Abstract
The resulting plant waste from R. idaeus, P. serotina, P. avium, and P. cerasus exhibits a complex chemical composition, depending on the variety from which it originates, with applications in multiple fields such as the food, pharmaceutical or dermato-cosmetic industry due to the presence of phytochemical compounds such as flavonoids, flavonols, tannins, cyanogenic glycosides, vitamins, aldehyde, and phenolic acids. The aim of this review was to summarize and analyze the most recent and significant data from literature on the importance of plant waste resulting from the pruning process of trees and shrubs, in the context of applying circular economy principles, with a focus on the pharmacological importance (antimicrobial, antioxidant, anti-inflammatory, anticoagulant, antiviral, and antitumoral) of some bioactive compounds identified in these species. Their applicability in various industries is closely linked to both the bioavailability of the final products and the study of their toxicity. The literature indicates that the isolation of these compounds can be carried out using conventional or modern methods, the last ones being favored due to the increased efficiency of the processes, as well as from the perspective of environmental protection. This review increases the attention and perspective of using plant waste as a linked source of pharmaceutical and dermato-cosmetic agents.
1. Introduction
The concept of the circular economy can be effectively applied in the management of plant waste by valorizing by-products in alternative industrial processes [1]. Specialized literature highlights that the plant waste resulting from orchard maintenance work can be utilized by mulching the land, thus supporting the principle of the circular economy, and, in addition to the benefit of ecological fertilization, financial sustainability is also added [2]. Although current technologies for their valorization are promising, their large-scale applicability is still limited and requires further studies on the technical-economic viability of the methods [3].
Additionally, there are other methods for valorizing these wastes, but not all of them have a minimally invasive or even positive impact on the environment. For example, the use of woody biomass as an energy source has a negative ecological impact on the environment, highlighting the need to research more ecological and efficient alternatives for its valorization [4].
Gas emissions and fine particles resulting from combustion contribute to global warming, but human health is also impacted by air pollution [2,5]. A study from the USA highlighted that emissions from the bioenergy sector contribute a maximum of 17% to total emissions from all energy [6].
A viable method of valorization on cherry and sour cherry woody biomass resulting from orchard renewal is to use the wood to make barrels that are used in the food industry for aging distillates, wines, or vinegar [7]. In addition, in the food industry, the use of cherry wood for smoking cheeses, such as Cheddar, is notable [8], as well as in smoking pork preparations [9]. However, this preservation method can promote contamination of food with carcinogenic polycyclic aromatic hydrocarbons [10].
Recent studies suggest that plant waste can significantly contribute to the development of sustainable alternatives in various industries, even as a substitute for lignocellulos. For example, Öncül et al. demonstrated the viability of replacing filler materials with woody biomass of P. avium to obtain environmentally sustainable polymeric materials. The results showed that the biocomposite containing only 5% filler material exhibits optimal mechanical properties [11].
Cherry trees and raspberries, plants belonging to the Rosaceae family, have a distinct distribution: genus Prunus ssp. is found across all continents except Antarctica, and genus Rubus ssp. is mainly distributed throughout the temperate zone of the northern hemisphere. These species, easily adaptable to different environmental conditions (climate, soil, etc.), with a variable chemical composition depending on numerous factors such as cultivation techniques, region, ripening, harvesting, and storage, are well represented in the northeastern area of Romania [12].
Plant waste from species such as red raspberry (Rubus idaeus) and cherry trees (Prunus avium—sweet cherry, Prunus serotina—bitter black cherry, Prunus cerasus—sour cherry) exhibits significant pharmacological potential, representing a path for sustainable and ecological valorization. Red raspberry stems contain compounds with antioxidant, anti-inflammatory [13], and antitumoral [14] actions, making them attractive to the pharmaceutical industry. According to specialized literature, calcium and magnesium are the most representative minerals, in terms of quantity, found in red raspberry stems [15]. Additionally, the twigs of bitter black cherry and sweet cherry are rich in phytochemical compounds [16] with beneficial effects on health, including antioxidant and anti-inflammatory activities [17,18]. These plant wastes can be transformed into valuable ingredients in the pharmaceutical and cosmetic industries, having applicability in medical treatments as well as in the development of new products [19].
Up to now, all the results suggest that Rubus and Prunus wastes possess significant potential for further research to generate value-added products which can be applied in multiple industries, offering both economic and health benefits.
However, there is a need for more in-depth research to fully understand the potential of these resources and to optimize the extraction and utilization process of bioactive compounds.
This review summarizes the research progress related to the bioactive compound content of certain Rubus and Prunus species, their extraction and isolation, as well as information regarding their pharmacological applicability and limitations. Also, aspects presented in the specialized literature regarding the structure–activity relationship of bioactive compounds and the synergistic effect they can have are highlighted, which could open the way to new applications.
2. Research Methodology
The selection of specialized literature was carried out with particular care, applying clear criteria to guarantee both thematic relevance and the scientific quality of the sources used in the following databases: PubMed, Science Direct, and SpringerLink.
Only those studies that specifically explored the chemical composition, the possibilities of valorization, and the practical uses of plant waste from the four investigated species were included in the analysis, with a special emphasis on twigs and shoots—plant residues frequently resulting from maintenance or harvesting activities. In addition, priority was given to works that provided detailed characterizations of the plant material and/or proposed solutions applicable in various fields, such as the pharmaceutical, food, cosmetic, or agricultural industries.
The initial documentation generated a considerable volume of information, exceeding 50,000 scientific articles. A large part of this resulted from the combination of different search terms such as:
- the expression “Rubus idaeus + shoots + bioactive compounds” produced over 1570 relevant results;
- the search “Prunus avium + twigs + bioactive compounds” provided approximately 2400 studies;
- “Prunus serotina + twigs + bioactive compounds” led to the identification of 540 articles;
- and the formula “Prunus cerasus + twigs + bioactive compounds” returned more than 1000 works.
More general terms, such as plant waste, twigs, biomass, or extraction of phenolic compounds, generated a very large number of results (over 26,000 for prunus woody biomass and over 16,000 for Rubus woody biomass). However, only those sources that were directly related to the species analyzed were retained for evaluation.
The narrowing of the search period was determined by the relatively recent interest of researchers in the sustainable valorization of plant waste. Also, the desire to present the latest experimental observations regarding the extraction and isolation of bioactive compounds, which are also viable from an ecological point of view, was also the basis for limiting the search interval.
Considering the graph presented in Figure 1, there is an upward trend in recent years in the interest shown by specialists in the field for the four previously mentioned species.
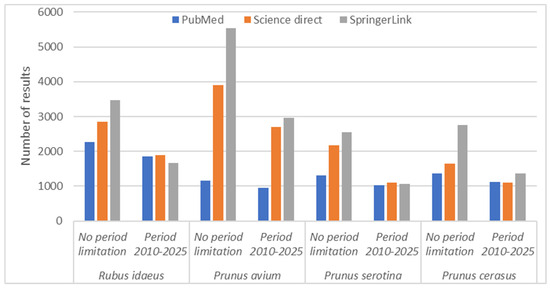
Figure 1.
The distribution of articles by species published between 2010 and 2025.
After applying all the selection filters-which targeted the relevance of the subject, methodological clarity, applicative value, and the elimination of redundancies-over 300 scientific sources were finally retained, with less than 10% of these published before 2010. This rigorous approach was the basis for a well-founded synthesis, which highlights the real potential for valorization of twigs and shoots from the R. idaeus, P. avium, P. serotina, and P. cerasus species, contributing to the promotion of sustainable practices and the consolidation of the circular economy in the agricultural and industrial sectors.
3. Bioactive Compounds from Plant Waste of Rubus idaeus, Prunus serotina, Prunus avium, and Prunus cerasus
From both an ecological and economic perspective, plant waste, a diverse mixture of woody and vegetable by-products generated mainly through deforestation and landscape maintenance, orchards, etc., requires appropriate management to transform it into a valuable and sustainable resource.
It is worth noting that woody biomass, a heterogeneous material, which is mainly considered a source of three natural polymers (cellulose, hemicelluloses and lignin), also contains an extractable fraction, which, although minor (<9%), includes important classes of compounds with potential biological activity (terpenoids, phenolic compounds, tannins, glycosides, vitamins, fatty acids, minerals, etc.).
A recent study by Newman and Cragg [20] on the impact of natural substances on pharmaceutical products introduced on the market between 1981 and 2019 highlights that over 37% of the 1881 molecules marketed during this period are based on natural sources: 71 are strictly natural, 356 are derivatives of natural substances obtained by semi-synthesis, and 272 are obtained by organic synthesis, having a pharmacophore inspired by natural substances. These data confirm the importance of focusing on the efficient valorization of the vegetative parts of Rubus and Prunus as sources of bioactive compounds (Figure 2).
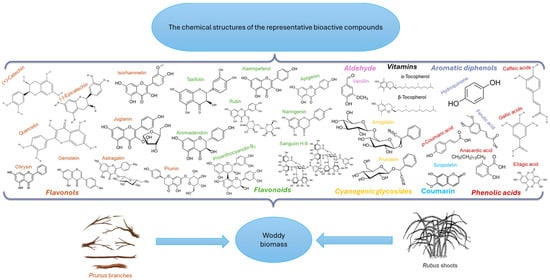
Figure 2.
The chemical structures of the representative bioactive compounds from Prunus and Rubus woody biomass with health benefits.
The studied literature showed that there is a relative similarity in the chemical composition of the three Prunus species, but specific bioactive compounds have also been identified, such as juglanin [17] and prunasin [19] in P. serotina, taxifolin and vanillin [7] in P. avium, and phlorizin [21] and galangin [22] in P. cerasus. On the other hand, R. idaeus stands out due to its content of sanguiin H6 [23] and casuarinin [24]. Among the biologically active chemical compounds identified in all plant sources are caffeic acid and p-coumaric acid, among others [25,26,27].
In the following table, several bioactive compounds found in the four plant sources are presented, for which concentrations have also been identified in the specialized literature.
According to the data presented in Table 1, it is observed that R. idaeus exhibits a higher concentration of bioactive compounds common with Prunus species, but there are also exceptions, such as chlorogenic and p-coumaric acids, which are found in higher concentrations in P. avium, as well as ferulic acid, which has a higher concentration in P. serotina.

Table 1.
Representative classes/bioactive compounds found in different plant parts of R. idaeus, P. serotina, P. avium, and P. cerasus.
The polyphenol concentration was investigated in 11 varieties of R. idaeus; the presence of pentoside quercetin was identified in a single variety, with a concentration of 23.9 g/100 g dry shoot sample, while the compound sanguiin was identified in 10 out of 11 varieties [23]. The rarity of quercetin pentoside may be justified by different climatic growing conditions or the earlier character of the variety. A specialized study aimed at identifying the impact of covering fruit trees with anti-hail netting on the phytochemical profile revealed that direct exposure to sunlight can lead to a lower content of quercetin pentoside compared to the content when the crop is covered [33]. The stems are notable for their content of chlorogenic acid and proanthocyanidin B1, but smaller amounts of catechin, salicylic acid, and astragalin are also present [15]. The dominant component in the leaf extract of R. idaeus is casuarinin, which constitutes 83% of all identified polyphenolic compounds in the extract [24]. Although the studies consulted did not explicitly highlight the presence of casuarinine in the extracts, it was noted that ellagitannins, a class to which casuarinine also belongs, are found in high concentrations in the leaf extract [34].
In contrast to Rubus, Prunus species show divergent trends in phenolic distribution. The total phenolic content, expressed in mg gallic acid/g dry sample, is significantly higher in P. avium branches (301.98) compared to leaves (100.71) or flowers (81.2). On the other hand, the flavonoid content is lower in branch samples, whether it is hydroethanolic extract or infusion [28].
Among the organic acids mentioned by various studies [31,35], the significant presence, both in fruits and in plant waste, of malic and quinic acids is noteworthy, while tartaric, citric, succinic, or oxalic acids showed a marginal content.
In a study that includes a comparative chemical analysis of the fruits and branches of P. avium, it was highlighted that the amount of oxalic acid in the branches is double that of the content in cherries, and the citric acid content is approximately 35 times higher in the branches. Significant differences were also identified in the tocopherol content, with γ-tocopherol being identified only in the branches [31].
Wojdyło et al. [35] demonstrated through the analysis of extracts obtained from fruits and leaves of P. avium and P. cerasus that there is a higher concentration of polyphenolic compounds in leaves compared to fruits, regardless of the analyzed variety. However, it is worth mentioning that anthocyanins were identified only in fruit extracts.
The polyphenolic compounds in the leaves were quite diverse; therefore, it can be considered that the leaves may represent an alternative, unconventional, and cheap source of antioxidants and preservatives, with various uses in the pharmaceutical, cosmetic, and food industries [35].
Overall, these results (Table 1) clearly indicate that the accumulation of bioactive compounds is closely dependent on the plant part analyzed.
Also, the differences between the results reported in various studies regarding the presence/absence of some compounds and their quantity could be explained by the stage of development of the analyzed parts, as well as by the different applied extraction/separation and identification methodologies.
4. Extraction and Isolation Methods
4.1. Extraction
The methods for extracting bioactive compounds from the woody biomass of various trees and shrubs are extremely diverse. The literature highlights that a number of solvents used for the extraction of compounds from different parts of berry plants are also effective for red raspberries: acidified ethanol–water extraction to obtain anthocyanidins, procyanidins, or caffeoylquinic acids [36]; methanolic extraction to obtain polyphenolic compounds and flavonoids; acetone–water mixture extraction to isolate tannins and methanol-hydrochloric acid solution for anthocyanins [37]. Considering that some extracts may have applicability in the food industry, the use of solvents such as methanol is not safe; therefore, the adoption of green technologies, such as subcritical water extraction, is necessary [38].
Regarding the extraction of bioactive compounds from cherry branches, a study comparing conventional extraction with ethanol (70 °C) to accelerated extraction (150 °C), also using ethanol as a solvent, concluded that the extraction temperature affects the quantity of bioactive compounds obtained more than the percentage of ethanol used. [39].
On the other hand, in light of the use of green extraction technologies for cherry plant parts, data from the literature has noted the use of supercritical carbon dioxide extraction [40], a technique that can also be applied to cherry woody biomass [41] or other plant samples [42].
To optimize this method, one can also resort to coupling it with microwave-assisted extraction. The application of the two combined techniques led to the optimization of extraction under the following conditions: particle size of approximately 0.3–0.4 mm, liquid–solid ratio of 54 mL/g, and extraction time of 30 min [43].
Table 2 presents the representative extraction methods applied to the four plant sources studied.

Table 2.
Extraction methods of bioactive compounds, applied to different types of plant waste from Rubus and Prunus species.
According to the data presented in the previous table, it is observed that, at least in the case of phenolic compounds, for all four plant sources, the extraction using ethanol or methanol as a solvent is among the most commonly used, also having satisfactory yields. Even if the extraction process can be optimized by using modern green methods, the presence of the alcoholic solvent could ensure performance regardless of the nature of the plant source.
The content of ellagic acid obtained in the extract from aerial parts of R. idaeus varies depending on the method used, as follows: 3.24 mg/g extract for decoction (solvent: water), 3.12 mg/g extract for infusion (solvent: water), 2.45 mg/g extract for maceration (hydroalcoholic solvent), and 2.38 mg/g extract for ultrasound-assisted extraction (hydroalcoholic solvent) [47]. The same study mentions that for phenol extraction, ultrasound-assisted extraction is more efficient, while for flavonoids, infusion and maceration are recommended. Knowing that the solubility of ellagic acid in water and alcohol is influenced by temperature, probably, these results obtained, better in the case of extraction by decoction and infusion, are due to the different working conditions: the high temperature of the decoction and infusion versus UAE in an ice bath and maceration at ambient temperature, respectively. Among the natural sources of ellagic acid, some studies mention residues from the wood industry as an attractive alternative in terms of commercial exploitation. But at the same time, it is emphasized that it is necessary to find an ecological extraction methodology, knowing that currently the production of commercial ellagic acid from natural sources is based on obtaining extracts from plants rich in ellagitannins using acid–methanol mixtures as solvents, followed by their hydrolysis in a strongly acidic environment (HCl or concentrated H2SO4) [54].
A study conducted in 2021 by Brozdowski et al. demonstrated that in the case of phenolic compounds extracted from dried cherry leaves/flowers (P. serotina), the content was significantly higher when methanol was used as the solvent, compared to aqueous extraction [27]. Ademović et al. showed that the use of ethanol as a solvent led to an increase in the total content of phenols and flavonoids compared to the aqueous extraction of dried P. avium branches, achieving concentrations 3 and 4 times higher in the first case, respectively [18]. Taking into account the field in which these extracts are intended to be utilized, even if the extraction yield when using methanol or alcohol has proven to be more efficient than an aqueous medium, it is desirable to use eco-friendly solvents.
Regarding the chemical composition of the branches of P. avium and P. cerasus, there are no significant differences in the case of extracts obtained with subcritical water, which also supports the relatively similar biological activity [55].
4.2. Isolation
The main techniques for refining and fractionating crude extracts in order to isolate one or more groups of bioactive molecules found in Rubus and Prunus species are presented in Table 3.

Table 3.
Isolation methods applicable to some representative bioactive compounds.
The data presented in the previous table highlight chromatographic methods as the most frequently used in the isolation of the bioactive compounds targeted in this study. Additionally, their lack of or low affinity for water is highlighted, and thermolabile or thermostable compounds appear in the isolate (Table 3). The literature has highlighted the fact that aglycone flavonoids degrade faster under the influence of temperature, compared to glycosylated ones [92]. It is important to mention the concern for a reproducible isolation and the application of specific structural confirmation methods (1H NMR, 13C NMR, HMBC, HSQC, COSY, NOESY, MS, UV absorption spectroscopy, etc.), robust and accurate to preserve the quality and activity of the phytocompounds and the extracts obtained.
5. Biological Activity
5.1. Pharmacological Importance, Bioavailability, and Toxicity
The biological activity of red raspberry, sweet cherry, sour cherry, and black cherry extracts can be determined both by the presence of common bioactive compounds identified in the four plant sources described previously, as well as by those specific to each species. Table 4 summarizes the information from the literature regarding their pharmacological activity, bioavailability, and toxicity.

Table 4.
Correlation of bioactive compound–pharmacological activity, bioavailability, and toxicity [17,65,67,70,75,78,87,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124,125,126,127,128,129,130,131,132,133,134,135,136,137,138,139,140,141,142,143,144,145,146,147,148,149,150,151,152,153,154,155,156,157,158,159,160,161,162,163,164,165,166,167,168,169,170,171,172,173,174,175,176,177,178,179,180,181,182,183,184,185,186,187,188,189,190,191,192,193,194,195,196,197,198,199,200,201,202,203,204,205,206,207,208,209,210,211,212,213,214,215,216,217,218,219,220,221,222,223,224,225,226,227,228,229,230,231,232,233,234,235,236,237,238,239,240,241,242,243,244,245,246,247,248,249,250].
As highlighted in the previous table, the bioactive compounds from the species R. idaeus, P. serotina, P. avium, and P. cerasus exhibit multiple bioactive activities in the medical or dermato-cosmetic field. Their applicability in skincare products is highlighted by the antioxidant capacity exhibited by many compounds, but they also display more complex activities, such as anticancer or neuroprotective effects (Figure 3).
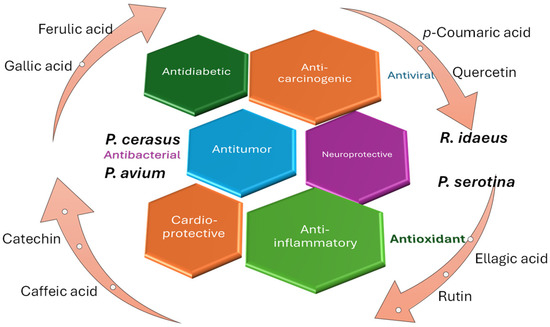
Figure 3.
Pharmacological and pro-health importance of several common compounds identified in Rubus and Prunus species.
For a product containing bioactive compounds to be declared effective, it is necessary to know both its bioavailability and its toxicity [251].
Despite the biological activities possessed by the compounds identified in the Rubus and Prunus species, some exhibit toxicity to the human body, as can be seen in Table 4. This aspect of toxicity necessitates a series of additional studies to fully understand the interactions between bioactive compounds, with the aim of identifying methods to mitigate or eliminate these adverse reactions, for the maximum enhancement of pharma-co-logical effects.
5.2. Synergistic Activity
Compounds such as salicylic acid and astragalin from R. idaeus [15]; juglanin and isorhamnetin from P. serotina [17,139]; taxifolin, chrysin, and vanillin from P. avium [7,22]; phloridzin and rutin from P. cerasus [21,29] could exhibit synergistic action, which would enhance the individual pharmacological activities.
Interest in synergistic actions between bioactive substances has increased in recent years due to recent paradigm shifts. In this context, Table 5 presents several synergistic actions of the aforementioned bioactive compounds, based on bibliographic references consulted for this study.

Table 5.
Synergistic activity of some bioactive compounds.
These findings suggest the need to continue investigations on the possibility of using extracts obtained from the four sources, in different mixtures, in order to improve their biological activity (anticarcinogenic, anti-inflammatory, antioxidant, etc.), to enhance the effect of certain medications or medical procedures, and/or minimize the adverse effects associated with the treatments.
5.3. Structure–Activity Relationship Study
Considering the multiple biological activities of the compounds present in the four investigated sources, data from the literature regarding the structure-activity relationship were taken into account, which can help to understand how the chemical structures of these molecules influence their biological activities.
5.3.1. Phenolic Compounds
Phenolic compounds exhibit antioxidant activity, among other things, which is determined by their ability to donate hydrogen atoms or electrons, but it can also be attributed to the possibility of metal chelation [269]. Additionally, studies in the field have shown that the antioxidant activity of phenols is influenced by the presence and position of the hydroxyl group [269,270].
Hydroxybenzoic acids exhibit a closer relationship between their antibacterial activity and hydrophobicity compared to hydroxycinnamic acids [271]. It is worth mentioning that this activity is influenced by the presence of hydroxyl groups, as well as by that of double bonds [272].
Antioxidant capacity assessment (ORAC assay) showed that extracts rich in polyphenolic and flavanol compounds, such as those from P. cerasus leaves and fruits, compared to those from P. avium, are useful for both cosmetic and pharmaceutical use [35].
- Simple Phenols
- Hydroquinone, in the phenolic structural form, called 1,4-dihydroxybenzene, due to the presence of the hydroxyl group in the para position, exhibits antioxidant activity [273].
- 2.
- Flavonoids
Some flavonoids have the ability to inhibit monoamine oxidase, an enzyme responsible for the onset of certain neurological disorders. An experimental study demonstrated that this inhibitory activity exhibits an ascending character for the following series of flavonoids: apigenin, luteolin, quercetin, aromadendrin, and taxifolin [274].
The presence of hydroxyl groups in the structure of flavonoids influences their antidepressant activity, specifically, the groups in positions 2 and 4 support this activity [275]. Additionally, the number of groups is particularly important; proanthocyanidins, which are polymerized monomers and therefore contain more hydroxyl groups, exhibit higher antioxidant activity, whereas the glycosylation of flavonoids reduces this activity [276]. On the other hand, methoxy or glycosidic groups attached to the flavonoid structure reduce their antioxidant activity [277].
- Quercetin, named 2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxy-4H-chromen-4-one [278], whose basic structure is represented by two phenyl groups connected by three carbon atoms, can be arranged in an open form or in the form of a heterocyclic ring [279]. The antioxidant activity of this compound is determined by the presence of the hydroxyl group, which is why some quercetin derivatives exhibit lower activity. On the other hand, obtaining methylated derivatives can lead to an increase in anti-inflammatory activity, and through glycosylation reactions, compounds with higher bioavailability regarding the antiobesity effect can be obtained [280]. Of the five hydroxyl groups in the structure of quercetin, only those at positions 3, 3′, and 4′ are responsible for the antioxidant activity of this compound, also being involved in its photolability [84]. The presence of the double bond in the heterocyclic ring of quercetin determines the manner in which this compound binds to DNA, by fitting into the helix of deoxyribonucleic acid, compared to naringenin, which does not have that double bond and exhibits a groove-type DNA binding [281]. The inhibitory activity on lipase is influenced by the structure of flavonoids as follows: it decreases through the hydrogenation of the double bond in the C ring, specifically through the glycosylation reaction, and increases with the presence of the carbonyl group or the hydroxylation reaction. Quercetin, due to its chemical structure, exhibits this activity, but it is lower than that of luteolin [282].
- Astragalin is also known as kaempferol 3-O-β-d-glucopyranoside. The substitution of phenolic hydroxyl groups influences the anti-inflammatory activity of astragalin, having a stronger effect than chrysin or luteolin [115].
- Rutin, known as 3′,4′,5,7-tetrahydroxyflavone-3-rutinoside or quercetin-3-rutinoside, is a flavonoid glycoside formed from quercetin and rutin [87]. The antioxidant activity of rutin can be enhanced by complexation with cyclodextrin [283]. On the other hand, glycosylation of this compound leads to an increase in antioxidant, antibacterial, and α-glucosidase inhibitory activities [88].
- Aromadendrin contains four hydroxyl groups in its structure and is also called (2R,3R)-3,5,7-trihydroxy-2-(4-hydroxyphenyl)-2,3-dihydrochromen-4-one. This compound exhibits multiple pharmacological activities, but in the case of antidiabetic and anticancer actions, the 7-O methylated derivative stands out, while methylation at the 4′-O position is noted to be effective for antiulcer activity [157].
- Juglanin (kaempferol 3-O-α-L-arabinofuranoside) contains multiple hydroxyl groups in its structure. This compound exhibits a lower antiradical effect compared to quercetin, the scientific justification being the presence of a single hydroxyl group on ring B [17].
- Kaempferol, also named 3,5,7-trihydroxy-2-(4-hydroxyphenyl)-4H-1-benzopyran-4-one, has a diphenylpropane structure and can be obtained through a series of reactions applied to naringenin [134,135]. In the study conducted by Rho et al., it was highlighted that depigmentation activity and cytotoxicity are enhanced by the presence of the hydroxyl group at position 3 [284].
- Isorhamnetin is a flavonoid, considered a methylated derivative of quercetin [261]. The methoxy group at position 3′ is associated with the antitumor activity of this compound [70].
- Prunin, a flavanone glycoside, is obtained following the hydrolysis process of naringenin. In the case of prunin laurate, a strong antibacterial activity against Porphyromonas gingivalis was shown by Wada et al. [285]. Additionally, in another study, when examining naringenin derivatives, it was highlighted that an aliphatic chain of 10–12 carbon atoms attached to ring A has the ability to enhance antimicrobial activity, with alkylprunin being an important representative [286].
- Apigenin or 4′,5,7–trihydroxyflavone, contains a 2-phenylchromen-4-one skeleton [63]. A study aimed at comparing the biological activity of apigenin and one of its derivatives, apigenin-7-O-glucoside, concluded that the presence of the sugar moiety in the derivative resulted in stronger antifungal activity against Candida albicans and Candida glabrata. Additionally, in vitro, the glycosidic derivative exhibits higher cytotoxic activity against cancer cells in the case of colon cancer, compared to apigenin [287].
- Chrysin (5,7-dihydroxyflavone) is a flavone that contains hydroxyl and keto functional groups [176]. The antioxidant activity of this compound is correlated with the lack of hydroxyl in rings B and C, as well as the presence of the carbonyl group on C4 and the double bond between C2 and C3 [288]. Liu et al. highlighted that halogenated derivatives exhibit stronger anticancer activity. Additionally, an enhancement of the effect was observed when the C7-OH of ring A was linked to various hydrophilic amines. Regarding the anti-inflammatory activity, a strong effect was demonstrated in the case of the derivative containing a cyclic pyridine at position 8 [289].
- Naringenin has two hydroxyl groups missing in its chemical structure compared to quercetin, which explains its lower antioxidant activity. Quercetin, on the other hand, has an antioxidant effect comparable to that of vitamin C, and the presence of two hydroxyl groups on ring C, instead of one as in the case of naringenin, leads to the formation of a stabilized quinone structure that contributes to enhancing the effect [281]. The antibacterial activity of naringenin is lower than that of other flavones that contain fewer hydroxyl groups; additionally, the position of these groups also influences the activity, so compounds that have hydroxyl groups in ring A but not in ring B exhibit significant activity. Methylation of hydroxyl groups may contribute to the reduction of the antibacterial effect [290].
- Taxifolin exhibits inhibitory activity against certain protein structures, such as amyloid fibrils, which have been highlighted in the literature as being responsible for the onset of Alzheimer’s disease. This inhibitory activity is due to the presence of the catechol group in ring B [291].
- Catechin is a flavan-3-ol, also named (2R,3S)-2-(3,4-dihydroxyphenyl)-3,4-dihydro-2H-chromene-3,5,7-triol. The position and number of hydroxyl groups influence the antibacterial activity of catechins. Additionally, the polymerization of catechin molecules enhances activity, as is the case with theaflavins [292]. The antioxidant activity is correlated with the presence of the hydroxyl group in position 3 [293].
- Genistein (4′,5,7-trihydroxyisoflavone) is a phytoestrogen that can be synthesized from naringenin in plants. It exhibits characteristics similar to those of the estrogen estradiol-17β, due to structural similarities consisting of the presence of the phenolic ring and the distance between the hydroxyl groups [294].
- Phlorizin, phloretin 2′-β-D-glucoside, according to Li et al., exhibits lower antioxidant activity than the parent compound because the glycosylation reaction reduces the number of phenolic hydroxyl groups [295].
Guerrero et al. investigated several flavonoids in terms of their inhibitory activity on the angiotensin-converting enzyme and highlighted the fact that at the structural level, there are some key elements underlying this activity, such as the presence of the catechol group at the B ring, the ketone group found at C4 in the C ring, and the double bond be-tween C2 and C3 of the C ring. It was concluded that this inhibitory activity has an upward trend for the following compounds:
- -
- at a concentration of 500 µM: hesperetin, genistein, epicatechin, naringenin, apigenin, kaempferol, quercetin, and rutin;
- -
- at a concentration of 100 µM: quercetin, rutin, kaempferol, and luteolin.
It should be mentioned that the highest activity was recorded at the minimum concentration [296].
Zhang et al. investigated the structure–activity relationship of some flavonoids responsible for inhibiting a breast cancer-resistant protein and concluded that, in this case as well, the double bond between C2 and C3 of ring C influences this interaction. Additionally, the importance of the B ring position, hydroxylation at position 5, and the absence of the hydroxyl group at position 3, as well as hydrophobic substitution at positions 6, 7, 8, or 4′, were mentioned as elements responsible for this protein–flavonoid interaction [297].
- 3.
- Tannins
- Proanthocyanidins contain significant monomeric units of flavan-3-ol, such as epicatechin or catechin, and are also referred to as condensed tannins. According to studies, these monomeric units influence biological activity, such as antioxidant or antidiabetic activity [247,298].
- Sanguiin H6, an ellagitannin derived from ellagic acid, has multiple biological activities that are influenced by the presence of hydroxyl groups and the galloyl configuration [299].
- 4.
- Phenolic Acids
A carboxyl group derived from an acid and at least one hydroxyl group that replaces hydrogen atoms in the benzene rings gives rise to phenolic acids [298].
The antioxidant activity of hydroxycinnamic acids is directly positively influenced by the presence of a double bond in the side chain, the number and position of hydroxyl groups on the aromatic ring, the esterification or amidation of the carboxyl group, as well as the presence of the catechol group [299].
- 3,4-Dihydroxycinnamic acid exhibits hepatoprotective activity that can be enhanced through methoxylation at positions 3 or 4 [300]. The esterification gives rise to derivatives that exhibit remarkable antileishmanial activity. Otero et al. highlighted in a study that the bioactivity of cinnamic acid derivatives depends on the degree of oxygenation at positions 3 and 4, the presence of a double bond in the side chain, and hydroxyl groups, as well as the length of the alkyl chain [301].
- Caffeic acid is a hydroxycinnamic acid that contains an aromatic ring and three hydroxyl groups, along with the double bond in the carbon chain, with anticancer activity [212]. Anilides and aliphatic amides of caffeic acid enhance its antioxidant activity [302]. The attachment of a naphthyl ring increases the capacity of caffeic acid to inhibit monoamine oxidase, an enzyme responsible for multiple neurological disorders [301,303].
- p-Coumaric acid, a phenolic acid, derived from cinnamic acid, is also known as 4-hydroxycinnamic acid [304]. Derivatives of p-coumaric acid exhibit higher antimicrobial activity, especially esters, anilides, and amides with bulky aromatic groups [305].
- Ferulic acid, also called 4-hydroxy-3-methoxycinnamic acid, is responsible for some biological activities [306]. The anticancer activity of some ferulic acid derivatives was investigated; thus, although some derivatives exhibit lower activity compared to caffeic acid derivatives, the phenylsulfonylfuroxan nitrates of ferulic acid stand out as having strong anticancer activity [307]. Ferulic acid, found in raspberry plant parts, has the ability to stabilize anthocyanins, but it is also recognized for its involvement in flavonoid catabolism, particularly in the spontaneous carboxylation of caffeic acid [308].
- Chlorogenic acid is derived from caffeic acid and quinic acid, and the hydroxyl groups present in its structure are responsible for the strong antioxidant effect it exhibits [58].
- Ellagic acid or 2,3,7,8-tetrahydroxy [1]-benzopyrano [5,4,3-cde] benzopyran-5,10-dione, structurally contains a hydrophilic part, represented by phenolic groups and lactone-type groups, as well as a lipophilic part represented by the four phenolic rings [50…60]. The anticancer activity of this compound is closely related to its chemical structure, specifically the presence of hydroxyl groups at positions 3 and 4, as well as the presence of lactone groups [309,310].
- Salicylic acid or 2-hydroxybenzoic acid is a plant hormone, being the main precursor of aspirin. From a structural perspective, it is notable for the ortho arrangement of the hydroxyl and carboxyl groups [311]. The inhibition of luciferase by salicylic acid is enhanced by the amidation of the carboxyl group or the substitution of chlorine at position 5 [312].
- Anacardic acid, a derivative of salicylic acid, has a side chain with different degrees of unsaturation, which is responsible for its varied biological activity. Regarding antioxidant activity, trienic anacardic acid (15:3) stands out, while for antifungal activity, monoenic anacardic acid (15:1) is highlighted [313]. The biological activity of anacardic acid is closely related to the structure of the side chain; thus, the presence of the trienic alkyl side chain determines a strong bactericidal activity against Streptococcus mutans and Staphylococcus aureus, while the saturated alkyl chain acts against Propionibacterium acnes. The antioxidant activity is synergistically influenced by the length of the alkyl chain, the presence of the salicylic acid moiety, as well as the stereochemistry of the side chain [56]. Some researchers have noted that the anticancer activity of anacardic acid largely depends on the molecular volume of the hydrophobic side chain, in addition to its metal-chelating ability and its action as a surfactant [93].
5.3.2. Coumarins
- Scopoletin, 6-methoxy-7-hydroxycoumarin, is characterized by the presence of a single hydroxyl group, a methoxy group, and a keto group [210]. Liu et al. demonstrated that derivatives containing a Δ3,4 olefinic bond, as well as naphthyl or phenyl groups with a sulfate ester at the C7 position, enhance insecticidal activity against Tetranychus cinnabarinus and Artemia salina, respectively [314].
5.3.3. Cyanogenic Glycosides
- Prunasin, the glucoside of (R)-mandelonitrile, can be glycosylated with the formation of amygdalin, and it can be converted into mandelonitrile by α-glucosidase or a hydrolase, and subsequently hydrolyzed into benzaldehyde and hydrocyanic acid [315].
5.3.4. Aldehyde
- Vanillin is an important flavor molecule, being named 4-hydroxy-3-methoxybenzaldehyde, and constitutes the major component of vanilla [316]. The aldehyde group in the structure of vanillin, as well as the position of the side group on the benzene ring, supports the antifungal activity exhibited by this compound [317]. Furthermore, this compound also exhibits antioxidant activity, stronger than that of ascorbic acid, justified by its self-dimerization in contact with free radicals [318].
5.3.5. Terpenoid
- Squalene is a precursor of cholesterol, and not only a triterpene that contains 30 carbon atoms in its structure. In the synthesis of cholesterol, the process was initially proposed to be described as a cyclization of squalene to lanosterol; later, it was demonstrated that it oxidizes to form monooxidosqualene before cyclization [319]. It exhibits a high detoxification capacity due to its ability to attach to uncharged substances, owing to its nonpolarity [320].
5.3.6. Vitamins
- Ascorbic acid, better known as vitamin C, is a compound with multiple bioactive activities, including antioxidant activity. This activity is justified on one hand by the acid’s ability to donate single hydrogen atoms, and on the other hand by the interaction between radicals and the monodehydroascorbate anion [321]. It is also worth mentioning the importance of vitamin C in collagen synthesis, a compound extremely important for human health, as well as in the fixation of vitamin E or iron [322]. The structure of lactone, with two ionizable hydroxyl groups, makes this compound an excellent reducing agent. It oxidizes successively, forming ascorbate radical and then dehydroascorbic acid, a mechanism that underlies many biological activities [323].
- Tocopherol, belonging to the vitamin E family, has a chemical structure that contains a polar chromanol ring and a lipophilic phytyl chain, and its antioxidant activity is justified by its ability to form tocopherol quinone [324]. Just like in the case of vitamin C, the presence of hydroxyl groups in the chemical structure of tocopherols, which act as hydrogen donors for peroxyl radicals, reveals other biological activities, such as cellular signaling properties [325].
Research in the field has highlighted the fact that Rubus and Prunus plant waste can be used to obtain extracts with broad applicability in the medical field. The antioxidant, antimicrobial, and anti-inflammatory actions have been explored both in vitro and in vivo. It is worth noting that the identified studies, both in vitro and in vivo, mainly reported on the leaf extract [326,327].
The reviewed literature underscores the importance of woody biomass of Prunus and Rubus species as a source of bioactive compounds with multiple pharmacological and cosmetic applications [328].
6. Conclusions
Plant waste, including red raspberry stems and cherry twigs, presents significant potential for the development of a sustainable valorization model through the lens of bio-active compound content. They are predominantly applicable in the pharmaceutical and cosmetic industries, thus contributing both to the reduction of industrial waste and to the enhancement of the sustainability of economic processes.
In this analysis, recent data from the specialized literature on the biological activity of the main compounds identified in the species R. idaeus, P. serotina, P. avium and P. cerasus are presented, emphasizing the following aspects:
- -
- presentation of the bioactive compounds representative of these species and highlighting their extraction and isolation methodology;
- -
- correlation between biological activity and their chemical structure, with emphasis on the possible synergistic action of some compounds common to the four species.
The diversity of available extraction technologies is noteworthy, from conventional methods such as ethanol extraction, to more environmentally friendly solutions such as subcritical water extraction and supercritical CO2 extraction that allow the obtaining of bioactive compounds with high yields.
In addition, an affinity for chromatographic methods for the isolation and purification of target compounds was observed, a fact justified both by the complexity of the chemical composition of the plant matrix and by the need to obtain high-purity products in accordance with the requirements of the pharmaceutical and cosmetic industries.
These aspects represent a starting point for in-depth research into the four plant sources rich in active phytochemical compounds, which can become a valuable source for the manufacture of pharmaceutical, cosmetic, and even food products.
7. Future Perspectives
In order to maximize the potential for valorization of plant waste, it is essential to continue exploring the correlation between the structure and biological activity, the mechanism of action, the bioavailability, and the potential toxicity of the identified compounds. It is also important to develop and improve technologies that increase the separation efficiency of bioactive compounds, applicable on an industrial scale and with low environmental impact.
In addition to these challenges, further developments are needed to increase the adoption of waste recovery technologies, economic feasibility, market potential, and policy incentives to support the superior recovery of plant waste.
Author Contributions
All the authors have equal contributions to this study.
Funding
The work was partially supported by the School of Doctoral Studies of “Vasile Alecsandri” University of Bacău through the Project SSD-0153–2025.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Brás, I.; Silva, E.; Raimondo, R.; Saetta, R.; Mignano, V.; Fabbricino, M.; Ferreira, J. Valorisation of forest and agriculture residual biomass—The application of life cycle assessment to analyse composting, mulching, and energetic valorisation strategies. Sustainability 2024, 16, 630. [Google Scholar] [CrossRef]
- Rousou, I.S. Importance of Reusing Wood from Pruning and Promotion of Circular Economy Principles in Agricultural Sector in Tripolis, Greece. Master Thesis, Agricultural University of Athens, Athens, Greece, 2025. Available online: http://hdl.handle.net/10329/8412 (accessed on 23 July 2025).
- Sanoja-López, K.A.; Guamán-Marquines, C.W.; Luque, R. Advanced processes in biomass/waste valorization: A Review. Sustain. Chem. Pharm. 2024, 41, 101704. [Google Scholar] [CrossRef]
- Syrodoy, S.V.; Yu, M.D.; Nigay, N.A.; Purin, M.V. Influence of the type of woody biomass on energy and environmental characteristics of the thermal preparation processes and ignition of bio-water-coal fuel particles. Process Saf. Environ. Protect. 2024, 184, 736–746. [Google Scholar] [CrossRef]
- Tomlin, A.S. Air quality and climate impacts of biomass use as an energy source: A review. Energy Fuels 2021, 35, 14213–14240. [Google Scholar] [CrossRef]
- Tran, H.; Jino, E.; Arunachalam, S. Emissions of wood pelletization and bioenergy use in the United States. Renew. Energy 2023, 219, 119536. [Google Scholar] [CrossRef]
- Aliaño-González, M.J.; Gabaston, J.; Ortiz-Somovilla, V.; Cantos-Villar, E. Wood waste from fruit trees: Biomolecules and their applications in agri-food industry. Biomolecules 2022, 12, 238. [Google Scholar] [CrossRef]
- Del Toro-Gipson, R.S.; Rizzo, P.V.; Hanson, D.J.; Drake, M. Sensory characterization of specific wood smoke aromas and their contributions to smoked Cheddar cheese flavor. J. Sens. Stud. 2020, 35, e12564. [Google Scholar] [CrossRef]
- Swaney-Stueve, M.; Talavera, M.; Jepsen, T.; Severns, B.; Wise, R.; Deubler, G. Sensory and consumer evaluation of smoked pulled pork prepared using different smokers and different types of wood. J. Food Sci. 2019, 84, 640–649. [Google Scholar] [CrossRef]
- Racovita, R.C.; Secuianu, C.; Ciuca, M.D.; Israel-Roming, F. Effects of smoking temperature, smoking time, and type of wood sawdust on polycyclic aromatic hydrocarbon accumulation levels in directly smoked pork sausages. J. Agric. Food Chem. 2020, 68, 9530–9536. [Google Scholar] [CrossRef]
- Öncül, M.; Atagür, M.; Atan, E.; Sever, K. A preliminary evaluation of bing cherry tree (Prunus avium L.) pruning waste as an alternative lignocellulosic filler for lightweight composite material applications. Polym. Compos. 2025, 46, 3655–3667. [Google Scholar] [CrossRef]
- Memete, A.R.; Sărac, I.; Teusdea, A.C.; Budău, R.; Bei, M.; Vicas, S.I. Bioactive compounds and antioxidant capacity of several blackberry (Rubus spp.) fruits cultivars grown in Romania. Horticulturae 2023, 9, 556. [Google Scholar] [CrossRef]
- Buczyński, K.; Kapłan, M.; Jarosz, Z. Review of the report on the nutritional and health-promoting values of species of the Rubus L. genus. Agriculture 2024, 14, 1324. [Google Scholar] [CrossRef]
- Azzini, E.; Barnaba, L.; Mattera, M.; Calina, D.; Sharifi-Rad, J.; Cho, W.C. Updated evidence on raspberries as functional foods: Anticancer bioactivity and therapeutic implications. Food Front. 2024, 5, 2351–2382. [Google Scholar] [CrossRef]
- Ispiryan, A.; Viškelis, J.; Viškelis, P.; Urbonavičienė, D.; Raudonė, L. Biochemical and antioxidant profiling of raspberry plant parts for sustainable processing. Plants 2023, 12, 2424. [Google Scholar] [CrossRef]
- Telichowska, A.; Kobus-Cisowska, J.; Szulc, P. Phytopharmacological possibilities of bird cherry Prunus padus L. and Prunus serotina L. species and their bioactive phytochemicals. Nutrients 2020, 12, 1966. [Google Scholar] [CrossRef]
- Rutkowska, M.; Witek, M.; Olszewska, M.A. A comprehensive review of molecular mechanisms, pharmacokinetics, toxicology and plant sources of juglanin: Current landscape and future perspectives. Int. J. Mol. Sci. 2024, 25, 10323. [Google Scholar] [CrossRef]
- Ademović, Z.; Hodžić, S.; Halilić-Zahirović, Z.; Husejnagić, D.; Džananović, J.; Šarić-Kundalić, B.; Suljagić, J. Phenolic compounds, antioxidant and antimicrobial properties of the wild cherry (Prunus avium L.) stem. Acta Period. Technol. 2017, 48, 1–13. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Chakraborty, S. A review on medicinal plants and its importance from glycosides. Int. J. Res. Appl. Sci. Eng. Technol. 2024, 12, 715–729. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef]
- Zymonė, K.; Liaudanskas, M.; Lanauskas, J.; Nagelytė, M.; Janulis, V. Variability in the qualitative and quantitative composition of phenolic compounds and the in vitro antioxidant activity of sour cherry (Prunus cerasus L.) leaves. Antioxidants 2024, 13, 553. [Google Scholar] [CrossRef]
- Tomar, O.; Akarca, G.; Gök, V.; İstek, Ö. Chemical composition and antifungal potential of apricot, sour cherry, and cherry tree bio-products (resins) against food-borne molds. Food Biosci. 2022, 47, 101627. [Google Scholar] [CrossRef]
- Krauze-Baranowska, M.; Głód, D.; Kula, M.; Majdan, M.; Hałasa, R.; Matkowski, A.; Kozłowska, W.; Kawiak, A. Chemical composition and biological activity of Rubus idaeus shoots—A traditional herbal remedy of Eastern Europe. BMC Complement. Altern. Med. 2014, 14, 480. [Google Scholar] [CrossRef]
- Cyboran-Mikołajczyk, S.; Męczarska, K.; Solarska-Ściuk, K.; Ratajczak-Wielgomas, K.; Oszmiański, J.; Jencova, V.; Bonarska-Kujawa, D. Protection of erythrocytes and microvascular endothelial cells against oxidative damage by Fragaria vesca L. and Rubus idaeus L. leaves extracts—The mechanism of action. Molecules 2022, 27, 5865. [Google Scholar] [CrossRef]
- Raal, A.; Vahtra, A.; Koshovyi, O.; Ilina, T.; Kovalyova, A.; Püssa, T. Polyphenolic compounds in the stems of raspberry (Rubus idaeus) growing wild and cultivated. Molecules 2024, 29, 5016. [Google Scholar] [CrossRef]
- Nastić, N.; Lozano-Sánchez, J.; Borrás-Linares, I.; Švarc-Gajić, J.; Segura-Carretero, A. New technological approaches for recovering bioactive food constituents from sweet cherry (Prunus avium L.) stems. Phytochem. Anal. 2020, 31, 119–130. [Google Scholar] [CrossRef]
- Brozdowski, J.; Waliszewska, B.; Gacnik, S.; Hudina, M.; Veberic, R.; Mikulic-Petkovsek, M. Phenolic composition of leaf and flower extracts of black cherry (Prunus serotina Ehrh.). Ann. For. Sci. 2021, 78, 66. [Google Scholar] [CrossRef]
- Jesus, F.; Gonçalves, A.C.; Alves, G.; Silva, L.R. Health benefits of Prunus avium plant parts: An unexplored source rich in phenolic compounds. Food Rev. Int. 2022, 38, 118–146. [Google Scholar] [CrossRef]
- Nunes, A.R.; Gonçalves, A.C.; Alves, G.; Falcão, A.; Garcia-Viguera, C.; Moreno, D.A.; Silva, L.R. Valorisation of Prunus avium L. by-products: Phenolic composition and effect on Caco-2 cells viability. Foods 2021, 10, 1185. [Google Scholar] [CrossRef]
- Costea, T.; Vlase, L.; Gostin, I.N.; Olah, N.K.; Predan, G.M.I. Botanical characterization, phytochemical analysis and antioxidant activity of indigenous red raspberry (Rubus idaeus L.) leaves. Stud. Univ. Vasile Goldis Ser. Stiintele Vietii 2016, 26, 463–472. [Google Scholar]
- Bastos, C.; Barros, L.; Dueñas, M.; Calhelha, R.C.; Queiroz, M.J.R.P.; Santos-Buelga, C.; Ferreira, I.C.F.R. Chemical characterisation and bioactive properties of Prunus avium L.: The widely studied fruits and the unexplored stems. Food Chem. 2015, 173, 1045–1053. [Google Scholar] [CrossRef]
- Dudzinska, D.; Luzak, B.; Boncler, M.; Rywaniak, J.; Sosnowska, D.; Podsedek, A.; Watala, C. CD39/NTPDase-1 expression and activity in human umbilical vein endothelial cells are differentially regulated by leaf extracts from Rubus caesius and Rubus idaeus. Cell. Mol. Biol. Lett. 2014, 19, 361–380. [Google Scholar] [CrossRef]
- Jakopič, J.; Štampar, F.; Veberič, R. Influence of hail net and reflective foil on cyanidin glycosides and quercetin glycosides in ‘Fuji’apple skin. HortScience 2010, 45, 1447–1452. [Google Scholar] [CrossRef]
- Alkhudaydi, H.M.S.; Muriuki, E.N.; Spencer, J.P. Determination of the polyphenol composition of raspberry leaf using LC-MS/MS. Molecules 2025, 30, 970. [Google Scholar] [CrossRef]
- Wojdyło, A.; Nowicka, P.; Turkiewicz, I.P.; Tkacz, K. Profiling of polyphenols by LC-QTOF/ESI-MS, characteristics of nutritional compounds and in vitro effect on pancreatic lipase, α-glucosidase, α-amylase, cholinesterase and cyclooxygenase activities of sweet (Prunus avium) and sour (P. cerasus) cherries leaves and fruits. Ind. Crops Prod. 2021, 174, 114214. [Google Scholar] [CrossRef]
- Tian, Y.; Liimatainen, J.; Alanne, A.L.; Lindstedt, A.; Liu, P.; Sinkkonen, J.; Kallio, H.; Yang, B. Phenolic compounds extracted by acidic aqueous ethanol from berries and leaves of different berry plants. Food Chem. 2017, 220, 266–281. [Google Scholar] [CrossRef]
- Kanoun, K.; Belyagoubi-Benhammou, N.; Ghembaza, N.; Atik Bekkara, F. Comparative studies on antioxidant activities of extracts from the leaf, stem and berry of Myrtus communis L. Int. Food Res. J. 2014, 21, 1957–1962. [Google Scholar]
- Cvetanović, A.; Zengin, G.; Zeković, Z.; Švarc-Gajić, J.; Ražić, S.; Damjanović, A.; Mašković, P.; Mitić, M. Comparative in vitro studies of the biological potential and chemical composition of stems, leaves and berries Aronia melanocarpa’s extracts obtained by subcritical water extraction. Food Chem. Toxicol. 2018, 121, 458–466. [Google Scholar] [CrossRef]
- Willig, G.; Brunissen, F.; Brunois, F.; Godon, B.; Magro, C.; Monteux, C.; Peyrot, C.; Ioannou, I. Phenolic compounds extracted from cherry tree (Prunus avium) branches: Impact of the process on cosmetic properties. Antioxidants 2022, 11, 813. [Google Scholar] [CrossRef]
- Zhang, H.; Li, Q.; Qiao, G.; Qiu, Z.; Wen, Z.; Wen, X. Optimizing the supercritical carbon dioxide extraction of sweet cherry (Prunus avium L.) leaves and UPLC-MS/MS analysis. Anal. Methods 2020, 12, 3004–3013. [Google Scholar] [CrossRef]
- Kniepkamp, K.; Errico, M.; Yu, M.; Roda-Serrat, M.C.; Eilers, J.G.; Wark, M.; van Haren, R. Lipid extraction of high-moisture sour cherry (Prunus cerasus L.) stones by supercritical carbon dioxide. J. Chem. Technol. Biotechnol. 2024, 99, 810–819. [Google Scholar] [CrossRef]
- Pavlić, B.; Aćimović, M.; Sknepnek, A.; Miletić, D.; Mrkonjić, Ž.; Kljakić, A.C.; Jerković, J.; Mišan, A.; Pojić, M.; Stupar, A.; et al. Sustainable raw materials for efficient valorization and recovery of bioactive compounds. Ind. Crops Prod. 2023, 193, 116167. [Google Scholar] [CrossRef]
- Zhang, H.; Yan, H.; Lu, C.; Lin, H.; Li, Q. Optimization of ultrasound and microwave-assisted extraction of sweet cherry tree branches and chemical component analysis by UPLC–MS/MS. Trees—Struct. Funct. 2021, 35, 1247–1256. [Google Scholar] [CrossRef]
- Wang, L.; Lin, X.; Zhang, J.; Zhang, W.; Hu, X.; Li, W.; Li, C.; Liu, S. Extraction methods for the releasing of bound phenolics from Rubus idaeus L. leaves and seeds. Ind. Crops Prod. 2019, 135, 1–9. [Google Scholar] [CrossRef]
- Hałasa, R.; Turecka, K.; Mizerska, U.; Krauze-Baranowska, M. Anti-Helicobacter pylori biofilm extracts from Rubus idaeus and Rubus occidentalis. Pharmaceutics 2024, 16, 501. [Google Scholar] [CrossRef]
- Kotuła, M.; Kapusta-Duch, J.; Smoleń, S.; Doskočil, I. Phytochemical composition of the fruits and leaves of raspberries (Rubus idaeus L.)—Conventional vs. organic and those wild grown. Appl. Sci. 2022, 12, 11783. [Google Scholar] [CrossRef]
- Plasencia, P.; Finimundy, T.C.; Carocho, M.; Calhelha, R.C.; Añibarro-Ortega, M.; Pires, T.C.S.P.; Barreiro, F.; Garcia, P.A.; Barros, L.; Heleno, S.A. Extraction of bioactive compounds from Rubus idaeus bioresidues: A full screening on phenolic composition and bioactive potential. Waste Biomass Valor. 2025, 16, 737–747. [Google Scholar] [CrossRef]
- Maslov, O.; Komisarenko, M.; Kolisnyk, S.; Kostina, T.; Golik, M.; Moroz, V.; Tarasenko, D.; Akhmedov, E. Investigation of the extraction dynamic of the biologically active substances of the raspberry (Rubus idaeus L.) shoots. Curr. Issues Pharm. Med. Sci. 2023, 36, 194–198. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, Z.; Li, X.; Abubaker, M.A.; Liu, X.; Li, Z.; Wang, X.; Zhu, X.; Zhang, J.; Chen, X. Comparative study of three raspberry cultivar (Rubus idaeus L.) leaves metabolites: Metabolome profiling and antioxidant activities. Appl. Sci. 2022, 12, 990. [Google Scholar] [CrossRef]
- Ruiz-Aquino, F.; Feria-Reyes, R.; Rutiaga-Quiñones, J.G.; Robledo-Taboada, L.H.; Gabriel-Parra, R. Characterization of tannin extracts derived from the bark of four tree species by HPLC and FTIR. For. Sci. Technol. 2023, 19, 38–46. [Google Scholar] [CrossRef]
- Agarwal, C.; Hofmann, T.; Vršanská, M.; Schlosserová, N.; Visi-Rajczi, E.; Voběrková, S.; Pásztory, Z. In vitro antioxidant and antibacterial activities with polyphenolic profiling of wild cherry, the european larch and sweet chestnut tree bark. Eur. Food Res. Technol. 2021, 247, 2355–2370. [Google Scholar] [CrossRef]
- Švarc-Gajić, J.; Clavijo, S.; Suárez, R.; Cvetanović, A.; Cerdà, V. Simultaneous dispersive liquid-liquid microextraction derivatisation and gas chromatography mass spectrometry analysis of subcritical water extracts of sweet and sour cherry stems. Anal. Bioanal. Chem. 2018, 410, 1943–1953. [Google Scholar] [CrossRef]
- Voss, M.; Gaudino, E.C.; Tabasso, S.; Forte, C.; Cravotto, G. Current emerging green technologies for the valorization of grape and cherry wastes. Curr. Food Sci. Technol. Rep. 2023, 1, 47–61. [Google Scholar] [CrossRef]
- Evtyugin, D.D.; Magina, S.; Evtuguin, D.V. Recent advances in the production and applications of ellagic acid and its derivatives. A review. Molecules 2020, 25, 2745. [Google Scholar] [CrossRef]
- Švarc-Gajić, J.; Cerdà, V.; Clavijo, S.; Suárez, R.; Mašković, P.; Cvetanović, A.; Delerue-Matos, C.; Carvalho, A.P.; Novakov, V. Bioactive compounds of sweet and sour cherry stems obtained by subcritical water extraction. J. Chem. Technol. Biotechnol. 2018, 93, 1627–1635. [Google Scholar] [CrossRef]
- Hamad, F.B.; Mubofu, E.B. Potential biological applications of bio-based anacardic acids and their derivatives. Int. J. Mol. Sci. 2015, 16, 8569–8590. [Google Scholar] [CrossRef]
- Li, Z.; Niu, L.; Chen, Y.; Qiu, X.; Du, T.; Zhu, M.; Wang, M.; Mo, H.; Xiao, S. Recent advance in the biological activity of chlorogenic acid and its application in food industry. Int. J. Food Sci. Technol. 2023, 58, 4931–4947. [Google Scholar] [CrossRef]
- Wang, L.; Pan, X.; Jiang, L.; Chu, Y.; Gao, S.; Jiang, X.; Zhang, Y.; Chen, Y.; Luo, S.; Peng, C. The biological activity mechanism of chlorogenic acid and its applications in food industry: A review. Front. Nutr. 2022, 9, 943911. [Google Scholar] [CrossRef]
- Peng, R.; Wu, Q.; Chen, J.; Ghosh, R.; Chen, X. Isolation of ellagic acid from pomegranate peel extract by hydrophobic interaction chromatography using graphene oxide grafted cotton fiber adsorbent. J. Sep. Sci. 2018, 41, 747–755. [Google Scholar] [CrossRef]
- García-Niño, W.R.; Zazueta, C. Ellagic acid: Pharmacological activities and molecular mechanisms involved in liver protection. Pharmacol. Res. 2015, 97, 84–103. [Google Scholar] [CrossRef]
- Tomou, E.M.; Papakyriakopoulou, P.; Skaltsa, H.; Valsami, G.; Kadoglou, N.P.E. Bio-actives from natural products with potential cardioprotective properties: Isolation, identification, and pharmacological actions of apigenin, quercetin, and silibinin. Molecules 2023, 28, 2387. [Google Scholar] [CrossRef]
- Kumar, K.S.; Sabu, V.; Sindhu, G.; Rauf, A.A.; Helen, A. Isolation, identification and characterization of apigenin from Justicia gendarussa and its anti-inflammatory activity. Int. Immunopharmacol. 2018, 59, 157–167. [Google Scholar] [CrossRef]
- Cvetanović, A.; Švarc-Gajić, J.; Gašić, U.; Tešić, Ž.; Zengin, G.; Zeković, Z.; Đurović, S. Isolation of apigenin from subcritical water extracts: Optimization of the process. J. Supercrit. Fluids 2017, 120, 32–42. [Google Scholar] [CrossRef]
- Mehdi, A.; Al-ani, W.M.K.; Raoof, A. Isolation of astragalin from IRAQI Chenopodium album. Asian J. Pharm. Clin. Res. 2018, 11, 530–535. [Google Scholar] [CrossRef]
- Ruan, J.; Shi, Z.; Cao, X.; Dang, Z.; Zhang, Q.; Zhang, W.; Wu, L.; Zhang, Y.; Wang, T. Research progress on anti-inflammatory effects and related mechanisms of astragalin. Int. J. Mol. Sci. 2024, 25, 4476. [Google Scholar] [CrossRef]
- Jeyanthi, V.; Anbu, P.; Vairamani, M.; Velusamy, P. Isolation of hydroquinone (benzene-1,4-diol) metabolite from halotolerant Bacillus methylotrophicus MHC10 and its inhibitory activity towards bacterial pathogens. Bioprocess Biosyst. Eng. 2016, 39, 429–439. [Google Scholar] [CrossRef]
- Enguita, F.J.; Leitão, A.L. Hydroquinone: Environmental pollution, toxicity, and microbial answers. BioMed Res. Int. 2013, 2013, 542168. [Google Scholar] [CrossRef]
- Raza, M.J.; Jalil, O.; Kumar, D.; Pandey, C.M. Highly sensitive electrochemical detection of hydroquinone in wastewater using ionic liquid grafted rGO-ZrO2 nanohybrid-based conducting paper. J. Electrochem. Soc. 2025, 172, 067508. [Google Scholar] [CrossRef]
- Cao, X.; Wei, Y.; Ito, Y. Preparative isolation of isorhamnetin from Stigma Maydis using high speed countercurrent chromatography. J. Liq. Chromatogr. Relat. Technol. 2009, 32, 273–280. [Google Scholar] [CrossRef]
- Mei, C.; Liu, Y.; Lyu, X.; Jiang, Z.; Liu, Z.; Zhi, Y.; Xu, X.; Wang, H. Advances in isorhamnetin treatment of malignant tumors: Mechanisms and applications. Nutrients 2025, 17, 1853. [Google Scholar] [CrossRef]
- Duan, M.; Wang, X.; Feng, J.; Xiao, X.; Zhang, L.; He, S.; Ma, L.; Wang, X.; Yang, S.; Rao, M.J. From agricultural waste to functional tea: Optimized processing enhances bioactive flavonoid recovery and antioxidant capacity with multifaceted health benefits in loquat (Eriobotrya japonica Lindl.) flowers. Horticulturae 2025, 11, 766. [Google Scholar] [CrossRef]
- Nguelefack, T.B.; Mbakam, F.H.K.; Tapondjou, L.A.; Watcho, P.; Nguelefack-Mbuyo, E.P.; Ponou, B.K.; Kamanyi, A.; Park, H.J. A dimeric triterpenoid glycoside and flavonoid glycosides with free radical-scavenging activity isolated from Rubus rigidus var. camerunensis. Arch. Pharmacal Res. 2011, 34, 543–550. [Google Scholar] [CrossRef]
- Gajjar, N.D.; Dhameliya, T.M.; Shah, G.B. In search of RdRp and Mpro inhibitors against SARS CoV-2: Molecular docking, molecular dynamic simulations and ADMET analysis. J. Mol. Struct. 2021, 1239, 130488. [Google Scholar] [CrossRef]
- Wahab, A.; Begum, S.; Mahmood, I.; Mahmood, T.; Ahmad, A.; Fayyaz, N. Luteolin and kaempferol from Cassia Alata. Antimicrobial and antioxidant activity of its methanolic extracts. FUUAST J. Biol. 2014, 4, 1–5. [Google Scholar]
- Bangar, S.P.; Chaudhary, V.; Sharma, N.; Bansal, V.; Ozogul, F.; Lorenzo, J.M. Kaempferol: A flavonoid with wider biological activities and its applications. Crit. Rev. Food Sci. Nutr. 2023, 63, 9580–9604. [Google Scholar] [CrossRef]
- Montenegro, I.; Pérez, C.; González, B.; Domínguez, Á.; Gómez, E. Thermal characterization and heat capacities of seven polyphenols. Molecules 2025, 30, 199. [Google Scholar] [CrossRef]
- Sudto, K.; Pornpakakul, S.; Wanichwecharungruang, S. An efficient method for the large scale isolation of naringin from pomelo (Citrus grandis) peel. Int. J. Food Sci. Technol. 2009, 44, 1737–1742. [Google Scholar] [CrossRef]
- Koirala, M.; Lee, Y.K.; Kim, M.S.; Chung, Y.C.; Park, J.S.; Kim, S.Y. Biotransformation of naringenin by Bacillus amyloliquefaciens into three naringenin derivatives. Nat. Prod. Commun. 2019, 14, 465–472. [Google Scholar] [CrossRef]
- Zhang, L.; Song, L.; Zhang, P.; Liu, T.; Zhou, L.; Yang, G.; Lin, R.; Zhang, J. Solubilities of naringin and naringenin in different solvents and dissociation constants of naringenin. J. Chem. Eng. Data 2015, 60, 932–940. [Google Scholar] [CrossRef]
- Kylli, P.; Nohynek, L.; Puupponen-Pimiä, R.; Westerlund-Wikström, B.; Leppänen, T.; Welling, J.; Moilanen, E.; Heinonen, M. Lingonberry (Vaccinium vitis-idaea) and European cranberry (Vaccinium microcarpon) proanthocyanidins: Isolation, identification, and bioactivities. J. Agric. Food Chem. 2011, 59, 3373–3384. [Google Scholar] [CrossRef]
- Chen, S.; Song, J.; Du, L.; Ma, Y.; Ren, S.; Ren, J.; Li, S. Quantitative analysis of solubility parameters and surface properties of larch bark proanthocyanidins. Polymers 2020, 12, 2800. [Google Scholar] [CrossRef]
- Choi, J.S.; Yokozawa, T.; Oura, H. Improvement of hyperglycemia and hyperlipemia in streptozotocin-diabetic rats by a methanolic extract of Prunus davidiana stems and its main component, prunin. Planta Med. 1991, 57, 208–211. [Google Scholar] [CrossRef]
- Céliz, G.; Daz, M. Biocatalytic preparation of alkyl esters of citrus flavanone glucoside prunin in organic media. Process Biochem. 2011, 46, 94–100. [Google Scholar] [CrossRef]
- Dall’Acqua, S.; Miolo, G.; Innocenti, G.; Caffieri, S. The photodegradation of quercetin: Relation to oxidation. Molecules 2012, 17, 8898–8907. [Google Scholar] [CrossRef]
- Ramešová, Š.; Sokolová, R.; Degano, I.; Bulíčková, J.; Žabka, J.; Gál, M. On the stability of the bioactive flavonoids quercetin and luteolin under oxygen-free conditions. Anal. Bioanal. Chem. 2012, 402, 975–982. [Google Scholar] [CrossRef]
- Yingyuen, P.; Sukrong, S.; Phisalaphong, M. Isolation, separation and purification of rutin from banana leaves (Musa balbisiana). Ind. Crops Prod. 2020, 149, 112307. [Google Scholar] [CrossRef]
- Baldisserotto, A.; Vertuani, S.; Bino, A.; De Lucia, D.; Lampronti, I.; Milani, R.; Gambari, R.; Manfredini, S. Design, synthesis and biological activity of a novel rutin analogue with improved lipid soluble properties. Bioorg. Med. Chem. 2015, 23, 264–271. [Google Scholar] [CrossRef]
- Choi, S.S.; Park, H.R.; Lee, K.A.A. Comparative study of rutin and rutin glycoside: Antioxidant activity, anti-inflammatory effect, effect on platelet aggregation and blood coagulation. Antioxidants 2021, 10, 1696. [Google Scholar] [CrossRef]
- Choe, U.; Li, Y.; Yu, L.; Gao, B.; Wang, T.T.Y.; Sun, J.; Chen, P.; Yu, L. Chemical composition of cold-pressed blackberry seed flour extract and its potential health-beneficial properties. Food Sci. Nutr. 2020, 8, 1215–1225. [Google Scholar] [CrossRef]
- Firmansyah, A.; Winingsih, W.; Manobi, J.D.Y. Review of scopoletin: Isolation, analysis process, and pharmacological activity. Biointerface Res. Appl. Chem. 2021, 11, 12006–12019. [Google Scholar] [CrossRef]
- Galán-Pérez, J.A.; Gámiz, B.; Celis, R. Determining the effect of soil properties on the stability of scopoletin and its toxicity to target plants. Biol. Fertil. Soils 2021, 57, 643–655. [Google Scholar] [CrossRef]
- Chaaban, H.; Ioannou, I.; Chebil, L.; Slimane, M.; Gérardin, C.; Paris, C.; Charbonnel, C.; Chekir, L.; Ghoul, M. Effect of heat processing on thermal stability and antioxidant activity of six flavonoids. J. Food Process. Preserv. 2017, 41, e13203. [Google Scholar] [CrossRef]
- Hemshekhar, M.; Sebastin Santhosh, M.; Kemparaju, K.; Girish, K.S. Emerging roles of anacardic acid and its derivatives: A pharmacological overview. Basic Clin. Pharmacol. Toxicol. 2012, 110, 122–132. [Google Scholar] [CrossRef]
- Rosa, M.E.P.; Rebouças, L.M.; Marques, S.P.D.; Silva, L.M.R.; Cunha, F.E.T.; Costa, P.M.S.; de Assis, D.A.; Silveira, K.B.; Muniz, C.R.; Trevisan, M.T.S.; et al. Sodium hyaluronate microcapsules to promote antitumor selectivity of anacardic acid. Int. J. Biol. Macromol. 2025, 296, 139616. [Google Scholar] [CrossRef]
- Schultz, D.J.; Krishna, A.; Vittitow, S.L.; Alizadeh-Rad, N.; Muluhngwi, P.; Rouchka, E.C.; Klinge, C.M. Transcriptomic response of breast cancer cells to anacardic acid. Sci. Rep. 2018, 8, 8063. [Google Scholar] [CrossRef]
- Anjum, M.M.; Patel, K.K.; Pandey, N.; Tilak, R.; Agrawal, A.K.; Singh, S. Development of anacardic acid/hydroxypropyl-β-cyclodextrin inclusion complex with enhanced solubility and antimicrobial activity. J. Mol. Liq. 2019, 296, 112085. [Google Scholar] [CrossRef]
- Jit, T.; Deb Roy, S.; Shil, D.; Chakraborty, J.; Paul, A.; Roy, S.; De, D. The potential of tannins from medicinal plants as anti-cancer agents. J. Med. Plants Stud. 2024, 12, 414423. [Google Scholar]
- Radha, R.; Prakash, S.; Kumari, N.; Sharma, N.; Puri, S.; Singh, J.; Thakur, M.; Kumar, M. Bioactives and bioactivities from food byproducts. Curr. Food Sci. Technol. Rep. 2024, 2, 297–308. [Google Scholar] [CrossRef]
- Ko, H.; Jeon, H.; Lee, D.; Choi, H.K.; Kang, K.S.; Choi, K.C. Sanguiin H6 suppresses TGF-β induction of the epithelial–mesenchymal transition and inhibits migration and invasion in A549 lung cancer. Bioorg. Med. Chem. Lett. 2015, 25, 5508–5513. [Google Scholar] [CrossRef]
- Gesek, J.; Jakimiuk, K.; Atanasov, A.G.; Tomczyk, M. Sanguiins—Promising molecules with broad biological potential. Int. J. Mol. Sci. 2021, 22, 12972. [Google Scholar] [CrossRef]
- Park, E.J.; Lee, D.; Baek, S.E.; Kim, K.H.; Kang, K.S.; Jang, T.S.; Lee, H.L.; Song, J.H.; Yoo, J.E. Cytotoxic effect of sanguiin H-6 on MCF-7 and MDA-MB-231 human breast carcinoma cells. Bioorg. Med. Chem. Lett. 2017, 27, 4389–4392. [Google Scholar] [CrossRef]
- Huang, Z.Q.; Chen, P.; Su, W.W.; Wang, Y.G.; Wu, H.; Peng, W.; Li, P.B. Antioxidant activity and hepatoprotective potential of quercetin 7-rhamnoside in vitro and in vivo. Molecules 2018, 23, 1188. [Google Scholar] [CrossRef]
- Deng, D.; Zhao, B.; Yang, H.; Wang, S.; Geng, Z.; Zhou, J.; Yang, G.; Han, L. Investigating the effect and potential mechanism of rhamnetin 3-o-α-rhamnoside on acute liver injury in vivo and in vitro. Pharmaceuticals 2025, 18, 116. [Google Scholar] [CrossRef]
- Li, L.; Lei, X.; Chen, L.; Ma, Y.; Luo, J.; Liu, X.; Xu, X.; Zhou, G.; Feng, X. Protective Mechanism of quercetin compounds against acrylamide-induced hepatotoxicity. Food Sci. Hum. Wellness 2024, 13, 225–240. [Google Scholar] [CrossRef]
- Choi, H.J.; Song, J.H.; Park, K.S.; Kwon, D.H. Inhibitory effects of quercetin 3-rhamnoside on influenza a virus replication. Eur. J. Pharm. Sci. 2009, 37, 329–333. [Google Scholar] [CrossRef]
- Meng, X.; Xia, C.; Wu, H.; Gu, Q.; Li, P. Metabolism of quercitrin in the colon and its beneficial regulatory effects on gut microbiota. J. Sci. Food Agric. 2024, 104, 9255–9264. [Google Scholar] [CrossRef]
- Reinboth, M.; Wolffram, S.; Abraham, G.; Ungemach, F.R.; Cermak, R. Oral bioavailability of quercetin from different quercetin glycosides in dogs. Br. J. Nutr. 2010, 104, 198–203. [Google Scholar] [CrossRef]
- Li, Y.; Yao, J.; Han, C.; Yang, J.; Chaudhry, M.T.; Wang, S.; Liu, H.; Yin, Y. Quercetin, inflammation and immunity. Nutrients 2016, 8, 167. [Google Scholar] [CrossRef]
- Cao, M.M.; Guo, Z.; Wang, J.; Ma, H.Y.; Qin, X.Y.; Hu, Y.; Lan, R. Astragalin alleviates lipopolysaccharide-induced depressive-like behavior in mice by preserving blood-brain barrier integrity and suppressing neuroinflammation. Free Radic. Biol. Med. 2025, 232, 340–352. [Google Scholar] [CrossRef]
- Li, Q.; Yang, Z.; Lu, H.; Liu, F.; Zhou, D.; Zou, Y. Astragalin Exerted hypoglycemic effect by both inhibiting α-glucosidase and modulating AMPK signaling pathway. Nutrients 2025, 17, 406. [Google Scholar] [CrossRef]
- Riaz, A.; Rasul, A.; Hussain, G.; Zahoor, M.K.; Jabeen, F.; Subhani, Z.; Younis, T.; Ali, M.; Sarfraz, I.; Selamoglu, Z. Astragalin: A bioactive phytochemical with potential therapeutic activities. Adv. Pharmacol. Pharm. Sci. 2018, 2018, 9794625. [Google Scholar] [CrossRef]
- Zeng, W.; Chen, L. Astragalin inhibits the proliferation of high-risk HPV-positive cervical epithelial cells and attenuates malignant cervical lesions. Cytotechnology 2025, 77, 80. [Google Scholar] [CrossRef]
- Li, C.; Hu, M.; Jiang, S.; Liang, Z.; Wang, J.; Liu, Z.; Wang, H.M.D.; Kang, W. Evaluation procoagulant activity and mechanism of astragalin. Molecules 2020, 25, 177. [Google Scholar] [CrossRef]
- Yang, C.Z.; Wang, S.H.; Zhang, R.H.; Lin, J.H.; Tian, Y.H.; Yang, Y.Q.; Liu, J.; Ma, Y.X. Neuroprotective effect of astragalin via activating PI3K/Akt-MTOR-mediated autophagy on APP/PS1 mice. Cell Death Discov. 2023, 9, 15. [Google Scholar] [CrossRef]
- Chen, J.; Zhong, K.; Qin, S.; Jing, Y.; Liu, S.; Li, D.; Peng, C. Astragalin: A food-origin flavonoid with therapeutic effect for multiple diseases. Front. Pharmacol. 2023, 14, 1265960. [Google Scholar] [CrossRef]
- Duszka, K.; Clark, B.F.C.; Massino, F.; Barciszewski, J. Biological activities of kinetin. In Herbal Drugs: Ethnomedicine to Modern Medicine; Ramawat, K.G., Ed.; Springer: Berlin/Heidelberg, Germany, 2009; pp. 369–380. [Google Scholar] [CrossRef]
- Jiang, X.; Yang, L.; Chen, G.; Feng, X.; Liu, Y.; Gao, Q.; Mai, M.; Chen, C.; Yu, C.; Ye, S.; et al. Discovery of kinetin in inhibiting colorectal cancer progression via enhancing PSMB1-mediated RAB34 degradation. Cancer Lett. 2024, 584, 216600. [Google Scholar] [CrossRef]
- Souza, T.M.L.; Pinho, V.D.; Setim, C.F.; Sacramento, C.Q.; Marcon, R.; Fintelman-Rodrigues, N.; Chaves, O.A.; Heller, M.; Temerozo, J.R.; Ferreira, A.C.; et al. Preclinical development of kinetin as a safe error-prone SARS-CoV-2 antiviral able to attenuate virus-induced inflammation. Nat. Commun. 2023, 14, 199. [Google Scholar] [CrossRef]
- Chandorkar, Y.; Valeske, M.; Kolrosova, B.; Elbs-Glatz, Y.; Zuber, F.; Schoeller, J.; Kummer, N.; Ren, Q.; Rottmar, M.; Maniura-Weber, K. Bioactive salicylic acid containing coating for dental implants to combat infection and inflammation. Adv. Mater. Interfaces 2024, 11, 2300750. [Google Scholar] [CrossRef]
- Miclaus, M.O.; Borodi, G.; Turza, A. Four polymorphs of the bioactive diuretic drug 4-chloro-5-chlorosulfonyl salicylic acid. Crystals 2025, 15, 136. [Google Scholar] [CrossRef]
- Nagelschmitz, J.; Blunck, M.; Kraetzschmar, J.; Ludwig, M.; Wensing, G.; Hohlfeld, T. Pharmacokinetics and pharmacodynamics of acetylsalicylic acid after intravenous and oral administration to healthy volunteers. Clin. Pharmacol.: Adv. Appl. 2014, 6, 51–59. [Google Scholar] [CrossRef]
- Madan, R.K.; Levitt, J. A review of toxicity from topical salicylic acid preparations. J. Am. Acad. Dermatol. 2014, 70, 788–792. [Google Scholar] [CrossRef]
- Varvara, M.; Bozzo, G.; Celano, G.; Disanto, C.; Pagliarone, C.N.; Celano, G.V. The use of ascorbic acid as a food additive: Technical-legal issues. Ital. J. Food Saf. 2016, 5, 4313. [Google Scholar] [CrossRef]
- Davis, J.L.; Paris, H.L.; Beals, J.W.; Binns, S.E.; Giordano, G.R.; Scalzo, R.L.; Schweder, M.M.; Blair, E.; Bell, C. Liposomal-encapsulated ascorbic acid: Influence on vitamin C bioavailability and capacity to protect against ischemia-reperfusion injury. Nutr. Metab. Insights 2016, 9, 25–30. [Google Scholar] [CrossRef]
- Stephenson, C.M.; Levin, R.D.; Spector, T.; Lis, C.G. Phase I clinical trial to evaluate the safety, tolerability, and pharmacokinetics of high-dose intravenous ascorbic acid in patients with advanced cancer. Cancer Chemother. Pharmacol. 2013, 72, 139–146. [Google Scholar] [CrossRef]
- Bhatt, S.C.; Naik, B.; Kumar, V.; Kumar Gupta, A.; Kumar, S.; Singh Preet, M.; Sharma, N.; Rustagi, S. Untapped potential of non-conventional Rubus species: Bioactivity, nutrition, and livelihood opportunities. Plant Methods 2023, 19, 114. [Google Scholar] [CrossRef]
- Borel, P.; Preveraud, D.; Desmarchelier, C. Bioavailability of vitamin E in humans: An update. Nutr. Rev. 2013, 71, 319–331. [Google Scholar] [CrossRef]
- Gad, S.C. Hydroquinone. In Encyclopedia of Toxicology, 4th ed.; Wexler, P., Ed.; Academic Press: Cambridge, MA, USA, 2024; Volume 5, pp. 425–430. [Google Scholar] [CrossRef]
- Fabian, I.M.; Sinnathamby, E.S.; Flanagan, C.J.; Lindberg, A.; Tynes, B.; Kelkar, R.A.; Varrassi, G.; Ahmadzadeh, S.; Shekoohi, S.; Kaye, A.D. Topical hydroquinone for hyperpigmentation: A narrative review. Cureus 2023, 15, e48840. [Google Scholar] [CrossRef]
- Banodkar, P.D.; Banodkar, K.P. History of hydroquinone. Indian J. Dermatol. Venereol. Leprol. 2022, 88, 696–699. [Google Scholar] [CrossRef]
- Shivaram, K.; Edwards, K.; Mohammad, T.F. An update on the safety of hydroquinone. Arch. Dermatol. Res. 2024, 316, 378. [Google Scholar] [CrossRef]
- Serrano, D.R.; Gordo, M.J.; Matji, A.; González, S.; Lalatsa, A.; Torrado, J.J. Tuning the transdermal delivery of hydroquinone upon formulation with novel permeation enhancers. Pharmaceutics 2019, 11, 167. [Google Scholar] [CrossRef]
- Imran, M.; Salehi, B.; Sharifi-Rad, J.; Gondal, T.A.; Saeed, F.; Imran, A.; Shahbaz, M.; Fokou, P.V.T.; Arshad, M.U.; Khan, H.; et al. Kaempferol: A key emphasis to its anticancer potential. Molecules 2019, 24, 2277. [Google Scholar] [CrossRef]
- Periferakis, A.; Periferakis, K.; Badarau, I.A.; Petran, E.M.; Popa, D.C.; Caruntu, A.; Costache, R.S.; Scheau, C.; Caruntu, C.; Costache, D.O. Kaempferol: Antimicrobial properties, sources, clinical, and traditional applications. Int. J. Mol. Sci. 2022, 23, 15054. [Google Scholar] [CrossRef]
- Calderón-Montaño, J.M.; Burgos-Morón, E.; Pérez-Guerrero, C.; López-Lázaro, M. A review on the dietary flavonoid kaempferol. Mini-Rev. Med. Chem. 2011, 11, 298–344. [Google Scholar] [CrossRef]
- Li, Q.; Ge, C.; Tan, J.; Sun, Y.; Kuang, Q.; Dai, X.; Zhong, S.; Yi, C.; Hu, L.F.; Lou, D.S.; et al. Juglanin protects against high fat diet-induced renal injury by suppressing inflammation and dyslipidemia via regulating NF-ΚB/HDAC3 signaling. Int. Immunopharmacol. 2021, 95, 107340. [Google Scholar] [CrossRef]
- Ren, Y.; Hu, S.; Pu, H.; Zhou, Y.; Jiang, M.; Li, Y.; Deng, C.; Gao, J.; Xu, M.; Ge, C. Juglanin Ameliorates depression-like behavior in chronic unpredictable mild stress-induced mice by improving AMPK signaling. J. Funct. Foods 2022, 98, 105263. [Google Scholar] [CrossRef]
- Wang, P.; Hu, M.; Wang, L.; Qu, J.; Liu, Y.; Li, C.; Liu, Z.; Ma, C.; Kang, W. Chemical constituents and coagulation effects of the flowers of Rosa chinensis Jacq. J. Future Foods 2023, 3, 155–162. [Google Scholar] [CrossRef]
- Gong, G.; Guan, Y.Y.; Zhang, Z.L.; Rahman, K.; Wang, S.J.; Zhou, S.; Luan, X.; Zhang, H. Isorhamnetin: A review of pharmacological effects. Biomed. Pharmacother. 2020, 128, 110301. [Google Scholar] [CrossRef]
- González-Arceo, M.; Gomez-Lopez, I.; Carr-Ugarte, H.; Eseberri, I.; González, M.; Cano, M.P.; Portillo, M.P.; Gómez-Zorita, S. Anti-obesity effects of isorhamnetin and isorhamnetin conjugates. Int. J. Mol. Sci. 2022, 24, 299. [Google Scholar] [CrossRef]
- Dayem, A.A.; Choi, H.Y.; Kim, Y.B.; Cho, S.G. Antiviral effect of methylated flavonol isorhamnetin against influenza. PLoS ONE 2015, 10, e0121610. [Google Scholar] [CrossRef]
- Ku, S.K.; Kim, T.H.; Bae, J.S. Anticoagulant activities of persicarin and isorhamnetin. Vascul. Pharmacol. 2013, 58, 272–279. [Google Scholar] [CrossRef]
- Scarlata, G.G.M.; Lopez, I.; Gambardella, M.L.; Milanović, M.; Milić, N.; Abenavoli, L. Preventive and therapeutic effects of baicalein, galangin, and isorhamnetin in chronic liver diseases: A narrative review. Molecules 2025, 30, 1253. [Google Scholar] [CrossRef]
- Huang, Z.R.; Lin, Y.K.; Fang, J.Y. Biological and pharmacological activities of squalene and related compounds: Potential uses in cosmetic dermatology. Molecules 2009, 14, 540–554. [Google Scholar] [CrossRef]
- Shalu, S.; Raveendranathan, P.K.; Vaidyanathan, V.K.; Blank, L.M.; Germer, A.; Balakumaran, P.A. Microbial squalene: A sustainable alternative for the cosmetics and pharmaceutical industry – A review. Eng. Life Sci. 2024, 24, e202400003. [Google Scholar] [CrossRef]
- Maxim, C.; Turcov, D.; Bulgariu, A.G.; Șuteu, D. Squalene—Background and perspectives in cosmeceuticals formulas. Bul. Inst. Polit. Iasi 2024, 70, 47–57. [Google Scholar] [CrossRef]
- Hussain, S.; Javed, M.; Abid, M.A.; Khan, M.A.; Syed, S.K.; Faizan, M.; Feroz, F. Prunus avium L.; Phytochemistry, nutritional and pharmacological review. Adv. Life Sci. 2021, 8, 307–314. [Google Scholar] [CrossRef]
- Kumari, N.; Radha; Kumar, M.; Puri, S.; Zhang, B.; Rais, N.; Pundir, A.; Chandran, D.; Raman, P.; Dhumal, S.; et al. Peach (Prunus persica (L.) Batsch) seeds and kernels as potential plant-based functional food ingredients: A review of bioactive compounds and health-promoting activities. Food Biosci. 2023, 54, 102914. [Google Scholar] [CrossRef]
- Kajla, V.; Singh, B.; Muskaan, S.K.D.; Sharma, P.; Vishali, M.; Sayam, A. Harnessing amygdalin in integrative medicine: Novel insights for endocrine disorders. Rev. Argent. Clin. Psicol. 2024, 33, 54–73. [Google Scholar] [CrossRef]
- Mungamuri, S.K.; Chatterjee, N.; Ara, D. Phytotherapy for liver fibrosis: Insights from the biology of hepatic stellate cells—A narrative review. Liver Int. Commun. 2025, 6, e70015. [Google Scholar] [CrossRef]
- Qin, Y.; Wang, S.; Wen, Q.; Xia, Q.; Wang, S.; Chen, G.; Sun, J.; Shen, C.; Song, S. Interactions between Ephedra sinica and Prunus armeniaca: From stereoselectivity to deamination as a metabolic detoxification mechanism of amygdalin. Front. Pharmacol. 2021, 12, 744624. [Google Scholar] [CrossRef]
- EFSA Panel on Contaminants in the Food Chain (CONTAM). Acute health risks related to the presence of cyanogenic glycosides in raw apricot kernels and products derived from raw apricot kernels. EFSA J. 2016, 14, e04424. [Google Scholar] [CrossRef]
- Das, A.; Baidya, R.; Chakraborty, T.; Samanta, A.K.; Roy, S. Pharmacological basis and new insights of taxifolin: A comprehensive review. Biomed. Pharmacother. 2021, 142, 112004. [Google Scholar] [CrossRef]
- Liu, Y.; Shi, X.; Tian, Y.; Zhai, S.; Liu, Y.; Xiong, Z.; Chu, S. An insight into novel therapeutic potentials of taxifolin. Front. Pharmacol. 2023, 14, 1173855. [Google Scholar] [CrossRef]
- Zu, Y.; Wu, W.; Zhao, X.; Li, Y.; Wang, W.; Zhong, C.; Zhang, Y.; Zhao, X. Enhancement of solubility, antioxidant ability and bioavailability of taxifolin nanoparticles by liquid antisolvent precipitation technique. Int. J. Pharm. 2014, 471, 366–376. [Google Scholar] [CrossRef]
- Rajnochová Svobodová, A.; Ryšavá, A.; Psotová, M.; Kosina, P.; Zálešák, B.; Ulrichová, J.; Vostálová, J. The phototoxic potential of the flavonoids, taxifolin and quercetin. Photochem. Photobiol. 2017, 93, 1240–1247. [Google Scholar] [CrossRef]
- Patel, K.; Patel, D.K. Biological potential of aromadendrin against human disorders: Recent development in pharmacological activities and analytical aspects. Pharmacol. Res. Mod. Chin. Med. 2024, 11, 100424. [Google Scholar] [CrossRef]
- El-Shiekh, R.A.; Radi, M.H.; Abdel-Sattar, E. Unveiling the therapeutic potential of aromadendrin (AMD): A promising anti-inflammatory agent in the prevention of chronic diseases. Inflammopharmacology 2025, 33, 1209–1220. [Google Scholar] [CrossRef]
- Fernandes, F.H.; Guterres, Z.D.R.; Corsino, J.; Garcez, W.S.; Garcez, F.R. Assessment of the mutagenicity of propolis compounds from the Brazilian Cerrado biome in somatic cells of Drosophila melanogaster. Orbital: Electron. J. Chem. 2019, 11, 307–313. [Google Scholar] [CrossRef]
- Salehi, B.; Fokou, P.V.T.; Sharifi-Rad, M.; Zucca, P.; Pezzani, R.; Martins, N.; Sharifi-Rad, J. The therapeutic potential of naringenin: A review of clinical trials. Pharmaceuticals 2019, 12, 11. [Google Scholar] [CrossRef]
- Orhan, I.E.; Nabavi, S.F.; Daglia, M.; Tenore, G.C.; Mansouri, K.; Nabavi, S.M. Naringenin and atherosclerosis: A review of literature. Curr. Pharm. Biotechnol. 2015, 16, 245–251. [Google Scholar] [CrossRef]
- Hernández-Aquino, E.; Muriel, P. Beneficial effects of naringenin in liver diseases: Molecular mechanisms. World J. Gastroenterol. 2018, 24, 1679. [Google Scholar] [CrossRef]
- Abdelkawy, Y.S.; Elharoun, M.; Sheta, E.; Abdel-Raheem, I.T.; Nematalla, H.A. Liraglutide and naringenin relieve depressive symptoms in mice by enhancing neurogenesis and reducing inflammation. Eur. J. Pharmacol. 2024, 971, 176525. [Google Scholar] [CrossRef]
- Rebello, C.J.; Beyl, R.A.; Lertora, J.J.L.; Greenway, F.L.; Ravussin, E.; Ribnicky, D.M.; Poulev, A.; Kennedy, B.J.; Castro, H.F.; Campagna, S.R.; et al. Safety and pharmacokinetics of naringenin: A randomized, controlled, single-ascending-dose clinical trial. Diabetes Obes. Metab. 2020, 22, 91–98. [Google Scholar] [CrossRef]
- Pérez-Coll, C.S.; Herkovits, J. Lethal and teratogenic effects of naringenin evaluated by means of an amphibian embryo toxicity test (AMPHITOX). Food Chem. Toxicol. 2004, 42, 299–306. [Google Scholar] [CrossRef]
- Monadi, T.; Mohajer, Z.; Soltani, A.; Khazeei Tabari, M.A.; Manayi, A.; Azadbakht, M. The influence of apigenin on cellular responses to radiation: From protection to sensitization. Biofactors 2024, 51, e2113. [Google Scholar] [CrossRef]
- Salehi, B.; Venditti, A.; Sharifi-Rad, M.; Kręgiel, D.; Sharifi-Rad, J.; Durazzo, A.; Lucarini, M.; Santini, A.; Souto, E.B.; Novellino, E.; et al. The therapeutic potential of apigenin. Int. J. Mol. Sci. 2019, 20, 1305. [Google Scholar] [CrossRef]
- Allemailem, K.S.; Almatroudi, A.; Alharbi, H.O.A.; AlSuhaymi, N.; Alsugoor, M.H.; Aldakheel, F.M.; Khan, A.A.; Rahmani, A.H. Apigenin: A bioflavonoid with a promising role in disease prevention and treatment. Biomedicines. 2024, 12, 1353. [Google Scholar] [CrossRef]
- DeRango-Adem, E.F.; Blay, J. Does oral apigenin have real potential for a therapeutic effect in the context of human gastrointestinal and other cancers? Front. Pharmacol. 2021, 12, 681477. [Google Scholar] [CrossRef]
- Lee, J.A.; Ha, S.K.; Kim, Y.C.; Choi, I. Effects of friedelin on the intestinal permeability and bioavailability of apigenin. Pharmacol. Rep. 2017, 69, 1044–1048. [Google Scholar] [CrossRef]
- Rana, J.N.; Mumtaz, S. Prunin: An emerging anticancer flavonoid. Int. J. Mol. Sci. 2025, 26, 2678. [Google Scholar] [CrossRef]
- Gunaseelan, S.; Wong, K.Z.; Min, N.; Sun, J.; Ismail, N.K.B.M.; Tan, Y.J.; Lee, R.C.H.; Chu, J.J.H. Prunin suppresses viral IRES activity and is a potential candidate for treating enterovirus A71 infection. Sci. Transl. Med. 2019, 11, eaar5759. [Google Scholar] [CrossRef]
- Patel, D.K.; Patel, K. Biological importance of prunin in the medicine for the treatment of diabetes related complication: Therapeutic benefit through data analysis. Metabolism 2022, 128, 155057. [Google Scholar] [CrossRef]
- Guo, F.; Yan, D.; Qin, Z.; Bais, S. Prunin modulates the expression of cerebral serotonin induced by anxiety-like behavior in mice. Nat. Prod. Commun. 2021, 16, 1–9. [Google Scholar] [CrossRef]
- Pan, L.; Ye, H.; Pi, X.; Liu, W.; Wang, Z.; Zhang, Y.; Zheng, J. Effects of several flavonoids on human gut microbiota and its metabolism by in vitro simulated fermentation. Front. Microbiol. 2023, 14, 1092729. [Google Scholar] [CrossRef]
- Mani, R.; Natesan, V. Chrysin: Sources, beneficial pharmacological activities, and molecular mechanism of action. Phytochemistry 2018, 145, 187–196. [Google Scholar] [CrossRef]
- Nabavi, S.F.; Braidy, N.; Habtemariam, S.; Orhan, I.E.; Daglia, M.; Manayi, A.; Gortzi, O.; Nabavi, S.M. Neuroprotective effects of chrysin: From chemistry to medicine. Neurochem. Int. 2015, 90, 224–231. [Google Scholar] [CrossRef]
- Siddiqui, A.; Badruddeen; Akhtar, J.; Shahab Uddin, M.S.; Khan, M.I.; Khalid, M.; Ahmad, M. A Naturally occurring flavone (chrysin): Chemistry, occurrence, pharmacokinetic, toxicity, molecular targets and medicinal properties. J. Biol. Act. Prod. Nat. 2018, 8, 208–227. [Google Scholar] [CrossRef]
- Islam, A.; Islam, M.S.; Uddin, M.N.; Hasan, M.M.I.; Akanda, M.R. The potential health benefits of the isoflavone glycoside genistin. Arch. Pharmacal Res. 2020, 43, 395–408. [Google Scholar] [CrossRef]
- Tang, X.; Liao, R.; Zhou, L.; Yi, T.; Ran, M.; Luo, J.; Huang, F.; Wu, A.; Mei, Q.; Wang, L.; et al. Genistin: A novel estrogen analogue targeting ERβ to alleviate thrombocytopenia. Int. J. Biol. Sci. 2024, 20, 2236–2260. [Google Scholar] [CrossRef]
- Jaiswal, N.; Akhtar, J.; Singh, S.P.; Ahsan, F. An overview on genistein and its various formulations. Drug Res. 2019, 69, 305–313. [Google Scholar] [CrossRef]
- Arya, S.S.; Rookes, J.E.; Cahill, D.M.; Lenka, S.K. Vanillin: A review on the therapeutic prospects of a popular flavouring molecule. Adv. Tradit. Med. 2021, 21, 1–17. [Google Scholar] [CrossRef]
- Huang, W.; Yang, Y.; Wen, W.; Luo, Y.; Wu, J.; Xiang, L.; Hu, Y.; Xu, S.; Chen, S.; Wang, P. Vanillin enhances the passive transport rate and absorption of drugs with moderate oral bioavailability in vitro and in vivo by affecting the membrane structure. Food Funct. 2020, 11, 700–710. [Google Scholar] [CrossRef]
- Ruwizhi, N.; Aderibigbe, B.A. Cinnamic acid derivatives and their biological efficacy. Int. J. Mol. Sci. 2020, 21, 5712. [Google Scholar] [CrossRef]
- Safaeian, L.; Asghari-Varzaneh, M.; Alavi, S.S.; Halvaei-Varnousfaderani, M.; Laher, I. Cardiovascular protective effects of cinnamic acid as a natural phenolic acid: A review. Arch. Physiol. Biochem. 2025, 131, 52–62. [Google Scholar] [CrossRef]
- Adisakwattana, S. Cinnamic acid and its derivatives: Mechanisms for prevention and management of diabetes and its complications. Nutrients 2017, 9, 163. [Google Scholar] [CrossRef]
- Bickers, D.; Calow, P.; Greim, H.; Hanifin, J.M.; Rogers, A.E.; Saurat, J.H.; Sipes, I.G.; Smith, R.L.; Tagami, H. A Toxicologic and dermatologic assessment of cinnamyl alcohol, cinnamaldehyde and cinnamic acid when used as fragrance ingredients. Food Chem. Toxicol. 2005, 43, 799–836. [Google Scholar] [CrossRef]
- Albuquerque, C.F.B.; de Souza, D.A.A.; Figueiredo, P.L.B.; Rocha, C.Q.; Maia, J.G.S.; Kato, M.J.; Chisté, R.C.; da Silva, J.K.R. Optimization of extraction conditions for improving gallic acid and quercetin content in Pouteria macrophylla fruits: A promising cosmetic ingredient. ACS Omega 2025, 10, 7371–7380. [Google Scholar] [CrossRef]
- Jiang, Y.; Pei, J.; Zheng, Y.; Miao, Y.J.; Duan, B.Z.; Huang, L.F. Gallic acid: A potential anti-cancer agent. Chin. J. Integr. Med. 2022, 28, 661–671. [Google Scholar] [CrossRef]
- Bhuia, M.S.; Rahaman, M.M.; Islam, T.; Bappi, M.H.; Sikder, M.I.; Hossain, K.N.; Akter, F.; Al Shamsh Prottay, A.; Rokonuzzman, M.; Gürer, E.S.; et al. Neurobiological effects of gallic acid: Current perspectives. Chin. Med. 2023, 18, 27. [Google Scholar] [CrossRef]
- Wianowska, D.; Olszowy-Tomczyk, M. A concise profile of gallic acid—From its natural sources through biological properties and chemical methods of determination. Molecules 2023, 28, 1186. [Google Scholar] [CrossRef]
- Verma, S.; Singh, A.; Mishra, A. Gallic acid: Molecular rival of cancer. Environ. Toxicol. Pharmacol. 2013, 35, 473–485. [Google Scholar] [CrossRef]
- Kahkeshani, N.; Farzaei, F.; Fotouhi, M.; Alavi, S.S.; Bahramsoltani, R.; Naseri, R.; Momtaz, S.; Abbasabadi, Z.; Rahimi, R.; Farzaei, M.H.; et al. pharmacological effects of gallic acid in health and diseases: A mechanistic review. Iran J. Basic Med. Sci. 2019, 22, 225. [Google Scholar] [CrossRef]
- Hadidi, M.; Liñán-Atero, R.; Tarahi, M.; Christodoulou, M.C.; Aghababaei, F. The potential health benefits of gallic acid: Therapeutic and food applications. Antioxidants 2024, 13, 1001. [Google Scholar] [CrossRef]
- Zhao, X.L.; Cao, Z.J.; Li, K.-D.; Tang, F.; Xu, L.-Y.; Zhang, J.-N.; Liu, D.; Peng, C.; Ao, H. Gallic acid: A dietary metabolite’s therapeutic potential in the management of atherosclerotic cardiovascular disease. Front. Pharmacol. 2024, 15, 1515172. [Google Scholar] [CrossRef]
- Kaliora, A.C.; Kanellos, P.T.; Kalogeropoulos, N. Gallic acid bioavailability in humans. In Handbook on Gallic Acid; Thompson, M.A., Collins, P.B., Eds.; Nova Science: New York, NY, USA, 2013; pp. 301–312. [Google Scholar]
- Sarimahmut, M.; Vekshari, S.; Karaali, D.; Çelikler, S. In vitro evaluation of antigenotoxic effects of phloridzin. Cumhur. Sci. J. 2022, 43, 358–364. [Google Scholar] [CrossRef]
- Lv, F.; Chen, Y.; Xie, H.; Gao, M.; He, R.; Deng, W.Y.; Chen, W. Therapeutic potential of phloridzin carbomer gel for skin inflammatory healing in atopic dermatitis. Arch. Dermatol. Res. 2025, 317, 352. [Google Scholar] [CrossRef]
- Wang, L.; Wu, X.; Wan, Q.; Yang, Y.; Gao, C. Phloridzin reduces synovial hyperplasia and inflammation in rheumatoid arthritis rat by modulating MTOR pathway. Int. Immunopharmacol. 2024, 133, 111727. [Google Scholar] [CrossRef]
- Tian, L.; Cao, J.; Zhao, T.; Liu, Y.; Khan, A.; Cheng, G. The bioavailability, extraction, biosynthesis and distribution of natural dihydrochalcone: Phloridzin. Int. J. Mol. Sci. 2021, 22, 962. [Google Scholar] [CrossRef]
- Londzin, P.; Siudak, S.; Cegieła, U.; Pytlik, M.; Janas, A.; Waligóra, A.; Folwarczna, J. Phloridzin, an apple polyphenol, exerted unfavorable effects on bone and muscle in an experimental model of type 2 diabetes in rats. Nutrients 2018, 10, 1701. [Google Scholar] [CrossRef]
- Rampogu, S.; Gajula, R.G.; Lee, K.W. A comprehensive review on chemotherapeutic potential of galangin. Biomed. Pharmacother. 2021, 141, 111808. [Google Scholar] [CrossRef]
- Thapa, R.; Afzal, O.; Alfawaz Altamimi, A.S.; Goyal, A.; Almalki, W.H.; Alzarea, S.I.; Kazmi, I.; Jakhmola, V.; Singh, S.K.; Dua, K.; et al. Galangin as an inflammatory response modulator: An updated overview and therapeutic potential. Chem. Biol. Interact. 2023, 378, 110482. [Google Scholar] [CrossRef]
- Khawaja, G.; El-Orfali, Y.; Shoujaa, A.; Abou Najem, S. Galangin: A promising flavonoid for the treatment of rheumatoid arthritis—Mechanisms, evidence, and therapeutic potential. Pharmaceuticals 2024, 17, 963. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, B.; Deng, H.; Zhang, C.; Wang, Y.; Chen, L.; Teng, H. Galangin alleviates alcohol-provoked liver injury associated with gut microbiota disorder and intestinal barrier dysfunction in mice. J. Agric. Food Chem. 2024, 72, 22336–22348. [Google Scholar] [CrossRef]
- Chen, F.; Tan, Y.F.; Li, H.L.; Qin, Z.M.; Cai, H.D.; Lai, W.Y.; Zhang, X.P.; Li, Y.H.; Guan, W.W.; Li, Y.B.; et al. Differential systemic exposure to galangin after oral and intravenous administration to rats. Chem. Cent. J. 2015, 9, 14. [Google Scholar] [CrossRef]
- Çakır, D.K.; Zannou, O.; Koca, I. Scopoletin contents and antioxidant properties of some edible plants of black sea regions. Discov. Food 2022, 2, 1–10. [Google Scholar] [CrossRef]
- Antika, L.D.; Tasfiyati, A.N.; Hikmat, H.; Septama, A.W. Scopoletin: A review of its source, biosynthesis, methods of extraction, and pharmacological activities. Z. Naturforsch. C J. Biosci. 2022, 77, 303–316. [Google Scholar] [CrossRef]
- Gao, X.Y.; Li, X.Y.; Zhang, C.Y.; Bai, C.Y. Scopoletin: A review of its pharmacology, pharmacokinetics, and toxicity. Front. Pharmacol. 2024, 15, 1268464. [Google Scholar] [CrossRef]
- Zeng, Y.C.; Li, S.; Liu, C.; Gong, T.; Sun, X.; Fu, Y.; Zhang, Z.R. Soluplus micelles for improving the oral bioavailability of scopoletin and their hypouricemic effect in vivo. Acta Pharmacol. Sin. 2017, 38, 424–433. [Google Scholar] [CrossRef]
- Parama, D.; Girisa, S.; Khatoon, E.; Kumar, A.; Alqahtani, M.S.; Abbas, M.; Sethi, G.; Kunnumakkara, A.B. An overview of the pharmacological activities of scopoletin against different chronic diseases. Pharmacol. Res. 2022, 179, 106202. [Google Scholar] [CrossRef]
- Monteiro Espíndola, K.M.; Ferreira, R.G.; Mosquera Narvaez, L.E.; Rocha Silva Rosario, A.C.; Machado Da Silva, A.H.; Bispo Silva, A.G.; Oliveira Vieira, A.P.; Chagas Monteiro, M. Chemical and pharmacological aspects of caffeic acid and its activity in hepatocarcinoma. Front. Oncol. 2019, 9, 467241. [Google Scholar] [CrossRef]
- Pavlíková, N. Caffeic acid and diseases—Mechanisms of action. Int. J. Mol. Sci. 2022, 24, 588. [Google Scholar] [CrossRef]
- Bhuia, M.S.; Ferdous, J.; Chowdhury, R.; Ansari, S.A.; Ansari, I.A.; Al Hasan, M.S.; Sheikh, S.; Islam, M.T. Exploring the antiemetic potential of caffeic acid: A combined in vivo and computational approach. Neurogastroenterol. Motil. 2025, 37, e70003. [Google Scholar] [CrossRef]
- Muhammad Abdul Kadar, N.N.; Ahmad, F.; Teoh, S.L.; Yahaya, M.F. Caffeic acid on metabolic syndrome: A review. Molecules 2021, 26, 5490. [Google Scholar] [CrossRef]
- Boo, Y.C. p-Coumaric acid as an active ingredient in cosmetics: A review focusing on its antimelanogenic effects. Antioxidants 2019, 8, 275. [Google Scholar] [CrossRef]
- Pei, K.; Ou, J.; Huang, J.; Ou, S. p-Coumaric acid and its conjugates: Dietary sources, pharmacokinetic properties and biological activities. J. Sci. Food Agric. 2016, 96, 2952–2962. [Google Scholar] [CrossRef]
- Zduńska, K.; Dana, A.; Kolodziejczak, A.; Rotsztejn, H. Antioxidant properties of ferulic acid and its possible application. Ski. Pharmacol. Physiol. 2018, 31, 332–336. [Google Scholar] [CrossRef]
- Markowska, A.; Markowska, J.; Stanisławiak-Rudowicz, J.; Kozak, K.; Roubinek, O.K.; Jasińska, M. The role of ferulic acid in selected malignant neoplasms. Molecules 2025, 30, 1018. [Google Scholar] [CrossRef]
- Zheng, M.; Liu, Y.; Zhang, G.; Yang, Z.; Xu, W.; Chen, Q. The antioxidant properties, metabolism, application and mechanism of ferulic acid in medicine, food, cosmetics, livestock and poultry. Antioxidants 2024, 13, 853. [Google Scholar] [CrossRef]
- Gortzi, O.; Patsios, S.I.; Pyrzynska, K. Ferulic acid—A brief review of its extraction, bioavailability and biological activity. Separations 2024, 11, 204. [Google Scholar] [CrossRef]
- Raj, N.D.; Singh, D. A critical appraisal on ferulic acid: Biological profile, biopharmaceutical challenges and nano formulations. Health Sci. Rev. 2022, 5, 100063. [Google Scholar] [CrossRef]
- Naveed, M.; Hejazi, V.; Abbas, M.; Kamboh, A.A.; Khan, G.J.; Shumzaid, M.; Ahmad, F.; Babazadeh, D.; FangFang, X.; Modarresi-Ghazani, F.; et al. Chlorogenic acid (CGA): A pharmacological review and call for further research. Biomed. Pharmacother. 2018, 97, 67–74. [Google Scholar] [CrossRef]
- Nguyen, V.; Taine, E.G.; Meng, D.; Cui, T.; Tan, W. Chlorogenic acid: A systematic review on the biological functions, mechanistic actions, and therapeutic potentials. Nutrients 2024, 16, 924. [Google Scholar] [CrossRef]
- Golmei, P.; Kasna, S.; Roy, K.P.; Kumar, S. A review on pharmacological advancement of ellagic acid. J. Pharmacol. Pharmacother. 2024, 15, 93–104. [Google Scholar] [CrossRef]
- Lu, G.; Wang, X.; Cheng, M.; Wang, S.; Ma, K. The multifaceted mechanisms of ellagic acid in the treatment of tumors: State-of-the-art. Biomed. Pharmacother. 2023, 165, 115132. [Google Scholar] [CrossRef]
- Naraki, K.; Ghasemzadeh Rahbardar, M.; Ajiboye, B.O.; Hosseinzadeh, H. The effect of ellagic acid on the metabolic syndrome: A review article. Heliyon 2023, 9, e21844. [Google Scholar] [CrossRef]
- Ríos, J.L.; Giner, R.M.; Marín, M.; Recio, M.C. A Pharmacological update of ellagic acid. Planta Med. 2018, 84, 1068–1093. [Google Scholar] [CrossRef]
- Lafay, S.; Gil-Izquierdo, A. Bioavailability of phenolic acids. Phytochem. Rev. 2008, 7, 301–311. [Google Scholar] [CrossRef]
- Przybylska-Balcerek, A.; Stuper-Szablewska, K. The effect of phenolic acids on living organisms. Indian J. Med. Res. Pharm. Sci. 2019, 6, 1–14. [Google Scholar] [CrossRef]
- Jian, X.; Shi, C.; Luo, W.; Zhou, L.; Jiang, L.; Liu, K. Therapeutic effects and molecular mechanisms of quercetin in gynecological disorders. Biomed. Pharmacother. 2024, 173, 116418. [Google Scholar] [CrossRef]
- Pei, J.; Kumarasamy, R.V.; Jayaraman, S.; Kanniappan, G.V.; Long, Q.; Palanisamy, C.P. Quercetin-functionalized nanomaterials: Innovative therapeutic avenues for Alzheimer’s disease management. Ageing Res. Rev. 2025, 104, 102665. [Google Scholar] [CrossRef]
- Kasahara, K.; Kerby, R.L.; Aquino-Martinez, R.; Evered, A.H.; Cross, T.-W.L.; Everhart, J.; Ulland, T.K.; Kay, C.D.; Bolling, B.W.; Bäckhed, F.; et al. Gut microbes modulate the effects of the flavonoid quercetin on atherosclerosis. NPJ Biofilms Microbiomes. 2025, 11, 12. [Google Scholar] [CrossRef]
- Bian, X.; Ge, Z.; Chen, X.; Zhong, S.; Li, L.; Xu, W.; Li, B.; Chen, S.; Lv, G. Protective effects and mechanisms of quercetin in animal models of hyperuricemia: A systematic review and meta-analysis. Pharmacol. Res. 2025, 213, 107665. [Google Scholar] [CrossRef]
- Carrillo-Martinez, E.J.; Flores-Hernández, F.Y.; Salazar-Montes, A.M.; Nario-Chaidez, H.F.; Hernández-Ortega, L.D. Quercetin, a flavonoid with great pharmacological capacity. Molecules 2024, 29, 1000. [Google Scholar] [CrossRef]
- Aghababaei, F.; Hadidi, M. Recent advances in potential health benefits of quercetin. Pharmaceuticals 2023, 16, 1020. [Google Scholar] [CrossRef]
- Zou, H.; Ye, H.; Kamaraj, R.; Zhang, T.; Zhang, J.; Pavek, P. A review on pharmacological activities and synergistic effect of quercetin with small molecule agents. Phytomedicine 2021, 92, 153736. [Google Scholar] [CrossRef]
- Harwood, M.; Danielewska-Nikiel, B.; Borzelleca, J.F.; Flamm, G.W.; Williams, G.M.; Lines, T.C. A critical review of the data related to the safety of quercetin and lack of evidence of in vivo toxicity, including lack of genotoxic/carcinogenic properties. Food Chem. Toxicol. 2007, 45, 2179–2205. [Google Scholar] [CrossRef]
- Kandemir, K.; Tomas, M.; McClements, D.J.; Capanoglu, E. Recent advances on the improvement of quercetin bioavailability. Trends Food Sci. Technol. 2022, 119, 192–200. [Google Scholar] [CrossRef]
- Isemura, M. Catechin in human health and disease. Molecules 2019, 24, 528. [Google Scholar] [CrossRef]
- Ganeshpurkar, A.; Saluja, A. The pharmacological potential of catechin. Indian J. Biochem. Biophys. 2020, 57, 505–511. [Google Scholar] [CrossRef]
- Bernal-Mercado, A.T.; Vazquez-Armenta, F.J.; Tapia-Rodriguez, M.R.; Islas-Osuna, M.A.; Mata-Haro, V.; Gonzalez-Aguilar, G.A.; Lopez-Zavala, A.A.; Ayala-Zavala, J.F. Comparison of single and combined use of catechin, protocatechuic, and vanillic acids as antioxidant and antibacterial agents against uropathogenic Escherichia coli at planktonic and biofilm levels. Molecules 2018, 23, 2813. [Google Scholar] [CrossRef]
- Peters, C.M.; Green, R.J.; Janle, E.M.; Ferruzzi, M.G. Formulation with ascorbic acid and sucrose modulates catechin bioavailability from green tea. Food Res. Int. 2010, 43, 95–102. [Google Scholar] [CrossRef]
- Ganeshpurkar, A.; Saluja, A.K. The pharmacological potential of rutin. Saudi Pharm. J. 2017, 25, 149–164. [Google Scholar] [CrossRef]
- Wang, X.; Xia, X.; Song, X.; Zhou, Y.; Ma, M.; Ren, Y.; Chen, X.; Xia, Z.; Guo, Y.; Song, C. Therapeutic potential of rutin in premenstrual depression: Evidence from in vivo and in vitro studies. Front. Pharmacol. 2024, 15, 1525753. [Google Scholar] [CrossRef]
- Hamid, Z.M.; Sahib, H.B. The acute toxicity of rutin in mice. Iraqi J. Pharm. Sci. 2021, 30, 231–240. [Google Scholar] [CrossRef]
- Rauf, A.; Imran, M.; Abu-Izneid, T.; Iahtisham-Ul-Haq; Patel, S.; Pan, X.; Naz, S.; Sanches Silva, A.; Saeed, F.; Rasul Suleria, H.A. Proanthocyanidins: A comprehensive review. Biomed. Pharmacother. 2019, 116, 108999. [Google Scholar] [CrossRef]
- Qi, Q.; Chu, M.; Yu, X.; Xie, Y.; Li, Y.; Du, Y.; Liu, X.; Zhang, Z.; Shi, J.; Yan, N. Anthocyanins and proanthocyanidins: Chemical structures, food sources, bioactivities, and product development. Food Rev. Int. 2023, 39, 4581–4609. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, Y.; Li, D.; Ho, C.T.; Li, J.; Wan, X. The absorption, distribution, metabolism and excretion of procyanidins. Food Funct. 2016, 7, 1273–1281. [Google Scholar] [CrossRef]
- Yamakoshi, J.; Saito, M.; Kataoka, S.; Kikuchi, M. Safety evaluation of proanthocyanidin-rich extract from grape seeds. Food Chem. Toxicol. 2002, 40, 599–607. [Google Scholar] [CrossRef]
- Rein, M.J.; Renouf, M.; Cruz-Hernandez, C.; Actis-Goretta, L.; Thakkar, S.K.; da Silva Pinto, M. Bioavailability of bioactive food compounds: A challenging journey to bioefficacy. Br. J. Clin. Pharmacol. 2013, 75, 588–602. [Google Scholar] [CrossRef]
- Colunga Biancatelli, R.M.L.; Berrill, M.; Catravas, J.D.; Marik, P.E. Quercetin and vitamin C: An experimental, synergistic therapy for the prevention and treatment of SARS-CoV-2 related disease (COVID-19). Front. Immunol. 2020, 11, 1451. [Google Scholar] [CrossRef]
- Ge, C.; Wei, X.; Xu, Y.; Jiang, Y.; Yang, X.; Lin, J.; Li, M.; Tian, Y.; Fan, S.; Ye, T.; et al. Natural ellagic acid-polyphenol ″sandwich biscuit″ self-assembled solubilizing system for formation mechanism and antibacterial synergia. ACS Appl. Mater. Interfaces 2025, 17, 27772–27787. [Google Scholar] [CrossRef]
- Liu, Y.; Guan, L.; Yang, D.; Luo, H.; Zhang, H. Investigating the synergistic antibacterial effects of chlorogenic and p-coumaric acids on Shigella dysenteriae. Food Chem. 2025, 462, 141011. [Google Scholar] [CrossRef]
- Guan, H.; Zhang, W.; Liu, H.; Jiang, Y.; Li, F.; Wang, D.; Liu, Y.; He, F.; Wu, M.; Waterhouse, G.I.N.; et al. Simultaneous binding of quercetin and catechin to FOXO3 enhances IKKα transcription inhibition and suppression of oxidative stress-induced acute alcoholic liver injury in rats. J. Adv. Res. 2025, 67, 71–92. [Google Scholar] [CrossRef]
- Mukai, K.; Mitani, S.; Ohara, K.; Nagaoka, S.I. Structure–activity relationship of the tocopherol-regeneration reaction by catechins. Free Radic. Biol. Med. 2005, 38, 1243–1256. [Google Scholar] [CrossRef]
- Jaramillo Carmona, S.M.; López Martín, S.; Abia, R.; Rodriguez-Arcos, R.; Jiménez Araujo, A.; Guillén Bejarano, R.; Muriana, F.J. Combination of quercetin and kaempferol enhances in vitro cytotoxicity on human colon cancer (HCT-116) cells. Rec. Nat. Prod. 2014, 8, 262–271. [Google Scholar]
- Mok, J.Y.; Jeong, S.I.; Kim, J.H.; Jang, S.I. Synergic effect of quercetin and astragalin from mulberry leaves on anti-inflammation. Korean J. Orient. Physiol. Pathol. 2011, 25, 830–836. [Google Scholar]
- Hajimehdipoor, H.; Shahrestani, R.; Shekarchi, M. Investigating the synergistic antioxidant effects of some flavonoid and phenolic compounds. Res. J. Pharmacogn. 2014, 1, 35–40. [Google Scholar]
- Liu, X.; Zhao, T.; Shi, Z.; Hu, C.; Li, Q.; Sun, C. Synergism antiproliferative effects of apigenin and naringenin in NSCLC cells. Molecules 2023, 28, 4947. [Google Scholar] [CrossRef]
- Biswas, P.; Kaium, M.A.; Islam Tareq, M.M.; Tauhida, S.J.; Hossain, M.R.; Siam, L.S.; Parvez, A.; Bibi, S.; Hasan, M.H.; Rahman, M.M.; et al. The experimental significance of isorhamnetin as an effective therapeutic option for cancer: A comprehensive analysis. Biomed. Pharmacother. 2024, 176, 116860. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, W.; Chen, F.; Lu, Z. Combined effect of chrysin and apigenin on inhibiting the development and progression of colorectal cancer by suppressing the activity of P38-MAPK/AKT pathway. IUBMB Life 2021, 73, 774–783. [Google Scholar] [CrossRef]
- Harasstani, O.A.; Tham, C.L.; Israf, D.A. Kaempferol and chrysin synergies to improve septic mice survival. Molecules 2017, 22, 92. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, M.; Tao, Q.; Li, Y.; Wang, H.; Zhang, M.; Liu, X.; Zhang, J. Betaine–salicylic acid cocrystal for enhanced skincare and acne treatment. RSC Med. Chem. 2025, 16, 1705–1714. [Google Scholar] [CrossRef]
- Bezerra, C.F.; Camilo, C.J.; do Nascimento Silva, M.K.; de Freitas, T.S.; Ribeiro-Filho, J.; Coutinho, H.D.M. Vanillin selectively modulates the action of antibiotics against resistant bacteria. Microb. Pathog. 2017, 113, 265–268. [Google Scholar] [CrossRef]
- Catanzaro, E.; Greco, G.; Potenza, L.; Calcabrini, C.; Fimognari, C. Natural products to fight cancer: A focus on Juglans regia. Toxins 2018, 10, 469. [Google Scholar] [CrossRef]
- Jafari, S.; Dabiri, S.; Mehdizadeh Aghdam, E.; Fathi, E.; Saeedi, N.; Montazersaheb, S.; Farahzadi, R. Synergistic effect of chrysin and radiotherapy against triple-negative breast cancer (TNBC) cell lines. Clin. Transl. Oncol. 2023, 25, 2559–2568. [Google Scholar] [CrossRef]
- Sarg, N.H.; Hersi, F.H.; Zaher, D.M.; Hamouda, A.O.; Ibrahim, S.I.; El-Seedi, H.R.; Omar, H.A. Unveiling the therapeutic potential of taxifolin in cancer: From molecular mechanisms to immune modulation and synergistic combinations. Phytomedicine 2024, 133, 155934. [Google Scholar] [CrossRef]
- Materska, M. Quercetin and its derivatives: Chemical structure and bioactivity - a review. Pol. J. Food Nutr. Sci. 2008, 58, 407–413. [Google Scholar]
- Pietta, P.G. Flavonoids as antioxidants. J. Nat. Prod. 2000, 63, 1035–1042. [Google Scholar] [CrossRef]
- Sanchez-Maldonado, A.F. Mode of Action, Interaction and Recovery of Plant Secondary Metabolites for Potential Applications as Food Preservatives. Ph.D. Thesis, University of Alberta, Edmonton, Canada, 2014. [Google Scholar] [CrossRef]
- Bhuyan, D.J.; Basu, A. Phenolic compounds potential health benefits and toxicity. In Utilisation of Bioactive Compounds from Agricultural and Food Waste, 1st ed.; Vuong, Q.V., Ed.; CRC Press: Boca Raton, FL, USA, 2017; pp. 27–59. [Google Scholar] [CrossRef]
- Pizzimenti, S.; Muzio, G.; Barrera, G.; Giner, R.M.; Ríos, J.L.; Máñez, S. Antioxidant activity of natural hydroquinones. Antioxidants 2022, 11, 343. [Google Scholar] [CrossRef]
- Xiang, H.H.; Seong, S.H.; Ji, S.H.; Myung, K.L.; Bang, Y.H.; Ro, J.S. Monoamine oxidase inhibitory components from Cayratia japonica. Arch. Pharm. Res. 2007, 30, 13–17. [Google Scholar] [CrossRef]
- Abou Baker, D.H. An ethnopharmacological review on the therapeutical properties of flavonoids and their mechanisms of actions: A comprehensive review based on up to date knowledge. Toxicol. Rep. 2022, 9, 445–469. [Google Scholar] [CrossRef]
- Procházková, D.; Boušová, I.; Wilhelmová, N. Antioxidant and prooxidant properties of flavonoids. Fitoterapia 2011, 82, 513–523. [Google Scholar] [CrossRef]
- Latos-Brozio, M.; Masek, A. Structure-activity relationships analysis of monomeric and polymeric polyphenols (quercetin, rutin and catechin) obtained by various polymerization methods. Chem. Biodivers. 2019, 16, e1900426. [Google Scholar] [CrossRef]
- Zahoor, M.; Shafiq, S.; Ullah, H.; Sadiq, A.; Ullah, F. Isolation of quercetin and mandelic acid from Aesculus indica fruit and their biological activities. BMC Biochem. 2018, 19, 5. [Google Scholar] [CrossRef]
- Bentz, A.B. A review of quercetin: Chemistry, antioxidant properties, and bioavailability. J. Young Investig. 2009. Available online: https://www.jyi.org/2009-april/2017/10/15/a-review-of-quercetin-chemistry-antioxidant-properties-and-bioavailability (accessed on 1 October 2024).
- Magar, R.T.; Sohng, J.K. A review on structure, modifications and structure-activity relation of quercetin and its derivatives. J. Microbiol. Biotechnol. 2020, 30, 11–20. [Google Scholar] [CrossRef]
- Tu, B.; Liu, Z.-J.; Chen, Z.-F.; Ouyang, Y.; Hu, Y.-J. Understanding the structure–activity relationship between quercetin and naringenin: In vitro. RSC Adv. 2015, 5, 106171–106181. [Google Scholar] [CrossRef]
- Li, M.M.; Chen, Y.T.; Ruan, J.C.; Wang, W.J.; Chen, J.G.; Zhang, Q.F. Structure-activity relationship of dietary flavonoids on pancreatic lipase. Curr. Res. Food Sci. 2023, 6, 100424. [Google Scholar] [CrossRef]
- Nguyen, T.A.; Liu, B.; Zhao, J.; Thomas, D.S.; Hook, J.M. An investigation into the supramolecular structure, solubility, stability and antioxidant activity of rutin/cyclodextrin inclusion complex. Food Chem. 2013, 136, 186–192. [Google Scholar] [CrossRef]
- Rho, H.S.; Ghimeray, A.K.; Yoo, D.S.; Ahn, S.M.; Kwon, S.S.; Lee, K.H.; Cho, D.H.; Cho, J.Y. Kaempferol and kaempferol rhamnosides with depigmenting and anti-inflammatory properties. Molecules 2011, 16, 3338–3344. [Google Scholar] [CrossRef]
- Wada, E.; Ito, C.; Shinohara, M.; Handa, S.; Maetani, M.; Yasugi, M.; Miyake, M.; Sakamoto, T.; Yazawa, A.; Kamitani, S. Prunin laurate derived from natural substances shows antibacterial activity against the periodontal pathogen Porphyromonas gingivalis. Foods 2024, 13, 1917. [Google Scholar] [CrossRef]
- Céliz, G.; Daz, M.; Audisio, M.C. Antibacterial activity of naringin derivatives against pathogenic strains. J. Appl. Microbiol. 2011, 111, 731–738. [Google Scholar] [CrossRef]
- Smiljkovic, M.; Stanisavljevic, D.; Stojkovic, D.; Petrovic, I.; Vicentic, J.M.; Popovic, J.; Golic Grdadolnik, S.G.; Markovic, D.; Sanković-Babić, S.; Glamoclija, J.; et al. Apigenin-7-O-glucoside versus apigenin: Insight into the modes of anticandidal and cytotoxic actions. EXCLI J. 2017, 16, 795–807. [Google Scholar] [CrossRef]
- Naz, S.; Imran, M.; Rauf, A.; Orhan, I.E.; Shariati, M.A.; Iahtisham-Ul-Haq; IqraYasmin; Shahbaz, M.; Qaisrani, T.B.; Shah, Z.A.; et al. Chrysin: Pharmacological and therapeutic properties. Life Sci. 2019, 235, 116797. [Google Scholar] [CrossRef]
- Liu, Y.; Song, X.; He, J.; Zheng, X.; Wu, H. Synthetic derivatives of chrysin and their biological activities. Med. Chem. Res. 2014, 23, 555–563. [Google Scholar] [CrossRef]
- Shamsudin, N.F.; Ahmed, Q.U.; Mahmood, S.; Shah, S.A.A.; Khatib, A.; Mukhtar, S.; Alsharif, M.A.; Parveen, H.; Zakaria, Z.A. Antibacterial effects of flavonoids and their structure-activity relationship study: A comparative interpretation. Molecules 2022, 27, 1149. [Google Scholar] [CrossRef]
- Sato, M.; Murakami, K.; Uno, M.; Ikubo, H.; Nakagawa, Y.; Katayama, S.; Akagi, K.; Irie, K. Structure-activity relationship for (+)-taxifolin isolated from silymarin as an inhibitor of amyloid β aggregation. Biosci. Biotechnol. Biochem. 2013, 77, 1100–1103. [Google Scholar] [CrossRef]
- Renzetti, A.; Betts, J.W.; Fukumoto, K.; Rutherford, R.N. Antibacterial green tea catechins from a molecular perspective: Mechanisms of action and structure–activity relationships. Food Funct. 2020, 11, 9370–9396. [Google Scholar] [CrossRef]
- Ahmadi, S.M.; Farhoosh, R.; Sharif, A.; Rezaie, M. Structure-antioxidant activity relationships of luteolin and catechin. J. Food Sci. 2020, 85, 298–305. [Google Scholar] [CrossRef]
- Dixon, R.A.; Ferreira, D. Genistein. Phytochemistry 2002, 60, 205–211. [Google Scholar] [CrossRef]
- Li, X.; Chen, B.; Xie, H.; He, Y.; Zhong, D.; Chen, D. Antioxidant structure–activity relationship analysis of five dihydrochalcones. Molecules 2018, 23, 1162. [Google Scholar] [CrossRef]
- Guerrero, L.; Castillo, J.; Quiñones, M.; Garcia-Vallvé, S.; Arola, L.; Pujadas, G.; Muguerza, B. Inhibition of angiotensin-converting enzyme activity by flavonoids: Structure-activity relationship studies. PLoS ONE 2012, 7, e49493. [Google Scholar] [CrossRef]
- Zhang, S.; Yang, X.; Coburn, R.A.; Morris, M.E. Structure activity relationships and quantitative structure activity relationships for the flavonoid-mediated inhibition of breast cancer resistance protein. Biochem. Pharmacol. 2005, 70, 627–639. [Google Scholar] [CrossRef]
- Li, Z.; Liu, J.; You, J.; Li, X.; Liang, Z.; Du, J. Proanthocyanidin structure-activity relationship analysis by path analysis model. Int. J. Mol. Sci. 2023, 24, 6379. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, L.; Wang, Y.; Chen, Z.; Zhang, M.; Panichayupakaranant, P.; Chen, H. Study on the active polyphenol constituents in differently colored Rubus chingii Hu and the structure-activity relationship of the main ellagitannins and ellagic acid. LWT 2020, 121, 108967. [Google Scholar] [CrossRef]
- Chen, J.; Yang, J.; Ma, L.; Li, J.; Shahzad, N.; Kim, C.K. Structure-antioxidant activity relationship of methoxy, phenolic hydroxyl, and carboxylic acid groups of phenolic acids. Sci. Rep. 2020, 10, 2611. [Google Scholar] [CrossRef]
- Otero, E.; Robledo, S.M.; Díaz, S.; Carda, M.; Muñoz, D.; Paños, J.; Vélez, I.D.; Cardona, W. Synthesis and leishmanicidal activity of cinnamic acid esters: Structure-activity relationship. Med. Chem. Res. 2014, 23, 1378–1386. [Google Scholar] [CrossRef]
- Fernandez-Martinez, E.; Bobadilla, R.A.; Morales-Rios, M.S.; Muriel, P.; Perez-Alvarez, V.M. Trans-3-phenyl-2-propenoic acid (cinnamic acid) derivatives: Structure-activity relationship as hepatoprotective agents. Med. Chem. 2007, 3, 475–479. [Google Scholar] [CrossRef]
- Razzaghi-Asl, N.; Garrido, J.; Khazraei, H.; Borges, F.; Firuzi, O. Antioxidant properties of hydroxycinnamic acids: A review of structure- activity relationships. Curr. Med. Chem. 2013, 20, 4436–4450. [Google Scholar] [CrossRef]
- De Armas-Ricard, M.; Ruiz-Reyes, E.; Ramírez-Rodríguez, O. Caffeates and caffeamides: Synthetic methodologies and their antioxidant properties. Int. J. Med. Chem. 2019, 2019, 2592609. [Google Scholar] [CrossRef]
- Dhiman, P.; Malik, N.; Khatkar, A. Hybrid caffeic acid derivatives as monoamine oxidases inhibitors: Synthesis, radical scavenging activity, molecular docking studies and in silico ADMET analysis. Chem. Cent. J. 2018, 12, 112. [Google Scholar] [CrossRef]
- Kiliç, I.; Yeşiloǧlu, Y. Spectroscopic studies on the antioxidant activity of p-coumaric acid. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2013, 115, 719–724. [Google Scholar] [CrossRef]
- Khatkar, A.; Nanda, A.; Kumar, P.; Narasimhan, B. Synthesis, antimicrobial evaluation and QSAR studies of p-coumaric acid derivatives. Arab. J. Chem. 2017, 10, S3804–S3815. [Google Scholar] [CrossRef]
- de Oliveira Silva, E.; Batista, R. Ferulic acid and naturally occurring compounds bearing a feruloyl moiety: A review on their structures, occurrence, and potential health benefits. Compr. Rev. Food Sci. Food Saf. 2017, 16, 580–616. [Google Scholar] [CrossRef]
- Li, W.; Li, N.; Tang, Y.; Li, B.; Liu, L.; Zhang, X.; Fu, H.; Duan, J.A. Biological activity evaluation and structure–activity relationships analysis of ferulic acid and caffeic acid derivatives for anticancer. Bioorg. Med. Chem. Lett. 2012, 22, 6085–6088. [Google Scholar] [CrossRef]
- Varzaru, I.; Oancea, A.G.; Vlaicu, P.A.; Saracila, M.; Untea, A.E. Exploring the antioxidant potential of blackberry and raspberry leaves: Phytochemical analysis, scavenging activity, and in vitro polyphenol bioaccessibility. Antioxidants 2023, 12, 2125. [Google Scholar] [CrossRef]
- Macario, A.; López, J.C.; Blanco, S. Molecular structure of salicylic acid and its hydrates: A rotational spectroscopy study. Int. J. Mol. Sci. 2024, 25, 4074. [Google Scholar] [CrossRef]
- Kim, J.; Kang, S.; Hong, S.; Yum, S.; Kim, Y.M.; Jung, Y. Structure–Activity relationship of salicylic acid derivatives on inhibition of TNF-α dependent NFκB activity: Implication on anti-inflammatory effect of n-(5-chlorosalicyloyl)phenethylamine against experimental colitis. Eur. J. Med. Chem. 2012, 48, 36–44. [Google Scholar] [CrossRef]
- Morais, S.M.; Silva, K.A.; Araujo, H.; Vieira, I.G.P.; Alves, D.R.; Fontenelle, R.O.S.; Silva, A.M.S. Anacardic acid constituents from cashew nut shell liquid: NMR characterization and the effect of unsaturation on its biological activities. Pharmaceuticals 2017, 10, 31. [Google Scholar] [CrossRef]
- Liu, C.; Zheng, P.; Wang, H.; Wei, Y.; Wang, C.; Hao, S. Design and synthesis of scopoletin sulfonate derivatives as potential insecticidal agents. Molecules 2023, 28, 530. [Google Scholar] [CrossRef]
- Shalayel, M.H.F.; Al-Mazaideh, G.M.; Alanezi, A.A.; Almuqati, A.F.; Alotaibi, M. The potential anti-cancerous activity of Prunus amygdalus var. amara extract. Processes 2023, 11, 1277. [Google Scholar] [CrossRef]
- Walton, N.J.; Mayer, M.J.; Narbad, A. Vanillin. Phytochemistry 2003, 63, 505–515. [Google Scholar] [CrossRef]
- Fitzgerald, D.J.; Stratford, M.; Gasson, M.J.; Narbad, A. Structure-function analysis of the vanillin molecule and its antifungal properties. J. Agric. Food Chem. 2005, 53, 1769–1775. [Google Scholar] [CrossRef]
- Tai, A.; Sawano, T.; Yazama, F.; Ito, H. Evaluation of antioxidant activity of vanillin by using multiple antioxidant assays. Biochim. Biophys. Acta 2011, 1810, 170–177. [Google Scholar] [CrossRef]
- Chua, N.K.; Coates, H.W.; Brown, A.J. Squalene monooxygenase: A journey to the heart of cholesterol synthesis. Prog. Lipid Res. 2020, 79, 101033. [Google Scholar] [CrossRef]
- Güneş, F.E. Medical use of squalene as a natural antioxidant. J. Marmara Univ. Inst. Health Sci. 2013, 3, 220–228. [Google Scholar] [CrossRef]
- Njus, D.; Kelley, P.M.; Tu, Y.J.; Schlegel, H.B. Ascorbic acid: The chemistry underlying its antioxidant properties. Free Radic. Biol. Med. 2020, 159, 37–43. [Google Scholar] [CrossRef]
- Hon, S.L. Vitamin C (ascorbic acid). In Encyclopedia of Toxicology, 4th ed.; Wexler, P., Ed.; Academic Press: Cambridge, MA, USA, 2024; Volume 9, pp. 805–807. [Google Scholar] [CrossRef]
- Du, J.; Cullen, J.J.; Buettner, G.R. Ascorbic acid: Chemistry, biology and the treatment of cancer. Biochim. Biophys. Acta 2012, 1826, 443–457. [Google Scholar] [CrossRef]
- Lushchak, V.I.; Semchuk, N.M. Tocopherol biosynthesis: Chemistry, regulation and effects of environmental factors. Acta Physiol. Plant. 2012, 34, 1607–1628. [Google Scholar] [CrossRef]
- Rimbach, G.; Moehring, J.; Huebbe, P.; Lodge, J.K. Gene-regulatory activity of α-tocopherol. Molecules 2010, 15, 1746–1761. [Google Scholar] [CrossRef]
- Mamoun, C.; GhanemI, F.Z.; Yelles, N.; Chenni, F.Z.; Essid, R.Y.M.; Patoli, D.; El Haci, I.A.; Benariba, K.; Rahmoun, A.; Kharoubi, O.; et al. Exploring the therapeutic potential of sour cherry leaf extract (Prunus cerasus L.): A comprehensive in vitro and in vivo evaluation. Farmacia 2025, 73, 2. [Google Scholar] [CrossRef]
- Nunes, A.R.; Gonçalves, A.C.; Falcão, A.; Alves, G.; Silva, L.R. Prunus avium L. (sweet cherry) by-products: A source of phenolic compounds with antioxidant and anti-hyperglycemic properties—A review. Appl. Sci. 2021, 11, 8516. [Google Scholar] [CrossRef]
- Roșcan, A.G.; Ifrim, I.-L.; Patriciu, O.-I.; Fînaru, A.-L. Bioactive Compounds from Orchard Waste: A Pharmacological Perspective on Rubus and Prunus Species. In Proceedings of the Conference Proceedings Abstracts of the 20th International Conference OPROTEH 2025, Bacău, Romania, 21–23 May 2025. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).