Utilization of All-Chitin Composite Films for High-Density Three-Dimensional Cell Cultivation
Abstract
1. Introduction
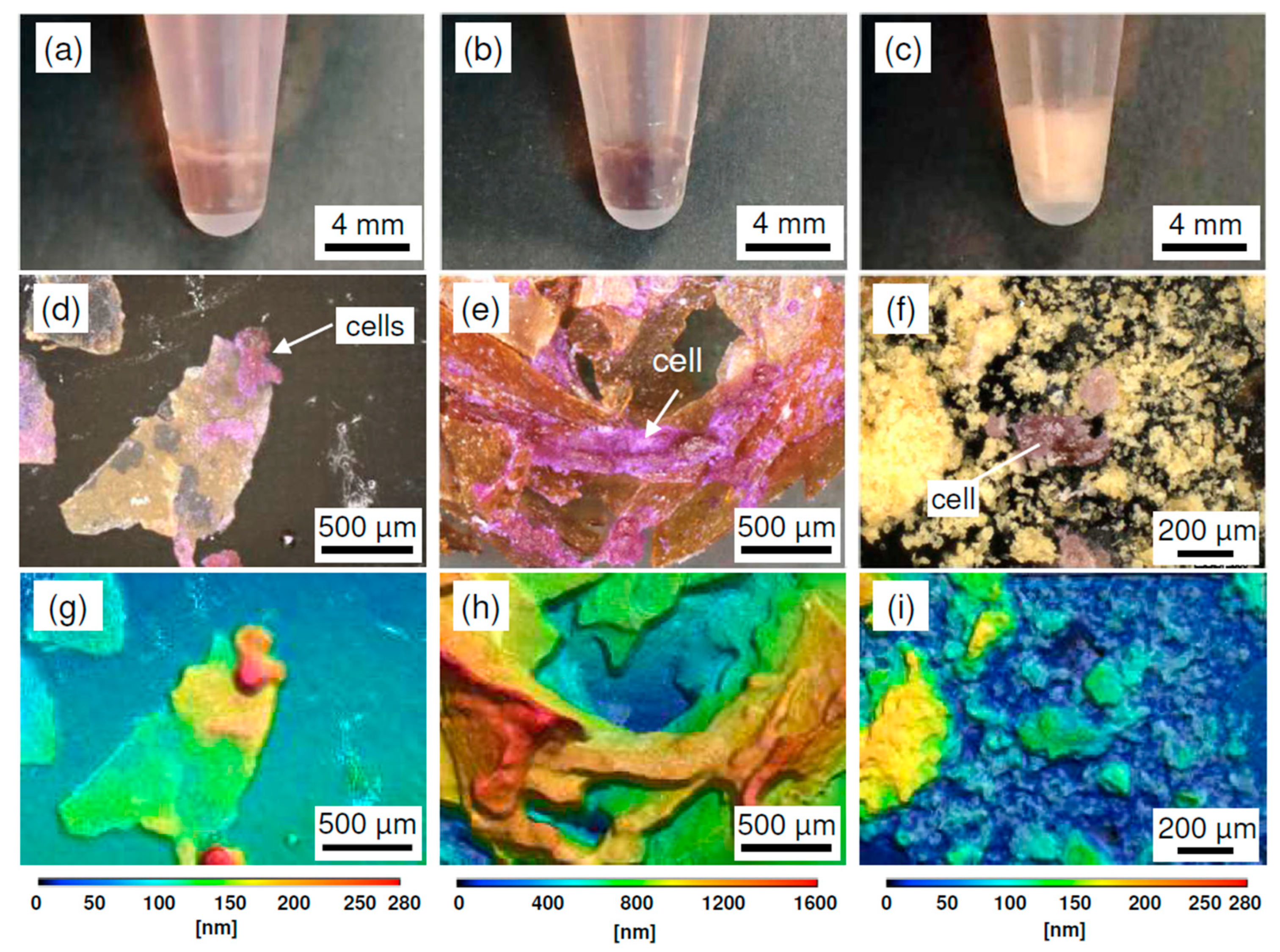
2. Results
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Materials
5.2. Protein Adsorption Test
5.3. Cell Adhesion Test
5.4. Measurements
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wasyłeczko, M.; Sikorska, W.; Chwojnowski, A. Review of Synthetic and Hybrid Scaffolds in Cartilage Tissue Engineering. Membranes 2020, 10, 348. [Google Scholar] [CrossRef] [PubMed]
- Suamte, L.; Tirkey, A.; Babu, P.J. Design of 3D smart scaffolds using natural, synthetic and hybrid derived polymers for skin regenerative applications. Smart Mater. Med. 2023, 4, 243–256. [Google Scholar] [CrossRef]
- Nair, S.A.; Gayathri, V. Scaffold Materials and Toxicity. In Biomedical Applications and Toxicity of Nanomaterials; Mohanan, P.V., Kappalli, S., Eds.; Springer Nature: Singapore, 2023; pp. 535–558. [Google Scholar] [CrossRef]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef]
- Rodríguez-Vázquez, M.; Vega-Ruiz, B.; Ramos-Zúñiga, R.; Saldaña-Koppel, D.A.; Quiñones-Olvera, L.F. Chitosan and Its Potential Use as a Scaffold for Tissue Engineering in Regenerative Medicine. Biomed. Res. Int. 2015, 2015, 821279. [Google Scholar] [CrossRef]
- Dong, C.; Lv, Y. Application of Collagen Scaffold in Tissue Engineering: Recent Advances and New Perspectives. Polymers 2016, 8, 42. [Google Scholar] [CrossRef]
- Xie, Z.-T.; Zeng, J.; Kang, D.-H.; Saito, S.; Miyagawa, S.; Sawa, Y.; Matsusaki, M. 3D Printing of Collagen Scaffold with Enhanced Resolution in a Citrate-Modulated Gellan Gum Microgel Bath. Adv. Healthc. Mater. 2023, 12, 2301090. [Google Scholar] [CrossRef]
- Xie, Z.-T.; Zeng, J.; Miyagawa, S.; Sawa, Y.; Matsusaki, M. 3D puzzle-inspired construction of large and complex organ structures for tissue engineering. Mater. Today Bio 2023, 21, 100726. [Google Scholar] [CrossRef]
- Mirsky, N.A.; Ehlen, Q.T.; Greenfield, J.A.; Antonietti, M.; Slavin, B.V.; Nayak, V.V.; Pelaez, D.; Tse, D.T.; Witek, L.; Daunert, S.; et al. Three-Dimensional Bioprinting: A Comprehensive Review for Applications in Tissue Engineering and Regenerative Medicine. Bioengineering 2024, 11, 777. [Google Scholar] [CrossRef]
- Matsunaga, Y.T.; Morimoto, Y.; Takeuchi, S. Molding Cell Beads for Rapid Construction of Macroscopic 3D Tissue Architecture. Adv. Mater. 2011, 23, H90–H94. [Google Scholar] [CrossRef]
- Yang, J.; Yamato, M.; Sekine, H.; Sekiya, S.; Tsuda, Y.; Ohashi, K.; Shimizu, T.; Okano, T. Tissue Engineering Using Laminar Cellular Assemblies. Adv. Mater. 2009, 21, 3404–3409. [Google Scholar] [CrossRef] [PubMed]
- Eltom, A.; Zhong, G.; Muhammad, A. Scaffold Techniques and Designs in Tissue Engineering Functions and Purposes: A Review. Adv. Mater. Sci. Eng. 2019, 2019, 3429527. [Google Scholar] [CrossRef]
- Suamte, L.; Tirkey, A.; Barman, J.; Jayasekhar Babu, P. Various manufacturing methods and ideal properties of scaffolds for tissue engineering applications. Smart Mater. Manuf. 2023, 1, 100011. [Google Scholar] [CrossRef]
- Noda, T.; Hatakeyama, M.; Kitaoka, T. Combination of Polysaccharide Nanofibers Derived from Cellulose and Chitin Promotes the Adhesion, Migration and Proliferation of Mouse Fibroblast Cells. Nanomaterials 2022, 12, 402. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Z.; Huang, Y.; Hu, Q.; He, W.; Duan, B.; Yan, X.; Yang, Z.; Liang, W.; Liu, Z.; Peng, Z.; et al. Elucidation of molecular pathways responsible for the accelerated wound healing induced by a novel fibrous chitin dressing. Biomater. Sci. 2019, 7, 5247–5257. [Google Scholar] [CrossRef] [PubMed]
- Satitsri, S.; Muanprasat, C. Chitin and Chitosan Derivatives as Biomaterial Resources for Biological and Biomedical Applications. Molecules 2020, 25, 5961. [Google Scholar] [CrossRef]
- Baharlouei, P.; Rahman, A. Chitin and Chitosan: Prospective Biomedical Applications in Drug Delivery, Cancer Treatment, and Wound Healing. Mar. Drugs 2022, 20, 460. [Google Scholar] [CrossRef] [PubMed]
- Tsurkan, M.V.; Voronkina, A.; Khrunyk, Y.; Wysokowski, M.; Petrenko, I.; Ehrlich, H. Progress in chitin analytics. Carbohydr. Polym. 2021, 252, 117204. [Google Scholar] [CrossRef]
- Phongying, S.; Aiba, S.-i.; Chirachanchai, S. A novel soft and cotton-like chitosan-sugar nanoscaffold. Biopolymers 2006, 83, 280–288. [Google Scholar] [CrossRef]
- Ifuku, S.; Saimoto, H. Chitin nanofibers: Preparations, modifications, and applications. Nanoscale 2012, 4, 3308–3318. [Google Scholar] [CrossRef]
- Lv, J.; Lv, X.; Ma, M.; Oh, D.-H.; Jiang, Z.; Fu, X. Chitin and chitin-based biomaterials: A review of advances in processing and food applications. Carbohydr. Polym. 2023, 299, 120142. [Google Scholar] [CrossRef]
- Wu, Y.; Sasaki, T.; Irie, S.; Sakurai, K. A novel biomass-ionic liquid platform for the utilization of native chitin. Polymer 2008, 49, 2321–2327. [Google Scholar] [CrossRef]
- Shamshina, J.L. Chitin in ionic liquids: Historical insights into the polymer’s dissolution and isolation. A review. Green Chem. 2019, 21, 3974–3993. [Google Scholar] [CrossRef]
- Kadokawa, J. Application of ionic liquids for the functional materialization of chitin. Mater. Adv. 2022, 3, 3355–3364. [Google Scholar] [CrossRef]
- Prasad, K.; Murakami, M.-a.; Kaneko, Y.; Takada, A.; Nakamura, Y.; Kadokawa, J. Weak gel of chitin with ionic liquid, 1-allyl-3-methylimidazolium bromide. Int. J. Biol. Macromol. 2009, 45, 221–225. [Google Scholar] [CrossRef]
- Kadokawa, J.; Takegawa, A.; Mine, S.; Prasad, K. Preparation of chitin nanowhiskers using an ionic liquid and their composite materials with poly(vinyl alcohol). Carbohydr. Polym. 2011, 84, 1408–1412. [Google Scholar] [CrossRef]
- Hashiguchi, T.; Yamamoto, K.; Kadokawa, J. Fabrication of highly flexible nanochitin film and its composite film with anionic polysaccharide. Carbohydr. Polym. 2021, 270, 118369. [Google Scholar] [CrossRef] [PubMed]
- Kadokawa, J.; Obama, Y.; Yoshida, J.; Yamamoto, K. Gel formation from self-assembled chitin nanofiber film by grafting of poly(2-methyl-2-oxazoline). Chem. Lett. 2018, 47, 949–952. [Google Scholar] [CrossRef]
- Kadokawa, J.; Egashira, N.; Yamamoto, K. Chemoenzymatic preparation of amylose-grafted chitin nanofiber network materials. Biomacromolecules 2018, 19, 3013–3019. [Google Scholar] [CrossRef] [PubMed]
- Kadokawa, J.; Kawano, A.; Yamamoto, K. Fabrication of semi-crystalline film by hexanoylation on self-assembled chitin nanofibers. ChemistrySelect 2019, 4, 797–801. [Google Scholar] [CrossRef]
- Egi, Y.; Kontani, A.; Kadokawa, J. Fabrication of all-chitin composite films. Int. J. Biol. Macromol. 2023, 253, 127512. [Google Scholar] [CrossRef]
- Sato, K.; Noguchi, T.; Kiyose, M. Deacetylation and gelation of amorphous chitin cake. Chitin Chitosan Res. 2019, 25, 136–137. [Google Scholar]
- Totani, M.; Tanihata, Y.; Egi, Y.; Kadokawa, J. Fabrication of self-reinforced chitin composites by double crystalline blend approach. Int. J. Biol. Macromol. 2025, 286, 138441. [Google Scholar] [CrossRef]
- Totani, M.; Shinchi, H.; Kadokawa, J. Cancer cell adhesion property on all-chitin composite films with reduced crystallinity. Carbohydr. Res. 2025, 549, 109373. [Google Scholar] [CrossRef] [PubMed]
- Antoni, D.; Burckel, H.; Josset, E.; Noel, G. Three-Dimensional Cell Culture: A Breakthrough in Vivo. Int. J. Mol. Sci. 2015, 16, 5517–5527. [Google Scholar] [CrossRef]
- Previtera, M.L.; Langhammer, C.G.; Firestein, B.L. Effects of substrate stiffness and cell density on primary hippocampal cultures. J. Biosci. Bioeng. 2010, 110, 459–470. [Google Scholar] [CrossRef]
- Venugopal, B.; Mogha, P.; Dhawan, J.; Majumder, A. Cell density overrides the effect of substrate stiffness on human mesenchymal stem cells’ morphology and proliferation. Biomater. Sci. 2018, 6, 1109–1119. [Google Scholar] [CrossRef]
- Su, J.; Song, Y.; Zhu, Z.; Huang, X.; Fan, J.; Qiao, J.; Mao, F. Cell-cell communication: New insights and clinical implications. Signal Transduct. Target. Ther. 2024, 9, 196. [Google Scholar] [CrossRef]
- Khoushab, F.; Yamabhai, M. Chitin research revisited. Mar. Drugs 2010, 8, 1988–2012. [Google Scholar] [CrossRef] [PubMed]
- Moraru, C.; Mincea, M.; Menghiu, G.; Ostafe, V. Understanding the Factors Influencing Chitosan-Based Nanoparticles-Protein Corona Interaction and Drug Delivery Applications. Molecules 2020, 25, 4758. [Google Scholar] [CrossRef]
- Pollard, T.D.; Cooper, J.A. Actin, a central player in cell shape and movement. Science 2009, 326, 1208–1212. [Google Scholar] [CrossRef] [PubMed]
- Lai, J.; Kong, D.; Liu, Y.; Dai, J.; Zhang, M. A Flexible Fibrin-Based Platform for Driving One-Day Self-Assembly of Millimeter-Sized Cell-Rich Spheroids and Their Applications: An Old Material with New Tricks. Adv. Funct. Mater. 2024, 34, 2403507. [Google Scholar] [CrossRef]
- Cárdenas, G.; Cabrera, G.; Taboada, E.; Miranda, S.P. Chitin characterization by SEM, FTIR, XRD, and 13C cross polarization/mass angle spinning NMR. J. Appl. Polym. Sci. 2004, 93, 1876–1885. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Totani, M.; Eda, M.; Shinchi, H.; Kadokawa, J.-i. Utilization of All-Chitin Composite Films for High-Density Three-Dimensional Cell Cultivation. Molecules 2025, 30, 4243. https://doi.org/10.3390/molecules30214243
Totani M, Eda M, Shinchi H, Kadokawa J-i. Utilization of All-Chitin Composite Films for High-Density Three-Dimensional Cell Cultivation. Molecules. 2025; 30(21):4243. https://doi.org/10.3390/molecules30214243
Chicago/Turabian StyleTotani, Masayasu, Mako Eda, Hiroyuki Shinchi, and Jun-ichi Kadokawa. 2025. "Utilization of All-Chitin Composite Films for High-Density Three-Dimensional Cell Cultivation" Molecules 30, no. 21: 4243. https://doi.org/10.3390/molecules30214243
APA StyleTotani, M., Eda, M., Shinchi, H., & Kadokawa, J.-i. (2025). Utilization of All-Chitin Composite Films for High-Density Three-Dimensional Cell Cultivation. Molecules, 30(21), 4243. https://doi.org/10.3390/molecules30214243






