Unraveling Mechanisms of Enzymatic Browning in Nuts and Their Relationship with Pre- and Post-Harvest Factors: Management Strategies for Mitigation
Abstract
1. Introduction
2. Morphological Characterization of the Enzymatic Browning of Fruits in Tree Nuts
| Tree Nuts | Description of Symptoms | Image | References |
|---|---|---|---|
| Almond (P. dulcis L.) | It covers 50% of the inner part of the kernel with a brown coloration, associated with bitter flavors, which can develop both during and after harvest. | [26] | |
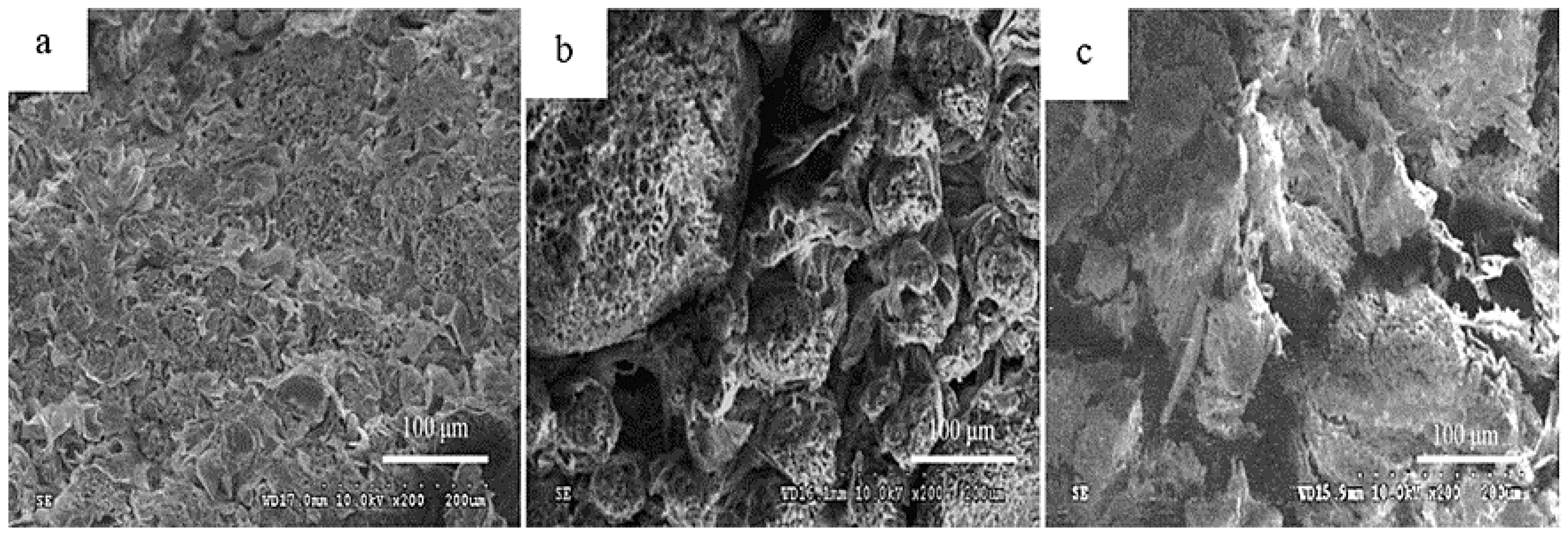
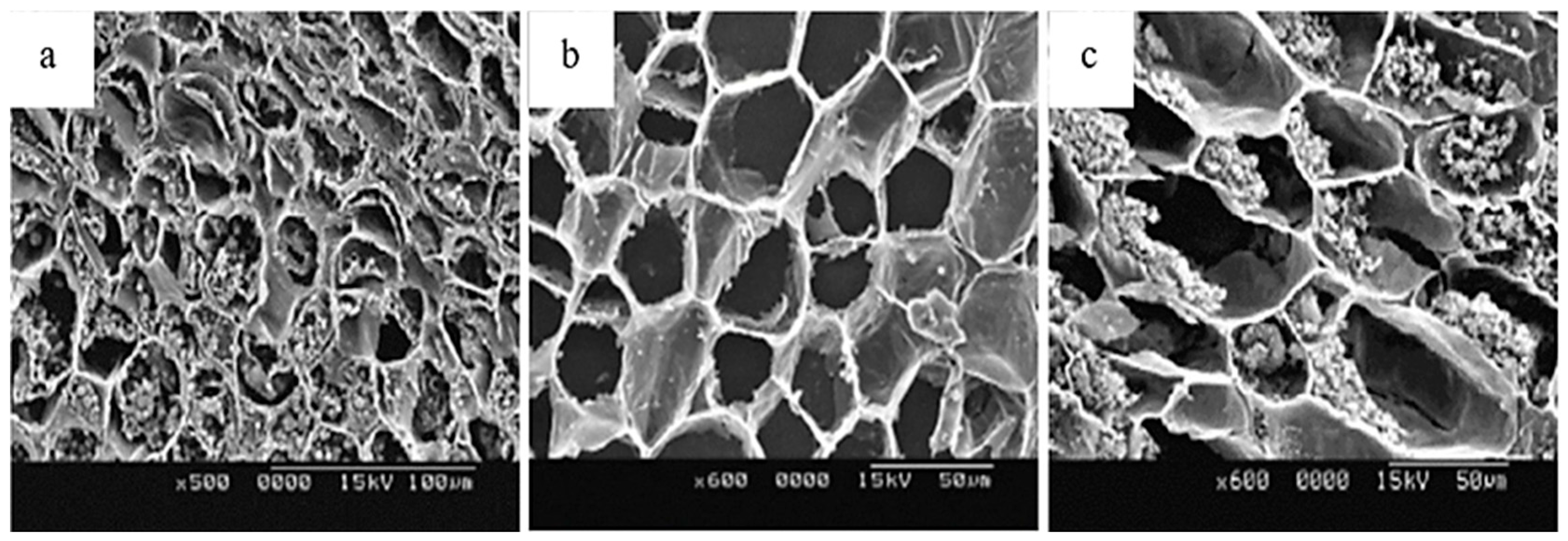
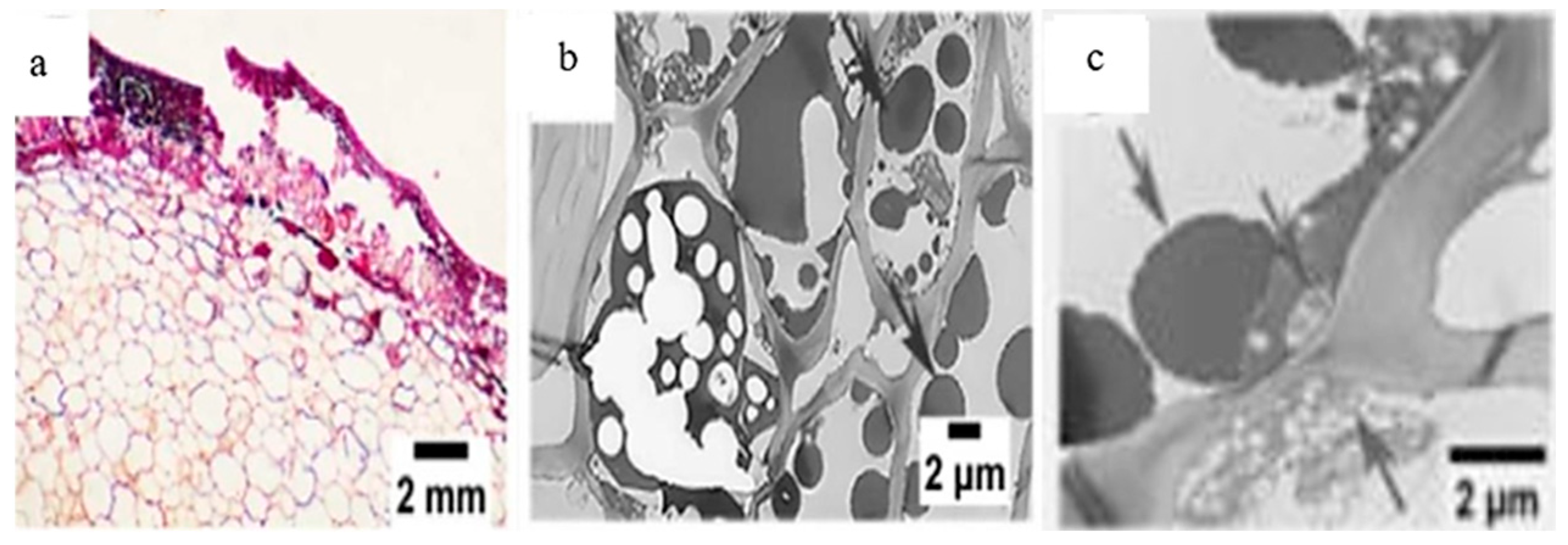
| Betel nut (B.catechu L.) | The kernel gradually decomposes with darker color changes; it is destroyed in the form of a honeycomb, decreasing lignification and increasing the interstitial space. |  1 1 | [27] |
| Chestnut (C. mollissima Blume) (C. henryi) | Kernels turn brown after processing, changing color and causing degradation of flavor and nutrients, thus reducing shelf life. |  2 2 | [6,7,21] |
| Halzenut (C. avellana L.) | The cells in the browning zone gradually lose their isodiametric shape until the tissue is destroyed by lysis which results in the appearance of brown watery exudates and generates a softer and less consistent nut. |  3 3 | [12,31] |
| Macadamia (M. integrifolia) (M. tetraphylla) | A thin line of brown discoloration around the equator of the kernel, which in severe cases affects the entire base of the kernel, accompanied by an unpleasant odor and taste. | [14,28,29] | |
| Pecan (C. illinoinensis L.) | Freshly harvested kernels have a golden yellow color, which gradually turns brown during storage, affecting flavor, nutritional value, and commercial appeal. |  4 4 | [8,32] |
| Pistachio (P. vera L.) | It is present in the hulls, causing a brown coloration followed by putrefaction, causing quality loss during processing and storage. | [10,20] | |
| Walnut (J. regia L.) | The kernels and hull are prone to darken, forming a black exudate that contaminates the inside of the nut, thus reducing quality. |  5 5 | [4,5,9,33] |
3. Mechanisms of EB of Commercial Tree Nuts and Their Relationship with the Different Systems Involved
4. Factors Contributing to EB and Quality Deterioration in Commercial Tree Nuts
4.1. Pre-Harvest Factors
4.1.1. Cultivar
4.1.2. Plant Nutrition
4.1.3. Climatic Conditions and Altitude
4.2. Post-Harvest Factors
4.2.1. Nut Shelling
4.2.2. Storage and Industrial Processing
5. Strategies to Prevent Browning in Tree Nuts
5.1. Synthetic Inhibitors by Mode of Action
5.1.1. Reduction
5.1.2. Competitive
5.1.3. Chelation
5.1.4. Complexation
5.2. Physical Treatments
5.2.1. Heat Treatment
5.2.2. Alternative Treatment (Non-Thermal)
5.2.3. Atmosphere Composition Management
| Treatment | Technique | Tree Nuts | Effect | References |
|---|---|---|---|---|
| Thermal | IR blanching | Walnut (J. regia L.) | It reduced drying time, peroxide values, browning, and quinone content, and inactivated LOX and PPO enzymatic activities. It also showed higher TP retention. | [32] |
| Thermal | Hot air drying | Walnut (J. regia L.) | Fourteen flavonoids were identified that showed a strong correlation with BI. In addition, LAC and ANR were the key enzymes involved in EB. | [18] |
| Non-thermal | High hydrostatic pressure (HHP) | Cashew (A. occidentale L.) | It produced losses of color parameters, in addition to the reduction of AA, polyphenols, and AC, causing EB. | [23] |
| Composition of the atmosphere | Controlled atmosphere (CA) storage | Walnut (J. regia L.) | It promoted enzymatic activity, preserved TPC, ASA, and GSH, inhibited ethylene production, and reduced ROS, acidity, and peroxide, delaying EB. It also increased quality by inhibiting color and moisture loss. | [4] |
| Composition of the atmosphere | Controlled atmosphere storage (CA) | Walnut (J. regia L.) | Increased GABA levels through increased activity of glutamate decarboxylase, GABA transaminase, and succinate dehydrogenase. Reducing changes in EB, respiratory rate, and color parameters. | [9] |
| Composition of the atmosphere | Modified atmosphere storage (MAP) | Almond (P. dulcis L.) | It showed no changes in film color, respiration intensity, or enzymatic activities of PPO and POD while fully maintaining their quality. | [46] |
| Composition of the atmosphere | Modified atmosphere storage (MAP) | Hazelnut (C. avellana L.) | Reduced lipid oxidation, BI, and quality deterioration. | [48] |
| Composition of the atmosphere | Modified atmosphere storage (MAP) active | Pistachio (P. vera L.) | It prevented weight loss and chlorophyll and carotenoid content. It also promoted higher levels of PAL while preserving levels of AC, TAA, TPC, and quality. | [47] |
| Composition of the atmosphere | Modified atmosphere storage (MAP) | Walnut (J. regia L.) | The levels of TP, total flavonoids, PAL, and antioxidant activity remained high, while the incidence of rot, PPO, and POD, in addition to peroxide value and acidity, was lower. | [34] |
| Low temperatures | Low temperatures | Walnut (J. regia L.) | Prevented loss of antioxidants and EB. In addition, increased TP and TAC in the stored walnut. | [33,36] |
| Low temperatures | Low temperatures | Walnut (J. regia L.) | It reduced oxidative metabolism, decreased PPO activity, suppressed antioxidant loss, and delayed EB. | [49] |
| Low temperatures | Low temperatures | Walnut (J. regia L.) | Decreased EB level, peroxide values, and PPO activity. As well as an increase in AC and TP levels, improving quality. | [24] |
5.2.4. Low Temperatures
5.3. Tree Nut Extracts as Potential Inhibitors of EB
6. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nuts Dried Fruits Statistical Yearbook. International Nut and Dried Fruit Council. Available online: https://inc.nutfruit.org/technical-projects/ (accessed on 1 May 2025).
- Pakrah, S.; Rahemi, M.; Nabipour, A.; Zahedzadeh, F.; Kakavand, F.; Vahdati, K. Sensory and nutritional attributes of Persian walnut kernel influenced by maturity stage, drying method, and cultivar. J. Food Process Preserv. 2021, 45, e15513. [Google Scholar] [CrossRef]
- Gavilán-CuiCui, G.; Padilla-Contreras, D.; Manterola-Barroso, C.; Morina, F.; Meriño-Gergichevich, C. Antioxidant Performance in Hazelnut (Corylus avellana L.) Cultivars Shell is Substantially Influenced by Season and Locality. Agronomy 2024, 14, 1412. [Google Scholar] [CrossRef]
- Ye, N.; Zhang, P.; Wang, Y.; Ma, H.; Zhang, T. Effects of controlled atmosphere on browning, redox metabolism and kernel quality of fresh in-hull walnut (Juglans regia L.). HEB 2021, 62, 397–409. [Google Scholar] [CrossRef]
- Ortiz, C.M.; Vicente, A.R.; Fields, R.P.; Grilo, F.; Labavitch, J.M.; Donis-Gonzalez, I.; Crisosto, C.H. Walnut (Juglans regia L.) kernel postharvest deterioration as affected by pellicle integrity, cultivar and oxygen concentration. Postharvest Biol. Technol. 2019, 156, 110948. [Google Scholar] [CrossRef]
- Zhou, D.; Li, L.; Wu, Y.; Fan, J.; Ouyang, J. Salicylic acid inhibits enzymatic browning of fresh-cut Chinese chestnut (Castanea mollissima) by competitively inhibiting polyphenol oxidase. Food Chem. 2015, 171, 19–25. [Google Scholar] [CrossRef]
- Teng, Y.; Murtaza, A.; Iqbal, A.; Fu, J.; Ali, S.W.; Iqbal, M.A.; Xu, X.; Pan, S.; Hu, W. Eugenol emulsions affect the browning processes, and microbial and chemical qualities of fresh-cut Chinese water chestnut. Food Biosci. 2020, 38, 100716. [Google Scholar] [CrossRef]
- Yang, X.; Wang, G.; Mo, Z.; Li, Y.; Zhang, Y.; Jia, X.; Xuan, J. Transcriptomics unravel the mechanism of CiPPO1 and CiPPO2 involved in regulating pecan kernel pellicle browning during storage. Sci. Hortic. 2024, 336, 113412. [Google Scholar] [CrossRef]
- Ye, N.; Zhang, D.; Liu, S.; Zhang, Y.; Wang, Y.; Tang, Y.; Yang, X.; Chai, J.; Ma, Y.; Ma, H. Controlled atmosphere storage alleviates the browning of walnut (Juglans regia L.) fruit through enhancing GABA-mediated energy metabolism. Postharvest Biol. Technol. 2024, 210, 112765. [Google Scholar] [CrossRef]
- Gheysarbigi, S.; Mirdehghan, S.H.; Ghasemnezhad, M.; Nazoori, F. The inhibitory effect of nitric oxide on enzymatic browning reactions of in-package fresh pistachios (Pistacia vera L.). Postharvest Biol. Technol. 2020, 159, 110998. [Google Scholar] [CrossRef]
- Zhang, S.; Tian, L.; Zhang, Y.; Zhao, H.; Zhao, J.; Guo, J.; Zhu, G.P. De novo transcriptome assembly of the fresh-cut white husk of Juglans cathayensis Dode: Insights for enzymatic browning mechanism of fresh-cut husk of walnut. Sci. Hortic. 2019, 257, 108654. [Google Scholar] [CrossRef]
- Romero, A.; Tous, J.; Durfort, M.; Rius, M. Histology of hazelnut (Corylus avellana L.) kernel affected by brown spots in kernel cavity phisiopathy. SJAR 2003, 1, 47–54. [Google Scholar]
- Wang, Y.; Guo, Z.; Zhou, R.; Tang, Y.; Ye, N.; Zhang, D.; Rasel, M.; Huang, N.; Qiu, L.; Wang, N.; et al. JrPPO1/2 play distinct roles in regulating walnut fruit browning by different spatiotemporal expression and enzymatic characteristics. Plant Physiol. Biochem. 2024, 215, 109018. [Google Scholar] [CrossRef]
- Le Lagadec, M.D. Kernel brown centres in macadamia: A review. Crop Pasture Sci. 2009, 60, 1117–1123. [Google Scholar] [CrossRef]
- Yuan, X.; Huang, S.; Ma, H.; Huang, N.; Ye, N. Differential responses of walnut cultivars to cold storage and their correlation with postharvest physiological parameters. HEB 2019, 60, 345–356. [Google Scholar] [CrossRef]
- Cacka, J.; Sanguankeo, P. Calcium influence on hazelnut quality and yields in Oregon. In Proceedings of the VIII International Congress on Hazelnut, Temuco City, Chile, 19–22 March 2012; Volume 1052, pp. 187–193. [Google Scholar]
- Romero, A.; Tous, J.; Ferran, X.; Rius, M.; Sentís, X.; Plana, J. The brown spots in kernel cavity disorder of hazelnut. In Proceedings of the Fifth International Congress on Hazelnut, Corvallis, OR, USA, 27–31 August 2000; Volume 556, pp. 397–402. [Google Scholar]
- Li, Q.; Mo, R.; Shen, D.; Sun, S.; Tang, F.; Guo, Y.; Liu, Y. External browning mechanism in walnut kernel pellicles under different drying conditions based on the combination of widely-targeted and anthocyanin-targeted metabolomics. Food Chem. 2024, 460, 140440. [Google Scholar] [CrossRef] [PubMed]
- Najjari, M.; Bodaghi, H. Inhibitory Effect of Ascorbic Acid in Enzymatic Browning and Preservation of Quality in Fresh Pistachios (Pistacia vera L.). Appl. Fruit Sci. 2024, 66, 609–620. [Google Scholar] [CrossRef]
- Hashemi, M.; Shakerardekani, A.; Mirzaalian Dastjerdi, A.; Mirdehghan, S. Effect of sodium alginate in combination with Zataria multiflora Boiss. On phenolic compounds, antioxidant activity, and Browning enzymes of fresh in-Hull pistachio (Pistacia vera L.). J. Food Qual. 2021, 2021, 3193573. [Google Scholar] [CrossRef]
- Li, G.P.; Zhou, D.; Kan, L.N.; Wu, Y.W.; Fan, J.F.; Ouyang, J. Competitive inhibition of phytic acid on enzymatic browning of chestnut (Castanea mollissima Blume). Acta Aliment. 2017, 46, 100–108. [Google Scholar] [CrossRef]
- Zhu, L.; Hu, W.; Murtaza, A.; Iqbal, A.; Li, J.; Zhang, J.; Li, J.; Kong, M.; Xu, X.; Pan, S. Eugenol treatment delays the flesh browning of fresh-cut water chestnut (Eleocharis tuberosa) through regulating the metabolisms of phenolics and reactive oxygen species. Food Chem. X 2022, 14, 100307. [Google Scholar] [CrossRef]
- Queiroz, C.; Lopes, M.L.M.; Da Silva, A.J.R.; Fialho, E.; Valente-Mesquita, V.L. Effect of high hydrostatic pressure and storage in fresh-cut cashew apple: Changes in phenolic profile and polyphenol oxidase activity. JFPP 2021, 45, e15857. [Google Scholar] [CrossRef]
- Shojaee, A.; Rastegar, S.; Tajeddin, B.; Sayyad-Amin, P. Quality Preservation of Walnut Kernels: Effect of Storage Temperature and Vacuum Packaging. Erwerbs-Obstbau 2023, 65, 2407–2418. [Google Scholar] [CrossRef]
- Habibi, A.; Yazdani, N.; Koushesh Saba, M.; Chatrabnous, N.; Molassiotis, A.; Sarikhani, S.; Vahdati, K. Natural preservation and improving lipid oxidation inhibition of fresh walnut. HEB 2023, 64, 133–142. [Google Scholar] [CrossRef]
- Sarami, F.; Shiran, B.; Rabiei, G.; Jafari, M. Morpho-physiological and molecular investigations of the effect of pollen grains on the browning of almond kernels. Heliyon 2025, 11, e42975. [Google Scholar] [CrossRef]
- Guo, Y.; Pan, Y.; Zhang, Z.; Zhang, W. Study on the browning mechanism of betel nut (Betel catechu L.) kernel. Food Sci. Nutr. 2020, 8, 1818–1827. [Google Scholar] [CrossRef]
- Walton, D.A.; Randall, B.W.; Le Lagadec, M.D.; Wallace, H.M. Maintaining high moisture content of macadamia nuts-in-shell during storage induces brown centres in raw kernels. J. Sci. Food Agric. 2013, 93, 2953–2958. [Google Scholar] [CrossRef]
- Srichamnong, W.; Srzednicki, G. Internal discoloration of various varieties of Macadamia nuts as influenced by enzymatic browning and Maillard reaction. Sci. Hortic. 2015, 192, 180–186. [Google Scholar] [CrossRef]
- Shi, J.; Li, J.; Zhu, S.; Zhou, J. Browning inhibition on fresh-cut chestnut kernel by exogenous nitric oxide. Int. J. Food Sci. Technol. 2011, 46, 944–950. [Google Scholar] [CrossRef]
- Gavilán-CuiCui, G.; González, F.; Ruiz, A.; Meriño-Gergichevich, C. Doctoral Program in Natural Resources Sciences, Universidad de La Frontera, Temuco, Chile. Laboratory of Physiology and Plant Nutrition for Fruit Trees, Faculty of Agricultural Sciences and Environment, Universidad de La Frontera, Temuco, Chile. Laboratory of Soil Fertility, Faculty of Agricultural Sciences and Environment, Universidad de La Frontera, Temuco, Chile, 2025. manuscript in preparation; to be submitted.
- Luo, S.Z.; Sun, Y.; Yuan, X.; Pan, L.H.; Zheng, Z.; Zhao, Y.Y.; Zhong, X.Y. Infrared radiation blanching–inhibited browning and extended shelf life of pecan kernels. J. Food Sci. 2023, 88, 1566–1579. [Google Scholar] [CrossRef] [PubMed]
- Christopoulos, M.V.; Tsantili, E. Effects of temperature and packaging atmosphere on total antioxidants and colour of walnut (Juglans regia L.) kernels during storage. Sci. Hortic. 2011, 131, 49–57. [Google Scholar] [CrossRef]
- Wang, J.; Li, P.; Gong, B.; Li, S.; Ma, H. Phenol metabolism and preservation of fresh in-hull walnut stored in modified atmosphere packaging. J. Sci. Food Agric. 2017, 97, 5335–5342. [Google Scholar] [CrossRef]
- Habibie, A.; Yazdani, N.; Saba, M.K.; Vahdati, K. Ascorbic acid incorporated with walnut green husk extract for preserving the postharvest quality of cold storage fresh walnut kernels. Sci. Hortic. 2019, 245, 193–199. [Google Scholar] [CrossRef]
- Christopoulos, M.V.; Tsantili, E. Storage of fresh walnuts (Juglans regia L.)–low temperature and phenolic compounds. Postharvest Biol. Technol. 2012, 73, 80–88. [Google Scholar] [CrossRef]
- Queiroz, C.; Da Silva, A.J.R.; Lopes, M.L.M.; Fialho, E.; Valente-Mesquita, V.L. Polyphenol oxidase activity, phenolic acid composition and browning in cashew apple (Anacardium occidentale, L.) after processing. Food Chem. 2011, 125, 128–132. [Google Scholar] [CrossRef]
- Schieber, A. Reactions of quinones—Mechanisms, structures, and prospects for food research. J. Agric. Food Chem. 2018, 66, 13051–13055. [Google Scholar] [CrossRef]
- Panis, F.; Rompel, A. Identification of the amino acid position controlling the different enzymatic activities in walnut tyrosinase isoenzymes (jrPPO1 and jrPPO2). Sci. Rep. 2020, 10, 10813. [Google Scholar] [CrossRef]
- Zhao, S.; Wang, H.; Liu, K.; Li, L.; Yang, J.; An, X.; Li, P.; Yun, L.; Zhang, Z. The role of JrPPOs in the browning of walnut explants. BMC Plant Biol. 2021, 21, 9. [Google Scholar] [CrossRef] [PubMed]
- Persic, M.; Mikulic-Petkovsek, M.; Halbwirth, H.; Solar, A.; Veberic, R.; Slatnar, A. Red walnut: Characterization of the phenolic profiles, activities and gene expression of selected enzymes related to the phenylpropanoid pathway in pellicle during walnut development. J. Agric. Food Chem. 2018, 66, 2742–2748. [Google Scholar] [CrossRef]
- Meriño-Gergichevich, C.; Luengo-Escobar, A.; Alarcón, D.; Reyes-Díaz, M.; Ondrasek, G.; Morina, F.; Ogass, K. Combined spraying of boron and zinc during fruit set and premature stage improves yield and fruit quality of European hazelnut cv. Tonda di Giffoni. Front. Plant Sci. 2021, 12, 661542. [Google Scholar] [CrossRef]
- Cacka, J.F.; Smith, F. Foliar nutrition applied at early hazelnut development shows positive yield and quality factors in the Willamette valley of Oregon. In Proceedings of the VII International Congress on Hazelnut, Viterbo, Italy, 23–27 June 2008; Volume 845, pp. 343–348. [Google Scholar]
- Rogiers, S.Y.; Quinlan, K.P.; Bright, J.D. Challenges to optimal macadamia (Macadamia spp.) kernel quality in a changing climate. Funct. Plant Biol. 2025, 52, FP24218. [Google Scholar] [CrossRef]
- Peng, L.; Jiang, Y. Exogenous salicylic acid inhibits browning of fresh-cut Chinese water chestnut. Food Chem. 2006, 94, 535–540. [Google Scholar] [CrossRef]
- Massantini, R.; Cristofori, V.; Frangipane, M.T. Storage of the early ripe almonds under modified atmosphere to preserve kernel qualitative and sensory traits. Agriculture 2022, 12, 974. [Google Scholar] [CrossRef]
- Sheikhi, A.; Mirdehghan, S.H.; Karimi, H.R.; Ferguson, L. Effects of passive-and active-modified atmosphere packaging on physio-chemical and quality attributes of fresh in-hull pistachios (Pistacia vera L. cv. Badami). Foods 2019, 8, 564. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Dag, D.; Jung, J.; Zhao, Y. Investigation of antioxidant edible coating and modified atmosphere packaging for enhancing storability of hazelnut kernels. J. Agric. Food Res. 2024, 17, 101246. [Google Scholar] [CrossRef]
- Shojaei, A.; Rastegar, S.; Sayyad-Amin, P. Shelf life extension of walnut kernel: Effect of temperature and vacuum packaging storage. J. Food Meas. Charact. 2023, 17, 3938–3949. [Google Scholar] [CrossRef]
- Chatrabnous, N.; Yazdani, N.; Tavallali, V.; Vahdati, K. Preserving quality of fresh walnuts using plant extracts. LWT 2018, 91, 1–7. [Google Scholar] [CrossRef]

| Tree Nuts | Enzymes | Phenolic Substrates | References |
|---|---|---|---|
| Betel nut (B. catechu L.) | PPO | Chlorogenic acid, dopamine and L-epicathechin | [27] |
| Cashew (A. occidentale L.) | PPO | caffeic acid, catechin, gallic acid and o-dihydroxyphenol catechol | [23,37] |
| Chestnut (C. mollissima L.) | PAL PPO POD | Catechol, chlorogenic acid, 2-hydroxy phenol and p-hydroxybenzoic acid | [7,30] |
| Hazelnut (C. avellana L.) | PPO POD | Coumaroylquinic and p-coumaric acid | [31] |
| Macadamia (M. integrifolia L.) | PPO | Chlorogenic acid, p-hydroxybenzoic acid | [29] |
| Pecan (C. illinoinensis L.) | PPO | Catechin, 2,3,4-trihydroxybenzoic acid, phloroglucinol, procyanidin, and protocatechuic acid | [8] |
| Pistachio (P. vera L.) | PAL PPO | - | [20] |
| Walnut (J. regia L.) | PPO | Caffeic acid, catechol, chlorogenic acid, coumaric acid, 4 hydroxyphenylacetic acid, epicatechin, ethyl gallate, gallic acid, guaiacol, L-DOPA, protocatechuic acid, and quercetin | [13,39,40] |
| Walnut (J. cathayensis Dode) | CHS 4CL PAL PPO | - | [11] |
| Mechanism | Treatment | Tree Nuts | Effect | References |
|---|---|---|---|---|
| Reduction | Ascorbic acid (AA) | Pistachio (P. vera L.) | It reduced browning, weight loss, electrolytes, and peroxide. In addition, it preserved TPC and inhibited PPO activity, reducing EB. | [19] |
| Reduction | Ascorbic acid (AA) | Walnut (J. regia L.) | Inhibited EB, peroxide oxidation, and PPO. In addition to increasing TPC, AC preservation, and sensory properties. | [35] |
| Competitive | Salicylic acid (SA) | Chinese chestnut (Eleocharis dulcis) | Delayed discoloration, maintained quality, and reduced PPO, POD, and PAL activities. | [45] |
| Competitive | Salicylic acid (SA) | Chinese chestnut (C. mollissima) | It delayed EB by inhibiting PPO activity, while POD was unchanged. | [6] |
| Competitive | Sodium alginate + thyme oil | Pistachio (P. vera L.) | Quality was maintained, since PPO activity decreased, and TPC and PAL increased. | [20] |
| Competitive | Phytic acid (PA) | Chestnut (C. mollissima blume) | Due to the inhibition of external and internal EB, PPO, and POD activities also decreased. | [21] |
| Complexation | Eugenol (EUG) | Water chestnut (Eleocharis tuberosa) | Maintained quality and inhibited EB by reducing PPO, POD, and PAL activities, as well as changes in PC and quinone formation. | [7] |
| Complexation | Eugenol (EUG) | Water chestnut (E. tuberosa) | Inactivated EB-related enzymes and reduced the phenolic content of the external tissue. It increased ROS enzymatic activity, decreasing O2. In addition, PC accumulation occurred, and internal tissue AC increased. | [22] |
| Complexation | Nitric oxide (NO) | Chestnut (C. henryi) | It delayed EB and inhibited PPO and POD activities while TPC increased. | [30] |
| Complexation | Sodium nitroprusside (SNP) | Pistachio (P. vera L.) | It showed a lower browning index and reduced PPO, PAL, and POD activity. In addition to maintaining the levels of TPC, flavonoids, and AC, prolonging post-harvest life. | [10] |
| Treatment | Technique | Tree Nuts | Effect | References |
|---|---|---|---|---|
| Walnut green husk extract (WGHE) | Natural agent | Walnut (J. regia L.) | Inhibited lipid peroxidation, increased acidity index, improved quality, and prolonged post-harvest life. | [50] |
| Walnut green husk extract (WGHE) | Natural agent | Walnut (J. regia L.) | Increased AC and TP levels prevented the increase of peroxide value and PPO activity, inhibited EB, and preserved quality attributes. | [25,35] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gavilán-CuiCui, G.; Lagos-Muñoz, R.; Ellena, F.M.; Di Matteo, A.; Morina, F.; Meriño-Gergichevich, C. Unraveling Mechanisms of Enzymatic Browning in Nuts and Their Relationship with Pre- and Post-Harvest Factors: Management Strategies for Mitigation. Molecules 2025, 30, 3866. https://doi.org/10.3390/molecules30193866
Gavilán-CuiCui G, Lagos-Muñoz R, Ellena FM, Di Matteo A, Morina F, Meriño-Gergichevich C. Unraveling Mechanisms of Enzymatic Browning in Nuts and Their Relationship with Pre- and Post-Harvest Factors: Management Strategies for Mitigation. Molecules. 2025; 30(19):3866. https://doi.org/10.3390/molecules30193866
Chicago/Turabian StyleGavilán-CuiCui, Gabriela, Ricardo Lagos-Muñoz, Felix Miguel Ellena, Antonio Di Matteo, Filis Morina, and Cristian Meriño-Gergichevich. 2025. "Unraveling Mechanisms of Enzymatic Browning in Nuts and Their Relationship with Pre- and Post-Harvest Factors: Management Strategies for Mitigation" Molecules 30, no. 19: 3866. https://doi.org/10.3390/molecules30193866
APA StyleGavilán-CuiCui, G., Lagos-Muñoz, R., Ellena, F. M., Di Matteo, A., Morina, F., & Meriño-Gergichevich, C. (2025). Unraveling Mechanisms of Enzymatic Browning in Nuts and Their Relationship with Pre- and Post-Harvest Factors: Management Strategies for Mitigation. Molecules, 30(19), 3866. https://doi.org/10.3390/molecules30193866










