Abstract
As an adsorbent, biomass activated carbon is effective at the removal of a wide range of organic and inorganic pollutants; however, its synthesis remains complex. Although spent coffee grounds (SCG) could be an effective material for the production of activated carbon, achieving a sufficient surface area has proven to be difficult. Here, this study presents a preliminary investigation into the optimal manufacturing conditions of activated-carbon adsorbents derived from SCG. SCG samples were characterized according to proximate analysis, elementary analysis, surface area, and pore volumes, then subjected to various processes (i.e., drying, carbonization, and chemical activation) with different operating parameters (temperature and time). The samples were optimized as follows: (1) Stable drying of SCG with a high moisture content of approximately 65% required consumption energy of 49 kWh/kg and drying at 105 °C for 20 h. (2) By comparing changes in the consumption energy and product yield with an increasing amount of carbon fraction, it was found that drying carbonization was more suitable than hydrothermal carbonization for SCG. The optimum drying carbonization temperature for achieving attractive biochar was 500 °C for 1 h. (3) Activated carbon with the optimum surface area (3687 m2/g) and mesopore volume fraction (approximately 70%) was achieved with a chemical activator agent ratio of approximately 3 and heating at 850 °C for 1 h. Furthermore, the butane working capacity of the activated carbon was related to the mesopore volume/surface area and reached 74.5% at a mesopore volume/surface area of 0.0004, indicating its suitability for activated carbon canisters. These findings can be used to optimize the synthesis of industrial-grade activated carbon from SCG.
1. Introduction
Coffee is a popular beverage worldwide, with its production reaching 10 million tons in 2019. In 2023/2024, global coffee consumption grew by 2.2% from the previous year to 176 million bags, following an average annual growth rate of 0.6% since 2017 [1,2]. The global production of instant coffee and coffee brewing generates approximately six million tons of spent coffee grounds (SCG), with this outcome being increasingly highlighted as an environmental issue [3,4].
Coffee bean brewing produces an estimated 98% residual coffee waste and 2% coffee. Residual coffee waste includes 60% liquids (moisture, oils, etc.) and 40% solids (spent coffee grounds, SCG) [5,6]. According to the World Bank, in high-income countries, approximately 39% of waste is landfilled, 36% is recycled or composted, and 22% is incinerated for energy recovery [7]. However, SCG dumping in high-load landfills drives air pollution via the emission of CO2 and CH4. Additionally, SCG dumping has also been shown to drive water pollution through the release of phenol, chlorogenic acid, hydroxycinnamic acid, and quinin acid. Therefore, demand is increasing for new methods of treating or recycling SCG as an alternative to landfilling [5,8].
Biomass has been shown to be useful in the production of liquid fuel [9], solid fuel [9,10], building materials [9,11,12], cosmetics [13], and adsorbents [14,15,16,17,18,19,20,21]. There has been a concerted global effort to shift toward biofuel and biochemical alternatives to fossil fuel. From the perspective of SCG, numerous studies have attempted to overcome the low yield of SCG in the bio-ethanol process and its high energy consumption in the bio-diesel process, as SCG contains high contents of carbohydrates and lipids when converted to bio-ethanol (0.15 g/g-SCG) or bio-diesel (0.22 g/g-SCG) [9]. Moreover, biomass solid fuel such as briquettes and pellets can be manufactured from compressed SCG, which contains a similar energy density to wood chip (20.1 MJ/kg) [10]; however, the direct combustion of SCG may lead to air pollution from nitrogen oxide and sulfur oxide. As a packing and building material, SCG feedstock exhibits good physical properties (e.g., tensile strength and impact resistance) that can be enhanced by approximately 10–20% by varying the particle size and distribution [11]; however, consistent application is hindered by technical limitations [9,11,12]. Furthermore, owing to its rough texture, SCG feedstock can also be used to produce cosmetics, such as scrubs and exfoliators, and has potential as an alternative to microplastics [13]. However, technical problems arise in relation to reducing residual caffeine and ensuring skin safety.
Many existing adsorbents either are made of relatively poor-quality materials or involve complex production, culminating in their commercialization being hindered by the high energy consumption and low product yields [14,15]. Conversely, biomass activated carbon (BAC) is a highly effective adsorbent that boasts a high surface area over 2000 m2/g as well as heterogeneity, making it effective at the removal of various organic and inorganic pollutants. However, various thermal and biochemical processes and combinations of processes have been proposed for manufacturing BAC, which affects the resulting adsorbent quality [15].
The many activation methods for producing BAC from SCG include three main stages: drying, carbonization, and activation. First, complete drying is required before carbonization because of the high moisture content of SCG. Second, SCG carbonization is performed at 300–700 °C to increase carbon content and reduce organic materials via thermal treatment in an oxygen-free environment [4,16]. However, recent studies have demonstrated that applying higher pyrolysis temperatures, such as 850 °C, can further improve the porosity and structural properties of the resulting biochar, albeit with increased energy consumption [17,18].
Generally, two carbonization methods can be employed, namely, hydrothermal carbonization or drying carbonization; the selection of these methods impacts consumption energy, recovery rate, and final product properties. Recently, flash pyrolysis with thermal shock has emerged as a promising carbonization technique, offering rapid heating and efficient pore development [19]. Third, the two different activation methods are physical activation and chemical activation. Physical activation uses steam or CO2 as activating agents, whereas chemical activation uses chemicals such as KOH, K2CO3, H3PO4, and ZnCl2 [20]. Physical activation is a simpler process but limited by a low recovery yield, high consumption energy, and the sensitivity of raw materials compared to chemical activation. Conversely, chemical activation leads to a higher surface area; therefore, biomass raw materials with low homogeneity are typically activated via chemical activation [21].
The importance of activation conditions has been highlighted in numerous studies. Such studies have investigated the effects of different activation agents on the porosity and adsorption properties of biomass-derived activated carbon. For instance, Pal et al. synthesized waste palm trunk-based activated carbon using KOH activation at 900 °C, achieving a specific surface area exceeding 2900 m2/g [22]. Minakshi et al. synthesized mango seed-based activated carbon using KOH activation at 800–1100 °C, achieving a specific surface area exceeding 1900 m2/g. Their findings highlight the crucial role of activation temperature and activator ratio in determining the final pore structure and adsorption performance of the carbon material and the need for process optimization to achieve high surface area and enhanced adsorption properties [23].
Importantly, the manufacturing method is ultimately reflective of the resultant product. The current cost of BAC manufacturing by physical activation is USD 1000–2000/ton, which results in a specific surface area of 1000–1500 m2/g, whereas that employing chemical activation is USD 1400–2500/ton, with an improved specific surface area of 2000–3000 m2/g [24]. Thus, the demand for high-surface-area adsorbents has made the BAC manufacturing process more complex, and limited research has been conducted on a laboratory scale.
Consequently, researchers are investigating alternative raw materials and activation techniques to enhance the efficiency of BAC production while minimizing costs and energy consumption. Among these, SCG has emerged as a promising precursor for activated carbon due to its abundance, economic viability, and environmental advantages.
SCG has been shown to be an excellent raw material for the production of adsorbents because it is a cost-effective and environmentally-friendly carbon-based precursor [25,26,27]. Specifically, activated carbon from SCG has been obtained using a chemical activation method, with KOH as the activating agent; the resultant material had a specific surface area of 1200 m2/g and adsorptive capacity similar to that of activated carbon when tested using similar methylene blue and chlorophenol [26]. In another study, activated carbon was prepared via the impregnation method using phosphoric acid; this study yielded a surface area of 721 m2/g and a high fraction of micropores [27]. Overall, the production of activated carbon with a high surface area from SCG remains a challenge. Nevertheless, activated carbon with a surface area of 2746 m2/g has been prepared using chemical activation by KOH and acid washing in HCl [8].
Despite these advances, systematic optimization of pretreatment conditions for SCG-derived activated carbon remains underexplored. Therefore, the aim of this study is to investigate the influence of various pretreatment conditions on the properties of activated carbon prepared from SCG. The following pretreatment conditions are examined: (1) drying holding time, (2) carbonization factors (method, temperature, and time), and (3) chemical activation factors (activator ratio, activation time, and activation temperature). Moreover, this study explores the viability of activated carbon prepared from SCG as an industrial adsorbent, considering its butane working capacity for n-butane.
2. Materials and Methods
2.1. Physical Properties
SCG samples were obtained from five different local coffee shops in Kyungido, Korea. First, the SCG was oven dried at 105 °C in a convection oven (LO-FP505, LKlab Korea Inc., Namyangju-si, Republic of Korea). Then, dried SCG was measured to detect the moisture content at different times. Proximate analysis was performed using American Standard Test Method (ASTM D1762-84) standards [28] to determine moisture, volatile matter, total ash, and fixed carbon contents using an oven (DF-3.5, Dae Heung Science Co., Incheon, Republic of Korea) [29]. Elemental analysis was performed by an EA analyzer (FlashSMART, ThermoFisher Scientific Inc., Waltham, MA, USA) to determine the fraction of carbon (C), hydrogen (H), oxygen (O), nitrogen (N), and sulfur (S). Sample weight loss was detected via thermogravimetric analysis (TGA, TGA 4000, PerkinElmer Inc., Waltham, MA, USA). The specific surface area (m2/g) and porosity (micropore (cm3/g), mesopore (cm3/g), and total pore volume (cm3/g)) of activated carbon were determined by N2 adsorption/desorption at −196 °C using a Brunauer–Emmett–Teller analyzer (Trixtar II, Micromeritics, Norcross, GA, USA) and the t-plot method. Energy consumption for the two carbonization methods was measured with a power meter (HPM-100A, Wattman, Seoul, Republic of Korea).
The morphologies of prepared activated carbons were observed using field emission scanning electron microscopy (Mira 3, Tescan, Brno, Czech). The crystal structure was analyzed via X-ray diffraction (SmartLab SE, Rigaku, Tokyo, Japan) and Raman spectra (RAMANtouch, Nanophoton, Seattle, WA, USA). XRD was measured using a Cu Kα (1.542 Å) source over a 20–80° range. The Raman spectra were measured in the range of 0–4000 cm−1 at room temperature and a wavelength of 523.05 nm.
2.2. Carbonization
Hydrothermal carbonization was performed in a 500 cm3/batch reactor with temperature control. For samples with drying pretreatment, 100 g of SCG was loaded into the reactor, and then 235 mL of water was added. The reactor was closed under N2 and heated using an electric heater to the required temperature (180–250 °C) based on previous research [30]. The sample was then stirred using a four-blade stirrer for 1 h. The hydrolysate samples from the reactor were collected and filtered using a Whatman nylon membrane filter in a drying convection oven. The samples were denoted as HTC-“temperature” as follows: HTC-180, HTC-200, HTC-220, and HTC-250. For drying carbonization, the dried SCG was loaded into a 1 kg/batch reactor with 1 L/min of N2 for 30 min to maintain the inner condition. Subsequently, the temperature was increased to 300–700 °C and maintained for 1, 3, or 5 h. The resulting sample names are shown in Table 1.

Table 1.
The denoted names of the carbonization samples.
2.3. Activation
Preparation of BAC from SCG was performed via chemical activation. The dried SCG and activating agent (KOH) were physically mixed and set in a stainless-steel reactor under nitrogen gas at 1 L/min for 30 min. The sample was activated at temperatures of 750–950 °C for 1–3 h at a heating rate of 5 °C/min. Upon completion of the activation process, the nitrogen flow was replaced with carbon dioxide at 1 L/min, and the CO2 flow was maintained until the reactor cooled to room temperature. The sample was then washed several times with deionized water via sonication until the pH of the filtrate became neutral. The washed samples were dried using hot-air convection (LO-FP505, LKlab KOREA Inc., Republic of Korea) to prepare the activated carbon. Three parameters affecting the properties of activated carbon were varied, including the chemical activation temperature (T = 750 °C, 850 °C, 950 °C), activation time (H = 1 h, 2 h, 3 h), and activator weight ratio (activator/precursor ratio = 1, 2, 3). Therefore, the activated carbon samples derived from chemical activation with KOH were denoted as CAC1–CAC9, as shown in Table 2.

Table 2.
The denoted name of the activated carbon depends on the different chemical activation conditions.
2.4. Adsorption Efficiency
To evaluate adsorption efficiency, butane was used as an adsorbate. Stock standard gas of n-butane containing 0.3 g of activated carbon with 99.9% purity (JC GAS Co., Gwangju, Republic of Korea) was prepared and stored in U-type glass at 25 °C. The flow rate of n-butane gas was 250 mL/min for 15 min, then 250 mL/min for 10 min, before purging the N2 gas (99%) at 300 mL/min for 40 min until no more weight change was observed. This procedure was performed according to American Standard Test Method (ASTM D5228) standards [31]. All measurements were conducted in triplicate, and the reported values represent the average of these three repeated cycles.
The butane activity (BA), butane retentivity (BR), and butane working capacity (BWC) were estimated from the following equations:
3. Results and Discussion
3.1. SCG Drying Conditions
The initial moisture content of SCG was measured at 50–60% (Table 3). Figure 1a presents the decrease in moisture content over drying time, while Figure 1b shows the corresponding mass loss rate, representing the rate at which mass decreased over time. During the early drying stage (0–150 min), the surface moisture evaporated rapidly, resulting in a high mass loss rate. Between 150 and 240 min, the process entered a transitional phase where the mass loss rate temporarily plateaued as heat gradually penetrated the interior of the sample. After 240 min, the internal moisture began evaporating more actively, leading to a renewed increase in the mass loss rate, which peaked around 600 min. Although the rate declined beyond this point, approximately 20% residual moisture remained, indicating that complete drying was not achieved within the observed timeframe. These results highlight the multi-stage drying dynamics of SCG and underscore how the mass loss rate reflects the combined effects of surface evaporation, internal heat transfer, and latent heat-driven moisture removal.

Table 3.
Moisture content of spend coffee grounds.
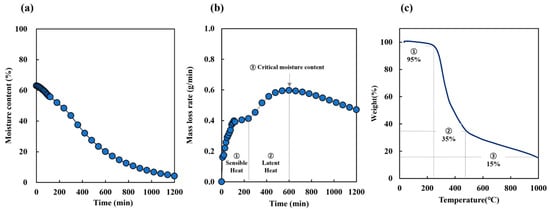
Figure 1.
The physical properties of SCG: (a) moisture content, (b) mass loss rate, (c) thermogravimetric analysis (TGA).
Accordingly, the theoretical energy consumption in convection was calculated as follows:
where Et is the total energy consumption (kWh), A is the cross-sectional area of the container, ν is the air velocity (m/s), ρa is the air density (kg/m3), t is the total drying time (h), Ca is the specific heat (kJ/kg°C), ΔT is the temperature difference (°C), Ekg is the specific energy required, and wo is the initial weight of the sample (kg). Therefore, owing to the high moisture content of the biomass, energy consumption during SCG drying was 49 kWh/kg, which is similar to the specific energy requirement for convection drying (51 kWh) [32].
Et (kWh) = AνρatCaΔT
Ekg = Et/wo
Omitting the carbonization of biomass precursor results in a low product yield [14]; therefore, most processes require carbonization to increase the carbon fraction. To select the carbonization temperature, TGA was performed (Figure 1c). The resulting thermal profile indicated three states: dehydration, devolatilization, and solid decomposition. The first stage of weight loss up to 220 °C corresponded to the moisture content of the raw materials (Figure 1b). The second stage of weight loss, between 220 °C and 450 °C, may be attributed to the decomposition of hemicellulose and the release of volatile matter in the SCG. The third stage, observed beyond 450 °C, is attributed to the gradual decomposition of lignin, which exhibits high thermal stability, as well as the rearrangement and condensation of the carbon matrix. This stage leads to the formation of a thermally stable carbon structure and accounts for the residual char observed at the end of the thermal analysis. Therefore, the drying carbonization temperature was set between 300 °C and 700 °C. For hydrothermal carbonization, the temperature range was set between 160 °C and 250 °C, based on our previous research [30].
Finally, the SCG composition in wt.% carbon, hydrogen, oxygen, nitrogen, and sulfur is shown in Table 4. The ratio of hydrogen/carbon was approximately 0.13 for all raw SCG samples. Generally, a lower H/C ratio indicates a higher carbon content and greater thermal stability, which can result in a stable carbon layer. This also means less carbon loss during activation, making it suitable as a precursor for activated carbon. Therefore, from the various SCG samples shown in Table 1, SCG #3 was selected for subsequent experiments owing to its lower H/C ratio.

Table 4.
The proximate analysis and elemental analysis of dried SCG.
3.2. Carbonization Properties
3.2.1. Hydrothermal Carbonization
As temperature is one of the primary determinants of product properties, its effect was investigated through hydrothermal carbonization experiments conducted at 180–250 °C for 1 h at a feedstock-to-water weight ratio of 1:2. The proportion of fixed carbon increased as the hydrothermal carbonization temperature increased (Figure 2a), to 15–32% more than that in the raw materials. According to elemental analysis, the carbon fraction also increased with increasing hydrothermal temperature, but the rate of increase was low. Finally, as observed in Figure 2b, energy consumption was in the range of 6.3–8.6 kWh/kg, and it increased with the hydrothermal temperature, followed by a decrease in product yield. Therefore, the increase in carbon fraction was associated with higher energy consumption and a reduction in product yield.
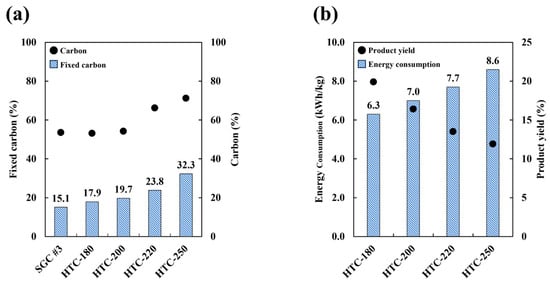
Figure 2.
(a) The physical properties (fixed carbon and carbon fraction) of HTC samples and (b) the consumption energy and product yield after HTC.
3.2.2. Drying Carbonization
Thermal analysis was performed to determine the thermal behavior of the product under carbonization. Table 5 shows the proximate and elemental analysis of dried SCG subjected to drying carbonization under different temperature and time conditions. The amount of fixed carbon increased with increasing carbonization temperature and time. The H/C atomic ratio generally decreased with heating temperature and time, which was consistent with the reported increasing aromaticity (=H/C) [33]. This means that the stability of biochar was enhanced as an adsorbent material, which is consistent with our research findings, where stability improves as the reaction temperature increases [34]. The carbonization temperature had a greater effect on the rate at which fixed carbon increased than time. Table 5 and Figure 3a show that the fixed carbon fraction and energy consumption were proportional to the carbonization temperature and time, but the product yield was inversely proportional to these parameters. Based on the TGA results, different thermal behavior was observed with different carbonization temperatures (Figure 3b). Specifically, the amount of solid residue at the end of the process increased with increasing carbonization temperature. During the carbonization process of SCG, the first stage of weight loss associated with hemicellulose decomposition was not observed for D-500/5 and D-700/5. During carbonization, the thermal decomposition of hemicellulose and cellulose at 200~400 °C led to the release of volatile compounds (e.g., CO2, CO, CH4), creating initial pore structures within the carbon matrix. At higher temperatures (400~600 °C), the gradual degradation of lignin and the rearrangement of fixed carbon further stabilized the porous framework. These physicochemical transformations collectively contributed to the development of both micropores and mesopores, enhancing the overall specific surface area and adsorption performance of the final material.

Table 5.
The proximate analysis and elemental analysis depend on different drying carbonization methods for dried SCG.
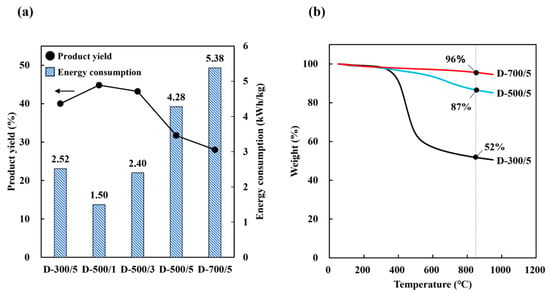
Figure 3.
(a) Solid yield and consumption energy with different drying carbonization methods and (b) TGA after carbonization.
Based on energy consumption, the proportion of fixed carbon, and the product yield values obtained, it is obvious that successful dry carbonization could be achieved by maintaining carbonization temperature at 500 °C for 1 h. Furthermore, drying carbonization is suitable for SCG carbonization because it can result in high product yields and increased amounts of fixed carbon with lower consumption energy than hydrothermal carbonization.
3.3. Properties and Characteristics of Chemically Activated Carbon
3.3.1. Morphology of Chemically Activated Carbon
Figure S1 presents the SEM images of SCG, D-500/1, and CAC samples (3, 6/1 h, 6, and 9). The SCG sample exhibited a relatively dense and compact structure with smooth surfaces and minimal porosity. The higher-magnification image of D-500/1 confirms that the carbonization process effectively removed non-carbon components. A comparative analysis of D-500/1, CAC 3, 6/1 h, and 6 indicates that chemical activation significantly enhanced pore formation and surface roughness. The well-developed honeycomb-like porous network observed in CAC 6 further supports the notion that chemical activation markedly improves surface area and adsorptive properties. Conversely, CAC 9 exhibited significantly reduced particle size with inadequate pore formation, suggesting suboptimal activation conditions.
3.3.2. Microstructural and Textural Properties
Figure 4 presents the Raman spectra and XRD patterns of CAC 3, 6/1 h, 6, and 9. The Raman spectra of CAC 3, 6/1 h, and 6 exhibited characteristic features of activated carbon (AC), including the D-band (~1350 cm−1) and G-band (~1580 cm−1). The intensities of the D-band and G-band in these samples were relatively balanced, indicating an appropriate level of disorder while preserving the graphitic structure. In contrast, CAC 9 displayed not only the D-band and G-band but also the 2D-band (~2700 cm−1). The Raman spectra of CAC 9 closely resemble those of reduced graphene oxide (rGO) [35], suggesting an increase in carbon crystallinity as activation progressed at high temperatures.
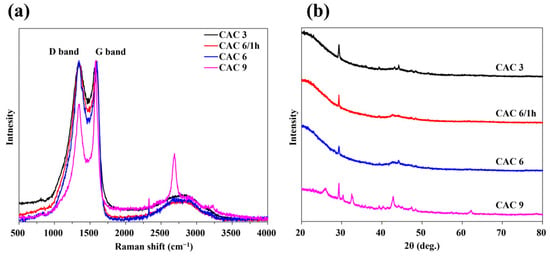
Figure 4.
The (a) Raman spectra and (b) X-ray diffraction patterns of CAC 3, 6/1 h, 6, and 9.
The broad diffraction peak around 20–25° (2θ) in XRD pattern is characteristic of amorphous carbon, reflecting the disordered carbon structure present in CAC3, CAC6/1 h, and CAC6. In contrast, CAC9 exhibited a sharper peak near 25°, indicating the development of graphitic domains and partial graphitization due to high-temperature activation. Minor additional peaks are attributed to residual inorganic compounds such as K2CO3 formed during the KOH activation process.
Several studies have reported the transformation of porous graphene-based materials through high-temperature heat treatment utilizing chemical activation agents as catalysts [36,37]. In the present study, the CAC9 sample underwent heat treatment at 950 °C, a higher temperature than that of other samples, which resulted in enhanced crystallinity and the progression of graphene-like structural formation within the porous framework. This trend was further corroborated by the comparison of Raman and XRD spectra. Notably, the sharp peak observed around 25° in the XRD pattern of the CAC9 sample suggests a transition from amorphous carbon to a more crystalline structure.
Table 6 and Figure 5 display the specific surface area and volumes of chemically activated carbon (CAC) samples according to different activation temperatures, times, and agent ratios for D-500/1 samples. After chemical activation, a significant increase in surface area was observed with increasing temperature and activation agent ratio, to more than two times that before chemical activation. The maximum specific surface area was achieved with an activation agent ratio of 3 and an activation temperature and time of 850 °C and 3 h, respectively (CAC6). Unexpectedly, the specific surface areas of CAC7–CAC9 decreased with increasing activated agent ratio, an outcome that may be attributable to collapsed pores [38,39,40].

Table 6.
The physical properties of CACs with different activation temperatures (750–950 °C) and agent ratio (1 to 3) after drying carbonization at 500 °C for 1 h.
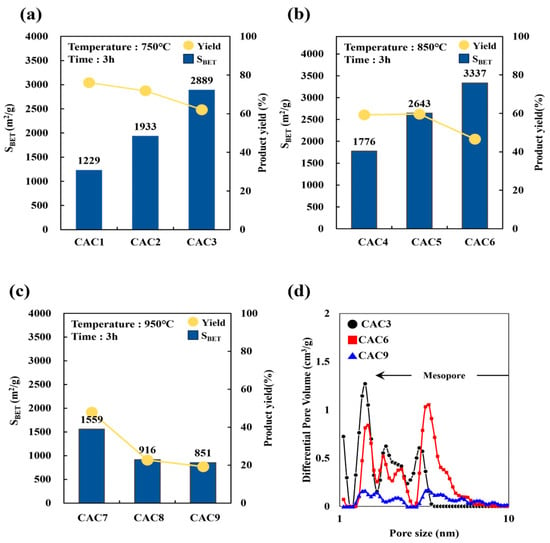
Figure 5.
The surface area and product yield with different activation temperatures (a) 750 °C, (b) 850 °C, (c) 950 °C for 3 h, and the (d) pore size distribution of CAC3, CAC6, and CAC9.
The micropore volume of CACs decreased with increasing activation agent ratio and activation temperature, whereas their mesopore volume increased until the activation temperature reached 850 °C, then decreased at higher temperatures. This indicates that temperatures higher than 850 °C led to pore collapses [20], likely due to excessive chemical activation under high KOH ratios and temperatures, which over-etches the carbon framework and causes pore wall collapse. This structural degradation reduces the effective micropore and mesopore network, ultimately lowering the available surface area for adsorption.
The BAC product yield of CAC1–CAC9 decreased from 76% to 19% with increasing temperature from 750 °C to 950 °C and activation agent ratio from 1 to 3, primarily due to increased carbon loss and volatilization under over-activation conditions. Thus, CAC6 was deemed the optimum condition for manufacturing low-cost, renewable BAC with a high surface area, balancing pore development and material integrity without sacrificing yield, which has implications for economic and environmental materials manufacturing. The surface area and pore volume measurements of CAC samples are presented in Figure S2. The isotherms of CAC3 and CAC6 corresponded to type I(b) (IUPAC classification), indicating the dense micropore–mesopore structure typical of activated carbon. However, the isotherms of CAC9 corresponded to type IV(a), which represents a dense mesopore–macropore structure [41]. This shift was likely due to the high-temperature-induced expansion and partial collapse of microporous frameworks, which enlarges pores into the mesopore and macropore ranges. Therefore, these results confirmed the production of mesoporous activated carbons with excellent adsorption characteristics [42].
Table 7 and Figure 6 compare the physical properties of CACs for different activation times from 1 h to 3 h at 850 °C and an activation agent ratio of 2 (CAC5 groups) and 3 (CAC6 groups), which represent the optimum conditions. As the agent ratio varied between the CAC5 and CAC6 groups, the surface area did not depend on the activation time. For both CAC5 and CAC6 groups, no significant increase in the surface area (3210–3687 m2/g and 2643–2819 m2/g, respectively) or product yield (60–62% and 54–59%, respectively) was observed with increasing activation time. These results further confirmed the production of mesoporous activated carbon with excellent adsorption characteristics, with mesopore fractions of 40% and 72% for CAC5 and CAC6 groups, respectively.

Table 7.
The physical properties of CACs with different activation times (1 h to 3 h) and agent ratios (2 to 3) at 850 °C after drying carbonization at 500 °C for 1 h.
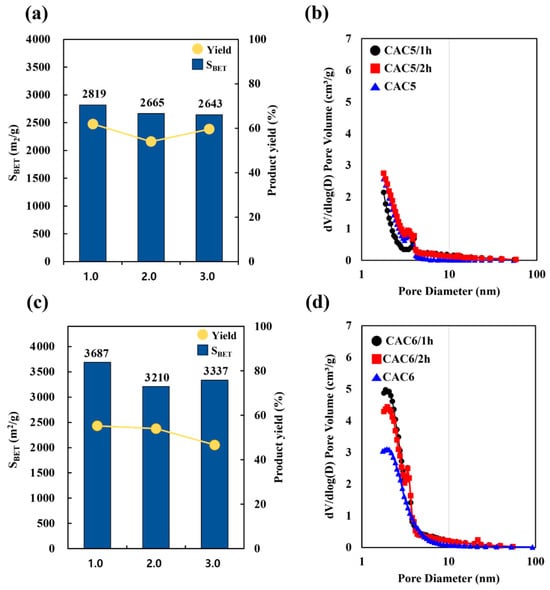
Figure 6.
The (a) surface area, product yield, and (b) pore diameter of the CAC5 groups (agent ratio 2 at 850 °C for 1 h to 3 h), and the (c) surface area, product yield, and pore diameter of the CAC6 groups (agent ratio 3 at 850 °C for 1 h to 3 h), and the (d) pore diameter of the CAC6 groups (agent ratio 3 at 850 °C for 1 h to 3 h).
Regarding the pore diameter and pore volume, the CAC5 groups showed similar results to the CAC6 group at an activation agent ratio of 3, even when the activation time was increased. In the CAC6 group, the micropore volume decreased rapidly as the activation time increased, and the peak in the region of 2–3 nm disappeared. The surface area and pore volume measurements are presented in Figure S3. The isotherms of the CAC5 and CAC6 groups corresponded to type I(b) (IUPAC classification), which indicates the dense micropore–mesopore structure typical of activated carbon. In this study, the high surface area (3687 m2/g) and high mesopore volume fraction (68%) of the prepared CAC6 samples indicate their applicability for gas-phase adsorption or gas storage.
As shown in Table 8, our SCG-derived activated carbon outperformed many biomass-based activated carbons in terms of surface area and mesoporous structure. Compared to previously reported SCG-based activated carbon (2746 m2/g) and mango seed-derived carbon (1944 m2/g), the optimized activation method in this study resulted in significantly higher porosity and mesopore volume (1.45 cm3/g), highlighting its enhanced adsorption potential. Additionally, the H3PO4-activated SCG (720 m2/g, 0.051 cm3/g mesopore volume) exhibited much lower porosity, further reinforcing the effectiveness of our KOH-based activation process. Furthermore, the H3PO4-activated coconut shell-derived carbon (891 m2/g, 0.46 cm3/g mesopore volume) and KOH-activated bamboo-derived carbon (3208 m2/g, 1.01 cm3/g mesopore volume) provide additional comparison points, demonstrating that while bamboo-derived carbon achieves a comparably high surface area, our SCG-based carbon exhibited superior mesoporous volume, making it highly suitable for applications requiring large pore structures. These results emphasize that the combination of optimized drying, carbonization, and chemical activation parameters enables the production of highly porous activated carbon suitable for industrial applications, particularly in gas-phase adsorption.

Table 8.
Comparative textural properties of biomass-derived activated carbons.
3.4. Butane Working Capacity Test
The BWC test, the most common analytical method for analyzing the performance of activated carbon canisters, analyzes the adsorption and desorption behavior of activated carbon on n-butane. Figure 7 presents the calculated BA, BR, and BWC of CACs according to Equations (1)–(3), respectively. With increasing surface area, which provides more sites for physical adsorption and desorption, BA and BR increased from 43% to 98% and from 26% to 53%, respectively. The BA and BR of activated carbon have a close relationship with the sub-mesopore volume in the range of 1.5–3.8 nm and with the mesopore volume in the range of 5.0–7.0 nm, respectively [41,45]. However, the mesopore volume fraction was calculated based on the surface area, and the correlation with BWC was confirmed because the surface area affects adsorption and desorption. Thus, the BWC decreased when the fraction of mesopore volume/surface area was higher or lower than 0.0004 cm3/m2, and the mesopore volume/surface area of 0.0004 was the maximum. Under high-temperature chemical activation (950 °C), the mesopore volume fraction was high at a low surface area, resulting in low BWC. Accordingly, a mesopore volume-to-surface area ratio of approximately 0.0004, such as that in CAC6, exhibited the highest BWC performance.
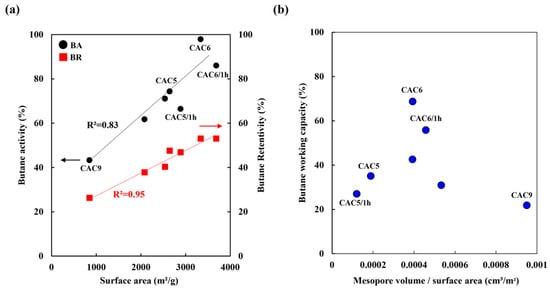
Figure 7.
Butane working capacity of CACs as a function of activation conditions. (a) BA, BR of CACs as a function of surface area, (b) Correlation between mesopore volume-to-surface area ratio and butane working capacity.
According to Lee et al. [41], the butane working capacity (BWC) of the commercial activated carbons WVA-1100 (Ingevity, North Charleston, SC, USA) and 2GK (Kuraray, Osaka, Japan) was reported to be 35.0 wt.% and 37.7 wt.%, respectively. Additionally, Lee et al. reported that the BWC of BAX-1500 (Ingevity, USA) was 42.0 wt.% [42].
Table 9 presents a comparative analysis of the butane adsorption performance of CAC6, BAX-1500, WVA1100, and 2GK. The results indicate that CAC6 demonstrated a significantly higher BWC (74.6 wt.%) compared to commercial activated carbons. The superior BWC of CAC6 indicates that its optimized pore structure and activation conditions enhanced both butane adsorption and retention, suggesting its potential for high-performance adsorption applications. Additionally, the BWC values were averaged over three repeated cycles, confirming the sufficient reusability of CAC6.

Table 9.
Comparison of butane working capacity among CAC6, WVA1100, and 2GK.
4. Conclusions
In this study, various SCG processing methods (drying, carbonization, and chemical activation) were optimized to develop high-performance activated carbon with a specific surface area of 3687 m2/g and a butane working capacity (BWC) of 74.5%. Compared to previously reported biomass-derived carbons, such as coconut shell-based (891 m2/g) [43], mango seed-based (1944 m2/g) [23], and bamboo-based activated carbon (3208 m2/g) [44], the material developed in this study exhibited superior mesoporosity and competitive adsorption performance, underscoring the effectiveness of the optimized pretreatment and activation strategies. Notably, the overall yield of 10.6% from raw SCG, coupled with high mesoporosity, highlights the material’s practical potential for industrial applications. Optimal processing conditions were identified as follows:
Drying: Stable drying was carried out over 20+ h at 105 °C in a convection oven with an energy input exceeding 49 kWh/kg, reducing moisture content below 57.1% (yield: 42.9%).
Carbonization: Drying carbonization was carried out at 500 °C for 1 h under an inert N2 atmosphere, yielding 44.8% carbonized SCG.
Chemical Activation: KOH activation was performed at 850 °C for 1 h with a 3:1 agent ratio, achieving a final surface area of 3687 m2/g, a mesopore volume of ≥70%, and an overall yield of 10.6% from raw SCG.
These results confirm the suitability of the synthesized activated carbon for canister and gas-phase adsorption applications. This work provides a scalable and energy-efficient pathway for transforming low-cost, renewable SCG waste into high-performance adsorbent materials, promoting sustainable waste valorization and circular economy practices.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules30122557/s1. Figure S1: The SEM images of (a) SCG, (b) D-500/1, (c) CAC 3, (d) CAC 6/1 h, (e) CAC 6, and (f) CAC 9. Figure S2: The isotherms with different activation temperatures of (a) CAC3-750 °C, (b) CAC6-850 °C, and (c) CAC9-950 °C for 3 h; Figure S3: The isotherms with different activation times: (a) CAC5 group and (b) CAC6 group.
Author Contributions
Conceptualization, J.-E.P.; methodology, G.-W.H., G.B.L. and J.-E.P.; validation, G.-W.H., G.B.L. and J.-E.P.; formal analysis, D.J.K.; investigation, S.E.L.; resources, S.E.L.; project discusstion, K.M.S.; data curation, J.-E.P.; writing—original draft preparation, G.-W.H.; writing—review and editing, G.-W.H., G.B.L. and J.-E.P.; visualization, J.-E.P.; supervision, J.-E.P.; project administration, J.-E.P.; funding acquisition, J.-E.P. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by HYUNDAI Motor Company (HMC) as “Research on thermal decomposition of organic waste resources and automotive materialization technology”.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
Author Kwang Mo Seong was employed by Hyundai Motor Group. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- McNutt, J.; He, Q. Spent coffee grounds: A review on current utilization. J. Ind. Eng. Chem. 2019, 71, 78–88. [Google Scholar] [CrossRef]
- Zhang, S.; Zhong, X.; Chen, J.; Nilghaz, A.; Yun, X.; Wan, X.; Tian, J. Manufacturing biodegradable lignocellulosic films with tunable properties from spent coffee grounds: A sustainable alternative to plastics. Int. J. Biol. Macromol. 2024, 273, 132918. [Google Scholar] [CrossRef]
- Ballesteros, L.F.; Teixeira, J.A.; Mussatto, S.I. Chemical, functional, and structural properties of spent coffee grounds and coffee silverskin. Food Bioprocess Technol. 2014, 7, 3493–3503. [Google Scholar] [CrossRef]
- Getachew, A.T.; Chun, B.S. Influence of pretreatment and modifiers on subcritical water liquefaction of spent coffee grounds: A green waste valorization approach. J. Clean. Prod. 2017, 142, 3719–3727. [Google Scholar] [CrossRef]
- Kim, M.S.; Koo, N.; Kim, J.G. Characteristics and recycling of spent coffee grounds. Life Sci. Nat. Resour. Res. 2012, 20, 59–69. [Google Scholar]
- Gottstein, V.; Bernhardt, M.; Dilger, E.; Keller, J.; Bretling-Utzmann, C.M.; Schwarz, S.; Kuballa, T.; Lachenmeier, D.W.; Bunzel, M. Coffee silver skin: Chemical characterization with special consideration of dietary fiber and heat-induced contaminants. Foods 2021, 10, 1705. [Google Scholar] [CrossRef]
- World Bank. What a Waste 2.0: A Global Snapshot of Solid Waste Management to 2050; World Bank: Washington, DC, USA, 2018; Available online: https://datatopics.worldbank.org/what-a-waste/ (accessed on 13 February 2025).
- Hwang, M.J.; Koh, S.C.; Chang, J.S. Characteristics of activated carbons made from coffee ground and their capacities for acid orange 7 removal. KIEAE J. 2016, 6, 38–39. [Google Scholar]
- Johnson, K.; Liu, Y.; Lu, M. A review of recent advances in spent coffee grounds upcycle technologies and practices. Front. Chem. Eng. 2022, 4, 838605. [Google Scholar] [CrossRef]
- Ibitoye, S.E.; Mahamood, R.M.; Jen, T.; Loha, C.; Akinlabi, E.T. An overview of biomass solid fuels: Biomass sources, processing methods, and morphological and microstructural properties. J. Bioresour. Bioprod. 2023, 8, 333–360. [Google Scholar] [CrossRef]
- Chen, Y.; Li, Q.; Feng, C.; Hu, Y.; Liu, Y.; Tian, J. Biocomposites Based on Spent Coffee Grounds and Application in Packaging. Interdiscip. Res. Print. Packag. 2022, 896, 455–461. [Google Scholar]
- Scalia, G.L.; Saeli, M.; Miglietta, P.P.; Micale, R. Coffee biowaste valorization within circular economy: An evaluation method of spent coffee grounds potentials for mortar production. Int. J. Life Cycle Assess. 2021, 26, 1805–1815. [Google Scholar] [CrossRef] [PubMed]
- Andrade, C.; Perestrelo, R.; Câmara, J.S. Valorization of spent coffee grounds as a natural source of bioactive compounds for several industrial applications—A volatilomic approach. Foods 2022, 11, 1731. [Google Scholar] [CrossRef] [PubMed]
- Rosson, E.; Garbo, F.; Marangoni, G.; Bertani, R. Activated carbon from spent coffee grounds: A good competitor of commercial carbons for water decontamination. Appl. Sci. 2020, 10, 5598. [Google Scholar] [CrossRef]
- Tran, T.K.N.; Ngo, T.C.Q.; Nguyen, Q.V.; Do, T.S.; Hoang, N.B. Chemistry potential and application of activated carbon manufactured from coffee grounds in the treatment of wastewater: A review. Mater. Today Proc. 2022, 60, 1914–1919. [Google Scholar] [CrossRef]
- Malini, K.; Selvakumar, D.; Kumar, N.S. Activated carbon from biomass: Preparation, factors improving basicity and surface properties for enhanced CO2 capture capacity–A review. J. CO2 Util. 2023, 67, 102318. [Google Scholar] [CrossRef]
- Li, J.; Wang, J.; Wang, S.; Qiao, M.; Zhang, X. Study on Pyrolysis Characteristics and Mechanism of Biomass Waste: Rice Husk, Corn Stalk, and Sawdust. Sustainability 2024, 16, 2889. [Google Scholar]
- Saba, A.; Iqbal, H.M.; Duman, G.; Jablonska, M.; Duman, F. Biomass-Derived Ni-Based Catalysts for Green Hydrogen Production by Glycerol Steam Reforming. Catalysts 2023, 13, 1004. [Google Scholar]
- Zhang, Y.; Huang, Z.; Xie, P.; Lacey, S.D.; Jacob, R.J.; Yao, Y. Flash Joule heating (FJH) is an emerging and profitable technology for converting inexhaustible biomass into flash graphene (FG). Nature 2020, 577, 647–651. [Google Scholar]
- Roychand, R.; Kilmartin-Lynch, S.; Saberian, M.; Li, J.; Zhang, G.; Li, C.Q. Transforming spent coffee grounds into a valuable resource for the enhancement of concrete strength. J. Clean. Prod. 2023, 419, 138205. [Google Scholar] [CrossRef]
- Tay, T.; Ucar, S.; Karagöz, S. Preparation and characterization of activated carbon from waste biomass. J. Hazard. Mater. 2009, 165, 481–485. [Google Scholar] [CrossRef]
- Pal, A.; Thu, K.; Mitra, S.; Ibrahim, I.E.; SaHa, B.B.; Kil, H.S.; Yoon, S.H.; Miyawaki, J. Study on biomass derived activated carbons for adsorptive heat pump application. Int. J. Heat Mass Transf. 2017, 110, 7–19. [Google Scholar] [CrossRef]
- Wickramaarachchi, W.K.P.; Minakshi, M.; Gao, X.; Dabare, R.; Wong, K.W. Hierarchical porous carbon from mango Seed husk for electro-chemcal energy storage. Chem. Eng. J. Adv. 2021, 8, 100158. [Google Scholar] [CrossRef]
- Ahmad, A.A.; Alwahbi, A.; Khatib, L.A.A.; Dammag, H. Biomass-Based Activated Carbon. In From Biomass to Biobased Products; IntechOpen: London, UK, 2024. [Google Scholar]
- Campos, G.A.F.; Perez, J.P.H.; Block, I.; Sagu, S.T.; Celis, P.S.; Taubert, A.; Rawel, H. Preparation of activated carbons from spent coffee grounds and coffee parchment and assessment of their adsorbent efficiency. Processes 2021, 9, 1396. [Google Scholar] [CrossRef]
- Ani, J.U.; Akpmie, K.G.; Okoro, U.C.; Onukwuli, O.D.; Ujam, O.T. Potentials of activated carbon produced from biomass materials for sequestration of dyes, heavy metals, and crude oil components from aqueous environment. Appl. Water Sci. 2020, 10, 1–11. [Google Scholar] [CrossRef]
- Zięzio, M.; Charmas, B.; Jedynak, K.; Hawryluk, M. Preparation and characterization of activated carbons obtained from the waste materials impregnated with phosphoric acid (V). Appl. Nanosci. 2020, 10, 4703–4716. [Google Scholar] [CrossRef]
- ASTM D1762-84; Standard Test Method for Chemical Analysis of Wood Charcoal. ASTM International: West Conshohocken, PA, USA, 2021.
- Aller, D.; Bakshi, S.; Laird, D.A. Modified method for proximate analysis of biochars. J. Anal. Appl. Pyrolysis 2017, 124, 335–342. [Google Scholar] [CrossRef]
- Park, J.E.; Lee, G.B.; Jeong, C.J.; Kim, H.; Kim, C.G. Determination of Relationship Between Higher Heating Value and Atomic Ratio of Hydrogen to Carbon in Spent Coffee Grounds by Hydrothermal Carbonization. Energies 2021, 14, 6551. [Google Scholar] [CrossRef]
- ASTM D5228-16; Standard Test Method for Determination of Butane Working Capacity of Activated Carbon. ASTM International: West Conshohocken, PA, USA, 2016.
- Saka, T.; San-Pedro, L.; Abubakar, A.M.; Sylvain, T. Evaluation of the physical properties of various biomass materials for the production of activated carbon. J. Chem. Environ. 2023, 1, 30–39. [Google Scholar] [CrossRef]
- Xiao, X.; Chen, Z.; Chen, B. H/C atomic ratio as a smart linkage between pyrolytic temperatures, aromatic clusters and sorption properties of biochars derived from diverse precursory materials. Sci. Rep. 2016, 6, 22644. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Luo, L.; Deng, S.; Shi, G.; Zhang, S.; Zhang, Y.; Deng, O.; Wang, L.; Zhang, J.; Wei, L. Sorption of tetracycline on H3PO4 modified biochar derived from rice straw and swine manure. Bioresour. Technol. 2018, 267, 431–437. [Google Scholar] [CrossRef]
- Scardaci, V.; Compagnini, G. Raman Spectroscopy Investigation of Graphene Oxide Reduction by Laser Scribing. C 2021, 7, 48. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, Y.; Konarov, A.; Gosselink, D.; Chen, P.; Adair, K.R.; Sun, Q.; Li, R.; Sun, X. High-Performance Potassium-Ion Batteries with Ultrafine MoO Nanoparticles Embedded in Carbon Nanosheets Anode. J. Power Sources 2020, 476, 229076. [Google Scholar]
- Helmi, M.; Moazami, F.; Hemmati, A.; Ghaemi, A. Synthesis and Characterization of KOH Graphene Oxide-Fe3O4 as a Magnetic Composite Adsorbent for CO2 Capture. J. Phys. Chem. Solids 2023, 178, 111338. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Chen, X. Modeling of experimental adsorption isotherm data. Information 2015, 6, 14–22. [Google Scholar] [CrossRef]
- Furmaniak, S.; Gauden, P.A.; Lezanska, M.; Miskiewicz, R.; Blajet-Kosicka, A.; Kowalczyk, P. The finite pore volume GAB adsorption isotherm model as a simple tool to estimate a diameter of cylindrical nanopores. Molecules 2021, 26, 1509. [Google Scholar] [CrossRef]
- Lee, H.; Lee, B.; An, K.; Park, S.; Kim, B. Facile preparation of activated carbon with optimal pore range for high butane working capacity. Carbon Lett. 2020, 30, 297–305. [Google Scholar] [CrossRef]
- Lee, B.-H.; Lee, H.-M.; Chung, D.C.; Kim, B.-J. Effect of Mesopore Development on Butane Working Capacity of Biomass-derived Activated Carbon for Automobile Canister. Nanomaterials 2021, 11, 673. [Google Scholar] [CrossRef]
- Wang, X.; Li, D.; Li, W.; Peng, J.; Xia, H.; Zhang, L.; Guo, S.; Chen, G. Optimization of Mesoporous Activated Carbon from Coconut Shells by Chemical Activation with Phosphoric Acid. BioResources 2013, 8, 6184–6195. [Google Scholar] [CrossRef]
- Zhao, W.; Luo, L.; Wang, H.; Fan, M. Synthesis of Bamboo-Based Activated Carbons with Super-High Specific Surface Area for Hydrogen Storage. BioResources 2017, 12, 1246–1262. [Google Scholar] [CrossRef]
- Lee, H.; Lee, B.; Park, S.; An, K.; Kim, B. Pitch-derived activated carbon fibers for emission control of low-concentration hydrocarbon. Nanomaterials 2019, 9, 1313. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).