Abstract
The synthesis of a new acyclic and cyclic series of D-Ala-AMP analogues was reported. Chemical modifications were introduced on the carbohydrate, the sulfamate linker, and/or the amino-acid N-terminal moiety in order to increase in vivo stability and cell permeability. These new compounds were evaluated in vitro as DltA inhibitors and also in vivo as adjuvant antibiotics to re-sensitize methicillin-resistant Staphylococcus aureus. Indeed, we showed that seven nucleosides containing either a fluorine atom, an azido group, a difluorophosphonylated allylic ether moiety onto the 2′-position, or a sulfamate and a triazole as the sulfamate linker had moderate to excellent IC50 values. Among all these new DltA inhibitors, two molecules functionalized by the fluorinated ether or the sulfamide linker were able to efficiently re-sensitize MRSA to imipenem. Quantification of D-alanyl esters confirmed that these two compounds reduced the level of bacterial cell wall D-alanyl residues by 50% and 80%.
1. Introduction
Antibiotics represent one of the most significant medical discoveries of the 20th century, revolutionizing the treatment of bacterial infections and saving countless lives worldwide for several decades [1,2]. Antibacterial agents were initially designed to either kill bacteria or inhibit their growth by counteracting essential biological functions such as cell wall synthesis, DNA replication, and protein synthesis. However, these modes of action exert a selective pressure that modifies the human microbiome and induces antimicrobial resistance (AMR) [3,4,5]. As a result, inappropriate utilization and overuse of antibiotics in the past have accelerated the emergence of antibiotic-resistant bacterial strains, rendering previously effective treatments obsolete, and posing grave threats to public health [6,7]. This phenomenon was further amplified with the COVID-19 pandemic, where, in addition to the increased use of antibacterial cleaning products [8], approximately 75% of patients were treated with antibiotics although bacterial co-infection was estimated at 8.6% [9]. In 2019, 4.95 million deaths worldwide were associated with bacterial resistance to antibiotics, including 1.27 million directly attributable to AMR, positioning it as a major global burden of diseases ahead of HIV and malaria [10]. The development of new therapeutic approaches involving active compounds towards new targets remains an important challenge and is an emergency requirement for human care. In this field, adjuvant antibiotics have emerged and represent a promising way to fight AMR infections [11]. These molecules are designed to improve antibiotic actions or re-sensitize pathogens to drugs from which they have become resistant by targeting the antibiotic resistance mechanism. D-alanylation of teichoic acids serves as an effective strategy for Gram-positive bacteria to develop antibioresistance [12]. This process, catalyzed by five proteins (DltXABCD) encoded by the dlt operon [13,14], transfers a D-alanine residue onto the cell wall, altering its net charge and permeability, thereby reducing susceptibility to cationic antimicrobial peptides and glycopeptides such as vancomycin (Figure 1) [15].
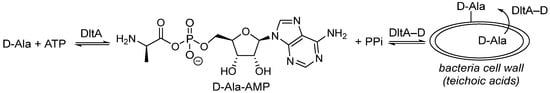
Figure 1.
D-alanylation of cell wall teichoic acids.
Indeed, nucleoside 1, designed as a D-Ala-AMP analog, was reported as the sole DltA inhibitor (Ki = 0.2 μM) and found to be efficient as an adjuvant to inhibit the growth of Bacillus subtilis, a non-pathogenic bacterium, in the presence of vancomycin [16]. We recently showed that compound 1 was also able to counteract the antibiotic resistance of methicillin-resistant Staphylococcus aureus (MRSA) and β-lactam-resistant enterococci, including vancomycin-resistant strains, when co-administered with the antibiotics [17,18,19]. However, high concentrations (500 μM to 1 mM) of inhibitor 1 were needed, suggesting that nucleoside 1 may suffer from low metabolic stability and/or weak cellular uptake. For this type of nucleoside, both the glycosidic bond and the sulfamate function were identified as being responsible for its enzymatic and chemical instability. As a result, N-acylsquaramides, N-acylsulfamides, and triazoles derived from acyclo-, morpholino-, and carba-nucleosides have already been proposed to overcome this main limitation but were never applied to 1 [20,21]. Thus, the design of new DltA inhibitors with improved stability and cell permeability still deserves to be developed for clinical use. In this context, synthesis of stable analogues of D-Ala-AMP, in which D-Alanine is attached to the nucleoside by a non-scissile bond in order to prevent its transfer and block the D-alanylation process, is of particular interest. In this paper, we report the synthesis, molecular docking, and biological studies of a new series of nucleosides derived from inhibitor 1 through chemical modifications of the carbohydrate, the sulfamate linker, and/or the amino-acid N-terminal moiety (Figure 2).
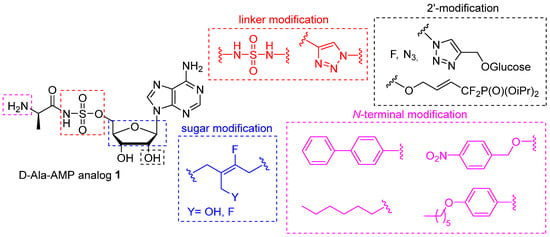
Figure 2.
Chemical modifications envisaged for D-Ala-AMP.
2. Results and Discussion
2.1. Chemistry
The introduction of electron-withdrawing groups onto the 2′-position of nucleosides is well known to increase the N-glycosidic bond stability. This chemical modification was first considered. Indeed, 2′-fluoro and 2′-azido N-acylsulfamate adenosine 8–9 were prepared according to the procedure reported by Herdewijn (Scheme 1) [22]. Known compounds 2 [22] and 3 [23] were reacted with NH2SO2Cl, affording the corresponding N-sulfamoyl derivatives 4–5 in 80% and 89% yield, respectively. The desired nucleosides 8 and 9 were finally obtained in two steps involving a coupling reaction with Boc-D-Ala-Osu, followed by amine and alcohol deprotection.
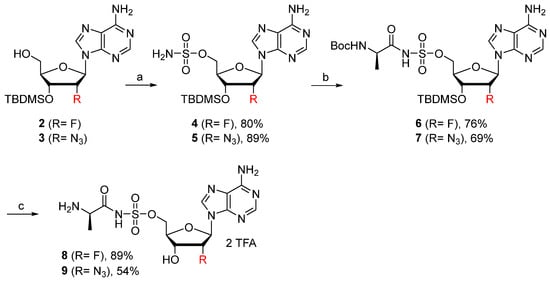
Scheme 1.
Reagents and conditions: (a) (i) NH2SO2Cl, DMA, MeCN, 20 °C, 2 h. (ii) Et3N, MeOH, 0 °C, 10 min. (b) Boc-D-Ala-OSu, DBU, DMF, 20 °C, 48 h for 6 and 16 h for 7. (c) TFA, H2O, CH2Cl2, 20 °C, 5 h for 8 and 12 h for 9.
Carbohydrate–drug conjugates have already been explored to enhance cellular uptake through specific glucose transporters such as GLUT proteins [24,25,26,27]. Indeed, glycoconjugates of various cytotoxic molecules were successfully developed to improve their penetration into cancer cells [28,29]. In the field of antibiotics, the use of β-cyclodextrin grafted with D-glucose and D-mannose potentiated the activity of erythromycin, rifampicin, and ciprofloxacin [30]. With respect to the structure–activity relationship of D-glucose and GLUT transporter [28] and inspired by the different linkers already used in conjugation [24,25,26,27,28,29,30,31], incorporation of a glucose moiety onto the 2′-position through a triazolyl linker was next envisaged (Scheme 2). D-glucose was introduced via a 1,3-dipolar cycloaddition reaction between 7 and 1-O-propargyl-2,3,4,-tetra-O-acetyl-D-glucopyranose. After 24 h of stirring at 20 °C in t-BuOH/H2O in the presence of CuSO4 and sodium ascorbate, triazole 10 was isolated in 64% yield. Finally, additional treatment with TFA, sodium methoxide, and Et3N.3HF led to the formation of the desired glucosyl-derived adenosine 11 in 32% yield over three steps.
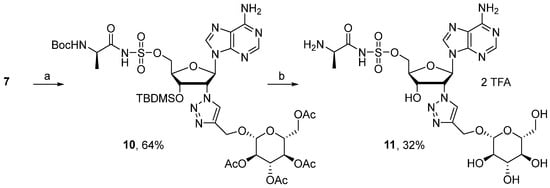
Scheme 2.
Reagents and conditions: (a) 1-O-propargyl-2,3,4,-tetra-O-acetyl-D-glucopyranose, CuSO4, NaAsc, t-BuOH, H2O, 20 °C, 24 h. (b) (i) TFA, H2O, 20 °C, 12 h. (ii) MeONa, MeOH, 20 °C, 1 h. (iii) Et3N.3HF, THF, 20 °C, 12 h.
The last 2′-modification realized was the introduction of a difluorophosphonylated allylic ether moiety that has already been used by our group to improve the metabolic stability and lipophilicity of homouridylates [32]. The synthesis of the corresponding fluorinated N-acylsulfamate adenosine 18 was accomplished in six steps starting from 12 [33] (Scheme 3). Nucleoside 13, easily obtained from 12, was first treated with methylamine, followed by an excess of Boc2O to furnish 14 in 78% yield. After selective 5′-deprotection occurring in TFA/H2O, the corresponding alcohol 15 was sulfamoylated with NH2SO2Cl and then engaged in a coupling reaction with Boc-D-Ala-OSu in the presence of DBU to afford 17, which was converted into the desired nucleoside 18 after a final deprotection conducted with TFA.
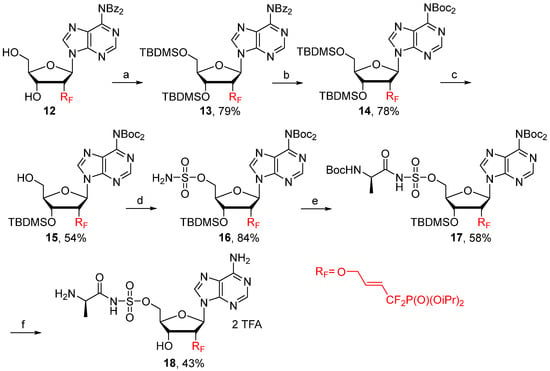
Scheme 3.
Reagents and conditions: (a) TBDMSCl, DMAP, Imidazole, DMF, 20 °C, 24 h. (b) (i) MeNH2, EtOH, 20 °C, 30 min. (ii) (Boc)2O, Et3N, DMAP, DMF, 20 °C, 12 h. (c) AcOH, H2O, THF, 20 °C, 64 h. (d) (i) NH2SO2Cl, DMAc, MeCN, 20 °C, 2 h. (ii) NEt3, MeOH, 0 °C, 10 min. (e) Boc-D-Ala-OSu, DBU, DMF, 20 °C, 24 h. (f) TFA, H2O, CH2Cl2, 20 °C, 24 h.
It was noticed that N-acylsulfamate adenosines could easily decompose into their corresponding cyclonucleosides via a nucleophilic substitution between the adenine N3-atom and the 5′-sulfamoyl group [34,35]. To avoid this competitive reaction, the bridge modification was investigated. Substitution of the acylsulfamate linkage by a stable isosteric acylphosphate mimic such as acylsquaramide was first envisaged (Scheme 4). Indeed, protected nucleoside 19 was first activated with diphenylphosphoryl azide and then reacted with sodium azide to provide 20 after hydrogenation [36,37]. Squaramide 21, easily obtained from 20 under standard conditions [21], was treated with activated alanylester in the presence of DBU. After 4 h of stirring at 60 °C in DMF, nucleoside 22 was isolated in 49% yield. Additional treatment with TFA produced the ammonium salt 23 in good yield.
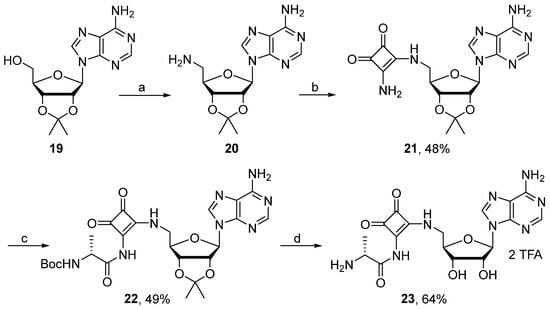
Scheme 4.
Reagents and conditions: (a) See ref [36,37] for reagents and conditions. (b) (i) Dimethyl squarate, MeOH, 20 °C, 4 h. (ii) NH3, MeOH, 20 °C, 8 h. (c) Boc-D-Ala-OSu, DBU, DMF, 60 °C, 4 h. (d) TFA, H2O, CH2Cl2, 20 °C, 5 h.
Substitution of the sulfamate oxygen with a nitrogen, leading to the more stable corresponding acylsulfamide derivatives [38], was also considered starting from 24 and 25, easily prepared according to reported procedures [20] (Scheme 5). Compound 26 was reacted with Boc-D-Ala-OSu in the presence of DBU to produce, after 16 h of stirring in DMSO, nucleoside 28 in 76% yield. Under the same conditions, the reaction with 27 was slower and reached completion after 48 h. In this case, 2′-fluoroadenosine 29 was isolated in 77% yield. N-acylsulfamide adenosine 30 and 2′-fluoro N-acylsulfamide adenosine 31 were finally obtained after Boc and TBDMS deprotection with TFA.
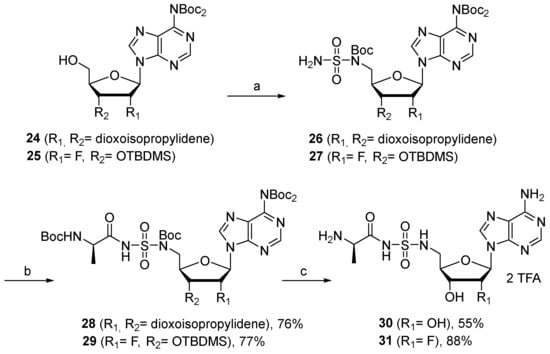
Scheme 5.
Reagents and conditions: (a) See ref [20] for reagents and conditions. (b) Boc-D-Ala-OSu, DBU, DMF, 20 °C, 16 h for 25 and 48 h for 26. (c) TFA, H2O, CH2Cl2, 20 °C, 5 h for 27 and 10 h for 28.
Agrofoglio showed that acyclonucleosides containing a transbutenyl motif were recognized by kinase as nucleoside mimics, and we recently reported that the fluorinated transbutenyl moiety can be used as stable nucleoside sugar surrogates [39,40]. Synthesis of N-acylsulfamides 38 was investigated to access a mimic of fluorinated nucleoside 8 (Scheme 6). The synthesis started from bromo-alcohol 33, easily obtained from 32 by oxetane ring-opening reaction [40]. This later was first treated with cesium fluoride to give the corresponding acyclonucleoside 34 in 17% yield. In this case, a competitive elimination reaction occurred, leading to diene 35 as a major product. The same results were obtained with KF and TBAF with or without the presence of 18-crown-6. To overcome this limitation, tert-butanol was used as a solvent in order to balance the basic character of the fluoride ion and emphasize its nucleophilicity [41,42]. Under these conditions, 19F NMR analysis of the crude mixture revealed the presence of 92% of 34 and 8% of 35, affording pure 34 in 43% yield. Compound 34 was next reacted with tert-butylsulfamoylcarbamate in the presence of PPh3 and DIAD. After 16 h of stirring at room temperature in THF, the corresponding fluoroalkene 36 was isolated in 74% yield. The desired acyclonucleoside 38 was finally obtained in two steps involving a coupling reaction with Boc-D-Ala-OSu, followed by a deprotection.
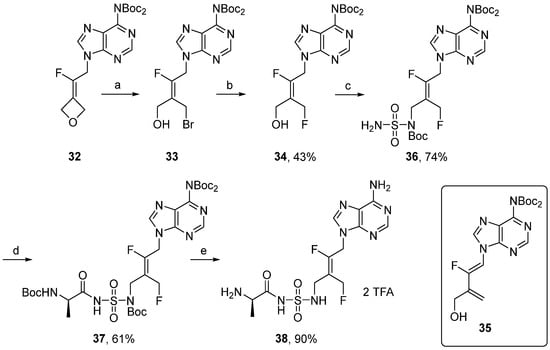
Scheme 6.
Reagents and conditions: (a) See ref [40] for reagents and conditions. (b) CsF, tBuOH, 70 °C, 4 h. (c) NH2SO2NHBoc, PPh3, DIAD, THF, 20 °C, 16 h. (d) Boc-D-Ala-OSu, DBU, DMF, 20 °C, 16 h. (e) TFA, H2O, CH2Cl2, 20 °C, 5h.
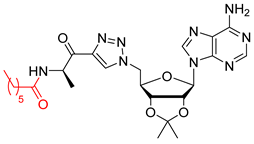
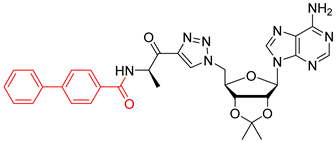
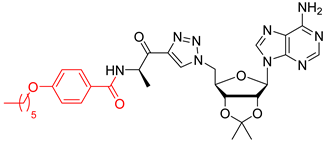
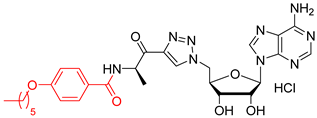
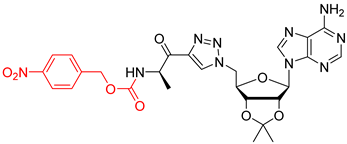
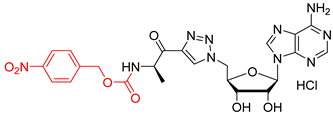
We showed that the introduction of a triazole ring to link D-alanine to adenosine or its fluorinated transbutenyl mimic was not detrimental for the inhibitory activity in vitro towards the DltA enzyme [40]. Indeed, nucleosides 39 and 40 were previously prepared (Scheme 7, Table 1, entry 1) and exhibited IC50 values in the same order of magnitude as those obtained from 1 [40]. To extend this series, analogs 50–54 were synthesized starting from 43 and 44, easily prepared following reported procedures [43,44] (Table 1). Indeed, azide 43 was reacted with 4-N-Boc-amino-pent-1-yn-2-one in the presence of CuSO4 and sodium ascorbate. After 24 h of stirring at 20 °C in t-BuOH/H2O, triazole 45 was isolated in 68% yield. Additional treatment with TFA afforded 50 in 88% yield (Table 1, entry 2). Sulfamate substitution by a triazole ring removes the zwitterionic form of the 5′-chain found with compound 1 in physiological media. To restore this neutral character, we envisaged functionalizing the amino group of the triazolyl moiety with various lipophilic acyl derivatives, which are also known to improve cell permeability in several bacteria [45]. These modifications will also enrich structure-activity studies and establish if the functionalization of the amino-group of D-Ala will affect recognition by the enzyme and the activity. To rapidly access these compounds, 1,3-dipolar cycloaddition reactions between azide 44 and keto-alkynes containing either a biphenyl, heptyl, hexyloxyphenyl or a 4-nitrobenzyl chain were chosen as the key step (Table 1, entries 3–6 and see SI). Under the same previous conditions (24 h at 20 °C), low conversions were observed, and 24 h of stirring at 40 °C was necessary to drive the reaction until completion. Indeed, triazoles 46–49 were obtained in good yield and then treated with HCl or TFA to produce the corresponding deprotected nucleosides 51–54 in 76–83% yield.
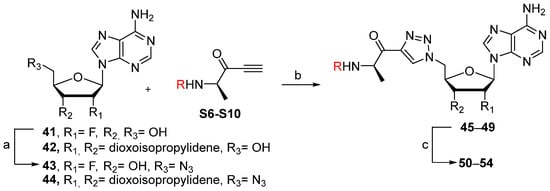
Scheme 7.
Reagents and conditions: (a) See ref [43,44] for reagents and conditions. (a) CuSO4.5H2O, Sodium Ascorbate, t-BuOH/H2O, 40 °C, 24 h. (b) TFA, H2O, CH2Cl2, 20 °C, 5 h for 44 or HClaq (3 M), MeOH, 20 °C, 20 h for 45–48.

Table 1.
N-terminal functionalization of triazolyl nucleoside derivatives.
2.2. Molecular Docking and Biological Studies
Nucleoside 1 has already been reported to effectively inhibit the growth of Bacillus subtilis in the presence of vancomycin by acting as a DltA inhibitor (Ki = 0.2 μM), disrupting the D-alanylation process [16]. To validate the various proposed chemical modifications, the in silico binding affinity of each molecule was measured towards B. Subtilis DltA (PDB 3FCC) and compared to the one obtained with 1. Docking scores listed in Table 2 are the average of nine different minimized energy states. Functionalization of the 2′-position did not disturb the binding affinity, which even seemed to increase with the size of the functional group. In fact, 2′-F and 2′-azido derivatives 8 (−8.2 kcal/mol) and 9 (−8.5 kcal/mol) showed binding affinities similar to 1 (−8.2 kcal/mol) while the introduction of bulky chains led to docking scores of −8.8 kcal/mol for 18 and −9.7 kcal/mol for 11 (Table 2, entries 1–5). Substitution of the sulfamate linkage by either a squaramide, a sulfamide or a triazole resulted in similar or slightly increased binding affinities with docking scores of −8.2 kcal/mol for 30, −8.7 kcal/mol for 23, and −8.9 kcal/mol for 40 (Table 2, entries 6, 7, and 11). N-terminal moiety functionalization of triazole 40 improved affinity, resulting in binding energies ranging from −8.9 kcal/mol to −10.5 kcal/mol (Table 2, entries 13–16). When the nucleosidic sugar pocket is replaced by the fluorinated transbutenyl moiety, the binding affinity of 38 (−7.7 kcal/mol) and 39 (−8.1 kcal/mol) was still close to 1 (Table 2, entries 1, 9, and 10). To ensure these docking results could be extended to E. faecalis, S. aureus, and S. epidermidis, we compared the 3D structures of DltA from these four bacteria (Figure 3). Structural superposition indicated a RMSD ranging from 2.25 Å (480 aa aligned) to 0.790 Å (387 aa aligned) (B. subtilis vs. S. epidermidis), from 4.13 Å (476 aa aligned) to 0.859 Å (368 aa aligned) (B. subtilis vs. E. faecalis), and from 3.45 Å (479 aa aligned) to 0.787 Å (377 aa aligned) (B. subtilis vs. S. aureus). This suggested that the overall structure was well conserved. Regarding the ATP and D-alanine pockets, all the structures were perfectly homologous and exhibited the same spatial distribution. These results of structural homologies supported the idea that the proposed chemical modifications should not disturb the molecular recognition of the different inhibitors by DltA from E. faecalis, S. aureus, and S. epidermidis.

Table 2.
In silico binding affinity and biological studies.
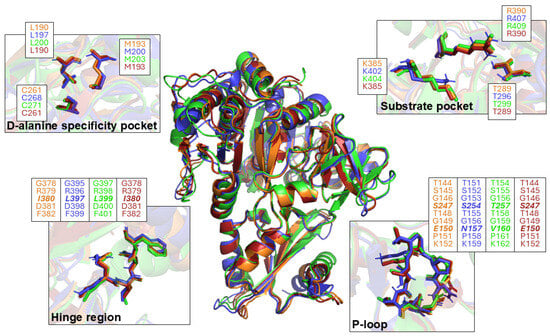
Figure 3.
Structural superposition of DltA from B. subtilis MR168 (blue), S. epidermidis ATCC 35984 (orange), S. aureus Mu50 (dark red), and E. faecalis V583 (green). The structure of DltA from S. epidermidis, S. aureus, and E. faecalis was predicted using AlphaFold Server [46]. The structure of DltA from B. subtilis was previously determined by Yonus [47]. The aa that constitute each region are indicated in the boxes. Residues in bold italic indicate the aa differing between the four structures.
All new compounds were then evaluated in vitro for enzyme inhibition against recombinant DltA from E. faecalis or S. epidermis. Half-maximal inhibitory concentrations (IC50) were then determined by measuring the amount of PPi released at different inhibitor concentrations (Table 2). Our results showed that the IC50 values of compound 1 against DltA from E. faecalis and S. epidermidis were similar, with values of 2.3 μM and 2.4 μM, respectively (Table 2, entry 1). The adenosine 2′-hydroxyl did not seem to be involved in the enzymatic recognition since its substitution by a fluorine atom, leading to compound 8, showed the same activity as 1 (IC50 = 2.4 μM) with an IC50 value of 2.6 μM (Table 2, entries 1 and 2). We noticed the DltA enzyme tolerated the presence of various modifications on the 2′-position, but the activity was inversely related to the size of the introduced group. Indeed, compounds 9 (IC50 = 3.8 μM) and 18 (IC50 = 7.6 μM), containing either an azide or a difluorophosphonylated allylic ether, were respectively 1.6 and 3 times less active than 1 while the activity of the glucose derivative 11 (IC50 = 39.5 μM) dropped 16-fold (Table 2, entries 1, 3–5). Substitution of the sulfamate linkage by a squaramide drastically decreased the enzyme activity with an IC50 value of 28.4 μM for 23 (Table 2, entry 6). Despite a good predicted binding affinity (−8.7 Kcal/mol), we hypothesize that the lower activity could be explained by the fact that the squaramide nitrogen atom might not be under its anionic form, as is the case with sulfamate 1. Compounds 30 (IC50 = 1.8 μM) and 31 (IC50 = 3.2 μM), both containing the more acidic sulfamide linker instead of the squaramide, were as efficient as 1 (Table 2, entries 7 and 8). Surprisingly, when this linkage was introduced onto the fluorinated transbutenyl derivative 38, a complete loss of the activity was observed (Table 2, entry 9). This result contrasted with our previous reported work in which triazolyl transbutenyl derivative 39 (IC50 = 7.5 μM) was found to inhibit DltA in the same order of magnitude as its corresponding nucleosidic analogue 40 (Table 2, entries 10 and 11) [40]. In this triazolyl series, the introduction of a fluorine atom onto the 2′-position did not improve the enzymatic activity (Table 2, entries 11 and 12). Instead, fluorinated compound 50 (IC50 = 14.7 μM) was three times less active than non-fluorinated derivative 40 (IC50 = 4.5 μM). The presence of the amino group at the 5′-end seemed to be necessary since 51–54 were three to five times less active than the amine-free derivative 40 (Table 2, entries 11, 13–16).
All these compounds were also tested in vivo against MRSA clinical isolates. None of them had a significant effect on bacterial growth at concentrations up to 1 mM, thus excluding that they can act as antibiotics. The minimum inhibitory concentrations (MICs) of imipenem (IPM) were next determined in the absence or presence of 1 mM of the different DltA inhibitors to evaluate their potential to re-sensitize MRSA to this β-lactam (Table 2). In the absence of an inhibitor, the MIC value of IPM against the tested MRSA strain was 32 µg/mL, which is well above the clinical breakpoint of resistance of 2 µg/mL. Among the best in vitro Dlt inhibitors with IC50 values ranging from 1.8 to 7.6 μM, only 18 (MIC = 0.25 μg/mL) and 30 (MIC = 1 μg/mL) acted as efficient adjuvants of IPM with MICs comparable with those previously reported with 1 (MIC = 0.25 µg/mL) (Table 2, entries 1, 5 and 7) [17,18,19], while compounds 8, 9, 31, 39, and 40 were 32 to 128 times less active than 1 in vivo emphasizing their potential poor cell permeability or metabolic instability (Table 2, entries 2, 3, 8, 10, and 11). Compounds with moderate and weak inhibitory activity such as 11, 23, 38, 50, 51, 53, and 54 did not re-sensitize the bacteria to IPM since MIC values, ranging from 16 to 64 μg/mL, are above the breakpoint (Table 2, entries 4, 6, 9, 12, 13, 15, and 16). However, we observed that 52 (MIC = 8 μg/mL) slightly restored IPM antimicrobial activity even though this compound was weakly active towards DltA in vitro (Table 2, entry 14). This result seems to indicate that 52 was able to slightly potentiate IPM against MRSA, probably by targeting an enzyme other than DltA. To clarify this hypothesis, quantification of D-alanyl ester of the MRSA cell wall was performed as previously described in the presence of inhibitors (Table 2) [48]. At 1 mM, 40 (MIC = 8 μg/mL) did not affect D-alanylation while 52 (MIC = 8 μg/mL) and 9 (MIC = 8 μg/mL) reduced the level of bacteria cell wall D-alanyl ester residues by 16 % and 74.9%, respectively (Table 2, entries 3, 11 and 14). This clearly supports that the IPM antimicrobial activity observed with the presence of 40 was not related to D-alanylation inhibition, in contrast with 52 and 9, which had an effect on this pathway. In addition to 9 (reduction of 74.9%), molecules exhibiting the best in vivo activities targeted the D-alanylation process to re-sensitize MRSA to IPM. Indeed, a reduction of 51.5% and 80.9% in D-alanyl residues was observed in the presence of compounds 18 and 30, which were as efficient as 1 (reduction of 86.7%) (Table 2, entries 1, 5, and 7). These new series represent a promising structure entry not reported yet for the inhibition of DltA and the re-sensitization of bacteria toward known antibiotics.
3. Materials and Methods
3.1. General Information and Materials
Unless otherwise specified, all reagents were obtained from commercial suppliers and were used without purification. For anhydrous conditions, the glassware was flamed under a continuous nitrogen flow and cooled to room temperature before performing the experiment. Anhydrous solvents (THF, MeCN, Toluene, Et2O, and CH2Cl2) were obtained with PURE SOLV, Innovative Technology Solvent Purification System, by passing the degassed solvents (N2) through a column of activated alumina. Anhydrous Pyridine and DMF were distilled from CaH2. Flash column chromatography was performed on silica gel (40–63 µm), and thin layer chromatography plates were revealed by UV light and/or KMnO4 solution. 1H NMR, 13C NMR, 31P NMR, and 19F NMR spectra were recorded on 500 or 600 MHz apparatus in deuterated solvent. All NMR spectra were calibrated by the residual peaks of the deuterated solvent according to Hugo E. Gottlieb’s values [49]. 19F and 31P NMR spectral lines are with respect to the internal references CFCl3 and H3PO4, respectively. All data are reported in the following order: chemical shifts (δ) in parts per million (ppm), multiplicity (s: singlet, d: doublet, t: triplet, q: quadruplet, m: multiplet, br s: broad signal), coupling constants (J) in Hertz (Hz), and number of protons. All proton and carbon assignments were realized by NMR methods involving DEPT-135, HSQC, HMBC, and COSY experiments. Mass spectra and high-resolution mass spectra (HRMS) were recorded on a Q-TOF (Quadrupole time-of-flight) micro instrument with an electrospray source in the ESI mode. Reversed phase preparative high-performance liquid chromatography was performed on Gemini® 5 μm C18 110 Å, LC Column 250 × 10 mm, equipped with a diode array detector. The exact HPLC-RP method: buffer TFA 0.05%, flow rate 5 mL/min; solvent A/B (MeCN/H2O): 0–3 min: 5%A, 3–7 min: linear gradient 5–100%A, 7–13 min: 100%A.
3.2. General Procedure
General procedure A for the sulfamoylation: (Method A1) To a solution of alcohol (1.0 equiv.) in DMF (0.5 M) were added triethylamine (1.1 equiv.) and sulfamoyl chloride (2.4 equiv.) at 0 °C. The reaction mixture was stirred at 0 °C for 2 h and overnight at 20 °C. The mixture was diluted with EtOAc, then washed three times with H2O and once with brine. The organic layer was dried over MgSO4 and concentrated under reduced pressure to give the desired product. (Method A2) To a solution of alcohol (1.0 equiv.) in DMAc (0.6 M) was added a cooled solution of sulfamoyl chloride (2.4 equiv.) in MeCN (1.9 M) at 0 °C. The mixture was stirred for 2 h at 20 °C, then triethylamine (4.0 equiv.) with MeOH was added at 0 °C. The mixture was stirred for 10 min and concentrated under reduced pressure. The residue was taken up with EtOAc, and the organic layer was washed twice with an aqueous solution of NaHCO3 (1 M) and brine. The organic layer was dried over MgSO4, filtered, and concentrated under reduced pressure to give the desired product.
General procedure B for the amino acid introduction: To a solution of sulfamoyl derivative (1.0 equiv.) and activated alanine (1.0 equiv.) in DMF (0.1 M), 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU) (1.5 equiv.) was added at 20 °C. The mixture was stirred for 16 h, then the solvent was evaporated under reduced pressure. The crude product was purified by column chromatography to give the desired product.
General procedure C for the nucleoside deprotection: (Method C1) To a solution of protected nucleoside analogue (1.0 equiv.) in MeOH (0.1 M), a solution of aqueous HCl (3 M) was added. The reaction mixture was stirred at 20 °C for 20 h, then the solvent was removed under reduced pressure. The residue was taken up with a small amount of MeOH and precipitated by the addition of Et2O. The desired product was isolated after filtration. Method C2: To a solution of protected nucleoside analogue (1.0 equiv.) in CH2Cl2/H2O (5:1), TFA was added, and the reaction mixture was stirred for 5 h at 20 °C. The solvent was removed under reduced pressure. The residue was taken up with a small amount of MeOH and precipitated by the addition of Et2O. The desired product was isolated after filtration.
General procedure D for the sulfamide introduction by Mitsunobu reaction: To a solution of alcohol (1.0 equiv.) in THF (0.05 M), tert-butyl sulfamoylcarbamate (3.0 equiv.) and triphenylphosphine (1.5 equiv.) were added. Then, diisopropyl azodicarboxylate (1.05 equiv.) was added dropwise. The mixture was stirred overnight at 20 °C and concentrated under reduced pressure. The crude mixture was purified by column chromatography to afford the desired product.
General procedure E for the copper-catalyzed azide-alkyne cycloaddition: To a solution of alkyl azide (1.0 equiv.) in t-BuOH/H2O (0.2 M, 1:1), alkyne (1.1 equiv.), followed by sodium ascorbate (10 mol%), was added, and CuSO4.5H2O (5 mol%) was added at 20 °C. The mixture was stirred for 24 h at 40 °C, then the solvent was removed under reduced pressure. The residue was taken up with CH2Cl2/H2O (1:1), and the aqueous layer was extracted three times with CH2Cl2. The combined organic layers were washed with brine, dried over MgSO4, filtered, and then concentrated under reduced pressure. The crude was purified by column chromatography to give the desired product.
General procedure F for the alcohol protection by silyl ether: To a solution of adenosine derivative (1.0 equiv.) in DMF (0.2 M), Imidazole (6.6 equiv.) and DMAP (0.1 equiv.) were added at 20 °C. TBDMSCl (3.3 equiv.) was then added by portion at 0 °C. The reaction mixture was stirred for 24 h at 20 °C, then quenched with an aqueous solution of NH4Cl. The aqueous layer was extracted three times with EtOAc, and the combined organic layers were washed with H2O and brine. The organic layer was dried over MgSO4, filtered, and concentrated under reduced pressure. The crude product was purified by column chromatography to give the desired product.
General procedure G for the selective deprotection of silyl ether: (Method G1) To a solution of protected nucleoside (1.0 equiv.) in H2O and THF (1:1), glacial acetic acid (3 v/v) was added at 0 °C. The mixture was stirred for 2 days at 20 °C, then quenched with a cooled aqueous solution of HCl (1 M). The aqueous layer was extracted three times with EtOAc, and the combined organic layers were washed once with brine. The organic layer was dried over MgSO4, filtered, and concentrated under reduced pressure. The crude product was purified by column chromatography to give the desired product. Method G2: To a solution of protected nucleoside (1.0 equiv.) in THF (4 v/v), an aqueous solution of TFA (1:1) was added at 0 °C. The mixture was stirred for 2 h at 0 °C, then quenched with a cooled aqueous solution of saturated NaHCO3. The aqueous layer was extracted three times with EtOAc, and the combined organic layers were washed once with H2O and brine. The organic layer was dried over MgSO4, filtered, and concentrated under reduced pressure. The crude product was purified by column chromatography to give the desired product.
3.3. Synthesis
((2R,3R,4R,5R)-5-(6-amino-9H-purin-9-yl)-3-((tert-butyldimethylsilyl)oxy)-4-fluorotetrahydrofuran-2-yl)methyl sulfamate (4). The general procedure A (Method A2) was followed with compound 2 (590 mg, 1.54 mmol), prepared as previously described [22], in DMAc (2.4 mL), sulfamoyl chloride (426 mg, 3.69 mmol) in MeCN (1.9 mL). The crude product was purified by column chromatography (Eluent CH2Cl2/MeOH 94:6) to give compound 4 (570 mg, 1.23 mmol, 80%) as a white solid. 1H NMR (CD3CN, 500 MHz): δ 0.00 (s, 3H), 0.04 (s, 3H), 0.84 (s, 9H), 3.87 (dd, J = 12.2 Hz, J = 3.4 Hz, 1H), 4.03 (dd, J = 12.2 Hz, J = 2.3 Hz, 1H), 4.30–4.33 (m, 1H), 5.51 (ddd, JHF = 18.5 Hz, J = 7.2 Hz, J = 4.6 Hz, 1H), 5.76 (ddd, JHF = 51.9 Hz, J = 4.6 Hz, J = 1.8 Hz, 1H), 6.05 (br s, 4H), 6.26 (dd, JHF = 18.3 Hz, J = 1.8 Hz, 1H), 8.11 (s, 1H), 8.24 (s, 1H). 19F NMR (CD3CN, 470 MHz): δ −202.0 (dt, JFH = 51.9 Hz, JFH = 18.5 Hz). 13C NMR (CD3CN, 150 MHz): δ −5.4, −5.3, 19.0, 26.2, 61.8, 74.8 (d, JCF = 14.8 Hz), 82.0, 87.9 (d, JCF = 33.6 Hz), 92.4 (d, JCF = 189.9 Hz), 120.7, 140.5, 150.3, 154.1, 157.0. HRMS-ESI (m/z) calcd for C16H28FN6O5SiS [M+H]+ 463.1595, found 463.1602. The NMR spectrum is identical to that previously reported [13].
((2R,3S,4S,5R)-5-(6-amino-9H-purin-9-yl)-4-azido-3-((tert-butyldimethylsilyl)oxy)tetrahydrofuran-2-yl)methyl sulfamate (5). The general procedure A (Method A2) was followed with compound 3 (250 mg, 0.61 mmol), prepared as previously described [23], in DMAc (1 mL), sulfamoyl chloride (170 mg, 1.47 mmol) in MeCN (0.75 mL) to give compound 5 (265 mg, 0.54 mmol, 89%) as a white solid. The compound was used in the next step without further purification. 1H NMR (MeOD, 500 MHz): δ 0.23 (s, 3H), 0.24 (s, 3H), 1.00 (s, 9H), 4.28–4.32 (m, 2H), 4.40–4.43 (m, 1H), 4.72–4.74 (m, 1H), 4.87–4.89 (m, 1H), 6.17 (d, J = 6.1 Hz, 1H), 8.23 (s, 1H), 8.30 (s, 1H). 13C NMR (MeOD, 125 MHz): δ −4.8, −4.6, 21.3, 26.3, 66.1, 68.8, 74.1, 84.6, 87.6, 120.6, 141.1, 150.6, 154.1, 157.5. HRMS-ESI (m/z) calcd for C16H28N9O5SSi [M+H]+ 486.1703, found 486.1710.
((2R,3R,4R,5R)-5-(6-amino-9H-purin-9-yl)-3-((tert-butyldimethylsilyl)oxy)-4-fluorotetrahydrofuran-2-yl)methyl ((tert-butoxycarbonyl)-D-alanyl)sulfamate (6). The general procedure B was followed with compound 4 (375 mg, 0.81 mmol), Boc-D-Ala-OSu (232 mg, 0.81 mmol), and DBU (0.18 mL, 1.22 mmol) in DMF (10.8 mL). The crude was purified by column chromatography (Eluent CH2Cl2/MeOH 94:6 to 9:1) to give compound 6 (392 mg, 0.61 mmol, 76%) as a white foam. 1H NMR (DMSO-d6, 600 MHz): δ −0.02 (s, 3H), 0.00 (s, 3H), 0.82 (s, 9H), 1.17 (d, J = 7.1 Hz, 3H), 1.33 (s, 9H), 3.75–3.83 (m, 2H), 3.91–3.95 (m, 1H), 4.16–4.20 (m, 1H), 4.37–4.46 (m, 1H), 5.66 (d, JHF = 52.1 Hz, 1H), 6.27 (dd, JHF = 17.3 Hz, J = 2.3 Hz, 1H), 6.46 (br s, 1H), 7.40 (br s, 2H), 8.14 (s, 1H), 8.27 (s, 1H). 19F NMR (DMSO-d6, 565 MHz): δ −201.5 (m). 13C NMR (DMSO-d6, 150 MHz): δ −5.6, −5.5, 18.1, 18.5, 25.8, 28.2, 51.3, 62.2, 74.0, 77.8, 81.5, 85.7 (d, JCF = 32.2 Hz), 91.2 (d, JCF = 190.2 Hz), 119.0, 139.1, 148.8, 152.7, 154.8, 156.1, 176.1. HRMS-ESI (m/z) calcd for C24H41FN7O8SiS [M+H]+ 634.2491, found 634.2489.
((2R,3S,4S,5R)-5-(6-amino-9H-purin-9-yl)-4-azido-3-((tert-butyldimethylsilyl)oxy)tetrahydrofuran-2-yl)methyl ((tert-butoxycarbonyl)-D-alanyl)sulfamate (7). The general procedure B was followed with compound 5 (438 mg, 0.90 mmol), Boc-D-Ala-OSu (258 g, 0.90 mmol), and DBU (0.20 mL, 1.35 mmol) in DMF (12 mL). The crude was purified by column chromatography (Eluent EtOAc with 1% AcOH) to give compound 7 (408 mg, 0.62 mmol, 69%) as a white foam. 1H NMR (CD3CN, 600 MHz): δ 0.18 (s, 3H), 0.19 (s, 3H), 0.96 (s, 9H), 1.27 (d, J = 7.1 Hz, 3H), 1.37 (s, 9H), 4.00–4.02 (m, 1H), 4.30–4.32 (m, 1H), 4.44 (dd, J = 11.2 Hz, J = 3.7 Hz, 1H), 4.55–4.60 (m, 2H), 4.78–4.79 (m, 1H), 5.82 (br s, 1H), 6.09 (d, J = 7.1 Hz, 1H), 8.34 (s, 1H), 8.38 (s, 1H). 13C NMR (CD3CN, 150 MHz): δ −4.7, −4.6, 18.6, 19.6, 26.1, 28.6, 53.7, 66.5, 68.1, 73.8, 79.7, 84.5, 86.5, 120.2, 141.1, 150.6, 154.1, 156.5, 156.7, 171.7. HRMS-ESI (m/z) calcd for C24H41N10O8SSi [M+H]+ 657.2599, found 657.2601.
((2R,3R,4R,5R)-5-(6-amino-9H-purin-9-yl)-4-fluoro-3-hydroxytetrahydrofuran-2-yl)methyl (D-alanyl)sulfamate salt (8). The general procedure C (Method C2) was followed with compound 6 (498 mg, 0.78 mmol), TFA (9.7 mL), and CH2Cl2/H2O (8/2 mL) to give compound 8 (450 mg, 0.69 mmol, 89%) as a white solid (2 TFA salt). 1H NMR (D2O, 500 MHz): δ 1.52 (d, J = 7.1 Hz, 3H), 3.81 (dd, J = 13.1 Hz, J = 3.7 Hz, 1H), 3.89 (q, J = 7.1 Hz, 1H), 3.98 (dd, J = 13.1 Hz, J = 2.3 Hz, 1H), 4.42–4.46 (m, 1H), 5.51 (ddd, JHF = 17.2 Hz, J = 7.6 Hz, J = 4.8 Hz, 1H), 5.76 (ddd, JHF = 51.1 Hz, J = 4.8 Hz, J = 2.0 Hz, 1H), 6.51 (dd, JHF = 18.4 Hz, J = 2.0 Hz, 1H), 8.45 (s, 1H), 8.51 (s, 1H). 19F NMR (D2O, 470 MHz): δ −200.8 (dt, JFH = 51.1 Hz, JFH = 17.6 Hz). 13C NMR (D2O, 150 MHz): δ 51.3, 59.2, 73.8 (d, JCF = 14.9 Hz), 81.5, 87.3 (d, JCF = 34.8 Hz), 91.4 (d, JCF = 191.9 Hz), 116.2 (q, JCF = 291.4 Hz), 117.2, 119.0, 143.3, 144.6, 147.9, 150.0, 162.9 (q, JCF = 35.3 Hz, TFA), 176.4. HPLC purity: (96.6%, tR = 4.48 min). HRMS-ESI (m/z) calcd for C13H18FN7O6S [M+H]+ 420.1102, found 420.1102.
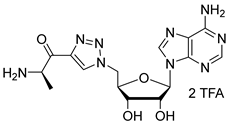
((2R,3S,4S,5R)-5-(6-amino-9H-purin-9-yl)-4-azido-3-hydroxytetrahydrofuran-2-yl)methyl (D-alanyl)sulfamate salt (9). To a solution of compound 7 (165 mg, 0.25 mmol, 1 equiv.) in H2O (2.5 mL) was added TFA (10 mL) at 20 °C. The mixture was stirred for 12 h and concentrated under reduced pressure. The residue was taken up with a small amount of MeOH. By addition of Et2O, a precipitate appeared, which was isolated by filtration to give compound 9 (91 mg, 0.13 mmol, 54%) as a white solid (2 TFA salt). 1H NMR (DMSO-d6, 500 MHz): δ 1.29 (d, J = 7.1 Hz, 3H), 3.48–3.50 (m, 1H), 4.07 (dd, J = 11.1 Hz, J = 4.3 Hz, 1H), 4.14–4.17 (m, 1H), 4.19 (dd, J = 11.1 Hz, J = 3.7 Hz, 1H), 4.57–4.59 (m, 1H), 4.67–4.69 (m, 1H), 6.06 (d, J = 6.1 Hz, 1H), 6.17 (d, J = 5.4 Hz, 1H), 7.35 (br s, 1H), 7.81 (br s, 2H), 8.16 (s, 1H), 8.39 (s, 1H). 13C NMR (DMSO-d6, 150 MHz): δ 17.2, 50.8, 64.2, 67.2, 71.5, 83.0, 84.8, 118.9, 139.3, 149.3, 152.9, 156.1, 173.4. HPLC purity: (94.9%, tR = 4.59 min). HRMS-ESI (m/z) calcd for C13H19N10O6S [M+H]+ 443.1210, found 443.1208.
(2R,3R,4S,5R,6R)-2-(acetoxymethyl)-6-((1-((2R,3R,4S,5R)-2-(6-amino-9H-purin-9-yl)-5-(((N-((tert-butoxycarbonyl)-D-alanyl)sulfamoyl)oxy)methyl)-4-((tert-butyldimethylsilyl)oxy)tetrahydrofuran-3-yl)-1H-1,2,3-triazol-4-yl)methoxy)tetrahydro-2H-pyran-3,4,5-triyl triacetate (10). The general procedure E was followed with compound 7 (269 mg, 0.41 mmol), glucosyl-O-propargyl ether (175 mg, 0.45 mmol), prepared as previously described [50], sodium ascorbate (8 mg, 0.04 mmol) and CuSO4.5H2O (5 mg, 0.02 mmol) in t-BuOH/H2O (4 mL). The crude was purified by column chromatography (Eluent EtOAc/MeOH 9:1) to give compound 10 (276 mg, 0.26 mmol, 64%) as a white solid. 1H NMR (CD3CN, 600 MHz): δ −0.18 (s, 3H), 0.01 (s, 3H), 0.73 (s, 9H), 1.23 (d, J = 7.4 Hz, 3H), 1.33 (s, 9H), 1.85 (s, 3H), 1.92 (s, 3H), 1.97 (s, 3H), 2.02 (s, 3H), 3.82 (ddd, J = 10.1 Hz, J = 4.7 Hz, J = 2.5 Hz, 1H), 3.98 (br s, 1H), 4.10 (dd, J = 12.4 Hz, J = 2.5 Hz, 1H), 4.25 (dd, J = 12.4 Hz, J = 4.7 Hz, 1H), 4.28 (dd, J = 10.4 Hz, J = 3.6 Hz, 1H), 4.38–4.39 (m, 1H), 4.44–4.46 (m, 1H), 4.68 (d, J = 12.4 Hz, 1H), 4.71 (d, J = 8.1 Hz, 1H), 4.81 (d, J = 12.4 Hz, 1H), 4.85–4.88 (m, 2H), 5.02 (t, J = 9.7 Hz, 1H), 5.18 (t, J = 9.7 Hz, 1H), 5.78 (br s, 1H), 5.96 (br s, 1H), 6.28 (br s, 2H), 6.80 (d, J = 5.6 Hz, 1H), 7.94 (s, 1H), 8.19 (s, 1H), 8.39 (br s, 1H). 13C NMR (CD3CN, 150 MHz): δ −4.9, −4.8, 18.3, 19.7, 20.8, 20.9 (2C), 21.0, 25.9, 28.6, 53.7, 62.7, 62.9, 66.2, 68.2, 69.2, 71.9, 72.5, 72.8, 73.4, 79.7, 85.1, 86.3, 100.3, 120.3, 126.0, 141.0, 144.3, 150.6, 154.1, 156.4, 156.8, 170.2, 170.5, 170.9, 171.4, 182.0. HRMS-ESI (m/z) calcd for C41H63N10O18SSi [M+H]+ 1043.3812, found 1043.3835.
((2R,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-3-hydroxy-4-(4-((((2R,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)tetrahydro-2H-pyran-2-yl)oxy)methyl)-1H-1,2,3-triazol-1-yl)tetrahydrofuran-2-yl)methyl (D-alanyl)sulfamate salt (11). A solution of compound 10 (70 mg, 0.06 mmol, 1 equiv.) in H2O (2 mL) and TFA (8 mL) was stirred for 12 h at 20 °C. Volatiles were removed under reduced pressure, and the residue was dissolved in MeOH (1 mL), and a solution of MeONa (5.4 M) in MeOH (0.05 mL, 0.3 mmol, 5 equiv.) was added at 20 °C. The mixture was stirred for 1 h and quenched with an aqueous solution of TFA (10%), then concentrated under reduced pressure. The residue was dissolved in THF (1 mL), and a solution of 37% triethylamine trihydrofluoride (0.1 mL, 0.24 mmol, 4 equiv.) was added at 20 °C. The mixture was stirred for 12 h, and volatiles were removed under reduced pressure. The crude was purified by HPLC RP-18 (MeCN/H2O, linear gradient) to give compound 11 (17 mg, 0.26 mmol, 32%) as a white solid (2 TFA salt). 1H NMR (D2O, 600 MHz): δ 1.48 (d, J = 7.2 Hz, 3H), 3.24–3.26 (m, 1H), 3.33–3.37 (m, 1H), 3.41–3.45 (m, 2H), 3.66 (dd, J = 12.7 Hz, J = 6.3 Hz, 1H), 3.85–3.87 (m, 1H), 3.89–3.93 (m, 1H), 4.46–4.48 (m, 1H), 4.51–4.57 (m, 2H), 4.62–4.64 (m, 1H), 4.88 (d, J = 12.7 Hz, 1H), 4.99 (d, J = 12.7 Hz, 1H), 5.05 (t, J = 5.6 Hz, 1H), 5.99 (t, J = 5.6 Hz, 1H), 6.99 (d, J = 4.9 Hz, 1H), 8.26 (s, 1H), 8.39 (s, 1H), 8.60 (s, 1H). 13C NMR (D2O, 150 MHz): δ 16.3, 51.0, 60.6, 61.7, 65.8, 68.3, 69.5, 69.8, 72.9, 75.6, 75.9, 83.2, 86.3, 101.4, 116.2 (q, JCF = 291.1 Hz), 119.2, 126.6, 142.7, 143.7, 144.6, 148.1, 149.9, 162.9 (q, JCF = 35.8 Hz), 175.4. HPLC purity: (88.5%, tR = 2.42 min). HRMS-ESI (m/z) calcd for C22H33N10O12S [M+H]+ 661.2000, found 661.2016.
diisopropyl ((E)-4-(((2R,3R,4R,5R)-2-(6-(N-benzoylbenzamido)-9H-purin-9-yl)-4-((tert-butyldimethylsilyl)oxy)-5-(((tert-butyldimethylsilyl)oxy)methyl)tetrahydrofuran-3-yl)oxy)-1,1-difluorobut-2-en-1-yl)phosphonate (13). The general procedure F was followed with compound 12 (320 mg, 0.44 mmol), prepared as previously described [33], TBDMSCl (218 mg, 1.45 mmol), imidazole (196 mg, 2.89 mmol), and DMAP (4.9 mg, 0.04 mmol) in DMF (5 mL). The crude product was purified by column chromatography (Eluent Pentane/EtOAc 7:3) to give compound 13 (333 mg, 0.35 mmol, 79%) as a white foam. 1H NMR (CDCl3, 500 MHz): δ 0.06 (s, 3H), 0.08 (s, 3H), 0.11 (s, 6H), 0.90 (s, 9H), 0.92 (s, 9H), 1.33 (t, J = 6.2 Hz, 6H), 1.35 (dd, J = 6.2 Hz, JHP = 1.7 Hz, 6H), 3.78 (dd, J = 11.7 Hz, J = 3.1 Hz, 1H), 3.98 (dd, J = 11.7 Hz, J = 3.4 Hz, 1H), 4.13 (dt, J = 5.7 Hz, J = 3.1 Hz, 1H), 4.22–4.31 (m, 2H), 4.36 (dd, J = 5.7 Hz, J = 3.6 Hz, 1H), 4.53–4.55 (m, 1H), 4.79–4.86 (m, 2H), 5.95–6.03 (m, 1H), 6.18 (d, J = 3.6 Hz, 1H), 6.26–6.33 (m, 1H), 7.33–7.36 (m, 4H), 7.46–7.49 (m, 2H), 7.84–7.86 (m, 4H), 8.37 (s, 1H), 8.65 (s, 1H). 19F NMR (CDCl3, 565 MHz): δ −109.5 (ddd, JFF = 298.3 Hz, JFP = 113.2 Hz, JFH = 12.4 Hz), −110.3 (ddd, JFF = 298.3 Hz, JFP = 113.2 Hz, JFH = 12.4 Hz). 31P NMR (CDCl3, 243 MHz): δ 4.3 (t, JPF = 113.2 Hz). 13C NMR (CDCl3, 150 MHz): δ −5.3, −5.2, −4.7, −4.5, 18.2, 18.6, 23.8 and 23.9 (d, JCP = 4.1 Hz), 24.3 (d, JCP = 3.4 Hz, 2C), 25.8, 26.2, 61.8, 69.3, 69.8, 73.9 (d, JCP = 7.1 Hz, 2C), 82.1, 85.1, 87.3, 116.8 (dt, JCF = 258.9 Hz, JCP = 220.7 Hz), 122.7 (dt, JCF = 22.3 Hz, JCP = 13.4 Hz), 128.3, 128.8 (4C), 129.6 (4C), 133.1 (2C), 134.2 (2C), 134.4 (dt, JCF = 9.8 Hz, JCP = 5.6 Hz), 143.7, 151.9, 152.3, 152.7, 172.4. HRMS-ESI (m/z) calcd for C46H67F2N5O9PSi2 [M+H]+ 958.4183, found 958.4179.
tert-butyl (tert-butoxycarbonyl)(9-((2R,3R,4R,5R)-4-((tert-butyldimethylsilyl)oxy)-5-(((tert-butyldimethylsilyl)oxy)methyl)-3-(((E)-4-(diisopropoxyphosphoryl)-4,4-difluorobut-2-en-1-yl)oxy)tetrahydrofuran-2-yl)-9H-purin-6-yl)carbamate (14). To a solution of compound 13 (300 mg, 0.44 mmol, 1 equiv.) in EtOH (3.5 mL), CH3NH2 (2.1 mL, 33% in EtOH) was added at 20 °C. The mixture was stirred for 1 h and followed by TLC. When the conversion was quantitative, the solvent was removed under reduced pressure. It was dissolved in DMF (4 mL), and triethylamine (0.17 mL, 1.20 mmol, 3 equiv.) and DMAP (5 mg, 0.04 mmol, 0.5 equiv.) were added at 20 °C. The solution was cooled, and (Boc)2O (0.27 mL, 1.20 mmol, 3 equiv.) was added dropwise at 0 °C. The reaction mixture was stirred for 12 h and quenched with an aqueous solution of HCl (1 M). The aqueous layer was extracted three times with EtOAc. The combined organic layers were washed with brine, dried over MgSO4, filtered, and concentrated under reduced pressure. The crude product was purified by column chromatography (Eluent Pentane/EtOAc 7:3) to give compound 14 (326 mg, 0.34 mmol, 78%) as a waxy oil. 1H NMR (CDCl3, 600 MHz): δ 0.07 (s, 3H), 0.08 (s, 3H), 0.12 (s, 3H), 0.13 (s, 3H), 0.91 (s, 9H), 0.94 (s, 9H), 1.33 (dd, J = 6.4 Hz, JHP = 4.3 Hz, 6H), 1.36 (dd, J = 6.4 Hz, JHP = 1.9 Hz, 6H), 1.45 (s, 18H), 3.81 (dd, J = 11.6 Hz, J = 2.6 Hz, 1H), 4.05 (dd, J = 11.6 Hz, J = 3.4 Hz, 1H), 4.16 (dt, J = 6.3 Hz, J = 2.6 Hz, 1H), 4.24 (dd, J = 4.7 Hz, J = 3.1 Hz, 1H), 4.26–4.30 (m, 1H), 4.36–4.40 (m, 1H), 4.52 (dd, J = 6.3 Hz, J = 4.7 Hz, 1H), 4.80–4.86 (m, 2H), 5.98–6.06 (m, 1H), 6.22 (d, J = 3.1 Hz, 1H), 6.29–6.33 (m, 1H), 8.54 (s, 1H), 8.84 (s, 1H). 19F NMR (CDCl3, 565 MHz): δ −109.6 (ddd, JFF = 298.4 Hz, JFP = 112.4 Hz, JFH = 12.2 Hz), −110.2 (ddd, JFF = 298.4 Hz, JFP = 112.4 Hz, JFH = 12.2 Hz). 31P NMR (CDCl3, 243 MHz): δ 4.3 (t, JPF = 112.4 Hz). 13C NMR (CDCl3, 150 MHz): δ −5.3, −5.2, −4.8, −4.5, 18.2, 18.6, 23.8 and 23.9 (d, JCP = 4.3 Hz), 24.3 (d, JCP = 3.4 Hz, 2C), 25.8, 26.2, 27.9, 61.5, 69.3, 69.4, 73.9 (d, JCP = 7.1 Hz, 2C), 82.5, 84.0, 84.7, 87.3, 116.7 (dt, JCF = 259.1 Hz, JCP = 220.8 Hz), 122.7 (dt, JCF = 21.3 Hz, JCP = 13.4 Hz), 129.4, 134.4 (dt, JCF = 9.8 Hz, JCP = 5.3 Hz), 143.4, 150.4, 150.6, 152.2, 152.6. HRMS-ESI (m/z) calcd for C42H75F2N5O11PSi2 [M+H]+ 950.4707, found 950.4697.
tert-butyl (tert-butoxycarbonyl)(9-((2R,3R,4R,5R)-4-((tert-butyldimethylsilyl)oxy)-3-(((E)-4-(diisopropoxyphosphoryl)-4,4-difluorobut-2-en-1-yl)oxy)-5-(hydroxymethyl)tetrahydrofuran-2-yl)-9H-purin-6-yl)carbamate (15). The general procedure G (method G1) was followed with compound 14 (666 mg, 0.70 mmol) in H2O (1.2 mL), THF (1.2 mL), and AcOH (3.75 mL). The crude product was purified by column chromatography (Eluent Cyclohexane/EtOAc 6:4 to 4:6) to give compound 15 (315 mg, 0.38 mmol, 54%) as a colorless oil. 1H NMR (CDCl3, 600 MHz): δ 0.12 (s, 6H), 0.93 (s, 9H), 1.31 (dd, J = 6.2 Hz, JHP = 2.6 Hz, 6H), 1.33 (d, J = 6.2 Hz, 6H), 1.46 (s, 18H), 3.73 (dd, J = 13.1 Hz, J = 1.4 Hz, 1H), 3.86–3.89 (m, 1H), 3.97 (dd, J = 13.1 Hz, J = 1.8 Hz, 1H), 4.08–4.11 (m, 1H), 4.21–4.23 (m, 1H), 4.57–4.58 (m, 1H), 4.72 (dd, J = 7.6 Hz, J = 4.5 Hz, 1H), 4.77–4.83 (m, 2H), 5.83–5.90 (m, 1H), 6.01 (d, J = 7.6 Hz, 1H), 6.10–6.15 (m, 1H), 8.22 (s, 1H), 8.82 (s, 1H). 19F NMR (CDCl3, 470 MHz): δ −109.7 (ddd, JFF = 315.6 Hz, JFP = 113.2 Hz, JFH = 12.3 Hz), −110.6 (ddd, JFF = 315.6 Hz, JFP = 113.2 Hz, JFH = 12.3 Hz). 31P NMR (CDCl3, 202 MHz): δ 4.2 (t, JPF = 113.2 Hz). 13C NMR (CDCl3, 150 MHz): δ −4.6, −4.5, 18.2, 23.8 and 23.9 (d, JCP = 2.3 Hz), 24.2 and 24.3 (d, JCP = 1.4 Hz), 25.8, 27.9, 62.9, 69.1, 71.6, 73.9 and 74.0 (d, JCP = 3.7 Hz), 80.9, 84.3, 89.5, 89.6, 116.6 (dt, JCF = 258.6 Hz, JCP = 220.6 Hz), 122.7 (dt, JCF = 21.2 Hz, JCP = 13.4 Hz), 130.4, 134.0 (dt, JCF = 10.1 Hz, JCP = 5.8 Hz), 144.9, 150.5, 151.4, 151.6, 152.0. HRMS-ESI (m/z) calcd for C36H61F2N5O11PSi [M+H]+ 836.3843, found 836.3840.
((2R,3R,4R,5R)-5-(6-(bis(tert-butoxycarbonyl)amino)-9H-purin-9-yl)-3-((tert-butyldimethylsilyl)oxy)-4-(((E)-4-(diisopropoxyphosphoryl)-4,4-difluorobut-2-en-1-yl)oxy)tetrahydrofuran-2-yl)methyl sulfamate (16). The general procedure A (Method A2) was followed with compound 15 (100 mg, 0.12 mmol) in DMAc (0.6 mL), sulfamoyl chloride (33 mg, 0.29 mmol) in MeCN (0.5 mL) to give compound 16 (105 mg, 0.10 mmol, 84%) with DMAc (13% w/w) as a colorless oil. This compound was used in the next step without further purification to avoid its degradation. 1H NMR (CDCl3, 600 MHz): δ 0.12 (s, 6H), 0.91 (s, 9H), 1.33 (dd, J = 6.2 Hz, JHP = 2.4 Hz, 6H), 1.36 (d, J = 6.2 Hz, 6H), 1.45 (s, 18H), 4.20–4.27 (m, 2H), 4.30–4.33 (m, 2H), 4.47–4.49 (m, 1H), 4.53–4.55 (m, 1H), 4.58–4.60 (m, 1H), 4.80–4.87 (m, 2H), 5.88 (br, s, 2H), 5.93–5.96 (m, 1H), 6.20 (d, J = 4.2 Hz, 1H), 6.20–6.24 (m, 1H), 8.44 (s, 1H), 8.85 (s, 1H). 19F NMR (CDCl3, 565 MHz): δ −109.7 (ddd, JFF = 334.1 Hz, JFP = 114.2 Hz, JFH = 12.8 Hz, 1F), −110.5 (ddd, JFF = 334.1 Hz, JFP = 114.2 Hz, JFH = 12.8 Hz, 1F). 31P NMR (CDCl3, 243 MHz): δ 4.0 (t, JPF = 114.2 Hz). 13C NMR (CDCl3, 150 MHz): δ −4.8, −4.6, 18.1, 23.8 and 23.9 (d, JCP = 4.7 Hz), 24.2 and 24.3 (d, JCP = 2.3 Hz), 25.8, 27.9, 67.9, 69.3, 70.5, 74.3 and 74.4 (d, JCP = 7.2 Hz), 80.6, 82.7, 84.3, 87.5, 116.5 (dt, JCF = 258.4 Hz, JCP = 220.7 Hz), 123.3 (dt, JCF = 21.3 Hz, JCP = 13.7 Hz), 128.9, 134.4 (dt, JCF = 9.8 Hz, JCP = 5.7 Hz), 143.8, 150.4, 150.6, 152.4, 152.7. HRMS-ESI (m/z) calcd for C36H62F2N6O13SiPS [M+H]+ 915.3571, found 915.3564.
((2R,3R,4R,5R)-5-(6-(bis(tert-butoxycarbonyl)amino)-9H-purin-9-yl)-3-((tert-butyldimethylsilyl)oxy)-4-(((E)-4-(diisopropoxyphosphoryl)-4,4-difluorobut-2-en-1-yl)oxy)tetrahydrofuran-2-yl)methyl ((tert-butoxycarbonyl)-D-alanyl)sulfamate (17). The general procedure B was followed with compound 16 (285 mg, 0.31 mmol), Boc-D-Ala-OSu (89 mg, 0.31 mmol), and DBU (0.07 mL, 0.46 mmol) in DMF (4.4 mL). The crude was purified by column chromatography (Eluent EtOAc/CycloHex 9:1) to give compound 17 (195 mg, 0.18 mmol, 58%) as a waxy oil. 1H NMR (Acetone-d6, 500 MHz): δ 0.19 (s, 3H), 0.21 (s, 3H), 0.97 (s, 9H), 1.29 (dd, J = 6.3 Hz, JHP = 1.8 Hz, 6H), 1.31 (d, J = 6.4 Hz, 3H), 1.33 (d, J = 6.2 Hz, 6H), 1.39 (s, 9H), 1.44 (s, 18H), 4.01–4.06 (m, 1H), 4.28–4.38 (m, 4H), 4.41–4.46 (m, 1H), 4.75–4.83 (m, 4H), 5.96–6.04 (m, 2H), 6.26–6.31 (m, 1H), 6.39–6.41 (m, 1H), 8.77 (s, 1H), 8.83 (s, 1H). 19F NMR (Acetone-d6, 470 MHz): δ −109.8 (ddd, JFF = 291.6 Hz, JFP = 113.4 Hz, JFH = 12.9 Hz), −110.5 (ddd, JFF = 291.6 Hz, JFP = 113.4 Hz, JFH = 12.9 Hz). 31P NMR (Acetone-d6, 202 MHz): δ 3.7 (t, JPF = 111.6 Hz). 13C NMR (Acetone-d6, 150 MHz): δ −4.5, −4.4, 18.6, 19.9, 23.9 and 24.0 (d, JCP = 3.1 Hz), 24.3 (d, JCP = 3.3 Hz, 2C), 26.2, 27.9, 28.7, 53.2, 68.9, 69.6, 71.9, 74.3 and 74.4 (d, JCP = 6.4 Hz), 79.0, 82.2, 84.0, 84.3, 87.4, 117.7 (dt, JCF = 257.6 Hz, JCP = 222.3 Hz), 122.7 (dt, JCF = 22.1 Hz, JCP = 13.5 Hz), 129.7, 135.8 (dt, JCF = 10.3 Hz, JCP = 5.6 Hz), 145.1, 151.0, 151.2, 152.7, 153.9, 155.9, 181.0. HRMS-ESI (m/z) calcd for C44H75F2N7O16PSSi [M+H]+ 1086.4466, found 1086.4462.
((2R,3R,4R,5R)-5-(6-amino-9H-purin-9-yl)-4-(((E)-4-(diisopropoxyphosphoryl)-4,4-difluorobut-2-en-1-yl)oxy)-3-hydroxytetrahydrofuran-2-yl)methyl (D-alanyl)sulfamate salt (18). The general procedure C (Method C2) was followed with compound 17 (317 mg, 0.29 mmol), TFA (3.6 mL), and CH2Cl2/H2O (3:0.7 mL). The crude was purified by HPLC RP-18 (MeCN/H2O, linear gradient) to give compound 18 (112 mg, 0.12 mmol, 43%) as a white solid (2 TFA salt). 1H NMR (MeOD, 600 MHz): δ 1.31 (d, J = 6.1 Hz, 6H), 1.34 (dd, J = 6.1 Hz, JHP = 1.8 Hz, 6H), 1.52 (d, J = 7.1 Hz, 3H), 3.87 (q, J = 7.1 Hz, 1H), 4.32–4.36 (m, 2H), 4.43–4.47 (m, 2H), 4.48–4.53 (m, 2H), 4.75–4.80 (m, 2H), 5.91–5.99 (m, 1H), 6.27 (d, J = 4.1 Hz, 1H), 6.29–6.33 (m, 1H), 8.42 (s, 1H), 8.62 (s, 1H). 19F NMR (MeOD, 470 MHz): δ −111.5 (m), −111.2 (m). 31P NMR (MeOD, 202 MHz): δ 4.2 (t, JPF = 114.6 Hz). 13C NMR (MeOD, 150 MHz): δ 17.3, 24.0 (d, JCP = 4.9 Hz, 2C), 24.3 and 24.4 (d, JCP = 1.2 Hz), 52.0, 70.2, 70.5, 72.2, 75.9 (d, JCP = 7.3 Hz, 2C), 83.7, 85.0, 88.8, 117.8 (dt, JCF = 252.8 Hz, JCP = 225.1 Hz), 120.4, 122.6 (dt, JCF = 21.9 Hz, JCP = 13.8 Hz), 136.9 (dt, JCF = 9.8 Hz, JCP = 5.6 Hz), 143.4, 146.3, 149.8, 152.4, 174.1. HPLC purity: (77.6%, tR = 10.53 min). HRMS-ESI (m/z) calcd for C23H37F2N7O10PS [M+H]+ 672.2028, found 672.2036.
2′,3′-O-isopropylidene-5′-N-(3-amino-cyclobut-3-ene-1,2-dione)aminodeoxyadenosine (21). To a solution of 20 (0.92 g, 3 mmol, 1 equiv.), prepared as previously described [36,37], in MeOH (20 mL), dimethyl squarate (0.89 g, 6.26 mmol, 2 equiv.) was added at 20 °C. The mixture was stirred for 4 h and concentrated under reduced pressure. The residue was dissolved in MeOH (70 mL), and a solution of NH3 (7 N) in MeOH (4.87 mL) was added at 20 °C. The mixture was stirred for 16 h, and the reaction mixture was filtered. The solid was washed with pentane to give compound 21 (0.58 g, 1.45 mmol, 48%) as a white foam. The product was used in the next step without further purification. 1H NMR (DMSO-d6, 500 MHz): δ 1.32 (s, 3H), 1.54 (s, 3H), 3.70–3.76 (m, 1H), 3.94 (br s, 1H), 4.24–4.27 (m, 1H), 5.00–5.02 (m, 1H), 5.43 (s, 1H), 6.20 (s, 1H), 7.37 (br s, 2H), 7.44 (br s, 1H), 8.19 (s, 1H), 8.33 (s, 1H). 13C NMR (DMSO-d6, 150 MHz): δ 25.7, 27.5, 27.3, 45.6, 81.6, 83.6, 89.2, 114.2, 119.6, 140.4, 149.2, 153.4, 156.6, 170.1, 183.8.
2′,3′-O-isopropylidene-5′-N-(N-tert-butoxycarbonyl-D-alanyl]-(3amino-cyclobut-3-ene-1,2-dione))aminodeoxy adenosine (22). The general procedure B was followed with compound 21 (200 mg, 0.5 mmol), Boc-D-Ala-OSu (180 mg, 0.63 mmol), and DBU (0.19 mL, 1.2 mmol) in DMF (5 mL) at 60 °C for 4h. The crude mixture was purified by column chromatography (Eluent EtOAc/MeOH 9:1) to give compound 22 (140 mg, 0.244 mmol, 49%) as a white solid. 1H NMR (DMSO-d6, 500 MHz): δ 1.19–1.20 (m, 5H), 1.31 (s, 3H), 1.36 (s, 18H), 1.53 (s, 3H), 3.69–3.74 (m, 1H), 3.81–3.87 (m, 2H), 4.23–4.27 (m, 1H), 5.01–5.03 (m, 1H), 5.42 (s, 1H), 6.18 (s, 1H), 6.87 (br s, 1H), 7.36 (s, 1H), 7.85 (br s, 1H), 8.17 (s, 1H), 8.33 (s, 1H). 13C NMR (DMSO-d6, 150 MHz): δ 18.1, 25.7, 27.5, 28.6, 45.5, 49.7, 78.2, 81.7, 83.2, 83.6, 89.2, 113.8, 114.2, 119.6, 140.3, 149.3, 153.4, 155.5, 156.6, 170.2, 175.5, 183.7. HRMS-ESI (m/z) calcd for C25H33N8O8 [M+H]+ 573.2421 found 573.2428.
5′-N-(N-D-alanyl]-(3-amino-cyclobut-3-ene-1,2-dione))aminodeoxy adenosine salt (23). The general procedure C (Method C2) was followed with compound 22 (210 mg, 0.37 mmol), TFA (5 mL), and CH2Cl2/H2O (4/1 mL). The crude mixture was purified by HPLC RP-C18 (MeCN/H2O, linear gradient) to give compound 23 (100.8 mg, 0.153 mmol, 64%) as a white solid (2 TFA salt). 1H NMR (DMSO-d6, 500 MHz): δ 1.38–1.43 (m, 3H), 3.86–3.94 (m, 1H), 4.05–4.13 (m, 3H),4.18–4.23 (m, 1H), 4.61–4.65 (m, 1H), 5.93 (d, J = 5.5 Hz, 1H), 7.80 (br s, 1H), 8.10 (br s, 2H), 8.26 (br s, 2H), 8.28 (s, 1H), 8.43 (s, 1H), 11.98 (s, 1H). 13C NMR (DMSO-d6, 150 MHz): δ 16.6, 45.8, 48.5, 70.8, 73.1, 83.2,87.7, 119.1, 140.7, 148.9, 150.1, 154.1, 169.3, 172.1, 172.8, 181.4, 189.3. HPLC purity: (97.1%, tR = 3.10 min). HRMS-ESI (m/z) calcd for C17H21N8O6 [M+H]+ 433.1584, found 433.1585.
tert-butyl (tert-butoxycarbonyl)(9-((3aR,4R,6R,6aR)-6-(((tert-butoxycarbonyl)(N-((tert-butoxycarbonyl)-D-alanyl)sulfamoyl)amino)methyl)-2,2-dimethyltetrahydrofuro[3,4-d][1,3]dioxol-4-yl)-9H-purin-6-yl)carbamate (28). The general procedure B was followed with compound 26 (1.2 g, 1.76 mmol), prepared as previously described [20], Boc-D-Ala-OSu (640 mg, 2.23 mmol), and DBU (0.6 mL, 4.22 mmol) in DMF (25 mL). The crude was purified by flash chromatography (Eluent CH2Cl2/MeOH 9:1) to give compound 28 (1.1 g, 1.33 mmol, 76%) as a white foam. 1H NMR (CDCl3, 500 MHz): δ 1.32 (d, J = 7.4 Hz, 3H), 1.39 (s, 3H), 1.43 (s, 18H), 1.44 (s, 9H), 1.62 (s, 3H), 4.04–4.15 (m, 3H), 4.20 (dd, J = 15.4 Hz, J = 5.9 Hz, 1H), 4.53–4.58 (m, 1H), 4.83–4.95 (m, 1H), 5.13–5.17 (m, 1H), 5.38–5.41 (m, 1H), 6.18 (d, J = 2.1 Hz, 1H), 8.21 (s, 1H), 8.87 (s, 1H). 13C NMR (CDCl3, 125 MHz): δ 16.4, 25.6, 27.4, 27.9, 28.0, 28.4, 50.3, 82.4, 83.9, 84.5, 85.3, 85.4, 90.7, 114.9, 129.6, 144.0, 150.5, 150.7, 152.4, 152.7, 171.7. HRMS-ESI (m/z) calcd for C31H48N8O12S [M+H]+ 757.3191, found 757.3193.
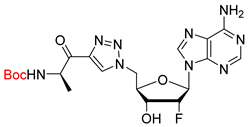
tert-butyl (tert-butoxycarbonyl)(9-((2R,3R,4R,5R)-5-(((tert-butoxycarbonyl)(N-((tert-butoxycarbonyl)-D-alanyl)sulfamoyl)amino)methyl)-4-((tert-butyldimethylsilyl)oxy)-3-fluorotetrahydrofuran-2-yl)-9H-purin-6-yl)carbamate (29). The general procedure B was followed with compound 27 (185 mg, 0.24 mmol), prepared as previously described [20], Boc-D-Ala-OSu (70 mg, 0.24 mmol), and DBU (55 µL, 0.36 mmol) in DMF (3.3 mL). The crude was purified by column chromatography (Eluent Pentane/EtOAc 1:1) to give compound 29 (173 mg, 0.18 mmol, 77%) as a white foam. 1H NMR (CD3CN, 600 MHz): δ 0.16 (s, 3H), 0.19 (s, 3H), 0.95 (s, 9H), 1.19 (d, J = 7.2 Hz, 3H), 1.25 (s, 9H), 1.40 (s, 18H), 1.41 (s, 9H), 3.97–4.03 (m, 2H), 4.14–4.17 (m, 1H), 4.34–4.35 (m, 1H), 4.80–4.83 (m, 1H), 5.53–5.63 (m, 1H), 5.77 (br s, 1H), 6.30 (dd, JFH = 17.5 Hz, J = 2.4 Hz, 1H), 8.44 (s, 1H), 8.81 (s, 1H). 19F NMR (CD3CN, 470 MHz): δ −205.6 (m). 13C NMR (CD3CN, 150 MHz): δ −4.7, −4.5, 18.2, 18.7, 26.1, 27.9, 28.1, 28.7, 50.4, 53.3, 73.0 (d, JCF = 15.9 Hz), 80.9, 83.1, 85.0, 85.9, 88.3 (d, JCF = 32.9 Hz), 93.3 (d, JCF = 190.8 Hz), 130.1, 146.0, 151.0, 151.5, 152.8, 153.6, 155.6, 157.2, 179.5. HRMS-ESI (m/z) calcd for C39H66FN8O13SiS [M+H]+ 933.4223, found 933.4211.
(R)-2-amino-N-(N-(((2R,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-3,4-dihydroxytetrahydrofuran-2-yl)methyl)sulfamoyl)propenamide salt (30). The general procedure C (Method C2) was followed with compound 28 (528 mg, 0.62 mmol), TFA (7.5 mL), and CH2Cl2/H2O (6/1.5 mL). The crude was purified by HPLC RP-C18 (MeCN/H2O, linear gradient) to give compound 30 (219 mg, 0.34 mmol, 55%) as a white solid (2 TFA salt). 1H NMR (D2O, 500 MHz): δ 1.52 (d, J = 7.2 Hz, 3H), 3.37–3.38 (m, 2H), 3.97 (q, J = 7.2 Hz, 1H), 4.31–4.34 (m, 1H), 4.41–4.44 (m, 1H), 4.80–4.83 (m, 1H), 6.04 (d, J = 5.8 Hz, 1H), 8.37 (s, 1H), 8.39 (s, 1H). 13C NMR (D2O, 125 MHz): δ 16.4, 44.3, 50.4, 71.0, 73.5, 83.7, 89.1, 119.3, 142.8, 147.1, 148.2, 151.7, 173.0. HRMS-ESI (m/z) calcd for C13H20N8O6S [M+H]+ 417.1305, found 417.1304.
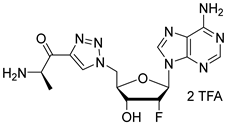
(R)-2-amino-N-(N-(((2R,3R,4R,5R)-5-(6-amino-9H-purin-9-yl)-4-fluoro-3-hydroxytetrahydrofuran-2-yl)methyl)sulfamoyl)propenamide salt (31). The general procedure C (Method C2) was followed with compound 29 (173 mg, 0.18 mmol), TFA (2.3 mL), and CH2Cl2/H2O (2/0.5 mL) to give compound 31 (105 mg, 0.16 mmol, 88%) as a white solid (2 TFA salt). 1H NMR (D2O, 500 MHz): δ 1.47 (d, J = 7.2 Hz, 3H), 3.33 (dd, J = 14.3 Hz, J = 4.5 Hz, 1H), 3.42 (dd, J = 14.3 Hz, J = 2.8 Hz, 1H), 3.85 (q, J = 7.2 Hz, 1H), 4.28–4.32 (m, 1H), 4.70 (ddd, JHF = 18.3 Hz, J = 7.2 Hz, J = 4.7 Hz, 1H), 5.50 (ddd, JHF = 52.1 Hz, J = 4.7 Hz, J = 2.4 Hz, 1H), 6.34 (dd, JHF = 17.3 Hz, J = 2.4 Hz, 1H), 8.28 (s, 1H), 8.32 (s, 1H). 19F NMR (D2O, 470 MHz): δ −203.9 (dt, JFH = 52.1 Hz, JFH = 17.3 Hz, 1F). 13C NMR (D2O, 125 MHz): 16.6, 43.4, 51.0, 69.5 (d, JCF = 17.1 Hz), 81.6, 87.1 (d, JCF = 33.9 Hz), 93.0 (d, JCF = 187.9 Hz), 119.1, 141.3, 148.3, 150.6, 154.1, 175.3. HPLC purity: (98.3%, tR = 2.08 min). HRMS-ESI (m/z) calcd for C13H19FN8O5S [M+H]+ 419.1261, found 419.1264.
tert-butyl (E)-(tert-butoxycarbonyl)(9-(2,4-difluoro-3-(hydroxymethyl)but-2-en-1-yl)-9H-purin-6-yl)carbamate (34). A solution of compound 33 (247 mg, 0.48 mmol, 1 equiv.), prepared as previously described [40], in t-BuOH (5 mL) was added CsF (111 mg, 0.96 mmol, 2.0 equiv.). The reaction mixture was stirred at 70 °C for 4 h, and the solvent was removed under reduced pressure. The residue was taken up with EtOAc/H2O, and the aqueous layers were extracted three times with EtOAc. The combined organic layers were washed with brine, dried over MgSO4, filtered, and concentrated under reduced pressure. The crude product was purified by column chromatography (Eluent Pentane/EtOAc 1:1 to 6:4) to give compound 34 (93 mg, 0.21 mmol, 43%) as a colorless oil. 1H NMR (CDCl3, 500 MHz): δ 1.44 (s, 18H), 2.33 (br s, 1H), 4.34 (s, 2H), 5.14 (dd, JHF = 21.7 Hz, JHF = 1.8 Hz, 2H), 5.35 (dd, JHF = 47.8 Hz, JHF = 2.2 Hz, 2H), 8.14 (s, 1H), 8.85 (s, 1H). 19F NMR (CDCl3, 470 MHz): δ −107.4 (t, JFH = 21.7 Hz), −212.1 (t, JFH = 47.8 Hz). 13C NMR (CDCl3, 125 MHz): δ 27.9, 41.0 (d, JCF = 29.6 Hz), 56.3 (dd, JCF = 8.7 Hz, JCF = 1.2 Hz), 78.6 (dd, JCF = 166.7 Hz, JCF = 10.1 Hz), 84.1, 118.8 (dd, JCF = 15.5 Hz, JCF = 13.4 Hz), 128.5, 144.5, 150.5, 150.6, 152.5, 153.2, 153.7 (dd, JCF = 269.6 Hz, JCF = 10.8 Hz). HRMS-ESI (m/z) calcd for C20H28F2N5O5 [M+H]+ 456.2059, found 456.2054.
tert-butyl (E)-(tert-butoxycarbonyl)(9-(4-((tert-butoxycarbonyl)(sulfamoyl)amino)-2-fluoro-3-(fluoromethyl)but-2-en-1-yl)-9H-purin-6-yl)carbamate (36). The general procedure D was followed with compound 34 (120 mg, 0.26 mmol), tert-butyl sulfamoylcarbamate (155 mg, 0.78 mmol), triphenylphosphine (102 mg, 0.39 mmol), and diisopropyl azodicarboxylate (0.05 mL, 0.27 mmol) in dry THF (5.2 mL). The crude product was purified by column chromatography (EtOAc/Pentane 1:2) to give compound 36 (123 mg, 0.19 mmol, 74%) as a white foam. 1H NMR (CDCl3, 600 MHz): δ 1.44 (s, 9H), 1.46 (s, 18H), 4.57 (br s, 2H), 5.16 (d, JHF = 21.1 Hz, 2H), 5.33 (d, JHF = 47.8 Hz, 2H), 5.40 (br s, 2H), 8.15 (s, 1H), 8.85 (s, 1H). 19F NMR (CDCl3, 565 MHz): δ −103.1 (t, JFH = 21.1 Hz), −208.1 (t, JFH = 47.8 Hz). 13C NMR (CDCl3, 150 MHz): δ 27.9, 28.1, 41.1 (d, JCF = 30.1 Hz), 43.1 (d, JCF = 8.4 Hz), 79.6 (dd, JCF = 165.9 Hz, JCF = 10.4 Hz), 84.1, 85.3, 114.9 (dd, JCF = 13.9 Hz, JCF = 12.1 Hz), 128.6, 144.4, 150.6, 150.7, 151.9, 152.6, 153.1, 155.6 (dd, JCF = 263.6 Hz, JCF = 11.7 Hz). HRMS-ESI (m/z) calcd for C25H38F2N7O8S [M+H]+ 634,2471 found 634.2474.
tert-butyl (E)-(tert-butoxycarbonyl)(9-(4-((N-((tert-butoxycarbonyl)-D-alanyl)sulfamoyl)amino)-2-fluoro-3-(fluoromethyl)but-2-en-1-yl)-9H-purin-6-yl)carbamate (37). The general procedure B was followed with compound 36 (130 mg, 0.20 mmol), Boc-D-Ala-OSu (59 mg, 0.20 mmol), and DBU (46 µL, 0.31 mmol) in DMF (2.6 mL). The crude was purified by column chromatography (Eluent EtOAc/Pentane 1:1 to 1:0) to give compound 37 (100 mg, 0.12 mmol, 61%) as a white foam. 1H NMR (CDCl3, 600 MHz): δ 1.31 (d, JHF = 6.9 Hz, 3H), 1.40 (s, 9H), 1.44 (s, 18H), 1.45 (s, 18H), 4.69 (br s, 2H), 5.17 (d, JHF = 21.3 Hz, 2H), 5.26 (d, JHF = 47.8 Hz, 2H), 8.18 (s, 1H), 8.86 (s, 1H). 19F NMR (CDCl3, 565 MHz): δ −103.9–103.6 (m), −209.6 (t, JFH = 47.8 Hz). 13C NMR (CDCl3, 150 MHz): δ 18.1, 27.8, 27.9, 28.4, 41.1 (d, JCF = 29.4 Hz), 44.1, 52.1, 78.4 (dd, JCF = 166.4 Hz, JCF = 9.6 Hz), 80.1, 84.1, 84.4, 116.2 (m), 128.4, 144.8, 150.2, 150.6, 152.3, 152.7, 153.3, 154.6 (dd, JCF = 262.3 Hz, JCF = 10.9 Hz), 156.0, 177.7. HRMS-ESI (m/z) calcd for C33H51F2N8O11S [M+H]+ 805.3366, found 805.3391.
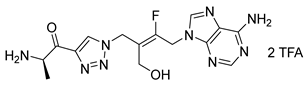
(R,E)-2-amino-N-(N-(4-(6-amino-9H-purin-9-yl)-3-fluoro-2-(fluoromethyl)but-2-en-1-yl)sulfamoyl)propenamide salt (38). The general procedure C (Method C2) was followed with compound 37 (100 mg, 0.12 mmol), TFA (1.4 mL) and CH2Cl2/H2O (1.2/0.3 mL) to give compound 38 (76 mg, 0.11 mmol, 90%) as a white solid (2 TFA salt). 1H NMR (MeOD, 600 MHz): δ 1.52 (d, J = 7.1 Hz, 3H), 3.88 (br s, 2H), 3.92 (q, J = 7.1 Hz), 5.28 (dd, JHF = 20.6 Hz, JHF = 1.6 Hz, 2H), 5.33 (dd, JHF = 47.6 Hz, JHF = 1.8 Hz, 2H), 8.24 (s, 1H), 8.32 (s, 1H). 19F NMR (CDCl3, 565 MHz): δ −105.5 (m), −211.3 (m).13C NMR (MeOD, 150 MHz): δ 17.1, 39.0 (d, JCF = 9.1 Hz), 41.8 (d, JCF = 28.8 Hz), 50.5, 79.3 (dd, JCF = 163.1 Hz, JCF = 9.2 Hz), 116.6 (dd, JCF = 15.1 Hz, JCF = 12.6 Hz), 118.1 (q, JCF = 292.1 Hz), 119.7, 143.8, 149.8, 150.6, 154.6, 156.5 (dd, JCF = 261.7 Hz, JCF = 10.8 Hz), 163.4 (q, JCF = 32.4 Hz), 170.1. HPLC purity: (95.8%, tR = 3.23 min). HRMS-ESI (m/z) calcd for C13H19N8O3F2S [M+H]+ 405.1269, found 405.1271.
tert-butyl ((R)-1-(1-(((2R,3R,4R,5R)-5-(6-amino-9H-purin-9-yl)-4-fluoro-3-hydroxytetrahydrofuran-2-yl)methyl)-1H-1,2,3-triazol-4-yl)-1-oxopropan-2-yl)carbamate (45). The general procedure E was followed with compound 43 (410 mg, 1.39 mmol), prepared as previously described [43], alkyne S6 (295 mg, 1.53 mmol), sodium ascorbate (28 mg, 0.14 mmol), and CuSO4.5H2O (17 mg, 0.07 mmol) in t-BuOH/H2O (9 mL). The crude was purified by column chromatography (Eluent EtOAc/MeOH 94:6) to give compound 45 (470 g, 0.94 mmol, 68%) as a white solid. 1H NMR (DMSO-d6, 500 MHz): δ 1.26 (d, J = 7.4 Hz, 3H), 1.32 (s, 9H), 4.34 (dt, J = 7.4 Hz, J = 3.2 Hz, 1H), 4.69–4.77 (m, 1H), 4.82 (dd, J = 14.8 Hz, J = 7.4 Hz, 1H), 4.88 (dd, J = 14.8 Hz, J = 2.8 Hz, 1H), 4.89–4.95 (m, 1H), 5.50 (ddd, JHF = 52.6 Hz, J = 4.5 Hz, J = 1.3 Hz, 1H), 6.08 (d, J = 6.4 Hz, 1H), 6.24 (dd, JHF = 20.6 Hz, J = 1.3 Hz, 1H), 7.24 (d, J = 6.8 Hz, 1H), 7.33 (br s, 2H), 8.10 (s, 1H), 8.23 (s, 1H), 8.60 (s, 1H). 19F NMR (DMSO-d6, 470 MHz): δ −201.2 (dt, JFH = 52.6 Hz, JFH = 20.6 Hz). 13C NMR (DMSO-d6, 125 MHz): δ 16.6, 28.1, 51.0, 52.3, 69.7 (d, JCF = 16.3 Hz), 78.0, 80.0, 86.5 (d, JCF = 34.8 Hz), 93.0 (d, JCF = 186.5 Hz), 119.2, 128.7, 140.0, 144.6, 148.6, 152.7, 155.2, 156.1, 193.4. HRMS-ESI (m/z) calcd for C20H27FN9O5 [M+H]+ 492.2119, found 492.2123.
N-((R)-1-(1-(((3aR,4R,6R,6aR)-6-(6-amino-9H-purin-9-yl)-2,2-dimethyltetrahydrofuro[3,4-d][1,3]dioxol-4-yl)methyl)-1H-1,2,3-triazol-4-yl)-1-oxopropan-2-yl)heptanamide (46). The general procedure E was followed with compound 44 (400 mg, 1.20 mmol), prepared as previously described [44], alkyne S7 (277 mg, 1.32 mmol), sodium ascorbate (24 mg, 0.12 mmol), and CuSO4.5H2O (15 mg, 0.06 mmol) in t-BuOH/H2O (8 mL). The crude was purified by column chromatography (Eluent EtOAc/MeOH 1:0 to 98:2) to give compound 46 (511 mg, 0.95 mmol, 79%) as a brown foam. 1H NMR (CDCl3, 500 MHz): δ 0.80 (t, J = 7.0 Hz, 3H), 1.19–1.25 (m, 6H), 1.35 (s, 3H), 1.44 (d, J = 7.6 Hz, 3H), 1.56 (s, 3H), 2.18 (t, J = 7.0 Hz, 2H), 4.56–4.61 (m, 1H), 4.84–4.86 (m, 2H), 5.18–5.20 (m, 1H), 5.38–5.39 (m, 1H), 5.44–5.52 (m, 1H), 6.07 (s, 1H), 6.61 (s, 1H), 6.67–6.72 (m, 1H), 7.86 (s, 1H), 8.03 (s, 1H), 8.25 (s, 1H). 13C NMR (CDCl3, 125 MHz): δ 14.1, 18.8, 22.5, 25.4, 25.6, 27.2, 28.9, 31.6, 36.6, 52.0, 81.8, 84.1, 84.2, 85.3, 90.7, 115.1, 120.4, 128.3, 140.4, 145.3, 148.8, 152.9, 155.9, 172.9, 193.4. HRMS-ESI (m/z) calcd for C25H36N9O5 [M+H]+ 542.2839, found 542.2841.
N-((R)-1-(1-(((3aR,4R,6R,6aR)-6-(6-amino-9H-purin-9-yl)-2,2-dimethyltetrahydrofuro[3,4-d][1,3]dioxol-4-yl)methyl)-1H-1,2,3-triazol-4-yl)-1-oxopropan-2-yl)-[1,1′-biphenyl]-4-carboxamide (47). The general procedure E was followed with compound 44 (400 mg, 1.20 mmol), prepared as previously described [44], alkyne S8 (367 mg, 1.32 mmol), sodium ascorbate (24 mg, 0.12 mmol), and CuSO4.5H2O (15 mg, 0.06 mmol) in t-BuOH/H2O (8 mL). The crude was purified by column chromatography (Eluent EtOAc/MeOH 1:0 to 99:1) to give compound 47 (561 mg, 0.92 mmol, 77%) as a brown foam. 1H NMR (CDCl3, 500 MHz): δ 1.33 (s, 3H), 1.55 (s, 3H), 1.59–1.62 (m, 3H), 4.56–4.61 (m, 1H), 4.80–4.88 (m, 1H), 5.17–5.19 (m, 1H), 5.36–5.37 (m, 1H), 5.66–5.73 (m, 1H), 6.06 (br s, 1H), 6.62 (br s, 2H), 7.31–7.34 (m, 1H), 7.38–7.41 (m, 2H), 7.50–7.58 (m, 5H), 7.85–7.88 (m, 3H), 8.04–8.05 (m, 1H), 8.27 (br s, 1H). 13C NMR (CDCl3, 125 MHz): δ 18.9, 25.4, 27.1, 29.7, 51.9, 52.7, 81.7, 84.1, 85.3, 90.6, 115.1, 120.4, 127.1 (2C), 127.2 (2C), 127.7 (2C), 128.4, 128.9 (2C), 132.8, 140.0, 140.2, 144.2, 145.2, 148.8, 153.3, 156.1, 166.6, 193.2. HRMS-ESI (m/z) calcd for C31H32N9O5 [M+H]+ 610.2526, found 610.2531.
N-((R)-1-(1-(((3aR,4R,6R,6aR)-6-(6-amino-9H-purin-9-yl)-2,2-dimethyltetrahydrofuro[3,4-d][1,3]dioxol-4-yl)methyl)-1H-1,2,3-triazol-4-yl)-1-oxopropan-2-yl)-4-(hexyloxy)benzamide (48). The general procedure E was followed with compound 44 (400 mg, 1.20 mmol), prepared as previously described [44], alkyne S9 (398 mg, 1.32 mmol), sodium ascorbate (24 mg, 0.12 mmol), and CuSO4.5H2O (15 mg, 0.06 mmol) in tBuOH/H2O (8 mL). The crude was purified by column chromatography (Eluent EtOAc/MeOH 99:1) to give compound 48 (617 mg, 0.97 mmol, 81%) as a brown foam. 1H NMR (CDCl3, 500 MHz): δ 0.87–0.90 (m, 3H), 1.30–1.36 (m, 4H), 1.40 (s, 3H), 1.41–1.45 (m, 2H), 1.58–1.61 (m, 6H), 1.72–1.79 (m, 2H), 3.92–3.97 (m, 2H), 4.59–4.61 (m, 1H), 4.84–4.91 (m, 2H), 5.13–5.20 (m, 1H), 5.33–5.39 (m, 1H), 5.64–5.70 (m, 1H), 6.06 (br s, 1H), 6.82–6.88 (m, 2H), 7.75–7.77 (m, 2H), 7.88 (s, 1H), 8.04 (s, 1H), 8.28 (s, 1H). 13C NMR (CDCl3, 125 MHz): δ 14.1, 19.1, 22.7, 25.4, 25.8, 27.2, 29.2, 31.6, 52.1, 52.7, 68.3, 81.8, 84.2, 85.4, 90.7, 114.2 (2C), 115.2, 120.5, 126.2, 128.4, 129.0 (2C), 129.7, 140.6, 145.4, 149.2, 152.6, 162.0, 166.4, 193.4. HRMS-ESI (m/z) calcd for C31H40N9O6 [M+H]+ 634.3102, found 634.3104.
4-nitrobenzyl ((R)-1-(1-(((3aR,4R,6R,6aR)-6-(6-amino-9H-purin-9-yl)-2,2-dimethyltetrahydrofuro[3,4-d][1,3]dioxol-4-yl)methyl)-1H-1,2,3-triazol-4-yl)-1-oxopropan-2-yl)carbamate (49). The general procedure E was followed with compound 44 (400 mg, 1.20 mmol), prepared as previously described [44], compound S10 (365 mg, 1.32 mmol), sodium ascorbate (24 mg, 0.12 mmol), and CuSO4.5H2O (15 mg, 0.06 mmol) in t-BuOH/H2O (8 mL). The crude was purified by column chromatography (Eluent EtOAc/MeOH 1:0 to 99:1) to give compound 49 (470 mg, 0.77 mmol, 64%) as a brown foam. 1H NMR (CDCl3, 500 MHz): δ 1.37 (s, 3H), 1.53 (t, J = 7.6 Hz, 3H), 1.58 (s, 3H), 4.58–4.61 (m, 1H), 4.87–4.88 (m, 2H), 5.13–5.22 (m, 3H), 5.32–5.35 (m, 1H), 5.40–5.42 (m, 1H), 6.04–6.07 (m, 1H), 6.35 (br s, 2H), 7.46–7.47 (m, 2H), 7.88 (s, 1H), 7.97 (s, 1H), 8.11–8.16 (m, 1H), 8.29 (s, 1H). 13C NMR (CDCl3, 125 MHz): δ 19.1, 25.4, 27.2, 29.8, 52.1, 53.7, 65.3, 81.9, 84.2, 85.4, 90.8, 115.2, 123.8 (2C), 128.1 (2C), 128.2, 140.6, 144.1, 145.2, 147.6, 152.9, 155.3, 155.8, 193.1. HRMS-ESI (m/z) calcd for C26H29N10O8 [M+H]+ 609.2170, found 609.2173.
(R)-2-amino-1-(1-(((2R,3R,4R,5R)-5-(6-amino-9H-purin-9-yl)-4-fluoro-3-hydroxytetrahydrofuran-2-yl)methyl)-1H-1,2,3-triazol-4-yl)propan-1-one salt (50). The general procedure C (Method C2) with compound 45 (380 mg, 0.77 mmol), TFA (9.5 mL), and CH2Cl2/H2O (8.7/1.8 mL) to give compound 50 (470 mg, 0.68 mmol, 88%) as a white solid (2 TFA). 1H NMR (D2O, 500 MHz): δ 1.65 (d, J = 7.2 Hz, 3H), 4.58–4.61 (m, 1H), 4.73 (ddd, JHF = 22.7 Hz, J = 9.2 Hz, J = 4.4 Hz, 1H), 4.93 (q, J = 7.2 Hz, 1H), 5.00 (d, J = 3.9 Hz, 2H), 5.58 (dd, JHF = 51.6 Hz, J = 4.8 Hz, 1H), 6.42 (d, JHF = 20.3 Hz, 1H), 8.23 (s, 1H), 8.30 (s, 1H), 8.55 (s, 1H). 19F NMR (D2O, 470 MHz): δ −201.5 (ddd, JFH = 51.6 Hz, JFH = 22.7 Hz, JFH = 20.3 Hz, 1F). 13C NMR (D2O, 150 MHz): δ 16.2, 50.1, 52.6, 69.3 (d, JCF = 16.9 Hz), 79.6, 87.3 (d, JCF = 36.1 Hz), 92.8 (d, JCF = 186.1 Hz), 115.3, 118.7, 130.5, 142.8, 145.6, 147.7, 150.7, 189.7. HPLC purity: (98.5%, tR = 10.24 min). HRMS-ESI (m/z) calcd for C15H19FN9O3 [M+H]+ 392.1595, found 392.1594.
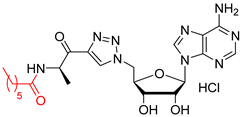
N-((R)-1-(1-(((2R,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-3,4-dihydroxytetrahydrofuran-2-yl)methyl)-1H-1,2,3-triazol-4-yl)-1-oxopropan-2-yl)heptanamide salt (51). The general procedure C (Method C1) was followed with compound 46 (250 mg, 0.46 mmol), HCl aq (1 mL, 3 M), and MeOH (2.5 mL). The crude was precipitated in MeOH/Et2O to give compound 51 (219 mg, 0.41 mmol, 89%) as a white solid (HCl salt). 1H NMR (DMSO-d6, 500 MHz): δ 0.83 (t, J = 7.1 Hz, 3H), 1.18–1.24 (m, 6H), 1.27–1.29 (m, 3H), 1.41–1.44 (m, 2H), 2.06–2.10 (m, 2H), 4.27–4.29 (m, 1H), 4.37–4.40 (m, 1H), 4.62–4.64 (m, 1H), 4.85–4.91 (m, 2H), 5.11–5.16 (m, 1H), 5.98 (d, J = 5.2 Hz, 1H), 8.27 (br s, 1H), 8.54 (s, 1H), 8.74 (s, 1H), 8.77 (s, 1H), 9.07 (br s, 1H), 9.78 (br s, 1H). 13C NMR (DMSO-d6, 125 MHz): δ 14.0, 16.7, 22.0, 25.2, 28.3, 31.1, 34.8, 50.9, 51.7, 70.8, 73.4, 82.5, 88.3, 118.9, 128.9, 142.8, 144.7, 144.9, 148.2, 150.3, 172.1, 193.0. HPLC purity: (93.6%, tR = 10.83 min). HRMS-ESI (m/z) calcd for C22H32N9O5 [M+H]+ 502.2526, found 502.2527.
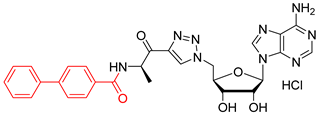
N-((R)-1-(1-(((2R,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-3,4-dihydroxytetrahydrofuran-2-yl)methyl)-1H-1,2,3-triazol-4-yl)-1-oxopropan-2-yl)-[1,1′-biphenyl]-4-carboxamide salt (52). The general procedure C (Method C1) was followed with compound 47 (250 mg, 0.41 mmol), HCl aq (1 mL, 3 M), and MeOH (2.5 mL). The crude was precipitated in MeOH/Et2O to give compound 52 (205 mg, 0.34 mmol, 83%) as a white solid (HCl salt). 1H NMR (DMSO-d6, 500 MHz): δ 1.46–1.48 (m, 3H), 4.29–4.31 (m, 1H), 4.39–4.43 (m, 1H), 4.63 (dt, J = 7.8 Hz, J = 5.1 Hz, 1H), 5.87–5.94 (m, 2H), 5.36–5.42 (m, 1H), 5.99–6.01 (m, 1H), 7.39–7.42 (m, 1H), 7.47–7.51 (m, 2H), 7.72–7.78 (m, 4H), 7.97–8.00 (m, 2H), 8.53 (d, J = 1.6 Hz, 1H), 8.75 (d, J = 4.8 Hz, 1H), 8.79 (d, J = 9.8 Hz, 1H), 8.90 (d, J = 6.2 Hz, 1H), 8.97 (br s, 1H), 9.67 (br s, 1H). 13C NMR (DMSO-d6, 125 MHz): δ 16.4, 51.7, 52.0, 70.8, 73.4, 82.5, 88.3, 118.9, 126.4, (2C), 126.9 (2C), 128.1, 128.3 (2C), 128.8, 129.1 (2C), 132.5, 139.1, 142.7, 142.9, 144.8, 145.3, 148.3, 150.5, 165.9, 192.7. HPLC purity: (98.8%, tR = 12.32 min). HRMS-ESI (m/z) calcd for C28H28N9O5 [M+H]+ 570.2213, found 570.2214.
N-((R)-1-(1-(((2R,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-3,4-dihydroxytetrahydrofuran-2-yl)methyl)-1H-1,2,3-triazol-4-yl)-1-oxopropan-2-yl)-4-(hexyloxy)benzamide salt (53). The general procedure C (Method C1) was followed with compound 48 (491 mg, 0.77 mmol), HCl aq (1.9 mL, 3 M), and MeOH (4.7 mL). The crude was precipitated in MeOH/Et2O to give compound 53 (370 mg, 0.58 mmol, 76%) as a white solid (HCl salt). 1H NMR (DMSO-d6, 500 MHz): δ 0.87 (t, J = 7.0 Hz, 3H), 1.28–1.31 (m, 4H), 1.39–1.44 (m, 5H), 1.68–1.73 (m, 2H), 4.01 (t, J = 6.6 Hz, 2H), 4.28–4.30 (m, 1H), 4.38–4.42 (m, 1H), 4.61–4.64 (m, 1H), 4.88–4.91 (m, 1H), 5.31–5.38 (m, 1H), 5.98–6.00 (m, 1H), 6.95–6.98 (m, 2H), 7.84–7.86 (m, 2H), 8.53 (s, 1H), 8.65–8.66 (m, 1H), 8.74–8.75 (m, 1H), 8.76–8.78 (m, 1H), 8.96 (br s, 1H), 9.66 (br s, 1H). 13C NMR (DMSO-d6, 125 MHz): δ 13.9, 16.4, 22.1, 25.2, 28.6, 31.0, 51.6, 51.8, 67.7, 70.8, 73.3, 82.4, 88.3, 113.8 (2C), 118.9, 125.7, 128.8, 129.4 (2C), 142.7, 144.8, 145.3, 148.3, 150.1, 161.2, 165.7, 193.1. HPLC purity: (96.5%, tR = 14.41 min). HRMS-ESI (m/z) calcd for C28H36N9O6 [M+H]+ 594.2789, found 594.2797.
4-nitrobenzyl ((R)-1-(1-(((2R,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-3,4-dihydroxytetrahydrofuran-2-yl)methyl)-1H-1,2,3-triazol-4-yl)-1-oxopropan-2-yl)carbamate salt (54). The general procedure C (Method C1) was followed with compound 49 (250 mg, 0.41 mmol), HCl aq (1 mL, 3 M), and MeOH (2.5 mL). The crude was precipitated in MeOH/Et2O to give compound 54 (195 mg, 0.32 mmol, 79%) as a white solid (HCl salt). 1H NMR (DMSO-d6, 500 MHz): δ 1.34 (t, J = 7.4 Hz, 3H), 4.27–4.29 (m, 1H), 4.38–4.41 (m, 1H), 4.62–4.64 (m, 1H), 4.89–4.91 (m, 2H), 5.02–5.07 (m, 1H), 5.15 (s, 2H), 5.98 (d, J = 4.9 Hz, 1H), 7.59 (d, J = 8.1 Hz, 2H), 7.94 (d, J = 7.4 Hz, 1H), 8.22 (d, J = 8.1 Hz, 2H), 8.52 (s, 1H), 8.75 (s, 1H), 8.77 (s, 1H), 9.01 (br s, 1H), 9.69 (br s, 1H). 13C NMR (DMSO-d6, 125 MHz): δ 16.8, 51.6, 52.8, 64.2, 70.8, 73.4, 82.4, 88.3, 118.8, 123.5 (2C), 128.1 (2C), 128.9, 142.7, 144.5, 145.0, 145.1, 146.9, 148.2, 150.4, 155.5, 193.0. HPLC purity: (98.3%, tR = 10.74 min). HRMS-ESI (m/z) calcd for C23H25N10O8 [M+H]+ 569.1857, found 569.1860.
3.4. Determination of the Binding Affinity (Molecular Docking)
The molecular docking was performed by AutoDock Vina 1.2.7 software, which uses a non-deterministic Docking Algorithm with 8 maximum exhaustiveness to determine the binding affinity for each ligand. Molecular docking was simulated with the crystal structure of Bacillus cereus D-alanyl carrier protein ligase [51] from the Protein Data Bank (PDB ID: 3FFC) using Research Collaboratory for Structural Bioinformatics. Crystallization cofactors such as ATP, Magnesium, as well as traces of residual water from the 3FCC structure, were removed using PyMol 3.1 software. Kollman and Gasteiger charges, as well as the polar hydrogens, were added, respectively, to the receptor and the ligands using AutoDockTools 1.5.7 software to prepare molecules in pdbqt format. The active site was defined by default through the ligand co-crystallized in the 3FCC protein, and the grid box was established with a dimension (Å) of X: 19.8903, Y: 20.2516, and Z: 24.5359; and for center, X: 18.9110, Y: 9.0989, and Z: 13.3228. Docking scores listed are the average of nine different minimized energy states.
3.5. Determination of the Half-Maximal Inhibitory Concentration (IC50)
The DltA-catalyzed ATP-hydrolysis reaction was monitored in vitro via PPi release (DltA +D-alanine +ATP → DltA-DAla-AMP +PPi) using a coupled-enzyme assay. The purified DltA was pretreated with DltC purified from E. coli to clear D-Ala-AMP already present in the enzyme preparation after expression in E. coli. Holo-DltC is the natural protein partner of DltA, and DltC purified from E. coli BL21 (DE3) contained 51% of this active form of DltC as determined by HPLC analysis. DltA was pretreated with DltC (ratio 1:1.2), incubated for 30 min at 37 °C and 30 min at 4 °C. Three successive washes were realized on Amicon Ultra-2 Centrifugal Filter Unit—Ultracel 30 membrane 30 kDa MWCO (Merck, Darmstadt, Germany) with elution buffer EB at 4 °C. The components of the coupled enzyme assay included 1.5 μM pre-treated DltA, 20 μM of ATP, and 14 μM of D-alanine. The half-maximal inhibitory concentration (IC50) of potential DltA inhibitors was determined by the addition of different concentrations of the synthesized molecules. PPi release was determined using the PiPer—Pyrophosphate assay kit (Invitrogen, Waltham, MA, USA) according to the recommendation of the supplier. Reactions were carried out in 96-well Chimney Style, non-binding Microplates with μClear Film Bottom (Greiner Bio-one, Frickenhausen, Germany). Plates were incubated at 37 °C for 5 h and read in a FlexStation 3 Multi-Mode plate reader (Molecular Devices, Silicon Valley, CA, USA), with λ excitation of 530 nm and λ emission of 590 nm. Experiments were conducted in sextuplicate, and the IC50 was determined by linear regression of the curve.
3.6. MIC Determination
Colonies from BHI agar plates were suspended in saline solution (0.9% NaCl) and adjusted to an OD600 of 0.5. For growth kinetics determination, 96-well microplates (Starlab, Orsay, France) containing 200 μL of fresh BHI medium were inoculated with bacterial suspensions to an OD600 of 0.02 with a wide range of DltA inhibitors (from 0 to 2 mM). Plates were incubated at 37 °C with shaking (orbital amplitude of 3 mm) in a microplate reader (InfiniteM Nano, Tecan, Seoul, Republic of Korean). OD600nm was measured every 10 min for 24 h. Determination of MIC/MBC of antibiotics and bacterial survival assays were performed as previously described using BHI medium [17,18,19]. The DltA inhibitor was diluted in pure water and added to the growth medium to a final concentration of 0, 0.1, 0.25, 0.5, 0.75, or 1 mM.
3.7. Quantification of D-Alanyl Ester in the Bacterial Cell Wall
Ester-linked D-alanines were quantified as described previously [48], with the following modifications. Cultures were grown in 5 mL of BHI supplemented with DltA inhibitor to a final concentration of 0.05, 0.1, 0.25, 0.5, 0.75, or 1 mM for 16 h at 37 °C under shaking (120 rpm). Briefly, the quantification is based on the oxidation of a chromogen by H2O2 generated when free D-alanines are oxidized by a D-amino acid oxidase. Three independent experiments with duplicate samples were performed.
4. Conclusions
In this paper, a new series of D-Ala-AMP analogues was synthesized and tested as adjuvants to re-sensitize MRSA to IPM. Modification of the 2′-position of adenosine was first envisaged to increase cell permeability and/or metabolic stability. Indeed, a fluorine atom, an azido group, a difluorophosphonylated allylic ether, and a glucose moiety were successfully introduced. Squaramide, sulfamide, and triazole derivatives were also prepared as phosphate surrogates, as well as fluorinated transbutenyl acyclonucleosides as sugar mimics. Functionalization of the N-terminal moiety to facilitate bacterial penetration was also realized to produce a series of N-acylated derivatives. The binding affinity of each molecule was measured towards B. Subtilis DltA (PDB 3FCC), revealing docking scores close to reference molecule 1. Each new compound was tested in vitro as a DltA inhibitor. Indeed, we observed seven nucleosides containing modifications onto the carbohydrate or the linker, which had IC50 values in the same order of magnitude as the reference. Among them, two molecules containing either a difluorophosphonylated allylic ether onto the 2′-position or the sulfamide linker were able to re-sensitize MRSA to imipenem as efficiently as the reference. Quantification of D-alanyl esters confirmed that these two compounds reduced the level of bacterial cell wall D-alanyl residues by 50% and 80%. These new series represent a promising structure entry not reported yet for DltA inhibition and re-sensitization of bacteria towards known antibiotics. Further investigations will be realized to evaluate these new DltA inhibitors in MRSA-infected larvae of Galleria mellonella and will be reported in due course.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/molecules30122569/s1, Experimental procedure for the preparation of 35, S1–S10, 1H, 13C, 31P and 19F NMR spectra of compounds 5–9, 10, 11, 13–18, 21–23, 28–31, 36–38, 45–54, S2–S5, S7–S10, HPLC chromatogram for final products 8, 9, 11, 18, 23, 30, 31, 38, 50–54.
Author Contributions
Conceptualization, E.P. and T.L.; methodology, D.L., X.F., A.M. and D.C.; software, D.L. and A.M.; validation, E.P., T.L., N.V. and A.B.-V.; formal analysis, F.L.C.; investigation, D.L., X.F., A.M. and D.C.; data curation, E.P., T.L., N.V. and A.B.-V.; writing—original draft preparation, E.P. and T.L.; writing—review and editing, E.P., T.L., N.V. and A.B.-V.; supervision, E.P., T.L., N.V. and A.B.-V.; project administration, E.P., T.L., N.V. and A.B.-V.; funding acquisition, E.P., T.L., N.V. and A.B.-V. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Agence National de la Recherche, the DALATAR project (ANR-19-CE18-0008) and the excellence laboratory LabEx SYNORG (ANR-11-LABX-0029), the Conseil Régional de Normandie, and ERDF funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data are contained within the article and Supplementary Materials.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Cook, M.A.; Wright, G.D. The Past, Present, and Future of Antibiotics. Sci. Transl. Med. 2022, 14, eabo7793. [Google Scholar] [CrossRef] [PubMed]
- Lima, L.M.; Silva, B.N.M.D.; Barbosa, G.; Barreiro, E.J. β-Lactam Antibiotics: An Overview from a Medicinal Chemistry Perspective. Eur. J. Med. Chem. 2020, 208, 112829. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, S.; Mehra, P.; Dhanjal, D.S.; Sharma, P.; Sharma, V.; Singh, R.; Nepovimova, E.; Chopra, C.; Kuča, K. Antibiotics and Antibiotic Resistance- Flipsides of the Same Coin. Curr. Pharm. Des. 2022, 28, 2312–2329. [Google Scholar] [CrossRef] [PubMed]
- Patangia, D.V.; Anthony Ryan, C.; Dempsey, E.; Paul Ross, R.; Stanton, C. Impact of Antibiotics on the Human Microbiome and Consequences for Host Health. MicrobiologyOpen 2022, 11, e1260. [Google Scholar] [CrossRef]
- Baran, A.; Kwiatkowska, A.; Potocki, L. Antibiotics and Bacterial Resistance—A Short Story of an Endless Arms Race. Int. J. Mol. Sci. 2023, 24, 5777. [Google Scholar] [CrossRef]
- Giurazza, R.; Mazza, M.C.; Andini, R.; Sansone, P.; Pace, M.C.; Durante-Mangoni, E. Emerging Treatment Options for Multi-Drug-Resistant Bacterial Infections. Life 2021, 11, 519. [Google Scholar] [CrossRef]
- Avershina, E.; Shapovalova, V.; Shipulin, G. Fighting Antibiotic Resistance in Hospital-Acquired Infections: Current State and Emerging Technologies in Disease Prevention, Diagnostics and Therapy. Front. Microbiol. 2021, 12, 707330. [Google Scholar] [CrossRef]
- Mahoney, A.R.; Safaee, M.M.; Wuest, W.M.; Furst, A.L. The Silent Pandemic: Emergent Antibiotic Resistances Following the Global Response to SARS-CoV-2. iScience 2021, 24, 102304. [Google Scholar] [CrossRef]
- Langford, B.J.; So, M.; Raybardhan, S.; Leung, V.; Soucy, J.P.R.; Westwood, D.; Daneman, N.; MacFadden, D.R. Antibiotic prescribing in patients with COVID-19: Rapid review and meta-analysis. Clin. Microbiol. Infect. 2021, 27, 520–531. [Google Scholar] [CrossRef]
- Murray, C.J.L.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Robles Aguilar, G.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E.; et al. Global Burden of Bacterial Antimicrobial Resistance in 2019: A Systematic Analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef]
- Kumar, V.; Yasmeen, N.; Pandey, A.; Ahmad Chaudhary, A.; Alawam, A.S.; Ahmad Rudayni, H.; Islam, A.; Lakhawat, S.S.; Sharma, P.K.; Shahid, M. Antibiotic Adjuvants: Synergistic Tool to Combat Multi-Drug Resistant Pathogens. Front. Cell. Infect. Microbiol. 2023, 13, 1293633. [Google Scholar] [CrossRef]
- Saar-Dover, R.; Bitler, A.; Nezer, R.; Shmuel-Galia, L.; Firon, A.; Shimoni, E.; Trieu-Cuot, P.; Shai, Y. D-Alanylation of Lipoteichoic Acids Confers Resistance to Cationic Peptides in Group B Streptococcus by Increasing the Cell Wall Density. PLoS Pathog. 2012, 8, e1002891. [Google Scholar] [CrossRef]
- Neuhaus, F.C.; Baddiley, J. A Continuum of Anionic Charge: Structures and Functions of d-Alanyl-Teichoic Acids in Gram-Positive Bacteria. Microbiol. Mol. Biol. Rev. 2003, 67, 686–723. [Google Scholar] [CrossRef]
- Schultz, B.J.; Snow, E.D.; Walker, S. Mechanism of D-Alanine Transfer to Teichoic Acids Shows How Bacteria Acylate Cell Envelope Polymers. Nat. Microbiol. 2023, 8, 1318–1329. [Google Scholar] [CrossRef]
- Percy, M.G.; Gründling, A. Lipoteichoic Acid Synthesis and Function in Gram-Positive Bacteria. Annu. Rev. Microbiol. 2014, 68, 81–100. [Google Scholar] [CrossRef]
- May, J.J.; Finking, R.; Wiegeshoff, F.; Weber, T.T.; Bandur, N.; Koert, U.; Marahiel, M.A. Inhibition of the D-alanine: D-alanyl Carrier Protein Ligase from Bacillus Subtilis Increases the Bacterium’s Susceptibility to Antibiotics That Target the Cell Wall. FEBS J. 2005, 272, 2993–3003. [Google Scholar] [CrossRef]
- Coupri, D.; Budin-Verneuil, A.; Hartke, A.; Benachour, A.; Léger, L.; Lequeux, T.; Pfund, E.; Verneuil, N. Genetic and Pharmacological Inactivation of D-Alanylation of Teichoic Acids Sensitizes Pathogenic Enterococci to β-Lactams. J. Antimicrob. Chemother. 2019, 74, 3162–3169. [Google Scholar] [CrossRef]
- Coupri, D.; Verneuil, N.; Hartke, A.; Liebaut, A.; Lequeux, T.; Pfund, E.; Budin-Verneuil, A. Inhibition of d-Alanylation of Teichoic Acids Overcomes Resistance of Methicillin-Resistant Staphylococcus Aureus. J. Antimicrob. Chemother. 2021, 76, 2778–2786. [Google Scholar] [CrossRef]
- Mahé, A.; Verneuil, N.; Coupri, D.; Hartke, A.; Cattoir, V.; Rincé, I.; Gueulle, S.; Feng, X.; Lequeux, T.; Pfund, E.; et al. D-alanylation of lipoteichoic acids inhibitor provides anti-virulence and anti-resistance effects against methicillin-resistant Staphylococcus epidermidis. Antimicrob. Agents Chemother. 2025, 69, e01822-24. [Google Scholar] [CrossRef]
- Bockman, M.R.; Kalinda, A.S.; Petrelli, R.; De la Mora-Rey, T.; Tiwari, D.; Liu, F.; Dawadi, S.; Nandakumar, M.; Rhee, K.Y.; Schnappinger, D.; et al. Targeting Mycobacterium tuberculosis Biotin Protein Ligase (MtBPL) with Nucleoside-Based Bisubstrate Adenylation Inhibitors. J. Med. Chem. 2015, 58, 7349–7369. [Google Scholar] [CrossRef]
- Evans, C.E.; Si, Y.; Matarlo, J.S.; Yin, Y.; French, J.B.; Tonge, P.J.; Tan, D.S. Structure-Based Design, Synthesis, and Biological Evaluation of Non-Acyl Sulfamate Inhibitors of the Adenylate-Forming Enzyme MenE. Biochemistry 2019, 58, 1918–1930. [Google Scholar] [CrossRef]
- Van De Vijver, P.; Ostrowski, T.; Sproat, B.; Goebels, J.; Rutgeerts, O.; Van Aerschot, A.; Waer, M.; Herdewijn, P. Aminoacyl-tRNA Synthetase Inhibitors as Potent and Synergistic Immunosuppressants. J. Med. Chem. 2008, 51, 3020–3029. [Google Scholar] [CrossRef]
- Kim, B.T.; Kim, S.K.; Lee, S.J.; Hwang, K.J. A Convenient and Versatile Synthesis of 2’ (and 3’)-Amino (and Azido)-2’ (and 3’)-Deoxyadenosine as Diverse Synthetic Precursors of Cyclic Adenosine Diphosphate Ribose (cADPR). Bull. Korean Chem. Soc. 2004, 25, 243–248. [Google Scholar] [CrossRef]
- Thorens, B.; Mueckler, M. Glucose Transporters in the 21st Century. Am. J. Physiol.-Endocrinol. Metab. 2010, 298, E141–E145. [Google Scholar] [CrossRef]
- Zhang, R.; Qin, X.; Kong, F.; Chen, P.; Pan, G. Improving cellular uptake of therapeutic entities through interaction with components of cell membrane. Drug Deliv. 2019, 26, 328–342. [Google Scholar] [CrossRef]
- Mullapudi, S.S.; Mitra, D.; Li, M.; Kang, E.T.; Chiong, E.; Neoh, K.G. Potentiating anti-cancer chemotherapeutics and antimicrobials via sugar-mediated strategies. Mol. Syst. Des. Eng. 2020, 5, 772–791. [Google Scholar] [CrossRef]
- Fu, J.; Yang, J.; Seeberger, P.H.; Yin, J. Glycoconjugates for glucose transporter-mediated cancer-specific targeting and treatment. Carbohydr. Res. 2020, 498, 108195–108203. [Google Scholar] [CrossRef]
- Calvaresia, E.C.; Hergenrother, P.J. Glucose conjugation for the specific targeting and treatment of cancer. Chem. Sci. 2013, 4, 2319–2333. [Google Scholar] [CrossRef]
- Hossain, F.; Andreana, P.R. Developments in Carbohydrate-Based Cancer Therapeutics. Pharmaceuticals 2019, 12, 84. [Google Scholar] [CrossRef]
- Li, M.; Neoh, K.G.; Xu, L.; Yuan, L.; Leong, D.T.; Kang, E.T.; Chua, K.L.; Hsu, L.Y. Sugar-Grafted Cyclodextrin Nanocarrier as a “Trojan Horse” for Potentiating Antibiotic Activity. Pharm. Res. 2016, 33, 1161–1174. [Google Scholar] [CrossRef]
- Dumont, E.; Vergalli1, J.; Pajovic, J.; Bhamidimarri, S.P.; Morante, K.; Wang, J.; Lubriks, D.; Suna, E.; Stavenger, R.A.; Winterhalter, M.; et al. Mechanistic aspects of maltotriose-conjugate translocation to the Gram-negative bacteria cytoplasm. Life Sci. Alliance 2018, 2, e201800242. [Google Scholar] [CrossRef]
- Pfund, E.; Dupouy, C.; Rouanet, S.; Legay, R.; Lebargy, C.; Vasseur, J.-J.; Lequeux, T. Difluorophosphonylated Allylic Ether Moiety as a 2′-Modification of RNA-Type Molecules: Synthesis, Thermal, and Metabolic Studies. Org. Lett. 2019, 21, 4803–4807. [Google Scholar] [CrossRef]
- Ceuninck, A.; Lequeux, T.; Pfund, E. Expanding the Scope of Group Transfer Radical Reaction: Toward the Synthesis of Fluorinated Nucleoside Analogues Incorporating Difluorophosphonylated Allylic Ether Moieties. J. Org. Chem. 2024, 89, 8099–8110. [Google Scholar] [CrossRef]
- Duckworth, B.P.; Geders, T.W.; Tiwari, D.; Boshoff, H.I.; Sibbald, P.A.; Barry, C.E.; Schnappinger, D.; Finzel, B.C.; Aldrich, C.C. Bisubstrate Adenylation Inhibitors of Biotin Protein Ligase from Mycobacterium Tuberculosis. Chem. Biol. 2011, 18, 1432–1441. [Google Scholar] [CrossRef]
- Shuman, D.A.; Robins, R.K.; Robins, M.J. The Synthesis of Adenine 5′-O-Sulfamoyl Nucleosides Related to Nucleocidin. J. Am. Chem. Soc. 1969, 9, 3391–4000. [Google Scholar] [CrossRef]
- Moreau, C.; Kirchberger, T.; Swarbrick, J.M.; Bartlett, S.J.; Fliegert, R.; Yorgan, T.; Bauche, A.; Harneit, A.; Guse, A.H.; Potter, B.V.L. Structure–Activity Relationship of Adenosine 5′-Diphosphoribose at the Transient Receptor Potential Melastatin 2 (TRPM2) Channel: Rational Design of Antagonists. J. Med. Chem. 2013, 56, 10079–10102. [Google Scholar] [CrossRef]
- Zhang, G.; Richardson, S.L.; Mao, Y.; Huang, R. Design, synthesis, and kinetic analysis of potent protein N-terminal methyltransferase 1 inhibitors. Org. Biomol. Chem. 2015, 13, 4149–4154. [Google Scholar] [CrossRef]
- Somu, R.V.; Boshoff, H.; Qiao, C.; Bennett, E.M.; Barry, C.E.; Aldrich, C.C. Rationally Designed Nucleoside Antibiotics That Inhibit Siderophore Biosynthesis of Mycobacterium tuberculosis. J. Med. Chem. 2006, 49, 31–34. [Google Scholar] [CrossRef]
- Topalis, D.; Pradère, U.; Roy, V.; Caillat, C.; Azzouzi, A.; Broggi, J.; Snoeck, R.; Andrei, G.; Lin, J.; Eriksson, S.; et al. Novel Antiviral C5-Substituted Pyrimidine Acyclic Nucleoside Phosphonates Selected as Human Thymidylate Kinase Substrates. J. Med. Chem. 2011, 54, 222–232. [Google Scholar] [CrossRef]
- Leparfait, D.; Xiao, F.; Coupri, D.; Gueulle, S.; Desriac, F.; Budin-Verneuil, A.; Verneuil, N.; Hartke, A.; Pfund, E.; Lequeux, T. Oxetane Ring-Opening Reaction: A Key Step for the Preparation of Substituted (Fluoro)Alkenyl as Acyclonucleoside Mimics. Eur. J. Org. Chem. 2023, 26, e202300258. [Google Scholar] [CrossRef]
- Kim, D.W.; Ahn, D.-S.; Oh, Y.-H.; Lee, S.; Kil, H.S.; Oh, S.J.; Lee, S.J.; Kim, J.S.; Ryu, J.S.; Moon, D.H.; et al. A New Class of SN2 Reactions Catalyzed by Protic Solvents: Facile Fluorination for Isotopic Labeling of Diagnostic Molecules. J. Am. Chem. Soc. 2006, 128, 16394–16397. [Google Scholar] [CrossRef]
- Lee, J.-W.; Oliveira, M.T.; Jang, H.B.; Lee, S.; Chi, D.Y.; Kim, D.W.; Song, C.E. Hydrogen-Bond Promoted Nucleophilic Fluorination: Concept, Mechanism and Applications in Positron Emission Tomography. Chem. Soc. Rev. 2016, 45, 4638–4650. [Google Scholar] [CrossRef]
- Dawadi, S.; Kawamura, S.; Rubenstein, A.; Remmel, R.; Aldrich, C.C. Synthesis and Pharmacological Evaluation of Nucleoside Prodrugs Designed to Target Siderophore Biosynthesis in Mycobacterium Tuberculosis. Bioorganic Med. Chem. 2016, 24, 1314–1321. [Google Scholar] [CrossRef]
- Liu, F.; Austin, D.J. A General Synthesis of 5‘-Azido-5‘-Deoxy-2‘,3‘- O -Isopropylidene Nucleosides. J. Org. Chem. 2001, 66, 8643–8645. [Google Scholar] [CrossRef]
- Davis, T.D.; Gerry, C.J.; Tan, D.S. General Platform for Systematic Quantitative Evaluation of Small-Molecule Permeability in Bacteria. ACS Chem. Biol. 2014, 9, 2535–2544. [Google Scholar] [CrossRef]
- Abramson, J.; Alder, J.; Dunger, J.; Evans, R.; Green, T.; Pritzel, A.; Ronneberger, O.; Willmore, L.; Ballard, A.J.; Bambrick, J.; et al. Accurate structure prediction of biomolecular interactions with AlphaFold 3. Nature 2024, 630, 493–500. [Google Scholar] [CrossRef]
- Yonus, H.; Neumann, P.; Zimmermann, S.; May, J.J.; Marahiel, M.A.; Stubbs, M.T. Crystal structure of DltA. Implications for the reaction mechanism of non-ribosomal peptide synthetase adenylation domains. J. Biol. Chem. 2008, 283, 32484–32491. [Google Scholar] [CrossRef]
- McBride, S.M.; Sonenshein, A.L. The Dlt Operon Confers Resistance to Cationic Antimicrobial Peptides in Clostridium Difficile. Microbiology 2011, 157, 1457–1465. [Google Scholar] [CrossRef]
- Gottlieb, H.E.; Kotlyar, V.; Nudelman, A. NMR Chemical Shifts of Common Laboratory Solvents as Trace Impurities. J. Org. Chem. 1997, 62, 7512–7515. [Google Scholar] [CrossRef]
- Morotti, A.L.M.; Lang, K.L.; Carvalho, I.; Schenkel, E.P.; Bernardes, L.S.C. Semi-Synthesis of New Glycosidic Triazole Derivatives of Dihydrocucurbitacin B. Tetrahedron Lett. 2015, 56, 303–307. [Google Scholar] [CrossRef]
- Osman, K.T.; Du, L.; He, Y.; Luo, Y. Crystal Structure of Bacillus Cereus D-Alanyl Carrier Protein Ligase (DltA) in Complex with ATP. J. Mol. Biol. 2009, 388, 345–355. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).