Eleutherococcus senticosus Fruit Extract Stimulates the Membrane Potential of the Trachea and Small Intestine in Rabbits
Abstract
1. Introduction
2. Results and Discussion
2.1. The Effect of the Extract on the Transepithelial Electric Potential in the Distal Section of the Trachea and Small Intestine
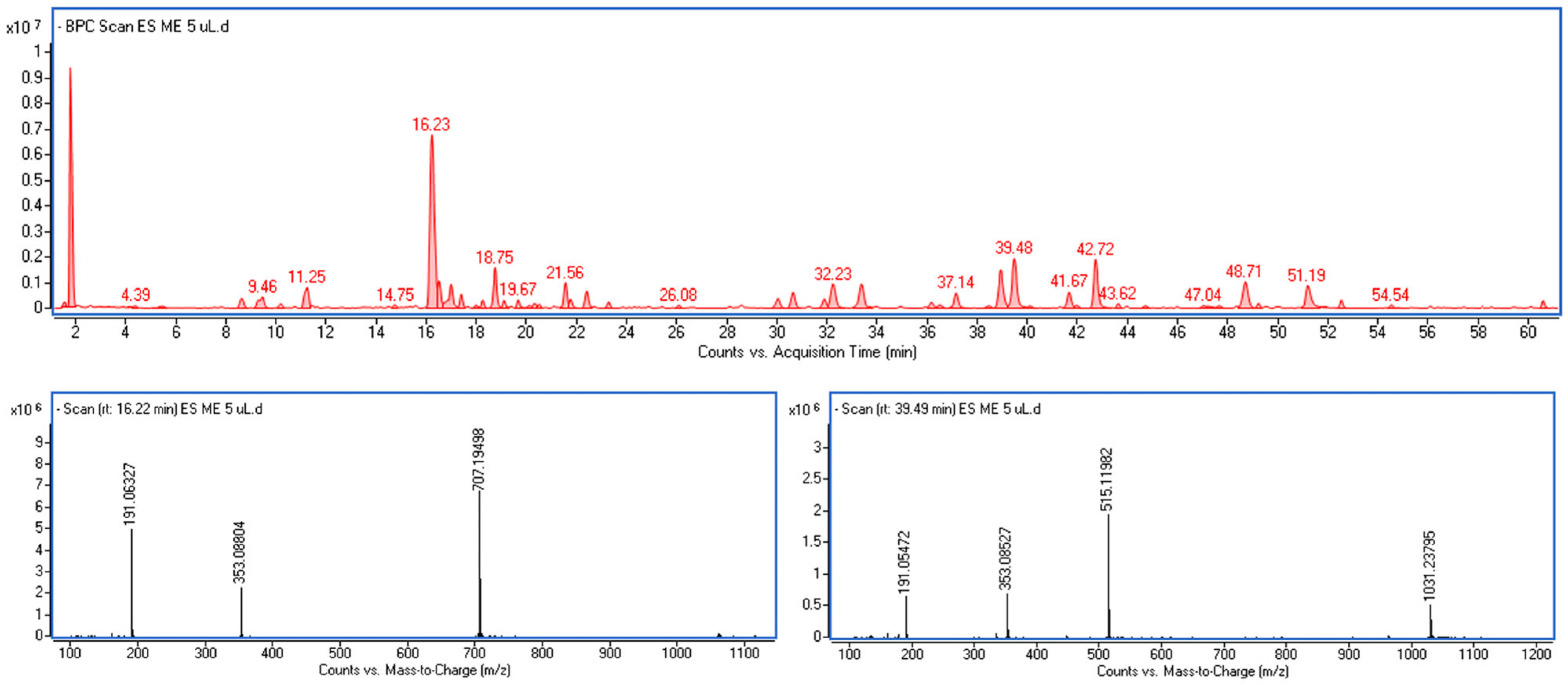
2.2. Ultra-High-Performance Liquid Chromatography (UHPLC)
3. Materials and Methods
3.1. Plant Material
3.2. The Effect of the Extract on the Transepithelial Electric Potential in the Section of the Rabbit Trachea and Small Intestine
Experimental Procedure
3.3. Chemicals and Solutions
- 0.001 mg/100 mL (INT-0.001);
- 0.1 mg/100 mL (INT-0.1);
- 10 mg/100 mL (INT-10);
- The Ringer solution (RH): 147.2 mM Na+, 4.0 mM K+, 2.2 mM Ca2+, 2.6 mM Mg2+, 155.6 mM Cl−, pH 7.4 (POCH, Poland).
3.4. Ultra-High-Performance Liquid Chromatography (UHPLC-DAD-MS)
3.5. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| dPD | changes in the transepithelial electrical potential difference after 15 s mechanical and mechanical-chemical stimulation (mV) |
| ES | Eleutherococcus senticosus |
| INT | Eleutherococcus senticosus extract |
| Me | median |
| n | number of specimens isolated from the rabbit trachea and small intestine wall |
| p | statistically significant difference at p < 0.05 |
| PD | transepithelial electrical potential difference (mV) |
| R | transepithelial electrical resistance (Ω/cm2) |
| RH | Ringer solution |
| RS | control stimulation |
References
- Li, W.; Luo, Q.; Jin, L.H. Acanthopanax senticosus Extracts Have a Protective Effect on Drosophila Gut Immunity. J. Ethnopharmacol. 2013, 146, 257–263. [Google Scholar] [CrossRef]
- Załuski, D.; Olech, M.; Verpoorte, R.; Khan, I.; Kuźniewski, R.; Nowak, R. Phytoconstituents and Nutritional Properties of the Fruits of Eleutherococcus divaricatus and Eleutherococcus sessiliflorus: A Study of Non-European Species Cultivated in Poland. Oxid. Med. Cell. Longev. 2017, 2017, 8374295. [Google Scholar] [CrossRef]
- Załuski, D.; Smolarz, H.D.; Gawlik-Dziki, U. Bioactive Compounds and Antioxidative, Antileukemic and Anti-MMPs Activity of Eleutherococcus Species Cultivated in Poland. Nat. Prod. Commun. 2012, 7, 1483–1486. [Google Scholar] [CrossRef]
- Zhang, N.; Van Crombruggen, K.; Holtappels, G.; Bachert, C. A Herbal Composition of Scutellaria Baicalensis and Eleutherococcus senticosus Shows Potent Anti-Inflammatory Effects in an Ex Vivo Human Mucosal Tissue Model. Evid. Based Complement. Altern. Med. 2012, 2012, 673145. [Google Scholar] [CrossRef]
- Miyauchi-Wakuda, S.; Kagota, S.; Maruyama-Fumoto, K.; Shiokawa, Y.; Yamada, S.; Shinozuka, K. Acanthopanax senticosus Root Extract Exerts Dual Action on Mouse Ileal Smooth Muscle Function, Leading to Modulation of Gastrointestinal Motility. Biol. Pharm. Bull. 2020, 43, 817–822. [Google Scholar] [CrossRef] [PubMed]
- Graczyk, F.; Gębalski, J.; Piskorska, E.; Małkowska, M.; Słomka, A.; Gawenda-Kempczyńska, D.; Kondrzycka-Dąda, A.; Olszewska-Słonina, D.; Styczyński, J.; Taglialatela-Scafati, O.; et al. The Eleutherococcus senticosus Fruits’ Intractum Affects Changes in the Transepithelial Electric Potential in the Distal Section of the Rabbit’s Large Intestine and Inhibits Hyaluronidase. J. Ethnopharmacol. 2024, 325, 117847. [Google Scholar] [CrossRef] [PubMed]
- Panossian, A.; Brendler, T. The Role of Adaptogens in Prophylaxis and Treatment of Viral Respiratory Infections. Pharmaceuticals 2020, 13, 236. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Guan, L.; Zhu, L.; Wang, K.; Gao, Y.; Li, J.; Yan, S.; Ji, N.; Zhou, Y.; Yao, X.; et al. A Review of the Extraction and Purification Methods, Biological Activities, and Applications of Active Compounds in Acanthopanax senticosus. Front. Nutr. 2024, 11, 1391601. [Google Scholar] [CrossRef]
- Graczyk, F.; Orzechowska, B.; Franz, D.; Strzemski, M.; Verpoorte, R.; Załuski, D. The Intractum from the Eleutherococcus senticosus Fruits Affects the Innate Immunity in Human Leukocytes: From the Ethnomedicinal Use to Contemporary Evidence-Based Research. J. Ethnopharmacol. 2021, 268, 113636. [Google Scholar] [CrossRef]
- Taylor, P.J.; Nengovhela, A.; Denys, C.; Scott, G.R.; Ivy, C.M. Adaptation in Brain Structure and Respiratory and Olfactory Structures across Environmental Gradients in African and North American Muroid Rodents. Integr. Zool. 2024, 19, 165–181. [Google Scholar] [CrossRef]
- Mirkov, I.; Tucovic, D.; Kulas, J.; Malesevic, A.; Kataranovski, D.; Kataranovski, M.; Popov Aleksandrov, A. Physiological Strategies in Wild Rodents: Immune Defenses of Commensal Rats. Integr. Zool. 2024, 19, 350–370. [Google Scholar] [CrossRef] [PubMed]
- Soriano, J.B.; Polverino, F.; Cosio, B.G. What Is Early COPD and Why Is It Important? Eur. Respir. J. 2018, 52, 1801448. [Google Scholar] [CrossRef] [PubMed]
- Spurzem, J.R.; Rennard, S.I. Pathogenesis of COPD. Semin. Respir. Crit. Care Med. 2005, 26, 142–153. [Google Scholar] [CrossRef] [PubMed]
- Dolinger, M.; Torres, J.; Vermeire, S. Crohn’s Disease. Lancet 2024, 403, 1177–1191. [Google Scholar] [CrossRef]
- Gryn-Rynko, A.; Hołyńska-Iwan, I.; Janiak, M.A.; Olszewska-Słonina, D.; Amarowicz, R.; Graczyk, R. The Impact of Morus alba L. Leaf Extract on Intestinal Ion Transport. An in Vitro Study. Biomed. Pharmacother. 2022, 150, 112939. [Google Scholar] [CrossRef]
- Hołyńska-Iwan, I.; Sobiesiak, M.; Kowalczyk, W.; Wróblewski, M.; Cwynar, A.; Szewczyk-Golec, K. Nickel Ions Influence the Transepithelial Sodium Transport in the Trachea, Intestine and Skin. Sci. Rep. 2023, 13, 6931. [Google Scholar] [CrossRef]
- Kunzelmann, K.; Mall, M. Electrolyte Transport in the Mammalian Colon: Mechanisms and Implications for Disease. Physiol. Rev. 2002, 82, 245–289. [Google Scholar] [CrossRef]
- Song, L.; Wu, T.; Zhang, L.; Wan, J.; Ruan, Z. Chlorogenic Acid Improves the Intestinal Barrier by Relieving Endoplasmic Reticulum Stress and Inhibiting ROCK/MLCK Signaling Pathways. Food Funct. 2022, 13, 4562–4575. [Google Scholar] [CrossRef]
- Zha, P.; Liu, W.; Zhou, Y.; Chen, Y. Protective Effects of Chlorogenic Acid on the Intestinal Barrier of Broiler Chickens: An Immunological Stress Model Study. Poult. Sci. 2024, 103, 103949. [Google Scholar] [CrossRef]
- Naveed, M.; Hejazi, V.; Abbas, M.; Kamboh, A.A.; Khan, G.J.; Shumzaid, M.; Ahmad, F.; Babazadeh, D.; FangFang, X.; Modarresi-Ghazani, F.; et al. Chlorogenic Acid (CGA): A Pharmacological Review and Call for Further Research. Biomed. Pharmacother. 2018, 97, 67–74. [Google Scholar] [CrossRef]
- Spagnol, C.M.; Assis, R.P.; Brunetti, I.L.; Isaac, V.L.B.; Salgado, H.R.N.; Corrêa, M.A. In Vitro Methods to Determine the Antioxidant Activity of Caffeic Acid. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2019, 219, 358–366. [Google Scholar] [CrossRef]
- Nardini, M.; D’Aquino, M.; Tomassi, G.; Gentili, V.; Di Felice, M.; Scaccini, C. Inhibition of Human Low-Density Lipoprotein Oxidation by Caffeic Acid and Other Hydroxycinnamic Acid Derivatives. Free. Radic. Biol. Med. 1995, 19, 541–552. [Google Scholar] [CrossRef] [PubMed]
- Chhetri, D.R. Myo-Inositol and Its Derivatives: Their Emerging Role in the Treatment of Human Diseases. Front. Pharmacol. 2019, 10, 1172. [Google Scholar] [CrossRef] [PubMed]
- Arefhosseini, S.; Roshanravan, N.; Asghari, S.; Tutunchi, H.; Ebrahimi-Mameghani, M. Expression of Inflammatory Genes, WBC-Derived Inflammatory Biomarkers and Liver Function Indices: Effects of Myo-Inositol Supplementation in Obese Patients with NAFLD. J. Funct. Foods 2023, 104, 105524. [Google Scholar] [CrossRef]
- Arefhosseini, S.; Roshanravan, N.; Tutunchi, H.; Rostami, S.; Khoshbaten, M.; Ebrahimi-Mameghani, M. Myo-Inositol Supplementation Improves Cardiometabolic Factors, Anthropometric Measures, and Liver Function in Obese Patients with Non-Alcoholic Fatty Liver Disease. Front. Nutr. 2023, 10, 1092544. [Google Scholar] [CrossRef]
- Quecchia, C.; Vianello, A. The Therapeutic Potential of Myo-Inositol in Managing Patients with Respiratory Diseases. Int. J. Mol. Sci. 2025, 26, 2185. [Google Scholar] [CrossRef]
- Ong, H.X.; Traini, D.; Salama, R.; Anderson, S.D.; Daviskas, E.; Young, P.M. The Effects of Mannitol on the Transport of Ciprofloxacin across Respiratory Epithelia. Mol. Pharm. 2013, 10, 2915–2924. [Google Scholar] [CrossRef]
- Düchler, M.; Pengg, M.; Brunner, S.; Müller, M.; Brem, G.; Wagner, E. Transfection of Epithelial Cells Is Enhanced by Combined Treatment with Mannitol and Polyethyleneglycol. J. Gene Med. 2001, 3, 115–124. [Google Scholar] [CrossRef]
- Shawkat, H.; Westwood, M.M.; Mortimer, A. Mannitol: A Review of Its Clinical Uses. Contin. Educ. Anaesth. Crit. Care Pain 2012, 12, 82–85. [Google Scholar] [CrossRef]
- Shi, J.; Qian, J.; Li, H.; Luo, H.; Luo, W.; Lin, Z. Renal Tubular Epithelial Cells Injury Induced by Mannitol and Its Potential Mechanism. Ren. Fail. 2018, 40, 85–91. [Google Scholar] [CrossRef]
- Miyano, T.; Suzuki, A.; Sakamoto, N. Hyperosmotic Stress Induces Epithelialmesenchymal Transition through Rearrangements of Focal Adhesions in Tubular Epithelial Cells. PLoS ONE 2021, 16, e0261345. [Google Scholar] [CrossRef]
- Zahid, N.; Schweiger, P.; Galinski, E.; Deppenmeier, U. Identification of Mannitol as Compatible Solute in Gluconobacter oxydans. Appl. Microbiol. Biotechnol. 2015, 99, 5511–5521. [Google Scholar] [CrossRef]
- Xu, W.; Hong, S.J.; Zhong, A.; Xie, P.; Jia, S.; Xie, Z.; Zeitchek, M.; Niknam-Bienia, S.; Zhao, J.; Porterfield, D.M.; et al. Sodium Channel Nax Is a Regulator in Epithelial Sodium Homeostasis. Sci. Transl. Med. 2015, 7, 312ra177. [Google Scholar] [CrossRef]
- Sunderman, F.W.; Hopfer, S.M.; Sweeney, K.R.; Marcus, A.H.; Most, B.M.; Creason, J. Nickel Absorption and Kinetics in Human Volunteers. Proc. Soc. Exp. Biol. Med. 1989, 191, 5–11. [Google Scholar] [CrossRef]
- Rizzi, A.; Nucera, E.; Laterza, L.; Gaetani, E.; Valenza, V.; Corbo, G.M.; Inchingolo, R.; Buonomo, A.; Schiavino, D.; Gasbarrini, A. Irritable Bowel Syndrome and Nickel Allergy: What Is the Role of the Low Nickel Diet? J. Neurogastroenterol. Motil. 2017, 23, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Brasier, A.R.; Zhao, Y.; Chung, K.F. Editorial: Mucosal Adaptations to Chronic Airway Injury: Mechanisms and Interrelationships of Epithelial Plasticity on Innate Immunity and Airway Remodeling. Front. Immunol. 2024, 15, 1435120. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.J.; Leung, J.M.; Sin, D.D. A Tale as Old as Time—The Importance of Accelerated Lung Aging in Chronic Obstructive Pulmonary Disease. Expert Rev. Respir. Med. 2025, 19, 597–608. [Google Scholar] [CrossRef] [PubMed]
- Neurath, M.F.; Artis, D.; Becker, C. The Intestinal Barrier: A Pivotal Role in Health, Inflammation, and Cancer. Lancet Gastroenterol. Hepatol. 2025, 10, 573–592. [Google Scholar] [CrossRef]
| n = 20 | dPD [mV] n = 20 | R [Ω×cm2] n = 20 | ||
|---|---|---|---|---|
| Median | IQR | Median | IQR | |
| RS-1 | −0.64 a | 1.97 | 216.75 | 140.00 |
| INT-0.001 | 0.00 ab | 0.72 | 224.25 | 177.50 |
| RS-2 | −0.24 bc | 1.39 | 225.75 | 206.00 |
| INT-0.1 | 0.00 b | 0.42 | 228.00 | 244.25 |
| RS-3 | −0.33 b | 0.53 | 227.25 | 276.00 |
| INT-10 | 0.00 c | 0.41 | 227.25 | 293.75 |
| RS-4 | −0.31 b | 0.28 | 227.25 | 302.75 |
| n = 20 | dPD [mV] n = 20 | R [Ω×cm2] n = 20 | ||
|---|---|---|---|---|
| Median | IQR | Median | IQR | |
| RS-1 | 0.00 ab | 0.06 | 66.25 | 41.00 |
| INT-0.001 | 0.00 ab | 0.06 | 78.75 | 54.75 |
| RS-2 | 0.00 a | 0.06 | 66.50 | 38.75 |
| INT-0.1 | 0.00 ab | 0.00 | 58.75 | 35.00 |
| RS-3 | 0.00 b | 0.12 | 67.75 | 38.00 |
| INT-10 | 0.00 ab | 0.20 | 58.00 | 31.25 |
| RS-4 | 0.00 ab | 0.12 | 61.00 | 37.25 |
| Rt (min) | Observed Ion Mass [M–H]−/(Characteristic Fragments) | Error (ppm) | Formula | Identified | Concentration (mg/g) |
|---|---|---|---|---|---|
| 1.66 | 179.05588 | −1.29 | C6H12O6 | myo-inositol | 4.84 ± 0.13 |
| 1.68 | 181.07122 | −2.98 | C6H14O6 | mannitol | + |
| 1.79 | 191.05656 | 2.33 | C7H12O6 | quininc acid | 11.81 ± 1.01 |
| 6.67 | 299.07761 (137) | 1.23 | C13H16O8 | hydroxybenzoic acid hexoside | + |
| 6.80 | 315.07254 (153) | 1.22 | C13H16O9 | protocatechuic acid hexoside | + |
| 8.70 | 153.01932 (109) | −0.08 | C7H6O4 | protocatechuic acid * | 0.41 ± 0.02 |
| 11.25 | 353.08842 (191, 179) | 1.73 | C16H18O9 | neochlorogenic acid * | 1.61 ± 0.08 |
| 14.27 | 387.12931 [M+HCOOH]−/(179) | −0.93 | C16H22O8 | coniferin | + |
| 14.82 | 451.12473 (289) | 0.32 | C21H24O11 | catechin hexoside | 0.23 ± 0.02 |
| 15.59 | 289.07182 | 0.20 | C15H14O6 | catechin * | 0.25 ± 0.02 |
| 16.18 | 353.08804 (191, 179) | 0.66 | C16H18O9 | chlorogenic acid * | 25.22 ± 1.12 |
| 16.88 | 579.1333 (285) | −3.87 | C26H28O15 | cyanidin derivative | 2.75 ± 0.12 |
| 16.99 | 179.03532 | 1.88 | C9H8O4 | caffeic acid * | 0.19 ± 0.01 |
| 18.75 | 353.08847 (191, 179) | 1.88 | C16H18O9 | cryptochlorogenic acid * | 0.61 ± 0.04 |
| 19.12 | 415.16043 | −1.3 | C19H28O10 | unknown | + |
| 19.7, 20.4, 21.6, 24.5 ** | 335.07756 (179) | 0.95 | C16H16O8 | caffeoylshikimic acid isomer | 0.96 ± 0.06 |
| 21.6,22.5, 24.1, 24.9 ** | 367.10366 (191, 173) | 0.55 | C17H20O9 | feruloylquinic acid isomer | 0.43 ± 0.03 |
| 26.06 | 787.26391 [m/z+HCOOH]− | −3.65 | C34H46O18 | eleutheroside E * | 0.20 ± 0.02 |
| 30.66 | 609.14703 | 1.51 | C27H30O16 | quercetin 7-O-rutinoside * | 0.47 ± 0.03 |
| 31.91 | 609.14667 (463, 300) | 0.92 | C27H30O16 | quercetin 3-O-rutinoside * | 0.25 ± 0.02 |
| 32.26 | 463.08857 (300) | 0.80 | C21H20O12 | quercetin 3-O-galactoside * | 0.41 ± 0.03 |
| 33.38 | 463.08876 (300) | 1.21 | C21H20O12 | quercetin 3-O-glucoside * | 0.43 ± 0.03 |
| 36.16 | 515.11967 (353, 191, 179) | 0.33 | C25H24O12 | dicaffeoylquinic acid isomer | 0.45 ± 0.02 |
| 37.14 | 515.11902 (353, 191, 179) | −0.93 | C25H24O12 | dicaffeoylquinic acid isomer | 0.62 ± 0.03 |
| 38.76 | 515.11916 (353, 191, 179) | −0.66 | C25H24O12 | 3,5-dicaffeoylquinic acid * | 3.81 ± 0.18 |
| 39.48 | 515.11981 (353, 191, 179) | 0.60 | C25H24O12 | 1,5-dicaffeoylquinic acid * | 5.61 ± 0.27 |
| 42.72 | 515.12037 (353, 191, 179) | 1.69 | C25H24O12 | 4,5-dicaffeoylquinic acid * | 2.38 ± 0.12 |
| 48.36 | 515.12099 (353, 191, 179) | 2.89 | C25H24O12 | dicaffeoylquinic acid isomer | 0.15 ± 0.01 |
| 48.71 | 207.06715 (179, 161, 133) | 4.17 | C11H12O4 | caffeic acid derivative | 0.39 ± 0.02 |
| 51.19 | 301.03656 | 3.92 | C15H10O7 | Quercetin * | 0.17 ± 0.01 |
| 60.58 | 577.13572 (193, 385) | 0.99 | C30H26O12 | diferulic acid derivative | + |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Graczyk, F.; Piskorska, E.; Gawenda-Kempczyńska, D.; Krolik, K.; Gębalski, J.; Olszewska-Słonina, D.; Kondrzycka-Dąda, A.; Wójciak, M.; Taglialatela-Scafati, O.; Verpoorte, R.; et al. Eleutherococcus senticosus Fruit Extract Stimulates the Membrane Potential of the Trachea and Small Intestine in Rabbits. Molecules 2025, 30, 4041. https://doi.org/10.3390/molecules30204041
Graczyk F, Piskorska E, Gawenda-Kempczyńska D, Krolik K, Gębalski J, Olszewska-Słonina D, Kondrzycka-Dąda A, Wójciak M, Taglialatela-Scafati O, Verpoorte R, et al. Eleutherococcus senticosus Fruit Extract Stimulates the Membrane Potential of the Trachea and Small Intestine in Rabbits. Molecules. 2025; 30(20):4041. https://doi.org/10.3390/molecules30204041
Chicago/Turabian StyleGraczyk, Filip, Elżbieta Piskorska, Dorota Gawenda-Kempczyńska, Krystian Krolik, Jakub Gębalski, Dorota Olszewska-Słonina, Aneta Kondrzycka-Dąda, Magdalena Wójciak, Orazio Taglialatela-Scafati, Robert Verpoorte, and et al. 2025. "Eleutherococcus senticosus Fruit Extract Stimulates the Membrane Potential of the Trachea and Small Intestine in Rabbits" Molecules 30, no. 20: 4041. https://doi.org/10.3390/molecules30204041
APA StyleGraczyk, F., Piskorska, E., Gawenda-Kempczyńska, D., Krolik, K., Gębalski, J., Olszewska-Słonina, D., Kondrzycka-Dąda, A., Wójciak, M., Taglialatela-Scafati, O., Verpoorte, R., & Załuski, D. (2025). Eleutherococcus senticosus Fruit Extract Stimulates the Membrane Potential of the Trachea and Small Intestine in Rabbits. Molecules, 30(20), 4041. https://doi.org/10.3390/molecules30204041








