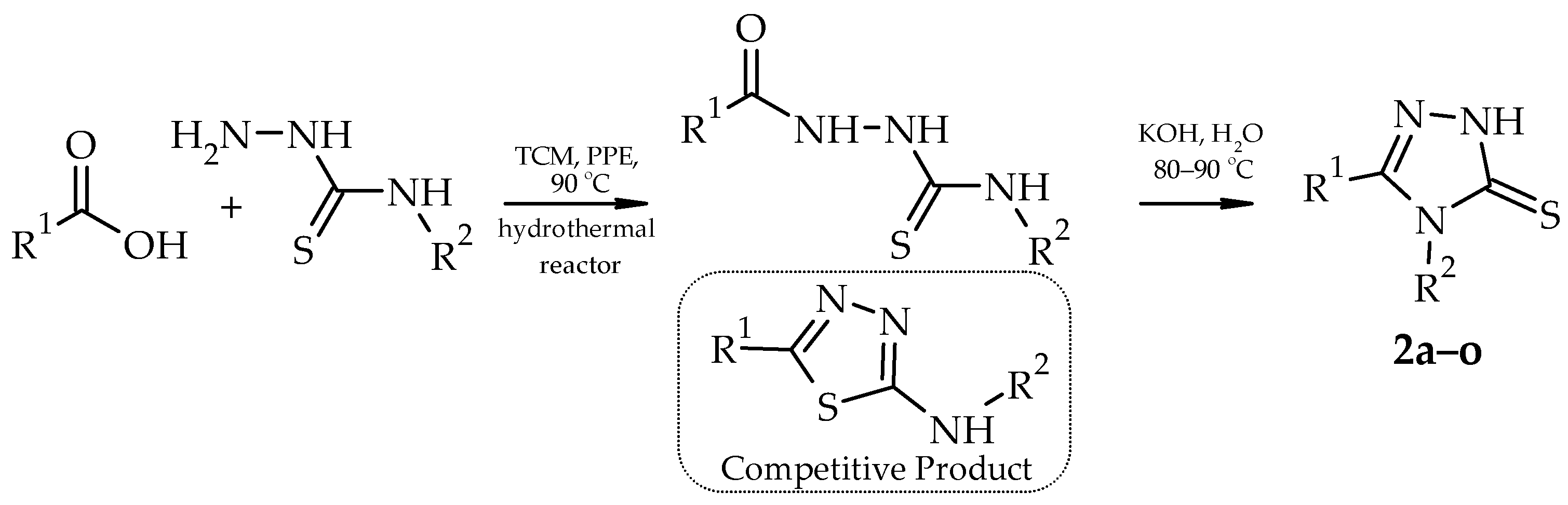
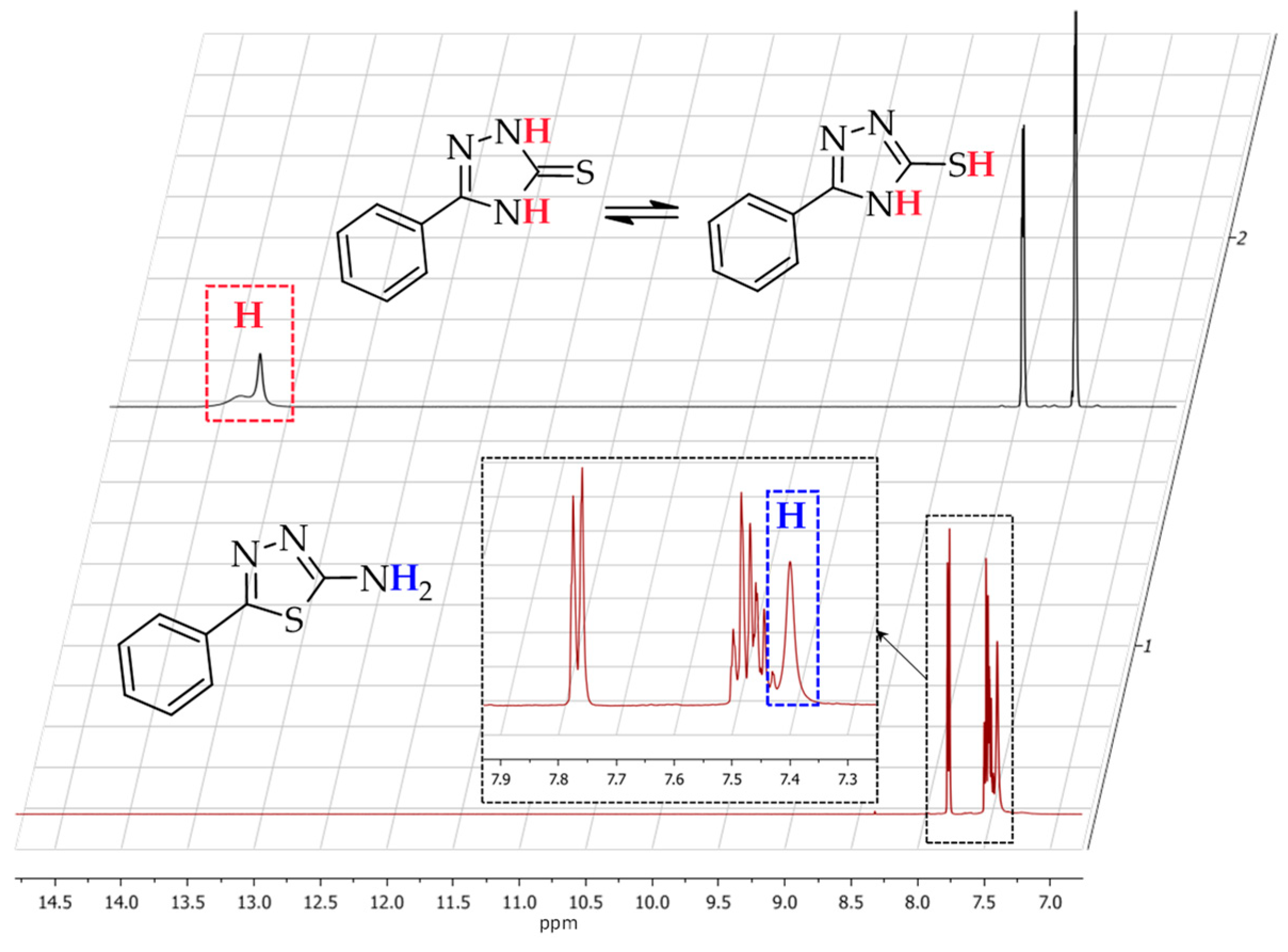
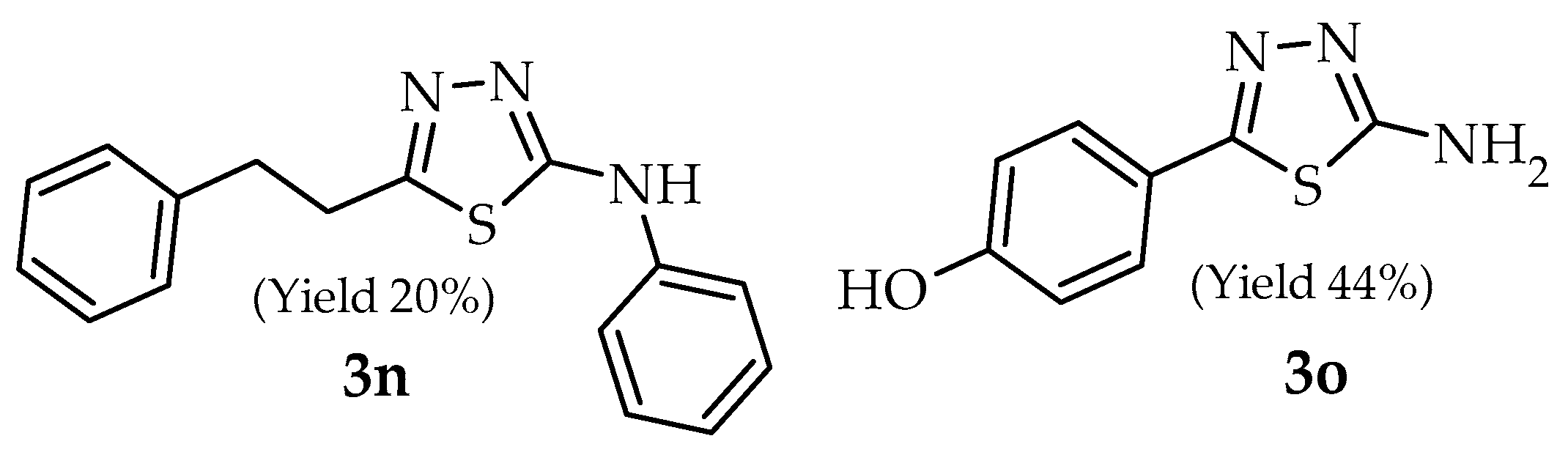
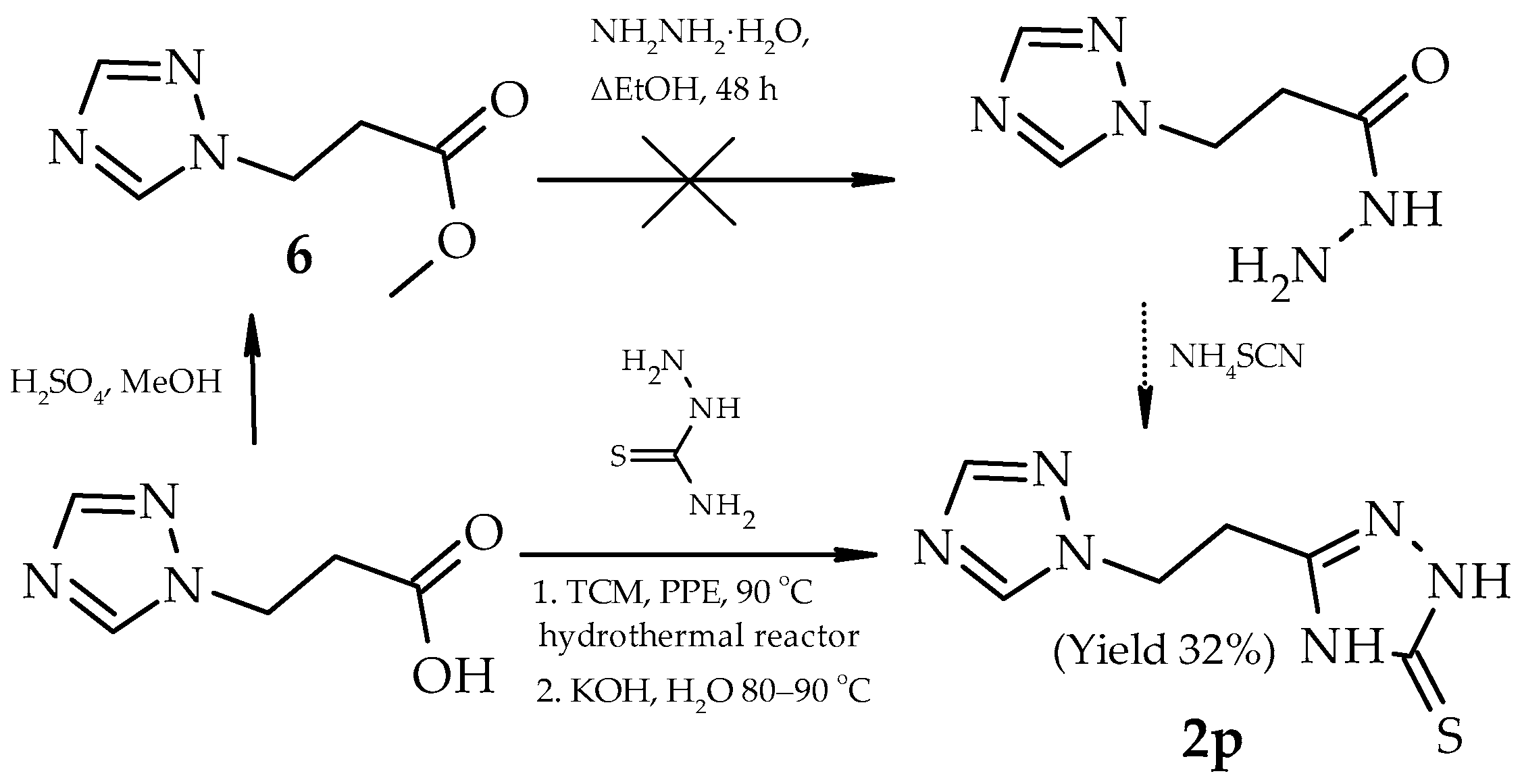
2. Results and Discussion
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Aman, K.; Seema, D.; Sanjeev, K.; Kashmiri, L. Recent advancements in the multifaceted biomedical efficacy of triazole based metal complexes. Coord. Chem. Rev. 2025, 536, 216675. [Google Scholar] [CrossRef]
- Isern, J.A.; Carlucci, R.; Labadie, G.R.; Porta, E.O.J. Progress and Prospects of Triazoles in Advanced Therapies for Parasitic Diseases. Trop. Med. Infect. Dis. 2025, 10, 142. [Google Scholar] [CrossRef]
- Glomb, T.; Minta, J.; Nowosadko, M.; Radzikowska, J.; Świątek, P. Search for New Compounds with Anti-Inflammatory Activity Among 1,2,4-Triazole Derivatives. Molecules 2024, 29, 6036. [Google Scholar] [CrossRef]
- Ameziane El Hassani, I.; Rouzi, K.; Ameziane El Hassani, A.; Karrouchi, K.; Ansar, M. Recent Developments Towards the Synthesis of Triazole Derivatives: A Review. Organics 2024, 5, 450–471. [Google Scholar] [CrossRef]
- Magnaghi, P.; D’Alessio, R.; Valsasina, B.; Avanzi, N.; Rizzi, S.; Asa, D.; Gasparri, F.; Cozzi, L.; Cucchi, U.; Orrenius, C.; et al. Covalent and allosteric inhibitors of the ATPase VCP/p97 induce cancer cell death. Nat. Chem. Biol. 2013, 9, 548–556. [Google Scholar] [CrossRef] [PubMed]
- Casciuc, L.; Horvath, D.; Gryniukova, A.; Tolmachova, K.A.; Vasylchenko, O.V.; Borysko, P.; Moroz, Y.S.; Bajorath, J.; Varnek, A. Pros and cons of virtual screening based on public “Big Data”: In silico mining for new bromodomain inhibitors. Eur. J. Med. Chem. 2019, 165, 258–272. [Google Scholar] [CrossRef] [PubMed]
- Aouad, M.R.; Almehmadi, M.A.; Albelwi, F.F.; Teleb, M.; Tageldin, G.N.; Abu-Serie, M.M.; Hagar, M.; Rezki, N. Targeting the interplay between MMP-2, CA II and VEGFR-2 via new sulfonamide-tethered isomeric triazole hybrids; Microwave-assisted synthesis, computational studies and evaluation. Bioorg. Chem. 2022, 124, 105816. [Google Scholar] [CrossRef]
- Malebari, A.M.; Ibrahim, T.S.; Salem, I.M.; Salama, I.; Khayyat, A.N.; Mostafa, S.M.; El-Sabbagh, O.I.; Darwish, K.M. The Anticancer Activity for the Bumetanide-Based Analogs via Targeting the Tumor-Associated Membrane-Bound Human Carbonic Anhydrase-IX Enzyme. Pharmaceuticals 2020, 13, 252. [Google Scholar] [CrossRef] [PubMed]
- Vats, L.; Kumar, R.; Bua, S.; Nocentini, A.; Gratteri, P.; Supuran, C.T.; Pawan, K.; Sharma, P.K. Continued exploration and tail approach synthesis of benzenesulfonamides containing triazole and dual triazole moieties as carbonic anhydrase I, II, IV and IX inhibitors. Eur. J. Med. Chem. 2019, 183, 111698. [Google Scholar] [CrossRef]
- Khan, F.M.; Abbasi, M.A.; Rehman, A.U.; Siddiqui, S.Z.; Butt, A.R.S.; Raza, H.; Zafar, A.; Shah, S.A.A.; Shahid, M.; Seo, S.-Y. Convergent synthesis of carbonic anhydrase inhibiting bi-heterocyclic benzamides: Structure–activity relationship and mechanistic explorations through enzyme inhibition, kinetics, and computational studies. J. Heterocycl. Chem. 2021, 58, 1089–1103. [Google Scholar] [CrossRef]
- Pitucha, M.; Janeczko, M.; Klimek, K.; Fornal, E.; Wos, M.; Pachuta-Stec, A.; Ginalska, G.; Kaczor, A.A. 1,2,4-Triazolin-5-thione derivatives with anticancer activity as CK1γ kinase inhibitors. Bioorg. Chem. 2020, 99, 103806. [Google Scholar] [CrossRef]
- Zhou, W.; Xu, C.; Dong, G.; Qiao, H.; Yang, J.; Liu, H.; Ding, L.; Sun, K.; Zhao, W. Development of phenyltriazole thiol-based derivatives as highly potent inhibitors of DCN1-UBC12 interaction. Eur. J. Med. Chem. 2021, 217, 113326. [Google Scholar] [CrossRef]
- Hassan, G.S.; El-Messery, S.M.; Al-Omary, F.A.M.; Al-Rashood, S.T.; Shabayek, M.I.; Abulfadl, Y.S.; Habib, E.-S.E.; El-Hallouty, S.M.; Fayad, W.; Mohamed, K.M.; et al. Nonclassical antifolates, part 4. 5-(2-Aminothiazol-4-yl)-4-phenyl-4H-1,2,4-triazole-3-thiols as a new class of DHFR inhibitors: Synthesis, biological evaluation and molecular modeling study. Eur. J. Med. Chem. 2013, 66, 135–145. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, F.; Petrella, A.; Chacón-Huete, F.; Covone, J.; Tsai, T.-W.; Yu, C.-C.; Forgione, P.; Kwan, D.H. A High-Throughput Glycosyltransferase Inhibition Assay for Identifying Molecules Targeting Fucosylation in Cancer Cell-Surface Modification. ACS Chem. Biol. 2019, 14, 715–724. [Google Scholar] [CrossRef]
- Vergani, B.; Sandrone, G.; Marchini, M.; Ripamonti, C.; Cellupica, E.; Galbiati, E.; Caprini, G.; Pavich, G.; Rocchio, G.P.; Lattanzio, M.; et al. Novel Benzohydroxamate-Based Potent and Selective Histone Deacetylase 6 (HDAC6) Inhibitors Bearing a Pentaheterocyclic Scaffold: Design, Synthesis, and Biological Evaluation. J. Med. Chem. 2019, 62, 10711–10739. [Google Scholar] [CrossRef]
- Vergani, B.; Caprini, G.; Fossati, G.; Lattanzio, M.; Marchini, M.; Pavich, G.; Pezzuto, M.; Ripamonti, C.; Sandore, G.; Steinkühler, C.; et al. Selective HDAC6 Inhibitors. International Patent WO 2018/189340 A1, 18 October 2018. [Google Scholar]
- Du, Z.; Foley, K. Method for Treating Proliferative Disorders Associated with Protooncogene Products. International Patent WO 2007139951 A2, 25 May 2007. [Google Scholar]
- Ying, W.; James, D.; Zhang, S.; Przewloka, T.; Chae, J.; Chimmanamada, D.U.; Lee, C.-W.; Kostik, E.; Ng, H.P.; Foley, K.; et al. Triazole Compounds That Modulate HSP90 Activity. United States Patent US 8901308 B2, 2 December 2014. [Google Scholar]
- Alsehli, M.; Aljuhani, A.; Ihmaid, S.K.; El-Messery, S.M.; Othman, D.I.A.; El-Sayed, A.-A.A.A.; Ahmed, H.E.A.; Rezki, N.; Aouad, M.R. Design and Synthesis of Benzene Homologues Tethered with 1,2,4-Triazole and 1,3,4-Thiadiazole Motifs Revealing Dual MCF-7/HepG2 Cytotoxic Activity with Prominent Selectivity via Histone Demethylase LSD1 Inhibitory Effect. Int. J. Mol. Sci. 2022, 23, 8796. [Google Scholar] [CrossRef] [PubMed]
- King, B.W.; Bell, B.; Sheppeck, J.; Gilmore, J.L. Triazolone and Triazolethione Derivatives as Inhibitors of Matrix Metalloproteinases and/or TNF-α Converting Enzyme. International Patent WO 2004032846 A2, 11 July 2006. [Google Scholar]
- Leyla Yurttaş, L.; Evren, A.E.; Kubilay, A.; Temel, H.E. Synthesis of New 1,2,4-Triazole Derivatives and Investigation of Their Matrix Metalloproteinase-9 (MMP-9) Inhibition Properties. Acta Pharm. Sci. 2021, 59, 215–232. [Google Scholar] [CrossRef]
- Youssef, M.F.; Nafie, M.S.; Salama, E.E.; Boraei, A.T.A.; Gad, E.M. Synthesis of New Bioactive Indolyl-1,2,4-Triazole Hybrids As Dual Inhibitors for EGFR/PARP-1 Targeting Breast and Liver Cancer Cells. ACS Omega 2022, 7, 45665–45677. [Google Scholar] [CrossRef]
- Boraei, A.T.A.; Singh, P.K.; Sechi, M.; Satta, S. Discovery of novel functionalized 1,2,4-triazoles as PARP-1 inhibitors in breast cancer: Design, synthesis and antitumor activity evaluation. Eur. J. Med. Chem. 2019, 15, 11162. [Google Scholar] [CrossRef] [PubMed]
- Munkuev, A.A.; Mozhaitsev, E.S.; Chepanova, A.A.; Suslov, E.V.; Korchagina, D.V.; Zakharova, O.D.; Ilina, E.S.; Dyrkheeva, N.S.; Zakharenko, A.L.; Reynisson, J.; et al. Novel Tdp1 Inhibitors Based on Adamantane Connected with Monoterpene Moieties via Heterocyclic Fragments. Molecules 2021, 26, 3128. [Google Scholar] [CrossRef] [PubMed]
- Shultz, M.D.; Kirby, C.A.; Stams, T.; Chin, D.N.; Blank, J.; Charlat, O.; Cheng, H.; Cheung, A.; Cong, F.; Feng, Y.; et al. [1,2,4]Triazol-3-ylsulfanylmethyl)-3-phenyl-[1,2,4]oxadiazoles: Antagonists of the Wnt Pathway That Inhibit Tankyrases 1 and 2 via Novel Adenosine Pocket Binding. J. Med. Chem. 2012, 55, 1127–1136. [Google Scholar] [CrossRef]
- Yu, M.; Yang, Y.; Sykes, M.; Wang, S. Small-Molecule Inhibitors of Tankyrases as Prospective Therapeutics for Cancer. J. Med. Chem. 2022, 65, 5244–5273. [Google Scholar] [CrossRef]
- Shahzad, S.A.; Yar, M.; Khan, Z.A.; Shahzadi, L.; Naqvi, S.A.R.; Mahmood, A.; Ullah, S.; Shaikh, A.J.; Sherazi, T.A.; Bale, A.T.; et al. Identification of 1,2,4-triazoles as new thymidine phosphorylase inhibitors: Future anti-tumor drugs. Bioorg. Chem. 2019, 85, 209–220. [Google Scholar] [CrossRef] [PubMed]
- Balba, M.; El-Hady, N.A.; Taha, N.; Rezki, N.; El Ashry, E.S.H. Inhibition of α-glucosidase and α-amylase by diaryl derivatives of imidazole-thione and 1,2,4-triazole-thiol. Eur. J. Med. Chem. 2011, 46, 2596–2601. [Google Scholar] [CrossRef] [PubMed]
- Rahim, F.; Ullah, H.; Hussain, R.; Taha, M.; Khan, S.; Nawaz, M.; Nawaz, F.; Gilani, S.J.; Jumah, M.N.B. Thiadiazole based triazole/hydrazone derivatives: Synthesis, in vitro α-glucosidase inhibitory activity and in silico molecular docking study. J. Mol. Struct. 2023, 1287, 135619. [Google Scholar] [CrossRef]
- Riaz, N.; Iftikhar, M.; Saleem, M.; Rehman, A.U.; Ahmed, I.; Ashraf, M.; Shahnawaz; Rehman, J.; al-Rashida, M. A novel method for the synthesis of 1,2,4-triazole-derived heterocyclic compounds: Enzyme inhibition and molecular docking studies. J. Iran. Chem. Soc. 2020, 17, 1183–1200. [Google Scholar] [CrossRef]
- Le, T.D.; Nguyen, T.C.; Nuong Bui, T.M.; Dung Hoang, T.K.; Vu, Q.T.; Pham, C.T.; Dinh, C.P.; Jibril Abdullahi Alhaji, J.A.; Meervelt, L.V. Synthesis, structure and α-glucosidase inhibitor activity evaluation of some acetamide derivatives starting from 2-(naphthalen-1-yl) acetic acid, containing a 1,2,4-triazole. J. Mol. Struct. 2023, 1284, 135321. [Google Scholar] [CrossRef]
- Su, X.; Vicker, N.; Thomas, M.P.; Pradaux-Caggiano, F.; Halem, H.; Culler, M.D.; Potter, B.V.L. Discovery of Adamantyl Heterocyclic Ketones as Potent 11β-Hydroxysteroid Dehydrogenase Type 1 Inhibitors. ChemMedChem. 2011, 6, 1439–1451. [Google Scholar] [CrossRef]
- Sharma, B.; Xie, L.; Yang, F.; Wang, W.; Zhou, Q.; Xiang, M.; Zhou, S.; Lv, W.; Jia, Y.; Pokhrel, L.; et al. Recent advance on PTP1B inhibitors and their biomedical applications. Eur. J. Med. Chem. 2020, 199, 112376. [Google Scholar] [CrossRef] [PubMed]
- Shahid, W.; Muhammad Ashraf, M.; Muhammad Saleem, M.; Bashir, B.; Muzaffar, S.; Ali, M.; Kaleem, A.; Rehman, A.U.; Amjad, H.; Bhattarai, K.; et al. Exploring phenylcarbamoylazinane-1,2,4-triazole thioethers as lipoxygenase inhibitors supported with in vitro, in silico and cytotoxic studies. Bioorg. Chem. 2021, 115, 105261. [Google Scholar] [CrossRef]
- Bivacqua, B.; Barreca, M.; Spano, V.; Raimondi, M.V.; Romeo, I.; Alcaro, S.; Graciela Andrei, G.; Barraja, P.; Montalbano, A. Insight into non-nucleoside triazole-based systems as viral polymerases inhibitors. Eur. J. Med. Chem. 2023, 249, 115136. [Google Scholar] [CrossRef]
- Abdellatif, K.R.A.; Abdelall, E.K.A.; Elshemy, H.A.H.; Philoppes, J.N.; Hassanein, E.H.M.; Kahk, N.M. Optimization of pyrazole-based compounds with 1,2,4-triazole-3-thiol moiety as selective COX-2 inhibitors cardioprotective drug candidates: Design, synthesis, cyclooxygenase inhibition, anti-inflammatory, ulcerogenicity, cardiovascular evaluation, and molecular modeling studies. Bioorg. Chem. 2021, 114, 105122. [Google Scholar] [CrossRef]
- Mohassab, A.M.; Hassan, H.A.; Dalia Abdelhamid, D.; Mohamed Abdel-Aziz, M.; Dalby, K.N.; Kaoud, T.S. Novel quinoline incorporating 1,2,4-triazole/oxime hybrids: Synthesis, molecular docking, anti-inflammatory, COX inhibition, ulceroginicity and histopathological investigations. Bioorg. Chem. 2017, 75, 242–259. [Google Scholar] [CrossRef] [PubMed]
- Butt, A.R.S.; Abbasi, M.A.; Rehman, A.U.; Siddiqui, S.Z.; Hassan, M.; Raza, H.; Shah, S.A.A.; Seo, S.-Y. Synthesis and structure-activity relationship of elastase inhibiting novel ethylated thiazole-triazole acetamide hybrids: Mechanistic insights through kinetics and computational contemplations. Bioorg. Chem. 2019, 86, 197–209. [Google Scholar] [CrossRef] [PubMed]
- Bulut, N.; Kocyigit, U.M.; Gecibesler, I.H.; Dastan, T.; Karci, H.; Taslimi, P.; Dastan, D.S.; Gulcin, I.; Cetin, A. Synthesis of some novel pyridine compounds containing bis-1,2,4-triazole/thiosemicarbazide moiety and investigation of their antioxidant properties, carbonic anhydrase, and acetylcholinesterase enzymes inhibition profiles. J. Biochem. Mol. Toxicol. 2018, 32, e22006. [Google Scholar] [CrossRef]
- Mishra, D.; Fatima, A.; Kumar, P.; Munjal, N.S.; Singh, B.K.; Singh, R. Synthesis of Benzothiazole Linked Triazole Conjugates and Their Evaluation Against Cholinesterase Enzymes. ChemistrySelect 2022, 7, e20220360. [Google Scholar] [CrossRef]
- Siddiqui, S.Z.; Arfan, M.; Abbasi, M.A.; Rehman, A.U.; Shah, S.A.A.; Ashraf, M.; Hussain, S.; Saleem, R.S.Z.; Rafique, R.; Khan, K.M. Discovery of Dual Inhibitors of Acetyl and Butrylcholinesterase and Antiproliferative Activity of 1,2,4-Triazole-3-thiol: Synthesis and In Silico Molecular Study. ChemistrySelect 2020, 5, 6430–6439. [Google Scholar] [CrossRef]
- Aslan, E.K.; Sağlık, B.N.; Özkay, Y.; Palaska, E. Synthesis and Biological Evaluation of Benzoxazolone−Thiosemicarbazide, 1,2,4-Triazole, 1,3,4-Thiadiazole Derivatives as Cholinesterase Inhibitors. ChemistrySelect 2023, 8, e202302069. [Google Scholar] [CrossRef]
- Riaz, N.; Iftikhar, M.; Saleem, M.; Rehman, A.U.; Hussain, S.; Rehmat, F.; Afzal, Z.; Khawar, S.; Ashraf, M.; al-Rashida, M. New synthetic 1,2,4-triazole derivatives: Cholinesterase inhibition and molecular docking studies. Results Chem. 2020, 2, 100041. [Google Scholar] [CrossRef]
- Amjad, H.; Abbasi, M.A.; Siddiqui, S.Z.; Iqbal, J.; Rasool, S.; Ashraf, M.; Hussain, S.; Shah, S.A.A.; Imran, S.; Shahid, M.; et al. In vitro and in silico assessment of bioactivity properties and pharmacokinetic studies of new 3,5-disubstituted-1,2,4-triazoles. J. Mol. Struct. 2023, 1275, 134720. [Google Scholar] [CrossRef]
- Xu, M.; Peng, Y.; Zhu, L.; Wang, S.; Ji, J.; Rakesh, K.P. Triazole derivatives as inhibitors of Alzheimer’s disease: Current developments and structure-activity relationships. Eur. J. Med. Chem. 2019, 180, 656–672. [Google Scholar] [CrossRef]
- Edwards, L.; Isaac, M.; Johansson, M.; Kers, A.; Malmberg, J.; Mcleod, D.; Minidis, A.; Staaf, K.; Slassi, A.; Stefanac, T.; et al. Additional Heteropolycyclic Compounds and Their Use as Metabotropic Glutamate Receptor Antagonists. US Patent US 20070179188 A1, 2 August 2007. [Google Scholar]
- Svensson, M.; Tiden, A.-K.; Turek, D. Use of Derivatives of 2,4-Dihydro-[1,2,4]Triazole-3-Thione as Inhibitors of FTEH Enzyme Myeloperoxidase (MPO). International Patent WO 2004096781 A, 11 November 2004. [Google Scholar]
- Zohar, O.; Alkon, D.L. Therapeutic Effects of Bryostatins, Bryologs, and Other Related Substances on Head Trauma-Induced Memory Impairment and Traumatic Brain Injury. International Patent WO 2008143880 A2, 27 November 2008. [Google Scholar]
- Shi, L.; Zhang, X.Y.; Li Ping Cheng, L.P. Design, synthesis and biological evaluation of 1,3,4-triazole-3-acetamide derivatives as potent neuraminidase inhibitors. Bioorg. Med. Chem. Lett. 2022, 61, 128590. [Google Scholar] [CrossRef]
- El-Aleam, R.H.A.; George, R.F.; Lee, K.J.; Keeton, A.B.; Piazza, G.A.; Kamel, A.A.; El-Daly, M.E.; Hassan, G.S.; Abdel-Rahman, H.M. Design and synthesis of 1,2,4-triazolo[1,5-a]pyrimidine derivatives as PDE 4B inhibitors endowed with bronchodilator activity. Arch. Pharm. 2019, 352, 1900002. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-Y.; Yu, Y.-F.; Zhang, C.; Chen, Y.; Zhou, Q.; Li, Z.; Zhou, S.; Li, Z.; Guo, L.; Wu, D.; et al. Validation of Phosphodiesterase-10 as a Novel Target for Pulmonary Arterial Hypertension via Highly Selective and Subnanomolar Inhibitors. J. Med. Chem. 2019, 62, 3707–3721. [Google Scholar] [CrossRef] [PubMed]
- Faridoon; Hussein, W.M.; Vella, P.; Islam, N.U.; Ollis, D.L.; Schenk, G.; McGeary, R.P. 3-Mercapto-1,2,4-triazoles and N-acylated thiosemicarbazides as metallo-b-lactamase inhibitors. Bioorg. Med. Chem. Lett. 2012, 22, 380–386. [Google Scholar] [CrossRef]
- Sevaille, L.; Gavara, L.; Bebrone, C.; Luca, F.D.; Nauton, L.; Achard, M.; Mercuri, P.; Tanfoni, S.; Borgianni, L.; Guyon, C.; et al. 1,2,4-Triazole-3-thione Compounds as Inhibitors of Dizinc Metallo-β-lactamases. ChemMedChem 2017, 12, 972–985. [Google Scholar] [CrossRef]
- Gavara, L.; Verdirosa, F.; Sevaille, L.; Legru, A.; Corsica, G.; Nauton, L.; Mercuri, P.S.; Sannio, F.; De Luca, F.; Hadjadj, M.; et al. 1,2,4-Triazole-3-thione analogues with an arylakyl group at position 4 as metallo-β-lactamase inhibitors. Bioorg. Med. Chem. 2022, 72, 116964. [Google Scholar] [CrossRef]
- Hernandez, J.-F.; Gavara, L.; Docquier, J.-D.; Sevaille, L. Inhibitors of Metallo-Beta-Lactamases. European Patent EP 3653611 A1, 15 November 2018. [Google Scholar]
- Xiang, Y.; Chang, Y.-N.; Ge, Y.; Kang, J.S.; Zhang, Y.-L.; Liu, X.-L.; Oelschlaeger, P.; Yang, K.-W. Azolylthioacetamides as a potent scaffold for the development of metallo-β-lactamase inhibitors. Bioorg. Med. Chem. Lett. 2017, 27, 5225–5229. [Google Scholar] [CrossRef] [PubMed]
- Helgren, T.R.; Chen, C.; Wangtrakuldee, P.; Edwards, T.E.; Staker, B.L.; Abendroth, J.; Sankaran, B.; Housley, N.A.; Myler, P.J.; Audia, J.P.; et al. Rickettsia prowazekii methionine aminopeptidase as a promising target for the development of antibacterial agents. Bioorg. Med. Chem. 2017, 25, 813–824. [Google Scholar] [CrossRef]
- Menteşe, E.; Emirik, M.; Sökmen, B.B. Design, molecular docking and synthesis of novel 5,6-dichloro-2-methyl-1H-benzimidazole derivatives as potential urease enzyme inhibitors. Bioorg. Chem. 2019, 86, 151–158. [Google Scholar] [CrossRef]
- Ceylan, S.; Bektas, H.; Bayrak, H.; Demirbas, N.; Alpay-Karaoglu, S.; Ülker, S. Syntheses and Biological Activities of New Hybrid Molecules Containing Different Heterocyclic Moieties. Arch. Pharm. Chem. Life Sci. 2013, 346, 743–756. [Google Scholar] [CrossRef] [PubMed]
- Rezki, N.; Mayaba, M.M.; Al-blewi, F.F.; Aouad, M.R.; El Ashry, E.S.H. Click 1,4-regioselective synthesis, characterization, and antimicrobial screening of novel 1,2,3-triazoles tethering fluorinated 1,2,4-triazole and lipophilic side chain. Res. Chem. Intermed. 2017, 43, 995–1011. [Google Scholar] [CrossRef]
- Jain, R.K.; Mishra, V.K.; Kashaw, V. Synthesis and Antimicrobial Activity of Some New 1,2,4-Trizoles. Asian J. Chem. 2017, 29, 1317–1322. [Google Scholar] [CrossRef]
- Faiz, S.; Zahoor, A.F.; Ajmal, M.; Kamal, S.; Ahmad, S.; Abdelgawad, A.M.; Elnaggar, M.E. Design, Synthesis, Antimicrobial Evaluation, and Laccase Catalysis Effect of Novel Benzofuran–Oxadiazole and Benzofuran–Triazole Hybrids. J. Heterocycl. Chem. 2019, 56, 2839–2852. [Google Scholar] [CrossRef]
- Sonawane, A.D.; Rode, N.D.; Nawale, L.; Joshi, R.R.; Joshi, R.A.; Likhite, A.P.; Dhiman Sarkar, D. Synthesis and biological evaluation of 1,2,4-triazole-3-thione and 1,3,4-oxadiazole-2-thione as anti-mycobacterial agents. Chem. Biol. Drug Des. 2017, 90, 200–209. [Google Scholar] [CrossRef]
- Kini, S.G.; Bhat, A.; Pan, Z.; Dayan, F.E. Synthesis and antitubercular activity of heterocycle substituted diphenyl ether derivatives. J. Enzym. Inhib. Med. Chem. 2010, 25, 730–736. [Google Scholar] [CrossRef]
- Karczmarzyk, Z.; Swatko-Ossor, M.; Wysocki, W.; Drozd, M.; Ginalska, G.; Pachuta-Stec, A.; Pitucha, M. New Application of 1,2,4-Triazole Derivatives as Antitubercular Agents. Structure, In Vitro Screening and Docking Studies. Molecules 2020, 25, 6033. [Google Scholar] [CrossRef]
- Koparir, M.; Orek, C.; Parlak, A.E.; Söylemez, A.; Koparir, P.; Karatepe, M.; Dastan, S.D. Synthesis and biological activities of some novel aminomethyl derivatives of 4-substituted-5-(2-thienyl)-2,4-dihydro-3H-1,2,4-triazole-3-thiones. Eur. J. Med. Chem. 2013, 63, 340–346. [Google Scholar] [CrossRef]
- Kokovina, T.S.; Gadomsky, S.Y.; Terentiev, A.A.; Sanina, N.A. A Novel Approach to the Synthesis of 1,3,4-Thiadiazole-2-amine Derivatives. Molecules 2021, 26, 5159. [Google Scholar] [CrossRef]
- Szécsényi, K.M.; Leovac, V.M.; Evans, I.R. Synthesis and characterisation of a novel polymeric Cd complex, catena-(l-thio)[bis(N-phenylthiourea] bis(dimethylsulphoxide)dichlorocadmium(II). J. Coord. Chem. 2006, 59, 523–530. [Google Scholar] [CrossRef]
- Tretyakov, B.A.; Gadomsky, S.Y.; Terentiev, A.A. A Reaction of N-Substituted Succinimides with Hydroxylamine as a Novel Approach to the Synthesis of Hydroxamic Acids. Organics 2023, 4, 186–195. [Google Scholar] [CrossRef]
- Rao, J.M.R.; Venkatesham, U.; Doppalapudi, S.R.; Kenchegowda, B.Y.; Fernand, G.; George, J.; Madhavan, G.R.; Naidu, G.P.; Kadambari, V.S.N.R.; Jagannath, S.; et al. Preparation of Azaadamantane Derivatives for Use as 11-β-Hydroxysteroid Dehydrogenase Type 1 Inhibitors. International Patent WO 201311115 0A1, 1 August 2013. [Google Scholar]
- Timokhin, B.V.; Golubin, A.I.; Vysotskaya, O.V.; Kron, V.A.; Oparina, L.A.; Gusarova, N.K.; Trofimov, B.A. Non-catalytic addition of 1,2,4-triazole to nucleophilic and electrophilic alkenes. Chem. Heterocycl. Compd. 2002, 38, 981–985. [Google Scholar] [CrossRef]
- Dixon, L.A. Polyphosphate Ester. In e-EROS Encyclopedia of Reagents for Organic Synthesis; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2001. [Google Scholar] [CrossRef]
- Zhang, M.; Zou, B.; Gunaratna, M.J.; Weerasekara, S.; Tong, Z.; Nguyen, T.D.T.; Koldas, S.; Cao, W.S.; Pascual, C.; Xie, X.S.; et al. Synthesis of 1,3,4-oxadiazoles as selective T-tupe calcium channel inhibitors. Heterocycles 2020, 101, 145–164. [Google Scholar] [CrossRef] [PubMed]
- Samanta, S.; Lim, T.L.; Lam, Y. Synthesis and in vitro Evaluation of West Nile Virus Protease Inhibitors Based on the 2-{6-[2-(5-Phenyl-4H-{1,2,4]triazol-3-ylsulfanyl)acetylamino]benzothiazol-2-ylsulfanyl}acetamide Scaffold. ChemMedChem 2013, 8, 994–1001. [Google Scholar] [CrossRef] [PubMed]
- Damião, M.C.F.C.B.; Galaverna, R.; Kozikowski, A.P.; Eubanksc, J.; Pastre, J.C. Telescoped continuous flow generation of a library of highly substituted 3-thio-1,2,4-triazoles. React. Chem. Eng. 2017, 2, 896–907. [Google Scholar] [CrossRef]
- Peng, K.; Li, Y.; Bai, Y.; Jiang, T.; Sun, H.; Zhu, Q.; Xu, Y. Discovery of novel nonpeptide small-molecule NRP1 antagonists: Virtual screening, molecular simulation and structural modification. Bioorg. Med. Chem. 2020, 28, 115183. [Google Scholar] [CrossRef]
- Wang, Z.; Shi, H.; Shi, H. Novel synthesis of condensed heterocyclic systems containing 1,2,4-triazole ring. Synth. Commun. 2001, 31, 2841–2848. [Google Scholar] [CrossRef]
- Xiao, S.; Wang, X.; Xu, L.; Li, T.; Cao, J.; Zhao, Y. Novel panaxadiol triazole derivatives induce apoptosis in HepG-2 cells through the mitochondrial pathway. Bioorg. Chem. 2020, 102, 104078. [Google Scholar] [CrossRef]
- Shi, H.; Shi, H.; Wang, Z. Efficient one-pot synthesis of S-triazolo[3,4-b]-[1,3,5]thiadiazines containing a chiral side chain by double Mannich type reaction. J. Heterocycl. Chem. 2001, 38, 929–932. [Google Scholar] [CrossRef]
- Beyzaei, H.; Ghanbari, K.M.; Samareh, D.H.; Aryan, R. Synthesis, antimicrobial and antioxidant evaluation, and molecular docking study of 4,5-disubstituted 1,2,4-triazole-3-thiones. J. Mol. Struct. 2020, 1215, 128273. [Google Scholar] [CrossRef]
- Katkevica, S.; Salun, P.; Jirgensons, A. Synthesis of 5-substituted 3-mercapto-1,2,4-triazoles via Suzuki-Miyaura reaction. Tetrahedron Lett. 2013, 54, 4524–4525. [Google Scholar] [CrossRef]
- Taylor, R.W.; Romaine, I.M.; Liu, C.; Murthi, P.; Jones, P.L.; Waterson, A.G.; Sulikowski, G.A.; Zwiebel, L.J. Structure–Activity Relationship of a Broad-Spectrum Insect Odorant Receptor Agonist. ACS Chem. Biol. 2012, 7, 1647–1652. [Google Scholar] [CrossRef]
- Cheng, H.; Chen, C.; Cheng, J.; Song, W.; Sang, W.; Wang, Z. A Kind of Oxadiazole Heterocyclic Compound and Its Preparation Method and Application. People’s Republic of China Patent CN112300091A, 2 February 2021. [Google Scholar]
- Omar, Y.M.; Abdu-Allah, H.H.M.; Abdel-Moty, S.G. Synthesis, biological evaluation and docking study of 1,3,4-thiadiazolethiazolidinone hybrids as anti-inflammatory agents with dual inhibition of COX-2 and 15-LOX. Bioorg. Chem. 2018, 80, 461–471. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.-Y.; Zhao, P.-S.; Li, R.-Q.; Zhou, S.-M. Synthesis, Crystal Structure and Quantum Chemical Study on 3-Phenylamino-4-Phenyl-1,2,4-Triazole-5-Thione. Molecules 2009, 14, 608–620. [Google Scholar] [CrossRef] [PubMed]
- Qian, C.; Xu, J.-M.; Wu, Q.; Lv, D.-S.; Lin, X.-F. Promiscuous acylase-catalyzed aza-Michael additions of aromatic N-heterocycles in organic solvent. Tetrahedron Lett. 2007, 48, 6100–6104. [Google Scholar] [CrossRef]


| Therapeutic Areas | Targets | ||
|---|---|---|---|
| Cancer | ATPase VCP/p97 [5] | DHFR [13], FUT6 [14] | PARP-1 [22,23] |
| BRD4 [6] | HDACs [15,16] | Tdp1 [24] | |
| CA I, II, IV and IX [7,8,9,10] | Hsp90 [17,18] | TNKS1/2 [25] | |
| CK1γ3 [11] | KDM1A [19] | PARP-5b [26] | |
| DCN1-UBC12 int. [12] | MMPs [7,20,21] | TYMP [27] | |
| Diabetes | α-AMY [28], AGase [28,29,30,31] | 11b-HSD1 [32] | PTP1B [33] |
| Inflammatory disorders | ALOX15 [34,35] | COX1 [36,37] | ELANE [38] |
| Neurological disorders | AChE [30,39,40,41,42,43,44] | AChRε [45] | MPO [47] |
| BChE [30,40,41,42,43,44] | mGlu5 [46] | PKC [48] | |
| Respiratory disorders | NA [49], PDE4B [50] | PDE10A [51] | RNA pol. [35] |
| Bacterial infections | MBLs [52,53,54,55,56] | METAP1 [57] | Urease [58] |
| Activity Against | Strains | |
|---|---|---|
| Gram-positive bacteria | A. oxydans [59] | M. smegmatis [59] |
| B. cereus [59] | M. tuberculosis [63,64,65] | |
| B. subtilis [13,60,61,62] | S. aureus. [13,59,60,61,62,66] | |
| E. faecalis [59] | S. pneumoniae [60] | |
| Gram-negative bacteria | Acinetobacter sp. [59] | P. aeruginosa [13,59,60,61] |
| E. coli [13,59,60,61,62,66] | P. vulgaris [59] | |
| K. oxytoca [59] | S. marcescens [59] | |
| K. pneumoniae [60,66] | YPTB [59] | |
| Fungi | A. flavus [66] | M. anisopliae [62] |
| A. fumigatus [60,66] | P. marneffei [66] | |
| A. niger [61,62] | S. cerevisiae [59] | |
| C. albicans [13,59,60,61] | T. harzianum [62] | |
| C. tropicalis [59] | T. mentagrophytes [66] | |
| Compound | R1 | R2 | Yield, % |
|---|---|---|---|
| 2a |  | H | 30 |
| 2b | Ethyl | 27 | |
| 2c | Phenyl | 22 | |
| 2d |  | H | 34 |
| 2e | 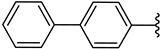 | H | 15 |
| 2f |  | H | 34 |
| 2g |  | H | 54 |
| 2h | 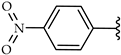 | Ethyl | 20 |
| 2i | 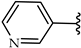 | H | 37 |
| 2j | Ethyl | 29 | |
| 2k |  | H | 35 |
| 2l | Ethyl | 13 | |
| 2m | Phenyl | 35 | |
| 2n |  | Phenyl | - |
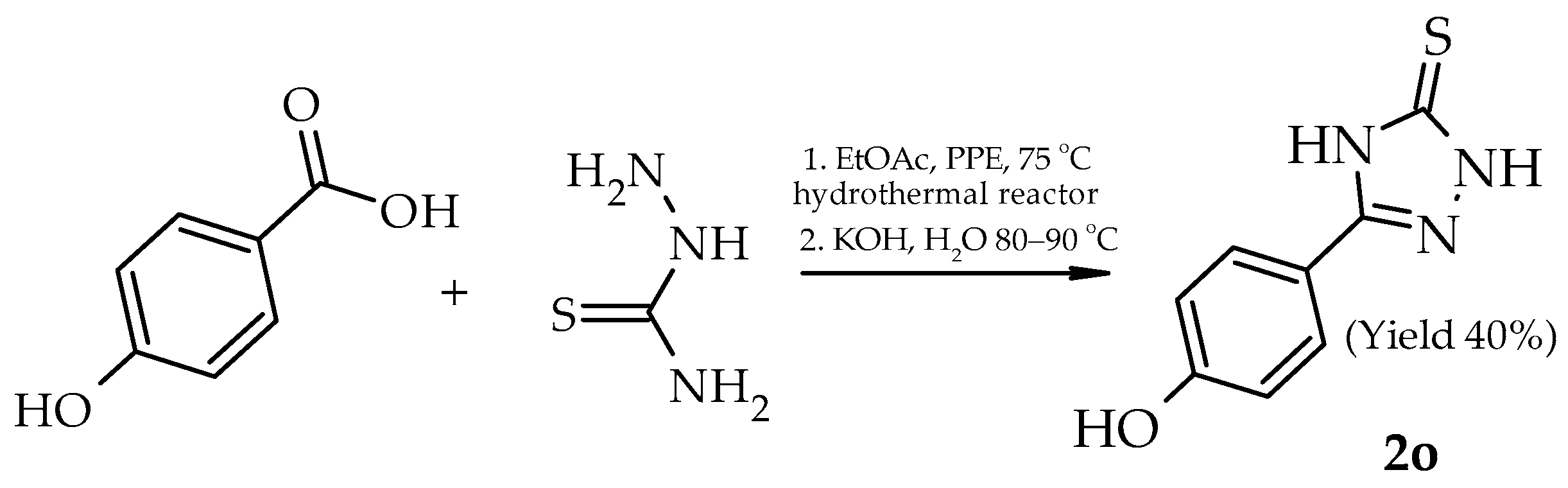
| 2o |  | H | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tretyakov, B.A.; Tikhonova, V.I.; Gadomsky, S.Y.; Sanina, N.A. Synthesis of 1,2,4-Triazole-3-Thiol Derivatives from Thiosemicarbazides and Carboxylic Acids Using Polyphosphate Ester. Molecules 2025, 30, 4422. https://doi.org/10.3390/molecules30224422
Tretyakov BA, Tikhonova VI, Gadomsky SY, Sanina NA. Synthesis of 1,2,4-Triazole-3-Thiol Derivatives from Thiosemicarbazides and Carboxylic Acids Using Polyphosphate Ester. Molecules. 2025; 30(22):4422. https://doi.org/10.3390/molecules30224422
Chicago/Turabian StyleTretyakov, Bogdan A., Viktoria I. Tikhonova, Svyatoslav Y. Gadomsky, and Nataliya A. Sanina. 2025. "Synthesis of 1,2,4-Triazole-3-Thiol Derivatives from Thiosemicarbazides and Carboxylic Acids Using Polyphosphate Ester" Molecules 30, no. 22: 4422. https://doi.org/10.3390/molecules30224422
APA StyleTretyakov, B. A., Tikhonova, V. I., Gadomsky, S. Y., & Sanina, N. A. (2025). Synthesis of 1,2,4-Triazole-3-Thiol Derivatives from Thiosemicarbazides and Carboxylic Acids Using Polyphosphate Ester. Molecules, 30(22), 4422. https://doi.org/10.3390/molecules30224422








