Abstract
Rheumatoid arthritis (RA) is a widespread autoimmune disease that significantly impacts the lives of RA patients. It is often typified as swelling and deformation of small joints, as well as systemic inflammation. Rhodiola rosea has been utilized for millennia to treat various ailments and is known to contain numerous active compounds, including saponins, volatile oils, coumarins, and flavonoids. Recent studies have underscored the pivotal role of salidroside (SAL), a key constituent of Rhodiola rosea L. Modern research indicates that SAL has various pharmacological activities, such as its antioxidant, anti-inflammatory, anti-fatigue, and anti-cancer effects. Despite this, the pathogenesis of RA remains highly complex, and a notable lack exists in overview studies investigating the anti-RA mechanisms of SAL. Therefore, the purpose of this article is to review the present research efforts on the anti-RA mechanisms of SAL and to explore future research prospects for this compound.
1. Introduction
RA is a long-term inflammatory disease of the joints that leads to cartilage and bone damage as well as disability [1]. Being a systemic autoimmune illness, RA commonly impacts other tissues and organs as well as the joints, including the heart, kidneys, gastrointestinal tract, nerves, and so on [2].
Therefore, a timely early evaluation of RA is crucial to avoid serious arthritic injuries such as physical disability. Autoantibodies as biomarkers can help practitioners make an efficient diagnosis and achieve the desired therapeutic outcome. Effective treatments for RA include oral administration of traditional synthetic modified DMARDs (smDMARDs), including methotrexate, targeted synthesis DMARDs (ts DMARDs) such as Janus kinase (JAK) inhibitors or tumor necrosis factor inhibitor (TNFi) biologics, and the recently discovered proteasome inhibitors (PIs) [3,4,5,6]. Although early and aggressive use of pharmacologically palliative antirheumatic drugs can significantly improve clinical and radiological outcomes, some patients have been reported to have significant problems with emotional and social functioning that reflect the unmet needs of RA patients [7]. Therefore, non-pharmacological treatments for RA patients are also a means of relieving the symptoms of arthritis. Patients with RA can benefit from dietary intervention with the Mediterranean diet as a complement and extension of drug therapy [8]. However, in relation to the disease itself, appropriate exercise and diet can only be considered a supplementary treatment. Tapping into an efficient and therapeutically effective anti-RA drug is essential for the treatment of RA. SAL is compared with the current RA treatment drugs, as shown in Table 1.

Table 1.
SAL versus the current RA medication.
Rhodiola rosea, a worldwide adaptogen, protects against inflammation in diseases like heart disease, neurodegenerative diseases, and cancer [9]. SAL is a tyrosol glucoside isolated from the roots of Rhodiola rosea Spp. [10]. It has attracted the attention of researchers because of its many biochemical and pharmacological features. During the latest decade, the medicinal properties of SAL have been gradually discovered in popular studies, such as anti-cancer, antioxidant, anti-inflammatory, and immunomodulatory agents [11]. SAL also promotes blood vessel growth, protects bone cells against glucocorticoids, and prevents steroid-induced osteonecrosis. It also fights inflammation by blocking TNF-α and IL-6 while encouraging IL-10 [12]. SAL has a pharmacological effect on the regulation of glial cell polarization and the reduction in neuroinflammation [13]. Based on the pharmacological effects of SAL and the current urgent need for a compound to treat RA, we summarized the induction mechanism of RA, the latest research results of SAL against RA, and prospects for SAL in anti-RA.
2. Mechanisms of RA
As an autoimmune disease, RA is characterized by chronic and systemic inflammation and has been linked to various complications in the cardiovascular and respiratory systems [14]. Rheumatoid arthritis is a multi-stage and complex process involving multiple aspects such as genetic, environmental, and behavioral risk factors. These factors may lead to damage to the immune system and induce autoimmune processes like autoantibody production and inflammation. Although diagnosed RA usually involves joints, the key to the pathogenesis of the disease may not be present in the joints: association with citrulline production, the gut microbiome, or adipose tissue in lung or periodontal tissues supports the normal findings in synovial tissues of patients with arthralgia. Anti-citrullinated protein antibody (ACPA) [15,16,17,18]. Years before the onset of RA, the presence of ACPA can be detected. Possible mechanisms involved in RA are summarized in Table 2 and Figure 1.

Table 2.
Possible mechanisms involved in RA.
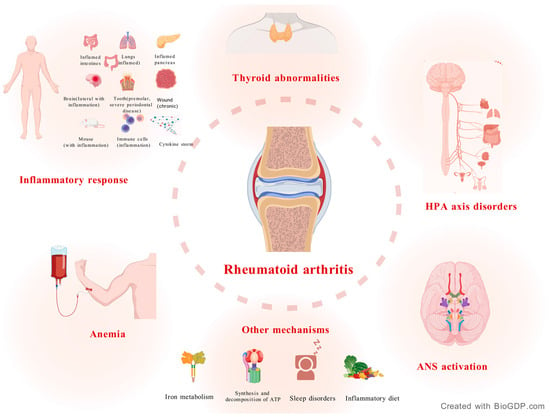
Figure 1.
Pathogenesis of RA. RA is strongly associated with an inflammatory response. HPA axis disorders, autonomic nervous system activation, anemia, and thyroid metabolic abnormalities are also major manifestations of RA. In addition, late nights, iron imbalance, and diet can also affect RA.
2.1. Inflammatory Response
RA is a chronic autoimmune disease [31]. Autoimmunity in RA may initially occur at mucosal sites. Serological and other tests may first detect autoimmunity in the blood, which later develops into inflammation and other symptomatic extensions during the dissemination phase [32]. It is generally accepted that the joints are mainly affected by RA. The basic pathological change in RA is synovitis. In the acute phase, the synovium shows exudative and cellular infiltration, with dilatation of small blood vessels in the sub-synovial layer, swelling of endothelial cells, and enlargement of the cellular spaces. While in the chronic phase, the synovium becomes hypertrophic and forms many villous protrusions that protrude into the joint cavity or invade into the cartilage and subchondral bone, which, if left untreated, can lead to joint destruction and disability [33]. The mechanistic links between RA and the inflammatory response are extremely complex, involving multiple dimensions of aberrant immune cell activation, cytokine network imbalance, and autoantibody-mediated tissue damage [34,35,36].
The core target cells of RA are SFs and SMs [37]. Synovial tissue macrophages are recognized as pro-inflammatory in RA, with the ability to release a large number of inflammatory cytokines, including IL-1, IL-6, and TNF-α [38]. A multitude of immune and non-immune cells are activated by inflammatory factors, and a variety of tissue-degrading enzymes secreted by these cells contribute to the chronic pro-inflammatory, tissue-destroying, and persistent pain response in RA [39]. TNF-α emerges as the principal inflammatory regulator, functioning through both autocrine stimulation and paracrine induction to orchestrate the production of crucial pro-inflammatory cytokines, including IL-1 and IL-6 [40]. Activation of TNF-α/NF-κB signaling causes the upregulation of NF-κB targets in foot and ankle RA patients, affecting the control genes that regulate cell growth, death, and inflammation [41]. The dysregulated release of IL-1β can be detrimental and is involved in the progression and pathophysiology of several chronic inflammatory diseases, especially RA [42]. IL-6, as one of the multi-effector cytokines, is a linchpin of the effector thymus [43]. Synovitis and joint destruction can be promoted by IL-6 [44]. A Mendelian randomized study suggested that long-term suppressive treatment of IL-1 and IL-6 might reduce the proportion of people who develop RA [45]. An advanced microenvironment-activating probe can target RA by conjugation with active and anti-IL-6R antibodies, manifesting NIR-II fluorescence signals at the lesion, enabling highly sensitive RA diagnosis and real-time treatment monitoring. The combination of ROS clearance and IL-6 signaling blockade leads to a potent treatment effect and synergistic immunomodulatory effects that significantly alleviate RA symptoms and prevent joint destruction [46]. Macrophage abundance and activation in the inflamed synovium/pannus have been shown to correlate with disease progression in RA. Therefore, targeting macrophage activation appears to be an effective therapeutic measure for reducing local and systemic inflammation and preventing irreversible joint damage [47].
T cells are the major effector cells of cellular immunity and are essential for synovial inflammation and joint destruction. Many cytokines are produced by T cells that play a role in the immune response. They directly or indirectly mediate inflammation and regulate other types of immune cells [48]. The Th1/Th2 immune response imbalance is critical in RA pathogenesis [49]. Th1 cytokines, for example, IL-2 and IFN-γ, promote the inflammatory microenvironment in RA patients and play a supportive role in the joints. In contrast, Th2 cytokines such as IL-4 and IL-10 have a beneficial effect against RA [50]. Th1 cytokines were demonstrated in all samples of RA synovium. Meanwhile, Th2 cytokines were detected in approximately 90% of samples [51]. Sophora decoction is expected to be an effective drug for the treatment of RA by decreasing the levels of Th1 cytokines and increasing Th2 cytokines in T cells to modulate the imbalance in RA rats, which is likely to be accomplished through the NF-KB pathway [52]. The animal drug dilong in traditional Chinese medicine may also have a therapeutic benefit in RA by blocking the NF-kb pathway [53]. A 12-week observation study was conducted on 40 patients with RA. In the etanercept combined with MTX treatment group, RA activity was improved by normalizing the distribution of Th17, Treg, and their related cytokines [54]. RNA sequencing was performed on the synovial tissues of several RA patients before and after tumor necrosis factor treatment. The baseline characteristic of RA patients is the activation of immune pathways, but this activation weakens after treatment. Moreover, several peripheral inflammatory proteins exhibit a decrease that is consistent with the corresponding changes in synovial gene expression [55].
For decades, researchers have commonly categorized the immune response into a Th1- and Th2-biased response. Yet, the discovery of new helper T cell subsets and the plasticity of helper T cells have changed the previous paradigm, suggesting that the immune process in this disease is the coordinated action of multiple T cells [56]. Tfh cells are important in the pathogenesis of various autoimmune diseases, not only in RA, due to their ability to promote B cell development, germinal center formation of lymphoid organs, and antibody production. Abnormal proliferation and other functional changes in Tfh cells can lead to pathological processes such as autoantibody production and tissue damage [57,58].
Another cell, Tph cells, instead of expressing BCL6, expresses molecules like CXCL13, IL-21, and ICOS to support B cells in peripheral tissues [59,60]. The role of T-cells in the course of RA has become clearer as research into a variety of helper T-cells has deepened. Understanding the role of immune cells and cytokines in RA will help us provide more convenient and feasible strategies for the mechanism and efficacy of RA treatment in Figure 2.
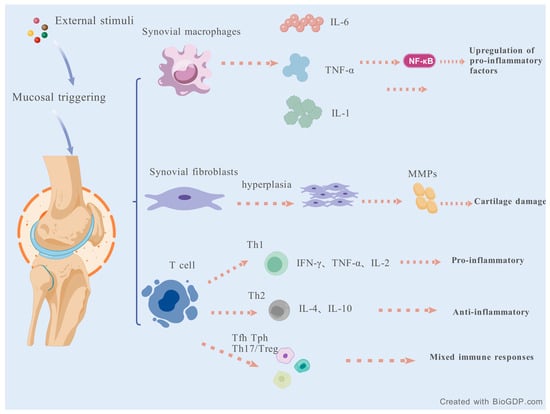
Figure 2.
The role of immune cells and cytokines in RA.
2.2. Hypothalamic-Pituitary-Adrenal (HPA) Axis Disorders
The HPA axis has an essential regulating and controlling role in immune responses. Chronic stress promotes comorbidity of both the hypothalamus–pituitary–thyroid (HPT) axis and the immune system through dysregulation of the HPA axis [61,62]. The HPA axis plays a pivotal role in regulating and controlling the immune response. Disorders of this axis are among the causes of rheumatic diseases, including RA [63].
Bidirectional communication exists between the neuroendocrine and immune systems. These systems exert a reciprocal influence on one another, in accordance with physiological conditions and inflammatory stimuli [64]. In chronic inflammatory conditions, including RA, the HPA axis shows three changes: inappropriate cortisol release, low inflammation-related ACTH release, and decreased adrenal androgens [65]. Inflammation-associated inadequate cortisol production and decreased adrenal androgen production are features of HPA-associated endocrine findings in RA [66]. DHEA and DHEAS are the major adrenal AAs produced in humans. Differences in AA production occur when immune function is activated or abnormal, such as in RA and SLE, where DHEA and DHEAS are relatively low [67]. Research has indicated the presence of low levels of DHEAS in a specific group of women experiencing premenopausal RA [68]. The increased release of endogenous cortisol and growth hormone is a critical component of the metabolic response induced by TNF-α. In the RA patient, long-term treatment with anti-TNF therapy was found to sensitize the pituitary gland and improve adrenal androgen secretion [69]. Non-invasive vagus nerve stimulation (VNS) inhibits inflammation through the release of HPA and adrenocortical steroids and the CAP and increases vagal nerve activity [70]. Female patients appear to be more dependent on the HPA axis than men, and steroid production of adrenal androgens is more significantly altered in premenopausal RA patients treated with nonglucocorticoids [71]. Synovial macrophages, monocytes, and lymphocytes possess functional androgen and estrogen receptors, which may influence metabolism. They play a crucial immunomodulatory role in the context of sex hormone-related RA. Try to combine antirheumatic drugs such as MTX with androgen replacement therapy, and sex hormones can play a valuable concomitant or auxiliary therapeutic role [72]. The relationship between RA and the HPA axis is multi-layered, involving developmental programming, gene regulation, immune interaction, and stress feedback. A better understanding of this network could provide a novel strategy for treating psychiatric disorders, autoimmune diseases, and metabolic syndromes.
2.3. Autonomic Nervous System (ANS) Activation
Among numerous chronic autoimmune diseases, researchers have observed a common pathological state, namely the imbalance of the ANS. Naturally, this phenomenon has also been widely found in the population of RA patients [73]. Studies in recent years stand out that disruption of the ANS is associated with the onset and activity of RA [74]. The ANS deals with issues such as the supposed sympathetic/vagal balance, organization into plexuses, and “little brains” that are active, for example, in the enteric system or around the heart [75]. Targeting the nervous system can have the effect of reducing inflammation-related neurological symptoms while promoting immunity [76,77]. Forty-seven percent of RA patients and 19% of systemic lupus erythematosus (SLE) patients have symptoms indicative of autonomic nervous system dysfunction.
The characteristics of several mood disorders, including depression, are dysfunctions of the ANS (i.e., increases or decreases in sympathetic or parasympathetic activity). ANS activity is part of the central autonomic network involved in stress responses and is related to the pathophysiology of mood disorders [78]. In a retrospective cohort analysis, depression predicted RA progression. Patients with MDD (major depressive disorder) have a 38% increased risk of developing RA [79]. Antidepressants that have been demonstrated to be efficacious in the treatment of RA-MDD comorbidities, with the caveat that their administration should be accompanied by cognitive-behavioral therapy [80]. Immunoregulatory drugs (such as anti-TNF-α Ozoralizumab and anti-IL-6 tocilizumab RoActemra) improve anxiety and depressive symptoms in RA patients [80,81,82].
The vagus nerve, the primary parasympathetic nerve, in addition to its role in nutrient transportation, also has certain inflammatory and analgesic properties [83]. Transcutaneous auricular vagus nerve stimulation (taVNS) was observed to be effective in alleviating articular cartilage and bone destruction in CIA rats, which may be related to the reduction in osteoclast production, downregulation of MMP family expression levels, and RANKL/OPG ratio in rats with CIA [84]. Electrical or physiological (deep breathing-DB) VNS may be a potential treatment for disorders related to impairment of the ANS and vagus nerve function. For example, it is used to treat the common autoimmune diseases RA and systemic lupus erythematosus. DB (17–31%) and taVNS (18–25%), after all, the HRV parameters of heart rate variability (area) showed no difference between the two kinds of VNS [85].
Acupuncture is considered to be a reflexology that has an effect on the body’s ANS. Just as stated in the ancient book “Treatise on Cold Damage Disorders”, acupuncture using classical diagnostic procedures to assign acupuncture points to patients can alleviate pain in RA patients to some extent and improve hand function [86]. It is well known that exercise can partially restore the activity of the ANS, especially its parasympathetic nerve components. Patients with RA who are very inactive and/or at high risk of CVD may benefit substantially from a physical activity program [87]. A 12-week exercise program improved cardiac autonomic function in women with RA, as shown by short-term HRV, aligning with prior results [88]. Biofeedback of heart rate variability biofeedback (HRVB) is conducive to restoring the balance of the ANS and has a positive effect on emotional self-regulation. Mindfulness meditation on the mind could reduce autonomic nervous system reactivity [89]. A growing body of evidence highlights the effectiveness of non-drug approaches, including yoga, mindfulness practices, and emotion-focused therapies, in significantly alleviating mood disturbances and reducing inflammation in rheumatoid arthritis patients [90].
Recent breakthroughs in the study of immune-autonomic neural pathways, which reveal the intricate interplay between the nervous and immune systems, are opening doors to groundbreaking approaches for managing rheumatoid arthritis.
2.4. Anemia
Anemia is one of the common symptoms in RA patients [91]. Anemia is a prevalent hematological disorder characterized by decreased RBCs in the body’s peripheral blood [92]. This will lead to insufficient oxygen supply to tissues and organs [93,94]. A multi-organizational cross-sectional observational study in tertiary care hospitals showed that in the study of 330 patients with RA seen from January 2023 to December 2023, 180 patients (54.55%) developed anemia [95]. A potent clinical association between anemia and RA has been confirmed by logistic regression analysis. Using GWAS analysis, the overall genetic correlation of the two diseases was positive, which confirmed that rheumatoid arthritis has a significant causal relationship with anemia [96]. To sum up, it is not difficult to find that anemia is a frequent symptom in RA patients. Anemia has also been shown to have deleterious effects on the cardiovascular system, the CNS, and the kidneys [97].
There are many causes of anemia, and the popular belief is that it is related to nutritional deficiencies (e.g., iron), blood loss, and hereditary disorders (e.g., thalassemia) [98]. Unlike general anemia, RA anemia is associated with inflammatory factors [99]. The pathological causes of combined anemia in patients with RA are mainly related to chronic inflammation and immune abnormalities, with the central mechanism being anemia of chronic disease (ACD) [100]. The chronic inflammatory state in RA patients leads to a massive release of pro-inflammatory cytokines, including IL-6, which interfere with iron metabolism and erythropoiesis, leading to anemia of inflammation (AI) or ACD [101,102]. Inflammatory factors inhibit renal erythropoietin (EPO) synthesis and decrease bone marrow responsiveness to EPO. IL-6 upregulates hepatic secretion of ferroportin (hepcidin), which inhibits intestinal iron absorption and macrophage iron release, resulting in impaired iron metabolism [103]. Meanwhile, oxidative stress and inflammation accelerate erythrocyte destruction. Inflammatory factors (e.g., TNF-α) directly inhibit the proliferation of red lineage progenitor cells, leading to decreased erythropoiesis [104].
In addition, there are many causes of RA anemia, such as disease medications and inflammatory diets. Many drugs are now often used clinically to treat RA, such as methotrexate, which may directly suppress bone marrow hemopoiesis, leading to megaloblastic anemia or pancytopenia [105]. Immunosuppressive agents such as azathioprine may cause bone marrow suppression, leading to pancytopenia [106]. Long-term use of NSAIDs may also trigger occult bleeding in the gastrointestinal tract and aggravate iron deficiency anemia [107]. In a 6-month-long study of celecoxib versus omeprazole and diclofenac in the Treatment of Patients with High-Risk Osteoarthritis and RA Pilot Study and the Long-Term Arthritis Safety Study of Celecoxib, hemoglobin was reduced by ≥2 g/dL in 1.9% and 2.0% of celecoxib-treated and 3.3% and 5.7% of diclofenac-treated patients in the two trials, respectively [108]. According to the latest WHO criteria, a columnar graphical model of key dietary factors showed that a total of 10.25% with RA developed anemia, and furthermore, anemic patients had higher levels of dietary inflammation index (DII) than non-anemic patients [109].
2.5. Thyroid Abnormalities
The connection between RA and the thyroid gland has long been extensively studied, focusing on a) functional and immune thyroid abnormalities with a prior history of RA and b) joint changes with prior antithyroid drug (ATD) [110]. Research has revealed a reciprocal causal link between thyroid function and RA. Genetic susceptibility to hypothyroidism appears linked to a higher risk of RA. Similarly, a link has been suggested between RA and hypothyroidism, as well as other types of hyperthyroidism [111]. The interaction between the thyroid gland and RA is a combined result of an imbalance in the immune–endocrine–metabolic network. A vicious circle is formed by genetic background, inflammatory factors, hormone axis interactions, and environmental factors [112,113,114].
NHANES data (2009–2012) suggest inflammation influences thyroid dysfunction onset and progression. Abnormal thyroid hormone levels can affect immune cell function promptly. Early anti-inflammatory intervention may mitigate thyroid irregularities [115]. The elevated T3 level in patients with hyperthyroidism promotes Th1 cell differentiation and tends to exacerbate the autoimmune response of RA. Similarly, in patients with hypothyroidism, insufficient thyroid hormone weakens regulatory T cell (Treg) function, leading to insufficient immunosuppression, which may exacerbate RA [111,116]. In the early stage, there is an obvious cross-talk between antigen-presenting cells and Th cells. As the disease progresses to the advanced stage, antigen-specific and non-specific immune cells migrate to the thyroid, forming inflammatory infiltrates [114]. The incidence of thyroid dysfunction is at least three times higher in women with RA than in women with non-inflammatory rheumatic diseases like osteoarthritis and fibromyalgia [117].
RA and autoimmune thyroid disorders could share a genetic connection. Of the 58 available screened members of 504 RA multi-case families, 6% of patients had thyroid disease, thyroglobulin antibodies were present in 5% of men and 11% of women, and thyroid microsomal antibodies were present in 5% of men and 15% of women. Clearly, these rates are higher than the general level of published data. More strikingly, this difference persisted after rigorous analysis of the age groups individually [118].
Medications and treatments may also affect the course of the disease. For example, methotrexate is effective in RA therapy and may improve the thyroid autoimmune response. However, long-term use will affect thyroid hormone metabolism to a certain extent, and the patient’s liver function needs to be monitored [119]. In addition, diet and lifestyle habits also play a role. Daily vitamin D intake is associated with elevated RA disease activity and a higher prevalence of thyroid autoantibodies [120]. Anti-TNF-α and anti-IL-17 therapies may be considered as reasonable treatment options for thyroid-related disorders [121].
2.6. Other Mechanisms
Enrichment analysis of the intersecting targets of anemia and RA targets resulted in highlighting pathways associated with inflammation, immune response, and iron metabolism. Further testing by SMR analysis showed that BLK and FAM167A were ranked as the top two most promising genes [96]. The regulation of iron homeostasis in RA is impaired, and dysregulation of iron balance can contribute to disease progression via mechanisms including the generation of ROS, fibrosis, inflammation, abnormalities in bone homeostasis, and cellular senescence [122]. Iron-induced anti-inflammatory macrophage M2 ferroptosis is highly susceptible, while pro-inflammatory M1 is to a lesser extent affected. Different GPX4 degradation in both cell types depends on p62/SQSTM1 and contributes greatly to progression [123].
In a pro-inflammatory environment, T cells and macrophages switch from oxidative phosphorylation to glycolysis (Warburg effect), resulting in a decrease in ATP production efficiency. When ATP is decomposed into ADP/AMP, the release of free energy decreases, which affects energy-consuming processes such as ion pumps and protein modification, resulting in cell dysfunction [123]. The reduction of adenosine, the ATP breakdown product, weakens its anti-inflammatory effects mediated through A2A receptors [124].
In addition to the above-mentioned pathogenesis, the pathogenesis of RA involves many layers and multi-level interactions and does not exist in isolation. Further work is required in the future to clarify the specific molecular functions of these pathways and explore their potential for clinical translation.
3. Current Research Progress of the RA Model
For better exploration of the pathogenesis of RA and the pharmacological effects of traditional Chinese medicine on RA, it is important to choose a suitable RA model construction method [125]. Animal models facilitate the study of RA, thereby enabling researchers to study complex systems involving inflammation, immune tolerance, and autoimmunity [126]. In addition to some conventional surgical modeling methods, the latest experimenters used some emerging methods.
Studies show that sinomenine reduces collagen-induced arthritis by stopping new blood vessel formation in mice. This supports its use in RA treatment [127]. An attempt was made to investigate the relationship between dietary patterns, circadian oscillations of the gut microbiota, and RA rhythmicity in CIA mice and RA patients using dietary timing-induced diurnal oscillations of the gut microbiota.
This showed that Parabacteroides distasonis influences RA inflammatory rhythms based upon the SIRT5-NF-κB axis [128]. CAIA modeling is emerging at the beginning of the 21st century and is particularly suitable for rapid pharmacodynamic evaluation [129]. In comparison with the original CIA model, the CAIA model has been demonstrated to elicit arthritis in the majority of mice, including those that are CIA insensitive. Moreover, CAIA has a short modeling period, accelerating the screening and evaluation of therapeutic agents for RA [130]. The model can be extended to transgenic mice to resolve the mechanisms of gene regulation of arthritis progression, as well as to support the systematic assessment of the function of RA-associated inflammatory mediators and causative factors such as microbial toxins [131,132]. Human SCID transplantation is a common technique for the long-term study of RA explants [133]. This model allows for the measurement of long-distance migration by synovial cells in human RA and these cell-induced cartilage destruction allows for the initial screening of human target-specific biologics [134].
In addition to the above chemical and physical methods for model construction, transgenic mice with self-deficiencies are also widely used in RA research. CIA modeling of transgenic mice lacking CD4 and CD8 molecules expressing the RA susceptibility gene HLA-DQ8 shows that CD4-deficient mice are resistant to CIA, CD8-deficient DQ8 transgenic mice have an increased susceptibility to CIA and auto-antibody development, and CD8(+) T cells fail to initiate CIA in the DQ8 transgenic mice but may have a regulatory/protective role [135]. Susceptible mice are an invaluable tool in mimicking the multifactorial pathogenesis of RA, inducing arthritis through microbial stimulation, which is closer to the complex etiology of human RA [136]. Abhinav Lamba et al. used mice expressing RA susceptibility and drug-resistant HLA class II genes to describe their interactions with gut dysbiosis in preclinical autoreactivity of sex bias [137]. The construction of more authoritative and efficient RA models will provide more convenience and feasible strategies for the investigation of the mechanism and efficacy of TCM in RA treatment.
4. The Potential Pharmacological Mechanism of SAL in Anti-RA
4.1. Improve Exercise Endurance
Rhodiola rosea improves endurance and fatigue during exercise. Acute and sustained 4-week supplementation with SAL in competitive rowers increased plasma antioxidant levels but did not affect exercise-induced oxidative damage [138]. Another double-blind experiment, which was a study investigating the impact of an acute intake of Rhodiola rosea on physical performance, muscle strength, and limb movement speed in athletes, was found to achieve an increase in endurance exercise capacity in volunteers [139]. In a detailed experiment investigating swimming-induced oxidative stress in rats, treatment with Rhodiola rosea (5, 25, 125 mg/day/rat) extract for 4 weeks significantly suppressed the production of O2(-)* in the blood and liver. Additionally, it increased the concentration of malondialdehyde in plasma. The four primary components of the extract, including SAL, were found to effectively eliminate O2(-)*, decrease the activity of H2O2 and HOCl, and exhibit a certain degree of dose dependence. The experiments further revealed that the expressions of Mn-superoxide dismutase, Cu/Zn-superoxide dismutase, and catalase were all upregulated in the livers of the rats. These findings collectively suggest that oxidative stress may be alleviated through the scavenging of reactive oxygen species and enhancement of antioxidative capacity by SAL [140].
4.2. Energy Metabolism Effects
4.2.1. Glycogen Storage Effects
SAL may exert its anti-fatigue effect by regulating cellular energy metabolism. In the weight-bearing experiment conducted on mice, SAL treatment (100 mg/day/rat) was found to markedly prolong swimming endurance compared to the control group (26.2 min vs. 10.5 min). Furthermore, the activity of mitochondrial respiratory chain enzyme complexes in the skeletal muscle tissue of SALt-treated mice was significantly increased [141]. SAL (0.1, 1, 10 μM) significantly downregulated LOX-1 and upregulated ATP-binding cassette transporter A. Furthermore, SAL significantly decreased the phosphorylation of JNK, ERK, and p38 MAPK, while increasing the phosphorylation of Akt, which resulted in an inhibition of oxidative LDL-induced formation and apoptosis of THP1-derived foam cells [142].
4.2.2. Promote Lipid Metabolism
SAL is not simply about “lowering blood lipids,” but rather involves a dual regulation of “immune and metabolism”. By reprogramming lipid metabolism, redox metabolism, and energy metabolism in both immune cells and metabolic organs through multi-system and multi-target approaches, SAL can correct pathological and pro-inflammatory metabolic conditions, restoring a healthy and stable metabolic state. SAL reduces lipid accumulation in macrophages and atherosclerotic plaques by directly binding to Hif-1α and inhibiting the thermal protein deposition pathway. SAL significantly reduces lipid accumulation in macrophages in a dose-dependent manner (EC50 = 50.49 μM) [143]. SAL demonstrates significant therapeutic effects by suppressing CD8 T cell infiltration, mitigating oxidative stress, and alleviating inflammatory factors. This multifaceted approach enhances mitochondrial metabolism, iron metabolism, lipid metabolism, and redox metabolism in the brains of SAMP8 mice [144]. Low-dose SAL (0.3 mg/mL) can inhibit the generation of ROS and lipid accumulation in the fatty liver model of laying hens in vitro through the PI3K/AKT/Gsk3-β pathway. Higher doses of SAL (20 mg/kg) can alleviate liver steatosis in laying hens induced by a high-fat diet [145]. SAL alleviates lipid accumulation and inflammatory response in primary hepatocytes after palmitic acid/oleic (OP) acid stimulation and can inhibit the progression of NASH induced by metabolic stress by activating AMPK signal transduction [146]. One hundred micromoles of SAL greatly increased the subsequent blastocyst formation rate and embryo quality of porcine oocytes, oocyte lipid content, and mitochondrial number [147]. SAL (30 mg/kg/day, 60 mg/kg/day) reduces the levels of ROS and LPO in the oxidative stress model induced by t-BHP. Further research and analysis in network biology and cell metabolomics indicate that galactose metabolism and fatty acid metabolism are the major ways in which it functions [148].
4.2.3. Repair Mitochondrial Dysfunction
Scientists have conducted various experiments to explore the potential mechanism of Rhodiola rosea in treating metabolic disorders. The mechanisms involved include multi-target effects that regulate oxidative stress, inflammation, mitochondria, autophagy, cell death, etc. They work together through multi-pathway and multi-level synergistic pathways [149]. The mitochondrial dysfunction is closely related to RA [150]. Imbalance of mitochondrial homeostasis is closely associated with cardiovascular disease, and SAL was found to have beneficial anti-inflammatory and anti-atherosclerotic effects, suggesting that SAL has potential in mitochondrial treatment for RA [150]. SAL intervention reduced IL-1β and TNF-α mRNA expression and inhibited the changes in mitochondrial membrane potential and mass, ROS level and nuclear translocation of NF-κB p65, but had no effect on the expression of iNOS. This finding demonstrates that SAL (400 μM) effectively counteracts M1 macrophage polarization; it accomplishes this by enhancing mitochondrial membrane potential while modulating the expression of both pro-inflammatory mediators and proteins critical to mitochondrial homeostasis [151]. SAL (100–400 μg/mL) effectively curbed melanoma growth by disrupting key cellular processes, including proliferation and migration. It also impaired metabolic functions by reducing both glycolysis and oxidative phosphorylation [152].
4.3. Regulate Amino Acid Metabolism
The regulation of amino acid metabolism by SAL is one of the important mechanisms by which it exerts its anti-RA effect. SAL demonstrates its potential as an effective anti-RA drug by regulating amino acid metabolism, alleviating endoplasmic reticulum stress, and correcting metabolic disorders caused by RA. Studies have shown that there are significant amino acid metabolic disorders in RA model mice [153]. Metabolomic profiling of CIA mouse plasma revealed a significant reduction in 20 out of 45 detected amino acid metabolites, linking these metabolic perturbations to RA-induced muscle atrophy and energy imbalance [154]. Pretreatment with SAL (1, 3, 30, 300 μmol/L) demonstrated a dose-dependent protective effect against Hcy-induced cytotoxicity in HUVECs. It primarily works through suppressing the expression of ER stress markers, including Bip and CHOP, as well as inhibiting the phosphorylation of PERK and IRE1α [155]. Rhodiola rosea extract (200 μg/mL) has been identified as a novel tyrosinase inhibitor; the inhibitory mechanism of it is mediated through modulation of the CREB/MITF/tyrosinase signaling pathway in B16F0 melanoma cells [156]. This also reflects from another perspective the potential of SAL and its source extracts in regulating amino acid metabolism-related signaling pathways.
However, the limited availability of plant sources restricts its large-scale production. To address this challenge, microbial biosynthesis of SAL via heterologous expression of plant-derived uridine UGT enzymes presents a viable and sustainable alternative [157].
4.4. Reduce Metabolite Accumulation
SAL has significant anti-fatigue properties, mechanistically linked to attenuating oxidative stress damage, reducing harmful metabolite accumulation, and increasing energy substrate stores. The metabolic signature of RA extends beyond energy provision to actively participate in disease pathogenesis by: (i) fueling hyperplastic synovial growth, (ii) maintaining pro-inflammatory cytokine production, (iii) recruiting immune cells to joints, (iv) activating osteoclast-mediated bone erosion, and (v) disrupting protein homeostasis in skeletal muscle, collectively contributing to RA etiology [158]. Samples were taken from the muscles of RA patients, and mRNA targeting measurements of the samples confirmed that both muscle energy metabolism and metabolic gene expression are modified within a CIA model, and RA contributes to dysregulation of metabolic homeostasis in skeletal muscle [159]. An important signaling pathway that could link inflammatory processes and RA is IDO. IDO is induced by inflammatory stimuli and catalyzes the catabolism of tryptophan to kynurenine for subsequent conversion to neuroactive metabolites [160]. In comparison with the model control group, both the Rhodiola rosea capsules and SAL treatment groups (5–20 mg/kg) exhibited a statistically significant prolongation of exhaustive swimming time in mice. Biochemical analyses revealed that SAL treatment effectively mitigated oxidative stress, as evidenced by decreased levels of MDA, H2O2, and lactic acid in hepatic and muscular tissues, concurrent with increased concentrations of GSH, hepatic glycogen, and muscle glycogen. Furthermore, SAL administration significantly enhanced the activities of total superoxide dismutase (T-SOD) and ATPase [161].
4.5. Antioxidant Activity
Oxidative stress occurs in many autoimmune diseases, including RA, along with overproduction of ROS and RNS; thus, antioxidant treatment appears to be the most valuable therapeutic approach [162]. SAL has significant antioxidant activity [163]. In vitro antioxidant studies also proved that it has excellent antioxidant properties [164]. By means of computer-aided methods and label-free interaction analysis, it was found that SAL directly inhibits HSP90, inhibits its ATPase activity, and affects the interaction of related proteins and the expression of downstream genes, effectively prolonging lifespan and regulating oxidative stress, and exerting anti-aging and antioxidant properties [165]. SAL (20, 40 mg/kg) protects against LPS-induced cardiac injury by targeting iNOS/COX-2 and NF-κB/PI3K/Akt/mTOR pathways. NAC pretreatment in H9C2 cells verified ROS-dependent mechanisms, supporting SAL’s potential for cardiovascular therapy [166].
People with RA may develop some brain disorders. Inflammatory factors produced during the RA pathological process, like IL-17, may affect the stroke process [167]. Endothelial cell function may be impaired, and endothelial dysfunction and cranial nerve barrier (BNB) may be pathological for RA patients [168]. In MCAO-induced CIRI rats after SAL treatment (50, 100 mg/kg), there was a significantly reduced infarction rate and improved histopathological changes. SAL was also effective in inhibiting apoptosis and reducing the level of oxidative stress in PC12 caused by OGD/R stimulation. The alleviation of apoptosis may be due to enhanced inhibition of cleaved caspase-3 and Bax/Bcl-2 proteins and also reduced MDA formation, and oxidative stress was strongly associated with upregulation of SOD and CAT activities and Nrf2 and Trx1 expression [169]. SAL (0.1,1,10 nM) possesses antioxidant functions and attenuates H2O2-induced apoptosis in cells, which is closely related to the activities of IGF1R and p-ERK1/2 [170].
SAL (0, 200, 400, and 600 mg/kg) enhances the activity of antioxidant enzymes and upregulates the expression of antioxidant genes in both the liver and muscle [171]. SAL (20 mg/kg) reduces reactive oxygen species levels, decreases malondialdehyde levels, and enhances superoxide dismutase and catalase protein expression in the ipsilateral testes, thereby ameliorating testicular ischemia-reperfusion injury by upregulating superoxide dismutase and catalase protein expression [172]. SAL (5,10,20μM) increases antioxidant capacity by lowering MDA, raising SOD, CAT, and GSH, and scavenging mitochondrial ROS. The salvage of mitochondrial ultrastructure, retention of MMP and ATP, inhibition of cytoplasmic cytochrome and cleaved caspase 3 expression, and inhibition of apoptosis suggest that Rhodiola rosea glycoside is a mitochondria-targeted antioxidant [173]. SAL has achieved remarkable results in neurological diseases because of its antioxidant activity.
SAL may have anti-RA effects, which are mediated by multiple pathways like antioxidant stress and reduced metabolite accumulation, as shown in Figure 3.
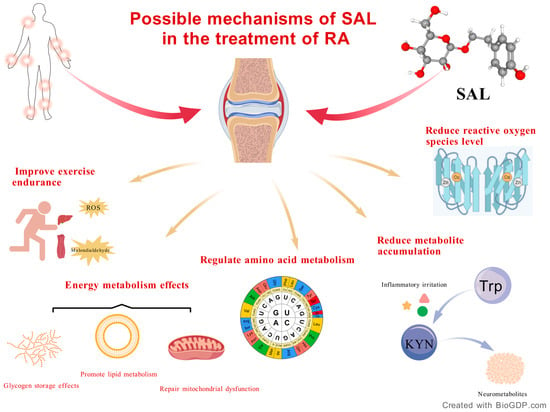
Figure 3.
Possible mechanisms of SAL in the treatment of RA.
4.6. Regulate Immune System
Immune system imbalances are thought to be connected with the symptoms of RA [174,175,176]. Network pharmacology and experiments have shown that GSZD (Guizhi-Shaoyao-Zhimu) may be able to partially reverse the imbalance of the inflammatory-immunomodulatory network that occurs during the progression of RA by modulating the HDAC1-HSP90AA1-NFKB2-IKBKB-TNF-α axis [177]. The CIA rat model and the TNF-α-induced HFLS-RA model were established. The Rosea-euonymus alatus drug of Rhodiola rosea can inhibit the PI3K/AKT signaling pathway and regulate the balance between matrix metalloproteinases and their inhibitors, thereby exerting therapeutic effects in RA [178]. After further integration with LC-MS/MS and bioinformatics, it was found that the onset of RA may be related to inflammation-associated immune cells involved in adaptive and innate immune responses. The potential key targets for treatment include seven, such as AKR1B10, MMP13, and FABP4. Further component research identified salidroside as one of the key components [179]. Comprehensive network pharmacology and experimental methods were explored to investigate the therapeutic mechanism of Jingulian capsules on RA, and JGL included the active ingredient of SAL that can inhibit inflammation through the IL-17/NF-κB pathway, thereby showing a protective effect on RA [180].
Pharmacological studies revealed that the inhibitory effect of SAL on BMDM pyroptosis was partially attenuated by TREM1 inhibition. In the context of adaptive immunity, SAL treatment restored gut microbiota diversity in DSS-induced colitis mice while rebalancing the Th17/Treg cell ratio. SAL ameliorates experimental colitis through dual modulation of macrophage proptosis and Th17/Treg homeostasis, highlighting its therapeutic potential for related immune disorders, like RA [181]. In addition to joint damage, RA can also have extra-articular manifestations, such as liver damage [182]. SAL demonstrates significant hepatoprotective properties. In bleomycin (BLM)-induced pulmonary injury models, characteristic EMT markers were observed, including decreased E-cadherin expression and upregulation of mesenchymal markers. Mechanistically, SAL (20, 40 mg/kg) treatment effectively attenuated these BLM-induced EMT alterations by enhancing the expression of TGF-β1 and phosphorylation of its downstream signaling molecules Smad-2/3 [183,184]. SAL (20 mg/kg/day) also improves hepatocyte death and apoptosis by activating GSK-3β/NrF2-dependent antioxidant responses and subsequently inhibiting MPTP [185].
Anemia is a significant symptom of RA [186]. The pathogenesis of acquired aplastic anemia (AA) is tightly correlated with the imbalance of Th17/Treg cells, and severe AA can even lead to death. The elevated level of HIF-1α in patients with AA constitutes a relatively important pathological feature. In the SAL group, blood cell counts were increased, bone marrow function was improved, the number of blood cells showed an increasing trend, the expression of FoxP3 was increased, and the imbalance of Th17/Treg was corrected. Meanwhile, in the SAL group, STAT3, RORγt, and IL-17a showed a downward trend, and the upward trend of HIF-1α level was also reversed. SAL corrects the Th17/Treg imbalance through the STAT3/HIF-1α/RORγt pathway and has the potential to be a novel therapeutic drug for AA and RA [187]. SAL can serve as an adjuvant for red blood cell production, effectively addressing anemia and hypoxia. SAL has been shown to enhance erythropoiesis in EPO-treated cells and decrease the rate of apoptosis in TF-1 erythroblasts following H2O2 treatment. This protective effect may be mediated through the upregulation of antioxidant defense proteins, specifically thioredoxin-1 (Trx1) and glutathione peroxidase-1 (GPx1) [187]. The SAL (75 μg/g body weight) activation of PARP-1 can affect the homeostasis and function of hematopoietic stem cells in mice under oxidative stress and prevent the loss of hematopoietic stem cells (HSCs). However, SAL does not prevent the production of reactive oxygen species but reduces the DNA strand breaks induced by hydrogen peroxide in hematopoietic stem cells [188]. SAL may also promote the recovery of hematopoietic function in mice by terminating G0/G1 phase arrest, accelerating the transitions between G0/G1-S and S-G2/M phases, upregulating Bcl-2 expression, and downregulating Bax expression [189].
The inhibitory effect of SAL on the IL-17/NF-κB and STAT3 pathways suggests that SAL may have therapeutic value in related diseases, based on the multiple central and peripheral effects of inflammatory factors in Figure 4.
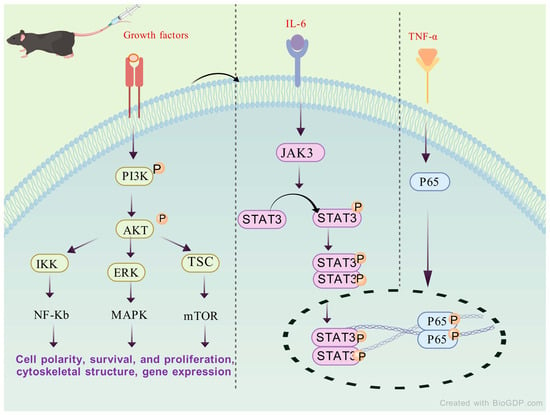
Figure 4.
The potential mechanism of SAL acting against RA by regulating STAT3 signaling.
4.7. Interfere with the Synthesis and Release of Neurotransmitters in the Brain
SAL demonstrates broad neuroprotective potential in various neurological disease models by synergistically exerting anti-inflammatory, antioxidant, and neuroregulatory effects. The inflammatory response induced by LPS was significantly reduced after pretreatment with SAL (12 mg/kg and 24 mg/kg). The expression levels of BDNF and TrkB increased, with a decrease in NE and 5-HT levels in the prefrontal cortex, suggesting that SAL exerts neuroprotective effects via the BDNF/TrkB signaling pathway [190]. The pathogenesis of attention deficit disorder is hypothesized to be associated with Aβ-induced oxidative stress and chronic neuroinflammation in the brain. This point is similar to the pathogenesis of RA in terms of inflammation and oxidative stress [191]. Oxidative stress mediated by NADPH oxidase in rats induced by Aβ1-40 increased, NF-κB was in an activated state, and the expressions of related inflammatory factors such as COX-2 and iNOS were significantly upregulated. SAL (25, 50, and 75 mg/kg p.o.) treatment group reversed all the aforementioned changes [192]. SAL (50 mg/kg) treatment activates the Nrf2-ARE signaling, inhibits oxidative stress and neuroinflammation, and achieves certain therapeutic effects in rats [193]. SAL ameliorates isoflurane-induced cognitive dysfunction by inhibiting TNF-α and IL-1β release, reducing oxidative stress, and restoring cholinergic balance in the hippocampus, such as acetylcholine and superoxide dismutase [194]. SAL) (60, 120, 180 mg/kg) has stress-protective properties that could also improve cognitive functions by inhibiting caspase-3 activity and antagonizing NO and NOS production during H2O2 stimulation [195].
After pretreatment of PC12 cells with SAL, the data showed that apoptosis was significantly reduced and the collapse of mitochondrial membrane potential was alleviated. Meanwhile, SAL (10,50,100μM) inhibits the increase in NO induced by MPP (+) and the overexpression of nNOS and iNOS and suppresses the accumulation of ROS and intracellular free Ca2+, disrupting key mediators of neurotoxic signaling cascades [196].
The iminodipropyl nitrile (IDPN)-induced Tourette syndrome model (TS) was created. Compared with the positive drug haloperidol treatment group, the tic behavior score in the SAL (0.5 mg/kg × d−1) treatment group increased, the DA level in plasma and striatum decreased, and the 5-HT level increased, resulting in a marked rebalancing of central neurotransmitter levels. Therefore, the therapeutic mechanism of SAL (50 mg/kg/d) is likely to be associated with the modulation of DA neurons in the striatum, potentially through presynaptic regulation or receptor interactions [197]. Long-term chronic stress can activate the peripheral and central immune systems, triggering the release of a large number of inflammatory cytokines, which is one of the important pathophysiological mechanisms for the occurrence of depression [198,199].
Both depressive states and inflammatory responses can lead to excessive generation of reactive oxygen species, thereby triggering oxidative stress and further damaging lipids, proteins, and DNA in neurons [200,201]. SAL (30, 90 mg/kg and 0.1 μg/mL) itself is an effective antioxidant that can activate the core antioxidant defense pathway Nrf2/ARE [202,203] in the body and may increase the availability of 5-HT, NE, and DA in the synaptic cleft through other indirect mechanisms (such as reducing inflammatory responses to improve neurotransmitter function), thereby improving emotional state, enhancing motivation, and restoring pleasure experience [190,204]. In traditional Chinese medicine, Rhodiola rosea is utilized to invigorate qi and promote blood circulation, demonstrating potential therapeutic effects in the treatment of depression. SAL (at least 20 mg/kg) stimulates hippocampal neurogenesis through the upregulation of the SIRT1/PGC-1α signaling pathway, inhibits the P2X7/NF-κB/NLRP3 axis to regulate NLRP3 inflammasome-mediated proptosis, reduces the expression of pro-inflammatory cytokines such as IL-6 and TNF-α, suppresses microglial activation, enhances monoaminergic neural transmission, and attenuates LPS-induced neuroinflammation [205].
SAL may have a therapeutic effect on RA through the above-mentioned mechanism. The detailed mechanism and the involved models are shown in Table 3.

Table 3.
Possible mechanisms of SAL in the treatment of RA.
5. Pharmacokinetics, Safety and Toxicity
After intravenous administration of SAL (7.5–30 mg/kg) in rats, it exhibited linear pharmacokinetics with a short t1/2 < 1 h and no sex-related differences. SAL was widely distributed within 15 min, with the highest levels in the liver, adipose tissue, skeletal muscle, ovary, and testis. Renal clearance was the major elimination pathway, with 53.67% of the dose excreted unchanged in urine within 48 h. In contrast, fecal and biliary excretion were minimal (<2%). The low total recovery (<54%) indicates significant metabolic clearance [206]. Salidroside underwent more extensive metabolism following gavage administration compared to intravenous (i.v.) administration. The total excretion rates of salidroside, including both salidroside itself and its metabolite p-tyrosol, were 68.85% after i.v. administration and 26.07% after gavage administration [207]. Rats were orally administered 12–48 mg/kg SAL for plasma concentration determination. The highest intestinal absorption was achieved at an oral dose of 24 mg/kg [208]. The method was utilized in a pharmacokinetic study of salidroside injection conducted on six beagle dogs. Following a single intravenous dose of 75 mg/kg, the primary pharmacokinetic parameters of salidroside in the dogs were as follows: Cmax was 96.16 ± 8.59 µg/mL, AUC0–24 was 180.3 ± 30.6 µg·h/mL, AUC0–∞ was 189.3 ± 32.1 µg·h/mL, and the half-life (t1/2) was 2.006 ± 0.615 h [209].
Rhodiola rosea polysaccharides, as a kind of natural polymer material, are characterized by their non-toxicity and non-irritation. They are often used as key components in drug formulations and made into various dosage forms such as injections and capsules [210]. In the safety evaluation of SAL in the treatment of viral myocarditis, including (bacterial reverse mutation test, chromosomal aberration test, and mouse micronucleus determination), no structural and numerical changes or increase in chromosomal aberration were observed at all treatment doses (500, 1000, and 2000 μg/kg) [211]. Administration of a 20 mg/kg body weight aqueous-alcoholic extract of Rhodiola rosea to pregnant and lactating mice may modulate prenatal and postnatal angiogenesis, potentially involving additional unidentified bioactive compounds that influence endothelial cell proliferation [212].
Based on the above mechanism, we speculate that the combination of salidroside and the following drugs may have adverse effects. Rhodiola rosea is known in traditional Chinese medicine for its properties of promoting blood circulation and resolving blood stasis [213]. Its concurrent use with anticoagulant medications, such as warfarin, may increase the risk of abnormal bleeding. Additionally, due to the presence of salidroside, which may influence the levels of monoamine neurotransmitters such as HT and NE [190], it is not recommended to use Rhodiola rosea in combination with monoamine oxidase inhibitors (MAOIs), including older antidepressants like phenylhydrazone and trans-phenyl cyclopropylamine. Rhodiola rosea is widely recognized for its adaptogenic properties and is commonly used to alleviate fatigue [190]. However, when taken alongside sedative-hypnotic drugs such as diazepam and barbiturates, it may exert antagonistic effects.
6. Conclusion and Perspectives
Rhodiola rosea, as a traditional Chinese medicinal herb, has a sweet, bitter, and neutral taste. It enters the lung and heart meridians. Thousands of years of clinical experience in traditional Chinese medicine have concluded that it has the effects of benefiting qi, promoting blood circulation, unblocking meridians, and relieving asthma. It is used in TCM to treat qi deficiency, blood stasis, chest pain, and heart pain. Rhodiola rosea has long been listed as a superior medicinal herb in the “Shennong’s Classic of Materia Medica” and is widely used in China and other Asian countries, benefiting the world. Phenolic compounds such as rhodioloside and tyrosol glucoside are responsible for R. rosea’s biological effects: antioxidant, immunomodulatory, antiaging, and anti-fatigue [214]. SAL has powerful antioxidative, anti-inflammatory, cancer-fighting, and age-fighting properties and has been used in the cosmetics industry [215,216]. In the medical field, the huge potential of SAL in multiple aspects, such as antioxidation, makes its development into immunomodulatory and anti-RA practical and promising.
In addition to its significant antioxidant effects, SAL also has strong anti-inflammatory effects. The strong anti-inflammatory effect has also become a significant advantage of SAL in treating RA symptoms.
Therefore, in the future, we can design a novel RA-targeted nano-preparation to directly exert a therapeutic effect at the site of RA lesions and, at the same time, achieve the purpose of alleviating inflammation, so as to achieve the dual therapeutic effect [217].
To summarize: SAL holds significant potential for application in the clinical treatment of RA. Nevertheless, further exploration and validation of its anti-RA mechanism are required through large-scale, systematic, and standardized preclinical animal experiments, as well as extensive clinical trials.
Author Contributions
J.G., writing—original draft, formal analysis, visualization, software, conceptualization; S.J., writing—review and editing, methodology, conceptualization, investigation; M.L., investigation; M.W., visualization; B.H., software; N.Z., supervision, resources; Y.L., supervision; Y.X., funding acquisition, resources; J.L., methodology, writing—review and editing, funding acquisition, resources, supervision, project administration; H.S., writing—review and editing, funding acquisition, resources, supervision, project administration. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Science and Technology Major Project of China grant number 2018ZX09201009, the National Natural Science Foundation of China grant number 8227417, the Major Project of Science, Technology Innovation of Hunan Provincial Department of Science and Technology grant number 2020SK1020, In-ternational Cooperative Project of Traditional Chinese Medicine grant number 2541STC72898, and the Jiangsu CM Clinical Innovation Center of Degenerative Bone & Joint Disease (General Project). The APC was funded by Huifeng Sun, National Science and Technology Major Project of China. The author would like to thank his colleagues at Hunan University of Medicine and Heilongjiang University of Chinese Medicine for their contributions and manuscript reviews. The author would like to express sincere gratitude to Xia Lei from the Wuxi Affiliated Hospital of Nanjing University of Chinese Medicine for her valuable support.
Acknowledgments
This research was funded by the National Science and Technology Major Project of China (Project No. 2018ZX09201009), the National Natural Science Foundation of China (Project No. 8227417), and the Major Project of Science, Technology Innovation of Hunan Provincial Department of Science and Technology (Project No. 2020SK1020) and Jiangsu CM Clinical Innovation Center of Degenerative Bone &Joint Disease (General Project). The author would like to thank his colleagues at Hunan University of Medicine and Heilongjiang University of Chinese Medicine for their contributions and manuscript reviews. The author would like to express sincere gratitude to Xia Lei from the Wuxi Affiliated Hospital of Nanjing University of Chinese Medicine for her valuable support. The figure in this manuscript was created with BioGDP.com [218].
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
5-HT: 5-hydroxyserotonin; DA, dopamine; ATP, adenosine triphosphate; ROS, reactive oxygen species; RNS, reactive nitrogen species; TNF-α, tumor necrosis factor-alpha; IL-1β, interleukin-1; IL-6, interleukin-6; HPA axis, hypothalamic–pituitary–adrenal axis; NF-κB, nuclear factor kappa B; MMP, matrix metalloproteinase; AD, Alzheimer’s disease; SAL, salidroside; SOD, superoxide dismutase; NASH, non-alcoholic steatohepatitis; OP, osteoporosis; Hcy, homocysteine; POF, premature ovarian failure; BBB, blood–brain barrier; TGF-β1, transforming growth factor-β1; IDO, Indoleamine 2,3-dioxygenase; AA, acquired aplastic anemia; DMARD, disease-modifying anti-rheumatic drugs; tsDMARD, including methotrexate, targeted synthetic; SF, synovial fibroblast; SM, synovial macrophage; Th1/2, type 1/2 helper T cell; ICOS, inducible T cell co-stimulators CIA, collagen-induced arthritis; VNS, vagus nerve stimulation; CVD, cardiovascular disease; HRVB, heart rate variability; RBC, red blood cells; ACD, anemia of chronic disease; NSAID, non-steroidal anti-inflammatory drugs; ATD, autoimmune thyroid disease; Aβ, beta-amyloid; UGT, uridine diphosphate glycosyltransferase; DHEA, dehydroepiandrosterone; DHEAS, dehydroepiandrosterone sulfate; AA, androgen; SLE, systemic lupus erythematosus; CAP, cholinergic anti-inflammatory pathway; TCM, traditional Chinese medicine; LPO, lipid peroxidation; HUVECs, human umbilical vein endothelial cells; ER, endoplasmic reticulum; Bip, binding immunoglobulin protein; CHOP, and C/EBP homologous protein; PERK, phosphorylation of extracellular regulated protein kinases; IRE1α inositol-requiring enzyme 1α; Tph, peripheral helper T; MDA, malondialdehyde; H2O2, hydrogen peroxide; GSH, glutathione; BMDM, bone marrow-derived macrophage; DSS, dextran sulfate sodium; EMT, epithelial–mesenchymal transition; t-BHP, tert-butyl hydrogen peroxide; GPX4, glutathione peroxidase 4; α-SMA, α-smooth muscle actin.
References
- Smolen, J.S.; Aletaha, D.; McInnes, I.B. Rheumatoid arthritis. Lancet 2016, 388, 2023–2038. [Google Scholar] [CrossRef]
- Wu, D.; Luo, Y.; Li, T.; Chen, Y.; Liu, F.; Wu, Y.; He, Y.; Sun, W.; Chen, J.; Li, Z.; et al. Systemic complications of rheumatoid arthritis: Focus on pathogenesis and treatment. Front. Immunol. 2022, 13, 1051082. [Google Scholar] [CrossRef]
- Singh, J.A. Treatment Guidelines in Rheumatoid Arthritis. Rheum. Dis. Clin. N. Am. 2022, 48, 679–689. [Google Scholar] [CrossRef]
- Tanaka, Y. Recent progress in treatments of rheumatoid arthritis: An overview of developments in biologics and small molecules, and remaining unmet needs. Rheumatology 2021, 60 (Suppl. S6), vi12–vi20. [Google Scholar] [CrossRef] [PubMed]
- Chuang, A.T.; Tsai, D.H.; Weng, M.Y.; Huang, H.K.; Lai, E.C. Effectiveness of JAK inhibitors in biologics-naïve patients with RA: A population-based study. Rheumatology 2025, 64, 4668–4677. [Google Scholar] [CrossRef] [PubMed]
- CTarjányi, O.; Olasz, K.; Rátky, F.; Sétáló, G.; Boldizsár, F. Proteasome Inhibitors: Potential in Rheumatoid Arthritis Therapy? Int. J. Mol. Sci. 2025, 26, 2943. [Google Scholar] [CrossRef] [PubMed]
- Küçükdeveci, A.A. Nonpharmacological treatment in established rheumatoid arthritis. Best Pract. Res. Clin. Rheumatol. 2019, 33, 101482. [Google Scholar] [CrossRef]
- Forsyth, C.; Kouvari, M.; D’Cunha, N.M.; Panagiotakos, D.B.; Mellor, D.D.; Kellett, J.; Naumovski, N. The effects of the Mediterranean diet on rheumatoid arthritis prevention and treatment: A systematic review of human prospective studies. Rheumatol. Int. 2018, 38, 737–747. [Google Scholar] [CrossRef]
- Pu, W.L.; Zhang, M.Y.; Bai, R.Y.; Sun, L.K.; Li, W.H.; Yu, Y.L. Anti-inflammatory effects of Rhodiola rosea L.: A review. Biomed. Pharmacother. 2020, 121, 109552. [Google Scholar] [CrossRef]
- Xue, H.; Li, P.; Luo, Y.; Fan, L.; Liu, X.; Zhang, L.; Liu, J.; Wang, N.; He, J.; Sun, S.; et al. Salidroside stimulates the Sirt1/PGC-1α axis and ameliorates diabetic nephropathy in mice. Phytomedicine 2019, 54, 240–247. [Google Scholar] [CrossRef]
- Magani, S.K.J.; Mupparthi, S.D.; Gollapalli, B.P.; Nile, S.H.; Garg, M.; Monga, J.; Mocan, A.; Atanasov, A.G. Salidroside—Can it be a Multifunctional Drug? Curr. Drug Metab. 2020, 21, 512–524. [Google Scholar] [CrossRef]
- Wojdasiewicz, P.; Brodacki, S.; Cieślicka, E.; Dobkowska, K.; Frasuńska, J.; Głuszko, P.; Paradowska-Gorycka, A. Salidroside: A Promising Agent in Bone Metabolism Modulation. Nutrients 2024, 16, 2387. [Google Scholar] [CrossRef]
- Wang, C.; Wang, Q.; Lou, Y.; Zhou, J.; Tang, Q.; Li, M.; Gu, P.; Li, X.; Hao, Y.; Yang, Z.; et al. Salidroside attenuates neuroinflammation and improves functional recovery after spinal cord injury through microglia polarization regulation. J. Cell. Mol. Med. 2018, 22, 1148–1166. [Google Scholar] [CrossRef]
- Shand, G.; Fuller, D.T.; Lufkin, L.; Katz, P.; Lanata, C.M.; Criswell, L.A.; Jolly, M. A stronger association of depression with rheumatoid arthritis in presence of obesity and hypertriglyceridemia. Front. Epidemiol. 2023, 3, 1216497. [Google Scholar] [CrossRef]
- Spagnolo, P.; Lee, J.S.; Sverzellati, N.; Rossi, G.; Cottin, V. The Lung in Rheumatoid Arthritis: Focus on Interstitial Lung Disease. Arthritis Rheumatol. 2018, 70, 1544–1554. [Google Scholar] [CrossRef]
- Willemze, A.; Trouw, L.A.; Toes, R.E.; Huizinga, T.W. The influence of ACPA status and characteristics on the course of RA. Nat. Rev. Rheumatol. 2012, 8, 144–152. [Google Scholar] [CrossRef]
- Shi, H.; Wu, H.; Winkler, M.A.; Chen, Y.; Li, T.; Han, X.; Zhao, Y.; Sun, L.; Zhang, Y.; Li, Y.; et al. Perivascular adipose tissue in autoimmune rheumatic diseases. Pharmacol. Res. 2022, 182, 106354. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Wei, Y.; Zhu, Y.; Xie, Z.; Hai, Q.; Li, Z.; Qin, D. Gut microbiota and rheumatoid arthritis: From pathogenesis to novel therapeutic opportunities. Front. Immunol. 2022, 13, 1007165. [Google Scholar] [CrossRef] [PubMed]
- Kuis, W.; Heijnen, C.J. Rheumatoid arthritis and juvenile chronic arthritis: The role of the neuro-endocrine system. Clin. Exp. Rheumatol. 1994, 12 (Suppl. S10), S29–S34. [Google Scholar] [PubMed]
- Sattler, J.; Tu, J.; Stoner, S.; Lue, Y.; Swerdloff, R.S.; Wang, C.; Hama, S.; Yuen, K.C.J.; Haddad, G.H. Role of 11β-HSD type 1 in abnormal HPA axis activity during immune-mediated arthritis. Endocr. Connect. 2018, 7, 385–394. [Google Scholar] [CrossRef]
- D’Haens, G.; Eberhardson, M.; Cabrijan, Z.; Danese, S.; van den Wall Bake, K.; Rijk, M.; Löwenberg, M.; Ponsioen, C.; van der Woude, J.; Bergmans, P.; et al. Neuroimmune Modulation Through Vagus Nerve Stimulation Reduces Inflammatory Activity in Crohn’s Disease Patients: A Prospective Open-label Study. J. Crohns Colitis 2023, 17, 1897–1909. [Google Scholar] [CrossRef]
- Campbell, K.L.; Winters-Stone, K.M.; Wiskemann, J.; May, A.M.; Schwartz, A.L.; Courneya, K.S.; Zucker, D.S.; Matthews, C.E.; Ligibel, J.A.; Gerber, L.H.; et al. Exercise Guidelines for Cancer Survivors: Consensus Statement from International Multidisciplinary Roundtable. Med. Sci. Sports Exerc. 2019, 51, 2375–2390. [Google Scholar] [CrossRef] [PubMed]
- Heath, E.I.; Weise, A.; Vaishampayan, U.; Danforth, D.; Ungerleider, R.S.; Urata, Y. Phase Ia dose escalation study of OBP-801, a cyclic depsipeptide class I histone deacetylase inhibitor, in patients with advanced solid tumors. Investig. New Drugs 2022, 40, 300–307. [Google Scholar] [CrossRef]
- Hockenberry, M.J.; Hooke, M.C.; Gregurich, M.; McCarthy, K. Carnitine plasma levels and fatigue in children/adolescents receiving cisplatin, ifosfamide, or doxorubicin. J. Pediatr. Hematol. Oncol. 2009, 31, 664–669. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.R.; Wolff, B.S.; Liwang, J.; Regan, J.; Alshawi, S.; Raheem, S.; Saligan, L.N. Cancer-related fatigue during combined treatment of androgen deprivation therapy and radiotherapy is associated with mitochondrial dysfunction. Int. J. Mol. Med. 2019, 45, 485–496. [Google Scholar] [CrossRef] [PubMed]
- Agca, R.; Heslinga, S.C.; Rollefstad, S.; Heslinga, M.; McInnes, I.B.; Peters, M.J.L.; Kvien, T.K.; Dougados, M.; Radner, H.; Atzeni, F.; et al. EULAR recommendations for cardiovascular disease risk management in patients with rheumatoid arthritis and other forms of inflammatory joint disorders: 2015/2016 update. Ann. Rheum. Dis. 2017, 76, 17–28. [Google Scholar] [CrossRef]
- Boissier, M.C.; Assier, E.; Falgarone, G.; Bessis, N. Shifting the imbalance from Th1/Th2 to Th17/treg: The changing rheumatoid arthritis paradigm. Joint Bone Spine 2008, 75, 373–375. [Google Scholar] [CrossRef]
- Khalaf, W.; Al-Rubaie, H.A.; Shihab, S. Studying anemia of chronic disease and iron deficiency in patients with rheumatoid arthritis by iron status and circulating hepcidin. Hematol. Rep. 2019, 11, 7708. [Google Scholar] [CrossRef]
- Behl, T.; Upadhyay, T.; Singh, S.; Chigurupati, S.; Alsubayiel, A.M.; Mani, V.; Vargas-De-La-Cruz, C.; Uivarosan, D.; Bustea, C.; Sava, C.; et al. Polyphenols Targeting MAPK Mediated Oxidative Stress and Inflammation in Rheumatoid Arthritis. Molecules 2021, 26, 6570. [Google Scholar] [CrossRef]
- Furst, D.E. The risk of infections with biologic therapies for rheumatoid arthritis. Semin. Arthritis Rheum. 2010, 39, 327–346. [Google Scholar] [CrossRef]
- Liu, H.; Zhu, Y.; Gao, Y.; Qi, D.; Zhao, L.; Zhao, L.; Liu, C.; Tao, T.; Zhou, C.; Sun, X.; et al. NR1D1 modulates synovial inflammation and bone destruction in rheumatoid arthritis. Cell Death Dis. 2020, 11, 129. [Google Scholar] [CrossRef]
- Deane, K.D.; Holers, V.M. The Natural History of Rheumatoid Arthritis. Clin. Ther. 2019, 41, 1256–1269. [Google Scholar] [CrossRef]
- Rivellese, F.; Pontarini, E.; Pitzalis, C. Tertiary Lymphoid Organs in Rheumatoid Arthritis. Curr. Top. Microbiol. Immunol. 2020, 426, 119–141. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.; Kwon, E.J.; Lee, J.J. Rheumatoid Arthritis: Pathogenic Roles of Diverse Immune Cells. Int. J. Mol. Sci. 2022, 23, 905. [Google Scholar] [CrossRef] [PubMed]
- Mateen, S.; Zafar, A.; Moin, S.; Khan, A.Q.; Zubair, S. Understanding the role of cytokines in the pathogenesis of rheumatoid arthritis. Clin. Chim. Acta 2016, 455, 161–171. [Google Scholar] [CrossRef]
- Pablos, J.L.; Cañete, J.D. Immunopathology of rheumatoid arthritis. Curr. Top. Med. Chem. 2013, 13, 705–711. [Google Scholar] [CrossRef]
- Cheng, L.; Wang, Y.; Wu, R.; Ding, T.; Xue, H.; Gao, C.; Li, X.; Wang, C. New Insights From Single-Cell Sequencing Data: Synovial Fibroblasts and Synovial Macrophages in Rheumatoid Arthritis. Front. Immunol. 2021, 12, 709178. [Google Scholar] [CrossRef] [PubMed]
- Kurowska-Stolarska, M.; Alivernini, S. Synovial tissue macrophages in joint homeostasis, rheumatoid arthritis and disease remission. Nat. Rev. Rheumatol. 2022, 18, 384–397. [Google Scholar] [CrossRef]
- Siouti, E.; Andreakos, E. The many facets of macrophages in rheumatoid arthritis. Biochem. Pharmacol. 2019, 165, 152–169. [Google Scholar] [CrossRef]
- Arango Duque, G.; Descoteaux, A. Macrophage cytokines: Involvement in immunity and infectious diseases. Front. Immunol. 2014, 5, 491. [Google Scholar] [CrossRef]
- Wen, X.; Chen, X.; Liang, X.; Wu, J.; Li, X.; Wang, X.; Huang, W.; Lin, J. The small molecule NSM00191 specifically represses the TNF-α/NF-κB axis in foot and ankle rheumatoid arthritis. Int. J. Biol. Sci. 2018, 14, 1732–1744. [Google Scholar] [CrossRef]
- Zwerina, J.; Redlich, K.; Polzer, K.; Joosten, L.; Krönke, G.; Distler, J.; Hess, A.; Pundt, N.; Pap, T.; Hoffmann, O.; et al. TNF-induced structural joint damage is mediated by IL-1. Proc. Natl. Acad. Sci. USA 2007, 104, 11742–11747. [Google Scholar] [CrossRef]
- Yao, X.; Huang, J.; Zhong, H.; Shen, N.; Faggioni, R.; Fung, M.; Yao, Y. Targeting interleukin-6 in inflammatory autoimmune diseases and cancers. Pharmacol. Ther. 2014, 141, 125–139. [Google Scholar] [CrossRef] [PubMed]
- Srirangan, S.; Choy, E.H. The role of interleukin 6 in the pathophysiology of rheumatoid arthritis. Ther. Adv. Musculoskelet. Dis. 2010, 2, 247–256. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.; Li, X.; Lin, A.; Larsson, S.C. Interleukins and rheumatoid arthritis: Bi-directional Mendelian randomization investigation. Semin. Arthritis Rheum. 2022, 53, 151958. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, D.; Chen, W.; Tao, Y.; Li, W.; Qi, J. Microenvironment-Activatable Probe for Precise NIR-II Monitoring and Synergistic Immunotherapy in Rheumatoid Arthritis. Adv. Mater. 2024, 36, 2409661. [Google Scholar] [CrossRef]
- Kinne, R.W.; Bräuer, R.; Stuhlmüller, B.; Palombo-Kinne, E.; Burmester, G.R. Macrophages in rheumatoid arthritis. Arthritis Res. 2000, 2, 189–202. [Google Scholar] [CrossRef]
- Dong, C. Cytokine Regulation and Function in T Cells. Annu. Rev. Immunol. 2021, 39, 51–76. [Google Scholar] [CrossRef]
- Wang, Z.; Zhuo, F.; Chu, P.; Yang, X.; Zhao, G. Germacrone alleviates collagen-induced arthritis via regulating Th1/Th2 balance and NF-κB activation. Biochem. Biophys. Res. Commun. 2019, 518, 560–564. [Google Scholar] [CrossRef]
- Ruschpler, P.; Stiehl, P. Shift in Th1 (IL-2 and IFN-gamma) and Th2 (IL-10 and IL-4) cytokine mRNA balance within two new histological main-types of rheumatoid-arthritis (RA). Cell. Mol. Biol. 2002, 48, 285–293. [Google Scholar]
- Cañete, J.D.; Martínez, S.E.; Farrés, J.; Sanmartí, R.; Blay, M.; Gómez, A.; Salvador, G.; Muñoz-Gómez, J. Differential Th1/Th2 cytokine patterns in chronic arthritis: Interferon gamma is highly expressed in synovium of rheumatoid arthritis compared with seronegative spondyloarthropathies. Ann. Rheum. Dis. 2000, 59, 263–268. [Google Scholar] [CrossRef]
- Niu, Y.; Dong, Q.; Li, R. Matrine regulates Th1/Th2 cytokine responses in rheumatoid arthritis by attenuating the NF-κB signaling. Cell Biol. Int. 2017, 41, 611–621. [Google Scholar] [CrossRef]
- Bao, Y.; Peng, J.; Yang, K.L.; Ma, Y.; Li, X.; Zou, Y.; Chen, H.; Zhou, P.; Liu, E.H.; Li, P.; et al. Therapeutic effects of Chinese medicine Di-Long (Pheretima vulgaris) on rheumatoid arthritis through inhibiting NF-κB activation and regulating Th1/Th2 balance. Biomed. Pharmacother. 2022, 147, 112643. [Google Scholar] [CrossRef]
- Lina, C.; Conghua, W.; Nan, L.; Ping, Z. Combined treatment of etanercept and MTX reverses Th1/Th2, Th17/Treg imbalance in patients with rheumatoid arthritis. J. Clin. Immunol. 2011, 31, 596–605. [Google Scholar] [CrossRef]
- Wang, J.; Conlon, D.; Rivellese, F.; Pereira, J.M.; Silverman, G.J.; Gregory, A.P.; Gravallese, E.M.; Weinblatt, M.E.; Shadick, N.A.; Su, L.; et al. Synovial Inflammatory Pathways Characterize Anti-TNF-Responsive Rheumatoid Arthritis Patients. Arthritis Rheumatol. 2022, 74, 1916–1927. [Google Scholar] [CrossRef] [PubMed]
- Rogozynski, N.P.; Dixon, B. The Th1/Th2 paradigm: A misrepresentation of helper T cell plasticity. Immunol. Lett. 2024, 268, 106870. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Niu, X. T follicular helper cells in autoimmune diseases. J. Autoimmun. 2023, 134, 102976. [Google Scholar] [CrossRef]
- Lu, J.; Wu, J.; Xia, X.; Peng, H.; Wang, S. Follicular helper T cells: Potential therapeutic targets in rheumatoid arthritis. Cell. Mol. Life Sci. 2021, 78, 5095–5106. [Google Scholar] [CrossRef] [PubMed]
- Yoshitomi, H. Peripheral helper T cells, mavericks of peripheral immune responses. Int. Immunol. 2024, 36, 9–16. [Google Scholar] [CrossRef]
- Weinand, K.; Sakaue, S.; Nathan, A.; Zhang, J.; Nasser, J.; Korsunsky, I.; Karjalainen, J.; Okada, Y.; Raychaudhuri, S. The chromatin landscape of pathogenic transcriptional cell states in rheumatoid arthritis. Nat. Commun. 2024, 15, 4650. [Google Scholar] [CrossRef] [PubMed]
- Fuite, J.; Vernon, S.D.; Broderick, G. Neuroendocrine and immune network re-modeling in chronic fatigue syndrome: An exploratory analysis. Genomics 2008, 92, 393–399. [Google Scholar] [CrossRef]
- Feldt-Rasmussen, U.; Effraimidis, G.; Klose, M. The hypothalamus-pituitary-thyroid (HPT)-axis and its role in physiology and pathophysiology of other hypothalamus-pituitary functions. Mol. Cell. Endocrinol. 2021, 525, 111173. [Google Scholar] [CrossRef] [PubMed]
- Spies, C.M.; Straub, R.H.; Cutolo, M.; Buttgereit, F. Circadian rhythms in rheumatology--a glucocorticoid perspective. Arthritis Res. Ther. 2014, 16 (Suppl. S2), S3. [Google Scholar] [CrossRef] [PubMed]
- Eijsbouts, A.M.; Murphy, E.P. The role of the hypothalamic-pituitary-adrenal axis in rheumatoid arthritis. Baillieres Best Pract. Res. Clin. Rheumatol. 1999, 13, 599–613. [Google Scholar] [CrossRef]
- Atzeni, F.; Straub, R.H.; Cutolo, M.; Sarzi-Puttini, P. Psoriatic arthritis: Clinical improvement and correlation with hormone axes in etanercept-treated patients. Ann. N. Y. Acad. Sci. 2010, 1193, 176–178. [Google Scholar] [CrossRef]
- Mravcova, M.; Chovanova, L.; Paulikova, L.; Lazurova, I. Genetics of neuroendocrine factors in rheumatoid arthritis. Horm. Metab. Res. 2015, 47, 411–417. [Google Scholar] [CrossRef]
- Chen, C.C.; Parker, C.R., Jr. Adrenal androgens and the immune system. Semin. Reprod. Med. 2004, 22, 369–377. [Google Scholar] [CrossRef]
- Imrich, R.; Rovenský, J. Hypothalamic-pituitary-adrenal axis in rheumatoid arthritis. Rheum. Dis. Clin. N. Am. 2010, 36, 721–727. [Google Scholar] [CrossRef] [PubMed]
- Bach, E.; Møller, A.B.; Jørgensen, J.O.L.; Vendelbo, M.H.; Jessen, N.; Olesen, J.L.; Pedersen, S.B.; Møller, N. Intact pituitary function is decisive for the catabolic response to TNF-α: Studies of protein, glucose and fatty acid metabolism in hypopituitary and healthy subjects. J. Clin. Endocrinol. Metab. 2015, 100, 578–586. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Abdullahi, A.; Wong, T.W.L.; Ng, S.S.M. Putative role of non-invasive vagus nerve stimulation in cancer pathology and immunotherapy: Can this be a hidden treasure, especially for the elderly? Cancer Med. 2023, 12, 19081–19090. [Google Scholar] [CrossRef]
- Cutolo, M.; Villaggio, B.; Foppiani, L.; Briata, M.; Sulli, A.; Pizzorni, C. The hypothalamic-pituitary-adrenal and gonadal axes in rheumatoid arthritis. Ann. N. Y. Acad. Sci. 2000, 917, 835–843. [Google Scholar] [CrossRef]
- Cutolo, M. Sex hormone adjuvant therapy in rheumatoid arthritis. Rheum. Dis. Clin. N. Am. 2000, 26, 881–895. [Google Scholar] [CrossRef]
- Koopman, F.A.; van Maanen, M.A.; Vervoordeldonk, M.J.; Tak, P.P. Balancing the autonomic nervous system to reduce inflammation in rheumatoid arthritis. J. Intern. Med. 2017, 282, 64–75. [Google Scholar] [CrossRef] [PubMed]
- Syngle, V.; Syngle, A.; Garg, N.; Krishan, P.; Verma, I. Predictors of autonomic neuropathy in rheumatoid arthritis. Auton. Neurosci. 2016, 201, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Karemaker, J.M. An introduction into autonomic nervous function. Physiol. Meas. 2017, 38, R89–R118. [Google Scholar] [CrossRef]
- Mendiola, A.S.; Ryu, J.K.; Bardehle, S.; Meyer-Franke, A.; Ang, K.K.H.; Wilson, C.; Baeten, K.M.; Hanspers, K.; Merlini, M.; Thomas, S.; et al. Transcriptional profiling and therapeutic targeting of oxidative stress in neuroinflammation. Nat. Immunol. 2020, 21, 513–524. [Google Scholar] [CrossRef] [PubMed]
- Louthrenoo, W.; Ruttanaumpawan, P.; Aramrattana, A.; Sukitawut, W. Cardiovascular autonomic nervous system dysfunction in patients with rheumatoid arthritis and systemic lupus erythematosus. QJM 1999, 92, 97–102. [Google Scholar] [CrossRef]
- Gullett, N.; Zajkowska, Z.; Walsh, A.; Harper, R.; Mondelli, V. Heart rate variability (HRV) as a way to understand associations between the autonomic nervous system (ANS) and affective states: A critical review of the literature. Int. J. Psychophysiol. 2023, 192, 35–42. [Google Scholar] [CrossRef]
- Vallerand, I.A.; Lewinson, R.T.; Frolkis, A.D.; Lowerison, M.W.; Kaplan, G.G.; Swain, M.G.; Patten, S.B.; Bulloch, A.G.M. Depression as a risk factor for the development of rheumatoid arthritis: A population-based cohort study. RMD Open 2018, 4, e000670. [Google Scholar] [CrossRef]
- Dhavale, H.S.; Gawande, S.; Bhagat, V.; Kalra, P.; Deo, S.S. Evaluation of efficacy and tolerability of dothiepin hydrochloride in the management of major depression in patients suffering from rheumatoid arthritis. J. Indian Med. Assoc. 2005, 103, 291–294. [Google Scholar]
- Hider, S.L.; Tanveer, W.; Brownfield, A.; Mattey, D.L.; Packham, J.C. Depression in RA patients treated with anti-TNF is common and under-recognized in the rheumatology clinic. Rheumatology 2009, 48, 1152–1154. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, D.; Salvadore, G.; Hsu, B.; Curran, M.; Casper, C.; Smolen, J.S. The effects of interleukin-6 neutralizing antibodies on symptoms of depressed mood and anhedonia in patients with rheumatoid arthritis and multicentric Castleman’s disease. Brain Behav. Immun. 2017, 66, 156–164. [Google Scholar] [CrossRef]
- Courties, A.; Berenbaum, F.; Sellam, J. Vagus nerve stimulation in musculoskeletal diseases. Joint Bone Spine 2021, 88, 105149. [Google Scholar] [CrossRef]
- Yan, Q.Q.; Sun, S.Y.; Tan, L.H.; Shi, Y.N.; Qiao, L.N.; Yang, Y.S. Effects of transcutaneous auricular vagus nerve stimulation on bone and cartilage destruction in rats with rheumatoid arthritis. Zhen Ci Yan Jiu 2022, 47, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Jensen, M.K.; Andersen, S.S.; Andersen, S.S.; Liboriussen, C.H.; Kristensen, S.; Jochumsen, M. Modulating Heart Rate Variability through Deep Breathing Exercises and Transcutaneous Auricular Vagus Nerve Stimulation: A Study in Healthy Participants and in Patients with Rheumatoid Arthritis or Systemic Lupus Erythematosus. Sensors 2022, 22, 7884. [Google Scholar] [CrossRef]
- Seca, S.; Kirch, S.; Cabrita, A.S.; Greten, H.J. Evaluation of the effect of acupuncture on hand pain, functional deficits and health-related quality of life in patients with rheumatoid arthritis—A study protocol for a multicenter, double-blind, randomized clinical trial. J. Integr. Med. 2016, 14, 219–227. [Google Scholar] [CrossRef]
- Hupin, D.; Sarajlic, P.; Venkateshvaran, A.; Börjesson, M.; Agewall, S.; Opava, C.H.; Brodin, N. Cardiovascular Autonomic Function Changes and Predictors During a 2-Year Physical Activity Program in Rheumatoid Arthritis: A PARA 2010 Substudy. Front. Med. 2021, 8, 788243. [Google Scholar] [CrossRef] [PubMed]
- Janse van Rensburg, D.C.; Ker, J.A.; Grant, C.C.; Fletcher, L. Effect of exercise on cardiac autonomic function in females with rheumatoid arthritis. Clin. Rheumatol. 2012, 31, 1155–1162. [Google Scholar] [CrossRef] [PubMed]
- Ngô, T.L. Review of the effects of mindfulness meditation on mental and physical health and its mechanisms of action. Sante Mentale Au Quebec 2013, 38, 19–34. [Google Scholar] [CrossRef]
- Zautra, A.J.; Davis, M.C.; Reich, J.W.; Nicassario, P.; Tennen, H.; Finan, P.; Kratz, A. Comparison of cognitive behavioral and mindfulness meditation interventions on adaptation to rheumatoid arthritis for patients with and without history of recurrent depression. J. Consult. Clin. Psychol. 2008, 76, 408–421. [Google Scholar] [CrossRef]
- Bloxham, E.; Vagadia, V.; Scott, K.; Francis, G.; Saravanan, V.; Heycock, C.; Rynne, M.; Hamilton, J.; Kelly, C.A. Anaemia in rheumatoid arthritis: Can we afford to ignore it? Postgrad. Med. J. 2011, 87, 596–600. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Liu, J.; Liu, S.; Wang, Y.; Wang, Y.; Li, J.; Wang, Y.; Zhang, X.; Li, Y.; Li, Z. Red Blood Cell-Related Parameters in Rheumatoid Arthritis: Clinical Value and Immunological Significance. J. Inflamm. Res. 2024, 17, 10641–10650. [Google Scholar] [CrossRef]
- Ford, J. Red blood cell morphology. Int. J. Lab. Hematol. 2013, 35, 351–357. [Google Scholar] [CrossRef]
- Mathru, M.; Solanki, D.R.; Woodson, L.C.; Funston, J.S.; Ozkan, A.K.; Roberts, P.R.; Lang, J.D.; Deyo, D.J. Splanchnic oxygen consumption is impaired during severe acute normovolemic anemia in anesthetized humans. Anesthesiology 2006, 105, 37–44. [Google Scholar] [CrossRef]
- Shah, J.; Farooq, A.; Zadran, S.; Kakar, Z.H.; Zarrar, M.; Bhatti, H. Prevalence of Anemia in Patients With Rheumatoid Arthritis Presenting at Multi-organization Tertiary Care Hospitals. Cureus 2024, 16, e72418. [Google Scholar] [CrossRef]
- Li, C.; Shi, X.; Chen, S.; Peng, X.; Zong, S. Novel mechanistic insights into the comorbidity of anemia and rheumatoid arthritis: Identification of therapeutic targets. Mol. Immunol. 2025, 180, 74–85. [Google Scholar] [CrossRef]
- Choudhury, C.; Sahib, A.; Karmakar, P.; Kar, S. Correlation of Serum Vitamin D and High-Density Lipoprotein (HDL) Cholesterol Levels With Disease Activity in Rheumatoid Arthritis: A Single-Center Experience From Eastern India. Cureus 2024, 16, e69333. [Google Scholar] [CrossRef]
- Vreugdenhil, G.; Wognum, A.W.; van Eijk, H.G.; Swaak, A.J. Anaemia in rheumatoid arthritis: The role of iron, vitamin B12, and folic acid deficiency, and erythropoietin responsiveness. Ann. Rheum. Dis. 1990, 49, 93–98. [Google Scholar] [CrossRef]
- Gluba-Brzózka, A.; Franczyk, B.; Olszewski, R.; Rysz, J. The Influence of Inflammation on Anemia in CKD Patients. Int. J. Mol. Sci. 2020, 21, 725. [Google Scholar] [CrossRef] [PubMed]
- Bertero, M.T.; Caligaris-Cappio, F. Anemia of chronic disorders in systemic autoimmune diseases. Haematologica 1997, 82, 375–381. [Google Scholar] [PubMed]
- Kheansaard, W.; Mas-Oo-Di, S.; Nilganuwong, S.; Tanyong, D.I. Interferon-gamma induced nitric oxide-mediated apoptosis of anemia of chronic disease in rheumatoid arthritis. Rheumatol. Int. 2013, 33, 151–156. [Google Scholar] [CrossRef]
- Weiss, G.; Ganz, T.; Goodnough, L.T. Anemia of inflammation. Blood 2019, 133, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Maury, C.P.; Andersson, L.C.; Teppo, A.M.; Partanen, S.; Juvonen, E. Mechanism of anaemia in rheumatoid arthritis: Demonstration of raised interleukin 1 beta concentrations in anaemic patients and of interleukin 1 mediated suppression of normal erythropoiesis and proliferation of human erythroleukaemia (HEL) cells in vitro. Ann. Rheum. Dis. 1988, 47, 972–978. [Google Scholar] [CrossRef]
- Stuart, C.M.; Jacob, C.; Varatharaj, A.; Grant, M.M.; Carden, M.J.; Spector, T.D.; Menni, C.; Berry, S.E.; Frost, G.S.; Chan, Q.; et al. Mild Systemic Inflammation Increases Erythrocyte Fragility. Int. J. Mol. Sci. 2024, 25, 7027. [Google Scholar] [CrossRef]
- Fulton, R.A. Megaloblastic anaemia and methotrexate treatment. Br. J. Dermatol. 1986, 114, 267–269. [Google Scholar] [CrossRef]
- Hurd, E.R.; Ziff, M. Studies on the anti-inflammatory action of 6-mercaptopurine. J. Exp. Med. 1968, 128, 785–800. [Google Scholar] [CrossRef] [PubMed]
- van Santen, S.; de Mast, Q.; Oosting, J.D.; van Ede, A.; Swinkels, D.W.; van der Ven, A.J. Hematologic parameters predicting a response to oral iron therapy in chronic inflammation. Haematologica 2014, 99, e171–e173. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, J.L.; Chan, F.K.; Lanas, A.; Peura, D.A.; Wilcox, C.M.; Fort, J.G.; Bello, A.E.; Huang, B.; Bauer, R.S.; Reasner, C.A.; et al. Haemoglobin decreases in NSAID users over time: An analysis of two large outcome trials. Aliment. Pharmacol. Ther. 2011, 34, 808–816. [Google Scholar] [CrossRef]
- Song, J.; Zhang, Y.; Li, A.; Wang, Y.; Chen, S.; Li, Y.; Wang, J. Prevalence of anemia in patients with rheumatoid arthritis and its association with dietary inflammatory index: A population-based study from NHANES 1999 to 2018. Medicine 2024, 103, e38471. [Google Scholar] [CrossRef]
- Staykova, N.D. Rheumatoid arthritis and thyroid abnormalities. Folia Med. 2007, 49, 5–12. [Google Scholar]
- Gu, P.; Pu, B.; Ma, Y.; Li, J.; Zhang, Y.; Wang, Y.; Zhang, Y.; Liu, Y.; Chen, Y.; Li, Z.; et al. Appraising the causal relationship between thyroid function and rheumatoid arthritis: A two-sample bidirectional Mendelian randomization study. Front. Immunol. 2023, 14, 1238757. [Google Scholar] [CrossRef]
- Lichtiger, A.; Fadaei, G.; Tagoe, C.E. Autoimmune thyroid disease and rheumatoid arthritis: Where the twain meet. Clin. Rheumatol. 2024, 43, 895–905. [Google Scholar] [CrossRef] [PubMed]
- Lazúrová, I.; Jochmanová, I.; Benhatchi, K.; Sotak, S. Autoimmune thyroid disease and rheumatoid arthritis: Relationship and the role of genetics. Immunol. Res. 2014, 60, 193–200. [Google Scholar] [CrossRef]
- Rasmussen, A.K. Cytokine actions on the thyroid gland. Dan. Med. Bull. 2000, 47, 94–114. [Google Scholar] [PubMed]
- Zhai, Y.; Wang, B.; Han, W.; Yu, B.; Ci, J.; An, F. Correlation between systemic inflammatory response index and thyroid function: 2009–2012 NHANES results. Front. Endocrinol. 2023, 14, 1305386. [Google Scholar] [CrossRef]
- Liu, X.; Yuan, J.; Wang, X.; Li, J.; Zhang, Y.; Li, Y.; Wang, J.; Chen, S.; Li, Z. Association between rheumatoid arthritis and autoimmune thyroid disease: Evidence from complementary genetic methods. Endocrine 2024, 84, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Shiroky, J.B.; Cohen, M.; Ballachey, M.L.; Neville, C. Thyroid dysfunction in rheumatoid arthritis: A controlled prospective survey. Ann. Rheum. Dis. 1993, 52, 454–456. [Google Scholar] [CrossRef]
- Silman, A.J.; Ollier, W.E.; Bubel, M.A. Autoimmune thyroid disease and thyroid autoantibodies in rheumatoid arthritis patients and their families. Br. J. Rheumatol. 1989, 28, 18–21. [Google Scholar] [CrossRef]
- Waldenlind, K.; Delcoigne, B.; Saevarsdottir, S.; Askling, J. Does autoimmune thyroid disease affect rheumatoid arthritis disease activity or response to methotrexate? RMD Open 2020, 6, e001282. [Google Scholar] [CrossRef]
- Lebiedziński, F.; Lisowska, K.A. Impact of Vitamin D on Immunopathology of Hashimoto’s Thyroiditis: From Theory to Practice. Nutrients 2023, 15, 3174. [Google Scholar] [CrossRef]
- Fedorchenko, Y.; Yessirkepov, M.; Doskaliuk, B.; Zaiats, L.; Mahmudov, K. Thyroid disease as a comorbidity in inflammatory rheumatic diseases. Rheumatol. Int. 2025, 45, 46. [Google Scholar] [CrossRef]
- Givian, A.; Azizan, A.; Jamshidi, A.; Mahmoudi, M.; Farhadi, E. Iron metabolism in rheumatic diseases. J. Transl. Autoimmun. 2025, 10, 100267. [Google Scholar] [CrossRef]
- Yang, Z.; Matteson, E.L.; Goronzy, J.J.; Weyand, C.M. T-cell metabolism in autoimmune disease. Arthritis Res. Ther. 2015, 17, 29. [Google Scholar] [CrossRef] [PubMed]
- Pal, Y.; Bandyopadhyay, N.; Pal, R.S.; Ahmed, S.; Bandopadhyay, S. Perspective and Potential of A2A and A3 Adenosine Receptors as Therapeutic Targets for the Treatment of Rheumatoid Arthritis. Curr. Pharm. Des. 2019, 25, 2859–2874. [Google Scholar] [CrossRef] [PubMed]
- Miyoshi, M.; Liu, S. Collagen-Induced Arthritis Models. Methods Mol. Biol. 2024, 2766, 3–7. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, N.; Bhatt, L.K.; Prabhavalkar, K.S. Experimental animal models for rheumatoid arthritis. Immunopharmacol. Immunotoxicol. 2018, 40, 193–200. [Google Scholar] [CrossRef]
- Feng, Z.T.; Yang, T.; Hou, X.Q.; Yan, L.H.; Wang, G.C. Sinomenine mitigates collagen-induced arthritis mice by inhibiting angiogenesis. Biomed. Pharmacother. 2019, 113, 108759. [Google Scholar] [CrossRef]
- Ma, F.; Li, Z.; Liu, H.; Zhang, X.; Jiang, H.; Li, X.; Zhao, Y.; Wang, Y.; Chen, W.; Liu, X.; et al. Dietary-timing-induced gut microbiota diurnal oscillations modulate inflammatory rhythms in rheumatoid arthritis. Cell Metab. 2024, 36, 2367–2382.e5. [Google Scholar] [CrossRef]
- Lange, F.; Bajtner, E.; Rintisch, C.; Nandakumar, K.S.; Sack, U.; Holmdahl, R. Methotrexate ameliorates T cell dependent autoimmune arthritis and encephalomyelitis but not antibody induced or fibroblast induced arthritis. Ann. Rheum. Dis. 2005, 64, 599–605. [Google Scholar] [CrossRef]
- Khachigian, L.M. Collagen antibody-induced arthritis. Nat. Protoc. 2006, 1, 2512–2516. [Google Scholar] [CrossRef]
- Okano, T.; Ashida, H.; Komatsu, N.; Nakano, Y.; Yamaguchi, T.; Oda, Y.; Ohya, K.; Itoh, S.; Okada, Y.; Matsushita, K. Caspase-11 mediated inflammasome activation in macrophages by systemic infection of A. actinomycetemcomitans exacerbates arthritis. Int. J. Oral Sci. 2024, 16, 54. [Google Scholar] [CrossRef]
- Tu, J.; Chen, W.; Huang, W.; Li, J.; Zhang, Y.; Wang, Y.; Chen, S.; Li, Z. Positive feedback loop PU.1-IL9 in Th9 promotes rheumatoid arthritis development. Ann. Rheum. Dis. 2024, 83, 1707–1721. [Google Scholar] [CrossRef]
- Liu, S. Human Xenograft Model. Methods Mol. Biol. 2024, 2766, 9–15. [Google Scholar] [CrossRef]
- Kong, J.S.; Jeong, G.H.; Yoo, S.A. The use of animal models in rheumatoid arthritis research. Yeungnam Univ. J. Med. 2023, 40, 23–29. [Google Scholar] [CrossRef]
- Taneja, V.; Taneja, N.; Paisansinsup, T.; Behrens, M.; Griffiths, M.; Luthra, H.; David, C.S. CD4 and CD8 T cells in susceptibility/protection to collagen-induced arthritis in HLA-DQ8-transgenic mice: Implications for rheumatoid arthritis. J. Immunol. 2002, 168, 5867–5875. [Google Scholar] [CrossRef] [PubMed]
- Muruganandam, A.; Migliorini, F.; Jeyaraman, M.; Maffulli, N.; Udayakumar, P.D.; Jeyaraman, N. Molecular Mimicry Between Gut Microbiome and Rheumatoid Arthritis: Current Concepts. Med. Sci. 2024, 12, 72. [Google Scholar] [CrossRef]
- Lamba, A.; Taneja, V. Gut microbiota as a sensor of autoimmune response and treatment for rheumatoid arthritis. Immunol. Rev. 2024, 325, 90–106. [Google Scholar] [CrossRef]
- Skarpanska-Stejnborn, A.; Pilaczynska-Szczesniak, L.; Basta, P.; Deskur-Smielecka, E. The influence of supplementation with Rhodiola rosea L. extract on selected redox parameters in professional rowers. Int. J. Sport Nutr. Exerc. Metab. 2009, 19, 186–199. [Google Scholar] [CrossRef] [PubMed]
- De Bock, K.; Eijnde, B.O.; Ramaekers, M.; Hespel, P. Acute Rhodiola rosea intake can improve endurance exercise performance. Int. J. Sport Nutr. Exerc. Metab. 2004, 14, 298–307. [Google Scholar] [CrossRef]
- Huang, S.C.; Lee, F.T.; Kuo, T.Y.; Yang, J.H.; Chien, C.T. Attenuation of long-term Rhodiola rosea supplementation on exhaustive swimming-evoked oxidative stress in the rat. Chin. J. Physiol. 2009, 52, 316–324. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Shen, F.; Wang, X.; Zhang, Y.; Li, Y.; Wang, J.; Chen, S.; Li, Z. Reshaped Gut Microbial Composition and Functions Associated with the Antifatigue Effect of Salidroside in Exercise Mice. Mol. Nutr. Food Res. 2023, 67, e2300015. [Google Scholar] [CrossRef]
- Ni, J.; Li, Y.; Li, W.; Guo, R. Salidroside protects against foam cell formation and apoptosis, possibly via the MAPK and AKT signaling pathways. Lipids Health Dis. 2017, 16, 198. [Google Scholar] [CrossRef]
- Guo, W.; Huang, R.; Bian, J.; Zhang, Y.; Li, Y.; Wang, J.; Chen, S.; Li, Z. Salidroside ameliorates macrophages lipid accumulation and atherosclerotic plaque by inhibiting Hif-1α-induced pyroptosis. Biochem. Biophys. Res. Commun. 2025, 742, 151104. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Wang, L.; Zeng, Y.; Zhang, Y.; Li, Y.; Wang, J.; Chen, S.; Li, Z. Salidroside alleviates cognitive impairment by inhibiting ferroptosis via activation of the Nrf2/GPX4 axis in SAMP8 mice. Phytomedicine 2023, 114, 154762. [Google Scholar] [CrossRef]
- Cui, Z.; Jin, N.; Amevor, F.K.; Du, X.; Zhang, Y.; Shu, G.; Zhao, X.; Tian, Y.; Zhu, Q. Dietary supplementation of salidroside alleviates liver lipid metabolism disorder and inflammatory response to promote hepatocyte regeneration via PI3K/AKT/Gsk3-β pathway. Poult. Sci. 2022, 101, 102034. [Google Scholar] [CrossRef]
- Hu, M.; Zhang, D.; Xu, H.; Zhang, Y.; Li, Y.; Wang, J.; Chen, S.; Li, Z. Salidroside Activates the AMP-Activated Protein Kinase Pathway to Suppress Nonalcoholic Steatohepatitis in Mice. Hepatology 2021, 74, 3056–3073. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, R.; Chen, Q.; Tian, Y.; Gao, L.; Lei, A. Salidroside improves porcine oocyte maturation and subsequent embryonic development by promoting lipid metabolism. Theriogenology 2022, 192, 89–96. [Google Scholar] [CrossRef]
- Wang, Y.F.; Chang, Y.Y.; Zhang, X.M.; Li, Y.; Wang, J.; Chen, S.; Li, Z. Salidroside protects against osteoporosis in ovariectomized rats by inhibiting oxidative stress and promoting osteogenesis via Nrf2 activation. Phytomedicine 2022, 99, 154020. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.L.; Deng, X.L.; Wu, G.J.; Li, W.J.; Jin, S. Rhodiola and salidroside in the treatment of metabolic disorders. Mini-Rev. Med. Chem. 2019, 19, 1611–1626. [Google Scholar] [CrossRef]
- Ma, C.; Wang, J.; Hong, F.; Yang, S. Mitochondrial Dysfunction in Rheumatoid Arthritis. Biomolecules 2022, 12, 1216. [Google Scholar] [CrossRef]
- Wang, X.L.; Sun, R.X.; Li, D.X.; Zhang, Y.; Li, Y.; Wang, J.; Chen, S.; Li, Z. Salidroside Regulates Mitochondrial Homeostasis After Polarization of RAW264.7 Macrophages. J. Cardiovasc. Pharmacol. 2023, 81, 85–92. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, M.; Zhang, Z.; Zhou, S. Salidroside induces mitochondrial dysfunction and ferroptosis to inhibit melanoma progression through reactive oxygen species production. Exp. Cell Res. 2024, 438, 114034. [Google Scholar] [CrossRef] [PubMed]
- Panfili, E.; Gerli, R.; Grohmann, U.; Pallotta, M.T. Amino Acid Metabolism in Rheumatoid Arthritis: Friend or Foe? Biomolecules 2020, 10, 1280. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Harms, A.C.; van Wijk, E.; Hankemeier, T.; van der Greef, J.; Wang, M. Role of amino acids in rheumatoid arthritis studied by metabolomics. Int. J. Rheum. Dis. 2019, 22, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Jia, F.; Wei, J.; Zhang, Y.; Li, Y.; Wang, J.; Chen, S.; Li, Z. Salidroside protects against homocysteine-induced injury in human umbilical vein endothelial cells via the regulation of endoplasmic reticulum stress. Cardiovasc. Ther. 2017, 35, 33–39. [Google Scholar] [CrossRef]
- Chiang, H.M.; Chien, Y.C.; Wu, C.H.; Kuo, Y.H.; Wu, W.C.; Pan, Y.Y.; Su, Y.H.; Wen, K.C. Hydroalcoholic extract of Rhodiola rosea L. (Crassulaceae) and its hydrolysate inhibit melanogenesis in B16F0 cells by regulating the CREB/MITF/tyrosinase pathway. Food Chem. Toxicol. 2014, 65, 129–139. [Google Scholar] [CrossRef]
- Xue, F.; Guo, H.; Hu, Y.; Liu, R.; Huang, L.; Lv, Z.; Liu, T. Expression of Codon-Optimized Plant Glycosyltransferase UGT72B14 in Escherichia coli Enhances Salidroside Production. BioMed Res. Int. 2016, 2016, 9845927. [Google Scholar] [CrossRef]
- Xu, L.; Chang, C.; Jiang, P.; Zhang, Y.; Li, Y.; Wang, J.; Chen, S.; Li, Z. Metabolomics in rheumatoid arthritis: Advances and review. Front. Immunol. 2022, 13, 961708. [Google Scholar] [CrossRef]
- Casanova-Vallve, N.; Constantin-Teodosiu, D.; Filer, A.; Hardy, R.S.; Greenhaff, P.L.; Chapman, V. Skeletal muscle dysregulation in rheumatoid arthritis: Metabolic and molecular markers in a rodent model and patients. PLoS ONE 2020, 15, e0235702. [Google Scholar] [CrossRef]
- Kim, S.; Miller, B.J.; Stefanek, M.E.; Miller, A.H. Inflammation-induced activation of the indoleamine 2,3-dioxygenase pathway: Relevance to cancer-related fatigue. Cancer 2015, 121, 2129–2136. [Google Scholar] [CrossRef]
- Qin, N.; Xie, H.; Zhao, A.; Li, Y.; Wang, J.; Chen, S.; Li, Z. Effects of salidroside on exercise tolerance of mice under high altitude hypoxia environment. J. Zhejiang Univ. Med. Sci. 2022, 51, 397–404. [Google Scholar] [CrossRef]
- Smallwood, M.J.; Nissim, A.; Knight, A.R.; Whiteman, M.; Haigh, R.; Winyard, P.G. Oxidative stress in autoimmune rheumatic diseases. Free Radic. Biol. Med. 2018, 125, 3–14. [Google Scholar] [CrossRef]
- Tinsley, G.M.; Jagim, A.R.; Potter, G.D.M.; Garner, D.; Galpin, A.J. Rhodiola rosea as an adaptogen to enhance exercise performance: A review of the literature. Br. J. Nutr. 2024, 131, 461–473. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Zhang, Y.; Jiang, S.; Li, Y.; Wang, J.; Chen, S.; Li, Z. Salidroside intensifies mitochondrial function of CoCl2-damaged HT22 cells by stimulating PI3K-AKT-MAPK signaling pathway. Phytomedicine 2023, 109, 154568. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, Z.; Song, J.; Li, Y.; Wang, J.; Chen, S.; Li, Z. Salidroside promotes healthy longevity by interfering with HSP90 activity. GeroScience 2024, 46, 1641–1655. [Google Scholar] [CrossRef]
- Chen, L.; Liu, P.; Feng, X.; Ma, C. Salidroside suppressing LPS-induced myocardial injury by inhibiting ROS-mediated PI3K/Akt/mTOR pathway in vitro and in vivo. J. Cell. Mol. Med. 2017, 21, 3178–3189. [Google Scholar] [CrossRef]
- Clanchy, F.I.L.; Williams, R.O. Ibudilast Inhibits Chemokine Expression in Rheumatoid Arthritis Synovial Fibroblasts and Exhibits Immunomodulatory Activity in Experimental Arthritis. Arthritis Rheumatol. 2019, 71, 703–711. [Google Scholar] [CrossRef]
- Maiuolo, J.; Muscoli, C.; Gliozzi, M.; Carresi, C.; Scarano, F.; Coppoletta, A.R.; Guarnieri, L.; Nusca, A.; Mollace, V. Endothelial Dysfunction and Extra-Articular Neurological Manifestations in Rheumatoid Arthritis. Biomolecules 2021, 11, 81. [Google Scholar] [CrossRef]
- Li, F.; Mao, Q.; Wang, J.; Li, Y.; Wang, J.; Chen, S.; Li, Z. Salidroside inhibited cerebral ischemia/reperfusion-induced oxidative stress and apoptosis via Nrf2/Trx1 signaling pathway. Metab. Brain Dis. 2022, 37, 2965–2978. [Google Scholar] [CrossRef]
- Ju, I.J.; Tsai, B.C.; Kuo, W.W.; Ho, T.J.; Day, C.H.; Mahalakshmi, B.; Huang, C.Y. Rhodiola and Salidroside Attenuate Oxidative Stress-Triggered H9c2 Cardiomyoblast Apoptosis Through IGF1R-Induced ERK1/2 Activation. Environ. Toxicol. 2024, 39, 5150–5161. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ge, H.; Yu, Y.; Li, Y.; Wang, J.; Chen, S.; Li, Z. Dietary salidroside supplementation improves meat quality and antioxidant capacity and regulates lipid metabolism in broilers. Food Chem. 2024, 22, 101406. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.M.; Huang, Y.M.; Qin, Z.Q. Salidroside Exerts Beneficial Effect on Testicular Ischemia-Reperfusion Injury in Rats. Oxid. Med. Cell. Longev. 2022, 2022, 8069152. [Google Scholar] [CrossRef]
- Sun, Y.M.; Wang, X.Y.; Zhou, X.R.; Li, Y.; Wang, J.; Chen, S.; Li, Z. Salidroside Ameliorates Radiation Damage by Reducing Mitochondrial Oxidative Stress in the Submandibular Gland. Antioxidants 2022, 11, 1414. [Google Scholar] [CrossRef]
- Gautam, S.; Kumar, R.; Kumar, U.; Kumar, S.; Luthra, K.; Dada, R. Yoga maintains Th17/Treg cell homeostasis and reduces the rate of T cell aging in rheumatoid arthritis: A randomized controlled trial. Sci. Rep. 2023, 13, 14924. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Tong, Y.; Wu, L.; Li, Y.; Wang, J.; Chen, S.; Li, Z. Alteration of Gut Microbiota in Individuals at High-Risk for Rheumatoid Arthritis Associated With Disturbed Metabolome and the Initiation of Arthritis Through the Triggering of Mucosal Immunity Imbalance. Arthritis Rheumatol. 2023, 75, 1736–1748. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Feng, Y.; Zheng, X.; Li, Y.; Wang, J.; Chen, S.; Li, Z. M2-type exosomes nanoparticles for rheumatoid arthritis therapy via macrophage re-polarization. J. Control. Release 2022, 341, 16–30. [Google Scholar] [CrossRef]
- Guo, Q.; Mao, X.; Zhang, Y.; Li, Y.; Wang, J.; Chen, S.; Li, Z. Guizhi-Shaoyao-Zhimu decoction attenuates rheumatoid arthritis partially by reversing inflammation-immune system imbalance. J. Transl. Med. 2016, 14, 165. [Google Scholar] [CrossRef]
- Zheng, Q.H.; Du, L.Y.; Zhao, Y.; Li, Y.; Wang, J.; Chen, S.; Li, Z. Mechanism of Rhodiola rosea-Euonymus alatus drug pair against rheumatoid arthritis: Network pharmacology and experimental validation. Immun. Inflamm. Dis. 2023, 11, e1127. [Google Scholar] [CrossRef]
- Tang, L.; Guo, D.; Jia, D.; Li, Y.; Wang, J.; Chen, S.; Li, Z. Exploring the therapeutic potential of “Tianyu” medicine pair in rheumatoid arthritis: An integrated study combining LC-MS/MS, bioinformatics, network pharmacology, and experimental validation. Front. Med. 2024, 11, 1475239. [Google Scholar] [CrossRef]
- Chen, T.; Li, S.; Lian, D.; Li, Y.; Wang, J.; Chen, S.; Li, Z. Integrated Network Pharmacology and Experimental Approach to Investigate the Protective Effect of Jin Gu Lian Capsule on Rheumatoid Arthritis by Inhibiting Inflammation via IL-17/NF-κB Pathway. Drug Des. Devel. Ther. 2023, 17, 3723–3748. [Google Scholar] [CrossRef]
- Liu, X.; Zhou, M.; Dai, Z.; Li, Y.; Wang, J.; Chen, S.; Li, Z. Salidroside alleviates ulcerative colitis via inhibiting macrophage pyroptosis and repairing the dysbacteriosis-associated Th17/Treg imbalance. Phytother. Res. 2023, 37, 367–382. [Google Scholar] [CrossRef] [PubMed]
- Mihai, I.R.; Rezus, C.; Burlui, M.A.; Cardoneanu, A.; Macovei, L.A.; Bratoiu, I.; Rezus, E. Autoimmune Liver Diseases and Rheumatoid Arthritis—Is There an Etiopathogenic Link? Int. J. Mol. Sci. 2024, 25, 3848. [Google Scholar] [CrossRef] [PubMed]
- Ding, C.; Guo, Z.; Liao, Q.; Li, Y.; Wang, J.; Chen, S.; Li, Z. Network Pharmacology and Machine Learning Reveal Salidroside’s Mechanisms in Idiopathic Pulmonary Fibrosis Treatment. J. Inflamm. Res. 2024, 17, 9453–9467. [Google Scholar] [CrossRef] [PubMed]
- Tong, Z.; Du, X.; Zhou, Y.; Li, Y.; Wang, J.; Chen, S.; Li, Z. Drp1-mediated mitochondrial fission promotes pulmonary fibrosis progression through the regulation of lipid metabolic reprogramming by ROS/HIF-1α. Cell. Signal. 2024, 117, 111075. [Google Scholar] [CrossRef]
- Cai, L.; Li, Y.; Zhang, Q.; Li, Y.; Wang, J.; Chen, S.; Li, Z. Salidroside protects rat liver against ischemia/reperfusion injury by regulating the GSK-3β/Nrf2-dependent antioxidant response and mitochondrial permeability transition. Eur. J. Pharmacol. 2017, 806, 32–42. [Google Scholar] [CrossRef]
- Wilson, A.; Yu, H.T.; Goodnough, L.T.; Nissenson, A.R. Prevalence and outcomes of anemia in rheumatoid arthritis: A systematic review of the literature. Am. J. Med. 2004, 116 (Suppl. S7A), 50S–57S. [Google Scholar] [CrossRef]
- Liu, Z.; Chen, L.; Xiong, D.; Li, Y.; Wang, J.; Chen, S.; Li, Z. Salidroside affects the Th17/Treg cell balance in aplastic anemia via the STAT3/HIF-1α/RORγt pathway. Redox Rep. 2023, 28, 2225868. [Google Scholar] [CrossRef]
- Li, X.; Sipple, J.; Pang, Q.; Du, W. Salidroside stimulates DNA repair enzyme Parp-1 activity in mouse HSC maintenance. Blood 2012, 119, 4162–4173. [Google Scholar] [CrossRef]
- Zhang, X.S.; Zhu, B.D.; Hung, X.Q.; Chen, Y.F. Effect of salidroside on bone marrow cell cycle and expression of apoptosis-related proteins in bone marrow cells of bone marrow depressed anemia mice. Sichuan Da Xue Xue Bao Yi Xue Ban 2005, 36, 820–823, 846. [Google Scholar]
- Zhu, L.; Wei, T.; Gao, J.; Chang, X.; He, H.; Miao, M.; Yan, T. Salidroside attenuates lipopolysaccharide (LPS) induced serum cytokines and depressive-like behavior in mice. Neurosci. Lett. 2015, 606, 1–6. [Google Scholar] [CrossRef]
- Doroszkiewicz, J.; Mroczko, J.; Winkel, I.; Mroczko, B. Metabolic and Immune System Dysregulation: Unraveling the Connections between Alzheimer’s Disease, Diabetes, Inflammatory Bowel Diseases, and Rheumatoid Arthritis. J. Clin. Med. 2024, 13, 5057. [Google Scholar] [CrossRef]
- Zhang, J.; Zhen, Y.F.; Pu Bu Ci, R.; Wang, W.; Li, Y.; Wang, J.; Chen, S.; Li, Z. Salidroside attenuates beta amyloid-induced cognitive deficits via modulating oxidative stress and inflammatory mediators in rat hippocampus. Behav. Brain Res. 2013, 244, 70–81. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, Y.; Wu, Y.; Li, T.; Wang, W. Salidroside shows anticonvulsant and neuroprotective effects by activating the Nrf2-ARE pathway in a pentylenetetrazol-kindling epileptic model. Brain Res. Bull. 2020, 164, 14–20. [Google Scholar] [CrossRef]
- Liang, L.; Ma, Z.; Dong, M.; Ma, J.; Jiang, A.; Sun, X. Protective effects of salidroside against isoflurane-induced cognitive impairment in rats. Hum. Exp. Toxicol. 2017, 36, 1295–1302. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, Q.; Cheng, Q.; Ding, F. Protective effect of salidroside against H2O2-induced cell apoptosis in primary culture of rat hippocampal neurons. Mol. Cell. Biochem. 2009, 332, 85–93. [Google Scholar] [CrossRef]
- Li, X.; Ye, X.; Li, X.; Li, X.; Li, H.; Zhang, Z.; Lin, A.; Liu, K.; Chen, X.; Xu, R. Salidroside protects against MPP+-induced apoptosis in PC12 cells by inhibiting the NO pathway. Brain Res. 2011, 1382, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Wang, Z.; Ji, Y.; Yin, J.; Yang, W.H.; Ren, L.M. Effects of Salidroside on Tic Behavior of Tourette Syndrome Model Rats. Zhongguo Zhong Xi Yi Jie He Za Zhi 2016, 36, 90–93. [Google Scholar] [PubMed]
- Cruz-Pereira, J.S.; Rea, K.; Nolan, Y.M.; O’Leary, O.F.; Dinan, T.G.; Cryan, J.F. Depression’s Unholy Trinity: Dysregulated Stress, Immunity, and the Microbiome. Annu. Rev. Psychol. 2020, 71, 49–78. [Google Scholar] [CrossRef] [PubMed]
- Hassamal, S. Chronic stress, neuroinflammation, and depression: An overview of pathophysiological mechanisms and emerging anti-inflammatories. Front. Psychiatry 2023, 14, 1130989. [Google Scholar] [CrossRef]
- Jomova, K.; Raptova, R.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Valko, M. Reactive oxygen species, toxicity, oxidative stress, and antioxidants: Chronic diseases and aging. Arch. Toxicol. 2023, 97, 2499–2574. [Google Scholar] [CrossRef]
- Jung, J.; Lee, S.M.; Lee, M.J.; Kim, J.E.; Lee, J.E.; Hahm, D.H.; Shim, I. Lipidomics reveals that acupuncture modulates the lipid metabolism and inflammatory interaction in a mouse model of depression. Brain Behav. Immun. 2021, 94, 424–436. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Ren, T.; Xiong, Q.; Lin, Z.; Lin, X.; Lin, G. Salidroside alleviates diet-induced obesity and insulin resistance by activating Nrf2/ARE pathway and enhancing the thermogenesis of adipose tissues. Food Sci. Nutr. 2023, 11, 4735–4744. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Xu, T.; Zhou, F.; Wang, Y.; Wang, J.; Chen, S.; Li, Z. Neuroprotective Effects of Four Phenylethanoid Glycosides on H2O2-Induced Apoptosis on PC12 Cells via the Nrf2/ARE Pathway. Int. J. Mol. Sci. 2018, 19, 1135. [Google Scholar] [CrossRef] [PubMed]
- Veenema, A.H.; Koolhaas, J.M.; de Kloet, E.R. Basal and stress-induced differences in HPA axis, 5-HT responsiveness, and hippocampal cell proliferation in two mouse lines. Ann. N. Y. Acad. Sci. 2004, 1018, 255–265. [Google Scholar] [CrossRef]
- Ao, W.; Gao, W.; Li, T. Research progress on the mechanism of antidepressant effect of salidroside. Int. Immunopharmacol. 2025, 162, 115205. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, L.; Lin, L.; Wang, Y.; Wang, J.; Chen, S.; Li, Z. Pharmacokinetics, tissue distribution, and excretion of salidroside in rats. Planta Med. 2013, 79, 1429–1433. [Google Scholar] [CrossRef]
- Guo, N.; Hu, Z.; Fan, X.; Zheng, J.; Zhang, Z.; Shang, L.; Wang, C.; Li, Y. Simultaneous determination of salidroside and its aglycone metabolite p-tyrosol in rat plasma by liquid chromatography-tandem mass spectrometry. Molecules 2012, 17, 4733–4754. [Google Scholar] [CrossRef]
- He, Y.-X.; Liu, X.-D.; Wang, X.-T.; Liu, X.; Wang, G.-J.; Xie, L. Sodium-dependent Glucose Transporter Was Involved in Salidroside Absorption in Intestine of Rats. Chin. J. Nat. Med. 2009, 7, 444–448. [Google Scholar] [CrossRef]
- Mao, Y.; Zhang, X.; Zhang, X.; Lu, G. Development of an HPLC method for the determination of salidroside in beagle dog plasma after administration of salidroside injection: Application to a pharmacokinetics study. J. Sep. Sci. 2007, 30, 3218–3222. [Google Scholar] [CrossRef]
- Gan, Z.; Fang, X.; Yin, C.; Li, Y.; Wang, J.; Chen, S.; Li, Z. Extraction, purification, structural characterization, and bioactivities of the genus Rhodiola L. polysaccharides: A review. Int. J. Biol. Macromol. 2024, 276, 133614. [Google Scholar] [CrossRef]
- Zhu, J.; Wan, X.; Zhu, Y.; Ma, X.; Zheng, Y.; Zhang, T. Evaluation of salidroside in vitro and in vivo genotoxicity. Drug Chem. Toxicol. 2010, 33, 220–226. [Google Scholar] [CrossRef]
- Zdanowski, R.; Skopińska-Różewska, E.; Wilczak, J.; Borecka, A.; Lewicka, A.; Lewicki, S. Different effects of feeding pregnant and lactating mice Rhodiola kirilowii aqueous and hydro-alcoholic extracts on their serum angiogenic activity and content of selected polyphenols. Cent. Eur. J. Immunol. 2017, 42, 17–23. [Google Scholar] [CrossRef]
- Wang, X.; Tang, Y.; Xie, N.; Li, Y.; Wang, J.; Chen, S.; Li, Z. Salidroside, a phenyl ethanol glycoside from Rhodiola crenulata, orchestrates hypoxic mitochondrial dynamics homeostasis by stimulating Sirt1/p53/Drp1 signaling. J. Ethnopharmacol. 2022, 293, 115278. [Google Scholar] [CrossRef]
- Bernatoniene, J.; Jakstas, V.; Kopustinskiene, D.M. Phenolic Compounds of Rhodiola rosea L. as the Potential Alternative Therapy in the Treatment of Chronic Diseases. Int. J. Mol. Sci. 2023, 24, 12293. [Google Scholar] [CrossRef]
- Liu, Q.; Chen, J.; Zeng, A.; Song, L. Pharmacological functions of salidroside in renal diseases: Facts and perspectives. Front. Pharmacol. 2023, 14, 1309598. [Google Scholar] [CrossRef]
- Liu, H.; Tian, Y.; Zhou, Y.; Li, Y.; Wang, J.; Chen, S.; Li, Z. Multi-modular engineering of Saccharomyces cerevisiae for high-titre production of tyrosol and salidroside. Microb. Biotechnol. 2021, 14, 2605–2616. [Google Scholar] [CrossRef]
- Di Felice, G.; Colombo, P. Nanoparticle-allergen complexes for allergen immunotherapy. Int. J. Nanomedicine 2017, 12, 4493–4504. [Google Scholar] [CrossRef]
- Jiang, S.; Li, H.; Zhang, L.; Li, Y.; Wang, J.; Chen, S.; Li, Z. Generic Diagramming Platform (GDP): A comprehensive database of high-quality biomedical graphics. Nucleic Acids Res. 2025, 53, D1670–D1676. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).