Abstract
Magnetite (Fe3O4) is an essential material for enhancing microwave absorption performance and is widespread and abundant as a solid solution in natural minerals and metallurgical slags. In this work, the effect of Mg2+ on the structure, stabilization, and microwave absorption performance of magnesium-containing magnetite (MgxFe3−xO4) was investigated. On the basis of experiments on the reactions of Fe2O3 and MgO under different levels of pCO/(pCO + pCO2), MgxFe3−xO4 (x=0.0,0.2,0.4,0.6,1.0) was synthesized, and Mg2+ was found to inhibit the re-oxidation of magnetite. On this basis, the microwave absorption performance of various synthesized MgxFe3−xO4 samples was measured and analyzed, where Mg2+ was found to enhance the microwave absorption performance of Fe3O4, and the RLmin value of Mg0.2Fe2.8O4 increased to −50.43 dB compared to that of −19.20 dB for Fe3O4. Furthermore, the enhancement mechanism of Mg2+ was revealed through impedance matching, dielectric and magnetic loss tangents, and magnetization curves, where the Mg2+ ions were found to accelerate the hopping of electrons and change the impedance matching of MgxFe3−xO4 to a more ideal state.
1. Introduction
The proliferation of 5G technology has propelled the application of electromagnetic waves to an unprecedented height [1,2]. While electromagnetic waves have brought much convenience to humanity, they have also introduced the increasingly prominent issues of electromagnetic radiation and electromagnetic interference (EMI) [3]. In this context, effectively reducing the impact of electromagnetic fields on device performance and addressing their potential human health effects has become of paramount importance. Consequently, research into electromagnetic-wave-absorbing materials, particularly the design, fabrication, and application of high-performance electromagnetic-wave-absorption materials, has emerged as a key focus in current studies [4,5].
Magnetite, with the primary chemical composition of Fe3O4, possesses a series of essential parameters for enhancing microwave absorption, including complex permeability, complex permittivity, high saturation magnetization, and a high Curie temperature [6,7,8,9,10]. This material offers advantages such as ease of fabrication, good biocompatibility, superparamagnetic properties, and favorable chemical stability. Furthermore, it exhibits significant magnetic loss under alternating electromagnetic fields. It has been verified that Fe3O4 crystal features a cubic inverse spinel structure, where oxygen (O) atoms form a face-centered cubic (fcc) close-packed arrangement, and the trivalent iron cations (Fe3+) occupy the tetrahedral interstitial sites (A-sites), whereas the octahedral interstitial sites (B-sites) are occupied by two Fe3+ cations and one Fe2+ cation. At room temperature, electron hopping occurs between the Fe3+ and Fe2+ cations at the B-sites, endowing magnetite with high electrical conductivity. Consequently, Fe3O4-based materials can simultaneously generate both dielectric loss and magnetic loss upon interaction with microwaves, resulting in effective wave absorption performance.
Today, some metallurgical ores and slag by-products containing relatively large amounts of magnetite, including nickel slag [11], steel slag [12], copper slag [13,14], titanium slag [15], and vanadium slag [16], have been utilized to prepare construction or building materials that possess microwave absorption properties. In addition to the mechanical strength of each individual mineral and slag product, the enhancement effect of magnetite on microwave absorption is an essential factor for enabling the higher-value application of various types of slag. In natural minerals, magnetite mainly exists in ore in the form of a solid solution [17,18]. Especially in some metallurgical ores and slags, where the bivalent ions of some gangue elements (Mg2+, Ni2+, Cu2+, et al.) tend to react with stoichiometric magnetite to replace the Fe2+ to form a non-stoichiometric M2+-doping magnetite solid solution. However, up to now, the definite structures, properties, and corresponding applications of these solid solutions in ores or slag were still an enormous challenge for the researchers, due to the lack of a database for the corresponding non-stoichiometric compounds. This would greatly inhibit the application of various ores or slags. Furthermore, the doping ions are confirmed to possess the ability to improve the dielectric polarization of the materials in some previous studies [19,20,21].
Among various slag by-products, magnesium ions are usually the most abundant and are the easiest to react with magnetite to form magnesium-containing magnetite solid solutions [22,23,24]. In this study, the effect of Mg2+ on the stabilization and microwave absorption properties of magnetite was investigated. Through the solid reaction experiments of MgO with Fe2O3, the single magnesium-containing magnetite solid solutions (MgxFe3−xO4) were synthesized, and the effect of Mg2+ on the structure of MgxFe3−xO4 was clarified. On the base, the microwave absorption properties, including reflection loss, dielectric and magnetic loss, electromagnetic performance, and impedance matching of MgxFe3−xO4 were tested and analyzed, and the enhancement mechanism of Mg2+ on the microwave absorption properties of magnetite was also revealed. This work aims to provide a new method for the performance research of solid solutions in metallurgical slags, to broaden the application field of metallurgical slag as a construction or building material.
2. Results and Discussion
2.1. Effect of Mg2+ on the Formation of MgxFe3−xO4
XRD patterns of different Mg-doping magnetite samples sintered at 900 °C for 2 h under different pCO/(pCO + pCO2) are shown in Figure 1. As shown in Figure 1a, for the sample of x = 0.0, when the pCO/(pCO + pCO2) was 0.15, all of the diffraction peaks were matched well with Fe3O4 (PDF#79-418). When the pCO/(pCO + pCO2) increased to 0.22, another kind of diffraction peak appeared, which was matched with Fe0.942O (PDF#73-2144). With the increase of pCO/(pCO + pCO2), the diffraction peak intensity of Fe0.942O was further increased, indicating that increasing pCO/(pCO + pCO2) was beneficial for the formation of Fe0.942O. While, as shown in Figure 1b, recording to Mg0.2Fe2.8O4, as the pCO/(pCO + pCO2) was increased to 0.17, the diffraction peaks of Fe0.942O appeared. Furthermore, compared with the XRD results of x = 0, there was a significant increase in the peak intensity of Fe0.942O under the same atmosphere, such as pCO/(pCO + pCO2) of 0.22 and 0.25. Furthermore, the pCO/(pCO + pCO2) for the appearance of Fe0.942O became 0.08 and 0.05, respectively, as shown in Figure 1d,e, recording to Mg0.6Fe2.4O4 and MgFe2O4. On the other hand, it can be seen that some hematite phase could appear under the air atmosphere in the sample of x = 0.6. On the contrary, there was no hematite phase in the sample of x = 1.0. It was obvious that the doping of Mg2+ in magnetite not only could inhibit the re-oxidation of magnetite, but also could promote the reduction of MgxFe3−xO4 to MgxFe1−xO, where the pCO/(pCO + pCO2) of reduction beginning for Fe3O4, Mg0.2Fe2.8O4, Mg0.4Fe2.6O4, Mg0.6Fe2.4O4, and MgFe2O4 varied from 0.22, 0.17, 0.10, 0.08, and 0.05, respectively. Furthermore, the results also indicate that the doping of Mg2+ in magnetite was also beneficial for the stabilization of magnetite in the air or oxidizing atmosphere, which was of great significance for utilization in the area of building materials or coatings.
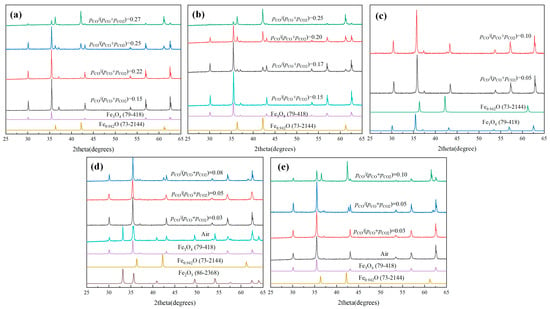
Figure 1.
XRD patterns of different MgxFe3−xO4 samples sintered at 900 °C for 2 h under different pCO/(pCO + pCO2): (a) x = 0.0, (b) x = 0.2, (c) x = 0.4, (d) x = 0.6, (e) x = 1.0.
Based on the XRD patterns of different MgxFe3−xO4 samples as above, the formation conditions of single MgxFe3−xO4 were obtained, and the corresponding SEM images of synthesized Fe3O4, Mg0.2Fe2.8O4, Mg0.6Fe2.4O4, and MgFe2O4 are shown in Figure 2. It can be seen that all four Mg2+-doping magnetites possessed a uniform state in a microscopic field of view, indicating single uniform MgxFe3−xO4 solid solutions have been synthesized through the reaction of Fe2O3 and MgO. Furthermore, the element distribution of synthesized MgxFe3−xO4 solid solutions was also investigated by the method of EDS, and the corresponding results are presented in Table 1. From the atomic percentage of Fe, Mg, and O at the three positions for the four MgxFe3−xO4 solid solutions, it was found that the variation of Fe, Mg, and O was small. Moreover, from the calculated average data as presented in Table 1, the average data for Fe, Mg, and O were close to the chemical formula of Fe3O4, Mg0.2Fe2.8O4, Mg0.6Fe2.4O4, and MgFe2O4, respectively. This further confirmed that the distribution of Mg2+ in the synthesized MgxFe3−xO4 solid solutions was basically homogeneous, which provides a guarantee for the investigation of the effect of Mg2+ on the microwave absorption properties of magnetite.
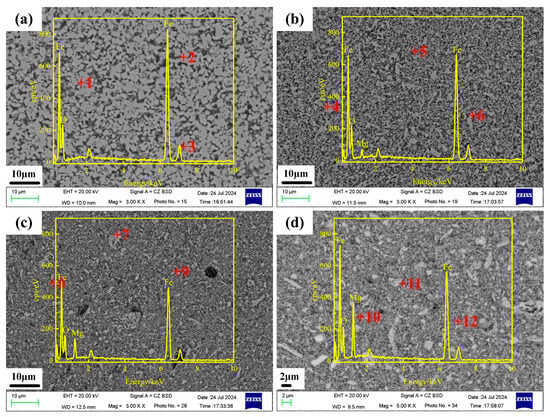
Figure 2.
SEM images of synthesized MgxFe3−xO4 samples (a) x = 0.0, pCO/(pCO + pCO2) = 0.15; (b) x = 0.2, pCO/(pCO + pCO2) = 0.15; (c) x = 0.6, pCO/(pCO + pCO2) = 0.03 (d) x = 1.0, air.

Table 1.
EDS data of synthesized MgxFe3−xO4 samples.
Due to the deficiency of systematic PDF cards for non-stoichiometric MgxFe3−xO4 solid solutions in the current XRD database, the XRD patterns of synthesized MgxFe3−xO4 were refined, and the Rietveld refinement results, final refined reliability factors, and cell parameters of synthesized MgxFe3−xO4 are shown in Figure 3 and Table 2. The reliability factor value (Chi2) for all of MgxFe3−xO4 was below 2, which demonstrated that the fitting refinement results for synthesized MgxFe3−xO4 are ideal. Furthermore, as can be seen from the structure parameters from Table 2, the synthesized MgxFe3−xO4 were all existed as a cubic space group with similar cell parameters (a = ~8.39 Å) and the same bond angle (α = β = γ = 90°), which indicated that the doping of Mg2+ in magnetite would not change the basic crystal structure. Figure 4a,b present the schematic diagram and structure parameters of the synthesized Fe3O4 and Mg0.6Fe2.4O4. It can be seen that when the Mg2+ was incorporated into the crystal lattice of Fe3O4, the Mg2+ ions could both replace the tetrahedron (A-site) and octahedron (B-site) of magnetite.
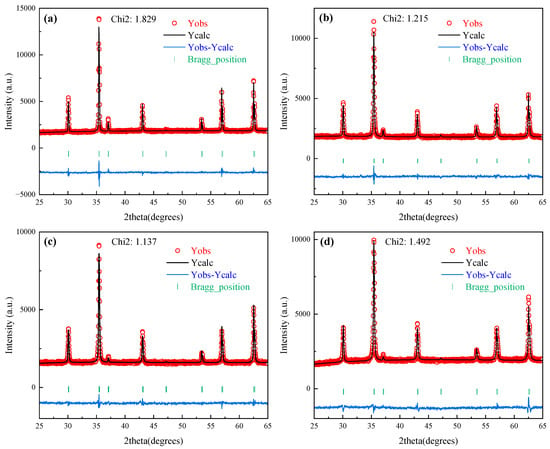
Figure 3.
Rietveld refinement results of synthesized MgxFe3−xO4: (a) x = 0.0, (b) x = 0.2, (c) x = 0.6, (d) x = 1.0.

Table 2.
Refined structure parameters and reliability factors of synthesized MgxFe3−xO4.
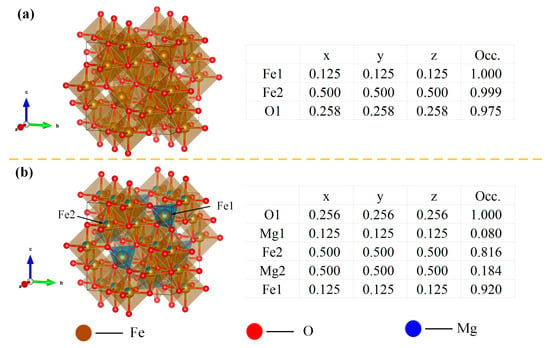
Figure 4.
Schematic diagram and structure parameters of synthesized MgxFe3−xO4: (a) x = 0.0, (b) x = 0.6.
2.2. Microwave Absorption Performance of MgxFe3−xO4
Figure 5 presents the permittivity and permeability values of MgxFe3−xO4 as a function of frequency, where the real part and imaginary part of permittivity, real part and imaginary part of permeability were shown in Figure 5a–d, respectively. It was obvious that for all the permittivity or permeability parts of MgxFe3−xO4, it showed a similar tendency as a function of frequency, indicating that the basic structure and properties of magnetite were not changed a lot. For the permittivity of MgxFe3−xO4, as shown in Figure 5a,b, it can be seen that the permittivity values for solid solutions including Mg0.2Fe2.8O4, Mg0.4Fe2.6O4, and Mg0.6Fe2.4O4 were far higher than those for stoichiometric Fe3O4 and MgFe2O4, indicating that an increase in electrical energy storage and consumption appeared after Mg2+ was doped into Fe3O4. For permeability parts of MgxFe3−xO4, as shown in Figure 5c,d, it showed a decreasing trend was observed as a function of frequency.
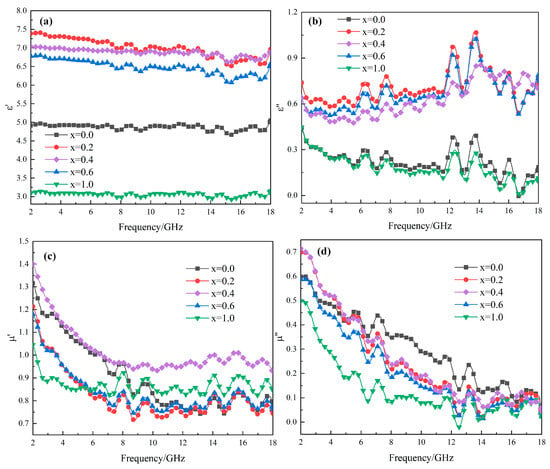
Figure 5.
Frequency dependences of permittivity and permeability of MgxFe3−xO4: (a) real part of permittivity; (b) imaginary part of permittivity; (c) real part of permeability; (d) imaginary part of permeability.
Figure 6 shows the RL-frequency curves and 2D contour RL maps of Fe3O4 and MgFe2O4 at different thicknesses (1–5 mm) in the frequency range of 1–18 GHz. It can be seen that the two stoichiometric compounds exhibited totally different microwave absorption properties. For the RL curves of Fe3O4 as shown in Figure 6a, except for the thickness of 1–2.5 mm, all of the other thicknesses possessed the EAB range (RL < −10 dB). Furthermore, it was obvious that with the increase in thickness, the EAB range was gradually moving towards a lower frequency direction. For a thickness of 4.5 mm, the RLmin value has reached −19.20 dB at the frequency of 7.26 GHz. However, for the RL curves of MgFe2O4 as shown in Figure 6c, the thickness of 3.5 mm possessed the RLmin peak of −4.72 dB at the frequency of 13.28 GHz. From the 2D contour RL maps as the functions of thickness and frequency, as shown in Figure 6b,d, it can be visually seen that the difference of absorption properties between Fe3O4 and MgFe2O4, where Fe3O4 possessed a relatively large area of EAB range, inversely, there was no EAB range for MgFe2O4.
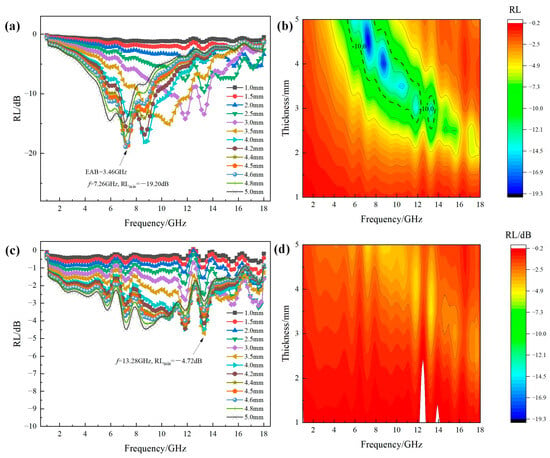
Figure 6.
(a,c) RL-frequency curves and (b,d) 2D contour RL maps at different thicknesses of Fe3O4 and MgFe2O4 in the frequency range of 1–18 GHz: (a,b) Fe3O4; (c,d) MgFe2O4.
RL-frequency curves and 2D contour RL maps at different thicknesses of MgxFe3−xO4 solid solution are shown in Figure 7. It can be seen that after Mg2+ has been doped into the magnetite, the microwave absorption properties were further enhanced compared to the single Fe3O4 and MgFe2O4. As shown in Figure 7a, for the reflection curves of Mg0.2Fe2.8O4, it can be seen that RLmin peak values for the thickness of 3–5 mm were further decreased to −25–−50 dB in the frequency range of 2–10 GHz compared to single Fe3O4, and the RLmin value of 4.4 mm has reached −50.43 dB. This confirmed that the MgxFe3−xO4 possessed the potential for application in the field of microwave absorbing coating [6]. As shown in Figure 7b, it can be seen that the area of EAB in 2D contour RL maps was significantly increased compared with single Fe3O4, especially in the lower thickness and frequency range. When the doping content of Mg2+ in the magnetite was increased to x = 0.4, as shown in Figure 7c,d, the RL value showed a slight decrease to −27.27 dB, and the area of EAB in the 2D contour RL map also showed a slight increase, especially in the higher frequency range. When the doping content of Mg2+ was increased to x = 0.6, as shown in Figure 7e,f, the RLmin value showed a significant increase, where the RLmin of −41.47 dB appeared at the frequency of 7.17 GHz with a match of 4.4 mm. From the 2D contour RL map of Mg0.6Fe2.4O4, it can also be found that the area possessing the RL value below −10 dB was also larger compared to Fe3O4 and MgFe2O4.
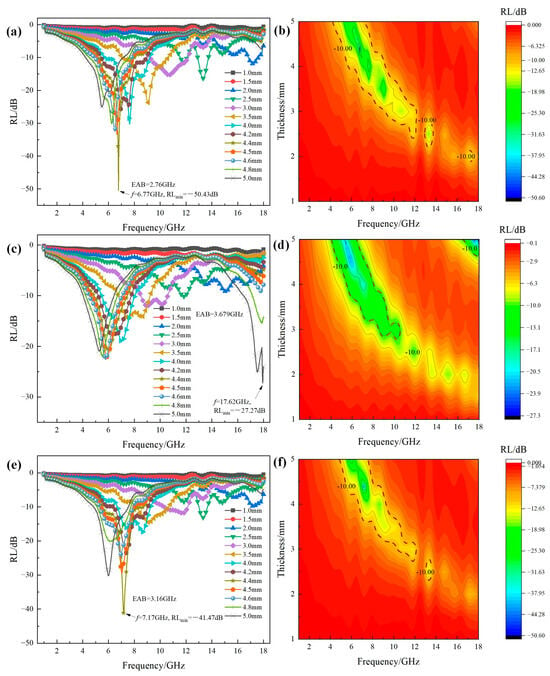
Figure 7.
(a,c,e) RL-frequency curves and (b,d,f) 2D contour RL maps at different thicknesses of MgxFe3−xO4 solid solution: (a,b) x = 0.2; (c,d) x = 0.4; (e,f) x = 0.6.
Figure 8 presents the variation curves of the RLmin value and EAB range for synthesized MgxFe3−xO4. It was obvious that the non-stoichiometric Mg2+-doping magnetite, such as Mg0.2Fe2.8O4, Mg0.4Fe2.6O4, Mg0.6Fe2.4O4, possessed the lower RLmin value and wider EAB range, where the MgFe2O4 possessed the poorest microwave absorption properties. Microwave absorption performance of Fe3O4-containing materials in some previous studies was also listed in Table 3. It was obvious that the RLmin and EAB value of Mg0.2Fe2.8O4 has been superior to Fe3O4 micro or nanoscale spheres and Fe3O4-based composite material.
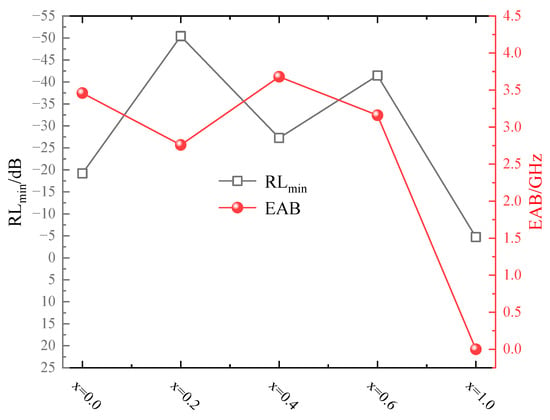
Figure 8.
Variation curves of RLmin value and EAB range for MgxFe3−xO4.

Table 3.
Microwave absorption performance of Fe3O4-containing materials in some previous studies.
2.3. Microwave Absorption Enhancement Mechanism of Mg2+ for Magnetite
In order to find the enhancement mechanism of Mg2+ for magnetite, the impedance matching of various synthesized MgxFe3−xO4 was calculated, where the impedance matching was determined based on Equation (1). As verified, the impedance matching is a fundamental principle in the design of high-performance microwave wave absorbing materials [30,31]. When the intrinsic impedance of the absorbing material approaches that of free space (Z → 1), electromagnetic waves can propagate into the material with minimal reflection at the interface, thereby allowing the material’s intrinsic loss mechanisms (dielectric or magnetic losses, e.g.,) to effectively dissipate the electromagnetic energy. Figure 9 presents the 2D impedance matching plots of synthesized MgxFe3−xO4, where the thickness, frequency, impedance matching, and corresponding color distribution are all presented in the same standard, and the regions that approach Z → 1 (0.75 < Z < 1.25) for all the MgxFe3−xO4 were highlighted. It can be seen that the impedance matching of Mg0.2Fe2.8O4 demonstrated a considerably large area, as shown in Figure 7b. In comparison, the impedance matching of Fe3O4 and Mg0.6Fe2.4O4 both possess an area with a greater impedance mismatch in the region of relatively high frequency and low thickness, as shown in Figure 9a,c. Moreover, the impedance matching of MgFe2O4 showed the greatest impedance mismatch area, as shown in Figure 7d. Combined with the RLmin results as above, the reflection loss and impedance matching of MgxFe3−xO4 exhibit the same trend of variation, which provides a good explanation for the variation trend of microwave absorption performance of MgxFe3−xO4.
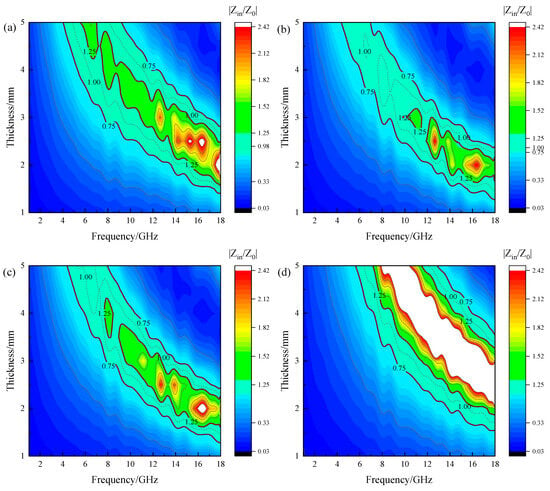
Figure 9.
2D impedance matching plots of synthesized MgxFe3−xO4: (a) x = 0.0, (b) x = 0.2, (c) x = 0.6, (d) x = 1.0.
Dielectric and magnetic loss tangents (tanδε = ε″/ε′, tanδμ = μ″/μ′) are also the criteria for intensifying the dielectric and magnetic losses in a material, where the contributions of the magnetic and dielectric loss mechanisms at different frequency ranges can be determined [32,33]. Figure 10 presents the dielectric loss tangent and magnetic loss tangent curves of MgxFe3−xO4. For the dielectric loss tangent as shown in Figure 10a, it was obvious that the stoichiometric Fe3O4, MgFe2O4 and three magnesium-containing magnetite solid solutions (Mg0.2Fe2.8O4, Mg0.4Fe2.6O4, Mg0.6Fe2.4O4) showed a completely different tendency as the function of frequency, where the variation of Fe3O4, MgFe2O4 showed a fluctuation decline tendency, inversely the magnetite solid solutions showed a rapidly rising trend, and the value of tan δε of solid solutions were much higher than that for Fe3O4 and MgFe2O4. For the magnetic loss tangent as shown in Figure 10b, the samples showed a trend of decreasing fluctuations, where the value of Fe3O4 was a little more than the other samples. As previously verified by previous studies, an increase in tangent value could significantly reveal the decrease the reflection loss and broaden the EAB through revealing loss mechanisms and optimizing impedance matching [34,35,36]. Therefore, it can be inferred that the doping of Mg2+ could mainly enhance the conversion of dielectric loss to thermal energy. Figure 11a shows the magnetization curves of Fe3O4, Mg0.6Fe2.4O4, and MgFe2O4. The saturation magnetization (Ms) of Fe3O4, Mg0.6Fe2.4O4, and MgFe2O4 was 89.94 emu/g, 61.35 emu/g, and 42.43 emu/g, respectively, indicating the magnetic properties of MgxFe3−xO4 were decreased with the increase of x. Figure 11b presents the conductivity of Fe3O4 and Mg0.2Fe2.8O4, where it is obvious that under the same pressure and similar density, the conductivity of Mg0.2Fe2.8O4 is far higher than that of Fe3O4, indicating the macroscopic current and eddy currents are triggered by charge carriers, facilitating the conversion of electromagnetic energy into heat energy. On the basis of the measured results above and previous studies, the enhancement mechanism of Mg2+ for microwave absorption performance in MgxFe3−xO4 could be revealed. The Mg2+ ions could replace the Fe3+ ions at the A-site of magnetite, and some of the Fe3+ ions migrate to the B-site, which may accelerate the hopping of electrons between Fe2+ and Fe3+ to a certain extent, thus increasing the dielectric loss of magnetite [37]. Although the magnetic properties and magnetic loss of Mg0.6Fe2.4O4 compared to magnetite were decreased, the impedance match was optimized to a more ideal value [38].
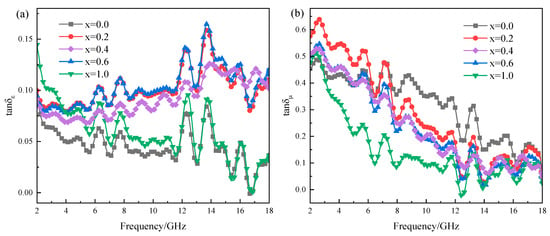
Figure 10.
Dielectric loss tangent and magnetic loss tangent curves of MgxFe3−xO4: (a) dielectric loss tangent, (b) magnetic loss tangent.
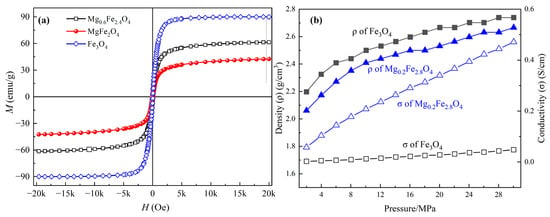
Figure 11.
Magnetization (a) and conductivity (b) curves of MgxFe3−xO4.
3. Materials and Methods
3.1. Synthesis Procedure
In this work, the Mg2+-doping magnetite was synthesized through the reaction of Fe2O3 with MgO. For stoichiometric hematite (Fe2O3) and magnetite (Fe3O4), the thermodynamic transition temperature and atmosphere could be determined based on the Fe-O phase diagram [39]. While for non-stoichiometric hematite and magnetite solid solutions, there is no exact thermodynamic database to achieve the transformation from hematite to magnetite. Therefore, in this study, the reaction behavior for Fe2O3 with MgO under different oxygen partial pressure (pCO/(pCO + pCO2)) at 900 °C was investigated to synthesize the MgxFe3−xO4 and reveal the effect of Mg2+ doping on MgxFe3−xO4.
The chemical pure reagents, including Fe2O3 and MgO from China National Pharmaceutical Group Corporation, were selected according to the Fe2O3/MgO mole ratio to synthesize different content of Mg-doping magnetite solid solutions (MgxFe3−xO4, x = 0.0, 0.2, 0.4, 0.6, 1.0) respectively. The reagents were homogenized for 30 min, followed by pressing into the tablets with about 15 mm in diameter and 5 mm in height under a pressure of 5 MPa by the electric briquetting machine. After that, the tablets were put into a hanging basket made of Fe-Cr-Al alloy, which was heated in an in-situ thermogravimetric testing device under the specified time and temperature, followed by quenching in water. The in-situ thermogravimetric testing device consisted of an electronic balance, a resistance furnace, and a gas flow control system, where more annotations about the device have been illustrated in our previous studies [40]. The atmosphere in this work was controlled by the gas flow control system, where the gas flow velocity of N2 (vN2) was set as 1.0 L/min, and the sum of the flowing velocity for CO and CO2 was set as 1.0 L/min (vCO + vCO2 = 1.0 L/min). The pCO/(pCO + pCO2) was controlled by the flowing velocity ratio of CO and CO2 to adjust the partial oxygen pressure.
3.2. Characterizations
The quenched samples were first dried at 60 °C and then separated into two parts, where one part was grinded to powders below 50 μm size in the agate mortar for half an hour for powder XRD determination, where mineral phase was identified by Rigaku Ultima IV X-ray diffractometer (Rigaku Corporation, Tokyo, Japan), and Cu Kα was used as the radiation source (40 kV, 400 mA) combined with the graphite monochromator. The continuous scanning speed was 10°/min, and the data was analyzed by Crystallographica Search-Match (version of 2.1.1.0). The other part was polished for SEM and optical observations. The microstructure observation was performed by FEI Quanta 250 scanning electron microscope (FEI Corporation, Hillsboro, OR, USA). The element distribution was obtained by the XFlash 5030 EDS detector (Bruker Nano GmbH, Berlin, Germany). Magnetic properties of MgxFe3−xO4 samples were investigated at room temperature using a vibrating sample magnetometer (VSM, LakeShore, Chicago, IL, USA). The conductivity of MgxFe3−xO4 samples was measured at room temperature using a four-probe resistivity tester (ST2742C, Suzhou Lattice Electronics Co., Ltd., Suzhou, China).
In this study, Rietveld refinement was conducted to obtain the structure variation of MgxFe3−xO4. The refinement program was Fullproof (Version of July2001). The detected diffraction peaks of XRD patterns were fitted by a pseudo-Voigt profile function. The background level is simulated by the six-coefficient polynomial function. The basic crystallographic information files of MgxFe3−xO4 were obtained from the ICSD database. Subsequently, the refinement was proceeded with a sequence of scale factor, lattice parameters, zero shift, shape and asymmetry parameters, full width at half maximum (FWHM), and atomic positions, respectively.
3.3. Microwave Absorption Test
In this study, the microwave absorption test was also conducted to assess the microwave absorption performance of MgxFe3−xO4. Before the measurement, the MgxFe3−xO4 was first ground into powder in the agate mortar for half an hour to maintain the MgxFe3−xO4 in the same particle size level. The microwave absorption measurements were carried out using the vector network analyzer (VNA, Agilent E5071C, Santa Clara, CA, USA) equipped with a coaxial transmission waveguide, where the frequency range was 1–18 GHz. For every measurement, the Short, Open, Load, and Thru standard components are confirmed to be clean and undamaged, and the coaxial cables and connectors are inspected for any physical defects are inspected. After that, the desired frequency range, the appropriate calibration type, and the calibration kit definitions corresponding to the physical standards in use are set. Subsequently, the calibration process is performed sequentially on each port. The mixture of magnetite powders and paraffin wax at a 1:1 mass ratio, which was pressed into an appropriate toroidal-shaped sample (Φouter = 7.00 mm and Φinner = 3.04 mm) with tunable layer thickness. The VNA was calibrated in both forward and reverse directions, and the microwave source was stabilized for 1.5 h. During the normal running of VNA, the electromagnetic parameters, including ε′, ε″, μ′, μ″ of as-obtained toroidal-shaped samples were determined. The ε′ and ε″ are the real part and imaginary part of complex permittivity, whereas the μ′ and μ″ are the real part and imaginary part of complex permeability, respectively. The relative complex permittivity (εr) and relative permeability (μr) of the absorb medium are expressed as εr = ε − jε″ and μr = μ′ − jμ″. The reflection loss (RL) values of samples with different layer thicknesses can be calculated using MATLAB (R2021b) based on the following formulas:
where Z0 represents the impedance of free space, Zin is the normalized input impedance of the microwave absorption layer, f is the frequency of the electromagnetic wave, c is the velocity of light in free space, d is the layer thickness, and RLmin is the minimal reflection loss value. The frequency range where the RL value was below −10 dB represents that over 90% of the electromagnetic waves can be absorbed, and can be defined as the effective absorption bandwidth (EAB).
4. Conclusions
In this work, the magnesium-containing magnetite solid solution (MgxFe3−xO4) was synthesized, and the effect of Mg2+ on microwave absorption performance was investigated, whereas the microwave absorption enhancement mechanism by Mg2+ was also revealed.
- (1)
- The reaction behaviors of Fe2O3 and MgO under various pCO/(pCO + pCO2) atmosphere were investigated. It was found that Mg2+ could not only inhibit the re-oxidation of magnetite, but also promote the reduction of MgxFe3−xO4 to MgxFe1−xO, where the pCO/(pCO + pCO2) of reduction beginning for Fe3O4, Mg0.2Fe2.8O4, Mg0.4Fe2.6O4, Mg0.6Fe2.4O4, and MgFe2O4 varied from 0.22, 0.17, 0.10, 0.08, and 0.05, respectively.
- (2)
- Microwave absorption performance of MgxFe3−xO4 for the thickness of 1–5 mm was measured and analyzed. It was found that Mg2+ could significantly improve the microwave absorption performance of Fe3O4, where the RLmin value of Mg0.2Fe2.8O4 has decreased to −50.43 dB compared to −19.20 dB for Fe3O4. When the content of Mg2+ in Fe3O4 increased to x = 1 (MgFe2O4), the performance suddenly deteriorated, where the RLmin value decreased to −4.72 dB.
- (3)
- The enhancement mechanism for microwave absorption performance of MgxFe3−xO4 by Mg2+ was revealed through impedance matching, dielectric loss tangent, and magnetic loss and magnetization curves, where the Mg2+ ions could accelerate the hopping of electrons to improve the dielectric loss of magnetite, thus the impedance match could be optimized to a more ideal value.
Author Contributions
Conceptualization, Y.D. and X.G.; methodology, Y.D. and J.S.; software, Y.D.; validation, X.G. and X.D.; formal analysis, Y.D. and B.L.; investigation, Y.D.; resources, X.G. and X.D.; data curation, Y.D.; writing—original draft preparation, Y.D.; writing—review and editing, X.G.; visualization, Y.Y.; supervision, X.G. and X.L.; project administration, X.G.; funding acquisition, X.G. and X.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China, grant numbers 52304317 and U22A20175.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
No new data were created, and no data are available due to privacy or ethical restrictions.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Liu, J.; Li, Y.; Jin, C.; Li, Y.; Yang, B.; Lin, H. Preparation and research of high electromagnetic wave transmission type concrete in 5G frequency band. Constr. Build. Mater. 2024, 443, 137769. [Google Scholar] [CrossRef]
- Cheon, S.J.; Choi, J.R.; Lee, S.B.; Lee, J.I.; Lee, H. Frequency tunable Ni–Ti-substituted Ba–M hexaferrite for efficient electromagnetic wave absorption in 8.2–75 GHz range. J. Alloy Compd. 2024, 976, 173019. [Google Scholar] [CrossRef]
- Cui, Z.; Yang, M.; Han, G.; Zhang, H.; Wang, Y.; Zhang, Y.; Li, Z.; He, J.; Yu, R.; Shui, J.; et al. Recent advances in carbon composite films for high-performance, multifunctional and intelligent electromagnetic interference shielding and electromagnetic wave absorption. Carbon 2024, 230, 119627. [Google Scholar] [CrossRef]
- Sharma, S.; Parne, S.R.; Panda, S.S.S.; Gandi, S. Progress in microwave absorbing materials: A critical review. Adv. Colloid. Interfac. 2024, 327, 103143. [Google Scholar] [CrossRef]
- Jia, Z.; Lan, D.; Lin, K.; Qin, M.; Kou, K.; Wu, G.; Wu, H. Progress in low-frequency microwave absorbing materials. J. Mater. Sci-Mater. El. 2018, 29, 17122–17136. [Google Scholar] [CrossRef]
- Wang, L.; Wang, Y.; Lu, J.; Yan, X.; Liu, D.; Zhang, X.; Huang, X.; Wen, G. Electromagnetic absorption by magnetic oxide nanomaterials: A review. ACS Appl. Nano Mater. 2023, 6, 22611–22634. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, Y.; Rehman, S.U.; Wang, L.; Chen, Y.; Shen, S.; Chen, C.; Liang, T. In situ synthesis of Fe3O4 coated on iron-based magnetic microwave absorbing materials and the influence of oxide magnetic materials on microwave absorption mechanism. Ceram. Int. 2023, 49, 12972–12979. [Google Scholar] [CrossRef]
- Wang, X.; Liu, Y.; Han, H.; Mølhave, K.; Sun, H. Enhanced high-frequency microwave absorption of Fe3O4 architectures based on porous nanoflake. Ceram. Int. 2017, 43, 16013–16017. [Google Scholar] [CrossRef]
- Liu, X.; Zhao, Y.; Wei, Z.; Zhang, D. Microwave absorption enhancement of asphalt concrete with SiC-Fe3O4 mixtures modifier. Constr. Build. Mater. 2020, 254, 119209. [Google Scholar] [CrossRef]
- Bleija, M.; Platnieks, O.; Starkova, O.; Macutkevič, J.; Tsyhanok, D.; Orlova, L.; Gaidukovs, S. Evaluation of thermal conductivity models and dielectric properties in metal oxide-filled poly (butylene succinate-co-adipate) composites. Sci. Rep. 2024, 14, 13629. [Google Scholar] [CrossRef]
- Zong, H.; Long, J.; Zheng, J.; Shen, Y.; Li, B.; Shen, Y.; Ren, X.; Lu, S.; Du, X. Preparation of nickel slag derived Fe3O4/conductive carbon black/natural rubber composites and enhanced microwave absorption. J. Mater. Sci. 2024, 35, 157. [Google Scholar] [CrossRef]
- Lou, B.; Liu, Z.; Sha, A.; Jia, M.; Li, Y. Microwave absorption ability of steel slag and road performance of asphalt mixtures incorporating steel slag. Materials 2020, 13, 663. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Li, P.; Zhu, Y.; Zhao, Q.; Zhang, H. Experimental study on microwave absorption properties of HMA containing copper slag. Constr. Build. Mater. 2022, 341, 127850. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, T.; Zhou, L. Investigation on electromagnetic and microwave absorption properties of copper slag-filled cement mortar. Cem. Concr. Comp. 2016, 74, 174–181. [Google Scholar] [CrossRef]
- Chen, G.; Chen, J.; Peng, J. Effects of mechanical activation on structural and microwave absorbing characteristics of high titanium slag. Powder Technol. 2015, 286, 218–222. [Google Scholar] [CrossRef]
- Li, K.; Jiang, Q.; Gao, L.; Chen, J.; Peng, J.; Koppala, S.; Omran, M.; Chen, G. Investigations on the microwave absorption properties and thermal behavior of vanadium slag: Improvement in microwave oxidation roasting for recycling vanadium and chromium. J. Hazard. Mater. 2020, 395, 122698. [Google Scholar] [CrossRef]
- Harrison, R.J.; Putnis, A. Magnetic properties of the magnetite-spinel solid solution: Curie temperatures, magnetic susceptibilities, and cation ordering. Am. Mineral. 1996, 81, 375–384. [Google Scholar] [CrossRef]
- Higuchi, K.; Tanaka, T.; Sato, T. Reaction behavior of dolomite accompanied with formation of magnetite solid solution in iron ore sintering process. ISIJ Int. 2007, 47, 669–678. [Google Scholar] [CrossRef]
- Li, Y.; Jin, Y.; Raza, H.; Wang, Y.; Chen, Q.; Zou, X.; Ren, Z.; Guo, J.; Zheng, G.; Cheng, J. Dual driving strategy from micro-polarization to macroscopic conductance: Tailoring optimized low-frequency and wide-band microwave absorption in high-entropy oxides. J. Mater. Sci. Technol. 2025, 235, 110–121. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, C.; Wang, D.; Ye, F.; Lu, F.; Hu, C.; Cheng, L. Effect of Sm doping on structure and microwave absorption properties of brownmillerite oxide Ca2Fe2O5. Ceram. Int. 2025, 51, 57997–58009. [Google Scholar] [CrossRef]
- Jiang, W.; Xu, S.; Lv, C.; Lan, D.; Zhang, S.; Gao, Z.; Jia, Z.; Wu, G. Multi-scale Engineering of N-doped Carbon Nanofibers with Co3O4/CeO2 Heterostructures: Tailoring Heterointerface Polarization for Microwave Absorption. Carbon 2025, 245, 120784. [Google Scholar] [CrossRef]
- Jastrzębska, I.; Przystaś, J.; Pająk, O.; Błachuta, T.; Drożdż, P.; Mandal, S. Corrosion and wettability of Magnesium Orthotitanate (Mg2TiO4) refractory aggregate by copper slags with varying iron/silica ratios. J. Eur. Ceram. Soc. 2025, 46, 117728. [Google Scholar] [CrossRef]
- Ren, G.; Wang, X.; Zheng, B.; Zhang, Z.; Yang, L.; Yang, X. Fabrication of Mg doped magnetite nanoparticles by recycling of titanium slag and their application of arsenate adsorption. J. Clean. Prod. 2020, 252, 119599. [Google Scholar] [CrossRef]
- Sun, L.; Wu, J.; Wang, J.; Yang, Y.; Xu, M.; Liu, J.; Yang, C.; Cai, Y.; He, H.; Du, Y.; et al. In-situ constructing nanostructured magnesium ferrite on steel slag for Cr (VI) photoreduction. J. Hazard. Mater. 2022, 422, 126951. [Google Scholar] [CrossRef] [PubMed]
- Ni, S.; Sun, X.; Wang, X.; Zhou, G.; Yang, F.; Wang, J.; He, D. Low temperature synthesis of Fe3O4 micro-spheres and its microwave absorption properties. Mater. Chem. Phys. 2010, 124, 353–358. [Google Scholar] [CrossRef]
- Li, L.G.; Lai, Q.; Zeng, G.X.; Li, Y.J.; Xie, H.Z.; Kwan, A.K.H. Combined effects of micro and nano Fe3O4 on workability, strength, packing, microstructure and EM wave absorbing properties of mortar. Constr. Build. Mater. 2023, 406, 133407. [Google Scholar] [CrossRef]
- Xu, J.; Tang, S.; Liu, D.; Bai, Z.; Xie, X.; Tian, X.; Xu, W.; Hou, W.; Meng, X.; Yang, N. Rational design of hollow Fe3O4 microspheres on Ti3C2Tx MXene nanosheets as highly-efficient and lightweight electromagnetic absorbers. Ceram. Int. 2022, 48, 2595–2604. [Google Scholar] [CrossRef]
- Zhu, L.; Zeng, X.; Chen, M.; Yu, R. Controllable permittivity in 3D Fe3O4/CNTs network for remarkable microwave absorption performances. RSC Adv. 2017, 7, 26801–26808. [Google Scholar] [CrossRef]
- Deng, B.; Wang, L.; Xiang, Z.; Liu, Z.; Pan, F.; Lu, W. Rational construction of MXene/Ferrite@C hybrids with improved impedance matching for high-performance electromagnetic absorption applications. Mater. Lett. 2021, 284, 129029. [Google Scholar] [CrossRef]
- Jazirehpour, M.; Ebrahimi, S.S. Effect of aspect ratio on dielectric, magnetic, percolative and microwave absorption properties of magnetite nanoparticles. J. Alloys Compd. 2015, 638, 188–196. [Google Scholar] [CrossRef]
- Xu, H.; Deng, J.; Bai, Z.; Zhao, B.; Wang, G.; Yang, L. Natural magnetite/coke composite: A novel promising microwave absorption material. J. Alloys Compd. 2023, 931, 167497. [Google Scholar] [CrossRef]
- Cao, Y.; Liu, C.; Xue, Z.; Jiang, T.; Fang, G.; Peng, K.; Zhang, Y. Excellent microwave absorption of Fe3O4/Ag composites attained by synergy of considerable magnetic loss and dielectric loss. Ceram. Int. 2022, 48, 5824–5830. [Google Scholar] [CrossRef]
- Jadav, M.; Bhatnagar, S.P. Particle size controlled magnetic loss in magnetite nanoparticles in RF-microwave region. IEEE Trans. Magn. 2020, 56, 2800208. [Google Scholar] [CrossRef]
- Hussain, A.; Gul, I.H.; Khan, M.Z. Enhancement of dielectric, magnetic and microwave absorption properties of Co2+-Zr4+ substituted SrFe12O19 nanoparticles. Ceram. Int. 2025, 51, 4768–4779. [Google Scholar] [CrossRef]
- Huang, X.; Qiao, M.; Lu, X.; Li, Y.; Ma, Y.; Kang, B.; Quan, B.; Ji, G. Evolution of dielectric loss-dominated electromagnetic patterns in magnetic absorbers for enhanced microwave absorption performances. Nano Res. 2021, 14, 4006–4013. [Google Scholar] [CrossRef]
- Zhu, S.; Guo, X.; Xiang, R.; Cheng, L.; Ye, F.; Li, Z.; Yao, Q.; Qi, H.; Long, Q. Balance dielectric and magnetic losses to enhance the microwave absorption performance of CaFe0. 5Mn0. 5O3−δ@ Co3O4@ Fe3O4. J. Alloys Compd. 2025, 1020, 179194. [Google Scholar] [CrossRef]
- Singh, N.; Agarwal, A.; Sanghi, S.; Singh, P. Effect of magnesium substitution on dielectric and magnetic properties of Ni–Zn ferrite. Physica. B 2011, 406, 687–692. [Google Scholar] [CrossRef]
- Abbas, S.M.; Dixit, A.K.; Chatterjee, R.; Goel, T.C. Complex permittivity, complex permeability and microwave absorption properties of ferrite–polymer composites. J. Magn. Magn. Mater. 2007, 309, 20–24. [Google Scholar] [CrossRef]
- Sundman, B. An assessment of the Fe-O system. J. Phase Equilibria 1991, 12, 127–140. [Google Scholar] [CrossRef]
- Du, Y.; Guo, X.M. Displacement and Migration Behavior of Al3+ in Ca2Fe2−xAlxO5 Solid Solution During Reduction Process. Metall. Mater. Trans. B 2024, 55, 2601–2616. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).