Abstract
The increasing number of antibiotic-resistant pathogens forces us to accelerate the search for new antimicrobial agents. Based on this, we chose to synthesize a library of 1-(2-hydroxy-5-methylphenyl)-5-oxopyrrolidine-3-carboxylic acid derivatives and evaluate their antibacterial activity against various pathogens. A series of (2-hydroxy-5-methylphenyl)-5-oxopyrrolidine-3-carboxylic acid and its hydrazide derivatives were prepared and identified by the methods of IR, 1H, and 13C NMR spectroscopy and a microanalysis technique. The resulting compounds were evaluated in vitro for their efficacy against the Gram-positive Staphylococcus aureus (ATCC 9144), Listeria monocytogenes (ATCC 7644), and Bacillus cereus (ATCC 11778) bacterial strains as well as the Gram-negative Escherichia coli (ATCC 8739) bacteria. Oxacillin, ampicillin, and cefuroxime were used as control antibiotics. Among the obtained compounds, hydrazone with a 5-nitrothien-2-yl fragment surpassed the control cefuroxime (7.8 μg/mL) against almost all strains tested. Hydrazone with a 5-nitrofuran-2-yl moiety showed a slightly lower but also potent effect on all bacterial strains. Moreover, hydrazone with a benzylidene moiety demonstrated very strong inhibition of S. aureus (3.9 μg/mL) in comparison with the antibacterial drug cefuroxime (7.8 μg/mL). In addition, some of these compounds exhibited remarkable bactericidal properties. In a complete biofilm disruption study, 5-nitrothienylhydrazone showed excellent results in disrupting S. aureus and E. coli biofilms. The test results show the potential of the newly obtained derivatives as a source of antibacterial agents. Therefore, further studies on the molecular optimization of these compounds are necessary for the development of new antibacterial drugs.
1. Introduction
Heterocyclic compounds are the largest and most diverse class of natural or synthetic organic compounds that play a key role in the development and discovery of modern drugs, as they provide a variety of scaffolds and derivatives through robust synthetic methods [1]. These compounds have a unique chemical behavior that allows them to be widely used in drug development. Their stability, diverse functionality, and drug-likeness characterize their significance.
Pharmaceutical medications, agrochemicals, and veterinary are the main areas of application of heterocycles [2]. Their ability to interact with specific biological targets determines their important therapeutic effects [3]. As a result, most approved drugs contain one or more heterocyclic rings. According to the published data, compounds with one or more heterocycles in their structure make up more than 85% of all biologically active compounds [2,4], and they are prevalent in more than 90% of newly approved drugs [5,6].
Heterocyclic rings are found to be abundant in macromolecules such as enzymes, vitamins, natural products, and biologically active chemicals [7,8]. Compounds of natural origin, such as alkaloids, morphine, vinblastine, and reserpine, and antibiotics, such as cephalosporin, penicillin, and others, include a heterocyclic scaffold [9]. Numerous studies have shown them to possess anticancer, antiallergic, anti-inflammatory, antimicrobial, antiviral, antioxidant, anti-HIV, antidiabetic, anticonvulsant, and other biological effects [2,10,11], and they are used in the creation of antibiotics, antidepressants, and anticancer, antiviral, and anti-inflammatory medical preparations [12].
Heterocycles have been recognized as important structural motifs in the development of new drugs [13], particularly in the field of antimicrobial agents [14]. The importance of heterocycles in drug development is demonstrated by their ability to modify the physicochemical properties, biological effects, pharmacokinetics, and toxicological profile of a drug candidate [15]. Much research in the past decade has focused on therapeutic agents whose structures are based on the presence of different heterocycles in their molecular structure [16,17,18,19]. Scientists have successfully synthesized a large number of heterocyclic compounds and developed a number of new antibacterial drugs that can treat infections caused by drug-resistant strains of bacteria [20]. Cefiderocol, cefditoren pivoxil, ceftobiprope, sulbactam/durlobactam, sulopenem, ensifentrine, ceftobiprole medocaril sodium, and others, are antibacterial pharmaceuticals that have been approved in recent decades [21,22]. This is very important because antibiotic resistance in the modern world has become a crucial global problem, posing major challenges to medicine and public health. Over the past few decades, the misuse and overuse of antibiotics has accelerated the development of pathogen resistance, making previously effective treatments virtually ineffective. All of this has led to the emergence of previously controlled diseases and an increase in untreated infections, leading to prolonged illness and increased mortality. Various forms of malignant bacteria have emerged, each with their own level of resistance to medical treatment. Escherichia coli, multidrug-resistant tuberculosis [23], methicillin-resistant Staphylococcus aureus [24], and others, can cause serious infections. Therefore, urgent and coordinated efforts are necessary to address this important problem. However, the growing understanding of bacterial pathogenesis and cellular communication has revealed many potential drug discovery strategies that can be used to treat bacterial infections [25].
In our previous studies, we revealed some five-membered heterocycle-based compounds with promising bioactivity against various cancer cell lines [26,27,28] and pathogenic strains [29,30]. Our research findings have led to further exploration of this area, and this time, we present our latest research in the search for promising antibacterial agents.
2. Results and Discussion
Derivatives obtained from aromatic amines are widely used in the production of various important and useful substances, such as drugs, polymers, dyes, surfactants, cosmetics, corrosion inhibitors, photosensitizers, and agricultural protection agents [31].
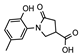
For this study, we chose 2-amino-4-methylphenol (1) as the starting compound, hoping to obtain a series of its derivatives with promising pharmacological properties. The target acid 2, in 88% yield, was synthesized by the modification of the amine 1 with itaconic acid using the aza-Michael method [32,33].
Several natural alkaloids containing a 2-pyrrolidone core have been reported to possess notable antibacterial and antimicrobial activities such as Acanthophoraine A and Salinosporamide A (Figure 1), and we were encouraged to design and synthesize novel analogues based on this scaffold with the aim of enhancing their biological efficacy [34,35].
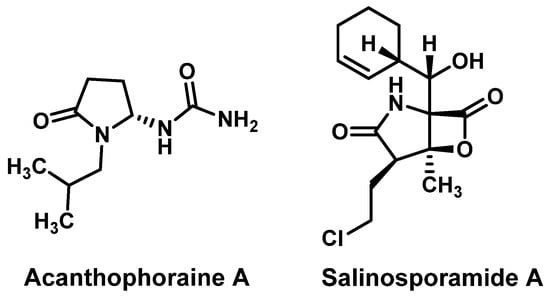
Figure 1.
Naturally occurring alkaloids featuring a 2-pyrrolidone core.
The formation of a 5-oxopyrrolidine-3-carboxylic acid fragment was confirmed by the corresponding signals of COCH2, CH, and NCH2 groups in the 1H NMR spectra as follows: 2.58–2.68, 3.26–3.42, and 3.76–3.93 ppm. A broad singlet at 12.65 ppm confirmed the COOH group present in the molecular structure. The carbon peaks of the corresponding groups in the 13C NMR spectrum were observed at 33.74, 36.22, and 50.98 ppm, and the COOH carbon resonance line was found at 174.41 ppm. (Supplementary Files, Figures S1 and S2).
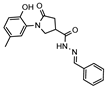
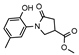
The intended acid hydrazide 4 was prepared by esterification of acid 2 followed by hydrazinolysis of the prepared ester 3 (Supplementary Files, Figures S3 and S4) with hydrazine monohydrate (Scheme 1) (Supplementary Files, Figures S5 and S6). In this work, the structural elucidation was mainly focused on the analysis of the 1H and 13C NMR spectra. The spectra of 3 and 4 were in excellent agreement with the desired structures.
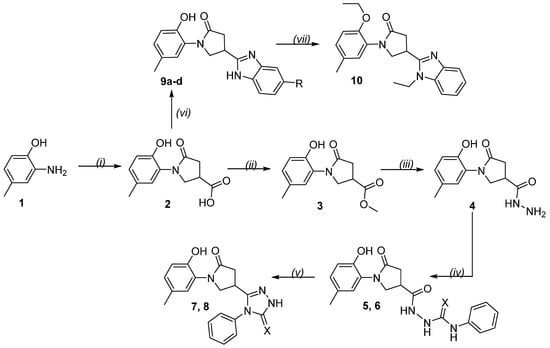
Scheme 1.
Synthesis of aza-heterocycles. Reagents and conditions: (i) itaconic acid, water, Δ, 2 h; (ii) MeOH, H2SO4, Δ, 8 h; (iii) N2H4·H2O, i-PrOH, Δ, 8 h; (iv) phenyl isothiocyanate (for 5) or phenyl isocyanate (for 6), MeOH, Δ, 4 (for 5) or 6 (for 6) h; (v) aqueous 4% NaOH, Δ, 3 (for 7) or 4 (for 8) h; (vi) unsubstituted (9a) or the corresponding substituted (9b–d) benzene-1,2-diamine, 4N HCl, Δ, 24 h; (vii) EtI, KOH, K2CO3, DMF, r.t. 6 h. 2–10. 5, 7 X = S; 6, 8 X = O; 9a R = H; 9b R = F; 9c R = Cl; 9d R = Me.
Further, according to Scheme 1, the preparation of the target thiosemicarbazide 5 and semicarbazide 6 was accomplished by interacting the acid hydrazide 4 with phenyl isothiocyanate or phenyl isocyanate, respectively. The reaction was performed in methanol at reflux for 4 or 6 h. The reactants were used in a 1:1 molar ratio, and the resulting compounds were isolated in 89% and 91% yields. The NMR spectra of thiosemicarbazide 5 showed three singlets at 9.58, 9.86, and 10.17 ppm for the three protons of 3 NH (1H) and an intense resonance line at 172.24 ppm for the two C=O carbon atoms, as well as a spectral line for C=S at 181 ppm. In the NMR spectra of semicarbazide 6, singlets at 8.11, 8.42, 8.79, 8.98, 9.62, and 9.91 ppm proved the presence of three NH groups (1H), and the peaks at 172.21, 172.61, and 176.79 ppm indicated three C=O groups (13C) (Supplementary Files, Figures S7–S10).
Next, the obtained phenylhydrazine-1-carbothioamide 5 and phenylhydrazine-1-carboxamide 6 were converted to the corresponding triazoles 7 and 8 by the classical technique for the cyclization of the appropriate semicarbazides. The alkaline cyclization of 5 and 6 in aqueous 4% sodium hydroxide for 3 or 4 h, respectively, led to the formation of triazolethione 7 or triazolone derivative 8 (Scheme 1). The desired compounds were isolated in good yields of 83% and 79%. The cyclic structure of both compounds was fully approved by the used structure identification techniques, and the 1H and 13C NMR data can be found in the Supplementary Files, Figures S11–S14. Analysis of the 1H NMR spectrum of triazolethione 7 showed a characteristic singlet at 13.41 ppm for the NH of the 1,2,4-triazole ring, and the 13C NMR spectrum also showed carbon peaks of the N=C and thiocarbonyl groups at 168.38 and 182.99 ppm, respectively.
If we analyze the NMR spectrum of compound 8, there is clear evidence for the formation of the 1,2,4-triazolone ring, i.e., the proton of the NH group resonates at the characteristic field of the 1H NMR spectrum, namely at 11.93 ppm, and the 13C NMR demonstrates resonance lines at 168.48 and 173.63 ppm for the carbons of the N=C and the carbonyl of the triazole ring.
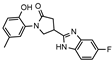
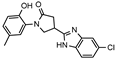
Starting from the common precursor, i.e., carboxylic acid 2, a series of benzimidazoles 9a–d were obtained by heating at reflux a mixture of initial acid 2 with the appropriate benzene-1,2-diamine in 4N hydrochloric acid for 24 h, as described in [36]. Depending on the applied benzene-1,2-diamine, unsubstituted benzimidazole 9a, 5-fluoro-9b, 5-chloro-9c, and 5-methyl-9d benzimidazoles were isolated from the reaction mixtures in the yield range of 54–67%. Based on the elemental analysis and spectral data, the structure of the compounds was fully confirmed. In the 1H NMR spectrum of compound 9a, the singlet at δ 12.57 ppm showed the presence of the NH proton. Five protons of the benzimidazole phenyl ring resonated in the range of 7.11–7.76 ppm. The remaining protons of the molecule resonated in excellent agreement with the proposed structure (Supplementary Files, Figure S15). As for the 13C NMR spectrum, the spectral peaks of the carbon atoms of the molecule resonated in the expected areas of the spectrum and confirmed the formation of the desired benzimidazole 9a (Supplementary Files, Figure S16).
Biological evaluation has demonstrated N-alkylated benzimidazoles to be promising compounds in drug design and discovery [37].
In this work, we performed the alkylation of compound 9a using a large (6-fold) excess of iodoethane to avoid the formation of a mixture of O- and N-alkylated compounds. The reaction was performed in dimethylformamide as a solvent and in the presence of potassium hydroxide and potassium carbonate in the reaction mixture. After stirring for 6 h at room temperature, the alkylated product 10 was isolated from the mixture. The 1H and 13C NMR spectra showed N- and O-alkylation. In the 1H NMR spectrum, triplets at 1.19 and 1.36 ppm and four protons in the range of 3.75–4.14 ppm confirmed the presence of two CH3CH2 fragments in the target compound. The absence of OH and NH proton singlets in the 1H NMR spectrum led to the conclusion that N- as well as O-alkylation occurred. The 13C NMR spectrum revealed resonance lines at 14.53, 14.57 (2CH3CH2), and 29.46, 63.79 (2CH3CH2) ppm, which were assigned to the carbons of the ethyl fragments (Supplementary Files, Figures S23 and S24).
A library of hydrazones 11a–d and 12a–j based on acid hydrazide 4 were also synthesized (Scheme 2). To achieve this, condensation of compound 4 with heterocyclic (for 11) and aromatic (for 12) aldehydes in propan-2-ol was caried out. All obtained structures 11 and 12 were confirmed by IR, 1H NMR, 13C NMR, and elemental analysis data. Analyzing the 1H NMR spectra of hydrazones 11a–d, the presence of two singlets in the ranges of 7.91–8.47 ppm for the CH=N group and 11.50–11.98 ppm for the NH of the hydrazone fragment was found. The 13C NMR spectra of hydrazones 11a–d showed additional resonance lines originating from the carbonyl, azomethine, and newly attached aryl ring carbons (Supplementary Files, Figures S25–S32). Compounds 12a–j were also fully confirmed using microanalysis and spectral results. The 1H and 13C NMR spectra can be found in the Supplementary Files, Figures S33–S50.
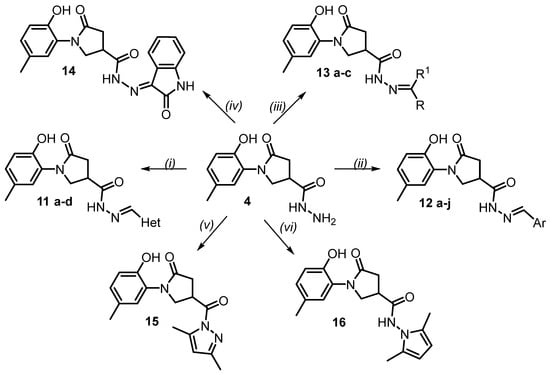
Scheme 2.
Synthesis of compounds 12–16. Reagents and conditions: (i) the corresponding carbaldehyde, i-PrOH, Δ, 2 h; (ii) the corresponding aromatic aldehyde, i-PrOH, Δ, 2 (for 12a–i) or 3 (for 12j) h; (iii) for 13a, b, acetone or ethyl methyl ketone, Δ, 4 h; for 13c, 4-aminoacetophenone, glacial AcOH, i-PrOH, Δ, 20 h; (iv) isatin, MeOH, AcOH, Δ, 3 h; (v) pentane-2,4-dione, conc. hydrochloric acid, i-PrOH, Δ, 2 h; (vi) hexane-2,5-dione, glacial AcOH, i-PrOH, Δ, 4 h. 11a Het = thien-2-yl; 11b Het = 5-nitro-2-thienyl; 11c Het = furan-2-yl; 11d Het = 5-nitrofuran-2-yl; 12a Ar = Ph; 12b Ar = 2,4-F2Ph; 12c Ar = 4-ClPh; 12d Ar = 4-BrPh; 12e Ar = 4-O2NPh; 12f Ar = 4-MePh; 12g Ar = 4-Me2NPh; 12h Ar = 2,3,4-(MeO)3Ph; 12i Ar = 3,4,5-(MeO)3Ph; 12j Ar = 1-naphthyl; 13a R = R1 = Me; 13b R = Me, R1 = Et; 13c R = Me, R1 = 4-H2N-Ph.
Hydrazones derived from acid hydrazides and aromatic aldehydes have mostly linear structures of stereoisomers arising from Z/E isomerism with respect to the double C=N bond and restricted rotation around the amide bond [38]. The hydrazones are usually obtained in an E configuration at the imine double bond and as a Z/E conformers mixture due to the amide bond [39]. The 1H NMR spectra in DMSO-d6 showed two sets of signals in a 65:35 intensity ratio (Z/E) for hydrazones 11a–d and in an intensity ratio of 60:40 (Z/E) for arylidene hydrazones 12a–j.
In the case of ketones (Scheme 2), their reactions with hydrazide 4 gave a set of expected hydrazones 13a–c. Reactions with acetone and ethyl methyl ketone afforded N′-propan-2-ylidene-13a or N′-butan-2-ylidene-13b carbohydrazides, respectively. Meanwhile, condensation with aminoacetophenone in propan-2-ol resulted in N′-(1-(4-aminophenyl)ethylidene)carbohydrazide derivative 13c. The NMR spectra of the obtained 13a–c fully confirmed their structures (Supplementary Files, Figures S51–S56). Compounds 13a and 13b were found to exist in DMSO-d6 solutions as a mixture of Z/E conformers with an intensity ratio of 57:43, whereas 12c had a Z/E rotamer ratio of 65:35.
In an extension of the present study, the reaction of acid hydrazide 4 with isatin was carried out. The reaction was realized in methanol using a molar ratio of reactants of 1 to 1.5, and a catalytic amount of glacial acetic acid was used to accelerate the reaction. Compound 14 was isolated in a good yield of 69%. The 1H NMR spectrum of isatin derivative 14 showed a proton signal of the amide group at 11.33 ppm, while the NH signal of the isatin core was visible at 10.81 ppm [40]. In the 13C NMR spectrum, the carbons of the isatin, amide, and 5-oxopyrrolidine carbonyls were observed as an intense peak at 172.11 ppm (Supplementary Files, Figures S57 and S58).
To synthesize small molecules suitable as antimicrobial agents, 3,5-dimethylpyrazole 15 and 2,5-dimethylpyrrole 16 derivatives were obtained. The condensation of acid hydrazide 4 with the appropriate diketone, as depicted in Scheme 2, was performed in propan-2-ol by catalytically accelerating the reaction with concentrated hydrochloric acid for 15 or glacial acetic acid for 16. In the 1H NMR spectrum of pyrazole 15, a proton singlet of a CHpyr was observed at 6.22 ppm, and an additional singlet at 2.19 ppm integrated for six protons indicated the presence of two new methyl groups in the molecule. In the 13C NMR spectrum, the carbons of the same groups were found as follows: 111.50, 13.54, and 14.07 ppm (Supplementary Files, Figures S59 and S60).
Examining the 1H NMR spectrum of compound 16, an intense singlet of the protons of two CH groups of the newly formed pyrrole ring was observed at 5.65 ppm, and peaks of two CH3 groups were found to resonate at 1.97 and 2.00 ppm. In the 13C NMR results, the pyrrole structure was affirmed by the presence of the spectral lines at 10.92, 10.96 (2 CH3), and 103.08 (2 CH), as well as at 126.74 and 126.76 (2 C–CH3) ppm.
The structures of the obtained compounds and their yields (%) are shown in Table 1.

Table 1.
Structures of the synthesized compounds and their yields.
The antibacterial activity of the synthesized compounds was evaluated as described in Section 3.2. The minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) values were determined against three Gram-positive bacterial strains—Staphylococcus aureus subsp. aureus (ATCC 9144), Listeria monocytogenes (ATCC 7644), and Bacillus cereus (ATCC 11778)—as well as the Gram-negative strain Escherichia coli (ATCC 8739). The results are graphically depicted in Figure 2, Figure 3, Figure 4 and Figure 5.
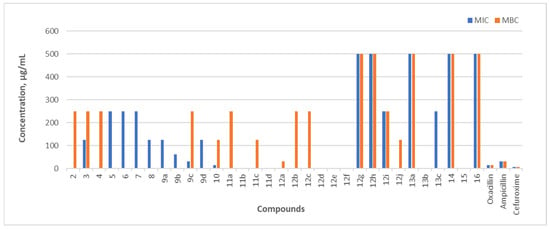
Figure 2.
In vitro MIC and MBC of the tested compounds (2–16) against Staphylococcus aureus (ATCC 9144), expressed in µg/mL. The antibacterial activity of the compounds was compared with standard antibiotics: oxacillin, ampicillin, and cefuroxime.
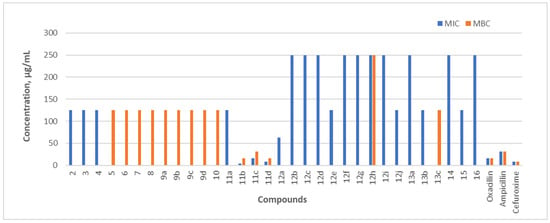
Figure 3.
In vitro MIC and MBC of the tested compounds (2–16) against expressed in L. monocytogenes (ATCC 7644) µg/mL. The antibacterial activity of the compounds was compared with standard antibiotics: oxacillin, ampicillin, and cefuroxime.
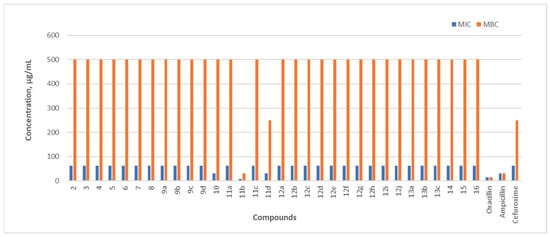
Figure 4.
In vitro MIC and MBC of the tested compounds (2–16) against expressed in B. cereus (ATCC 11778) µg/mL. The antibacterial activity of the compounds was compared with standard antibiotics: oxacillin, ampicillin, and cefuroxime.
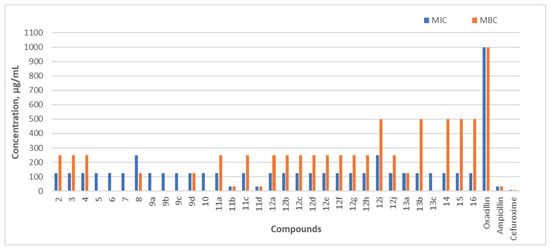
Figure 5.
In vitro MIC and MBC of the tested compounds (2–16) against expressed in E. coli (ATCC 8739) µg/mL. The antibacterial activity of the compounds was compared with standard antibiotics: oxacillin, ampicillin, and cefuroxime.
Staphylococcus aureus is a Gram-positive coccus and is a pathogen responsible for hospital-acquired (nosocomial) infections, often causing skin and upper respiratory tract infections. A key factor contributing to its pathogenicity is its ability to form biofilms, which protect it from host immune responses and antimicrobial treatments [41]. Three compounds from the hydrazone class, containing 5-nitro-2-thiophene, 5-nitro-2-furfural, or an additional benzene ring in their structure—11b, 11d, and 12a—exhibited bacteriostatic activity against S. aureus. The MIC value of these compounds against this pathogenic S. aureus was 3.90 µg/mL, which is 2 times lower than that of cefuroxime, 4 times lower than that of oxacillin, and 8 times lower than that of ampicillin. Compounds 11b and 11d also demonstrated strong bactericidal properties against S. aureus. The MBC of compound 11d was comparable to cefuroxime, which showed the best bactericidal effect among the control antibiotics, while the MBC value for compound 11b was half as low. The bactericidal and bacteriostatic activities of the other unmentioned compounds were much weaker. Hydrazone 13b, the pyrazole-containing compound 15, the compound containing an isatin fragment 14, hydrazinecarbo(thio)amides 5, 6, triazole derivatives 7, 8, and benzimidazoles 9a did not exhibit bactericidal activity against S. aureus.
L. monocytogenes is a Gram-positive, non-spore-forming, motile, rod-shaped facultative intracellular bacterium. It is one of the most virulent foodborne pathogens [42]. Among all the new compounds synthesized during the study, only those containing thiophene and furfural heterocyclic rings (compounds 11b–d) exhibited strong antibacterial properties. The MIC of compound 11b (7.8 µg/mL) was equivalent to that of the second-generation cephalosporin antibiotic cefuroxime; however, its MBC (15.23 µg/mL) was two-fold higher. The activity of this compound and others with antibacterial properties may be related to the inhibition of DNA, RNA, cell wall, or protein synthesis. Compounds containing a lactam ring are characterized by an inhibition of the synthesis of the peptidoglycan layer of the cell wall [43]. These compounds interfere with the normal functioning of enzymes such as transpeptidase, transglycosylase, and/or carboxypeptidase, which are responsible for the final stages of peptidoglycan synthesis, leading to bacterial cell death.
B. cereus is a Gram-positive, rod-shaped bacterium that forms endospores and produces biofilms [44]. It is commonly found in soil and food. The MIC of compound 11b (7.8 µg/mL) was two-fold lower (15.23 µg/mL) compared with oxacillin and four-fold lower compared with ampicillin—31.20 µg/mL. The MIC of compound 11d and the N-alkylated benzimidazole fragment-containing compound 10 was equal to the MIC of ampicillin (31.25 µg/mL) and 2 times lower than that of cefuroxime (62.4 µg/mL). The compound with the strongest bactericidal activity against B. cereus was the 5-nitrothiophen-2-yl-containing hydrazone 11b, with a minimum bactericidal concentration (MBC) of 31.25 µg/mL.
During the study, the antibacterial activity of the synthesized compounds was also tested against E. coli, a Gram-negative, non-spore-forming, and biofilm-forming intestinal rod [45]. The compounds with the highest antibacterial activity were hydrazones 11b and 11d, which contain 5-nitro-2-thiophene and 5-nitro-2-furfural fragments in their structure. The MIC and MBC values of the latter compounds were equal to the MIC and MBC values of ampicillin (31.25 µg/mL) and 4 times higher than those of cefuroxime (7.80 µg/mL). The MIC values of the other compounds were ≤125 µg/mL. The minimum bactericidal concentration of many compounds was 250 µg/mL, or they did not exhibit bactericidal activity against E. coli.
The results show that compounds 11b and 11d exhibited the widest spectrum of activity. They demonstrated antibacterial activity against all tested bacterial strains, with the strongest bacteriostatic effect observed against Gram-positive S. aureus (3.90 µg/mL) and L. monocytogenes (3.90 µg/mL for compound 11b and 7.80 µg/mL for compound 11d). The most potent antibacterial properties were found in the hydrazones containing 5-nitro-2-thiophene and 5-nitro-2-furfural structures in their composition.
Compounds 11b and 11d demonstrated the broadest spectrum of activity in the results. Consequently, these compounds were selected to assess their ability to disrupt biofilms produced by the Gram-positive coccus S. aureus and the Gram-negative rod E. coli.
Using the tube method, moderate biofilm formation was observed for both S. aureus and E. coli. After treating the bacterial biofilms with compounds 11b and 11d for 1 h, no biofilm was detected, except when compound 11d was used, as it did not disrupt the biofilm of S. aureus.
These findings highlight the importance of identifying compounds that can interfere with the early stages of biofilm formation and slow its progression. Additionally, it is important to note that the concentration of compounds required for biofilm removal is typically higher than that needed to target planktonic bacteria [46,47].
The ability of S. aureus and E. coli to form biofilms plays a critical role in the persistence and severity of the infections they cause. For example, the extracellular matrix of the biofilm prevents the diffusion of antibiotics, and the bacteria within the biofilm adopt a slower metabolic rate, which reduces the efficacy of antibiotics.
Compound 11b appears to be more effective in terms of biofilm disruption, which is consistent with findings from similar studies investigating biofilm inhibitors. For instance, a range of small molecules, including natural and synthetic compounds, have been found to disrupt biofilm formation in S. aureus and E. coli [48].
The failure of compound 11d to disrupt S. aureus biofilms may suggest that it is less effective against the specific mechanisms of biofilm formation in this Gram-positive bacterium. Research has shown that biofilm formation can vary significantly between bacterial species, and different bacteria utilize distinct strategies to form and maintain biofilms. In Gram-positive bacteria like S. aureus, factors such as surface protein adhesins, teichoic acids, and the accessory gene regulator (Agr) system are crucial for biofilm formation [49]. Compound 11d might not sufficiently target these specific mechanisms in S. aureus, whereas compound 11b could be more broadly effective due to its mechanism of action.
Our results also indicate that the concentrations of compounds required to disrupt biofilms are higher than those needed for planktonic bacteria. This is consistent with previous studies showing that biofilms are more resistant to antimicrobial agents than their free-living (planktonic) counterparts [50]. The dense extracellular matrix and altered metabolic state of bacteria within biofilms contribute to their enhanced resistance. In fact, treatment strategies often require significantly higher doses of antibiotics or biofilm-disrupting agents to overcome the protective barrier [51].
For example, a study by Chapman et al. (2024) [52] demonstrated that higher concentrations of vancomycin were required to treat S. aureus biofilm infections compared to planktonic S. aureus infections. Similarly, a review by Høiby et al. (2011) and Stewart et al. (2001) [50,53] emphasized the need for higher antibiotic concentrations or adjunct therapies, such as biofilm-disrupting enzymes, to effectively treat biofilm-associated infections.
3. Materials and Methods
3.1. Synthesis
Reagents and solvents were purchased from Sigma-Aldrich (St. Louis, MO, USA) and used without further purification. The reaction course and purity of the synthesized compounds were monitored by TLC using Merc Silica gel 60 F254 aluminum plates (Merck KGaA, Darmstadt, Germany).
NMR spectra were recorded on a Bruker BioSpin GmbH (1H 400 MHz, 13C 101 MHz), spectrometer (Bruker BioSpin AG, Fällanden, Switzerland). Chemical shifts were reported in δ ppm relative to tetramethylsilane (TMS), with the residual solvent as an internal reference (DMSO-d6, δ 2.50 ppm for 1H and δ 39.52 ppm for 13C). Data are reported as follows: chemical shift, multiplicity, coupling constant, Hz, integration, and assignment.
IR spectra (ν, cm−1) were recorded on a Perkin–Elmer Spectrum BX FT–IR spectrometer (Perkin–Elmer Inc., Waltham, MA, USA) using KBr pellets.
The C, H, and N elemental analysis was conducted on an Elemental Analyzer CE-440 (Exeter Analytical, Inc., Chelmsford, MA, USA). The results were found to be in good agreement (±0.3%) with the calculated values.
Melting points were determined in an opened capillary tube with an APA1 melting point analyzer and were uncorrected.
- 1-(2-Hydroxy-5-methylphenyl)-5-oxopyrrolidine-3-carboxylic acid (2)
A mixture of itaconic acid (12.36 g, 95 mmol) and 2-amino-4-methylphenol (1) (10 g, 81 mmol) was refluxed in water (40 mL) for 2 h and then cooled down; the formed crystalline precipitate was filtered off, washed with water, and purified by dissolving it in aqueous 5% sodium hydroxide solution (60 mL), filtering and acidifying the filtrate with hydrochloric acid to pH 2 to give the title compound 2 (greyish solid, yield 16.75 g, 88%, m. p. 172–173 °C).
1H NMR (400 MHz, DMSO-d6) δ 2.18 (s, 3H, CH3), 2.58–2.68 (m, 2H, COCH2), 3.26–3.42 (m, 1H, CH, overlaps with the peak of the H2O), 3.76–3.93 (m, 2H, NCH2), 6.79 (d, J = 8.7 Hz, 1H, HAr), 6.72 (s, 1H, HAr), 6.74 (s, 1H, HAr), 9.31 (br s, 1H, OH), 12.65 (br s, 1H, COOH) ppm.
13C NMR (101 MHz, DMSO-d6) δ 19.96 (CH3), 33.74, 36.22, 50.98 (COCH2, CH, NCH2), 116.65, 125.15, 127.88, 128.31, 128.71, 150.26 (CAr). 172.19, 174.41 (2 CO) ppm.
IR (KBr), ν 3302 (2 OH); 1699, 1641 (2 C=O) cm−1.
Calcd for C12H13NO4, %: C 61.27; H 5.57; N 5.95. Found, %: C 61.20; H 5.54; N 5.92.
- Methyl-1-(2-hydroxy-5-methylphenyl)-5-oxopyrrolidine-3-carboxylate (3)
To a solution of carboxylic acid 2 (8 g, 340 mol) in methanol (130 mL), a catalytic amount of concentrated sulfuric acid (2.5 mL) was added dropwise, and the mixture was heated at reflux for 8 h. The solvent was then evaporated under reduced pressure, and the residue was neutralized with 5% sodium carbonate solution to pH 8–9. After cooling, the obtained solid was filtered off, washed with plenty of water and hexane, and recrystallized from 2-propanol (25 mL) to give the title compound 3 (greenish solid, yield 8.26 g, 97%, m. p. 116–117 °C).
1H NMR (400 MHz, DMSO-d6) δ 2.19 (s, 3H, CH3), 2.59–2.72 (m, 2H, COCH2), 3.43–3.52 (m, 1H, CH2CH), 3.68 (s, 3H, OCH3), 3.79–3.92 (m, 2H, NCH2), 6.76–6.81 (m, 1H, HAr), 6.93 (s, 2H, HAr), 9.31 (s, 1H, OH) ppm.
13C NMR (101 MHz, DMSO-d6) δ 19.94 (CH3), 33.60 (COCH2), 36.00 (CH2CH), 50.73 (NCH2), 52.13 (OCH3), 116.61, 125.01, 127.86, 128.34, 128.75, 150.26 (CAr), 171.86, 173.30 (2 C=O) ppm.
IR (KBr), ν 3287 (OH); 1732, 1682 (2 C=O); 1213 (COOCH3) cm−1.
Calcd for C13H15NO4, %: C 62.64; H 6.07; N 5.62. Found, %: C 62.70; H 6.02; N 5.68.
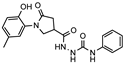
- 1-(2-Hydroxy-5-methylphenyl)-5-oxopyrrolidine-3-carbohydrazide (4)
To a cooled solution of methyl ester 3 (2.85 g, 12 mmol) in 2-propanol (10 mL), hydrazine monohydrate (1.2 g, 24 mmol) was added, and the mixture was heated at reflux for 8 h. After completion of the reaction (TLC), the mixture was cooled to room temperature, and the obtained precipitate was filtered off, washed with propan-2-ol, and recrystallized from 2-propanol to give the title compound 4 (brownish solid, yield 1.98 g, 67%, m. p. 190–191 °C).
1H NMR (400 MHz, DMSO-d6) δ 2.18 (s, 3H, CH3), 2.45–2.63 (m, 2H, COCH2), 3.14–3.26 (m, 1H, CH), 3.62–3.71 (m, 1H, NCH2), 3.74–3.84 (m, 1H, NCH2), 4.31 (br s, 2H, NH2), 6.72–6.82 (m, 1H, HAr), 6.86–7.00 (m, 2H, HAr), 8.59 (s, 1H, NH), 9.29 (s, 1H, OH) ppm.
13C NMR (101 MHz, DMSO-d6) δ 19.96 (CH3), 34.42, 35.56, 51.65 (COCH2, CH, NCH2), 116.62, 125.13, 127.89, 128.39, 128.78, 150.36 (CAr), 171.82, 172.43 (2 C=O) ppm.
IR (KBr), ν 3345, 3295, 3144 (OH, NH2, NH); 1687, 1646 (2 C=O) cm−1.
Calcd for C12H15N3O3, %: C 57.82; H 6.07; N 16.86. Found, %: C 55.98; H 5.93; N 16.63.
- General procedure for the preparation of compounds 5 and 6
To a solution of hydrazide 4 (0.55 g, 2.2 mmol) in methanol (10 mL), phenyl isothiocyanate (0.30 g, 2.2 mmol, 5) or phenyl isocyanate (0.26 g, 2.2 mmol, 6) was added, and the mixture was heated at reflux for 4 or 6 h, respectively. After completion of the reaction, mixture 5 was diluted with methanol (20 mL), and water was added drop-wise while stirring the mixture. The obtained oily mass was separated and dissolved in methanol and water was added dropwise. After cooling, the obtained crystalline solid was filtered off, washed with methanol, and recrystallized from 1,4-dioxane.
For mixture 6, part of the solvent was evaporated under reduced pressure, and the obtained solid was filtered off, washed with methanol, and recrystallized from 1,4-dioxane.
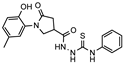
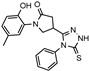
- 2-(1-(2-Hydroxy-5-methylphenyl)-5-oxopyrrolidine-3-carbonyl)-N-phenylhydrazine-1-carbothioamide (5)
Brownish solid, yield 0.22 g, 69%, mp 165–166 °C.
1H NMR (400 MHz, DMSO-d6) δ 2.19 (s, 3H, CH3), 2.54–2.79 (m, 2H, COCH2), 3.32–3.37 (m, 1H, CH), 3.70–4.02 (m, 2H, NCH2), 6.80 (d, J = 8.1 Hz, 1H, HAr), 6.68–6.73 (m, 2H, HAr), 7.17 (t, J = 6.9 Hz, 1H, HAr), 7.34 (t, J = 7.4 Hz, 2H, HAr), 7.38–7.51 (m, 2H, HAr), 9.31 (s, 1H, OH), 9.58, 9.86, 10.17 (3s, 3H, 3 NH) ppm.
13C NMR (101 MHz, DMSO-d6) δ 19.97 (CH3), 34.07, 35.44, 48.65, 51.35 (COCH2, CH, NCH2), 116.63, 125.14, 126.07, 126.26, 127.91, 128.20, 128.29, 128.35, 128.73, 139.08, 150.26 (CAr), 172.24 (2 C=O), 181.04 (C=S) ppm.
IR (KBr), ν 3470, 3281, 3224, 3031 (OH, NH); 1664, 1622 (2 C=O) cm−1. Calcd for C19H20N4O3S, %: C 59.36; H 5.24; N, 14.57. Found, %: C 59.30; H 5.28; N 14.52.
- 2-(1-(2-Hydroxy-5-methylphenyl)-5-oxopyrrolidine-3-carbonyl)-N-phenylhydrazine-1-carboxamide (6)
White, solid, yield 1.07 g, 91%, mp 98–99 °C.
1H NMR (400 MHz, DMSO-d6) δ 2.19 (s, 3H, CH3), 2.54–2.74 (m, 2H, COCH2), 3.31–3.36 (m, 1H, CH), 3.70–3.96 (m, 2H, NCH2), 6.80 (d, J = 8.3 Hz, 1H, HAr), 6.92–6.97 (m, 2H, HAr), 7.16–7.31 (m, 2H, HAr), 7.44 (d, J = 7.8 Hz, 2H, HAr); 8.11, 8.42, 8.79, 8.98, 9.62, 9.91 (3s, 3H, 3 NH), 9.24, 9.30 (s, 1H, OH) ppm.
13C NMR (101 MHz, DMSO-d6) δ 19.94 (CH3), 34.07, 35.18, 51.43, 51.63 (COCH2, CH, NCH2), 116.61, 118.18, 118.51, 121.98, 122.38, 125.12, 127.88, 128.34, 128.69, 128.76, 139.16, 139.58, 148.33, 150.27, 154.02, 155.27 (CAr), 172.21, 172.61, 172.79 (3 C=O) ppm.
IR (KBr), ν 3299, 3040, 3027, 2923 (OH, NH); 1686, 1642, 1602 (3 C=O) cm−1.
Calcd for C19H20N4O4, %: C 61.95; H 5.47; N 15.21. Found, %: C 61.90; H 5.48; N 15.18.
- General procedure for the preparation of compounds 7 and 8
A mixture of thiosemicarbazide 5 (0.45 g, 1.2 mmol) or semicarbazide 6 (0.3 g, 0.81 mmol) and aqueous 4% sodium hydroxide solution (11 mL) was refluxed for 3 or 4 h, respectively. The reaction mixture was cooled and neutralized with dropwise glacial acetic acid to pH 6 while cooling in an ice bath. The formed solid was filtered off (7) or the solvent was decanted and the remaining solid (8) washed with water and recrystallized from propan-2-ol to give the title compounds 7 or 8.
- 1-(2-Hydroxy-5-methylphenyl)-4-(4-phenyl-5-thioxo-4,5-dihydro-1H-1,2,4-triazol-3-yl)pyrrolidin-2-one (7)
Brownish solid, yield 0.36 g, 83%, mp 261–262 °C.
1H NMR (400 MHz, DMSO-d6) δ 2.16 (s, 3H, CH3), 2.40–2.47 (m, 1H, COCH2), 2.65–2.74 (m, 1H, COCH2), 3.51–3.98 (m, 3H, CH, NCH2), 6.75 (d, J = 8.1 Hz, 1H, HAr), 6.81–6.99 (m, 2H, HAr), 7.20–7.70 (m, 5H, HAr), 9.51 (br s, 1H, OH), 13.41 (br s, 1H, NH) ppm.
13C NMR (101 MHz, DMSO-d6) δ 19.90 (CH3), 29.03, 34.59, 51.39 (COCH2, CH, NCH2), 116.54, 124.73, 126.17, 127.68, 128.38, 128.56, 128.76, 129.55, 129.63, 133.77, 150.33, 152.72 (CAr), 168.38 (NC), 171.45 (C=O), 182.99 (C=S) ppm.
IR (KBr), ν 3385, 3139 (OH, NH); 1667 (C=O); 1519 (C=N) cm−1.
Calcd for C19H18N4O2S, %: C 62.28; H 4.95; N 15.29. Found, %: C 62.35; H 4.97; N 15.32.
- 5-(1-(2-Hydroxy-5-methylphenyl)-5-oxopyrrolidin-3-yl)-4-phenyl-2,4-dihydro-3H-1,2,4-triazol-3-one (8)
Brownish solid, yield 0.11 g, 38%, mp 217–218 °C.
1H NMR (400 MHz, DMSO-d6) δ 2.15 (s, 3H, CH3), 2.40–2.47 (m, 1H, COCH2), 2.60–2.76 (m, 1H, COCH2), 3.48–3.83 (m, 3H, NCH2, CH), 6.77 (d, J = 8.1 Hz, 1H, HAr), 6.81–6.94 (m, 2H, HAr), 7.38–7.63 (m, 5H, HAr), 11.93 (br s, 2H, OH, NH) ppm.
13C NMR (101 MHz, DMSO-d6) δ 19.90 (CH3), 29.02 (COCH2), 33.96 (CH2CH), 51.00 (NCH2), 116.58, 124.80, 127.30, 127.82, 128.34, 128.64, 128.96, 129.60, 132.77, 147.33, 150.63, 154.70, 168.48 (N=C, CAr), 171.57, 173.63 (2 C=O) ppm.
IR (KBr), ν 3157, 3025 (OH, NH); 1697, 1663 (2 C=O); 1519 (C=N) cm−1.
Calcd for C19H18N4O3, %: C 65.13; H 5.18; N 15.99. Found, %: C 65.20; H 5.15; N 15.94.
- General procedure for the preparation of benzimidazoles 9a–d
To a cooled solution of carboxylic acid 2 (0.7 g, 3 mmol) and 4N hydrochloric acid (14 mL), the corresponding benzene-1,2-diamine (6 mmol) was added, and the mixture was heated at reflux for 24 h. Afterwards, the mixture was cooled and neutralized with 5% sodium carbonate to pH 8 in an ice bath. The formed precipitate was filtered off and washed with 5% sodium carbonate. Purification was carried out by dissolving it in 5% sodium hydroxide (15 mL), filtering and acidifying the filtrate with glacial acetic acid to pH 6, and then the obtained crystalline solid was recrystallized from propan-2-ol.
For 9b and 9c, purification from 5% sodium carbonate was repeated twice, and then recrystallization from propan-2-ol was performed.
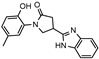
- 4-(1H-benzo[d]imidazol-2-yl)-1-(2-hydroxy-5-methylphenyl)pyrrolidin-2-one (9a)
Brownish solid, yield 0.35 g, 45%, mp 239–240 °C.
1H NMR (400 MHz, DMSO-d6) δ 2.20 (s, 3H, CH3), 2.64–2.77, 2.90–3.04 (2m, 2H, COCH2), 3.93–4.04 (m, 2H, NCH2), 4.06–4.15 (m, 1H, CH), 6.83 (m, J = 8.2 Hz, 1H, HAr), 6.90–7.03 (m, 2H, HAr), 7.11–7.26 (m, 2H, HAr), 7.35–7.76 (br s, 2H, HAr), 10.19 (s, 1H, OH), 12.57 (s, 1H, NH) ppm.
13C NMR (101 MHz, DMSO-d6) δ 19.96 (CH3); 32.12, 36.72, 53.31 (COCH2, CH, NCH2), 111.29, 116.80, 118.17, 121.57, 122.18, 124.89, 127.76, 128.58, 129.07, 134.19, 142.29, 150.93 (CAr), 172.29 (C=O) ppm.
IR (KBr), ν 3183, 3152, 3061 (OH, NH); 1663 (C=O); 1506 (C=N) cm−1.
Calcd for C18H17N3O2, %: C 70.34; H 5.58; N 13.67. Found, %: C 70.28; H 5.54; N 13.62.
- 4-(6-Fluoro-1H-benzo[d]imidazol-2-yl)-1-(2-hydroxy-5-methylphenyl)pyrrolidin-2-one (9b)
Brownish solid, yield 0.10 g, 11%, mp 246–247 °C.
1H NMR (400 MHz, DMSO-d6) δ 2.20 (s, 3H, CH3), 2.69–2.82, 2.88–3.03 (2m, 2H, COCH2), 3.92–4.03 (m, 2H, NCH2), 4.05–4.16 (m, 1H, CH), 6.82 (d, J = 8.0 Hz, 1H, HAr), 6.90–7.12 (m, 3H, HAr), 7.34 (d, J = 9.2 Hz, 1H, HAr), 7.46–7.61 (m, 1H, HAr), 10.10 (br s, 1H, OH), 12.48 (s, 1H, NH) ppm.
13C NMR (101 MHz, DMSO-d6) δ 19.93 (CH3); 32.04, 36.42, 53.17 (COCH2, CH, NCH2); 109.61, 109.86, 116.71, 120.67, 124.94, 127.75, 128.46, 128.92, 150.69, 157.18, 157.72, 157.26 (CAr), 172.20 (C=O) ppm.
IR (KBr), ν 3159, 3137 (OH, NH); 1660 (C=O); 1507 (C=N) cm−1.
Calcd for C18H16FN3O2, %: C 66.45; H 4.96; N 12.92. Found, %: C 66.52; H 4.94; N 12.89.
- 4-(6-Chloro-1H-benzo[d]imidazol-2-yl)-1-(2-hydroxy-5-methylphenyl)pyrrolidin-2-one (9c)
Brownish solid, yield 0.16 g, 15%, mp 239–240 °C.
1H NMR (400 MHz, DMSO-d6) δ 2.20 (s, 3H, CH3), 2.64–2.84, 2.88–3.05 (2m, 2H, COCH2), 3.90–4.04 (m, 2H, NCH2), 4.05–4.22 (m, 1H, CH), 6.81 (d, J = 8.0 Hz, 1H, HAr); 6.88–7.04 (m, 2H, HAr), 7.18 (d, J = 8.5 Hz, 1H, HAr), 7.53 (d, J = 8.5 Hz, 1H, HAr), 7.59 (s, 1H, HAr), 11.27 (br s, 2H, OH, NH) ppm.
13C NMR (101 MHz, DMSO-d6) δ 19.93 (CH3); 32.11, 36.46, 53.17 (COCH2, CH, NCH2); 114.60, 115.84, 116.76, 121.83, 124.96, 125.98, 127.54, 128.47, 128.91, 137.11, 139.71, 150.95 (CAr), 172.18 (C=O) ppm.
IR (KBr), ν 3227, 3115 (OH, NH); 1670 (C=O); 1521 (C=N) cm−1.
Calcd for C18H16ClN3O2, %: C 63.25; H 4.72; N 12.29. Found, %: C 63.31; H 4.72; N 12.26.
- 1-(2-Hydroxy-5-methylphenyl)-4-(6-methyl-1H-benzo[d]imidazol-2-yl)pyrrolidin-2-one (9d)
Brown solid, yield 1.38 g, 67%, mp 247–248 °C.
1H NMR (400 MHz, DMSO-d6) δ 2.20 (s, 3H, CH3), 2.47 (s, 3H, CH3), 3.00 (d, J = 8.8 Hz, 2H, COCH2); 4.09 (t, J = 8.3 Hz, 1H, NCH2), 4.17 (t, J = 8.9 Hz, 1H, NCH2), 4.24–4.40 (m, 1H, CH), 6.85 (d, J = 8.2 Hz, 1H, HAr), 6.96 (d, J = 8.2 Hz, 1H, HAr), 7.03 (s, 1H, HAr), 7.28 (d, J = 8.3 Hz, 1H, HAr); 7.53 (s, 1H, HAr), 7.62 (d, J = 8.3 Hz, 1H, HAr), 12.43 (br s, 2H, OH, NH) ppm.
13C NMR (101 MHz, DMSO-d6) δ 19.96 (CH3), 21.15 (CH3), 30.65, 35.62, 52.38 (COCH2, CH, NCH2), 113.46, 113.70, 116.65, 124.65, 126.22, 127.73, 128.53, 128.99, 130.77, 132.79, 134.58, 150.61, 153.84 (CAr), 171.27 (C=O) ppm.
IR (KBr), ν 3051, 2987 (OH, NH); 1654 (C=O); 1519 (C=N) cm−1.
Calcd for C19H19N3O2, %: C 71.01; H 5.96; N 13.08. Found, %: C 71.11; H 5.95; N 13.05.
- 1-(2-Ethoxy-5-methylphenyl)-4-(1-ethyl-1H-benzo[d]imidazol-2-yl)pyrrolidin-2-one (10)
To a solution of benzimidazole 9a (0.15 g, 0.5 mmol) in DMF (3 mL), ground potassium hydroxide (0.06 g, 1.05 mmol) and potassium carbonate (0.05 g, 0.35 mmol) were added, and the mixture was stirred at room temperature for 15 min. Then, ethyl iodide (0.47 g, 3 mmol) was added dropwise, and the reaction was performed at room temperature for 6 h. After completion of the reaction (TLC), inorganic precipitate was filtered off, ethyl iodide was evaporated under reduced pressure, and the residue was diluted with water (10 mL) while keeping the flask in an ice bath. The formed crystalline solid was filtered off and recrystallized from the mixture of propan-2-ol and water (2:1) to give the title compound 10 (brown solid, yield 61 mg, 34%, mp 210–211 °C).
1H NMR (400 MHz, DMSO-d6) δ 1.19 (t, J = 7.1 Hz, 3H, CH2CH3), 1.36 (t, J = 6.9 Hz, 3H, CH2CH3), 2.24 (s, 3H, CH3), 2.87–3.04 (m, 2H, COCH2), 3.75–4.44 (m, 7H, NCH2, 2 CH2CH3, CH2CH), 6.81–7.12 (m, 3H, HAr), 7.21–7.39 (m, 2H, HAr), 7.57–7.74 (m, 2H, HAr) ppm.
13C NMR (101 MHz, DMSO-d6) δ 14.53, 14.57 (2 CH2CH3), 19.93 (CH3), 29.46, 35.77, 36.60, 53.01, 63.79 (CH, COCH2, 2CH2CH3, NCH2), 110.76, 113.42, 116.71, 122.61, 122.85, 123.55, 124.67, 126.69, 128.70, 129.01, 129.28, 151.58, 154.31 (CAr); 171.79 (C=O) ppm.
IR (KBr), ν 1692 (C=O); 1510 (C=N) cm−1.
Calcd for C22H25N3O2, %: C 72.70; H 6.93; N 11.56. Found, %: C 72.78; H 6.94; N 11.51.
- General procedure for the synthesis of hydrazones 11a–d
To a cooled solution of hydrazide 4 (0.3 g, 1.2 mmol) in propan-2-ol (13 mL), the corresponding carbaldehyde was added, and the reaction mixture was heated at reflux for 2 h. Then, the mixture was cooled, and the formed solids 11a, b, and d were filtered off, washed with 2-propanol, and recrystallized from 1,4-dioxane to give the title compounds 11a, b, and d. For 11c, the cooled reaction mixture was diluted with hexane (5 mL) to isolate the target compound, and then the separation procedure of 11a, b, and d was repeated.
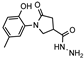
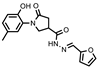
- 1-(2-Hydroxy-5-methylphenyl)-5-oxo-N′-(thien-2-ylmethylene)pyrrolidine-3-carbohydrazide (11a)
White solid, yield 0.30 g, 73%, mp 203–204 °C.
1H NMR (400 MHz, DMSO-d6) δ (Z/E isomers mixture, 65/35) 2.19 (s, 3H, CH3), 2.58–2.79 (m, 2H, COCH2), 3.33–3.38, 3.96–3.99 (2m, 1H, CH), 3.70–3.96 (s, 2H, NCH2), 6.70–6.84 (m, 1H, HAr), 6.86–7.02 (m, 2H, HAr), 7.04–7.17 (m, 1H, HAr), 7.36–7.50 (m, 1H, HAr), 7.53–7.70 (m, 1H, HAr), 8.19, 8.42 (2s, 1H, N=CH), 9.30, 9.31 (2s, 1H, OH), 11.53, 11.55 (2s, 1H, NH) ppm.
13C NMR (101 MHz, DMSO-d6) δ 19.92 (CH3); 33.16, 34.15, 34.17, 36.17, 51.05, 51.44 (COCH2, CH, NCH2), 116.62, 125.18, 127.83, 127.97, 128.37, 128.37, 128.43, 128.64, 129.00, 130.37, 138.55, 138.88, 138.93, 142.18, 150.24, 168.61 (CAr, N=CH), 172.13, 172.29, 173.27 (2 C=O) ppm.
IR (KBr), ν 3274, 3110 (OH, NH); 1692, 1653 (2x C=O); 1509 (C=N) cm−1.
Calcd for C17H17N3O3S, %: C 59.46; H 4.99; N 12.24. Found, %: C 59.53; H 5.03; N 12.20.
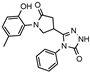
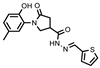
- 1-(2-Hydroxy-5-methylphenyl)-N′-((5-nitrothien-2-yl)methylene)-5-oxopyrrolidine-3-carbohydrazide (11b)
Yellowish solid, yield 0.34 g, 73%, mp 210–211 °C.
1H NMR (400 MHz, DMSO-d6) δ (Z/E isomers mixture, 65/35) 2.19 (s, 3H, CH3); 2.60–2.80 (m, 2H, COCH2), 3.35–3.44, 3.75–3.81 (2m, 1H, CH), 3.87–4.09 (m, 2H, NCH2), 6.74–6.83 (m, 1H, HAr). 6.89–6.99 (m, 2H, HAr). 7.53, 7.56 (2d, J = 4.2 Hz, 1H, HAr). 8.06–8.14 (m, 1H, HAr). 8.19, 8.47 (2s, 1H, N=CH), 9.30, 9.33 (2s, 1H, OH), 11.92, 11.96 (2s, 1H, NH) ppm.
13C NMR (101 MHz, DMSO-d6) δ 19.92 (CH3); 33.18, 34.04, 36.23, 50.94, 51.26 (COCH2, CH, NCH2), 116.63, 125.11, 127.85, 128.36, 128.68, 129.20, 129.77, 130.65, 136.82, 140.55, 146.57, 150.26, 150.52, 150.87, 169.27 (N=CH, CAr), 172.01, 172.17, 173.93 (2 C=O) ppm.
IR (KBr), ν 3324, 3191 (OH, NH); 1703, 1675 (2 C=O); 1509 (C=N) cm−1.
Calcd for C17H16N4O5S, %: C 52.57; H 4.15; N 14.43. Found, %: C 52.50, H 4.17; N 14.39.
- N′-(furan-2-ylmethylene)-1-(2-hydroxy-5-methylphenyl)-5-oxopyrrolidine-3-carbohydrazide (11c)
Brownish solid, yield 0.22 g, 57%, mp 153–154 °C.
1H NMR (400 MHz, DMSO-d6) δ (Z/E isomers mixture, 65/35) 2.19 (s, 3H, CH3), 2.58–2.79 (m, 2H, COCH2), 3.30–3.35, 3.98–4.07 (2m, 2H, CH), 3.72–4.06 (m, 2H, NCH2), 6.53–6.99 (m, 5H, HAr), 7.80, 7.83 (2s, 1H, HAr), 7.91, 8.10 (2s, 1H, N=CH), 9.28, 9.32 (2s, 1H, OH), 11.50, 11.54 (2s, 1H, NH) ppm.
13C NMR (101 MHz, DMSO-d6) δ 19.93 (CH3), 34.04, 34.15, 36.21, 51.12, 51.45 (COCH2, CH, NCH2), 112.13, 112.19, 113.42, 113.71, 116.59, 116.64, 125.09, 125.20, 127.86, 128.28, 128.37, 128.65, 128.70, 133.70, 136.88, 144.98, 145.98, 145.25, 149.14, 149.24, 150.23, 150.23, 150.27, 168.72 (N=CH, CAr), 172.17, 172.34, 173.57 (2 C=O) ppm.
IR (KBr), ν 3263, 3153 (OH, NH); 1702, 1655 (2 C=O); 1509 (C=N) cm−1.
Calcd for C17H17N3O4, %: C 62.38; H 5.23; N 12.84. Found, %: C 62.43; H 5.21; N 12.88.
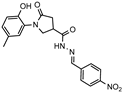
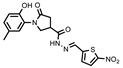
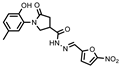
- 1-(2-Hydroxy-5-methylphenyl)-N′-((5-nitrofuran-2-yl)methylene)-5-oxopyrrolidine-3-carbohydrazide (11d)
Yellow solid, yield 0.28 g, 63%, mp 116–117 °C.
1H NMR (400 MHz, DMSO-d6) δ (Z/E isomers mixture, 65/35) 2.19 (s, 3H, CH3), 2.58–2.80 (m, 2H, COCH2), 3.38–3.45, 4.00–4.12 (2m, 1H, CH), 3.75–3.97 (m, 2H, NCH2), 6.2–6.84 (m, 1H, HAr), 6.87–7.03 (m, 2H, HAr), 7.19–7.32 (m, 1H, HAr); 7.78 (d, J = 2.5 Hz, 1H, HAr), 7.97, 8.18 (2s, 1H, N=CH), 9.29, 9.33 (2s, 1H, OH), 11.94, 11.98 (2s, 1H, NH) ppm.
13C NMR (101 MHz, DMSO-d6) δ 19.92 (CH3), 33.25, 33.94, 33.98, 36.26, 50.96, 51.27 (COCH, CH, NCH2), 114.60, 114.73, 115.45, 116.57, 116.62, 125.03, 125.12, 127.86, 128.34, 128.34, 128.69, 131.72, 134.90, 150.27, 151.57, 151.76, 169.33 (N=CH, CAr), 172.00, 172.17, 174.16 (2 C=O) ppm.
IR (KBr), ν 3351, 3204 (OH, NH); 1673 (2 C=O); 1510 (C=N) cm−1.
Calcd for C17H16N4O6, %: C 54.84; H 4.33; N 15.05. Found, %: C 54.89; H 4.31; N 15.01.
- General procedure for the preparation of hydrazones 12a–j
To a cooled solution of hydrazide 4 (0.3 g, 1.2 mmol) in propan-2-ol (15 mL), the corresponding aromatic aldehyde (1.4 mmol) was added, and the mixture was heated at reflux for 2 (a–i) or 3 (j) h. Then, the mixture (a–i) was cooled, and the formed solid was filtered off, washed with 2-propanol, and recrystallized from the mixture of methanol and water (2:1) to give the title compounds 12a–i. For 12j, the cooled reaction mixture was diluted with hexane (20 mL), and then the obtained solid was recrystallized from the mixture of methanol and water (2:1) to give the title compound 12j.
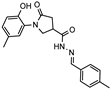
- N′-benzylidene-1-(2-hydroxy-5-methylphenyl)-5-oxpyrrolidine-3-carbohydrazide (12a)
White solid, yield 0.25 g, 69%, mp 223–224 °C.
1H NMR (400 MHz, DMSO-d6) δ (mixture of the Z/E isomers, 60/40) 2.19 (s, 3H, CH3), 2.60–2.83 (m, 2H, COCH2), 3.36–3.43, 4.06–4.16 (m, 1H, CH), 3.73–4.05 (m, 2H, NCH2), 6.80 (dd, J = 7.6, 3.8 Hz, 1H, HAr), 6.87–7.03 (m, 2H, HAr), 7.33–7.55 (m, 3H, HAr), 7.57–7.80 (m, 2H, HAr), 8.03, 8.21 (2s, 1H, N=CH), 9.31, 9.33 (2s, 1H, OH), 11.56, 11.61 (2s, 1H, NH) ppm.
13C NMR (101 MHz, DMSO-d6) δ 19.94 (CH3), 34.15, 34.19, 36.20, 51.11, 51.46 (COCH2, CH, NCH2), 116.59, 116.65, 125.11, 125.,21, 126.82, 127.10, 127.86, 128.29, 128.37, 128.70, 128.83, 128.87, 129.90, 130.13, 134.14, 143.57, 147.02, 150.21, 150.28, 168.79, 172.19, 172.39, 173.69 (N=CH, CAr, 2 C=O) ppm.
IR (KBr), ν 3374, 3227 (OH, NH); 1694, 1655 (2 C=O); 1519 (C=N) cm−1.
Calcd for C19H19N3O3, %: C 67.64; H 5.68; N 12.46. Found, %: C 67.69; H 5.66; N 12.42.
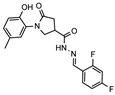
- N′-(2,4-difluorobenzylidene)-1-(2-hydroxy-5-methylphenyl)-5-oxopyrrolidine-3-carbohydrazide (12b)
White solid, yield 0.26 g, 66%, mp 258–259 °C.
1H NMR (400 MHz, DMSO-d6) δ (mixture of the Z/E isomers, 60/40) 2.19 (s, 3H, CH3), 2.60–2.80 (m, 2H, COCH2), 3.34–3.41, 4.05–4.15 (m, 1H, CH), 3.77–4.01 (m, 2H, NCH2), 6.79 (dd, J = 7.9, 3.9 Hz, 1H, HAr), 6.84–7.02 (m, 2H, HAr), 7.06–7.23 (m, 1H, HAr), 7.25–7.41 (m, 1H, HAr), 7.83–8.01 (m, 1H, HAr), 8.17, 8.38 (2s, 1H, N=CH), 9.30, 9.32 (2s, 1H, OH), 11.65, 11.74 (2s, 1H, NH) ppm.
13C NMR (101 MHz, DMSO-d6) δ 19.93 (CH3), 33.27, 34.07, 36.23, 51.00, 51.36 (COCH2, CH, NCH2), 104.26, 104.51, 104.77, 112.51, 112,54, 112.72, 112.76, 116.58, 116.64, 118.51, 118.54, 118.61, 118.64, 125.08, 125.19, 127.84, 127.89, 127.93, 127.98, 128.28, 128.36, 128.62, 128.69, 135.77, 139.04, 150.19, 150.26, 160.85 (1J = 253 Hz, C–F), 163.04 (1J = 252.5 Hz, C–F), 168.83, 172.11, 172.31, 173.77 (N=CH, CAr, 2 C=O) ppm.
IR (KBr), ν 3186, 3072 (OH, NH); 1671, 1613 (2 C=O); 1520 (C=N) cm−1. Calcd for C19H17F2N3O3, %: C 61.12; H 4.59; N 11.25. Found, %: C 61.14; H 4.57; N 11.28.
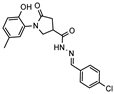
- N′-(4-chlorobenzylidene)-1-(2-hydroxy-5-methylphenyl)-5-oxpyrrolidine-3-carbohydrazide (12c)
White solid, yield 0.39 g, 87%, mp 246–247 °C.
1H NMR (400 MHz, DMSO-d6) δ (mixture of the Z/E isomers, 60/40) 2.19 (s, 3H, CH3); 2.59–2.81 (m, 2H, COCH2); 3.36–3.46, 4.07–4.16 (m, 1H, CH); 3.75–4.03 (m, 2H, NCH2); 6.80 (dd, J = 8.4, 3.4 Hz, 1H, HAr); 6.85–7.05 (m, 2H, HAr), 7.40–7.57 (m, 2H, HAr); 7.64–7.82 (m, 2H, HAr); 8.01, 8.20 (2s, 1H, NCH); 9.30, 9.32 (2s, 1H, OH); 11.61, 11.67 (2s, 1H, NH) ppm.
13C NMR (101 MHz, DMSO-d6) δ 19.93 (CH3), 33.32, 34.11, 36.19, 51.05, 51.42 (COCH2, CH, NCH2), 116.59, 116.64, 125.09, 125.19, 127.85, 128.27, 128.36, 128.47, 128.61, 128.72, 128.93, 133.10, 134.30, 134.56, 142.28, 145.69, 150.18, 150.27, 168.86, 172.15, 172.34, 173.75 (N=CH, CAr, 2 C=O) ppm.
IR (KBr), ν 3199, 3068 (OH, NH); 1671 (2x C=O); 1520 (C=N) cm−1.
Calcd for C19H18ClN3O3, %: C 61.38; H 4.88; N 11.30. Found, %: C 61.42; H 4.87; N 11.26.
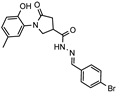
- N′-(4-brombenzylidene)-1-(2-hydroxy-5-methylphenyl)-5-oxpyrrolidine-3-carbohydrazide (12d)
White solid, yield 0.45 g, 92%, mp 244–245 °C.
1H NMR (400 MHz, DMSO-d6) δ (mixture of the Z/E isomers, 60/40) 2.19 (s, 3H, CH3); 2.59–2.81 (m, 2H, COCH2); 3.38–3.43, 4.06–4.15 (m, 1H, CH); 3.76–4.03 (m, 2H, NCH2); 6.79 (dd, J = 7.8, 2.7 Hz, 1H, HAr); 6.85–7.02 (m, 2H, HAr); 7.57–7.68 (m, 4H, HAr); 8.00, 8.18 (2s, 1H, N=CH); 9.31, 9.33 (2s, 1H, OH); 11.61, 11.68 (2s, 1H, NH) ppm.
13C NMR (101 MHz, DMSO-d6) δ 19.95 (CH3), 33.32, 34.13, 36.19, 51.06, 51.43 (COCH2, CH, NCH2), 116.59, 116.65, 123.09, 123.23, 125.10, 125.19, 127.87, 128.29, 128.72, 128.97, 130.24, 131.86, 132.05, 133.45, 142.41, 145.80, 150.19, 150.28, 160.78, 168.89, 172.17, 172.36, 173.77 (N=CH, CAr, 2 C=O) ppm.
IR (KBr), ν 3198, 3078 (OH, NH); 1671 (2x C=O); 1519 (C=N) cm−1.
Calcd for C19H18BrN3O3, %: C 54.82; H 4.36; N 10.09. Found, %: C 54.90; H 4.36; N 10.13.
- 1-(2-Hydroxy-5-methylphenyl)-N′-(4-nitrobenzylidene)-5-oxopyrrolidine-3-carbohydrazide (12e)
White solid, yield 0.46 g, 98%, mp 261–262 °C.
1H NMR (400 MHz, DMSO-d6) δ (mixture of the Z/E isomers, 60/40) 2.19 (s, 3H, CH3), 2.60–2.88 (m, 2H, COCH2), 3.39–3.46, 4.11–4.19 (m, 1H, CH), 3.77–4.05 (m, 2H, NCH2), 6.70–6.86 (m, 1H, HAr), 6.87–7.09 (m, 2H, HAr), 7.86–8.04 (m, 2H, HAr), 8.09–8.34 (m, 3H, N=CH, HAr), 9.35 (s, 1H, OH), 11.84, 11.91 (2s, 1H, NH) ppm.
13C NMR (101 MHz, DMSO-d6) δ 20.39 (CH3), 33.73, 34.54, 34.61, 36.67, 51.40, 51.81 (COCH2, CH, NCH2), 117.07, 124.53, 125.51, 125.59, 128.24, 128.31, 128.49, 128.77, 128.84, 129.10, 129.18, 140.90, 140.95, 141.71, 145.02, 148.17, 148.35, 150.63, 150.72, 169.69, 172.56, 172.74, 174.54 (N=CH, CAr, 2 C=O) ppm.
IR (KBr), ν 3207, 3083 (OH, NH); 1671 (2 C=O); 1520 (C=N); 1342, 1317 (NO2) cm−1.
Calcd for C19H18N4O5, %: C 59.68; H 4.75; N 14.65. Found, %: C 59.59; H 4.73; N 14.71.
- 1-(2-Hydroxy-5-methylphenyl)-N′-(4-methylbenzylidene)-5-oxopyrrolidine-3-carbohydrazide (12f)
Greyish solid, yield 0.37 g, 87%, mp 263–264 °C.
1H NMR (400 MHz, DMSO-d6) δ (mixture of the Z/E isomers, 60/40) 2.19 (s, 3H, NCCHCCH3), 2.32 (s, 3H, CH3), 2.58–2.82 (m, 2H, COCH2), 3.35–3.42, 4.06–4.16 (m, 1H, CH), 3.72–4.04 (m, 2H, NCH2), 6.79 (dd, J = 8.0, 3.6 Hz, 1H, HAr), 6.84–7.10 (m, 2H, HAr), 7.11–7.35 (m, 2H, HAr), 7.39–7.77 (m, 2H, HAr), 7.99, 8.17 (2s, 1H, N=CH), 9.30, 9.32 (2s, 1H, OH), 11.49, 11.54 (2s, 1H, NH) ppm. 13C NMR (101 MHz, DMSO-d6) δ 19.93 (CH3); 21.02 (CH3); 33.33, 34.12, 34.20, 36.19, 51.11, 51.47 (COCH2, CH, NCH2), 116.64, 125.11, 125.21, 126.79, 127.08, 127.84, 128.27, 128.36, 128.61, 128.69, 129.46, 131.44, 139.67, 139.95, 147.04, 150.20, 150.27, 168.65 172.19, 172.39, 173.57 (N=CH, CAr, 2 C=O) ppm.
IR (KBr), ν 3210, 3083 (OH, NH); 1671 (2x C=O); 1520 (C=N) cm−1.
Calcd for C20H21N3O3, %: C 68.36; H 6.02; N 11.96. Found, %: C 68.42; H 6.05; N 11.91.
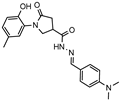
- N′-(4-(dimethylamino)benzylidene)-1-(2-hydroxy-5-methylphenyl)-5-oxopyrrolidine-3-carbohydrazide (12g)
Brownish solid, yield 0.38 g, 83%, mp 262–263 °C.
1H NMR (400 MHz, DMSO-d6) δ (mixture of the Z/E isomers, 60/40) 2.19 (s, 3H, CH3), 2.58–2.83 (m, 2H, COCH2), 2.95, 2.96 (2s, 6H, N(CH3)2), 3.29–3.34, 4.02–4.16 (2m, 1H, CH), 3.72–4.01 (m, 2H, NCH2), 6.73 (t, J = 7.5 Hz, 2H, HAr), 6.80 (dd, J = 8.2, 2.6 Hz, 1H, HAr), 6.82–7.05 (m, 2H, HAr), 7.34–7.67 (m, 2H, HAr), 7.89, 8.06 (2s, 1H, N=CH), 9.30, 9.32 (2s, 1H, OH), 11.27, 11.31 (2s, 1H, NH) ppm.
13C NMR (101 MHz, DMSO-d6) δ 19.93 (CH3), 33.31, 34.18 36.18 (COCH2, CH), 39.76 (N(CH3)2), 51.12, 51.58 (NCH2), 111.77, 111.84, 115.58, 116.65, 121.35, 121.51, 125.12, 125.24, 127.85, 128.06, 128.26, 128.36, 128.36, 128.43, 128.60, 128.69, 144.36, 147.84, 150.20, 150.28, 151.36, 151.54 168.17, 172.27, 172.49, 173.06 (NCH, CAr, 2 C=O) ppm.
IR (KBr), ν 3207, 3099 (OH, NH); 1672, 1660 (2 C=O); 1520 (C=N) cm−1.
Calcd for C21H24N4O3, %: C 66.30; H 6.36; N 14.73. Found, %: C 66.22; H 6.36; N 14.76.
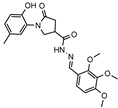
- 1-(2-Hydroxy-5-methylphenyl)-5-oxo-N′-(2,3,4-trimethoxybenzylidene)pyrrolidine-3-carbohydrazide (12h)
White solid, yield 0.45 g, 88%, mp 226–227 °C.
1H NMR (400 MHz, DMSO-d6) δ (mixture of the Z/E isomers, 60/40) 2.19 (s, 3H, CH3), 2.58–2.81 (m, 2H, COCH2), 3.31–3.38, 4.03–4.14 (m, 1H, CH), 3.68–3.84 (m, 9H, 3OCH3), 3.84–4.02 (m, 2H, NCH2), 6.80 (dd, J = 8.0, 3.6 Hz, 1H, HAr), 6.84–7.03 (m, 4H, HAr), 7.56 (dd, J = 8.4, 6.7 Hz, 1H, HAr), 8.21, 8.37 (2s, 1H, N=CH), 9.30, 9.32 (2s, 1H, OH), 11.42, 11.55 (2s, 1H, NH) ppm.
13C NMR (101 MHz, DMSO-d6) δ 19.93 (CH3), 33.29, 34.19, 36.21, 51.17, 51.49 (COCH2, CH, NCH2), 56.00, 60.48, 61.73 (3 OCH3), 108.74, 108.77, 116.60, 116.65, 120.10, 120.31, 120.37, 120.53, 125.12, 125.22, 127.86, 128.28, 128.34, 128.62, 128.69, 139.45, 141.53, 141.60, 142.50, 150.20, 150.27, 152.47, 152.57, 154.98, 155.21, 168.43, 172.21, 172.42, 173.34 (CAr, NCH, 2 C=O) ppm.
IR (KBr), ν 3325, 3059 (OH, NH); 1700 (2 C=O); 1519 (C=N) cm−1.
Calcd for C22H25N3O6, %: C 61.82; H 5.90; N 9.83. Found, %: C 61.76; H 5.91; N 9.85.
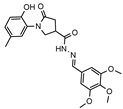
- 1-(2-Hydroxy-5-methylphenyl)-5-oxo-N′-(3,4,5-trimethoxybenzylidene)pyrrolidine-3-carbohydrazide (12i)
White solid, yield 0.39 g, 75%, mp 185–186 °C.
1H NMR (400 MHz, DMSO-d6) δ (mixture of the Z/E isomers, 60/40) 2.18, 2.20 (2s, 3H, CH3), 2.62–2.85 (m, 2H, COCH2); 3.35–3.43 (m, 0.4H, CH), 3.68. 3.70 (2s, 3H, OCH3), 3.72–4.13 (m, 2H, NCH2 + 2s, 6H, OCH3 + 0.6H, CH), 6.80 (t, J = 6.7 Hz, 1H, HAr), 6.84–7.14 (m, 4H, HAr), 7.93, 8.13 (2s, 1H, N=CH), 9.28, 9.33 (2s, 1H, OH); 11.58, 11.59 (2s, 1H, NH) ppm.
13C NMR (101 MHz, DMSO-d6) δ 19.91 (CH3), 33.19, 34.22, 36.17, 51.28, 51.50 (COCH2, CH, NCH2); 55.91, 55.95, 60.11 (3 OCH3); 104.03, 104.32, 116.57, 116.65, 125.10, 125.17 127.86, 128.37, 128.67, 128.69, 129.65, 129.68, 139.02, 139.23, 143.27, 147.27, 147.00, 150.27, 153.19 168.77, 172.18, 172.46, 173.68 (N=CH, CAr, 2 C=O) ppm.
IR (KBr), ν 3400, 3181 (OH, NH); 1663 (2 C=O); 1505 (C=N) cm−1.
Calcd for C22H25N3O6, %: C 61.82; H 5.90; N 9.83. Found, %: C 61.91; H 5.90; N 9.86.
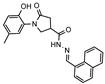
- 1-(2-Hydroxy-5-methylphenyl)-N′-(naphthalen-1-ylmethylene)-5-oxopyrrolidine-3-carbohydrazide (12j)
White solid, yield 0.23 g, 50%, mp 196–197 °C.
1H NMR (400 MHz, DMSO-d6) δ (Z/E isomers mixture, 60/40) 2.18 (s, 3H, CH3), 2.68–2.86 (m, 2H, COCH2), 3.39–3.49, 4.15–4.24 (2m, 1H, CH), 3.80–4.11 (m, 2H, NCH2), 6.74–7.00 (m, 3H, HAr), 7.55–7.70 (m, 3H, HAr), 7.86–8.04 (m, 3H, HAr), 8.59, 8.87 (2d, J = 8.5 Hz, 1H, HAr), 8.73, 8.82 (2s, 1H, N=CH), 9.32, 9.35 (2s, 1H, OH), 11.61, 11.72 (2s, 1H, NH) ppm.
13C NMR (101 MHz, DMSO-d6) δ 19.93 (CH3), 33.31, 34.24, 36.25, 51.14, 51.48 (COCH2, CH, NCH2), 116.63, 123.48, 124.38, 125.13, 125.17, 125.54, 125.62, 126.29, 126.32, 126.98, 127.39, 127.85, 128.27, 128.34, 128.65, 128.70, 128.80, 128.89, 129.34, 129.37, 130.10, 130.37, 130.65, 133.51, 133.55, 143.30, 147.11, 150.25, 150.28, 168.81, 172.20, 172.40, 173.62 (N=CH, CAr, 2 C=O) ppm.
IR (KBr), ν 3190, 3057 (OH, NH); 1663 (2 C=O); 1509 (C=N) cm−1.
Calcd for C23H21N3O3, %: C 71.30; H 5.46; N 10.85. Found, %: C 71.20; H 5.44; N 10.82.
- General procedure for the preparation of hydrazones 13a, b
A solution of hydrazide 4 (0.3 g, 1.2 mmol) in acetone (6 mL) or ethylmethyl ketone (6 mL) was heated at reflux for 4 h for both reactions. Then, the mixture was cooled, and the formed solid was filtered off and washed with acetone and recrystallized from the mixture propan-2-ol and water (1:1) to give the title compounds 13a (white solid, 0.3 g, 86%, mp 199–200 °C) or 13b (white solid, yield 0.28 g, 77%, mp 172–173 °C).
- 1-(2-Hydroxy-5-methylpnenyl)-5-oxo-N′-(propan-2-ylidene)pyrrolidine-3-carbohydrazide (13a)
1H NMR (400 MHz, DMSO-d6) δ (Z/E isomers mixture, 57/43) 1.86, 1.87 (2s, 3H, CH3), 1.91, 1.93 (2s, 3H, CH3), 2.19 (s, 3H, CH3), 2.53–2.76 (m, 2H, COCH2), 3.40–4.04 (m, 3H, NCH2, CH), 6.79 (dd, J = 8.1, 3.4 Hz, 1H, HAr), 6.92 (s, 1H, HAr), 6.94 (s, 1H, HAr), 9.26, 9.30 (2s, 1H, OH), 10.20, 10.29 (2s, 1H, NH) ppm.
13C NMR (101 MHz, DMSO-d6) δ 17.11, 17.63 (CH3), 19.93 (CH3), 24.98, 25.25 (CH3), 34.26, 34.38 (COCH2), 35.76 (CH), 51.31, 51.80 (NCH2), 116.57, 116.63, 125.15, 125.25, 127.85, 128.24, 128.35, 128.61, 128.67, 150.21, 150.28, 151.23, 156.34, 168.74, 172.37, 172.50, 173.82 (CAr, N=C, 2 C=O) ppm.
IR (KBr), ν 3184, 3051 (OH, NH); 1682, 1660 (2x C=O); 1522 (C=N) cm−1. Calcd for C15H19N3O3, %: C 62.27; H 6.62; N 14.52. Found, %: C 62.18; H 6.64; N 14.50.
- N′-(butan-2-ylidene)-1-(2-hydroxy-5-methylphenyl)-5-oxopyrrolidine-3-carbohydrazide (13b)
1H NMR (400 MHz, DMSO-d6) δ (Z/E isomers mixture, 57/43) 0.9, 0.97, 1.02 (3t, J = 7.3 Hz, 3H, CH2CH3), 1.84, 1.86 (2s, 3H, CH3), 2.09–2.36 (m, 5H, CH3, CH2CH3), 2.54–2.81 (m, 2H, COCH2), 3.40–4.03 (m, 3H, NCH2, CH), 6.79 (dd, J = 8.1, 3.5 Hz, 1H, HAr), 6.92 (s, 1H, HAr), 6.94 (s, 1H, HAr), 9.27, 9.31 (2s, 1H, OH), 10.17, 10.24 10.32, 10.40 (4s, 1H, NH) ppm.
13C NMR (101 MHz, DMSO-d6) δ 9.78, 9.81, 10.35, 10.77 (CH2CH3), 16.05, 16.11 (CH3), 19.92 (CH3), 31.43, 31.56 (CH2CH3), 33.25, 34.42 (COCH2), 35.81 (CH), 51.19, 51.79 (NCH2), 116.57, 116.62, 125.16, 125.23, 127.84, 128.26, 128.35, 128.60, 128.66, 150.21, 150.27, 154.48, 155.35, 159.82, 160.16, 168.80, 172.37, 172.51, 173.91 (CAr, N=C, 2 C=O) ppm.
IR (KBr), ν 3200, 3055 (OH, NH); 1682, 1659 (2x C=O); 1522 (C=N) cm−1. Calcd for C16H21N3O3, %: C 63.35; H 6.98; N 13.85. Found, %: C 63.27; H 6.97; N 13.81.
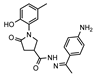
- N′-(1-(4-aminophenyl)ethylidene)-1-(2-hydroxy-5-methylphenyl)-5-oxopyrrolidine-3-carbohydrazide (13c)
To a solution of hydrazide 4 (0.30 g, 1.2 mmol) in propan-2-ol (14 mL), 4-aminoacetophenone (0.18 g, 1.3 mmol) and a catalytic amount of glacial acetic acid (3 drops) were added, and the mixture was heated at reflux for 20 h. Then, the reaction mixture was cooled, and the obtained solid was filtered off, washed with diethyl ether, and recrystallized from 1,4-dioxane.
White solid, yield 0.24 g, 55%, mp 178–179 °C.
1H NMR (400 MHz, DMSO-d6) δ (Z/E isomers mixture, 65/35) 2.15, 2.18, 2.19 (3s, 6H, 2 CH3), 2.56–2.83 (m, 2H, COCH2), 3.48–3.62, 4.05–4.20 (m, 1H, CH), 3.70–4.04 (m, 2H, NCH2), 5.43, 5.46 (2s, 2H, NH2), 6.56 (d, J = 8.2 Hz, 2H, HAr), 6.79 (t, J = 6.9 Hz, 1H, HAr), 6.93 (d, J = 7.1 Hz, 1H, HAr), 6.96 (s, 1H, HAr), 7.51 (t, J = 8.6 Hz, 2H, HAr), 9.29, 9.33 (2s, 1H, OH), 10.34, 10.47 (2s, 1H, NH) ppm.
13C NMR (101 MHz, DMSO-d6) δ 13.24, 13.81 (CH3), 19.94 (CH3), 34.42, 34.53, 35.99, 51.32, 51.88 (COCH2, CH, NCH2), 112.46, 113.13, 113.28, 116.58, 116.66, 125.05, 125.18, 125.24, 127.21, 127.62, 127.86, 128.26, 128.38, 128.61, 128.68, 130.58, 149.00, 149.98, 150.03, 150.20, 150.24, 150.29, 153.63, 153.79, 168.87 (N=C, CAr), 172.42, 172.58, 174.05 (2 C=O).
IR (KBr), ν 3461, 3360, 3176 (OH, NH2, NH); 1669 (2 C=O); 1519 (C=N) cm−1. Calcd for C20H22N4O3, %: C 65.56; H 6.05; N 15.29. Found, %: C 65.61; H 6.07; N 15.21.
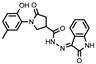
- 1-(2-Hydroxy-5-methylphenyl)-5-oxo-N′-(2-oxoindolin-3-ylidene)pyrrolidine-3-carbohydrazide (14)
To a cooled solution of hydrazide 4 (0.5 g, 2 mmol) in methanol (10 mL), isatin (0.44 g, 3 mmol) was added, followed by the dripping of a catalytic amount of glacial acetic acid (3 drops), and the reaction mixture was heated at reflux for 3 h. Then, the mixture was cooled, and the obtained solid was filtered off, washed with propan-2-ol, and recrystallized from 2-propanol to give the title compound 14 (yellow solid, yield 0.36 g, 48%, mp 210–211 °C).
1H NMR (400 MHz, DMSO-d6) δ 2.19 (s, 3H, CH3), 2.61–2.84 (m, 2H, COCH2), 3.80–4.02 (m, 2H, NCH2), 4.16 (br s, 1H, CH), 6.79 (d, J = 8.1 Hz, 1H, HAr), 6.86–6.95 (m, 2H, HAr), 6.96 (s, 1H, HAr), 7.05 (t, J = 7.0 Hz, 1H, HAr), 7.38 (t, J = 7.6 Hz, 1H, HAr), 8.12 (s, 1H, HAr), 9.33 (s, 1H, OH), 10.81, 11.33 (2s, 2H, 2 NH) ppm.
13C NMR (101 MHz, DMSO-d6) δ 19.94 (CH3), 33.70, 34.71, 51.27 (COCH2, CH, NCH2); 110.63, 115.23, 116.62, 121.71, 125.12, 126.28, 127.87, 128.39, 128.72, 132.67, 143.84, 150.31, 164.60 (N=C, CAr), 172.11 (3 C=O) ppm.
IR (KBr), ν 3208, 3199, 3139 (OH, NH); 1732, 1717, 1691 (3 C=O); 1510 (C=N) cm−1.
Calcd for C20H18N4O4, %: C 63.49; H, 4.80; N 14.81. Found, %: C 63.41; H 4.82; N 14.79.
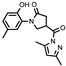
- 4-(3,5-Dimethyl-1H-pyrazol-1-yl)-1-(2-hydroxy-5-methylphenyl)pyrrolidin-2-one (15)
To a cooled solution of hydrazide 4 (0.5 g, 2 mmol) in propan-2-ol (15 mL), pentane-2,4-dione (0.24 g, 2.4 mmol) and a catalytic amount of concentrated hydrochloric acid (3 drops) were added, and the mixture was heated at reflux for 2 h. When completed, the mixture was cooled, and the obtained crystalline solid was filtered off, washed with propan-2-ol, and recrystallized from 1,4-dioxane to give the title compound 15 (white solid, yield 0.29 g, 46%, mp 170–171 °C).
1H NMR (400 MHz, DMSO-d6) δ 2.19 (s, 6H, 2 CH3), 2.50 (s, 3H, CH3, overlaps with the DMSO-d6); 2.68–2.81 (m, 2H, COCH2), 3.79–4.09 (m, 2H, NCH2), 4.43–4.53 (m, 1H, CH), 6.22 (s, 1H, HPyr), 6.79 (d, J = 8.1 Hz, 1H, HAr), 6.93 (d, J = 8.3 Hz, 1H, HAr), 6.96 (s, 1H, HAr); 9.32 (s, 1H, OH) ppm.
13C NMR (101 MHz, DMSO-d6) δ 13.54, 14.07 (2 CH3 Pyr), 19.91, 33.47, 36.69, 51.22 (COCH2, CH, NCH2), 111.50 (CHPyr), 116.57, 124.97, 127.82, 128.39, 128.70, 143.81, 150.26, 152.06 (CAr), 171.80, 172.61 (2 C=O) ppm.
IR (KBr), ν 3211 (OH); 1729, 1664 (2 C=O); 1512 (C=N) cm−1.
Calcd for C17H19N3O3, %: C 65.16; H 6.11; N 13.41. Found, %: C 65.21; H 6.11; N 13.46.
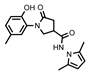
- N-(2,5-dimethyl-1H-pyrrol-1-yl)-1-(2-hydroxy-5-methylphenyl)-5-oxopyrrolidine-3-carboxamide (16)
To a solution of hydrazide 4 (0.55 g, 2.2 mmol) in propan-2-ol (22 mL), hexane-2,5-dione (0.44 g, 3.9 mmol) and glacial acetic acid (3 drops) were added dropwise, and the mixture was heated at reflux for 4 h. Then, the reaction mixture was cooled, and the formed crystalline solid was filtered off, washed with propan-2-ol, and recrystallized from 1,4-dioxane to give the title compound 16 (brownish solid, yield 0.34 g, 48%, mp 208–1209 °C).
1H NMR (400 MHz, DMSO-d6) δ 1.97, 2.00 (2s, 6H, 2 CH3), 2.19 (s, 3H, NCCHCCH3), 2.61–2.78 (m, 2H, COCH2), 3.43–3.57 (m, 1H, CH), 3.77–3.87 (m, 1H, NCH2), 3.89–4.07 (m, 1H, NCH2), 5.65 (s, 2H, HPyrr), 6.80 (d, J = 7.9 Hz, 1H, HAr), 6.87–7.02 (m, 2H, HAr), 9.35 (s, 1H, OH), 10.86 (s, 1H, NH) ppm.
13C NMR (101 MHz, DMSO-d6) δ 10.92, 10.96 (2 CH3), 19.93 (CH3), 33.82, 35.28, 51.41 (COCH2, CH, NCH2), 103.08 (CHPyrr), 116.55, 124.99, 126.74, 126.76, 127.85, 128.46, 128.75, 150.32 (CAr), 171.89, 171.99 (2 C=O) ppm.
IR (KBr), ν 3265, 3117 (OH, NH); 1667, 1608 (2 C=O) cm−1.
Calcd for C18H21N3O3, %: C 66.04; H 6.47; N 12.84. Found, %: C 66.11; H 6.50; N 12.79.
3.2. Determination of Antimicrobial Activity
3.2.1. Preparation of Bacterial Inoculum
Clinically important reference bacterial strains were sourced from the American Type Culture Collection (ATCC). In this study, the Gram-positive bacteria Staphylococcus aureus subsp. aureus (ATCC 9144), Listeria monocytogenes (ATCC 35152), and Bacillus cereus (ATCC 11778), as well as the Gram-negative bacterium Escherichia coli (ATCC 8739), were used to evaluate the compounds in vitro.
Bacteria were cultured on Tryptone Soya Agar (OXOID, Hampshire, UK) 24 h prior to testing. A turbidity equivalent to 0.5 McFarland standard (108 CFU/mL) was prepared from the cultured bacterial suspensions.
3.2.2. Determination of Minimum Inhibitory Concentration (MIC)
The broth microdilution method [54] was used to study the antibacterial activity of the derivatives. Bacterial growth was assessed in 96-well microplates (OXOID, Hampshire, UK) in Muller–Hinton (MH) broth. Serial two-fold dilutions of the compounds were used to determine the MIC, ranging from 500 µg/mL to 0.244 µg/mL concentrations. The concentration of bacteria used for the study was 5 × 105 CFU/mL. Each well contained 100 µL of the mixture (different concentrations of compounds and bacteria at 5 × 104 CFU). The sterility of the medium, the growth of the tested bacteria, and the sensitivity to the antibiotics oxallin, ampicillin, and cefuroxime were also controlled. Microtitration plates with the test mixture were incubated at 37 °C for 24 h. The MIC was determined as the lowest concentration of the compound at which no bacterial growth (turbidity) could be seen in the plate wells. Cefuroxime was used as a control (C) for antibacterial activity screening. The experiment was performed in triplicate.
3.2.3. Determination of Minimum Bactericidal Concentration (MBC)
The MBC was considered as the lowest concentration of the compound causing a ≥3 log10 reduction (≥99.9% kill) in the number (5 × 104 CFU/100 µL) of bacteria [54]. Ten microliters of mixture was taken from the well in which the MIC value was determined and from up to three wells in which the concentration of the compound was 2, 4, and 8 times higher. The tested mixtures were spread on Mueller–Hinton agar. The growth of bacteria and number of colonies were evaluated after 24 h of incubation at 37 °C. The MBC was considered as the lowest concentration of a compound when bacteria did not grow or formed up to 5 colonies. The experiment was conducted in triplicate.
3.2.4. Biofilm Assay
Biofilm formation was investigated using the tube adherence test, a qualitative assay originally described by Christensen et al. (1985) [14]. The bacterial strains included in the study were Staphylococcus aureus subsp. aureus (ATCC 9144), Listeria monocytogenes (ATCC 7644), Bacillus cereus (ATCC 11778), and the Gram-negative bacterium Escherichia coli (ATCC 8739). Following this assay, biofilm formation was confirmed for S. aureus and E. coli. Based on these findings and previous studies demonstrating antibacterial activity, two compounds, 11b and 11d, were selected to evaluate their capacity to disrupt biofilms formed by the Gram-positive coccus S. aureus and the Gram-negative rod E. coli.
A loopful of test bacteria was inoculated into 10 mL of Tryptic Soy Broth (Liofilchem, Roseto degli Abruzzi, Italy) containing 1% glucose in test tubes. The tubes were incubated for 24 h at 37 °C. After incubation, the tubes were decanted, washed with phosphate-buffered saline (PBS, pH 7.3), and dried.
The ability of compounds 11b and 11d to disrupt biofilms was evaluated at a concentration of 10.0 µg/mL. The compounds were added to the tubes and incubated for 1 h before staining. The tubes were then stained with 0.1% crystal violet. Excess stain was removed by washing with deionized water. The tubes were dried in an inverted position.
Biofilm formation was scored based on the results from the control strains. Bacteria were considered biofilm-producing when a visible layer of biofilm was observed on the walls of the tube. The scoring for biofilm formation was as follows: (1) negative, (2) weak positive, (3) moderate positive, and (4) strong positive. The experiment was conducted in triplicate [55].
4. Conclusions
In the current study, a library of 1-(2-hydroxy-5-methylphenyl)-5-oxopyrrolidine-3-carboxylic acid derivatives were obtained, characterized, and assessed for their antibacterial activity against Staphylococcus aureus, Listeria monocytogenes, Bacillus cereus, and Escherichia coli bacteria strains. In summary, hydrazones 11b and 11d demonstrate the highest antibacterial properties and show promise as potential biofilm-disrupting agents. The ability of compound 11b to disrupt biofilms of both S. aureus and E. coli suggests that it could be a valuable candidate for further investigation. However, the differential activity of compound 11d against S. aureus biofilms indicates that its mechanism of action may be species-specific, highlighting the complexity of biofilm-targeting strategies. Further studies examining the mechanism of action of these compounds and optimizing their concentrations could provide valuable insights into their potential as adjunct therapies for treating chronic infections caused by biofilm-forming pathogens.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules30122639/s1, Figure S1: 1H NMR of compound 2; Figure S2: 13C NMR of compound 2; Figure S3: 1H NMR of compound 3; Figure S4: 13C NMR of compound 3; Figure S5: 1H NMR of compound 4; Figure S6; 13C NMR of compound 4; Figure S7: 1H NMR of compound 5; Figure S8: 13C NMR of compound 5; Figure S9: 1H NMR of compound 6; Figure S10: 13C NMR of compound 6; Figure S11: 1H NMR of compound 7; Figure S12: 13C NMR of compound 7; Figure S13: 1H NMR of compound 8; Figure S14: 13C NMR of compound 8; Figure S15: 1H NMR of compound 9a; Figure S16: 13C NMR of compound 9a; Figure S17: 1H NMR of compound 9b; Figure S18: 13C NMR of compound 9b; Figure S19: 1H NMR of compound 9c; Figure S20: 13C NMR of compound 9c; Figure S21: 1H NMR of compound 9d; Figure S22; 13C NMR of compound 89; Figure S23; 1H NMR of compound 10; Figure S24: 13C NMR of compound 10; Figure S25: 1H NMR of compound 11a; Figure S26: 13C NMR of compound 11a; Figure S27: 1H NMR of compound 11b; Figure S28: 13C NMR of compound 11b; Figure S29: 1H NMR of compound 11c; Figure S30: 13C NMR of compound 11c; Figure S31: 1H NMR of compound 11d; Figure S32: 13C NMR of compound 11d; Figure S33: 1H NMR of compound 12a; Figure S34: 13C NMR of compound 12a; Figure S35: 1H NMR of compound 12b; Figure S36: 13C NMR of compound 12b; Figure S37: 1H NMR of compound 12c; Figure S38: 13C NMR of compound 12c; Figure S39: 1H NMR of compound 12d; Figure S40: 13C NMR of compound 12d; Figure S41: 1H NMR of compound 12e; Figure S42: 13C NMR of compound 12e; Figure S43: 1H NMR of compound 12f; Figure S44: 13C NMR of compound 12f; Figure S45: 1H NMR of compound 12g; Figure S46: 13C NMR of compound 12g; Figure S47: 1H NMR of compound 12h; Figure S48: 13C NMR of compound 12h; Figure S49: 1H NMR of compound 12i; Figure S50: 13C NMR of compound 12i; Figure S51: 1H NMR of compound 12j; Figure S52: 13C NMR of compound 12j; Figure S53: 1H NMR of compound 13a; Figure S54: 13C NMR of compound 13a; Figure S55: 1H NMR of compound 13b; Figure S56: 13C NMR of compound 13b; Figure S57: 1H NMR of compound 13c; Figure S58: 13C NMR of compound 13c; Figure S59: 1H NMR of compound 14; Figure S60: 13C NMR of compound 14; Figure S61: 1H NMR of compound 15; Figure S62: 13C NMR of compound 15; Figure S63: 1H NMR of compound 16; Figure S64: 13C NMR of compound 16; Figure S65: IR spectrum of compound 2; Figure S66: IR spectrum of compound 3; Figure S67: IR spectrum of compound 4; Figure S68: IR spectrum of compound 5; Figure S69: IR spectrum of compound 6; Figure S70: IR spectrum of compound 7; Figure S71: IR spectrum of compound 8; Figure S72: IR spectrum of compound 9a; Figure S73: IR spectrum of compound 9b; Figure S74: IR spectrum of compound 9c; Figure S75: IR spectrum of compound 9d; Figure S76: IR spectrum of compound 10; Figure S77: IR spectrum of compound 11a; Figure S78: IR spectrum of compound 11b; Figure S79: IR spectrum of compound 11c; Figure S80: IR spectrum of compound 11d; Figure S81: IR spectrum of compound 12a; Figure S82: IR spectrum of compound 12b; Figure S83: IR spectrum of compound 12c; Figure S84: IR spectrum of compound 12d; Figure S85: IR spectrum of compound 12e; Figure S86: IR spectrum of compound 12f; Figure S87: IR spectrum of compound 12g; Figure S88: IR spectrum of compound 12h; Figure S89: IR spectrum of compound 12i; Figure S90: IR spectrum of compound 12j; Figure S91: IR spectrum of compound 13a; Figure S92: IR spectrum of compound 13b; Figure S93: IR spectrum of compound 13c; Figure S94: IR spectrum of compound 14; Figure S95: IR spectrum of compound 15; Figure S96: IR spectrum of compound 16.
Author Contributions
Conceptualization, B.G., V.M., and J.Š.; methodology, B.G. and K.K.; validation, R.V. and J.Š.; formal analysis, K.K., J.Š., R.V., and V.M.; investigation, K.K., J.Š., B.G., and V.M.; resources, J.Š. and V.M.; data curation, B.G. and V.M.; writing—original draft preparation, K.K. and V.M.; writing—review and editing, B.G., R.V., and V.M.; visualization, K.K. and R.V.; supervision, B.G. and V.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Acknowledgments
This study is based upon work from COST Action EURESTOP, CA21145, supported by COST (European Cooperation in Science and Technology).
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| O | Oxacillin |
| A | Ampicillin |
| C | Cefuroxime |
| MIC | Minimum Inhibitory Concentration |
| MBC | Minimum Bactericidal Concentration |
| S. aureus | Staphylococcus aureus subsp. aureus |
References
- Borah, P.; Hazarika, S.; Chettri, A.; Sharma, D.; Deka, S.; Venugopala, K.N.; Shinu, P.; Al-Shar’i, N.A.; Bardaweel, S.K.; Deb, P.K. Heterocyclic compounds as antimicrobial agents. In Viral, Parasitic, Bacterial, and Fungal Infections; Academic Press: Cambridge, MA, USA, 2023; pp. 781–804. [Google Scholar] [CrossRef]
- Qadir, T.; Amin, A.; Sharma, P.K.; Jeelani, I.; Abe, H. A Review on Medicinally Important Heterocyclic Compounds. Open J. Med. Chem. 2022, 16, e187410452202280. [Google Scholar] [CrossRef]
- Anwer, K.E.; El-Hddad, S.S.A.; Abd El-Sattar, N.E.A.; El-Morsy, A.; Khedr, F.; Mohamady, S.; Keshek, D.E.; Salama, S.A.; El-Adl, K.; Hanafy, N.S. Five and Six Membered Heterocyclic Rings Endowed with Azobenzene as Dual EGFRT790M and VEGFR-2 Inhibitors: Design, Synthesis, in Silico ADMET Profile, Molecular Docking, Dynamic Simulation and Anticancer Evaluations. RSC Adv. 2023, 13, 35321–35338. [Google Scholar] [CrossRef] [PubMed]
- Heravi, M.M.; Zadsirjan, V. Prescribed Drugs Containing Nitrogen Heterocycles: An Overview. RSC Adv. 2020, 10, 44247–44311. [Google Scholar] [CrossRef]
- Kabir, E.; Monir Uzzaman, M. A review on biological and medicinal impact of heterocyclic compounds. Results Chem. 2022, 4, 100606. [Google Scholar] [CrossRef]
- Peerzada, M.N.; Hamel, E.; Bai, R.; Supuran, C.T.; Azam, A. Deciphering the key heterocyclic scaffolds in targeting microtubules, kinases and carbonic anhydrases for cancer drug development. Pharmacol. Ther. 2021, 225, 107860. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, A.R.; Farooqui, M.; Satpute, R.H.; Abed, S. Overview on nitrogen containing compounds and their assessment based on International Regulatory Standards. J. Drug Delivery Therap. 2018, 8, 424–428. [Google Scholar] [CrossRef]
- Arora, P.; Arora, V.; Lamba, H.S.; Wadhwa, D. Importance of heterocyclic chemistry: A review. Int. J. Pharm. Sci. Res. 2012, 8, 2947–2954. [Google Scholar] [CrossRef]
- Sharma, P.K.; Qadir, T.; Amin, A.; Sarkar, D. Synthesis of medicinally important indole derivatives: A Review. Open Med. Chem. J. 2021, 15, 1–16. [Google Scholar] [CrossRef]
- Hilal, H.S.; Ali-Shtayeh, M.S.; Arafat, R.; Al-Tel, T.; Voelter, W.; Barakat, A. Synthesis of a new series of heterocyclic scaffolds for medicinal purposes. Eur. J. Med. Chem. 2006, 41, 1017–1024. [Google Scholar] [CrossRef]
- Barbuceanu, S.-F.; Olaru, O.T. Synthesis and Evaluation of Biologically Active Compounds from Heterocycles Class. Molecules 2025, 30, 394. [Google Scholar] [CrossRef]
- Available online: https://www.reachemchemicals.com/blog/applications-of-heterocyclic-compounds-in-pharmaceuticals/ (accessed on 3 March 2025).
- Cebeci, Y.U.; Batur, Ö.Ö.; Boulebd, H. Design, synthesis, theoretical studies, and biological activity evaluation of new nitrogen-containing poly heterocyclic compounds as promising antimicrobial agents. J. Mol. Struct. 2024, 1299, 137115. [Google Scholar] [CrossRef]
- Sadek, K.U.; Mekheimer, R.A.; Abd-Elmonem, M.; Abo-Elsoud, F.A.; Hayallah, A.M.; Mostafa, S.M.; Abdellattif, M.H.; Abourehab, M.A.S.; Farghaly, T.A.; Elkamhawy, A. Recent developments in the synthesis of hybrid heterocycles, a promising approach to develop multi-target antibacterial agents. J. Mol. Struct. 2023, 1286, 135616. [Google Scholar] [CrossRef]
- Gomtsyan, A. Heterocycles in Drugs and Drug Discovery. Chem. Heterocycl. Comp. 2012, 48, 7–10. [Google Scholar] [CrossRef]
- Barreca, M.; Spanò, V.; Rocca, R.; Bivacqua, R.; Gualtieri, G.; Raimondi, M.V.; Gaudio, E.; Bortolozzi, R.; Manfreda, L.; Bai, R.; et al. Identification of Pyrrolo [3′,4′:3,4]Cyclohepta[1,2-d][1,2]Oxazoles as Promising New Candidates for the Treatment of Lymphomas. Eur. J. Med. Chem. 2023, 254, 115372. [Google Scholar] [CrossRef]
- Grillone, K.; Riillo, C.; Rocca, R.; Ascrizzi, S.; Spanò, V.; Scionti, F.; Polerà, N.; Maruca, A.; Barreca, M.; Juli, G.; et al. The New Microtubule-Targeting Agent SIX2G Induces Immunogenic Cell Death in Multiple Myeloma. Int. J. Mol. Sci. 2022, 23, 10222. [Google Scholar] [CrossRef]
- Lee, B.; Kim, D.G.; Lee, A.; Kim, Y.M.; Cui, L.; Kim, S.; Choi, I. Synthesis and Discovery of the First Potent Proteolysis Targeting Chimaera (PROTAC) Degrader of AIMP2-DX2 as a Lung Cancer Drug. J. Enzym. Inhib. Med. Chem. 2023, 38, 51–66. [Google Scholar] [CrossRef]
- Bivacqua, R.; Barreca, M.; Spanò, V.; Raimondi, M.V.; Romeo, I.; Alcaro, S.; Andrei, G.; Barraja, P.; Montalbano, A. Insight into Non-Nucleoside Triazole-Based Systems as Viral Polymerases Inhibitors. Eur. J. Med. Chem. 2023, 249, 115136. [Google Scholar] [CrossRef]
- Fesatidou, M.; Petrou, A.; Athina, G. Heterocycle Compounds with Antimicrobial Activity. Curr. Pharm. Des. 2020, 26, 867–904. [Google Scholar] [CrossRef] [PubMed]
- Rusu, A.; Moga, I.-M.; Uncu, L.; Hancu, G. The Role of Five-Membered Heterocycles in the Molecular Structure of Antibacterial Drugs Used in Therapy. Pharmaceutics 2023, 15, 2554. [Google Scholar] [CrossRef]
- Shi, Z.; Zhang, J.; Tian, L.; Xin, L.; Liang, C.; Ren, X.; Li, M. A Comprehensive Overview of the Antibiotics Approved in the Last Two Decades: Retrospects and Prospects. Molecules 2023, 28, 1762. [Google Scholar] [CrossRef]
- Cheddie, A.; Shintre, S.A.; Bantho, A.; Mocktar, C.; Koorbanally, N.A. Synthesis and antibacterial activity of a series of 2-trifluoromethylbenzimidazole-thiazolidinone derivatives. J. Heterocycl. Chem. 2020, 57, 299–307. [Google Scholar] [CrossRef]
- Hassoun, A.; Linden, P.K.; Friedman, B. Incidence, prevalence, and management of MRSA bacteremia across patient populations—A review of recent developments in MRSA management and treatment. Crit. Care 2017, 21, 211–221. [Google Scholar] [CrossRef]
- Abd El-Aleam, R.H.; George, R.F.; Georgey, H.H.; Hamdy, M.; Abdel-Rahman, H.M. Bacterial virulence factors: A target for heterocyclic compounds to combat bacterial resistance. RSC Adv. 2021, 11, 36459–36482. [Google Scholar] [CrossRef]
- Kavaliauskas, P.; Sapijanskaitė-Banevič, B.; Grybaitė, B.; Mickevičiūtė, E.; Anusevičius, K.; Garcia, A.; Naing, E.; Petraitienė, R.; Petraitis, V.; Grigalevičiūtė, R.; et al. Synthesis and In Vitro Anticancer Activity of Pyrrolidone Derivatives Bearing 3,4,5-Trimethoxyphenyl Moiety as a Promising Anticancer Scaffold. Appl. Sci. 2024, 14, 11784. [Google Scholar] [CrossRef]
- Kairytė, K.; Vaickelionienė, R.; Grybaitė, B.; Anusevičius, K.; Mickevičius, V.; Petrikaitė, V. The Effect of 4-(Dimethylamino)phenyl-5-oxopyrrolidines on Breast and Pancreatic Cancer Cell Colony Formation, Migration, and Growth of Tumor Spheroids. Int. J. Mol. Sci. 2024, 25, 1834. [Google Scholar] [CrossRef]
- Balandis, B.; Mickevičius, V.; Petrikaitė, V. Exploration of Benzenesulfonamide-Bearing Imidazole Derivatives Activity in Triple-Negative Breast Cancer and Melanoma 2D and 3D Cell Cultures. Pharmaceuticals 2021, 14, 1158. [Google Scholar] [CrossRef] [PubMed]
- Kavaliauskas, P.; Grybaite, B.; Mickevicius, V.; Petraitiene, R.; Grigaleviciute, R.; Planciuniene, R.; Gialanella, P.; Pockevicius, A.; Petraitis, V. Synthesis, ADMET Properties, and In Vitro Antimicrobial and Antibiofilm Activity of 5-Nitro-2-thiophenecarbaldehyde N-((E)-(5-Nitrothienyl)methylidene)hydrazone (KTU-286) against Staphylococcus aureus with Defined Resistance Mechanisms. Antibiotics 2020, 9, 612. [Google Scholar] [CrossRef]
- Šiugždaitė, J.; Lelešius, R.; Grybaitė, B.; Vaickelionienė, R.; Mickevičius, V. Synthesis and biological studies of new 2-benzoxazolinone derivatives as antibacterial agents. Appl. Sci. 2024, 14, 4783. [Google Scholar] [CrossRef]
- Ferraz, E.R.A.; de Oliveira, G.A.R.; de Oliveira, D.P. The impact of aromatic amines on the environment: Risks and damages. Front. Biosci. 2012, 4, 914–923. [Google Scholar] [CrossRef]
- Sirviö, J.A.; Kantola, A.M.; Komulainen, S.; Filonenko, S. Aqueous Modification of Chitosan with Itaconic Acid to Produce Strong Oxygen Barrier Film. Biomacromolecules 2021, 22, 2119–2128. [Google Scholar] [CrossRef]
- Paytash, P.L.; Sparrow, E.; Gathe, J.C. The reaction of itaconic acid with primary amines. J. Am. Chem. Soc. 1950, 72, 1415–1416. [Google Scholar] [CrossRef]
- Lin, J.L.; Liang, Y.Q.; Liao, X.J.; Yang, J.T.; Li, D.C.; Huang, Y.L.; Jiang, Z.H.; Xu, S.H.; Zhao, B.X. Acanthophoraine A, a new pyrrolidine alkaloid from the red alga Acanthophora spicifera. Nat. Prod. Res 2020, 34, 2065–2070. [Google Scholar] [CrossRef] [PubMed]
- Ahankar, H.; Ramazani, A.; Ślepokura, K.; Lis, T.; Joo, S.W. Synthesis of pyrrolidinone derivatives from aniline, an aldehyde and diethyl acetylenedicarboxylate in an ethanolic citric acid solution under ultrasound irradiation. Green. Chem. 2016, 18, 3582–3593. [Google Scholar] [CrossRef]
- Mickevicius, M.; Beresnevicius, Z.J.; Mickevicius, V.; Mikulskiene, G. Condensation products of 1-Aryl-4-carboxy2-pyrrolidinones with o-diaminoarenes, o-aminophenol, and their structural studies. Heteroat. Chem. 2006, 17, 47–56. [Google Scholar] [CrossRef]
- Srivastava, R.; Gupta, S.K.; Naaz, F.; Gupta, P.S.S.; Yadav, M.; Singh, V.K.; Singh, A.; Rana, M.K.; Gupta, S.K.; Schols, D.; et al. Alkylated benzimidazoles: Design, synthesis, docking, DFT analysis, ADMET property, molecular dynamics and activity against HIV and YFV. Comput. Biol. Chem. 2020, 89, 107400. [Google Scholar] [CrossRef]
- Ershov, A.Y.; Lagoda, I.V.; Yakimovich, S.I.; Zerova, I.V.; Pakalnis, V.V.; Shamanin, V.V. Structure of the condensation products of 3-sulfanylpropionic acid hydrazide with aldehydes, ketones, and aldoses. Russ. J. Org. Chem. 2009, 45, 1488–1495. [Google Scholar] [CrossRef]
- Levrand, B.; Fieber, W.; Lehn, J.-M.; Herrmann, A. Controlled Release of Volatile Aldehydes and Ketones from Dynamic Mixtures Generated by Reversible Hydrazone Formation. Helv. Chim. Acta 2007, 90, 2281–2314. [Google Scholar] [CrossRef]
- Czelen, P.; Skotnicka, A.; Szefler, B. Designing and Synthesis of New Isatin Derivatives as Potential CDK2 Inhibitors. Int. J. Mol. Sci. 2022, 23, 8046. [Google Scholar] [CrossRef]
- Xu, J.; Lin, Q.; Sheng, M.; Ding, T.; Li, B.; Gao, Y.; Tan, Y. Antibiofilm Effect of Cinnamaldehyde-Chitosan Nanoparticles against the Biofilm of Staphylococcus Aureus. Antibiotics 2022, 11, 1403. [Google Scholar] [CrossRef]
- Fotopoulou, E.T.; Jenkins, C.; Paiset, A.; Amar, C. Listeria monocytogenes: The silent assassin. J. Med. Microbiol. 2024, 73, 001800. [Google Scholar] [CrossRef]
- Kohanski, A.A.; Dwyer, D.J.; Collins, J.J. How antibiotics kill bacteria: From targets to networks. Nat. Rev. Microbiol. 2010, 8, 423–435. [Google Scholar] [CrossRef] [PubMed]
- Pitt, T.L.; McClure, J.; Parker, M.D.; Amezquita, A.; McClure, P.J. Bacillus cereus in personal care products: Risk to consumers. Int. J. Cosmet. Sci. 2015, 37, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Ballén, V.; Ratia, V.; Gabasa, Y.; Soto, S.M. Clinical Escherichia coli: From Biofilm Formation to New Antibiofilm Strategies. Microorganisms 2022, 10, 1103. [Google Scholar] [CrossRef] [PubMed]
- Kashi, M.; Noei, M.; Chegini, Z.; Shariati, A. Natural Compounds in the Fight against Staphylococcus Aureus Biofilms: A Review of Antibiofilm Strategies. Front. Pharmacol. 2024, 15, 1491363. [Google Scholar] [CrossRef]
- Didehdar, M.; Chegini, Z.; Tabaeian, S.P.; Razavi, S.; Shariati, A. Cinnamomum: The New Therapeutic Agents for Inhibition of Bacterial and Fungal Biofilm-Associated Infection. Front. Cell. Infect. Microbiol. 2022, 12, 930624. [Google Scholar] [CrossRef]
- Christensen, G.D.; Simpson, W.A.; Binso, A.L.; Beachey, E.H. Adherence of slime-producing strains of Staphylococcus epidermidis to smooth surfaces. Infect. Immun. 1982, 37, 318–326. [Google Scholar] [CrossRef]
- Tan, L.; Huang, Y.; Shang, W.; Yang, Y. Accessory Gene Regulator (agr) Allelic Variants in Cognate Staphylococcus aureus Strain Display Similar Phenotypes. Front. Microbiol. 2022, 13, 2022. [Google Scholar] [CrossRef]
- Høiby, N.; Ciofu, O.; Johansen, H.K.; Song, Z. The clinical impact of bacterial biofilms. Int. J. Oral Sci. 2011, 3, 55–65. [Google Scholar] [CrossRef]
- Dostert, M.; Trimble, M.J.; Hancock, R.E.W. Antibiofilm peptides: Overcoming biofilm-related treatment failure. RSC Adv. 2021, 11, 2718–2728. [Google Scholar] [CrossRef]
- Chapman, J.E.; George, S.E.; Wolz, C.; Olson, M.E. Biofilms: A developmental niche for vancomycin-intermediate Staphylococcus aureus. Infect. Genet. Evol. 2024, 117, 105545. [Google Scholar] [CrossRef]
- Stewart, P.S.; Costerton, J.W. Antibiotic resistance of bacteria in biofilms. Lancet 2001, 358, 135–138. [Google Scholar] [CrossRef] [PubMed]
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.K. Methods for in Vitro Evaluating Antimicrobial Activity: A Review. J. Pharm. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Shree, P.; Singh, C.K.; Sodhi, K.K.; Surya, J.N.; Singh, D.K. Biofilms: Understanding the structure and contribution towards bacterial resistance in antibiotics. Med. Microecol. 2023, 16, 100084. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).