Abstract
Andrographis paniculata, a medicinal plant widely found in Asia, contains andrographolide as its main active compound, known for its wide-ranging pharmacological effects, including anti-inflammatory, anti-cancer, anti-obesity, and anti-diabetic properties. Recent investigations have highlighted the anti-infective potential of andrographolide and its derivatives, with demonstrated antiviral, antibacterial, and antimalarial activities. This review summarizes progress in andrographolide’s anti-infective applications, focusing on its structure–activity relationship (SAR) and mechanisms of action. Researchers have used semi-synthetic methods, such as esterification, oxidation, Michael addition, salification, and hybrid design, to enhance andrographolide’s physicochemical properties and biological activity. These derivatives show potent antiviral activity against RNA and DNA viruses, antibacterial activity against Gram-positive and Gram-negative bacteria, antifungal effects, and antiparasitic activity against Plasmodium spp. and Leishmania spp. Nevertheless, poor solubility and limited bioavailability still hinder their clinical translation. Strategies such as nano delivery systems and β-cyclodextrin complexes are discussed to improve bioavailability. Although andrographolide itself has not received regulatory approval as a stand-alone drug, several andrographolide-containing preparations have been clinically used in certain countries. Overall, this review brings together evidence on antiviral, antibacterial, antifungal, and antiparasitic activities, linking them with structure–activity trends and pharmacokinetic insights, thereby providing a consolidated foundation for future development and clinical translation.
1. Introduction
Andrographis paniculata [1], a medicinal plant widely distributed across Asia, has been traditionally employed for the treatment of inflammatory disorders [2] and infectious diseases [3]. Its main active ingredient, andrographolide, the principal bioactive constituent, exhibits anti-inflammatory activity [4], battling cancer [5,6,7], curbing obesity [8,9], and managing diabetes [10,11]. In anti-infective contexts, andrographolide and selected derivatives modulate pathogen-induced inflammatory signaling (e.g., NF-κB/TLR pathways) and, in several models, directly inhibit pathogen replication [12,13,14].
The major bioactive constituent, andrographolide, is isolated primarily from the leaves and stems and features a bicyclic diterpene scaffold with an α,β-unsaturated γ-lactone and conjugated double bonds—structural motifs associated with its biological activities [15,16]. Recent research highlights how it combats infections by boosting the immune system [17], dialing down inflammation [18], and clearing out oxidative stress [19]. Considerable progress has also been made in elucidating its antiviral, antibacterial, and antimalarial mechanisms of action [20].
We provide an integrated appraisal of the anti-infective properties of andrographolide and its derivatives across viruses, bacteria, fungi, and parasites, explicitly connecting mechanistic insights and SAR with formulation strategies and PK liabilities—a scope that, to our knowledge, has not been comprehensively consolidated. Inclusion criteria were original research articles reporting in vitro, in vivo, or clinical outcomes for andrographolide or chemically defined derivatives within the last ~20 years. Exclusion criteria comprised narrative reviews, studies unrelated to infectious indications, derivatives not based on the andrographolide scaffold, duplicate records, and reports lacking primary quantitative endpoints (e.g., EC50/IC50, MIC, CC50, SI).
Literature was identified from the Web of Science Core Collection (cross-checked with PubMed/Scopus) using keywords related to andrographolide, derivatives, and infectious indications over 2005–2025. Screening followed the inclusion/exclusion criteria stated above. Data were organized by pathogen class with consistent reporting of EC50/IC50 (μM), CC50 (μM), MIC (μg·mL−1), and SI (CC50/EC50 or IC50).
In anti-infective research, andrographolide and its derivatives play important roles by inhibiting pathogen-induced inflammation, modulating the immune system, and scavenging oxidative stress. For example, andrographolide can inhibit inflammation caused by bacterial infection by regulating the mitogen-activated protein kinase (MAPK) and nuclear factor-κB (NF-κB) signaling pathways [21], thereby reducing the expression of pro-inflammatory cytokines. In antiviral activity, andrographolide can inhibit viral replication and regulate the expression of Nrf2 and its downstream genes [22], reducing lung inflammation caused by viral infection. In antimalarial activity, andrographolide, in combination with curcumin, has shown significant antiplasmodial activity against Plasmodium falciparum [23,24,25].
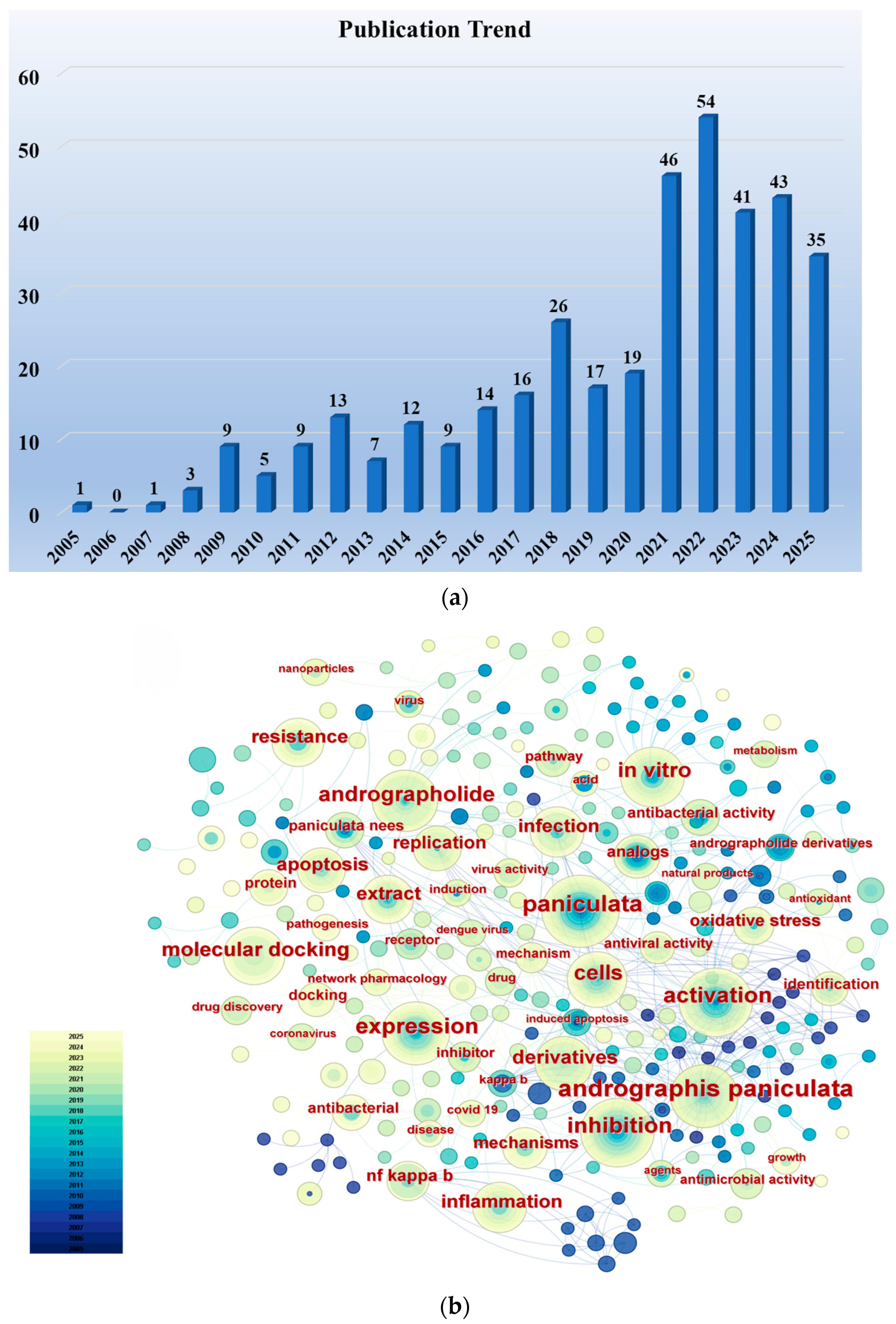
We conducted a literature search in the Web of Science Core Collection using the keywords “Andrographolide” and “Anti-infective (including antiviral, antibacterial, antiparasitic)” (considering only research articles from the past two decades), identifying a total of 380 relevant research papers. Figure 1a illustrates the publication trend over the past two decades for research papers concerning the anti-infective activity of andrographolide. Although the number of papers published in certain years showed a slight decrease compared to the preceding year, the overall trend is upward, reflecting researchers’ increasingly intense interest in the anti-infective activity of andrographolide. Using CiteSpace software, an analysis of keyword frequency was conducted on these publications. Figure 1b clearly shows that keywords such as “infection”, “antibacterial activity”, “antiviral activity”, “derivatives”, “extract”, and “molecular docking” appear with high frequency. This indicates that current researchers’ interests are primarily focused on the biological activity of andrographolide, the synthesis of its derivatives, and structure–activity relationships.
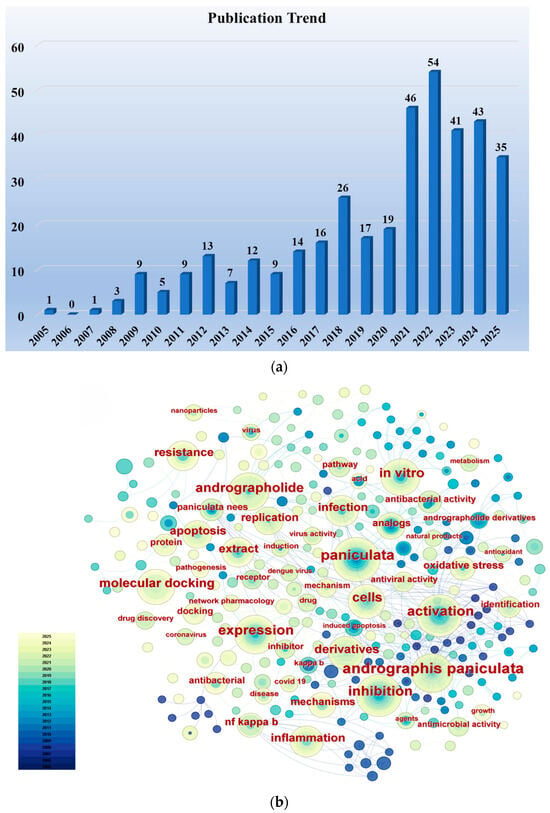
Figure 1.
(a) Publication trends in research articles concerning the anti-infective activity of andrographolide over the past two decades. (b) Frequency distribution of keywords in research articles on the anti-infective activity of andrographolide over the past two decades. Supported by CiteSpace 6.4.R1.
The scope of this review covers viruses, bacteria, fungi, and parasites, and the reporting standards for potency and selectivity are summarized in Table 1.

Table 1.
Pathogen coverage and reporting standards used in this review.
2. Structural Diversity and Representative Synthetic Derivatives
2.1. Parent Skeleton and Key Functional Groups
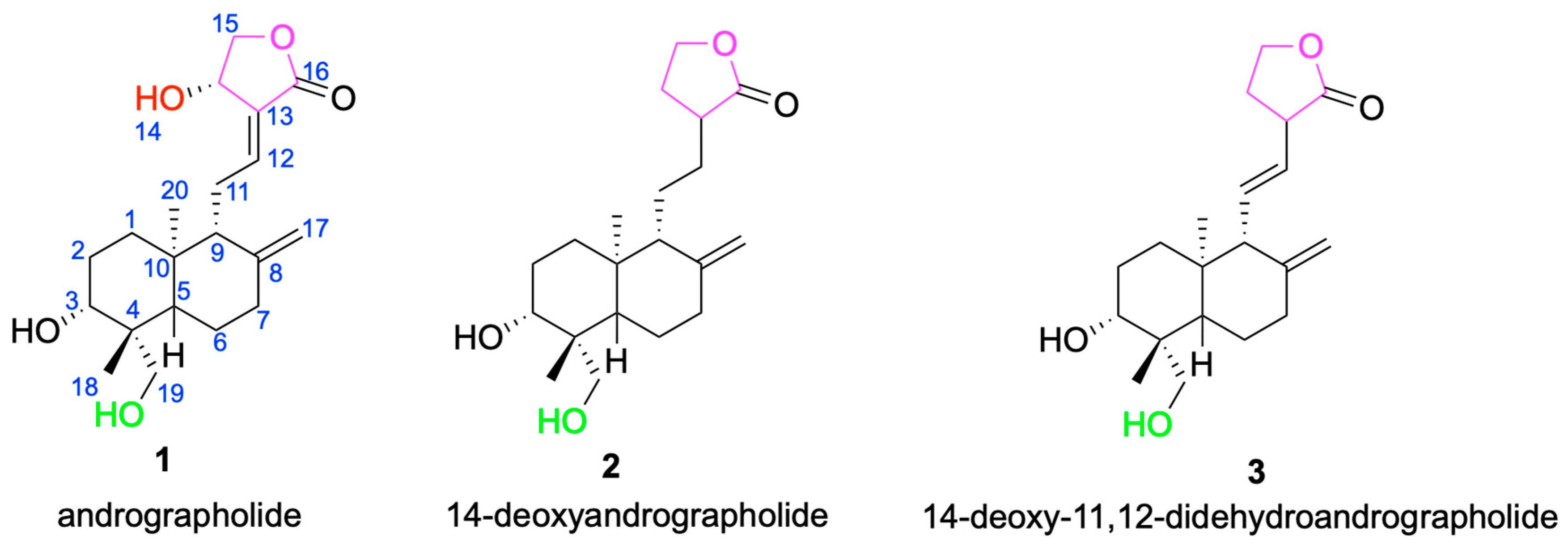
The core framework of andrographolide is a rigid, lipophilic C20-labdane-γ-lactone scaffold, in which a decahydronaphthalene ring system is fused to a γ-butyrolactone moiety through a C-8/C-17 conjugated double bond [33]. This architecture influences molecular conformation and membrane permeability and provides a stable stereochemical platform for downstream derivatization [34]. In addition to the parent scaffold andrographolide 1, other compounds such as 14-deoxyandrographolide 2 and 14-deoxy-11,12-didehydroandrographolide 3 have also been isolated from Andrographis paniculata [35] (Figure 2). These compounds share structural similarities with andrographolide but exhibit differences at certain positions, which may influence their biological activities. For example, 14-deoxyandrographolide 2 lacks the hydroxyl group at C-14, while 14-deoxy-11,12-didehydroandrographolide 3 has a double bond at the C-11 and C-12 positions. These structural features may endow them with unique antiviral and antibacterial activities [36,37].
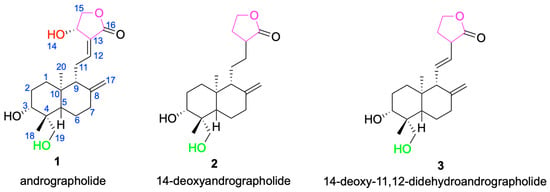
Figure 2.
Chemical structures of andrographolide 1 and its analogs 2 and 3.
The primary hydroxyl group at C-14 is readily esterified or oxidized to form α, β- unsaturated diketone or sulfonate esters, which can form covalent bonds with viral proteases such as the cysteine residue of SARS-CoV-2 [38]. The primary hydroxyl group at C-19 can be converted into sodium sulfonate or phosphate salts [39]. For example, the sulfonated preparation Xiyanping injection (sodium andrographolide sulfonate) is used clinically in China for acute upper respiratory tract infections (e.g., acute pharyngotonsillitis) and acute bronchitis. This herbal-derived product is not equivalent to regulatory approval of pure andrographolide, and the supporting clinical evidence remains limited in scope [40].
2.2. Overview of Semi-Synthetic Strategies: Esterification, Oxidation, Michael Addition, Salification, and Hybrid Design
2.2.1. Esterification
Esterification at C-14/C-19 can increase aqueous solubility and tune lipophilicity; C-14 ester chain length correlates with influenza A antiviral potency in multiple series. This modification markedly improves the aqueous solubility of andrographolide and enhances its bioavailability [41,42]. For instance, compound 12 (AL-1), a C-14/C-19–modified conjugate, markedly reduced the viral burden of influenza A (H1N1) (EC50 = 7.2 μM; SI = 109) in vitro and protected mice at high dose (see Section 3.1, H1N1).
2.2.2. Oxidation
Oxidations that install α,β-unsaturated carbonyls can enhance cysteine-targeting electrophilicity, with selectivity and safety trade-offs. This strategy can introduce a variety of active functional groups, thereby modulating the pharmacological properties and pharmacokinetic characteristics of the drug [43,44]. For example, the α, β-unsaturated carbonyl derivative 14-deoxy-11,12-didehydroandrographolide exhibited enhanced cysteine-targeting reactivity in biochemical assays, illustrating the activity–selectivity trade-off (see Section 2.1 and Section 3.1).
2.2.3. Michael Addition
Michael acceptor-containing analogues may improve enzyme inhibition but require reactivity control to mitigate off-target liabilities. This strategy can significantly enhance the biological activity of andrographolide, especially its antibacterial and antiviral activities [45,46]. For example, compound 10 (Andro-NBD) inhibits SARS-CoV-2 Mpro (IC50 = 2.79 ± 0.30 μM), whereas the non-Michael Compound 11 (NCTU-048) shows no measurable inhibition (IC50 > 240 μM), underscoring the need to control electrophilic reactivity (Section 3.1, SARS-CoV-2).
2.2.4. Salification
Salification forms salts to improve aqueous solubility and sometimes solid-state stability. However, improved solubility does not guarantee better pharmacokinetics (e.g., oral bioavailability or half-life), which remain route- and formulation-dependent and require empirical confirmation [47]. For example, the sulfonated preparation Xiyanping improves aqueous solubility and is used clinically in certain regions as a herbal-derived product; however, this does not constitute regulatory approval of pure andrographolide, and any PK improvement requires empirical confirmation (Section 2.1 and Section 4).
2.2.5. Hybrid Design
Hybridization (e.g., quinoline– or oxindole–labdane) enables dual-mechanism profiles and broader spectra while retaining the andrographolide pharmacophore. This strategy can significantly enhance the biological activity of andrographolide and expand its range of applications [48]. For example, the quinolinoxy–labdane compounds 14/15 display anti-flavivirus activity (ZIKV EC50 = 4.5 μM and 1.3 μM; SI = 19.7 and 17.5), and the oxindole–labdane Compound 16 exhibits anti-CHIKV activity (see Section 3.1, DENV/ZIKV and CHIKV).
By employing these semi-synthetic strategies, the physicochemical properties and biological activities of andrographolide can be effectively improved, providing more possibilities for its application in anti-infective research. Collectively, these strategies enrich the structural diversity of andrographolide derivatives and provide a rational framework for their clinical application.
3. Pathogen-Classified Anti-Infective Profile and Mechanisms
3.1. Antiviral Activity
Pathogen coverage and reporting standards: This section organizes findings by pathogen class, including viruses (SARS-CoV-2, influenza A/H1N1, DENV, ZIKV, CHIKV, EV-A71, HBV, HSV-1/2), bacteria (Staphylococcus aureus [MRSA], Enterococcus faecalis, Escherichia coli, Pseudomonas aeruginosa), and representative fungi (Candida albicans, Candida auris, Aspergillus fumigatus) and parasites (Plasmodium falciparum, Leishmania donovani). Potency is reported as EC50/IC50 (μM) or MIC (μg·mL−1) with corresponding CC50 (μM) when available. Selectivity index (SI) is defined as CC50/EC50 (or IC50), unless otherwise stated. To facilitate cross-study comparisons, we note key assay parameters (cell line, MOI, exposure time) where provided.
3.1.1. RNA Viruses
Anti-SARSCoV2 Activity
SARS-CoV-2, first recognized in late 2019, is a betacoronavirus that caused the COVID-19 pandemic. A related bat coronavirus (RaTG13) shares ~96% genome sequence identity, and SARS-CoV-2 uses the angiotensin-converting enzyme 2 (ACE2) as the entry receptor. The virus uses the angiotensin-converting enzyme 2 (ACE2) as its receptor to enter human cells. It has an incubation period of about 5 days, with symptoms like fever, dry cough, and fatigue. Severe cases may develop into acute respiratory distress syndrome (ARDS) and require intensive care. SARS-CoV-2 spreads primarily through respiratory droplets and close contact between hosts As of late September 2025, the WHO COVID-19 Dashboard reports approximately 7.7 × 108 confirmed cases and 7.1 × 106 deaths worldwide (World Health Organization, 2025) [49,50,51].
Andrographolide inhibits the SARS-CoV-2 main protease (Mpro) in enzymatic assays with low-micromolar IC50 values, and available probe/labeling studies are consistent with cysteine engagement at Cys145. However, biochemical potency alone does not establish in vivo antiviral efficacy [52].
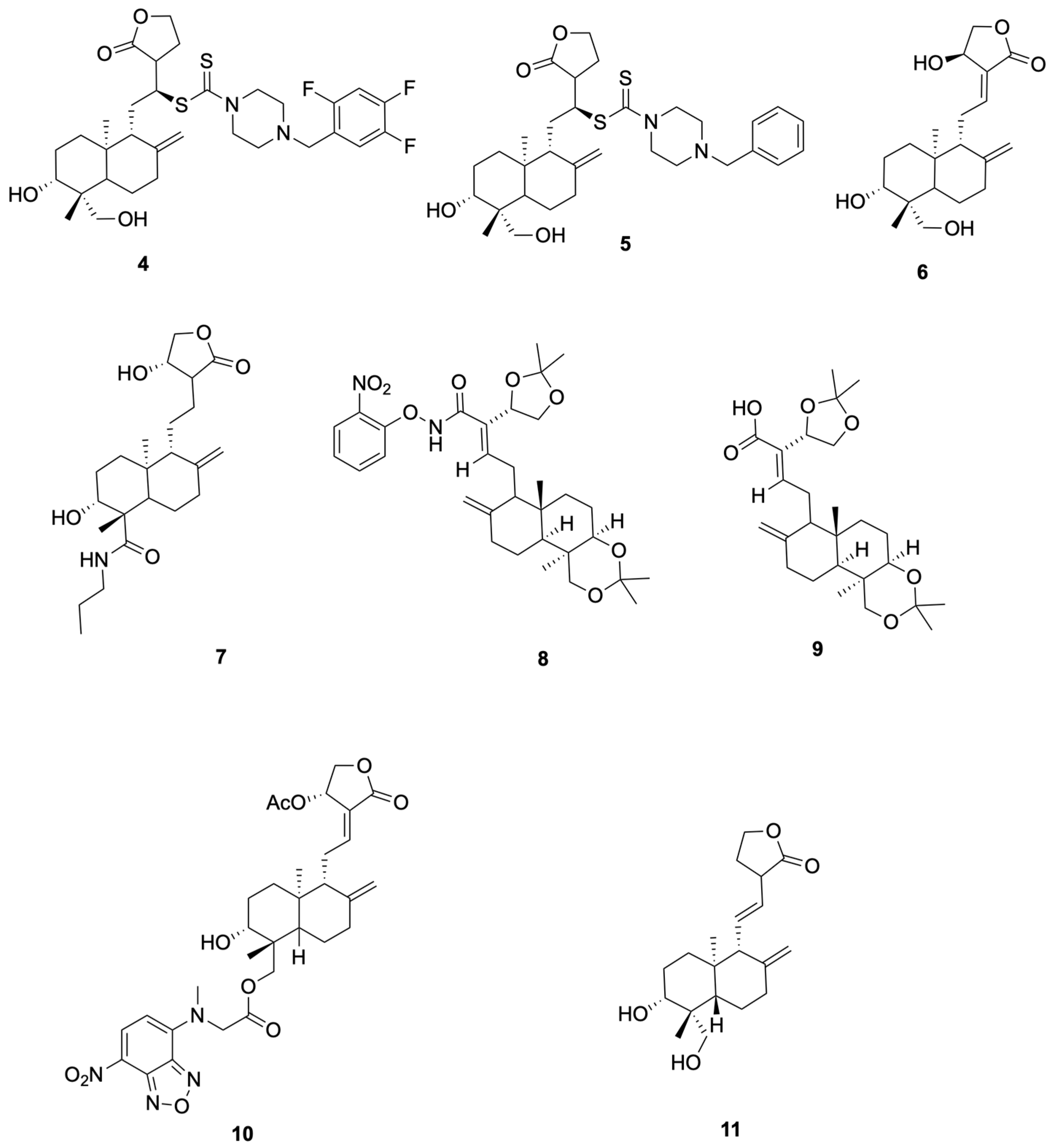
Schulte et al. [53] synthesized compounds 4 and 5, which showed anti-SARS-CoV-2 activity (representative antiviral andrographolide derivatives are shown in Figure 3). In Vero-E6 assays, Compound 4 reduced SARS-CoV-2 replication with NT50 = 8.1 μM and showed no detectable cytotoxicity at the tested concentrations (CC50 > 10 μM). It activates the KEAP1–NRF2 pathway with an ARE specificity ratio (SR) of 10.8. Compound 5 was an NRF2 activator (SR = 13.3) and showed no measurable antiviral effect up to 10 μM (NT50 > 10 μM), with CC50 > 10 μM. This derivative also exhibited improved solubility with low cytotoxicity, supporting further evaluation rather than establishing clinical candidacy.
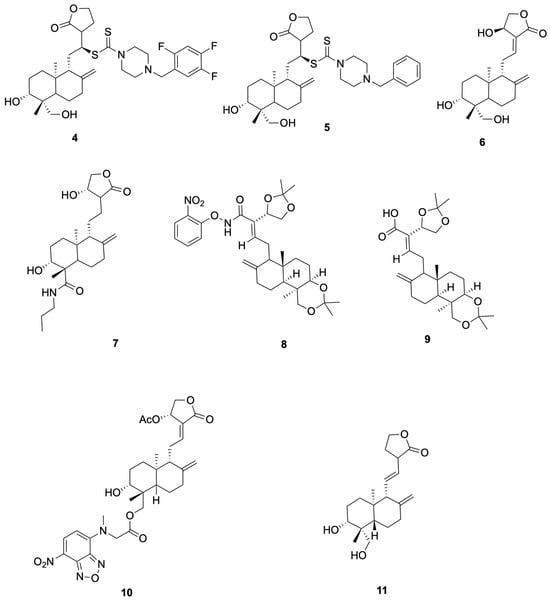
Figure 3.
Compounds 4–11.
Suriya et al. [54] synthesized and evaluated Compound 6 (14β-andrographolide) and Compound 7 showed cell-based antiviral activity against SARS-CoV-2 in Vero E6 plaque-reduction assays (NT50 = 2.1 μM and 3.7 μM, respectively), without cytotoxicity ≤ 10 μM (representative antiviral andrographolide derivatives are shown in Figure 3). Mechanistic data in that study associated the antiviral effect with KEAP1/NRF2 pathway modulation, rather than direct main protease inhibition. We therefore use 6 and 7 as representative examples in this section and describe their activity as host-directed antiviral effects in cell assays.
Rahman et al. [55] synthesized and profiled Compound 8 and Compound 9 as β-glucosidase inhibitors (representative antiviral andrographolide derivatives are shown in Figure 3). Compound 8 showed the best activity in the series (IC50 = 92.4 μM), outperforming castanospermine (108.6 μM), consistent with an electron-withdrawing o-NO2 effect on the aryl group. Compound 9 exhibited moderate inhibition (IC50 = 102.4 μM), comparable to the simple phenyl amide (106.5 μM) and better than andrographolide (142.5 μM). As these readouts derive from a biochemical model (sweet-almond β-glucosidase), enzyme-level potency is hypothesis-generating; translation to anti-infective efficacy requires cellular and in vivo confirmation.
Shi et al. [52] reported that compound 10 (Andro-NBD) inhibits SARS-CoV-2 Mpro in a biochemical assay (IC50 = 2.79 ± 0.30 μM) and labels the enzyme in gel; the signal is abolished by the C145A active-site mutant, and MS indicates an adduct on Cys145, consistent with a putative covalent mechanism. In contrast, the thiol-insensitive analogue compound 11 (NCTU-048) shows weak inhibition (>~240 μM), supporting cysteine dependence. As these are enzyme-level readouts, potency and labeling are hypothesis-generating; antiviral efficacy requires cellular and in vivo confirmation (see Figure 4 for representative antiviral derivatives).
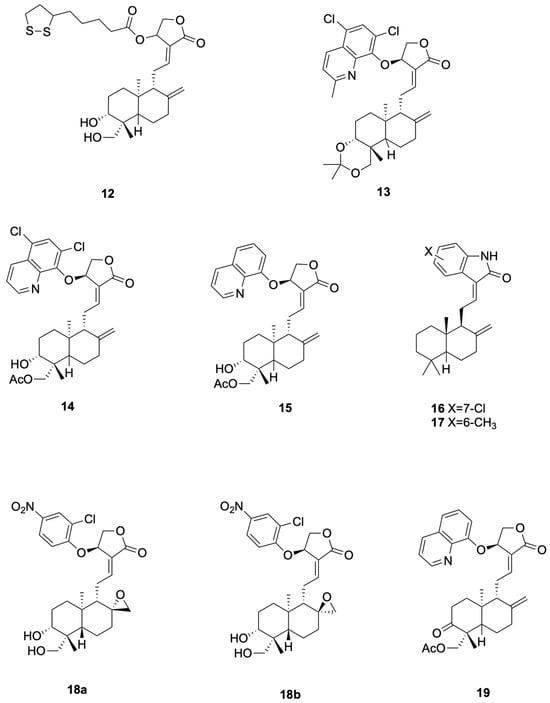
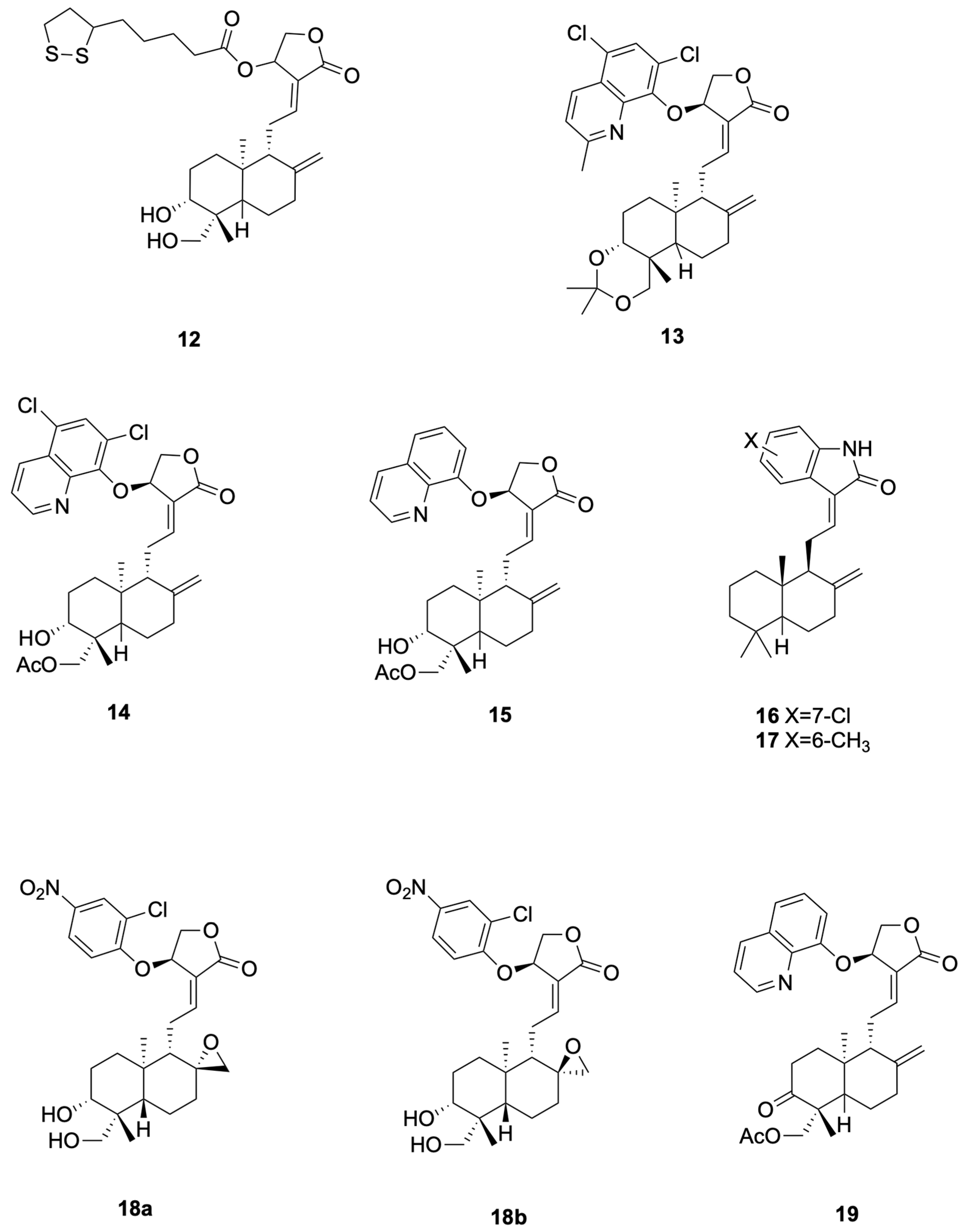
Figure 4.
Compounds 12–19.
Anti-Influenza A (H1N1) Virus Activity
Influenza A (H1N1), which caused the 2009 global pandemic, remains a major respiratory pathogen worldwide. It originated from pigs and is also known as “swine flu.” The virus is highly contagious and can cause respiratory infections with symptoms like fever, cough, and sore throat. The 2009 H1N1 pandemic was notable for its rapid spread and affected many countries. Vaccines and antiviral medications were used to control its impact. Since then, H1N1 has become a seasonal flu virus and continues to circulate globally [56].
In the case of influenza A virus, andrographolide derivatives have demonstrated the capacity to inhibit the RNA-dependent RNA polymerase (RdRp), which is a key enzyme for viral RNA synthesis. By targeting RdRp, these compounds can effectively halt the production of viral RNA, thus limiting the spread of the virus within the host. The C-14 ester chain length of andrographolide derivatives appears to correlate with antiviral potency against influenza A, with optimal chain lengths enhancing the interaction with the viral enzyme.
Chen et al. [57] synthesized compounds 12, which demonstrated potent anti-H1N1 activity (Figure 4 presents representative antiviral andrographolide derivatives). 12 (CC50 = 784 μM, EC50 = 7.2 μM, SI = 109) completely prevented mortality in mice at 200 mg kg−1 day−1, reduced lung virus titers by 46%, and inhibited viral adsorption to erythrocytes by directly interfering with hemagglutinin–receptor interactions. 12 showed CC50 = 784 μM and SI = 109 (EC50 ≈ 7.2 μM in the same assay). The 200 mg·kg−1·day−1 dose used in mice represents a relatively high pharmacological exposure and warrants pharmacokinetic characterization to establish clinical relevance.
Anti-Dengue and Anti-Zika Viruses’ Activity
Dengue is a significant infectious disease affecting public health across tropical and subtropical areas worldwide. It presents clinically with a biphasic fever pattern, accompanied by symptoms such as severe headache, muscle and joint pain, profound fatigue, skin rash, swollen lymph nodes, and a marked reduction in white blood cell count [58,59].
Zika virus (ZIKV), a member of the Flavivirus genus within the Flaviviridae family, is classified as an arthropod-borne virus (arbovirus). Its primary mode of transmission to humans is through the bite of infected mosquitoes. Clinically, Zika fever often presents with nonspecific symptoms, making it challenging to distinguish from other arboviral diseases such as dengue and chikungunya, and frequently leading to potential misdiagnosis [60].
For dengue virus and zika virus, andrographolide derivatives have been found to interfere with the viral RdRp and Mpro, like their action against other RNA viruses. These interactions prevent the virus from efficiently replicating its RNA genome and processing its polyproteins. The α, β-unsaturated lactone moiety of andrographolide derivatives is particularly effective in covalently binding to viral targets, thereby inactivating them and reducing viral replication.
In this review, SI is defined as CC50/EC50 (or IC50), unless otherwise stated. Where EC50 values are ≥10–20 μM (e.g., DENV 22.6 μM; ZIKV 27.9 μM for the 14-aryloxy analogue), we describe the antiviral activity as weak-to-moderate rather than “potent”, and we report EC50, CC50, and SI (=CC50/EC50) explicitly to avoid ambiguity.
Zhou et al. [61] synthesized the 14-aryloxy analogue compounds 13 (Figure 4 presents representative antiviral andrographolide derivatives), which showed dual activity with weak-to-moderate potency against DENV (EC50 = 22.6 μM, SI = 6.6) and ZIKV (EC50 = 27.9 μM, SI = 9.8). Compounds 13 significantly reduced viral production in HEK293T/17 (DENV) and A549 (ZIKV) cells, and proteomics revealed down-regulation of HSPA1A and up-regulation of PGK1 as key mechanistic differences from the parent andrographolide. Proteomics changes (e.g., HSPA1A, PGK1) are associative and do not prove causality without orthogonal validation.
Li et al. [62] designed compounds 14 (19-acetylated 14α-(5,7-dichloro-8-quinolyloxy)andrographolide, EC50 4.5 μM, SI 19.7) and compounds 15 (14β-(8-quinolyloxy)-3,19-diol andrographolide, EC50 1.3 μM, SI 17.5) as anti-ZIKV hybrids with low- to single-digit micromolar potency (Figure 4 presents representative antiviral andrographolide derivatives); the 5,7-dichloroquinoline and C-19 acetate of 14 lower cytotoxicity while the C-14 8-quinolyloxy and 3,19-diol pattern of 15 delivers maximal antiviral efficacy, jointly defining the andrographolide–quinoline platform for next-generation flavivirus inhibitors.
Anti-Chikungunya Virus Activity
Chikungunya is a mosquito-borne viral illness primarily spread by Aedes species mosquitoes. Initially identified in Tanzania in 1953, the causative agent—Chikungunya virus—belongs to the Alphavirus genus of the Togaviridae family. Typical clinical manifestations of the disease include an acute febrile phase accompanied by skin rash and severe joint pain, which often leads to significant immobility [63].
Against chikungunya virus, andrographolide derivatives exhibit antiviral activity by targeting viral enzymes and proteins that are essential for viral replication and assembly. The C-15 imine group and C-19 sulfonate group of these derivatives are crucial for their antiviral effects, as they can form covalent bonds with viral targets, thereby disrupting the virus life cycle.
Tran et al. [64] designed and synthesized compounds 16 (see Figure 4 for representative antiviral derivatives), EC50 = 0.14 μM, SI = 714, and compounds 17, EC50 = 0.002 μM, SI = 12,715, as potent anti-CHIKV agents; both compounds effected ≥3.7-log viral reduction in HeLa CCL2 cells at 10–80 μM, maintained CC50 > 100 μM, and exhibited prophylactic as well as therapeutic efficacy against clinical CHIKV-122508 and CHIKV-6708 isolates, validating the oxindole-labdane hybrids as next-generation andrographolide-derived antivirals.
Anti-Enterovirus Virus Activity
Enterovirus 71 (EV71) was first isolated in California, USA, in 1969. This virus is closely related to poliovirus, with its specific receptors present on leukocytes, respiratory and gastrointestinal cells, and dendritic cells [65].
In the context of enteroviruses, andrographolide derivatives have been shown to inhibit the viral RdRp and 3CLpro, which are essential for viral RNA replication and polyprotein processing. The α, β-unsaturated lactone group of andrographolide derivatives is particularly effective in covalently binding to these viral targets, thereby inactivating them and reducing viral replication.
Kun Dai et al. [66]. synthesized the 14-aryloxy-8,17-epoxy andrographolide compound 18 (see Figure 4 for representative antiviral derivatives), which displayed potent anti-EV-A71 activity (IC50 = 0.95 μM, SI = 12.3). Compound 18 specifically inhibited post-entry viral RNA replication, significantly reduced VP0 and VP2 protein expression in RD cells, and exhibited broad-spectrum efficacy against CV-A16 (IC50 = 0.46 μM), CV-A6 (IC50 = 1.12 μM) and EV-D68 (IC50 = 1.59 μM); repeated passaging failed to yield resistant mutants, indicating host-factor targeting.
Jie Kai Tan et al. [67] prepared compound 19 (see Figure 4 for representative antiviral derivatives), bearing 14-quinolinoxy and 8,9-olefin modifications, that potently suppressed EV-A71 infection (IC50 = 2.06 μM, SI = 14.4). Compound 19 acted between 4–6 hpi to block viral RNA replication without affecting IRES-mediated translation, leading to marked loss of VP2 and 35-fold reduction in VP0; it also inhibited CV-A16, CV-A6, Echo7, CV-B5, CV-A24 and EV-D68, and no resistant mutants emerged after 21 passages, consistent with host-directed antiviral action.
3.1.2. DNA Viruses
Anti-Hepatitis B Virus Activity
Since its identification in 1966, the hepatitis B virus (HBV) has been recognized as a major global health challenge, with over 350 million individuals affected. Chronic HBV infection is a primary etiological factor for serious liver conditions, including persistent hepatitis, liver cirrhosis, and hepatocellular carcinoma, contributing to approximately one million deaths each year [68].
Andrographolide derivatives have demonstrated the ability to inhibit the RdRp of HBV, which is crucial for the replication of the viral DNA. By targeting this enzyme, these compounds can effectively reduce the production of viral DNA, thereby limiting the spread of the virus within the host. Additionally, andrographolide derivatives may interfere with the virus–host protein interactions that are necessary for the virus to establish a persistent infection.
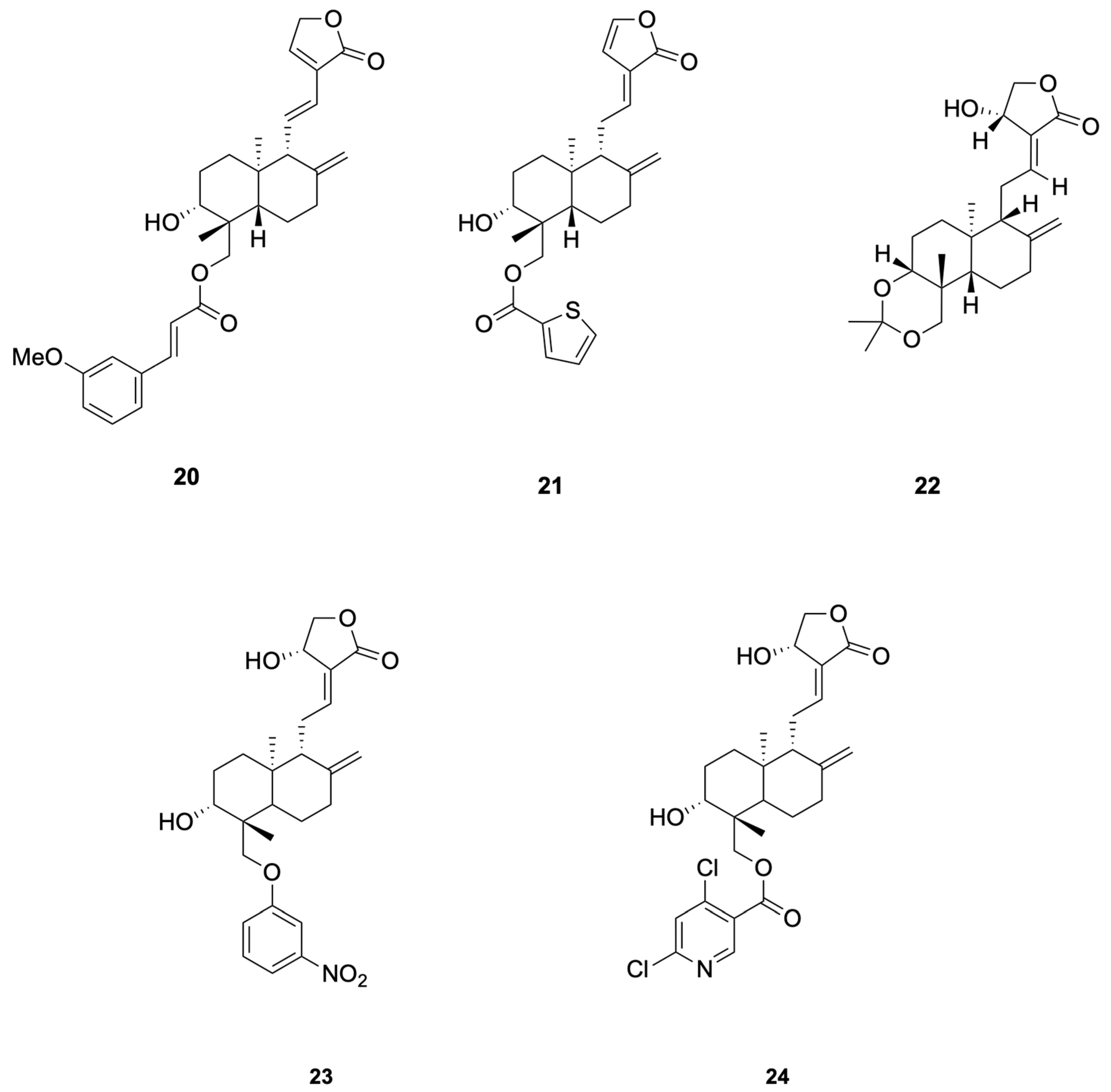
Chen et al. [69] synthesized 48 dehydroandrographolide and andrographolide derivatives and identified compound 20 as the most potent inhibitor of HBV DNA replication (IC50 = 10.3 μM, SI > 165.1), while compound 21 simultaneously suppressed HBsAg, HBeAg secretion and DNA replication (SI = 20.3–104.9) (see Figure 5 for representative antiviral derivatives), corroborating the hypothesis that andrographolide scaffolds can interfere with HBV RdRp and viral–host protein interactions essential for persistent infection.
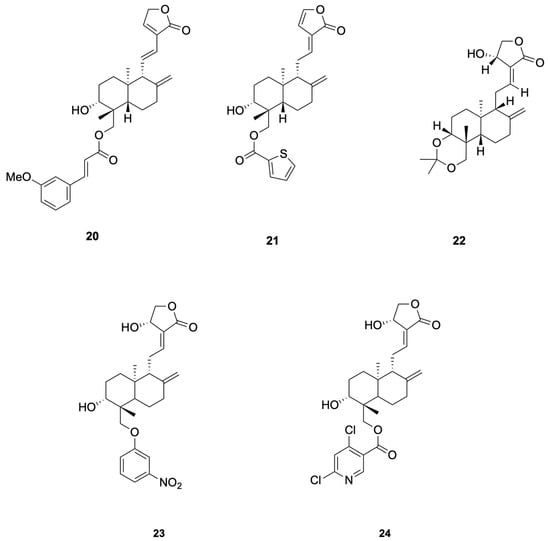
Figure 5.
Compounds 20–24.
Anti-Herpes Simplex Virus Activity
Herpes simplex virus (HSV), a pathogen belonging to the Herpesviridae family, has been documented as early as ancient Greece. It commonly infects humans and can lead to diverse clinical outcomes, ranging from mild, self-limiting skin and mucous membrane lesions to severe, life-threatening conditions [70].
For herpes simplex viruses, andrographolide derivatives have been found to inhibit the viral RdRp and Mpro, similar to their action against other DNA viruses. These interactions prevent the virus from efficiently replicating its DNA genome and processing its polyproteins. The C-15 imine group and C-19 sulfonate group of andrographolide derivatives are particularly effective in covalently binding to viral targets, thereby inactivating them and reducing viral replication.
Priengprom et al. [71] synthesized Compound 22 and evaluated its anti-HSV activity (see Figure 5 for representative antiviral derivatives). Compound 22 suppressed HSV-1/HSV-2 cytopathic effects in Vero cells at non-cytotoxic concentrations (~20.5 μM) and lowered viral DNA and late proteins (e.g., ICP8/gD); time-of-addition/stage assays indicated a post-entry mechanism. Potency is in the low-to-single-digit tens micromolar range; standardized EC50/CC50/SI reporting and in vivo confirmation are still needed.
3.1.3. Retroviruses
Anti-Human Immunodeficiency Virus Activity
The acquired immunodeficiency syndrome (AIDS) was initially documented in the summer of 1981. The causative agent, human immunodeficiency virus (HIV), was identified two years after the initial reports and subsequently confirmed in 1984 as the definitive etiology of AIDS [72].
Andrographolide derivatives have shown promise in inhibiting the RdRp and Mpro of HIV-1, which are essential for viral RNA replication and polyprotein processing. By targeting these enzymes, these compounds can effectively reduce the production of viral RNA and proteins, thereby limiting the spread of the virus within the host. Additionally, andrographolide derivatives may interfere with the virus–host protein interactions that are necessary for the virus to establish a persistent infection.
Uttekar et al. [73] synthesized a series of andrographolide derivatives and evaluated their anti-HIV activity. Among them, the 3-nitrobenzylidene derivative 23 exhibited higher in vitro anti-HIV activity (IC50 = 0.51 μM) compared to andrographolide (IC50 = 0.59 μM), while the 2,6-dichloro-nicotinoyl ester derivative 24 showed a higher Therapeutic Index (TI = 12,474) due to its lower cytotoxicity (see Figure 5 for representative antiviral derivatives). These derivatives inhibited gp120-mediated cell fusion, suggesting they interfere with early events in HIV entry by interacting with the V3 loop of gp120. Molecular docking studies revealed that these compounds bind to the V3 loop region of HIV-1 envelope protein, gp120, which is crucial for viral entry.
3.2. Antibacterial Activity
3.2.1. Against Gram-Positive Bacteria
Staphylococcus aureus (MRSA)
Staphylococcus aureus is a pathogenic bacterium capable of inducing a wide spectrum of diseases, which may arise from either pyogenic mechanisms or toxin-mediated pathways. It is also a common causative agent in serious systemic and localized infections, including septicemia, infective endocarditis, pneumonia, as well as infections affecting the eyes and the central nervous system [74].
Andrographolide derivatives exhibit antibacterial activity against MRSA by increasing the production of reactive oxygen species (ROS) within the bacterial cells. This increase in ROS can lead to oxidative stress and damage to bacterial cellular components, ultimately resulting in cell death. Additionally, the membrane potential of MRSA cells is disrupted by andrographolide derivatives, which further contributes to their antibacterial effects. The C-14 ester chain length of andrographolide derivatives plays a significant role in determining their antibacterial potency against MRSA, with optimal chain lengths enhancing the interaction with the bacterial cell membrane.
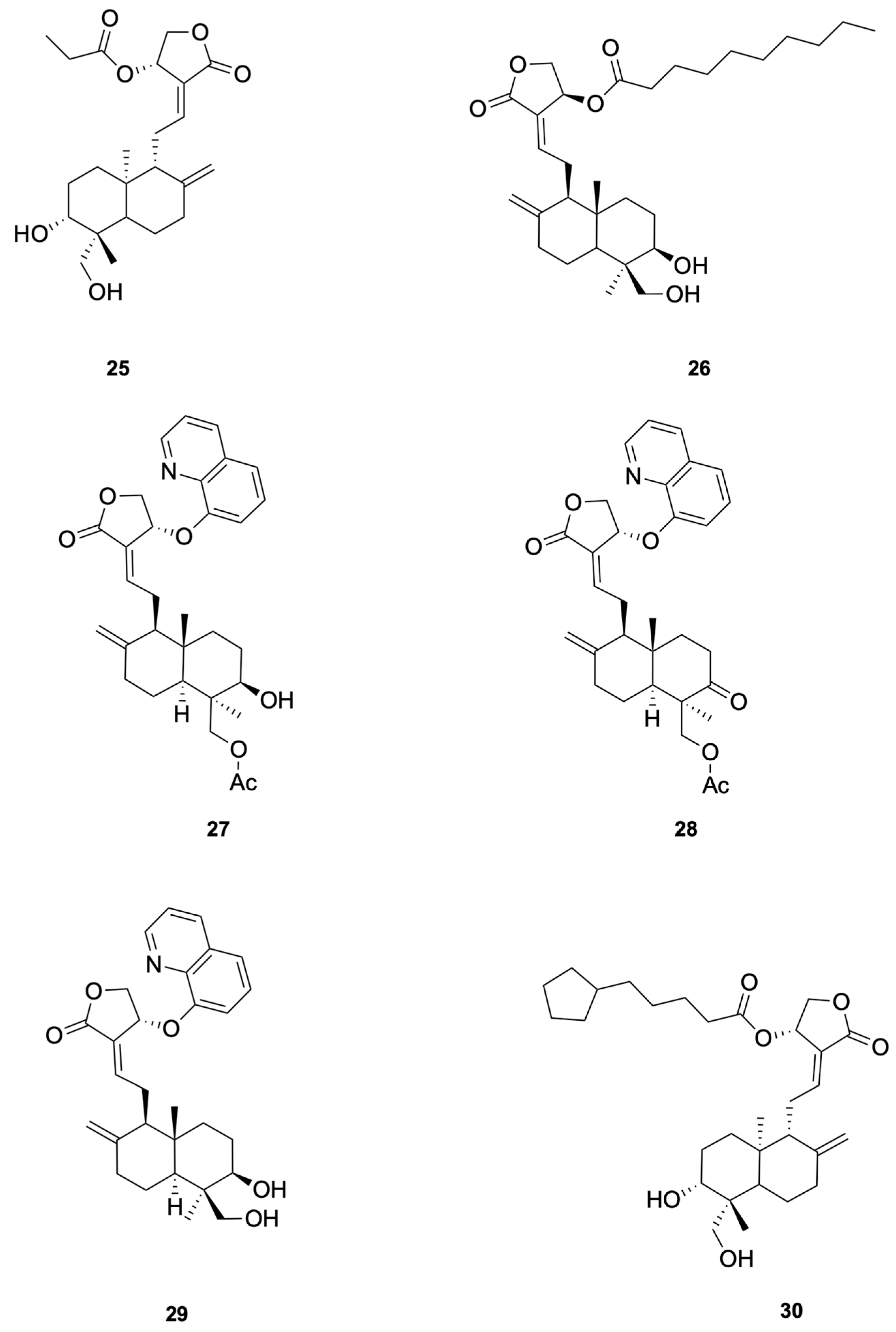
Patil et al. [75] synthesized a series of andrographolide derivatives using Amano lipase AK (Pseudomonas fluorescens) as a biocatalyst to achieve stereoselective esterification, preferentially forming the propionyl ester of the S-isomer. Among the synthesized derivatives, compound 25 and compound 26 exhibited antimicrobial activity against Staphylococcus aureus with low minimal inhibitory concentration (MIC) values of 4 mg/mL and 16 mg/mL, respectively (see Figure 6 for representative antiviral derivatives). These derivatives also showed low hemolysis activity at their respective MICs and increased the permeability of the bacterial cell membrane, as demonstrated by FITC uptake and SEM imaging studies.
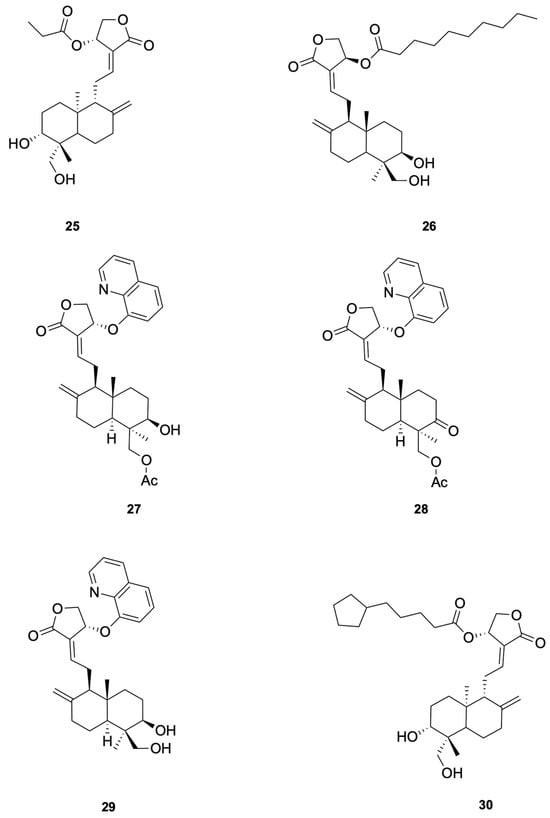
Figure 6.
Compounds 25–30.
Hemolysis was evaluated at the MIC or multiples thereof; any hemolysis at MIC raises a toxicity concern for lead selection.
Enterococcus faecalis
Enterococcus faecalis, while naturally occurring as a Gram-positive symbiont in the intestinal flora of numerous species, has become a prominent cause of healthcare-associated infections. Its current status as a multidrug-resistant pathogen is a direct consequence of selective pressures from antibiotic use [76].
For Enterococcus faecalis, Feng et al. [77] synthesized a series of 14-aryloxy andrographolide derivatives. These derivatives were found to exhibit significant antibacterial activity against E. faecalis. The andrographolide skeleton was identified as a key structural feature for selectivity against E. faecalis, with the 14-aryloxy group, particularly the 14-(8-quinolinyloxy) group, emerging as a crucial pharmacophore. For example, compounds 27 and 28 demonstrated good selective inhibitory activity against E. faecalis (see Figure 6 for representative antiviral derivatives). These findings highlight the potential of andrographolide derivatives with specific structural modifications to serve as novel antibacterial agents against E. faecalis.
In our assays, this molecule was active mainly against Gram-positive bacteria and inactive/limited against the Gram-negative panel at the tested concentrations. However, related andrographolide derivatives bearing C-14 aryloxy substitution have shown Gram-negative activity (e.g., E. coli), with potency tracking with the C-14 ester/aryloxy chain length [77]. Thus, the current molecule appears narrow under our test conditions, whereas the andrographolide scaffold is substitution-dependent and tunable rather than universally narrow.
3.2.2. Against Gram-Negative Bacteria
Escherichia coli
Escherichia coli (E. coli) commonly colonizes the human intestine as a nonpathogenic, facultative anaerobic commensal. Despite this, certain strains have acquired virulence factors that enable them to induce infections—including those affecting the gastrointestinal and urinary tracts, as well as the central nervous system—even in immunocompetent individuals [78].
To study the antibacterial activity against Escherichia coli, Feng et al. [77] synthesized a series of andrographolide derivatives. These derivatives were found to exhibit antibacterial activity against E. coli by increasing ROS production and disrupting membrane potential. These effects lead to oxidative stress and damage to bacterial cellular components, ultimately resulting in cell death. The C-14 ester chain length appears to correlate with antibacterial potency, although additional matched-pair studies are needed to confirm causality. For example, compound 29 (see Figure 6 for representative antiviral derivatives), which has an optimal C-14 ester chain length, demonstrated significant inhibitory activity against E. coli. These findings highlight the potential of andrographolide derivatives with specific structural modifications to serve as novel antibacterial agents against E. coli.
Pseudomonas aeruginosa
Pseudomonas aeruginosa is a bacterium that is widespread in the environment and can cause opportunistic infections in humans. Its diverse metabolic pathways and regulatory genes give it remarkable adaptability to different growth conditions. The bacterium’s nutritional flexibility, diverse virulence factors, and high antibiotic resistance make it extremely difficult to eradicate in infected hosts, especially in lung infections affecting patients with cystic fibrosis [79].
To study the anti-Pseudomonas aeruginosa activity, Wang et al. [80] designed and synthesized a series of andrographolide derivatives that inhibit bacterial quorum sensing (QS) and the production of virulence factors. These derivatives do not directly inhibit bacterial growth but interfere with QS signaling pathways, which are crucial for the regulation of virulence factors such as pyocyanin and protease. The study found that compound 30 (see Figure 6 for representative antiviral derivatives), a previously synthesized derivative, significantly inhibited the production of both pyocyanin and protease, suggesting that these compounds can disrupt QS-mediated virulence without directly killing the bacteria. The results highlight the potential of andrographolide derivatives as novel antibacterial agents that target QS pathways in P. aeruginosa.
3.3. Antifungal Activity
Derivatives caused a measurable increase in mitochondrial ROS (e.g., DCFH-DA assays) and modulated cell-wall integrity/MAPK signaling, consistent with antifungal activity. These derivatives primarily exert their antifungal effects through the induction of mitochondrial ROS production, which causes oxidative stress and damages fungal cell components. They also inhibit the cell wall integrity (CWI) pathway regulated by the MAPK signaling cascade, weakening the fungal cell wall. Additionally, the C-19 aryl ether substitution enhances their interaction with ergosterol in the fungal cell membrane, further disrupting membrane integrity. These mechanisms collectively contribute to the antifungal efficacy of andrographolide derivatives.
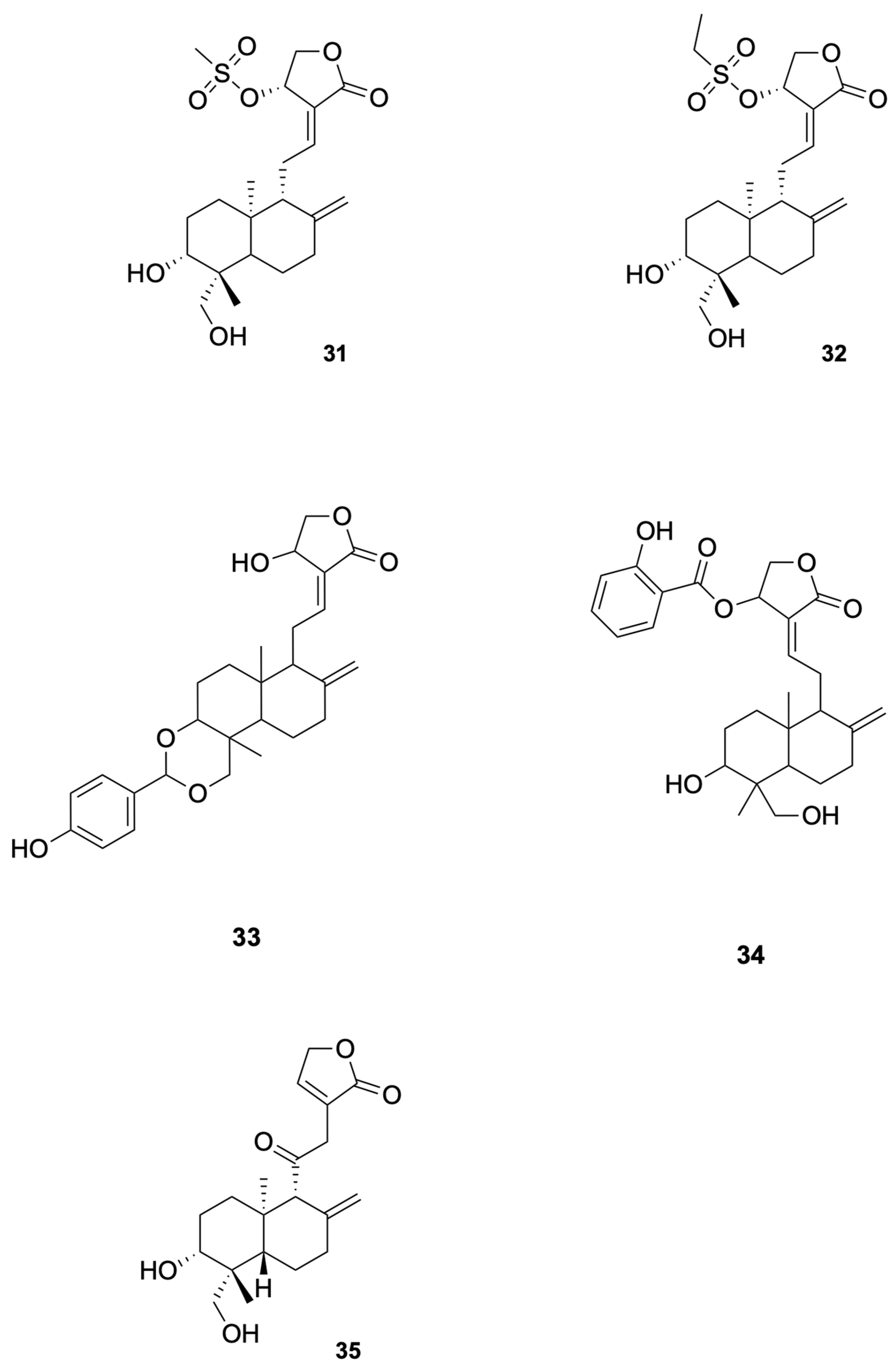
Agarwal et al. [81] synthesized a series of C (14)-sulfonylester-type andrographolide derivatives and evaluated their antimicrobial activity against various bacterial and fungal strains. Among these derivatives, the methyl sulfonyl derivative compound 31 and ethyl sulfonyl derivative compound 32 exhibited significant inhibitory activity against fungal strains (see Figure 7 for representative antiviral derivatives), demonstrating the potential of structural modifications to improve antifungal efficacy. These results suggest that the introduction of sulfonylester groups at the C-14 position can enhance the antifungal activity of andrographolide derivatives.
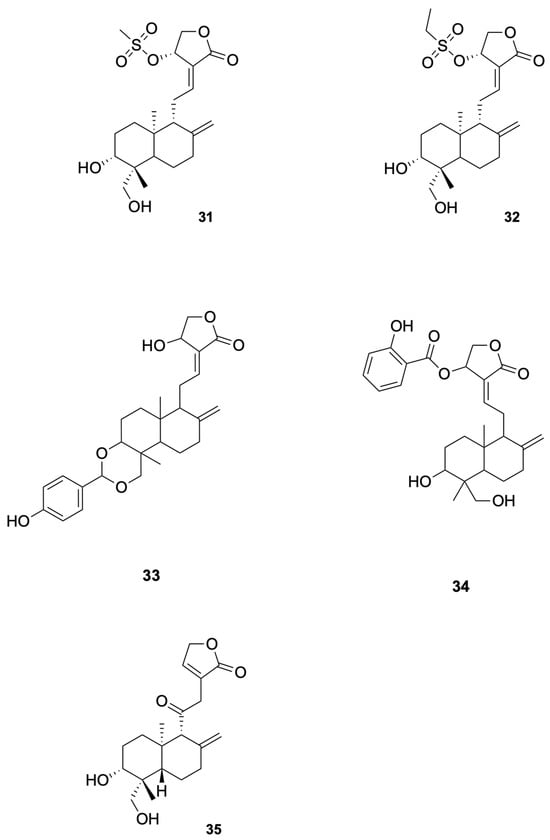
Figure 7.
Compounds 31–35.
3.4. Antiparasitic Activity
3.4.1. Plasmodium spp. (Malaria)
Malaria, a life-threatening infectious disease with significant global impact on public health, is triggered by protozoan parasites from the Plasmodium genus. The transmission and propagation of this disease rely on an intricate life cycle that involves both mosquito vectors and vertebrate hosts [82]. Artemisinin-based combination therapies (ACTs) are the current frontline treatment for P. falciparum malaria. They combine a fast-acting artemisinin derivative with a partner drug [83]. Resistance has emerged especially in Southeast Asia, and cases are being increasingly reported in Africa. Resistance doesn’t always mean full failure of therapy, but often partial resistance manifested by delayed parasite clearance or persistence of parasites after certain time points in treatment [84].
Andrographolide derivatives have shown antiparasitic activity against Plasmodium spp., which are the causative agents of malaria. These derivatives can inhibit the mitochondrial electron transport chain of the parasite, thereby disrupt its energy metabolism and leading to parasite death. Additionally, andrographolide derivatives can inhibit the activity of topoisomerase II (TOPO II), which is essential for the replication of the parasite’s DNA. The C-15 long-chain alkyl substitution of andrographolide derivatives enhances their hydrophobicity, allowing them to more effectively insert into the parasite’s membrane and exert their antiparasitic effects [85,86,87].
Megantara et al. [88] reported that andrographolide derivatives, namely, compound 33 and compound 34, exhibited high binding affinity with plasmepsin I, II, and IV (see Figure 7 for representative antiviral derivatives), which are key enzymes involved in the initial cleavage of hemoglobin in Plasmodium spp. The binding affinity values for compound 34 were −8.80 kcal/mol (plasmepsin I), −8.80 kcal/mol (plasmepsin II), and −8.30 kcal/mol (plasmepsin IV), indicating strong interactions with these enzymes. These results suggest that compound 33 and compound 34 have the potential to be developed into new antimalarial drugs. Docking/MD binding energies are hypothesis-generating and require confirmation by biochemical and cellular assays.
3.4.2. Leishmania spp. (Leishmaniasis)
Leishmaniasis is a parasitic infection resulting from the transmission of intracellular Leishmania parasites through sand fly bites. The clinical presentation of the disease varies significantly based on the infecting species and the host’s immune status, spanning from self-limiting cutaneous lesions to systemic visceral involvement that can be life-threatening [89].
In the case of Leishmania spp., andrographolide derivatives exhibit antiparasitic activity by inhibiting the mitochondrial electron transport chain of the parasite. This inhibition disrupts the parasite’s energy metabolism, leading to its death. Additionally, andrographolide derivatives can inhibit topoisomerase II (TOPO II), which is essential for the replication of the parasite’s DNA. The C-15 long-chain alkyl substitution of andrographolide derivatives enhances their hydrophobicity, allowing them to more effectively insert into the parasite’s membrane and exert their antiparasitic effects. For example, studies have shown that andrographolide derivatives with C-15 alkyl chains significantly reduce the viability of Leishmania parasites by disrupting their mitochondrial function and inhibiting DNA replication. These mechanisms collectively contribute to the antiparasitic efficacy of andrographolide derivatives against Leishmania spp. [90,91].
Lala et al. [92] reported that the andrographolide derivative compound 35 showed significant antileishmanial activity (see Figure 7 for representative antiviral derivatives). In their study, the free form of the drug reduced the spleen parasite load by 39%, while the drug incorporated in liposomes, niosomes, and microspheres reduced the parasite load by 78%, 91%, and 59%, respectively. The highest efficacy was observed with niosomes, which reduced the parasite load by 91%. These results suggest that compound 35 has the potential to be developed into a new antileishmanial drug with enhanced efficacy and reduced toxicity.
The representative antiviral, antibacterial, antifungal, and antiparasitic data of andrographolide derivatives discussed in Section 3.1, Section 3.2, Section 3.3 and Section 3.4 are consolidated in Table S1 (see in Supplementary Materials).
4. Pharmacokinetics & Formulation Challenges
4.1. Oral Bioavailability: First-Pass Effect & β-Glucuronidation
Following oral administration, andrographolide exhibits low systemic exposure owing to extensive first-pass metabolism and limited solubility–permeability. [93,94,95]. In the intestine and liver, phase II conjugation (including β-glucuronidation) and oxidative biotransformation have been reported; in vitro interaction data indicate liabilities with UGT/CYP pathways that can further constrain exposure [95,96,97,98,99]. Microbiota-related transformations have also been observed in specific studies but may not be generalizable across settings [100,101]. Consequently, short half-life and relatively high clearance are frequently noted in preclinical models [93,94,95]. For oral dosing, the low observed toxicity may reflect limited systemic exposure due to poor PK (e.g., low solubility/permeability and first-pass metabolism); definitive safety assessment requires PK measurements (Cmax/AUC) at efficacious exposures.
4.2. Enabling Delivery to Enhance Exposure and Tissue Targeting
Liposomes. Phospholipid liposomes can encapsulate hydrophilic and lipophilic forms of andrographolide, stabilize the payload and prolong circulation, thereby increasing apparent exposure in vivo [102,103,104,105].
PLGA nanoparticles. Biodegradable PLGA-based systems (e.g., emulsion–solvent evaporation, nanoprecipitation) enable high loading, controlled release, and improved tissue distribution, increasing drug availability at infected sites in preclinical models [106,107,108,109,110].
β-Cyclodextrin inclusion. β-Cyclodextrin (β-CD) forms inclusion complexes with andrographolide that raise aqueous solubility and improve thermal stability [104].
Translation caveat. Andrographolide derivatives represent a versatile class of molecules with broad anti-infective potential and well-defined structure–activity relationships; biodistribution, hemocompatibility/hemolysis, complement activation, RES uptake, sterility/endotoxin, and dose proportionality should be characterized prior to translation [102,103,104,105,106,107,108,109,110,111].
4.3. Parenteral and Alternative Routes
Intravenous/intraperitoneal administration can circumvent first-pass metabolism and achieve exposures unattainable by oral dosing; formulation choice materially affects CL, V, and t½ [87,98]. Inhaled/intranasal delivery is conceptually attractive for respiratory infections (local lung exposure) but requires dedicated pulmonary safety and device/drug compatibility assessments before clinical application [95,96,97,98].
4.4. Exposure–Response and On-Target Engagement
To relate in vitro potency (EC50/IC50 in μM) to in vivo efficacy, studies should quantify free (unbound) plasma/tissue exposure, target engagement, and, where appropriate, biomarkers of pathway modulation. Minimal PK information includes IV/PO crossover, CL, V, t½, plasma protein binding (fu), metabolite identification (UGT/SULT/CYP), transporter liability, and tissue distribution in the relevant infection model [86,87,88,89,90,91]. Exposure-aware study design is essential to avoid over-interpreting biochemical/cellular potency.
4.5. Clinical and Regulatory Context
Andrographolide-containing preparations (e.g., andrographolide sulfonate/Xiyanping injection) are used clinically in some regions as herbal-derived products; this does not constitute regulatory approval of the purified parent compound as a stand-alone drug [33,40].
5. Conclusions
Andrographolide derivatives exhibit broad anti-infective potential with tractable structure–activity relationships (e.g., C-14/C-19 modifications and hybrid scaffolds). However, solubility and oral pharmacokinetics remain limited. Future efforts should focus on exposure-aware lead optimization, validation of delivery systems to achieve pharmacologically relevant concentrations, and bridging biochemical potency to in vivo efficacy.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules30214273/s1, Table S1: Anti-infective activities of andrographolide derivatives (Compounds 4–35).
Author Contributions
Conceptualization, Z.R. and P.C.; methodology, Z.R. and P.C.; software, Z.C.; validation, Z.R., Z.C. and Y.X.; writing—original draft preparation, Z.R.; writing—review and editing, Z.R. and P.C.; visualization, Z.R., Z.C. and Y.X.; supervision, P.C.; project administration, P.C.; funding acquisition, P.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by FDCT grants from Macau Science and Technology University to P.C. (Project Code: 0005-2023-RIA1).
Data Availability Statement
The original contributions presented in this study are included in the article and Supplementary Materials. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| ARDS | Acute Respiratory Distress Syndrome |
| ACE2 | Angiotensin-Converting Enzyme 2 |
| Mpro | Main Protease |
| RdRp | RNA-dependent RNA Polymerase |
| HSPA1A | Heat Shock Protein A1A |
| PGK1 | Phosphoglycerate Kinase 1 |
| 3CLpro | 3C-like Protease |
| VP0 | Viral Protein 0 |
| VP2 | Viral Protein 2 |
| IRES | Internal Ribosome Entry Site |
| HBsAg | Hepatitis B Surface Antigen |
| HBeAg | Hepatitis B e Antigen |
| gp120 | Glycoprotein 120 |
| FITC | Fluorescein Isothiocyanate |
| SEM | Scanning Electron Microscopy |
| QS | Quorum Sensing |
| CWI | Cell Wall Integrity |
| TOPO II | Topoisomerase II |
| UGT | UDP-Glucuronosyltransferase |
| PLGA | Poly(Lactic-co-Glycolic Acid) |
| PEG | Polyethylene Glycol |
| DTA | Differential Thermal Analysis |
| TGA | Thermogravimetric Analysis |
References
- Chao, W.-W.; Lin, B.-F. Isolation and identification of bioactive compounds in Andrographis paniculata (Chuanxinlian). Chin. Med. 2010, 5, 17. [Google Scholar] [CrossRef]
- Sheeja, K.; Shihab, P.; Kuttan, G. Antioxidant and anti-inflammatory activities of the plant Andrographis paniculata Nees. Immunopharmacol. Immunotoxicol. 2006, 28, 129–140. [Google Scholar] [CrossRef]
- Hossain, S.; Urbi, Z.; Karuniawati, H.; Mohiuddin, R.B.; Qrimida, A.M.; Allzrag, A.M.M.; Ming, L.C.; Pagano, E.; Capasso, R. Andrographis paniculata (burm. F.) wall. Ex nees: An updated review of phytochemistry, antimicrobial pharmacology, and clinical safety and efficacy. Life 2021, 11, 348. [Google Scholar] [CrossRef]
- Chao, W.-W.; Kuo, Y.-H.; Lin, B.-F. Anti-inflammatory activity of new compounds from Andrographis paniculata by NF-κB transactivation inhibition. J. Agric. Food Chem. 2010, 58, 2505–2512. [Google Scholar] [CrossRef] [PubMed]
- Harikrishnan, A.; Veena, V.; Kancharla, R.; Chavan, S.; Rajabathar, J.R.; Al-Lohedan, H.; Pandiaraj, S.; Karuppiah, P.; Arokiyaraj, S. Anti-breast cancer activity of bioactive metabolites from Andrographis paniculata through inhibition of PI3K activity in triple negative breast cancer (MDA-MB-231) cells. J. Mol. Struct. 2023, 1294, 136506. [Google Scholar] [CrossRef]
- Deshmukh, R.; Jain, A.K.; Singh, R.; Das Paul, S.; Harwansh, R.K. Andrographis paniculata and andrographolide-a snapshot on recent advances in nano drug delivery systems against cancer. Curr. Drug Deliv. 2024, 21, 631–644. [Google Scholar] [CrossRef]
- Subarmaniam, T.; Rusli, R.N.M.; Perumal, K.V.; Yong, Y.K.; Hadizah, S.; Othman, F.; Salem, K.; Shafie, N.H.; Hasham, R.; Yin, K.B.; et al. The potential chemopreventive effect of Andrographis paniculata on 1, 2-dimethylhydrazine and high-fat-diet-induced colorectal cancer in sprague dawley rats. Int. J. Mol. Sci. 2023, 24, 5224. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-C.; Lii, C.-K.; Lin, Y.-H.; Shie, P.-H.; Yang, Y.-C.; Huang, C.-S.; Chen, H.-W. Andrographis paniculata improves insulin resistance in high-fat diet-induced obese mice and TNFα-treated 3T3-L1 adipocytes. Am. J. Chin. Med. 2020, 48, 1073–1090. [Google Scholar] [CrossRef]
- Islam, M.T.; Ali, E.S.; Mubarak, M.S. Anti-obesity effect of plant diterpenes and their derivatives: A review. Phytother. Res. 2020, 34, 1216–1225. [Google Scholar] [CrossRef]
- Akhtar, M.T.; Sarib, M.S.B.M.; Ismail, I.S.; Abas, F.; Ismail, A.; Lajis, N.H.; Shaari, K. Anti-diabetic activity and metabolic changes induced by Andrographis paniculata plant extract in obese diabetic rats. Molecules 2016, 21, 1026. [Google Scholar] [CrossRef]
- Komalasari, T.; Harimurti, S. A Review on the Anti-Diabetic Activity of Andrographis paniculata (Burm. f.) Nees Based In-Vivo Study. Int. J. Public Health Sci. 2015, 4, 256–263. [Google Scholar] [CrossRef]
- Jadhav, A.K.; Karuppayil, S.M. Andrographis paniculata (Burm. F) Wall ex Nees: Antiviral properties. Phytother. Res. 2021, 35, 5365–5373. [Google Scholar]
- Berteina-Raboin, S. Comprehensive overview of antibacterial drugs and natural antibacterial compounds found in food plants. Antibiotics 2025, 14, 185. [Google Scholar] [CrossRef] [PubMed]
- Apsari, P.I.B.; Suryana, I.K.; Satriyasa, B.K.; Laksmi, D.A.A.S.; Wati, D.K.; Wirasuta, I.M.A.G. Towards Andrographolide as Antimalarial: A Systematic Review. Biomed. Pharmacol. J. 2025, 18, 499–515. [Google Scholar] [CrossRef]
- Amanda, L.P.; Azhari, H.M.; Ferdi, R.; Iqbal, I.; Andhini, P.; Azzya, P.A.; Permata, S.I. A Scoping Review pharmacology of Andrographis paniculata. Indones. J. Health Pharm. 2025, 1, 33–45. [Google Scholar]
- Dai, Y.; Chen, S.-R.; Chai, L.; Zhao, J.; Wang, Y.; Wang, Y. Overview of pharmacological activities of Andrographis paniculata and its major compound andrographolide. Crit. Rev. Food Sci. Nutr. 2019, 59 (Suppl. S1), S17–S29. [Google Scholar] [CrossRef]
- Cai, Q.; Zhang, W.; Sun, Y.; Xu, L.; Wang, M.; Wang, X.; Wang, S.; Ni, Z. Study on the mechanism of andrographolide activation. Front. Neurosci. 2022, 16, 977376. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yuan, W.; Wu, J.; Zhen, J.; Sun, Q.; Yu, M. Andrographolide, a natural anti-inflammatory agent: An Update. Front. Pharmacol. 2022, 13, 920435. [Google Scholar] [CrossRef] [PubMed]
- Mussard, E.; Cesaro, A.; Lespessailles, E.; Legrain, B.; Berteina-Raboin, S.; Toumi, H. Andrographolide, a natural antioxidant: An update. Antioxidants 2019, 8, 571. [Google Scholar] [CrossRef]
- Shan, X.; Li, S.; Liu, J.; Wang, C.; Wei, Y.; Shi, J.; Zhang, X.; Gu, L. The Therapeutic Potential of Andrographolide and Its Derivatives in Inflammatory Diseases. Pharmacol. Res. Perspect. 2025, 13, e70161. [Google Scholar] [CrossRef]
- Yang, C.-H.; Yen, T.-L.; Hsu, C.-Y.; Thomas, P.-A.; Sheu, J.-R.; Jayakumar, T. Multi-targeting andrographolide, a novel NF-κB inhibitor, as a potential therapeutic agent for stroke. Int. J. Mol. Sci. 2017, 18, 1638. [Google Scholar] [CrossRef] [PubMed]
- Lee, C. Therapeutic modulation of virus-induced oxidative stress via the Nrf2-dependent antioxidative pathway. Oxidative Med. Cell Longev. 2018, 2018, 6208067. [Google Scholar] [CrossRef]
- Shakib, P.; Kalani, H.; Ho, J.; Dolatshah, M.; Amiri, S.; Cheraghipour, K. A Systematic Review on Curcumin and Anti-Plasmodium berghei Effects. Curr. Drug Discov. Technol. 2022, 19, 67–72. [Google Scholar]
- Rahul, M.; Boini, T.; Lakshminarayana, M.; Radhakrishnan, T.; Sudayadas, R.K. Approaches to improve solubility, stability and the clinical potential of andrographolide: A review. J. Young Pharm. 2022, 14, 15. [Google Scholar] [CrossRef]
- Zeng, B.; Wei, A.; Zhou, Q.; Yuan, M.; Lei, K.; Liu, Y.; Song, J.; Guo, L.; Ye, Q. Andrographolide: A review of its pharmacology, pharmacokinetics, toxicity and clinical trials and pharmaceutical researches. Phytother. Res. 2022, 36, 336–364. [Google Scholar] [CrossRef]
- Wang, M.; Cao, R.; Zhang, L.; Yang, X.; Liu, J.; Xu, M.; Shi, Z.; Hu, Z.; Zhong, W.; Xiao, G. Remdesivir and Chloroquine Effectively Inhibit the Recently Emerged Novel Coronavirus (2019-nCoV) In Vitro. Cell Res. 2020, 30, 269–271. [Google Scholar] [CrossRef]
- Wu, C.; Liu, Y.; Yang, Y.; Zhang, P.; Zhong, W.; Wang, Y.; Wang, Q.; Xu, Y.; Li, M.; Li, X.; et al. Analysis of Therapeutic Targets for SARS-CoV-2 and Discovery of Potential Drugs by Computational Methods. Acta Pharm. Sin. B 2020, 10, 766–788. [Google Scholar] [CrossRef] [PubMed]
- EUCAST. Breakpoint Tables for Interpretation of MICs and Zone Diameters, Version 14.0. 2024. Available online: https://www.eucast.org/clinical_breakpoints (accessed on 17 October 2025).
- CLSI. Performance Standards for Antimicrobial Susceptibility Testing, 32nd ed.; CLSI Supplement M100; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2022; Available online: https://clsi.org/shop/standards/m100/ (accessed on 17 October 2025).
- CDC. Candida auris: Antifungal Susceptibility Testing. 2024. Available online: https://www.cdc.gov/candida-auris/hcp/laboratories/antifungal-susceptibility-testing.html (accessed on 17 October 2025).
- Fairhurst, R.M.; Dondorp, A.M. Artemisinin-Resistant Plasmodium falciparum Malaria. Microbiol. Spectr. 2016, 4, 409–429. [Google Scholar] [CrossRef] [PubMed]
- Purkait, B.; Kumar, A.; Nandi, N.; Sardar, A.H.; Das, S.; Kumar, S.; Pandey, K.; Ravidas, V.; Kumar, M.; De, T.; et al. Mechanism of Amphotericin B Resistance in Clinical Isolates of Leishmania donovani. Antimicrob. Agents Chemother. 2012, 56, 1031–1041. [Google Scholar] [CrossRef]
- Lim, J.C.W.; Chan, T.K.; Ng, D.S.; Sagineedu, S.R.; Stanslas, J.; Wong, W.F. Andrographolide and its analogues: Versatile bioactive molecules for combating inflammation and cancer. Clin. Exp. Pharmacol. Physiol. 2012, 39, 300–310. [Google Scholar] [CrossRef]
- Ren, X.; Xu, W.; Sun, J.; Dong, B.; Awala, H.; Wang, L. Current trends on repurposing and pharmacological enhancement of andrographolide. Curr. Med. Chem. 2021, 28, 2346–2368. [Google Scholar] [CrossRef]
- Intharuksa, A.; Arunotayanun, W.; Yooin, W.; Sirisa-Ard, P. A comprehensive review of Andrographis paniculata (Burm. f.) Nees and its constituents as potential lead compounds for COVID-19 drug discovery. Molecules 2022, 27, 4479. [Google Scholar] [CrossRef]
- Aromdee, C. Modifications of andrographolide to increase some biological activities: A patent review (2006–2011). Expert Opin. Ther. Pat. 2012, 22, 169–180. [Google Scholar] [CrossRef]
- Lim, X.Y.; Chan, J.S.W.; Tan, T.Y.C.; Teh, B.P.; Razak, M.R.M.A.; Mohamad, S.; Mohamed, A.F.S. Andrographis paniculata (Burm. F.) wall. ex nees, andrographolide, and andrographolide analogues as SARS-CoV-2 antivirals? A rapid review. Nat. Prod. Commun. 2021, 16, 1934578X211016610. [Google Scholar] [CrossRef]
- He, Z.; Yuan, J.; Zhang, Y.; Li, R.; Mo, M.; Wang, Y.; Ti, H. Recent advances towards natural plants as potential inhibitors of SARS-Cov-2 targets. Pharm. Biol. 2023, 61, 1186–1210. [Google Scholar] [CrossRef]
- Aromdee, C. Andrographolide: Progression in its modifications and applications–a patent review (2012–2014). Expert Opin. Ther. Pat. 2014, 24, 1129–1138. [Google Scholar] [CrossRef]
- Chen, X.; Ren, J.; Yang, J.; Zhu, Z.; Chen, R.; Zhang, L. A critical review of Andrographis paniculata. Med. Plant Biol. 2023, 2, 15. [Google Scholar] [CrossRef]
- Messire, G.; Rollin, P.; Gillaizeau, I.; Berteina-Raboin, S. Synthetic modifications of andrographolide targeting new potential anticancer drug candidates: A comprehensive overview. Molecules 2024, 29, 2884. [Google Scholar] [CrossRef]
- Menon, V.; Bhat, S. Anticancer activity of andrographolide semisynthetic derivatives. Nat. Prod. Commun. 2010, 5, 717–720. [Google Scholar] [CrossRef]
- Preet, R.; Chakraborty, B.; Siddharth, S.; Mohapatra, P.; Das, D.; Satapathy, S.R.; Das, S.; Maiti, N.C.; Maulik, P.R.; Kundu, C.N.; et al. Synthesis and biological evaluation of andrographolide analogues as anti-cancer agents. Eur. J. Med. Chem. 2014, 85, 95–106. [Google Scholar] [CrossRef] [PubMed]
- Kasemsuk, T.; Piyachaturawat, P.; Bunthawong, R.; Sirion, U.; Suksen, K.; Suksamrarn, A.; Saeeng, R. One-pot three steps cascade synthesis of novel isoandrographolide analogues and their cytotoxic activity. Eur. J. Med. Chem. 2017, 138, 952–963. [Google Scholar] [CrossRef]
- Singh, M.; Ravichandiran, V.; Bharitkar, Y.P.; Hazra, A. Natural products containing olefinic bond: Important substrates for semi-synthetic modification towards value addition. Curr. Org. Chem. 2020, 24, 709–745. [Google Scholar] [CrossRef]
- Wang, W.; Yang, Y.; Liu, Y.; Sun, D.; Li, H.; Chen, L. Recent Progress in Andrographolide Derivatization: Structural Modification and Biological Activity. Mini Rev. Med. Chem. 2022, 22, 1559–1584. [Google Scholar] [CrossRef]
- Jing, M.; Li, Z.; Zhang, Z.; Wang, Y.; Xu, L.; Yu, P. Synthesis and biological evaluation of andrographolide derivatives as potential anti-inflammatory agents. J. Pharm. Biomed. Sci. 2017, 7, 94–99. [Google Scholar]
- Gao, L.; Wang, F.; Chen, Y.; Li, F.; Han, B.; Liu, D. The antithrombotic activity of natural and synthetic coumarins. Fitoterapia 2021, 154, 104947. [Google Scholar] [CrossRef]
- Hu, B.; Guo, H.; Zhou, P.; Shi, Z.L. Characteristics of SARS-CoV-2 and COVID-19. Nat. Rev. Microbiol. 2021, 19, 141–154. [Google Scholar] [CrossRef]
- Zhang, D.-Q.; Ma, Q.-H.; Yang, M.-C.; Belyakova, Y.Y.; Yang, Z.-F.; Radulov, P.S.; Chen, R.-H.; Yang, L.-J.; Wei, J.-Y.; Peng, Y.-T.; et al. Peroxide derivatives as SARS-CoV-2 entry inhibitors. Virus Res. 2024, 340, 199295. [Google Scholar] [CrossRef]
- Wiersinga, W.J.; Rhodes, A.; Cheng, A.C.; Peacock, S.J.; Prescott, H.C. Pathophysiology, Transmission, Diagnosis, and Treatment of Coronavirus Disease 2019 (COVID-19): A Review. Jama 2020, 324, 782–793. [Google Scholar] [CrossRef]
- Shi, T.-H.; Huang, Y.-L.; Chen, C.-C.; Pi, W.-C.; Hsu, Y.-L.; Lo, L.-C.; Chen, W.-Y.; Fu, S.-L.; Lin, C.-H. Andrographolide and its fluorescent derivative inhibit the main proteases of 2019-nCoV and SARS-CoV through covalent linkage. Biochem. Biophys. Res. Commun. 2020, 533, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Schulte, B.; König, M.; Escher, B.I.; Wittenburg, S.; Proj, M.; Wolf, V.; Lemke, C.; Schnakenburg, G.; Sosič, I.; Streeck, H.; et al. Andrographolide Derivatives Target the KEAP1/NRF2 Axis and Possess Potent Anti-SARS-CoV-2 Activity. ChemMedChem 2022, 17, e202100732. [Google Scholar] [CrossRef] [PubMed]
- Suriya, U.; Intamalee, P.; Saeeng, R.; Wilasluck, P.; Deetanya, P.; Wangkanont, K.; Kanjanasirirat, P.; Wongwitayasombat, C.; Nutho, B. Design and Evaluation of Andrographolide Analogues as SARS-CoV-2 Main Protease Inhibitors: Molecular Modeling and in vitro Studies. Drug Des. Dev. Ther. 2025, 19, 3907–3924. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.U.; Ayoob, I.; Rehman, S.U.; Bhat, K.A.; Ara, T. Microwave-Assisted Synthesis of Andrographolide Derivatives and Evaluation of β-Glucosidase Inhibition. SynOpen 2018, 2, 330–342. [Google Scholar] [CrossRef]
- Novel Swine-Origin Influenza A (H1N1) Virus Investigation Team. Emergence of a novel swine-origin influenza A (H1N1) virus in humans. N. Engl. J. Med. 2009, 360, 2605–2615. [Google Scholar]
- Chen, J.-X.; Xue, H.-J.; Ye, W.-C.; Fang, B.-H.; Liu, Y.-H.; Yuan, S.-H.; Yu, P.; Wang, Y.-Q. Activity of andrographolide and its derivatives against influenza virus in vivo and in vitro. Biol. Pharm. Bull. 2009, 32, 1385–1391. [Google Scholar] [CrossRef]
- Murugesan, A.; Manoharan, M. Dengue Virus, in Emerging and Reemerging Viral Pathogens; Elsevier: Amsterdam, The Netherlands, 2020; pp. 281–359. [Google Scholar]
- Henchal, E.A.; Putnak, J.R. The dengue viruses. Clin. Microbiol. Rev. 1990, 3, 376–396. [Google Scholar] [CrossRef] [PubMed]
- Petersen, L.R.; Jamieson, D.J.; Powers, A.M.; Honein, M.A. Zika virus. N. Engl. J. Med. 2016, 374, 1552–1563. [Google Scholar] [CrossRef]
- Li, F.; Khanom, W.; Sun, X.; Paemanee, A.; Roytrakul, S.; Wang, D.; Smith, D.R.; Zhou, G.-C. Andrographolide and its 14-aryloxy analogues inhibit zika and dengue virus infection. Molecules 2020, 25, 5037. [Google Scholar] [CrossRef]
- Li, F.; Lee, E.M.; Sun, X.; Wang, D.; Tang, H.; Zhou, G.-C. Design, synthesis and discovery of andrographolide derivatives against Zika virus infection. Eur. J. Med. Chem. 2020, 187, 111925. [Google Scholar] [CrossRef]
- Pialoux, G.; Gaüzère, B.-A.; Jauréguiberry, S.; Strobel, M. Chikungunya, an epidemic arbovirosis. Lancet Infect. Dis. 2007, 7, 319–327. [Google Scholar] [CrossRef]
- Tran, Q.T.N.; Lee, R.C.H.; Liu, H.J.; Ran, D.; Low, V.Z.L.; To, D.Q.; Chu, J.J.H.; Chai, C.L.L. Discovery and development of labdane-oxindole hybrids as small-molecule inhibitors against chikungunya virus infection. Eur. J. Med. Chem. 2022, 230, 114110. [Google Scholar] [CrossRef] [PubMed]
- Solomon, T.; Lewthwaite, P.; Perera, D.; Cardosa, M.J.; McMinn, P.; Ooi, M.H. Virology, epidemiology, pathogenesis, and control of enterovirus 71. Lancet Infect. Dis. 2010, 10, 778–790. [Google Scholar] [CrossRef]
- Dai, K.; Tan, J.K.; Qian, W.; Lee, R.C.H.; Chu, J.J.H.; Zhou, G.-C. Discovery of 14S-(2′-chloro-4′-nitrophenoxy)-8R/S,17-epoxy andrographolide as EV-A71 infection inhibitor. Biochem. Pharmacol. 2021, 194, 114820. [Google Scholar] [CrossRef]
- Tan, J.K.; Chen, R.; Lee, R.C.H.; Li, F.; Dai, K.; Zhou, G.-C.; Chu, J.J.H. Discovery of Novel Andrographolide Derivatives as Antiviral Inhibitors against Human Enterovirus A71. Pharmaceuticals 2022, 15, 115. [Google Scholar] [CrossRef]
- Lee, W.M. Hepatitis B virus infection. N. Engl. J. Med. 1997, 337, 1733–1745. [Google Scholar] [CrossRef]
- Chen, H.; Ma, Y.-B.; Huang, X.-Y.; Geng, C.-A.; Zhao, Y.; Wang, L.-J.; Guo, R.-H.; Liang, W.-J.; Zhang, X.-M.; Chen, J.-J. Synthesis, structure-activity relationships and biological evaluation of dehydroandrographolide and andrographolide derivatives as novel anti-hepatitis B virus agents. Bioorganic Med. Chem. Lett. 2014, 24, 2353–2359. [Google Scholar] [CrossRef]
- Whitley, R.J.; Roizman, B. Herpes simplex virus infections. Lancet 2001, 357, 1513–1518. [Google Scholar] [CrossRef] [PubMed]
- Priengprom, T.; Ekalaksananan, T.; Kongyingyoes, B.; Suebsasana, S.; Aromdee, C.; Pientong, C. Synergistic effects of acyclovir and 3, 19-isopropylideneandrographolide on herpes simplex virus wild types and drug-resistant strains. BMC Complement. Altern. Med. 2015, 15, 56. [Google Scholar] [CrossRef] [PubMed]
- Ruelas, D.S.; Greene, W.C. An integrated overview of HIV-1 latency. Cell 2013, 155, 519–529. [Google Scholar] [CrossRef] [PubMed]
- Uttekar, M.M.; Das, T.; Pawar, R.S.; Bhandari, B.; Menon, V.; Nutan; Gupta, S.K.; Bhat, S.V. Anti-HIV activity of semisynthetic derivatives of andrographolide and computational study of HIV-1 gp120 protein binding. Eur. J. Med. Chem. 2012, 56, 368–374. [Google Scholar] [CrossRef] [PubMed]
- Ondusko, D.S.; Nolt, D. Staphylococcus aureus. Pediatr. Rev. 2018, 39, 287–298. [Google Scholar] [CrossRef]
- Patil, H.S.; Jadhav, D.D.; Paul, A.; Mulani, F.A.; Karegaonkar, S.J.; Thulasiram, H.V. Regioselective and efficient enzymatic synthesis of antimicrobial andrographolide derivatives. Bioorganic Med. Chem. Lett. 2018, 28, 1132–1137. [Google Scholar] [CrossRef]
- Van Tyne, D.; Martin, M.J.; Gilmore, M.S. Structure, function, and biology of the Enterococcus faecalis cytolysin. Toxins 2013, 5, 895–911. [Google Scholar] [CrossRef]
- Li, F.; Li, X.-M.; Sheng, D.; Chen, S.-R.; Nie, X.; Liu, Z.; Wang, D.; Zhao, Q.; Wang, Y.; Wang, Y.; et al. Discovery and preliminary SAR of 14-aryloxy-andrographolide derivatives as antibacterial agents with immunosuppressant activity. RSC Adv. 2018, 8, 9440–9456. [Google Scholar] [CrossRef]
- Nataro, J.P.; Kaper, J.B. Diarrheagenic Escherichia coli. Clin. Microbiol. Rev. 1998, 11, 142–201. [Google Scholar] [CrossRef]
- Wu, W.; Jin, Y.; Bai, F.; Jin, S. Pseudomonas aeruginosa. In Molecular Medical Microbiology; Elsevier: Amsterdam, The Netherlands, 2015; pp. 753–767. [Google Scholar]
- Wang, Z.; Yu, P.; Zhang, G.; Xu, L.; Wang, D.; Wang, L.; Zeng, X.; Wang, Y. Design, synthesis and antibacterial activity of novel andrographolide derivatives. Bioorganic Med. Chem. 2010, 18, 4269–4274. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, D.; Singh, P.; Vihar, V.C.V.; Neelbud, B. Synthesis, characterization and antibacterial activity of C (14)-sulfonyl ester-type andrographolide derivatives. J. Global. Biosci 2015, 4, 2348–2354. [Google Scholar]
- Tuteja, R. Malaria—An overview. FEBS J. 2007, 274, 4670–4679. [Google Scholar] [CrossRef] [PubMed]
- Haynes, R.H.; Cheu, K.W.; N’Da, D.; Coghi, P.; Monti, D. Considerations on the mechanism of action of artemisinin antimalarials: Part 1-The’carbon radical’and’heme’hypotheses. Infect. Disord. Drug Targets Disord. 2013, 13, 217–277. [Google Scholar] [CrossRef]
- Tiwari, M.K.; Coghi, P.; Agrawal, P.; Shyamlal, B.R.K.; Yang, L.J.; Yadav, L.; Peng, Y.; Sharma, R.; Yadav, D.K.; Sahal, D.; et al. Design, synthesis, Structure-Activity relationship and docking studies of novel functionalized Arylvinyl-1, 2, 4-Trioxanes as potent antiplasmodial as well as anticancer agents. ChemMedChem 2020, 15, 1216–1228. [Google Scholar] [CrossRef]
- Limsong, J.; Benjavongkulchai, E.; Kuvatanasuchati, J. Inhibitory effect of some herbal extracts on adherence of Streptococcus mutans. J. Ethnopharmacol. 2004, 92, 281–289. [Google Scholar] [CrossRef]
- Chander, R.; Srivastava, V.; Tandon, J.S.; Kapoor, N.K. Antihepatotoxic activity of diterpenes of Andrographis paniculata (Kal-Megh) against Plasmodium berghei-induced hepatic damage in Mastomys natalensis. Int. J. Pharmacogn. 1995, 33, 135–138. [Google Scholar] [CrossRef]
- Misra, P.; Pal, N.L.; Guru, P.Y.; Katiyar, J.C.; Srivastava, V.; Tandon, J.S. Antimalarial activity of Andrographis paniculata (Kalmegh) against Plasmodium berghei NK 65 in Mastomys natalensis. Int. J. Pharmacogn. 1992, 30, 263–274. [Google Scholar] [CrossRef]
- Megantara, S.; Fauzan, M.A.; Saputri, F.A.; Sofian, F.F. Pharmacophore Screening and Molecular Docking of Andrographolide and Its Derivatives on Plasmepsin as Anti-Malarial Drug. J. Glob. Pharma Technol. 2020, 12, 62–71. [Google Scholar]
- Torres-Guerrero, E.; Quintanilla-Cedillo, M.R.; Ruiz-Esmenjaud, J.; Arenas, R. Leishmaniasis: A review. F1000Research 2017, 6, 750. [Google Scholar] [CrossRef] [PubMed]
- Sinha, J.; Mukhopadhyay, S.; Das, N.; Basu, M.K. Targeting of liposomal andrographolide to L. donovani-infected macrophages in vivo. Drug Deliv. 2000, 7, 209–213. [Google Scholar]
- Gupta, P.; Tandon, J.; Patnaik, G. Antiallergic activity of andrographolides isolated from Andrographis paniculata (Burm. F) Wall. Pharm. Biol. 1998, 36, 72–74. [Google Scholar] [CrossRef]
- Lala, S.; Nandy, A.K.; Mahato, S.B.; Basu, M.K. Delivery in vivo of 14-deoxy-11-oxoandrographolide, an antileishmanial agent, by different drug carriers. Indian J. Biochem. Biophys. 2003, 40, 169–174. [Google Scholar]
- Panossian, A.; Hovhannisyan, A.; Mamikonyan, G.; Abrahamian, H.; Hambardzumyan, E.; Gabrielian, E.; Goukasova, G.; Wikman, G.; Wagner, H. Pharmacokinetic and oral bioavailability of andrographolide from Andrographis paniculata fixed combination Kan Jang in rats and human. Phytomedicine 2000, 7, 351–364. [Google Scholar] [CrossRef]
- Lin, J.H.; Chiba, M.; Baillie, T.A. Is the role of the small intestine in first-pass metabolism overemphasized? Pharmacol. Rev. 1999, 51, 135–157. [Google Scholar] [CrossRef]
- Pawar, A.; Rajalakshmi, S.; Mehta, P.; Shaikh, K.; Bothiraja, C. Strategies for formulation development of andrographolide. RSC Adv. 2016, 6, 69282–69300. [Google Scholar] [CrossRef]
- Pekthong, D.; Martin, H.; Abadie, C.; Bonet, A.; Heyd, B.; Mantion, G.; Richert, L. Differential inhibition of rat and human hepatic cytochrome P450 by Andrographis paniculata extract and andrographolide. J. Ethnopharmacol. 2008, 115, 432–440. [Google Scholar] [CrossRef]
- Ooi, J.P.; Kuroyanagi, M.; Sulaiman, S.F.; Muhammad, T.S.T.; Tan, M.L. Andrographolide and 14-deoxy-11, 12-didehydroandrographolide inhibit cytochrome P450s in HepG2 hepatoma cells. Life Sci. 2011, 88, 447–454. [Google Scholar] [CrossRef]
- Ma, H.-Y.; Sun, D.-X.; Cao, Y.-F.; Ai, C.-Z.; Qu, Y.-Q.; Hu, C.-M.; Jiang, C.; Dong, P.-P.; Sun, X.-Y.; Hong, M.; et al. Herb–drug interaction prediction based on the high specific inhibition of andrographolide derivatives towards UDP-glucuronosyltransferase (UGT) 2B7. Toxicol. Appl. Pharmacol. 2014, 277, 86–94. [Google Scholar] [CrossRef]
- Wang, D.W.; Xiang, Y.J.; Wei, Z.L.; Yao, H.; Shen, T. Andrographolide and its derivatives are effective compounds for gastrointestinal protection: A review. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 2367–2382. [Google Scholar]
- Wu, H.; Wu, X.; Huang, L.; Ruan, C.; Liu, J.; Chen, X.; Liu, J.; Luo, H. Effects of andrographolide on mouse intestinal microflora based on high-throughput sequence analysis. Front. Vet. Sci. 2021, 8, 702885. [Google Scholar] [CrossRef]
- Tang, D.; Hu, W.; Fu, B.; Zhao, X.; You, G.; Xie, C.; Wang, H.Y.; Guo, X.; Zhang, Q.; Liu, Z.; et al. Gut microbiota-mediated C-sulfonate metabolism impairs the bioavailability and anti-cholestatic efficacy of andrographolide. Gut Microbes 2024, 16, 2387402. [Google Scholar] [CrossRef]
- Akbarzadeh, A.; Rezaei-Sadabady, R.; Davaran, S.; Joo, S.W.; Zarghami, N.; Hanifehpour, Y.; Samiei, M.; Kouhi, M.; Nejati-Koshki, K. Liposome: Classification, preparation, and applications. Nanoscale Res. Lett. 2013, 8, 102. [Google Scholar] [CrossRef]
- Szoka, F., Jr.; Papahadjopoulos, D. Comparative properties and methods of preparation of lipid vesicles (liposomes). Annu. Rev. Biophys. Bioeng. 1980, 9, 467–508. [Google Scholar] [CrossRef]
- Piazzini, V.; Landucci, E.; Graverini, G.; Pellegrini-Giampietro, D.E.; Bilia, A.R.; Bergonzi, M.C. Stealth and cationic nanoliposomes as drug delivery systems to increase andrographolide BBB permeability. Pharmaceutics 2018, 10, 128. [Google Scholar] [CrossRef]
- Jain, P.K.; Khurana, N.; Pounikar, Y.; Gajbhiye, A.; Kharya, M.D. Enhancement of absorption and hepatoprotective potential through soya-phosphatidylcholine-andrographolide vesicular system. J. Liposome Res. 2013, 23, 110–118. [Google Scholar] [CrossRef]
- Sur, S.; Rathore, A.; Dave, V.; Reddy, K.R.; Chouhan, R.S.; Sadhu, V. Recent developments in functionalized polymer nanoparticles for efficient drug delivery system. Nano-Struct. Nano-Objects 2019, 20, 100397. [Google Scholar] [CrossRef]
- Roy, P.; Das, S.; Bera, T.; Mondol, S.; Mukherjee, A. Andrographolide nanoparticles in leishmaniasis: Characterization and in vitro evaluations. Int. J. Nanomed. 2010, 5, 1113–1121. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, Y.; Gao, W.; A Repka, M.; Wang, Y.; Chen, M. Andrographolide-loaded PLGA-PEG-PLGA micelles to improve its bioavailability and anticancer efficacy. Expert Opin. Drug Deliv. 2014, 11, 1367–1380. [Google Scholar] [CrossRef] [PubMed]
- Mondal, S.; Roy, P.; Das, S.; Halder, A.; Mukherjee, A.; Bera, T. In vitro susceptibilities of wild and drug resistant Leishmania donovani amastigote stages to andrographolide nanoparticle: Role of vitamin E derivative TPGS for nanoparticle efficacy. PLoS ONE 2013, 8, e81492. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Pradhan, G.K.; Das, S.; Nath, D.; Saha, K.D. Enhanced protective activity of nano formulated andrographolide against arsenic induced liver damage. Chem. Biol. Interact. 2015, 242, 281–289. [Google Scholar] [CrossRef]
- Zhao, D.; Liao, K.; Ma, X.; Yan, X. Study of the supramolecular inclusion of β-cyclodextrin with andrographolide. J. Incl. Phenom. Macrocycl. Chem. 2002, 43, 259–264. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).