Pharmacological Investigation of a Novel Resveratrol-like SIRT1 Activator Endowed with a Cardioprotective Profile
Abstract
1. Introduction
2. Results and Discussion
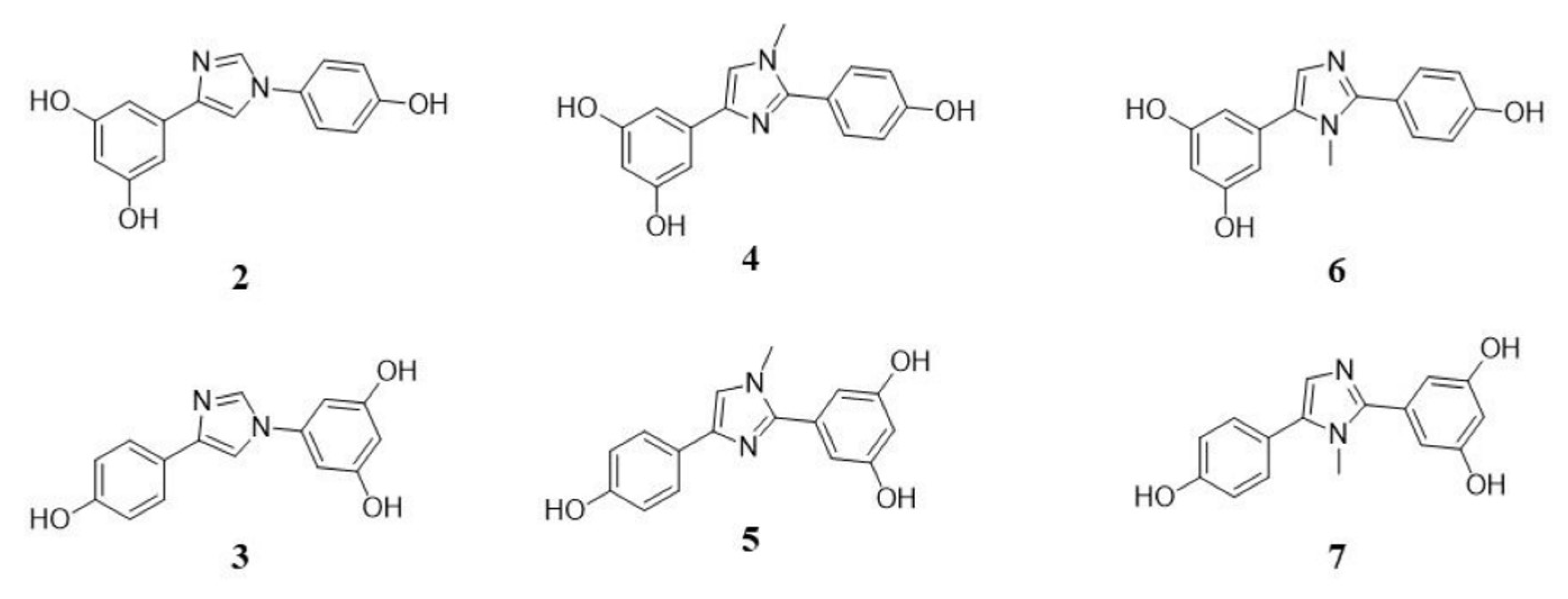
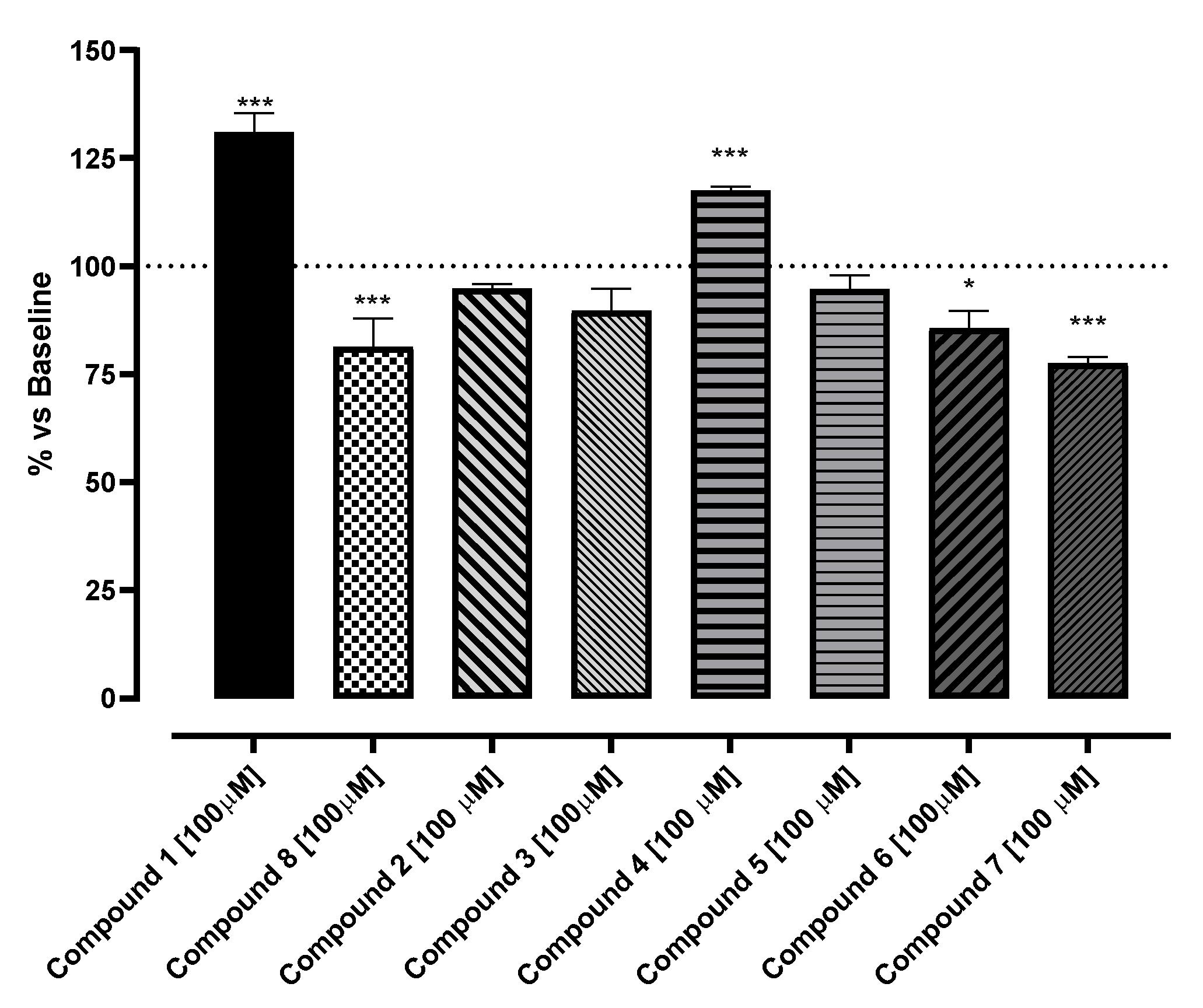
2.1. In Vitro Evaluation of Potential SIRT1 Activators
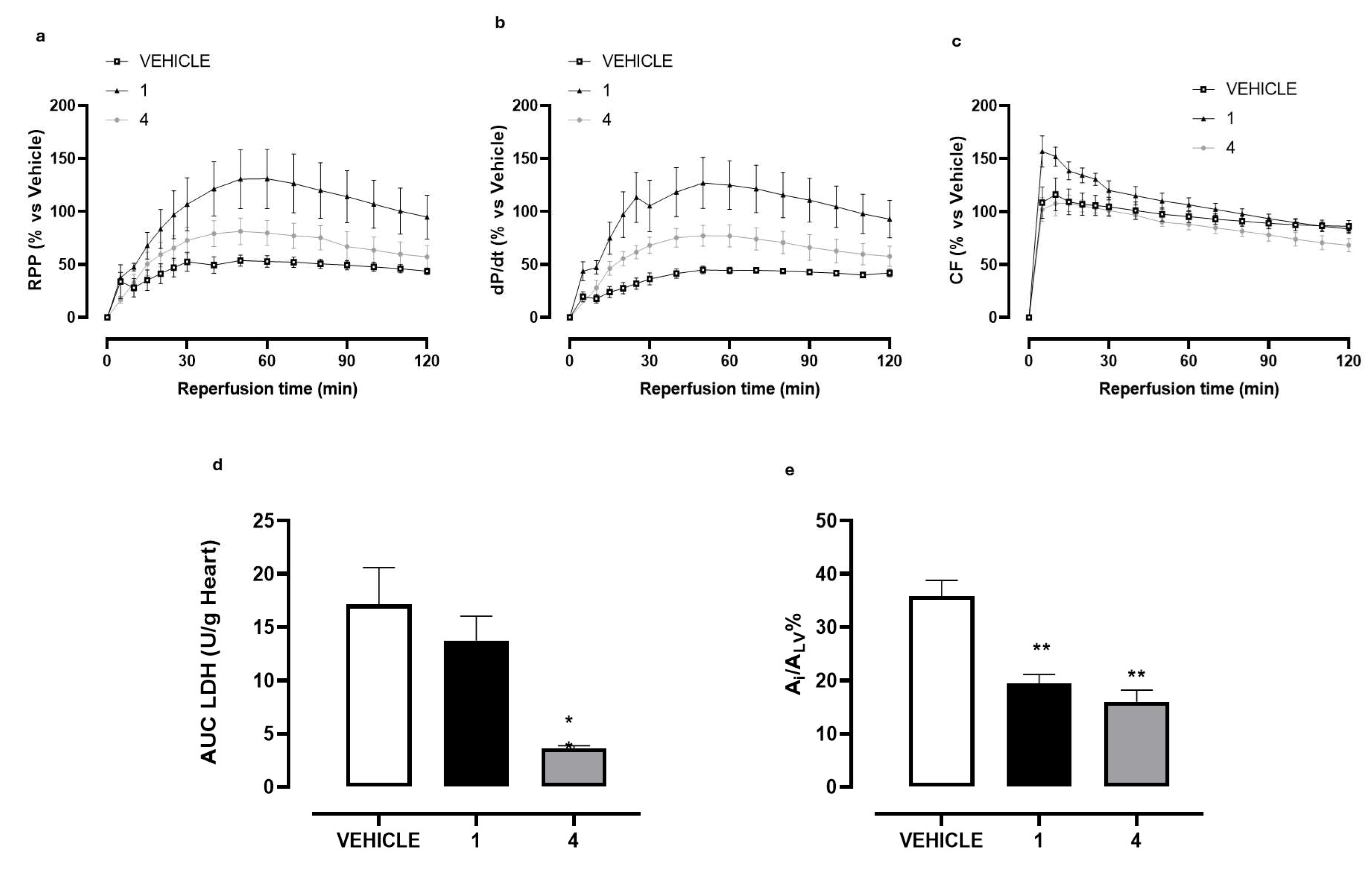
2.2. Ex Vivo Evaluation of 4
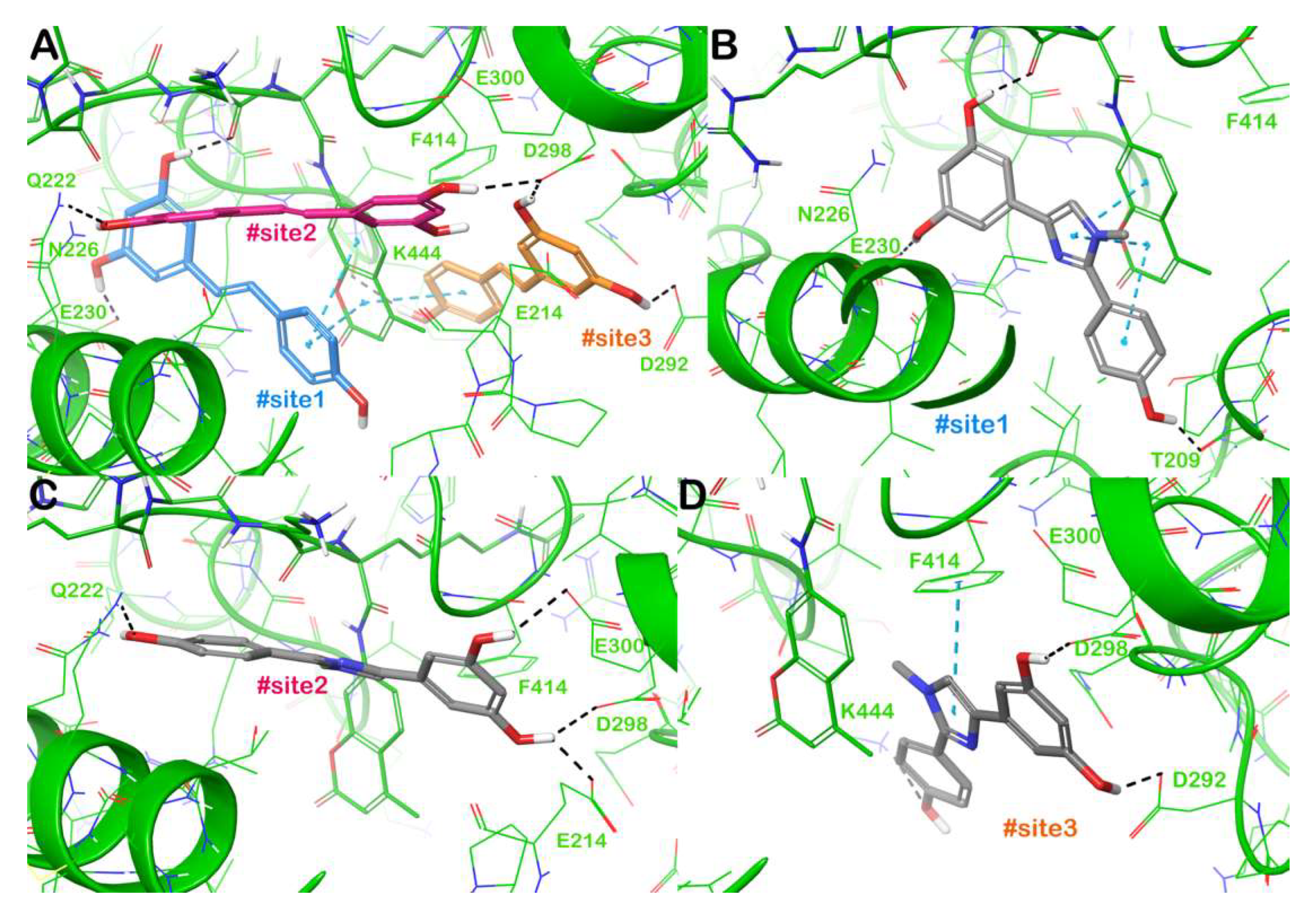
2.3. Binding Mode of 4 Within SIRT1
3. Materials and Methods
3.1. Diarylimidazole Synthesis
3.2. In Vitro Assay
3.3. Ex Vivo Studies
3.4. Computational Details
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Flori, L.; Petrarolo, G.; Brogi, S.; La Motta, C.; Testai, L.; Calderone, V. Identification of novel SIRT1 activators endowed with cardioprotective profile. Eur. J. Pharm. Sci. 2021, 165, 105930. [Google Scholar] [CrossRef]
- Bononi, G.; Flori, L.; Citi, V.; Acciai, C.; Nocilla, V.; Martelli, A.; Poli, G.; Tuccinardi, T.; Granchi, C.; Testai, L.; et al. New Synthetic Analogues of Natural Polyphenols as Sirtuin 1-Activating Compounds. Pharmaceuticals 2022, 15, 339. [Google Scholar] [CrossRef]
- Pyo, I.S.; Yun, S.; Yoon, Y.E.; Choi, J.-W.; Lee, S.-J. Mechanisms of Aging and the Preventive Effects of Resveratrol on Age-Related Diseases. Molecules 2020, 25, 4649. [Google Scholar] [CrossRef]
- Bononi, G.; Citi, V.; Martelli, A.; Poli, G.; Tuccinardi, T.; Granchi, C.; Testai, L.; Calderone, V.; Minutolo, F. Sirtuin 1-activating derivatives belonging to the anilinopyridine class displaying in vivo cardioprotective activities. RSC Med. Chem. 2024, 15, 267–282. [Google Scholar] [CrossRef]
- Bononi, G.; Citi, V.; Lapillo, M.; Martelli, A.; Poli, G.; Tuccinardi, T.; Granchi, C.; Testai, L.; Calderone, V.; Minutolo, F. Sirtuin 1-Activating Compounds: Discovery of a Class of Thiazole-Based Derivatives. Molecules 2022, 27, 6535. [Google Scholar] [CrossRef] [PubMed]
- Badawi, W.A.; Ezz-ElDien, E.S.; El-Atawy, M.A.; Omar, A.Z.; Hamed, E.A.; Ahmed, H.A.; Jaremko, M.; Emwas, A.H.; Okda, T.M.; El-Readi, M.Z.; et al. Design and synthesis of multitarget triazinoindole derivatives with potent antiproliferative activity: Targeting EGFR, SIRT1, MDM2, Hsp90, PI3K, p53, and caspase-9. Bioorg Chem. 2025, 165, 108943. [Google Scholar] [CrossRef] [PubMed]
- Ciccone, L.; Piragine, E.; Brogi, S.; Camodeca, C.; Fucci, R.; Calderone, V.; Nencetti, S.; Martelli, A.; Orlandini, E. Resveratrol-like Compounds as SIRT1 Activators. Int. J. Mol. Sci. 2022, 23, 15105. [Google Scholar] [CrossRef] [PubMed]
- Patra, S.; Praharaj, P.P.; Singh, A.; Bhutia, S.K. Targeting SIRT1-regulated autophagic cell death as a novel therapeutic avenue for cancer prevention. Drug Discov. Today 2023, 28, 103692. [Google Scholar] [CrossRef]
- Scisciola, L.; Sarno, F.; Carafa, V.; Cosconati, S.; Di Maro, S.; Ciuffreda, L.; De Angelis, A.; Stiuso, P.; Feoli, A.; Sbardella, G.; et al. Two novel SIRT1 activators, SCIC2 and SCIC2.1, enhance SIRT1-mediated effects in stress response and senescence. Epigenetics 2020, 15, 664–683. [Google Scholar] [CrossRef]
- Li, Z.; Xing, J. Role of sirtuins in cerebral ischemia-reperfusion injury: Mechanisms and therapeutic potential. Int. J. Biol. Macromol. 2025, 310, 143591. [Google Scholar] [CrossRef]
- Dai, H.; Ellis, J.L.; Sinclair, D.A.; Hubbard, B.P. Synthesis and Assay of SIRT1-Activating Compounds. Methods Enzymol. 2016, 574, 213–244. [Google Scholar] [CrossRef]
- Ding, X.; Zhu, C.; Wang, W.; Li, M.; Ma, C.; Gao, B. SIRT1 is a regulator of autophagy: Implications for the progression and treatment of myocardial ischemia-reperfusion. Pharmacol. Res. 2024, 199, 106957. [Google Scholar] [CrossRef]
- Mishra, D.; Mohapatra, L.; Tripathi, A.S.; Paswan, S.K. The influential responsibility of sirtuins in senescence and associated diseases: A review. J. Biochem. Mol. Toxicol. 2024, 38, e23812. [Google Scholar] [CrossRef]
- Yu, C.; Shin, Y.G.; Chow, A.; Li, Y.; Kosmeder, J.W.; Lee, Y.S.; Hirschelman, W.H.; Pezzuto, J.M.; Mehta, R.G.; van Breemen, R.B. Human, rat, and mouse metabolism of resveratrol. Pharm. Res. 2002, 19, 1907–1914. [Google Scholar] [CrossRef]
- Testai, L.; Piragine, E.; Piano, I.; Flori, L.; Da Pozzo, E.; Miragliotta, V.; Pirone, A.; Citi, V.; Di Cesare Mannelli, L.; Brogi, S.; et al. The Citrus Flavonoid Naringenin Protects the Myocardium from Ageing-Dependent Dysfunction: Potential Role of SIRT1. Oxidative Med. Cell. Longev. 2020, 2020, 4650207. [Google Scholar] [CrossRef]
- Cui, Z.; Zhao, X.; Amevor, F.K.; Du, X.; Wang, Y.; Li, D.; Shu, G.; Tian, Y.; Zhao, X. Therapeutic application of quercetin in aging-related diseases: SIRT1 as a potential mechanism. Front. Immunol. 2022, 13, 943321. [Google Scholar] [CrossRef] [PubMed]
- Vergara, D.; De Domenico, S.; Tinelli, A.; Stanca, E.; Del Mercato, L.L.; Giudetti, A.M.; Simeone, P.; Guazzelli, N.; Lessi, M.; Manzini, C.; et al. Anticancer effects of novel resveratrol analogues on human ovarian cancer cells. Mol. Biosyst. 2017, 13, 1131–1141. [Google Scholar] [CrossRef] [PubMed]
- Bellina, F.; Guazzelli, N.; Lessi, M.; Manzini, C. Imidazole analogues of resveratrol: Synthesis and cancer cell growth evaluation. Tetrahedron 2015, 71, 2298–2305. [Google Scholar] [CrossRef]
- Walle, T. Bioavailability of resveratrol. Ann. N. Y. Acad. Sci. 2011, 1215, 9–15. [Google Scholar] [CrossRef]
- Stivala, L.A.; Savio, M.; Carafoli, F.; Perucca, P.; Bianchi, L.; Maga, G.; Forti, L.; Pagnoni, U.M.; Albini, A.; Prosperi, E.; et al. Specific structural determinants are responsible for the antioxidant activity and the cell cycle effects of resveratrol. J. Biol. Chem. 2001, 276, 22586–22594. [Google Scholar] [CrossRef]
- Breschi, M.C.; Calderone, V.; Martelli, A.; Minutolo, F.; Rapposelli, S.; Testai, L.; Tonelli, F.; Balsamo, A. New benzopyran-based openers of the mitochondrial ATP-sensitive potassium channel with potent anti-ischemic properties. J. Med. Chem. 2006, 49, 7600–7602. [Google Scholar] [CrossRef] [PubMed]
- Citi, V.; Corvino, A.; Fiorino, F.; Frecentese, F.; Magli, E.; Perissutti, E.; Santagada, V.; Brogi, S.; Flori, L.; Gorica, E.; et al. Structure-activity relationships study of isothiocyanates for H(2)S releasing properties: 3-Pyridyl-isothiocyanate as a new promising cardioprotective agent. J. Adv. Res. 2021, 27, 41–53. [Google Scholar] [CrossRef]
- Zhang, L.X.; Li, C.X.; Kakar, M.U.; Khan, M.S.; Wu, P.F.; Amir, R.M.; Dai, D.F.; Naveed, M.; Li, Q.Y.; Saeed, M.; et al. Resveratrol (RV): A pharmacological review and call for further research. Biomed. Pharmacother. 2021, 143, 112164. [Google Scholar] [CrossRef] [PubMed]
- Ren, B.; Kwah, M.X.; Liu, C.; Ma, Z.; Shanmugam, M.K.; Ding, L.; Xiang, X.; Ho, P.C.; Wang, L.; Ong, P.S.; et al. Resveratrol for cancer therapy: Challenges and future perspectives. Cancer Lett. 2021, 515, 63–72. [Google Scholar] [CrossRef]
- Meng, T.; Xiao, D.; Muhammed, A.; Deng, J.; Chen, L.; He, J. Anti-Inflammatory Action and Mechanisms of Resveratrol. Molecules 2021, 26, 229. [Google Scholar] [CrossRef]
- Parsamanesh, N.; Asghari, A.; Sardari, S.; Tasbandi, A.; Jamialahmadi, T.; Xu, S.; Sahebkar, A. Resveratrol and endothelial function: A literature review. Pharmacol. Res. 2021, 170, 105725. [Google Scholar] [CrossRef] [PubMed]
- Cao, D.; Wang, M.; Qiu, X.; Liu, D.; Jiang, H.; Yang, N.; Xu, R.M. Structural basis for allosteric, substrate-dependent stimulation of SIRT1 activity by resveratrol. Genes Dev. 2015, 29, 1316–1325. [Google Scholar] [CrossRef]
- Mokni, M.; Limam, F.; Elkahoui, S.; Amri, M.; Aouani, E. Strong cardioprotective effect of resveratrol, a red wine polyphenol, on isolated rat hearts after ischemia/reperfusion injury. Arch. Biochem. Biophys. 2007, 457, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Bradamante, S.; Barenghi, L.; Piccinini, F.; Bertelli, A.A.; De Jonge, R.; Beemster, P.; De Jong, J.W. Resveratrol provides late-phase cardioprotection by means of a nitric oxide- and adenosine-mediated mechanism. Eur. J. Pharmacol. 2003, 465, 115–123. [Google Scholar] [CrossRef]
- Dernek, S.; Ikizler, M.; Erkasap, N.; Ergun, B.; Koken, T.; Yilmaz, K.; Sevin, B.; Kaygisiz, Z.; Kural, T. Cardioprotection with resveratrol pretreatment: Improved beneficial effects over standard treatment in rat hearts after global ischemia. Scand. Cardiovasc. J. 2004, 38, 245–254. [Google Scholar] [CrossRef]
- da Silva, E.R.; Brogi, S.; Lucon-Júnior, J.F.; Campiani, G.; Gemma, S.; Maquiaveli, C.D.C. Dietary polyphenols rutin, taxifolin and quercetin related compounds target Leishmania amazonensis arginase. Food Funct. 2019, 10, 3172–3180. [Google Scholar] [CrossRef] [PubMed]
- Brogi, S.; Fiorillo, A.; Chemi, G.; Butini, S.; Lalle, M.; Ilari, A.; Gemma, S.; Campiani, G. Structural characterization of Giardia duodenalis thioredoxin reductase (gTrxR) and computational analysis of its interaction with NBDHEX. Eur. J. Med. Chem. 2017, 135, 479–490. [Google Scholar] [CrossRef]
- Brindisi, M.; Ulivieri, C.; Alfano, G.; Gemma, S.; de Asís Balaguer, F.; Khan, T.; Grillo, A.; Chemi, G.; Menchon, G.; Prota, A.E.; et al. Structure-activity relationships, biological evaluation and structural studies of novel pyrrolonaphthoxazepines as antitumor agents. Eur. J. Med. Chem. 2019, 162, 290–320. [Google Scholar] [CrossRef] [PubMed]
- Paolino, M.; Brindisi, M.; Vallone, A.; Butini, S.; Campiani, G.; Nannicini, C.; Giuliani, G.; Anzini, M.; Lamponi, S.; Giorgi, G.; et al. Development of Potent Inhibitors of the Mycobacterium tuberculosis Virulence Factor Zmp1 and Evaluation of Their Effect on Mycobacterial Survival inside Macrophages. ChemMedChem 2018, 13, 422–430. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carbonetti, L.; Brogi, S.; D’Orsi, R.; Lessi, M.; Calderone, V.; Testai, L.; Bellina, F. Pharmacological Investigation of a Novel Resveratrol-like SIRT1 Activator Endowed with a Cardioprotective Profile. Molecules 2025, 30, 4378. https://doi.org/10.3390/molecules30224378
Carbonetti L, Brogi S, D’Orsi R, Lessi M, Calderone V, Testai L, Bellina F. Pharmacological Investigation of a Novel Resveratrol-like SIRT1 Activator Endowed with a Cardioprotective Profile. Molecules. 2025; 30(22):4378. https://doi.org/10.3390/molecules30224378
Chicago/Turabian StyleCarbonetti, Leonardo, Simone Brogi, Rosarita D’Orsi, Marco Lessi, Vincenzo Calderone, Lara Testai, and Fabio Bellina. 2025. "Pharmacological Investigation of a Novel Resveratrol-like SIRT1 Activator Endowed with a Cardioprotective Profile" Molecules 30, no. 22: 4378. https://doi.org/10.3390/molecules30224378
APA StyleCarbonetti, L., Brogi, S., D’Orsi, R., Lessi, M., Calderone, V., Testai, L., & Bellina, F. (2025). Pharmacological Investigation of a Novel Resveratrol-like SIRT1 Activator Endowed with a Cardioprotective Profile. Molecules, 30(22), 4378. https://doi.org/10.3390/molecules30224378









