Abstract
The cationic chloro-P-{[4-(diphenylphosphanyl)phenyl]-N,N-dimethylmethanammonio(norbornadiene)rhodium(I) complex was encapsulated inside a self-assembled hexameric capsule. This capsule was obtained through a reaction involving 2,8,14,20-tetra-undecyl-resorcin[4]arene and water in chloroform. The formation of an inclusion complex was deduced from a combination of spectral measurements (UV-visible spectroscopy, 1H, 31P{1H} NMR and DOSY). The rhodium complex was evaluated in the [2+2+2] cycloaddition between N,N-dipropargyl-p-toluenesulfonamide and arylacetylene derivatives. In the presence of two equivalents of arylacetylenes in water-saturated chloroform at 60 °C for 24 h, the 4-methyl-N-(prop-2-yn-1-yl)-N-((2-tosylisoindolin-5-yl)methyl)benzenesulfonamide, the homocycloaddition product of 1,6-diyne is predominantly formed. In the presence of the supramolecular capsule, a selectivity inversion in favor of 5-aryl-2-tosylisoindoline is observed, with heterocycloaddition products formed in proportions between 53 and 69%.
1. Introduction
Reactivity and selectivity have always been problems in catalytic reactions. Improving the performance of catalytic systems takes time and involves the development of new catalysts. Drawing inspiration from nature, particularly enzymes, the use of confined spaces has emerged as a promising solution for enhancing the efficiency of catalytic systems, especially in cases where targeted reactions result in the formation of multiple products. Indeed, the confinement of a species can induce a modification of the reaction products in relation to free systems, resulting in some cases in a more active system, better selectivity and even the formation of new products [1,2,3,4].
In this context, the integration of supramolecular capsules into homogeneous catalysis has emerged as an innovative approach. These capsules, which are formed by self-assembly, have the capacity to encapsulate substrates or catalysts, thereby creating a well-defined microenvironment. These structures have been shown to mimic the function of enzymes, thereby enabling the local accumulation of reagents, control of reactivity and even acceleration or direction of specific chemical transformations through stabilization of the transition state [5,6,7,8,9,10,11,12].
One of the most widely used capsules, which was discovered by Atwood, consists of six units of 2,8,14,20-tetra-undecyl-resorcin[4]arene (1) and eight water molecules (Figure 1) [13]. The process of host diffusion within the hexameric capsule is achieved by the temporary dissociation of a resorcinarene subunit, thereby exposing the internal cavity to substrates in the solution [14]. The encapsulation of metal complexes within 16 is predominantly predicated on the positive charge carried by the metal center or a ligand [15,16,17,18]. While this method has proven effective, its applications in catalysis are limited. The objective of this study is to utilize a readily available phosphine that can be rendered cationic. Consequently, the triphenylphosphine derivative 2 was selected for further investigation (Figure 1). The phosphorus atom facilitates coordination with a metal center, while the amine provides a cationic charge precursor site through protonation. This positive charge should facilitate π-cation interactions with the inner aromatic walls of the cavity, thereby immobilizing the complex within the capsule, as reported for numerous organometallic complexes [15,16,17,18,19,20,21,22,23,24,25,26].
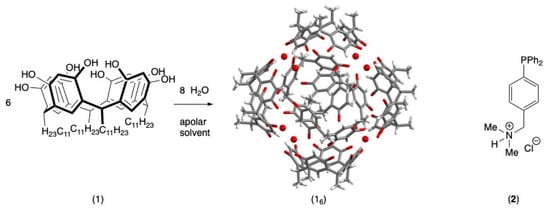
Figure 1.
Formation of the self-assembled capsule 16 and the 4-(diphenylphosphanyl)-phenyl]-N,N-dimethylmethanamine (2) employed in the present study.
The rhodium(I) complex [RhCl(2)(nbd)] (3; nbd = bicyclo[2.2.1]hepta-2,5-diene) will undergo testing in the [2+2+2] cycloaddition of alkynes [27,28,29,30,31,32] in the supramolecular host. For this cycloaddition, it can be seen that the generally used ligands of choice are mono- or bis-triarylphosphines, such as triphenylphosphine and (biphenyl-2,2′-diyl)bis(diphenylphosphine) (BIPHEP) derivatives. As illustrated in Scheme 1, the reaction of N,N-dipropargyl-p-toluenesulfonamide (4) with arylacetylene 5 results in the formation of three distinct products. It was determined that these products correspond to the heterocycloaddition product 6, as well as to mono- and bis-homocycloaddition products 7 and 8, respectively.
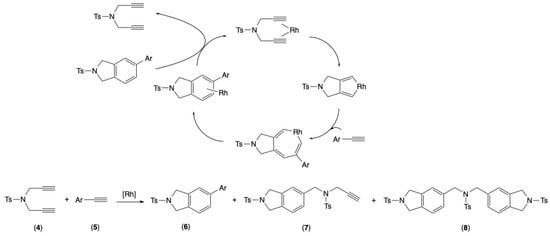
Scheme 1.
Rhodium-catalyzed [2+2+2] cycloaddition of N,N-dipropargyl-p-toluenesulfonamide (4) and arylacetylene 5.
In the case of phenylacetylene (5a), the formation of the product of interest (6a) has been described in the literature as catalyzed by rhodium complexes. For instance, Wu, Zhu and co-workers reported the formation of product 6a in a 52% yield using 5 mol % of the dimeric [Rh2(OAc)2] complex in 1,2-dichloroethane at 80 °C for 24 h. However, the employment of five equivalents of phenylacetylene 5a is imperative to facilitate the heterocycloaddition reaction [33]. On the other hand, Wu and co-workers explored the synthesis of 6a catalyzed by the commercially available [Rh(cod)Cl]2 complex (1 mol %; cod = 1,5-octadiene). After one hour in a mixture of water/THF (1:1) at room temperature, the targeted product, 6a, was isolated in a 97% yield using only two equivalents of phenylacetylene 5a [34]. Cobalt complexes have also been shown to function as catalysts; however, the problem of the substoichiometric use of monoalkyne 5a has been observed, with values ranging from 4 to 5 equivalents [35,36]. In the recent literature, iron- and nickel-based catalysts have been described for this reaction. The selectivity for the desired product, 6a, is optimized with minimal monoalkyne loading (ranging from 1 to 1.5 equivalents). However, it is imperative to note that achieving a catalyst loading of 10% is necessary to ensure optimal performance [37,38,39].
It is hypothesized that the alkyne trimerization reaction, when carried out in a confined space, will modify the product distribution in favor of the sterically less bulky product of interest, 6a. This hypothesis is based on our experience in catalysis with inclusion complexes generated from neutral complexes and supramolecular capsule 16 [40,41].
2. Results and Discussion
2.1. Synthesis of the Rhodium (I) Complex
As a preliminary undertaking, the synthesis of 4-(diphenylphosphanyl)-phenyl]-N,N-dimethylmethanamine was initiated. The synthesis was adapted from the protocol reported by Togo and co-workers [42] and began with the amination of 4-bromobenzyl bromide by the addition of dimethyl amine in the presence of K2CO3. The bromine atom of 1-(4-bromophenyl)-N,N-dimethylmethanamine was substituted by a halogen–metal exchange with n-BuLi at −78 °C, following by a reaction with chlorodiphenylphosphine. Due to the practical constraints associated with the handling of the resultant phosphine, its oxidation was carried out using H2O2. Subsequent to the purification process, the phosphine was reduced with an excess of trichlorosilane. The phosphorus ligand was obtained in three steps, resulting in an overyield of 63%.
The chloro-P-{[4-(diphenylphosphanyl)phenyl]-N,N-dimethylmethanammonio} (norbornadiene)rhodium(I) chloride complex (3) was obtained by reacting the phosphine previously obtained and the [RhCl(nbd)]2 complex (nbd = bicyclo[2.2.1]hepta-2,5-diene) in CH2Cl2. The cationic complex 3 was subsequently obtained by protonation with HCl and isolated by precipitation with a yield of 94% (Scheme 2). The coordination of the metal center was confirmed through the use of 31P{1H} NMR spectroscopy, which displayed a substantial weak-field shift of the phosphorus atom following its coordination with the rhodium precursor (31P{1H} NMR chemical shifts of −6.0 and 31.3 ppm for free and coordinated phosphine, respectively). Furthermore, the signal associated with complex 3 is consistent with a doublet that exhibits a coupling constant indicative of rhodium complexes (1JRhP = 170.7 Hz) [43]. The 1H NMR spectrum revealed the presence of a signal in the weak fields at 12.53 ppm, indicative of the acid proton attributed to the amine, thereby confirming its protonation.
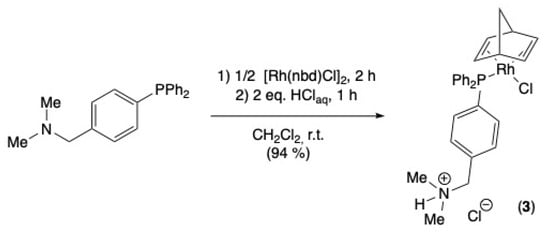
Scheme 2.
Synthesis of cationic [RhCl(2)(nbd)] complex (3).
2.2. Formation of the Inclusion Complex
The encapsulation of cationic complex 3 was studied in the presence of 16 ([1] = 26 mM and [3] = 4 mM) in H2O-saturated CDCl3. Following a 30 min stirring period, the formation of the inclusion complex 3⊂16 was observed by 1H NMR spectroscopy (Figure 2). Indeed, the appearance of signals in the negative zone (at −0.29 and −1.75 ppm), attributed to the methyl groups of the amine, was observed, synonymous with a species interacting with the internal walls of the cavity of 16. In addition to the substantial observed shielding (δ = 2.96 ppm in the “free” complex 3), desymmetrization and signal splitting were also observed. The loss of symmetry can be attributed to the distinct electronic environment in which each methyl was localized. With regard to the signal splitting, the observed intensities of the peaks appear to be in direct correspondence with the intensities of the phenolic groups of 16(8-H2O) and 16(15-H2O) [44]. This observation suggests the encapsulation of 3 within these two capsules. The addition of NEt4Cl (5 equivalents), a competitor to encapsulation, to the solution of the inclusion complex 3⊂16 resulted in the release of the rhodium(I) complex 3.
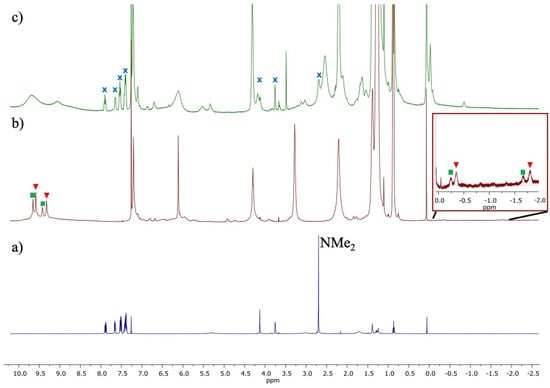
Figure 2.
1H NMR spectra of the following: free complex 3 (a); encapsulated complex 3 (3⊂16; with 3⊂16(8-H2O) red triangle and 3⊂16(15-H2O) green square) (b); after addition of NEt4Cl to 3⊂16 with signals from free 3 marked X (c).
The transition to a novel electronic environment was demonstrated to induce a modification of the 31P{1H} chemical shift. The initial doublet of 3 is divided into two tangled large doublets in the presence of 16. The observed signals can be attributed to the two inclusion complexes generated with capsules 16(8-H2O) and 16(15-H2O). The incorporation of five equivalents of NEt4Cl was demonstrated to induce the regeneration of the original spectrum, as previously observed (Figure 3).
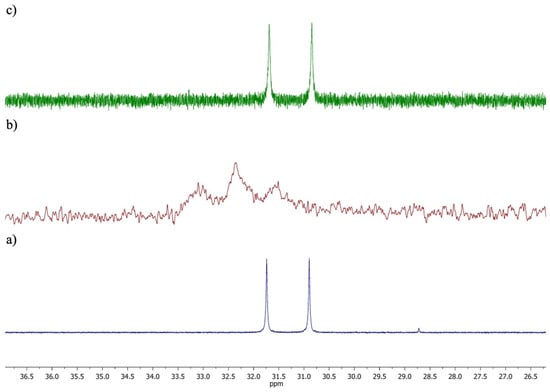
Figure 3.
1P{1H} NMR spectra of the following: free complex 3 (a); encapsulated complex 3 (3⊂16) (b); after addition of NEt4Cl to 3⊂16 (c).
Additionally, signals in the negative part of the 1H NMR spectrum were also observed in the DOSY NMR experiment. The analysis revealed the presence of a single species attributed to the inclusion complex 3⊂16 (diffusion coefficient 225 μm2s−1; hydrodynamic volume 18,800 Å3; Figure 4). This value was found to be equivalent to that of capsule 16 (225 μm2s−1; hydrodynamic volume 18,800 Å3) and distinctly different from that of “free” complex 3 (diffusion coefficient 625 μm2s−1; hydrodynamic volume 877 Å3). It was determined that the presence of capsule 16 results in the absence of signals corresponding to “free” complex 3. Given the finding that the values of hydrodynamic volumes 16 and 3⊂16 were identical, it could be deduced that the encapsulation of 3, with an estimated volume of 610 Å3 and an occupancy factor of the inner cavity of 44%, close to the ideal value of 55 ± 9% [45], did not result in substantial modification of the structure of the supramolecular capsule.
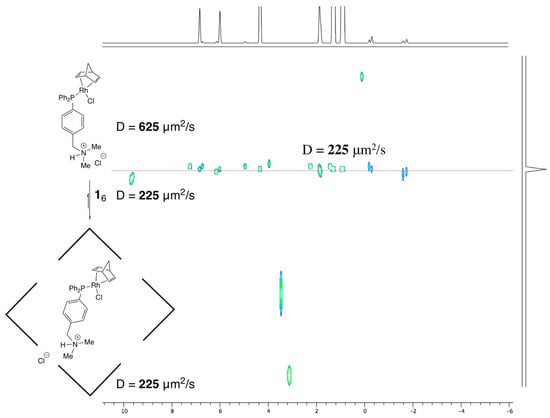
Figure 4.
DOSY NMR of inclusion complex 3⊂16.
The formation of the inclusion complex was subsequently confirmed through the utilization of UV-visible spectroscopy (Figure 5). The maximum of the extinction coefficient of complex 3 (λ = 431 nm) underwent a weak bathochromic effect of 4 nm by the addition of 16 (λ = 427 nm), in agreement with the formation of an encapsulation phenomenon (3⊂16) [23,24,25,40]. The release of 3 in solution by the addition of NEt4Cl was also confirmed by an absorbance maximum corresponding to the free complex (λ = 431 nm), once again demonstrating the reversibility of the encapsulation of complex 3 in the hexameric capsule 16.
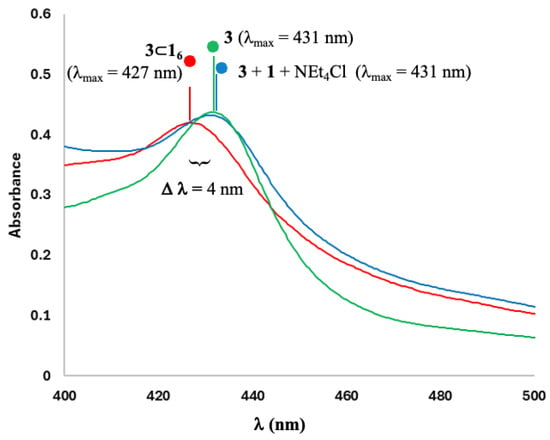
Figure 5.
UV/Vis measurements of free complex 3 (in green with λmax = 431 nm), encapsulated complex 3 (3⊂16; in red with λmax = 427 nm) and after addition of NEt4Cl to 3⊂16 (in blue with λmax = 431 nm).
2.3. Rhodium-Catalyzed [2+2+2] Cycloaddition
The well-documented reactivity of rhodium(I) complexes with [2+2+2] cycloaddition prompted the investigation of the inclusion complex 3⊂16 as a catalyst for this reaction. The model reaction studied is the [2+2+2] cycloaddition of N,N-dipropargyl-p-toluenesulfonamide 4 with phenylacetylene 5a. Consequently, this reaction yields three distinct products, each corresponding to a specific chemical outcome. The first product, designated as 6a, is a heterocycloaddition product, while the second product, labeled 7, is a homocycloaddition product. The third product, designated as 8, is a bishomocycloaddition adduct, which is formed through the reaction of 4 and 7. To investigate the distribution of the products, conditions to achieve full conversion were used, with 100 µmol of 4, an excess of 5a and a catalytic loading of 3⊂16 of 4 mol % in H2O-saturated CHCl3 at 60 °C for 24 h (Scheme 3).
Scheme 3.
Rhodium-catalyzed [2+2+2] cycloaddition of N,N-dipropargyl-p-toluenesulfonamide 4 with arylacetylene 5.
Complex 3 was initially evaluated in the presence of two equivalents of phenylacetylene 5a, and its activity was observed, irrespective of the presence of capsule 16 (Table 1, entries 1 and 2). Full conversions could be observed, with heterocycloaddition product formation of 44% and 62% for 3 and 3⊂16, respectively. In the absence of the rhodium complex, no reactivity was observed, with or without capsule 16 (Table 1, entries 3 and 4). Furthermore, the addition of a competitor to the encapsulation, corresponding to NEt4Cl (20 mol %) to 3⊂16, resulted in a product distribution intermediate to those observed with 3 and 3⊂16 as precatalysts (Table 1, entry 5). The observed phenomenon could be attributed to the incomplete release of the catalytic species into the solution, which remained entrapped within capsule 16. Slight discrepancies in the distribution of products resulting from a test conducted with D2O-saturated solvent as opposed to H2O, with no deuterium incorporation into the products (Table 1, entry 6), imply that the capsule should not affect the conventional mechanism for this reaction (see Scheme 1). The insertion of the carbon–carbon triple bond into an Rh-H intermediate is unequivocally ruled out. It is noteworthy that in the presence of methanol (25% v/v) in the reaction medium, the distribution of catalysis products remains unaltered by the presence of capsule 76 (Table 1, entries 7 and 8). This outcome can be attributed to the disruption of the hydrogen bonding network, likely partially due to the amount of MeOH utilized, which results in the opening of the capsule [46]. These tests demonstrated that the presence of the capsule promoted the formation of the product of interest, 6a, by enabling cycloaddition to take place inside the cavity formed by 16.

Table 1.
Control experiments 1.
Subsequently, an investigation was conducted into the impact of the phenylacetylene 5a equivalent number on this reaction. Consequently, the unencapsulated complex 3 was initially assessed (Figure 6a). The predominant formation of homocycloaddition product 7 was observed for stoichiometry below two equivalents of 5a. At the critical value of two equivalents of 5a, a similar formation of product 7 and the product of interest, 6a, was obtained (46% and 44%, respectively). At higher stoichiometries, the 6a product exhibited a marginal predominance. The bishomocycloaddition product 8 was observed in the minority under different conditions (for one equivalent of 5a: 15% formation of 8, for three equivalents of 5a: 9% formation of 8).
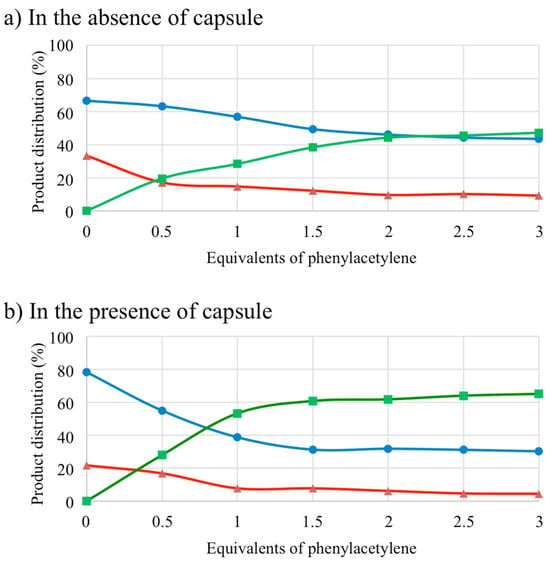
Figure 6.
Rhodium-catalyzed [2+2+2] cycloaddition of N,N-dipropargyl-p-toluenesulfonamide 4 with different proportions of phenylacetylene 5a, formation of heterocycloaddition product 6a (in green) and homocycloaddition products 7 (in blue) and 8 (in red).
In a subsequent step, the incorporation of capsule 16 served to reverse the selectivity of this reaction (Figure 6b). In accordance with the experimental findings, the heterocycloaddition product 6a was predominantly formed from one equivalent of 5a. The formation of 53% of 6a and 39% of 7 was measured. This trend remained constant for a higher number of 5a equivalents. As anticipated, the formation of bishomocycloaddition product 8 was diminished in the presence of 16 (for one equivalent of 5a: 8% formation of 8, for three equivalents of 5a: 5% formation of 8). The hypothesis that this product was formed in hexameric capsule 16 was not supported by the size of the reagents involved. The decomposition of the hexameric capsule into a pentameric capsular species [47], i.e., a more accessible catalytic active species, or a minor release of rhodium complex into solution, could provide a potential explanation for the formation of 8. The increase in the product of interest, 6a, when the capsule 16 was added may be explained by the difference in size of the reagents, which favored diffusion of the less sterically hindered alkyne. Furthermore, the varying degrees of affinity exhibited by the reagents for the cavity may be a subject of debate. It was evident that 1,6-diyne 4 exhibited a higher degree of reactivity in comparison to phenylacetylene 5a. The heightened reactivity of 4 resulted in the predominance of homocycloaddition product 7 under stoichiometric conditions, particularly when the catalyst was “free” in solution. The incorporation of the capsule 16 modified the diffusion of the substrates toward the metal, thereby affecting the reactivity of the alkynes with the catalytic species. Consequently, the predominant formation of the heterocycloaddition product 6 was observed. It is noteworthy that the homocycloaddition product [2+2+2] of phenylacetylene 5a was only formed in trace amounts. No oligomerization products were detected.
It was demonstrated that the presence of the supramolecular capsule 16 favors the formation of the heterocycloaddition product 6. Consequently, a series of thirteen alkynes were subjected to testing. The alkynes included in the study were phenylacetylene (5a), 4-ethynyltoluene (5b), 4-ethynylanisole (5c), 1-tert-butyl-4-ethynylbenzene (5d), 1-chloro-4-ethynylbenzene (5e), 1-fluoro-4-ethynylbenzene (5f), 2-ethynyltoluene (5g), 1-fluoro-2-ethynylbenzene (5h), 2-ethynylnaphthalene (5i), 2-ethynyl-6-methoxynaphthalene (5j), 9-ethynylphenanthrene (5k), ethynyltrimethylsilane (5l) and propargyl alcohol (5m). Catalytic tests were carried out with two equivalents of alkylene 5a–l, corresponding to the stoichiometry, in the case of phenylacetylene 5a, for which in the absence of 16, the formation of 6a and 7 became similar (Table 2).

Table 2.
Rhodium-catalyzed [2+2+2] cycloaddition with different alkynes 1.
The employment of the inclusion complex 3⊂16 for the series of arylacetylenes 5a–k resulted in an enhancement of the heterocycloaddition products 6a–k, which became the predominant product for all the distinct tested alkynes. In this series, the increase in product 6 varied by a factor of 1.2 to 1.7 times greater compared with the “free” catalytic system. The most significant increase was observed in the case of 2-ethynyltoluene 5g, where the methyl substituent was positioned in close proximity to the alkyne (6g formation of 32% and 54% with, respectively, 3 and 3⊂16; Table 2, entries 13 and 14). For catalysis by unencapsulated 3, this proximity resulted in a reduction of alkyne reactivity due to steric hindrance induced by the methyl group. This phenomenon was less evident when 16 was present, attributable to the close proximity of reagents 4 and 5g induced by the cavity of 16. A comparable effect was observed for 1-fluoro-2-ethynylbenzene 5h (6h formation of 37% and 59% with, respectively, 3 and 16; Table 2, entries 15 and 16). The presence of a bulky substituent, as with 1-tert-butyl-4-ethynylbenzene 5d, which hindered the diffusion of the substrate into the cavity of 16, did not inhibit the formation of the product 6e (6e formation of 38% and 58% with, respectively, 3 and 3⊂16; Table 2, entries 7 and 8). In the case of extended aromatic systems 5i–k, the formation of products 6i–k with the “free” precatalyst 3 was predominant, with the exception of 9-ethynylphenanthrene 5k. Nevertheless, it was observed that the incorporation of 16 led to an enhancement in the formation of products 6i–k (Table 2, entries 17–22).
However, it should be noted that this system was not without limitations. For instance, in the case of ethynyltrimethylsilane (5l), the selectivity toward heterocycloaddition product 6l diminished, and homocycloaddition product 7 was formed in the majority of cases, whether in the presence (46%) or absence (47%) of the capsule. It was noteworthy that this substrate exhibited a higher quantity (12%) of bishomocycloaddition product 8 (Table 2, entries 23 and 24). This observation indicated that substrate 5l could potentially contribute to the destabilization of the inclusion complex 3⊂16, which could result in the release of the catalytic center or the opening of the capsule leading to the formation of a pentameric structure. Consequently, these may facilitate the formation of the sterically hindered product 8. It has been observed that the utilization of propargyl alcohol (5m) as a substrate results in a modification of the 60-hydrogen bond network of the capsule 76. It was observed that, in the presence or absence of the supramolecular capsule 76, the heterocycloaddition product 6m was formed in a proportion of 85% with the latter reagent (Table 2, entries 25 and 26).
3. Materials and Methods
3.1. General
All the manipulations were carried out under dry argon. Routine 1H, 13C{1H}, 31P{1H} and DOSY spectra were recorded with Bruker FT instruments (AC 300, 500 and 600). CDCl3 was degassed, passed down a 5 cm thick basic alumina column and stored under argon. NMR spectra were calibrated according to residual protonated solvent (δ = 7.26 ppm and 77.16 ppm for 1H and 13C{1H} NMR, respectively). 31P{1H} NMR spectroscopic data are given relative to external H3PO4. Chemical shifts and coupling constants are reported in ppm and Hz, respectively.
UV-visible spectra were recorded on an Agilent Technologies Cary 60 UV-Vis spectrometer. Mass spectra were recorded on a Bruker MicroTOF spectrometer (ESI-TOF).
4-Bromobenzyl bromide, dimethylamine, tetraethylammonium chloride, [RhCl(nbd)]2, phenylacetylene, 4-ethynyltoluene, 2-ethynyltoluene, 4-ethynylanisole, 4-tert-butylphenylacetylene, 1-chloro-4-ethynylbenzene, 1-fluoro-4-ethynylbenzene, 1-fluoro-2-ethynylbenzene, 2-ethynyl-naphthalene, 2-ethynyl-6-methoxynaphthalene, 9-ethynylphenanthrene, ethynyltrimethylsilane and propargyl alcohol are commercially available products (Alfa Aesar, Sigma-Aldrich or Abcr) and were used as received without any further purification. 2,8,14,20-Tetra-undecyl-resorcin[4]arene (1) [48], 4-(diphenylphosphanyl)-phenyl]-N,N-dimethylmethanamine [42] and N,N-dipropargyl-p-toluenesulfonamide (4) [49] were prepared by following the literature procedures.
3.2. Synthesis of Chloro-P-{[4-(Diphenylphosphanyl)phenyl]-N,N-dimethylmethanammonio} (Norbornadiene)rhodium(I) Chloride (3)
The 1-(4-(diphenylphosphaneyl)phenyl)-N,N-dimehylmethanamine (227 mg, 0.711 mmol) and [RhCl(nbd)]2 (163 mg, 0.354 mmol) were dissolved in CH2Cl2 (10 mL) and the resulting solution was stirred at room temperature. After 2 h, HCl (37% in water, 0.118 mL, 1.423 mmol) was added and the reaction mixture was stirred for 1 h before the addition of MgSO4, which was eliminated by filtration. The filtrate was concentrated to ca. 2 mL and n-hexane (40 mL) was added. The precipitate was filtered, washed with n-hexane (3 × 8 mL) and dried under vacuum, yielding a brown solid (392 mg, 94%). 1H NMR (500 MHz, CDCl3): δ = 12.53 (s, 1H, NH(CH3)2), 7.92–7.88 (m, 2H, arom CH of P(C6H4)), 7.66 (d, 2H, arom CH of P(C6H4), 3JHH = 8.5 Hz), 7.54–7.50 (m, 4H, arom CH of P(C6H5)2), 7.44–7.38 (m, 6H, arom CH of P(C6H5)2), 5.32 (brs, 2H, CH of nbd), 4.13 (d, 2H, NCH2, 3JHH = 5.0 Hz), 3.76 (s, 2H, CH of nbd), 3.02 (brs, 2H, CH of nbd), 2.71 (d, 6H, NH(CH3)2, 3JHH = 5.0 Hz), 1.39 (s, 2H, CH2 of nbd); 13C{1H} NMR (126 MHz, CDCl3): δ = 135.84 (d, arom CH of P(C6H4), 2JCP = 12.2 Hz), 134.59 (d, arom Cquat of P(C6H4), 1JCP = 38.8 Hz), 134.19 (d, arom CH of P(C6H5), 2JCP = 11.7 Hz), 130.93 (d, arom CH of P(C6H4), 3JCP = 10.2 Hz), 130.87 (d, arom CH of P(C6H4), 4JCP = 2.5 Hz), 130.64 (d, arom Cquat of P(C6H5)2, 1JCP = 42.7 Hz), 130.64 (d, arom CH of P(C6H5)2, 4JCP = 2.3 Hz), 128.70 (d, arom CH of P(C6H5)2, 3JCP = 10.0 Hz), 85.69 (brs, CH of nbd, CH=CH), 64.03 (d, CH2 of nbd, 4JCP = 5.4 Hz), 60.87 (s, NCH2), 51.85 (brs, CH of nbd, CH=CH), 50.79 (d, CH of nbd, 3JCP = 1.5 Hz), 42.61 (s, NH(CH3)2); 31P{1H} NMR (202 MHz, CDCl3): δ = 31.3 (d, PPh2, 1JPRh = 170.7 Hz) ppm. MS (ESI-TOF): m/z = 550.09 [M − Cl]+, 514.12 [M − HCl − Cl]+ (expected isotopic profiles). Elemental analysis (%): calcd for C28H31NCl2PRh (586.34): C: 57.36; H: 5.33; N: 2.39; found C: 57.54; H: 5.51 N: 2.25.
3.3. General Procedure for the Rhodium-Catalyzed Cycloaddition
Under argon, the rhodium complex (3) (2.4 mg, 4 µmol, 4 mol %) was dissolved in H2O-saturated CHCl3 (1 mL). When necessary, resorcinarene (1) (28.7 mg, 26 µmol, 26 mol %) was added and the resulting solution was stirred at room temperature for 0.5 h. Then, N,N-dipropargyl-p-toluenesulfonamide (4; 24.7 mg, 100 µmol) and the corresponding amount of the desired alkyne were added. The reaction mixture was heated at 60 °C for 24 h. An aliquot of the solution was analyzed by 1H NMR. The products were unambiguously identified by 1H and 13C{1H} NMR after their isolation by flash chromatography on silica gel (eluent: petroleum ether/AcOEt: 7/3 v/v) (see Supplementary Materials).
4. Conclusions
In the present article, we reported on the use of a triphenylphosphine analogue, 1-(4-(diphenylphosphaneyl)phenyl)-N,N-dimethylmethanimine for intra-cavity catalytic application. Following coordination of the [RhCl(nbd)]2 and subsequent protonation of its amine function, this protonated phosphine demonstrated the capacity to stabilize the inclusion complex with the self-assembled capsule generated with six molecules of 2,8,14,20-tetra-undecyl-resorcin[4]arene and eight molecules of water. The formation of an inclusion complex was deduced from a combination of spectral measurements (UV-visible spectroscopy, 1H, 31P{1H} NMR and DOSY). The formation of the rhodium(I)-based inclusion complex was facilitated by π-cation interactions between the complex ligand and the aromatic inner rings of the cavity.
The inclusion complex was demonstrated to promote the formation of the heterocycloaddition product [2+2+2] between N,N-dipropargyl-p-toluenesulfonamide and arylacetylenic derivatives when compared with the “free” rhodium catalyst. In optimal conditions and with the utilization of merely two equivalents of arylacetylenes, the formation of these products of interest was found to be augmented by up to 69% in the case of 4-ethynylanisole, which corresponds to 1.4 times the amount observed with the “free” rhodium complex.
The employment of this readily accessible phosphine is expected to facilitate the immobilization of diverse organometallic complexes within supramolecular capsules comprising aromatic rings. Subsequent research will employ this strategy to examine the impact of confining catalytic centers within self-assembled capsules.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules30143052/s1, characterizing data of chloro-P-{[4-(diphenylphosphanyl) phenyl]-N,N-dimethylmethanammonio}(norbornadiene)rhodium(I) chloride (3) with Figure S1: 1H NMR spectrum, Figure S2: 13C{1H} NMR spectrum, Figure S3: 31P{1H} NMR spectrum, Figure S4: Mass spectrum (ESI-TOF) and Figure S5: DOSY NMR; characterizing data of 5-phenyl-2-tosylisoindoline (6a) with Figure S6: 1H NMR spectrum and Figure S7: 13C{1H} NMR spectrum; characterizing data of 5-(p-tolyl)-2-tosylisoindoline (6b) with Figure S8: 1H NMR spectrum and Figure S9: 13C{1H} NMR spectrum; characterizing data of 5-(4-methoxyphenyl)-2-tosylisoindoline (6c) with Figure S10: 1H NMR spectrum and Figure S11: 13C{1H} NMR spectrum; characterizing data of 5-(4-(tert-butyl)phenyl)-2-tosylisoindoline (6d) with Figure S12: 1H NMR spectrum and Figure S13: 13C{1H} NMR spectrum; characterizing data of 5-(4-chlorophenyl)-2-tosylisoindoline (6e) with Figure S14: 1H NMR spectrum and Figure S15: 13C{1H} NMR spectrum; characterizing data of 5-(4-fluorophenyl)-2-tosylisoindoline (6f) with Figure S16: 1H NMR spectrum, Figure S17: 13C{1H} NMR spectrum and Figure S18: 19F{1H} NMR spectrum; characterizing data of 5-(o-tolyl)-2-tosylisoindoline (6g) with Figure S19: 1H NMR spectrum and Figure S20: 13C{1H} NMR spectrum; characterizing data of 5-(2-fluorophenyl)-2-tosylisoindoline (6h) with Figure S21: Mass spectrum, Figure S22: 1H NMR spectrum, Figure S23: 13C{1H} NMR spectrum and Figure S24: 19F{1H} NMR spectrum; characterizing data of 5-(naphthalen-2-yl)-2-tosylisoindoline (6i) with Figure S25: Mass spectrum, Figure S26: 1H NMR spectrum and Figure S27: 13C{1H} NMR spectrum; characterizing data of 5-(6-methoxynaphthalen-2-yl)-2-tosylisoindoline (6j) with Figure S28: Mass spectrum, Figure S29: 1H NMR spectrum and Figure S30: 13C{1H} NMR spectrum; characterizing data of 5-(phenanthren-9-yl)-2-tosylisoindoline (6k) with Figure S31: Mass spectrum, Figure S32: 1H NMR spectrum and Figure S33: 13C{1H} NMR spectrum; characterizing data of 2-tosyl-5-(trimethylsilyl)isoindoline (6l) with Figure S34: Mass spectrum, Figure S35: 1H NMR spectrum and Figure S36: 13C{1H} NMR spectrum; characterizing data of (2-tosylisoindolin-5-yl)methanol (6m) with Figure S37: 1H NMR spectrum and Figure S38: 13C{1H} NMR spectrum; characterizing data of 4-methyl-N-(prop-2-yn-1-yl)-N-((2-tosylisoindolin-5-yl)methyl)benzenesulfonamide (7) with Figure S39: 1H NMR spectrum and Figure S40: 13C{1H} NMR spectrum; characterizing data of 4-methyl-N,N-bis((2-tosylisoindolin-5-yl)methyl)benzenesulfonamide (8) with Figure S41: 1H NMR spectrum and Figure S42: 13C{1H} NMR spectrum.
Author Contributions
Conceptualization, M.S. and D.S.; methodology, M.S. and D.S.; validation, D.S.; formal analysis, M.S. and D.S.; investigation, M.S.; resources, D.S.; data curation, D.S.; writing—original draft preparation, M.S.; writing—review and editing, D.S.; supervision, D.S. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Agence Nationale de la Recherche (HEXCAPS Programme, ANR-24-CE07-1738).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available upon request from the corresponding author.
Acknowledgments
M.S. thanks M.R.T. for the research fellowship.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Diao, D.; Simaan, A.J.; Martinez, A.; Colomban, C. Bioinspired complexes confined in well-defined capsules: Getting closer to metalloenzyme functionalities. Chem. Commun. 2023, 59, 4288–4299. [Google Scholar] [CrossRef] [PubMed]
- Shteinman, A.A. Metallocavitins as advanced enzyme mimics and promising chemical catalysts. Catalysts 2023, 13, 415. [Google Scholar] [CrossRef]
- Li, W.-L.; Head-Gordon, T. Catalytic principles from natural enzymes and translational design strategies for synthetic catalysts. ACS Cent. Sci. 2021, 7, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Raynal, M.; Ballester, P.; Vidal-Ferran, A.; van Leeuwen, P.W.N.M. Supramolecular catalysis. Part 2: Artificial enzyme mimics. Chem. Soc. Rev. 2014, 43, 1734–1787. [Google Scholar] [CrossRef] [PubMed]
- Iuliano, V.; Della Sala, P.; Talotta, C.; De Rosa, M.; Soriente, A.; Gaeta, C.; Neri, P. Supramolecular control on reactivity and selectivity inside the confined space of H-bonded hexameric capsules. Curr. Opin. Colloid Interface Sci. 2023, 65, 101692. [Google Scholar] [CrossRef]
- Olivo, G.; Capocasa, G.; Del Giudice, D.; Lanzalunga, O.; Di Stefano, S. New horizons for catalysis disclosed by supramolecular chemistry. Chem. Soc. Rev. 2021, 50, 7681–7724. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Catti, L.; Tiefenbacher, K. Catalysis inside the hexameric resorcinarene capsule. Acc. Chem. Res. 2018, 51, 2107–2114. [Google Scholar] [CrossRef] [PubMed]
- Gaeta, C.; Talotta, C.; De Rosa, M.; La Manna, P.; Soriente, A.; Neri, P. The hexameric resorcinarene capsule at work: Supramolecular catalysis in confined spaces. Chem. Eur. J. 2019, 25, 4899–4913. [Google Scholar] [CrossRef] [PubMed]
- Pappalardo, A.; Puglisi, R.; Sfrazzetto, G.T. Catalysis inside supramolecular capsules: Recent developments. Catalysts 2019, 9, 630. [Google Scholar] [CrossRef]
- Catti, L.; Zhang, Q.; Tiefenbacher, K. Advantages of catalysis in self-assembled molecular capsules. Chem. Eur. J. 2016, 22, 9060–9066. [Google Scholar] [CrossRef] [PubMed]
- Lorenzetto, T.; Bordignon, F.; Munarin, L.; Mancin, F.; Fabris, F.; Scarso, A. Substrate selectivity imparted by self-assembled molecular containers and catalysts. Chem. Eur. J. 2024, 30, e202301811. [Google Scholar] [CrossRef] [PubMed]
- Steinmetz, M.; Sémeril, D. Molecular modeling is key to understanding supramolecular resorcinarenyl capsules, inclusion complex formation and organic reactions in nanoconfined space. Molecules 2025, 30, 2549. [Google Scholar] [CrossRef] [PubMed]
- MacGillivray, L.R.; Atwood, J.L. A chiral spherical molecular assembly held together by 60 hydrogen bonds. Nature 1997, 389, 469–472. [Google Scholar] [CrossRef]
- Yamanaka, M.; Shivanyuk, A.; Rebek, J., Jr. Kinetics and thermodynamics of hexameric capsule formation. J. Am. Chem. Soc. 2004, 126, 2939–2943. [Google Scholar] [CrossRef] [PubMed]
- Cavarzan, A.; Scarso, A.; Sgarbossa, P.; Strukul, G.; Reek, J.N.H. Supramolecular control on chemo- and regioselectivity via encapsulation of (NHC)-Au catalyst within a hexameric self-assembled host. J. Am. Chem. Soc. 2011, 133, 2848–2851. [Google Scholar] [CrossRef] [PubMed]
- Cavarzan, A.; Reek, J.N.H.; Trentin, F.; Scarso, A.; Strukul, G. Substrate selectivity in the alkyne hydration mediated by NHC-Au(I) controlled by encapsulation of the catalyst within a hydrogen bonded hexameric host. Catal. Sci. Technol. 2013, 3, 2898–2901. [Google Scholar] [CrossRef]
- Jans, A.C.H.; Gómez-Suárez, A.; Nolan, S.P.; Reek, J.N.H. A switchable gold catalyst by encapsulation in a self-assembled cage. Chem. Eur. J. 2016, 22, 14836–14839. [Google Scholar] [CrossRef] [PubMed]
- Jongkind, L.J.; Rahimi, M.; Poole III, D.; Ton, S.J.; Fogg, D.E.; Reek, J.N.H. Protection of ruthenium olefin metathesis catalysts by encapsulation in a self-assembled resorcinarene capsule. ChemCatChem 2020, 12, 4019–4023. [Google Scholar] [CrossRef]
- Adriaenssens, L.; Escribano-Cuesta, A.; Homs, A.; Echavarren, A.M.; Ballester, P. Encapsulation studies of cationic gold complexes within a self-assembled hexameric resorcin[4] arene capsule. Eur. J. Org. Chem. 2013, 2013, 1494–1500. [Google Scholar] [CrossRef]
- Philip, I.E.; Kaifer, A.E. Electrochemically driven formation of a molecular capsule around the ferrocenium ion. J. Am. Chem. Soc. 2002, 124, 12678–12679. [Google Scholar] [CrossRef] [PubMed]
- Horiuchi, S.; Yamaguchi, T.; Tessarolo, J.; Tanaka, H.; Sakuda, E.; Arikawa, Y.; Meggers, E.; Clever, G.H.; Umakoshi, K. Symmetry-breaking host-guest assembly in a hydrogen-bonded supramolecular system. Nat. Commun. 2023, 14, 155. [Google Scholar] [CrossRef] [PubMed]
- Philip, I.; Kaifer, A.E. Noncovalent encapsulation of cobaltocenium inside resorcinarene molecular capsules. J. Org. Chem. 2005, 70, 1558–1564. [Google Scholar] [CrossRef] [PubMed]
- Bianchini, G.; Scarso, A.; Sorella, G.L.; Strukul, G. Switching the activity of a photoredox catalyst through reversible encapsulation and release. Chem. Commun. 2012, 48, 12082. [Google Scholar] [CrossRef] [PubMed]
- Horiuchi, S.; Tanaka, H.; Sakuda, E.; Arikawa, Y.; Umakoshi, K. Encapsulation and enhanced luminescence properties of IrIII complexes within a hexameric self-assembled capsule. Chem. Eur. J. 2016, 22, 17533–17537. [Google Scholar] [CrossRef] [PubMed]
- Horiuchi, S.; Matsuo, C.; Sakuda, E.; Arikawa, Y.; Clever, G.H.; Umakoshi, K. Anion-mediated encapsulation-induced emission enhancement of an IrIII complex within a resorcin[4]arene hexameric capsule. Dalton Trans. 2020, 49, 8472–8477. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Le Corre, L.; Reinaud, O.; Colasson, B. A promising approach for controlling the second coordination sphere of biomimetic metal complexes: Encapsulation in a dynamic hydrogen-bonded capsule. Chem. Eur. J. 2021, 27, 434–443. [Google Scholar] [CrossRef] [PubMed]
- Chopade, P.R.; Louie, J. [2+2+2] Cycloaddition reactions catalyzed by transition metal complexes. Adv. Synth. Catal. 2006, 348, 2307–2327. [Google Scholar] [CrossRef]
- Pla-Quintana, A.; Roglans, A. [2+2+2] Cycloaddition reactions of macrocyclic systems catalyzed by transition metals. A review. Molecules 2010, 15, 9230–9251. [Google Scholar] [CrossRef] [PubMed]
- Shibata, Y.; Tanaka, K. Rhodium-catalyzed [2+2+2] cycloaddition of alkynes for the synthesis of substituted benzenes: Catalysts, reaction scope, and synthetic applications. Synthesis 2012, 44, 323–350. [Google Scholar] [CrossRef]
- Transition-Metal-Mediated Aromatic Ring Construction; Tanaka, K., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 2013. [Google Scholar]
- Roglans, A.; Pla-Quintana, A.; Solà, M. Mechanistic Ssudies of transition-metal-catalyzed [2+2+2] cycloaddition reactions. Chem. Rev. 2021, 121, 1894–1979. [Google Scholar] [CrossRef] [PubMed]
- Pla-Quintana, A.; Roglans, A. The choice of rhodium catalysts in [2+2+2] cycloaddition reaction: A personal account. Molecules 2022, 27, 1332. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Li, W.; Chen, Y.; Wu, R.; Zhu, S. Dirhodium-catalyzed [2+2+2] cycloaddition of 1,6-diynes and alkynes. J. Org. Chem. 2024, 89, 17248–17259. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Zhang, X.Y.; Kang, S.X. Rhodium-catalyzed selective [2+2+2] cyclizations of 1,6-diynes with monoynes leading to isoindolines and isobenzofurans. Chin. Chem. Lett. 2010, 21, 18–22. [Google Scholar] [CrossRef]
- García-Lacuna, J.; Domínguez, G.; Blanco-Urgoiti, J.; Pérez-astells, J. Cobalt octacarbonyl-catalyzed scalable alkyne cyclotrimerization and crossed [2+2+2]-cycloaddition reaction in a plug flow reactor. Org. Lett. 2018, 20, 5219–5223. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Si, X.J.; Wang, X.N.; Kou, H.D.; Chen, D.M.; Liu, C.S.; Du, M. A high-activity cobalt-based MOF catalyst for [2+2+2] cycloaddition of diynes and alkynes: Insights into alkyne affinity and selectivity control. RSC Adv. 2018, 8, 4895–4899. [Google Scholar] [CrossRef] [PubMed]
- Féo, M.; Bakas, N.J.; Radović, A.; Parisot, W.; Clisson, A.; Chamoreau, L.M.; Haddad, M.; Ratovelomanana-Vidal, V.; Neidig, M.L.; Lefèvre, G. Thermally stable redox noninnocent bathocuproine-iron complex for cycloaddition reactions. ACS Catal. 2023, 13, 4882–4893. [Google Scholar] [CrossRef]
- Parisot, W.; Haddad, M.; Phansavath, P.; Lefèvre, G.; Ratovelomanana-Vidal, V. A versatile, functional group-tolerant, and bench-stable Iron precatalyst for building arene and triazine rings by [2+2+2] cycloadditions. Chem. Eur. J. 2024, 30, e202400096. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Mao, Y.; Sun, Y.; Ma, B.; Wang, Y.; Zhou, G.; Zhang, Y.; Zeng, F.; Wang, Z.; Li, B. Bench-stable nickel(II)-aryl complex-catalyzed cyclotrimerisation of diynes and alkynes to synthesis multisubstituted benzene derivatives. Results Chem. 2024, 7, 101523. [Google Scholar] [CrossRef]
- Hkiri, S.; Steinmetz, M.; Schurhammer, R.; Sémeril, D. Encapsulated neutral ruthenium catalyst for substrate-selective oxidation of alcohols. Chem. Eur. J. 2022, 28, e202201887. [Google Scholar] [CrossRef] [PubMed]
- Steinmetz, M.; Sémeril, D. Confined catalysis involving a palladium complex and a self-assembled capsule for the dimerization of vinyl arenes and the formation of indane and tribenzo-pentaphene derivatives. Catalysts 2025, 15, 585. [Google Scholar] [CrossRef]
- Kawagoe, Y.; Moriyama, K.; Togo, H. Facile preparation of amides from carboxylic acids and amines with ion-supported Ph3P. Tetrahedron 2013, 69, 3971–3977. [Google Scholar] [CrossRef]
- Takenaka, Y.; Osakada, K. Preparation and structure of [RhCl(nbd)(dmap)] (nbd = bicyclo[2.2.1]hepta-2,5-diene; dmap = 4-(N,N-dimethylamino)pyridine). Bull. Chem. Soc. Jpn. 2000, 73, 129–130. [Google Scholar] [CrossRef]
- Poole III, D.A.; Mathew, S.; Reek, J.N.H. Just add water: Modulating the structure-derived acidity of catalytic hexameric resorcinarene capsules. J. Am. Chem. Soc. 2021, 143, 16419–16427. [Google Scholar] [CrossRef] [PubMed]
- Mecozzi, S.; Rebek, J., Jr. The 55 % solution: A formula for molecular recognition in the liquid state. Chem. Eur. J. 1998, 4, 1016–1022. [Google Scholar] [CrossRef]
- Avram, L.; Cohen, Y. Self-recognition, structure, stability, and guest affinity of pyrogallol[4]arene and resorcin[4]arene capsules in solution. J. Am. Chem. Soc. 2004, 126, 11556–11563. [Google Scholar] [CrossRef] [PubMed]
- Gaeta, C.; Manna, P.L.; Rosa, M.D.; Talotta, C.; Rescifina, A.; Floresta, G.; Soriente, A.; Neri, P. Synergic interplay between halogen bonding and hydrogen bonding in the activation of a neutral substrate in a nanoconfined space. Angew. Chem. Int. Ed. 2019, 59, 811–818. [Google Scholar]
- Merget, S.; Catti, L.; Piccini, G.; Tiefenbacher, K. Requirements for terpene cyclizations inside the supramolecular resorcinarene capsule: Bound water and its protonation determine the catalytic activity. J. Am. Chem. Soc. 2020, 142, 4400–4410. [Google Scholar] [CrossRef] [PubMed]
- Vila, J.; Vinardell, R.; Solà, M.; Pla-Quintana, A.; Roglans, A. A Rh(I)-catalyzed cascade cyclization of 1,5-bisallenes and alkynes for the formation of cis-3,4-arylvinyl pyrrolidines and cyclopentanes. Adv. Synth. Catal. 2022, 364, 206–217. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).