Abstract
Our review systematically outlines the bioactivity and industrial applications of key functional compounds in roses, with a particular focus on their potential in food and pharmaceutical industries from technical, economic, and commercialization perspectives. We summarize the evidence supporting the efficacy of rose-derived polyphenols, flavonoids, and essential oils in areas including antioxidant, anti-inflammatory, antibacterial, and neuroprotective effects, which provide a scientific basis for their use in functional foods and preventive medicines. We further evaluated the commercial viability of processing rose by-products. Additionally, we analyze current and potential applications of rose bioactive compounds in natural food preservatives, functional dietary supplements, herbal medicines, and cosmetic products. Finally, we discuss the remaining challenges and future directions for the industrial utilization of roses, including standardization, efficacy validation in humans, and scalable economic models, to facilitate the transition from experimental research to commercially sustainable applications.
1. Introduction
Roses (Rosa spp.) are the most romantic and widely used ornamental plant worldwide. They have long been valued for their broad applications in landscaping, ecological conservation, perfumery, cosmetics, food processing, as well as medical and health care [1,2]. In recent years, there has been growing scientific and commercial interest in roses due to their rich profile of bioactive compounds, including essential oils, polyphenols, flavonoids, polysaccharides, and vitamins [3,4], which are associated with a wide range of health benefits such as antioxidant, anti-inflammatory, antimicrobial, and antidiabetic activities, as well as protective effects on different tissues [5,6].
Rosa spp., a genus within the family Rosaceae, comprise approximately 317 species and thousands of cultivated varieties. Many of these were previously world-famous ornamental plants without biological applications. They are rich in natural antioxidants and functionally active compounds, which contribute to significant nutritional and bioactivities [1]. This review focuses on five species that are most widely used in industrial applications: Rosa damascena Mill., Rosa rugosa Thunb., Rosa canina L., Rosa roxburghii Tratt., and R. laevigata Michx. We elaborate on the major bioactive compounds derived mainly from their petals and fruits. Moreover, the potential for the industrial exploitation of roses, particularly in functional foods and pharmaceuticals, remains underexplored, and large quantities of rose by-products generated during essential oil and floral water extraction are often discarded, leading to resource waste and environmental concerns. Therefore, this review presents new findings on the bioactive compounds and health benefits of roses and their by-products.
Herein, we reviewed high-quality literature from the Web of Science, PubMed, and Scopus published in recent years. This review summarizes the advances in the principal bioactive compounds and key biological functions of Rosa species, with a particular focus on rose bioactive ingredients and their practical, economically viable applications in industry. Ultimately, this study seeks to provide a roadmap for the integrated and sustainable utilization of rose resources, supporting the transition from laboratory evidence to industrial applications.
2. Bioactive Compounds in Rosa spp.
Rose species are rich in a diverse array of bioactive compounds, which contribute significantly to their nutritional, medicinal, and functional properties. The major classes of bioactive constituents include phenolic compounds, polysaccharides, vitamins, carotenoids, and essential oils [7,8]. These phytochemicals are primarily concentrated in the petals and fruits, which represent the most studied and commercially utilized parts, driving applications in functional foods, nutraceuticals, cosmetics, and pharmaceuticals.
Rose essential oil is a valuable aromatic oil extracted from roses (Figure 1); its composition, however, varies considerably across different rose species [9]. Geraniol (42.08%), heneicosane (22.07%), n-heptadecane (16.70%), and linalool (11.55%) are the main constituents of the essential oil from R. canina flowers [10,11]. The total monoterpenoid content of R. rugosa essential oil is 70.31%, of which the linalool content is 33.73% [6]. In addition, the relative contents of geraniol and citral are relatively high in the four types of rose essential oils [12]. GC-MS analysis identified citronellol and geraniol as the predominant constituents within the essential oil composition, although their relative abundances exhibited significant quantitative fluctuations in response to variations in temperature and precipitation levels [13].
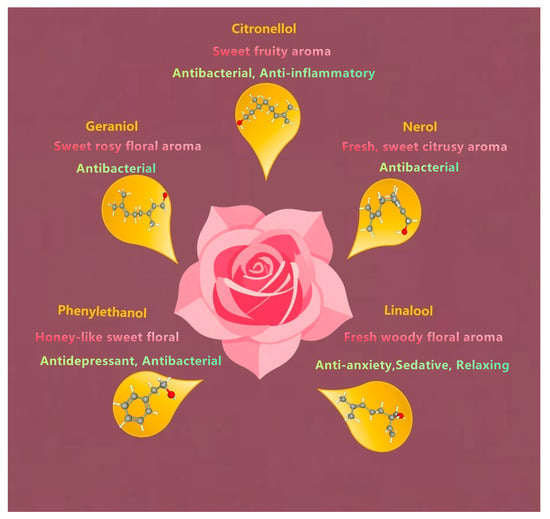
Figure 1.
The main components and effects of rose essential oil.
In the past decade, there has been a new research focus in the rose industry on the recovery and utilization of by-products after the extraction of rose essential oils. Rose waste has potential applications in food production owing to its rich in bioactive substances, including flavonoids, anthocyanins, polysaccharides, and dietary fiber [14,15]. It not only extends the industrial chain and enhances industrial value, but also facilitates the recycling of waste materials and reduces environmental impact.
In R. damascena residue water, the main compound is phenylethyl alcohol, which is also rich in polyphenols, including epicatechin and hesperidin. This indicates that the composition of polyphenols in R. damascena wastewater is similar to that of rose essential oils [16], with quercetin and kaempferol glycosides being the major polyphenol compounds. After freeze-drying, R. rugosa residue also contains high levels of polyphenols and anthocyanins [17]. Additionally, polysaccharides are essential components of rose residues, especially water-soluble pectic extracts of R. damascena residues [18]. Two pectic polysaccharide components, WSRP-2A and WSRP-2B, have been identified in R. setate × R. rugosa waste [19]. However, the soluble dietary fiber content of rose residue is relatively low, and most dietary fiber is insoluble. Thus, improving the utilization of this dietary fiber remains a problem to be addressed [20].
3. Bioactivities
The bioactive compounds in roses demonstrate a wide range of biological effects, such as antioxidant, anti-inflammatory, antibacterial, anti-diabetic, anti-cancer activities, as well as protective effects on the nervous system, gastrointestinal tract, liver, and skin (Table 1).

Table 1.
The bioactive components and potential mechanisms of Rosa in vivo and in clinical trials.
3.1. Antioxidant Effect
The antioxidant activity of roses mainly comes from essential oil and extracts. Most studies have focused on the antioxidant activities of rose petals. The antioxidant activity of rose essential oils mainly arises due to terpene compounds, especially citronellol, geraniol, nerol, linalool, and phenylethanol. Meanwhile, the extracts of rose (such as water extracts, ethanol extracts, and supercritical CO2 extracts) are rich in phenolic compounds, such as flavonoids (quercetin, kaempferol glycoside), phenolic acids (gallic acid), and tannic acid and its derivatives.
Studies show that the phenol contents are positively correlated with antioxidant capacity, with phytochemicals being most prominent in unfolded petals [43]. It has also been reported that antioxidant activity is highly correlated with the total phenolic content, but it is not affected by the harvest seasons of the R. rugosa petals [44]. Moreover, two new isoflavones isolated from the flowers of R. damascena have been shown to exhibit good antioxidant activity with IC50 values of 4.2 and 4.0 μg/mL [45]. However, there are five different Rosa extracts that effectively reduced malondialdehyde levels and enhanced the activities of key antioxidant enzymes, superoxide dismutase, and glutathione peroxidase in the H2O2-induced HaCaT cell model, highlighting their significant potential for development as novel antioxidant agents [46]. Research indicates that antioxidant-rich R. damascena significantly reduces oxidative stress in high-risk pregnant women, supporting its potential as an herbal-based preventive intervention for the management of high-risk pregnancies [22]. A study demonstrated that R. rugosa extract exhibits significant, dose-dependent antioxidant activity, scavenging up to 82% of free radicals at 50 mg/mL in vitro [47]. Studies in Nrf2-knockout zebrafish model demonstrated that R. rugosa extracts confers oxidative stress resistance and upregulates antioxidant gene expression (gstp1, prdx1) through an Nrf2-dependent mechanism [21]. Further analysis revealed that, in human endothelial cells, the polyphenolic composition of R. canina fruits significantly increased the levels of the antioxidant glutathione molecule while decreasing reactive oxygen species [48].
Several studies have analyzed the antioxidant effects of polysaccharides. One study focused on polysaccharides from R. rugosa petals (RRPS) and determined their in vitro antioxidant activities. This study suggests that RRPS-2 has good potential for scavenging radicals [49]. Antioxidant activity assays revealed that R. roxburghii polysaccharides possess significant free radical scavenging capabilities, with IC50 values of 0.45 mg/mL and 0.53 mg/mL against DPPH and ABTS radicals, respectively [46]. Furthermore, a novel water-soluble polysaccharide (RRTP1-1) has been obtained from R. roxburghii fruit using ultrasonic-assisted extraction. In vitro antioxidant tests have shown that RRTP1-1 exhibits significant scavenging activity against DPPH, hydroxyl, and superoxide radicals. Moreover, in vivo antioxidant assays have shown that RRTP1-1 at 200 or 400 mg/kg significantly enhances the activities of antioxidant enzymes, increases total antioxidant capacity values, and decreases lipid peroxidation level and Malonaldehyde (MDA) levels to different degrees in the serum of D-Gal aging-induced mice [50].
3.2. Anti-Inflammatory Activity
Inflammation is a basic pathogenic factor involved in tissue injury and pain as well as in acute and chronic diseases. Previously, the structural types of anti-inflammatory natural products discovered from plants mainly included terpenoids, flavonoids, alkaloids, and polysaccharides. Roses are known as medicinal plants in Asia and have protective effects against inflammation-related diseases that have been widely reported, including lung inflammation [51], allergic inflammation [24], skin inflammation [52], and other immunomodulatory activities [53]. Rugosic acid A, extracted from R. rugosa petals, has been shown to exhibit anti-inflammatory effects in LPS-mediated RAW 264.7 cells and a lung injury model. It can disrupt IL-6-mediated STAT3 activation and ameliorate LPS-induced nitric oxide (NO) production and nuclear translocation of NF-κB [51]. Epidermal-growth-factor-induced A549 cells have been used to investigate inflammation in allergic asthma and extracts from R. laevigata have been found to inhibit NF-kappa B activity and COX-2 expression [24]. JB6 P+ cells have been used to evaluate the anti-inflammatory activity of the skin and the results demonstrated that rose petal extract (RPE) reduced both cytokine production and expression of SUV-induced COX-2 [52]. In addition, Rosa extracts also reduce mouse thymus inflammation [25]. The anti-inflammatory effect of the extract was dose dependent, as it inhibited NO production in LPS-stimulated RAW 264.7 macrophages [54]. Because severe adverse effects are caused by the long-term use of synthetic steroids and non-steroidal anti-inflammatory drugs, novel effective materials with minimal side effects are required [26]. RT50 could reduce the pro-inflammatory cytokines and chemokines stimulated by tumor necrosis factor-α/interferon-γ in HaCaT cells and lower the elevated levels of Immunoglobulin E/Immunoglobulin G caused by DNCB exposure, demonstrating promising therapeutic potential for inflammatory skin conditions by upregulating key skin barrier proteins (filaggrin, involucrin, and loricrin) [23]. RLPa-2 inhibited the production of inflammatory factors (NO, IL-6, and TNF-α), and the expression of pro-inflammatory proteins (p-STAT3/STAT3) in LPS and IFN-γ-induced M1 macrophage [55]. The anti-inflammatory mechanism of roses is mainly related to their various bioactive components. These components exert anti-inflammatory effects by regulating inflammatory signaling pathways, inhibiting the release of pro-inflammatory factors, and scavenging free radicals. Therefore, Rosa could be a natural biomaterial resource for the development of safe and effective pharmacological immunomodulatory agents.
3.3. Antibacterial Activity
Treating infections caused by antibiotic-resistant bacteria is challenging, and researchers are looking for new antimicrobial compounds. The antibacterial activity of rose essential oil has mainly been determined according to the total content of phenylethyl alcohol, citronellol, geraniol, neroli, linalool, and eugenol [56], while Rosa extracts and their natural products, such as phenols, linalool, phenylethyl alcohol, citronellol, and bisabol, show a broad spectrum of antibacterial activity [57]. This makes it a valuable ingredient in natural skincare, food preservation, and pharmaceutical products.
Different extraction approaches, such as ethyl acetate [58,59], ethanol [57], and aqueous [60] extraction, have been studied to evaluate their influence on antimicrobial activity. The antibacterial effects of different extracts of R. canina have been examined against pathogenic Gram-negative bacilli [4]. The ethyl acetate extract from the petals of R. damascena has shown in vitro anti-plasmodial activity [59]. The aqueous extract of R. rugosa fruit exhibited activity against over 10 bacteria, including Bacillus cereus, Escherichia coli, Klebsiella pneumoniae, Enterococcus faecalis, Proteus mirabilis, Pseudomonas aeruginosa, Proteus mirabilis, and Enterococcus faecalis. However, bacteria such as Salmonella enteritidis, Staphylococcus aureus, Proteus mirabilis, Listeria innocua, Enterococcus faecalis, and Staphylococcus epidermidis have shown the highest levels of resistance to ethanolic extracts [60].
The antibacterial activity of rose extracts also varies among different parts of the plant. An interesting finding was that, in the antimicrobial evaluation against one fungal and eight bacterial strains, the leaf extracts demonstrated the greatest efficacy, followed by petal and hip extracts, whereas root extracts showed the weakest activity [61]. Due to the unique physical and chemical properties, metal-based nanoparticles have gained special attention for development as antimicrobial nanomaterials. Rosehip-extract-functionalized magnesium-based nanoparticles showed enhanced antibacterial activity against both Gram-positive (S. epidermis and S. aureus) and Gram-negative (E. coli) bacteria, as well as enhanced in vivo efficacy in an invertebrate model infected with S. aureus bacteria [27].
Roses and their derived extracts exhibit significant potential for application as natural preservatives within the cosmetic and food industries, as well as formulations for pet care. Their historical and ongoing use in traditional medicine for the management of infections and dermatological conditions underscores their therapeutic relevance. However, the realization of this potential necessitates the targeted selection of specific rose varieties and appropriate extraction methodologies based on the intended target microorganism species. Consequently, rigorous validation of their demonstrated efficacy under relevant conditions is imperative prior to deployment in these applications.
3.4. Anti-Diabetic Effect
Diabetes mellitus is a multisystemic and common metabolic disorder characterized by chronic hyperglycemia that may affect the eyes, kidneys, vessels, and heart. Chronic hyperglycemia causes the non-enzymatic glycation of proteins and elevation of the polyol pathway, resulting in oxidative stress that damages organs [62]. The main active components contributing to roses’ anti-diabetic effects include polyphenolic compounds (such as ellagic acid and gallic acid), flavonoids (including quercetin and kaempferol), terpenoids (notably citronellol and geraniol), and polysaccharides.
There has been a surge in research on the use of natural products to manage diabetes [3,63]. Polysaccharides in R. roxburghii exhibit strong antioxidant and α-d-glucosidase-inhibitory activities [64]. Selenium nanoparticles functionalized with polysaccharides from R. roxburghii fruit have also shown greater protective effects on INS-1 cells against H2O2-induced cell apoptosis [65]. The flavonoids isolated from R. roxburghii have great inhibitory effects on α-glucosidase in vitro, and research has shown that the inhibitory effects of flavonoids decrease after simulated gastric digestion [66]. Water-soluble polysaccharides extracted from R. roxburghii exhibit favorable inhibitory activities against α-glucosidase in a mixed-inhibition manner [67]. An in vivo study has indicated that the levels of fasting blood glucose, serum insulin, and serum lipids in type-2 diabetic db/db mice are decreased by the oral administration of a polysaccharide isolated from R. roxburghii fruit [28]. Polyphenols extracted from R. rugosa Thunb regulate lipid metabolism in diabetic rats by activating the AMPK pathway and increasing the expression of FGF21 in the liver [29]. Another diabetes study conducted on animal models revealed that purified oligosaccharide isolated from R. canina can stimulate the regeneration of β-cells in the islands of langerhans, resulting in a significant increase in insulin levels [30].
The anti-diabetic effect of roses is centered on polyphenols and flavonoids, and it is achieved through multiple pathways such as AMPK/PI3K-Akt signaling activation, β-cell protection, inhibition of glycometabolic enzymes, and anti-inflammation. Future research should focus on the mechanism of component synergy and clinical translation to promote its application as an adjuvant therapy.
3.5. Anti-Cancer Effect
Currently, tumor monotherapy cannot meet clinical needs, such as high doses, poor efficacy, and the emergence of drug resistance. Combination therapy is a new approach to overcoming these problems [68]. The active components in roses exhibit significant anti-cancer effects and possess considerable potential application as an anti-cancer dietary supplement.
Various polysaccharide-rich extracts of roses have shown in vitro antiproliferative activity against human lung cancer and colon cancer [69]. The extract of R. canina induced cell cycle arrest at the G1 phase and triggered apoptosis by reducing mitochondrial membrane potential and increasing caspase activity in A549 and prostate cancer (PC-3) cells [70]. Research shows that R. rugosa seed oil significantly inhibited the spontaneous migration of fibroblasts and A549 cells and reduced the formation of reactive oxygen species, with a higher emulsion activity in A549 cultures [71]. Polysaccharide-rich extracts from petals of R. rugosa have been studied for their composition and influence on various cellular processes involved in the development of cancer and other civilization diseases [69].
In vivo experiments showed that, as the concentration of the R. laevigata fruits-derived polysaccharide (JYP70–1) increased, the expression levels of phosphorylated FAK (Tyr397) and matrix metalloproteinase-2 decreased, which indicates that JYP70-1 regulates the FAK signaling pathway to inhibit the migration of HepG2 cells [31]. The anti-cancer effect and the underlying mechanism of action of the ethanolic extract of R. cymosa fruits was investigated both in vitro and in a xenograft animal model. R. cymosa fruits’ ethanolic extracts significantly reduced the tumor size by activating phosphatase and tensin homolog and impairing the PI3K/Akt/Foxo and Jak/Stat activation pathways [72]. When treated with an herbal combination, including R. rugosa, Cyperus rotundus L., and Citrus medica L., the tumor weight and volume, and the level of estradiol in serum were reduced in a breast cancer rat model, and the protein expression of SNCG, ER-α, p-AKT, and p-ERK was decreased in breast cancer (MCF-7 and T47D) cells [32]. A randomized controlled clinical trial reported that different concentrations of R. damascena essential oil improved the sleep quality of patients with cancer [73].
The anti-cancer mechanisms of roses are characterized by a dual-core system: the polyphenol/flavonoid-mediated induction of apoptosis and cell cycle arrest, coupled with polysaccharide-driven immunomodulation. This core activity is augmented by multidimensional effects, including the inhibition of metastasis and angiogenesis. Overcoming bioavailability limitations and advancing clinical validation remain critical for future translational research.
3.6. Neuroprotection
Human life expectancy increases as living conditions improve significantly, and an increasing number of people are suffering from neurodegenerative diseases such as Parkinson’s, Alzheimer’s, and senile dementia [74]. New approaches are needed to treat these pathologies because most neurodegenerative disease clinical trials do not obtain efficacious results [75].
Researchers have investigated the neuroprotective effect of selenium-containing polysaccharides from the fruit of R. laevigata against oxidative stress induced by H2O2 in SH-SY5Y neuroblastoma cells, finding that Se-RLFP-1 exhibited obvious neuroprotective activity at a concentration of 100 μg/mL [76]. Two pure polyhydroxy triterpenoids isolated from R. laevigata fruit have shown high acetylcholinesterase (AChE) inhibitory activities, with IC50 values of 29.22 and 45.47 μg/mL, as well as exhibiting potential neuroprotective activities against H2O2-induced SHSY5Y cell death [76]. Researchs have also evaluated five cultivars of R. damascena, all of which had high potency for scavenging free radicals and inhibiting acetylcholinesterase activity, with a high IC50 value of 3.92 μg/mL [77]. According to the above findings, the compound in Rosa extracts can inhibit the activity of acetylcholinesterase and may have the potential to be used as a drug to prevent and treat senile dementia.
Aaquatic R. damascena extract has a protective effect against the oxidative damage induced by AlCl3 intoxication in an Alzheimer’s model [33]. This is because the R. damascena extract increased catalase and glutathione levels, attenuated MDA levels, and regulated acetylcholinesterase activity. A clinical trial assay evaluated the effects of aromatherapy with R. damascena on pain and anxiety reduction during the first stage of labor and revealed that pain severity and anxiety levels were significantly lower in the treatment group [34]. An ultrasonic nebulizer was used to apply aromatherapy to patients for 15 min before they went to the operating room for surgery. The treatment group showed a significant difference from the control group in the second STAI-S test [35].
The neuroprotective effects of rose, particularly its petals and rosehips, result from multi-target and multi-pathway synergistic actions. The core mechanisms lie in its exceptional antioxidant and anti-inflammatory capacities, which directly counteract the two primary contributors to neuronal damage, including oxidative stress and neuroinflammation. Furthermore, by activating endogenous protective pathways, safeguarding critical cellular organelles, inhibiting detrimental enzyme activities, and potentially modulating neurotransmitter systems, rose constituents provide a more comprehensive protective barrier for neurons. These mechanisms underpin its potential therapeutic applications in the prevention and adjunctive treatment of neurodegenerative diseases, cerebral ischemic injury, and neuroinflammatory disorders, as well as in the improvement of cognitive function.
3.7. Gastrointestinal Protection
Lifestyle and diet affect the occurrence and development of gastrointestinal diseases [78]. Polysaccharides from roses have been found to exhibit excellent gastrointestinal protective properties.
One study investigated the digestibility and prebiotic potential of a novel polysaccharide from R. roxburghii fruit using an in vitro fermentation model and found it improved some beneficial gut microbiota via modulating the microbial structure, lowering the ratio of Firmicutes to Bacteroidetes from 14.89 to 4.68 after 48 h of fermentation [79]. Furthermore, a fecal microbiota transplantation study was conducted to demonstrate that polysaccharides from R. roxburghii fruit exhibited prebiotic effects on mice with high-fat-diet-induced colitis. The fecal microbiota of R. roxburghii fruit-polysaccharide-treated donor mice significantly alleviated body weight loss, gut microbiota dysbiosis, loss of barrier integrity, and colonic inflammation, while upregulating the expression of tight junction proteins [37]. Oral supplementation of R. laevigata polysaccharides ameliorated the typical symptoms of ulcerative colitis, such as body weight loss and diarrhea, by increasing the abundance of beneficial bacteria and reducing the abundance of harmful bacteria [36]. The contractile responses of the intestinal smooth muscles are due to periodic depolarization and repolarization. The juice mixture of R. canina had a protective effect against indomethacin-induced gastric lesions and antagonized the effects of indomethacin on apoptosis and lipid peroxidation in a rat mode [80].
The gastrointestinal protective effects of rose center on reinforcement of the mucosal barrier and dual-pathway inhibition of inflammation and oxidative stress. By inhibiting gastric acid secretion, promoting tissue repair, modulating gut microbiota, and regulating smooth muscle function, rose acts multi-dimensionally to maintain gastrointestinal homeostasis. Its natural multi-component synergistic properties confer significant development potential for functional gastrointestinal disorders and mucosal injury repair, although further standardized research is necessary to validate clinical applications.
3.8. Hepatorenal Protection
Recent evidence highlights the efficacy of rose polyphenols and polysaccharides in mitigating diabetic nephropathy, cisplatin nephrotoxicity, non-alcoholic fatty liver disease, and drug-induced liver injury.
R. roxburghii fruit is rich in multiple bioactive compounds with hepatorenal protective activities. It has previously been shown to possess antifibrotic properties in chronic renal diseases. Freeze-dried Rosa fruit powder efficiently alleviated pathological changes in the kidneys of unilateral ureteral obstruction rats by inhibiting oxidative stress and TGF-β1/Smads signaling [38]. R. laevigata polysaccharide modulated tryptophan metabolism, inhibited ferroptosis in the kidneys, and prevented apoptosis mediated by the PI3K/AKT pathway, showing significant therapeutic effects on diabetic nephropathy mice [39].
Total flavonoids from R. laevigata fruit exhibited protective activity against lipopolysaccharide-induced liver injury [40]. R. Laevigata extract intragastrically administered for seven days improved liver pathological changes by altering farnesoid X receptor-mediated oxidative stress, inflammation, and lipid metabolism in the liver injury mice models [40].
The hepatorenal protective effects of rose are primarily mediated through Nrf2-driven antioxidant defense, suppression of NF-κB/NLRP3-dependent inflammation, and inhibition of TGF-β-induced fibrotic signaling. However, clinical translation requires enhanced bioavailability of active constituents and validation in human trials.
3.9. Skin Protection
Current evidence shows that different extracts from Rosa are used in skin protection as antioxidation [56], anti-aging [81,82], whitening [83], moisturizing, anti-acne [84], anti-allergy, and sunscreen agents [56], inhibiting all kinds of melanin enzymes [57], blocking the melanocyte signaling pathway [52], inhibiting melanocyte proliferation, and reducing ultraviolet radiation [85].
Research found that the flower cell sap of R. rugosa exhibited strong tyrosinase inhibitory activities [56]. Kojic acid has also been detected in ethanol extracts of R. damascena as a tyrosinase inhibitor [57]. In vitro studies showed that the RRPS significantly reduced nitric oxide (NO) production on RAW264.7 macrophages, indicating anti-inflammatory effects [42]. Furthermore, ultrasonic extracts from R. canina L. are excellent sources of natural anti-tyrosinase inhibitors. In a study of the skin-aging-related activities of Rosa spp., flavonoid content extracted from R. gallica petals was found to significantly suppress tyrosinase activity, melanin production, and solar-UV-induced matrix metalloproteinase [82]. A polyphenol from R. rugosa flower tea increases the mean lifespan and enhances thermotolerance and resistance to oxidative stress in C. elegans, owing to its powerful antioxidant effects in vitro and strong protection against oxidative DNA damage [81]. Recently, studies performed in vitro and in vivo, as well as clinical trials, have investigated the skin-whitening and anti-wrinkle activities of R. gallica L. petal extracts [83]. In an in vitro study, RPE reduced melanin accumulation in human B16F10 melanoma cells and exhibited anti-wrinkle activity in human dermal fibroblasts. In an in vivo study, RPE inhibited solar-ultraviolet-stimulated MMP-1 expression via c-Jun regulation. In a clinical trial, volunteers who underwent facial skin RPE treatment exhibited significant skin brightness. R. roxburghii could significantly prevent the skin aging in D-galactose-induced mice by enhancing the activity of SOD, reducing the accumulation of MDA [41]. To evaluate moisture-preserving activities, the moisture absorption and moisture retention activities of polysaccharides from R. rugosa petals were tested in vitro by controlling the relative humidity of the desiccators, and they were found to exhibit strong moisture-preserving activity [49]. Extracts from R. rugosa were able to alleviate excessive androgenic-induced acne in a golden hamster acne model [84], which may also affect the regulation of sex hormone levels.
4. Cascade Utilization of Rosa spp.
Roses have medicinal, edible, and ornamental value and are increasingly loved by people. Since the raw materials of roses, rose essential oil, hydrosol, and the remaining rose residues contain various bioactive components, a cascading utilization approach has been proposed to maximize the application of this resource (Figure 2).

Figure 2.
Cascade utilization of Rosa spp.
4.1. Utilization of Raw Materials
The raw materials of roses are widely used in the production of various foods and beverages, such as tea, sauces, jam, wine, vinegar, and cake.
Owing to their richness in alcohols, terpenes, and various volatile aromatic components, rose flowers yield a tea with a pleasant fragrance. Regular consumption of rose flower tea is associated with the benefits of clearing heat, detoxification, promoting metabolism, nourishing the liver and stomach, and regulating hormones [86]. An analysis of rose flower tea and methanol extracts revealed significantly higher copper content in rose flower tea than in methanol extracts, accompanied by notable antioxidant effects [81].
Rose flower sauce, which contains volatile compounds such as aldehydes, alcohols, and esters, boasts a natural rose aroma that is widely appreciated for its taste and flavor [87]. Rose flowers are rich in nutrients and novel methods have been adopted to preserve these nutrients during processing. For instance, using fresh flower petals as the base material and adding ingredients such as white sugar, citric acid, pectin, and salt results in a new type of rose sauce [88]. This approach offers a simple processing technique that enhances the color, aroma, taste, and appearance of the product compared with traditional methods. It not only maintains nutritional value but also elevates flavor. Sweet products produced using rose flower sauce, such as mooncakes, bread, and pastries, enhance the color and fragrance of food and provide a unique flavor [89].
Rose vinegar is naturally fermented with plant materials, resulting in a natural rose-red color, mild flavor, and strong vinegar aroma. This has made it popular among the public. It is possible to increase aroma components and vinegar content by improving the processing techniques and fermentation methods [90]. Additionally, these modifications can positively affect the taste, color, nutritional value, and production efficiency of vinegar [91].
Rose wine, which has deep historical and cultural significance, features a rich and aromatic flavor characterized by its sweetness and aftertaste. Current research on the craftsmanship of rose wine has matured, resulting in products not only rich in rose aroma but also imbued with amino acids and phenolic substances from the fermentation process [92]. It imparts an appealing aroma and provides both nutritional and health benefits [93].
Roses are also used in a variety of desserts, pastries, and snacks and are valued for their numerous beneficial components. This has led to their extensive use in various forms, including dishes such as rock candy rose tea [94], which provides a sweet and fragrant experience while helping to disperse blood stasis. Rose porridge is another popular health food that promotes the dispersion of blood stasis. Rose flower buds combined with brown sugar can be made into a paste for consumption to support blood nourishment and skin rejuvenation.
4.2. Utilization of Rose Essential Oil and Rose Hydrosol
Rose essential oil is a precious natural fragrance that serves as a vital ingredient in the production of high-grade cosmetics, perfumes, and food products, and it ranks among elite flower essential oils [95]. The oil exhibits properties such as promoting digestion, improving blood circulation, and relieving pain; it has potential benefits for peripheral and visceral microcirculation [96].
The diverse chemical composition of rose essential oils, including terpenes, alcohols, esters, ethers, aldehydes, and alkanes, contributes to cell regeneration, enhances blood circulation, and provides soothing and sedative effects to aid sleep [97]. In addition, it possesses antibacterial, anti-thrombotic, and antioxidant properties. Its high antimicrobial activity, along with its substantial content of tocopherols and carotenoids, makes rose essential oil a natural preservative in the food industry, capable of reducing costs and mitigating the risks associated with the extensive use of artificial preservatives [5].
Rose hydrosol, a by-product of rose essential oil distillation, is primarily composed of compounds such as citronellol, nerol, and eugenol, imparting a rich and fragrant aroma [98]. It is notable for its pronounced efficacy in alleviating stress and is suitable as a component of composite preservatives, demonstrating favorable application effects. Research has demonstrated the potent inhibitory effects of rose hydrosol on bacteria [99], whereas its effects on molds and yeasts are less significant.
4.3. Applications of High-Value Compounds Extracted from Rosa spp. By-Products
Rose residues are the waste produced during the extraction of rose essential oils. The main products of rose processing are rose essential oil and rose hydrosol, of which 80% of the by-products are rose residues. The extraction of rose essential oil only extracts volatile components, such as rose aroma, whereas other non-volatile components (such as proteins and minerals) remain in the residue, with prominent nutrients and high reuse value.
Rose residues are mostly discarded as garbage, wasting resources and polluting the environment. Studies have shown that rose residues are rich in nutrients [100], with a relatively high content, and that they contain essential amino acids for humans. Additionally, they exhibit elevated levels of crude fat and crude fiber and a substantial amount of vitamin E. The mineral element profiles are also diverse. The analysis of rose petal residues reveals significant nutritional components. They include crude fat, crude fiber, vitamin E, calcium, zinc, iron, copper, magnesium, manganese, and phosphorus. This nutritional information highlights the potential benefits of using rose petal residues in animal diets and their positive impact on health due to their rich content of various essential nutrients and minerals.
Rose residue is a natural raw material that can be further developed and utilized. Currently, the extraction, separation, and determination of the active compounds in rose residues primarily focus on polyphenols, polysaccharides, and flavonoids. Studies on the efficacy of rose residues have primarily focused on their antioxidant activity, with most studies conducted at the national level rather than internationally. Thus, rose residues are a natural resource that is worthy of further exploration.
Rose residues can also be used to develop products such as rose petal jams by incorporating rose petals into pomace. Studies have shown that they can be used to produce cookies, candies, tea, and animal feed additives. This allows for the transformation of pomace from waste to a valuable resource, and the reutilization of rose residue for the production of rose essential oil. Such practices not only yield economic benefits but also address waste management concerns. The rational and comprehensive utilization of large quantities of rose pomace has emerged as a pressing challenge in essential oil production for roses.
Traditional waste handling methods incur costs related to disposal and pollution mitigation. However, advanced valorization pathways can transform waste into high-value products, improving profitability. Technologies for extracting bioactive compounds from rose waste, developing functional foods, and producing animal feed additives have demonstrated success [14]. Additionally, bioremediation approaches using algal–fungal systems degrade organic pollutants in wastewater, achieving COD removal and ammonia nitrogen reduction [101]. These processes reduce environmental impact while generating revenue streams.
Cascading utilization technologies we mentioned in our review can increase the value of rose products. Despite progress, gaps remain in global waste volume data and detailed cost–benefit analyses, underscoring the need for further research. Embracing circular economy principles in the rose industry not only mitigates waste but also unlocks new markets for nutraceuticals, cosmetics, and functional materials, aligning sustainability with economic growth.
5. Conclusions
The COVID-19 pandemic has increased people’s awareness of healthcare. Roses have attracted increasing attention due to their diverse biological functions as well as their dual medicinal and edible properties; however, the medicinal and food-related effects of roses have not yet been fully explored. Moreover, there remains a lack of systematic explorations of the pharmacological research and development process for studying the medicinal properties of roses and their by-products. Further research is needed to explore processed products and improve the related processing technologies.
Author Contributions
Conceptualization, visualization, writing—original draft, writing—review and editing, X.Z.; investigation, writing—original draft, writing—review and editing, Y.J.; writing—review and editing, M.Q.; conceptualization, validation, writing—review and editing, F.L.; project administration, funding acquisition, writing—review and editing, B.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Agricultural Science and Technology Innovation Program (No. ASTIP2023-34-IUA-05, No. ASTIP-S202402), Local Financial Funds of National Agricultural Science and Technology Center, Chengdu (No. NASC2024TD02).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare that they have no known competing financial interest or personal relationships that could have appeared to influence the work reported in this study.
References
- Kalisz, A.; Włodarczyk, Z.; Bieniasz, M.; Smoleń, S.; Neugebauerová, J.; Szewczyk Taranek, B.; Pawłowska, B. Petals of different ornamental rose cultivars as a rich source of bioactive compounds for functional foods. Sci. Hortic. 2023, 321, 112240. [Google Scholar] [CrossRef]
- Noh, Y.M.; Ait Hida, A.; Raymond, O.; Comte, G.; Bendahmane, M. The scent of roses, a bouquet of fragrance diversity. J. Exp. Bot. 2024, 75, 1252–1264. [Google Scholar] [CrossRef]
- Gandhi, G.R.; Hillary, V.E.; Antony, P.J.; Zhong, L.L.; Yogesh, D.; Krishnakumar, N.M.; Ceasar, S.A.; Gan, R.Y. A systematic review on anti-diabetic plant essential oil compounds: Dietary sources, effects, molecular mechanisms, and safety. Crit. Rev. Food Sci. Nutr. 2024, 64, 6526–6545. [Google Scholar] [CrossRef]
- Haghparasti, A.; Sichani, M.M.; Tavakoli, M. Chemical composition and antibacterial activity of wild rose (Rosa canina L.) gall extracts against gram-negative pathogenic bacteria. J. Adv. Biomed. Sci. 2023, 13, 13–22. [Google Scholar] [CrossRef]
- Bhavaniramya, S.; Vishnupriya, S.; Al Aboody, M.S.; Vijayakumar, R.; Baskaran, D. Role of essential oils in food safety: Antimicrobial and antioxidant applications. Grain Oil Sci. Technol. 2019, 2, 49–55. [Google Scholar] [CrossRef]
- Li, C.L.; Luo, Y.; Zhang, W.; Cai, Q.; Wu, X.; Tan, Z.; Chen, R.; Chen, Z.; Wang, S.; Zhang, L. A comparative study on chemical compositions and biological activities of four essential oils: Cymbopogon citratus (DC.) Stapf, Cinnamomum cassia (L.) Presl, Salvia japonica Thunb. and Rosa rugosa Thunb. J. Ethnopharmacol. 2021, 280, 114472. [Google Scholar] [CrossRef]
- An, B.F.; Chen, C.Y.; Qiao, G.F. Research progress on the extraction and application of rose essential oil. J. Anhui Agric. Sci. 2024, 52, 6–10. [Google Scholar]
- Gidik, B.; Akar, Z.; Can, Z.; Sefali, A.; Erturk, O. Determination of antioxidant, antimicrobial activities, phenolic compounds of wild Rosa L. species Bayburt Turkey. Fresenius Environ. Bull. 2019, 28, 9973–9982. [Google Scholar]
- Xiao, Z.; Luo, J.; Niu, Y.; Wu, M. Characterization of key aroma compounds from different rose essential oils using gas chromatography-mass spectrometry, gas chromatography-olfactometry and partial least squares regression. Nat. Prod. Res. 2018, 32, 1567–1572. [Google Scholar] [CrossRef]
- Elhawary, E.A.; Mostafa, N.M.; Labib, R.M.; Singab, A.N. Metabolomic profiles of essential oils from selected Rosa varieties and their antimicrobial activities. Plants 2021, 10, 1721. [Google Scholar] [CrossRef]
- Oz, M.; Deniz, I.; Okan, O.T.; Baltaci, C.; Karatas, S.M. Determination of the chemical composition, antioxidant and antimicrobial activities of different parts of Rosa canina L. and Rosa pimpinellifolia L. essential oils. J. Essent. Oil Bear. Plants 2021, 24, 519–537. [Google Scholar] [CrossRef]
- Tao, Y.X.; An, B.F.; Chen, C.Y.; Qiao, G.F.; Feng, Z.S. Detection and component analysis of essential oils of four different varieties of roses. China Condiment 2024, 49, 149–154. [Google Scholar]
- Rathore, S.; Kundlas, K.; Kumar, R. Variability in essential oil content and constituent profile of damask rose (Rosa damascena Mill.) at altered intervals of harvest in the Indian Western Himalaya. J. Appl. Res. Med. Aromat. Plants 2024, 39, 100537. [Google Scholar] [CrossRef]
- Zhai, Y.M.; Li, Y.Y.; Liu, L.X.; Liu, Y.G. Extraction methods, physiological activities, and applications of rose residue and its bioactive components: A comprehensive review. J. Food Meas. Charact. 2025, 19, 5183–5196. [Google Scholar] [CrossRef]
- Liu, W.; Chen, L.; Huang, Y.; Fu, L.; Song, L.; Wang, Y.; Bai, Z.; Meng, F.; Bi, Y. Antioxidation and active constituents analysis of flower residue of Rosa damascena. Chin. Herb. Med. 2020, 12, 336–341. [Google Scholar] [CrossRef]
- Boso, S.; Gago, P.; Santiago, J.L.; Alvarez-Acero, I.; Bartolomé, M.A.M.; Martínez, M.C. Polyphenols in the waste water produced during the hydrodistillation of ‘Narcea Roses’ cultivated in the cibea river valley (northern Spain). Horticulturae 2022, 8, 376. [Google Scholar] [CrossRef]
- Yu, Y.; Lv, Y. Degradation kinetic of anthocyanins from rose (Rosa rugosa) as prepared by microencapsulation in freeze-drying and spray-drying. Int. J. Food Prop. 2019, 22, 2009–2021. [Google Scholar] [CrossRef]
- Slavov, A.; Panchev, I.; Kovacheva, D.; Vasileva, I. Physico-chemical characterization of water-soluble pectic extracts from Rosa damascena, Calendula officinalis and Matricaria chamomilla wastes. Food Hydrocoll. 2016, 61, 469–476. [Google Scholar] [CrossRef]
- Wu, M.; Li, W.; Zhang, Y.; Shi, L.; Xu, Z.; Xia, W.; Zhang, W. Structure characteristics, hypoglycemic and immunomodulatory activities of pectic polysaccharides from Rosa setate x Rosa rugosa waste. Carbohydr. Polym. 2021, 253, 117190. [Google Scholar] [CrossRef]
- Liu, Y.; Ao, H.; Zheng, J.; Liang, Y.; Ren, D. Improved functional properties of dietary fiber from Rosa roxburghii Tratt residue by steam explosion. J. Food Process. Preserv. 2021, 46, e16119. [Google Scholar] [CrossRef]
- Nguyen, V.T.; Bui Thị Thu, V.; Vo Thi Minh, T.; Huynh Vinh, K.; Lam Vy, N.; Nguyen Hoang Thuy, V.; Luu Pham, T.; Xuan Nguyen, T.; Thi Thuy, D.; Huynh, H.D.; et al. UPLC-QTOF-MS/MS-Guided phytochemical characterization and molecular docking of Rosa rusoga extract for Keap1-Nrf2 modulation. Nat. Prod. Res. 2025, 1–9. [Google Scholar] [CrossRef]
- Laila, A.; Alyensi, F.; Pertiwi, R. Effect of natural antioxidant supplementation of rose cider (Rosa damascena) on oxidative stress in high-risk pregnant women: An analysis of malondialdehyde (MDA) biomarkers. J. Health Nutr. Res. 2025, 4, 633–640. [Google Scholar] [CrossRef]
- Bak, S.G.; Chandimali, N.; Park, E.J.; Lee, S.W.; Bae, J.; Rho, M.C.; Lee, S.J. Effectiveness of ethanol extract of Rosa rugosa Thunb. on ear edema. Mol. Nutr. Food Res. 2025, e70214. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Choi, S.H.; Lee, I.S.; Kim, Y.; An, E.J.; Jang, H.J. Anti-inflammatory effect of Rosa laevigata extract on in vitro and in vivo model of allergic asthma via the suppression of IgE and related cytokines. Mol. Cell. Toxicol. 2020, 16, 119–127. [Google Scholar] [CrossRef]
- Xu, S.; Zhang, F.; Wang, L.; Hao, M.; Yang, X.; Li, N.; Ji, H.; Xu, P. Flavonoids of Rosa roxburghii Tratt offers protection against radiation induced apoptosis and inflammation in mouse thymus. Apoptosis 2018, 23, 470–483. [Google Scholar] [CrossRef]
- Wang, C.; Kim, I.J.; Seong, H.R.; Noh, C.H.; Park, S.; Kim, T.M.; Jeong, H.S.; Kim, K.Y.; Kim, S.T.; Yuk, H.G.; et al. Antioxidative and anti-inflammatory activities of rosebud extracts of newly crossbred roses. Nutrients 2023, 15, 2376. [Google Scholar] [CrossRef]
- Alves, M.M.; Batista, C.; Mil-Homens, D.; Grenho, L.; Fernandes, M.H.; Santos, C.F. Enhanced antibacterial activity of Rosehip extract-functionalized Mg(OH)2 nanoparticles: An in vitro and in vivo study. Colloids Surf. B Biointerfaces 2022, 217, 112643. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, C.; Huang, Q.; Fu, X. Polysaccharide from Rosa roxburghii Tratt fruit attenuates hyperglycemia and hyperlipidemia and regulates colon microbiota in diabetic db/db mice. J. Agric. Food Chem. 2020, 68, 47–159. [Google Scholar] [CrossRef]
- Liu, L.; Yasen, M.; Tang, D.; Ye, J.; Aisa, H.A.; Xin, X. Polyphenol-enriched extract of Rosa rugosa Thunb regulates lipid metabolism in diabetic rats by activation of AMPK pathway. Biomed. Pharmacother. 2018, 100, 29–35. [Google Scholar] [CrossRef]
- Rahimi, M.; Sajadimajd, S.; Mahdian, Z.; Hemmati, M.; Malekkhatabi, P.; Bahrami, G.; Mohammadi, B.; Miraghaee, S.; Hatami, R.; Mansouri, K.; et al. Characterization and anti-diabetic effects of the oligosaccharide fraction isolated from Rosa canina in STZ-Induced diabetic rats. Carbohydr. Res. 2020, 489, 107927. [Google Scholar] [CrossRef]
- Guo, X.; Nie, F.; Jiang, H.; Che, S.; Liao Hb Xu, J.; Guo, Y. A bioactive polysaccharide derived from Rosa laevigata fruits: Structural properties, antitumor efficacy, and potential mechanisms. Int. J. Biol. Macromol. 2025, 304, 140382. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhao, L.; Wang, T.; Liu, Z.; Tang, S.; Huang, H.; Wu, L.; Sun, Y. The herbal combination Shu Gan Jie Yu regulates the SNCG/ER-a/AKT-ERK pathway in DMBA-induced breast cancer and breast cancer cell lines based on RNA-Seq and IPA analysis. Integr. Cancer Ther. 2024, 23, 15347354241233258. [Google Scholar] [CrossRef]
- Beigom Hejaziyan, L.; Hosseini, S.M.; Taravati, A.; Asadi, M.; Bakhshi, M.; Moshaei Nezhad, P.; Gol, M.; Mououdi, M. Effect of Rosa damascena extract on rat model Alzheimer’s disease: A histopathological, behavioral, enzyme activities, and oxidative stress study. Evid.-Based Complement. Altern. Med. 2023, 12, 4926151. [Google Scholar] [CrossRef]
- Hamdamian, S.; Nazarpour, S.; Simbar, M.; Hajian, S.; Mojab, F.; Talebi, A. Effects of aromatherapy with Rosa damascena on nulliparous women’s pain and anxiety of labor during first stage of labor. J. Integr. Med. 2018, 16, 120–125. [Google Scholar] [CrossRef]
- Dagli, R.; Avcu, M.; Metin, M.; Kiymaz, S.; Ciftci, H. The effects of aromatherapy using rose oil (Rosa damascena Mill.) on preoperative anxiety: A prospective randomized clinical trial. Eur. J. Integr. Med. 2019, 26, 37–42. [Google Scholar] [CrossRef]
- Peng, S.; Lu, X.; Lin, F.; Mao, N.; Yu, L.; Zhu, T.; He, J.; Yang, Y.; Liu, Z.; Wang, D. Rosa laevigata polysaccharides ameliorate sextran sulfate sodium-induced ulcerative colitis of beagles through regulating gut microbiota. Chem. Biodivers. 2024, 21, e202302102. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, P.; Chen, J.; Li, C.; Tian, Y.; Xu, F. Prebiotic properties of the polysaccharide from Rosa roxburghii Tratt fruit and its protective effects in high-fat diet-induced intestinal barrier dysfunction: A fecal microbiota transplantation study. Food Res. Int. 2023, 164, 112400. [Google Scholar] [CrossRef]
- Zhan, J.; Liu, M.; Pan, L.; He, L.; Guo, Y. Oxidative stress and TGF-β1/smads signaling are involved in Rosa roxburghii fruit extract alleviating renal fibrosis. Evid.-Based Complement. Altern. Med. 2019, 2019, 4946580. [Google Scholar] [CrossRef]
- Zhang, T.; Sun, W.; Wang, L.; Zhang, H.; Wang, Y.; Pan, B.; Li, H.; Ma, Z.; Xu, K.; Cui, H.; et al. Rosa laevigata Michx. polysaccharide ameliorates diabetic nephropathy in mice through inhibiting ferroptosis and PI3K/AKT pathway-mediated apoptosis and modulating tryptophan metabolism. J. Diabetes Res. 2023, 2023, 9164883. [Google Scholar] [CrossRef]
- Dong, L.; Han, X.; Tao, X.; Xu, L.; Xu, Y.; Fang, L.; Yin, L.; Qi, Y.; Li, H.; Peng, J. Protection by the total flavonoids from Rosa laevigata Michx fruit against lipopolysaccharide-induced liver injury in mice via modulation of FXR signaling. Foods 2018, 7, 88. [Google Scholar] [CrossRef]
- Liu, S.T.; Yin, R.F.; Wei, Y.Y.; Xia, X.; Li, Y.Q. Study on the effect of Rosa roxburghii tratt on preventing skin aging induced by D-galactose in mice. Food Res. Dev. 2020, 41, 1–5. [Google Scholar]
- Chen, M.; Peng, Y.; Zhu, R.; Luo, X.; Yang, X.; Chen, J.; Chen, H.; Zhou, W.; Du, Z. Therapeutic potential of Rosa rugosa polysaccharide and its nanofiber membrane in psoriasis via PI3K-AKT/mTOR pathway inhibition. Int. J. Biol. Macromol. 2025, 320, 145724. [Google Scholar] [CrossRef]
- Bian, Y.L.; Pan, J.J.; Gao, D.L.; Feng, Y.Z.; Zhang, B.J.; Song, L.; Wang, L.; Ma, X.G.; Liang, L. Bioactive metabolite profiles and quality of Rosa rugosa during its growing and flower-drying process. Food Chem. 2024, 450, 139388. [Google Scholar] [CrossRef]
- Cendrowski, A.; Ścibisz, I.; Mitek, M.; Kieliszek, M. Influence of harvest seasons on the chemical composition and antioxidant activity in Rosa rugosa petals. Agrochimica 2018, 62, 157–165. [Google Scholar]
- Xiang, H.; Xing, H.H.; Li, J.; Ye, L.; Kong, W.S.; Liu, X.; Li, Y.P.; Jiang, C.Q.; Wang, M.F.; Hu, Q.F.; et al. Two new isoflavones from the flowers of Rosa damascena and their biological activities. Chem. Nat. Compd. 2019, 55, 449–452. [Google Scholar] [CrossRef]
- Ni, M.; Chen, J.; Fu, M.; Li, H.; Bu, S.; Hao, X.; Gu, W. UPLC-ESI-MS/MS-based analysis of various edible Rosa fruits concerning secondary metabolites and evaluation of their antioxidant activities. Foods 2024, 13, 796. [Google Scholar] [CrossRef] [PubMed]
- Jeong, M.; Cho, J.; Lim, D.; Choi, M.; Park, Y.; Cheong, Y.; Kang, Y.; Kang, I.; Kim, S.; Kim, D. The biological effects of Rosa rugosa extract on keratinocyte differentiation and enhancement of skin barrier function. Adv. Tradit. Med. 2025, 25, 451–457. [Google Scholar] [CrossRef]
- Kerasioti, E.; Apostolou, A.; Kafantaris, I.; Chronis, K.; Kokka, E.; Dimitriadou, C.; Tzanetou, E.N.; Priftis, A.; Koulocheri, S.D.; Haroutounian, S.A.; et al. Polyphenolic composition of Rosa canina, Rosa sempervivens and Pyrocantha coccinea extracts and assessment of their antioxidant activity in human endothelial cells. Antioxidants 2019, 8, 92. [Google Scholar] [CrossRef]
- Zhang, C.; Zhao, F.; Li, R.; Wu, Y.; Liu, S.; Liang, Q. Purification, characterization, antioxidant and moisture-preserving activities of polysaccharides from Rosa rugosa petals. Int. J. Biol. Macromol. 2019, 124, 938–945. [Google Scholar] [CrossRef]
- Chen, G.; Kan, J. Characterization of a novel polysaccharide isolated from Rosa roxburghii Tratt fruit and assessment of its antioxidant in vitro and in vivo. Int. J. Biol. Macromol. 2018, 107, 166–174. [Google Scholar] [CrossRef]
- Kim, K.H.; Park, Y.J.; Jang, H.J.; Lee, S.J.; Lee, S.; Yun, B.S.; Lee, S.W.; Rho, M.C. Rugosic acid A, derived from Rosa rugosa Thunb., is novel inhibitory agent for NF-κB and IL-6/STAT3 axis in acute lung injury model. Phytother. Res. 2020, 34, 3200–3210. [Google Scholar] [CrossRef]
- Lee Mh Nam, T.G.; Lee, I.; Shin, E.J.; Han Ar Lee, P.; Lee, S.Y.; Lim, T.G. Skin anti-inflammatory activity of rose petal extract (Rosa gallica) through reduction of MAPK signaling pathway. Food Sci. Nutr. 2018, 6, 2560–2567. [Google Scholar]
- Roloff, S.J.; Scholten, J.D.; Chuang, J.; Hu, C.; Fast, D.J. Traditional Chinese medicine ingredients Rosa damascena and Poria cocos promote phagocytosis and a dendritic cell phenotype in THP-1 cells. Pharmacogn. Mag. 2018, 14, 567. [Google Scholar] [CrossRef]
- Yeddes, W.; Reguez, S.; Rebey, I.B.; Wannes, W.A.; Majdi, H.; Dakhlaoui, S.; Sawsen, S.; Msaada, K.; Tounsi, M.S. Valorisation of hydrodistillation by-products from Damask Rose (Rosa damascena): Extraction, characterization, and bioactivity of phenolic compounds with biological properties. Int. J. Environ. Health Res. 2025, 1–9. [Google Scholar] [CrossRef]
- Peng, S.; Gu, P.F.; Mao, N.N.; Yu, L.; Zhu, T.Y.; He, J.; Yang, Y.; Liu, Z.G.; Wang, D.Y. Structural characterization and in vitro anti-inflammatory activity of polysaccharides isolated from the fruits of Rosa laevigata. Int. J. Mol. Sci. 2024, 25, 2133. [Google Scholar] [CrossRef] [PubMed]
- Ren, G.; Xue, P.; Sun, X.; Zhao, G. Determination of the volatile and polyphenol constituents and the antimicrobial, antioxidant, and tyrosinase inhibitory activities of the bioactive compounds from the by-product of Rosa rugosa Thunb. var. plena Regal tea. BMC Complement. Altern. Med. 2018, 18, 307. [Google Scholar] [CrossRef] [PubMed]
- Akin, M.; Saki, N. Antimicrobial, DPPH scavenging and tyrosinase inhibitory activities of Thymus vulgaris, Helichrysum arenarium and Rosa damascena mill. ethanol extracts by using TLC bioautography and chemical screening methods. J. Liq. Chromatogr. Relat. Technol. 2019, 42, 204–216. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, Y.; Zhang, H.; Sun, W.; Li, Z.; Zhang, F.; Zhang, H.; Chen, F.; Zhang, H.; An, J.; et al. Antimicrobial mechanism of strictinin isomers extracted from the root of Rosa roxburghii Tratt (Ci Li Gen). J. Ethnopharmacol. 2020, 250, 112498. [Google Scholar] [CrossRef]
- Khare, S.; Gupta, M.; Cheema, H.S.; Maurya, A.K.; Rout, P.; Darokar, M.P.; Pal, A. Rosa damascena restrains Plasmodium falciparum progression in vitro and impedes malaria pathogenesis in murine model. Biomed. Pharmacother. 2018, 97, 1654–1662. [Google Scholar] [CrossRef]
- Cendrowski, A.; Kraśniewska, K.; Przybył, J.L.; Zielińska, A.; Kalisz, S. Antibacterial and antioxidant activity of extracts from rose fruits (Rosa rugosa). Molecules 2020, 25, 1365. [Google Scholar] [CrossRef]
- Maželienė, Ž.; Kirvaitienė, J.; Kaklauskienė, K.; Volskienė, R.; Aleksandravičienė, A. Antifungal and antibacterial activity of aqueous and ethanolic extracts of different Rosa rugosa parts. Microbiol. Res. 2025, 16, 26. [Google Scholar] [CrossRef]
- Demirbolat, I.; Ekinci, C.; Nuhoğlu, F.; Kartal, M.; Yıldız, P.; Geçer, M.Ö. Effects of orally consumed Rosa damascena Mill. hydrosol on hematology, clinical chemistry, Lens enzymatic activity, and lens pathology in streptozotocin-Induced diabetic Rats. Molecules 2019, 24, 4069. [Google Scholar] [CrossRef]
- Javid, H.; Sajadimajd, S.; Bahrami, M.; Bahrami, G.; Mohammadi, B.; Khazayel, S.; Miraghaee, S.S. Rosa canina extract relieves methylation alterations of pancreatic genes in STZ-induced diabetic rats: Gene methylation in diabetic rats treated with an extract. Mol. Biol. Rep. 2024, 51, 711. [Google Scholar] [CrossRef]
- Wang, H.; Li, Y.; Ren, Z.; Cong, Z.; Chen, M.; Shi, L.; Han, X.; Pei, J. Optimization of the microwave-assisted enzymatic extraction of Rosa roxburghii Tratt. polysaccharides using response surface methodology and its antioxidant and α-d-glucosidase inhibitory activity. Int. J. Biol. Macromol. 2018, 112, 473–482. [Google Scholar] [CrossRef]
- Wang, L.; Li, C.; Huang, Q.; Fu, X. Biofunctionalization of selenium nanoparticles with a polysaccharide from Rosa roxburghii fruit and their protective effect against H2O2-induced apoptosis in INS-1 cells. Food Funct. 2019, 10, 539–553. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Zhang, B.; Wang, B.; Li, C.; Fu, X.; Huang, Q. In-vitro inhibitory effects of flavonoids in Rosa roxburghii and R. sterilis fruits on α-glucosidase: Effect of stomach digestion on flavonoids alone and in combination with acarbose. J. Funct. Foods 2019, 54, 13–21. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, B.; Xiao, J.; Huang, Q.; Li, C.; Fu, X. Physicochemical, functional, and biological properties of water-soluble polysaccharides from Rosa roxburghii Tratt fruit. Food Chem. 2018, 249, 127–135. [Google Scholar] [CrossRef]
- Jin, H.; Wang, L.; Bernards, R. Rational combinations of targeted cancer therapies: Background, advances and challenges. Nat. Rev. Drug Discov. 2023, 22, 213–234. [Google Scholar] [CrossRef]
- Olech, M.; Nowacka-Jechalke, N.; Masłyk, M.; Martyna, A.; Pietrzak, W.; Kubiński, K.; Załuski, D.; Nowak, R. Polysaccharide-rich fractions from Rosa rugosa Thunb.—Composition and chemopreventive potential. Molecules 2019, 24, 1354. [Google Scholar] [CrossRef]
- Kilinc, K.; Demir, S.; Turan, I.; Mentese, A.; Orem, A.; Sonmez, M.; Aliyazicioglu, Y. Rosa canina extract has antiproliferative and proapoptotic effects on human lung and prostate cancer cells. Nutr. Cancer 2020, 72, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Grajzer, M.; Wiatrak, B.; Gębarowski, T.; Matkowski, A.; Grajeta, H.; Rój, E.; Kulma, A.; Prescha, A. Chemistry, oxidative stability and bioactivity of oil extracted from Rosa rugosa (Thunb.) seeds by supercritical carbon dioxide. Food Chem. 2021, 335, 127649. [Google Scholar] [CrossRef]
- Wang, K.; Liu, Y.C.; El-Shazly, M.; Shih, S.P.; Du, Y.C.; Hsu, Y.M.; Lin, H.Y.; Chen, Y.C.; Wu, Y.C.; Yang, S.C.; et al. The antioxidant from ethanolic extract of Rosa cymosa fruits activates phosphatase and tensin homolog in vitro and in vivo: A new insight on its antileukemic effect. Int. J. Mol. Sci. 2019, 20, 1935. [Google Scholar] [CrossRef]
- Heydarirad, G.; Keyhanmehr, A.S.; Mofid, B.; Nikfarjad, H.; Mosavat, S.H. Efficacy of aromatherapy with Rosa damascena in the improvement of sleep quality of cancer patients: A randomized controlled clinical trial. Complement. Ther. Clin. Pract. 2019, 35, 57–61. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.U.; Akram, M.; Daniyal, M.; Zainab, R. Awareness and current knowledge of parkinson’s disease: A neurodegenerative disorder. Int. J. Neurosci. 2019, 129, 55–93. [Google Scholar] [CrossRef]
- Si, Z.; Sun, L.; Wang, X. Evidence and perspectives of cell senescence in neurodegenerative diseases. Biomed. Pharmacother. 2021, 137, 111327. [Google Scholar] [CrossRef]
- Gao, P.; Han, T.; Jin, M.; Li, D.; Jiang, F.; Zhang, L.; Liu, X. Extraction and isolation of polyhydroxy triterpenoids from Rosa laevigata Michx. fruit with anti-acetylcholinesterase and neuroprotection properties. RSC Adv. 2018, 8, 38131–38139. [Google Scholar] [CrossRef]
- Tarbiat, S.; Türütoğlu, A.S.; Ekingen, M. Acetylcholinesterase inhibitory potential and antioxidant activities of five cultivars of Rosa Damascena Mill. from Isparta, Turkey. Curr. Top. Nutraceutical Res. 2020, 18, 354–359. [Google Scholar] [CrossRef]
- Kim, M.J.; Lee, S.; Kim, S.N. Effects of acupuncture on gastrointestinal diseases and its underlying mechanism: A literature review of animal studies. Front. Med. 2023, 10, 1167356. [Google Scholar] [CrossRef]
- Wang, L.; Li, C.; Huang, Q.; Fu, X.; Liu, R.H. In vitro digestibility and prebiotic potential of a novel polysaccharide from Rosa roxburghii Tratt fruit. J. Funct. Foods 2019, 52, 408–417. [Google Scholar] [CrossRef]
- Valcheva-Kuzmanova, S.; Denev, P.; Eftimov, M.; Georgieva, A.; Kuzmanova, V.; Kuzmanov, A.; Kuzmanov, K.; Tzaneva, M. Protective effects of Aronia melanocarpa juices either alone or combined with extracts from Rosa canina or Alchemilla vulgaris in a rat model of indomethacin-induced gastric ulcers. Food Chem. Toxicol. 2019, 132, 110739. [Google Scholar] [CrossRef]
- Zhang, J.; Xiao, Y.; Guan, Y.; Rui, X.; Zhang, Y.; Dong, M.; Ma, W. An aqueous polyphenol extract from Rosa rugosa tea has antiaging effects on Caenorhabditis elegans. J. Food Biochem. 2019, 43, e12796. [Google Scholar] [CrossRef]
- Shin, E.J.; Han, A.; Lee, M.H.; Song, Y.R.; Lee, K.M.; Nam, T.G.; Lee, P.; Lee, S.Y.; Lim, T.G. Extraction conditions for Rosa gallica petal extracts with anti-skin aging activities. Food Sci. Biotechnol. 2019, 28, 1439–1446. [Google Scholar] [CrossRef]
- Song, Y.R.; Lim, W.C.; Han, A.; Lee, M.H.; Shin, E.J.; Lee, K.M.; Nam, T.G.; Lim, T.G. Rose petal extract (Rosa gallica) exerts skin whitening and anti-skin wrinkle effects. J. Med. Food 2020, 23, 870–878. [Google Scholar] [CrossRef]
- Zhao, L.; Qiu, J.; Yin, X.; Zhang, N.; Wu, W.; Wang, C.; Ji, B.; Zhang, L.; Zhou, F. Blossom and bee pollen from Rosa rugosa as potential intervention for acne caused by excessive androgen secretion in golden hamster acne model. Food Agric. Immunol. 2019, 30, 1174–1188. [Google Scholar] [CrossRef]
- Kwak, C.S.; Yang, J.; Shin, C.Y.; Chung, J.H. Rosa multiflora Thunb flower extract attenuates ultraviolet-induced photoaging in skin cells and hairless mice. J. Med. Food 2020, 23, 988–997. [Google Scholar] [CrossRef]
- Zhou, L.; Yu, C.; Cheng, B.; Wan, H.; Luo, L.; Pan, H.; Zhang, Q. Volatile compound analysis and aroma evaluation of tea-scented roses in China. Ind. Crops Prod. 2020, 155, 112735. [Google Scholar] [CrossRef]
- Xia, A.; Tang, X.; Dong, G.; Lei, S.; Liu, Y.; Tian, X. Quality assessment of fermented rose jams based on physicochemical properties, HS-GC-MS and HS-GC-IMS. LWT 2021, 151, 112153. [Google Scholar]
- Xia, A.; Liu, L.; Tang, X.; Lei, S.; Meng, X.; Liu, Y. Dynamics of microbial communities, physicochemical factors and flavor in rose jam during fermentation. LWT 2022, 155, 112920. [Google Scholar] [CrossRef]
- Amrouche, T.A.; Yang, X.; Capanoglu, E.; Huang, W.; Chen, Q.; Wu, L.; Zhu, Y.; Liu, Y.; Wang, Y.; Lu, B. Contribution of edible flowers to the Mediterranean diet: Phytonutrients, bioactivity evaluation and applications. Food Front. 2022, 3, 92–630. [Google Scholar] [CrossRef]
- Zhao, G.; Kuang, G.; Li, J.; Hadiatullah, H.; Chen, Z.; Wang, X.; Yao, Y.; Pan, Z.; Wang, Y. Characterization of aldehydes and hydroxy acids as the main contribution to the traditional Chinese rose vinegar by flavor and taste analyses. Food Res. Int. 2020, 129, 108879. [Google Scholar] [CrossRef]
- Özdemir, N.; Budak, N.H. Bioactive compounds and volatile aroma compounds in rose (Rosa damascena Mill.) vinegar during the aging period. Food Biosci. 2022, 50, 102062. [Google Scholar] [CrossRef]
- Ma, T.; Sam, F.E.; Didi, D.A.; Atuna, R.A.; Amagloh, F.K.; Zhang, B. Contribution of edible flowers on the aroma profile of dealcoholized pinot noir rose wine. LWT 2022, 170, 114034. [Google Scholar] [CrossRef]
- Cendrowski, A.; Królak, M.; Kalisz, S. Polyphenols, L-ascorbic acid, and antioxidant activity in wines from rose fruits (Rosa rugosa). Molecules 2021, 26, 2561. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, L.X.; Han, Q.D.; Dong, G.Z.; Wang, B.; Zhang, J.F.; Lei, S.M.; Liu, Y.G. Quality assessment of rose tea with different drying methods based on physicochemical properties, HS–SPME–GC–MS, and GC–IMS. J. Food Sci. Technol. 2023, 88, 1378–1391. [Google Scholar] [CrossRef]
- Mileva, M.; Ilieva, Y.; Jovtchev, G.; Gateva, S.; Zaharieva, M.M.; Georgieva, A.; Dimitrova, L.; Dobreva, A.; Angelova, T.; Vilhelmova-Ilieva, N.; et al. Rose flowers-A delicate perfume or a natural healer? Biomolecules 2021, 11, 127. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, R.; Shi, J.; Zhang, R.; Tang, H.; Xie, C.; Wang, F.; Han, J.; Jiang, L. Chitosan/esterified chitin nanofibers nanocomposite films incorporated with rose essential oil: Structure, physicochemical characterization, antioxidant and antibacterial properties. Food Chem. X 2023, 18, 100714. [Google Scholar] [CrossRef]
- Koreti, D.; Kosre, A.; Mahish, P.K.; Chandrawanshi, N.K.; Kunjam, S.R. Application of essential oils in alternative medicine. In Essential Oils: Sources, Production and Applications; De Gruyter: Berlin, Germany, 2023; pp. 237–252. [Google Scholar]
- Tanjga, B.B.; Lončar, B.; Aćimović, M.; Kiprovski, B.; Šovljanski, O.; Tomić, A.; Travičić, V.; Cvetković, M.; Raičević, V.; Zeremski, T. Volatile profile of garden rose (Rosa hybrida) hydrosol and evaluation of its biological activity in vitro. Horticulturae 2022, 8, 895. [Google Scholar] [CrossRef]
- Bayhan, G.I.; Gumus, T.; Alan, B.; Savas, I.K.; Cam, S.A.; Sahin, E.A.; Arslan, S.O. Influence of Rosa damascena hydrosol on skin flora (contact culture) after hand-rubbing. GMS Hyg. Infect. Control 2020, 15, Doc21. [Google Scholar] [PubMed]
- De Nijs, E.; Maas, L.; Bol, R.; Tietema, A. Assessing the potential of co-composting rose waste as a sustainable waste management strategy: Nutrient availability and disease control. J. Clean. Prod. 2023, 399, 136685. [Google Scholar] [CrossRef]
- Dubey, S.; Chen, C.-W.; Haldar, D.; Tambat, V.S.; Kumar, P.; Tiwari, A.; Singhania, R.R.; Dong, C.-D.; Patel, A.K. Advancement in algal bioremediation for organic, inorganic, and emerging pollutants. Environ. Pollut. 2023, 317, 120840. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).