Abstract
Ruscus aculeatus L. (butcher’s broom), a member of the Asparagaceae family, is a perennial shrub native to the Mediterranean and Black Sea regions and naturalized in parts of Europe and North America. Traditionally, the rhizome and root of this species have been employed in folk medicine for the treatment of venous insufficiency, hemorrhoids, edema, and various dermatological and urinary ailments. These therapeutic applications are attributed primarily to the presence of steroidal saponins such as ruscogenin and neoruscogenin, as well as flavonoids and other bioactive compounds. In recent decades, R. aculeatus extracts have been incorporated into numerous pharmaceutical and cosmetic preparations, particularly those intended to improve venous tone, reduce swelling, and alleviate symptoms of chronic venous disorders. However, despite its widespread use, studies regarding R. aculeatus remain limited. Many investigations have focused on complex formulations such as Cyclo 3 Fort, which also contains hesperidin methylchalcone and ascorbic acid, making it difficult to attribute the observed effects solely to R. aculeatus. This review provides an overview of the current knowledge on the phytochemistry and pharmacological activities of R. aculeatus. The available data support the plant’s traditional use, yet further well-designed experimental and clinical studies are needed to clarify its mechanisms of action, confirm its therapeutic potential, and ensure safety and standardization in medicinal preparations.
1. Introduction
Ruscus L. is a genus of plants in the family Asparagaceae, which includes six species distributed from Macaronesia through Europe and North Africa [1]. Plants of this genus are primarily found in forests, often growing in deep shade. Among these species, Ruscus aculeatus L. is the most widespread. It occurs in forests on calcareous soils, in shrubland formations, shaded rocky areas, and moist habitats [2]. R. aculeatus occurs naturally in countries bordering the Mediterranean and Black Sea, as well as in southeastern Great Britain. The species has also been introduced to Mexico, Germany, and Ireland [3]. The scientific name of the genus derives from the ancient Latin word ruscum, referring to a thorny plant [4]. In English, R. aculeatus is commonly known as “butcher’s broom,” a name originating from the traditional use of its branches to clean butchers’ wooden blocks of blood and fat residues. Similarly, its Italian name (Pungitopo), its German name (Mausedorn)—both literally meaning “mouse sting”—and its Polish name (Myszopłoch kolczasty) refer to the custom of placing the plant’s shoots over food to keep rodents and other pests away [5].
The root and rhizome of R. aculeatus have traditionally been used in folk medicine for the treatment of venous insufficiency, hemorrhoids, and edema due to their vasoconstrictive, anti-inflammatory, and diuretic properties. Infusions and decoctions have also been employed to alleviate symptoms of colitis and diarrhea [6,7]. In Palestinian, South Balkan, and Eastern Mediterranean traditional medicine, R. aculeatus is mentioned as a remedy for various skin-related ailments [8,9]. In Turkey, decoctions prepared from the rhizomes are traditionally applied to relieve eczema [10], while infusions or decoctions from the aerial parts are used in Greece to treat itching and skin cracks [11]. Additionally, root and stem infusions are reported to be used as treatments for kidney stones and nephritis in Turkey [10,12]. In central Italy, topical applications of the plant have been used to manage warts and frostbite [6,13]. In addition, the species has been cultivated as an ornamental plant and as a food plant. Young shoots of R. aculeatus are eaten like asparagus in countries of the Istria region and Italy [14]. The seeds were also used in the past as a coffee substitute [5].
Despite its wide range of traditional uses, the most recognized property of R. aculeatus is its venoactive effect, which has found application in modern phytotherapy, particularly in the management of disorders associated with venous insufficiency. The rhizome is described in the EU herbal monograph as a remedy used to relieve leg discomfort and heaviness and to support the treatment of hemorrhoids [15]. Over recent decades, extracts of R. aculeatus have been incorporated into different preparations aimed at improving venous tone, reducing swelling, and alleviating symptoms of chronic venous disorders [16,17,18]. It has also been included as one of the components of a nanoemulsion tested in patients with advanced hemorrhoidal disease [19]. In medicine, the underground parts of the plant are used, as they are rich in active compounds such as steroidal saponins, which are believed to contribute to its therapeutic effects [5].
However, despite its widespread use, the number of studies evaluating its efficacy remains limited, and most research articles focus on Cyclo 3 Fort, which is actually a mixture of dry extract of butcher’s broom, hesperidin methylchalcone (HMC), and ascorbic acid (vitamin C) [20]. Therefore, the observed effects are difficult to attribute specifically to the activity of R. aculeatus itself.
Given the ongoing popularity of R. aculeatus in various formulations, there is a clear need to summarize the existing scientific data and identify gaps in current knowledge. This review aims to provide an up-to-date overview of the phytochemistry, pharmacological activities, and clinical applications of R. aculeatus, helping to improve the understanding of its potential therapeutic role and guide future research.
A comprehensive literature survey was conducted using the Scopus, PubMed, ScienceDirect, Web of Science, and Google Scholar databases using the term “Ruscus aculeatus” or “butcher’s broom”. Studies for which the full text was not available in English were excluded, as were studies in which the reported biological activity could not be attributed solely to R. aculeatus but rather to multi-component mixtures containing the plant.
2. Botanical Description
Ruscus aculeatus (Figure 1) is an evergreen, perennial herbaceous species, growing up to 80 cm. The representatives of this species are sometimes described as subshrubs, or shrubs; however, the shoots of Ruscus are not woody. It develops a short, underground rhizome forming dense clusters, which represents the pharmacognostically relevant part of the plant (Ruscus rhizoma) [2]. The aerial shoots are unbranched and bear green, rigid phylloclades—flattened, leaf-like branches arising from the axils of reduced leaves. These phylloclades, ovate to lanceolate, leathery, and ending in a sharp spine, perform an assimilative function [5,21]. True leaves occur only in seedlings or occasionally directly from the rhizome. Small, sessile bracts subtend the solitary, dioecious flowers located on the lower surface of the phylloclades [2]. The flowers are small and yellowish-white, with six pale green tepals, and either a violet ovary or stamens (the species is dioecious). Flowers develop between March and April. Butcher’s broom produces bright red berries, ripening in the autumn and winter, up to 15 mm in diameter, each containing one or two seeds [5,22]. The combination of an underground rhizome and phylloclades with axillary flowers and spiny tips is characteristic and allows easy identification of R. aculeatus in pharmacognostic analysis.

Figure 1.
Photograph of the Ruscus aculeatus plant taken at the Botanical Garden, UMCS, Lublin, Poland.
3. Phytochemistry
3.1. Steroidal Saponins
Phytochemical investigations of R. aculeatus have revealed a complex chemical profile dominated by steroidal saponins of both the spirostanol and furostanol types. In general, steroidal saponins possess a C27 carbon skeleton, usually derived from oxidized cholesterol, and contain one or more sugar residues attached at various positions. Spirostanol saponins are characterized by a bicyclic ketal structure at C-22, whereas furostanol saponins typically contain a hemiketal group at C-22 and an additional sugar moiety (most often a single β-d-glucose unit) linked to C-26. The principal aglycone of the spirostanol saponins identified in R. aculeatus is ruscogenin (Figure 2).
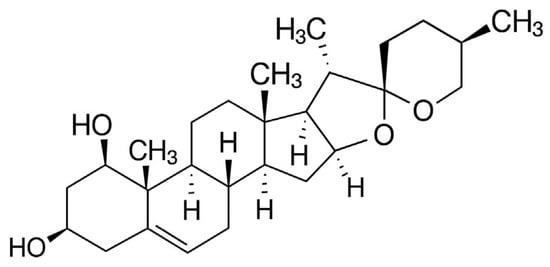
Figure 2.
Chemical structure of ruscogenin.
Among the numerous glycosidic derivatives isolated from the underground parts are ruscoside, ruscin, deglucoruscin, deglucoruscoside, desglucoruscoside, and a series of complex saponins such as ruscoponticosides (C–E), ceparosides (A, B), aculeosides (A, B), and spilacleosides (A, B), often containing sugar chains composed of α-l-rhamnose, β-d-galactose, and β-d-glucose units, variably acetylated at positions 3, 4, or 6 [23,24,25,26]. A characteristic feature of several metabolites is the presence of sulfated moieties [27,28]. The saponin profile also includes glycosides with molecular formulas ranging from approximately C39H62O13 to C58H92O27, comprising mono-, di-, and tri-acetylated sugar chains and furostanol-type derivatives glycosylated at C-26 [29,30].
Because the structure of steroidal saponins is very complex, hydrolysis is often performed to release free aglycones. Alkaline hydrolysis with KOH under elevated temperature conditions (4 h) yielded stigmasterol, ruscogenin, and neoruscogenin [31]. Ruscogenin was also identified following acid hydrolysis with 1% sulfuric acid for 12 h [32]. Free ruscogenin and neoruscogenin were also detected after treatment with 37% HCl (heating for 1 h) or 1 M H2SO4 (heating for 8 h) [33,34].
Interestingly, tissue culture systems (in vitro callus and regenerant cultures) of R. aculeatus retain the biosynthetic capacity to produce the same phytochemical markers found in the natural plant, including ruscogenin, neoruscogenin, ruscoside, ruscin, and desglucoruscin. Their qualitative and quantitative composition can be modulated by culture conditions and elicitation with methyl jasmonate [35,36,37].
3.2. Phenolic Compounds
Beyond saponins, R. aculeatus contains a diverse array of phenolic compounds and flavonoids, particularly in its aerial parts. HPLC–MS and UHPLC–MS/MS analyses identified derivatives of apigenin, quercetin, and kaempferol, including apigenin-C-Hexoside-C-pentoside, quercetin-O-deoxyhexoside-hexoside, caffeic acid hexoside, and kaempferol-O-deoxyhexoside-hexoside, found in hydroalcoholic extracts prepared from stems and rhizomes [38,39,40]. In the berries, anthocyanins were detected, primarily pelargonidin derivatives such as pelargonidin-3-glucoside, pelargonidin-3-rutinoside, and pelargonidin-3-p-coumaroylglucoside [41].
3.3. Other Minor Components
Additionally, ethanol and chloroform extracts of rhizomes yielded non-saponin constituents, including 12-docosenoic acid and euparone [42,43]. More recent studies reported the presence of phenethylamine-type alkaloids, such as aculebiphenyl A and aculebiphenyl B, together with mesembrine and mesembrenone [44].
Overall, the phytochemical profile of R. aculeatus represents a rich spectrum of spirostanol and furostanol saponins—often sulfated and/or acetylated—complemented by polyphenols, anthocyanins, fatty acids, and minor alkaloids. However, quantitative data are limited. Some studies report content per dried extract or as isolation yields [26,28,38,39], which does not reflect total plant content. Only a few studies provide quantitative data, but these results should be interpreted with caution, as compound identification was based solely on retention time comparison with standards. It has been reported that the contents of p-coumaric acid and quercetin in the aerial parts of the plant are 3.0 and 4.1 mg/g, respectively [40]. The ruscogenin content in the aerial and underground parts of four Ruscus taxa ranged from 0.06 to 0.22% in the herb and 0.04 to 0.19% in the rhizome [45]. Walasek-Januszet al. reported a content of rusogenin in the range of 0.3–0.38 and of neorusogenin in the range of 0.83–1.02% in rhizomes [46]. In turn, Tansi et al. found that the sum of ruscogenin and neoruscogenin in the aerial parts was 0.03–0.05%, and in the underground parts, it was 0.02–0.12% [47].
The main findings regarding the phytochemical constituents of R. aculeatus are summarized in Table 1.

Table 1.
Main chemical constituents found in R. aculeatus and identification techniques.
4. Biological Activity
4.1. Venotonic and Vasoprotective Effects
R. aculeatus is best known for its venotonic and vasoprotective effects. In this context, it is frequently mentioned in numerous review articles discussing the use of herbal drugs in chronic venous disease (CVD) [16,17,54,55,56].
CVD is a pathological condition caused by venous blood stasis resulting from incompetence of the venous valves. The disease is very common in adults and is responsible for skin changes and for approximately 40% of venous leg ulcers [57,58]. In the initiation of primary CVD, the process begins with leukocyte adhesion, followed by degranulation and migration beneath the venous endothelium, leading to chronic inflammation and subsequent remodeling of the venous valves and wall [59]. In the development of secondary CVD, persistent vein obstruction or recurrent deep vein thrombosis results in valve damage and venous hypertension [60]. This leads to increased leukocyte adhesion, excessive transcapillary filtration, and edema. Dilated, tortuous capillaries with impaired flow reduce oxygen delivery, leading to hyperpigmentation and lipodermatosclerosis [61]. In advanced CVD, fluid accumulation and venous abnormalities manifest as telangiectasias, varicose veins, pigmentation changes, pain, cramps, and restless legs [62].
Scientific reports verifying the venoactive and vasoprotective properties of R. aculeatus alone are scarce and originate mainly from the 1980s and 1990s. In turn, most recent studies have focused on combinations—particularly the mixture of R. aculeatus, hesperidin methyl chalcone (HMC), and vitamin C (Cyclo 3 Fort; Pierre Fabre, Paris, France). This formulation was developed in 1959 and is currently used as a first-line treatment for alleviating CVD symptoms. However, this review focuses specifically on the activity of R. aculeatus alone and will not discuss this combination, as several review articles have already addressed the topic in detail [20,56,63].
Regarding R. aculeatus, several studies have examined its impact on the vascular system. However, these studies are outdated, and many lack detailed information. A critical review of these papers was conducted in a report by the Committee on Herbal Medicinal Products (EMA/HMPC/188805/2017) [15]. The main directions of the investigated activities included venous and lymphatic contractility, vascular permeability, and effects on the endothelium.
Overall, the available studies consistently indicate that the extract of R. aculeatus exerts a contractile effect on venous vessels. In vitro studies demonstrated that its venoconstrictive effect is concentration-dependent and is mediated both by activation of postjunctional α1- and α2-adrenergic receptors and by stimulation of norepinephrine release. The response was shown to be independent of the endothelium and depended on temperature—it was enhanced by warming and inhibited by cooling [64,65,66,67,68]. The activity of R. aculeatus was further confirmed in in vivo models [69,70].
Furthermore, the study by Nemcova et al., based on veins collected from patients with primary varicose veins, demonstrated that extract of R. aculeatus exerts venotonic effects through modulation of cyclic nucleotide signaling in venous tissue. Experiments comparing varicose and non-varicose human saphenous veins showed that the extract selectively increased intracellular cAMP levels without affecting cGMP concentrations. This response was associated with normalization of the prostacyclin/thromboxane ratio, suggesting a regulatory influence on vascular tone and endothelial function [71].
The main feature of CVD is increased permeability of the venous endothelium, which leads to edema. Studies have demonstrated that R. aculeatus exerts a contractile effect on lymphatic vessels, thereby enhancing lymphatic flow [72]. Furthermore, it inhibits microvascular permeability. This effect was demonstrated in a hamster cheek pouch model, and the response was mediated by calcium and α1-adrenoreceptors [69]. These findings were further supported by in vivo experiments in anesthetized cats, where intravenous or oral administration of the extract prior to edema induction significantly reduced protein content and water accumulation in edematous tissue [73]. Moreover, a recent study showed that the extract can inhibit permeability changes induced by both histamine and ischemia/reperfusion. It also reduced leukocyte–endothelium interactions. In most assays, the Ruscus extract exhibited greater activity than diosmin [74]. The anti-edematous activity of Ruscus extract is most likely attributable to spirostanol saponins. Barbič et al. investigated the effects of isolated compounds from the rhizome on thrombin-induced hyperpermeability in human microvascular endothelial cells and found that spirostanol saponins and esculin reduced endothelial hyperpermeability. The strongest effects were observed for deglucoruscin, ruscin, and esculin at a concentration of 100 µM, with reductions of 41.9%, 42.6%, and 53.3%, respectively [50].
Another important mechanism of R. aculeatus involves its effects on the vascular endothelium. Experimental findings indicate that R. aculeatus can counteract hypoxia-induced endothelial activation. The extract inhibited the hypoxia-induced decrease in ATP content, the activation of phospholipase A2, and increase in neutrophil adhesion. Additionally, Ruscus extract and HMC exhibited additive effects in preventing ATP depletion [75]. In turn, Lestienne et al. reported that Ruscus extract exhibits binding affinity for muscarinic receptors, acting as a partial agonist, particularly at the M1 and M3 subtypes. This muscarinic activity appears to contribute to its vasoprotective and anti-inflammatory effects by reducing vascular permeability and leukocyte–endothelium interactions [76].
It should be mentioned that in most reports, the Ruscus extracts were not standardized, their chemical composition was not provided, and no information was given on how the extracts were prepared; therefore, the interpretation and comparison of the results should be made with caution. Table 2 summarizes the main studies on the effects of R. aculeatus on the venous and lymphatic system.

Table 2.
Pharmacological effects of R. aculeatus on venous and lymphatic vessels.
It is worth emphasizing that only one clinical study has evaluated the efficacy of Ruscus extract alone in CVD. The study, which included 166 women, showed that R. aculeatus extract significantly reduced leg volume, as well as ankle and lower leg circumference, during the study. In addition, the extract alleviated subjective symptoms such as heaviness, tiredness, tension, and tingling in the legs [77].
Beyond its phlebotherapeutic effects, R. aculeatus also appears to relieve symptoms of orthostatic hypotension [78]. Furthermore, it may be beneficial in diabetic retinopathy, a condition in which the small blood vessels of the retina are damaged due to elevated glucose levels. Evaluation of the fundus of the eye in patients treated with Ruscus showed a clear improvement in 23.1% of cases and slight improvement in visual field sensitivity after three months of therapy. Treatment significantly reduced blood glucose, fructosamine, glycosylated hemoglobin, and total cholesterol while raising HDL cholesterol. These findings suggest that Ruscus extract exerts a beneficial influence on both metabolic and vascular parameters in diabetic patients [79].
4.2. Antimicrobial Effects
Research on the antimicrobial activity of R. aculeatus is scarce, with only four studies addressing this topic to date. These works, summarized in Table 3, focus primarily on alcoholic extracts or infusions prepared from both aerial and underground parts of the plant. Unfortunately, most reports do not provide the phytochemical composition of the extracts, and in the case of the aerial parts, they do not clearly specify which plant sections were used. The only exception is the study by Rodrigues et al. [38], who characterized the phenolic composition of extracts from the aerial parts and specified that cladodes or laminar stems and lateral branches were used for extract preparation. They tested hydroethanolic extracts (80:20, v/v), infusions, and decoctions from aerial parts and underground organs against eight bacterial strains [38]. Preparations from the roots showed similar activity, with minimal inhibitory concentrations (MICs) of 20 mg/mL or higher. Only the decoction exhibited greater efficacy against MRSA (MIC = 10 mg/mL) compared to the ethanolic extract and infusion. These root preparations were also less effective than those obtained from aerial parts, for which MIC values ranged from 5 to >20 mg/mL.
Hadžifejzović et al. examined a 70% methanolic extract of rhizomes and aerial parts, as well as the fractions obtained by partitioning the herb extract. In general, all extracts exhibited similar antimicrobial activity, with no significant differences. The ethyl acetate fraction demonstrated stronger antibacterial activity against the five tested bacterial strains compared to the butanol fraction and exhibited superior antifungal activity against all tested fungi, except Aspergillus niger [80]. However, the weakness of the paper lies in the characterization of the phenolic composition of the extract. The authors refer to unpublished research results that were described in a doctoral dissertation (not accessible).
Ali-Shtayeh et al. investigated the antifungal activity of a water extract from the aerial parts of the plant. At 15 µg/mL, the extract showed significant antimycotic effects against several dermatophyte species, including Microsporum canis, Trichophyton mentagrophytes, and Trichophyton violaceum, with mycelial inhibition percentages of 83.6% (MIC = 35 µg/mL), 86.3% (MIC = 29 µg/mL), and 100% (MIC = 15 µg/mL), respectively [81]. In another study, the same authors evaluated the antimicrobial activity of an ethanolic extract and an infusion. The infusion demonstrated more pronounced activity than the ethanolic extract, which inhibited the growth of only two out of the six tested strains. In contrast, the infusion inhibited five bacterial species, with the exception of Klebsiella pneumoniae [8]. In turn, Festa et al. investigated the antimicrobial activity of saponins isolated from the underground parts of the plant. Among the 15 isolated components, only deglucoruscin showed mild activity. At a concentration of 128 µg/mL, it reduced the growth of S. aureus by approximately 30–36%, and it inhibited the growth of C. albicans by 31% at 64 µg/mL. Interestingly, the compound showed strong synergistic effects when combined with conventional drugs [52].
Furthermore, Sanna et al. evaluated the antiviral potential of R. aculeatus. The ethyl acetate fraction, obtained from the acetone extract of R. aculeatus leaves through sequential solvent extraction (n-hexane, ethyl acetate, acetone, and methanol), was tested against several RNA and DNA viruses, including Coxsackievirus B5 (CVB-5), Human Immunodeficiency Virus type 1 (HIV-1), Bovine Viral Diarrhea Virus (BVDV), and Yellow Fever Virus (YFV). The extract demonstrated notable activity against BVDV (EC50 = 58 µg/mL) and YFV (EC50 = 66 µg/mL), without cytotoxic effects on the Madin–Darby Bovine Kidney (MDBK) and Baby Hamster Kidney (BHK) host cell lines [82].
In addition, a recent study has shown that ethanolic extract from the rhizome possesses immunomodulatory and antimicrobial properties in skin cells. In primary human keratinocytes, the extract was found to induce the expression of the antimicrobial peptide RNase 7 through activation of the ERK signaling pathway, without affecting cell viability or triggering an inflammatory response. This suggests that R. aculeatus may enhance innate skin defense mechanisms under noninflammatory conditions [83].


Table 3.
Antibacterial and antifungal activity of extracts from R. aculeatus.
Table 3.
Antibacterial and antifungal activity of extracts from R. aculeatus.
| Part of Plant/Extract | Antibacterial/Antifungal Effect | Ref. |
|---|---|---|
| roots and rhizomes/ maceration with stirring 1 h using 80% ethanol (e), infusions (i), or decoctions (d) | MIC/MBC (mg/mL) Escherichia coli: 20/>20 (e, d), >20/>20 (i) Klebsiella pneumoniae: >20/>20 (e, i, d); Morganella morganii: >20/>20 (e, i, d); Proteus mirabilis: >20/>20 (e, i, d); Pseudomonas aeruginosa: >20/>20 (e, i, d); Enterococcus faecalis: 20/>20 (e, i), >20/>20 (d); Listeria monocytogenes: >20/>20 (e, i, d); MRSA: >20/>20 (e), 20/>20 (i), 10/>20 (d) | [38] |
| rhizome/ 70% MeOH | MIC/MBC or * MIC/MFC (mg/mL) Staphylococcus aureus: 0.2/0.5; Bacillus cereus: 0.5/1.0; Micrococcus flavus: 1.0/2.0; Listeria monocytogenes: 1.0/1.0; Pseudomonas aeruginosa: 0.2/0.5; Enterobacter cloacae: 1.0/1.0; Salmonella typhimurium: 0.2/0.5; Escherichia coli: 0.5/1.0; Trichoderma viride *: 1.0/2.0; Penicillium funiculosum *: 0.5/2.0; Aspergillus fumigatus *: 2.0/3.0; Aspergillus niger *: 2.0/2.0; Aspergillus versicolor *: 2.0/3.0 | [80] |
| aerial parts/infusion for 72 h | Microsporum canis: MIC 35 µg/mL Trichophyton mentagrophytes MIC 29 µg/mL, Trichophyton violaceum MIC 15 µg/mL | [81] |
| aerial parts/infusion (w) maceration with 95% ethanol (e) tested concentration: 200 mg/mL | ZOI (mm); disk diameter = 6 mm Staphylococcus aureus: 9.9 (w), 7.5 (e); Escherichia coli: 8.0 (w), 6.0 (e); Klebsiella pneumoniae: 6.0 (w), 6.0 (e); Proteus vulgaris: 11.0 (w), 9.0 (e); Pseudomonas aeruginosa: 8.5 (w), 6.0 (e); Candida albicans: 7.6 (w), 6.0 (e) | [8] |
| aerial part/maceration with stirring 1 h using 80% ethanol (e) infusions (i) decoctions (d) | MIC/MBC mg/mL Escherichia coli: 10/>20 (e), >20/>20 (i), 20/>20 (d); Klebsiella pneumoniae: 20/>20 (e, i, d); Morganella morganii: 10/>20 (e, i), 20/>20 (d); Proteus mirabilis: 20/>20 (e), >20/>20 (i, d); Pseudomonas aeruginosa: >20/>20 (e, i, d); Enterococcus faecalis: 10/>20 (e, i), 20/>20 (d); Listeria monocytogenes: 10/>20 (e, i, d); MRSA: 10/>20 (e, i), 5/>20 (d) | [38] |
| herb/70% MeOH (m)ethyl acetate fraction (ea)butanol fraction (b) | MIC/MBC or * MIC/MFC (mg/mL) Staphylococcus aureus: 0.1/0.2 (m, ea), 0.5/4.0 (b); Bacillus cereus: 1.0/4.0 (m, b), 0.5/2.0 (ea) Micrococcus flavus: 2.0/4.0 (m), 0.5/2.0 (ea), 2.0/2.0 (b); Listeria monocytogenes: 0.2/1.0 (m), 0.2/0.5 (ea), 1.0/2.0 (b); Pseudomonas aeruginosa: 1/4.0 (m), 0.5/2.0 (ea), 1.0/2.0 (b); Enterobacter cloacae: 1/4.0 (m), 0.5/2.0 (ea), 1.0/2.0 (b); Salmonella typhimurium: 0.2/0.5 (m, ea, b); Escherichia coli: 1.0/1.0 (m), 0.5/1.0 (ea, b); Trichoderma viride *: 1.0/2.0 (m), 0.25/0.5 (ea), 0.5/2.0 (b); Penicillium funiculosum *: 1.0/2.0 (m), 0.5/2.0 (ea, b); Aspergillus fumigatus *: 1.0/2.0 (m), 1.0/3.0 (ea, b); Aspergillus niger *: 2.0/2.0 (m), 1.0/3.0 (ea), 0.2/0.5 (b); Aspergillus versicolor *: 1.0/2.0 (m, ea), 1.0/3.0 (b) | [80] |
| aerial part/maceration with MeOH (40 °C, 3 h)tested concentration: 100 mg/mL | Escherichia coli: ZOI 6 mm Klebsiella pneumoniae: ZOI 10 mm Staphylococcus aureus: ZOI 15 mm Candida albicans: ZOI 15 mm | [84] |
MRSA—methicyllin-resistant Staphylococcus aureus; MIC—minimum inhibitory concentration; ZOI—zone of inhibition (mm); MBC—minimum bactericidal concentration; MFC—minimum fungicidal concentration; *— for the fungal strains, MFC values were provided instead of MBC.
4.3. Antioxidant Effect
Antioxidant activity is one of the most extensively investigated biological effects of plant extracts, as antioxidants exert beneficial effects on human health both internally and externally. When consumed, they can neutralize free radicals, reduce oxidative stress, and support the proper functioning of the cardiovascular system, immune response, and metabolic processes. When applied topically, they may accelerate wound healing, protect the skin from oxidative damage, and delay premature aging by limiting the harmful effects of reactive oxygen species.
Overall, the available data indicate that R. aculeatus, including both aerial and underground parts, shows moderate to weak free radical scavenging activity (Table 4).
Jakovljević et al. investigated the antioxidant activity of three extracts (ethanolic, acetone, and ethyl acetate) obtained from the aerial parts of the plants and found that the results of the DPPH and ABTS assays were notably weaker compared to ascorbic acid (AA). Interestingly, all extracts exhibited significant reducing power and metal-chelating effects, with FRAP assay results even surpassing those of ascorbic acid (AA) [85]. Similarly, various extracts from the aerial parts of R. aculeatus, obtained through successive maceration and Soxhlet extraction with petroleum ether, chloroform, and ethanol, were evaluated by Taşkin et al. using DPPH, ABTS, FRAP, and CUPRAC assays. Soxhlet extraction yielded higher antioxidant activity than maceration; however, in agreement with other reports, the results were considerably weaker than those of the standards used as positive controls [86]. In turn, Rodrigues et al. [38] evaluated the antioxidant activity of hydromethanolic and aqueous extracts from both the aerial and underground parts of R. aculeatus using two complementary in vitro assays: (i) the Thiobarbituric Acid Reactive Substances (TBARS) formation inhibition capacity, reflecting the ability to prevent lipid peroxidation, and (ii) the OxHLIA assay, which measures the protective effect of extracts on erythrocytes against oxidative hemolysis. The hydroethanolic extract from the aerial parts exhibited the highest activity in the TBARS assay. In the OxHLIA assay, the most active extracts were the ethanolic root extract and the infusion from aerial parts, with comparable IC50 values of 230 µg/mL and 236 µg/mL (∆t = 60 min), respectively. Nevertheless, in both assays, Ruscus extracts demonstrated significantly lower activity than ascorbic acid and Trolox, which were used as positive controls.


Table 4.
Antioxidant activity of extracts from Ruscus aculeatus.
Table 4.
Antioxidant activity of extracts from Ruscus aculeatus.
| Plant Part/Extract | Test (Control) | Ref. |
|---|---|---|
| Underground part/ 80% ethanol (e) Infusions (i) Decoctions (d) | TBARS EC50 (mg/mL): 0.78 (e), 1.0 (i), 1.55 (d) (Trolox: 0.0058) OxHLIA IC50 (µg/mL): ∆ t = 60 min 230 (e), 646 (i), 661 (d) (Trolox: 21.8) ∆ t = 120 min 383 (e), 1389 (i), 1198 (d) (Trolox: 43.5) | [38] |
| Rhizome/70% MeOH | DPPH IC50 (µg/mL): 386 (no control) | [80] |
| Aerial part/ Ethanolic (e) Ethyl acetate (ea) Acetone (a) | DPPH IC50 (µg/mL): 502 (e), 182.5 (ea), 227.2 (a) (AA: 6.05) ABTS IC50 (µg/mL): 3.5 (e), 3.4 (ea), 3.4 (a) (AA: 2.85) FRAP IC50 (µg/mL): 209 (e), 223 (ea), 239 (a) (AA: 881) FIC IC50 (µg/mL): 150 (e), 165 (ea), 170 (a) (AA: 352.9) LPI (mg/mL): 1.0–0.79 (e), 1.05–0.81 (ea), 1.2–0.84 (a) (AA: 0.4–0.25) | [85] |
| Aerial part/ 80% ethanol (e) Infusions (i) Decoctions (d) | TBARS EC50 (mg/mL): 0.28 (e), 0.49 (i), 0.88 (d) (Trolox: 0.0058) OxHLIA IC50 (µg/mL): ∆ t = 60 min 0 (e), 236 (i), 427 (d) (Trolox: 21.8) ∆ t = 120 min 0 (e, i, d) (Trolox: 43.5) | [38] |
| Stems, leaves/methanol | DPPH IC50 (µg/mL): 171.9 (AA: 0.3) | [40] |
| Herb/70% MeOH (m) Ethyl acetate fraction (ea) Butanol fraction (b) | DPPH IC50 (µg/mL): 206 (m), 158 (ea), 173 (b) (no control) | [80] |
| Aerial part/successive Soxhlet: petroleum ether (pe), chloroform (chl), Ethanol (e) | DPPH IC50 (mg/mL): 2.32 (pe), 0.18 (chl), 0.26 (e) (AA: 0.005) ABTS (mM trox/g): 1.63 (pe), 1.92 (chl), 3.24 (e) (AA: 13.01) FRAP (mM Fe2+/mg): 0.15 (pe), 0.37 (chl), 0.12 (e), (BHT: 1.1) CUPRAC (mM trolox/mg): 0.35 (pe), 0.86 (chl), 0.1 (e), (BHA: 1.62) | [86] |
| Aerial part/successive maceration: petroleum ether (pe), chloroform (chl), ethanol (e) | DPPH IC50 (mg/mL): 0.83 (pe), 0.81 (chl), 0.79 (e) (AA: 0.005) ABTS (mM trox/g): 1.56 (pe), 3.1 (chl), 3.22 (e) (AA: 13.01) FRAP (mM Fe2+/mg): 0.1 (pe), 0.33 (chl), 0.86 (e), (BHT: 1.1) CUPRAC (mM trox/mg): 0.25 (pe), 0.5 (chl), 0.15 (e), (BHA: 1.62) | [86] |
| Shoot/decoctions (d), maceration: 40% ethanol (e40), 96% ethanol (e96) | ABTS (mmol trox/100 g DW): 0.3 (d), 1.7 (e40), 0.1 (e96) DPPH (mmol trox/100 g DW): 0.1 (d), 2.2 (e40), 0.1 (e96) FRAP (mmol FeSO4+ DW): 1.2 (d), 4.8 (e40), 0.8 (e96) | [39] |
| Aerial parts/maceration with methanol (40 °C, 3 h) | TPC (mg GAE/g): 229.7 DPPH IC50 (mg/mL): 0.209 (BHA: 0.009, BHT: 0.365) β-CAR–LA (% inhibition): 58.6 (BHA: 66.08, BHT: 67.9) | [84] |
FIC—ferrous ion chelating; LPI—inhibitory activity against lipid peroxidation during 48–96 h; AA—ascorbic acid; BHT—butylated hydroxytoluene; BHA—butylated hydroxyanisole; trox—trolox; GAE—gallic acid equivalent; β-CAR–LA—β-carotene–linoleic acid assay.
4.4. Anticancer Activity
Research on the anticancer properties of R. aculeatus remains limited, and a major limitation of these studies is the lack of, or only partial, phytochemical characterization of the extracts. However, existing reports suggest that both aerial and underground parts of the plant possess cytotoxic potential (Table 5). Rodrigues et al. investigated the anticancer activity of hydroethanolic extracts, decoctions, and infusions from the aerial and underground parts of the plant against several cancer cell lines, including HeLa (cervical carcinoma), NCI-H460 (non-small cell lung cancer), MCF-7 (breast adenocarcinoma), and HepG2 (hepatocellular carcinoma), using the sulforhodamine B (SRB) assay. They observed that most of the extracts effectively inhibited the growth of the tested cell lines, with the hydroethanolic extract of the aerial parts being the most effective. However, both the hydroethanolic extracts and the decoction of the underground parts of the plant also showed toxicity toward the primary liver cell culture (PLP2) [38].

Table 5.
Cytotoxic activity of extracts from R. aculeatus.
In turn, Bassil et al. conducted extensive studies on the effects of ethanol extracts from the aerial parts of R. aculeatus on human acute T-cell lymphoblastic leukemia cells (Jurkat cell line). The cytotoxicity of each extract was assessed using lactate dehydrogenase (LDH) and MTT assays. R. aculeatus showed a cytotoxic effect on Jurkat cells, with IC50 values between 10 and 16 mg/mL at 24 h and between 8 and 10 mg/mL at 48 h (LDH). Unfortunately, R. aculeatus also exhibited a similar cytotoxic effect on normal lymphocytes. Furthermore, molecular studies evaluating apoptosis- and cell cycle-related pathways were performed. It was found that proteins directly involved in apoptosis (Caspase-8, Smac/DIABLO) and those indirectly involved (Bcl-2, Bax) did not show significant changes. R. aculeatus also did not significantly alter CDK-4 expression; however, it caused a decrease in p53 levels. Taken together, these results showed that R. aculeatus extract induces a non-apoptotic form of Jurkat cell death [87].
Bilušić et al. investigated the effect of a shoot water extract, prepared by heating at 100 °C for 5 min, on human bladder cancer (T24) and lung cancer (A549) cell lines, as well as a control line: the human embryonic kidney cell line HEK 293. The effects were evaluated at 4, 24, 48, and 72 h using the MTT assay. For T24 and A549 cells, a time-dependent increase in cancer cell inhibition was observed. After 72 h, 35–38% inhibition was recorded at the highest tested concentration, compared to cisplatin (positive control), which caused approximately 50% inhibition in T24 cells and 13–14% in A549 cells. In turn, for HEK 293 cells, the highest cytotoxicity was only observed at 4 and 24 h, with 32–36% inhibition, while cisplatin showed no inhibition [88].
The effect of saponins isolated from the underground part on human promyelocytic leukemia HL-60 cells was evaluated using the MTT assay. Among the 12 tested saponins, a furostanol saponin with a diglycoside moiety modified by a (2S,3S)-2-Hydroxy-3-methylpentanoic acid group and an acetic acid group, as well as its corresponding spirostanol saponin, exhibited significant cytotoxic activity after 72 h of treatment, showing 92.4% and 98.2% inhibition at 10 μg/mL, respectively. The IC50 values of these two compounds were approximately 3.5 and 3.0 μg/mL, respectively. Mimaki et al. suggested that the acetyl and 2-Hydroxy-3-methylpentanoyl groups attached to the diglycoside moiety are crucial for the observed cytostatic activity [23,24].
4.5. Anti-Inflammatory Activity
Rodrigues et al. evaluated the anti-inflammatory activity of hydroethanolic extracts, decoctions, and infusions prepared from the aerial and underground parts of R. asuleatus by measuring NO levels in LPS-activated murine macrophages. Nitric oxide (NO) is widely used as a marker of inflammation, as it is synthesized by inducible nitric oxide synthase (iNOS) during immune responses. Among the tested extracts, only the hydroethanolic extract from the aerial parts and the decoction from the root and rhizome showed anti-inflammatory activity, with EC50 values of 60 and 129 μg/mL, respectively (compared to dexamethasone with an EC50 of 16 μg/mL) [38].
Furthermore, fractions of steroidal saponins isolated from the rhizomes were investigated for their anti-inflammatory activity using the rat paw edema model. The saponins were administered orally 1 h before induction of inflammation with carrageenan or kaolin. At a dose of 500 mg/kg, the saponins significantly reduced carrageenan-induced edema by 43–69% within 1–5 h, and the effect was comparable to or even greater than that of diclofenac. In the kaolin model, edema was reduced by 64.4% and 40% at 4 h and 24 h, respectively (compared to 52.1% and 41% for diclofenac) [89]. An in vivo study using rats with inflammation induced by turpentine oil also confirmed the anti-inflammatory effect of the saponin mixture on the acute-phase bone marrow response, phagocytic capacity, and total antioxidant response. It was found that oral administration of the saponins decreased the leukocyte count and the percentage of neutrophils, indicating a reduction in inflammation. Additionally, the treatment reduced the phagocytic index and increased the total antioxidant response (TAR) [90].
4.6. Other Biological Activities
There are also a few individual studies investigating other pharmacological activities of butcher’s broom.
4.6.1. Anti-Urease and Anticholinesterase Effects
Taşkin et al. investigated the anti-urease and anticholinesterase activities of extracts from the aerial parts of R. aculeatus, obtained through successive maceration and Soxhlet extraction with petroleum ether, chloroform, and ethanol. They reported that both chloroform and ethanol extracts exhibited inhibitory effects on these enzymes. The ethanol extract showed the highest anti-urease activity, achieving approximately 29% urease inhibition at a concentration of 12.5 µg/mL (positive control: thiourea, 78.8%). Conversely, the chloroform extract demonstrated the strongest anticholinesterase activity, with 94.4% inhibition at 500 µg/mL, a value comparable to that of the reference inhibitor galantamine (96.5%) [86]. Unfortunately, they did not provide any information on the components of the extracts.
The anti-urease assay is particularly relevant in the context of Helicobacter pylori infection, as this bacterium is responsible for various gastrointestinal disorders. Urease is a key virulence factor of H. pylori because it hydrolyzes urea into ammonia, which neutralizes gastric acid and enables the pathogen to survive in the highly acidic environment of the stomach. Inhibiting urease activity can therefore reduce bacterial colonization and help prevent gastritis, peptic ulcers, and even gastric cancer associated with chronic H. pylori infection.
Similarly, the anticholinesterase activity assay is commonly used in the search for agents that protect against neurodegenerative disorders, particularly Alzheimer’s disease. Acetylcholinesterase catalyzes the breakdown of acetylcholine, a neurotransmitter essential for memory and learning. Inhibitors of this enzyme can elevate acetylcholine levels in the synaptic cleft, thereby enhancing cholinergic transmission, improving cognitive function, and potentially slowing disease progression.
4.6.2. Diuretic Activity
The steroidal saponin fractions extracted from the rhizomes of R. aculeatus have been shown to exert a diuretic effect. After oral administration in rats, they increased urinary volume as well as the excretion of Na+, K+, and Cl− ions. The resulting activity produced a less intense but more sustained diuretic response compared to furosemide, the reference diuretic drug. The highest activity was observed 24 h after administration. According to the authors, the mechanism of action may be at least partially tubular, involving a reduction in the reabsorption of water and electrolytes within the renal tubules [90].
4.6.3. Anti-Osteoporotic Activity
Chakuleska et al. [91] reported that R. aculeatus steroidal saponins may mimic the activity of sex hormones and help reduce risk of osteoporosis. In their study, an extract was prepared by combining 80% ethanolic and aqueous fractions from the roots and rhizomes, standardized to contain 20% saponins. This extract was administered orally to estrogen-deficient female rats subjected to ovariectomy (OVX), and markers of bone turnover (osteocalcin, alkaline and acid phosphatase, β-CrossLaps) and calcium–phosphate homeostasis (Ca, P, 25-Hydroxy vitamin D, parathyroid hormone (PTH), and estradiol) were evaluated. After 10 weeks of treatment with R. aculeatus extract, the altered parameters were restored to control levels, showing effects comparable to diosgenin and alendronate, which served as positive controls. It is worth noting that, after this period, there were also no statistically significant differences between untreated OVX rats and the sham-operated group. However, statistically significant differences between untreated OVX and OVX rats treated with the extract were observed after 35 days of treatment, confirming its impact on these parameters. Moreover, radiographs of animals treated with higher doses of the extract showed no signs of bone mineral content (BMC) deformities or expansion, in contrast to the untreated OVX group. Histological analysis revealed that OVX rats treated with 100 and 200 mg/kg of the extract showed signs of regeneration of the disturbed bone structure. Furthermore, the authors found that malondialdehyde (MDA), an indicator of lipid peroxidation, was significantly elevated in OVX rats, whereas reduced glutathione (GSH), a key antioxidant, was decreased. Treatment with the extract significantly lowered MDA levels and restored GSH to near-control values in the liver, intestines, and bones, demonstrating the extract’s ability to prevent oxidative stress. Cell line assays on the human osteoblast-like SaOS-2 cells confirmed the lack of cytotoxicity of R. aculeatus. Moreover, at a concentration of 200 μg/mL, it even demonstrated a stimulatory effect on cell proliferation.
Molecular docking studies with estrogen receptors revealed that ruscogenin and neoruscogenin, the key constituents of R. aculeatus, formed stable complexes with both estrogen receptor alpha and beta. Their binding scores were comparable to those of estradiol and diosgenin, suggesting that ERA could be developed as a potential candidate for the prevention of postmenopausal osteoporotic complications.
4.7. Side Effects
There are only a few studies on the adverse effects of Ruscus extract, and they mostly involve isolated case reports. Sadarmin et al. described the case of a 39-year-old woman who developed diabetic ketoacidosis (DKA) five days after initiating therapy with R. aculeatus. The patient experienced four episodes of vomiting and one episode of diarrhea within a 48 h period. The authors suggested that the concurrent use of butcher’s broom and metformin was the most probable trigger of her metabolic deterioration and led to hospitalization [92]. Ramírez-Hernández et al. reported an allergic reaction after the application of topical preparations in a 34-year-old woman, which, as further tests showed, was associated with ruscogenin [93].
5. Biological Activity of Ruscogenin
It is widely believed that one of the main active ingredients of butcher’s broom responsible for its biological effects is ruscogenin (molecular formula: C27H42O4; IUPAC name: (1β,3β,25R)-spirost-5-ene-1,3-diol). Ruscogenin is a bioactive compound that has been isolated from the rhizome of R. aculeatus, the radix of Ophiopogon japonicus, and the bulb stems of Allium cepa. Ruscogenin is a steroidal saponin of the spirostan type [36,94]. Its structure consists of two oxygen-containing heterocycles connected through a spiroacetal carbon atom located on the D ring of the steroid core, and the hydroxyl group at C-10 is glycosylated with a sugar chain.
However, as demonstrated by a review of the literature on the phytochemistry of R. aculeatus, ruscogenin is present in this plant material as an aglycone only in minor amounts, whereas its various derivatives constitute the principal constituents. Nevertheless, this low content does not diminish its pharmacological relevance, as ruscogenin may be generated through metabolic transformation following oral administration of Ruscus extract. Therefore, its pharmacological activity will be briefly discussed below.
Ruscogenin exhibits a broad spectrum of biological activities, including antioxidant, anti-inflammatory, neuroprotective, and anticancer properties. It has been found that it can inhibit neutrophil activation [95] and modulate key inflammatory mediators. For example, in mice with gastric ulcers, ruscogenin significantly decreased the levels of TNF-α, IL-6, IL-8, lipid peroxidation (LPO), and myeloperoxidase (MPO) while enhancing the activities of glutathione (GSH) and glutathione peroxidase (GSH-Px) [96]. A decrease in inflammatory mediators, including the expression of NF-κB target genes such as ICAM-1, inducible nitric oxide synthase (iNOS), cyclooxygenase-2 (COX-2), tumor necrosis factor-α (TNF-α), and interleukin-1β (IL-1β), was also observed in the mouse model of experimental stroke [97]. Ruscogenin also mitigates IL-1β-induced cartilage destruction and inflammation in osteoarthritis [98,99] and LPS-induced lung injury by inhibiting inflammatory mediators [100]. Furthermore, it displays neuroprotective properties across several neurological disease models. In models of Parkinson’s disease, ruscogenin reduces oxidative stress and nitric oxide production, suppresses IL-1β and IL-6 expression, and promotes dopaminergic neuron survival. In vivo, ruscogenin lowers the levels of TNF-α, IL-1β, IL-6, COX-2, and iNOS while improving motor coordination and grip strength in mice [101]. It inhibits amyloid-β oligomerization and aggregation, a critical process in the pathogenesis of Alzheimer’s disease [102].
In models of acute lung injury, ruscogenin was found to reduce endothelial cell apoptosis [103] and stabilize pulmonary endothelial barrier integrity [104]. Interestingly, it has shown potential in mitigating SARS-CoV-2 E protein-induced cell death, suggesting possible therapeutic applications in the management of COVID-19 complications [105].
In a rat model of monocrotaline-induced pulmonary arterial hypertension, ruscogenin effectively attenuated vascular remodeling and inflammation, demonstrating significant endothelial-protective activity [106]. Moreover, it has been shown to improve cardiac function, reduce myocardial fibrosis, and alleviate morphological damage, further supporting its cardioprotective potential [107].
Furthermore, ruscogenin demonstrates notable anticancer potential. Hua et al. reported that it inhibited the metastasis of hepatocellular carcinoma [108]. In another study using a lung cancer model, ruscogenin restored antioxidant balance, modulated immune responses, and normalized tumor-promoting markers to baseline levels [109]. The main directions of action of ruscogenin are presented in Figure 3.
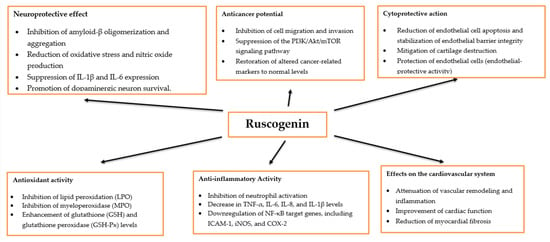
Figure 3.
Examples of biological activities and related effects of ruscogenin.
6. Regulatory Status of R. aculeatus
Following the discussion of R. aculeatus, it is important to consider the regulatory frameworks that govern its use and marketing. Due to its broad range of potential applications, R. aculeatus is subject to various legal acts. Within the European Union (EU), the regulation of this plant depends on the product category. When incorporated into medicinal products, its use is governed by Directive 2001/83/EC on the Community code relating to medicinal products for human use [110] and by the Pharmaceutical Law [111]. In the case of cosmetic products, the relevant framework is Regulation (EC) No 1223/2009 of the European Parliament and of the Council of 30 November 2009 on cosmetic products [112]. When used in herbal medicinal preparations, R. aculeatus falls under Decision 2008/911/EC [113]. For its inclusion in dietary supplements, the applicable legislation includes Directive 2002/46/EC of the European Parliament and of the Council of 10 June 2002 on the approximation of the laws of the Member States relating to food supplements [114], together with Regulation (EC) No 1924/2006 on nutrition and health claims made on foods [115] and Regulation (EU) No 1169/2011 on the provision of food information to consumers [116]. R. aculeatus has been approved by the European Medicines Agency (EMA). The plant is also included in the European Pharmacopoeia and is subject to regulations governing herbal medicinal products, including registration as a Traditional Herbal Medicinal Product (THMP).
In the United States, the regulation of pharmaceuticals, cosmetics, and other related products falls under the jurisdiction of the Food and Drug Administration (FDA) [117]. Prior to marketing, products must receive approval depending on their classification and associated risk level. However, Ruscus has not yet been reviewed by the FDA.
Overall, R. aculeatus represents a species with applications spanning pharmaceuticals, dietary supplements, herbal medicines, and cosmetics. The selection of the appropriate legal framework depends on the intended application, and understanding these regulations clarifies how herbal medicines are standardized, labeled, and evaluated for safety and efficacy across jurisdictions.
7. Conclusions and Future Perspectives
R. aculeatus L. remains an important medicinal plant with a long history of traditional use. A review of the existing literature confirms its broad biological activities, including venoactive, vasoprotective, anti-inflammatory, antioxidant, and antibacterial effects, which justify its ethnomedicinal applications and indicate its relevance in modern phytotherapy. However, this review has also revealed several limitations, such as the scarcity of recent, well-designed studies investigating R. aculeatus as a single botanical entity. Moreover, toxicological and pharmacokinetic data for Ruscus extract and its major saponins remain incomplete, with limited information regarding its metabolism, bioavailability, and potential drug interactions. Many older studies also lack phytochemical standardization in terms of chemical composition, solvent system, extraction method, and quantification of active compounds. Furthermore, there has been limited exploration of molecular mechanisms underlying its biological effects.
Future research should therefore focus on the following: (I) comprehensive phytochemical profiling of standardized extracts using advanced analytical techniques (e.g., LC–MS/MS, NMR); (II) mechanistic studies aimed at elucidating the molecular targets and signaling pathways of steroidal saponins; (III) well-controlled preclinical experiments employing chemically defined preparations; and (IV) rigorous clinical trials assessing the efficacy, safety, and pharmacokinetics of R. aculeatus extracts or purified constituents.
In conclusion, R. aculeatus holds considerable pharmacological promise, but further research is warranted to fill the existing knowledge gaps. The use of modern phytochemical and pharmacological approaches will be essential to fully substantiate its therapeutic potential and ensure its safe, evidence-based application in contemporary medicine
Author Contributions
Conceptualization, W.P., M.W., I.S. and M.F.; methodology, W.P., M.W., I.S., F.G., R.P. and M.F.; software, M.W., W.P. and I.S.; investigation, W.P., M.W., I.S., F.G., R.P. and M.F.; data curation, M.W., W.P. and I.S.; writing—original draft preparation, W.P., M.W., I.S., F.G., R.P. and M.F.; writing—review and editing, W.P., M.W. and I.S.; visualization, W.P., M.W. and I.S.; supervision, W.P., M.W. and I.S.; project administration, W.P., M.W. and I.S.; funding acquisition, M.W. and I.S. All authors have read and agreed to the published version of the manuscript.
Funding
The study was funded by the Medical University of Lublin (DS 51 and DS 52).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Available online: https://powo.science.kew.org/taxon/urn:lsid:ipni.org:names:24720-1#distributions (accessed on 10 September 2025).
- Flowering Plants · Monocotyledons; Kubitzki, K., Ed.; Springer: Berlin/Heidelberg, Germany, 1998. [Google Scholar] [CrossRef]
- Available online: https://powo.science.kew.org/taxon/urn:lsid:ipni.org:names:540443-1 (accessed on 10 September 2025).
- Quattrocchi, U. CRC World Dictionary of Plant Names: Common Names, Scientific Names, Eponyms, Synonyms, and Etymology; CRC Press: Boca Raton, FL, USA, 1999. [Google Scholar]
- Masullo, M.; Pizza, C.; Piacente, S. Ruscus Genus: A Rich Source of Bioactive Steroidal Saponins. Planta Med. 2016, 82, 1513–1524. [Google Scholar] [CrossRef] [PubMed]
- Savo, V.; Giulia, C.; Maria, G.P.; David, R. Folk Phytotherapy of the Amalfi Coast (Campania, Southern Italy). J. Ethnopharmacol. 2011, 135, 376–392. [Google Scholar] [CrossRef]
- Admuthe, N.B.; Karajgi, S.; Uikey, J.; Bhimraj, N.M.; Prakash, V.; Babar, T.P.; Karra, T.; Shrivastava, R. Natural Products in Hemorrhoid Management: A Comprehensive Literature Review of Traditional Herbal Remedies and Evidence-Based Therapies. Cureus 2025, 17, e83397. [Google Scholar] [CrossRef]
- Ali-Shtayeh, M.S.; Yaghmour, R.M.-R.; Faidi, Y.R.; Salem, K.; Al-Nuri, M.A. Antimicrobial Activity of 20 Plants Used in Folkloric Medicine in the Palestinian Area. J. Ethnopharmacol. 1998, 60, 265–271. [Google Scholar] [CrossRef]
- Tsioutsiou, E.E.; Amountzias, V.; Vontzalidou, A.; Dina, E.; Stevanović, Z.D.; Cheilari, A.; Aligiannis, N. Medicinal Plants Used Traditionally for Skin Related Problems in the South Balkan and East Mediterranean Region—A Review. Front. Pharmacol. 2022, 13, 936047. [Google Scholar] [CrossRef] [PubMed]
- Tuzlacı, E.; Aymaz, P.E. Turkish Folk Medicinal Plants, Part IV: Gönen (Balıkesir). Fitoterapia 2001, 72, 323–343. [Google Scholar] [CrossRef]
- Hanlidou, E.; Karousou, R.; Kleftoyanni, V.; Kokkini, S. The Herbal Market of Thessaloniki (N Greece) and Its Relation to the Ethnobotanical Tradition. J. Ethnopharmacol. 2004, 91, 281–299. [Google Scholar] [CrossRef]
- Güneş, S.; Savran, A.; Paksoy, M.Y.; Koşar, M.; Çakılcıoğlu, U. Ethnopharmacological Survey of Medicinal Plants in Karaisalı and Its Surrounding (Adana-Turkey). J. Herb. Med. 2017, 8, 68–75. [Google Scholar] [CrossRef]
- Guarrera, P.M. Traditional Phytotherapy in Central Italy (Marche, Abruzzo, and Latium). Fitoterapia 2005, 76, 1–25. [Google Scholar] [CrossRef]
- Dolina, K.; Łuczaj, Ł. Wild Food Plants Used on the Dubrovnik Coast (South-Eastern Croatia). Acta Soc. Bot. Pol. 2014, 83, 175–181. [Google Scholar] [CrossRef]
- European Medicines Agency; EMA/HMPC/188805/2017 Committee on Herbal Medicinal Products (HMPC). Assessment Report on Ruscus aculeatus L. Rhizoma. Available online: https://www.ema.europa.eu/en/documents/herbal-report/draft-assessment-report-ruscus-aculeatus-l-rhizome-revision-1_en.pdf (accessed on 1 September 2025).
- Bencsik, T.; Balázs, V.L.; Farkas, Á.; Csikós, E.; Horváth, A.; Ács, K.; Kocsis, M.; Doseděl, M.; Fialová, S.B.; Czigle, S.; et al. Herbal Drugs in Chronic Venous Disease Treatment: An Update. Fitoterapia 2024, 179, 106256. [Google Scholar] [CrossRef]
- Lichota, A.; Gwozdzinski, L.; Gwozdzinski, K. Therapeutic Potential of Natural Compounds in Inflammation and Chronic Venous Insufficiency. Eur. J. Med. Chem. 2019, 176, 68–91. [Google Scholar] [CrossRef]
- Nocera, R.; Eletto, D.; Santoro, V.; Parisi, V.; Bellone, M.L.; Izzo, M.; Tosco, A.; Dal Piaz, F.; Donadio, G.; De Tommasi, N. Design of an Herbal Preparation Composed by a Combination of Ruscus aculeatus L. and Vitis vinifera L. Extracts, Magnolol and Diosmetin to Address Chronic Venous Diseases through an Anti-Inflammatory Effect and AP-1 Modulation. Plants 2023, 12, 1051. [Google Scholar] [CrossRef]
- Orefice, R.; Litta, F.; Parello, A.; De Simone, V.; Campennì, P.; Marra, A.A.; Ratto, C. A Prospective Study on the Efficacy of Two Different Phlebotonic Therapies as a Bridge to Surgery in Patients with Advanced Hemorrhoidal Disease. J. Clin. Med. 2021, 10, 1549. [Google Scholar] [CrossRef]
- Kakkos, S.K.; Allaert, F.A. Efficacy of Ruscus Extract, HMC and Vitamin C, Constituents of Cyclo 3 Fort®, on Improving Individual Venous Symptoms and Edema: A Systematic Review and Meta-Analysis of Randomized Double-Blind Placebo-Controlled Trials. Int. Angiol. 2017, 36, 93–106. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Pallé, E.; Aronne, G. Pollination Failure in Mediterranean Ruscus aculeatus L. Bot. J. Linn. Soc. 2000, 134, 443–452. [Google Scholar] [CrossRef]
- Abascal, K.; Yarnell, E. Butcher’s Broom: Herb’s Potentials Too-Often Swept Under the Rug. Altern. Complement. Ther. 2002, 8, 177–185. [Google Scholar] [CrossRef]
- Mimaki, Y.; Kuroda, M.; Kameyama, A.; Yokosuka, A.; Sashida, Y. Steroidal Saponins from the Underground Parts of Ruscus aculeatus and Their Cytostatic Activity on HL-60 Cells. Phytochemistry 1998, 48, 485–493. [Google Scholar] [CrossRef]
- Mimaki, Y.; Kuroda, M.; Kameyama, A.; Yokosuka, A.; Sashida, Y. New Steroidal Constituents of the Underground Parts of Ruscus aculeatus and Their Cytostatic Activity on HL-60 Cells. Chem. Pharm. Bull. 1998, 46, 298–303. [Google Scholar] [CrossRef]
- Mari, A.; Napolitano, A.; Perrone, A.; Pizza, C.; Piacente, S. An Analytical Approach to Profile Steroidal Saponins in Food Supplements: The Case of Ruscus aculeatus. Food Chem. 2012, 134, 461–468. [Google Scholar] [CrossRef]
- Mimaki, Y.; Kuroda, M.; Kameyama, A.; Yokosuka, A.; Sashida, Y. Aculeoside B, a New Bisdesmosidic Spirostanol Saponin from the Underground Parts of Ruscus aculeatus. J. Nat. Prod. 1998, 61, 1279–1282. [Google Scholar] [CrossRef]
- Oulad-Ali, A.; Guillaume, D.; Belle, R.; David, B.; Anton, R. Sulphated Steroidal Derivatives from Ruscus aculeatus. Phytochemistry 1996, 42, 895–897. [Google Scholar] [CrossRef]
- Marino, S.; Festa, C.; Zollo, F.; Iorizzi, M. Novel Steroidal Components from the Underground Parts of Ruscus aculeatus L. Molecules 2012, 17, 14002–14014. [Google Scholar] [CrossRef]
- Mimaki, Y.; Kuroda, M.; Yokosuka, A.; Sashida, Y. A Spirostanol Saponin from the Underground Parts of Ruscus aculeatus. Phytochemistry 1999, 51, 689–692. [Google Scholar] [CrossRef]
- De Combarieu, E.; Falzoni, M.; Fuzzati, N.; Gattesco, F.; Giori, A.; Lovati, M.; Pace, R. Identification of Ruscus Steroidal Saponins by HPLC-MS Analysis. Fitoterapia 2002, 73, 583–596. [Google Scholar] [CrossRef] [PubMed]
- Ozer, G.; Guzelmeric, E.; Sezgin, G.; Ozyurek, E.; Arslan, A.; Sezik, E.; Yesilada, E. Comparative Determination of Ruscogenins Content in Butcher’s Broom Rhizome Samples Gathered from the Populations Grown in Different Soil Conditions in the Marmara Region and Attempts for Pilot Field Cultivation of Rhizomes. J. Chem. Metrol. 2018, 12, 79–88. [Google Scholar] [CrossRef]
- Sahin, I.; Ceylan, Ç.; Bayraktar, O. Ruscogenin Interacts with DPPC and DPPG Model Membranes and Increases the Membrane Fluidity: FTIR and DSC Studies. Arch. Biochem. Biophys. 2023, 733, 109481. [Google Scholar] [CrossRef]
- Mangas, S.; Bonfill, M.; Osuna, L.; Moyano, E.; Tortoriello, J.; Cusido, R.M.; Teresa Piñol, M.; Palazón, J. The Effect of Methyl Jasmonate on Triterpene and Sterol Metabolisms of Centella asiatica, Ruscus aculeatus and Galphimia glauca Cultured Plants. Phytochemistry 2006, 67, 2041–2049. [Google Scholar] [CrossRef]
- Vlase, L.; Kiss, B.; Balica, G.; Tămas, M.; Crisan, G.; Leucuta, S.E. High-Throughput LC/MS/MS Analysis of Ruscogenin and Neoruscogenin in Ruscus aculeatus L. J. AOAC Int. 2009, 92, 1055–1059. [Google Scholar] [CrossRef]
- Palazón, J.; Moyano, E.; Bonfill, M.; Osuna, L.T.; Cusidó, R.M.; Piñol, M.T. Effect of Organogenesis on Steroidal Saponin Biosynthesis in Calli Cultures of Ruscus aculeatus. Fitoterapia 2006, 77, 216–220. [Google Scholar] [CrossRef] [PubMed]
- Khojasteh, A.; Sanchez-Muñoz, R.; Moyano, E.; Bonfill, M.; Cusido, R.M.; Eibl, R.; Palazon, J. Biotechnological Production of Ruscogenins in Plant Cell and Organ Cultures of Ruscus aculeatus. Plant Physiol. Biochem. 2019, 141, 133–141. [Google Scholar] [CrossRef]
- Ivanova, T.; Dimitrova, D.; Gussev, C.; Bosseva, Y.; Stoeva, T. Ex Situ Conservation of Ruscus aculeatus L.—Ruscogenin Biosynthesis, Genome-Size Stability and Propagation Traits of Tissue-Cultured Clones. Biotechnol. Biotechnol. Equip. 2015, 29, 27–32. [Google Scholar] [CrossRef]
- Rodrigues, J.P.B.; Fernandes, Â.; Dias, M.I.; Pereira, C.; Pires, T.C.S.P.; Calhelha, R.C.; Carvalho, A.M.; Ferreira, I.C.F.R.; Barros, L. Phenolic Compounds and Bioactive Properties of Ruscus aculeatus L. (Asparagaceae): The Pharmacological Potential of an Underexploited Subshrub. Molecules 2021, 26, 1882. [Google Scholar] [CrossRef]
- Poljuha, D.; Šola, I.; Bilić, J.; Dudaš, S.; Bilušić, T.; Markić, J.; Rusak, G. Phenolic Composition, Antioxidant Capacity, Energy Content and Gastrointestinal Stability of Croatian Wild Edible Plants. Eur. Food Res. Technol. 2015, 241, 573–585. [Google Scholar] [CrossRef]
- Luís, Â.; Domingues, F.; Duarte, A.P. Bioactive Compounds, RP-HPLC Analysis of Phenolics, and Antioxidant Activity of Some Portuguese Shrub Species Extracts. Nat. Prod. Com. 2011, 6, 1934578X1100601219. [Google Scholar] [CrossRef]
- Longo, L.; Vasapollo, G. Determination of Anthocyanins in Ruscus aculeatus L. Berries. J. Agric. Food Chem. 2005, 53, 475–479. [Google Scholar] [CrossRef] [PubMed]
- Oulad-Ali, A.; David, B.; Charrouf, Z.; Bellé, R.; Hatinguais, P.; Anton, R. 12-Docosenoic Acid from Ruscus aculeatus. Int. J. Pharmacogn. 1997, 35, 382–384. [Google Scholar] [CrossRef]
- Elsohly, M.A.; Knapp, J.E.; Slatkin, D.J.; Schiff, P.L.; Doorenbos, N.J.; Quimby, M.W. Euparone, a New Benzofuran from Ruscus aculeatus L. J. Pharm. Sci. 1974, 63, 1623–1624. [Google Scholar] [CrossRef] [PubMed]
- Niu, S.-L.; Hao, J.-H.; Xu, J.-Y.; Guan, Q.; Zhou, Z.-C.; Lv, T.-M.; Sun, Y.-T. Aculebiphenyl A–B, New Biphenyl Derivatives from Ruscus aculeatus. J. Asian Nat. Prod. Res. 2023, 25, 1076–1084. [Google Scholar] [CrossRef]
- Güvenç, A.; Şatır, E.; Coşkun, M. Determination of Ruscogenin in Turkish ruscus L. Species by UPLC. Chromatographia 2007, 66, 141–145. [Google Scholar] [CrossRef]
- Walasek-Janusz, M.E.; Bajena, A.; Nurzyńska-Wierdak, R.; Skalicka-Woźniak, K. Extraction and Analysis of Ruscogenins from Butcher’s Broom (Ruscus aculeatus L.) Rhizomes Using HPLC. Acta Sci. Pol. Hortorum Cultus 2022, 21, 143–154. [Google Scholar] [CrossRef]
- Tansi, S.; Kokdil, G.; Karaman, S.; Toncer, O.; Yilmaz, H. Variation in Ruscogenin Contents in Ruscus aculeatus L. Growing Wild in Southern Turkey. Asian J. Chem. 2007, 19, 3015–3022. [Google Scholar]
- Kite, G.C.; Porter, E.A.; Simmonds, M.S.J. Chromatographic Behaviour of Steroidal Saponins Studied by High-Performance Liquid Chromatography–Mass Spectrometry. J. Chromatogr. A 2007, 1148, 177–183. [Google Scholar] [CrossRef]
- Kameyama, A.; Shibuya, Y.; Kusuoku, H.; Nishizawa, Y.; Nakano, S.; Tatsuta, K. Isolation and Structural Determination of Spilacleosides A and B Having a Novel 1,3-Dioxolan-4-One Ring. Tetrahedron Lett. 2003, 44, 2737–2739. [Google Scholar] [CrossRef]
- Barbič, M.; Willer, E.A.; Rothenhöfer, M.; Heilmann, J.; Fürst, R.; Jürgenliemk, G. Spirostanol Saponins and Esculin from Rusci rhizoma Reduce the Thrombin-Induced Hyperpermeability of Endothelial Cells. Phytochemistry 2013, 90, 106–113. [Google Scholar] [CrossRef]
- Mimaki, Y.; Kuroda, M.; Yokosuka, A.; Sashida, Y. Two New Bisdesmosidic Steroidal Saponins from the Underground Parts of Ruscus aculeatus. Chem. Pharm. Bull. 1998, 46, 879–881. [Google Scholar] [CrossRef]
- Festa, C.; Finamore, C.; Cammarota, M.; Panza, E.; Marchianò, S.; Fiorucci, S.; Zampella, A.; Buommino, E.; De Marino, S. Synergistic Effect of Plant-Derived Deglucoruscin in Combination with Conventional Antimicrobials against Staphylococcus Aureus and Candida Albicans. Fitoterapia 2025, 187, 106895. [Google Scholar] [CrossRef] [PubMed]
- Baranowska, I.; Bajkacz, S. A New UHPLC-MS/MS Method for the Determination of Flavonoids in Supplements and DPPH -UHPLC-UV Method for the Evaluation of the Radical Scavenging Activity of Flavonoids. Food Chem. 2018, 256, 333–341. [Google Scholar] [CrossRef]
- Raposo, A.; Saraiva, A.; Ramos, F.; Carrascosa, C.; Raheem, D.; Bárbara, R.; Silva, H. The Role of Food Supplementation in Microcirculation—A Comprehensive Review. Biology 2021, 10, 616. [Google Scholar] [CrossRef] [PubMed]
- Krasiński, Z.; Krasińska, B. Treatment of Early-Stage Chronic Venous Disease—Management Standards for 2023. Pol. J. Surg. 2023, 95, 64–69. [Google Scholar] [CrossRef]
- Bihari, I.; Guex, J.-J.; Jawien, A.; Szolnoky, G. Clinical Perspectives and Management of Edema in Chronic Venous Disease—What about Ruscus? Medicines 2022, 9, 41. [Google Scholar] [CrossRef] [PubMed]
- Bergan, J.J.; Schmid-Schönbein, G.W.; Smith, P.D.C.; Nicolaides, A.N.; Boisseau, M.R.; Eklof, B. Chronic Venous Disease. N. Engl. J. Med. 2006, 355, 488–498. [Google Scholar] [CrossRef] [PubMed]
- Beebe-Dimmer, J.L.; Pfeifer, J.R.; Engle, J.S.; Schottenfeld, D. The Epidemiology of Chronic Venous Insufficiency and Varicose Veins. Ann. Epidemiol. 2005, 15, 175–184. [Google Scholar] [CrossRef]
- Castro-Ferreira, R.; Cardoso, R.; Leite-Moreira, A.; Mansilha, A. The Role of Endothelial Dysfunction and Inflammation in Chronic Venous Disease. Ann. Vasc. Surg. 2018, 46, 380–393. [Google Scholar] [CrossRef]
- Nicolaides, A.; Bouskela, E. Pathophysiological Mechanisms of Chronic Venous Disease and Their Role in C0s Clinical Class. Vasc. Investig. Ther. 2018, 1, 103–109. [Google Scholar] [CrossRef]
- Raffetto, J.D.; Khalil, R.A. Mechanisms of Varicose Vein Formation: Valve Dysfunction and Wall Dilation. Phlebology 2008, 23, 85–98. [Google Scholar] [CrossRef] [PubMed]
- Langer, R.D.; Ho, E.; Denenberg, J.O.; Fronek, A.; Allison, M.; Criqui, M.H. Relationships Between Symptoms and Venous Disease: The San Diego Population Study. Arch. Intern. Med. 2005, 165, 1420–1424. [Google Scholar] [CrossRef]
- Jawien, A.; Bouskela, E.; Allaert, F.A.; Nicolaïdes, A.N. The Place of Ruscus Extract, Hesperidin Methyl Chalcone, and Vitamin C in the Management of Chronic Venous Disease. Int. Angiol. 2017, 36, 31–41. [Google Scholar] [CrossRef]
- Rubanyi, G.; Marcelon, G.; Vanhoutte, P.M. Effect of Temperature on the Responsiveness of Cutaneous Veins to the Extract of Ruscus aculeatus. Gen. Pharmacol. Vasc. Syst. 1984, 15, 431–434. [Google Scholar] [CrossRef]
- Miller, V.M.; Rud, K.; Gloviczki, P. Interactions of Ruscus-Extract with Endothelin-Receptors in Human Varicose Veins. Clin. Hemorheol. 1994, 14, S37–S45. [Google Scholar]
- Harker, C.T.; Marcelon, G.; Vanhoutte, P.M. Temperature, Oestrogens and Contractions of Venous Smooth Muscle of the Rabbit. Phlebology 1988, 3, 77–82. [Google Scholar]
- Marcelon, G.; Verbeuren, T.J.; Lauressergues, H.; Vanhoutte, P.M. Effect of Ruscus aculeatus on Isolated Canine Cutaneous Veins. Gen. Pharmacol. 1983, 14, 103–106. [Google Scholar] [CrossRef]
- Marcelon, G.; Vieu, S.; Pouget, G.; Tisné-Versailles, J. Oestrogenous Impregnation and Ruscus Action on the Human Vein In Vitro, Depending on Preliminary Results. Phlebology 1988, 3, 83–85. [Google Scholar]
- Bouskela, E.; Cyrino, F.Z.; Marcelon, G. Possible Mechanisms for the Inhibitory Effect of Ruscus Extract on Increased Microvascular Permeability Induced by Histamine in Hamster Cheek Pouch. J. Cardiovasc. Pharmacol. 1994, 24, 281–285. [Google Scholar] [CrossRef]
- Bouskela, E.; Cyrino, F.Z.G.A.; Marcelon, G. Effects of Ruscus Extract on the Internal Diameter of Arterioles and Venules of the Hamster Cheek Pouch Microcirculation. J. Cardiovasc. Pharmacol. 1993, 22, 221–224. [Google Scholar] [CrossRef] [PubMed]
- Nemcova, S.; Gloviczki, P.; Rud, K.S.; Miller, V.M. Cyclic Nucleotides and Production of Prostanoids in Human Varicose Veins. J. Vasc. Surg. 1999, 30, 876–884. [Google Scholar] [CrossRef]
- Marcelon, G.; Pouget, G.; Tisné-Versailles, J. Effect of Ruscus on the Adrenoceptors of the Canine Lymphatic Thoracic Duct. Phlebology 1988, 3, 109–112. [Google Scholar]
- Felix, W.; Schmidt, Y.; Nieberle, J. Protective Effect of Ruscus Extract against Injury of Vascular Endothelium and Vascular Smooth Muscle Caused by Ethracrynic Acid. Int. Angiol. 1984, 3, 77–79. [Google Scholar]
- De Almeida Cyrino, F.Z.G.; Balthazar, D.S.; Sicuro, F.L.; Bouskela, E. Effects of Venotonic Drugs on the Microcirculation: Comparison between Ruscus Extract and Micronized Diosmine1. Clin. Hemorheol. Microcirc. 2018, 68, 371–382. [Google Scholar] [CrossRef]
- Bouaziz, N.; Michiels, C.; Janssens, D.; Berna, N.; Eliaers, F.; Panconi, E.; Remacle, J. Effect of Ruscus Extract and Hesperidin Methylchalcone on Hypoxia-Induced Activation of Endothelial Cells. Int. Angiol. 1999, 18, 306–312. [Google Scholar]
- Rauly-Lestienne, I.; Heusler, P.; Cussac, D.; Lantoine-Adam, F.; De Almeida Cyrino, F.Z.G.; Bouskela, E. Contribution of Muscarinic Receptors to In Vitro and In Vivo Effects of Ruscus Extract. Microvasc. Res. 2017, 114, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Vanscheidt, W.; Jost, V.; Wolna, P.; Lücker, P.; Müller, A.; Theurer, C.; Patz, B.; Grützner, K. Efficacy and Safety of a Butcher’s Broom Preparation (Ruscus aculeatus L. Extract) Compared to Placebo in Patients Suffering from Chronic Venous Insufficiency. Arzneimittelforschung 2011, 52, 243–250. [Google Scholar] [CrossRef]
- Redman, D.A. Ruscus aculeatus (Butcher’s Broom) as a Potential Treatment for Orthostatic Hypotension, with a Case Report. J. Altern. Complement. Med. 2000, 6, 539–549. [Google Scholar] [CrossRef]
- Archimowicz-Cyryłowska, B.; Adamek, B.; Droździk, M.; Samochowiec, L.; Wójcicki, J. Clinical Effect of Buckwheat Herb, Ruscus Extract and Troxerutin on Retinopathy and Lipids in Diabetic Patients. Phytother. Res. 1996, 10, 659–662. [Google Scholar] [CrossRef]
- Hadžifejzović, N.; Kukić-Marković, J.; Petrović, S.; Soković, M.; Glamočlija, J.; Stojković, D.; Nahrstedt, A. Bioactivity of the Extracts and Compounds of Ruscus aculeatus L. and Ruscus hypoglossum L. Ind. Crops Prod. 2013, 49, 407–411. [Google Scholar] [CrossRef]
- Ali-Shtayeh, M.S.; Abu Ghdeib, S.I. Antifungal Activity of Plant Extracts against Dermatophytes. Mycoses 1999, 42, 665–672. [Google Scholar] [CrossRef]
- Sanna, G.; Farci, P.; Busonera, B.; Murgia, G.; La Colla, P.; Giliberti, G. Antiviral Properties from Plants of the Mediterranean Flora. Nat. Prod. Res. 2015, 29, 2065–2070. [Google Scholar] [CrossRef] [PubMed]
- Ono, S.; Kawasaki, A.; Tamura, K.; Minegishi, Y.; Mori, T.; Ota, N. Ruscus aculeatus Extract Promotes RNase 7 Expression through ERK Activation Following Inhibition of Late-Phase Autophagy in Primary Human Keratinocytes. PLoS ONE 2024, 19, e0314873. [Google Scholar] [CrossRef]
- Göycıncık, S.; Dolap, H. A Study on Antioxidant and Antimicrobial Activities of Phlomis Amanica, Echinops Viscosus and Ruscus aculeatus from Hatay, Türkiye. Int. J. Second. Metab. 2025, 12, 886–897. [Google Scholar] [CrossRef]
- Jakovljevic, V.; Milicevic, J.; Ðelic, G.; Vrvic, M. Antioxidant Activity of Ruscus Species from Serbia: Potential New Sources of Natural Antioxidants. Hem. Ind. 2016, 70, 99–106. [Google Scholar] [CrossRef]
- Taşkın, T.; Güler, E.; Şahin, T.; Bulut, G. Enzyme Inhibitory and Antioxidant Activities of Different Extracts from Ruscus aculeatus l. Acta Pharm. Sci. 2020, 58, 497. [Google Scholar] [CrossRef]
- Bassil, N.M.; Abdel-Massih, R.; El-Chami, N.; Smith, C.A.; Baydoun, E. Pleurotus Ostreatus and Ruscus aculeatus Extracts Cause Non-Apoptotic Jurkat Cell Death. JPS 2012, 1, p14. [Google Scholar] [CrossRef][Green Version]
- Bilušić, T.; Šola, I.; Rusak, G.; Poljuha, D.; Čikeš Čulić, V. Antiproliferative and Pro-apoptotic Activities of Wild Asparagus (Asparagus acutifolius L.), Black Bryony (Tamus communis L.) and Butcher’s Broom (Ruscus aculeatus L.) Aqueous Extracts against T24 and A549 Cancer Cell Lines. J. Food Biochem. 2019, 43, e12781. [Google Scholar] [CrossRef] [PubMed]
- Balica, G.; Vostinaru, O.; Tamas, M.; Crisan, G.; Mogosan, C. Anti-Inflammatory Effect of the Crude Steroidal Saponin from the Rhizomes of Ruscus aculeatus L. (Ruscaceae) in Two Rat Models of Acute Inflammation. J. Food Agric. Environ. 2013, 11, 106–108. [Google Scholar]
- Voștinaru, O.; Balica, G.; Pârvu, A.; Mogoșan, C.; Cazacu, I.; Pop, C.; Tămaș, M.; Crișan, G. Diuretic and Anti-Inflammatory Effects of a Crude Steroidal Saponin from the Rhizomes of Ruscus aculeatus L. in Rats. Farmacia 2017, 65, 467–471. [Google Scholar]
- Chakuleska, L.; Michailova, R.; Shkondrov, A.; Manov, V.; Zlateva-Panayotova, N.; Marinov, G.; Petrova, R.; Atanasova, M.; Krasteva, I.; Danchev, N.; et al. Bone Protective Effects of Purified Extract from Ruscus aculeatus on Ovariectomy-Induced Osteoporosis in Rats. Food Chem. Toxicol. 2019, 132, 110668. [Google Scholar] [CrossRef]
- Sadarmin, P.P.; Timperley, J. An Unusual Case of Butcher’s Broom Precipitating Diabetic Ketoacidosis. J. Emerg. Med. 2013, 45, e63–e65. [Google Scholar] [CrossRef]
- Ramírez-Hernández, M.; García-Sellés, J.; Mérida-Fernández, C.; Martínez-Escribano, J.A. Allergic Contact Dermatitis to Ruscogenins. Contact Dermat. 2006, 54, 60. [Google Scholar] [CrossRef]
- Xu, Y.; Liu, J.-H.; Wang, J.; Zhang, J.; Yu, B.-Y. A Monoclonal Antibody-Based Competitive ELISA for the Determination of Ruscogenin in Chinese Traditional Medicines and Biological Samples. Chin. J. Nat. Med. 2014, 12, 794–799. [Google Scholar] [CrossRef]
- Lin, Y.N.; Jia, R.; Liu, Y.H.; Gao, Y.; Wang, L.L.; Kou, J.P.; Yu, B.Y. Ruscogenin Suppresses Mouse Neutrophil Activation: Involvement of Protein Kinase A Pathway. J. Steroid Biochem. Mol. Biol. 2015, 154, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Ercan, G.; Ilbar Tartar, R.; Solmaz, A.; Gulcicek, O.B.; Karagulle, O.O.; Meric, S.; Cayoren, H.; Kusaslan, R.; Kemik, A.; Gokceoglu Kayali, D.; et al. Potent Therapeutic Effects of Ruscogenin on Gastric Ulcer Established by Acetic Acid. Asian J. Surg. 2020, 43, 405–416. [Google Scholar] [CrossRef]
- Guan, T.; Liu, Q.; Qian, Y.; Yang, H.; Kong, J.; Kou, J.; Yu, B. Ruscogenin Reduces Cerebral Ischemic Injury via NF-κB-Mediated Inflammatory Pathway in the Mouse Model of Experimental Stroke. Eur. J. Pharmacol. 2013, 714, 303–311. [Google Scholar] [CrossRef]
- Ruan, Q.; Wang, C.; Zhang, Y.; Sun, J. Ruscogenin Attenuates Cartilage Destruction in Osteoarthritis through Suppressing Chondrocyte Ferroptosis via Nrf2/SLC7A11/GPX4 Signaling Pathway. Chem.-Biol. Interact. 2024, 388, 110835. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, W.; Tang, H.; Yang, Z.; Wei, M.; Zhou, W.; Li, Z.; Huang, W. Ruscogenin Attenuates Osteoarthritis by Modulating Oxidative Stress-Mediated Macrophage Reprogramming via Directly Targeting Sirt3. Int. Immunopharmacol. 2024, 143, 113336. [Google Scholar] [CrossRef]
- Sun, Q.; Chen, L.; Gao, M.; Jiang, W.; Shao, F.; Li, J.; Wang, J.; Kou, J.; Yu, B. Ruscogenin Inhibits Lipopolysaccharide-Induced Acute Lung Injury in Mice: Involvement of Tissue Factor, Inducible NO Synthase and Nuclear Factor (NF)-κB. Int. Immunopharmacol. 2012, 12, 88–93. [Google Scholar] [CrossRef]
- Wu, Q.; Wang, M.; Chen, W.; Wang, K.; Wang, Y. Assessing Neuroprotective Efficacy of Phytochemical Saponin Ruscogenin in Both In Vitro and In Vivo Model. Arab. J. Chem. 2023, 16, 104693. [Google Scholar] [CrossRef]
- Tiwari, A.; Singh, R.; Kumar, S.; Sunkaria, A.; Jain, A. From Plant to Pathway: Molecular Mechanisms of Ruscogenin in Preventing Amyloid-Beta Aggregation through Computational and Experimental Approaches. ACS Chem. Neurosci. 2025, 16, 500–512. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Wang, Y.; Gong, S.; Tang, J.; Zhang, J.; Li, F.; Yu, B.; Zhang, Y.; Kou, J. Ruscogenin Alleviates LPS-Induced Pulmonary Endothelial Cell Apoptosis by Suppressing TLR4 Signaling. Biomed. Pharmacother. 2020, 125, 109868. [Google Scholar] [CrossRef]
- Wu, Y.; Yu, X.; Wang, Y.; Huang, Y.; Tang, J.; Gong, S.; Jiang, S.; Xia, Y.; Li, F.; Yu, B.; et al. Ruscogenin Alleviates LPS-Triggered Pulmonary Endothelial Barrier Dysfunction through Targeting NMMHC IIA to Modulate TLR4 Signaling. Acta Pharm. Sin. B 2022, 12, 1198–1212. [Google Scholar] [CrossRef]
- Huang, H.; Li, X.; Zha, D.; Lin, H.; Yang, L.; Wang, Y.; Xu, L.; Wang, L.; Lei, T.; Zhou, Z.; et al. SARS-CoV-2 E Protein-Induced THP-1 Pyroptosis Is Reversed by Ruscogenin. Biochem. Cell Biol. 2023, 101, 303–312. [Google Scholar] [CrossRef]
- Bi, L.-Q.; Zhu, R.; Kong, H.; Wu, S.-L.; Li, N.; Zuo, X.-R.; Zhou, S.-M.; Kou, J.-P.; Yu, B.-Y.; Wang, H.; et al. Ruscogenin Attenuates Monocrotaline-Induced Pulmonary Hypertension in Rats. Int. Immunopharmacol. 2013, 16, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Lai, Q.; Zhu, X.; Zhang, L.; Kou, J.; Liu, F.; Yu, B.; Li, F. Inhibition of OAT1/3 and CMPF Uptake Attenuates Myocardial Ischemia-Induced Chronic Heart Failure via Decreasing Fatty Acid Oxidation and the Therapeutic Effects of Ruscogenin. Transl. Res. 2023, 261, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Hua, H.; Zhu, Y.; Song, Y.-H. Ruscogenin Suppressed the Hepatocellular Carcinoma Metastasis via PI3K/Akt/mTOR Signaling Pathway. Biomed. Pharmacother. 2018, 101, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; He, B.; Seshadri, V.D.; Xu, S. Anticancer Effect of Ruscogenin in B(a)P-Induced Lung Cancer in Mice via Modulation of Proinflammatory Cytokines and Mitochondrial Enzymes. Appl. Biochem. Biotechnol. 2022, 194, 5862–5877. [Google Scholar] [CrossRef]
- Directive 2001/83/EC of the European Parliament and of the Council of 6 November 2001 on the Community Code Relating to Medicinal Products for Human Use. 2001. Available online: https://eur-lex.europa.eu/eli/dir/2001/83/oj/eng (accessed on 1 September 2025).
- Prawo Farmaceutyczne [Pharmaceutical Law]; Act of 6 September 2001. 2001. Available online: https://isap.sejm.gov.pl/isap.nsf/DocDetails.xsp?id=wdu20011261381 (accessed on 1 September 2025).
- Regulation (EC) No 1223/2009 of the European Parliament and of the Council of 30 November 2009 on Cosmetic Products. Official Journal of the European Union. 2009. Available online: https://health.ec.europa.eu/system/files/2016-11/cosmetic_1223_2009_regulation_en_0.pdf (accessed on 1 September 2025).
- Commission Decision 2008/911/EC Establishing a List of Herbal Substances, Preparations and Combinations Thereof for Use in Traditional Herbal Medicinal Products. Available online: https://sip.lex.pl/akty-prawne/dzienniki-UE/decyzja-2008-911-we-ustanawiajaca-wykaz-substancji-ziolowych-preparatow-i-67835363 (accessed on 3 October 2025).
- Directive 2002/46/EC of the European Parliament and of the Council of 10 June 2002 on the Approximation of the Laws of the Member States Relating to Food Supplements. Available online: https://eur-lex.europa.eu/eli/dir/2002/46/oj/eng (accessed on 1 September 2025).
- Regulation (EC) No 1924/2006 of the European Parliament and of the Council of 20 December 2006 on Nutrition and Health Claims Made on Foods. Available online: https://eur-lex.europa.eu/eli/reg/2006/1924/oj/eng (accessed on 1 September 2025).
- Regulation (EU) No 1169/2011 of the European Parliament and of the Council of 25 October 2011 on the provision of food information to consumers, amending Regulations (EC) No 1924/2006 and (EC) No 1925/2006 of the European Parliament and of the Council, and repealing Commission Directive 87/250/EEC, Council Directive 90/496/EEC, Commission Directive 1999/10/EC, Directive 2000/13/EC of the European Parliament and of the Council, Commission Directives 2002/67/EC and 2008/5/EC and Commission Regulation (EC) No 608/2004 Text with EEA Relevance. Available online: https://eur-lex.europa.eu/eli/reg/2011/1169/oj/eng (accessed on 1 September 2025).
- The United States Food and Drug Administration (FDA). Available online: https://www.productcomplianceinstitute.com/pl/united-states-fda/ (accessed on 9 October 2025).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).