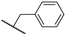
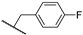
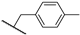
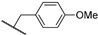
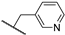
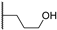
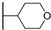
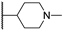
Abstract
6-Aryl-7H-pyrrolo[2,3-d]pyrimidin-4-amines have promising properties as colony-stimulating factor 1 receptor (CSF1R) inhibitors. Inspired by these antagonists, two series of 1,2,3-triazole analogues (28 compounds) were synthesized and evaluated as CSF1R inhibitors. Enzymatic IC50 profiling showed that 27 of the 28 derivatives had lower IC50 than the reference drug PLX-3397. Three derivatives displayed CSF1R Ba/F3 cellular IC50 well below 1 µM. Profiling of the most promising triazole analogue (compound 27a) toward a panel of kinases reveals a high selectivity for CSF1R with respect to its family kinases, but 27a also inhibits ABL, SRC, and YES kinases. Molecular docking of 27a toward two CSF1R X-ray structures identified two different ligand-inverted binding poses, which triggers interest for further investigations.
1. Introduction
Colony-stimulating factor 1 receptor (CSF1R) is a membrane-bound tyrosine kinase primarily expressed on the cell surface of monocyte/macrophage lineages, bone-resorbing osteoclasts, and microglial cells [1,2]. Upon binding of its cytokine ligands, colony-stimulating factor 1 (CSF1), and interleukin 34 (IL-34), CSF1R mediates signaling via downstream kinase pathways [3,4] necessary for the differentiation, proliferation, and normal function of these immune cells. However, in some diseases, the overexpression of CSF1 and/or elevated activity of CSF1R cause a misbalance of the immune cell phenotypes [5], leading to aggressive disease and poor prognosis [6,7]. Taking macrophages as examples, the simplified view is that they can have a tumoricidal (often called M1) phenotype, or a phenotype that promotes tumor growth, angiogenesis, invasion, and metastasis (often denoted as M2). Thus, finding ways of correcting the misbalance in the population of immune cells is of interest. The use of CSF1R inhibitors is one option with prospects in the treatment of cancers [8], inflammation-driven diseases [9], CNS diseases [10,11,12], and bone diseases [4,13]. There are currently two FDA-approved CSF1R inhibitors, pexidartinib (PLX-3397) and vimseltinib (DCC-3014), both used as a treatment for tenosynovial giant-cell tumors. The structures are shown in Figure 1. Clinical trials are also ongoing with other small molecular inhibitors and monoclonal antibodies [14,15]. CSF1R activity is also prominent in a few other FDA-approved kinase inhibitors such as dasatinib (multi-kinase inhibitor), imatinib (ABL-BCR), and surufatinib [15] (fibroblast growth factor receptor 1 inhibitor).
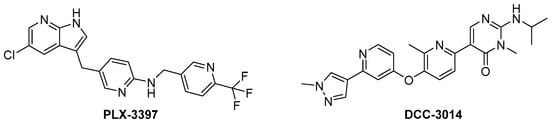
Figure 1.
Structure of the FDA-approved CSF1R inhibitors PLX-3397 and DCC-3014.
The kinase selectivity among small molecular CSF1R inhibitors varies, with some of the more selective inhibitors being DCC-3014) [16], sotuletinib (BLZ945) [17], and edicotinib (JNJ-40346527) [18,19]. Our CSF1R drug discovery efforts have focused on pyrrolopyrimidines [20,21,22] and purines [23]. Despite showing high enzymatic inhibition and excellent kinase selectivity, none of these derivatives have reached animal efficacy studies due to low metabolic stability in mice or low permeability [20]. These inhibitors feature two carbon-based aromatic substituents. While the presence of multiple carboaromatic rings in drug candidates is often associated with suboptimal physicochemical properties [24], these limitations can be mitigated by incorporating heterocycles such as triazoles [25,26]. Triazoles are widely utilized in medicinal chemistry [27,28,29] and are frequently found in kinase inhibitors [30,31,32,33,34,35,36]. In this study, we report the design, synthesis, and biological evaluation of a series of pyrrolopyrimidine-based CSF1R inhibitors bearing two distinct amines at the C4 position and eighteen different 1,2,3-triazole moieties at C6. The compounds were synthesized via Sonogashira cross-coupling followed by copper-catalyzed azide–alkyne cycloaddition (CuAAC). All derivatives were assessed in both enzymatic and cellular assays, and selected compounds were further evaluated for metabolic stability, permeability, and kinase selectivity. Additionally, molecular modeling was employed to explore the binding modes of the inhibitors.
2. Results and Discussion
2.1. Designs of the Inhibitors
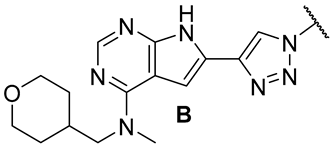
In our previous series of pyrrolopyrimidine-based CSF1R inhibitors, we identified two privileged substructures (Scaffold A (I) and Scaffold B (II), Figure 2A). An X-ray co-crystal structure of inhibitor III (Figure 2B) with CSF1R attributed its high activity to the hydrogen bonding of N1 and HN7 to Cys-666 in the hinge region, a water bridging interaction involving N3 and Thr-663, and lipophilic interactions for the N-methyl-N-(3-methylbenzyl) group at C4 with Leu-785 and Ala-800 (Figure 2B). It was assumed that the high selectivity was due to the placement of the meta-methyl group in a cavity in the front binding cleft [20]. Substructure II, which leads to even more potent inhibitors, is assumed to have more intimate contact with the protein alongside a hydrogen bond interaction from the tetrahydropyran (THP) oxygen to Arg-801 [22]. The benefit of inhibitors based on substructure II is higher solubility, though kinase selectivity seems to be somewhat reduced [22]. Aromatic groups at C6 also increase potency. Further, an N-methyl group at the 4-position is necessary to suppress epidermal growth factor receptor kinase’s (EGFR) inhibitory activity. When the aromatic structure at C6 was decorated with electron-withdrawing groups, a drop in activity was seen [22], possibly weakening cation-π interactions with Lys-586. Thus, we wanted to see how the replacement of carbo-aromatics with triazoles affected CSF1R inhibitory activity and molecular properties (Figure 2A).
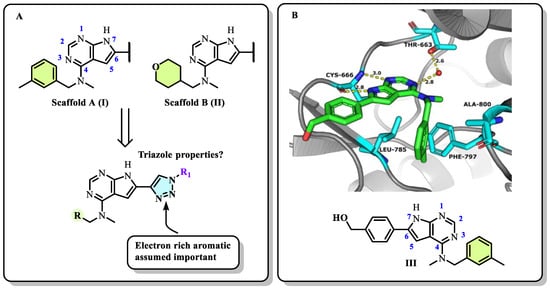
Figure 2.
(A) Design concept for the triazole-based CSF1R inhibitors. (B) X-ray co-crystal structure of CSF1R (PDB 8CGC). The inhibitor is shown in green and hydrogen bonds to the protein is shown with dashed yellow lines. The structure of inhibitor III is shown with numbering system.
2.2. Chemistry
The chemistry of these triazoles is shown in Scheme 1. First, the 2-(trimethylsilyl)ethoxymethyl (SEM)-protected building blocks 1a [20] and 1b [22] were reacted with ethynyltrimethylsilane at C6 in a Sonogashira cross-coupling reaction using Pd(PPh3)4 in combination with CuI in 87% and 73% isolated yields, respectively.
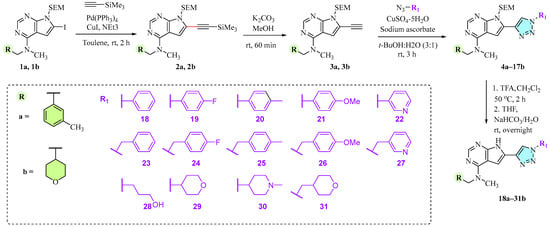
Scheme 1.
Synthesis of 1,2,3-triazole containing pyrrolo[2,3-d]pyrimidines 18a–31b.
Deprotection of the trimethylsilyl (TMS) group in 2a and 2b with K2CO3/MeOH produced the key intermediates 3a and 3b in 84% and 85% yields. These alkynes were then submitted to CuAAC with 14 different azides in t-BuOH/water (3:1) to give the triazoles 4a–17b in 40–86% yields. We also attempted CuAAC on the non-protected pyrrolopyrimidines using the same conditions. However, possibly due to Cu-coordination to the heterocycles, no product was formed. Finally, the SEM-protected triazoles 4a–17b were deprotected with trifluoroacetic acid (TFA) followed by treatment with aqueous NaHCO3 in THF to give 28 triazoles (18a–31b) in 62–98% isolated yields.
2.3. Enzymatic and Cellular Characterisation
The initial biological profiling (Table 1 and Table 2) was performed with enzymatic inhibition using a time-resolved Förster resonance energy transfer (TR-FRET) assay at an ATP concentration of 25 µM.

Table 1.
Enzymatic and Ba/F3 IC50 and solubility of aryl-substituted derivatives (A-series).

Table 2.
Enzymatic and Ba/F3 IC50 and solubility of THP-substituted derivatives (Series B).
This was followed by cellular profiling with CSF1R Ba/F3 cells and in an IL-3 dependent Ba/F3 cell line (CSF1R independent) to indicate cellular toxicity. Table 1 summarizes the calculated logP (cLogP), the calculated polar surface area (PSA), and the measure kinetic solubility, enzymatic IC50 values, and the Ba/F3 cellular IC50 for the series A of triazoles (18a–31a). The data for series B of triazoles are shown in Table 2.
Profiling of 18a–31a in enzymatic studies showed that except for the para-methyl analogue 20a, the compounds have lower IC50 than the reference drug PLX-3397. For triazoles having directly attached aryl groups (comp. 18a–22a), para-substitution lowers the enzymatic potency. The same trend is also seen in the benzylic series of triazoles (comp. 23a–27a), while compounds having groups with higher polarity (comp 28a–31a) were all extremely potent in the enzymatic assay. However, although clogP was below 4 for all compounds, they had limited kinetic solubility. The low solubility prevents the identification of meaningful cellular IC50 for derivatives 18a–21a and 23a–25a. Of the more soluble compounds, the 3-pyridyl analogue 27a appeared most promising with a cellular IC50 of 0.3 µM. None of the compounds showed any major effect in the IL-3-dependent Ba/F3 (CSF1R-independent) assay, which indicates low general cellular toxicity. On average, higher solubility and slightly more potent derivatives were seen for the THP-containing inhibitors (series B) than for series A. The enzymatic IC50 ranged from 1–9 nM. As in the A-series, para-substitution of the aryl group (comp. 19b–21b) lowers potency and, again, the para-methyl analogue (comp. 20b) was the least active. Triazoles substituted with benzylic type substituents (comp. 23b–27b) and polar substituents (28b–31b) were all highly active in the enzymatic assay. The cell activity of the series B triazoles correlates poorly with the enzymatic data. These studies indicated the para-methoxy 21b (IC50 = 0.6 µM) and the para-methylbenzyl 25b (IC50 = 1.0 µM) as most interesting for further evaluation. Both these compounds show higher cell potency than seen for their series A-analogues (Table 1, comp. 21a and 35a). No measurable cell activity was seen for 18b, 19b, and 29b, in line with that seen in series A. More surprisingly, the propanol-substituted 28b, in contrast to that seen for 28a (Table 1, IC50 = 1.0 µM), was also inactive.
Overall, 27 of the 28 derivatives exhibited lower enzymatic IC50 values than PLX-3397, but none achieved the same high level of cellular potency as the reference compound. While this can partly be attributed to the physicochemical properties of the inhibitors, differences in off-target profiles cannot be entirely ruled out. For instance, PLX-3397 has also been reported to inhibit KIT, CFK19, KDR, LCK, FLT1, and TRKC [37]. Another possible explanation involves the different activation forms or conformations adopted by CSF1R [38,39]. It is possible that PLX-3397 and the triazoles preferentially bind to different activation forms, with the form targeted by PLX-3397 being more prevalent in cellular environments. If this is the case, the truncated enzymatic model used in the assay may be a suboptimal mimic of the membrane-bound enzyme.
2.4. Initial ADME and Kinase Off-Targets
Assuming that the cellular Ba/F3 CSF1R assay is a better model of in vivo conditions than the enzymatic assay, the para-methoxyphenyl derivative 21b, the para-methylbenzyl 25b, and the 3-pyridylmethyl 27a were selected for further profiling (Table 3). The data are compared with two previously identified inhibitors III and IV [22], and PLX-3397. Firstly, the high enzymatic potency was confirmed by IC50 measurement using an alternative protocol (Z-LYTE) [38]. All compounds were also highly potent using this method (IC50 0.4–1.3 nM). A typical off-target for CSF1R inhibitors is the structurally related KIT kinase. KIT enzymatic inhibition (KD) was measured using the TR-FRET acceptor dye, kinase tracer 236, and an anti-GST antibody containing a TR-FRET donor dye, which binds to the GST tag at the N-terminus of KIT. The binding of an inhibitor to the kinase displaces the tracer resulting in a loss of FRET. Cellular proliferation for KIT activity was performed using the KIT-positive cell line GIST-T1 [39]. The enzymatic profiling compiled in Table 3 shows that the triazoles have low activity toward KIT (KD > 100 nM), in contrast to that of PLX-3397 (KD = 28 nM µM). The reference compounds III and IV also have low KIT activity, but KIT profiling in the case of III was performed by an alternative assay [22]. Cellular profiling in the KIT-positive cell line confirmed the data from the enzymatic assay. The 3-pyridyl analogue 27a was also profiled toward a panel of 49 kinases at a 1000 nM test concentration (Figure 3). The selection of kinases includes the structurally related kinases KIT, FLT3, and PDGFRB, some linked to cardiac toxicity [40], some previously identified off-targets for pyrrolopyrimidines [41,42], and common oncogenic targets [43]. In line with the KIT assay, lower activity was noted for the PDGFR family of kinases (FLT3, KIT, and PDGFRB). However, in contrast to the previously identified inhibitors with carbon-based aromatics at C6, 27a appears to be a multi-kinase inhibitor with especially high activity toward YES, ABL1, ABL2, SRC, and EPHA2 kinases. Calculation of the selectivity score (S-score) [43] using 50% and 30% inhibition as thresholds gave S-scores of 0.35 and 0.51, respectively. We had previously assumed that the meta-methyl at the N-methyl-N-(3-methylbenzyl) group at C4 was a major contributor to high kinase selectivity [20], but in light of the current investigation, this appears to be wrong. Pyrrolopyrimidines substituted with triazoles at C6 demonstrate potential as multi-kinase inhibitors rather than selective CSF1R inhibitors. Notably, the primary off-target kinases identified (ABL, SRC, YES, and EPHA2) are all of significant interest in oncology. ABL inhibitors are clinically established in the treatment of leukemia [44], while the inhibition of SRC and YES can mitigate the acquired resistance mechanism in cancer therapy [45,46,47]. Although no EPHA2 inhibitors have yet received regulatory approval, EPHA2 is recognized as a key regulator of tumorigenesis and disease progression across multiple cancer types [48].

Table 3.
Addition enzymatic, cellular, and ADME profiling of 21b, 25b, and 27a compared with that of structures III, IV, and PLX-3397.
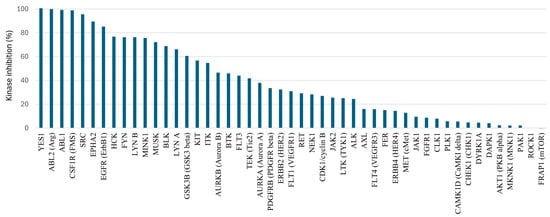
Figure 3.
Profiling of compound 27a toward a panel of kinases at 1000 nM using ATP level equal to KM.
To indicate the potential of 21b, 25b, and 27a for further development as multi-kinase inhibitors, a limited set of in vitro ADME assays was performed (see Table 3). Unfortunately, all three inhibitors had high metabolic clearance in human liver microsome (HML) and mouse liver microsome (MLM) assays. The triazoles were especially unstable in the MLM assay, with an intrinsic clearance of 288–1980 µL/min/mg compared to PLX-3397, with structures III and IV having a clearance in the range of 22–41 µL/min/mg. Permeability, as measured by the MDCK assay, was appropriate for 21b (Ratio Papp: 1.3), while for 25b and 27a, a higher rate in the B-A direction was observed, indicating active transport. The protein binding was mediocre for 21b (76%) and high for 25b (97.5%) and 27b (>99%). Overall, these assays did not motivate more advanced animal studies. Obviously, in this compound series, the metabolic soft spot must be identified, and appropriate measures must be taken to block degradation.
2.5. Molecular Modelling
To understand the binding interactions of the triazole series, compounds 21b, 25b, and 27a, structure III (Figure 2), and PLX-3397 were docked using Glide (Schrödinger Release 2025-1) [49] into two different X-ray structures of PDB 8CGC [20], originally obtained as a co-crystal with structure III (Figure 2), and PDB 4R7H [37] co-crystallized with PLX-3397. The docking scores are shown in Table 4. Structure III and PLX-3397 naturally docked with high docking scores toward their respective X-ray structures but with much lower affinity toward the other CSF1R X-ray structure. Based on this, we assumed that all triazoles would follow the pattern displayed by structure III and show better binding to 8CGC than to 4R7H. This was also the case for 21b and 25b.

Table 4.
Comparison of docking scores (Kcal/mol) for 21b, 25b, 27a, structure III, and PLX-3397, and docking toward two different CSF1R X-ray structures.
However, surprisingly, inhibitor 27a was found to bind almost equally well to both X-ray constructs. Using our previously obtained X-ray co-crystal structure (8CGC), 27a aligned well with the co-crystallized inhibitor I. In this pose, the N-methyl-N-(3-methylbenzyl) group at C-4 is directed inwards and the substituted triazole unit points toward the solvent-exposed region (Supplementary Materials, Figures S85 and S86). In docking with 4R7H, the PLX-3397-derived co-crystal, compound 27a occupies approximately the same space as PLX-3397. Figure 4A shows the docking pose of 27a superimposed by the X-ray pose of PLX-3397. In contrast to that seen when docking with 8CGC, the substituted triazole is now pointing inwards, and the N-methyl-N-(3-methylbenzyl) group at C-4 is directed toward the solvent-exposed area (see Figure 4B). In the alternative binding pose, compound 27a is predicted to engage in a water-mediated hydrogen bond between the pyridyl nitrogen and Asp-796, a residue within the DFG motif. Additionally, π–π stacking interactions are observed between both the triazole and pyridine moieties of 27a and the aromatic side chain of Trp-550. The nitrogen atoms N1 and N7 are positioned near the positively charged Arg-548, suggesting potential electrostatic interactions. Furthermore, the N-methyl-N-(3-methylbenzyl) substituent at the C4 position participates in hydrophobic interactions with the surrounding nonpolar residues.
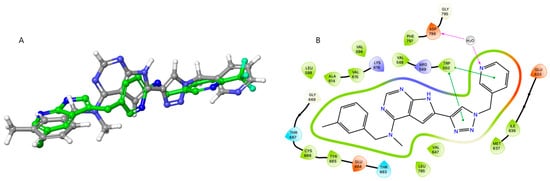
Figure 4.
(A) Superposition of docked 27a on CSF1R-PLX-3397 cocrystal structure 4R7H. (B) Suggested binding pose of 27a using crystal structure 4R7H.
In follow-up simulations, we performed docking of 27a toward 8CGC and 4R7H with an induced fit protocol using Glide. The experiment with 8CGC identified 14 similar poses corresponding to that seen in Figure 2. Induced-fit docking with the construct 4R7H resulted in 7 “inverted” poses, as seen in Figure 4, with small variations in the ligand position and docking score (−13.195 to −12.310 Kcal/mol). This is despite a relatively large variation in the position of Phe-797 in the DFG motif (see Supplementary Materials, Figure S90). Additionally, one pose with apparent low binding (docking score: −7.807 Kcal/mol) was found to correspond to the binding mode shown in Figure 2. We cannot firmly conclude the validity of these two different poses. However, it is known that CSF1R is a dynamic protein existing in different conformations [50,51], and it is likely that CSF1R structure 8CGC [20] is a representative model of the autoinhibited form of the kinase, while 4R7H might be a better model for the non-autoinhibited form, in line with previous in vitro enzymatic assays [20]. In living cells, there will be an equilibrium of activation states, and 27a is possibly able to inhibit several of these states.
3. Materials and Methods
3.1. Chemicals and Analysis
The solvents were purchased from Merck and VWR. The pyrrolopyrimidine intermediates 1a and 1b were made as previously described [20,22]. Ethynyltrimethylsilane, azidobenzene, 1-azido-4-fluorobenzene, 1-azido-4-methylbenzene, 1-azido-4-methoxybenzene, and benzyl azide were from Merck (Sigma-Aldrich), while the rest of the azides were from SynthonX. Nuclear magnetic resonance spectra were obtained with a Bruker Avance III HD 400 or 600 MHz instrument with DMSO-d6 as the solvent. High-resolution mass spectroscopy was performed on a Synapt G2-Q-TOF instrument from Waters (Milford, MA, USA) in positive mode using an electrospray (ES) probe. Processing was performed using Waters Software Masslynx V4.1 SCN871. The HPLC purity of the inhibitor candidates was determined with two methods: Method A [22]: Waters Acquity UPLC system, a Waters Acquity BEH C18 (50 × 2.1 mm, 1.7 μm) column, flow rate: 0.5 mL/min, column temperature: 60 °C; gradient elution with MeCN/H2O was performed as follows: 5% MeCN for 0.5 min, then a linear gradient up to 95% MeCN over 7.5 min, and finally 95% MeCN for 1.5 min; Method B: Agilent 1200 series instrument with an Agilent ObenLab ChemStation software version C.01.07(27). Column: Kinetex® 5 µM C18 100 Å, 150 × 4.6 mm, eluent: H2O/CH3CN (10:90) to 100% CH3CN over 15 min, 5 min hold time at 100% CH3CN. Molecular modeling was performed as previously described [22].
3.2. General Procedures
3.2.1. General Procedure A: Synthesis of Triazole Intermediates
To a round-bottom flask containing t-BuOH/H2O (3:1 by vol, 8 mL), the ethynyl compound (3a or 3b, 0.25 mmol), azide (1.3 equiv.), CuSO4 pentahydrate (0.1 equiv.), and sodium ascorbate (0.2 equiv.) were added. The reaction mixture was stirred at rt overnight. Upon reaction completion, the solvent was removed by evaporation. The residue was diluted with water and the mixture was extracted with CH2Cl2. The combined organic layers were washed with brine, dried over Na2SO4, and concentrated. The crude product was purified by silica-gel column chromatography to achieve the desired products as stated for each specific compound.
3.2.2. General Procedure B: SEM-Deprotection
To the SEM-protected triazole intermediate (4a–17b; 60–120 mg, 1 equiv.), CH2Cl2 (10 mL) TFA (2 mL) were added and stirred at 50 °C for 2 h. The reaction mixture was then concentrated in vacuo before it was taken up in THF (10 mL) and NaHCO3 (20 mL, 25% aqueous) and stirred at rt overnight. For most of the compounds, the solvent was removed by evaporation, resulting in a precipitate. The precipitate was isolated by filtration and washed with water (10 mL) and n-pentane (2 × 10 mL). Some compounds, as specified, were purified by silica-gel column chromatography.
3.3. Inhibitor Candidates
3.3.1. N-Methyl-N-(3-methylbenzyl)-6-(1-phenyl-1H-1,2,3-triazol-4-yl)-7H-pyrrolo[2,3-d]pyrimidin-4-amine (18a)
The reaction was run as described in General Procedure B using 4a (60 mg, 0.11 mmol). This gave 35 mg (0.08 mmol, 78%) of a white powder, mp. 292–294 °C; HPLC method A: 99%; 1H NMR (400 MHz, DMSO-d6) δ: 12.37 (s, 1H), 9.03 (s, 1H), 8.18 (s, 1H), 7.89 (d, J = 7.3 Hz, 2H), 7.64 (t, J = 7.9 Hz, 2H), 7.55–7.51 (m, 1H), 7.22 (t, J = 7.5 Hz, 1H), 7.11–7.02 (m, 4H), 5.02 (s, 2H), 3.35 (s, 3H), 2.27 (s, 3H); 13C NMR (101 MHz, DMSO-d6) δ: 156.6, 152.5, 151.4, 141.0, 138.2, 137.6, 136.4, 130.0 (2C), 128.9, 128.4, 127.6, 127.5, 124.5, 124.0, 120.1 (2C), 119.1, 102.7, 99.2, 52.6, 37.1, 21.0; HRMS (ES+, m/z): found 396.1939, calcd for C23H22N7, [M+H]+, 396.1937.
3.3.2. N-Methyl-6-(1-phenyl-1H-1,2,3-triazol-4-yl)-N-((tetrahydro-2H-pyran-4-yl)methyl)-7H-pyrrolo[2,3-d]pyrimidin-4-amine (18b)
The reaction was run as described in General Procedure B using 4b (80 mg, 0.15 mmol). This gave 50 mg (0.12 mmol, 85%) of a white powder, mp. 294–296 °C; HPLC method A: 97%; 1H NMR (600 MHz, DMSO-d6) δ: 12.30 (s, 1H), 9.04 (s, 1H), 8.14 (s, 1H), 7.91 (d, J = 7.4 Hz, 2H), 7.67–7.64 (m, 2H), 7.54 (t, J = 7.4 Hz, 1H), 7.06 (s, 1H), 3.84 (dd, J = 11.5, 2.6 Hz, 2H), 3.69 (d, J = 7.4 Hz, 2H), 3.41 (s, 3H), 3.26 (td, J = 11.7, 2.1 Hz, 2H), 2.10–2.06 (m, 1H), 1.57–1.54 (m, 2H), 1.34–1.27 (m, 2H); 13C NMR (151 MHz, DMSO-d6) δ: 156.4, 152.4, 151.3, 141.1, 136.4, 130.0 (2C), 128.9, 124.2, 120.1 (2C), 119.1, 102.7, 99.4, 66.7 (2C), 55.4, 38.9, 33.9, 30.3 (2C), 25.1; HRMS (ES+, m/z): found 390.2042, calcd for C21H24N7O, [M+H]+, 390.2042.
3.3.3. 6-(1-(4-Fluorophenyl)-1H-1,2,3-triazol-4-yl)-N-methyl-N-(3-methylbenzyl)-7H-pyrrolo[2,3-d]pyrimidin-4-amine (19a)
The reaction was run as described in General Procedure B using 5a (110 mg, 0.20 mmol). This gave 67 mg (0.16 mmol, 81%) of a white powder, mp. 285–287 °C; HPLC method A: 98%; 1H NMR (600 MHz, DMSO-d6) δ: 12.36 (s, 1H), 8.99 (s, 1H), 8.18 (s, 1H), 7.95–7.93 (m, 2H), 7.50 (t, J = 8.7 Hz, 2H), 7.22 (t, J = 7.6 Hz, 1H), 7.10 (s, 1H), 7.07 (d, J = 7.4 Hz, 2H), 7.01 (s, 1H), 5.01 (s, 2H), 3.35 (s, 3H), 2.27 (s, 3H); 13C NMR (151 MHz, DMSO-d6) δ: 161.7 (d, J = 246.1 Hz), 156.6, 152.5, 151.4, 141.0, 138.2, 137.6, 133.0 (d, J = 2.8 Hz), 128.4, 127.6, 127.5, 124.4, 124.0, 122.60 (d, J = 8.8 Hz, 2C), 119.5, 116.9 (d, J = 23.3 Hz, 2C), 102.7, 99.3, 52.6, 37.1, 21.0.; HRMS (ES+, m/z): found 414.1845, calcd for C23H21FN7, [M+H]+, 414.1842.
3.3.4. 6-(1-(4-Fluorophenyl)-1H-1,2,3-triazol-4-yl)-N-methyl-N-((tetrahydro-2H-pyran-4-yl)methyl)-7H-pyrrolo[2,3-d]pyrimidin-4-amine (19b)
The reaction was run as described in General Procedure B using 5b (115 mg, 0.21 mmol). This gave 76 mg (0.18 mmol, 87%) of a white powder, mp. >300 °C; HPLC method A: 99%; 1H NMR (600 MHz, DMSO-d6) δ: 12.31 (s, 1H), 9.01 (s, 1H), 8.14 (s, 1H), 7.96–7.94 (m, 2H), 7.51 (t, J = 8.8 Hz, 2H), 7.05 (s, 1H), 3.84 (dd, J = 11.5, 2.6 Hz, 2H), 3.69 (d, J = 7.4 Hz, 2H), 3.40 (s, 3H), 3.25 (td, J = 11.7, 2.1 Hz, 2H), 2.10–2.05 (m, 1H), 1.57–1.54 (m, 2H), 1.34–1.27 (m, 2H); 13C NMR (151 MHz, DMSO-d6) δ: 161.7 (d, J = 246.0 Hz), 156.4, 152.4, 151.3, 141.1, 133.0 (d, J = 3.0 Hz), 124.1, 122.6 (d, J = 8.8 Hz, 2C), 119.4, 116.9 (d, J = 23.4 Hz, 2C), 102.7, 99.4, 66.7 (2C), 55.4, 38.8, 33.9, 30.3 (2C); HRMS (ES+, m/z): found 408.1947, calcd for C21H23FN7O, [M+H]+, 408.1948.
3.3.5. N-Methyl-N-(3-methylbenzyl)-6-(1-(p-tolyl)-1H-1,2,3-triazol-4-yl)-7H-pyrrolo[2,3-d]pyrimidin-4-amine (20a)
The reaction was run as described in General Procedure B using 6a (76 mg, 0.14 mmol). This gave 38 mg (0.09 mmol, 67%) of a white solid, mp. 250–252 °C; HPLC method A: 99%; 1H NMR (400 MHz, DMSO-d6) δ: 12.32 (s, 1H), 8.96 (s, 1H), 8.17 (s, 1H), 7.76 (d, J = 8.5 Hz, 2H), 7.43 (d, J = 8.2 Hz, 2H), 7.22 (t, J = 7.5 Hz, 1H), 7.11–7.06 (m, 3H), 7.00 (s, 1H), 5.01 (s, 2H), 3.35 (s, 3H), 2.40 (s, 3H), 2.27 (3H); 13C NMR (101 MHz, DMSO-d6) δ: 156.5, 152.8, 151.2, 141.3, 138.5, 138.3, 137.6, 134.2, 130.3 (2C), 128.4, 127.6, 127.5, 125.1, 124.0, 120.0 (2C), 119.0, 102.8, 98.9, 52.6, 37.1, 21.0, 20.5; HRMS (ES+, m/z): found 410.2097, calcd for C24H24N7, [M+H]+, 410.2093.
3.3.6. N-Methyl-N-((tetrahydro-2H-pyran-4-yl)methyl)-6-(1-(p-tolyl)-1H-1,2,3-triazol-4-yl)-7H-pyrrolo[2,3-d]pyrimidin-4-amine (20b)
The reaction was run as described in General Procedure B using 6b (80 mg, 0.15 mmol). This gave 40 mg (0.09 mmol, 67%) of a white powder, mp. > 300 °C; HPLC method A: 99%; 1H NMR (400 MHz, DMSO-d6) δ: 12.27 (s, 1H), 8.99 (s, 1H), 8.12 (s, 1H), 7.78 (d, J = 8.3 Hz, 2H), 7.45 (d, J = 8.1 Hz, 2H), 7.04 (s, 1H), 3.86–3.83 (m, 2H), 3.69 (d, J = 7.4 Hz, 2H), 3.40 (s, 3H), 3.28–3.23 (m, 2H), 2.41 (s, 3H), 2.08 (m, 1H), 1.56 (d, J = 12.9 Hz, 2H), 1.35–1.25 (m, 2H); 13C NMR (101 MHz, DMSO-d6) δ: 156.4, 152.6, 151.2, 141.2, 138.5, 134.2, 130.3 (2C), 124.6, 120.0 (2C), 119.0, 102.8, 99.1, 66.7 (2C), 55.4, 38.8, 33.9, 30.3 (2C), 20.6; HRMS (ES+, m/z): found 404.2197, calcd for C22H26N7O, [M+H]+, 404.2199.
3.3.7. 6-(1-(4-Methoxyphenyl)-1H-1,2,3-triazol-4-yl)-N-methyl-N-(3-methylbenzyl)-7H-pyrrolo[2,3-d]pyrimidin-4-amine (21a)
The reaction was run as described in General Procedure B using 7a (60 mg, 0.11 mmol). This gave 32 mg (0.074 mmol, 69%), mp. 248–250 °C; HPLC method A: 99%; 1H NMR (600 MHz, DMSO-d6) δ: 12.32 (s, 1H), 8.91 (s, 1H), 8.18 (s, 1H), 7.79 (d, J = 9.0 Hz, 2H), 7.22 (t, J = 7.6 Hz, 1H), 7.18 (d, J = 9.0 Hz, 2H), 7.10 (s, 1H), 7.07 (d, J = 7.6 Hz, 2H), 7.00 (s, 1H), 5.01 (s, 2H), 3.84 (s, 3H), 3.35 (s, 3H), 2.28 (s, 3H); 13C NMR (151 MHz, DMSO-d6) δ: 159.4, 156.5, 152.5, 151.3, 140.8, 138.2, 137.6, 129.8, 128.4, 127.6, 127.5, 124.6, 124.0, 121.8 (2C), 119.2, 115.0 (2C), 102.7, 99.0, 55.6, 52.6, 37.1, 21.0; HRMS (ES+, m/z): found 426.2040, calcd for C24H24N7O, [M+H]+, 426.2042.
3.3.8. 6-(1-(4-Methoxyphenyl)-1H-1,2,3-triazol-4-yl)-N-methyl-N-((tetrahydro-2H-pyran-4-yl)methyl)-7H-pyrrolo[2,3-d]pyrimidin-4-amine (21b)
The reaction was run as described in General Procedure B using 7b (65 mg, 0.16 mmol). This gave 40 mg (0.09 mmol, 81%) of a white powder, mp. > 300 °C; HPLC method A: 97%; HPLC method B: 99%; 1H NMR (600 MHz, DMSO-d6) δ: 12.28 (s, 1H), 8.93 (s, 1H), 8.14 (s, 1H), 7.81 (d, J = 9.0 Hz, 2H), 7.18 (d, J = 8.9 Hz, 2H), 7.04 (s, 1H), 3.85 (s, 3H), 3.84–3.83 (m, 2H), 3.69 (d, J = 7.4 Hz, 2H), 3.40 (s, 3H), 3.25 (td, J = 11.7, 2.0 Hz, 2H), 2.10–2.05 (m, 1H), 1.56–1.54 (m, 2H), 1.31–1.29 (m, 2H); 13C NMR (151 MHz, DMSO-d6) δ: 159.4, 156.4, 152.3, 151.2, 140.9, 129.8, 124.3, 121.8 (2C), 119.2, 115.0 (2C), 102.7, 99.2, 66.7 (2C), 55.6 (2C), 38.8, 33.9, 30.3 (2C); HRMS (ES+, m/z): found 420.2150, calcd for C22H26N7O2, [M+H]+, 420.2148.
3.3.9. N-Methyl-N-(3-methylbenzyl)-6-(1-(pyridin-3-yl)-1H-1,2,3-triazol-4-yl)-7H-pyrrolo[2,3-d]pyrimidin-4-amine (22a)
The reaction was run as described in General Procedure B using 8a (110 mg, 0.21 mmol). This gave 70 mg (0.17 mmol, 85%) of a white powder, mp. 275–277 °C; HPLC method A: 95%; 1H NMR (600 MHz, DMSO-d6) δ: 12.41 (s, 1H), 9.14 (d, J = 2.6 Hz, 1H), 9.10 (s, 1H), 8.73 (dd, J = 4.8, 1.4 Hz, 1H), 8.34–8.32 (m, 1H), 8.19 (s, 1H), 7.71–7.68 (s, 1H), 7.22 (t, J = 7.6 Hz, 1H), 7.11–7.03 (m, 4H), 5.01 (s, 2H), 3.35 (s, 3H), 2.27 (s, 3H); 13C NMR (151 MHz, DMSO-d6) δ: 156.6, 152.5, 151.5, 149.9, 141.3, 141.2, 138.2, 137.6, 133.1, 128.5, 128.1, 127.6, 127.5, 124.7, 124.2, 124.0, 119.5, 102.7, 99.5, 52.6, 37.2, 21.0; HRMS (ES+, m/z): found 397.1888, calcd for C22H21N8, [M+H]+, 397.1889.
3.3.10. N-Methyl-6-(1-(pyridin-3-yl)-1H-1,2,3-triazol-4-yl)-N-((tetrahydro-2H-pyran-4-yl)methyl)-7H-pyrrolo[2,3-d]pyrimidin-4-amine (22b)
The reaction was run as described in General Procedure B using 8b (100 mg, 0.22 mmol). This gave 60 mg (0.15 mmol, 81%) of a white powder, mp. > 300 °C; HPLC method A: 96%; 1H NMR (600 MHz, DMSO-d6) δ: 12.36 (s, 1H), 9.15 (d, J = 2.7 Hz, 1H), 9.12 (s, 1H), 8.74 (dd, J = 4.8, 1.4 Hz, 1H), 8.36–8.33 (m, 1H), 8.14 (s, 1H), 7.72–7.70 (m, 1H), 7.06 (s, 1H), 3.84 (dd, J = 11.5, 2.6 Hz, 2H), 3.69 (d, J = 7.4 Hz, 2H), 3.41 (s, 3H), 3.26 (td, J = 11.7, 2.1 Hz, 2H), 2.10–2.06 (m, 1H), 1.57–1.54 (m, 2H), 1.34–1.27 (m, 2H); 13C NMR (151 MHz, DMSO-d6) δ: 156.5, 152.4, 151.4, 149.9, 141.4, 141.3, 133.1, 128.1, 124.7, 123.9, 119.5, 102.7, 99.6, 66.7 (2C), 55.4, 38.9, 33.9, 30.3 (2C); HRMS (ES+, m/z): found 391.1995, calcd for C20H23N8O, [M+H]+, 391.1995.
3.3.11. 6-(1-Benzyl-1H-1,2,3-triazol-4-yl)-N-methyl-N-(3-methylbenzyl)-7H-pyrrolo[2,3-d]pyrimidin-4-amine (23a)
The reaction was run as described in General Procedure B using 9a (75 mg, 0.14 mmol). This gave 51 mg (0.12 mmol, 91%) of a white solid; mp. 288–290 °C; HPLC method A: 99%; 1H NMR (400 MHz, DMSO-d6) δ: 12.20 (s, 1H), 8.40 (s, 1H), 8.14 (s, 1H), 7.42–7.33 (m, 5H), 7.21 (t, J = 7.5 Hz, 1H), 7.08–7.03 (m, 3H), 6.92 (s, 1H), 5.67 (s, 2H), 4.99 (s, 2H), 3.32 (s, 3H), 2.26 (s, 3H); 13C NMR (101 MHz, DMSO-d6) δ: 156.5, 152.4, 151.1, 140.4, 138.2, 137.6, 135.8, 128.8 (2C), 128.4, 128.2, 128.0 (2C), 127.6, 127.5, 125.1, 124.0, 121.0, 102.6, 98.5, 52.9, 52.6, 37.1, 21.0; HRMS (ES+, m/z): found 410.2093, calcd for C24H24N7, [M+H]+, 410.2093.
3.3.12. 6-(1-Benzyl-1H-1,2,3-triazol-4-yl)-N-methyl-N-((tetrahydro-2H-pyran-4-yl)methyl)-7H-pyrrolo[2,3-d]pyrimidin-4-amine (23b)
The reaction was run as described in General Procedure B using 9b (70 mg, 0.13 mmol). This gave 38 mg (0.09 mmol, 78%) of a white solid, mp. 298–300 °C; HPLC method A: 99%; 1H NMR (400 MHz, DMSO-d6) δ: 12.24 (s, 1H), 8.41 (s, 1H), 8.09 (s, 1H), 7.43–7.34 (m, 5H), 6.97 (s, 1H), 5.69 (s, 2H), 3.83 (dd, J = 11.8, 3.5 Hz, 2H), 3.66 (d, J = 7.4 Hz, 2H), 3.37 (s, 3H), 3.24 (td, J = 11.7, 2.1 Hz, 2H), 2.08–2.02 (m, 1H), 1.55–1.51 (m, 2H), 1.33–1.23 (m, 2H); 13C NMR (101 MHz, DMSO-d6) δ: 156.4, 152.2, 151.1, 140.4, 135.8, 128.8 (2C), 128.2, 128.0 (2C), 124.7, 120.9, 102.6, 98.7, 66.7 (2C), 55.3, 52.9, 33.9, 30.2 (2C); The N-Me group is within the DMSO-d5 signal; HRMS (ES+, m/z): found 404.2196, calcd for C22H26N7O, [M+H]+, 404.2199.
3.3.13. 6-(1-(4-Fluorobenzyl)-1H-1,2,3-triazol-4-yl)-N-methyl-N-(3-methylbenzyl)-7H-pyrrolo[2,3-d]pyrimidin-4-amine (24a)
The reaction was run as described in General Procedure B using 10a (110 mg, 0.20 mmol). This gave 72 mg (0.16 mmol, 86%), mp. 274–276 °C; HPLC method A: 99%; 1H NMR (600 MHz, DMSO-d6) δ: 12.21 (s, 1H), 8.37 (s, 1H), 8.14 (s, 1H), 7.44–7.41 (m, 2H), 7.25–7.19 (m, 3H), 7.08–7.04 (m, 3H), 6.92 (s, 1H), 5.67 (s, 2H), 4.99 (s, 2H), 3.32 (s, 3H), 2.26 (s, 3H); 13C NMR (151 MHz, DMSO-d6) δ: 161.9 (d, J = 244.5 Hz), 156.5, 152.4, 151.2, 140.3, 138.2, 137.6, 132.0 (d, J = 3.0 Hz), 130.4 (d, J = 8.4 Hz, 2C), 128.4, 127.6, 127.5, 125.0, 124.0, 120.9, 115.7 (d, J = 21.7 Hz, 2C), 102.6, 98.6, 52.6, 52.1, 37.1, 21.0; HRMS (ES+, m/z): found 428.2002, calcd for C24H23FN7, [M+H]+, 428.1999.
3.3.14. 6-(1-(4-Fluorobenzyl)-1H-1,2,3-triazol-4-yl)-N-methyl-N-((tetrahydro-2H-pyran-4-yl)methyl)-7H-pyrrolo[2,3-d]pyrimidin-4-amine (24b)
The reaction was run as described in General Procedure B using 11b (120 mg, 0.22 mmol). This gave 76 mg (0.18 mmol, 83%) of a white powder, mp. 281–283 °C; HPLC method A: 98%; 1H NMR (600 MHz, DMSO-d6) δ: 12.15 (s, 1H), 8.40 (s, 1H), 8.10 (s, 1H), 7.45–7.43 (m, 2H), 7.25 (t, J = 8.9 Hz, 2H), 6.97 (s, 1H), 5.68 (s, 2H), 3.83 (dd, J = 11.5, 2.6 Hz, 2H), 3.66 (d, J = 7.4 Hz, 2H), 3.37 (s, 3H), 3.24 (td, J = 11.7, 2.1 Hz, 2H), 2.07–2.03 (m, 1H), 1.54–1.52 (m, 2H), 1.32–1.25 (m, 2H); 13C NMR (151 MHz, DMSO-d6) δ: 161.9 (d, J = 244.4 Hz), 156.4, 152.2, 151.1, 140.4, 132.0 (d, J = 3.2 Hz), 130.4 (d, J = 8.5 Hz, 2C), 124.7, 120.9, 115.7 (d, J = 21.6 Hz, 2C), 102.7, 98.7, 66.7 (2C), 55.3, 52.1, 38.8, 33.9, 30.2 (2C); HRMS (ES+, m/z): found 422.2102, calcd for C22H25FN7O, [M+H]+, 422.2105.
3.3.15. N-Methyl-N-(3-methylbenzyl)-6-(1-(4-methylbenzyl)-1H-1,2,3-triazol-4-yl)-7H-pyrrolo[2,3-d]pyrimidin-4-amine (25a)
The reaction was run as described in General Procedure B using 11a (120 mg, 0.22 mmol). This gave 90 mg (0.21 mmol, 99%) of a white powder, mp. 253–255 °C; HPLC method A: 98%; 1H NMR (600 MHz, DMSO-d6) δ: 12.15 (s, 1H), 8.31 (s, 1H), 8.10 (s, 1H), 7.21 (d, J = 8.1 Hz, 2H), 7.18–7.16 (m, 3H), 7.04 (s, 1H), 7.03–7.00 (m, 2H), 6.88 (s, 1H), 5.57 (s, 2H), 4.95 (s, 2H), 3.28 (s, 3H), 2.25 (s, 3H), 2.23 (s, 3H); 13C NMR (151 MHz, DMSO-d6) δ: 156.5, 152.4, 151.1, 140.3, 138.2, 137.6, 132.7, 129.3 (3C), 128.4, 128.1 (32), 127.6, 127.5, 125.1, 124.0, 120.8, 102.6, 98.5, 52.8, 52.6, 37.1, 21.0, 20.7; HRMS (ES+, m/z): found 424.2249, calcd for C25H26N7, [M+H]+, 424.2250.
3.3.16. N-Methyl-6-(1-(4-methylbenzyl)-1H-1,2,3-triazol-4-yl)-N-((tetrahydro-2H-pyran-4-yl)methyl)-7H-pyrrolo[2,3-d]pyrimidin-4-amine (25b)
The reaction was run as described in General Procedure B using 11b (105 mg, 0.19 mmol). This gave 67 mg (0.16 mmol, 84%) of a white powder, mp. 279–281 °C; HPLC method A: 98%; HPLC method B > 99%; 1H NMR (600 MHz, DMSO-d6) δ: 12.13 (s, 1H), 8.37 (s, 1H), 8.09 (s, 1H), 7.26 (d, J = 8.1 Hz, 2H), 7.21 (d, J = 7.7 Hz, 2H), 6.96 (s, 1H), 5.62 (s, 2H), 3.83 (dd, J = 11.5, 2.6 Hz, 2H), 3.66 (d, J = 7.4 Hz, 2H), 3.37 (s, 3H), 3.24 (td, J = 11.7, 2.1 Hz, 2H), 2.29 (s, 3H), 2.07–2.03 (m, 1H), 1.54–1.52 (m, 2H), 1.32–1.25 (m, 2H); 13C NMR (151 MHz, DMSO-d6) δ: 156.4, 152.2, 151.1, 140.4, 137.6, 132.7, 129.3 (2C), 128.1 (2C), 124.8, 120.8, 102.7, 98.6, 66.7 (2C), 55.3, 52.8, 38.8, 33.9, 30.2 (2C), 20.7. HRMS (ES+, m/z): found 418.2351, calcd for C23H28N7O, [M+H]+, 418.2355.
3.3.17. 6-(1-(4-Methoxybenzyl)-1H-1,2,3-triazol-4-yl)-N-methyl-N-(3-methylbenzyl)-7H-pyrrolo[2,3-d]pyrimidin-4-amine (26a)
The reaction was run as described in General Procedure B using 12a (132 mg, 0.23 mmol). This gave 70 mg (0.15 mmol, 69%) of a white solid, mp. 240–242 °C; HPLC method A: 82%; 1H NMR (600 MHz, DMSO-d6) δ: 12.18 (s, 1H), 8.33 (s, 1H), 8.14 (s, 1H), 7.33 (d, J = 8.6 Hz, 2H), 7.21 (t, J = 7.6 Hz, 1H), 7.08–7.03 (m, 3H), 6.96 (d, J = 8.6 Hz, 2H), 6.91 (s, 1H), 5.58 (s, 2H), 4.98 (s, 2H), 3.74 (s, 3H), 3.31 (s, 3H), 2.26 (s, 3H); 13C NMR (151 MHz, DMSO-d6) δ: 159.2, 156.5, 152.3, 151.2, 140.2, 138.2, 137.6, 129.7 (3C), 128.4, 127.6, 127.5, 125.1, 124.0, 120.7, 114.2 (2C), 102.6, 98.5, 55.1, 52.6, 52.5, 37.1, 21.0; HRMS (ES+, m/z): found 440.2197, calcd for C25H26N7O, [M+H]+, 440.2199.
3.3.18. 6-(1-(4-Methoxybenzyl)-1H-1,2,3-triazol-4-yl)-N-methyl-N-((tetrahydro-2H-pyran-4-yl)methyl)-7H-pyrrolo[2,3-d]pyrimidin-4-amine (26b)
The reaction was run as described in General Procedure B using 12b (132 mg, 0.23 mmol). This gave 89 mg (0.20 mmol, 88%) of a white solid, mp. 279–281 °C; HPLC method A: 94%; 1H NMR (600 MHz, DMSO-d6) δ: 12.13 (s, 1H), 8.35 (s, 1H), 8.09 (s, 1H), 7.34 (d, J = 8.7 Hz, 2H), 6.97–6.96 (m, 3H), 5.59 (s, 2H), 3.83 (dd, J = 11.4, 2.7 Hz, 2H), 3.75 (s, 3H), 3.66 (d, J = 7.4 Hz, 2H), 3.36 (s, 3H), 3.24 (td, J = 11.7, 2.0 Hz, 2H), 2.07–2.03 (m, 1H), 1.54–1.51 (m, 2H), 1.31–1.25 (m, 2H); 13C NMR (151 MHz, DMSO-d6) δ: 159.2, 156.4, 152.2, 151.1, 140.3, 129.7 (2C), 127.6, 124.8, 120.6, 114.2 (2C), 102.7, 98.6, 66.7 (2C), 55.1 (2C), 52.5, 38.8, 33.9, 30.2 (2C); HRMS (ES+, m/z): found 434.2310, calcd for C23H28N7O2, [M+H]+, 434.2304.
3.3.19. N-Methyl-N-(3-methylbenzyl)-6-(1-(pyridin-3-ylmethyl)-1H-1,2,3-triazol-4-yl)-7H-pyrrolo[2,3-d]pyrimidin-4-amine (27a)
The reaction was run as described in General Procedure B using 13a (114 mg, 0.21 mmol). This gave 60 mg (0.14 mmol, 70%) of a white solid, mp. 273–275 °C; HPLC method A: 98%; HPLC method B > 98%; 1H NMR (400 MHz, DMSO-d6) δ: 12.20 (s, 1H), 8.65 (s, 1H), 8.57 (d, J = 4.8 Hz, 1H), 8.43 (s, 1H), 8.14 (s, 1H), 7.76 (d, J = 7.9 Hz, 1H), 7.45–7.41 (m, 1H), 7.20 (t, J = 7.5 Hz, 1H), 7.08–7.03 (m, 3H), 6.91 (s, 1H), 5.74 (s, 2H), 4.99 (s, 2H), 3.31 (s, 3H), 2.26 (s, 3H); 13C NMR (101 MHz, DMSO-d6) δ: 156.5, 152.5, 151.1, 149.5, 149.3, 140.5, 138.2, 137.6, 135.9, 131.5, 128.4, 127.6, 127.4, 125.1, 124.0, 123.9, 121.1, 102.7, 98.6, 52.6, 50.5, 37.1, 25.1, 21.0; HRMS (ES+, m/z): found 411.2049, calcd for C23H23N8, [M+H]+, 411.2046.
3.3.20. N-Methyl-6-(1-(pyridin-3-ylmethyl)-1H-1,2,3-triazol-4-yl)-N-((tetrahydro-2H-pyran-4-yl)methyl)-7H-pyrrolo[2,3-d]pyrimidin-4-amine (27b)
The reaction was run as described in General Procedure B using 13b (100 mg, 0.19 mmol). This gave 60 mg (0.14 mmol, 80%) of a white powder, mp. 293–295 °C; HPLC method A: 98%; 1H NMR (400 MHz, DMSO-d6) δ: 12.46 (s, 1H), 8.66 (d, J = 2.3 Hz, 1H), 8.58 (dd, J = 4.8, 1.7 Hz, 1H), 8.45 (s, 1H), 8.10 (s, 1H), 7.79–7.76 (m, 1H), 7.46–7.42 (m, 1H), 6.97 (s, 1H), 5.76 (s, 2H), 3.83 (dd, J = 11.6, 3.1 Hz, 2H), 3.66 (d, J = 7.4 Hz, 2H), 3.37 (s, 3H), 3.24 (td, J = 11.7, 2.1 Hz, 2H), 2.08–2.02 (m, 1H), 1.55–1.52 (m, 2H), 1.33–1.23 (m, 2H); 13C NMR (101 MHz, DMSO-d6) δ: 156.4, 152.2, 151.1, 149.5, 149.3, 140.5, 135.9, 131.5, 124.6, 123.9, 121.0, 102.6, 98.8, 66.7 (2C), 55.3, 50.5, 38.8, 33.9, 30.2 (2C); HRMS (ES+, m/z): found 405.2157, calcd for C21H25N8O, [M+H]+, 405.2151.
3.3.21. 3-(4-(4-(Methyl(3-methylbenzyl)amino)-7H-pyrrolo[2,3-d]pyrimidin-6-yl)-1H-1,2,3-triazol-1-yl)propan-1-ol (28a)
The reaction was run as described in General Procedure B using 14a (100 mg, 0.20 mmol). The solvent was removed by evaporation, and the remaining residue was added to water (10 mL) and extracted with CH2Cl2 (4 × 10 mL). Purification by silica-gel flash column chromatography (CH2Cl2/MeOH 9:1, Rf = 0.38) gave 47 mg (0.12 mmol, 64%) of a white powder, mp. 250–252 °C; HPLC method A: 99%; 1H NMR (600 MHz, DMSO-d6) δ: 12.23 (s, 1H), 8.36 (s, 1H), 8.15 (s, 1H), 7.21 (t, J = 7.6 Hz, 1H), 7.09 (s, 1H), 7.06 (t, J = 7.6 Hz, 2H), 6.90 (s, 1H), 4.99 (s, 2H), 4.69 (t, J = 5.0 Hz, 1H), 4.47 (t, J = 7.1 Hz, 2H), 3.43 (q, J = 6.1 Hz, 2H), 3.32 (s, 3H), 2.27 (s, 3H), 1.99 (p, J = 6.5 Hz, 2H); 13C NMR (151 MHz, DMSO-d6) δ: 156.5, 152.3, 151.1, 139.9, 138.2, 137.6, 128.4, 127.6, 127.5, 125.2, 124.0, 121.0, 102.6, 98.4, 57.3, 52.6, 46.7, 37.1, 32.8, 21.0; HRMS (ES+, m/z): found 378.2044, calcd for C20H24N7O, [M+H]+, 378.2042.
3.3.22. 3-(4-(4-(Methyl((tetrahydro-2H-pyran-4-yl)methyl)amino)-7H-pyrrolo[2,3-d]pyrimidin-6-yl)-1H-1,2,3-triazol-1-yl)propan-1-ol (28b)
The reaction was run as described in General Procedure B using 14b (80 mg, 0.16 mmol). The solvent was removed by evaporation, and the remaining residue was added to water (10 mL) and extracted with CH2Cl2 (4 × 10 mL). Purification by silica-gel flash column chromatography (CH2Cl2/MeOH, 9:1, Rf = 0.32) gave 40 mg (0.10 mmol, 68%) of a white powder, mp. 291–293 °C; HPLC method A: 99%; 1H NMR (600 MHz, DMSO-d6) δ: 12.19 (s, 1H), 8.39 (s, 1H), 8.11 (s, 1H), 6.95 (s, 1H), 4.70 (t, J = 5.0 Hz, 1H), 4.48 (t, J = 7.1 Hz, 2H), 3.83 (dd, J = 11.5, 2.6 Hz, 2H), 3.67 (d, J = 7.4 Hz, 2H), 3.44 (q, J = 5.8 Hz, 2H), 3.37 (s, 3H), 3.24 (td, J = 11.7, 2.1 Hz, 2H), 2.08–2.04 (m, 1H), 2.00 (p, J = 6.6 Hz, 2H), 1.55–1.52 (m, 2H), 1.32–1.25 (m, 2H); 13C NMR (151 MHz, DMSO-d6) δ: 156.4, 152.2, 151.0, 140.0, 124.9, 121.0, 102.7, 98.5, 66.7 (2C), 57.4, 55.3, 46.8, 38.8, 33.9, 32.9, 30.3 (2C); HRMS (ES+, m/z): found 372.2147, calcd for C18H26N7O2, [M+H]+, 372.2148.
3.3.23. N-Methyl-N-(3-methylbenzyl)-6-(1-(tetrahydro-2H-pyran-4-yl)-1H-1,2,3-triazol-4-yl)-7H-pyrrolo[2,3-d]pyrimidin-4-amine (29a)
The reaction was run as described in General Procedure B using 15a (120 mg, 0.23 mmol). This gave 65 mg (0.16 mmol, 72%) of a white powder, mp. 273–275 °C; HPLC method A: 87%; 1H NMR (600 MHz, DMSO-d6) δ: 12.23 (s, 1H), 8.47 (s, 1H), 8.15 (s, 1H), 7.21 (t, J = 7.6 Hz, 1H), 7.09 (s, 1H), 7.06 (t, J = 7.3 Hz, 2H), 6.90 (s, 1H), 4.99 (s, 2H), 4.84–4.79 (m, 1H), 4.00–3.98 (m, 2H), 3.52 (td, J = 11.8, 2.1 Hz, 2H), 3.33 (s, 3H), 2.27 (s, 3H), 2.12–2.09 (m, 2H), 2.02–1.95 (m, 2H); 13C NMR (151 MHz, DMSO-d6) δ: 156.5, 152.3, 151.1, 139.8, 138.2, 137.6, 128.4, 127.6, 127.5, 125.2, 124.0, 119.3, 102.7, 98.4, 65.6 (2C), 56.2, 52.6, 37.1, 32.8 (2C), 21.0; HRMS (ES+, m/z): found 404.2197, calcd for C22H26N7O, [M+H]+, 404.2199.
3.3.24. N-Methyl-6-(1-(tetrahydro-2H-pyran-4-yl)-1H-1,2,3-triazol-4-yl)-N-((tetrahydro-2H-pyran-4-yl)methyl)-7H-pyrrolo[2,3-d]pyrimidin-4-amine (29b)
The reaction was run as described in General Procedure B using 15b (140 mg, 0.27 mmol). The solvent was removed by evaporation, and the remaining residue was added to water (10 mL) and extracted with CH2Cl2 (4 × 10 mL). Purification by silica-gel flash column chromatography (CH2Cl2/MeOH 9:1) gave 85 mg (0.21 mmol, 81%) of a white powder, mp. > 300 °C; HPLC method A: 97%; 1H NMR (600 MHz, DMSO-d6) δ: 12.18 (s, 1H), 8.50 (s, 1H), 8.11 (s, 1H), 6.95 (s, 1H), 4.85–4.80 (m, 1H), 4.00 (dd, J = 10.4, 4.4 Hz, 2H), 3.84 (dd, J = 11.5, 2.7 Hz, 2H), 3.67 (d, J = 7.4 Hz, 2H), 3.53 (td, J = 11.8, 2.1 Hz, 2H), 3.38 (s, 3H), 3.24 (td, J = 11.8, 2.1 Hz, 2H), 2.12 (dd, J = 12.4, 2.4 Hz, 2H), 2.08–2.05 (m, 1H), 2.04–1.97 (m, 2H), 1.55–1.52 (m, 2H), 1.32–1.26 (m, 2H); 13C NMR (151 MHz, DMSO-d6) δ: 156.4, 152.2, 151.1, 139.9, 124.9, 119.2, 102.7, 98.5, 66.7 (2C), 65.6 (2C), 56.2, 55.3, 38.8, 33.9, 32.8 (2C), 30.2 (2C); HRMS (ES+, m/z): found 398.2303, calcd for C20H28N7O2, [M+H]+, 398.2304.
3.3.25. N-Methyl-N-(3-methylbenzyl)-6-(1-(1-methylpiperidin-4-yl)-1H-1,2,3-triazol-4-yl)-7H-pyrrolo[2,3-d]pyrimidin-4-amine (30a)
The reaction was run as described in General Procedure B using 16a (120 mg, 0.22 mmol). The solvent was removed by evaporation, and the remaining residue was added to water (10 mL) and extracted with CH2Cl2 (4 × 10 mL). Purification by silica-gel flash column chromatography (CH2Cl2/MeOH 4:1, Rf = 0.43) gave 60 mg (0.14 mmol, 66%) of a white powder, mp. 245–247 °C; HPLC method A: 99%; 1H NMR (600 MHz, DMSO-d6) δ: 12.22 (s, 1H), 8.45 (s, 1H), 8.15 (s, 1H), 7.21 (t, J = 7.6 Hz, 1H), 7.09 (s, 1H), 7.06 (t, J = 7.5 Hz, 2H), 6.90 (s, 1H), 4.99 (s, 2H), 4.54–4.49 (m, 1H), 3.32 (s, 3H), 2.87 (d, J = 11.9 Hz, 2H), 2.27 (s, 3H), 2.22 (s, 3H), 2.13–2.09 (m, 4H), 2.02–1.96 (m, 2H); 13C NMR (151 MHz, DMSO-d6) δ: 157.0, 152.8, 151.6, 140.2, 138.7, 138.1, 128.9, 128.1, 128.0, 125.7, 124.5, 119.7, 103.1, 98.8, 57.5, 54.1 (2C), 53.1, 46.1, 37.6, 32.4 (2C), 21.5. HRMS (ES+, m/z): found 417.2516, calcd for C23H29N8, [M+H]+, 417.2515.
3.3.26. N-Methyl-6-(1-(1-methylpiperidin-4-yl)-1H-1,2,3-triazol-4-yl)-N-((tetrahydro-2H-pyran-4-yl)methyl)-7H-pyrrolo[2,3-d]pyrimidin-4-amine (30b)
The reaction was run as described in General Procedure B using 16b (120 mg, 0.22 mmol). The solvent was removed by evaporation, and the remaining residue was added to water (10 mL) and extracted with CH2Cl2 (4 × 10 mL). Purification by silica-gel flash column chromatography (CH2Cl2/MeOH 4:1, Rf = 0.36) gave 60 mg (0.14 mmol, 66%) of a white powder, mp. > 300 °C; HPLC method A: 98%; 1H NMR (600 MHz, DMSO-d6) δ: 12.25 (s, 1H), 8.47 (s, 1H), 8.10 (s, 1H), 6.94 (s, 1H), 4.54–4.50 (s, 1H), 3.84 (dd, J = 11.4, 2.7 Hz, 2H), 3.67 (d, J = 7.4 Hz, 2H), 3.38 (s, 3H), 3.25 (td, J = 11.7, 2.1 Hz, 2H), 2.87 (d, J = 12.7 Hz, 2H), 2.22 (s, 3H), 2.13–2.09 (m, 4H), 2.08–2.04 (m, 1H), 2.03–1.97 (2H), 1.55–1.53 (m, 2H), 1.33–1.26 (m, 2H); 13C NMR (151 MHz, DMSO-d6) δ: 156.4, 152.2, 151.0, 139.8, 125.0, 119.2, 102.7, 98.5, 66.7 (2C), 57.1, 55.3, 53.7 (2C), 45.6, 38.8, 33.9, 32.0 (2C), 30.2 (2C); HRMS (ES+, m/z): found 411.2616, calcd for C21H31N8O, [M+H]+, 411.2621.
3.3.27. N-Methyl-N-(3-methylbenzyl)-6-(1-((tetrahydro-2H-pyran-4-yl)methyl)-1H-1,2,3-triazol-4-yl)-7H-pyrrolo[2,3-d]pyrimidin-4-amine (31a)
The reaction was run as described in General Procedure B using 17a (90 mg, 0.16 mmol). This gave 55 mg (0.13 mmol, 81%) of a white powder, mp. 295–297 °C; HPLC method A: 98%; 1H NMR (600 MHz, DMSO-d6) δ: 12.24 (s, 1H), 8.35 (s, 1H), 8.15 (s, 1H), 7.21 (t, J = 7.6 Hz, 1H), 7.09 (s, 1H), 7.06 (t, J = 7.3 Hz, 2H), 6.90 (s, 1H), 4.99 (s, 2H), 4.34 (d, J = 7.1 Hz, 2H), 3.84 (dd, J = 11.6, 2.7 Hz, 2H), 3.33 (s, 3H), 3.26 (td, J = 11.6, 2.1 Hz, 2H), 2.27 (s, 3H), 2.10–2.06 (m, 1H), 1.44 (d, J = 12.9 Hz, 2H), 1.31–1.24 (m, 2H); 13C NMR (151 MHz, DMSO-d6) δ: 156.5, 152.4, 151.2, 139.9, 138.2, 137.6, 128.4, 127.6, 127.4, 125.1, 124.0, 121.4, 102.6, 98.4, 66.4 (2C), 54.7, 52.6, 37.1, 35.6, 29.7 (2C), 21.0; HRMS (ES+, m/z): found 404.2197, calcd for C23H28N7O, [M+H]+, 404.2199.
3.3.28. N-Methyl-N-((tetrahydro-2H-pyran-4-yl)methyl)-6-(1-((tetrahydro-2H-pyran-4-yl)methyl)-1H-1,2,3-triazol-4-yl)-7H-pyrrolo[2,3-d]pyrimidin-4-amine (31b)
The reaction was run as described in General Procedure B using 17b (120 mg, 0.22 mmol). This gave 65 mg (0.15 mmol, 71%) of a with powder, mp. 288–290 °C; HPLC method A: 99%; 1H NMR (600 MHz, DMSO-d6) δ: 12.18 (s, 1H), 8.38 (s, 1H), 8.11 (s, 1H), 6.95 (s, 1H), 4.36–4.35 (m, 2H), 3.85–3.83 (m, 4H), 3.67 (d, J = 7.5 Hz, 2H), 3.38 (s, 3H), 3.29–3.23 (m, 4H), 2.08 (s, 2H), 1.50 (dd, J = 5.0, 12.9 Hz, 4H), 1.30–1.27 (m, 4H); 13C NMR (151 MHz, DMSO-d6) δ: 156.4, 152.2, 151.1, 140.0, 124.9, 121.3, 102.7, 98.5, 66.7 (2C), 66.4 (2C), 55.3, 54.7, 38.8, 35.6, 33.9, 30.2 (2C), 29.7 (2C); HRMS (ES+, m/z): found 412.2461, calcd for C21H30N7O2, [M+H]+, 412.2461.
3.4. Biochemical Assays
The CSF1R enzymatic inhibitory assays, the kinase panel screen, the cell viability assay with Ba/F3 cells, solubility, microsomal stability, plasma protein binding, and the MDCK permeability assay were performed as previously described [22]. The Caco-2 assay was described by Aarhus et al. [20].
3.4.1. KIT Inhibition (KD)
The principle behind this time-resolved FRET (TR-FRET) assay is based on the binding of a tracer containing a TR-FRET acceptor dye to the active site of cKIT kinase. It is detected by using an anti-GST antibody containing a TR-FRET donor dye, which binds to the GST tag at the N-terminus of cKIT. The binding of an inhibitor to the kinase displaces the tracer, resulting in a loss of FRET. For every sample, 2 µL of an assay mixture containing cKIT (0.9375 nM, Carna Biosciences, Kobe, Japan) and a buffer (50 mM HEPES pH 7,5, 10 mM MgCl2, 1 mM EGTA, 0.01% Brij35) were transferred into an assay plate (e.g., Corning #4513). Compounds were added in a concentration range of 1 µM to 0.00025 µM using an acoustic dispenser (Echo520, BeckmanCoulter, Brea, CA, USA) equipped with Echo Dose Response software version 2.4,15. After that, the kinase anti-GST antibody Eu-conjugate mix (8 µL, diluted 1/400, Revvity, Waltham, MA, USA) was added. After incubation for 30 min at 4 °C in a pre-cooled box containing wet tissue (to prevent edge effects), the reaction was started via the addition of Kinase Tracer 236 (5 µL, 4.5 nM, ThermoFisher) and mixed using a Bioshake 5000 microplate shaker (Q Instruments, Jena, Germany). After 60 min of incubation at 4 °C (pre-cooled box), the FRET signal was measured at 340 nm excitation, 665 nm, and 615 nm emission with a Pherastar FSX microplate reader (BMG Labtech, Ortenberg, Germany) with a 60 µs delay and a 200 µs integration time. KD values were determined from the sigmoidal dose–response curves with Scigilian Analyze software version 5.8.5.15 (Scigilian, Montreal, QC, Canada).
3.4.2. GIST-T1 CTG Assay
The CellTiter-Glo Luminescent Cell Viability Assay (Promega, Madison, WI, USA) is a homogeneous method of determining the number of viable cells in a culture. It is based on the quantification of ATP, indicating the presence of metabolically active cells. The GIST-T1 cell line (CosmoBio, Tokyo, Japan, # PMC-GIST01C) was cultivated in GIST-T1 cell culture medium without antibiotics (#GIST01C, CosmoBio) according to the product manual. For CellTiter-Glo Luminescent Cell Viability Assays, 25 µL of the GIST-T1 cell suspension are seeded at a concentration of 400 cells/384well plate on day 1. In previous assays, this cell number was shown to ensure assay linearity and optimal signal intensity. After incubation for 24 h at 37 °C/5% CO2, the compounds are added at different concentrations by Echo Liquid Handling Technology. Cells are further incubated in humidified chambers for 72 h at 37 °C and 5% CO2. Cells treated with the compound vehicle DMSO are used as positive controls and cells treated with 10 µM Staurosporine serve as negative controls. At day 5, 72 h after compound addition, the CellTiter Glo Reagent is prepared according to the instructions of the kit (Promega Inc.). The reagent is mixed 1:1 with the cell culture medium. Thereafter, the mixture and assay plates are equilibrated at room temperature for 20 min. Equal volumes of the reagent–medium mixture is added to the volume of the culture medium present in each well. The plates are mixed at ~300 rpm for 2 min on an orbital shaker. The microplates are then incubated at room temperature for 10 min for stabilization of the luminescent signal. Following incubation, the luminescence is recorded on a Victor microplate reader (Perkin Elmer) using a 200 ms integration time. The data are then analyzed with Excel using the XLFIT Plugin (dose–response Fit 205) for IC50 determination. As a quality control, the Z′-factor is calculated from 16 positive and negative control values. Only assay results showing a Z′-factor ≥ 0.5 are used for further analysis.
4. Conclusions
Two series of pyrrolopyrimdines decorated with substituted 1,2,3-triazoles (28 compounds) were synthesized using Sonogashira cross-coupling and copper-catalyzed azide–alkyne cycloaddition in the key steps. The CSF1R enzymatic inhibition studies showed 27 of the derivatives to have lower IC50 than the reference drug. Although the calculated logP is <4, several derivatives had low solubility, complicating development. Three derivatives showed promising activity in Ba/F3 cells (IC50: 0.3–0.6 µM) and no cellular toxicity. These derivatives have low activity toward the related PDGFR family of kinases, but profiling in a panel of kinases showed the front-runner compound 27a to also have high activity toward ABL1, ABL2, EPHA2, SRC, and YES. ADME profiling revealed the compounds to be unstable in phase I metabolic studies using both human and liver microsomes. These triazoles can be a starting point for the design of a new multi-kinase inhibitor if measures are taken to identify and block the metabolic labile site.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules30122641/s1, This file contains information on synthesis and characterization of intermediates, enzymatic inhibition titration curves, kinase profiling, HPLC trace, NMR and HRMS spectra, and additional illustrations of modeling. Figure S1–S84: 1H and 13C NMR spectra and HRMS of compounds 18a-31b; Figure S85–S90: graphics illustrating docking of 27a and PLX-3397; Table S1: Profiling of compound 27a towards a panel of kinases; Table S2: Examples of IC50 curves.
Author Contributions
Conceptualization, B.H.H., E.S. and S.C.; methodology, S.C.; software, E.S.; formal analysis, S.C., C.D., P.H. and A.U.; investigation, S.C.; resources, B.H.H., E.S. and J.E.; data curation, J.E., C.D., P.H. and A.U.; writing—original draft preparation, B.H.H.; writing—review and editing, B.H.H. and E.S.; visualization, B.H.H., E.S. and S.C.; supervision, B.H.H. and E.S.; project administration, B.H.H., E.S. and J.E.; funding acquisition, E.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Trond Mohn Foundation, grant number TMS2019TMT07.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Experimental data can be found in the Supplementary Materials.
Acknowledgments
Assistance from the NTNU arm of the Norwegian NMR Platform and the Mass Spectrometry Lab at the NV Faculty at NTNU is acknowledged. Roger Aarvik (NTNU) and Gaute Brede (NTNU Technology Transfer AS) are also thanked for their contributions.
Conflicts of Interest
Several of the authors contributing to this work is employed at Lead Discovery Center GmbH (LDC) a translational drug discovery organization. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Lin, H.; Lee, E.; Hestir, K.; Leo, C.; Huang, M.; Bosch, E.; Halenbeck, R.; Wu, G.; Zhou, A.; Behrens, D.; et al. Discovery of a cytokine and its receptor by functional screening of the extracellular proteome. Science 2008, 320, 807–811. [Google Scholar] [CrossRef] [PubMed]
- Wynn, T.A.; Chawla, A.; Pollard, J.W. Macrophage biology in development, homeostasis and disease. Nature 2013, 496, 445–455. [Google Scholar] [CrossRef]
- Cersosimo, F.; Lonardi, S.; Ulivieri, C.; Martini, P.; Morrione, A.; Vermi, W.; Giordano, A.; Giurisato, E. CSF-1R in Cancer: More than a Myeloid Cell Receptor. Cancers 2024, 16, 282. [Google Scholar] [CrossRef]
- Mun, S.H.; Park, P.S.U.; Park-Min, K.-H. The M-CSF receptor in osteoclasts and beyond. Exp. Mol. Med. 2020, 52, 1239–1254. [Google Scholar] [CrossRef] [PubMed]
- Yadav, S.; Priya, A.; Borade, D.R.; Agrawal-Rajput, R. Macrophage subsets and their role: Co-relation with colony-stimulating factor-1 receptor and clinical relevance. Immunol. Res. 2023, 71, 130–152. [Google Scholar] [CrossRef] [PubMed]
- Mo, H.; Hao, Y.; Lv, Y.; Chen, Z.; Shen, J.; Zhou, S.; Yin, M. Overexpression of macrophage-colony stimulating factor-1 receptor as a prognostic factor for survival in cancer: A systematic review and meta-analysis. Medicine 2021, 100, e25218. [Google Scholar] [CrossRef]
- Riaz, N.; Burugu, S.; Cheng, A.S.; Leung, S.C.Y.; Gao, D.; Nielsen, T.O. Prognostic Significance of CSF-1R Expression in Early Invasive Breast Cancer. Cancers 2021, 13, 5769. [Google Scholar] [CrossRef]
- Mantovani, A.; Allavena, P. The interaction of anticancer therapies with tumor-associated macrophages. J. Exp. Med. 2015, 212, 435–445. [Google Scholar] [CrossRef] [PubMed]
- Xiang, C.; Li, H.; Tang, W. Targeting CSF-1R represents an effective strategy in modulating inflammatory diseases. Pharmacol. Res. 2023, 187, 106566. [Google Scholar] [CrossRef]
- Olmos-Alonso, A.; Gomez-Nicola, D.; Askew, K.; Mancuso, R.; Sri, S.; Schetters, S.T.T.; Perry, V.H.; Vargas-Caballero, M.; Holscher, C. Pharmacological targeting of CSF1R inhibits microglial proliferation and prevents the progression of Alzheimer’s-like pathology. Brain 2016, 139, 891–907. [Google Scholar] [CrossRef]
- Sosna, J.; Philipp, S.; Albay, R., III; Reyes-Ruiz, J.M.; Baglietto-Vargas, D.; LaFerla, F.M.; Glabe, C.G. Early long-term administration of the CSF1R inhibitor PLX3397 ablates microglia and reduces accumulation of intraneuronal amyloid, neuritic plaque deposition and pre-fibrillar oligomers in 5XFAD mouse model of Alzheimer’s disease. Mol. Neurodegener. 2018, 13, 11. [Google Scholar] [CrossRef] [PubMed]
- Weyer, M.-P.; Strehle, J.; Schäfer, M.K.E.; Tegeder, I. Repurposing of pexidartinib for microglia depletion and renewal. Pharmacol. Ther. 2024, 253, 108565. [Google Scholar] [CrossRef] [PubMed]
- Hume, D.A.; Batoon, L.; Sehgal, A.; Keshvari, S.; Irvine, K.M. CSF1R as a therapeutic target in bone diseases: Obvious but not so simple. Curr. Osteoporos. Rep. 2022, 20, 516–531. [Google Scholar] [CrossRef]
- Alkubaisi, B.O.; Aljobowry, R.; Ali, S.M.; Sultan, S.; Zaraei, S.-O.; Ravi, A.; Al-Tel, T.H.; El-Gamal, M.I. The latest perspectives of small molecules FMS kinase inhibitors. Eur. J. Med. Chem. 2023, 261, 115796. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.; Wang, S.; Guo, R.; Liu, D. CSF1R inhibitors are emerging immunotherapeutic drugs for cancer treatment. Eur. J. Med. Chem. 2023, 245, 114884. [Google Scholar] [CrossRef]
- Smith, B.D.; Kaufman, M.D.; Wise, S.C.; Ahn, Y.M.; Caldwell, T.M.; Leary, C.B.; Lu, W.-P.; Tan, G.; Vogeti, L.; Vogeti, S.; et al. Vimseltinib: A Precision CSF1R Therapy for Tenosynovial Giant Cell Tumors and Diseases Promoted by Macrophages. Mol. Cancer Ther. 2021, 20, 2098–2109. [Google Scholar] [CrossRef]
- Krauser, J.A.; Jin, Y.; Walles, M.; Pfaar, U.; Sutton, J.; Wiesmann, M.; Graf, D.; Pflimlin-Fritschy, V.; Wolf, T.; Camenisch, G.; et al. Phenotypic and metabolic investigation of a CSF-1R kinase receptor inhibitor (BLZ945) and its pharmacologically active metabolite. Xenobiotica 2015, 45, 107–123. [Google Scholar] [CrossRef]
- Genovese, M.C.; Hsia, E.; Belkowski, S.M.; Chien, C.; Masterson, T.; Thurmond, R.L.; Manthey, C.L.; Yan, X.; Ge, T.; Franks, C.; et al. Results from a Phase IIA Parallel Group Study of JNJ-40346527, an Oral CSF-1R Inhibitor, in Patients with Active Rheumatoid Arthritis despite Disease-modifying Antirheumatic Drug Therapy. J. Rheumatol. 2015, 42, 1752–1760. [Google Scholar] [CrossRef]
- Manthey, C.L.; Moore, B.A.; Chen, Y.; Loza, M.J.; Yao, X.; Liu, H.; Belkowski, S.M.; Raymond-Parks, H.; Dunford, P.J.; Leon, F.; et al. The CSF-1-receptor inhibitor, JNJ-40346527 (PRV-6527), reduced inflammatory macrophage recruitment to the intestinal mucosa and suppressed murine T cell mediated colitis. PLoS ONE 2019, 14, e0223918. [Google Scholar] [CrossRef]
- Aarhus, T.I.; Bjørnstad, F.; Wolowczyk, C.; Larsen, K.U.; Rognstad, L.; Leithaug, T.; Unger, A.; Habenberger, P.; Wolf, A.; Bjørkøy, G.; et al. Synthesis and Development of Highly Selective Pyrrolo[2,3-d]pyrimidine CSF1R Inhibitors Targeting the Autoinhibited Form. J. Med. Chem. 2023, 66, 6959–6980. [Google Scholar] [CrossRef]
- Aarhus, T.I.; Teksum, V.; Unger, A.; Habenberger, P.; Wolf, A.; Eickhoff, J.; Klebl, B.; Wolowczyk, C.; Bjørkøy, G.; Sundby, E.; et al. Negishi Cross-Coupling in the Preparation of Benzyl Substituted Pyrrolo[2,3-d]pyrimidine Based CSF1R Inhibitors. Eur. J. Org. Chem. 2023, 26, e202300052. [Google Scholar] [CrossRef]
- Bjørnstad, F.; Havik, S.; Aarhus, T.I.; Mahdi, I.; Unger, A.; Habenberger, P.; Degenhart, C.; Eickhoff, J.; Klebl, B.M.; Sundby, E.; et al. Pyrrolopyrimidine based CSF1R inhibitors: Attempted departure from Flatland. Eur. J. Med. Chem. 2024, 265, 116053. [Google Scholar] [CrossRef]
- Aarhus, T.I.; Eickhoff, J.; Klebl, B.; Unger, A.; Boros, J.; Choidas, A.; Zischinsky, M.-L.; Wolowczyk, C.; Bjørkøy, G.; Sundby, E.; et al. A highly selective purine-based inhibitor of CSF1R potently inhibits osteoclast differentiation. Eur. J. Med. Chem. 2023, 255, 115344. [Google Scholar] [CrossRef]
- Meanwell, N.A. Improving Drug Candidates by Design: A Focus on Physicochemical Properties As a Means of Improving Compound Disposition and Safety. Chem. Res. Toxicol. 2011, 24, 1420–1456. [Google Scholar] [CrossRef]
- Ritchie, T.J.; Macdonald, S.J.F. Heterocyclic replacements for benzene: Maximising ADME benefits by considering individual ring isomers. Eur. J. Med. Chem. 2016, 124, 1057–1068. [Google Scholar] [CrossRef]
- Subbaiah, M.A.M.; Meanwell, N.A. Bioisosteres of the Phenyl Ring: Recent Strategic Applications in Lead Optimization and Drug Design. J. Med. Chem. 2021, 64, 14046–14128. [Google Scholar] [CrossRef]
- Khan, J.; Rani, A.; Aslam, M.; Maharia, R.S.; Pandey, G.; Nand, B. Exploring triazole-based drugs: Synthesis, application, FDA approvals, and clinical trial updates–A comprehensive review. Tetrahedron 2024, 162, 134122. [Google Scholar] [CrossRef]
- Bozorov, K.; Zhao, J.; Aisa, H.A. 1,2,3-Triazole-containing hybrids as leads in medicinal chemistry: A recent overview. Bioorg. Med. Chem. 2019, 27, 3511–3531. [Google Scholar] [CrossRef] [PubMed]
- Dixit, D.; Verma, P.K.; Marwaha, R.K. A review on ‘triazoles’: Their chemistry, synthesis and pharmacological potentials. J. Iran. Chem. Soc. 2021, 18, 2535–2565. [Google Scholar] [CrossRef]
- Arioli, F.; Borrelli, S.; Colombo, F.; Falchi, F.; Filippi, I.; Crespan, E.; Naldini, A.; Scalia, G.; Silvani, A.; Maga, G.; et al. N-[2-Methyl-5-(triazol-1-yl)phenyl]pyrimidin-2-amine as a Scaffold for the Synthesis of Inhibitors of Bcr-Abl. ChemMedChem 2011, 6, 2009–2018. [Google Scholar] [CrossRef]
- Mortazavi, M.; Eskandari, M.; Moosavi, F.; Damghani, T.; Khoshneviszadeh, M.; Pirhadi, S.; Saso, L.; Edraki, N.; Firuzi, O. Novel quinazoline-1,2,3-triazole hybrids with anticancer and MET kinase targeting properties. Sci. Rep. 2023, 13, 14685. [Google Scholar] [CrossRef] [PubMed]
- Srinivas, M.; Singh Pathania, A.; Mahajan, P.; Verma, P.K.; Chobe, S.S.; Malik, F.A.; Nargotra, A.; Vishwakarma, R.A.; Sawant, S.D. Design and synthesis of 1,4-substituted 1H-1,2,3-triazolo-quinazolin-4(3H)-ones by Huisgen 1,3-dipolar cycloaddition with PI3Kγ isoform selective activity. Bioorg. Med. Chem. Lett. 2018, 28, 1005–1010. [Google Scholar] [CrossRef]
- Safavi, M.; Ashtari, A.; Khalili, F.; Mirfazli, S.S.; Saeedi, M.; Ardestani, S.K.; Rashidi Ranjbar, P.; Barazandeh Tehrani, M.; Larijani, B.; Mahdavi, M. Novel quinazolin-4(3H)-one linked to 1,2,3-triazoles: Synthesis and anticancer activity. Chem. Biol. Drug Des. 2018, 92, 1373–1381. [Google Scholar] [CrossRef] [PubMed]
- Kettle, J.G.; Anjum, R.; Barry, E.; Bhavsar, D.; Brown, C.; Boyd, S.; Campbell, A.; Goldberg, K.; Grondine, M.; Guichard, S.; et al. Discovery of N-(4-{[5-Fluoro-7-(2-methoxyethoxy)quinazolin-4-yl]amino}phenyl)-2-[4-(propan-2-yl)-1H-1,2,3-triazol-1-yl]acetamide (AZD3229), a Potent Pan-KIT Mutant Inhibitor for the Treatment of Gastrointestinal Stromal Tumors. J. Med. Chem. 2018, 61, 8797–8810. [Google Scholar] [CrossRef]
- Menet, C.J.; Fletcher, S.R.; Van Lommen, G.; Geney, R.; Blanc, J.; Smits, K.; Jouannigot, N.; Deprez, P.; van der Aar, E.M.; Clement-Lacroix, P.; et al. Triazolopyridines as Selective JAK1 Inhibitors: From Hit Identification to GLPG0634. J. Med. Chem. 2014, 57, 9323–9342. [Google Scholar] [CrossRef] [PubMed]
- Kulukian, A.; Lee, P.; Taylor, J.; Rosler, R.; de Vries, P.; Watson, D.; Forero-Torres, A.; Peterson, S. Preclinical Activity of HER2-Selective Tyrosine Kinase Inhibitor Tucatinib as a Single Agent or in Combination with Trastuzumab or Docetaxel in Solid Tumor Models. Mol. Cancer Ther. 2020, 19, 976–987. [Google Scholar] [CrossRef]
- Tap, W.D.; Wainberg, Z.A.; Anthony, S.P.; Ibrahim, P.N.; Zhang, C.; Healey, J.H.; Chmielowski, B.; Staddon, A.P.; Cohn, A.L.; Shapiro, G.I.; et al. Structure-guided blockade of CSF1R kinase in tenosynovial giant-cell tumor. N. Engl. J. Med. 2015, 373, 428–437. [Google Scholar] [CrossRef]
- Pollok, B.A.; Hamman, B.D.; Rodems, S.M.; Makings, L.R. Optical Probes and Assays. U.S. Patent No. 6,410,255, 30 March 2004. [Google Scholar]
- Taguchi, T.; Sonobe, H.; Toyonaga, S.-i.; Yamasaki, I.; Shuin, T.; Takano, A.; Araki, K.; Akimaru, K.; Yuri, K. Conventional and Molecular Cytogenetic Characterization of a New Human Cell Line, GIST-T1, Established from Gastrointestinal Stromal Tumor. Lab. Investig. 2002, 82, 663–665. [Google Scholar] [CrossRef]
- Force, T.; Kolaja, K.L. Cardiotoxicity of kinase inhibitors: The prediction and translation of preclinical models to clinical outcomes. Nat. Rev. Drug Discovery 2011, 10, 111–126. [Google Scholar] [CrossRef]
- Kaspersen, S.J.; Han, J.; Nørsett, K.G.; Rydså, L.; Kjøbli, E.; Bugge, S.; Bjørkøy, G.; Sundby, E.; Hoff, B.H. Identification of new 4-N-substituted 6-aryl-7H-pyrrolo[2,3-d]pyrimidine-4-amines as highly potent EGFR-TK inhibitors with Src-family activity. Eur. J. Pharm. Sci. 2014, 59, 69–82. [Google Scholar] [CrossRef]
- Han, J.; Henriksen, S.; Nørsett, K.G.; Sundby, E.; Hoff, B.H. Balancing potency, metabolic stability and permeability in pyrrolopyrimidine-based EGFR inhibitors. Eur. J. Med. Chem. 2016, 124, 583–607. [Google Scholar] [CrossRef] [PubMed]
- Karaman, M.W.; Herrgard, S.; Treiber, D.K.; Gallant, P.; Atteridge, C.E.; Campbell, B.T.; Chan, K.W.; Ciceri, P.; Davis, M.I.; Edeen, P.T.; et al. A quantitative analysis of kinase inhibitor selectivity. Nat. Biotechnol. 2008, 26, 127–132. [Google Scholar] [CrossRef]
- Khatri, A.; Wang, J.; Pendergast, A.M. Multifunctional Abl kinases in health and disease. J. Cell Sci. 2016, 129, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Sun, D.; Tao, J.; Xu, M.; Zhang, X.; Hou, H. Role of YES1 signaling in tumor therapy resistance. Cancer Innov. 2023, 2, 210–218. [Google Scholar] [CrossRef]
- Ortiz, M.A.; Mikhailova, T.; Li, X.; Porter, B.A.; Bah, A.; Kotula, L. Src family kinases, adaptor proteins and the actin cytoskeleton in epithelial-to-mesenchymal transition. Cell Commun. Sig. 2021, 19, 67. [Google Scholar] [CrossRef]
- Garmendia, I.; Redin, E.; Montuenga, L.M.; Calvo, A. YES1: A Novel Therapeutic Target and Biomarker in Cancer. Mol. Cancer Ther. 2022, 21, 1371–1380. [Google Scholar] [CrossRef] [PubMed]
- Wilson, K.; Shiuan, E.; Brantley-Sieders, D.M. Oncogenic functions and therapeutic targeting of EphA2 in cancer. Oncogene 2021, 40, 2483–2495. [Google Scholar] [CrossRef]
- Schrödinger_Release_2025-1 Glide; Schrödinger, LLC: New York, NY, USA, 2025.
- Wodicka, L.M.; Ciceri, P.; Davis, M.I.; Hunt, J.P.; Floyd, M.; Salerno, S.; Hua, X.H.; Ford, J.M.; Armstrong, R.C.; Zarrinkar, P.P.; et al. Activation state-dependent binding of small molecule kinase inhibitors: Structural insights from biochemistry. Chem. Biol. 2010, 17, 1241–1249. [Google Scholar] [CrossRef]
- Kitagawa, D.; Gouda, M.; Kirii, Y.; Sugiyama, N.; Ishihama, Y.; Fujii, I.; Narumi, Y.; Akita, K.; Yokota, K. Characterization of kinase inhibitors using different phosphorylation states of colony stimulating factor-1 receptor tyrosine kinase. J. Biochem. 2012, 151, 47–55. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).