Phthalocyanines Conjugated with Small Biologically Active Compounds for the Advanced Photodynamic Therapy: A Review
Abstract
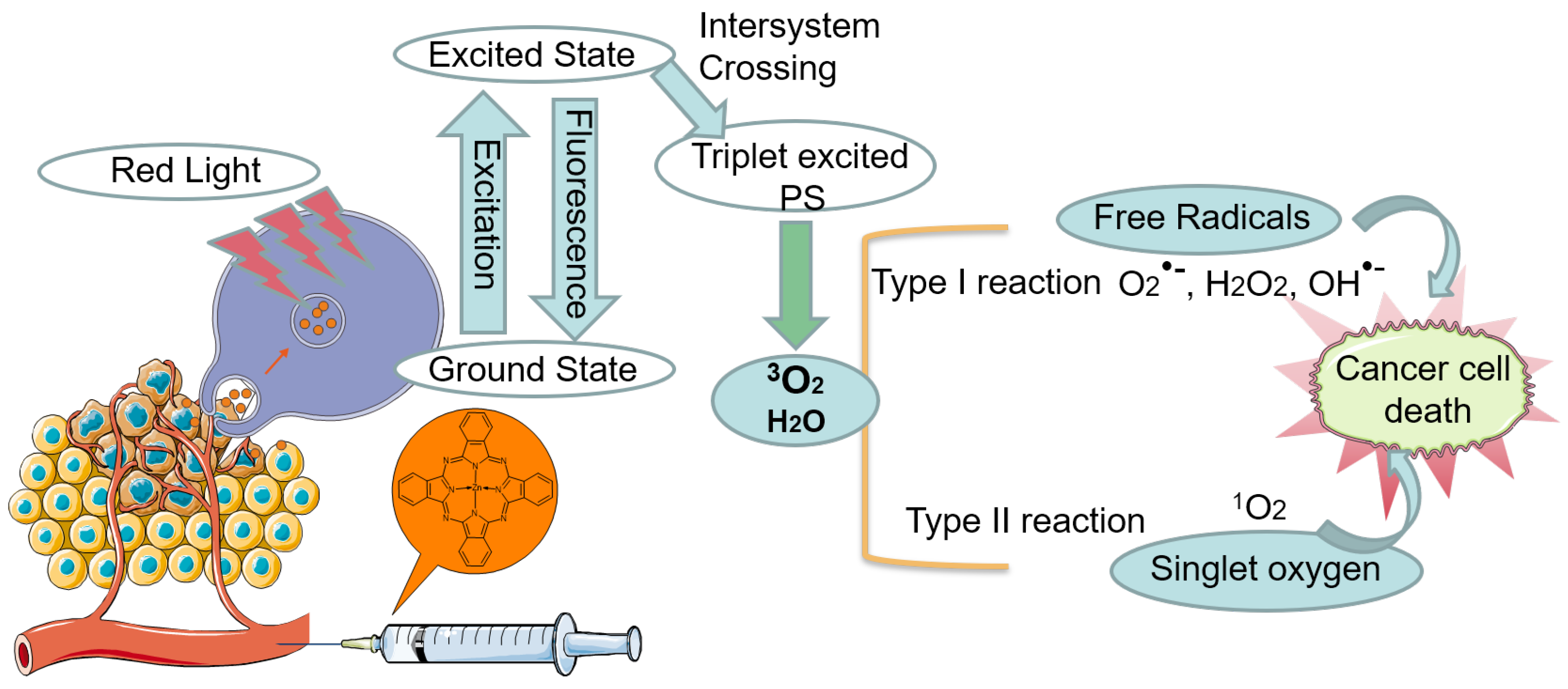
1. Introduction
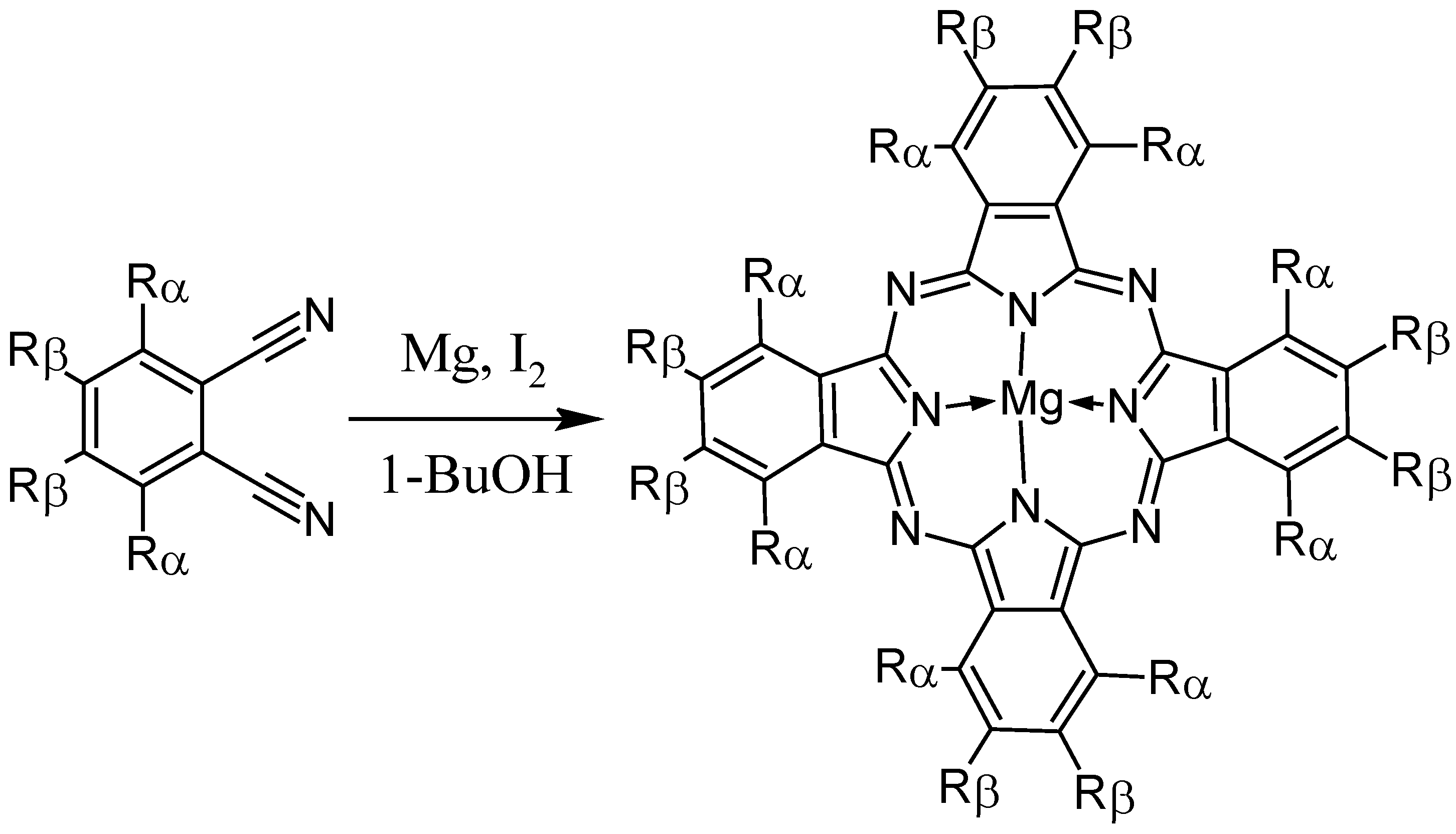
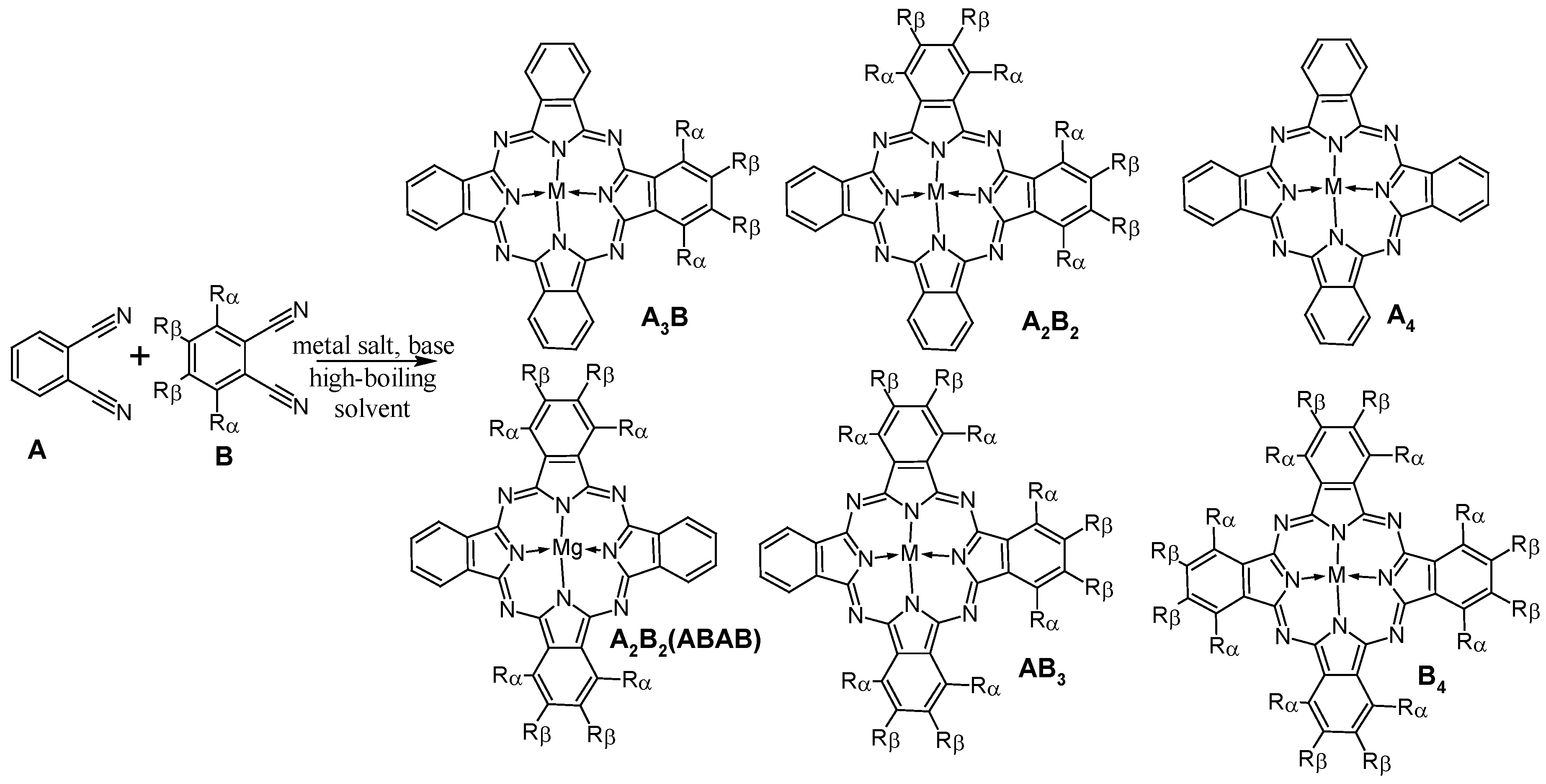
2. Synthesis of Phthalocyanines
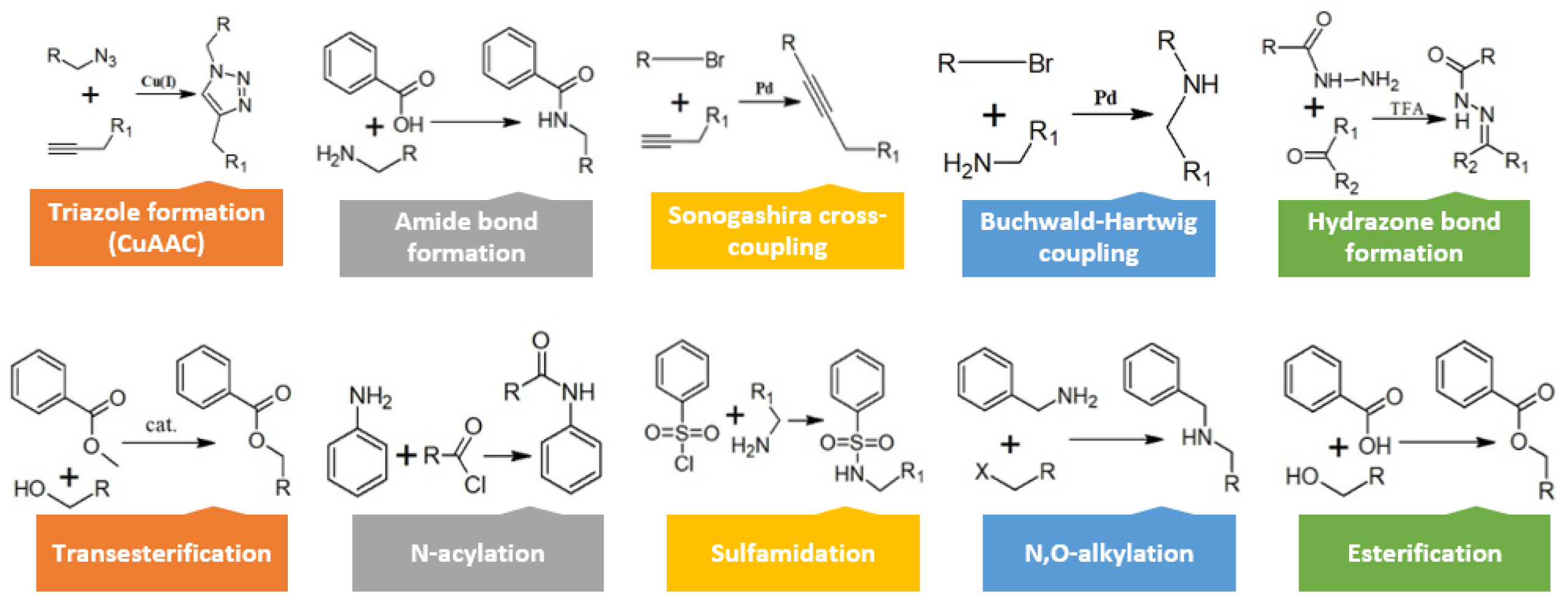
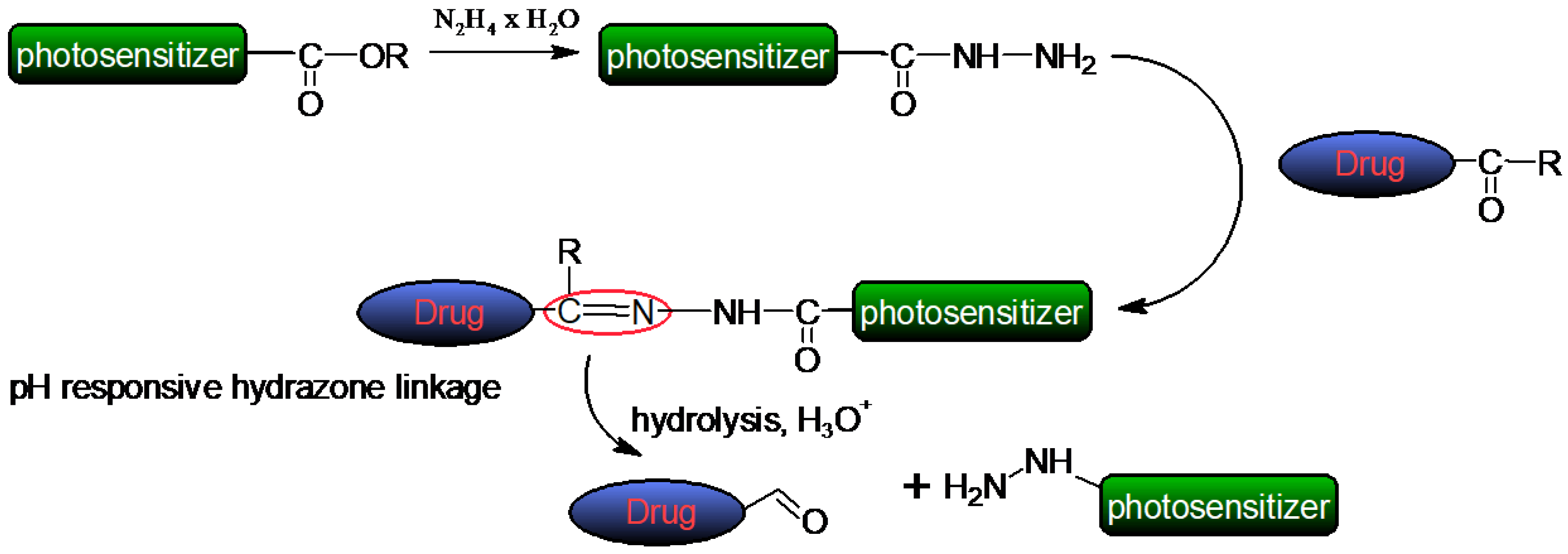
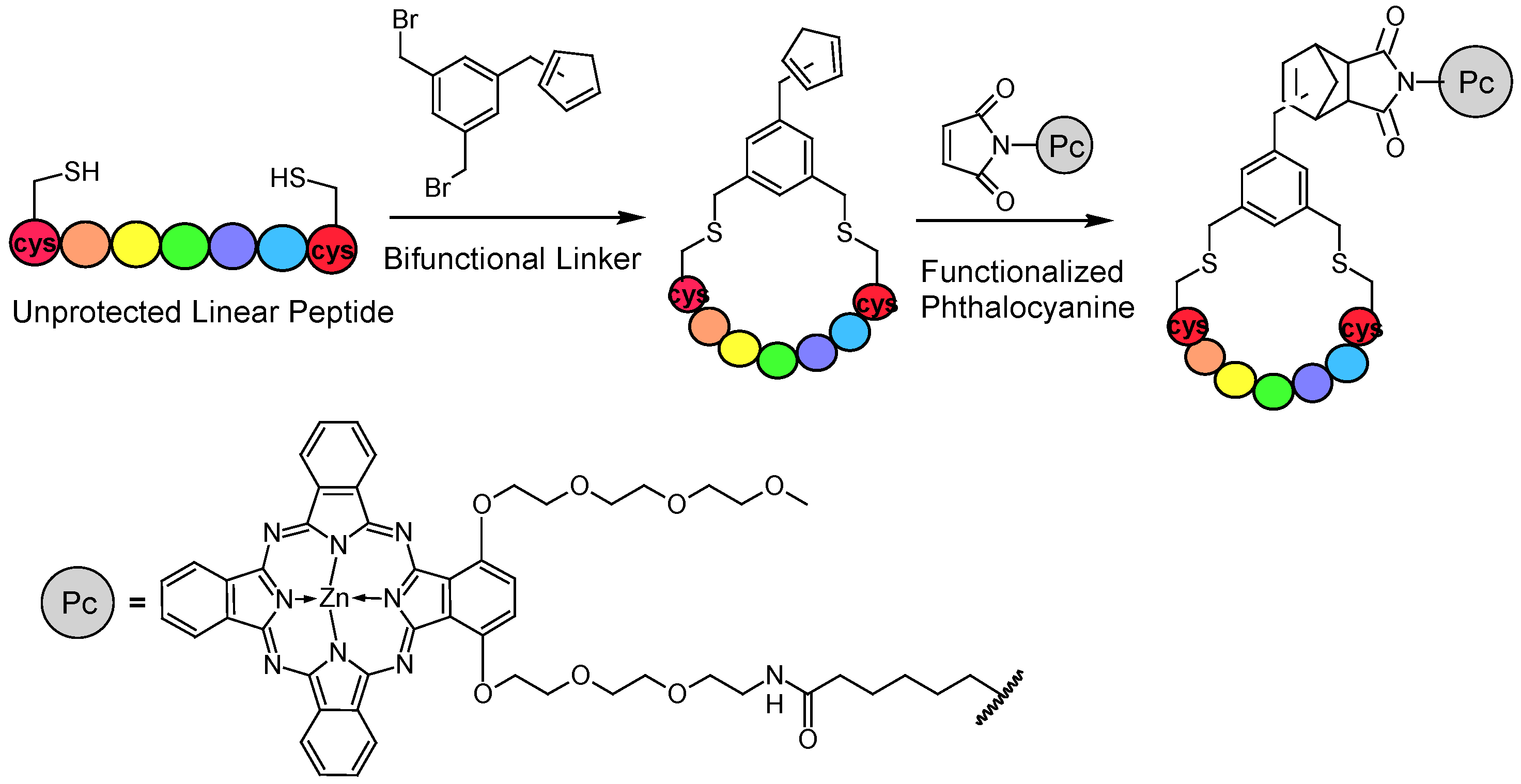
3. Strategies for Conjugate Synthesis
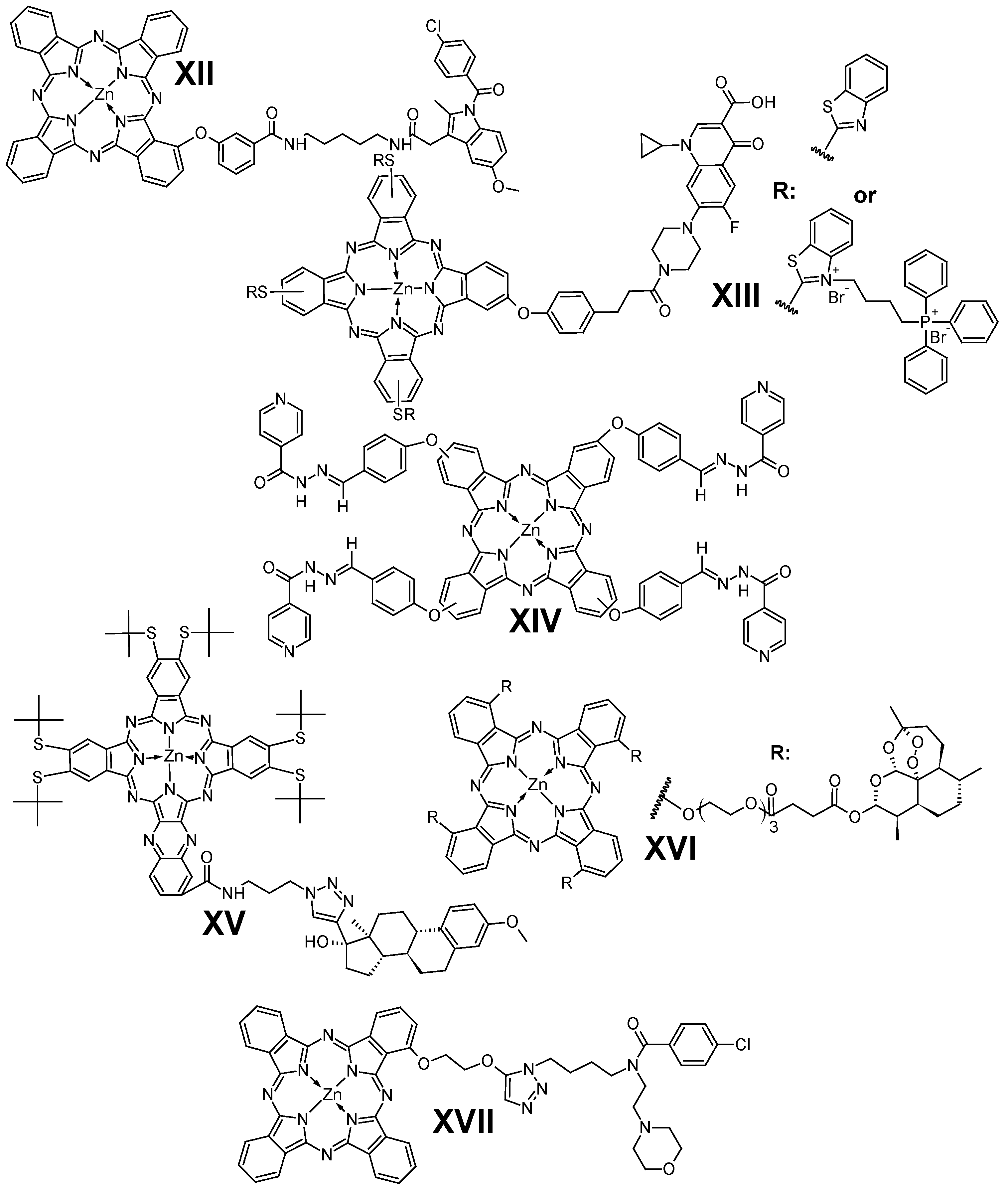
4. Phthalocyanines Conjugated with Therapeutic Agents
4.1. Anticancer Agents
4.2. Other Therapeutic Drugs
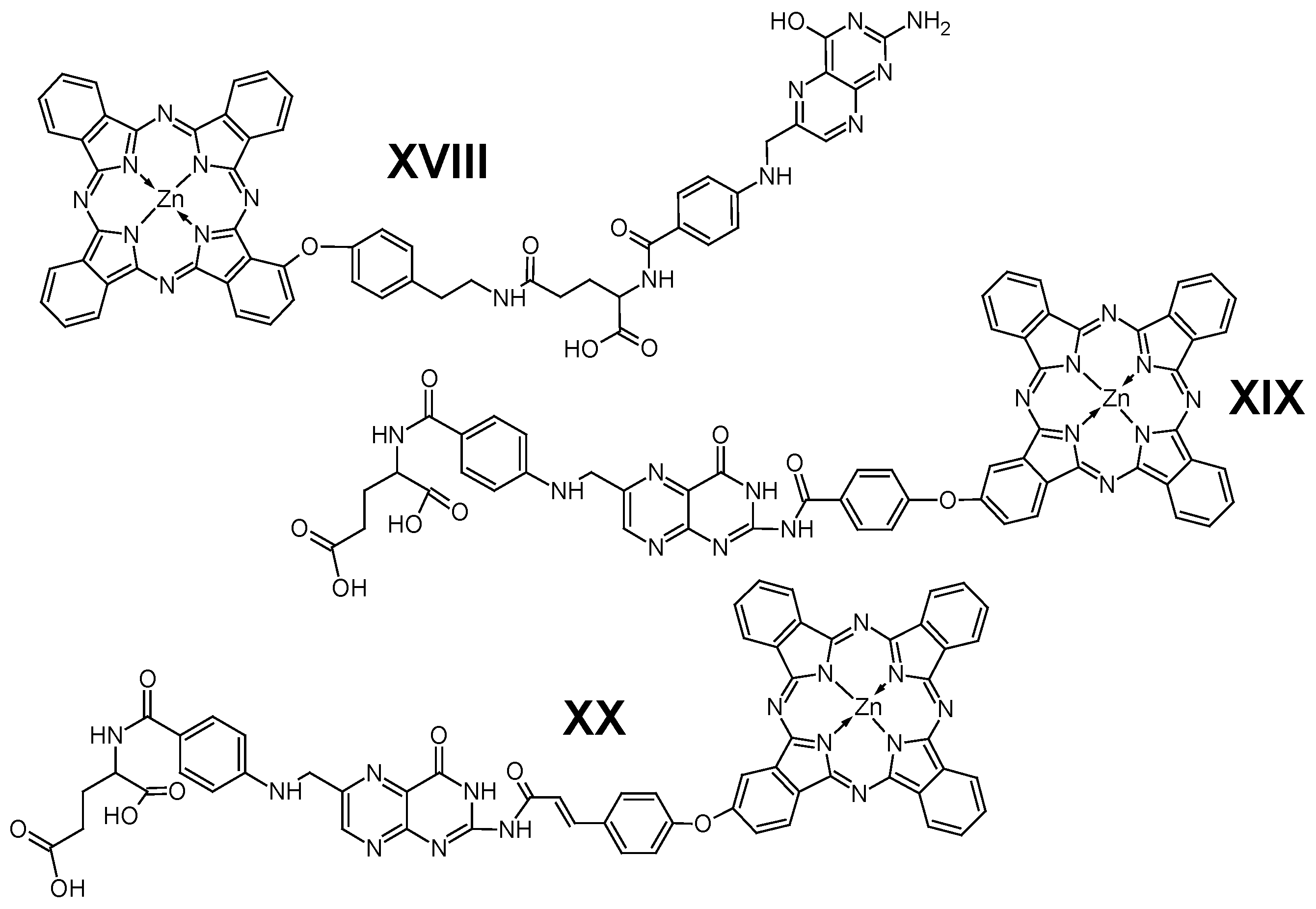
5. Phthalocyanines Conjugated with Targeting Biomolecules
5.1. Folic Acid
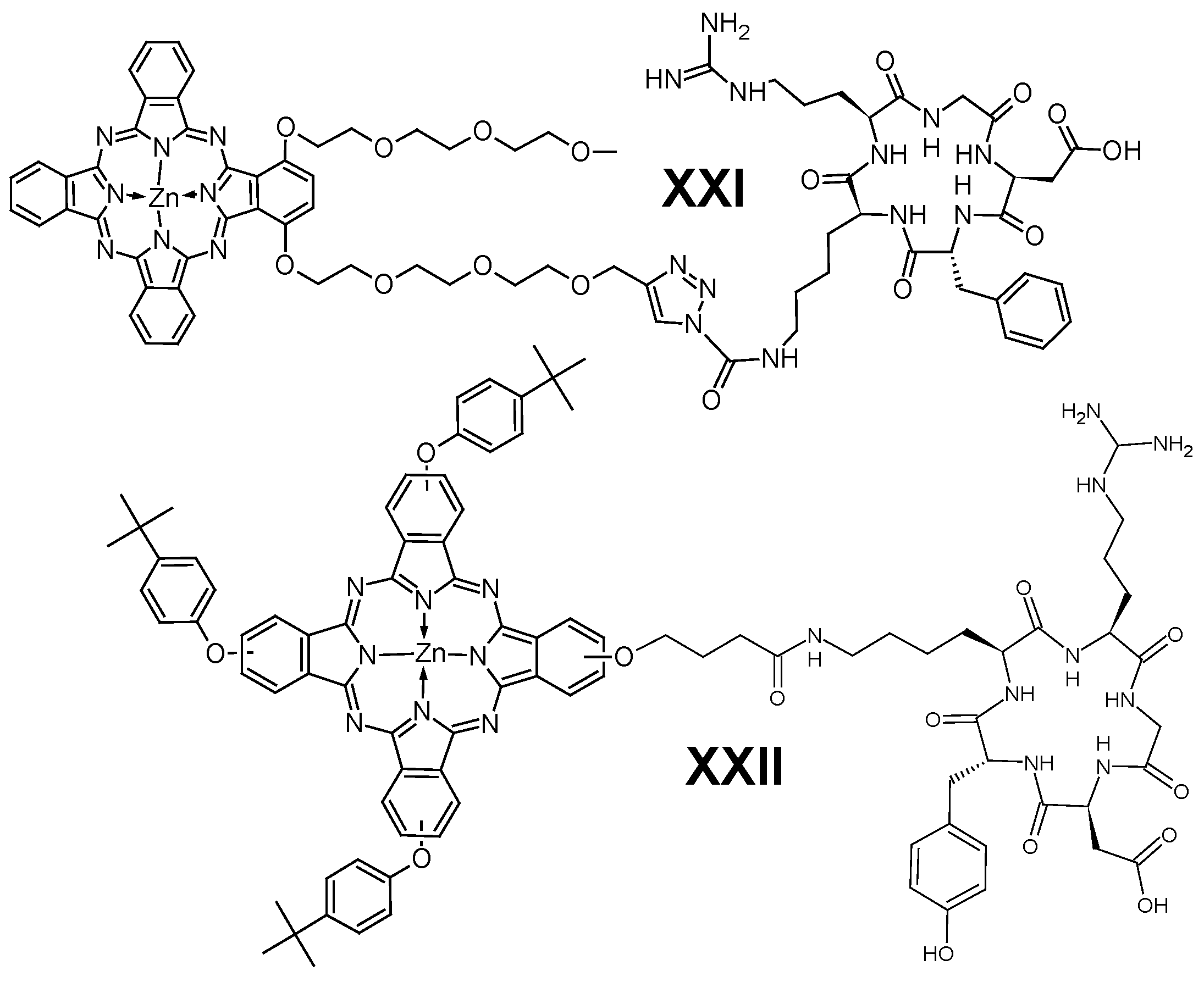
5.2. RGD Peptide
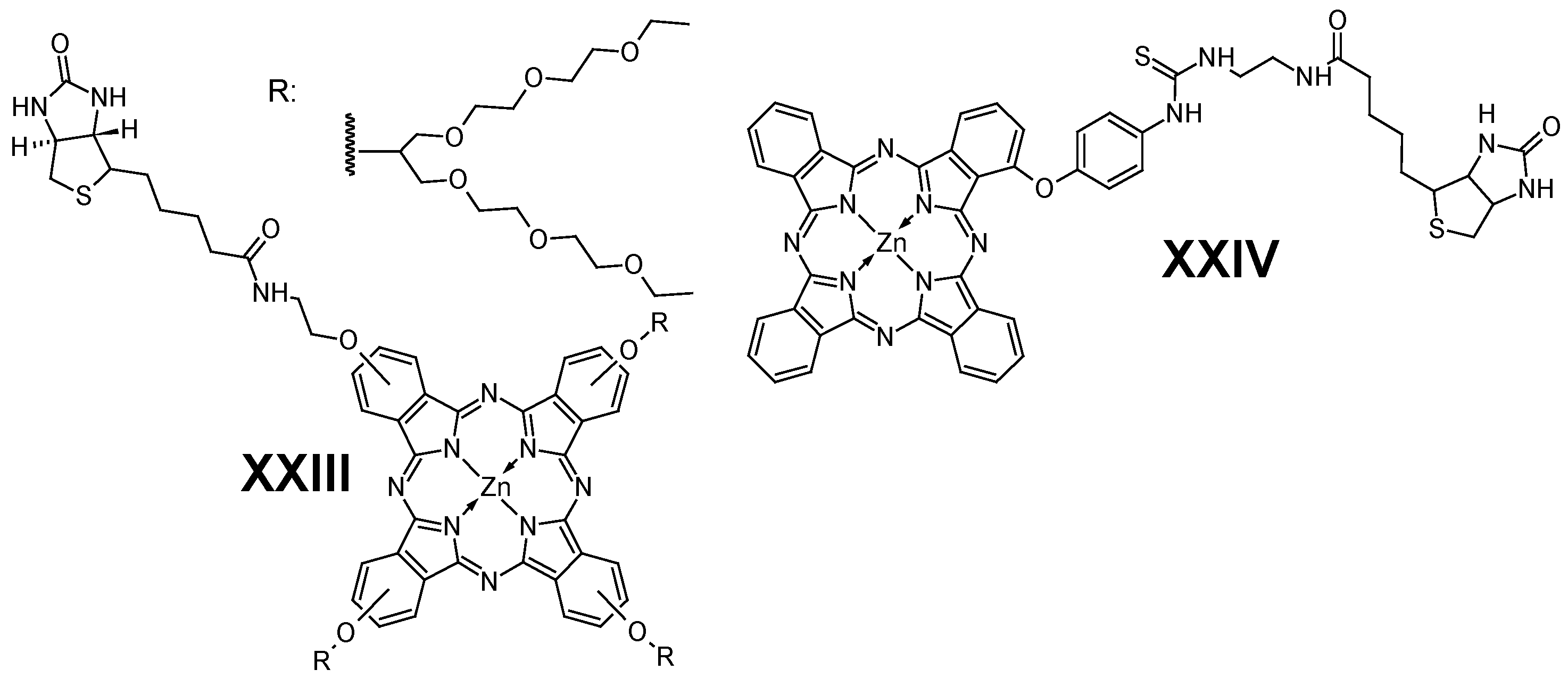
5.3. Biotin
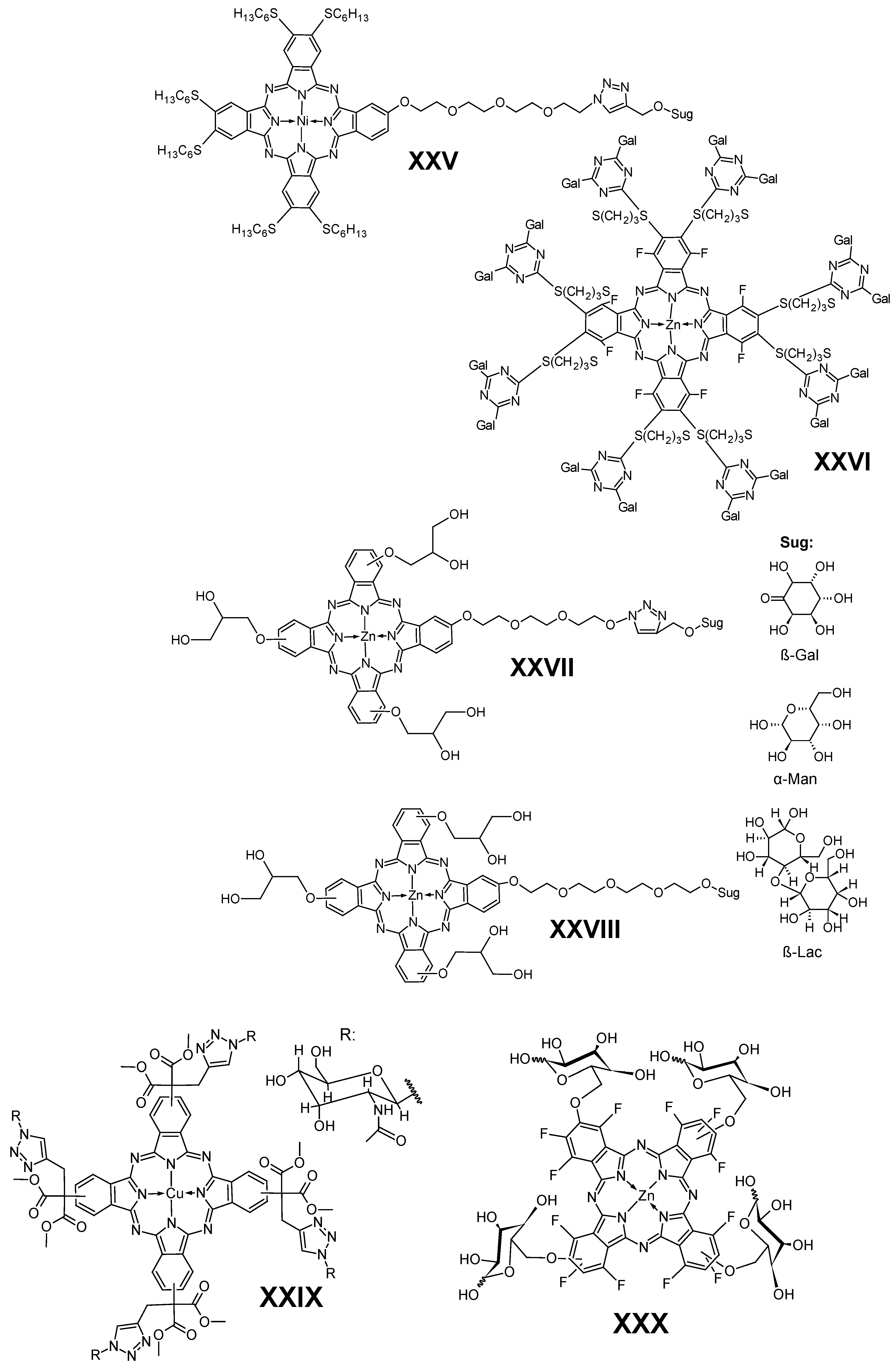
5.4. Carbohydrates
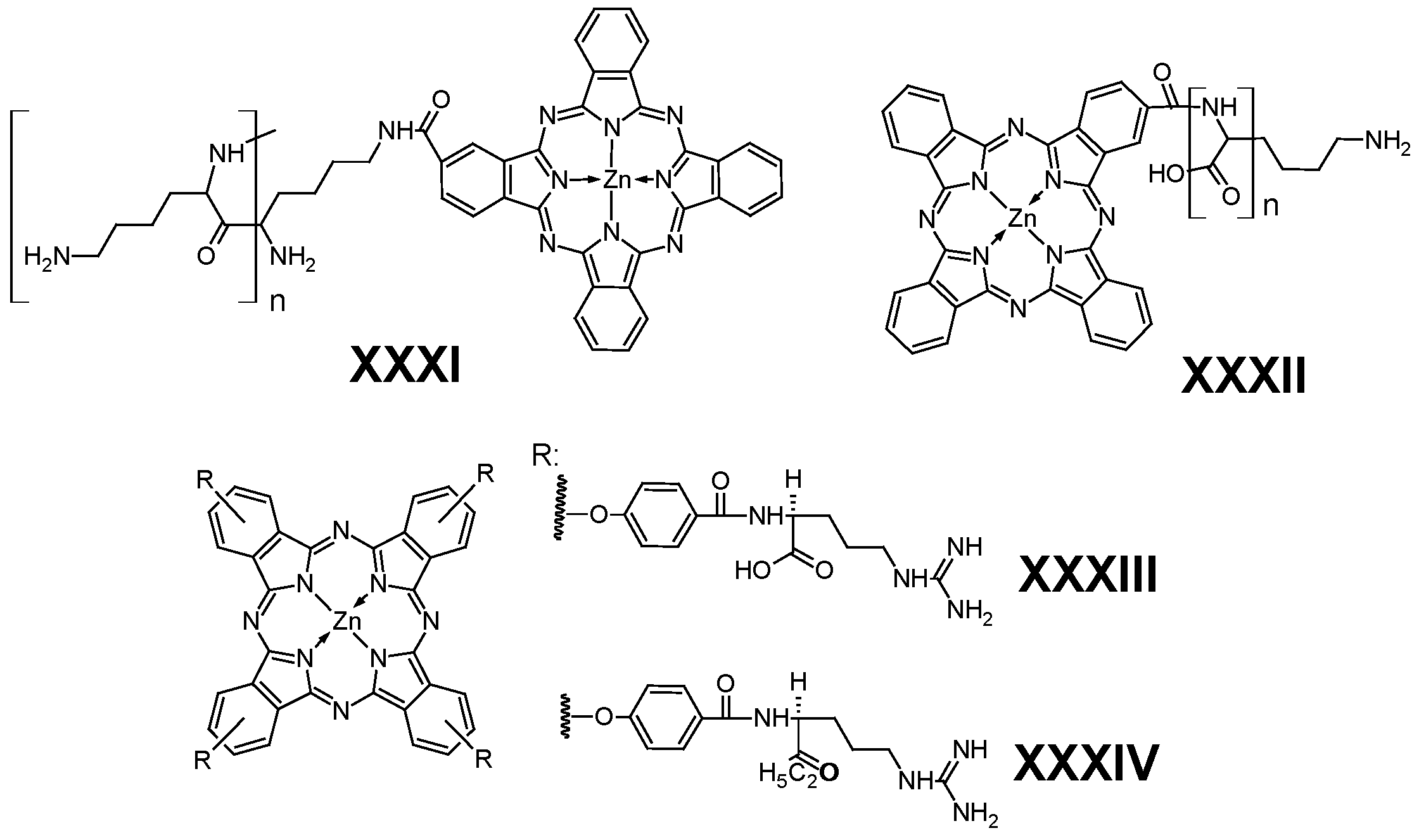
5.5. Amino Acids
6. Phthalocyanines with Other Photosensitizers and Imaging Agents
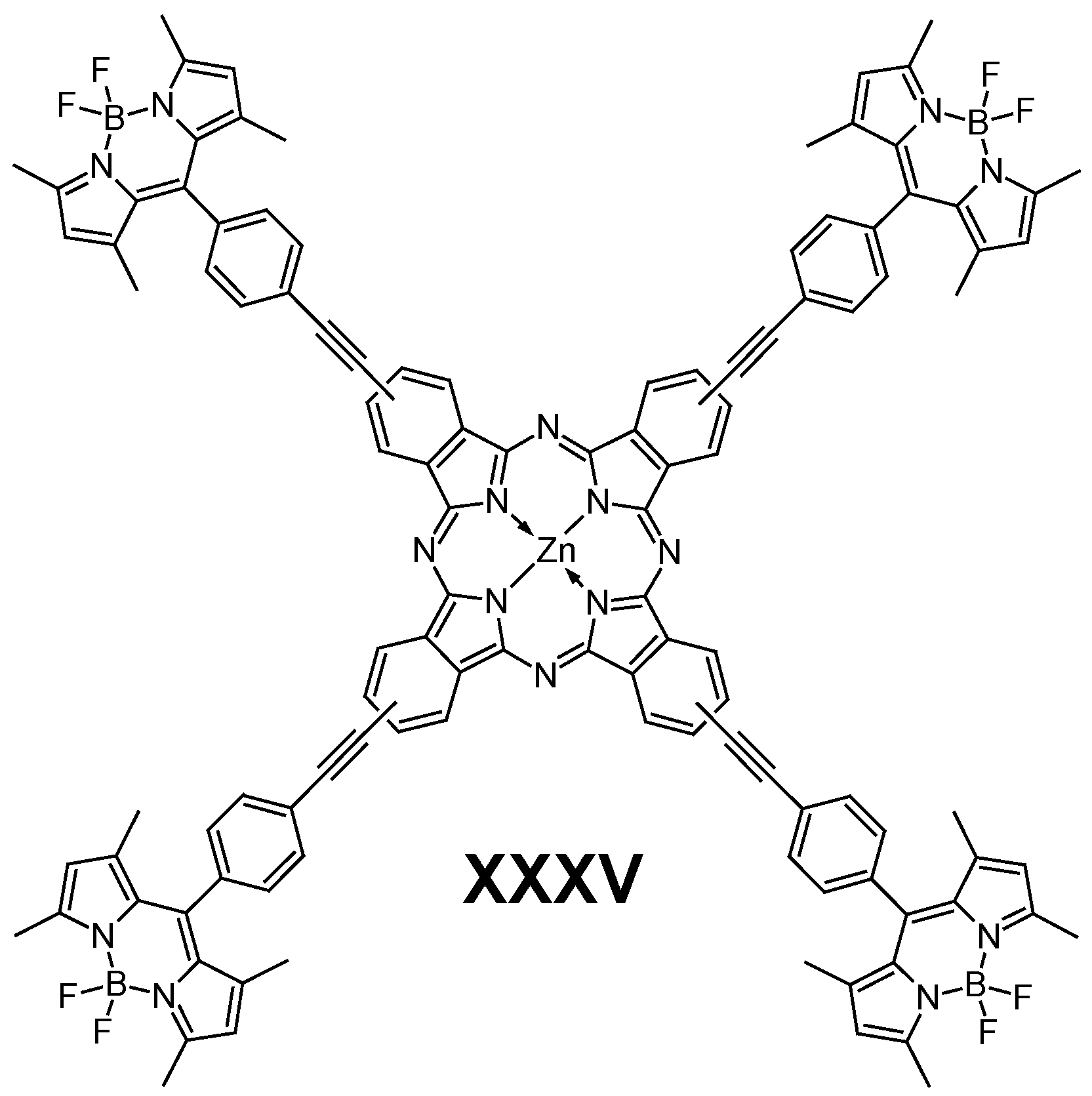
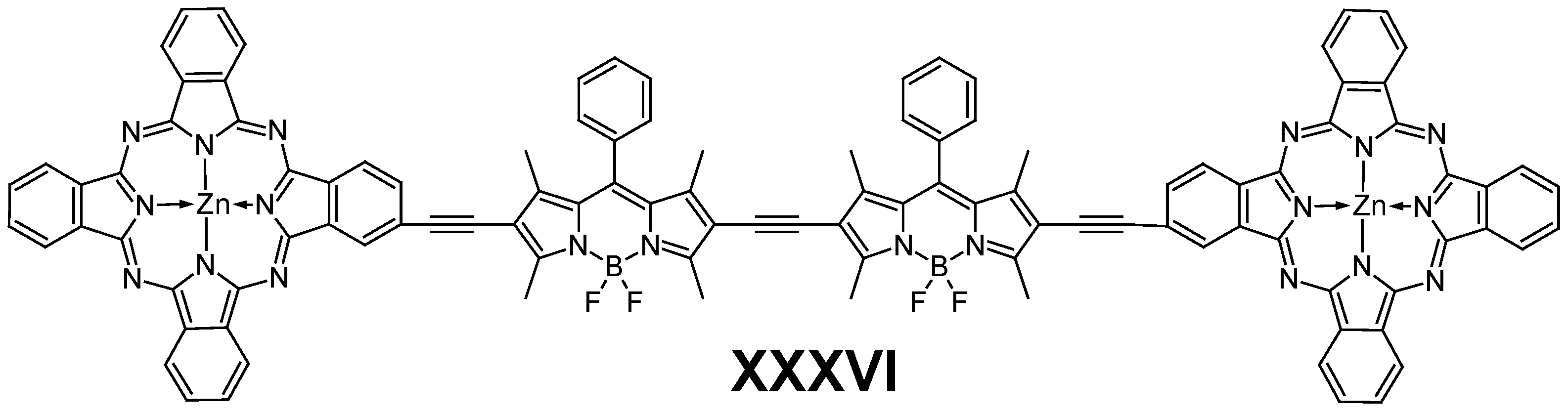
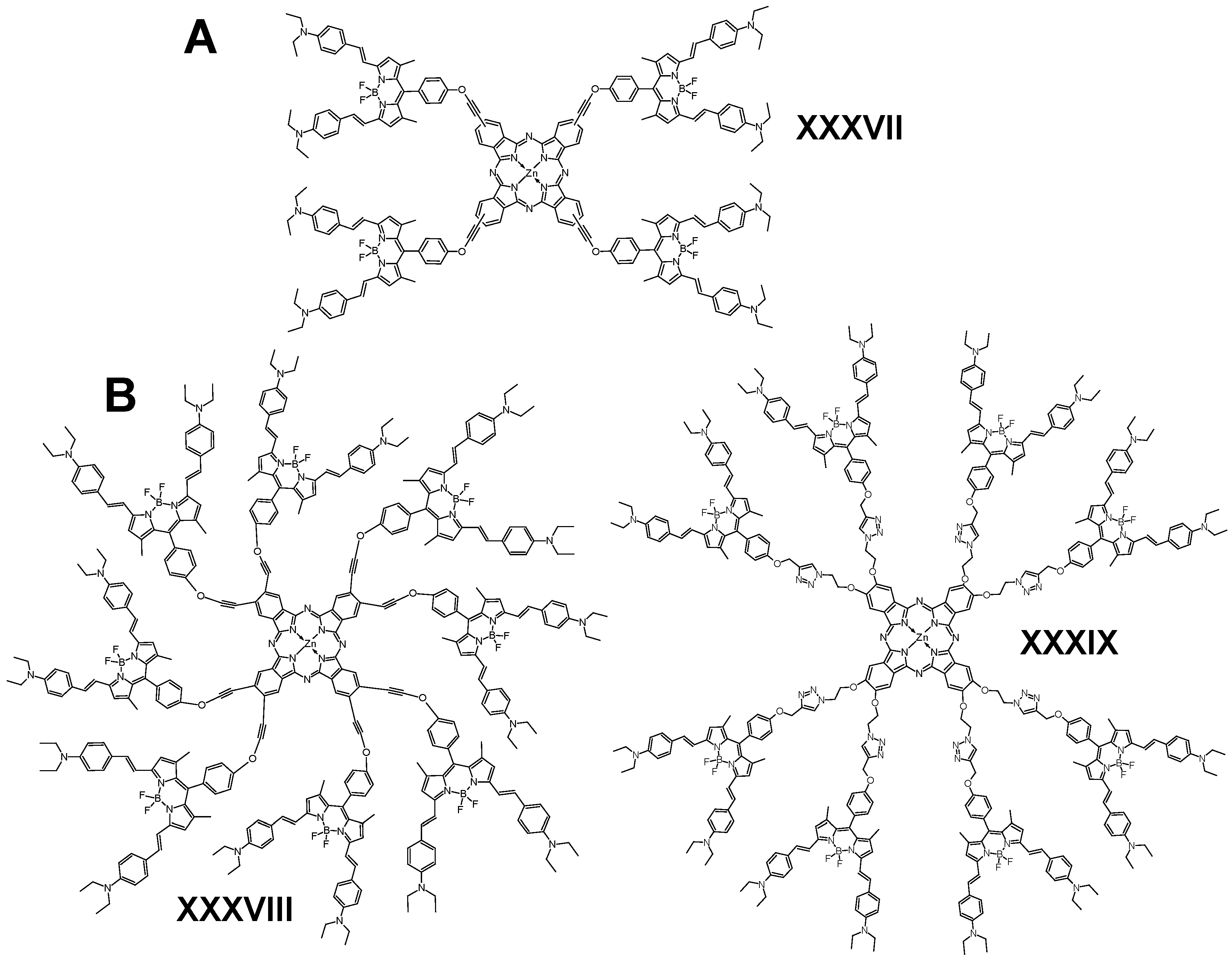
6.1. BODIPY
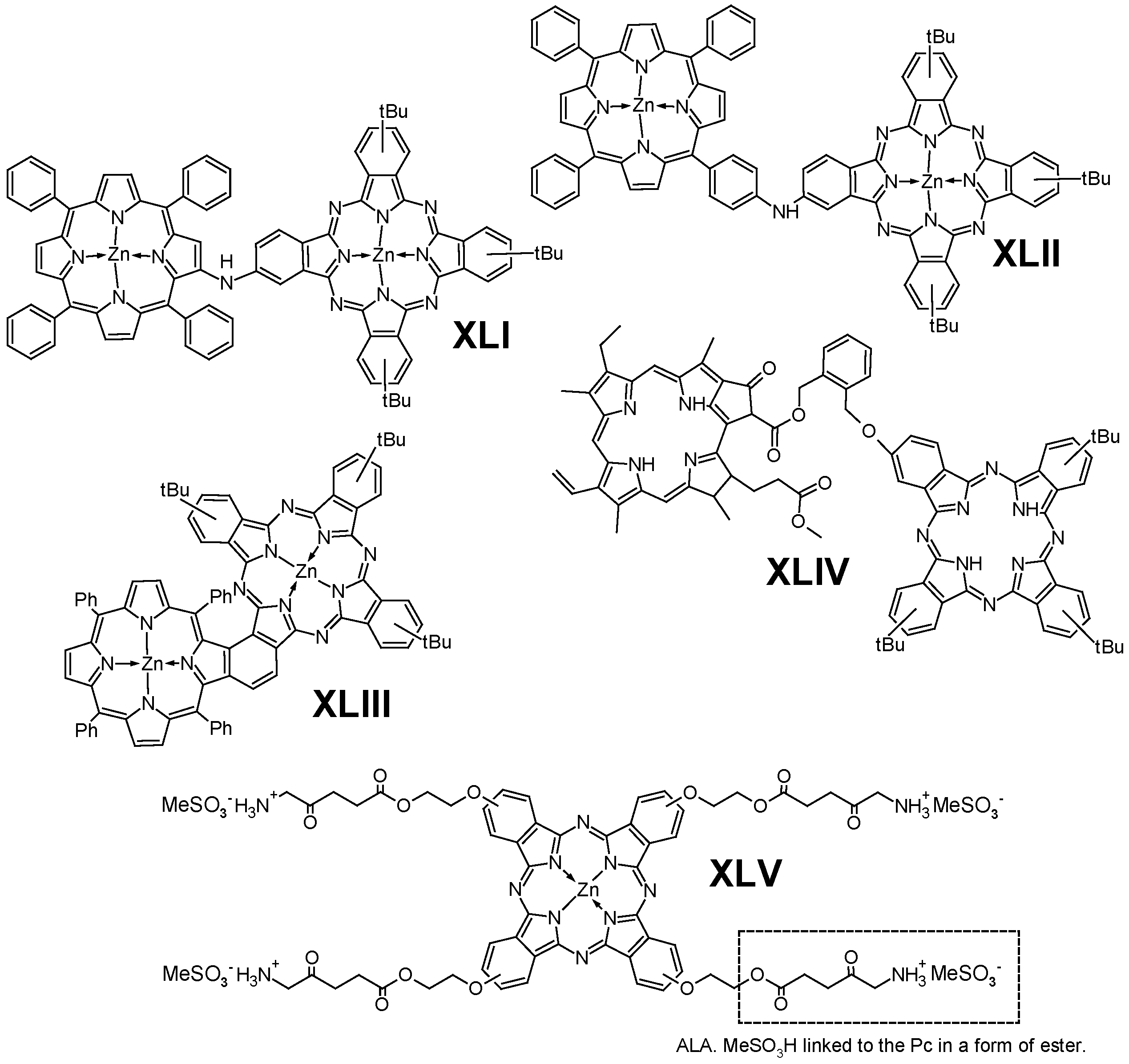
6.2. Porphyrinoids
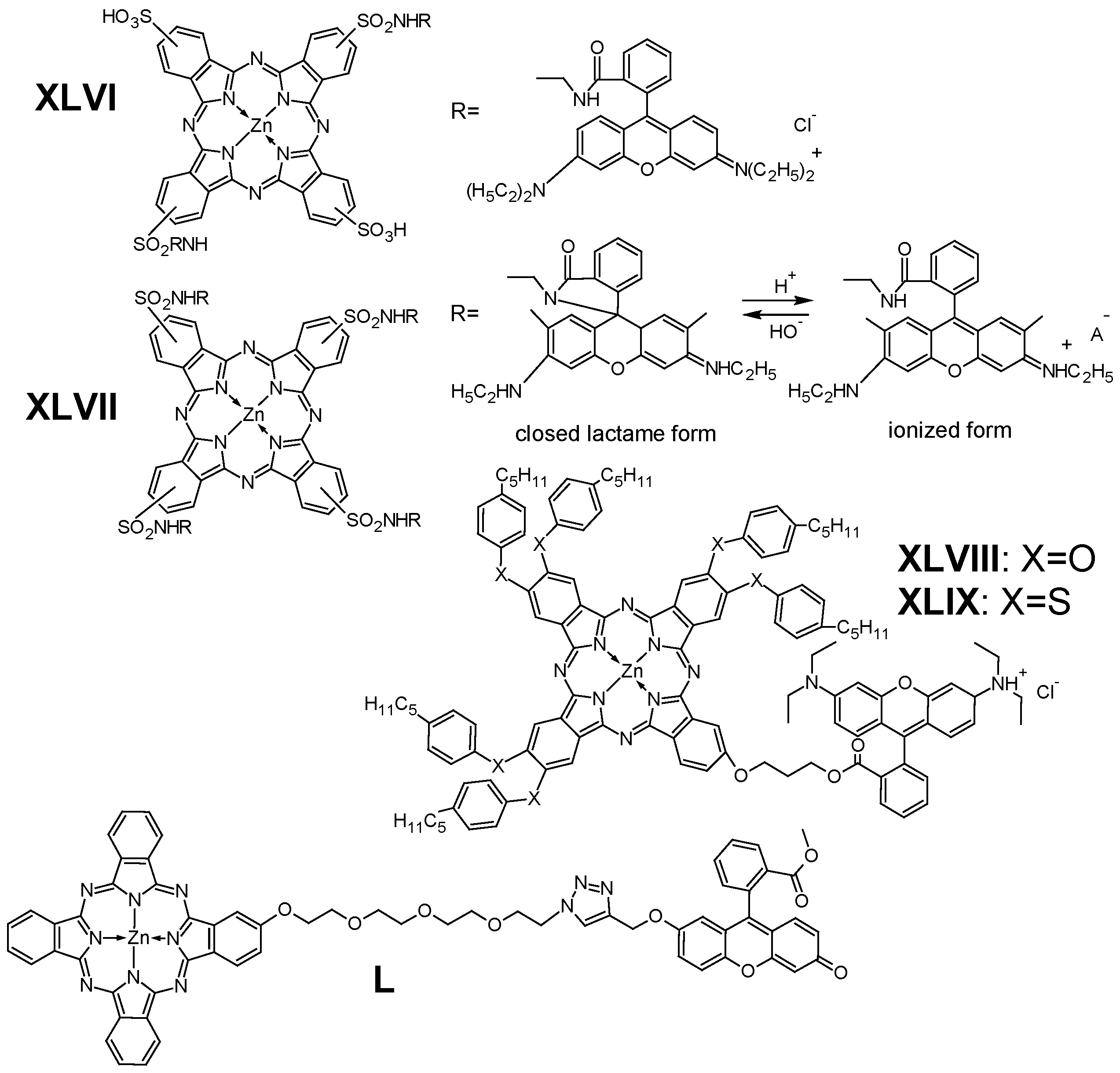
6.3. Other Fluorescent Dyes
7. Other Bioconjugates and Multifunctional Agents
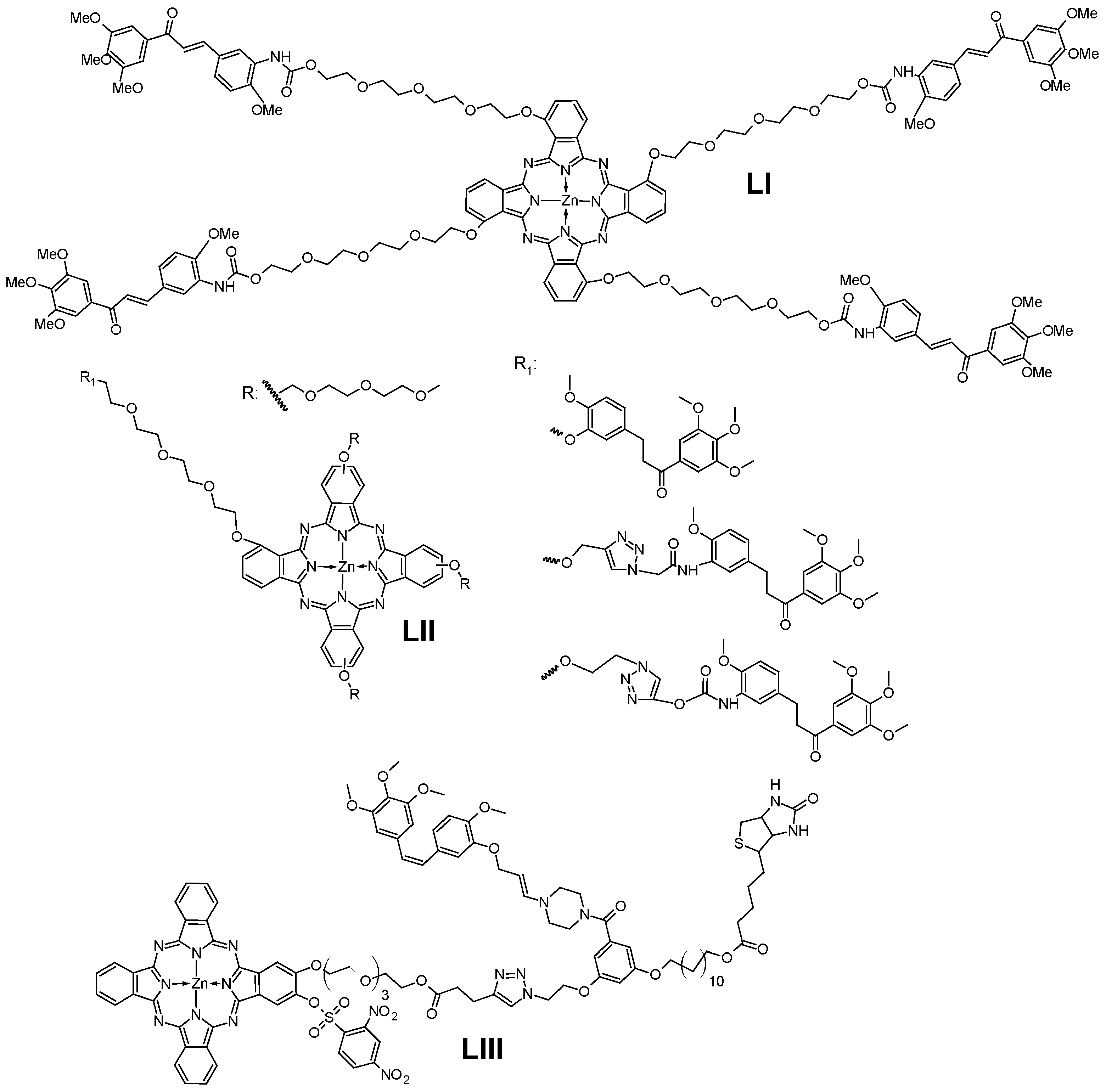
7.1. Chalcones
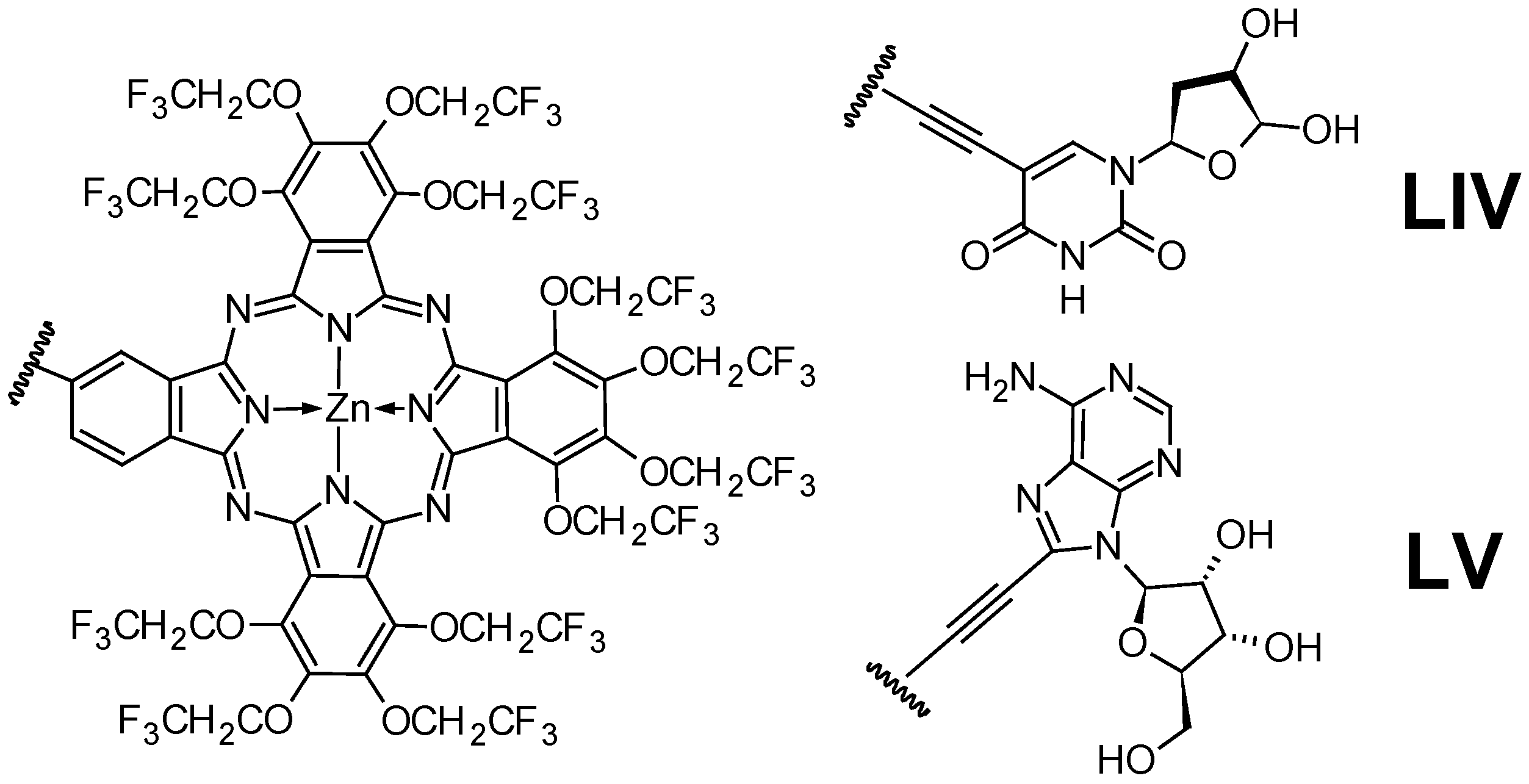
7.2. Other Compounds
8. Drawbacks and Limitations
9. Summary and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AESA | aggregation-enhanced sonodynamic activity |
| ALA | 5-aminolevulinic acid |
| ARS | artesunate |
| CI | combination index |
| COX | cyclooxygenase |
| CuAAC | copper(I)-catalyzed azide–alkyne cycloaddition |
| DMAP | 4-dimethylaminopyridine |
| DPBF | 1,3-diphenylisobenzofuran |
| Dox | doxorubicin |
| EDC | 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide |
| EGF | epidermal growth factor |
| EGFR | epidermal growth factor receptor |
| ER | estrogen receptor |
| EPR | enhanced permeability and retention |
| FAP | fibroblast activation protein |
| FA | folic acid |
| FR | folate receptor |
| GSH | glutathione |
| HAS | human serum albumin |
| Hsp90 | heat shock protein 90 |
| IC50 | half-maximal inhibitory concentration |
| INH | isoniazid |
| MDR | multidrug resistance |
| MLB | moclobemide |
| MSNs | mesoporous silica nanoparticles |
| NIR | near-infrared |
| OEG | oligoethylene glycol |
| PACT | photodynamic antimicrobial chemotherapy |
| PBS | phosphate-buffered saline |
| PEG | polyethylene glycol |
| Pc | phthalocyanine |
| PDT | photodynamic therapy |
| PET | photoinduced electron transfer |
| P-gp | P-glycoprotein |
| PS | photosensitizer |
| PVP | polyvinylpyrrolidone |
| RIMA | reversible inhibitor of monoamine oxidase A |
| RGD | arginylglycylaspartic acid |
| ROS | reactive oxygen species |
| SPDT | sonodynamic-photodynamic therapy |
| TPE | two-photon excitation |
| TSPO | translocator protein |
| VEGFR | vascular endothelial growth factor receptor |
| ZnPc | zinc phthalocyanine |
References
- Taquet, J.-P.; Frochot, C.; Manneville, V.; Barberi-Heyob, M. Phthalocyanines Covalently Bound to Biomolecules for a Targeted Photodynamic Therapy. Curr. Med. Chem. 2007, 14, 1673–1687. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Cai, S.; Wang, P.; Cheng, H.; Cheng, B.; Zhang, Y.; Liu, G. Innovative Design Strategies Advance Biomedical Applications of Phthalocyanines. Adv. Heal. Mater. 2023, 12, 2300263. [Google Scholar] [CrossRef] [PubMed]
- Plekhova, N.; Shevchenko, O.; Korshunova, O.; Stepanyugina, A.; Tananaev, I.; Apanasevich, V. Development of Novel Tetrapyrrole Structure Photosensitizers for Cancer Photodynamic Therapy. Bioengineering 2022, 9, 82. [Google Scholar] [CrossRef] [PubMed]
- Anas, A.; Sobhanan, J.; Sulfiya, K.M.; Jasmin, C.; Sreelakshmi, P.K.; Biju, V. Advances in Photodynamic Antimicrobial Chemotherapy. J. Photochem. Photobiol. C Photochem. Rev. 2021, 49, 100452. [Google Scholar] [CrossRef]
- Benov, L. Photodynamic Therapy: Current Status and Future Directions. Med. Princ. Pract. 2015, 24, 14–28. [Google Scholar] [CrossRef]
- Correia, J.H.; Rodrigues, J.A.; Pimenta, S.; Dong, T.; Yang, Z. Photodynamic Therapy Review: Principles, Photosensitizers, Applications, and Future Directions. Pharmaceutics 2021, 13, 1332. [Google Scholar] [CrossRef]
- Pham, T.C.; Nguyen, V.N.; Choi, Y.; Lee, S.; Yoon, J. Recent Strategies to Develop Innovative Photosensitizers for Enhanced Photodynamic Therapy. Chem. Rev. 2021, 121, 13454–13619. [Google Scholar] [CrossRef]
- Kwiatkowski, S.; Knap, B.; Przystupski, D.; Saczko, J.; Kędzierska, E.; Knap-Czop, K.; Kotlińska, J.; Michel, O.; Kotowski, K.; Kulbacka, J. Photodynamic Therapy—Mechanisms, Photosensitizers and Combinations. Biomed. Pharmacother. 2018, 106, 1098–1107. [Google Scholar] [CrossRef]
- Garland, M.J.; Cassidy, C.M.; Woolfson, D.; Donnelly, R.F. Designing Photosensitizers for Photodynamic Therapy: Strategies, Challenges and Promising Developments. Future Med. Chem. 2009, 1, 667–691. [Google Scholar] [CrossRef]
- Chinna Ayya Swamy, P.; Sivaraman, G.; Priyanka, R.N.; Raja, S.O.; Ponnuvel, K.; Shanmugpriya, J.; Gulyani, A. Near Infrared (NIR) Absorbing Dyes as Promising Photosensitizer for Photo Dynamic Therapy. Coord. Chem. Rev. 2020, 411, 213233. [Google Scholar] [CrossRef]
- Rennie, C.C.; Edkins, R.M. Targeted Cancer Phototherapy Using Phthalocyanine-Anticancer Drug Conjugates. Dalton Trans. 2022, 51, 13157–13175. [Google Scholar] [CrossRef]
- Gunaydin, G.; Gedik, M.E.; Ayan, S. Photodynamic Therapy—Current Limitations and Novel Approaches. Front. Chem. 2021, 9, 1697. [Google Scholar] [CrossRef] [PubMed]
- Lan, M.; Zhao, S.; Liu, W.; Lee, C.S.; Zhang, W.; Wang, P. Photosensitizers for Photodynamic Therapy. Adv. Heal. Mater. 2019, 8, 1900132. [Google Scholar] [CrossRef] [PubMed]
- Moreira, L.M.; Dos Santos, F.V.; Lyon, J.P.; Maftoum-Costa, M.; Pacheco-Soares, C.; Soares Da Silva, N. Photodynamic Therapy: Porphyrins and Phthalocyanines as Photosensitizers. Aust. J. Chem. 2008, 61, 741–754. [Google Scholar] [CrossRef]
- Galstyan, A. Turning Photons into Drugs: Phthalocyanine-Based Photosensitizers as Efficient Photoantimicrobials. Chem.—A Eur. J. 2021, 27, 1903–1920. [Google Scholar] [CrossRef]
- Mehraban, N.; Musich, P.R.; Freeman, H.S. Synthesis and Encapsulation of a New Zinc Phthalocyanine Photosensitizer into Polymeric Nanoparticles to Enhance Cell Uptake and Phototoxicity. Appl. Sci. 2019, 9, 401. [Google Scholar] [CrossRef]
- Wang, R.; Li, X.; Yoon, J. Organelle-Targeted Photosensitizers for Precision Photodynamic Therapy. ACS Appl. Mater. Interfaces 2021, 13, 19543–19571. [Google Scholar] [CrossRef]
- Bugaj, A.M. Targeted Photodynamic Therapy—A Promising Strategy of Tumor Treatment. Photochem. Photobiol. Sci. 2011, 10, 1097–1109. [Google Scholar] [CrossRef]
- Gierlich, P.; Mata, A.I.; Donohoe, C.; Brito, R.M.M.; Senge, M.O.; Gomes-Da-Silva, L.C. Ligand-Targeted Delivery of Photosensitizers for Cancer Treatment. Molecules 2020, 25, 5317. [Google Scholar] [CrossRef]
- Vale, N.; Ramos, R.; Cruz, I.; Pereira, M. Application of Peptide-Conjugated Photosensitizers for Photodynamic Cancer Therapy: A Review. Organics 2024, 5, 429–442. [Google Scholar] [CrossRef]
- Moret, F.; Reddi, E. Strategies for Optimizing the Delivery to Tumors of Macrocyclic Photosensitizers Used in Photodynamic Therapy (PDT). J. Porphyr. Phthalocyanines 2017, 21, 239–256. [Google Scholar] [CrossRef]
- Tripodo, G.; Mandracchia, D.; Collina, S.; Riu, M.; Rossi, D. New Perspectives in Cancer Therapy: The Biotin-Antitumor Molecule Conjugates. Med. Chem. 2014, 1–8. [Google Scholar] [CrossRef]
- Jurczyk, M.; Jelonek, K.; Musiał-kulik, M.; Beberok, A.; Wrześniok, D.; Kasperczyk, J. Single- versus Dual-Targeted Nanoparticles with Folic Acid and Biotin for Anticancer Drug Delivery. Pharmaceutics 2021, 13, 1–42. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Tiwari, A.; Verma, A.; Jain, S.K. Vitamins for Cancer Prevention and Treatment: An Insight. Curr. Mol. Med. 2017, 17, 321–340. [Google Scholar] [CrossRef] [PubMed]
- Linstead, R.P. Discoveries among Conjugated Macrocydic Compounds. J. Chem. Soc. 1953, 2873–2884. [Google Scholar] [CrossRef]
- Oliver, S.W.; Smith, T.D. Oligomeric Cyclization of Dinitriles in the Synthesis of Phthalocyanines and Related Compounds: The Role of the Alkoxide Anion. J. Chem. Soc. Perkin Trans. 2 1987, 1579–1582. [Google Scholar] [CrossRef]
- O’Shea, D.F.; Miller, M.A.; Matsueda, H.; Lindsey, J.S. Investigation of the Scope of Heterogeneous and Homogeneous Procedures for Preparing Magnesium Chelates of Porphyrins, Hydroporphyrins, and Phthalocyanines. Inorg. Chem. 1996, 35, 7325–7338. [Google Scholar] [CrossRef]
- Tomoda, H.; Saito, S.; Shiraishi, S. Synthesis Of Metallophthalocyanines From Phthalonitrile With Strong Organic Bases. Chem. Lett. 1983, 12, 313–316. [Google Scholar] [CrossRef]
- Chauhan, S.M.S.; Srinivas, K.A.; Srivastava, P.K.; Sahoo, B. Solvent-Free Synthesis of Phthalocyanines. J. Porphyr. Phthalocyanines 2003, 7, 548–550. [Google Scholar] [CrossRef]
- Szczolko, W.; Kryjewski, M.; Koczorowski, T.; Zuchowska, E.; Popenda, L.; Mlynarczyk, D.T. Pyrrole Dicarboxylate Substituted Porphyrazine, Microwave Assisted Synthesis and Properties. Sci. Rep. 2025, 15, 16668. [Google Scholar] [CrossRef]
- De La Torre, G.; Claessens, C.G.; Torres, T. Phthalocyanines: The Need for Selective Synthetic Approaches. Eur. J. Org. Chem. 2000, 2821–2830. [Google Scholar] [CrossRef]
- Chan, J.Y.M.; Ng, D.K.P. Mixed Cyclization: A Synthetic Route to Prepare Low-Symmetry Phthalocyanines. J. Org. Chem. 2022, 87, 7213–7218. [Google Scholar] [CrossRef]
- Bareschino, M.A.; Schettino, C.; Troiani, T.; Martinelli, E.; Morgillo, F.; Ciardiello, F. Erlotinib in Cancer Treatment. Ann. Oncol. 2007, 18, vi35–vi41. [Google Scholar] [CrossRef] [PubMed]
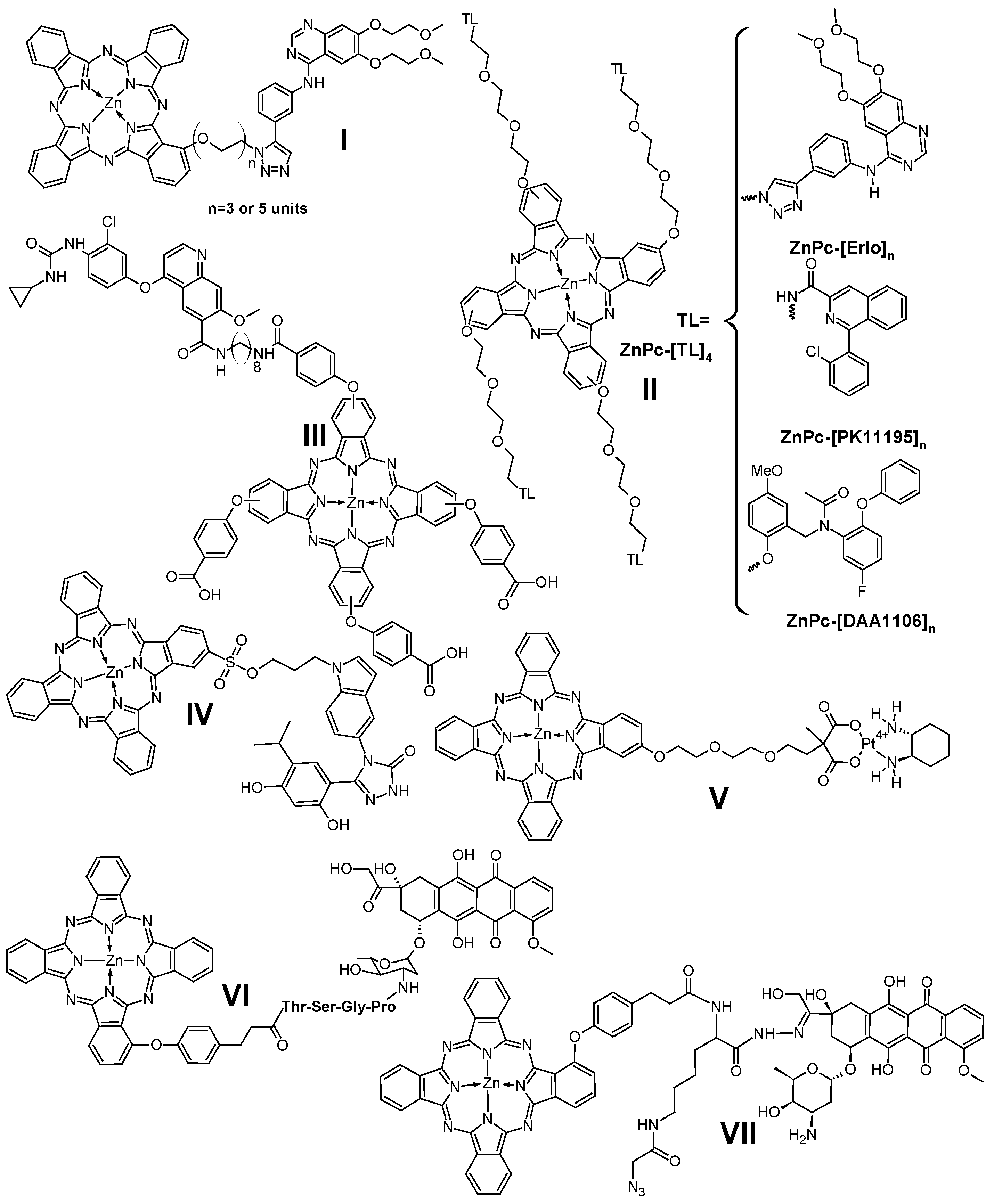
- Zhang, F.L.; Huang, Q.; Zheng, K.; Li, J.; Liu, J.Y.; Xue, J.P. A Novel Strategy for Targeting Photodynamic Therapy. Molecular Combo of Photodynamic Agent Zinc(II) Phthalocyanine and Small Molecule Target-Based Anticancer Drug Erlotinib. Chem. Commun. 2013, 49, 9570–9572. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.L.; Huang, Q.; Liu, J.Y.; Huang, M.D.; Xue, J.P. Molecular-Target-Based Anticancer Photosensitizer: Synthesis and in Vitro Photodynamic Activity of Erlotinib-Zinc(II) Phthalocyanine Conjugates. ChemMedChem 2015, 10, 312–320. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Ye, H.; Zhang, M.; Li, J.; Liu, J.; Xue, J. Erlotinib Analogue-Substituted Zinc(II) Phthalocyanines for Small Molecular Target-Based Photodynamic Cancer Therapy. Chin. J. Chem. 2016, 34, 983–988. [Google Scholar] [CrossRef]
- Toubia, I.; Nguyen, C.; Diring, S.; Onofre, M.; Daurat, M.; Gauthier, C.; Gary-Bobo, M.; Kobeissi, M.; Odobel, F. Development of Targeted Photodynamic Therapy Drugs by Combining a Zinc Phthalocyanine Sensitizer with TSPO or EGFR Binding Groups: The Impact of the Number of Targeting Agents on Biological Activity. Org. Biomol. Chem. 2023, 21, 6509–6523. [Google Scholar] [CrossRef]
- Wei, G.; Huang, L.; Jiang, Y.; Shen, Y.; Huang, Z.; Huang, Y.; Sun, X.; Zhao, C. Lenvatinib-Zinc Phthalocyanine Conjugates as Potential Agents for Enhancing Synergistic Therapy of Multidrug-Resistant Cancer by Glutathione Depletion. Eur. J. Med. Chem. 2019, 169, 53–64. [Google Scholar] [CrossRef]
- Youssef, M.E.; Cavalu, S.; Hasan, A.M.; Yahya, G.; Abd-Eldayem, M.A.; Saber, S. Role of Ganetespib, an HSP90 Inhibitor, in Cancer Therapy: From Molecular Mechanisms to Clinical Practice. Int. J. Mol. Sci. 2023, 24, 5014. [Google Scholar] [CrossRef]
- Huang, L.; Wei, G.; Sun, X.; Jiang, Y.; Huang, Z.; Huang, Y.; Shen, Y.; Xu, X.; Liao, Y.; Zhao, C. A Tumor-Targeted Ganetespib-Zinc Phthalocyanine Conjugate for Synergistic Chemo-Photodynamic Therapy. Eur. J. Med. Chem. 2018, 151, 294–303. [Google Scholar] [CrossRef]
- Lau, J.T.F.; Lo, P.C.; Fong, W.P.; Ng, D.K.P. A Zinc(II) Phthalocyanine Conjugated with an Oxaliplatin Derivative for Dual Chemo- and Photodynamic Therapy. J. Med. Chem. 2012, 55, 5446–5454. [Google Scholar] [CrossRef]
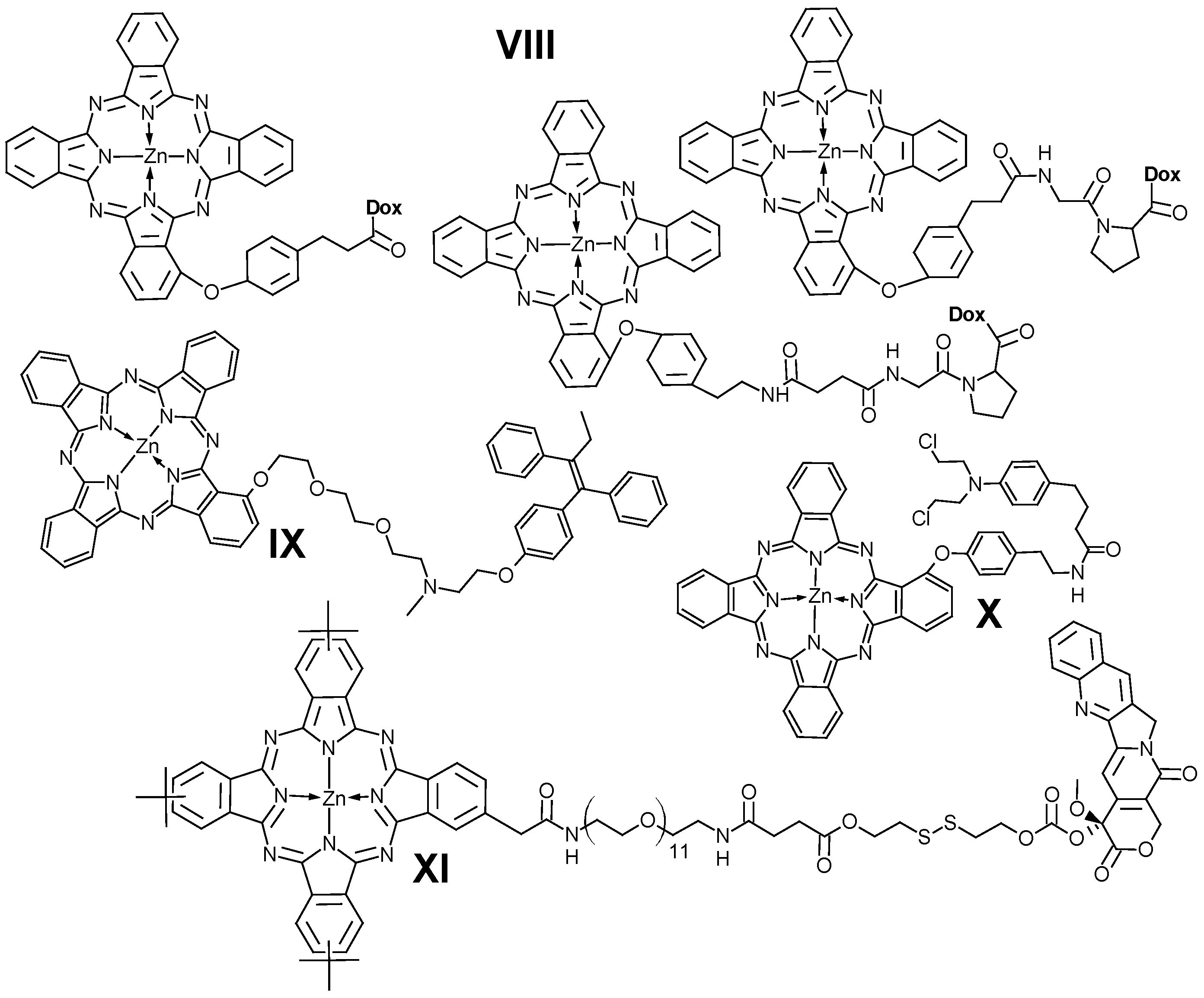
- Ke, M.R.; Chen, S.F.; Peng, X.H.; Zheng, Q.F.; Zheng, B.Y.; Yeh, C.K.; Huang, J.D. A Tumor-Targeted Activatable Phthalocyanine-Tetrapeptide-Doxorubicin Conjugate for Synergistic Chemo-Photodynamic Therapy. Eur. J. Med. Chem. 2017, 127, 200–209. [Google Scholar] [CrossRef]
- Wong, R.C.H.; Ng, D.K.P.; Fong, W.P.; Lo, P.C. Encapsulating PH-Responsive Doxorubicin–Phthalocyanine Conjugates in Mesoporous Silica Nanoparticles for Combined Photodynamic Therapy and Controlled Chemotherapy. Chem.—A Eur. J. 2017, 23, 16505–16515. [Google Scholar] [CrossRef]
- Peng, X.H.; Chen, S.F.; Xu, C.H.; Zheng, B.Y.; Ke, M.R.; Huang, J.D. Synthesis, Spectroscopic and Fibroblast Activation Protein (FAP)-Responsive Properties of Phthalocyanine-Doxorubicin Conjugates. ChemistrySelect 2018, 3, 5405–5411. [Google Scholar] [CrossRef]
- Zhang, F.L.; Song, M.R.; Yuan, G.K.; Ye, H.N.; Tian, Y.; Huang, M.D.; Xue, J.P.; Zhang, Z.H.; Liu, J.Y. A Molecular Combination of Zinc(II) Phthalocyanine and Tamoxifen Derivative for Dual Targeting Photodynamic Therapy and Hormone Therapy. J. Med. Chem. 2017, 60, 6693–6703. [Google Scholar] [CrossRef]
- Zhang, F.L.; Huang, N.; Weng, H.L.; Xue, J.P. Tamoxifen-Zinc(II) Phthalocyanine Conjugates for Target-Based Photodynamic Therapy and Hormone Therapy. J. Porphyr. Phthalocyanines 2019, 23, 1073–1083. [Google Scholar] [CrossRef]
- Peng, X.H.; Chen, S.F.; Zheng, B.Y.; Zheng, B.D.; Zheng, Q.F.; Li, X.S.; Ke, M.R.; Huang, J.D. Comparison between Amine-Terminated Phthalocyanines and Their Chlorambucil Conjugates: Synthesis, Spectroscopic Properties, and in Vitro Anticancer Activity. Tetrahedron 2017, 73, 378–384. [Google Scholar] [CrossRef]
- Martínez-Edo, G.; Xue, E.Y.; Ha, S.Y.Y.; Pontón, I.; González-Delgado, J.A.; Borrós, S.; Torres, T.; Ng, D.K.P.; Sánchez-García, D. Nanoparticles for Triple Drug Release for Combined Chemo- and Photodynamic Therapy. Chem.—A. Eur. J. 2021, 27, 14610–14618. [Google Scholar] [CrossRef] [PubMed]
- da Silva, R.N.; Cunha, Â.; Tomé, A.C. Phthalocyanine–Sulfonamide Conjugates: Synthesis and Photodynamic Inactivation of Gram-Negative and Gram-Positive Bacteria. Eur. J. Med. Chem. 2018, 154, 60–67. [Google Scholar] [CrossRef]
- Bacchi, S.; Palumbo, P.; Sponta, A.; Coppolino, M.F. Clinical Pharmacology of Non-Steroidal Anti-Inflammatory Drugs: A Review. Antiinflamm. Antiallergy Agents Med. Chem. 2012, 11, 52–64. [Google Scholar] [CrossRef]
- Huang, K.; Zhang, H.; Yan, M.; Xue, J.; Chen, J. A Novel Zinc Phthalocyanine-Indometacin Photosensitizer with “Three-in-One” Cyclooxygenase-2-Driven Dual Targeting and Aggregation Inhibition for High-Efficient Anticancer Therapy. Dye. Pigment. 2022, 198, 109997. [Google Scholar] [CrossRef]
- Magadla, A.; Mpeta, L.S.; Britton, J.; Nyokong, T. Photodynamic Antimicrobial Chemotherapy Activities of Phthalocyanine-Antibiotic Conjugates against Bacterial Biofilms and Interactions with Extracellular Polymeric Substances. Photodiagn. Photodyn. Ther. 2023, 44, 103878. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, G.F.d.S.; Salgado, H.R.N.; Santos, J.L. dos Isoniazid: A Review of Characteristics, Properties and Analytical Methods. Crit. Rev. Anal. Chem. 2017, 47, 298–308. [Google Scholar] [CrossRef] [PubMed]
- Nkanga, C.I.; Krause, R.W.M. Conjugation of Isoniazid to a Zinc Phthalocyanine via Hydrazone Linkage for PH-Dependent Liposomal Controlled Release. Appl. Nanosci. 2018, 8, 1313–1323. [Google Scholar] [CrossRef]
- Nkanga, C.I.; Krause, R.W.M. Encapsulation of Isoniazid-Conjugated Phthalocyanine-In-Cyclodextrin-In-Liposomes Using Heating Method. Sci. Rep. 2019, 9, 11485. [Google Scholar] [CrossRef]
- Schmider, J.; Greenblatt, D.J.; Von Moltke, L.L.; Karsov, D.; Vena, R.; Friedman, H.L.; Shader, R.I. Biotransformation of Mestranol to Ethinyl Estradiol in Vitro: The Role of Cytochrome P-450 2C9 Metabolic Inhibitors. J. Clin. Pharmacol. 1997, 37, 193–200. [Google Scholar] [CrossRef]
- Novakova, V.; Zimcik, P.; Miletin, M.; Kopecky, K.; Ivincová, J. A Phthalocyanine-Mestranol Conjugate for Photodynamic Therapy Prepared via Click Chemistry. Tetrahedron Lett. 2010, 51, 1016–1018. [Google Scholar] [CrossRef]
- Zhao, P.H.; Wu, Y.L.; Li, X.Y.; Feng, L.L.; Zhang, L.; Zheng, B.Y.; Ke, M.R.; Huang, J.D. Aggregation-Enhanced Sonodynamic Activity of Phthalocyanine–Artesunate Conjugates. Angew. Chem.—Int. Ed. 2022, 61, e202113506. [Google Scholar] [CrossRef]
- Bonnet, U. Moclobemide: Therapeutic Use and Clinical Studies. CNS Drug Rev. 2003, 9, 97–140. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, Q.; Jia, X.; Xue, J.; Chen, J. A Monoamine Oxidase-A Inhibitor Phthalocyanine Conjugate for Targeted Photodynamic Therapy and Inhibition of Prostate Cancer Metastasis in Vitro. Dye Pigment 2022, 207, 110717. [Google Scholar] [CrossRef]
- Stallivieri, A.; Baros, F.; Jetpisbayeva, G.; Myrzakhmetov, B.; Frochot, C. The Interest of Folic Acid in Targeted Photodynamic Therapy. Curr. Med. Chem. 2015, 22, 3185–3207. [Google Scholar] [CrossRef]
- Wang, M.; Long, J.; Zhang, S.; Liu, F.; Zhang, X.; Zhang, X.; Sun, L.; Ma, L.; Yu, C.; Wei, H. Folate-Targeted Anticancer Drug Delivery via a Combination Strategy of a Micelle Complex and Reducible Conjugation. ACS Biomater. Sci. Eng. 2020, 6, 1565–1572. [Google Scholar] [CrossRef]
- Ivanova-Radkevich, V.I. Biochemical Basis of Selective Accumulation and Targeted Delivery of Photosensitizers to Tumor Tissues. Biochemistry 2022, 87, 1226–1242. [Google Scholar] [CrossRef]
- Lin, A.L.; Chen, J.H.; Hong, J.W.; Zhao, Y.Y.; Zheng, B.Y.; Ke, M.R.; Huang, J.D. A Phthalocyanine-Based Self-Assembled Nanophotosensitizer for Efficient in Vivo Photodynamic Anticancer Therapy. J. Inorg. Biochem. 2021, 217, 111371. [Google Scholar] [CrossRef] [PubMed]
- Matlou, G.G.; Kobayashi, N.; Kimura, M.; Nyokong, T. Physicochemical Properties of Water Soluble Unsymmetrical Phthalocyanine-Folic Acid Conjugates. Dye Pigment 2018, 149, 393–398. [Google Scholar] [CrossRef]
- Matlou, G.G.; Oluwole, D.O.; Prinsloo, E.; Nyokong, T. Photodynamic Therapy Activity of Zinc Phthalocyanine Linked to Folic Acid and Magnetic Nanoparticles. J. Photochem. Photobiol. B. 2018, 186, 216–224. [Google Scholar] [CrossRef] [PubMed]
- Ogbodu, R.O.; Ndhundhuma, I.; Karsten, A.; Nyokong, T. Photodynamic Therapy Effect of Zinc Monoamino Phthalocyanine-Folic Acid Conjugate Adsorbed on Single Walled Carbon Nanotubes on Melanoma Cells. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2015, 137, 1120–1125. [Google Scholar] [CrossRef]
- Wang, B.S.; Wang, J.; Chen, J.Y. Conjugates of Folic Acids with Zinc Aminophthalocyanine for Cancer Cell Targeting and Photodynamic Therapy by One-Photon and Two-Photon Excitations. J. Mater. Chem. B. 2014, 2, 1594–1602. [Google Scholar] [CrossRef]
- Garanger, E.; Boturyn, D.; Dumy, P. Tumor Targeting with RGD Peptide Ligands-Design of New Molecular Conjugates for Imaging and Therapy of Cancers. Anticancer Agents Med. Chem. 2007, 7, 552–558. [Google Scholar] [CrossRef]
- Wang, Y.; Xiao, W.; Zhang, Y.; Meza, L.; Tseng, H.; Takada, Y.; Ames, J.B.; Lam, K.S. Optimization of RGD-Containing Cyclic Peptides against Avβ3 Integrin. Mol. Cancer Ther. 2016, 15, 232–240. [Google Scholar] [CrossRef]
- Ke, M.R.; Ng, D.K.P.; Lo, P.C. Synthesis and in Vitro Photodynamic Activities of an Integrin-Targeting CRGD-Conjugated Zinc(II) Phthalocyanine. Chem. Asian J. 2014, 9, 554–561. [Google Scholar] [CrossRef]
- Luan, L.; Fang, W.; Liu, W.; Tian, M.; Ni, Y.; Chen, X.; Yu, X.; He, J.; Yang, Y.; Li, X. 4-Tert-Butylphenoxy Substituted Phthalocyanine with RGD Motif as Highly Selective One-Photon and Two-Photon Imaging Probe for Mitochondria and Cancer Cell. J. Porphyr. Phthalocyanines 2016, 20, 397–406. [Google Scholar] [CrossRef]
- Luan, L.; Fang, W.; Liu, W.; Tian, M.; Ni, Y.; Chen, X.; Yu, X. Phthalocyanine-CRGD Conjugate: Synthesis, Photophysical Properties and in Vitro Biological Activity for Targeting Photodynamic Therapy. Org. Biomol. Chem. 2016, 14, 2985–2992. [Google Scholar] [CrossRef] [PubMed]
- Chu, J.C.H.; Fong, W.P.; Wong, C.T.T.; Ng, D.K.P. Facile Synthesis of Cyclic Peptide-Phthalocyanine Conjugates for Epidermal Growth Factor Receptor-Targeted Photodynamic Therapy. J. Med. Chem. 2021, 64, 2064–2076. [Google Scholar] [CrossRef]
- Ren, W.X.; Han, J.; Uhm, S.; Jang, Y.J.; Kang, C.; Kim, J.H.; Kim, J.S. Recent Development of Biotin Conjugation in Biological Imaging, Sensing, and Target Delivery. Chem. Commun. 2015, 51, 10403–10418. [Google Scholar] [CrossRef]
- Maiti, S.; Paira, P. Biotin Conjugated Organic Molecules and Proteins for Cancer Therapy: A Review. Eur. J. Med. Chem. 2018, 145, 206–223. [Google Scholar] [CrossRef]
- Balçik-Erçin, P.; Çetin, M.; Göksel, M.; Durmuş, M. Improved Targeting for Photodynamic Therapy: Via a Biotin-Phthalocyanine Conjugate: Synthesis, Photophysical and Photochemical Measurements, and in Vitro Cytotoxicity Assay. New J. Chem. 2020, 44, 3392–3401. [Google Scholar] [CrossRef]
- Okoth, E.A.; Zhou, Z.; Ongarora, B.; Stutes, A.; Mathis, J.M.; Vicente, M.G.H. Synthesis and Investigation of Phthalocyanine-Biotin Conjugates. J. Porphyr. Phthalocyanines 2020, 23, 125–135. [Google Scholar] [CrossRef]
- Ermeydan, M.A.; Dumoulin, F.; Basova, T.V.; Bouchu, D.; Gürek, A.G.; Ahsen, V.; Lafont, D. Amphiphilic Carbohydrate-Phthalocyanine Conjugates Obtained by Glycosylation or by Azide-Alkyne Click Reaction. New J. Chem. 2010, 34, 1153–1162. [Google Scholar] [CrossRef]
- Silva, S.; Pereira, P.M.R.; Silva, P.; Almeida Paz, F.A.; Faustino, M.A.F.; Cavaleiro, J.A.S.; Tomé, J.P.C. Porphyrin and Phthalocyanine Glycodendritic Conjugates: Synthesis, Photophysical and Photochemical Properties. Chem. Commun. 2012, 48, 3608–3610. [Google Scholar] [CrossRef]
- Lafont, D.; Zorlu, Y.; Savoie, H.; Albrieux, F.; Ahsen, V.; Boyle, R.W.; Dumoulin, F. Monoglycoconjugated Phthalocyanines: Effect of Sugar and Linkage on Photodynamic Activity. Photodiagnosis Photodyn. Ther. 2013, 10, 252–259. [Google Scholar] [CrossRef]
- Yilmaz, Y.; Zengin, H.; Sönmez, M. Synthesis and Photoluminescence Properties of Saccharide Conjugated Copper Phthalocyanine via Click Reaction. Synth. React. Inorg. Met. Org. Nano-Met. Chem. 2014, 45, 337–341. [Google Scholar] [CrossRef]
- Mori, S.; Yoshiyama, H.; Tokunaga, E.; Iida, N.; Hayashi, M.; Obata, T.; Tanaka, M.; Shibata, N. Design, Synthesis, Spectral Investigations and Biological Activity of Fluorinated Phthalocyanine Conjugated with Galactose and Comparison to Its Non-Fluorinated Counterpart. J. Fluor. Chem. 2015, 174, 137–141. [Google Scholar] [CrossRef]
- Makuch, S.; Kupczyk, P.; Woźniak, M.; Makarec, A.; Lipińska, M.; Klyta, M.; Sulecka-Zadka, J.; Szeja, W.; Gani, M.; Rapozzi, V.; et al. In Vitro and In Vivo Antipsoriatic Efficacy of Protected and Unprotected Sugar–Zinc Phthalocyanine Conjugates. Pharmaceutics 2024, 16, 838. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, Z.; Chen, J.; Chen, Z.; Huang, M. Phthalocyanine-Biomolecule Conjugated Photosensitizers for Targeted Photodynamic Therapy and Imaging. Curr. Drug Metab. 2015, 16, 816–832. [Google Scholar] [CrossRef]
- Liu, K.; Xing, R.; Zou, Q.; Ma, G.; Möhwald, H.; Yan, X. Simple Peptide-Tuned Self-Assembly of Photosensitizers towards Anticancer Photodynamic Therapy. Angew. Chem. 2016, 128, 3088–3091. [Google Scholar] [CrossRef]
- Lalatsa, A.; Schätzlein, A.G.; Mazza, M.; Le, T.B.H.; Uchegbu, I.F. Amphiphilic Poly(l-Amino Acids)—New Materials for Drug Delivery. J. Control. Release 2012, 161, 523–536. [Google Scholar] [CrossRef]
- Chen, J.; Chen, Z.; Zheng, Y.; Zhou, S.; Wang, J.; Chen, N.; Huang, J.; Yan, F.; Huang, M. Substituted Zinc Phthalocyanine as an Antimicrobial Photosensitizer for Periodontitis Treatment. J. Porphyr. Phthalocyanines 2011, 15, 293–299. [Google Scholar] [CrossRef]
- Li, L.; Luo, Z.; Chen, Z.; Chen, J.; Zhou, S.; Xu, P.; Hu, P.; Wang, J.; Chen, N.; Huang, J.; et al. Enhanced Photodynamic Efficacy of Zinc Phthalocyanine by Conjugating to Heptalysine. Bioconjug. Chem. 2012, 23, 2168–2172. [Google Scholar] [CrossRef]
- Nombona, N.; Antunes, E.; Chidawanyika, W.; Kleyi, P.; Tshentu, Z.; Nyokong, T. Synthesis, Photophysics and Photochemistry of Phthalocyanine-ε- Polylysine Conjugates in the Presence of Metal Nanoparticles against Staphylococcus Aureus. J. Photochem. Photobiol. A Chem. 2012, 233, 24–33. [Google Scholar] [CrossRef]
- Wang, A.; Zhou, R.; Zhou, L.; Sun, K.; Jiang, J.; Wei, S. Positively Charged Phthalocyanine-Arginine Conjugates as Efficient Photosensitizer for Photodynamic Therapy. Bioorg. Med. Chem. 2017, 25, 1643–1651. [Google Scholar] [CrossRef]
- Awuah, S.G.; You, Y. Boron Dipyrromethene (BODIPY)-Based Photosensitizers for Photodynamic Therapy. RSC Adv. 2012, 2, 11169–11183. [Google Scholar] [CrossRef]
- Göl, C.; Malkoç, M.; Yeşilot, S.; Durmuş, M. Novel Zinc(II) Phthalocyanine Conjugates Bearing Different Numbers of BODIPY and Iodine Groups as Substituents on the Periphery. Dye Pigment 2014, 111, 81–90. [Google Scholar] [CrossRef]
- Osati, S.; Ali, H.; Van Lier, J.E. Synthesis and Spectral Properties of Phthalocyanine-BODIPY Conjugates. Tetrahedron Lett. 2015, 56, 2049–2053. [Google Scholar] [CrossRef]
- Yanik, H.; Göksel, M.; Yeşilot, S.; Durmuş, M. Novel Phthalocyanine-BODIPY Conjugates and Their Photophysical and Photochemical Properties. Tetrahedron Lett. 2016, 57, 2922–2926. [Google Scholar] [CrossRef]
- Yanık, H.; Yeşilot, S.; Durmuş, M. Synthesis and Properties of Octa-Distyryl-BODIPY Substituted Zinc(II) Phthalocyanines. Dye Pigment 2017, 140, 157–165. [Google Scholar] [CrossRef]
- Ha, S.Y.Y.; Ng, D.K.P. Constructing a Four-Input Molecular Keypad Lock with a Multi-Stimuli-Responsive Phthalocyanine. Chem. Commun. 2020, 56, 14601–14604. [Google Scholar] [CrossRef] [PubMed]
- Soares, A.R.M.; Martínez-Díaz, M.V.; Bruckner, A.; Pereira, A.M.V.M.; Tomé, J.P.C.; Alonso, C.M.A.; Faustino, M.A.F.; Neves, M.G.P.M.S.; Tomé, A.C.; Silva, A.M.S.; et al. Synthesis of Novel N-Linked Porphyrin—Phthalocyanine Dyads. Org. Lett. 2007, 9, 1557–1560. [Google Scholar] [CrossRef]
- Hausmann, A.; Soares, A.R.M.; Martínez-Díaz, M.V.; Neves, M.G.P.M.S.; Tomé, A.C.; Cavaleiro, J.A.S.; Torres, T.; Guldi, D.M. Transduction of Excited State Energy between Covalently Linked Porphyrins and Phthalocyanines. Photochem. Photobiol. Sci. 2010, 9, 1027–1032. [Google Scholar] [CrossRef]
- Balashova, I.O.; Pushkarev, V.E.; Shestov, V.I.; Tomilova, L.G.; Koifman, O.I.; Ponomarev, G.V. Synthesis and Spectral Properties of Phthalocyanine-Methylpheophorbide a Covalently Linked Dyad. Macroheterocycles 2015, 8, 233–238. [Google Scholar] [CrossRef][Green Version]
- Balashova, I.O.; Tolbin, A.Y.; Tarakanov, P.A.; Krot, A.R.; Fedorova, K.V.; Sergeeva, I.A.; Trashin, S.A.; De Wael, K.; Pushkarev, V.E.; Koifman, M.O.; et al. A Covalently Linked Dyad Based on Zinc Phthalocyanine and Methylpheophorbide a: Synthetic and Physicochemical Study. Macroheterocycles 2021, 14, 40–50. [Google Scholar] [CrossRef]
- Pereira, A.M.V.M.; Soares, A.R.M.; Hausmann, A.; Neves, M.G.P.M.S.; Tomé, A.C.; Silva, A.M.S.; Cavaleiro, J.A.S.; Guldi, D.M.; Torres, T. Distorted Fused Porphyrin-Phthalocyanine Conjugates: Synthesis and Photophysics of Supramolecular Assembled Systems with a Pyridylfullerene. Phys. Chem. Chem. Phys. 2011, 13, 11858–11863. [Google Scholar] [CrossRef]
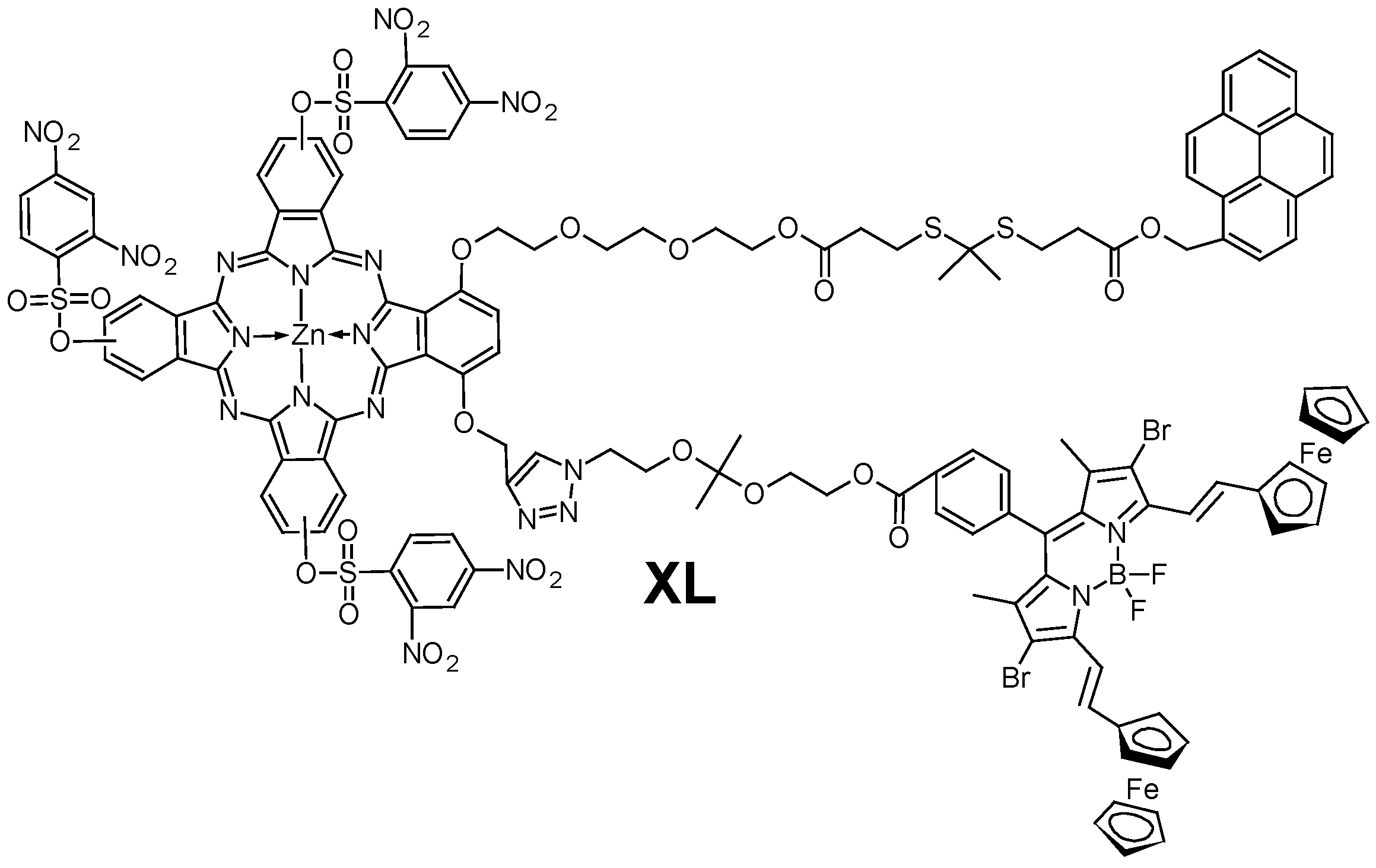
- De Oliveira, K.T.; De Assis, F.F.; Ribeiro, A.O.; Neri, C.R.; Fernandes, A.U.; Baptista, M.S.; Lopes, N.P.; Serra, O.A.; Iamamoto, Y. Synthesis of Phthalocyanines-ALA Conjugates: Water-Soluble Compounds with Low Aggregation. J. Org. Chem. 2009, 74, 7962–7965. [Google Scholar] [CrossRef]
- Pavani, C.; Francisco, C.M.L.; Gobo, N.R.S.; De Oliveira, K.T.; Baptista, M.S. Improved Photodynamic Activity of a Dual Phthalocyanine-ALA Photosensitiser. New J. Chem. 2016, 40, 9666–9671. [Google Scholar] [CrossRef]
- Kuznetsova, N.; Makarov, D.; Derkacheva, V.; Savvina, L.; Alekseeva, V.; Marinina, L.; Slivka, L.; Kaliya, O.; Lukyanets, E. Intramolecular Energy Transfer in Rhodamine-Phthalocyanine Conjugates. J. Photochem. Photobiol. A Chem. 2008, 200, 161–168. [Google Scholar] [CrossRef]
- Muli, D.K.; Rajaputra, P.; You, Y.; McGrath, D.V. Asymmetric ZnPc-Rhodamine B Conjugates for Mitochondrial Targeted Photodynamic Therapy. Bioorg. Med. Chem. Lett. 2014, 24, 4496–4500. [Google Scholar] [CrossRef] [PubMed]
- Un, I.; Zorlu, Y.; Ibisoglu, H.; Dumoulin, F.; Ahsen, V. A Phthalocyanine-Fluorescein Conjugate. Turk. J. Chem. 2013, 37, 394–404. [Google Scholar] [CrossRef]
- Zhuang, C.; Zhang, W.; Sheng, C.; Zhang, W.; Xing, C.; Miao, Z. Chalcone: A Privileged Structure in Medicinal Chemistry. Chem. Rev. 2017, 117, 7762–7810. [Google Scholar] [CrossRef] [PubMed]
- Sahu, N.K.; Balbhadra, S.S.; Choudhary, J.; Kohli, D.V. Exploring Pharmacological Significance of Chalcone Scaffold: A Review. Curr. Med. Chem. 2012, 19, 209–225. [Google Scholar] [CrossRef]
- Batovska, D.I.; Todorova, I.T. Trends in Utilization of the Pharmacological Potential of Chalcones. Curr. Clin. Pharmacol. 2010, 5, 1–29. [Google Scholar] [CrossRef]
- Tuncel, S.; Trivella, A.; Atilla, D.; Bennis, K.; Savoie, H.; Albrieux, F.; Delort, L.; Billard, H.; Dubois, V.; Ahsen, V.; et al. Assessing the Dual Activity of a Chalcone-Phthalocyanine Conjugate: Design, Synthesis, and Antivascular and Photodynamic Properties. Mol. Pharm. 2013, 10, 3706–3716. [Google Scholar] [CrossRef]
- Tuncel, S.; Fournier-Dit-Chabert, J.; Albrieux, F.; Ahsen, V.; Ducki, S.; Dumoulin, F. Towards Dual Photodynamic and Antiangiogenic Agents: Design and Synthesis of a Phthalocyanine-Chalcone Conjugate. Org. Biomol. Chem. 2012, 10, 1154–1157. [Google Scholar] [CrossRef] [PubMed]
- Aribi, F.; Vey, C.; Topkaya, D.; Kostakoglu, S.T.; Fournier-Dit-Chabert, J.; Büyükeksi, S.I.; Taskln, G.C.; Alpugan, S.; Albrieux, F.; Gürek, A.G.; et al. Phthalocyanine-Chalcone Conjugates. J. Porphyr. Phthalocyanines 2016, 20, 497–504. [Google Scholar] [CrossRef]
- Ha, S.Y.Y.; Zhou, Y.; Fong, W.P.; Ng, D.K.P. Multifunctional Molecular Therapeutic Agent for Targeted and Controlled Dual Chemo- And Photodynamic Therapy. J. Med. Chem. 2020, 63, 8512–8523. [Google Scholar] [CrossRef] [PubMed]
- Reddy, M.R.; Shibata, N.; Kondo, Y.; Nakamura, S.; Toru, T. Design, Synthesis, and Spectroscopic Investigation of Zinc Dodecakis(Trifluoroethoxy)-Phthalocyanines Conjugated with Deoxyribonucleosides. Angew. Chem.—Int. Ed. 2006, 45, 8163–8166. [Google Scholar] [CrossRef]
- Sessler, J.L.; Jayawickramarajah, J.; Gouloumis, A.; Dan Pantos, G.; Torres, T.; Guldi, D.M. Guanosine and Fullerene Derived De-Aggregation of a New Phthalocyanine-Linked Cytidine Derivative. Tetrahedron 2006, 62, 2123–2131. [Google Scholar] [CrossRef]
- Das, B.; Tokunaga, E.; Tanaka, M.; Sasaki, T.; Shibata, N. Perfluoroisopropyl Zinc Phthalocyanines Conjugated with Deoxyribonucleosides: Synthesis, Photophysical Properties and in Vitro Photodynamic Activities. Eur. J. Org. Chem. 2010, 2878–2884. [Google Scholar] [CrossRef]
- Ogbodu, R.O.; Limson, J.L.; Prinsloo, E.; Nyokong, T. Photophysical Properties and Photodynamic Therapy Effect of Zinc Phthalocyanine-Spermine-Single Walled Carbon Nanotube Conjugate on MCF-7 Breast Cancer Cell Line. Synth. Met. 2015, 204, 122–132. [Google Scholar] [CrossRef]
- Tolbin, A.Y.; Sukhorukov, A.Y.; Ioffe, S.L.; Lobach, O.A.; Nosik, D.N.; Tomilova, L.G. Synthesis of a Phthalocyanine-1,4,6,10-Tetraazaadamantane Conjugate and Its Activity against the Human Immunodeficiency Virus. Mendeleev Commun. 2010, 20, 25–27. [Google Scholar] [CrossRef]
| Oligoethylene glycol (OEG) | 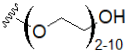 | Polyethylene glycol (PEG) | 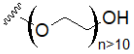 |
|---|---|---|---|
| Tyramine | 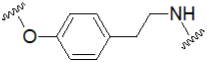 | Peptides (cleavable/non-cleavable) | Thr-Ser-Gly-Pro |
| Hydrazone (pH-sensitive linker) | 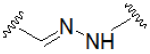 | Triazole | 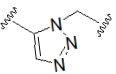 |
| Alkyl chain |  | Aryl-alkyl linker | 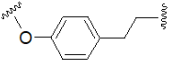 |
| Thiourea | 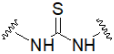 | Aminoacrylate | 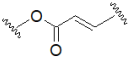 |
| Cyclopentadiene | 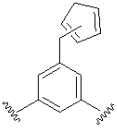 | ||
| Thioketal (ROS-cleavable) | 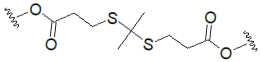 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chornovolenko, K.; Koczorowski, T. Phthalocyanines Conjugated with Small Biologically Active Compounds for the Advanced Photodynamic Therapy: A Review. Molecules 2025, 30, 3297. https://doi.org/10.3390/molecules30153297
Chornovolenko K, Koczorowski T. Phthalocyanines Conjugated with Small Biologically Active Compounds for the Advanced Photodynamic Therapy: A Review. Molecules. 2025; 30(15):3297. https://doi.org/10.3390/molecules30153297
Chicago/Turabian StyleChornovolenko, Kyrylo, and Tomasz Koczorowski. 2025. "Phthalocyanines Conjugated with Small Biologically Active Compounds for the Advanced Photodynamic Therapy: A Review" Molecules 30, no. 15: 3297. https://doi.org/10.3390/molecules30153297
APA StyleChornovolenko, K., & Koczorowski, T. (2025). Phthalocyanines Conjugated with Small Biologically Active Compounds for the Advanced Photodynamic Therapy: A Review. Molecules, 30(15), 3297. https://doi.org/10.3390/molecules30153297







