Abstract
Leucaena leucocephala (Lam.) de Wit is listed in the world’s 100 worst alien invasive species because of the risks it poses to native plant communities. Life history traits, such as high growth and reproductive rates, and a high capacity to adapt to different environmental conditions may contribute to its invasive properties. Biotic stressors, such as herbivores, pathogens, and competing plant species are known to exert significant selective pressure on the plant’s survival, distribution, and abundance. L. leucocephala has been reported to contain several compounds involved in the defense functions against these biotic stressors. A large amount of L-mimosine, a non-protein amino acid, was found in all plant parts of L. leucocephala, including its flowers. L-Mimosine is toxic to herbivorous mammals and insects, parasitic nematodes, pathogenic fungi, and neighboring competing plant species by inactivating various essential enzymes and blocking DNA replication, and/or inducing oxidative stress conditions. Several flavonoids, polyphenolic compounds, and/or derivatives of benzoic and cinnamic acids are toxic to parasitic nematodes, pathogenic fungi and bacteria, and competing plant species by disrupting plasma membrane structures and functions, and various metabolic processes. These compounds may represent the invasive traits of L. leucocephala that have undergone natural selection during the evolution of the species. They may contribute to the defense functions against the biotic stressors, and increase its survival, distribution, and abundance in the introduced ranges. This is the first review to focus on the compounds involved in the defense functions against biotic stressors.
Keywords:
allelochemical; biotic stressor; herbivore; insecticide; invasive species; mimosine; nematicide; pathogen; pesticide 1. Introduction
Leucaena leucocephala (Lam.) de Wit, belonging to the Fabaceae family, is a perennial tree, 3–10 m, rarely 20 m, in height, with a cylindrical stem of 5–50 cm in diameter and branched. The leaves are alternate and bipinnate, consisting of 4–9 pairs of pinnae per leaf, and 13–21 pairs of leaflets per pinna [1,2,3,4]. The lifespan of L. leucocephala is over 30 years [5], and the annual biomass productivity has been estimated to be at 20–60 tons per hectare in the tropical regions [6] (Figure 1).

Figure 1.
Trees and leaves of L. leucocephala.
The native range of L. leucocephala is Mexico and Central America, and the species has been introduced worldwide for its economic benefits, such as fodder for livestock [7,8,9,10], shade trees in nurseries and plantations [11,12,13], timber, paper pulp and biochar production [14,15,16,17], and rehabilitation and erosion control to cover degraded lands and slopes [18,19,20]. However, L. leucocephala is an aggressive colonizer, easily escaping to unintended sites, and forming dense monospecific stands in riparian areas, grasslands, hillsides, forest edges, abandoned agricultural fields, disturbed areas, roadsides, and protected areas, including reserved forests [5,21,22]. The expansion rate of the L. leucocephala population on the main island of Taiwan was estimated to be 3.4 hectare per year [23]. L. leucocephala has already spread into more than 130 countries in the tropical, subtropical, and warm temperate regions of South America, Africa, South and East Asia, Southern Europe, Australia, and the islands in the Caribbean, Indian, and Pacific Oceans [2,5,24].
L. leucocephala threatens native plant communities, reducing the density and richness of the native plant species, including endemic and endangered plant species in the infested areas [5,21,25]. The establishment of the native tree species in areas infested by L. leucocephala was hardly observed [25,26,27,28]. Field experiments have confirmed that the presence of L. leucocephala suppressed the regeneration process, such as germination and growth, of the native woody plant species Erythrina velutina Willd. [25]. The plant communities in the islands are much more vulnerable to the infestation of L. leucocephala, and the native woody species, including protected plant species, were replaced by L. leucocephala due to the interruption of their regeneration processes [2,26,27,28,29,30,31,32,33,34,35]. The infestation of L. leucocephala has also altered the plant successional pathway, and reduced the plant diversity in the mid- and late-successional forests. The dominance of L. leucocephala has persisted for a long time, and the composition of the native forests have not recovered [26,34,35].
Soil nitrogen levels under the L. leucocephala stands were high due to the symbiotic nitrogen fixation [36,37,38]. The increase in soil nitrogen availability may affect the composition of the existing plant species, and the structure and function of the ecosystem. As a result, the food webs and invertebrate and vertebrate habitats may be altered [39]. Because of the risk of invasion into native plant communities, L. leucocephala has been listed in the world’s 100 worst alien invasive species by the International Union for Conservation of Nature and Natural Resources [40]. The global warming trend may further increase the potential risk of L. leucocephala invasion [41].
To understand the invasion mechanism, the life history traits of several invasive plant species, such as their ability to adapt to different environmental conditions and their growth and reproductive capabilities, have been documented [42,43,44,45,46,47]. This information may also be necessary to understand the invasion mechanism of L. leucocephala. L. leucocephala grows well in tropical and subtropical climates, between 15 and 25 degrees of north and south latitude of the globe, with annual precipitation ranging from 650 mm to 3000 mm, and temperatures from 18 to 30 °C, and on drained, neutral to slightly alkaline soils [2,4]. However, the species tolerates annual precipitation as low as 300 mm, and at 0 °C for the mean temperature of the coldest month, and up to 40 degrees of north and south of the equator, including a warm temperate climate [2,5]. L. leucocephala has drought tolerance through a strong ability to maintain leaf water content by reducing the transpiration without defoliation. The species can survive for several months under drought stress conditions, and can recover soon after receiving the available water. Its leaflets hold together with the opposite leaflets of the pinnae to avoid water transpiration from the leaves under drought stress conditions during the daytime, and the leaves also show nyctinasty to avoid transpiration during the nighttime [48,49,50]. This tolerance allows the species to adapt to different environmental conditions (Figure 2).

Figure 2.
Closure of the leaves of L. leucocephala during daytime.
L. leucocephala grows rapidly and reaches its reproductive stages within 4–12 months after germination. The species flowers and produces seeds mainly by self-fertilization throughout the year under suitable climatic conditions [51,52,53]. The flat fruit pods contain 8–30 (average 18) seeds per pod [53,54]. The annual production rate of pods was recorded as 277–388 per plant, containing 4000–6000 seeds in tropical coastal Australia [53]. The species also dropped 5500 seeds per m2 per year on the forest floor in Brazil [55]. Seeds are carried by wind, water flow, and the activities of birds, rodents, and humans. Some seeds were carried by wind over 100 m from the parent plants [2,53]. Germination occurs over a long period of time after seed dispersal. The germination rate of fresh seeds was 59% under optimal conditions, and the seeds retained the ability to germinate for 20 years under good seed bank conditions [5,55,56]. In light of these observations, the life history traits, such as year-round flowering and fruiting, self-fertility, prolific seed production, seed bank establishment, and adaptability to different environmental conditions, may contribute to the infestation and expansion of L. leucocephala populations in new habitats (Figure 3).

Figure 3.
Capitulum and pods of L. leucocephala.
L. leucocephala has also been reported to exhibit toxic activity against herbivorous mammals and insects, parasitic nematodes, pathogenic fungi and bacteria, and neighboring competing plant species, and to contain the compounds responsible for these toxicities. These compounds may be involved in the defense functions against biotic stressors, such as herbivores, pathogens, and competing plant species. Such defense functions may contribute to the successful infestation, naturalization, and expansion of invasive plants in the introduced range [57,58,59,60,61,62]. However, no review has focused on the toxicity and related compounds of L. leucocephala for the understanding of its invasive properties. This is the first review to provide an overview of the toxicity of L. leucocephala to biotic stressors and the specific compounds associated with the toxicity. The possible mechanisms of action of these compounds, and their involvement in the invasive characteristics of L. leucocephala were also discussed. The literature was searched using a combination of major online search engines, including Scopus, Google Scholar, and ScienceDirect, and the combinations of L. leucocephala with the following keywords: ecology, distribution, invasion, impact, habitat, adaptation, growth, reproduction, herbivore, mammal, pathogen, nematicide, insecticide, fungicide, pesticide, and allelopathy.
2. Toxicity Against Herbivorous Mammals
L. leucocephala poisoning has been reported in ruminants, such as cattle (Bos taurus L.) [63,64,65], goats (Capra hircus L.) [66,67], and sheep (Ovis aries L.) [68], and in nonruminants, such as horses (Equus caballus L.) [69], pigs (Sus scrofa domesticus Erxleben) [70], rabbits (Oryctolagus cuniculus L.) [71,72], rats (Rattus norvegicus Berkenhout) [73,74,75], mice (Mus musculus L.) [76], and ring-tailed lemurs (Lemur catta L.) [77]. An active component of L. leucocephala poisoning has been identified as a non-protein amino acid, L-mimosine (hereafter, mimosine). Mimosine has been found in several species of the genera Mimosa and Leucaena [78,79,80] (Figure 4).
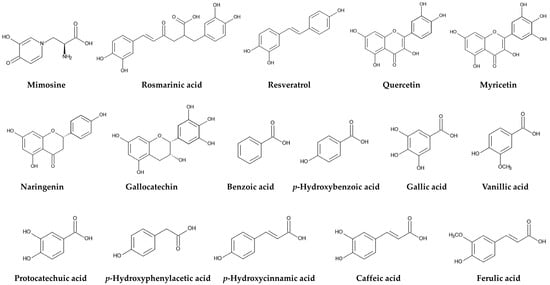
Figure 4.
Compounds involved in defense functions of L. leucocephala against herbivorous mammals and insects, parasitic nematodes, pathogenic fungi and bacteria, and competing plant species. Polyphenolic compounds: rosmarinic acid, resveratrol. Flavonoids: quercetin, myricetin, naringenin, gallocatechin. Benzoic acid and cinnamic acid derivatives: benzoic acid, p-hydroxybenzoic acid, gallic acid, vanillic acid, protocatechuic acid, p-hydroxyphenylacetic acid, p-hydroxycinnamic acid, caffeic acid, ferulic acid.
L. leucocephala contains a large amount of mimosine in all parts of the plant. The concentration of mimosine was 2.4–13.6% (mimosine weight/dry weight of the plant) in mature seeds, 0.47–8.6% in leaves, 1.2–2.7% in flowers, 0.15–0.68% in stems, and 0.16–0.66% in roots [81,82,83]. It was 22.2% in young shoot tips, 14.7% in mature shoot tips, 6.4% in young leaves, and 1.4% in senescent leaves, indicating that newly developing tissues contain more mimosine than old tissues. Mimosine concentration was also increased during germination and seedling growth. The concentration was 3.9% in seeds, and increased to 6.9% at 6 days after the sowing, and was 15.2% in 2-week-old seedlings [82,83], indicating that most of the mimosine is synthesized de novo during germination and development. Mimosine is synthesized by mimosine synthase from O-acetyl-L-serine and 3-hydroxy-4-pyridone (3H4P) [84,85]. O-acetyl-L-serine is formed from serine and acetyl-CoA by serine acetyltransferase [84,85,86,87]. However, the pathway of 3-hydroxy-4-pryridone synthesis has not yet been confirmed in L. leucocephala (Figure 5).
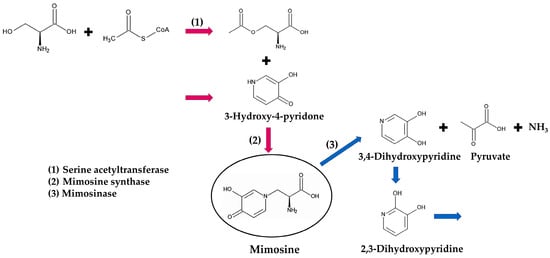
Figure 5.
Mimosine biosynthesis and degradation pathway. Red arrows: biosynthesis; blue arrows: degradation.
Ingestion of the L. leucocephala leaves and seeds, and the administration of mimosine has caused decreases in the food intake, body weight gain, and leukocytes, and increases in alopecia, cataracts, hepatic congestion, and infant mortality in ruminants and nonruminants [64,65,66,67,68,69,70,71,72,77]. L. leucocephala and mimosine has also caused increases in infertility, abortion, and abnormal embryo development in female mammals, and decreases in the serum testosterone levels and sexual behavior in male mammals [73,75,76,88,89].
Goiter is also a major symptom of feeding on L. leucocephala and mimosine, which has been well documented in ruminants [90,91,92,93]. Rumen bacteria, such as Synergistes jonesii Allison, Butyrivibrio fibrisolvens Bryant & Small, and Clostridium butyricum Prazmowski, produce mimosinase, which metabolizes mimosine to 3,4-dihydroxypyridine (3,4-DHP), pyruvate, and ammonia, and certain rumen bacteria, possibly including S. jonesii, then convert 3,4-DHP to its isomer 2,3-dihydroxypyridine (2,3-DHP) [94,95,96,97]. The 3,4-DHP and 2,3-DHP are still toxic and cause goiter in ruminants [90,96,98]. Additional rumen bacteria may be involved in the further metabolism of 2,3-DHP to unidentified non-toxic substances [97,98,99,100]. The ingestion of L. leucocephala and mimosine has also caused the enlargement of the thyroid gland in rabbits, and abnormal thyroid cells, and/or decreased thyroid hormone levels in rats, rabbits, and horses [69,100,101,102,103], the mimosine metabolism has not yet been documented in nonruminants (Figure 5).
Some of the toxic effects of mimosine are thought to be caused by its chelating potential due to its pyridine ring and α-amino acid residue. Mimosine is capable of forming stable complexes with bivalent transition metal ions, such as Fe (II), Cu (II), Ni (II), and Zn (II) [98,104]. Mimosine inactivates several metal ion-dependent enzymes, such as alkaline phosphatase, ribonucleotide reductase, tyrosinase, and dopamine β-hydroxylase, by limiting the availability of the metal ions to the metabolic cofactors of these enzymes by chelating these ions [94,105,106]. Mimosine is also able to form a strong complex with pyridoxal-5′-phosphate (PLP), and inactivates PLP-dependent enzymes, such as cystathionine γ-lyase, aspartate aminotransferase, cysteine synthase, tyrosine decarboxylase, and L-DOPA decarboxylase [107,108,109,110]. Therefore, mimosine is able to interrupt several important metabolic processes by inhibiting the activity of the related enzymes.
Mimosine also inhibits the process of cell proliferation, specifically the transition from the G1 to the S phase. Mimosine is an effective cell cycle arresting reagent. It works by synchronizing cells within the late G1 phase by blocking entry into the S phase through the activation of the Ataxia-telangiectasia-mutated (ATM) gene, and interrupting the binding of the chromatin-associated protein Ctf4 to chromatin. It also prevents the formation of replication forks in the S phase, thereby blocking DNA replication [111,112,113]. As previously mentioned, mimosine inhibits ribonucleotide reductase activity [106]. Ribonucleotide reductase catalyzes the formation of deoxyribonucleotides from ribonucleotides, and regulates the elongation process of DNA replication in the S phase [114,115].
Considering these findings, it can be concluded that mimosine inactivates several essential enzymes and blocks the DNA replication process. Inactivation of the essential enzymes and inhibition of DNA replication may result in a variety of pathological symptoms, including alopecia, cataracts, hepatic congestion, goiter, increased infertility, abortion, abnormal embryo development in female mammals, and decreased serum testosterone levels in male mammals [64,65,66,67,68,69,70,71,72,73,74,75,76,77,88,89]. L. leucocephala contains a significant amount of the toxic substance mimosine in all parts of the plant. Therefore, mimosine may function to protect L. leucocephala from herbivory by herbivorous mammals (Figure 5).
3. Toxicity Against Herbivorous Insects
Herbivorous insects often cause significant limitations to the host plants, suppressing the growth, seed production, and survival [116,117,118,119]. Aqueous extracts of L. leucocephala leaves increased the mortality of the eggs and larvae of the silverleaf whitefly Bemisia tabaci Gennadius [120]. Hexane and dichloromethane extracts of L. leucocephala seeds increased the mortality of adults of the plant-feeding mite Tetranychus urticae C.L. Koch, and decreased their oviposition activity [121].
Mimosine inhibited the growth and development of the larvae of the Australian bollworm Heliothis punctiger Wallengren [122] and the red flour beetle Tribolium castaneum Herbst [123], and increased the mortality of the adult termite Coptotermes formosanus Shiraki with an LD50 value of 1.2 μM [124]. Mimosine also inhibited the activity of acetylcholinesterase and tyrosinase in the adult termite C. formosanus. The concentration of mimosine, required for 50% inhibition of these activities, was determined to be 31.4 and 46.1 μM for acetylcholinesterase and tyrosinase, respectively [124]. Acetylcholinesterase catalyzes the breakdown of acetylcholine, a neurotransmitter, to acetate and choline at the synapses in the nervous system [125,126]. Suppression of acetylcholinesterase results in the prolonged retention of this neurotransmitter at the synapses of the acetylcholine receptors. This prolonged retention leads to the sustained excitation of neurons, resulting in abnormal behavior, including death [126,127,128]. Tyrosinase is a copper-containing enzyme involved in tyrosine metabolism, which is responsible for cuticle hardening and immune responses in insects [129,130,131]. Therefore, the inhibition of acetylcholinesterase and tyrosinase by mimosine may inhibit the growth and development of insects, and increase their mortality. Mimosine has also been reported to arrest cell division between the G1 and S phases of the mosquito Aedes albopictus [132]. The inhibition of cell division, and the activity of acetylcholinesterase and tyrosinase by mimosine in these insects may be caused by a mechanical action similar to that described in the mammalian cells. The extracts of the seeds and leaves of L. leucocephala increased the mortality of the insects, and mimosine may be an active principal of the extracts. Therefore, mimosine may function to protect L. leucocephala from herbivory by herbivorous insects.
4. Toxicity Against Parasitic Nematodes
The root-knot nematodes Meloidogyne spp. are parasitic on various host plant species. The juveniles of Meloidogyne spp. invade plant root cells, and form permanent feeding sites and root-knot galls, known as root-knot disease. The infested nematodes absorb nutrients from the host plant through the feeding sites. The feeding process of the nematodes causes severe disease, such as yellowing, wilting and/or stunting. The feeding process also causes significant damages to the host plant’s root system, reducing its ability to absorb water and nutrients from the soil and to resist infection by other pathogens [133,134,135,136,137].
Aqueous, methanol, and ethanol extracts of the leaves, roots, stems, and seeds of L. leucocephala increased the mortality of the root-knot nematode Meloidogyne incognita Kofoid & White and decreased its egg hatching, population, and parasitism [138,139,140]. Aqueous extracts of L. leucocephala leaves also increased the mortality of another root-knot nematode Meloidogyne exigua Goeldi, and decreased its parasitism and oviposition activity [141]. Aqueous ethanol extracts of L. leucocephala seeds increased the mortality of the laboratory model nematode Caenorhabditis elegans Maupas with an LC50 value of 73 mg of the plants/mL, and decreased its movement, feeding, and oviposition activity [142]. Mimosine also increased the mortality of C. elegans with an LC50 value of 16.8 μM [124], and decreased its movement, feeding, and oviposition activity [142]. Quercetin (flavonoid) isolated from the leaves of L. leucocephala suppressed the eggs hatching of M. incognita and increased their mortality [143] (Figure 4). Quercetin also showed inhibitory activity on the eggs hatching of Cooperia spp., which is the common intestinal parasitic nematode of cattle [144], and increased the mortality of the larvae of the dog parasitic roundworm Toxocara canis Johnston [145]. Based on these findings, L. leucocephala has nematocidal activity and protects against nematode parasitism.
Given its effectiveness against mammals and insects, mimosine in L. leucocephala may contribute to the nematocidal activity of the extracts by inactivating enzymes and/or inhibiting cell proliferation. However, no direct research has been conducted on its effects on parasitic nematodes. The mode of action of quercetin on nematocidal activity has also not yet been reported.
5. Toxicity Against Pathogenic Fungi and Bacteria
Pathogenic microbes cause various significant diseases on the infested host plants, reducing their growth, biomass, reproduction, and survival [146,147,148]. Aqueous extracts of L. leucocephala leaves suppressed the spore germination and growth of the pathogenic fungi Fusarium solani (Mart.) Sacc., Alternaria solani Sorauer, and Colletotrichum circinans (Berkeley) Voglino [149]. Aqueous extracts of L. leucocephala seeds inhibited the growth of the pathogenic fungus Sclerotium rolfsii Saccardo. The extracts and mimosine caused the reduction of the mycelial protein and nucleic acid levels in S. rolfsii [150].
Aqueous methanol extracts of the seeds, leaves, stems, and roots of L. leucocephala also inhibited the growth of the pathogenic fungi Rhizoctonia solani J.G. Kühn, F. solani, and A. solani, as well as the pathogenic bacteria Agrobacterium tumefaciens (Smith & Townsend) Conn, and Erwinia amylovora Winslow. The main constituents of these extracts were as follows: rosmarinic acid (4.8 mg/g of dried plant part), resveratrol (3.0 mg/g), quercetin (2.1 mg/g), myricetin (1.4 mg/g), and naringenin (1.2 mg/g) in the leaf extracts; benzoic acid (0.6 mg/g) and naringenin (0.4 mg/g) in the root extracts; rosmarinic acid (2.2 mg/g), resveratrol (1.6 mg/g), and benzoic acid (1.1 mg/g) in the twig, stem, and bark extracts; and benzoic acid (1.5 mg/g), myricetin (0.8 mg/g), and rosmarinic acid (0.8 mg/g) in the fruit and seed extracts [151] (Figure 4). The mechanisms of the antifungal and antibacterial activity of flavonoids, such as quercetin, naringenin, and myricetin, have been reported to be the disruption of plasma membrane structures, resulting in the interruption of several physiological functions, including ATP synthesis [152,153]. The polyphenolic compound, resveratrol acts as a phytoalexin and inhibits the growth of various bacteria and fungi. Resveratrol interacts with more than 20 proteins, including ATP synthase in these microbes, resulting in the inhibition of ATP synthesis, induction of DNA fragmentation, and disruption of the membrane structure [154,155,156]. The other polyphenolic compound, rosmarinic acid has been reported to disrupt mitochondrial activity and cell membrane structures [157]. A possible mechanism of the antimicrobial activity of benzoic acid is considered to be hyper-acidification at the plasma membrane of the microbes due to the transport of its undissociated form across the cytoplasmic membrane into the cells, and the release of H+ ions. Hyper-acidification alters the functions of the cell membrane, making it more permeable, and disrupting the ATPase pump, leading to cell death [158,159,160]. Therefore, L. leucocephala has antifungal and antibacterial activity. Mimosine, benzoic acid, flavonoids, such as quercetin, naringenin, and myricetin, and polyphenolic compounds, such as rosmarinic acid and resveratrol, may be involved in these activities.
6. Toxicity Against Competing Plant Species
Plants fight with neighboring plants for niches and resources, such as light, nutrients, and water. Stronger competitive ability of the plants guarantees better conditions for survival [161,162,163,164,165,166,167]. Allelopathy is the phenomenon whereby the germination and growth of neighboring plants is affected by the release of allelochemicals. Several invasive plants have been reported to suppress the germination, growth, and fitness of neighboring competing plant species by releasing allelochemicals, resulting in the acquisition of many more resources [168,169,170,171,172]. Allelochemicals are produced and reserved in the host plant tissues, and released into the environment, including rhizosphere soils, as necessary through the volatilization, secretion, and degradation processes of plant residues in the soil [173,174,175,176,177]. The growth and survival of a woody plant species, Erythrina velutina Willd, was observed to be limited by the presence of L. leucocephala under relatively controlled field conditions [178]. The competition, between both species, for light, water, and nutrients was elucidated from the observations due to the relatively controlled experimental conditions, suggesting the possible involvement of the allelopathy of L. leucocephala in limiting the growth and survival of E. velutina. The allelopathic activity of L. leucocephala was determined in its extracts, plant residues, and rhizosphere soils. Several allelochemicals have also been identified.
Aqueous extracts of the whole aerial parts of L. leucocephala suppressed the growth of Amaranthus hybridus L. and Bidens pilosa L. under laboratory and greenhouse conditions [179], and aqueous extracts of the leaves and seeds of L. leucocephala inhibited the germination and growth of Tridax procumbens L., Ageratum conyzoides L., Emilia sonchifolia (L.) DC. ex Wight, Pterogyne nitens Tul., and Peltophorum dubium (Spreng.) Taub. [180,181]. Thus, the extracts of the aerial parts and seeds of L. leucocephala may contain certain allelochemicals that cause growth inhibition.
When the seeds of a woody plant, Albiza procera (Roxb.) Benth., were sown into a mixture of the soil and leaves of L. leucocephala, their germination and growth were suppressed [182]. Soil mixed with the decomposing leaves of L. leucocephala caused a decrease in survival of five woody plant species, including Mimosa pudica L., Liquidambar formosana Hance, Casuarina glauca Sieber ex Spreng., Alnus formosana (Burkill) Makino, and Acacia confusa Marr. [183]. Aqueous extracts of the litter accumulated on the soil beneath L. leucocephala plants suppressed the germination and growth of four herbaceous plant species, namely Lolium multiflorum Lam., E. sonchifolia, T. procumbens, and A. conyzoides [183,184]. Soil collected from under L. leucocephala stands suppressed the germination and growth of E. sonchifolia, T. procumbens, and A. conyzoides. Exudates from the roots of L. leucocephala also suppressed the germination and growth of these plant species [184]. Therefore, certain allelochemicals may be released into the rhizosphere soil through the root exudation and the decomposition process of plant litter and residues, causing the growth inhibition of these plant species.
Mimosine has also been identified as an allelochemical in L. leucocephala for its growth inhibitory activity against several plant species [183,184,185,186]. At a concentration of 100 ppm, mimosine inhibited the root growth of B. pilosa, M. pudica, Phaseolus vulgaris L., Brassica rapa L., and L. multiflorum by 40–95%, and their shoot growth by 31–93% [81]. Mimosine also inhibited the root and shoot growth of Senna obtusifolia (L.) H.S. Irwin & Barneby and Sesbania herbacea (Mill.) McVaugh [187], as well as A. conyzoides, T. procumbens, and E. sonchifolia [188]. Mimosine reduced the vigor of the A. conyzoides seedlings and wilted the seedling 7 days after application at 50 ppm [183].
On the other hand, mimosine suppressed the growth of the roots and shoots of L. leucocephala by only 2.4% and 12%, respectively, at a concentration of 100 ppm [81]. Thus, mimosine has relatively little inhibitory effect on L. leucocephala itself. The enzyme, mimosinase (Figure 5), which is responsible for the degradation of mimosine, was found in L. leucocephala, and the gene expression of the enzyme was high [189,190,191,192]. Therefore, mimosinase degrades mimosine, and the degradation process may attenuate the toxicity of the mimosine to L. leucocephala itself.
L. leucocephala seedlings released mimosine into the growth medium at 1–5 μg/g dry weight of the plant per day [193], and mimosine accumulated at 7.4 μg/g dry weight of the rhizosphere soil [194], suggesting that L. leucocephala may release mimosine into its rhizosphere soil. In addition, L. leucocephala contains a large amount of mimosine in whole plant parts. Some of the mimosine may also be released into the soil during the decomposition process of the plant residues, and accumulate in the soil under L. leucocephala trees. The accumulated mimosine in the soil may act as an allelochemical to suppress the germination and growth of neighboring plant species.
Aqueous extracts of the aerial parts of L. leucocephala inhibited cell division in the roots of Zea mays L. and Pisum sativum L. [195,196]. Mimosine also suppressed the cell division between the G1 and S phases of the protoplasts of Petunia hybrida Juss. [197] and the phytoplankton Rhodomonas salina Karsten [198]. Mimosine suppressed rooting by disrupting the root cell division in Allium cepa L. [199]. Therefore, mimosine may also disrupt the cell division process in plants, resulting in growth suppression.
Aqueous extracts of the aerial parts of L. leucocephala increased the peroxidase activity in the roots of Z. mays [179]. Rinsed water from L. leucocephala leaves increased the activity of ascorbate peroxidase and catalase in the leaves of Eichhornia crassipes (Martius) Solms-Laubach, and induced electrolyte leakage from their leaf cell membranes [200]. These enzymes are known to increase in plants under oxidative stress conditions [201,202,203]. Oxidative stress induces the alteration of the physiological processes and structures in plant cells, including the plasma membrane [204,205,206,207,208]. Mimosine also induced the abnormal cytoplasmic organelles and necrosis in the meristem cells of A. cepa root tips, and increased catalase and superoxide dismutase activities, and malondialdehyde levels in the roots [199,200,201,202,203]. Therefore, mimosine may induce oxidative stress conditions in the target plant species, and disrupt their physiological processes and cell structures.
Jasmonic acid, and ethephon which is metabolized into ethylene in plants after absorption, increased mimosine production and concentrations in L. leucocephala seedlings [209]. Salicylic acid, mechanical injury, UV irradiation, and NaCl treatment also increased the mimosine concentrations in the seedlings [82,209,210]. Salicylic acid, jasmonic acid, and ethylene are plant hormones that act as stress signaling molecules, and induce the expression of stress-related genes [211,212,213]. The stress conditions caused by the mechanical injury, UV irradiation, and NaCl treatment, as well as the stress signaling molecules, including salicylic acid, jasmonic acid, and ethylene, may stimulate the production of mimosine in L. leucocephala. Therefore, the stress conditions caused by the competition with the neighboring plant species may increase the production of mimosine, and the increased mimosine levels may also enhance the competitive ability of L. leucocephala.
Benzoic acid and cinnamic acid derivatives, such as p-hydroxybenzoic acid, gallic acid, vanillic acid, protocatechuic acid, p-hydroxyphenylacetic acid, p-hydroxycinnamic acid, caffeic acid, and ferulic acid, have been identified as allelochemicals in the leaves of L. leucocephala [183] (Figure 4). These compounds have also been identified as allelochemicals in various plant extracts and their rhizosphere soils [214,215,216]. They alter the structure and function of the plasma membranes, including their lipid and protein composition and transmembrane electrochemical potential. This results in membrane depolarization. These changes cause the non-specific influx and efflux of cations and anions, such as potassium, magnesium, phosphate, and nitrate ions, and affect the water balance of the plant cells [217,218]. These compounds also interfere with various metabolic processes, such as protein synthesis, photosynthesis, plant hormone synthesis, phytohormone synthesis, and various secondary metabolites, and inhibit cell division, resulting in reduced germination, growth, and development of the target plant species [214,215,216].
In addition, methanol extracts of L. leucocephala roots inhibited the nitrification process in soil, with gallocatechin being the most active component [219] (Figure 4). The first step in nitrification is the oxidation of ammonia (NH3) or ammonium (NH4) to nitrate (NO2−) by ammonia-oxidizing bacteria and archaea. The second step is the oxidation of nitrate to nitrite (NO3−) by nitrite-oxidizing bacteria, such as Nitrobacter spp. Nitrification is an important process for nitrogen cycling in ecosystems [220,221]. Gallocatechin from L. leucocephala may inhibit the nitrification process by inhibiting the associated bacterial activity, although the mechanism of action remains unknown. Inhibition of nitrification may reduce nitrate availability in the soil, resulting in the growth suppression of the neighboring plant species. Conversely, soil nitrogen levels under L. leucocephala stands were reported to be high due to its symbiotic nitrogen fixation [36,37,38]. Therefore, it will be necessary to evaluate the actual amount of nitrogen available in the soil under L. leucocephala stands in the future.
Based on these findings, L. leucocephala exhibits allelopathic activity and contains several allelochemicals, including mimosine, gallocatechin, and derivatives of benzoic and cinnamic acids. Mimosine accumulates in the medium and soil through the root exudation of L. leucocephala. Mimosine may also be released into the soil during the decomposition process of the plant residues. These allelochemicals described here can suppress the germination, growth, and fitness of neighboring competing plant species. As a result, L. leucocephala may obtain much more nutrients and water, increasing its growth and population.
L. leucocephala contains various other compounds, including terpenoids, benzenoids, flavonoids, saponins, tannins, steroids, and glycerides in the leaves, roots, stems and seeds [222,223,224,225,226,227,228,229,230,231,232]. Some of them have pharmacological effects, such as anti-tumor, anti-diabetic, anti-malaria, anti-inflammatory, anti-diarrhea, and antioxidant activities [225,227,230,231,232]. It is possible that some of these compounds are responsible for the unknown defense functions and contribute to the invasive properties of L. leucocephala.
7. Conclusions
L. leucocephala is listed in the world’s 100 worst alien invasive species, and has been spread to over 130 countries in the tropical, subtropical, and warm temperate regions. The species threatens native plant communities by reducing their density and richness in the infested areas. Its life history traits, such as year-round flowering and fruiting, self-fertility, prolific seed production, seed bank establishment, and adaptability to diverse environmental conditions may contribute to its infestation and population expansion.
Biotic stressors, such as herbivores, pathogens, and competing plant species, affect plant germination, growth, biomass, seed production, and senescence. These stressors exert significant selective pressures on plant survival, distribution, and abundance [233,234,235,236]. Defense mechanisms against these biotic stressors may be necessary for the invasive plant species to increase their distribution and abundance in new habitats [237,238,239]. L. leucocephala produces several toxic compounds that protect against herbivorous mammals and insects, parasitic nematodes, pathogenic fungi and bacteria, and neighboring competing plant species e.g., [81,82,143,151,183,216].
Mimosine is toxic to herbivorous mammals and insects, parasitic nematodes, pathogenic fungi, and competing plant species e.g., [79,124,132,142,150]. L. leucocephala contains high levels of mimosine in the entire plant [81,82,83]. Consumption of the plant parts and the administration of mimosine cause various pathological symptoms, including alopecia, cataracts, hepatic congestion, goiter, increases in the infertility, abortion, and abnormal embryo development in female mammals, and decrease in the serum testosterone levels in male mammals. These symptoms caused by mimosine may be due to the inactivation of several essential enzymes and the blockade of DNA replication [94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113]. Mimosine increases the mortality of insects and parasitic nematodes, and suppresses the growth of pathogenic fungi e.g., [123,124,132,150]. The activity of acetylcholinesterase and tyrosinase in insects was also suppressed by mimosine application [124]. In addition, mimosine acts as an allelochemical, inhibiting the growth of the competing plant species by blocking DNA replication and causing oxidative stress conditions [81,183] (Figure 6).
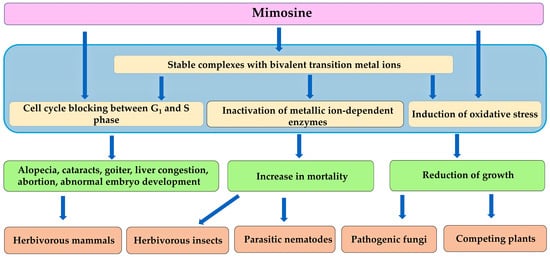
Figure 6.
Mechanism of action of mimosine.
Quercetin exhibits nematocidal activity, inhibiting egg hatching, and increasing nematode mortality [143]. Benzoic acid, flavonoids, such as quercetin, naringenin, and myricetin, and polyphenolic compounds, such as rosmarinic acid and resveratrol, are toxic to pathogenic fungi and bacteria [151]. Several benzoic and cinnamic acid derivatives, including p-hydroxybenzoic acid, gallic acid, vanillic acid, protocatechuic acid, p-hydroxyphenylacetic acid, p-hydroxycinnamic acid, caffeic acid, and ferulic acid, act as allelochemicals, and inhibit the growth of neighboring competing plant species [183]. Gallocatechin suppresses the nitrification process by inhibiting related bacterial activity, reducing nitrate availability in the soil, and causing the growth suppression of neighboring plant species [216]. These compounds may cause the inhibition by disrupting the structures and functions of plasma membranes and/or various metabolic processes (Figure 7).
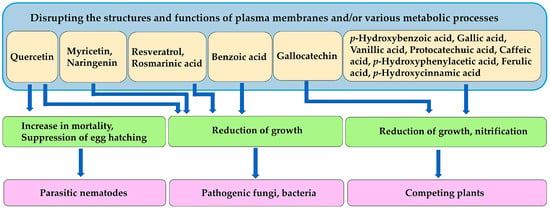
Figure 7.
Mechanism of action of other compounds involved in the defense functions against parasitic nematodes, pathogenic fungi and bacteria, and competing plant species.
These compounds described herein may play a role in the defense functions against biotic stressors, such as herbivores, pathogens, and competing plant species. Since these biotic stressors exert significant selective pressure on plant survival, these compounds may represent the invasive traits of L. leucocephala that have undergone natural selection during the evolution of the species. They may contribute to the increased survival, growth, and abundance of L. leucocephala as an invasive plant species in its introduced ranges.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Global Invasive Species Database, Species Profile: Leucaena leucocephala. Available online: https://www.iucngisd.org/gisd/species.php?sc=23 (accessed on 18 April 2025).
- Invasive Species Compendium, Leucaena leucocephala (Leucaena). Available online: https://www.cabidigitallibrary.org/doi/full/10.1079/cabicompendium.31634 (accessed on 18 April 2025).
- Walton, C. The biology of Australian weeds. 42. Leucaena leucocephala (Lamark) de Wit. Plant Prot. Quart. 2003, 18, 90–98. [Google Scholar]
- Sharma, P.; Kaur, A.; Batish, D.R.; Kaur, S.; Chauhan, B.S. Critical insights into the ecological and invasive attributes of Leucaena leucocephala, a tropical agroforestry species. Front. Agron. 2022, 4, 890992. [Google Scholar] [CrossRef]
- Campbell, S.; Vogler, W.; Brazier, D.; Vitelli, J.; Brooks, S. Weed leucaena and its significance, implications and control. Trop. Grassl. 2019, 7, 280–289. [Google Scholar] [CrossRef]
- Rengsirikul, K.; Kanjanakuha, A.; Ishii, Y.; Kangvansaichol, K.; Sripichitt, P.; Punsuvon, V.; Vaithanomsat, P.; Nakamenee, G.; Tudsri, S. Potential forage and biomass production of newly introduced varieties of leucaena (Leucaena leucocephala (Lam.) de Wit.) in Thailand. Grassl. Sci. 2011, 57, 94–100. [Google Scholar] [CrossRef]
- Wanapat, M.; Kang, S.; Polyorach, S. Development of feeding systems and strategies of supplementation to enhance rumen fermentation and ruminant production in the tropics. J. Anim. Sci. Biotechnol. 2013, 4, 32. [Google Scholar] [CrossRef]
- de Angelis, A.; Gasco, L.; Parisi, G.; Danieli, P.P. A multipurpose leguminous plant for the mediterranean countries: Leucaena leucocephala as an alternative protein source: A review. Animals 2021, 11, 2230. [Google Scholar] [CrossRef]
- Durango, S.G.; Barahona, R.; Bolívar, D.; Chirinda, N.; Arango, J. Feeding strategies to increase nitrogen retention and improve rumen fermentation and rumen microbial population in beef steers fed with tropical forages. Sustainability 2021, 13, 10312. [Google Scholar] [CrossRef]
- Verdecia, D.M.; Herrera, R.S.; Ramírez, J.L.; Leonard, I.; Bodas, R.; Andrés, S.; Giráldez, F.J.; Valdés, C.; Arceo, Y.; Paumier, M.; et al. Effect of age of regrowth, chemical composition and secondary metabolites on the digestibility of Leucaena leucocephala in the Cauto Valley, Cuba. Agrofor. Syst. 2020, 94, 1247–1253. [Google Scholar] [CrossRef]
- Mac Dicken, K.G. Nitrogen Fixing Trees for Wastelands; FAO Regional Office for Asia and the Pacific—Food and Agriculture Organization of the United Nations: Bangkok, Thailand, 1988; pp. 1–104. [Google Scholar]
- Hansted, A.L.S.; Nakashima, G.T.; Martins, M.P.; Yamamoto, H.; Yamaji, F.M. Comparative analyses of fast growing species in different moisture content for high quality solid fuel production. Fuel 2016, 184, 180–184. [Google Scholar] [CrossRef]
- Nguyen, M.P.; Vaast, P.; Pagella, T.; Sinclair, F. Local knowledge about ecosystem services provided by trees in coffee agroforestry practices in northwest Vietnam. Land 2020, 9, 486. [Google Scholar] [CrossRef]
- Prasad, J.V.N.S.; Korwar, G.R.; Rao, K.V.; Mandal, U.K.; Rao, G.R.; Srinivas, I.; Venkateswarlu, B.; Rao, S.N.; Kulkarni, H.D. Optimum stand density of Leucaena leucocephala for wood production in Andhra Pradesh, Southern India. Biomass Bioenergy 2011, 35, 227–235. [Google Scholar] [CrossRef]
- Khanna, N.K.; Shukla, O.P.; Gogate, M.G.; Narkhede, S.L. Leucaena for paper industry in Gujarat, India: Case study. Trop. Grassl.-Forrajes Trop. 2019, 7, 200–209. [Google Scholar] [CrossRef]
- Anupam, K.; Swaroop, V.; Deepika; Lal, P.S.; Bist, V. Turning Leucaena leucocephala bark to biochar for soil application via statistical modelling and optimization technique. Ecol. Eng. 2015, 82, 26–39. [Google Scholar] [CrossRef]
- Jha, P.; Neenu, S.; Rashmi, I.; Meena, B.P.; Jatav, R.C.; Lakaria, B.L.; Biswas, A.L.; Singh, M.; Patra, A.K. Ameliorating effects of Leucaena biochar on soil acidity and exchangeable Ions. Commun. Soil Sci. Plant Anal. 2016, 47, 1252–1262. [Google Scholar] [CrossRef]
- Yu, G.; Huang, H.Q.; Wang, Z.; Brierley, G.; Zhang, K. Rehabilitation of a debris-flow prone mountain stream in southwestern China—Strategies, effects and implications. J. Hydrol. 2012, 414-415, 231–243. [Google Scholar] [CrossRef]
- Adhikary, P.P.; Hombegowda, H.C.; Barman, D.; Jakhar, P.; Madhu, M. Soil erosion control and carbon sequestration in shifting cultivated degraded highlands of eastern India: Performance of two contour hedgerow systems. Agrof. Syst. 2017, 91, 757–771. [Google Scholar] [CrossRef]
- Ishihara, K.L.; Honda, M.D.H.; Bageel, A.; Borthakur, D. Leucaena leucocephala: A leguminous tree suitable for eroded habitats of Hawaiian Islands. In Ravine Lands: Greening for Livelihood and Environmental Security; Dagar, J., Singh, A., Eds.; Springer: Singapore, 2018; pp. 413–431. [Google Scholar]
- Badalamenti, E.; Pasta, S.; Sala, G.; Catania, V.; Quatrini, P.; La Mantia, T. The paradox of the alien plant Leucaena leucocephala subsp. glabrata (Rose) S. Zárate in Sicily: Another threat for the native flora or a valuable resource? Int. J. Plant Biol. 2020, 11, 8637. [Google Scholar]
- Iqbal, I.M.; Balzter, H.; Firdaus-e-Bareen; Shabbir, A. Mapping Lantana camara and Leucaena leucocephala in protected areas of Pakistan: A geo-spatial approach. Remote Sens. 2023, 15, 1020. [Google Scholar] [CrossRef]
- Chen, J.C.; Chen, C.T.; Jump, A.S. Forest disturbance leads to the rapid spread of the invasive Leucaena leucocephala in Taiwan. J. Rakuno Gakuen Univ. 2014, 38, 101–109. [Google Scholar] [CrossRef]
- Kew Royal Botanic Gardens, Leucaena leucocephala (Lam.) de Wit. Available online: https://powo.science.kew.org/taxon/urn:lsid:ipni.org:names:138955-2#distributions (accessed on 18 April 2025).
- Mello, T.J.; Oliveira, A.A. Making a bad situation worse: An invasive species altering the balance of interactions between local species. PLoS ONE 2016, 11, e0152070. [Google Scholar] [CrossRef]
- Hata, K.; Suzuki, J.I.; Kachi, N. Effects of an alien shrub species, Leucaena leucocephala, on establishment of native mid-successional tree species after disturbance in the national park in the Chichijima island, a subtropical oceanic island. Tropics 2007, 16, 283–290. [Google Scholar] [CrossRef]
- Jurado, E.; Flores, J.; Navar, J.; Jiménez, J. Seedling establishment under native tamaulipan thornscrub and Leucaena leucocephala plantation. For. Ecol. Manag. 1998, 105, 151–157. [Google Scholar] [CrossRef]
- Yoshida, K.; Oka, S. Invasion of Leucaena leucocephala and its effects on the native plant community in the Ogasawara (Bonin) Islands. Weed Technol. 2004, 18, 1371–1375. [Google Scholar] [CrossRef]
- Colón, S.M. Recovery of a subtropical dry forest after abandonment of different land. Biotropica 2006, 38, 354–364. [Google Scholar] [CrossRef]
- Francis, J.K.; Parrotta, J.A. Vegetation response to grazing and planting of Leucaena leucocephala in a Urochloa maximum-dominated grassland in Puerto Rico. Caribb. J. Sci. 2006, 42, 67–74. [Google Scholar]
- Hwang, C.Y.; Hsu, L.M.; Liou, Y.J.; Wang, C.Y. Distribution, growth, and seed germination ability of lead tree (Leucaena leucocephala) plants in Penghu Islands, Taiwan. Weed Technol. 2010, 24, 574–582. [Google Scholar] [CrossRef]
- Marler, T.N.; Nirmala Dongol, N.; Cruz, G.M. Leucaena leucocephala and adjacent native limestone forest habitats contrast in soil properties on Tinian Island. Commu. Integr. Biol. 2016, 9, e1212792. [Google Scholar]
- Njunge, J.T.; Kaholongo, I.K.; Amutenya, M.; Hove, K. Invasiveness and biomass production of Leucaena leucocephala under harsh ecological conditions of north-central Namibia. J. Trop. For. Sci. 2017, 29, 297–304. [Google Scholar]
- Yoshida, K.; Oka, S. Impact of biological invasion of Leucaena leucocephala on successional pathway and species diversity of secondary forest on Hahajima Island, Ogasawara (Bonin) Islands, northwestern Pacific. Jpn. J. Ecol. 2000, 50, 111–119. [Google Scholar]
- Idol, T. A short review of Leucaena as an invasive species in Hawaii. Trop. Grassl.-Forrajes Trop. 2019, 7, 290–294. [Google Scholar] [CrossRef]
- Brewbaker, J.L.; Hegde, N.; Hutton, E.M.; Jones, R.J.; Lowry, J.B.; Moog, F.; van den Beldt, R. Leucaena—Forage Production and Use; Nitrogen Fixing Tree Association: Waimanalo, HI, USA, 1985; pp. 1–39. [Google Scholar]
- Sandhu, J.; Sinha, M.; Ambasht, R.S. Nitrogen release from decomposing litter of Leucaena leucocephala in the dry tropics. Soil Biol. Biochem. 1990, 22, 859–863. [Google Scholar] [CrossRef]
- Valiente, C.A. The invasion ecology of Leucaena leucocephala on Moorea, French Polynesia. Biol. Geomorphol. Trop. Islands 2010, 19, 65–72. [Google Scholar]
- te Beest, M.; Le Roux, J.J.; Richardson, D.M.; Brysting, A.K.; Suda, J.; Kubešová, M.; Pyšek, P. The more the better? The role of polyploidy in facilitating plant invasions. Ann. Bot. 2012, 109, 19–45. [Google Scholar] [CrossRef] [PubMed]
- IUCN. 100 of the World’s Worst Invasive Alien Species. Available online: https://portals.iucn.org/library/sites/library/files/documents/2000-126.pdf (accessed on 18 April 2025).
- Marifatul, H.S.; Mohammed, D.; Muhammad, W.; Manoj, K.; Manzer, S.H.; Rainer, B.W. Predicting potential invasion risks of Leucaena leucocephala (Lam.) de Wit in the arid area of Saudi Arabia. J. Arid Land 2024, 16, 983–999. [Google Scholar] [CrossRef]
- Mack, R.M. Predicting the identity and fate of plant invaders: Emergent and emerging approaches. Biol. Conserv. 1996, 78, 107–121. [Google Scholar] [CrossRef]
- Theoharides, K.A.; Dukes, J.S. Plant invasion across space and time: Factors affecting nonindigenous species success during four stages of invasion. New Phytol. 2007, 176, 256–273. [Google Scholar] [CrossRef]
- Warren, R.J.; Matt Candeias, M.; Labatore, A.; Olejniczak, M.; Yang, L. Multiple mechanisms in woodland plant species invasion. J. Plant Ecol. 2019, 12, 201–209. [Google Scholar] [CrossRef]
- Kato-Noguchi, H. The impact and invasive mechanisms of Pueraria montana var. lobata, one of the world’s worst alien species. Plants 2023, 12, 3066. [Google Scholar]
- Kato-Noguchi, H. Invasive mechanisms of one of the world’s worst alien plant species Mimosa pigra and its management. Plants 2023, 12, 1960. [Google Scholar] [CrossRef]
- Clements, D.R.; Kato-Noguchi, H. Defensive mechanisms of Mikania micrantha likely enhance its invasiveness as one of the world’s worst alien species. Plants 2025, 14, 269. [Google Scholar] [CrossRef]
- Sohtome, Y.; Tokunaga, T.; Ueda, K.; Yamamura, S.; Ueda, M. Leaf-closing substance in Leucaena leucocephala. Biosci. Biotechnol. Biochem. 2002, 66, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Rao, P.B.; Kaur, A.; Tewari, A. Drought resistance in seedlings of five important tree species in Tarai region of Uttarakhand. Trop. Ecol. 2008, 49, 43. [Google Scholar]
- Yige, C.; Fangqing, C.; Lei, L.; Shunbo, Z. Physiological responses of Leucaena leucocephala seedlings to drought stress. Procedia Eng. 2012, 28, 110–116. [Google Scholar] [CrossRef]
- Bageel, A.; Honda, M.D.; Carrillo, J.T.; Borthakur, D. Giant leucaena (Leucaena leucocephala subsp. glabrata): A versatile tree-legume for sustainable agroforestry. Agrofor. System. 2020, 94, 251–268. [Google Scholar]
- Abair, A.; Hughes, C.E.; Bailey, C.D. The evolutionary history of Leucaena: Recent research, new genomic resources and future directions. Trop. Grassl.-Forrajes Trop. 2019, 7, 65–73. [Google Scholar] [CrossRef]
- Walton, C. Leucaena (Leucaena leucocephala) in Queensland; Department of Natural Resources and Mines: Brisbane, QLD, Australia, 2003; pp. 1–51. [Google Scholar]
- Olckers, T. Biological control of Leucaena leucocephala (Lam.) de Wit (Fabaceae) in South Africa: A tale of opportunism, seed feeders and unanswered questions. Afr. Entomol. 2011, 19, 356–365. [Google Scholar] [CrossRef]
- Marques, A.R.; Costa, C.F.; Atman, A.P.F.; Garcia, Q.S. Germination characteristics and seedbank of the alien species Leucaena leucocephala (Fabaceae) in Brazilian forest: Ecological implications. Weed Res. 2014, 54, 576–583. [Google Scholar] [CrossRef]
- Fonseca, N.G.D.; Jacobi, C.M. Germination performance of the invader Leucaena leucocephala (Lam.) de Wit. compared to Caesalpinia ferrea Mart. ex Tul. and C. pulcherrima (L.) Sw. (Fabaceae). Acta Bot. Bras. 2011, 25, 191–197. [Google Scholar] [CrossRef]
- Keane, R.M.; Crawley, M.L. Exotic plant invasions and the enemy release hypothesis. Trend. Ecol. Evol. 2002, 17, 164–170. [Google Scholar] [CrossRef]
- Callaway, R.M.; Aschehoug, E.T. Invasive plants versus their new and old neighbors: A mechanism for exotic invasion. Science 2000, 290, 521–523. [Google Scholar] [CrossRef]
- Kato-Noguchi, H.; Kato, M. Evolution of the secondary metabolites in invasive plant species Chromolaena odorata for the defense and allelopathic functions. Plants 2023, 12, 521. [Google Scholar] [CrossRef] [PubMed]
- Kato-Noguchi, H.; Kato, M. Defense molecules of the invasive plant species Ageratum conyzoides. Molecules 2024, 29, 4673. [Google Scholar] [CrossRef] [PubMed]
- Kato-Noguchi, H.; Kato, M. Compounds involved in the invasive characteristics of Lantana camara. Molecules 2025, 30, 411. [Google Scholar] [CrossRef] [PubMed]
- Kato-Noguchi, H.; Kato, M. Defensive compounds involved in the invasiveness of Tithonia diversifolia. Molecules 2025, 30, 1946. [Google Scholar] [CrossRef]
- Hammond, A.C. Leucaena toxicosis and its control in ruminants. J. Anim. Sci. 1995, 73, 1487–1492. [Google Scholar] [CrossRef]
- Dalzell, S.A.; Burnett, D.J.; Dowsett, J.E.; Forbes, V.E.; Shelton, H.M. Prevalence of mimosine and DHP toxicity in cattle grazing Leucaena leucocephala pastures in Queensland, Australia. Anim. Prod. Sci. 2012, 52, 365–372. [Google Scholar] [CrossRef]
- Radrizzani, A.; Nasca, J.A. The effect of Leucaena leucocephala on beef production and its toxicity in the Chaco Region of Argentina. Trop. Grassl.-Forrajes Trop. 2014, 2, 127–129. [Google Scholar] [CrossRef]
- Phaikaew, C.; Suksaran, W.; Ted-Arsen, J.; Nakamanee, G.; Saichuer, A.; Seejundee, S.; Kotprom, N.; Shelton, H.M. Incidence of subclinical toxicity in goats and dairy cows consuming leucaena (Leucaena leucocephala) in Thailand. Anim. Prod. Sci. 2012, 52, 283–286. [Google Scholar] [CrossRef]
- Peixoto, P.V.; França, T.N.; Cunha, B.M.; Tavares, D.V.A.M.; Brito, M.F. Spontaneous poisoning by Leucaena leucocephalai in a goat from Rio de Janeiro State, Brazil. Ciência Rural. 2008, 38, 551–555. [Google Scholar] [CrossRef]
- Halliday, M.J.; Padmanabha, J.; McSweeney, C.S.; Kerven, G.; Shelton, H.M. Leucaena toxicity: A new perspective on the most widely used forage tree legume. Trop. Grassl.-Forrajes Trop. 2013, 1, 1–11. [Google Scholar] [CrossRef]
- Machado, M.; Queiroz-Machado, C.R.; Gardner, D.R.; Castro, M.B.; Câmara, A.C.L.; Pimentel, L.A.; Galixa, G.J.N.; Riet-Correa, F. Leucaena leucocephala toxicity in Brazilian horses. Toxicon 2024, 240, 107655. [Google Scholar] [CrossRef] [PubMed]
- Anyanwu, G.A.; Okoro, V.M.O.; Mbajiorgu, C.A. Optimum inclusion levels of Leucaena leucocephala pasture leaf-meal on growth, haematology and physiological performance of growing pigs. Trop. Anim. Health Prod. 2021, 53, 116. [Google Scholar] [CrossRef]
- Fayemi, P.O.; Onwuka, C.F.I.; Isah, O.A.; Jegede, A.V.; Arigbede, O.M.; Muchenje, V. Effects of mimosine and tannin toxicity on rabbits fed processed Leucaena leucocephala (Lam) de Wit. leaves. Afr. J. Agric. Res. 2011, 6, 4081–4085. [Google Scholar]
- Mtenga, L.A.; Laswai, G.D. Leucaena leucocephala as feed for rabbits and pigs: Detailed chemical composition and effect of level of inclusion on performance. For. Ecol. Manag. 1994, 64, 249–257. [Google Scholar] [CrossRef]
- Burawat, J.; Uabandit, N.; Arun, S.; Nualkaew, S.; Iamsaard, S. Effects of Leucaena leucocephala (Lamk.) shoot tips plus young leaf extract containing mimosine on reproductive system of male rats. Int. J. Morphol. 2018, 36, 1062–1069. [Google Scholar] [CrossRef]
- de Almeida, E.R.; Martinelli, E.C.; Pereira, E.C.; Raspantini, L.E.; Hueza, I.M. Alternative method for oral administration of insoluble toxins to rats. A prenatal study of L-mimosine. Toxicon 2021, 202, 82–89. [Google Scholar] [CrossRef]
- de Almeida, E.R.; Górniak, S.L.; Momo, C.; Ferreira, V.L.; Pereira, E.C.; Hueza, I.M. Prenatal toxicity of L-mimosine in Wistar rats. Toxicon 2025, 254, 108223. [Google Scholar] [CrossRef]
- Kanla, P.; Burawat, J.; Arun, S.; Sawatpanich, T.; Chaichun, A.; Iamsaard, S. Acute effects of mimosine purified from Leucaena leucocephala on male reproductive system of adult mice. Int. J. Morphol. 2018, 36, 507–512. [Google Scholar] [CrossRef]
- Crawford, G.; Puschner, B.; Affolter, V.; Stalis, I.; Davidson, A.; Baker, T.; Tahara, J.; Jolly, A.; Ostapak, S. Systemic effects of Leucaena leucocephala ingestion on ringtailed lemurs (Lemur catta) at Berenty Reserve, Madagascar. Am. J. Primatol. 2015, 77, 633–641. [Google Scholar] [CrossRef]
- Brewbaker, J.L.; Hylin, J.W. Variations in mimosine content among Leucaena species and related mimosaceae. Crop Sci. 1965, 5, 348–349. [Google Scholar] [CrossRef]
- Smith, I.K.; Fowden, L.A. Study of mimosine toxicity in plants. J. Exp. Bot. 1996, 17, 750–761. [Google Scholar] [CrossRef]
- Soedarjo, M.; Borthakur, D. Mimosine produced by the tree-legume Leucaena provides growth advantages to some Rhizobium strains that utilize it as a source of carbon and nitrogen. Plant Soil 1996, 186, 87–92. [Google Scholar] [CrossRef]
- Xuan, T.D.; Elzaawely, A.A.; Deba, F.; Tawata, S. Mimosine in Leucaena as a potent bio-herbicide. Agron. Sustain. Dev. 2006, 26, 89–97. [Google Scholar] [CrossRef]
- Honda, M.D.H.; Borthakur, D. Mimosine concentration in Leucaena leucocephala under various environmental conditions. Trop. Grassl.-Forrajes Trop. 2019, 7, 164–172. [Google Scholar] [CrossRef]
- Honda, M.; Borthakur, D. Mimosine concentration in giant leucaena (Leucaena leucocephala subsp. glabrata) fluctuates with age and plant part: Mimosine concentration in giant leucaena. Trop. Grassl.-Forrajes Trop. 2024, 12, 11–23. [Google Scholar] [CrossRef]
- Ikegami, F.; Mizuno, M.; Kihara, M.; Murakoshi, I. Enzymatic synthesis of the thyrotoxic amino acid mimosine by cysteine synthase. Phytochemistry 1990, 29, 3461–3465. [Google Scholar] [CrossRef]
- Harun-Ur-Rashid, M.; Iwasaki, H.; Parveen, S.; Oogai, S.; Fukuta, M.; Amzad Hossain, M.A.; Anai, T.; Oku, H. Cytosolic cysteine synthase switch cysteine and mimosine production in Leucaena leucocephala. Appl. Biochem. Biotechnol. 2018, 186, 613–632. [Google Scholar] [CrossRef]
- Murakoshi, I.; Ikegami, F.; Hinuma, Y.; Hanma, Y. Purification and characterization of L-mimosine synthase from Leucaena leucocephala. Phytochemistry 1984, 23, 1905–1908. [Google Scholar] [CrossRef]
- MetaCyc. Pathway: Mimosine Biosynthesis. Available online: https://biocyc.org/gene?orgid=META&id=MONOMER-11365 (accessed on 18 April 2025).
- Gotardo, A.T.; Dipe, V.V.; de Almeida, E.R.M.; Hueza, I.M.; Pfister, J.A.; Górniak, S.L. Potential toxic effects produced by L-mimosine in the thyroid and reproductive systems. Evaluation in male rats. Toxicon 2021, 203, 121–128. [Google Scholar] [CrossRef]
- Hueza, I.M.; Dipe, V.V.; Gotardo, A.T.; Gardner, D.R.; de Almeida, E.R.M.; Górniak, S.L. Potential immunomodulatory response associated with L-mimosine in male Wistar rats. Toxicon 2023, 226, 107084. [Google Scholar] [CrossRef]
- Holmes, J.H.G.; Humphrey, J.D.; Walton, E.A.; O’Shea, J.D. Cataracts, goitre and infertility in cattle grazed on an exclusive diet of Leucaena leucocephala. Aust. Vet. J. 1981, 57, 257–261. [Google Scholar] [CrossRef] [PubMed]
- Zayed, M.Z.; Sallam, S.M.A.; Shetta, N.D. Review article on Leucaena leucocephala as one of the miracle timber trees. Int. J. Pharm. Pharmaceut. Sci. 2018, 10, 1–7. [Google Scholar] [CrossRef]
- Shelton, H.; Kerven, G.L.; Dalzell, S.A. An update on leucaena toxicity: Is inoculation with Synergistes jonesii necessary? Trop. Grassl.-Forrajes Trop. 2019, 7, 146–153. [Google Scholar] [CrossRef]
- Yanuartono, Y.; Indarjulianto, S.; Nururrozi, A.; Raharjo, S.; Purnamaningsih, H. Brief review: The negative impact of mimosin in L. leucocephala in ruminant animals and processing methods to reduce poisoning effects on ruminant livestock. J. Livest. Sci. Prod. 2019, 3, 199. [Google Scholar] [CrossRef]
- Negi, V.S.; Bingham, J.P.; Li, Q.X.; Borthakur, D. A carbon-nitrogen lyase from Leucaena leucocephala catalyzes the first step of mimosine degradation. Plant Physiol. 2014, 164, 922–934. [Google Scholar] [CrossRef]
- Jones, R.J.; Hegarty, M.P. The effect of different proportions of Leucaena leucocephala in the diet of cattle on growth, feed intake, thyroid function and urinary excretion of 3-hydroxy-4(1H)-pyridone. Aust. J. Agric. Res. 1984, 35, 317–325. [Google Scholar] [CrossRef]
- Gupta, H.K.; Atreja, P.P. Influence of feeding increasing levels of leucaena leaf meal on the performance of milch goats and metabolism of mimosine and 3-hydroxy-4 (1H) pyridone. Anim. Feed Sci. Technol. 1999, 78, 159–167. [Google Scholar] [CrossRef]
- Loh, Z.H.; Ouwerkerk, D.; Klieve, A.V.; Hungerford, N.L.; Fletcher, M.T. Toxin degradation by rumen microorganisms: A review. Toxins 2020, 12, 664. [Google Scholar] [CrossRef]
- Paul, S.S. Detoxification of mimosine and dihydroxypyridone: A review. Agric. Rev. 2000, 21, 104–109. [Google Scholar]
- Derakhshani, H.; Corley, S.W.; Jassim, R.A. Isolation and characterization of mimosine, 3, 4 DHP and 2, 3 DHP degrading bacteria from a commercial rumen inoculum. J. Basic Microbiol. 2016, 56, 580–585. [Google Scholar] [CrossRef]
- Allison, M.J.; Hammond, A.C.; Jones, R.J. Detection of ruminal bacteria that degrade toxic dihydroxypyridine compounds produced from mimosine. Appl. Environ. Microbiol. 1990, 56, 590–594. [Google Scholar] [CrossRef] [PubMed]
- Wiratmini, N.I.; Narayani, I.; Setiasih, N.L. The effect of dietary inclusion of detoxified Leucaena leucocephala leaf meal on thyroidal activity of rats during gestation-lactation period. Int. J. Biosc. Biotech. 2018, 2, 103–110. [Google Scholar] [CrossRef]
- Malafaia, P.; Armién, A.G.; Peixoto, P.V. Experimental poisoning of rabbits by Leucaena leucocephala. Pesqui. Vet. Bras. 1994, 4, 105–109. [Google Scholar]
- Porto, M.R.; Moscardini, A.R.; Novais, E.P.; Filho, S.L.C.; Lima, E.M.; Castro, M.B. Natural and experimental Leucaena leucocephala poisoning in horses. Pesqui. Vet. Bras. 2017, 37, 829–834. [Google Scholar] [CrossRef]
- Nguyen, B.C.; Tawata, S. The chemistry and biological activities of mimosine: A review. Phytother. Res. 2016, 30, 1230–1242. [Google Scholar] [CrossRef]
- Hashiguchi, H.; Takahashi, H. Inhibition of two copper-containing enzymes, tyrosinase and dopamine β-hydroxylase, by L-mimosine. Mol. Pharmacol. 1977, 13, 362–367. [Google Scholar] [CrossRef]
- Dai, Y.; Gold, B.; Vishwanatha, J.K.; Rhode, S.L. Mimosine inhibits viral DNA synthesis through ribonucleotide reductase. Virology 1994, 205, 210–216. [Google Scholar] [CrossRef]
- Crounse, R.G.; Maxwell, J.D.; Blank, H. Inhibition of growth of hair by mimosine. Nature 1962, 194, 694–695. [Google Scholar] [CrossRef]
- Lin, J.Y.; Shih, Y.M.; Ling, K.H. Studies on the mechanism of toxicity of mimosine (β-(N-[3-hydroxypyridone])-α-aminopropionic acid. Studies of the reactions of mimosine and pyridoxal 5-phosphate using the spectrophotometric method. J. Formos. Med. Assoc. 1962, 61, 997–1003. [Google Scholar]
- Lin, J.Y.; Lin, K.T.; Ling, K.H. Studies on the mechanism of toxicity of mimosine (β-(N-[3-hydroxypyridone])-α-aminopropionic acid. The effect of mimosine on the activity of L-dopa decarboxylase, in vitro. J. Formos. Med. Assoc. 1963, 62, 587–1003. [Google Scholar]
- Hylin, J.W. Toxic peptides and amino acids in foods and feeds. J. Agric. Food Chem. 1969, 17, 492–496. [Google Scholar] [CrossRef]
- Krude, T. Mimosine arrests proliferating human cells before onset of DNA replication in a dose-dependent manner. Expe. Cell Res. 1999, 247, 148–159. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Im, J.S.; Park, S.R.; Kim, S.E.; Wang, H.J.; Lee, J.K. Mimosine arrests the cell cycle prior to the onset of DNA replication by preventing the binding of human Ctf4/And-1 to chromatin via Hif-1α activation in HeLa cells. Cell Cycle 2012, 11, 761–766. [Google Scholar] [CrossRef] [PubMed]
- Kubota, S.; Fukumoto, Y.; Ishibashi, K.; Soeda, S.; Kubota, S.; Yuki, R.; Nakayama, Y.; Aoyama, K.; Yamaguchi, N.; Yamaguchi, N. Activation of the prereplication complex is blocked by mimosine through reactive oxygen species-activated ataxia telangiectasia mutated (ATM) protein without DNA damage. J. Biol. Chem. 2014, 289, 5730–5746. [Google Scholar] [CrossRef]
- Nordlund, P.; Reichard, P. Ribonucleotide reductases. Annu. Rev. Biochem. 2006, 75, 681–706. [Google Scholar] [CrossRef]
- Greene, B.L.; Kang, G.; Cui, C.; Bennati, M.; Nocera, D.G.; Drennan, C.L.; Stubbe, J. Ribonucleotide reductases: Structure, chemistry, and metabolism suggest new therapeutic targets. Annu. Rev. Biochem. 2020, 89, 45–75. [Google Scholar] [CrossRef]
- Blumenthal, D.M. Interactions between resource availability and enemy release in plant invasion. Ecol. Lett. 2006, 9, 887–895. [Google Scholar] [CrossRef]
- Blossey, B.; Notzold, R. Evolution of increased competitive ability in invasive nonindigenous plants—A hypothesis. J. Ecol. 1995, 83, 887–889. [Google Scholar] [CrossRef]
- Coley, P.D.; Barone, J.A. Herbivory and plant defenses in tropical forests. Annu. Rev. Ecol. Systemat. 1996, 27, 305–335. [Google Scholar] [CrossRef]
- Mithöfer, A.; Boland, W. Plant defense against herbivores: Chemical aspects. Annu. Rev. Plant Biol. 2012, 63, 431–450. [Google Scholar] [CrossRef]
- Cavalcante, G.M.; Moreira, A.F.C.; Vasconcelos, S.D. Insecticidal potential of aqueous extracts from arboreous species against whitefly. Pesqui. Agropecu. Bras. 2006, 41, 9–14. [Google Scholar] [CrossRef]
- Auamcharoen, W.; Chandrapatya, A. Acaricidal and ovicidal efficacies of Leucaena glauca Benth. seed crude extracts on Tetranychus urticae Koch (Acari: Tetranychidae). J. Biopestic. 2015, 8, 68. [Google Scholar] [CrossRef]
- Elliott, R.; Milton, J.T.B.; Gordon, G.H.; Blamey, F.P.C. Effect of L-mimosine in artificial diet on the growth and development of Heliothis punctiger larvae. J. Agric. Sci. 1984, 103, 477–479. [Google Scholar] [CrossRef]
- Ishaaya, I.; Hirashima, A.; Yablonski, S.; Tawata, S.; Eto, M. Mimosine, a nonprotein amino acid, inhibits growth and enzyme systems in Tribolium castaneum. Pestic. Biochem. Physiol. 1991, 39, 35–42. [Google Scholar] [CrossRef]
- Nguyen, B.C.Q.; Chompoo, J.; Tawata, S. Insecticidal and nematicidal activities of novel mimosine derivatives. Molecules 2015, 20, 16741–16756. [Google Scholar] [CrossRef]
- Yu, Q.Y.; Lu, C.; Li, W.L.; Xiang, Z.H.; Zhang, Z. Annotation and expression of carboxylesterases in the silkworm. BMC Genom. 2009, 10, 553. [Google Scholar] [CrossRef]
- Colovic, M.B.; Krstic, D.Z.; Lazarevic-Pasti, T.D.; Bondzic, A.M.; Vasic, V.M. Acetylcholinesterase inhibitors: Pharmacology and toxicology. Curr. Neuropharmacol. 2013, 11, 315–335. [Google Scholar] [CrossRef]
- Labbé, P.; Alout, H.; Djogbénou, L.; Pasteur, N.; Weill, M.G. Evolution of resistance to insecticide in disease vectors. In Genetics and Evolution of Infectious Diseases; Tibayrenc, M., Ed.; Elsevier: London, UK, 2011; pp. 363–409. [Google Scholar]
- Sidhu, G.K.; Singh, S.; Kumar, V.; Dhanjal, D.S.; Datta, S.; Singh, J. Toxicity, monitoring and biodegradation of organophosphate pesticides: A review. Crit. Rev. Environ. Sci. Technol. 2019, 49, 1135–1187. [Google Scholar] [CrossRef]
- Vavricka, C.J.; Han, Q.; Mehere, P.; Ding, H.; Christensen, B.M.; Li, J. Tyrosine metabolic enzymes from insects and mammals: A comparative perspective. Insect Sci. 2014, 21, 13–19. [Google Scholar] [CrossRef]
- Sterkel, M.; Oliveira, P.L. Developmental roles of tyrosine metabolism enzymes in the blood-sucking insect Rhodnius prolixus. Proc. Royal Soc. B Biol. Sci. 2017, 284, 20162607. [Google Scholar] [CrossRef]
- Arakane, Y.; Noh, M.Y.; Asano, T.; Kramer, K.J. Tyrosine metabolism for insect cuticle pigmentation and sclerotization. In Extracellular Composite Matrices in Arthropods; Cohen, E., Moussian, B., Eds.; Springer: Berlin/Heidelberg, Germany, 2016; pp. 165–220. [Google Scholar]
- Fallon, A.M. Effects of mimosine on Wolbachia in mosquito cells: Cell cycle suppression reduces bacterial abundance. Vitr. Cell. Dev. Biol. Animal 2015, 51, 958–963. [Google Scholar] [CrossRef] [PubMed]
- Lambert, K.; Bekal, S. Introduction to Plant-Parasitic Nematodes. Plant Health Instr. 2002, 2. Available online: https://www.apsnet.org/edcenter/disandpath/nematode/intro/Pages/IntroNematodes.aspx (accessed on 18 April 2025). [CrossRef]
- den Akker, S.E. Plant–nematode interactions. Curr. Opin. Plant Biol. 2021, 62, 102035. [Google Scholar]
- Seid, A.; Fininsa, C.; Mekete, T.; Decraemer, W.; Wesemael, W.M. Tomato (Solanum lycopersicum) and root-knot nematodes (Meloidogyne spp.)—A century-old battle. Nematology 2015, 17, 995–1009. [Google Scholar] [CrossRef]
- Sikandar, A.; Zhang, M.Y.; Wang, Y.Y.; Zhu, X.F.; Liu, X.Y.; Fan, H.Y.; Xuan, Y.H.; Chen, L.J.; Duan, Y.X. Meloidogyne incognita (root-knot nematode) a risk to agriculture. Appl. Ecol. Environ. Res. 2020, 18, 1. [Google Scholar] [CrossRef]
- Pires, D.; Vicente, C.S.L.; Menéndez, E.; Faria, J.M.S.; Rusinque, L.; Camacho, M.J.; Inácio, M.L. The fight against plant-parasitic nematodes: Current status of bacterial and fungal biocontrol agents. Pathogens 2022, 11, 1178. [Google Scholar] [CrossRef]
- Adekunle, O.K.; Akinlua, A. Nematicidal effects of Leucaena leucocephala and Gliricidia sepium extracts on Meloidogyne incognita infecting okra. J. Agric. Sci. 2007, 52, 53–63. [Google Scholar] [CrossRef]
- Ahmed, Z.M.; Dawar, S.; Tariq, M.; Zaki, M.J. Effect of local tree seeds in the control of root knot nematode Meloidogyne javanica (Treub) chitwood and growth promotion of chickpea (Cicer arietinum L.) and mung bean (Vigna radiata L.). Acta Agrobot. 2010, 63, 197–203. [Google Scholar] [CrossRef][Green Version]
- El-Nuby, A.S.M.; Alam, E.A. Phytochemical and nematicidal activity studies of some extracts of different plant parts of Leucaena leucocephala against Meloidogyne incognita. Int. J. Chem. Pharm. Sci. 2020, 11, 1–17. [Google Scholar]
- Amaral, D.R.; Oliveira, D.F.; Campos, V.P.; Pantaleão, J.A.; Carvalho, D.A.D.; Nunes, A.D.S. Effect of plant and fungous metabolites on Meloidogyne exigua. Cienc. Agrotec. 2009, 33, 1861–1865. [Google Scholar] [CrossRef]
- Widaad, A.; Zulkipli, I.N.; Petalcorin, M.I.R. Anthelmintic effect of Leucaena leucocephala extract and its active compound, mimosine, on vital behavioral activities in Caenorhabditis elegans. Molecules 2022, 27, 1875. [Google Scholar] [CrossRef] [PubMed]
- Adekunle, O.K.; Aderogba, M.A. Characterisation of an antinematicidal compound from Leucaena leucocephala. Australas. Plant Dis. Notes 2008, 3, 168–170. [Google Scholar]
- von Son-de Fernex, E.; Alonso-Díaz, M.Á.; Mendoza-de Gives, P.; Valles-de la Mora, B.; González-Cortazar, M.; Zamilpa, A.; Gallegos, E.C. Elucidation of Leucaena leucocephala anthelmintic-like phytochemicals and the ultrastructural damage generated to eggs of Cooperia spp. Vet. Parasitol. 2015, 214, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Elmahy, R.A.; Moustafa, A.Y.; Radwan, N.A. Toxocara canis: Prospective activity of Quercetin and venom of Cassiopea andromeda (Cnidaria: Cassiopeidae) against third-stage larvae in vitro. J. Exp. Zool. A Ecol. Integr. Physiol. 2024, 341, 991–1001. [Google Scholar] [CrossRef]
- Abramovitch, R.B.; Martin, G.B. Strategies used by bacterial pathogens to suppress plant Defenses. Curr. Opi. Plant Biol. 2004, 7, 356–364. [Google Scholar] [CrossRef]
- Rojas, C.M.; Senthil-Kumar, M.; Tzin, V.; Mysore, K.S. Regulation of primary plant metabolism during plant-pathogen interactions and its contribution to plant defense. Front. Plant Sci. 2014, 5, 17. [Google Scholar] [CrossRef]
- Pandey, P.; Irulappan, V.; Bagavathiannan, M.V.; Senthil-Kumar, M. Impact of combined abiotic and biotic stresses on plant growth and avenues for crop improvement by exploiting physio-morphological traits. Front. Plant Sci. 2017, 8, 537. [Google Scholar] [CrossRef]
- Abbas, A.M.; Novak, S.J.; Fictor, M.; Mostafa, Y.S.; Alamri, S.A.; Alrumman, S.A.; Taher, M.A.; Hashem, M.; Khalaphallah, R. Initial in vitro assessment of the antifungal activity of aqueous extracts from three invasive plant species. Agriculture 2022, 12, 1152. [Google Scholar] [CrossRef]
- Serrano, E.P.; Ilag, L.; Mendoza, E.M.T. Biochemical mechanisms of mimosine toxicity to Sclerotium rolfsii Sacco. Aust. J. Biol. Sci. 1983, 36, 445–454. [Google Scholar] [CrossRef]
- Elbanoby, N.E.; El-Settawy, A.A.; Mohamed, A.A.; Salem, M.Z. Phytochemicals derived from Leucaena leucocephala (Lam.) de Wit (Fabaceae) biomass and their antimicrobial and antioxidant activities: HPLC analysis of extracts. Biomass Convers. Biorefin. 2024, 14, 14593–14609. [Google Scholar] [CrossRef]
- Gorniak, I.; Bartoszewski, R.; Kroliczewski, J. Comprehensive review of antimicrobial activities of plant flavonoids. Phytochem. Rev. 2019, 18, 241–272. [Google Scholar] [CrossRef]
- Taheri, Y.; Suleria, H.A.R.; Martins, N.; Sytar, O.; Beyatli, A.; Yeskaliyeva, B.; Seitimova, G.; Salehi, B.; Semwal, P.; Painuli, S.; et al. Myricetin bioactive effects: Moving from preclinical evidence to potential clinical applications. BMC Complement. Med. Ther. 2020, 20, 241. [Google Scholar] [CrossRef] [PubMed]
- Gledhill, J.R.; Montgomery, M.G.; Leslie, A.G.; Walker, J.E. Mechanism of inhibition of bovine F1-ATPase by resveratrol and related polyphenols. Proc. Nat. Acad. Sci. USA 2007, 104, 13632–13637. [Google Scholar] [CrossRef] [PubMed]
- Hotra, A.; Suter, M.; Biuković, G.; Ragunathan, P.; Kundu, S.; Dick, T.; Grüber, G. Deletion of a unique loop in the mycobacterial F-ATP synthase γ subunit sheds light on its inhibitory role in ATP hydrolysis-driven H+ pumping. FEBS J. 2016, 283, 1947–1961. [Google Scholar] [CrossRef] [PubMed]
- Vestergaard, M.; Ingmer, H. Antibacterial and antifungal properties of resveratrol. Int. J. Antimicrob. Agents 2019, 53, 716–723. [Google Scholar] [CrossRef]
- Ivanov, M.; Kostić, M.; Stojković, D.; Soković, M. Rosmarinic acid-modes of antimicrobial and antibiofilm activities of a common plant polyphenol. S. Afr. J. Bot. 2022, 146, 521–527. [Google Scholar] [CrossRef]
- Choi, S.H.; Gu, M.B. Phenolic toxicity-detection and classification through the use of a recombinant bioluminescent Escherichia coli. Environ. Toxicol. Chem. 2001, 20, 248–255. [Google Scholar] [CrossRef]
- Cueva, C.; Moreno-Arribas, M.V.; Martín-Álvarez, P.J.; Bills, G.; Vicente, M.F.; Basilio, A.; Rivas, C.L.; Requena, T.; Rodríguez, J.M.; Bartolomé, B. Antimicrobial activity of phenolic acids against commensal, probiotic and pathogenic bacteria. Res. Microbiol. 2010, 161, 372–382. [Google Scholar] [CrossRef]
- Kim, H.W.; Seok, Y.S.; Rhee, M.S. Synergistic staphylocidal interaction of benzoic acid derivatives (benzoic acid, 4-hydroxybenzoic acid and β-resorcylic acid) and capric acid: Mechanism and verification study using artificial skin. J. Antimicrob. Chemother. 2020, 75, 571–575. [Google Scholar] [CrossRef]
- Kato-Noguchi, H. Allelopathy of knotweeds as invasive plants. Plants 2022, 11, 3. [Google Scholar] [CrossRef]
- Kato-Noguchi, H. Involvement of allelopathy in the invasive potential of Tithonia diversifolia. Plants 2020, 9, 766. [Google Scholar] [CrossRef] [PubMed]
- Kato-Noguchi, H.; Kurniadie, D. Allelopathy and allelochemicals of Leucaena leucocephala as an invasive plant species. Plants 2022, 11, 1672. [Google Scholar] [CrossRef] [PubMed]
- Callaway, R.M.; Ridenour, W.M. Novel weapons: Invasive success and the evolution of increased competitive ability. Front. Ecol. Environ. 2004, 2, 419–426. [Google Scholar] [CrossRef]
- Cappuccino, N.; Arnason, J.T. Novel chemistry of invasive exotic plants. Biol. Lett. 2006, 2, 189–193. [Google Scholar] [CrossRef]
- Muller-Scharer, H.; Schaffner, U.; Steinger, T. Evolution in invasive plants: Implications for biological control. Trends Ecol. Evol. 2004, 19, 417–422. [Google Scholar] [CrossRef]
- Wang, C.; Zhu, M.; Chen, X.; Qu, B. Review on allelopathy of exotic invasive plants. Procedia. Engin. 2011, 18, 240–246. [Google Scholar]
- Rice, E.L. Allelopathy, 2nd ed.; Academic Press: Orlando, FL, USA, 1984; pp. 1–422. [Google Scholar]
- Belz, R.G. Allelopathy in crop/weed interactions—An update. Pest Manag. Sci. 2007, 63, 308–326. [Google Scholar] [CrossRef]
- Kato-Noguchi, H. Bioactive compounds involved in the formation of the sparse understory vegetation in pine forests. Curr. Org. Chem. 2021, 25, 1731–1738. [Google Scholar] [CrossRef]
- Kato-Noguchi, H. Defensive molecules momilactones A and B: Function, biosynthesis, induction and occurrence. Toxins 2023, 15, 241. [Google Scholar] [CrossRef]
- Macías, F.A.; Molinillo, J.M.; Varela, R.M.; G167]alindo, J.C. Allelopathy—A natural alternative for weed control. Pest Manag. Sci. 2007, 63, 327–348. [Google Scholar] [CrossRef]
- Kato-Noguchi, H.; Kurniadie, D. Allelopathy of Lantana camara as an invasive plant. Plants 2021, 10, 1028. [Google Scholar] [CrossRef] [PubMed]
- Kato-Noguchi, H.; Saito, Y.; Suenaga, K. Involvement of allelopathy in the establishment of pure colony of Dicranopteris linearis. Plant Ecol. 2012, 213, 1937–1944. [Google Scholar] [CrossRef]
- Kato-Noguchi, H.; Nakamura, K.; Ohno, O.; Suenaga, K.; Okuda, N. Asparagus decline: Autotoxicity and autotoxic compounds in asparagus rhizomes. Plant Physiol. 2017, 213, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Kato-Noguchi, H.; Saito, Y.; Ohno, O.; Suenaga, K. A phytotoxic active substance in the decomposing litter of the fern Gleichenia japonica. J. Plant Physiol. 2015, 176, 55–60. [Google Scholar] [CrossRef]
- Kato-Noguchi, H.; Kimura, F.; Ohno, O.; Suenaga, K. Involvement of allelopathy in inhibition of understory growth in red pine forests. J. Plant Physiol. 2017, 218, 66–73. [Google Scholar] [CrossRef]
- Didham, R.K.; Tylianakis, J.M.; Gemmell, N.J.; Rand, T.A.; Ewers, R.M. Interactive effects of habitat modification and species invasion on native species decline. Trends Ecol. Evol. 2007, 22, 489–496. [Google Scholar] [CrossRef]
- Pires, N.M.; Prates, H.T.; Filho, I.A.P.; de Oliveira, R.S., Jr.; de Faria, T.C.L. Allelopathic activity of Leucaena on weed species. Sci. Agric. 2001, 58, 61–65. [Google Scholar] [CrossRef]
- Ishak, M.S.; Sahid, I. Allelopathic effects of the aqueous extract of the leaf and seed of Leucaena leucocephala on three selected weed species. AIP Conf. Proc. 2016, 1744, 020029. [Google Scholar]
- Alvim, S.; Boöhm, F.M.L.; Pastorin, L.H. Allelopathic potential of Leucaena leucocephala (Lam.) de Wit leaf extracts on native species. Braz. J. Biol. 2023, 83, e272274. [Google Scholar] [CrossRef]
- Ahmed, T.; Hoque, A.T.M.R.; Hossain, M.K. Allelopathic effects of Leucaena leucocephala leaf litter on some forest and agricultural crops grown in nursery. J. For. Res. 2008, 19, 298–302. [Google Scholar] [CrossRef]
- Chou, C.H.; Kuo, Y.L. Allelopathic research of subtropical vegetation in Taiwan. J. Chem. Ecol. 1986, 12, 1431–1448. [Google Scholar] [CrossRef] [PubMed]
- Ishak, M.S.; Ismail, B.S.; Yusoff, N. Allelopathic potential of Leucaena leucocephala (Lam.) de Wit on the germination and seedling growth of Ageratum conyzoides L., Tridax procumbens L. and Emilia sonchifolia (L.) DC. Allelopathy J. 2016, 37, 109–122. [Google Scholar]
- John, J.; Narwal, S.S. Allelopathic plants. 9. Leucaena leucocephala (Lam.) de Wit. Allelopathy J. 2003, 12, 13–36. [Google Scholar]
- Hussain, M.I.; Gonzalez-Rodriguez, L.; Roger, M.R. Germination and growth response of four plant species to different allelochemicals and herbicides. Allelopathy J. 2008, 22, 101–110. [Google Scholar]
- Williams, R.D.; Hoagland, R.E. Phytotoxicity of mimosine and albizziine on seed germination and seedling growth of crops and weeds. Allelopathy J. 2007, 19, 423–430. [Google Scholar]
- Sahid, I.; Ishak, S.M.; Bajrai, F.S.; Jansar, K.M.; Yusoff, N. Quantification and herbicidal activity of mimosine from Leucaena leucocephala (Lam.) de Wit. Trans Sci. Technol. 2017, 4, 62–67. [Google Scholar]
- Honda, M.D.H.; Ishihara, L.L.; Pham, D.T.; Borthakur, D. Highly expressed genes in the foliage of giant Leucaena (Leucaena leucocephala subsp. glabrata), a nutritious fodder legume in the tropics. Plant Biosyst. 2020, 154, 107–116. [Google Scholar] [CrossRef]
- Negi, V.S.; Borthakur, D. Heterologous expression and characterization of mimosinase from Leucaena leucocephala. In Biotechnology of Plant Secondary Metabolism: Methods and Protocols, Methods in Molecular Biology; Fett-Neto, A., Ed.; Humana Press: New York, NY, USA, 2016; pp. 59–77. [Google Scholar]
- Wee, K.L.; Wang, S. Effect of post-harvest treatment on the degradation of mimosine in Leucaena leucocephala leaves. J. Sci. Food Agric. 1987, 39, 195–201. [Google Scholar] [CrossRef]
- Lowry, J.B.; Tangendjaja, B. Autolysis of mimosine to 3-hydroxy-4-1 (H) pyridone in green tissues of Leucaena leucocephala. J. Sci. Food Agric. 1983, 34, 529–533. [Google Scholar] [CrossRef]
- Soedarjo, M.; Borthakur, D. Mimosine, a toxin produced by the tree-legume Leucaena provides a nodulation competition advantage to mimosine-degrading Rhizobium strains. Soil Biol. Biochem. 1998, 30, 1605–1613. [Google Scholar] [CrossRef]
- Honda, M.D.H.; Borthakur, D. Mimosine is a stress-response molecule that serves as both an antioxidant and osmolyte in giant leucaena (Leucaena leucocephala subsp. glabrata) during environmental stress conditions. Plant Stress 2021, 2, 100015. [Google Scholar]
- Pires, N.M.; de Souza, I.R.P.; Prates, H.T.; Faria, T.C.L.; Filho, I.A.P. Effect of Leucaena aqueous extract on the development, mitotic index, and peroxidase activity in maize seedlings. R. Bras. Fisiol. Veg. 2001, 13, 55–65. [Google Scholar] [CrossRef]
- Siddiqui, S.; Alamri, S.; Al-Rumman, S.; Moustafa, M. Allelopathic and cytotoxic effects of medicinal plants on vegetable crop pea (Pisum sativum). Cytologia 2018, 83, 277–282. [Google Scholar] [CrossRef]
- Perennes, C.; Qin, L.X.; Glab, N.; Bergounioux, C. Petunia p34cdc2 protein kinase activity in G2/M cells obtained with a reversible cell cycle inhibitor, mimosine. FEBS Lett. 1993, 333, 141–145. [Google Scholar] [CrossRef]
- Yeung, P.K.; Wong, F.T.; Wong, J.T. Mimosine, the allelochemical from the leguminous tree Leucaena leucocephala, selectively enhances cell proliferation in dinoflagellates. Appl. Eviron. Mcrobiol. 2002, 68, 5160–5163. [Google Scholar] [CrossRef]
- Çavuşoğlu, D.; Çavuşoğlu, K. Effect of exogenous application of L-mimosine on physiological, cytogenetic, biochemical and anatomical characteristics of Allium cepa L. S. Afr. J. Bot. 2024, 175, 744–754. [Google Scholar] [CrossRef]
- Chai, T.T.; Ooh, K.F.; Ooi, P.W.; Chue, P.S.; Wong, F.C. Leucaena leucocephala leachate compromised membrane integrity, respiration and antioxidative defense of water hyacinth leaf tissues. Bot. Stud. 2013, 54, 8. [Google Scholar] [CrossRef]
- Miao, L.; Clair, D.K.S. Regulation of superoxide dismutase genes: Implications in disease. Free Radic. Biol. Med. 2009, 47, 344–356. [Google Scholar] [CrossRef]
- Heck, D.E.; Shakarjian, M.; Kim, H.D.; Laskin, J.D.; Vetrano, A.M. Mechanisms of oxidant generation by catalase. Ann N. Y. Acad. Sci. 2010, 1203, 120–125. [Google Scholar] [CrossRef]
- Perry, J.J.P.; Shin, D.S.; Getzoff, E.D.; Tainer, J.A. The structural biochemistry of the superoxide dismutases. Biochim. Biophys. Acta 2010, 1804, 245–262. [Google Scholar] [CrossRef]
- Møller, I.M. Plant mitochondria and oxidative stress: Electron transport, NADPH turnover, and metabolism of reactive oxygen species. Annu. Rev. Plant Biol. 2001, 52, 561–591. [Google Scholar] [CrossRef] [PubMed]
- Arora, A.; Sairam, R.K.; Srivastava, G.C. Oxidative stress and antioxidative system in plants. Curr. Sci. 2002, 82, 1227–1238. [Google Scholar]
- Demidchik, V. Mechanisms of oxidative stress in plants: From classical chemistry to cell biology. Environ. Exp. Bot. 2015, 109, 212–228. [Google Scholar] [CrossRef]
- Alscher, R.G.; Erturk, N.; Heath, L.S. Role of superoxide dismutases (SODs) in controlling oxidative stress in plants. J. Exp. Bot. 2002, 53, 1331–1341. [Google Scholar] [CrossRef]
- Itri, R.; Junqueira, H.C.; Mertins, O.; Baptista, M.S. Membrane changes under oxidative stress: The impact of oxidized lipids. Biophys. Rev. 2014, 6, 47–61. [Google Scholar] [CrossRef]
- Rodrigues-Corrêa, K.C.D.S.; Honda, M.D.H.; Borthakur, D.; Fett-Neto, A.G. Mimosine accumulation in Leucaena leucocephala in response to stress signaling molecules and acute UV exposure. Plant Physiol. Biochem. 2019, 135, 432–440. [Google Scholar] [CrossRef]
- Vestena, S.; Fett-Neto, A.G.; Duarte, R.C.; Ferreira, A. Regulation of mimosine accumulation in Leucaena leucocephala seedlings. Plant Sci. 2001, 161, 597–604. [Google Scholar] [CrossRef]
- Khan, M.I.R.; Fatma, M.; Per, T.S.; Anjum, N.A.; Khan, N.A. Salicylic acid-induced abiotic stress tolerance and underlying mechanisms in plants. Front. Plant Sci. 2015, 6, 462. [Google Scholar] [CrossRef]
- Wang, X.; Pan, Y.-J.; Chang, B.-W.; Hu, Y.-B.; Guo, X.-R.; Tang, Z.H. Ethylene induced vinblastine accumulation is related to activated expression of downstream TIA pathway genes in Catharanthus roseus. BioMed Res. Int. 2016, 2016, 3708187. [Google Scholar]
- Wasternack, C.; Strnad, M. Jasmonate signaling in plant stress responses and development—Active and inactive compounds. New Biotechnol. 2016, 33, 604–613. [Google Scholar] [CrossRef]
- Inderjit. Plant phenolics in allelopathy. Bot. Rev. 1996, 62, 186–202. [Google Scholar] [CrossRef]
- Einhellig, F.A. Mode of action of allelochemical action of phenolic compounds. In Chemistry and Mode of Action of Allelochemicals; Macías, F.A., Galindo, J.C.G., Molino, J.M.G., Cutler, H.G., Eds.; CRC Press: Boca Raton, FL, USA, 2004; pp. 217–238. [Google Scholar]
- Dalton, B.R. The occurrence and behavior of plant phenolic acids in soil environments and their potential involvement in allelochemical interference interactions: Methodological limitations in establishing conclusive proof of allelopathy. In Principals and Practices in Plant Ecology: Allelochemical Interactions; Inderjit, Dakshini, K.M.M., Foy, C.L., Eds.; CRC Press: Boca Raton, FL, USA, 1999; pp. 57–74. [Google Scholar]
- Li, Z.H.; Wang, Q.; Ruan, X.; Pan, C.D.; Jiang, D.A. Phenolics and plant allelopathy. Molecules 2010, 15, 8933–8952. [Google Scholar] [CrossRef] [PubMed]
- Widhalm, J.R.; Dudareva, N. A familiar ring to it: Biosynthesis of plant benzoic acids. Mol. Plant 2015, 8, 83–97. [Google Scholar] [CrossRef] [PubMed]
- Erickson, A.J.; Ramsewak, R.S.; Smucker, A.J.; Nair, M.G. Nitrification Inhibitors from the Roots of Leucaena leucocephala. J. Agric. Food Chem. 2000, 48, 6174–6177. [Google Scholar] [CrossRef]
- Gujer, W. Nitrification and me—A subjective review. Water Res. 2000, 44, 1–19. [Google Scholar] [CrossRef]
- Hiatt, W.C.; Grady, C.L. An updated process model for carbon oxidation, nitrification, and denitrification. Water Environ. Res. 2008, 80, 2145–2156. [Google Scholar] [CrossRef]
- Chen, C.Y.; Wang, Y.D. Secondary metabolites from Leucaena leucocephala. Chem. Nat. Compd. 2011, 47, 145–146. [Google Scholar] [CrossRef]
- Salem, A.Z.M.; Salem, M.Z.; Gonzalez-Ronquillo, M.; Camacho, L.M.; Cipriano, M. Major chemical constituents of Leucaena leucocephala and Salix babylonica leaf extracts. J. Trop. Agric. 2011, 49, 95–98. [Google Scholar]
- Herrera, R.S.; Verdecia, D.M.; Ramírez, J.L.; García, M.; Cruz, A.M. Secondary metabolites of Leucaena leucochephala. Their relationship with some climate elements, different expressions of digestibility and primary metabolites. Cub. J. Agric. Sci. 2017, 51, 107–116. [Google Scholar]
- She, L.C.; Liu, C.M.; Chen, C.T.; Li, H.T.; Li, W.J.; Chen, C.Y. The anti-cancer and anti-metastasis effects of phytochemical constituents from Leucaena leucocephala. Biomed. Res. 2017, 28, 2893–2897. [Google Scholar]
- Deivasigamani, R. Phytochemical analysis of Leucaena leucocephala on various extracts. J. Phytopharm. 2018, 7, 480–482. [Google Scholar] [CrossRef]
- Zayed, M.Z.; Wu, A.; Sallam, S. Comparative phytochemical constituents of Leucaena leucocephala (Lam.) leaves, fruits, stem barks, and wood branches grown in Egypt using GC-MS method coupled with multivariate statistical approaches. BioResources 2019, 14, 996–1013. [Google Scholar] [CrossRef]
- Xu, Y.; Tao, Z.; Jin, Y.; Yuan, Y.; Dong, T.T.; Tsim, K.W.; Zhou, Z. Flavonoids, a potential new insight of Leucaena leucocephala foliage in ruminant health. J. Agric. Food Chem. 2018, 66, 7616–7626. [Google Scholar] [CrossRef] [PubMed]
- Chargui, H.; Ghazghazi, H.; Essghaier, B.; Fradj, M.K.B.; Feki, M.; Charfi, I.; Salem, R.B.; Rigane, G.; Bejaoui, Z. Investigation on the chemical composition of phenolic, fatty acid profiles (GC-FID) and biological activities from Leucaena leucocephala (Lam de wit) seed oil and leaves extracts: Effect of geographical location and maturation stage. Chem. Afr. 2023, 6, 819–826. [Google Scholar] [CrossRef]
- Mohammed, R.S.; El Souda, S.S.; Taie, H.A.; Moharam, M.E.; Shaker, K.H. Antioxidant, antimicrobial activities of flavonoids glycoside from Leucaena leucocephala leaves. J. Appl. Pharm. Sci. 2005, 5, 138–147. [Google Scholar] [CrossRef]
- Chaurasia, S.; Sharma, P. Evaluation of antibacterial and antimutagenic potential of Acokanthera oppositifolia and Leucaena leucocephala. Am. J. Pharm. Health. Res. 2015, 3, 246–258. [Google Scholar]
- Septina, E.; Yetti, R.D.; Rivai, H. Overview of traditional use, phytochemical, and pharmacological activities of chinese petai (Leucaena leucocephala). Int. J. Pharm. Sci. Med. 2020, 5, 1–10. [Google Scholar] [CrossRef]
- Clark, D.B.; Clark, D.A. Seedling dynamics of a tropical tree: Impacts of herbivory and meristem damage. Ecology 1985, 66, 1884–1892. [Google Scholar] [CrossRef]
- Karban, R.; Myers, J.H. Induced plant responses to herbivory. Annu. Rev. Ecol. Syst. 1989, 20, 331–348. [Google Scholar] [CrossRef]
- Maron, J.L.; Crone, E. Herbivory: Effects on plant abundance, distribution and population growth. Proc. R. Soc. B Biol. Sci. 2006, 273, 2575–2584. [Google Scholar] [CrossRef]
- Gong, B.; Zhang, G. Interactions between plants and herbivores: A review of plant defense. Acta Ecol. Sinica 2014, 34, 325–336. [Google Scholar] [CrossRef]
- Kato-Noguchi, H.; Kato, M. Allelopathy and allelochemicals of Solidago canadensis L. and S. altissima L. for their naturalization. Plants 2022, 11, 3235. [Google Scholar] [CrossRef] [PubMed]
- Kato-Noguchi, H.; Kato, M. Invasive characteristics of Robinia pseudoacacia and its impacts on the species diversity. Diversity 2024, 16, 773. [Google Scholar] [CrossRef]
- Kato-Noguchi, H. Allelopathy and allelochemicals of Imperata cylindrica as an invasive plant species. Plants 2022, 11, 2551. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).