Stimulation of Maize Growth and Development and Improvement of Soil Properties Using New Specialised Organic-Mineral Materials
Abstract
1. Introduction
2. Results
2.1. Maize
2.1.1. SPAD Index of Maize Leaves
2.1.2. Height of Maize
2.1.3. Yield of Maize
2.1.4. Macroelement Contents of Maize
2.1.5. Microelement Contents of Maize
2.2. Soil Chemical Properties After Maize Harvest
2.2.1. pH of Soil
2.2.2. Macroelement Contents of Soil
2.2.3. Microelement Contents of Soil
2.3. PCA Analysis
2.3.1. Maize
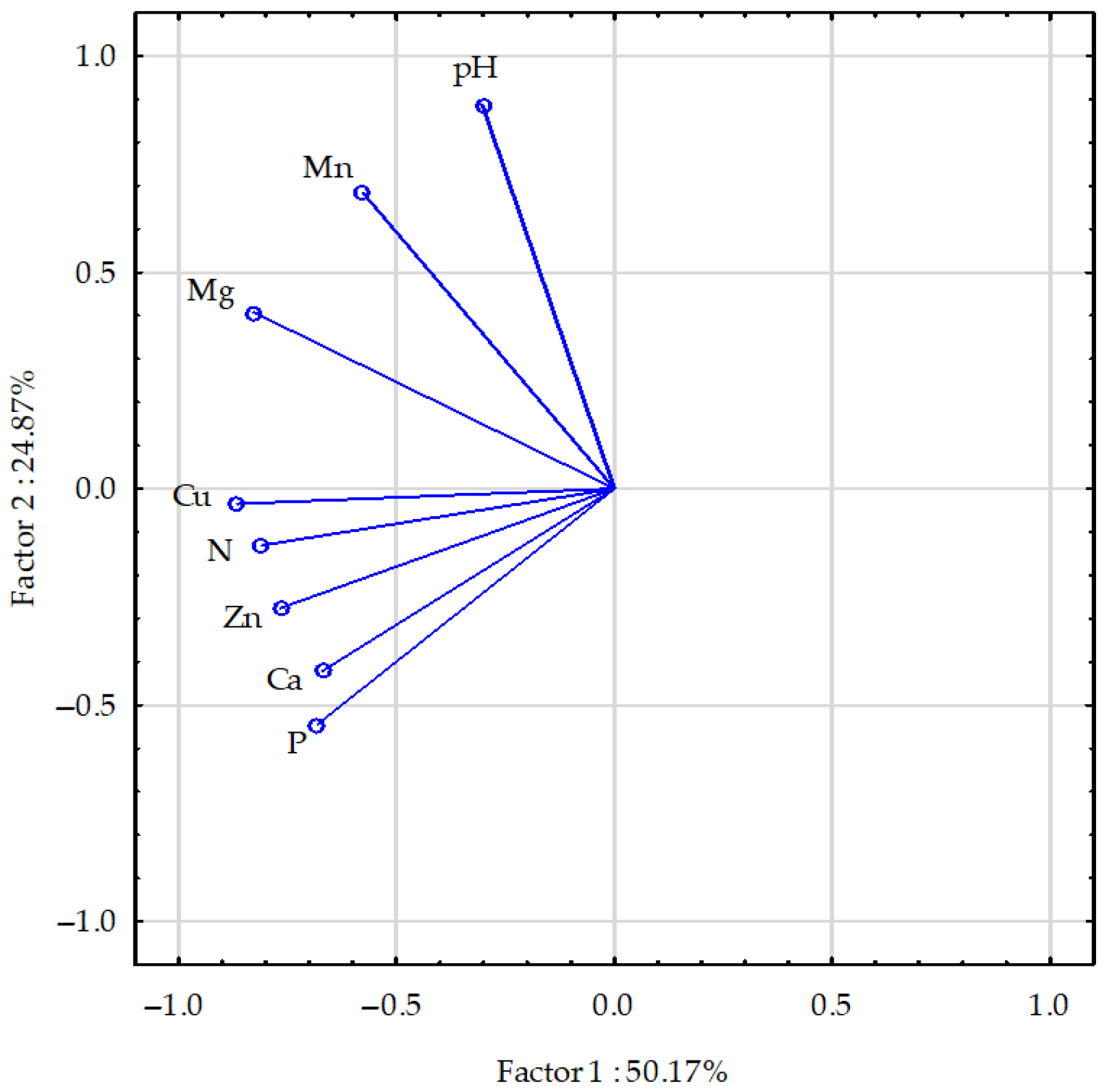
2.3.2. Soil After Maize Harvesting
3. Discussion
4. Materials and Methods
4.1. Methodological Assumptions
4.2. Laboratory and Statistical Analysis Methods
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Elbl, J.; Brtnicka, H.; Kintl, A.; Holatko, J.; Brtnicky, M. Use of organic-mineral fertilizers as alternative to conventional organic and mineral fertilizers: Effect on soil quality. Int. Multidiscip. Sci. GeoConf. SGEM 2019, 19, 583–590. [Google Scholar] [CrossRef]
- Directive, C. Concerning the protection of waters against pollution caused by nitrates from agricultural sources. Off. J. 1991, 375, 1–8. Available online: https://eur-lex.europa.eu/eli/dir/1991/676/oj/eng (accessed on 1 April 2025).
- Syed, S.; Wang, X.; Prasad, T.N.V.K.V.; Lian, B. Bio-organic mineral fertilizer for sustainable agriculture: Current trends and future perspectives. Minerals 2021, 11, 1336. [Google Scholar] [CrossRef]
- Brodowska, M.S.; Wyszkowski, M.; Karsznia, M. Application of urea and ammonium nitrate solution with potassium thiosulfate as a factor determining macroelement contents in plants. Agronomy 2024, 14, 1097. [Google Scholar] [CrossRef]
- Hao, D.-L.; Zhou, J.-Y.; Li, L.; Qu, J.; Li, X.-H.; Chen, R.-R.; Kong, W.-Y.; Li, D.-D.; Li, J.-J.; Guo, H.-L.; et al. An appropriate ammonium: Nitrate ratio promotes the growth of centipede grass: Insight from physiological and micromorphological analyses. Front. Plant Sci. 2023, 14, 1324820. [Google Scholar] [CrossRef] [PubMed]
- Hou, P.-F.; Chang, Y.-T.; Lai, J.-M.; Chou, K.-L.; Tai, S.-F.; Tseng, K.-C.; Chow, C.-N.; Jeng, S.-L.; Huang, H.-J.; Chang, W.-C. Long-term effects of fertilizers with regional climate variability on yield trends of sweet corn. Sustainability 2020, 12, 3528. [Google Scholar] [CrossRef]
- Galindo, F.S.; Pagliari, P.H.; Silva, E.C.; Lima, B.H.; Fernandes, G.C.; Thiengo, C.C.; Bernardes, J.V.S.; Jalal, A.; Oliveira, C.E.S.; Vilela, L.S.; et al. Impact of nitrogen fertilizer sustainability on corn crop yield: The role of beneficial microbial inoculation interactions. BMC Plant Biol. 2024, 24, 268. [Google Scholar] [CrossRef]
- Strachan, S.D.; Jeschke, M. Water and Nutrient Uptake During the Corn Growing Season. Pioneer Corteva Agrisci. 2018. Available online: https://www.pioneer.com/us/agronomy/water-nutrient-uptake-corn.html. (accessed on 20 May 2025).
- de Morais, E.G.; Silva, C.A.; Maluf, H.J.G.M.; De Oliveira Paiva, I.; De Paula, L.H.D. How do NPK-organomineral fertilizers affect the soil availability and uptake of iron, manganese, copper, and zinc by maize cultivated in red and yellow oxisols? J. Soil Sci. Plant Nutr. 2023, 23, 6284–6298. [Google Scholar] [CrossRef]
- Brodowska, M.S.; Wyszkowski, M.; Grzesik, R. New fertilisers with innovative chelates in wheat cultivation. Agronomy 2024, 14, 1832. [Google Scholar] [CrossRef]
- Berzsenyi, Z.; Árendás, T.; Bónis, P.; Micskei, G.; Sugár, E. Long-term effect of farmyard manure and mineral fertiliser on the yield and yield stability of maize (Zea mays L.) in dry and wet years. Acta Agron. Hung. 2011, 59, 303–315. [Google Scholar] [CrossRef]
- Merzlaya, G.; Afanasyev, R.; Mukhina, M.; Mozharova, I.; Bereznov, A.; Astarkhanova, T.; Zargar, M. Comparative efficiency of organic, mineral and organomineral fertilizer on soil properties and crops. Res. Crops 2021, 22, 841–848. [Google Scholar] [CrossRef]
- Baćmaga, M.; Wyszkowska, J.; Borowik, A.; Kucharski, J.; Paprocki, Ł. 2021. Microbiological and biochemical properties in Eutric/Dystric Brunic Arenosols, Eutric/Endocalcaric Cambisols, and Haplic/Albic Luvisols Soils. J. Soil Sci. Plant Nutr. 2021, 21, 1277–1292. [Google Scholar] [CrossRef]
- Barquero, M.; Cazador, C.; Ortiz-Liébana, N.; Zotti, M.; Brañas, J.; González-Andrés, F. Fertilising maize with bio-based mineral fertilisers gives similar growth to conventional fertilisers and does not alter soil microbiome. Agronomy 2024, 14, 916. [Google Scholar] [CrossRef]
- Chojnacka, K.; Baltrusaitis, J. Organo-mineral fertilizers for sustainable agriculture. Sustain. Sci. Technol. 2025, 2, 022001. [Google Scholar] [CrossRef]
- de Morais, E.G.; Silva, C.A.; Maluf, H.J.G.M.; de Oliveira Paiva, I.; De Paula, L.H.D. Effects of compost-based organomineral fertilizers on the kinetics of NPK release and maize growth in contrasting oxisols. Waste Biomass Valor. 2023, 14, 2299–2321. [Google Scholar] [CrossRef]
- Popin, G.V.; dos Santos, A.K.B.; Ferrao, G.E.; Lourenco, D.A.; Siqueira-Neto, M. Effect of organic N-sources on maize yield components. Australian J. Crop Sci. 2019, 13, 1215–1222. [Google Scholar] [CrossRef]
- Bożek, K.S.; Żuk-Gołaszewska, K.; Bojarczuk, J.; Gołaszewski, J. The effect of different nitrogen fertilizer rates, sowing density, and plant growth regulator application on the quality and milling value of Triticum durum Desf. grain. Agronomy 2022, 12, 1622. [Google Scholar] [CrossRef]
- Han, P.; Zhang, W.; Wang, G.; Sun, W.; Huang, Y. Changes in soil organic carbon in croplands subjected to fertilizer management: A global meta-analysis. Sci. Rep. 2016, 6, 27199. [Google Scholar] [CrossRef]
- Vakal, S.; Vakal, V.; Artyukhov, A.; Shkola, V.; Yanovska, A. Granulated organo-mineral fertilizers: The process of formation and investigation of porous phosphate-diatomite shell. Appl. Nanosci. 2023, 13, 5157–5164. [Google Scholar] [CrossRef]
- De Sousa, R.N.; Alleoni, L.R.F. Performance of struvite and organomineral fertilizers compared to traditional source of phosphorus in maize cultivation on tropical soils. J. Soil Sci. Plant Nutr. 2024, 24, 5250–5271. [Google Scholar] [CrossRef]
- Xiao, J.; Guo, G.; Jeong, B.R. Iron supplement-enhanced growth and development of Hydrangea macrophylla in vitro under normal and high pH. Cells 2021, 10, 3151. [Google Scholar] [CrossRef]
- Hoffmann, S.; Lepossa, A. Impact of mineral and organic fertilization on yield, C content in the soil, as well as on C, N and energy balances in a long-term field experiment. Arch. Agron. Soil Sci. 2013, 59, 1133–1141. [Google Scholar] [CrossRef]
- Elbl, J.; Hunady, I.; Koukalova, V.; Vaverkova, M.D.; Losak, T. Influence of alternative organic-mineral fertilisers application on soil organic matter and plant biomass yield. Int. Multidiscip. Sci. GeoConference SGEM 2020, 20, 433–440. [Google Scholar] [CrossRef]
- Kandel, B.P. SPAD value varies with age and leaf of maize plant and its relationship with grain yield. BMC Res. Notes 2020, 13, 475. [Google Scholar] [CrossRef]
- Zarzecka, K.; Gugała, M.; Mystkowska, I.; Sikorska, A. The Leaf Greenness Index SPAD and selected features of potato following an application of herbicides and biostimulants. J. Ecol. Eng. 2021, 22, 54–63. [Google Scholar] [CrossRef]
- Brodowska, M.S.; Wyszkowski, M.; Kordala, N. Use of organic materials to limit the potential negative effect of nitrogen on maize in different soils. Materials 2022, 15, 5755. [Google Scholar] [CrossRef] [PubMed]
- Wapa, J.M. Effects of mineral fertilizer and different sources of combination on the growth and grain yield of maize in Sudano-Sahelian Savanna, Nigeria. Internat. Agric. Innovat. Res. 2014, 2, 1096–1100. Available online: https://www.ijair.org/administrator/components/com_jresearch/files/publications/IJAIR_652_Final.pdf (accessed on 22 May 2025).
- Romanowska-Duda, Z.; Grzesik, M.; Janas, R. The Usefulness of nano-organic-mineral fertilizer stymjod in intensification of growth, physiological activity and yield of the Jerusalem Artichoke biomass. In Renewable Energy Sources: Engineering, Technology, Innovation; Wróbel, M., Jewiarz, M., Szlęk, A., Eds.; Springer Proceedings in Energy; Springer International Publishing: Cham, Switzerland, 2020; pp. 331–339. [Google Scholar] [CrossRef]
- El-Habbak, A.K.; El-Deepah, H.R.; Salwau, M.I.; El-Gizawy, N.K. Effect of organic, inorganic and nano fertilizers on agronomic traits of maize. Ann. Agric. Sci. Moshtohor 2019, 57, 11–20. [Google Scholar] [CrossRef]
- Boscaro, R.; Panozzo, A.; Piotto, S.; Moore, S.S.; Barion, G.; Wang, Y.; Vamerali, T. Effects of foliar-applied mixed mineral fertilizers and organic biostimulants on the growth and hybrid seed production of a male-sterile inbred maize line. Plants 2023, 12, 2837. [Google Scholar] [CrossRef]
- Wyszkowski, M.; Brodowska, M.S.; Kordala, N. Trace element contents in maize following the application of organic materials to reduce the potential adverse effects of nitrogen. Materials 2023, 16, 215. [Google Scholar] [CrossRef]
- Olu-Ogbera, O.A.; Adetomiwa, K.T.; Adejoro, S.A. Soil residual microbial population and chemical fertility following maize cropping as influenced by an organo-mineral fertilizer formulation. East Afr. Sch. J. Agri. Life Sci. 2025, 8, 20–27. [Google Scholar] [CrossRef]
- Wyszkowski, M.; Kordala, N.; Brodowska, M.S. Role of humic acids-based fertilisers and nitrogen fertilisers in the regulation of the macroelement content in maize biomass. J. Elem. 2023, 28, 1289–1309. [Google Scholar] [CrossRef]
- Jaskulska, I.; Lemanowicz, J.; Breza-Boruta, B.; Siwik-Ziomek, A.; Radziemska, M.; Jaskulski, D.; Białek, M. Chemical and biological properties of sandy loam soil in response to long-term organic–mineral fertilisation in a warm-summer humid continental climate. Agronomy 2020, 10, 1610. [Google Scholar] [CrossRef]
- Kabata-Pendias, A.; Szteke, B. Trace Elements in Soils and Plants, 1st ed.; CRC Press, Taylor and Francis Group: Boca Raton, FL, USA, 2015; p. 468. [Google Scholar] [CrossRef]
- Wyszkowski, M.; Brodowska, M.S.; Karsznia, M. Innovative fertiliser based on urea and ammonium nitrate solution with potassium thiosulphate as a crucial factor in shaping plant yield and its parameters. Agronomy 2024, 14, 802. [Google Scholar] [CrossRef]
- Mahmoud, A.W.M.; Ayad, A.A.; Abdel-Aziz, H.S.M.; Williams, L.L.; El-Shazoly, R.M.; Abdel-Wahab, A.; Abdeldaym, E.A. Foliar Application of different iron sources improves morpho-physiological traits and nutritional quality of broad bean grown in sandy soil. Plants 2022, 11, 2599. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Ruan, Y.; Wang, S.; Liu, X.; Lian, B. Effects of organic mineral fertiliser on heavy metal migration and potential carbon sink in soils in a Karst Region. Acta Geochim. 2017, 36, 539–543. [Google Scholar] [CrossRef]
- Litvinova, O.; Dehodiuk, S.E. Effect of systematic fertilisation on soil fertility in the cultivation of corn for grain. Plant Soil Sci. 2021, 12, 76–84. [Google Scholar] [CrossRef]
- Plett, D.C.; Ranathunge, K.; Melino, V.J.; Kuya, N.; Uga, Y.; Kronzucker, H.J. The intersection of nitrogen nutrition and water use in plants: New paths toward improved crop productivity. J. Exp. Bot. 2020, 71, 4452–4468. [Google Scholar] [CrossRef]
- Mahmood, F.; Khan, I.; Ashraf, U.; Shahzad, T.; Hussain, S.; Shahid, M.; Abid, M.; Ullah, S. Effects of organic and inorganic manures on maize and their residual impact on soil physico-chemical properties. J. Soil Sci. Plant Nutr. 2017, 17, 22–32. [Google Scholar] [CrossRef]
- Pandey, V.; Gautam, P.; Singh, A.P. Correlation between physical, chemical and biological properties of soil under different land use systems. Int. J. Chem. Stud. 2019, 7, 469–471. [Google Scholar]
- De Magalhães, C.A.S.; Morales, M.M.; de Rezende, F.A.; Langer, J. Eficiência de fertilizantes organominerais fosfatados em mudas de eucalipto. Sci. Agrar. 2017, 18, 80–85. [Google Scholar] [CrossRef]
- Elrys, A.S.; Chen, S.; Kong, M.; Liu, L.; Zhu, Q.; Dan, X.; Tang, S.; Wu, Y.; Meng, L.; Zhang, J.; et al. Organic fertilization strengthens multiple internal pathways for soil mineral nitrogen production: Evidence from the meta-analysis of long-term field trials. Biol. Fertil. Soils 2024, 60, 1173–1180. [Google Scholar] [CrossRef]
- Aguilar, A.S.; Cardoso, A.F.; Lima, L.C.; Queiroz Luz, J.M.; Rodrigues, T.; Quintão Lana, R.M. Influence of organomineral fertilization in the development of the potato crop cv. Cupid. Biosci. J. 2019, 35, 199–210. [Google Scholar] [CrossRef]
- Law-Ogbomo, K.E.; Remison, S.U.; Jombo, E.O. Effects of organic and inorganic fertilizer on the productivity of Amaranthus cruentus in an ultisol environment. Int. J. Plant Physiol. Biochem. 2011, 3, 247–252. [Google Scholar] [CrossRef]
- Iderawumi Abdulraheem, M.; Hu, J.; Ahmed, S.; Li, L.; Muhammad Zaigham Abbas Naqvi, S. Advances in the use of organic and organomineral fertilizers in sustainable agricultural production. In Organic Fertilizers-New Advances and Applications; IntechOpen: Rijeka, Croatia, 2023. [Google Scholar] [CrossRef]
- IUSS Working Group WRB. World Reference Base for Soil Resources 2014; International Soil Classification System for Naming Soils and Creating Legends for Soil Maps; Update 2015. In World Soil Resources Reports No. 106; FAO: Rome, Italy, 2015; p. 182. Available online: https://www.fao.org/3/i3794en/I3794en.pdf (accessed on 28 May 2024).
- ISO 11261; Soil Quality—Determination of Total Nitrogen—Modified Kjeldahl Method. International Organization for Standardization: Geneva, Switzerland, 1995.
- Ostrowska, A.; Gawliński, S.; Szczubiałka, Z. Methods of Analysis and Assessment of Soil and Plants Properties, 1st ed.; Institute of Environmental Protection: Warsaw, Poland, 1991; pp. 1–334. [Google Scholar]
- ISO 10390; Soil Quality—Determination of pH. International Organization for Standardization: Geneva, Switzerland, 2005.
- PN-R-04032; Soil and Mineral Materials—Sampling and Determination of Particle Size Distribution. Polish Committee for Standardization: Warszawa, Poland, 1998.
- Statistica (Data Analysis Software System), Version 13.3; TIBCO Software Inc.: Palo Alto, CA, USA, 2017.




| Fertiliser | Yield FM | Yield DM | N | P | Mg | Ca | Zn | Cu | Mn |
|---|---|---|---|---|---|---|---|---|---|
| g Plant−1 | Content in g kg−1 DM | Content in mg kg−1 DM | |||||||
| Control | 24.80 ± 0.36 | 3.61 ± 0.24 | 7.09 ± 0.12 | 2.157 ± 0.065 | 1.538 ± 0.128 | 3.033 ± 0.129 | 32.77 ± 1.29 | 1.867 ± 0.115 | 48.00 ± 3.00 |
| OMF NP24-12 | 78.38 ± 0.60 | 9.41 ± 0.10 | 15.59 ± 0.27 | 3.077 ± 0.180 | 2.341 ± 0.111 | 3.953 ± 0.414 | 54.73 ± 5.00 | 5.700 ± 0.693 | 72.33 ± 5.39 |
| OMF NP10-10 | 58.38 ± 1.01 | 6.95 ± 0.40 | 16.80 ± 0.12 | 3.947 ± 0.100 | 2.403 ± 0.048 | 4.263 ± 0.283 | 71.73 ± 4.38 | 6.600 ± 0.200 | 88.33 ± 5.03 |
| OMF NP10-10 Zn+ | 85.35 ± 0.98 | 12.71 ± 0.31 | 13.81 ± 0.43 | 3.050 ± 0.095 | 2.459 ± 0.062 | 3.430 ± 0.156 | 49.95 ± 2.65 | 6.133 ± 0.513 | 74.33 ± 6.43 |
| MCF NP24-12 | 91.28 ± 1.13 | 13.13 ± 0.39 | 13.81 ± 0.43 | 3.070 ± 0.166 | 2.243 ± 0.277 | 3.893 ± 0.380 | 45.97 ± 0.81 | 2.500 ± 0.173 | 59.33 ± 6.43 |
| MCF NP10-10 | 107.25 ± 1.50 | 15.48 ± 0.34 | 14.28 ± 0.36 | 3.523 ± 0.220 | 2.143 ± 0.137 | 3.863 ± 0.327 | 40.23 ± 5.36 | 3.533 ± 0.231 | 75.67 ± 4.16 |
| LSD0.01 | 0.19 | 0.14 | 0.79 | 0.626 | 0.369 | 0.752 | 7.52 | 0.957 | 13.18 |
| Fertiliser | pH | N | P | Mg | Ca | Zn | Cu | Mn |
|---|---|---|---|---|---|---|---|---|
| Content in g kg−1 DM | Content in mg kg−1 DM | |||||||
| Control | 5.48 ± 0.02 | 0.485 ± 0.032 | 66.57 ± 2.26 | 0.989 ± 0.015 | 1.253 ± 0.023 | 17.55 ± 1.09 | 1.807 ± 0.147 | 101.7 ± 1.1 |
| OMF NP24-12 | 5.20 ± 0.06 | 0.504 ± 0.010 | 73.33 ± 2.22 | 0.962 ± 0.017 | 1.287 ± 0.012 | 18.74 ± 0.28 | 2.640 ± 0.072 | 100.7 ± 3.3 |
| OMF NP10-10 | 5.18 ± 0.03 | 0.523 ± 0.032 | 94.40 ± 2.19 | 0.998 ± 0.022 | 1.360 ± 0.040 | 21.95 ± 1.43 | 3.147 ± 0.196 | 103.0 ± 2.1 |
| OMF NP10-10 Zn+ | 5.02 ± 0.05 | 0.560 ± 0.005 | 91.44 ± 1.83 | 1.010 ± 0.014 | 1.313 ± 0.012 | 24.22 ± 0.55 | 3.987 ± 0.172 | 98.9 ± 3.3 |
| MCF NP24-12 | 4.94 ± 0.03 | 0.429 ± 0.033 | 68.03 ± 2.49 | 0.946 ± 0.013 | 1.260 ± 0.035 | 19.17 ± 0.59 | 1.680 ± 0.072 | 91.5 ± 1.2 |
| MCF NP10-10 | 4.84 ± 0.04 | 0.523 ± 0.032 | 97.97 ± 2.47 | 0.970 ± 0.014 | 1.313 ± 0.061 | 18.29 ± 0.48 | 1.593 ± 0.050 | 93.4 ± 1.7 |
| LSD0.01 | 0.23 | 0.067 | 5.63 | 0.041 | 0.087 | 2.09 | 0.327 | 5.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brodowska, M.S.; Wyszkowski, M.; Grzesik, R. Stimulation of Maize Growth and Development and Improvement of Soil Properties Using New Specialised Organic-Mineral Materials. Molecules 2025, 30, 3050. https://doi.org/10.3390/molecules30143050
Brodowska MS, Wyszkowski M, Grzesik R. Stimulation of Maize Growth and Development and Improvement of Soil Properties Using New Specialised Organic-Mineral Materials. Molecules. 2025; 30(14):3050. https://doi.org/10.3390/molecules30143050
Chicago/Turabian StyleBrodowska, Marzena S., Mirosław Wyszkowski, and Ryszard Grzesik. 2025. "Stimulation of Maize Growth and Development and Improvement of Soil Properties Using New Specialised Organic-Mineral Materials" Molecules 30, no. 14: 3050. https://doi.org/10.3390/molecules30143050
APA StyleBrodowska, M. S., Wyszkowski, M., & Grzesik, R. (2025). Stimulation of Maize Growth and Development and Improvement of Soil Properties Using New Specialised Organic-Mineral Materials. Molecules, 30(14), 3050. https://doi.org/10.3390/molecules30143050







