Chemical Modification Methods for Inulin- and Agavin-Type Fructans: Synthesis, Characterization, and Biofunctional Activity: A Review
Abstract
1. Introduction

2. Fructans Structure Modification Methods: A Description of Chemical Approaches
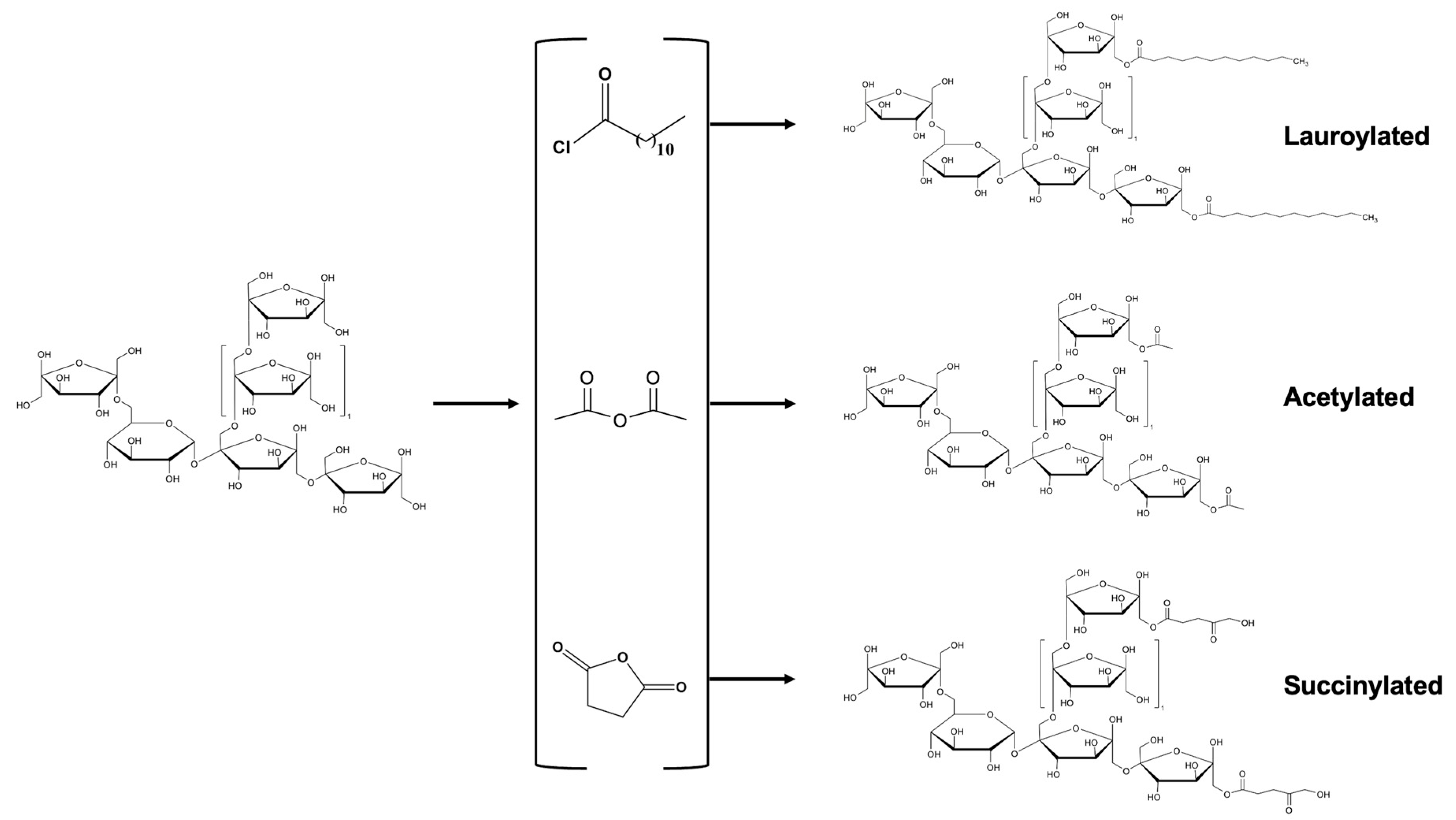
2.1. Esterification
2.1.1. Acetylation
2.1.2. Succinylation
2.2. Crosslinking
2.3. Carboxymethylation
2.4. Sulfation
2.5. Schiff Base Reactions
3. Structural Characterization of Modified Fructans
3.1. FTIR Analysis
3.2. NMR Analysis
- 0.80–2.65 ppm for esterification;
- 1.20–2.03 ppm for acetylation;
- 1.26–1.94 ppm for succinylation;
- 4.51 ppm for carboxymethylation.
3.3. Diffraction Analysis
4. Effect of Chemical Modifications on the Physicochemical and Functional Properties of Modified Fructans
4.1. Physicochemical Properties
4.2. Functional Properties
5. Effect of Chemical Modifications on the Biological of Modified Fructans
6. Prospects and Challenges
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Luna-Solís, H.A.; Ochoa-Martínez, L.A.; González-Herrera, S.M.; Rutiaga-Quiñones, O.M.; Wong-Paz, J.E. Extraction of Agavins from Agave Durangensis Leaves: Structural, Thermal and Techno-Functional Characterization. Food Biosci. 2024, 57, 103563. [Google Scholar] [CrossRef]
- Aldrete-Herrera, P.I.; López, M.G.; Ceja-Medina, L.I.; Medina-Torres, L.; Ortiz-Basurto, R.I. Study of the Rheological and Physicochemical Properties of Fructan Fractions of Agave Tequilana Cv. Cenizo. Agrociencia 2023, 57, 763–778. [Google Scholar] [CrossRef]
- Santiago-García, P.A.; Mellado-Mojica, E.; León-Martínez, F.M.; López, M.G. Evaluation of Agave Angustifolia Fructans as Fat Replacer in the Cookies Manufacture. LWT 2017, 77, 100–109. [Google Scholar] [CrossRef]
- García-Villalba, W.G.; Rodríguez-Herrera, R.; Ochoa-Martínez, L.A.; Rutiaga-Quiñones, O.M.; Gallegos-Infante, J.A.; González-Herrera, S.M. Agave Fructans: A Review of Their Technological Functionality and Extraction Processes. J. Food Sci. Technol. 2023, 60, 1265–1273. [Google Scholar] [CrossRef]
- Vijn, I.; Smeekens, S. Fructan: More than a Reserve Carbohydrate? Plant Physiol. 1999, 120, 351–360. [Google Scholar] [CrossRef] [PubMed]
- Tungland, B. Nondigestible Fructans as Prebiotics. In Human Microbiota in Health and Disease: From Pathogenesis to Therapy; Academic Press: London, UK, 2018. [Google Scholar]
- Ritsema, T.; Smeekens, S. Fructans: Beneficial for Plants and Humans. Curr. Opin. Plant Biol. 2003, 6, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Apolinário, A.C.; De Lima Damasceno, B.P.G.; De Macêdo Beltrão, N.E.; Pessoa, A.; Converti, A.; Da Silva, J.A. Inulin-Type Fructans: A Review on Different Aspects of Biochemical and Pharmaceutical Technology. Carbohydr. Polym. 2014, 101, 368–378. [Google Scholar] [CrossRef]
- Mellado-Mojica, E.; López, M.G. Fructan Metabolism in A. Tequilana Weber Blue Variety along Its Developmental Cycle in the Field. J. Agric. Food Chem. 2012, 60, 11704–11713. [Google Scholar] [CrossRef]
- Rodriguez Furlán, L.T.; Lecot, J.; Pérez Padilla, A.; Campderrós, M.E.; Zaritzky, N. Stabilizing Effect of Saccharides on Bovine Plasma Protein: A Calorimetric Study. Meat Sci. 2012, 91, 478–485. [Google Scholar] [CrossRef]
- Liang, X.; Lin, D.; Zhang, W.; Chen, S.; Ding, H.; Zhong, H.-J. Progress in the Preparation and Application of Inulin-Based Hydrogels. Polymers 2024, 16, 1492. [Google Scholar] [CrossRef]
- Afinjuomo, F.; Abdella, S.; Youssef, S.H.; Song, Y.; Garg, S. Inulin and Its Application in Drug Delivery. Pharmaceuticals 2021, 14, 855. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.-W.; Du, Z.-M.; Wang, Y.-W.; Feng, Y.-X.; Zhang, R.; Yan, X.-B. Chemical Modification, Characterization, and Activity Changes of Land Plant Polysaccharides: A Review. Polymers 2022, 14, 4161. [Google Scholar] [CrossRef]
- Jahangiri, F.; Mohanty, A.K.; Misra, M. Sustainable Biodegradable Coatings for Food Packaging: Challenges and Opportunities. Green Chem. 2024, 26, 4934–4974. [Google Scholar] [CrossRef]
- Moreno-Vilet, L.; Camacho-Ruiz, R.M.; Portales-Pérez, D.P. Prebiotic Agave Fructans and Immune Aspects. In Probiotics, Prebiotics, and Synbiotics; Elsevier: Amsterdam, The Netherlands, 2016; pp. 165–179. [Google Scholar]
- Wang, M.; Cheong, K.-L. Preparation, Structural Characterisation, and Bioactivities of Fructans: A Review. Molecules 2023, 28, 1613. [Google Scholar] [CrossRef]
- Díaz-Ramos, D.I.; Ortiz-Basurto, R.I.; García-Barradas, O.; Chacón-López, M.A.; Montalvo-González, E.; Pascual-Pineda, L.A.; Valenzuela-Vázquez, U.; Jiménez-Fernández, M. Lauroylated, Acetylated, and Succinylated Agave Tequilana Fructans Fractions: Structural Characterization, Prebiotic, Antibacterial Activity and Their Effect on Lactobacillus Paracasei under Gastrointestinal Conditions. Polymers 2023, 15, 3115. [Google Scholar] [CrossRef] [PubMed]
- Baig, M.T.; Abdel-Azeim, S.; Ganie, S.A.; Warsame, M.; Mady, M.F. Phosphonated Inulin as an Eco-Friendly Thermally Stable Scale Inhibitor for the Oil and Gas Industry: Synthesis, Characterization, Efficacy, and Molecular Insights. ACS Sustain. Chem. Eng. 2025, 13, 1762–1774. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, Y.; Liu, C.; Yi, S.; Sun, W.; He, J. Catalytic Ethanolysis of Fructan-Rich and Starchy Expired Food into Biofuel 5-Ethoxymethylfurfural. Bioresources 2021, 16, 6186–6200. [Google Scholar] [CrossRef]
- Segovia-Hernández, J.G.; Hernández, S.; Cossío-Vargas, E.; Sánchez-Ramírez, E. Challenges and Opportunities in Process Intensification to Achieve the UN’s 2030 Agenda: Goals 6, 7, 9, 12 and 13. Chem. Eng. Process. Process Intensif. 2023, 192, 109507. [Google Scholar] [CrossRef]
- Cravotto, G. Reshaping Chemical Manufacturing Towards Green Process Intensification: Recent Findings and Perspectives. Processes 2025, 13, 459. [Google Scholar] [CrossRef]
- Karimi, I.; Ghowsi, M.; Mohammed, L.J.; Haidari, Z.; Nazari, K.; Schiöth, H.B. Inulin as a Biopolymer; Chemical Structure, Anticancer Effects, Nutraceutical Potential and Industrial Applications: A Comprehensive Review. Polymers 2025, 17, 412. [Google Scholar] [CrossRef]
- Padilla-Camberos, E.; Arrizon, J.; Sandoval, G. Effect of Agave Fructan Bioconjugates on Metabolic Syndrome Parameters in a Murine Model. Pharmaceuticals 2023, 16, 412. [Google Scholar] [CrossRef] [PubMed]
- Cumpstey, I. Chemical Modification of Polysaccharides. ISRN Org. Chem. 2013, 2013, 1–27. [Google Scholar] [CrossRef]
- Prasad, K.S.; Patel, H.; Patel, T.; Patel, K.; Selvaraj, K. Biosynthesis of Se Nanoparticles and Its Effect on UV-Induced DNA Damage. Colloids Surf. B Biointerfaces 2013, 103, 261–266. [Google Scholar] [CrossRef]
- Falsafi, S.R.; Maghsoudlou, Y.; Rostamabadi, H.; Rostamabadi, M.M.; Hamedi, H.; Hosseini, S.M.H. Preparation of Physically Modified Oat Starch with Different Sonication Treatments. Food Hydrocoll. 2019, 89, 311–320. [Google Scholar] [CrossRef]
- López-Salazar, H.; Camacho-Díaz, B.H.; Ocampo, M.L.A.; Jiménez-Aparicio, A.R. Microwave-Assisted Extraction of Functional Compounds from Plants: A Review. Bioresources 2023, 18, 6614–6638. [Google Scholar] [CrossRef]
- Srivastava, N.; Mukhopadhyay, M. Biosynthesis and Structural Characterization of Selenium Nanoparticles Mediated by Zooglea Ramigera. Powder Technol. 2013, 244, 26–29. [Google Scholar] [CrossRef]
- Punia Bangar, S.; Ashogbon, A.O.; Singh, A.; Chaudhary, V.; Whiteside, W.S. Enzymatic Modification of Starch: A Green Approach for Starch Applications. Carbohydr. Polym. 2022, 287, 119265. [Google Scholar] [CrossRef]
- Karaki, N.; Aljawish, A.; Humeau, C.; Muniglia, L.; Jasniewski, J. Enzymatic Modification of Polysaccharides: Mechanisms, Properties, and Potential Applications: A Review. Enzyme Microb. Technol. 2016, 90, 1–18. [Google Scholar] [CrossRef]
- Zhao, T.; Yang, M.; Ma, L.; Liu, X.; Ding, Q.; Chai, G.; Lu, Y.; Wei, H.; Zhang, S.; Ding, C. Structural Modification and Biological Activity of Polysaccharides. Molecules 2023, 28, 5416. [Google Scholar] [CrossRef]
- Shah, I.A.; Kavitake, D.; Tiwari, S.; Devi, P.B.; Reddy, G.B.; Jaiswal, K.K.; Jaiswal, A.K.; Shetty, P.H. Chemical Modification of Bacterial Exopolysaccharides: Antioxidant Properties and Health Potentials. Curr. Res. Food Sci. 2024, 9, 100824. [Google Scholar] [CrossRef]
- Nataraj, A.; Govindan, S.; Rajendran, A.; Ramani, P.; Subbaiah, K.A.; Munekata, P.E.S.; Pateiro, M.; Lorenzo, J.M. Effects of Carboxymethyl Modification on the Acidic Polysaccharides from Calocybe Indica: Physicochemical Properties, Antioxidant, Antitumor and Anticoagulant Activities. Antioxidants 2022, 12, 105. [Google Scholar] [CrossRef]
- Mahmoud, Y.A.-G.; El-Naggar, M.E.; Abdel-Megeed, A.; El-Newehy, M. Recent Advancements in Microbial Polysaccharides: Synthesis and Applications. Polymers 2021, 13, 4136. [Google Scholar] [CrossRef] [PubMed]
- Brandon, A.G.; Scheller, H.V. Engineering of Bioenergy Crops: Dominant Genetic Approaches to Improve Polysaccharide Properties and Composition in Biomass. Front. Plant Sci. 2020, 11, 282. [Google Scholar] [CrossRef]
- Laurent, J.M.; Jain, A.; Kan, A.; Steinacher, M.; Enrriquez Casimiro, N.; Stavrakis, S.; deMello, A.J.; Studart, A.R. Directed Evolution of Material-Producing Microorganisms. Proc. Natl. Acad. Sci. USA 2024, 121, e2403585121. [Google Scholar] [CrossRef]
- Dass, R.S.; Anand, K.R.; Saha, D.; Dhinakar, J.E.; Thorat, P. Polysaccharides of Biomedical Importance from Genetically Modified Microorganisms. In Polysaccharides of Microbial Origin; Springer International Publishing: Cham, Switzerland, 2022; pp. 649–674. [Google Scholar]
- Verma, A.; Chauhan, A.; Sharma, T.; Thakur, S.; Bhatia, R.; Singh, T.G.; Awasthi, A. Advances in Gene Therapy: Polysaccharide Nanoparticles as Pioneers in Safe and Effective Gene Delivery. J. Drug Deliv. Sci. Technol. 2024, 102, 106287. [Google Scholar] [CrossRef]
- Xu, Y.; Wu, Y.; Sun, P.; Zhang, F.; Linhardt, R.J.; Zhang, A. Chemically Modified Polysaccharides: Synthesis, Characterization, Structure Activity Relationships of Action. Int. J. Biol. Macromol. 2019, 132, 970–977. [Google Scholar] [CrossRef]
- Simsek, M.; Asiyanbi-Hammed, T.T.; Rasaq, N.; Hammed, A.M. Progress in Bioactive Polysaccharide-Derivatives: A Review. Food Rev. Int. 2023, 39, 1612–1627. [Google Scholar] [CrossRef]
- Choi, J.; Hwang, D.S.; Lim, C.; Lee, D.W. Interaction Mechanism between Low Molecular Weight Chitosan Nanofilm and Functionalized Surfaces in Aqueous Solutions. Carbohydr. Polym. 2024, 324, 121504. [Google Scholar] [CrossRef]
- Díaz-Ramos, D.I.; Jiménez-Fernández, M.; García-Barradas, O.; Chacón-López, M.A.; Montalvo-González, E.; López-García, U.M.; Beristain-Guevara, C.I.; Ortiz-Basurto, R.I. Structural, Thermal, and Functional Properties of Agave Tequilana Fructan Fractions Modified by Acylation. Rev. Mex. Ing. Quim. 2023, 22, 1–23. [Google Scholar] [CrossRef]
- Miramontes-Corona, C.; Escalante, A.; Delgado, E.; Corona-González, R.I.; Vázquez-Torres, H.; Toriz, G. Hydrophobic Agave Fructans for Sustained Drug Delivery to the Human Colon. React. Funct. Polym. 2020, 146, 104396. [Google Scholar] [CrossRef]
- Ignot-Gutiérrez, A.; Ortiz-Basurto, R.I.; García-Barradas, O.; Díaz-Ramos, D.I.; Jiménez-Fernández, M. Physicochemical and Functional Properties of Native and Modified Agave Fructans by Acylation. Carbohydr. Polym. 2020, 245, 116529. [Google Scholar] [CrossRef]
- Han, L.; Ratcliffe, I.; Williams, P.A. Self-Assembly and Emulsification Properties of Hydrophobically Modified Inulin. J. Agric. Food Chem. 2015, 63, 3709–3715. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Ratcliffe, I.; Williams, P.A. Synthesis, Characterisation and Physicochemical Properties of Hydrophobically Modified Inulin Using Long-Chain Fatty Acyl Chlorides. Carbohydr. Polym. 2017, 178, 141–146. [Google Scholar] [CrossRef]
- Huang, S.; Chen, F.; Cheng, H.; Huang, G. Modification and Application of Polysaccharide from Traditional Chinese Medicine Such as Dendrobium Officinale. Int. J. Biol. Macromol. 2020, 157, 385–393. [Google Scholar] [CrossRef] [PubMed]
- Xue, H.; Li, P.; Bian, J.; Gao, Y.; Sang, Y.; Tan, J. Extraction, Purification, Structure, Modification, and Biological Activity of Traditional Chinese Medicine Polysaccharides: A Review. Front. Nutr. 2022, 9, 1005181. [Google Scholar] [CrossRef]
- Heinze, T.; Liebert, T.; Koschella, A. Esterification of Polysaccharides; Springer: Berlin/Heidelberg, Germany, 2006. [Google Scholar]
- Li, S.; Xiong, Q.; Lai, X.; Li, X.; Wan, M.; Zhang, J.; Yan, Y.; Cao, M.; Lu, L.; Guan, J.; et al. Molecular Modification of Polysaccharides and Resulting Bioactivities. Compr. Rev. Food Sci. Food Saf. 2016, 15, 237–250. [Google Scholar] [CrossRef]
- Xie, J.-H.; Zhang, F.; Wang, Z.-J.; Shen, M.-Y.; Nie, S.-P.; Xie, M.-Y. Preparation, Characterization and Antioxidant Activities of Acetylated Polysaccharides from Cyclocarya Paliurus Leaves. Carbohydr. Polym. 2015, 133, 596–604. [Google Scholar] [CrossRef] [PubMed]
- Lim, C.; Hwang, D.S.; Lee, D.W. Intermolecular Interactions of Chitosan: Degree of Acetylation and Molecular Weight. Carbohydr. Polym. 2021, 259, 117782. [Google Scholar] [CrossRef]
- Buitrago-Arias, C.; Londoño-Moreno, A.; Avila-Reyes, S.V.; Arenas-Ocampo, M.L.; Alamilla-Beltran, L.; Jimenez-Aparicio, A.R.; Camacho-Diaz, B.H. Evaluation of the Fermentation of Acetylated Agave Fructans (Agavins), with Saccharomyces Boulardii as a Probiotic. Rev. Mex. Ing. Quim. 2021, 20, 1–13. [Google Scholar] [CrossRef]
- Walz, M.; Hagemann, D.; Trentzsch, M.; Weber, A.; Henle, T. Degradation Studies of Modified Inulin as Potential Encapsulation Material for Colon Targeting and Release of Mesalamine. Carbohydr. Polym. 2018, 199, 102–108. [Google Scholar] [CrossRef]
- Petkova, N.; Tumbarski, Y.; Ivanov, I.; Denev, P. Design of Inulin Acetates with Potential Antimicrobial Activity. Bulg. J. Vet. Med. 2017, 20, 13–17. [Google Scholar]
- Shah, N.N.; Soni, N.; Singhal, R.S. Modification of Proteins and Polysaccharides Using Dodecenyl Succinic Anhydride: Synthesis, Properties and Applications—A Review. Int. J. Biol. Macromol. 2018, 107, 2224–2233. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Xiang, Z.; Lu, F. Synthesis and Emulsifying Properties of Long-Chain Succinic Acid Esters of Glucuronoxylans. Cellulose 2019, 26, 3713–3724. [Google Scholar] [CrossRef]
- Starbird, R.; Zuñiga, V.; Delgado, E.; Saake, B.; Toriz, G. Design of Microspheres from Blue Agave Fructans for Drug Delivery to the Colon. Part 1. Esterification of Agave Fructans. J. Biobased Mater. Bioenergy 2007, 1, 238–244. [Google Scholar] [CrossRef]
- Han, L.; Hu, B.; Ratcliffe, I.; Senan, C.; Yang, J.; Williams, P.A. Octenyl-Succinylated Inulin for the Encapsulation and Release of Hydrophobic Compounds. Carbohydr. Polym. 2020, 238, 116199. [Google Scholar] [CrossRef]
- Kokubun, S.; Ratcliffe, I.; Williams, P.A. Synthesis, Characterization and Self-Assembly of Biosurfactants Based on Hydrophobically Modified Inulins. Biomacromolecules 2013, 14, 2830–2836. [Google Scholar] [CrossRef]
- Han, L.; Sun, J.; Williams, P.A.; Yang, J.; Zhang, S. Octenyl-Succinylated Inulins for the Delivery of Hydrophobic Drug. Int. J. Biol. Macromol. 2022, 221, 1112–1120. [Google Scholar] [CrossRef]
- Haq, F.; Yu, H.; Wang, L.; Teng, L.; Haroon, M.; Khan, R.U.; Mehmood, S.; Bilal-Ul-Amin; Ullah, R.S.; Khan, A.; et al. Advances in Chemical Modifications of Starches and Their Applications. Carbohydr. Res. 2019, 476, 12–35. [Google Scholar] [CrossRef]
- Tavakoli, J.; Tang, Y. Hydrogel Based Sensors for Biomedical Applications: An Updated Review. Polymers 2017, 9, 364. [Google Scholar] [CrossRef]
- Li, Y.; Wu, B.; Li, Y.; Li, H.; Ji, S.; Xia, Q. PH-Responsive Pickering Emulsions-Pectin Hydrogel Beads for Loading of Resveratrol: Preparation, Characterization, and Evaluation. J. Drug Deliv. Sci. Technol. 2023, 79, 104008. [Google Scholar] [CrossRef]
- Flory, P.J.; Rehner, J. Statistical Mechanics of Cross-Linked Polymer Networks I. Rubberlike Elasticity. J. Chem. Phys. 1943, 11, 512–520. [Google Scholar] [CrossRef]
- Afinjuomo, F.; Barclay, T.G.; Song, Y.; Parikh, A.; Petrovsky, N.; Garg, S. Synthesis and Characterization of a Novel Inulin Hydrogel Crosslinked with Pyromellitic Dianhydride. React. Funct. Polym. 2019, 134, 104–111. [Google Scholar] [CrossRef]
- Ahmad, M.M. Recent Trends in Chemical Modification and Antioxidant Activities of Plants-Based Polysaccharides: A Review. Carbohydr. Polym. Technol. Appl. 2021, 2, 100045. [Google Scholar] [CrossRef]
- Chakka, V.P.; Zhou, T. Carboxymethylation of Polysaccharides: Synthesis and Bioactivities. Int. J. Biol. Macromol. 2020, 165, 2425–2431. [Google Scholar] [CrossRef]
- Zhang, J.; Tan, W.; Zhao, P.; Mi, Y.; Guo, Z. Facile Synthesis, Characterization, Antioxidant Activity, and Antibacterial Activity of Carboxymethyl Inulin Salt Derivatives. Int. J. Biol. Macromol. 2022, 199, 138–149. [Google Scholar] [CrossRef]
- Castañeda-Salazar, A.; Figueroa-Cárdenas, J.D.; López, M.G.; Mendoza, S. Physicochemical and Functional Characterization of Agave Fructans Modified by Cationization and Carboxymethylation. Carbohydr. Polym. Technol. Appl. 2023, 5, 100284. [Google Scholar] [CrossRef]
- Mi, Y.; Zhang, J.; Han, X.; Tan, W.; Miao, Q.; Cui, J.; Li, Q.; Guo, Z. Modification of Carboxymethyl Inulin with Heterocyclic Compounds: Synthesis, Characterization, Antioxidant and Antifungal Activities. Int. J. Biol. Macromol. 2021, 181, 572–581. [Google Scholar] [CrossRef]
- Łączkowski, K.Z.; Motylewska, K.; Baranowska-Łączkowska, A.; Biernasiuk, A.; Misiura, K.; Malm, A.; Fernández, B. Synthesis, Antimicrobial Evaluation and Theoretical Prediction of NMR Chemical Shifts of Thiazole and Selenazole Derivatives with High Antifungal Activity against Candida spp. J. Mol. Struct. 2016, 1108, 427–437. [Google Scholar] [CrossRef]
- Shi, F.; Li, C.; Xia, M.; Miao, K.; Zhao, Y.; Tu, S.; Zheng, W.; Zhang, G.; Ma, N. Green Chemoselective Synthesis of Thiazolo[3,2-a]Pyridine Derivatives and Evaluation of Their Antioxidant and Cytotoxic Activities. Bioorg. Med. Chem. Lett. 2009, 19, 5565–5568. [Google Scholar] [CrossRef]
- Dananjaya, S.H.S.; Erandani, W.K.C.U.; Kim, C.-H.; Nikapitiya, C.; Lee, J.; De Zoysa, M. Comparative Study on Antifungal Activities of Chitosan Nanoparticles and Chitosan Silver Nano Composites against Fusarium Oxysporum Species Complex. Int. J. Biol. Macromol. 2017, 105, 478–488. [Google Scholar] [CrossRef]
- Liu, Y.; Tang, Q.; Duan, X.; Tang, T.; Ke, Y.; Zhang, L.; Li, C.; Liu, A.; Su, Z.; Hu, B. Antioxidant and Anticoagulant Activities of Mycelia Polysaccharides from Catathelasma Ventricosum after Sulfated Modification. Ind. Crops Prod. 2018, 112, 53–60. [Google Scholar] [CrossRef]
- Shao, T.; Yuan, P.; Zhang, W.; Dou, D.; Wang, F.; Hao, C.; Liu, C.; Han, J.; Chen, K.; Wang, G. Preparation and Characterization of Sulfated Inulin-Type Fructans from Jerusalem Artichoke Tubers and Their Antitumor Activity. Carbohydr. Res. 2021, 509, 108422. [Google Scholar] [CrossRef]
- Schiff, H. Mittheilungen Aus Dem Universitätslaboratorium in Pisa: Eine Neue Reihe Organischer Basen. Justus Liebigs Ann. Chem. 1864, 131, 118–119. [Google Scholar] [CrossRef]
- Moss, G.P.; Smith, P.A.S.; Tavernier, D. Glossary of Class Names of Organic Compounds and Reactivity Intermediates Based on Structure (IUPAC Recommendations 1995). Pure Appl. Chem. 1995, 67, 1307–1375. [Google Scholar] [CrossRef]
- Raczuk, E.; Dmochowska, B.; Samaszko-Fiertek, J.; Madaj, J. Different Schiff Bases—Structure, Importance and Classification. Molecules 2022, 27, 787. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Mi, Y.; Li, Q.; Dong, F.; Guo, Z. Synthesis of Schiff Bases Modified Inulin Derivatives for Potential Antifungal and Antioxidant Applications. Int. J. Biol. Macromol. 2020, 143, 714–723. [Google Scholar] [CrossRef]
- Berhanu, A.L.; Gaurav; Mohiuddin, I.; Malik, A.K.; Aulakh, J.S.; Kumar, V.; Kim, K.-H. A Review of the Applications of Schiff Bases as Optical Chemical Sensors. TrAC Trends Anal. Chem. 2019, 116, 74–91. [Google Scholar] [CrossRef]
- Ansari, R.M.; Bhat, B.R. Copper (II) Schiff Base-Graphene Oxide Composite as an Efficient Catalyst for Suzuki-Miyaura Reaction. Chem. Phys. 2019, 517, 155–160. [Google Scholar] [CrossRef]
- Zhang, Z.; Song, Q.; Jin, Y.; Feng, Y.; Li, J.; Zhang, K. Advances in Schiff Base and Its Coating on Metal Biomaterials—A Review. Metals 2023, 13, 386. [Google Scholar] [CrossRef]
- Odularu, A.T. Ease to Challenges in Achieving Successful Synthesized Schiff Base, Chirality, and Application as Antibacterial Agent. BioMed Res. Int. 2023, 2023, 1626488. [Google Scholar] [CrossRef]
- Malik, U.S.; Niazi, M.B.K.; Jahan, Z.; Zafar, M.I.; Vo, D.-V.N.; Sher, F. Nano-Structured Dynamic Schiff Base Cues as Robust Self-Healing Polymers for Biomedical and Tissue Engineering Applications: A Review. Environ. Chem. Lett. 2022, 20, 495–517. [Google Scholar] [CrossRef]
- Sato, T.; Yang, J.; Terao, K. Micellar Structure of Hydrophobically Modified Polysaccharides in Aqueous Solution. Polym. J. 2022, 54, 403–412. [Google Scholar] [CrossRef]
- Taresco, V.; Suksiriworapong, J.; Creasey, R.; Burley, J.C.; Mantovani, G.; Alexander, C.; Treacher, K.; Booth, J.; Garnett, M.C. Properties of Acyl Modified Poly(Glycerol-Adipate) Comb-like Polymers and Their Self-Assembly into Nanoparticles. J. Polym. Sci. A Polym. Chem. 2016, 54, 3267–3278. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wang, X.; Xiong, Q.; Yu, Y.; Peng, L. Sulfated Modification, Characterization, and Potential Bioactivities of Polysaccharide from the Fruiting Bodies of Russula Virescens. Int. J. Biol. Macromol. 2020, 154, 1438–1447. [Google Scholar] [CrossRef]
- Liu, H.; Xu, J.; Xu, X.; Yuan, Z.; Song, H.; Yang, L.; Zhu, D. Structure/Function Relationships of Bean Polysaccharides: A Review. Crit. Rev. Food Sci. Nutr. 2023, 63, 330–344. [Google Scholar] [CrossRef] [PubMed]
- Sikorski, P.; Mo, F.; Skjåk-Bræk, G.; Stokke, B.T. Evidence for Egg-Box-Compatible Interactions in Calcium−Alginate Gels from Fiber X-Ray Diffraction. Biomacromolecules 2007, 8, 2098–2103. [Google Scholar] [CrossRef]
- Seidi, F.; Yazdi, M.K.; Jouyandeh, M.; Habibzadeh, S.; Munir, M.T.; Vahabi, H.; Bagheri, B.; Rabiee, N.; Zarrintaj, P.; Saeb, M.R. Crystalline Polysaccharides: A Review. Carbohydr. Polym. 2022, 275, 118624. [Google Scholar] [CrossRef]
- Kumar, D.; Pandey, J.; Raj, V.; Kumar, P. A Review on the Modification of Polysaccharide Through Graft Copolymerization for Various Potential Applications. Open Med. Chem. J. 2017, 11, 109–126. [Google Scholar] [CrossRef]
- Jain, A.K.; Sood, V.; Bora, M.; Vasita, R.; Katti, D.S. Electrosprayed Inulin Microparticles for Microbiota Triggered Targeting of Colon. Carbohydr. Polym. 2014, 112, 225–234. [Google Scholar] [CrossRef]
- Fernández-Lainez, C.; Akkerman, R.; Oerlemans, M.M.P.; Logtenberg, M.J.; Schols, H.A.; Silva-Lagos, L.A.; López-Velázquez, G.; de Vos, P. β(2→6)-Type Fructans Attenuate Proinflammatory Responses in a Structure Dependent Fashion via Toll-like Receptors. Carbohydr. Polym. 2022, 277, 118893. [Google Scholar] [CrossRef]
- Wang, Z.; Xie, J.; Shen, M.; Nie, S.; Xie, M. Sulfated Modification of Polysaccharides: Synthesis, Characterization and Bioactivities. Trends Food Sci. Technol. 2018, 74, 147–157. [Google Scholar] [CrossRef]
- Liu, X.; Xie, J.; Jia, S.; Huang, L.; Wang, Z.; Li, C.; Xie, M. Immunomodulatory Effects of an Acetylated Cyclocarya Paliurus Polysaccharide on Murine Macrophages RAW264.7. Int. J. Biol. Macromol. 2017, 98, 576–581. [Google Scholar] [CrossRef]
- Padzil, A.M.; Azi, A.A.; Muhamad, I.I. Physicochemical Properties of Encapsulated Purple Sweet Potato Extract; Effect of Maltodextrin Concentration, and Microwave Drying Power. Malays. J. Anal. Sci. 2018, 22, 612–618. [Google Scholar]
- Mathlouthi, M. Water Content, Water Activity, Water Structure and the Stability of Foodstuffs. Food Control 2001, 12, 409–417. [Google Scholar] [CrossRef]
- Bashir, M.; Haripriya, S. Physicochemical and Structural Evaluation of Alkali Extracted Chickpea Starch as Affected by γ-Irradiation. Int. J. Biol. Macromol. 2016, 89, 279–286. [Google Scholar] [CrossRef]
- Biswas, A.; Kumar, V.; Bhosle, S.; Sahoo, J.; Chatli, M.K. Dietary Fibers as Functional Ingredients in Meat Products and Their Role in Human Healt. Int. J. Livest. Prod. 2011, 2, 45–54. [Google Scholar]
- Acharya, P.T.; Bhavsar, Z.A.; Jethava, D.J.; Patel, D.B.; Patel, H.D. A Review on Development of Bio-Active Thiosemicarbazide Derivatives: Recent Advances. J. Mol. Struct. 2021, 1226, 129268. [Google Scholar] [CrossRef]
- Tang, S.; Wang, T.; Huang, C.; Lai, C.; Fan, Y.; Yong, Q. Sulfated Modification of Arabinogalactans from Larix Principis-Rupprechtii and Their Antitumor Activities. Carbohydr. Polym. 2019, 215, 207–212. [Google Scholar] [CrossRef]
- Kabara, J.J.; Swieczkowski, D.M.; Conley, A.J.; Truant, J.P. Fatty Acids and Derivatives as Antimicrobial Agents. Antimicrob. Agents Chemother. 1972, 2, 23–28. [Google Scholar] [CrossRef]
- Yarley, O.P.N.; Kojo, A.B.; Zhou, C.; Yu, X.; Gideon, A.; Kwadwo, H.H.; Richard, O. Reviews on Mechanisms of in Vitro Antioxidant, Antibacterial and Anticancer Activities of Water-Soluble Plant Polysaccharides. Int. J. Biol. Macromol. 2021, 183, 2262–2271. [Google Scholar] [CrossRef]
- He, F.; Yang, Y.; Yang, G.; Yu, L. Studies on Antibacterial Activity and Antibacterial Mechanism of a Novel Polysaccharide from Streptomyces Virginia H03. Food Control 2010, 21, 1257–1262. [Google Scholar] [CrossRef]

| Type of Modification | Improved Property | Observed Effect | Proposed Application | Reference(s) |
|---|---|---|---|---|
| Esterification (general) | Emulsifying capacity | Improved encapsulation and emulsion stabilization. | Food, cosmetic, and pharmaceutical industries | [45,46] |
| Acetylation | Enzymatic degradability | High encapsulation efficiency and targeted release | Encapsulation material for oral delivery | |
| Antifungal activity | Inhibition of Beauveria bassiana at 5 mg/mL | Natural pesticide use | [55] | |
| Succinylation | Hydrophobicity and modulated solubility | Encapsulation of antitumor drugs; release at neutral pH | Nutraceutical, medical, and pharmaceutical applications | [58,59,61] |
| Crosslinking (with PMDA) | Network density and pH sensitivity | Swelling decreases with increased crosslinking. pH-sensitive behavior | Modified hydrogels for colonic drug delivery | [66] |
| Schiff bases | Antifungal and antioxidant activity | Broad antifungal spectrum and free radical scavenging | Bioactive and biocompatible biomaterials | [80] |
| Carboxymethylation | Biocompatibility and bioactivity | High antioxidant and antibacterial capacity; enhanced antifungal effect via heterocycle modification | Medical, food, and cosmetic applications | [69,71] |
| Sulfation | Antiproliferative activity | Inhibition of tumor cell growth | Medical therapies with antitumor potential | [75] |
| Type of Modification | Improved Property | Observed Effect | Proposed Application | Reference(s) |
|---|---|---|---|---|
| Esterification | Hydrophobicity | Increased amphiphilic character; enhanced interaction with hydrophobic compounds | Food additives, emulsifiers, cosmetics, pharmaceuticals | [42,44] |
| High drug encapsulation and bioavailability | Drug delivery systems for colonic release | [43] | ||
| Acetylation | Prebiotic activity | Improved survival of L. paracasei; fermentation by S. boulardii | Development of prebiotic and symbiotic formulations | [17,53] |
| Hydrophobicity | Enhanced stability and prolonged drug release | Colonic drug transporters | [43] | |
| Succinylation | Prebiotic and antibacterial activity | Enhanced bacterial viability and antimicrobial potential | Prebiotic, symbiotic, and antiseptic applications | [17] |
| Carboxymethylation | Thermal behavior and antimicrobial activity | Improved melting temperature; inhibition of Gram-negative bacteria | Functional food ingredients or bioactive additives | [70] |
| Fructan/Modification | Characteristic Absorption Peak | Reference |
|---|---|---|
| Agavin/esterification | New signs at 2921 and 2847 cm−1 (CH3-CH2) and 1557 cm−1 (C=O) | [42] |
| New signs at 2921 m−1 (CH3-CH2) and 1556, 1421 cm−1 (C=O) | [44] | |
| Increase in the intensity of bands a 2852 and 2923 cm−1 (CH3) and new signs at 1740 (C=O) and 12181187 cm−1 (C-O) | [43] | |
| Agavin/acetylation | New signs at 1739.82 cm−1 (C=O) and 1368 cm−1 (CH3) | [17] |
| New signs at 1700–1750 cm−1 (C=O) | [53] | |
| Increased signals at 1220, 1370 and 1740 cm−1 (CH3, C-O, C=O, respectively) | [43] | |
| Inulin/acetylation | New peaks at 2900 cm−1 (CH3-CH2) and 1750 cm−1 (C=O) | [54] |
| New peaks at 1745 cm−1 (C=O), 1370 cm−1 (C-H) and 1220 cm−1 (C-O) | [55] | |
| Agavin/succinylation | New peaks at 2931 (OH) and 1643 cm−1 (C=O) that form the COOH and another sign at 1724 cm−1 (C=O) of the ester | [17] |
| Two new peaks at 1576 (COO-) and 1734 cm−1 (C=O) | [59] | |
| Inulin/crosslinking | New bands at 1729, and 1395 cm−1 (C=O), 1592 remains of COOH and 713 cm−1 aromatic ring (C-H) pyromellitic group | [66] |
| Agavina/carboxymethylation | New bands at 1596 and 1411 cm−1 (-COOH) | [70] |
| Inulin/carboxymethylation | New peaks at 1746 cm−1 and 1216 cm−1 COOH | [71] |
| Inulin/Schiff bases | Acetyl group at 1743 cm−1. New peak at 2107 cm−1 azido and other derivatives 1670–1680 cm−1 imine, 3060 and 1450–1600 cm−1 benzene ring | [80] |
| Inulin/sulfation | New peaks at 1250 cm−1 S=O, 814 cm−1 (C-O-S) and 3430 cm−1 | [76] |
| Fructan/Modification | Sings NMR 1H (ppm) | Reference |
|---|---|---|
| Agavin/esterification | 0.89, 2.65, 2.05, 2.19, 1.47, 1.28 methyl and methylene groups | [42] |
| 0.80, 1.25 methyl and methylene groups | [44] | |
| 0.86, 1.2, 1.9 methyl and methylene groups | [43] | |
| Agavin/acetylation | 2.0 methyl group | [17] |
| 1.2 methyl group | [53] | |
| 1.2 methyl and methylene groups | [43] | |
| Inulin/acetylation | 2.03 acetate group | [54] |
| Agavin/succinylation | 1.91 methylene group | [17] |
| 1.26 and 1.94 methyl and methylene groups | [59] | |
| Inulin/carboxymethylation | 4.51 methylene groups | [71] |
| Inulin/Schiff bases | 2.0 hydrogen of the acetyl, 3.7 H–C6–N, 8.4 H–C7=N and 6.5–8.0 aromatic rings | [80] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Díaz-Ramos, D.I.; Jiménez-Fernández, M.; García-Barradas, O.; Ortiz-Basurto, R.I.; Fouconnier, B. Chemical Modification Methods for Inulin- and Agavin-Type Fructans: Synthesis, Characterization, and Biofunctional Activity: A Review. Molecules 2025, 30, 2672. https://doi.org/10.3390/molecules30132672
Díaz-Ramos DI, Jiménez-Fernández M, García-Barradas O, Ortiz-Basurto RI, Fouconnier B. Chemical Modification Methods for Inulin- and Agavin-Type Fructans: Synthesis, Characterization, and Biofunctional Activity: A Review. Molecules. 2025; 30(13):2672. https://doi.org/10.3390/molecules30132672
Chicago/Turabian StyleDíaz-Ramos, Dafne I., Maribel Jiménez-Fernández, Oscar García-Barradas, Rosa Isela Ortiz-Basurto, and Benoit Fouconnier. 2025. "Chemical Modification Methods for Inulin- and Agavin-Type Fructans: Synthesis, Characterization, and Biofunctional Activity: A Review" Molecules 30, no. 13: 2672. https://doi.org/10.3390/molecules30132672
APA StyleDíaz-Ramos, D. I., Jiménez-Fernández, M., García-Barradas, O., Ortiz-Basurto, R. I., & Fouconnier, B. (2025). Chemical Modification Methods for Inulin- and Agavin-Type Fructans: Synthesis, Characterization, and Biofunctional Activity: A Review. Molecules, 30(13), 2672. https://doi.org/10.3390/molecules30132672









