Abstract
Visceral leishmaniasis caused by Leishmania donovani is one of the major neglected tropical diseases attributable to parasitic protozoa. In the absence of an effective vaccine, chemotherapy remains the only available therapeutic option. However, current treatments rely on a limited number of drugs that are largely obsolete, highly toxic or require intravenous administration, and their extensive use has led to the emergence of drug resistance. Consequently, the discovery of new antileishmanial agents is an urgent priority. In this study, a commercial library of 449 alkaloids in a high-throughput screening format was evaluated against both axenic bone marrow-derived amastigotes and intramacrophagic amastigotes from mice infected with L. donovani IRFP, a strain engineered to emit infrared fluorescence in its viable form. Six isoquinoline-type alkaloids showed the best antileishmanial efficacy against intramacrophagic amastigotes while exhibiting minimal cytotoxicity toward RAW 264.7 and HepG2 cell lines, with a promising selective index higher than four, and good mouse intestinal tolerance in mouse organoids. Among these compounds, the protoberberine scaffold emerged as the most promising candidate for further drug development.
1. Introduction
Neglected tropical diseases (NTDs) comprise twenty primarily infectious and parasitic diseases that disproportionately affect populations in low- and middle-income countries. These diseases can be fatal and not only impose significant health problems but also impede economic and social development in endemic regions due to enormous health costs and loss of workforce productivity among adults and education disruption among children. NTDs affect nearly one-sixth of the global population, with Africa bearing the greatest health, economic and social burden [1]. In response to this challenge, the African Union and Uniting to Combat NTDs adopted a declaration at the Kigali Summit on Malaria and NTDs in March 2022, aiming to reduce NTD infections by 90% and eliminate at least one NTD in 100 countries by 2030, in alignment with the WHO roadmap [2]. However, NTD control remains difficult due to limited or absent availability of vaccines, outdated drug treatments and the emergence of drug resistance, underscoring the urgent need for novel therapeutic interventions [3].
Visceral leishmaniasis (VL) is an important vector-borne protozoan NTD that remains a serious public health concern, particularly in sub-Saharan Africa, where the highest prevalence and mortality rates are reported [4,5]. According to the 2023 WHO report on VL, 40% of the 200 reporting countries were classified as endemic, collectively reporting over 11,000 new cases [6]. In the absence of an effective vaccine, current VL treatment relies on a limited number of drugs, including pentavalent antimonials, amphotericin B, miltefosine and paromomycin. Most of these drugs are outdated and associated with significant toxicity, complex administration requirements, and, in some cases, chemical instability at the point of care [7]. These limitations have led to reduced treatment efficacy and the emergence of drug-resistant strains. In recent years, no new therapeutic alternatives have been developed, apart from combinations of existing drugs, which have shown acceptable results [5].
In the urgent search for new active compounds to address this global health challenge, two main strategies have been employed in drug discovery: (i) phenotypic screening based on antileishmanial activity, and (ii) target-based approaches that rely on the identification of specific pharmacological targets [8,9]. Both strategies have been adapted to high-throughput screening (HTS) platforms, enabling large-scale evaluation of chemical libraries. In addition, computational approaches—traditionally considered complementary tools—are now playing an increasingly role in drug discovery workflows by optimizing resource allocation, reducing costs and timelines, and improving overall outcomes [10,11].
Natural plant-based products have long been recognized as an important source of secondary metabolites with diverse pharmacological activities [12]. Among these, alkaloids—nitrogen-containing heterocyclic compounds biosynthesized from amino acids and distributed across various botanical families—have demonstrated a wide range of bioactivities, including antiproliferative (antitumor), antibacterial, antiparasitic, anticoagulant and antioxidant properties. These characteristics make them promise candidates for drug development [13]. For decades, alkaloids have served as chemical templates for the design of numerous synthetic and semi-synthetic derivatives, many of which display potent biological activity both in vitro and in vivo [14,15,16].
The objective of the present study was to identify new antileishmanial drug candidates by screening a commercial library of plant-derived alkaloids using a platform based on axenic amastigotes of L. donovani IRFP—the causative agent of VL—genetically modified to emit infrared fluorescence during their viable stage (hereafter referred to as L. donovani IRFP) [17]. To this end, single-dose assays at 10 μM and 1 μM, as well as dose–response assays, were performed. Compounds showing significant activity in the initial screening were subsequently clustered according to chemical structure using the DataWarrior tool [18], and representative compounds were further evaluated in dose response assays against axenic amastigotes. The efficacy of the most potent compounds was subsequently assessed in ex vivo intramacrophagic amastigotes isolated from mice infected with L. donovani IRFP. In parallel, cytotoxicity of the most active compounds was evaluated in RAW 264.7 and HepG2 cell lines, as well as in mouse intestinal organoids. Finally, chemoinformatic analysis was conducted to assess the most favorable structural features of the most effective and safest alkaloids.
2. Results
2.1. Strategy of Compound Screening Against Leishmania
Non-target-based platforms of drug discovery (phenotypic screening platforms) are generally more relevant than those based on specific targets, as they can avoid a high rate of false positives. In this regard, some good practices have been recommended for designing reproducible phenotypic platforms for the detection and prioritization of results [19]. The strategy followed in this work to identify compounds effective against L. donovani IRFP from the commercial collection of alkaloid drugs (MedChemExpress, Sollentuna, Sweden. Ref.: HY−L071), is shown in Figure 1. In a first step, two single concentrations (10 µM and 1 µM) were tested in 96-well plates with 25,000 axenic amastigotes [20] in two independent experiments to individually evaluate the activity of these compounds. The percentage of inhibition at 72 h was determined using a cut-off value of ≥70% growth inhibition in at least one of the two experiments to classify the compound as active or inactive. Amphotericin B at 10 µM was used as the positive control, and 0.1% DMSO as the negative control. Plates with a Z’ factor ≥ 0.5 were considered valid, according to the formula described by Zhang and co-workers [21] (Figure S1, Supplementary Material).
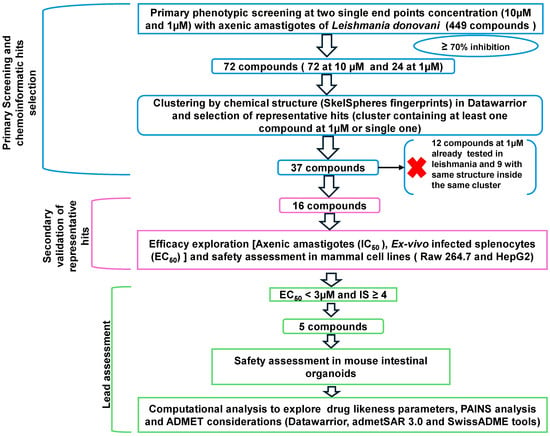
Figure 1.
A stepwise screening cascade was applied to 449 alkaloids from the MedChemExpress library (MedChemExpress, Sollentuna, Sweden. Ref.: HY−L071). Compounds were first tested in single-shot experiments at 10 μM and 1 μM, identifying a total of 72 and 24 hits, respectively, using a ≥70% inhibition cut-off. Chemoinformatic clustering by structural similarity reduced the set to 16 representative compounds. These were validated through dose/response assays on axenic amastigotes and ex-vivo infected splenic explants, followed by cytotoxicity testing in mammalian cells to determine safety and Selectivity Index (SI). Each open arrowhead represents subsequent steps in the screening process. A black arrow and a red cross indicate that those compounds were not further studied.
2.2. Primary Screening and Cheminformatics Hit Selection
The 449 compounds in the Alkaloids Compound Library (MedChemExpress, Sollentuna, Sweden. Ref.: HY−L071) were initially screened at single concentrations of 10 µM and 1 µM in two independent assays against L. donovani IRFP. Seventy-two and 24 compounds—corresponding to 16.04% and 5.35% of the collection, respectively—met the previously established cut-off value of 70% growth inhibition in at least one of the experimental replicates at 10 µM and 1 µM (Figure 2a,b). A list of active compounds identified in these two primary screens is provided in Table S1 (Supplementary Material).
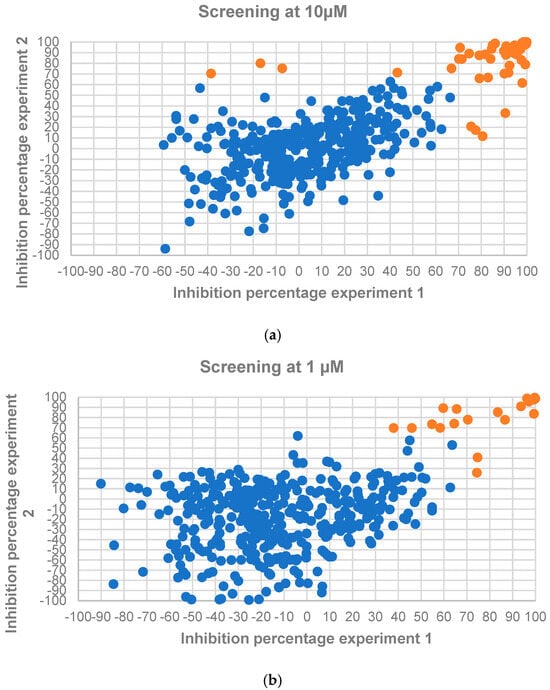
Figure 2.
L. donovani IRFP axenic amastigote growth inhibition caused by the 449 employed screening compounds from the Alkaloids Library (MedChemExpress, Sollentuna, Sweden. Ref.: HY−L071) at 10 µM (a) and 1 µM (b). Orange dots represent compounds surpassing the established cut-off threshold of ≥ 70% inhibition in at least one of the experimental replicates, while blue dots represent those molecules not passing the threshold.
The 72 active compounds at 10 µM were grouped using the DataWarrior tool based on their chemical structure (SkelSpheres fingerprint), to guide the selection of some representative results from some clusters containing at least one active hit at 1 µM (Figure 3a and Table S1). From this step, we selected compounds belonging to clusters that contained at least one active or isolated compound at 1 µM, resulting in a total of 37 compounds. Next, the alkaloids derived from camptothecin (7 molecules) were discarded, as our group had described the antileishmanial effect of these compounds in previous studies [22]. We also excluded an additional 7 compounds showing activity at 1 µM, since their antileishmanial effect had already been described by other authors. Finally, for those compounds whose active ingredient was present in multiple salt forms, only one was selected for analysis, typically the chloride salt.
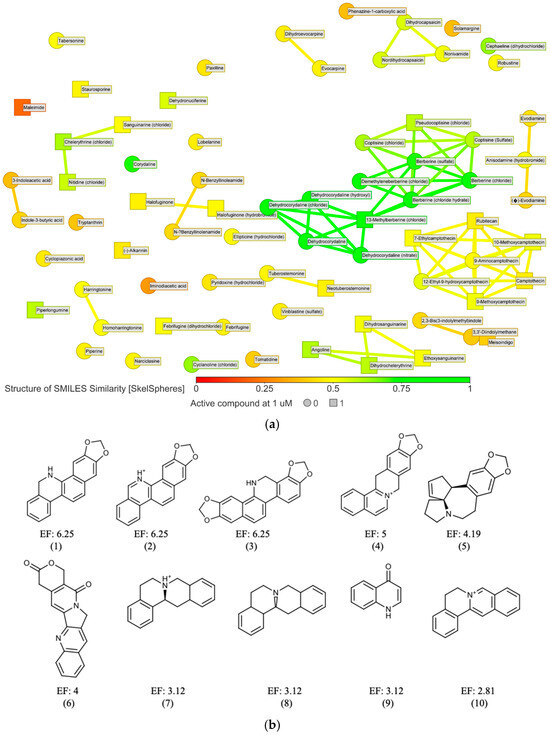
Figure 3.
(a) 2D similarity chart of the 72 active compounds at 10 µM from the phenotypic screening. Markers are dynamically colored based on structural similarity to the selected reference compound dehydrocorydaline. Similar neighbors are connected by lines. Squares indicate compounds active at 1 µM, while circles represent those that were not; (b) Murcko scaffolds were generated using the criteria described in Section 4. Only those with an enrichment factor (EF) ≥ 2 and containing at least two unique active molecules (excluding duplicates due to different salt forms) were included (represented by numbers from 1 to 10 below each scaffold). Total scaffolds found are listed in Table S2 (Supplementary Material).
The DataWarrior tool was used to filter, group, and prioritize compounds based on their chemical structures [18] (Figure 3a and Figure S2). A subsequent analysis of the basic molecular scaffolds of all 449 compounds of the library using the Murcko framework algorithm (Figure 3b) revealed a series of privileged pharmacophores among the active molecules [23]. These were classified into different groups, notably including compounds containing the isoquinoline ring (such as benzo[c]phenanthridines and proto-berberines), as well as derivatives of stemonin and indurubin (Table S2 in Supplementary Material).
Subsequently, compounds with EC50 values below 3 μM against intramacrophagic amastigotes were evaluated for adequate safety margin, as determined by the selectivity index (SI). To this end, we calculated SIs from 16 preselected results from the ex vivo system. With this aim, dose–response curves were performed in duplicate across at least three independent experiments using RAW 264.7 mouse macrophages (as a model of host cells harboring amastigotes) and HepG2 (as a model for assessing systemic toxicity). The results are shown in Table 1, and the corresponding dose–response curves are represented in Figure S3 (Supplementary Material).

Table 1.
Efficacy and safety of the 16 compounds that reduce the viability of L. donovani IRFP by ≥ 70% at 10 µM or 1µM in at least one experimental replicate.
With a few exceptions (dehydrocorydaline, coptisine and, to a lesser extent, 13-methylberberine), the compounds exhibited significantly higher antileishmanial activity against axenic amastigotes than against intramacrophagic amastigotes. This affected the value of the SI, which was lower when the CC50 values were compared to the IC50 values obtained in axenic amastigotes versus intramacrophagic amastigotes, likely reflecting differences in compound uptake. Some compounds exhibited unacceptable EC50 values, close to or exciding 10 μM, including dihydrochelerythrine, dihydrosanguinarine, neotuberostemonine, tuberostemomine, dehydronuciferine and meisoindigo. Other compounds were toxic or extremely toxic to cells, with SI < 4, including sanguinarine and nitidine. Consequently, we selected a group of six compounds, namely angoline, 13-methylberberine, pseudocoptisine, dehydrocorydaline and coptisine (despite its EC50 value being slightly higher than threshold), that met the dual criteria of EC50 < 3 μM and SI ≥ 4, and whose dose–response curves are shown in Figure 4. Additionally, berberine was included to compare its effects with those of its analogue, 13-methylberberine.
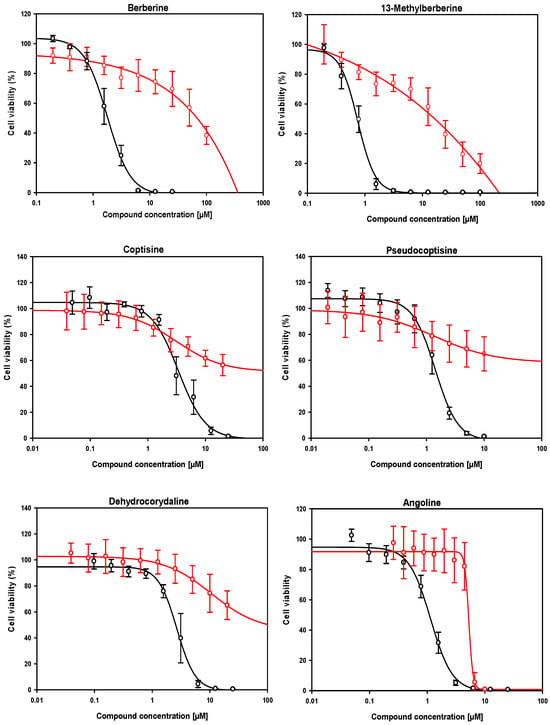
Figure 4.
Dose–response curves of the six compounds meeting the lead criteria of EC50 < 3 µM and SI ≥ 4, identified from the Alkaloids Compound Library (MedChemExpress, Sollentuna, Sweden. Ref.: HY−L071). The effect on intramacrophagic amastigotes in primary cultures of infected mouse splenocytes and the cytotoxic effect in RAW 264.7 mouse macrophages are shown in black and red, respectively. EC50 and CC50 values, listed in Table 1, were calculated using the SigmaPlotTM statistical software version 10.0. Each point represents the mean ± SD of at least three different experiments performed in triplicate.
Interestingly, the tolerance of these compounds for oral administration was good as demonstrated using mouse intestinal organoids (see Figure S4 in Supplementary Material). With the exception of angoline, which showed significant toxicity, the remaining compounds exhibited low toxicity, thus suggesting that future formulations may be feasible without major safety concerns.
Of the six selected compounds, we explored druglikeness and ADMET properties using three different computational chemistry tools. All three tools consistently indicated that the compounds comply with Lipinski’s rules [24]. Furthermore, SwissADME showed that the compounds also passed other druglikeness filters. No alerts related to the PAINS filter were observed across the three tools. Key pharmacokinetic parameters are summarized in Table 2 (other druglikeness parameters are listed in Table S3).

Table 2.
Predictive druglikeness and ADMET of the six compounds meeting the lead criteria of EC50 < 3 µM and SI ≥ 4 identified in the alkaloid library (MedChemExpress, Sollentuna, Sweden. Ref.: HY−L071).
3. Discussion
The need for drugs to treat visceral leishmaniasis is one of the key priorities in the WHO’s objectives, highlighting the outdated nature of current treatments and the lack of new chemical entities with good efficacy and safety profiles. Given that the compounds identified so far as leads and second-line drug candidates are still far from clinical approval, the only option currently validated by DNDi is the combination of known drugs, which has shown acceptable results to date [25]. Urgent research is therefore needed to identify chemical compounds with antileishmanial activity from diverse sources, to expand the pool of candidates for further preclinical and clinical studies. Plants are a rich source of natural products and plant-derived alkaloids have played a significant role over the past few decades in drug discovery. In fact, their multiple antitumor, antimicrobial, and anti-inflammatory properties, among others, suggest that these compounds hold great promise for the development of new drugs with antileishmanial properties [13].
Of the 16 alkaloids identified as having strong antileishmanial effect at an IC50 < 3 μM against axenic amastigotes of L. donovani IRFP, 11 share the well-characterized benzo[c]phenanthridine structure. This pharmacophore consisting of a four-fused-ring structure that includes an isoquinoline group, has been described as a promising antiparasitic scaffold [26], although not all their members have acceptable selective toxicity. Within this group, only angoline and, to a lesser extent chelerythrine, met the lead compound criteria of EC50 < 3 μM in intramacrophagic amastigotes and a SI ≥ 4 (chelerythrine barely had a SI = 3.4). These results are consistent with those reported for the orally administered antileishmanial drug miltefosine (IC50 for axenic amastigotes ranging from 0.4 to 3.8 µM), although they are higher than those observed for amphotericin B (0.6 to 0.7 µM), which has the drawback of requiring intravenous administration [27]. The rest of the compounds in this cluster either failed due to toxicity issues (ethoxysanguinarine, sanguinarine, and nitidine) or exhibited a significant loss of antileishmanial activity against intramacrophagic amastigotes (dihydrochelerytrine and dihydrosanguinarine), likely due to the necessity of crossing multiple permeability barriers to reach the parasite [28]. Benzo[c]phenantridines are known to be cytotoxic agents for cancer cells, and some of them have been shown to induce apoptosis. The mechanism of action of these compounds may be attributed to their flat structure, as they are considered intercalating agents capable of interfering with the activity of type I and II DNA-topoisomerases [29,30]. However, these effects have been poorly studied in trypanosomatids [31]. Castillo and co-workers [32] demonstrated that chelerythrine and nitidine exhibited in vitro IC50 values very similar to those of glucantime against axenic amastigotes of L. amazonensis, although their antileishmanial effect in vivo was moderate. Induction of apoptosis like death by inhibition of Protein Phosphatase 2C (PP2C) has been proposed as potential mechanism of action of these compounds [33].
Other alkaloids also derived from the isoquinoline heterocycle and featuring a four-ring fused system, were proto-berberine derivatives, such as berberine, 13-methylberberine, pseudocoptisine, and dehydrocorydaline. All of these compounds met the established criteria for efficacy against L. donovani IRFP intramacrophagic amastigotes and selectivity in cell cultures. Among the compounds evaluated, coptisin showed a slight reduction in efficacy against intramacrophagic amastigotes, with a moderate increase in EC50 of 3.39 µM. However, its favorable SI justified its consideration as a possible lead, especially given the lack of existing literature on its antileishmanial activity. The mechanism of action of berberine has been linked to the depolarization of the mitochondrial membrane of the parasite, causing cell arrest [34]. In addition, berberine chloride triggers apoptosis-like death following increased generation of reactive oxygen species [35] and induces immunomodulatory processes in the host [36]. However, to the best of our knowledge, no data regarding the potential antileishmanial effect of the remaining proto-berberine compounds is currently available, although several molecular targets have been suggested [37].
Two derivatives of stemonin, an alkaloid from traditional Chinese medicine with anti-inflammatory properties [38] and containing the characteristic pyrrole-[1,2-α]azepine scaffold, namely tuberostemonin and neotuberostemonin, were identified on our screening platform. Despite showing interesting effects against axenic amastigotes, they lost their antiparasitic activity against intramacrophagic amastigotes. Similar results were obtained with the indole alkaloid meisoindigo, a compound currently under investigation for its antitumor efficacy against leukemia [39], with no prior reports on antileishmanial activity. According to the results shown in Table 1, mesoindigo exhibited a marked loss of antileishmanial activity in the ex vivo model of intramacrophagic amastigotes and showed high cytotoxicity. Accordingly, we decided not to pursue further research on this compound.
It is worth highlighting a group of seven alkaloids derived from the quinoline pharmacophore isolated from the Chinese tree Camptotheca acuminata. These compounds, containing five fused pyrano indolizino quinoline rings, are exemplified by camptothecin, the benchmark molecule of this class. These compounds were identified in the primary screenings at both 1 μM and 10 μM concentrations but were subsequently discarded because of their toxicity. Camptothecin derivatives are well-known anti-tumor agents, and some semisynthetic derivatives, such as topotecan and irinotecan, are currently used to treat different types of cancer [40]. All these compounds irreversibly target DNA topoisomerase I, a nuclear enzyme responsible for unwinding DNA prior to gene transcription and DNA replication [41], and were previously tested by our group, showing interesting antileishmanial efficacy in vitro [22]. DNA topoisomerase I was identified as an important druggable target against trypanosomatids (including Leishmania) due to its peculiar structure as a heterodimeric enzyme (unlike the monomeric conformation characteristic of the other species) [42]. Nevertheless, due to the high cytotoxicity of this type of compound, further studies on this cluster were discontinued.
After evaluating efficacy and safety, we retained six compounds that met acceptable criteria for predictive pharmacokinetic analysis. Angoline, pseudocoptisine, coptisine, dehydrocorydaline, and 13-methylberberine, the analogue of berberine, were evaluated in silico to predict their ADMET profile. All six compounds complied with Lipinski’s rules according to the two applications described in Section 4, suggesting good gastrointestinal absorption. Furthermore, our in vitro results on the tolerability of these compounds in mouse intestinal organoids showed that none of them pose toxicological concerns, supporting their suitability for oral administration in future formulations. Angoline demonstrated a high predicted potential to inhibit key cytochrome P450 isoforms (CYP1A2, CYP2C9, CYP2D6, CYP2C19, and CYP3A4). However, CYP3A4, the most abundant isoform in the liver and intestine of adults and responsible for the metabolism of approximately 50% of small molecules [43], showed a predicted inhibition probability below 0.4 for the remaining compounds, suggesting that their potential for clinically relevant interactions may be low. Unfortunately, the low tolerance observed in mouse organoids and the bad toxicological predictions from both tools revealed that angoline has a high probability of mutagenicity and carcinogenicity, which precludes its further development. Nevertheless, considering all evaluated parameters, dehydrocorydaline was selected as the lead compound from the initial set of six candidates.
4. Materials and Methods
4.1. Preparation of the Drug Library
The alkaloids library (Ref.: HY−L071), purchased from MedChemExpress (MedChemExpress, Sollentuna, Sweden) in 2023, contains 449 structurally diverse molecules, including indoles, quinolines, isoquinolines, pyrrolidines, pyridines, pyrrolizidines, tropanes, terpenoids and steroids. Most compounds were dissolved in DMSO at a stock concentration of 10 mM and stored at −80 °C. Intermediate dilution plates were prepared at the required concentrations, stored at −20 °C, and subsequently used for the main parasite screening assays (Table S4).
4.2. Experimental Animals and Ethical Statement
To obtain bone marrow axenic amastigotes and/or intramacrophagic amastigotes, six- to eight-week-old female Balb/c mice were purchased from Janvier Labs (St Berthevin Cedex, France). All animal procedures were conducted in accordance with the Spanish legislation (RD 53/2013) and the European Union Directive (2010/63/UE). All protocols were approved by the Junta de Castilla y Leon (authorization number OEBA 010-2023).
4.3. Leishmania donovani Strain
The L. donovani IRFP strain was previously engineered in our laboratory [17] to constitutively express the iRFP gene, which encodes the infrared bacteriohytochrome protein from Rhodopseudomonas palustris [44]. The strain was maintained as free-living promastigotes in Schneider’s insect medium (Sigma-Aldrich, Merck, Darmstadt, Germany) supplemented with 20% (v/v) fetal bovine serum (FBS; Gibco, ThermoFisher Scientific, Waltham, MA, USA) and an antibiotic mixture containing 10,000 units/mL penicillin and 10,000 µg/mL streptomycin (HycloneTM, ThermoFisher Scientific, Waltham, MA, USA). Cultures were incubated at 26 °C following previously described conditions [45].
4.4. Isolation of Axenic Amastigotes of L. donovani IRFP from Bone Marrow
Female Balb/c mice aged from 6 to 8 weeks were intraperitoneally inoculated with 1.5 × 109 infective L. donovani IRFP metacyclic promastigotes. Between 8 and 12 weeks after infection, mice were humanely euthanised and bone marrow was collected from the femurs and tibias of both hind limbs. The bones were cut at both ends, and prewarmed PBS was flushed through the medullary cavity using a syringe fitted with a 27G needle, to extract the bone marrow cells. The resulting cell suspension was filtered through a 100 µm cell strainer and centrifuged at 2500 rpm for 10 min at room temperature. To obtain free amastigotes, the pelleted cells were resuspended within a 75 mL ventilated flask in amastigote culture medium (15 mM KCl; 136 mM KH2PO4; 10 mM K2HPO4.3H2O; 0.5 mM MgSO4.7 H2O; 24 mM NaHCO3; 22 mM glucose; 1 mM glutamine, 1x RPMI 1640 vitamin mix (Sigma-Aldrich, Merck, Darmstadt, Germany), 10 mM folic acid, 100 mM adenosine, 1x RPMI amino acid mix (Sigma-Aldrich, Merck, Darmstadt, Germany), 5 mg/mL hemin, antibiotic mixture, 25 mM MES, 10% FBS (Gibco, ThermoFisher Scientific, Waltham, MA, USA) and incubated at 37 °C in a humidified atmosphere containing 5% CO2 [46].
4.5. Culture of Ex Vivo Splenic Explants Infected with L. donovani IRFP
The isolation of intramacrophagic L. donovani IRPF amastigotes was performed as previously described [47]. Briefly, 1.5 × 109 infectious metacyclic promastigotes were injected intraperitoneally into Balb/c mice. Once infection was established (8–12 weeks post-inoculation), animals were humanely euthanized, and their spleens were aseptically removed, cut into small pieces, and digested with collagenase and biliverdin under sterile conditions. The cell pellet was resuspended in RPMI medium (Gibco) enriched with 20% FBS, 1 mM sodium pyruvate, 24 mM NaHCO3, 2 mM L-glutamine, 1x RPMI vitamins, 25 mM HEPES, and antibiotic mixture.
4.6. In Vitro Cytotoxicity and Tolerance Assessment
The efficacy of the selected compounds was assessed by measuring near-infrared fluorescence (700 nm) using an Odyssey Infrared Imaging System (Li-Cor, Lincoln, NE, USA) following the previously described protocols [47].
To evaluate the safety of the selected compounds, two cell lines were employed: RAW 264.7 (a mouse macrophage-derived cell line) and HepG2 (a human hepatoma cell line). Furthermore, to determine the intestinal tolerance relevant to potential oral administration, mouse intestinal organoids were prepared.
RAW 264.7 and HepG2 were routinely cultured under standard conditions. RAW 264.7 cells were maintained in RPMI medium (Gibco) supplemented with, 25 mM HEPES, 24 mM NaHCO3, 2 mM L-glutamine, mixture of antibiotics and 10% FBS. HepG2 cells were cultured in DMEM/F-12 (Gibco) and supplemented 10% of FBS. For cytotoxicity assays, 10,000 cells per well were seeded into 96-well plates and incubated overnight at 37 °C with 5% of CO2. Subsequently, 2-fold serial dilutions of the compounds were added and incubated for 72 h. Cell viability using the alamarBlue™ assay according to the manufacturer’s instructions, and fluorescence was measured with a Varioskan™ LUX microplate reader (ThermoFisher Scientific, Waltham, MA, USA). Non-linear regression analyses were performed using SigmaPlotTM software (version 10.0) to calculate CC50. Data were obtained from at least three independent experiments performed in duplicate.
Mouse intestinal organoids were generated following protocols standardized previously [48]. Briefly, to obtain adult intestine stem cells, C57BL/6 mice were humanely euthanized. A section of the duodenum was excised, rinsed in ice-cold PBS, and cut into small pieces for gentle dissociation using Gentle Cell Dissociation Reagent. Tissue fragments enriched in duodenal crypts and little tissue debris were microscopically selected and resuspended in a 1:1 mixture of Geltrex™ and IntestiCult™ Mouse Medium (Gibco, ThermoFisher Scientific, Waltham, MA, USA /Stemcell TechnologiesTM, Vancouver, BC, Canada). Aliquots of 50 µL of this suspension were dispensed into the center of each well of a prewarmed 24-well plate to form domes containing organoids, which were then incubated to allow matrix polymerization. Then, 500 mL of IntestiCult™ Mouse Medium was added to each well. The plate was incubated at 37 °C in a humidified atmosphere containing 5% CO2, and the culture medium was refreshed every 48 h. During this period, organoids continued to proliferate, and passaging was performed at a 1:4 ratio every 7–10 days.
To determine the in vitro tolerance to oral administration of the active compounds, a 384-well assay was adapted from the protocol described by Du and co-workers [49]. Briefly, following medium removal, organoids were collected from the extracellular matrix using the same procedure as for passaging. The resulting pellet was resuspended in 3.2 mL of a 1:1 mixture of Geltrex™ LDEV-Free Reduced Growth Factor Basement Membrane Matrix (without phenol red, Gibco) and IntestiCult™ Mouse Medium. Using a chilled pipette dispenser, 8 µL of the organoid suspension was dispensed into pre-cooled 384-well plates, which were then gently agitated to evenly distribute the matrix. The plates were incubated at 37 °C for 10 min and then, 32 µL of IntestiCult™ Mouse Medium was added to each well. To prevent edge effects, 100 µL of sterile water was added to the perimeter wells. After 3 days of culture, 10 µL per well of the test compounds dissolved in IntestiCult™ Mouse Medium were added. Positive (0.03% H2O2) and negative (0.2% DMSO) controls were included in each assay. After 72 h of incubation, 5 µL of alamarBlue™ HS were added to each well, and fluorescence was measured after 24 h using a Varioskan™ LUX microplate reader. All assays were conducted in triplicate and repeated in at least three independent experiments.
4.7. Chemo-Informatics
4.7.1. Chemical Clustering
DataWarrior version V06.04.02—an open-source cheminformatics software tool developed by Sander and coworkers and www.openmolecules.org (accessed on 31 March 2025) [18]—was used to group compounds based on chemical structure similarity. The SMILES of the 72 active compounds were compiled into a .csv file and used to calculate the SkelSpheres molecular descriptors for each molecule in the dataset. Clustering was then performed using the default parameters. The SkelSpheres descriptor is particularly suitable for fine-grained chemical graph similarity analysis, as it accounts for both aromatic and stereochemical features.
4.7.2. Murcko Scaffold Enrichment
The Murcko Scaffold, which represents the core ring systems and the direct connections among them within a molecule, was extracted from the complete list of alkaloids analyzed by the DataWarrior software [23]. To identify enriched Murcko frameworks in the active compound dataset, a two-step filtering approach was applied. First, scaffolds with a frequency greater than 1 were selected. For the enrichment analysis, only those scaffolds containing at least one active compound were retained. The Enrichment Factor (EF) was then calculated to quantify the relative abundance of active compounds within each retained Murcko scaffold. EF was defined as the ratio between the proportion of active compounds within a given scaffold and the overall proportion of active compounds in the dataset [50,51].
4.7.3. Predictive Druglikeness and ADMET
ADMET and druglikeness properties were evaluated using three open-source cheminformatic tools: DataWarrior [18], SwissADME [52] and admetSAR3.0 [53]. In DataWarrior, physicochemical and toxicity-related properties were calculated using the SMILES entered through chemical structure menu. The selected druglikeness parameters included the number of hydrogen bond acceptors (nHA), number of hydrogen bond donors (nHD), number of rotatable bonds (nRot), topological polar surface area (TPSA), lipophilicity (cLogP); water solubility (cLogS), molecular weight (Mw) and overall druglikeness score. The evaluated toxicity parameters comprised mutagenic, tumorigenic, reproductive effects, irritation and PAINS (Pan-Assay Interference Compounds) patterns. In the SwissADME, the SMILES of our six compounds were introduced to obtain additional data on physicochemical properties, lipophilicity, water solubility, pharmacokinetic behaviour, druglikeness and medicinal chemistry features. Finally, the same SMILES strings were uploaded to the admetSAR3.0 platform to predict ADMET profiles.
5. Conclusions
Screening of the commercial alkaloid collection HY L071 identified a group of 16 compounds with antileishmanial activity and well-defined chemical scaffolds. In silico prediction for the six most promising compounds—those with the highest efficacy, safety, and intestinal tolerability—revealed that all were derived from isoquinoline—one benzo[c]phenanthridine and five protoberberines. These compounds were predicted to be suitable for oral administration and, with the exception of angoline, did not present significant toxicological problems for further development.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules30214210/s1, Figure S1: Z’-factor; Figure S2: 2D similarity chart; Figure S3: Dose–response curves of the remaining hit compounds; Figure S4: Dose–response curves of the lead compounds in organoids; Table S1: List of the 72 hit compounds; Table S2: Total Murcko scaffolds. Table S3: Other druglikeness rules; Table S4: List of 449 alkaloids compounds tested.
Author Contributions
Conceptualization, R.B.-F. and R.M.R.; methodology, C.S.-K., E.M.-F. and M.-C.G.-M.; software, C.S.-K. and C.F.-R.; validation, C.S.-K., E.M.-F. and M.-C.G.-M.; formal analysis, R.B.-F. and R.M.R.; investigation, C.S.-K.; data curation, C.G.-E.; writing—original draft preparation, C.S.-K.; writing—review and editing, R.B.-F. and C.G.-E.; visualization, C.G.-E. and Y.P.-P.; supervision, R.B.-F. and R.M.R.; funding acquisition, R.B.-F. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The animal protocol used in this study complies with the Spanish Act (RD 53/2013) and the European Union Directive (2010/63/UE). All protocols were approved by the Junta de Castilla y Leon under the authorization OEBA 10-2023 (protocol code: OEBA 10-2023, date of approval: 14 April 2023).
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Acknowledgments
We express our sincere gratitude to the Fundación Mujeres por África for awarding C.S.-K. her PhD scholarship. Maria-Cristina Gonzalez-Montero received a fellowship from Universidad de León. Sincere gratitude goes to the laboratory technicians Irene Pilar Martínez Torres and Guillermo Peñalba González for supporting this research by performing reagent preparation and instrument maintenance.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| NTD | Neglected Tropical Diseases |
| VL | Visceral Leishmaniasis |
| HTS | High-Throughput Screening |
| IRPF | Infrared Fluorescent Protein |
| DNDi | Drugs for Neglected Diseases Initiative |
| FBS | Fetal Bovine Serum |
| ADMET | Absorption, Distribution, Metabolism, Excretion and Toxicity |
| SMILES | Simplified Molecular Input Line Entry System |
| PAINS | Pan-Assay Interference Compounds |
References
- World Health Organization. Ending the Neglect to Attain the Sustainable Development Goals: A Road Map for Neglected Tropical Diseases 2021–2030; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- Hietanen, H.; Pfavayi, L.T.; Mutapi, F. Unlocking the blueprint to eliminating neglected tropical diseases: A review of efforts in 50 countries that have eliminated at least 1 NTD. PLoS Negl. Trop. Dis. 2025, 19, e0013424. [Google Scholar] [CrossRef]
- DNDi-Annual Report 2024. Available online: https://dndi.org/wp-content/uploads/2025/07/DNDi-AnnualReport-2024.pdf (accessed on 1 September 2025).
- Burza, S.; Croft, S.L.; Boelaert, M. Leishmaniasis. Lancet 2018, 392, 951–970. [Google Scholar] [CrossRef]
- Mann, S.; Frasca, K.; Scherrer, S.; Henao-Martínez, A.F.; Newman, S.; Ramanan, P.; Suarez, J.A. A review of leishmaniasis: Current knowledge and future directions. Curr. Trop. Med. Rep. 2021, 8, 121–132. [Google Scholar] [CrossRef]
- Jain, S.; Madjou, S.; Farah, J.; Agua, V.; Maia-Elkhoury, A.N.; Valadas, S.; Warusavithana, S.; Osman, M.; Yajima, A.; Beshahe, A.; et al. Global leishmaniasis surveillance updates 2023: 3 years of the NTD road map. Wkly. Epidemiol. Rec. 2024, 45, 653–669. [Google Scholar]
- Reguera, R.M.; Pérez-Pertejo, Y.; Gutiérrez-Corbo, C.; Domínguez-Asenjo, B.; Ordóñez, C.; García-Estrada, C.; Martínez-Valladares, M.; Balaña-Fouce, R. Current and promising novel drug candidates against visceral leishmaniasis. Pure Appl. Chem. 2019, 91, 1385–1404. [Google Scholar] [CrossRef]
- Swinney, D.C. Phenotypic vs. target-based drug discovery for first-in-class medicines. Clin. Pharm. Ther. 2013, 93, 299–301. [Google Scholar] [CrossRef] [PubMed]
- Reguera, R.M.; Calvo-Álvarez, E.; Alvarez-Velilla, R.; Balaña-Fouce, R. Target-based vs. phenotypic screenings in Leishmania drug discovery: A marriage of convenience or a dialogue of the deaf? Int. J. Parasitol. Drugs Drug Resist. 2014, 4, 355–357. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Leelananda, S.P.; Lindert, S. Computational methods in drug discovery. Beilstein J. Org. Chem. 2016, 12, 2694–2718. [Google Scholar] [CrossRef] [PubMed]
- Frye, L.; Bhat, S.; Akinsanya, K.; Abel, R. From computer-aided drug discovery to computer-driven drug discovery. Drug Discov. Today Technol. 2021, 39, 111–117. [Google Scholar] [CrossRef]
- Harvey, A.L.; Edrada-Ebel, R.; Quinn, R.J. The re-emergence of natural products for drug discovery in the genomics era. Nat. Rev. Drug Discov. 2015, 14, 111–129. [Google Scholar] [CrossRef]
- Qiu, S.; Sun, H.; Zhang, A.H.; Xu, H.Y.; Yan, G.L.; Han, Y.; Wang, X.J. Natural alkaloids: Basic aspects, biological roles, and future perspectives. Chin. J. Nat. Med. 2014, 12, 401–406. [Google Scholar] [CrossRef] [PubMed]
- Cockram, P.E.; Smith, T.K. Active natural product scaffolds against trypanosomatid parasites: A review. J. Nat. Prod. 2018, 81, 2138–2154. [Google Scholar] [CrossRef] [PubMed]
- Daley, S.K.; Cordell, G.A. Alkaloids in contemporary drug discovery to meet global disease needs. Molecules 2021, 26, 3800. [Google Scholar] [CrossRef] [PubMed]
- Scotti, M.T.; Scotti, L.; Ishiki, H.; Ribeiro, F.F.; Cruz, R.M.; Oliveira, M.P.; Mendonça, F.J. Natural products as a source for antileishmanial and antitrypanosomal agents. Comb. Chem. High. Throughput Screen. 2016, 19, 537–553. [Google Scholar] [CrossRef]
- Calvo-Álvarez, E.; Stamatakis, K.; Punzón, C.; Álvarez-Velilla, R.; Tejería, A.; Escudero-Martínez, J.M.; Pérez-Pertejo, Y.; Fresno, M.; Balaña-Fouce, R.; Reguera, R.M. Infrared fluorescent imaging as a potent tool for in vitro, ex vivo and in vivo models of visceral leishmaniasis. PLoS Negl. Trop. Dis. 2015, 9, e0003666. [Google Scholar] [CrossRef]
- Sander, T.; Freyss, J.; von Korff, M.; Rufener, C. DataWarrior: An open-source program for chemistry aware data visualization and analysis. J. Chem. Inf. Model. 2015, 55, 460–473. [Google Scholar] [CrossRef]
- Berg, E.L. The future of phenotypic drug discovery. Cell Chem. Biol. 2021, 28, 424–430. [Google Scholar] [CrossRef]
- Ravinder; Bhaskar; Gangwar, S.; Goyal, N. Development of luciferase expressing Leishmania donovani axenic amastigotes as primary model for in vitro screening of antileishmanial compounds. Curr. Microbiol. 2012, 65, 696–700. [Google Scholar] [CrossRef]
- Zhang, J.H.; Chung, T.D.Y.; Oldenburg, K.R. A simple statistical parameter for use in evaluation and validation of high throughput screening assays. J. Biomol. Screen. 1999, 4, 67–73. [Google Scholar] [CrossRef]
- Balaña-Fouce, R.; Redondo, C.M.; Pérez-Pertejo, Y.; Díaz-González, R.; Reguera, R.M. Targeting atypical trypanosomatid DNA topoisomerase I. Drug Discov. Today 2006, 11, 733–740. [Google Scholar] [CrossRef]
- Bemis, G.W.; Murcko, M.A. The properties of known drugs. 1. Molecular frameworks. J. Med. Chem. 1996, 39, 2887–2893. [Google Scholar] [CrossRef]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 2001, 46, 3–26. [Google Scholar] [CrossRef] [PubMed]
- DNDi. Available online: https://dndi.org/diseases/visceral-leishmaniasis/projects-achievements/ (accessed on 1 September 2025).
- Yaluff, G.; Herrera, L.; Rolón, M.S.; Vega, C.; Cerecetto, H. The quinoline framework and related scaffolds in natural products with anti-Leishmania properties. Front. Chem. 2025, 13, 1571067. [Google Scholar] [CrossRef] [PubMed]
- Vermeersch, M.; da Luz, R.I.; Toté, K.; Timmermans, J.P.; Cos, P.; Maes, L. In vitro susceptibilities of Leishmania donovani promastigote and amastigote stages to antileishmanial reference drugs: Practical relevance of stage-specific differences. Antimicrob. Agents Chemother. 2009, 53, 3855–3859. [Google Scholar] [CrossRef] [PubMed]
- Fotie, J.; Bohle, D.S.; Olivier, M.; Adelaida Gómez, M.; Nzimiro, S. Trypanocidal and antileishmanial dihydrochelerythrine derivatives from Garcinia lucida. J. Nat. Prod. 2007, 70, 1650–1653. [Google Scholar] [CrossRef]
- Han, N.; Yang, Z.; Liu, Z.; Liu, H.; Yin, J. Research progress on natural benzophenanthridine alkaloids and their pharmacological functions: A review. Nat. Prod. Commun. 2016, 11, 1181–1188. [Google Scholar] [CrossRef]
- Peng, R.; Xu, M.; Xie, B.; Min, Q.; Hui, S.; Du, Z.; Liu, Y.; Yu, W.; Wang, S.; Chen, X.; et al. Insights on antitumor activity and mechanism of natural benzophenanthridine alkaloids. Molecules 2023, 28, 6588. [Google Scholar] [CrossRef]
- Fuchino, H.; Kawano, M.; Mori-Yasumoto, K.; Sekita, S.; Satake, M.; Ishikawa, T.; Kiuchi, F.; Kawahara, N. In vitro leishmanicidal activity of benzophenanthridine alkaloids from Bocconia pearcei and related compounds. Chem. Pharm. Bull. 2010, 58, 1047–1050. [Google Scholar] [CrossRef]
- Castillo, D.; Sauvain, M.; Rivaud, M.; Jullian, V. In vitro and in vivo activity of benzo[c]phenanthridines against Leishmania amazonensis. Planta Med. 2014, 80, 902–906. [Google Scholar] [CrossRef]
- Ornelas-Cruces, M.; Escalona-Montaño, A.R.; Salaiza-Suazo, N.; Sifontes-Rodríguez, S.; Aguirre-García, M.M. The potential role of sanguinarine as an inhibitor of leishmania pp2c in the induction of apoptosis. Acta Parasitol. 2025, 70, 35. [Google Scholar] [CrossRef]
- De Sarkar, S.; Sarkar, D.; Sarkar, A.; Dighal, A.; Staniek, K.; Gille, L.; Chatterjee, M. Berberine chloride mediates its antileishmanial activity by inhibiting Leishmania mitochondria. Parasitol. Res. 2019, 118, 335–345. [Google Scholar] [CrossRef] [PubMed]
- Saha, P.; Sen, R.; Hariharan, C.; Kumar, D.; Das, P.; Chatterjee, M. Berberine chloride causes a caspase-independent, apoptotic-like death in Leishmania donovani promastigotes. Free Radic. Res. 2009, 43, 1101–1110. [Google Scholar] [CrossRef] [PubMed]
- Saha, P.; Bhattacharjee, S.; Sarkar, A.; Manna, A.; Majumder, S.; Chatterjee, M. Berberine chloride mediates its anti-leishmanial activity via differential regulation of the mitogen activated protein kinase pathway in macrophages. PLoS ONE 2011, 6, e18467. [Google Scholar] [CrossRef] [PubMed]
- Alamzeb, M.; Saqib, A.; Mamoon-Ur-Rashid; Khan, B.; Ihsanullah; Adnan; Omer, M.; Ullah, A.; Ali, J.; Setzer, W.N.; et al. Antileishmanial potential of berberine alkaloids from berberis glaucocarpa roots: Molecular docking suggests relevant leishmania protein targets. Nat. Prod. Commun. 2021, 16, 805–812. [Google Scholar] [CrossRef]
- Zhang, N.; Xu, Y.; Yue, X.; Xiong, L.; Li, H.; Chen, L. Isolation, characterization and anti-inflammatory effect of alkaloids from the roots of Stemona tuberosa Lour. Phytochemistry 2024, 220, 114013. [Google Scholar] [CrossRef]
- Zhang, Z.X.; Li, S.Y.; Li, F.F.; Shi, Q.Y.; Tan, C.Y.; Wang, X.J.; Li, M.; Liu, Y.B.; Jin, J.; Li, Y.; et al. Meisoindigo acts as a molecular glue to target PKMYT1 for degradation in chronic myeloid leukemia therapy. Adv. Sci. 2025, 12, e2413676. [Google Scholar] [CrossRef]
- Venditto, V.J.; Simanek, E.E. Cancer therapies utilizing the camptothecins: A review of the in vivo literature. Mol. Pharm. 2010, 7, 307–349. [Google Scholar] [CrossRef]
- Thomas, A.; Pommier, Y. Targeting topoisomerase I in the era of precision medicine. Clin. Cancer Res. 2019, 25, 6581–6589. [Google Scholar] [CrossRef]
- Villa, H.; Otero Marcos, A.R.; Reguera, R.M.; Balaña-Fouce, R.; García-Estrada, C.; Pérez-Pertejo, Y.; Tekwani, B.L.; Myler, P.J.; Stuart, K.D.; Bjornsti, M.A.; et al. A novel active DNA topoisomerase I in Leishmania donovani. J. Biol. Chem. 2003, 278, 3521–3526. [Google Scholar] [CrossRef]
- Lee, J.; Beers, J.L.; Geffert, R.M.; Jackson, K.D. A review of CYP-mediated drug interactions: Mechanisms and in vitro drug-drug interaction assessment. Biomolecules 2024, 14, 99. [Google Scholar] [CrossRef]
- Yang, X.; Stojkovic, E.A.; Kuk, J.; Moffat, K. Crystal structure of the chromophore binding domain of an unusual bacteriophytochrome, RpBphP3, reveals residues that modulate photoconversion. Proc. Natl. Acad. Sci. USA 2007, 104, 12571–12576. [Google Scholar] [CrossRef]
- Reguera, R.M.; Fouce, R.B.; Cubría, J.C.; Bujidos, M.L.; Ordóñez, D. Fluorinated analogues of L-ornithine are powerful inhibitors of ornithine decarboxylase and cell growth of Leishmania infantum promastigotes. Life Sci. 1995, 56, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Melcón-Fernández, E.; Galli, G.; García-Estrada, C.; Balaña-Fouce, R.; Reguera, R.M.; Pérez-Pertejo, Y. Miltefosine and nifuratel combination: A promising therapy for the treatment of Leishmania donovani visceral leishmaniasis. Int. J. Mol. Sci. 2023, 24, 1635. [Google Scholar] [CrossRef] [PubMed]
- Domínguez-Asenjo, B.; Gutiérrez-Corbo, C.; Álvarez-Bardón, M.; Pérez-Pertejo, Y.; Balaña-Fouce, R.; Reguera, R.M. Ex vivo phenotypic screening of two small repurposing drug collections identifies nifuratel as a potential new treatment against visceral and cutaneous leishmaniasis. ACS Infect. Dis. 2021, 7, 2390–2401. [Google Scholar] [CrossRef] [PubMed]
- Kuratnik, A.; Giardina, C. Intestinal organoids as tissue surrogates for toxicological and pharmacological studies. Biochem. Pharmacol. 2013, 85, 1721–1726. [Google Scholar] [CrossRef]
- Du, Y.; Li, X.; Niu, Q.; Mo, X.; Qui, M.; Ma, T.; Kuo, C.J.; Fu, H. Development of a miniaturized 3D organoid culture platform for ultra-high-throughput screening. J. Mol. Cell Biol. 2020, 12, 630–643. [Google Scholar] [CrossRef]
- Manelfi, C.; Gemei, M.; Talarico, C.; Cerchia, C.; Fava, A.; Lunghini, F.; Beccari, A.R. “Molecular Anatomy”: A new multi-dimensional hierarchical scaffold analysis. J. Cheminform 2021, 13, 54. [Google Scholar] [CrossRef]
- Yu, T.; Nantasenamat, C.; Kachenton, S.; Anuwongcharoen, N.; Piacham, T. Cheminformatic analysis and machine learning modeling to investigate androgen receptor antagonists to combat prostate cancer. ACS Omega 2023, 8, 6729–6742. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef]
- Gu, Y.; Yu, Z.; Wang, Y.; Chen, L.; Lou, C.; Yang, C.; Li, W.; Liu, G.; Tang, Y. admetSAR3.0: A comprehensive platform for exploration, prediction and optimization of chemical ADMET properties. Nucleic Acids Res. 2024, 52, W432–W438. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).