Abstract
The genus Clinopodium L. (Lamiaceae) comprises perennial herbaceous plants known for their diverse pharmacological properties. Clinically, these plants are mainly used for the treatment of various hemorrhagic disorders. This review systematically summarizes the research progress on the chemical composition, pharmacological activity, quality control, toxicity, and pharmacokinetics of the genus Clinopodium by searching Google Scholar, Scopus-Elsevier, Wiley, Springer, Taylor & Francis, Medline, Web of Science, CNKI, Weipu, Wanfang, and other academic databases over the last decade (March 2015–February 2025). To date, more than one hundred and thirty structurally diverse secondary metabolites have been isolated and identified from this genus, including flavonoids, triterpenoid saponins, diterpenoid glycosides, lignans, and phenylpropanoids. In addition, numerous volatile oil constituents have been identified in over forty species of the genus Clinopodium. Crude extracts and purified compounds exhibit a variety of pharmacological activities, including hemostatic, anti-myocardial cell injury, cardiovascular protective, anti-inflammatory, antimicrobial, antitumor, hypoglycemic, and insecticidal properties. However, current quality assessment protocols in the genus Clinopodium are limited to flavonoid- and saponin-based evaluations in C. chinense (Benth.) O. Kuntze and C. gracile (Benth.) O. Matsum. Further research is needed to elucidate the pharmacological mechanisms, toxicity, and possible interactions with other drugs. Therefore, the genus Clinopodium has a wide range of biologically active compounds with potential applications in drug development for hemostasis and cardiovascular protection. Nevertheless, there is also an urgent need to establish standardized methodologies to address uncertainties concerning the safety and efficacy of injectable extracts or compounds.
1. Introduction
The genus Clinopodium L. is a class of perennial herbaceous plants in the Lamiaceae family. They are widely distributed in Asia and Europe, typically growing in wet areas, such as fertile forest margins, stream banks, and river valley grasslands. The Lamiaceae family is highly diverse, and the generic boundaries of the genera Satureja, Micromeria, Clinopodium, and Acinos are unclear [1]. Of these, Satureja and Micromeria are accepted genera, while Calamintha and Acinos are considered synonyms of Clinopodium following a comprehensive taxonomic revision and phylogenetic study of Old World Clinopodium [2]. Currently, there are approximately 192 Clinopodium species worldwide [3].
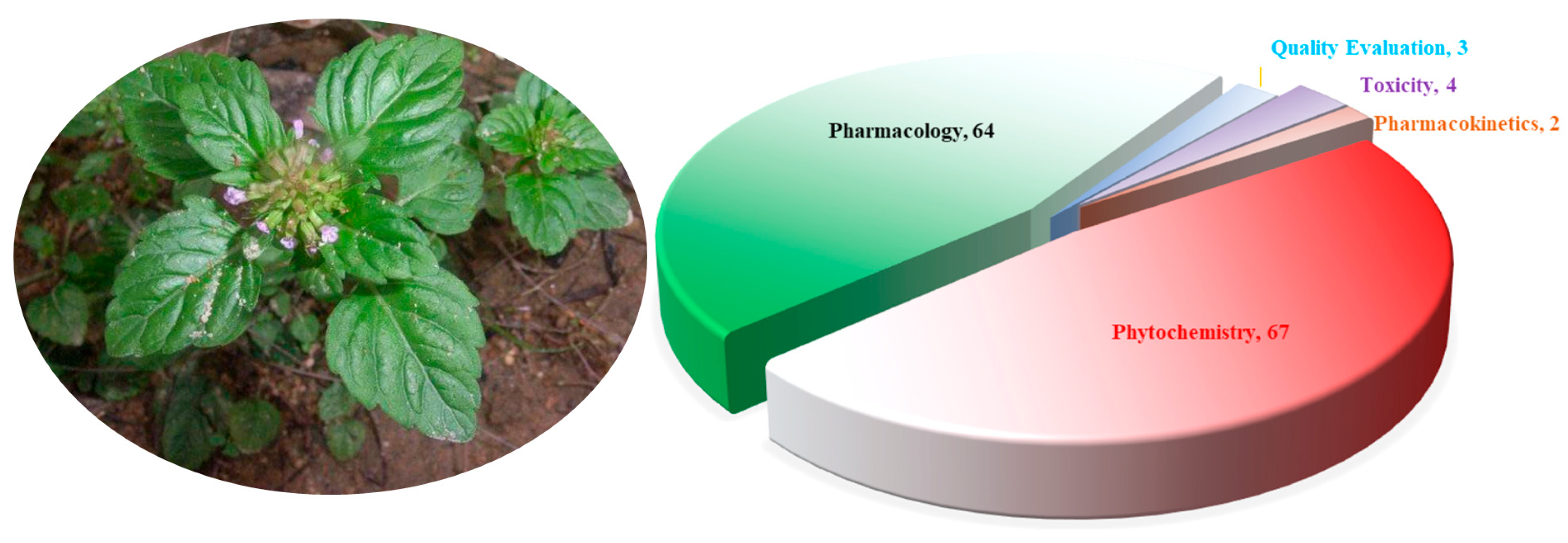
The morphological differences between species in the genus Clinopodium are relatively minor. Plants in this genus often have creeping stems that are finely longitudinally striate and densely glandular pubescent. They have ovate, opposite leaves with crenate serrations. They also have verticillasters, with narrowly tubular calyxes and purple-red corollas. The apex of the upper lip of the corolla is emarginate. Most Clinopodium plants flower from April to May and bear fruit from May to June. The nutlets are dark brown, oblong/oval, and smooth or pimply. They have a faint scent and an astringent, slightly bitter taste. The genus Clinopodium contains various pharmacologically active compounds, including terpenoids, phenylpropanoids, flavonoids, polyphenols, and fatty acids. This genus is also rich in essential oils [4]. Plants of Clinopodium have long been used in folk medicine to prevent and treat various diseases due to, for example, their hemostatic, blood circulation, uterine contraction, antibacterial, anti-inflammatory, immunomodulatory, hypoglycemic, endothelial cell protection, antitumor, sedative, hypnotic, analgesic, and anticonvulsant properties [5,6]. They have been proven to positively affect the cardiovascular system, and to clinically cure gynecological bleeding and oral bleeding diseases. They also have positive effects on hereditary telangiectasia, allergic purpura, and simple purpura. To better discover the current state of research on the genus Clinopodium, electronic databases (Google Scholar, Scopus-Elsevier, Wiley, Springer, Taylor & Francis, Medline, Web of Science, CNKI, Weipu, Wanfang, and other academic databases) were extensively searched using keywords including “Clinopodium”, “Calamintha”, “Acinos”, and “Fengluncai” (the Chinese name: 风轮菜). Over the past decade, a total of 119 articles on the chemical composition, pharmacological activity, quality control, toxicity, and pharmacokinetics of the genus Clinopodium were retrieved (Figure 1).
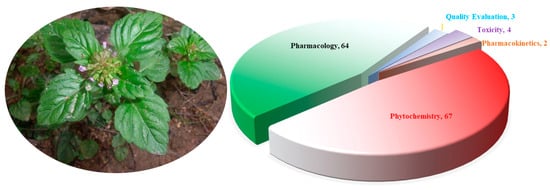
Figure 1.
Article distribution of the genus Clinopodium L.
This review summarizes the results of research conducted over the past decade on the phytochemistry and activity of Clinopodium plants. This research can provide a basis for developing drugs derived from Clinopodium plants.
2. Phytochemistry
From March 2015 to February 2025, a total of one hundred and thirty-six isolated and purified compounds were reported from the genus Clinopodium. These compounds included sixty-seven triterpenoid saponins, twenty-six flavonoids, fifteen phenylpropanoids, and sixteen uncategorizable compounds (compound names are shown in Table 1). These structures were elucidated using infrared spectroscopy (IR), mass spectroscopy (MS), and nuclear magnetic resonance (NMR, including 1H NMR, 13C NMR, COSY, NOESY, HSQC, 2D TOCSY, HSQC-TOCSY, and HMBC) analysis, as well as electrochemical circular dichroism (ECD) and data reported in the literature. For the new compounds containing the sugar moiety, the type and absolute configuration of the sugars were also determined by acid hydrolysis. In addition, most of the monoterpenes and sesquiterpenes were identified from essential oils (EOs), including piperidone oxides and piperidone epoxides. The LC-MS methodology was also employed to investigate the compounds. It is worth noting that some previously isolated compounds, including naphthoquinones and fatty acids, were not identified as new constituents during this period [7].

Table 1.
Compounds from the genus Clinopodium L.
2.1. Compounds Isolated and Identified from the Genus Clinopodium
2.1.1. Terpenoids
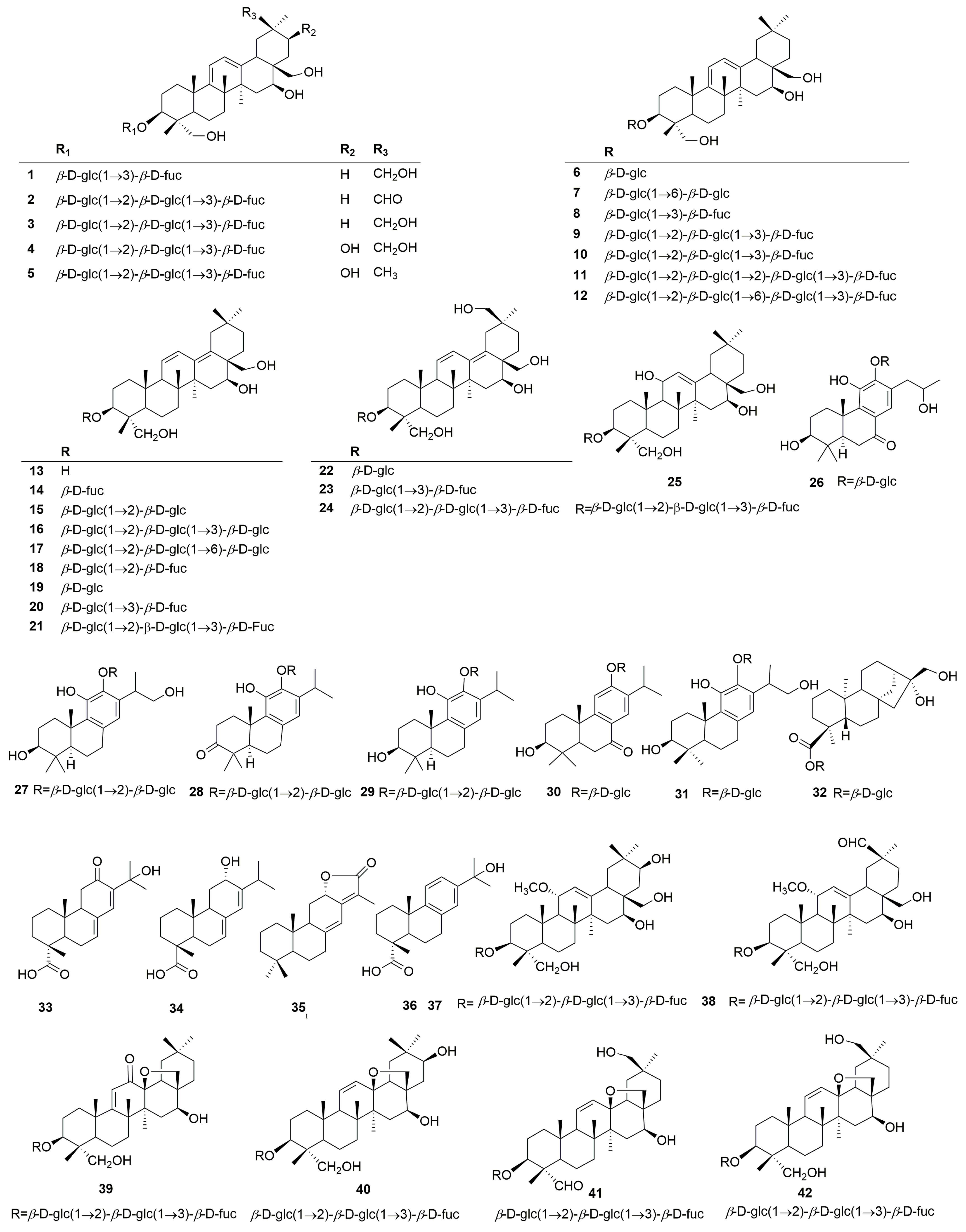
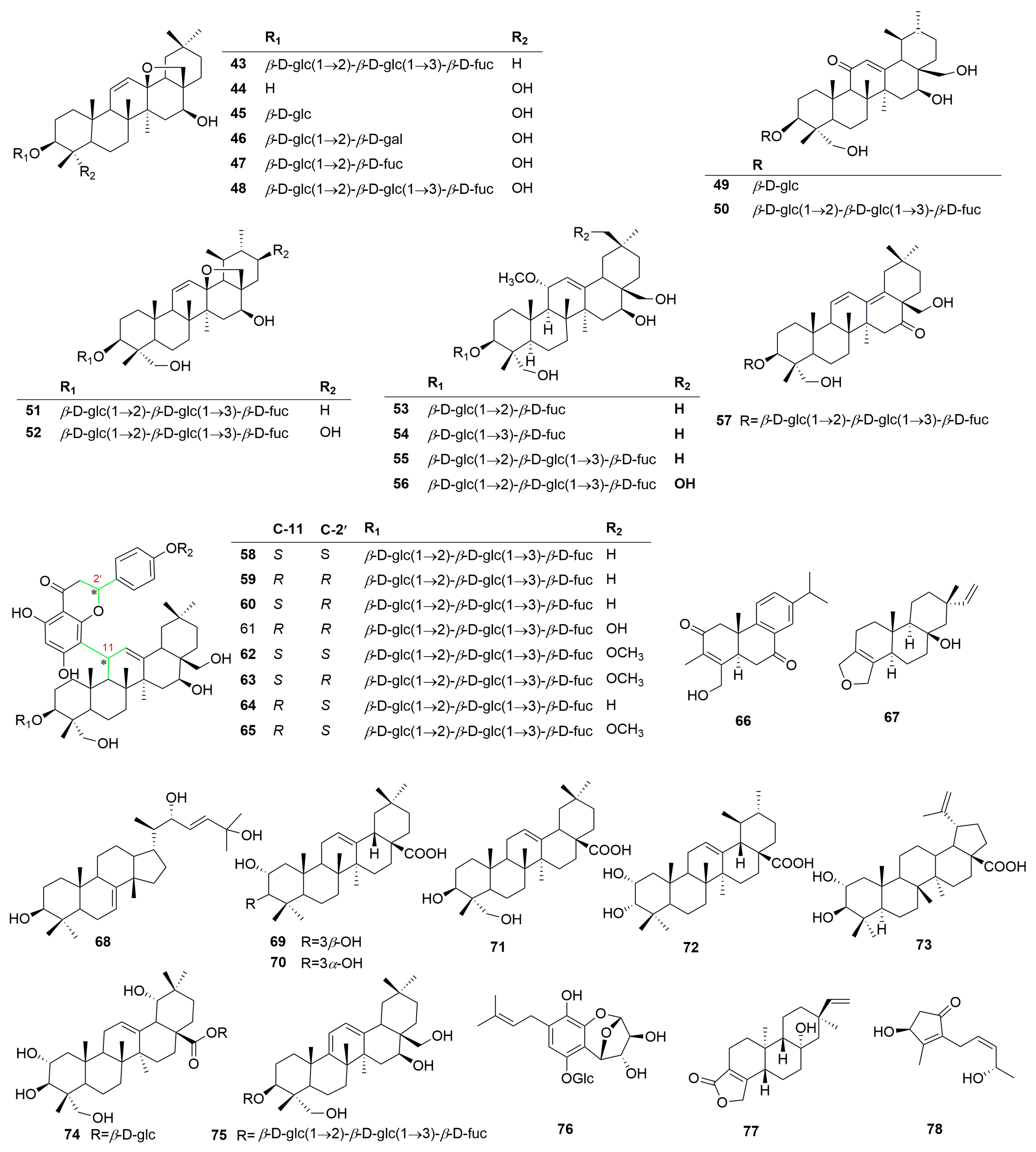
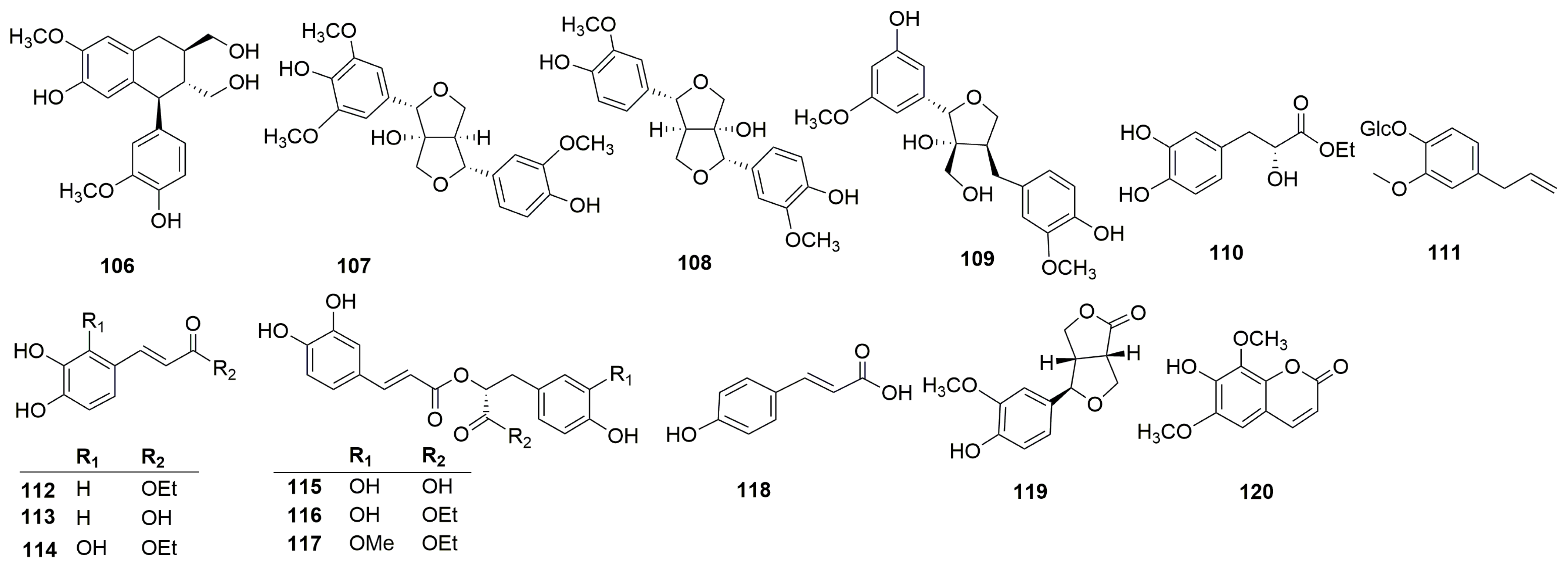
Terpenoids are the main active constituents of the genus Clinopodium. Seventy-eight terpenoids or their saponins have been isolated from plants of this genus, including thirty-six new compounds. Figure 2 shows the structures of the terpenoids and their saponins. The main structures of triterpenoids are oleanane, ursane, lupine, and saikosaponin (containing 13, 28-oxygen rings) skeletons. In addition to the triterpenes, there are fourteen diterpenoids and one monoterpenoid. Most terpenoids exist as glycosides. The sugar composition is mainly glucose, fucose, and rhamnose, and the number of sugars ranges from one to five, typically two to three. Of the seventy-eight terpenoids that were isolated and identified, fifty-one were found in C. chinense, seventeen in C. polycephalum, fourteen in C. gracile, four in C. bolivianum, and two in C. umbrosum. This suggests that C. chinense, C. polycephalum, and C. gracile are used more frequently in developing applications. Furthermore, the simultaneous isolation of buddlejasaponin IV (48), buddlejasaponin IVa (55), and buddlejasaponin IVb (21) from C. gracile, C. chinense, C. umbrosum, and C. polycephalum indicates that these compounds may be the characteristic components of the genus Clinopodium. These saponins were mostly extracted from the whole herb or the plant’s aerial parts using 70–80% ethanol as the extraction solvent.
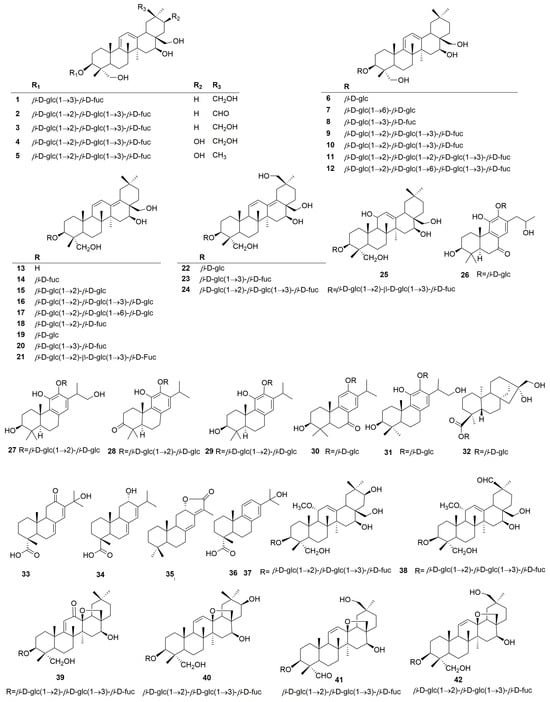
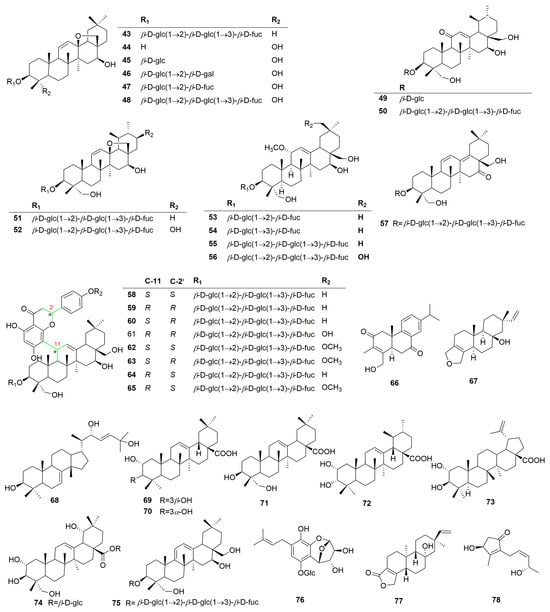
Figure 2.
Structures of terpenoids or their saponins from the genus Clinopodium.
2.1.2. Flavonoids
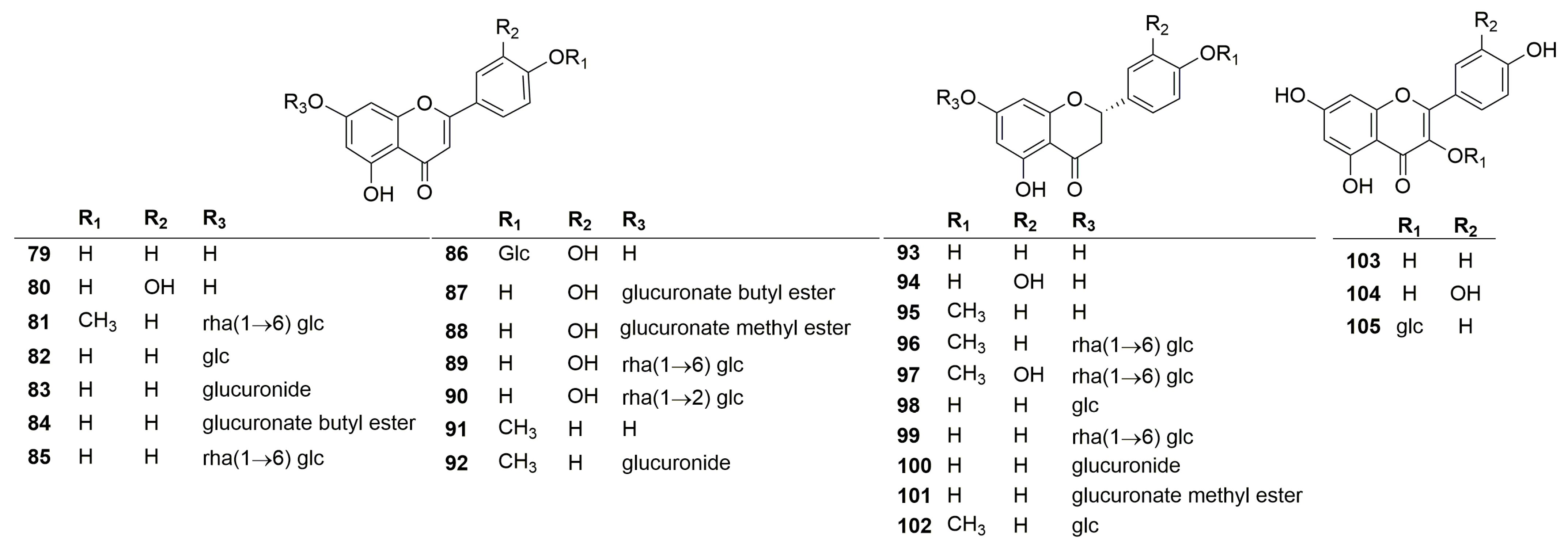
A total of twenty-seven flavonoids have been identified in the plants of the genus Clinopodium. Their chemical structures are shown in Figure 3. According to the structure of the aglycone, the flavonoids are mainly divided into three categories, including flavonoids (79–92), dihydroflavonoids (93–102), and flavonols (103–105). Among these compounds, polycephalum B (101) is a new compound derived from C. polycephalum [19], and the rest were obtained from C. chinense. In addition, most of these compounds are derivatives of luteolin, quercetin, and kaempferol. Notably, an artificial compound, luteolin 7-O-β-D-glucuronide butyl ester (82), was incorrectly written as apigenin 7-O-β-D-pyranglycuronate butyl ester [26].
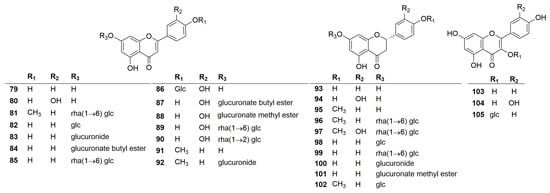
Figure 3.
Structures of flavonoids from the genus Clinopodium.
2.1.3. Phenylpropanoids
Phenylpropanoids are natural compounds consisting of a benzene ring linked to three straight-chain carbons (C6-C3 groups). Phenylpropanoids mainly include phenylpropionic acid, lignin, and coumarin. Thirty phenylpropanoids have been isolated and identified from C. chinense (Figure 4). Xu et al. isolated four lignin compounds—(+)-isolariciresinol (106), fraxiresinol (107), 8-hydroxy-7′-epipinoresinol (108), and deltoignan A (109)—as well as a phenylpropanoid compound salicifoliol (119) and a coumarin isofraxidin (120) from C. chinense [12]. Zeng et al. obtained the following compounds from C. chinense: ethyl (2R)-3-(3,4-dihydroxyphenyl)-2-hydroxypropanoate (110), ethyl (2E)-3-(3,4-dihydroxyphenyl)-prop-2-enoate (112), caffeic acid (113), ethyl (2E)-3-(2,3,4-trihydroxyphenyl)-prop-2-enoate (114), ethyl rosmarinate (116), and clinopodic acid B (117) [25]. Wang et al. identified two phenylpropionic acids named caffeic acid (113) and p-hydroxycinnamic acid (112) [26].
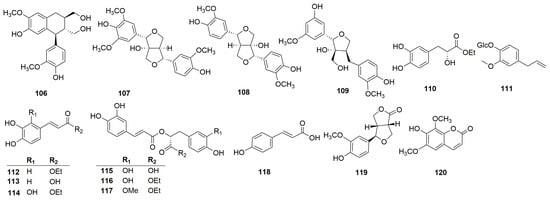
Figure 4.
Structures of phenylpropanoids from the genus Clinopodium.
2.1.4. Other Compounds
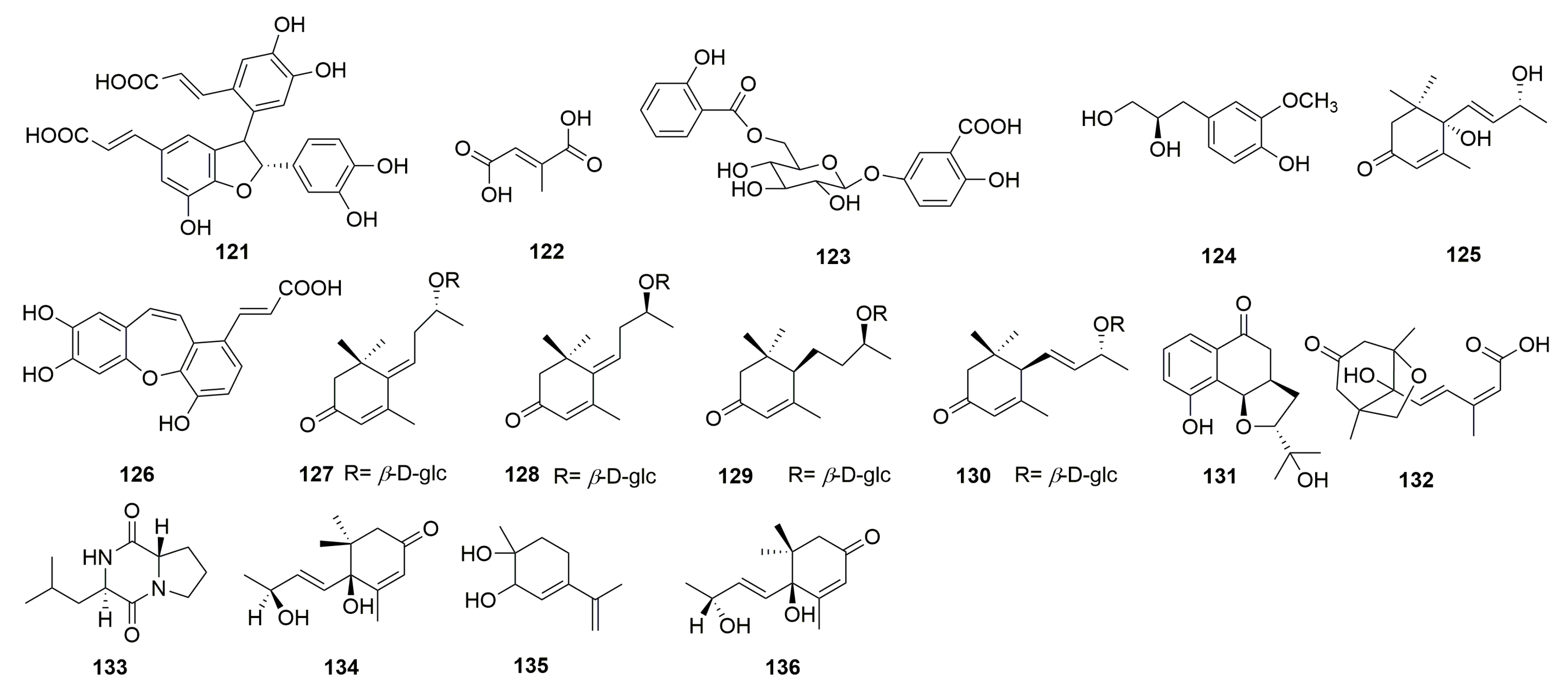
In addition, sixteen other compounds were discovered from C. chinense, including cis-3-[2-[1-(3,4-dihydroxy-phenyl)-1-hydroxymethyl]-1,3-ben-zodioxol-5-yl]-(E)-2-propenoic acid (121), mesaconic acid (122), gentisic acid 5-O-β-D-(6′-salicylyl)-glucopyranoside (123) [26], 4-hydroxyl-3-methoxyphenyl-1-propane-1,2-diol (124) [12], blumenol A (125) [12], tournefolic acid B (126) [27], (E)-6-[9R-(β-D-glucopyranosyloxy) butylidene]-1,1,5-trimethyl-4-cyclohexen-3-one (127), (E)-6-[9S-(β-D-glucopyranosyloxy) butylidene]-1,1,5-trimethyl-4-cyclohexen-3-one (128), blumenol C 9-O-β-D-glucopyranoside (129), (6R,9R)-3-oxo-α-ionol-9-O-β-D-glucopyranoside (130) [23], and Chinense B (131) (Figure 5). Compounds 121 and 122 are a pair of isomers and compounds 127 and 128 are known megastigmane-type glycosides. Chinense B (131) is a rare compound with a ringed prenylated naphthoquinoid skeleton [23]. Phaseic acid (132), cyclo-(S-Pro-R-Leu) (133), vomifoliol (134), p-mentha-3,8-dien-1,2-diol (135), and corchoionol C (136) were isolated and purified from the n-butanol extract of C. chinense [24].
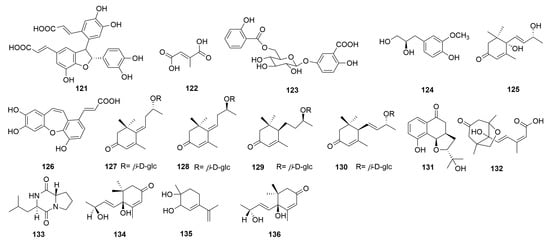
Figure 5.
Structures of other compounds from the genus Clinopodium.
2.2. Compounds Identified by GC-MS
The genus Clinopodium represents a rich source of chemically diverse essential oils (EOs) with significant pharmacological potential [28]. Gas chromatography–mass spectrometry (GC-MS) combined with a computerized search is an important tool for the analysis and identification of EOs. More than forty articles have reported on the chemical composition of EOs from nearly thirty Clinopodium species (Table 2), indicating that genus Clinopodium EOs are a popular research topic.
As can be seen from Table 2, the chemical composition of genus Clinopodium EOs is predominantly characterized by monoterpenes, oxygenated monoterpenes, sesquiterpene hydrocarbons, and oxygenated sesquiterpenes, such as pulegone, menthone, piperidone oxide, menthol, piperidone, E-caryophyllene, α-pinene, and their derivatives, which serve as dominant markers [29,30,31]. EOs extracted from different species in various regions of the globe reveal that the chemical type of EOs depends on the species category, geography, extraction solvent, collection time, and other factors [32,33,34]. EOs extracted from S. calamintha nepeta with water vapor contain high concentrations of 1,8-cineole (34.34%) and cis-pinene (11.87%) [35], while those extracted with supercritical CO2 contain high concentrations of piperone oxide [36]. Although the compositions of the same species were somewhat similar, their content varied greatly [37]. Environmental stressors, such as temperature and UV exposure, direct terpene biosynthesis. Cultivation introduces additional variability. For instance, wild S. calamintha has higher levels of pulegone (72.93% vs. 68.58% in cultivated plants [38]), which suggests that domestication may dilute certain bioactive compounds. Geographical origin profoundly shapes EO composition, often overriding phylogenetic boundaries. Mediterranean species (e.g., C. nepeta from Italy [39]) accumulate 1,8-cineole (34.09%) and eugenol (14.66%), whereas alpine populations (e.g., C. nubigenum from Ecuador [40]) prioritize carvacrol (32.9%) and pulegone (25.4%). C. rouyanum from the Mountains of the island of Majorca in Spain, belongs to both Mediterranean species and alpine populations, are pulegone-dominant, containing 73–82.2% pulegone [41]. Altitudinal shifts also drive variability. C. thymifolium from Serbia exhibits a decrease in pulegone content from 75.9% at low elevations to 50.4% in the mountains, alongside an increase in isomenthone content from 3.1% to 17.8% [42].
It is worth noting that terpinene-4-ol, α-terpineol, carvone, isopiperitenone, 4-hydroxyisopiperitenone, and 4-hydroxypiperitone were obtained from limonene, as well as that 8-hydroxythymol was obtained from thymol by biotransformation. Piperitenone, diosphenolene, and 1-hydroxy-p-menthan-3-one emerged as artifacts due to the opening of the epoxide rings in acidic conditions caused by fungi from piperitenone- and piperitone-epoxides, respectively [43].

Table 2.
Chemical constituents of genus Clinopodium EOs.
Table 2.
Chemical constituents of genus Clinopodium EOs.
| No. | Name | Collected Areas | Identified Compounds and Major Components | Ref. |
|---|---|---|---|---|
| 1 | C. axillare | Quiriría, San José, Province of Esteban Arce | Seventy-five compounds were identified. The major constituents were piperidone oxide (20–30%), piperidone epoxide (15–19%), piperidone (13%), pulerone (3–5%), and piperidone (4–5%), as well as limonene (8–12%) and -pinene (1–5%). | [37] |
| 2 | C. brevicalyx | The southern high Andean regions of Peru | The most abundant compounds were isomaltone (44.25%), menthol (22.22%), and prunone (8.23%). | [44] |
| 3 | C. brownei | Amazonian region of Ecuador | Non-polar constituents: ethyl cinnamate (21.4%), pridon (20.76%), methyl cinnamate (16.68%), caryophyllene (8.17%), β-chromoselenene (7.92%), and menthone (7.51%).Polar constituents: pridon (29.90%), ethyl cinnamate (18.75%), methyl cinnamate (13.82%), caryophyllene (10.0%), and menthone (8.04%). | [45] |
| 4 | Calamintha baborensis | Jijel eastern region of Algeria | The major constituent is eugenol (27.04%), followed by 3-methoxy acetophenone (26.4%) and phenyl ethyl alcohol (6.58%). | [46] |
| 5 | C. candidissimum | Region of Djebel Murdjadjo, Oran, Northwestern Algeria | Thirty-eight compounds were identified, including oxygenated monoterpenes pulegone (44.8%), piperitenone 1, * (6.6%), isopulegone (5.8%), and neo-menthol (3.8%). Among them, the sesquiterpene hydrocarbons germacrene D (16.2%) and bicyclogermacrene (3.0%) were the most abundant. | [47] |
| 6 | C. chinense | Lishui, Zhejiang Province, China | Thirty-five compounds were identified, accounting for 99.18% of the total oil. The major components were phorbol (18.54%), piperitone (18.9%), caryophyllene (12.04%), and bornyl acetate (8.14%), followed by caryophyllene oxide (4.19%), piperitone (4.09%), and carvacrol (4.01%). | [48] |
| 7 | Satureja calamintha | Faculty of Sciences Semlalia, Marrakech, Morocco | Fifteen compounds accounted for 99.88% and 98.14% of the total oils obtained from wild and cultivated plants, respectively. Pulegone (72.93–68.58%), menthone (12.07–10.15%), and menthol (6.31–9.83%) were found as the main constituents. | [38] |
| 8 | Satureja calamintha | Taounate, Morocco | Twenty-four compounds were identified. The main constituents were pulegone (21.48%), piperitenone * oxide (17.71%), and eucalyptol (11.99%). | [49] |
| 9 | Satureja calamintha | Jijel region of Algeria | Three most abundant compounds identified were l-menthone (32.10%), neo-menthol (32.07%), and pulegone (22.35%). | [50] |
| 10 | Calamintha fenzlii | Nablus region of Palestine | The chemical constituents were dominated by oxygenated monoterpenoid (96.91%). The major chemical components were represented by menthone 68.93% and pulegone 23.1%. | [51] |
| 11 | Calamintha glandulosa | Luštica in Stari Krašići (Montenegro) | Seventeen compounds were identified. The major compounds were pulegone (35.1%), piperitenone * (23.4%), menthone (15.7%) and piperitone (11.5%). | [30] |
| 12 | Calamintha incana (Sm.) Boiss. | Kestel, Bursa, Turkey | The oxygenated monoterpenes trans-piperitone oxide (41.37%), piperitenone oxide (34.47%), piperitenone * (6.67%), and monoterpene phenol thymol (3.37%) were found to be the major constituents. | [52] |
| 13 | Calamintha incana | Ajloun county in Jordan | The main constituents were benzenamine-4-methyl-3-nitro-(34.11%) and (2S,4R)-p-mentha-6,8-diene 2-hydroperoxide (31.48%). | [53] |
| 14 | C. macrostemum | San Andrés, Paxtlán, Oaxaca, México | Twenty-six compounds were identified, including menthone (approximately 35%) and piperitone oxide (approximately 30%). | [54] |
| 15 | C. menthifolium | AinDraham, Babouch, and Tabarka, Tunisia | Sixty-three different compounds were identified: piperitone (34.5%), cis-piperitone oxide (26.1%), and piperitone (47.9%). | [55] |
| 16 | Calamintha nepeta | Vratarnica near Zaječar (Serbia) | Fourteen compounds were identified. The major compounds were pulegone (58.0%) and piperitenone * (27.4%). | [30] |
| 17 | Calamintha nepeta | Morano Calabro, Cosenza, Italy | Thirty-four compounds were identified. The major components were 1,8-cineole (34.09%), eugenol (14.66%) and linalool acetate (11.25%), followed by sabinene (6.97%) and linalool (6.64%). | [39] |
| 18 | Calamintha nepeta | Beni-Saf region in the northwest of Algeria | The primary components included oxygenated monoterpenes, notably pulegone (58.36%), isoborneol (10.40%), menthone (8.91%), and piperitenone * (3.86%). | [56] |
| 19 | Calamintha nepeta | Basilicata region, Southeastern Italy | Twenty-four compounds were identified, accounting for 90.17% of total oil composition. Pulegone (44.7%), menthone (16.4%), piperitenone * (13.3%), and piperitone (6.01%) were the major constituents. | [57] |
| 20 | Calamintha nepeta | Tarquinia, Viterbo, Italy | Thirty-nine different chemical constituents have different concentrations in various fractions. Pulegone (37.7–77.7%) and crysanthenone (14.4–27.3%) were the most abundant components. | [32] |
| 21 | Calamintha nepeta | Alentejo region, Herdade da Mitra, Évora | Twenty-nine compounds were identified, representing 91% of oxygenated monoterpenes, 7% of hydrocarbon monoterpenes, and 1% of sesquiterpenes. The major components were 1,8-cineole (28%), menthone (22%), menthol (16%), and pulegone (5%). | [58] |
| 22 | Calamintha nepeta | Tengalti village and the region near the Velvelechay river of Quba | Seventy-eight compounds were identified; the major components were thymol (19.81%), cyclopropane, 1,1-diethyl-(19.77%), cyclohexanone, 3-vinyl3-methyl-(18.66%), D-limonene (7.45%), and caryophyllene (6.16%). | [59] |
| 23 | Satureja calamintha subsp. nepeta Briq. | Medea region, South Algiers and Chlef region, western Algiers | Seventy compounds were identified, representing 97.4% of the oil. 1,8-cineole (28.4%), pulegone (10.2%), menthone (9.7%), and isomenthone (9.6%) were the most important constituents. | [60] |
| 24 | S. calamintha nepeta | Mountains of the Skikda region located in northeastern Algeria | One hundred and ten compounds were identified. Piperitenone oxide, trans-piperitenone oxide, caryophyllene oxide, 3-methyldiphenyl ether, (E)-caryophyllene, gensmin, germacrene D, (Z)-jasmone, trans-calamenene, γ-gurjunene, and pulegone are the main constituents. | [61] |
| 25 | S. calamintha nepeta | Mountainous terrain of the Moroccan province of Ouazzane | Twenty-seven compounds were identified, making up 99.2% of the essential oil, with 1,8-cineole (34.34%) and cis-pinocamphone (11.87%) being the most significant. | [35] |
| 26 | C. nepeta | Béni-Mtir (Aîn Draham, Jendouba), North-western Tunisia | Forty-seven compounds were identified: the main components were piperitone oxide (16.3–51.7%) and piperitenone oxide (23.4–39.3%). | [33] |
| 27 | C. nepeta | Bilecik, Turkey | Forty-four compounds were identified. The main components were piperitenone oxide (47.8%), limonene (18.6%), and piperitone oxide II (13.6%). | [62] |
| 28 | C. nepeta | Antalya-Finike, in southwestern Turkey | Thirty-five compounds were identified and quantified. The major compounds were sabine (34.2%), β-pinene (25.9%), α-pinene (13.8%), and caryophyllene oxide (3.7%). | [63] |
| 29 | C. nepeta | sub-Mediterranean area of Bosnia and Herzegovina | The EOs contained 42 compounds, including pulegone (44.8%), piperitenone * (48.8%), and piperitenone oxide (60.2%) as the major compounds. | [64] |
| 30 | C. nubigenum | Mountains near Hacienda Zuleta, Imbaburra, Ecuador | Thirty-three compounds were identified. The major chemical constituents were carvacrol (32.9%), followed by pulegone (25.4%). Other important volatiles were p-cymene (9.1%) and iso-menthone (6.4%). Monoterpenes, both in their oxygenated and hydrocarbon forms (74 and 19.7%, respectively), were the major chemical class. | [40] |
| 31 | Calamintha officinalis | Northern Iran (Guilan, Lahijan) | Forty-one components were isolated, constituting 23.09% of the total oil. The major constituents were trans-caryophyllene (8.55%), isomenthol (2.98%), tetrahydrolinalyl acetate (2.96%), and pinene (2.24%). | [65] |
| 32 | C. pulegium | Svrljiški Timok gorge, Serbia | Nineteen previously described mono- and sesquiterpenes were found. The major compound was menthone (47.1%), followed by β-pinene (19.8%), isomenthone (12.3%), and pulegone (12, 8.5%). | [66] |
| 33 | C. rouyanum | Mountains of the island of Majorca, Spain | Twenty-seven compounds were identified from five samples of C. rouyanum, among which pulegone (73.0–82.2%), menthone (6.5–11.8%), and limonene (3.5–6.0%) were the major compounds. | [41] |
| 34 | C. serpyllifolium | BERC Experimental Station, Til, Nablus, Palestine | Twenty-three compounds were identified. Pulegone (50.22–81.51%), menthol (1.91–15.68%), and p-menth-3-en-8-ol (1.64–11.94%) were the major compounds. | [67] |
| 35 | C. serpyllifolium | The Newe Ya’ar living germplasm, Israel | The major constituents were oxygenated monoterpenes pulegone (10.4–50.6%), piperitenone oxide (3.2–28.6%), piperitenone * (0.9–14.6%), trans-piperitone oxide (0.3–11.2%), iso-menthol (0.3–8.8%), and sesquiterpene β-caryophyllene (7.4–13.7%). | [68] |
| 36 | C. sericeum | Region of Cajamarca (Perú) | Seventy-three compounds were identified. The major compounds were β-germacrene D (15%), β-caryophyllene (13.8%), and sabinene (11.2%). | [69] |
| 37 | Calamintha sylvatica | Morano Calabro, Cosenza, Italy | Twenty compounds were identified. The major compounds were piperitone oxide (37.70%), pulegone (20.91%), and piperitenone oxide (18.26%), iso-menthone (7.5%), and limonene (6.58%). | [39] |
| 38 | Calamintha sylvatica | The edge of a beech and hornbeam forest, under Mt. Rudnik (Serbia) | Twenty-eight compounds were identified. The major compounds were cis-piperitone epoxide (63.3%) and menthone (10.8%). | [30] |
| 39 | C. taxifolium | Province of Loja, Mount Villonaco | Thirty-seven compounds were identified, mainly including (E)-β-caryophyllene (17.8%), α-copperene (10.5%), β-bourbonene (9.9%), δ-carpentene (6.6%), cis-cadina-1(6),4-diene (6.4%), and myricene D (4.9%). | [70] |
| 40 | C. thymifolium | Limestone habitat near Tutin, SW Serbia | Fifty-six compounds were identified, mainly including pulegone (75.9% in vegetative stage and 50.4% in late flowering stage), piperitenone * (6.2% in vegetative stage and 10.4% in late flowering stage), isomenthone (3.1% in vegetative stage and 17.8% in late flowering stage), and limonene (vegetative stage). | [42] |
| 41 | C. umbrosum | Kheyroud forest near Noshahr, Mazandaran, Iran | Sixteen compounds were identified. The major compounds were tolualdehyde (29.16%), palmitic acid (17.57%), and acetophenone (13.44%). | [18] |
| 42 | Calamintha vardarensis | The underbrush in Radika Canyon (FYR Macedonia) | Twenty-five compounds were identified. The major compounds were pulegone (51.6%) and menthone (19.9%) | [30] |
1 Compounds with * are artifacts.
2.3. Compounds Identified by LC-MS
High-performance liquid chromatography–Orbitrap high-resolution mass spectrometry (UHPLC-HRMS) is widely used in plant metabolite studies because of its high sensitivity, accuracy, and rapidity. The constituents of Clinopodium plant extracts were easily identified using the UHPLC-Q-TOF-MS methodology.
Twenty-five compounds, including twelve flavonoids, nine saponins, and four organic acids, were identified in different batches of C. chinense, such as didymin (96), hesperidin (97), kaempferol (103), quercetin (104), naringenin (93), apigenin (79), saikosaponin a (46), buddlejasaponin IVb (21), acacetin (91), and isosakuranetin (95) [71]. Cluster analysis of these major constituents revealed similarities among C. chinensis, and those analyzed by LC-MS were consistent with the isolated and identified compounds. In contrast, the ethanolic or methanolic extracts of C. incana predominantly contained linolenic acid, myristic acid, and p-cymene [72,73]. These fatty acids accounted for 31.3% of the identified compounds (31.3%), followed by sesquiterpenes (23.9%) and monoterpenes (20.7%).
The major compounds identified in C. nepeta were caffeic acid (113), quercetin (104), and rosmarinic acid [29]. Similar organic acid constituents were also found in C. vulgare. LC-MS analysis revealed hundreds of compounds in C. vulgare, including organic acids such as coumaric acid and chlorogenic acid, as well as a high content of triterpene saponins [74]. Additionally, C. vulgare extracts also contained flavonoids and monomers, dimers, trimers, and tetramers of caffeic acid, as detected in both lyophilized aqueous and methanol extracts [75,76]. The relative abundance of caffeic acid derivatives was closely related to the harvest time. Plants collected in winter contained nearly four times more acetoacetic acid and its derivatives than they did caffeic acid derivatives [77].
Therefore, the genus Clinopodium contains three major classes of bioactive constituents: polyphenols, flavonoids, and saponins. These compounds are key markers for both pharmacological activity screening and quality control assessments. They provide a reliable chemical basis for evaluating the therapeutic potential and standardization of Clinopodium species.
3. Pharmacology
The various species of the genus Clinopodium have very rich chemical compositions, resulting in numerous pharmacological activities. These activities mainly include hemostatic, anti-myocardial cell injury, cardiovascular protection, anti-inflammatory, antimicrobial, antitumor, hypoglycemic, and insecticidal properties (Table 3).

Table 3.
Pharmacological activity of the extract, fraction, or compounds from the genus Clinopodium.
3.1. Hemostatic Activity
C. chinense and C. polycephalum exhibited significant hemostatic activity and are considered legitimate sources of “Duan Xue Liu” [105]. C. chinense extract primarily promoted platelet adhesion, shortened plasma recalcification and bleeding times, and reduced bleeding volume [78]. Investigating the mechanism by which C. chinense extract reduced uterine bleeding revealed that it inhibits the inflammatory response and promotes endometrial repair. The extract also increased microvessel density (MVD) and the levels of thromboxane B2 (TXB2), vascular endothelial growth factor (VEGF), and transforming growth factor-beta (TGF-β). Additionally, it also reduced the levels of interleukin-6 (IL-6)/tumor necrosis factor-alpha (TNF-α) and the expression of matrix metalloproteinases (MMPs) 2/9. These effects promote endometrial recovery [79]. Total saponins of C. chinense increased coagulation function and promoted endometrial repair when used to treat functional uterine bleeding. They also significantly increased TXB2 levels and the TXB2/6-keto-PGF_(1α) ratio. They also increased progesterone and FSH levels.
Similarly, total flavonoids were found to significantly increase E2, FSH, and LH levels. They also regulated estrogen levels and reduced inflammatory responses. Total flavonoids significantly decreased the amount of uterine bleeding, reduced pathological damage to the endometrium, and increased microvessel density in the endometrial tissue. Total extracts have the best therapeutic efficacy, revealing the synergistic effects of Chinese medicine on abnormal uterine bleeding [80]. Further research confirmed that buddlejasaponin IVb (21), hesperidin (97), naringenin (93), apigenin (79), and saikosaponin a (46) were the major active constituents in C. chinense responsible for reducing uterine bleeding [71]. In addition, buddlejasaponin IV (48) and prosaikogenin A (14) from C. chinense can significantly promote platelet aggregation, with EC50 values of 53.4 and 12.2 μM, respectively. Buddlejasaponin IVb (21) and saikogenin F (45) at 200 μM shortened TT by 20.6% and 25.1%, respectively [11]. However, the methanolic fraction of C. officinalis has an antithrombotic effect in experimental model mice. Further detailed phytochemical and pharmacological studies are necessary to identify the antithrombotic compounds and their mechanism of action [106].
3.2. Anti-Cardiomyocyte Damage and Cardiovascular Protection
Flavonoids are important metabolites that can improve cardiovascular risk factors [107,108]. Total flavonoids from C. chinense exert myocardial protective effects through multi-target mechanisms. Studies have shown that total flavonoids from C. chinense can significantly increase the activity of antioxidant enzymes such as SOD, CAT, and GSH-Px, while decreasing the levels of oxidative damage markers MDA and LDH. They can also enhance cardiomyocytes by activating the nuclear translocation of Nrf2 and the downstream expression of HO-1 antioxidant defense [81,82]. Total flavonoids from C. chinense inhibited the expression of pro-apoptotic factors p53, caspase-3, and P21. They also blocked the phosphorylation of oxidative stress-related MAPK signaling pathways (JNK, p38, and ERK), and activated the PI3K/AKT pathway, thereby increasing the viability of H9c2 cardiomyocyte [83]. Further studies have demonstrated that C. chinense total flavonoids also attenuate hypoxia/reoxygenation-induced cardiomyocyte injury by inhibiting miR-702-5p expression in concert with miRNA inhibitors [84].
Monomeric compounds were obtained for further screening of cardioprotective activity. The cell viabilities of clinopodiside X (3), clinopodiside XI (4), clinoposaponin XIX (42) (at 50.0 μg·mL–1), clinopoditerpene B (26) (at 12.5 μg·mL−1), and prunin (98) (at 25.0 mg·L–1) isolated from C. chinense were 78.46%, 80.77%, 79.55%, 73.7%, and 84.25%, respectively. These results suggest that these compounds have cardioprotective effects against A/R or H2O2-induced apoptosis in H9c2 cells [8,14,26]. Similarly, the terpene constituents of C. polycephalum, clinopodiside VI (6), saikosaponin c (18), and arjunglucoside I (74) increased cell viability by 77.8%, 80.9%, and 79.8% at 100.0 μg·mL−1, respectively, showing a moderate inhibitory effect on H2O2-induced H9c2 cell damage [9]. Clinoposides G (53) and H (54), two new flavonoid–triterpenoid saponin subterpenoids from C. chinense, significantly increased mitochondrial membrane potential, increased antioxidant enzyme activities, decreased inflammatory cytokine levels, decreased p65 protein levels, and increased nuclear Nrf2 levels, which inhibited hypoxia/reoxygenation (A/R)-induced injury and apoptosis in H9c2 cells [21]. Tournefolic acid B (TAB, 126) from C. chinense ameliorated hemodynamic parameters in isolated rat hearts and inhibited cardiomyocyte apoptosis. Conversely, TAB prevented myocardial I/R injury by inhibiting PI3K/AKT-mediated endoplasmic reticulum stress, oxidative stress, and apoptosis [27].
In addition, constituents of the genus Clinopodium also play an important role in the repair and functional regulation of the vascular system. Flavonoids and polyphenols, such as luteolin (80), naringenin (93), eriodictyol (94), ethyl (2R)-3-(3, 4-dihydroxyphenyl)-2-hydroxypropanate (110), caffeic acid (113), ethyl rosmarinate (116), and clinopodic acid B (117) from C. chinense, were involved in vascular endothelial-protective effects and significantly ameliorated high glucose-induced injury of HUVECs with EC50 values of approximately 3–36 μM [25]. Ethanolic extract of C. tomentosum improved the migration ability and angiogenic capacity of aortic endothelial cell pAEC, significantly increased the expression of FLK-1 mRNA, and ameliorated LPS-induced cell injury with angiogenic capacity [85]. An aqueous extract of C. vulgare increased CAT, GSH-Px, and SOD activities in the liver, kidneys, and brain. It also maintained the depletion of reduced glutathione GSH, and slightly decreased systolic blood pressure [86].
3.3. Anti-Inflammatory Activity
The anti-inflammatory effects of C. chinense were characterized by multi-target regulation, involving the inhibition of key signaling pathways and regulation of metabolic homeostasis. Ethyl acetate extract of C. chinense attenuated PA-induced inflammation through a dual pathway, exerting vasculo-protective effects. On the one hand, it inhibited TLR4 expression in HUVECs and blocked the downstream MyD88/TRIF/TRAF6 signaling pathway. This inhibited the phosphorylation of IκB kinase β, NF-κB and the MAPK family (JNK/ERK/p38), and reduced the release of inflammatory factors such as TNF-α, IL-1β, and IL-6. On the other hand, restoring eNOS activity by modulating the phosphorylation pattern of IRS-1 stimulated insulin-mediated NO production in PA-treated HUVECs and ameliorated impaired insulin signaling in the vascular endothelium [87]. This multi-pathway synergistic effect was further validated by evaluating the anti-inflammatory activity of camphor, menthol, and their equimolar combination. Camphor and menthol, the major compounds in C. nepeta EO, showed that the combination was more effective in inhibiting 5-lipoxygenase (72.5% vs. 48.3% for camphor and 52.9% for menthol), as well as COX-1 and COX-2 cyclooxygenases (78.1% and 79.4%, respectively, vs. 60.4% and 62.7% for camphor, 64.2% and 66.3% for menthol)[109].
Network pharmacological predictions, combined with experimental confirmation, show that the genus inhibits macrophage inflammation via the TLR4-NF-κB-iNOS/COX-2 axis. It also modulates key metabolic pathways such as arachidonic acid metabolism, histidine metabolism, alanine, aspartate and glutamate metabolism, and the pentose phosphate pathway. This results in reduced MDA levels and decreased pro-inflammatory factors, such as IL-6 and TNF-α in colon tissues of the UC model. In conclusion, C. chinense extract may alleviate ulcerative colitis by reducing systemic inflammation and modulating metabolism [88]. Caffeic acid, chlorogenic acid, and catechin from C. vulgare were proven to inhibit zymosan-induced COX-2 expression in bone marrow neutrophils [110]. Notably, specific targets in aqueous extracts of different C. vulgare species selectively inhibited p38/JNK MAPKs phosphorylation and MMP-9 activation, but had a weak effect on the COX-2/PGE2 pathway, whereas C. bolivianum inhibited the adhesion, invasion and biofilm formation of uroepithelial pathogenic E. coli by upregulating caveolin-1 [90]. The novel diterpene compounds, imbricatusol I (66) from C. polycephalum and buddlejasaponin IV (48) from C. chinense, were found to inhibit NO production in LPS-induced RAW 264.7 cells without affecting cell viability. These findings provide a new direction for the development of anti-inflammatory drugs targeting extracellular matrix protection [17,22].
The anti-inflammatory benefits of aqueous/alcoholic extracts of C. gracile are also demonstrated by a multimodal mechanism of action that is effective against acute neurogenic pain, inflammatory pain, and acute inflammation. These extracts can simultaneously decrease the levels of mediators, such as NO, MDA, PGE2, etc., in brain tissue and peripheral blood, and reduce intracellular ROS to baseline levels by inhibiting xanthine oxidase activity. This mechanism is associated with the suppression of the brain’s expression of NO, MDA, and PGE2, as well as the expression of NO, MDA, PGE2, IL-6, and TNF-α in peripheral blood [89]. Additionally, C. nepeta EO also exhibited strong anti-inflammatory properties against carrageenan-induced paw edema, with an IC50 value of 17.23 ± 0.32 μg·mL−1 compared to diclofenac [56].
3.4. Antimicrobial Activity
The genus Clinopodium exhibited broad-spectrum antimicrobial activity. The antimicrobial activity of EOs from more than 12 species of the genus Clinopodium has become a hot topic. In the area of antimicrobial resistance, C. nepeta EO demonstrated unique advantages against multi-drug resistant strains. For ampicillin-/ciprofloxacin-/gentamicin-resistant Escherichia coli JM109, its minimal inhibitory concentrations (MICs) were as low as 0.300–0.966 μL·mL−1 and the ratio of bactericidal concentration (MBC) to MIC was ≤2, confirming its potent bactericidal properties [91]. Notably, C. nepeta EO showed the highest activity against Salmonella typhimurium at 1250 µg·mL−1. The EO was more effective against B. cereus (2500 μg·mL−1) and S. sanguinis (2500 µg·mL−1). However, it was least active against E. coli and Pseudomonas aeruginosa [62]. An analysis of chemical fractions reveals conformational relationships: the synergistic effect of menthone (44.8%) and isomenthone (5.8%) from C. candidissimum EO resulted in a low MIC at 0.145 g·L−1 against phytopathogenic bacteria (e.g., Pseudomonas lilacis) and multi-targeting effects through the competitive inhibition of acetylcholinesterase (IC50 = 0.17–0.43 g·mL−1) [54]. Calamintha nepeta EO exhibited minimum inhibitory concentrations and bactericidal/fungicidal concentrations ranging from 0.937 to 3.75 µL·mL−1 and 0.937 to 15 µL·mL−1, respectively [56]. Satureja calamintha nepeta exhibits antifungal potency indicating that the methanolic extract is more active (>69.04% ± 2.06) than the aqueous extract (>29.76% ± 2.06) against all tested molds [111]. Furthermore, its EO shows strong activity against E. coli and E. vekanda strains, with MIC values of approximately 2.80 µg·mL−1 [35].
Regarding the potential for topical application, C. sericeum EO with β-stilbene (13.8%) and mangosteen (11.2%) as main constituents showed antimicrobial activity against Gram-negative and Gram-positive bacterial strains with MIC of 50–200 μg·mL−1 [69]. C. chinense extract also exhibited good inhibitory effects against Escherichia coli, Staphylococcus aureus, and Proteus vulgaris with MICs of 0.0625 g·mL−1; however, it showed no inhibition against Sarcina lutea and Aspergillus niger. This suggests that its antimicrobial spectrum is selective [112].
This selectivity was further elucidated in C. menthifolium. EO from the Babouch region showed the strongest antifungal activity against Aspergillus terreuss, Microsporum canis, and Candida albicans (MIC = 40–400 μg·mL−1), while EO from the Tabarka region showed a 42.5% repellency rate against the storage pest Tribolium confusum. These results indicate that geographical origin significantly influences biological activity by modulating terpene constituents [55]. EO from Calamintha menthifolia had strong antibacterial properties with Staphylococcus albicans, Escherichia coli, and P. fluorescens being the most susceptible strains and P. vulgaris being the least susceptible. However, it was less active than ciprofloxacin and L. angustifolia EO [113].
C. brevicaly EO showed an MIC of 125 μg·mL−1 against Trichophyton rubrum. Its antifungal activity was mechanistically related to the transmembrane permeability of terpene constituents [44]. The caryophyllene content of C. brownei EO has been reported to be positively correlated with the antibacterial activity. The bacterial growth inhibitory concentration was 3.11 mg·mL−1 for Candida albicans ATCC 10231, significantly lower than that of the positive control. This suggests that the antifungal activity is primarily due to the terpene constituents [45].
Besides the genus Clinopodium EOs, polyphenolic compounds in extracts are active antifungal constituents. C. nubigenum extracts obtained by supercritical fluid extraction contain thymol, carvacrol, (±)-6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid, 5-hydroxyflavone, and icarrin. These extracts exhibit the most potent antifungal activity against Colletotrichum musae, Mucor racemosus, and Fusarium verticillioides with MICs of 400, 400, and 825 mg·L−1, respectively [114]. AcOEt extract of C. nepeta inhibited the swarming motility of Pseudomonas aeruginosa PA01 with an MIC of 12.0 ± 0.5 mm, showed the best activity of the violacein group sensing 35.42 ± 1.00% at 100 μg·mL−1, and disrupted bacterial biofilm formation [92]. Furthermore, C. bolivianum polysaccharides have more powerful activity against HIV-1 infection, with an IC50 of 4.72 µg·mL−1 [115]. The low polar fraction of Calamintha baborensis showed good inhibition against staphylococci and E. coli, with inhibition zone diameters of 19 mm and 19.2 mm, and MIC values of about 43 and 43.4 μg·mL−1, respectively. The main active compound against staphylococci was phenyl-β-D-glucuronide rhamnoside [46,116].
In addition to the different effects of species type and extraction method, the origin of the species also affects antimicrobial activity. For example, hexane extracts of Calamintha sylvatica from natural and in vitro propagated plantlets showed activity only against Staphylococcus aureus with MIC values at 6.25 and 3.33 m·mL−1, respectively. This activity may be correlated with the content of rosmarinic acid [117]. S. calamintha EO showed the best activity against E. coli K12 compared to the antibiotic streptomycin sulfate. Similarly, wild and domesticated S. calamintha showed differences with inhibitory diameters of 25.67 ± 0.58 mm and 48.67 ± 1.15 mm and MIC values of 1.49 ± 0.00 μg·mL−1 and 0.373 ± 0.00 μg·mL−1, respectively [97]. The combined action of Lactobacillus (LB) exopolysaccharides and Satureja calamintha extracts reduced the aggregation ability of enteropathogenic Escherichia coli and decreased the rate of their adhesion [118]. C. vardarensis EO exhibits selective antimicrobial activity against Staphylococcus aureus (MIC 21.25 µg·mL−1). The overall effect of EO–antibiotic combinations varied from synergistic (FICI ≤ 0.5) to antagonistic (FICI ≥ 2) depending on the bacterial strain tested [30]. Thus, this genus is a diverse source of candidates for developing novel antimicrobial agents through the multimodal action of terpenoid, phenolic acid and glycoside components.
3.5. Antitumor Activity
The antitumor activity of C. chinense is closely related to the structural diversity of its specific secondary metabolites. Compound 45, a triterpenoid saponin with C-11 carbonyl/C-3 glucose modification from C. chinense, exhibited comparable cytotoxicity to the positive control, 10-hydroxycamptothecin, against mouse mammary adenocarcinoma 4T1 cells (IC50 = 7.4 μM and 7.6 μM, respectively) [8]. However, other terpenes from C. chinense showed no direct cytotoxicity (IC50 > 100 μM) against A549 and HepG2 cancer cell lines, which may be due to the lack of a C-11 carbonyl skeleton in the compounds or to the selectivity of the anticancer activity of the genus Clinopodium [119]. Nevertheless, compounds 28–30 and 32 significantly improved the insulin resistance index in HepG2 cells (42.7 ± 3.5% reduction in HOMA-IR), suggesting that they may indirectly affect the tumor microenvironment through metabolic regulatory pathways [13].
Similarly, petroleum ether, chloroform, and methanol extracts of C. umbraculum showed cytotoxic activity against HN-5 oral cancer cells with IC50 values of >250, >167, and 239.5 μg·mL−1, respectively. Buddlejasaponin IVa (55) and buddlejasaponin IV (48) isolated from C. umbraculum significantly increased the Bax/Bcl-2 ratio and activated caspase-9, inhibited the migration of HN-5 cells, and enhanced the cytotoxicity of compound 48 (IC50 = 58.3 μM) compared to the crude extract (IC50 > 167 μg·mL−1), indicating that the two saponins exerted their anticancer activities via the mitochondrial apoptotic pathway [94]. Compound 48 also showed considerable anticancer activity against A549, HCT116, MDA-MB-231, SNU638, and SK-HeP-1 cells [17].
In addition to triterpenoid saponins, EOs from the genus Clinopodium are also an important class of tumor-active constituents. The toxicity of C. fenzlii EO on HeLa cells showed concentration-dependent death. C. fenzlii VO at 1.4 and 0.7 mg·mL−1 significantly induced cell cytotoxicity with mortality rates of approximately 50% and 40%, respectively, while concentrations ranging from 2.9 to 23.22 mg·mL−1 significantly induced more than 70% of the cells to die [51]. C. sericeum EO (β-germacrene D 15%) exerted selective cytotoxic effects through non-oxidative stress pathways on T24 bladder cancer (IC50 = 0.19 mg·mL−1) and MCF-7 breast cancer (IC50 = 0.21 mg·mL−1) cells. Its constituents were also shown to exert cytotoxicity through the mitochondrial apoptotic pathway; they produced selective cytotoxicity, and the toxicity threshold for normal HEK-293 cells was high (IC50 = 0.38 mg·mL−1) [69]. C. nepeta EO showed anticancer activity against MCF-7, MDA-MB-231, and MDA-MB-436 breast cancer lines with IC50 values of 27.6, 31.2, and 36.5 µg·mL−1, respectively, demonstrating remarkable selectivity for cancer cells [109]. Calamintha nepeta EO showed cytotoxic activity against non-small cell lung cancer (A549) cells with IC50 values of 442.9 μg·mL−1 and 133.9 μg·mL−1 at 24 h and 48 h, respectively [120].
3.6. Antioxidant Activity
The antioxidant activity of the genus Clinopodium is significantly dependent on the extraction solvent. Aqueous extracts and organic solvent extracts (e.g., different concentrations of ethanol and methanol) showed significant differences in antioxidant capacity [95,98,101]. The n-BuOH extract of Calamintha baborensis was superior to the EtOAC extract in ABTS (81.7% inhibition), DPPH (80.99%), and FRAP (19.52 μM/mL), suggesting that high-polarity solvents are more conducive to the solubilization of its antioxidant components [46]. Similarly, the ethanolic extracts of C. serpyllifolium flower excelled in DPPH radical scavenging (92.14%) and FRAP reducing power (3.138 ± 0.08 mg TE/g). However, its aqueous extract was less active in ABTS and CUPRAC tests, which may be related to the extraction efficiency of polar solvents for phenolic compounds [7]. The methanolic extract of C. vulgare demonstrated broad-spectrum antioxidant activity, with CUPRAC and FRAP values of 44.32 and 87.25 mg TE/g, respectively. The CV3 fractions, which were separated by reversed-phase chromatography, showed an extremely strong free radical scavenging activity, with an IC50 value of 0.02 mg·mL−1 in the DPPH test and 0.0002 mg·mL−1 in the ABTS test. This suggests that methanol is a more suitable solvent for extracting highly active polyphenols [99,100].
In addition, the polyphenol content and antioxidant strength are influenced by the growing environment, collection time, extraction method, and cultivation method [77,121]. The EOs of wild Satureja calamintha (EOSS) showed higher activity in both the DPPH and FRAP assays than that of the cultivated variety (EOSD), possibly due to variations in the accumulation of secondary metabolites [97]. C. bolivianum extracts produced using various extraction methods revealed the technical benefits of ultrasound-assisted extraction. This method increased the total phenolic content by up to 182.2 mg GAE/g and the antioxidant capacity by up to 1470.0 µmol TE/g, which is an 83% increase in efficiency compared to the traditional methods [95]. Notably, the species-specific difference is reflected in the fact that the butanol extract of C. nepeta exceeded the α-tocopherol activity twofold in the FRAP test (A0.5 = 17.42 vs. 34.93 µg·mL−1) [92]. Furthermore, C. nepeta EO revealed a synergistic effect when combined equimolarly, achieving IC50 values of 10.3 µg·mL−1 in the DPPH test and 8.9 µg·mL−1 in the ABTS test. This surpasses the efficacy of ascorbic acid with an IC50 of 12.4 µg·mL−1 [109]. In contrast, EOs from C. sericeum and C. brownie showed very low antioxidant activity [45,52,69]. These findings systematically reveal the potential of C. nepeta as a natural antioxidant for the development of the genus Clinopodium, while suggesting the key role of germplasm resource screening and extraction process optimization for acquiring its active components [122].
The total phenolic content was shown to be significantly correlated with DPPH (r = −0.974), ABTS (r = −0.944), and FRAP (r = 0.957) activities. For example, C. vulgare aqueous extracts exhibited strong radical scavenging due to the high polyphenol content (174.42 mg GAE/g) [123]. Flavonoids such as quercetin (104) and rutin were found in C. serpyllifolium flower extracts at a high concentration of 214.03 mg QE/g, which was directly correlated with its DPPH activity [98]. The enrichment characteristics of rosemarinic acid (34.21 mg·g−1) and ellagic acid (29.31 mg·g−1), as well as protocatechuic acid and chlorogenic acid, are of particular interest, as they may be the key material basis for its significant lipid antioxidant protection [99,101]. In conclusion, the antioxidant capacity of the genus Clinopodium is dominated by polyphenols and flavonoids, and the characteristic compounds (e.g., rosemarinic acid) play a key role.
3.7. Insecticidal Effect
EOs from the genus Clinopodium exhibit significant insecticidal activity. C. nubigenum EO demonstrated remarkably inhibited oviposition of Lavandula sericata at 0.8 μL·cm–2 for 3 h, and oviposition inhibition of C. nubigenum EO was 89.5% after 24 h, better than that of Lavandula angustifolia Mill, a well-known medicinal plant. C. nubigenum EO showed potent toxicity with an LC50 value of 0.07 μL·cm–2 against the eggs of L. sericata by contact/fumigation, and LD50 value of 0.278 μL per individual by topical application on the adults. It is suggested that regarding C. nubigenum EO on acetylcholine esterase of L. sericata, with its IC50 value of 67.450 mg·L–1, the toxicity target of C. nubigenum EO is neural sites [40].
EO from C. chinense displayed both fumigant toxicity (LC50 = 423.39 μg·L−1 against Liposcelis bostrychophila) and contact toxicity (LC₅₀ = 215.25 μg·cm−2). Its major components, bornyl acetate and piperitone, showed enhanced activity with fumigant LC₅₀ values of 351.69 and 311.12 μg·L−1, respectively, and contact LC₅₀ values of 321.42 and 139.74 μg·cm−2, respectively [48]. C. menthifolium EO showed moderate contact toxicity against Tribolium confusum, resulting in 27.5–32.5% mortality at a 5% concentration [55].
Acaricidal activity was observed for C. nepeta (52%) and C. sylvatica (60%) at 2 mg·mL−1, with no apparent toxicity to honeybees [39]. Cultivation methods influenced chemical composition, increasing menthol content while decreasing pulegone content. Wild and cultivated plant oils exhibited strong insecticidal activity against stored-product pests (Tribolium confusum, Rhyzopertha dominica, Sitophilus oryzae), with LD₅₀ values of 0.004–0.011 μL·cm−2 (contact) and 1.988–10.817 μL·L−1 (fumigation) [38]. Notably, S. calamintha EO showed sex-dependent toxicity against Callosobruchus maculatus (100% male mortality vs. 86.66% female mortality at LC₅₀ = 2.17 μL·L−1) and strong repellent activity (91.67% repellency) [49]. Therefore, EOs of the genus Clinopodium have the potential to be developed as natural insecticides or fumigants for controlling insects in stored grains.
3.8. Others
In addition to the above mentioned compounds, luteolin (80), eriodictyol (94), ethyl rosmarinate (116), and clinopodic acid B (117), which are found in C. chinense, are potential lead compounds for antidiabetic drugs due to their α-glucosidase inhibitory activity with IC50 values ranging from 0.6 to 2.0 μM [25]. Moreover, aqueous extracts of Calamintha officinalis exhibited antidiabetic and hypolipidemic effects, indicating that these activities are primarily mediated by hydrophilic constituents [124]. C. vulgare extracts alleviated the scopolamine-induced downregulation of p-CREB/BDNF signaling, exhibit recognition memory retention and acetylcholinesterase inhibitory activity [125]. Beyond medicinal applications, EO from Calamintha leaves has also displayed anticorrosive potential for protecting mild steel, highlighting its industrial relevance [126].
4. Quality Evaluation
The Chinese Pharmacopoeia has always used buddlejasaponin IVb (21) as a reference compound. It was first developed in chloroform–methanol–glacial acetic acid–water (7:2.5:1:0.5), then developed with 10% sulfuric acid ethanol solution, and inspected under sunlight and ultraviolet light with a wavelength of 365 nm. However, the Pharmacopoeia still lacks method to determine the content and evaluate the quality of C. chinense. Quality control of the genus Clinopodium reported in the references is mainly for C. chinense and C. gracile, with the active components, flavonoids and saponins, as the predominant quantitative criteria. The HPLC characteristic spectrum of C. chinense was established with the following reference compounds: buddleoside (81), didymin (96), naringenin (93), apigenin (79), isosakuranetin (95), acacetin (91), and buddlejasaponin IVb (21). The contents of 95 and 96 (wavelength at 290 nm) and 81 (wavelength at 330 nm) were 4.19–12.14, 1.65–2.87, and 0.90–5.93 mg·g–1, respectively [127]. Qi et al. established a fingerprint spectrum of C. chinense extract using UPLC-Q-TOF-MS and identified 25 common peaks. They identified seven major components with anti-AUB activity, including compounds 21, 79, 93, hesperidin (97), kaempferol (103), quercetin (104), and saikosaponin a (46) [71].
In the thin-layer chromatography of C. gracile, a spot of the same color appeared at the corresponding position in the chromatogram of saikosaponin a (46) as the reference compound. The average moisture content of C. gracile was determined to be 10.10%, the total ash content to be 9.73%, the acid-insoluble ash content to be 1.06%, and the dilute ethanol extract to be 23.54%. The average rosmarinic acid content was determined to be 0.56% [128].
5. Toxicity
The acute and sub-acute toxicity of C. vulgare lyophilized aqueous extract (CVE) was demonstrated by intraperitoneal injection (i.p.), resulting in death due to difficulty breathing in mice and rats. The LD50 values were 675 mg·kg–1 (mice) and 500 mg·kg–1 (rats), respectively. These results indicate that acute intraperitoneal injection resulted in central nervous system toxic effects. In contrast, the LD50 for oral administration was higher than 2000 mg·kg–1 in both mice and rats. Furthermore, oral administration of CVE did not produce any toxic effects on hematology, blood and urine biochemistry, or histomorphometry of the pancreas, liver, spleen, or kidneys. This indicates that the flavonoids in the meso-extract, caffeic acid oligomers, saponins, and rosemarinic acid, which are the major compounds in the meso-extract, did not cause any hematological, biochemical, or histopathological changes [75]. Oral toxicity tests also showed that the LD50 of C. nepeta EO was 2500 mg/kg in Wistar rats [56] and 1500 mg/kg in Swiss mice [58].
Similarly, the total flavonoids in C. chinense, as the major active constituents, did not cause toxicity or death at the maximum oral doses of 4000 mg/kg in rats and 5000 mg/kg in mice, respectively. Moreover, no changes in food intake, water intake, body weight, chemical and hematological parameters, organ weights, gross pathology or histopathology were observed in rats fed continuously by gavage for four weeks [129]. The toxicity experiments provide a practical guide for selecting safe doses of C. vulgare, C. nepeta, and C. chinense for further studies in animal research or clinical trials.
6. Pharmacokinetics
As a traditional Chinese herbal medicine, C. chinense has been used to treat various gynecological bleeding disorders. However, the in vivo metabolism of its complex chemical components still needs to be analyzed in depth. Li et al. developed an LC-MS/MS method that simultaneously quantified five components in rat plasma, including buddlejasaponin IVb (21), saikosaponin a (46), apigenin (79), acacetin 7-O-glucuronide (87), and didymin (96). The lowest limits of quantification (LLOQs) were 5.3, 10.5, 1.85, 1, and 4.7 ng·mL–1, respectively. This method is simple, rapid, and stable. It also lays a methodological foundation for the systematic development of multi-component pharmacokinetic studies of C. chinense extracts [130]. It is worth noting that the existing studies have only monitored specific polar components, while the metabolic profiles of possible nonpolar-soluble components (e.g., sterols and volatile oils) and bound components of C. chinense remain unknown. This may lead to a biased perception of its overall pharmacokinetic behavior.
Changes in the expression and activity of drug metabolizing enzymes (DMEs) are key factors affecting the disposition of herbal components in vivo. A recent study showed that the ethanolic extract of Calamintha incana, a herb commonly used in the Middle East, significantly increased the hepatic level of the CYP3A11 gene—more than 10-fold—in mice after one month of low-dose intervention. Neither high nor low doses induced hepatic histopathological damage [131]. These results suggest that C. incana may mildly activate the CYP3A subfamily of enzymes via specific constituents, thereby affecting the metabolic clearance rate of its own or co-administered drugs. In addition, the expression of other CYP isoforms, such as CYP2C29, CYP2D9, and CYP1A1, did not change significantly, suggesting that C. incana selectively regulates metabolizing enzymes, which is consistent with the action characteristics of traditional Chinese medicine: “multi-component—precise target”.
7. Conclusions and Perspectives
This paper summarizes the latest findings on the chemical composition, pharmacological activity, quality evaluation, toxicity, and pharmacokinetics of the genus Clinopodium over the past decade. The active constituents include terpenoids and their saponins, flavonoids, and phenylpropanoids. Of these, 78 triterpenoids were identified, 36 of which were new. In addition, numerous studies on the chemical constituents of EOs and their antioxidant and antimicrobial activities have been reported. These findings suggest that terpenoids and EOs from different species types are two hot topics in the research of the genus Clinopodium. So far, most studies have focused on the important role of the genus Clinopodium in hemostasis, cardiovascular and myocardial cell protection, and anti-inflammatory analgesia, as well as on its antimicrobial, antitumor, and antioxidant properties; however, the mechanism of their biological activity has not been fully elucidated. The EOs of the genus Clinopodium also have fumigation toxicity against insects. However, there are no references proving their toxicity and side effects on humans. Thus, further research is needed to evaluate these effects. In addition, although the flavonoids of the genus Clinopodium have been reported in pharmacokinetic studies, pharmacokinetic and pharmacodynamic analyses of other components such as terpenoids need to be further understood. The various functions of the bioactive compounds of the genus Clinopodium are crucial for its drug development, especially for the application of hemostatic drugs.
Author Contributions
Conceptualization and methodology, X.O.; validation, W.L., J.P. and X.C.; investigation, W.L.; data curation, W.L., J.P. and X.C.; Formal analysis, X.C.; writing—original draft preparation, W.L.; funding acquisition, W.L., writing—review and editing, X.O.; visualization, S.G.; supervision, X.O.; project administration, X.O. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Ganzhou Municipal Key Research and Development Program, grant number 2023PNS27159.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the authors.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Kaya, A. Comparative root and stem anatomy of six Clinopodium (Lamiaceae) taxa. Biologia 2016, 71, 1330–1337. [Google Scholar] [CrossRef]
- Bräuchler, C. And now for something completely different-new names in Clinopodium with comments on some types. Phytotaxa 2018, 356, 71–80. [Google Scholar] [CrossRef]
- Clinopodium, L. Plants of the World Online|Kew Science. Available online: https://powo.science.kew.org/taxon/urn:lsid:ipni.org:names:30008690-2 (accessed on 15 May 2025).
- Qureshi, K.A.; Parvez, A.; Uzzaman Khan, M.M.; Aspatwar, A.; Atiya, A.; Elhassan, G.O.; Khan, R.A.; Erattil Ahammed, S.Y.; Khan, W.U.; Jaremko, M. Exploring nature’s hidden treasure: Unraveling the untapped phytochemical and pharmacological potentials of Clinopodium vulgare L.—A hidden gem in the Lamiaceae family. Heliyon 2024, 10, e24781. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.; Li-Min, L.; Yuan-Gen, X.; Xue-Bin, S.; Huan, L.; Si-Hui, N. Research progress on genus Clinopodium. China J. Chin. Mater. Med. 2020, 45, 4349–4357. [Google Scholar]
- Dobrev, H.P. Treatment of numerous hand warts with Clinopodium vulgare tea. Wien. Med. Wochenschr. 2021, 171, 82–83. [Google Scholar] [CrossRef]
- Zhong, M.; Sun, G.; Zhang, X.; Sun, G.; Xu, X.; Yu, S. A new prenylated naphthoquinoid from the aerial parts of Clinopodium chinense (Benth.) O. Kuntze. Molecules 2012, 17, 13910–13916. [Google Scholar] [CrossRef]
- Zhu, Y.-D.; Hong, J.-Y.; Bao, F.-D.; Xing, N.; Wang, L.-T.; Sun, Z.-H.; Luo, Y.; Jiang, H.; Xu, X.-D.; Zhu, N.-L.; et al. Triterpenoid saponins from Clinopodium chinense (Benth.) O. Kuntze and their biological activity. Arch. Pharmacal Res. 2018, 41, 1117–1130. [Google Scholar] [CrossRef]
- Hu, Y.-X.; Zhang, W.; Zhang, W.; Zhu, Y.-D.; Ma, G.-X.; Zhu, N.-L.; Sun, W.; Ma, Z.-X.; Yu, S.-C.; Xu, X.-D. Oleanane triterpene saponins with cardioprotective activity from Clinopodium polycephalum. J. Asian Nat. Prod. Res. 2017, 19, 697–703. [Google Scholar] [CrossRef]
- Zhao, T.; Liu, Y.N.; Sun, X.Y.; Li, J.Y.; Lei, C.; Hou, A.J. Two new oleanane-triterpenoid saponins from Clinopodium gracile. Chem. Biodivers. 2021, 18, e2100672. [Google Scholar] [CrossRef]
- Zeng, B.; Liu, G.-D.; Zhang, B.-B.; Wang, S.-S.; Ma, R.; Zhong, B.-S.; He, B.-Q.; Liang, Y.; Wu, F.-H. A new triterpenoid saponin from Clinopodium chinense (Benth.) O. Kuntze. Nat. Prod. Res. 2016, 30, 1001–1008. [Google Scholar] [CrossRef]
- Xu, M.-J.; Wu, Y.-W.; Zhao, D.; Xu, Y.-Y.; Pan, Q.-L.; Zhao, T.-T.; Zhou, W.-S.; Yuan, Y.-Y.; Xu, T.-H.; Zhu, Y.-D. Two new oleanane-type triterpenoid saponins from aerial part of Clinopodium chinense. Mod. Chin. Med. 2022, 24, 1447–1455. [Google Scholar]
- Qu, L.-X.; Liu, Y.-Q.; Wang, Y.; Wang, H.; Huang, X.-L.; Zhang, M.-L.; Mou, Y.-X.; Xu, T.-H.; Zhu, Y.-D. Diterpenoid and triterpenoid glycosides from Clinopodium chinense. Nat. Prod. Res. 2021, 35, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.-D.; Zhang, J.-Y.; Li, P.-F.; Wu, H.-F.; Zhu, N.-L.; Jiang, H.; Lv, C.-Y.; Wu, L.-L.; Ma, Z.-X.; Xu, X.-D.; et al. Two new abietane diterpenoid glycosides from Clinopodium chinense. Nat. Prod. Res. 2016, 30, 1075–1080. [Google Scholar] [CrossRef]
- Ticona, L.A.; Noguerón, A.M.; Sánchez-Corral, J.S.; Lozano, N.M.; Domenech, M.O. Anti-inflammatory, antibacterial, anti-biofilm, and anti-quorum sensing activities of the diterpenes isolated from Clinopodium bolivianum. Pharmaceutics 2024, 16, 1094. [Google Scholar] [CrossRef]
- Wang, Y.; He, L.; Li, L.-Y.; Xu, M.-J.; Wu, Y.-W.; Li, Y.-S.; Chu, X.-Y.; Pan, Q.; Zhao, T.-T.; Ye, X.-X.; et al. A new triterpenoid saponin from the aerial parts of Clinopodium chinense. Mod. Chin. Med. 2020, 22, 1596–1606. [Google Scholar]
- Kim, D.; Lee, S.K.; Park, K.-S.; Park, H.-J. Isolation of the constituents from Clinopodium chinense var. shibetchense and inhibition activity on cancer cell growth and nitric oxide production. Korean J. Pharmacogn. 2020, 51, 93–99. [Google Scholar]
- Fathiazad, F.; Kaboudi, N.; Esfahanizadeh, M.; Hamedeyazdan, S. Oleanane-type triterpenoid saponins and rosmarinic acid from Clinopodium umbrosum. Iran. J. Chem. Chem. Eng. 2023, 42, 2211–2220. [Google Scholar]
- Tao, M.K.; Liu, Y.X.; Zhou, X.M.; Yu, X.L.; Zhang, Y.; Guo, L.; Meng, D.L. Polycephalums A and B, New anticoagulant constituents isolated from Clinopodium polycephalum. Chem. Biodivers. 2023, 20, e202300448. [Google Scholar] [CrossRef]
- Zhu, Y.D.; Wu, H.F.; Ma, G.X.; Chen, R.C.; Long, H.L.; Zuo, Z.L.; Luo, Y.; Zhu, N.L.; Hou, B.; Xu, X.D.; et al. Clinoposides A-F: Meroterpenoids with protective effects on H9c2 cardiomyocyte from Clinopodium chinense. RSC Adv. 2016, 6, 7260–7266. [Google Scholar] [CrossRef]
- Zhu, Y.-D.; Chen, R.-C.; Wang, H.; Jiang, H.; Huang, X.-L.; Zhang, M.-L.; Li, L.-Y.; Hu, Z.; Xu, X.-D.; Wang, C.-J. Two new flavonoid–triterpene saponin meroterpenoids from Clinopodium chinense and their protective effects against anoxia/reoxygenation-induced apoptosis in H9c2 cells. Fitoterapia 2018, 128, 180–186. [Google Scholar] [CrossRef]
- Liu, Y.; Song, H.; Xu, J.; Bi, G.; Meng, D. Anti-inflammatory abietanes diterpenes and triterpenoids isolated from Clinopodium polycephalum. Fitoterapia 2022, 161, 105244. [Google Scholar] [CrossRef] [PubMed]
- Shen, M.-Y.; Zheng, C.-W.; Hao, Z.-J.; Song, H.-J.; Wang, Y.-M.; Meng, D.-L. Undescribed compounds from Clinopodium chinense (Benth.) O. Kuntze and their coagulation activity studies. Fitoterapia 2024, 172, 105736. [Google Scholar] [CrossRef]
- Zhao, D.; Wu, Y.-W.; Xu, Y.-Y.; Fan, J.-P.; Zhang, Y.-H.; Yang, R.-Y.; Wang, H.; Wu, L.-L.; Xu, T.-H.; Zhu, Y.-D. A new monoterpenoid from Clinopodium chinense (Benth.) O. Kuntze. Mod. Chin. Med. 2023, 25, 1918–1924. [Google Scholar]
- Zeng, B.; Chen, K.; Du, P.; Wang, S.S.; Ren, B.; Ren, Y.L.; Yan, H.S.; Liang, Y.; Wu, F.H. Phenolic compounds from Clinopodium chinense (Benth.) O. Kuntze and their inhibitory effects on α-glucosidase and vascular endothelial cells injury. Chem. Biodivers. 2016, 13, 596–601. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.-T.; Sun, Z.-H.; Zhong, M.-L.; Wu, H.-F.; Zhang, H.-J.; Zhu, N.-L.; Sun, G.-B.; Ye, X.-X.; Xu, X.-D.; Zhu, Y.-D.; et al. Studies on chemical constituents of Clinopodium chinense. China J. Chin. Mater. Med. 2017, 42, 2510–2517. [Google Scholar]
- Yu, Y.; Xing, N.; Xu, X.; Zhu, Y.; Wang, S.; Sun, G.; Sun, X. Tournefolic acid B, derived from Clinopodium chinense (Benth.) Kuntze, protects against myocardial ischemia/reperfusion injury by inhibiting endoplasmic reticulum stress-regulated apoptosis via PI3K/AKT pathways. Phytomedicine 2019, 52, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Gezici, S.; Turkmen, M.; Karahan, F. Exploring the anti-cancer properties of essential oils from some Lamiaceae species against human cancer cells with multivariate analysis. S. Afr. J. Bot. 2024, 166, 287–296. [Google Scholar] [CrossRef]
- Khodja, N.K.; Boulekbache, L.; Chegdani, F.; Dahmani, K.; Bennis, F.; Madani, K. Chemical composition and antioxidant activity of phenolic compounds and essential oils from Calamintha nepeta L. J. Complement. Integr. Med. 2018, 15, 20170080. [Google Scholar] [CrossRef]
- Milenković, M.; Stošović, J.; Slavkovska, V. Synergy between essential oils of Calamintha species (Lamiaceae) and antibiotics. Nat. Prod. Commun. 2018, 13, 371–374. [Google Scholar] [CrossRef]
- Kremer, D.; Dunkic, V.; Radosavljevic, I.; Bogunic, F.; Ivanova, D.; Ballian, D.; Stesevic, D.; Matevski, V.; Randelovic, V.; Eleftheriadou, E.; et al. Phytochemicals and their correlation with Molecular Data in Micromeria and Clinopodium (Lamiaceae) Taxa. Plants 2022, 11, 3407. [Google Scholar] [CrossRef]
- Božović, M.; Garzoli, S.; Sabatino, M.; Pepi, F.; Baldisserotto, A.; Andreotti, E.; Romagnoli, C.; Mai, A.; Manfredini, S.; Ragno, R. Essential oil extraction, chemical analysis and anti-candida activity of Calamintha nepeta (L.) Savi subsp. glandulosa (Req.) Ball—New approaches. Molecules 2017, 22, 203. [Google Scholar]
- Debbabi, H.; Mokni, R.E.; Chaieb, I.; Nardoni, S.; Maggi, F.; Caprioli, G.; Hammami, S. Chemical composition, antifungal and insecticidal activities of the essential oils from Tunisian Clinopodium nepeta Subsp. nepeta and Clinopodium nepeta Subsp. glandulosum. Molecules 2020, 25, 2137. [Google Scholar] [PubMed]
- Vlachou, G.; Papafotiou, M.; Daferera, D.J.; Tarantilis, P.A. Yield and composition of the essential oil of Clinopodium nepeta subsp. spruneri as affected by harvest season and cultivation method, i.e., outdoor, greenhouse and in vitro culture. Plants 2023, 12, 4098. [Google Scholar]
- Hayani, M.; Benabbouha, T.; Nachit, W.; Byadi, S.; Chefira, K.; Aboulmouhajir, A.; Tounsi, A.; Zair, T. Unveiling the potential of Satureja calamintha nepeta: A study on its phytochemical composition and antibacterial activity. Vietnam J. Chem. 2024, 62, 772–779. [Google Scholar] [CrossRef]
- Debbabi, H.; El Mokni, R.; Majdoub, S.; Aliev, A.; Hammami, S. The effect of pressure on the characteristics of supercritical carbon dioxide extracts from Calamintha nepeta subsp. nepeta. Biomed. Chromatogr. 2020, 34, e4871. [Google Scholar] [CrossRef] [PubMed]
- Arze, J.B.L.; Collin, G.; Jean, F.-I.; Gagnon, H. Essential oils from Bolivia. xiv. Lamiaceae: Clinopodium axillare. Am. J. Essent. Oils Nat. Prod. 2019, 7, 1–5. [Google Scholar]
- Abbad, I.; Soulaimani, B.; Abbad, A. Chemical composition, insecticidal and allelopathic properties of essential oils obtained from wild and cultivated Moroccan Satureja calamintha (L.). J. Nat. Pestic. Res. 2023, 3, 100021. [Google Scholar] [CrossRef]
- Bava, R.; Castagna, F.; Lupia, C.; Ruga, S.; Conforti, F.; Marrelli, M.; Argentieri, M.P.; Musella, V.; Britti, D.; Statti, G.; et al. Phytochemical Composition and Pharmacological Efficacy Evaluation of Calamintha nepeta, Calamintha sylvatica, Lavandula austroapennina and Mentha piperita Essential Oils for the Control of Honeybee (Apis mellifera) Varroosis. Animals 2024, 14, 69. [Google Scholar] [CrossRef]
- Bedini, S.; Flamini, G.; Cosci, F.; Ascrizzi, R.; Echeverria, M.C.; Gomez, E.V.; Guidi, L.; Landi, M.; Lucchi, A.; Conti, B. Toxicity and oviposition deterrence of essential oils of Clinopodium nubigenum and Lavandula angustifolia against the myiasis-inducing blowfly Lucilia sericata. PLoS ONE 2019, 14, e0212576. [Google Scholar] [CrossRef]
- Tomas, J.; Gil, L.; Llorens-Molina, J.; Cardona, C.; García, M.; Llorens, L. Biogenic volatiles of rupicolous plants act as direct defenses against molluscs: The case of the endangered Clinopodium rouyanum. Flora 2019, 258, 151428. [Google Scholar] [CrossRef]
- Dekić, M.; Radulović, N.; Antonijević, M.; Dekić, D.; Ličina, B. The essential oil of the condiment species Clinopodium thymifolium (Scop.) Kuntze: New natural products and seasonal variation. J. Sci. Food Agric. 2022, 102, 2437–2444. [Google Scholar] [CrossRef]
- Novakovic, M.; Bukvicki, D.; Vajs, V.; Tesevic, V.; Milosavljevic, S.; Marin, P.; Asakawa, Y. Microbial transformation of Calamintha glandulosa essential oil by Aspergillus Niger. Nat. Prod. Commun. 2018, 13, 479–482. [Google Scholar] [CrossRef]
- Merma Ccana, C.; Tomaylla Cruz, C.; del Carpio Jiménez, C. Anti-Trichophyton rubrum activity of the essential oil of Clinopodium brevicalyx and elaboration of a topical emulsion. J. High Andean Res. 2020, 22, 182–190. [Google Scholar]
- Noriega, P.; Calderón, L.; Ojeda, A.; Paredes, E. Chemical composition, antimicrobial and antioxidant bioautography activity of essential oil from leaves of Amazon plant Clinopodium brownei (Sw.). Molecules 2023, 28, 1741. [Google Scholar] [CrossRef]
- Seraoui, R.; Benkiniouar, R.; Akkal, S.; Ros, G.; Nieto, G. Phytochemical investigation, antioxidant and antimicrobial assays of Algerian Plant Calamintha baborensis Batt. Pharm. Chem. J. 2018, 52, 347–356. [Google Scholar] [CrossRef]
- Hamdi, B.; Peron, G.; Miara, M.D.; Bouriah, N.; Flamini, G.; Maggi, F.; Sut, S.; Dall’Acqua, S. Phytochemical analysis of Clinopodium candidissimum (Munby) Kuntze growing in Algeria by an integrated HS-SPME-GC-MS, NMR and HPLC-DAD-MSn approach: Valorisation of an endemic natural source of bioactive compounds. Nat. Prod. Res. 2024, 38, 140–145. [Google Scholar] [CrossRef] [PubMed]
- Li, H.-Y.; Liu, X.-C.; Chen, X.-B.; Liu, Q.-Z.; Liu, Z.-L. Chemical composition and insecticidal activities of the essential oil of Clinopodium chinense (Benth.) Kuntze aerial parts against Liposcelis bostrychophila Badonnel. J. Food Prot. 2015, 78, 1870–1874. [Google Scholar] [CrossRef]
- Baghouz, A.; Bouchelta, Y.; Es-safi, I.; El Brahimi, R.; Imtara, H.; AlZain, M.N.; Noman, O.M.; Shahat, A.A.; Guemmouh, R. Biocidal activity of Ziziphora hispanica L and Satureja calamintha Scheele L essential oils against the Callosobruchus maculatus (Fabricius) pest on cowpea seeds during storage. Front. Sustain. Food Sys. 2024, 8, 1329100. [Google Scholar] [CrossRef]
- Boudjema, K.; Bouanane, A.; Gamgani, S.; Djeziri, M.; Abou Mustapha, M.; Fazouane, F. Phytochemical profile and antimicrobial properties of volatile compounds of Satureja calamintha (L) Scheel from northern Algeria. Trop. J. Pharm. Res. 2018, 17, 857–864. [Google Scholar] [CrossRef]
- Jaradat, N.; Al-lahham, S.; Abualhasan, M.N.; Ghannam, D.; Mousa, K.; Kolayb, H.; Hussein, F.; Issa, L.; Mousa, A. Chemical fingerprinting, anticancer, anti-inflammatory and free radical scavenging properties of Calamintha fenzlii Vis. volatile oil from Palestine. Arab. J. Sci. Eng. 2020, 45, 63–70. [Google Scholar] [CrossRef]
- Popović-Djordjević, J.; Cengiz, M.; Ozer, M.S.; Sarikurkcu, C. Calamintha incana: Essential oil composition and biological activity. Ind. Crop. Prod. 2019, 128, 162–166. [Google Scholar] [CrossRef]
- Althaher, A.R.; Oran, S.A.; Bustanji, Y.K. Chemical composition, in vitro evaluation of antioxidant properties and cytotoxic activity of the essential oil from Calamintha incana (Sm.) Helder (Lamiaceae). Trop. J. Nat. Prod. Res. 2021, 5, 1333–1339. [Google Scholar]
- Villa-Ruano, N.; Pacheco-Hernández, Y.; Cruz-Durán, R.; Lozoya-Gloria, E. Volatiles and seasonal variation of the essential oil composition from the leaves of Clinopodium macrostemum var. laevigatum and its biological activities. Ind. Crop. Prod. 2015, 77, 741–747. [Google Scholar] [CrossRef]
- Debbabi, H.; El Mokni, R.; Nardoni, S.; Chaieb, I.; Maggi, F.; Nzekoue, F.K.; Caprioli, G.; Hammami, S. Chemical diversity and biological activities of essential oils from native populations of Clinopodium menthifolium subsp. ascendens (Jord.) Govaerts. Environ. Sci. Pollut. Res. 2021, 28, 13624–13633. [Google Scholar] [CrossRef] [PubMed]
- Bahri, F.; Benhassaini, H.; Szumny, A.; Bentaiba, K. Chemical composition and pharmacological activities of Calamintha nepeta essential oil. Trop. J. Nat. Prod. Res. 2024, 8, 7097–7105. [Google Scholar]
- Ambrico, A.; Trupo, M.; Martino, M.; Sharma, N. Essential oil of Calamintha nepeta (L.) Savi subsp. nepeta is a potential control agent for some postharvest fruit diseases. Org. Agric. 2020, 10, 35–48. [Google Scholar]
- Arantes, S.M.; Piçarra, A.; Guerreiro, M.; Salvador, C.; Candeias, F.; Caldeira, A.T.; Martins, M.R. Toxicological and pharmacological properties of essential oils of Calamintha nepeta, Origanum virens and Thymus mastichina of Alentejo (Portugal). Food Chem. Toxicol. 2019, 133, 110747. [Google Scholar] [CrossRef]
- Zeynalova, S.A. Chemical composition of the essential oil from Calamintha nepeta (L.) Savi plants growing in the flora Azerbaijan. Plant Fungal Res. 2018, 1, 62–68. [Google Scholar] [CrossRef]
- Benchaa, S.; Hazzit, M.; Zermane, N.; Abdelkrim, H. Chemical composition and herbicidal activity of essential oils from two Labiatae species from Algeria. J. Essent. Oil Res. 2019, 31, 335–346. [Google Scholar] [CrossRef]
- Medjdoub, A.R.; Benmehdi, H.; Oukali, Z. Chemical composition and antifungal activity of essential oil of Satureja calamintha spp. nepeta (L.) Briq against some toxinogenous mold. Nat. Volatiles Essent. Oils 2022, 9, 1981–2000. [Google Scholar]
- Öztürk, G.; Yilmaz, G.; Gülnur, E.; Demirci, B. Chemical composition and antibacterial activity of Clinopodium nepeta subsp. glandulosum (Req.) Govaerts essential oil. Nat. Volatiles Essent. Oils 2021, 8, 75–80. [Google Scholar]
- Çelik, G.; Kılıç, G.; Kanbolat, Ş.; Özlem Şener, S.; Karaköse, M.; Yaylı, N.; Karaoğlu, Ş.A. Biological activity, and volatile and phenolic compounds from five Lamiaceae species. Flavour Frag. J. 2021, 36, 223–232. [Google Scholar] [CrossRef]
- Boskailo, E.; Dzudzevic-Cancar, H.; Dedic, A.; Marijanovic, Z.; Alispahic, A.; Cancar, I.F.; Vidic, D.; Jerkovic, I. Clinopodium nepeta (L.) Kuntze from Bosnia and Herzegovina: Chemical characterisation of headspace and essential oil of fresh and dried samples. Rec. Nat. Prod. 2023, 17, 300–311. [Google Scholar]
- Shams Moattar, F.; Sariri, R.; Giahi, M.; Yaghmaee, P. Essential oil composition and antioxidant activity of Calamintha officinalis Moench. J. Appl. Biotechnol. Rep. 2018, 5, 55–58. [Google Scholar] [CrossRef]
- Stojičić, D.; Tošić, S.; Stojanović, G.; Zlatković, B.; Jovanović, S.; Budimir, S.; Uzelac, B. Volatile organic compound composition and glandular trichome characteristics of in vitro propagated Clinopodium pulegium (Rochel) Bräuchler: Effect of carbon source. Plants 2022, 11, 198. [Google Scholar] [CrossRef] [PubMed]
- Ali-Shtayeh, M.S.; Jamous, R.M.; Abu-Zaitoun, S.Y.; Akkawi, R.J.; Kalbouneh, S.R.; Bernstein, N.; Dudai, N. Chemical profile and bioactive properties of the essential oil isolated from Clinopodium serpyllifolium (M.Bieb.) Kuntze growing in Palestine. Ind. Crop. Prod. 2018, 124, 617–625. [Google Scholar] [CrossRef]
- Sharma, R.; Shachter, A.; Almog, L.; Oren, G.; Roynik-Toshner, H.; Dudai, N. Genetic chemical variability of the volatiles and phenolic compounds in Clinopodium serpyllifolium subsp. fruticosum (L.) Bräuchler syn. Micromeria fruticosa (L.) Druce grown in Israel. Israel J. Plant Sci. 2021, 68, 142–150. [Google Scholar]
- Benites, J.; Ríos, D.; Guerrero-Castilla, A.; Enríquez, C.; Zavala, E.; Ybañez-Julca, R.O.; Quispe-Díaz, I.; Jara-Aguilar, R.; Calderon, P.B. Chemical composition and assessment of antimicrobial, antioxidant and antiproliferative activities of essential oil from Clinopodium sericeum, a Peruvian medicinal plant. Rec. Nat. Prod. 2021, 15, 175–186. [Google Scholar]
- Espinosa, S.; Bec, N.; Larroque, C.; Ramírez, J.; Sgorbini, B.; Bicchi, C.; Cumbicus, N.; Gilardoni, G. A novel chemical profile of a selective In vitro cholinergic essential oil from Clinopodium taxifolium (Kunth) Govaerts (Lamiaceae), a native Andean species of Ecuador. Molecules 2021, 26, 45. [Google Scholar] [CrossRef]
- Qi, J.-J.; Zhang, Q.-Q.; Li, L.-L.; Huang, Q.; Yao, M.; Wang, N.; Peng, D.-Y. Spectrum-effect relationship between UPLC-Q-TOF-MS fingerprint and anti-AUB effect of Clinopodium chinense (Benth.) O. Kuntze. J. Pharm. Biomed. Anal. 2022, 217, 114828. [Google Scholar] [CrossRef]
- Althaher, A.R.; Mastinu, A. Calamintha incana (Sm.) Helder: A New Phytoextract with In Vitro Antioxidant and Antidiabetic Action. Appl. Sci. 2023, 13, 3966. [Google Scholar] [CrossRef]
- Althaher, A.R.; Shehabi, R.F.; Ameen, H.H.; Awadallah, M.W.; Mastinu, A.; Proestos, C. Calamintha incana methanolic extract: Investigation of phytochemical composition and antioxidant and antibacterial activities. J. Food Biochem. 2024, 2024, 6634969. [Google Scholar] [CrossRef]
- Bardarov, K.; Todorova, T.; Miteva, D.; Bardarov, V.; Atanassov, A.; Chankova, S. Preliminary screening for study of the chemical composition of Clinopodium vulgare L. water extract. Comptes Rendus Acad. Bulg. Sci. 2016, 69, 717–724. [Google Scholar]
- Zheleva-Dimitrova, D.; Simeonova, R.; Gevrenova, R.; Savov, Y.; Balabanova, V.; Nasar-Eddin, G.; Bardarov, K.; Danchev, N. In vivo toxicity assessment of Clinopodium vulgare L. water extract characterized by UHPLC-HRMS. Food Chem. Toxicol. 2019, 134, 110841. [Google Scholar] [CrossRef]
- Yumruta, N.; Pehlivan, M.; Yumruta, P.; Kahraman, D. Possible induction of apoptotic and necrotic death pathways in lung cancer cells by Clinopodium vulgare L. and phenolic acids and flavonoids detection by LC-MS-MS. J. Boletín Latinoam. Caribe Plantas Med. Aromáticas 2024, 23, 740. [Google Scholar] [CrossRef]
- Pacifico, S.; Galasso, S.; Piccolella, S.; Kretschmer, N.; Pan, S.-P.; Marciano, S.; Bauer, R.; Monaco, P. Seasonal variation in phenolic composition and antioxidant and anti-inflammatory activities of Calamintha nepeta (L.) Savi. Food Res. Int. 2015, 69, 121–132. [Google Scholar] [CrossRef]
- Bai, S.-Y.; Li, M.-T.; Cao, Z.-Q.; Shan, S.; Wu, J.-B.; He, N. Evaluation in vitro and vivo of hemostatic properties of extract from Chinese Clinopodium herb. J. Shanxi Med. Univ. 2022, 53, 1590–1593. [Google Scholar]
- Li, L.-L.; Huang, Q.; Duan, X.-C.; Han, L.; Peng, D.-Y. Protective effect of Clinopodium chinense (Benth.) O. Kuntze against abnormal uterine bleeding in female rats. J. Pharmacol. Sci. 2020, 143, 1–8. [Google Scholar] [CrossRef]
- Li, L.-L.; Huang, Q.; Peng, D.-Y.; Qi, J.-J.; Yao, M. Anti-inflammatory and hemostatic effects of total extract, saponins, and flavonoids of Clinopodium chinense in female rats with abnormal uterine bleeding and the mechanism. China J. Chin. Mater. Med. 2022, 47, 3372–3379. [Google Scholar]
- Xing, N.; Zhang, H.-J.; Shu, Z.-P.; Xu, X.-D.; Zhu, Y.-D.; Sun, G.-B.; Wang, Q.-H.; Sun, X.-B. Antioxidant effect of different isolated polar fractions in total flavones of Clinopodium chinense. World Chin. Med. 2015, 10, 1169–1172. [Google Scholar]
- Chen, R.-C.; Xu, X.-D.; Liu, X.-Z.; Sun, G.-B.; Zhu, Y.-D.; Dong, X.; Wang, J.; Zhang, H.-J.; Zhang, Q.; Sun, X.-B. Total flavonoids from Clinopodium chinense (Benth.) O. Ktze protect against doxorubicin-induced cardiotoxicity in vitro and in vivo. Evid. Based Complement. Altern. Med. 2015, 2015, 472565. [Google Scholar]
- Zhang, H.-J.; Chen, R.-C.; Sun, G.-B.; Yang, L.-P.; Zhu, Y.-D.; Xu, X.-D.; Sun, X.-B. Protective effects of total flavonoids from Clinopodium chinense (Benth.) O. Ktze on myocardial injury in vivo and in vitro via regulation of Akt/Nrf2/HO-1 pathway. Phytomedicine 2018, 40, 88–97. [Google Scholar] [CrossRef]
- Zhang, N.; Ma, J.; Wu, G.-L. Effects of total flavonoids of Clinopodium chinense combined with miR-702-5p in-hibitor on myocardial cell injury induced by hypoxia / reoxygenation. Pract. Pharm. Clin. Remedies 2021, 24, 23–28. [Google Scholar]
- Tubon, I.; Bernardini, C.; Antognoni, F.; Mandrioli, R.; Potente, G.; Bertocchi, M.; Vaca, G.; Zannoni, A.; Salaroli, R.; Forni, M. Clinopodium tomentosum (Kunth) Govaerts leaf extract influences in vitro cell proliferation and angiogenesis on primary cultures of porcine aortic endothelial cells. Oxidative Med. Cell. Longev. 2020, 2020, 2984613. [Google Scholar] [CrossRef] [PubMed]
- Nasar-Eddin, G.; Simeonova, R.; Zheleva-Dimitrova, D.; Gevrenova, R.; Savov, I.; Bardarov, K.; Danchev, N. Beneficial effects of Clinopodium vulgare water extract on spontaneously hypertensive rats. Bulg. Chem. Commun 2019, 51, 156–160. [Google Scholar]
- Shi, X.-J.; Wang, S.-S.; Luan, H.-L.; Tuerhong, D.; Lin, Y.-N.; Liang, J.-Y.; Xiong, Y.; Rui, L.-Y.; Wu, F.-H. Clinopodium chinense attenuates palmitic acid-induced vascular endothelial inflammation and insulin resistance through TLR4-mediated NF-κ B and MAPK pathways. Am. J. Chin. Med. 2019, 47, 97–117. [Google Scholar] [CrossRef]
- Wang, Y.; Shao, Z.; Song, C.; Zhou, H.; Zhao, J.; Zong, K.; Zhou, G.; Meng, D. Clinopodium chinense Kuntze ameliorates dextran sulfate sodium-induced ulcerative colitis in mice by reducing systematic inflammation and regulating metabolism. J Ethnopharmacol. 2023, 309, 116330. [Google Scholar] [CrossRef]
- Wang, S.-L.; Chen, L.-M.; Jin, T.; Lin, J.-M.; Shi, Y. The effect and mechanism of polyphenolic extract from Lonicera japonica on apoptosis promotion of lung cancer cells in vitro. Pharm. Clin. Chin. Mater. Med. 2018, 34, 93–98. [Google Scholar]
- Mohanty, S.; Kamolvit, W.; Zambrana, S.; Sandström, C.; Gonzales, E.; Östenson, C.-G.; Brauner, A. Extract of Clinopodium bolivianum protects against E. coli invasion of uroepithelial cells. J. Ethnopharmacol. 2017, 198, 214–220. [Google Scholar] [CrossRef]
- D’Aquila, P.; Paparazzo, E.; Crudo, M.; Bonacci, S.; Procopio, A.; Passarino, G.; Bellizzi, D. Antibacterial activity and epigenetic remodeling of essential oils from Calabrian aromatic plants. Nutrients 2022, 14, 391. [Google Scholar] [CrossRef]
- Beddiar, H.; Boudiba, S.; Benahmed, M.; Tamfu, A.N.; Ceylan, O.; Hanini, K.; Kucukaydin, S.; Elomri, A.; Bensouici, C.; Laouer, H.; et al. Chemical composition, anti-quorum sensing, enzyme inhibitory, and antioxidant properties of phenolic extracts of Clinopodium nepeta L. Kuntze. Plants 2021, 10, 1955. [Google Scholar] [CrossRef] [PubMed]
- Bouzidi, N.; Kemieg, M. Antioxidant and antimicrobial activities of essential oil of Satureja calamintha ssp. nepeta (L.) Briq. from the northwest of Algeria. Agric. Conspec. Sci. 2021, 86, 349–356. [Google Scholar]
- Sharifi, S.; Naseri, N.; Fathiazad, F.; Asnaashari, S.; Hamedeyazdan, S. Anticancer effect of buddlejasaponin IV and buddlejasaponin IVa from Clinopodium umbrosum on oral cancer cells (HN-5). Toxicon 2022, 220, 106939. [Google Scholar] [CrossRef]
- Campos, D.; García-Ríos, D.; Aguilar-Galvez, A.; Chirinos, R.; Pedreschi, R. Comparison of conventional and ultrasound-assisted extractions of polyphenols from Inca muña (Clinopodium bolivianum) and their characterization using UPLC–PDA-ESI–Q/TOF–MSn technique. J. Food Process. Preserv. 2022, 46, e16310. [Google Scholar] [CrossRef]
- Hodaj-Çeliku, E.; Tsiftsoglou, O.; Shuka, L.; Abazi, S.; Hadjipavlou-Litina, D.; Lazari, D. Antioxidant activity and chemical composition of essential oils of some aromatic and medicinal plants from Albania. Nat. Prod. Commun. 2017, 12, 785–790. [Google Scholar] [CrossRef] [PubMed]
- El Brahimi, R.; El Barnossi, A.; El Moussaoui, A.; Chebaibi, M.; Kachkoul, R.; Baghouz, A.; Nafidi, H.-A.; Salamatullah, A.M.; Bourhia, M.; Bari, A. Phytochemistry and biological activities of essential oils from Satureja calamintha nepeta. Separations 2023, 10, 344. [Google Scholar] [CrossRef]
- Gezici, S.; Koçum, D.; Yayla, F.; Sekeroglu, N.; Khan, A.A. Screening for in vitro antioxidant activities, polyphenolic contents and neuroprotective potentials of Clinopodium serpyllifolium subsp. serpyllifolium endemic to Turkey. . Ann. Phytomedicine Int. J. 2020, 9, 181–186. [Google Scholar]
- Sarikurkcu, C.; Ozer, M.S.; Tepe, B.; Dilek, E.; Ceylan, O. Phenolic composition, antioxidant and enzyme inhibitory activities of acetone, methanol and water extracts of Clinopodium vulgare L. subsp. vulgare L. Ind. Crop. Prod. 2015, 76, 961–966. [Google Scholar] [CrossRef]
- Nassar-Eddin, G.; Zheleva-Dimitrova, D.; Danchev, N.; Vitanska-Simeonova, R. Antioxidant and enzyme-inhibiting activity of lyophilized extract from Clinopodium vulgare L. (Lamiaceae). Pharmacia 2021, 68, 259–263. [Google Scholar] [CrossRef]
- Bektašević, M.; Politeo, O.; Roje, M.; Jurin, M. Polyphenol composition, anticholinesterase and antioxidant potential of the extracts of Clinopodium vulgare L. Chem. Biodivers. 2022, 19, e202101002. [Google Scholar] [CrossRef]
- Khan, S.; Khan, T.; Shah, A.J. Total phenolic and flavonoid contents and antihypertensive effect of the crude extract and fractions of Calamintha vulgaris. Phytomedicine 2018, 47, 174–183. [Google Scholar] [CrossRef] [PubMed]
- Azzane, A.; Azzaoui, B.; Akdad, M.; Bouadid, I.; Eddouks, M. Effect of calamintha officinalis on vascular contractility and angiotensinconverting enzyme-2. Cardiovasc. Hematol. Agents Med.Chem. 2022, 20, 219–236. [Google Scholar] [PubMed]
- Arantes, S.; Piçarra, A.; Candeias, F.; Teixeira, D.; Caldeira, A.T.; Martins, M.R. Antioxidant activity and cholinesterase inhibition studies of four flavouring herbs from Alentejo. Nat. Prod. Res. 2017, 31, 2183–2187. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-M.; Du, K.-C.; Wang, Q.-Y.; Yang, X.-Y.; Meng, D.-L. A multidimensional strategy for characterization, distinction, and quality control of two Clinopodium medicinal plants. J. Ethnopharmacol. 2024, 327, 118019. [Google Scholar] [CrossRef]
- Zoheir, K.; Bnouham, M.; Legssyer, A.; Ziyyat, A.; Berrabah, M.; Aziz, M.; Bensaid, M.; Mekhfi, H. Antithrombotic, antiaggregant and anticoagulant effect of methanolic fraction of Calamintha officinalis: In vitro and ex vivo experiments. J. Nat. Remedies 2018, 18, 131–142. [Google Scholar] [CrossRef]
- Ciumarnean, L.; Milaciu, M.V.; Runcan, O.; Vesa, S.C.; Rachisan, A.L.; Negrean, V.; Perne, M.G.; Donca, V.I.; Alexescu, T.G.; Para, I.; et al. The effects of flavonoids in cardiovascular diseases. Molecules 2020, 25, 4320. [Google Scholar] [CrossRef]
- Sánchez, M.; Romero, M.; Gómez-Guzmán, M.; Tamargo, J.; Pérez-Vizcaino, F.; Duarte, J. Cardiovascular effects of flavonoids. Curr. Med. Chem. 2019, 26, 6991–7034. [Google Scholar] [CrossRef]
- Taibi, M.; Elbouzidi, A.; Haddou, M.; Baraich, A.; Gharsallaoui, A.; Mothana, R.A.; Alqahtani, A.M.; Asehraou, A.; Bellaouchi, R.; Addi, M.; et al. Evaluation of the interaction between menthol and camphor, major compounds of Clinopodium nepeta essential oil: Antioxidant, anti-inflammatory and anticancer activities against breast cancer cell lines. Chem. Biodivers. 2025, 22, e202403098. [Google Scholar] [CrossRef]
- Amirova, K.M.; Dimitrova, P.; Marchev, A.S.; Aneva, I.Y.; Georgiev, M.I. Clinopodium vulgare L. (wild basil) extract and its active constituents modulate cyclooxygenase-2 expression in neutrophils. Food Chem. Toxicol. 2019, 124, 1–9. [Google Scholar] [CrossRef]
- Medjdoub, A.R.; Moussaoui, A.; Benzina, F.T.; Benmehdi, H. Qualitative and quantitative analysis and antifungal effects of Satureja calamintha spp. (nepeta) Briq from eastern Algeria. Adv. Food Sci. 2021, 43, 25–35. [Google Scholar]
- Gao, J.-Y.; Cai, G.; Liao, J.; Pan, F.-L.; Peng, X.-L.; Liu, S.-B.; Zhang, L. Bacteriostasis effects of extract from Clinopodium chinense and Leptopus chinensis, two folk medicinal herbs. Hunan Agric. Sci. 2019, 2, 7–10. [Google Scholar] [CrossRef]
- Vorobets, N.; Yavorska, H.; Svydenko, L. Antibacterial activity of Calamintha mentifolia Host. essential oils. Planta Medica 2022, 88, 1530. [Google Scholar]
- Jaramillo, L.I.; Bouajila, J.; Vera, E.; Camy, S. Influence of process parameters on yield, chemical composition and biological activities of Clinopodium nubigenum (Kunth) Kuntze (sunfo) extracts obtained by supercritical CO2 extraction and other techniques. J. Supercrit. Fluids 2025, 218, 106492. [Google Scholar] [CrossRef]
- Hernández, E.; Bermejo, P.; Abad, M.J.; Beltrán, M.; Alcamí, J.; Prieto, A.; Guerra, J.A.; Bedoya, L.M. Characterization of heterogeneous polysaccharides from the aerial parts of Clinopodium bolivianum (Benth.) with neutralizing activity against HIV-1 infection. Polysaccharides 2025, 6, 18. [Google Scholar] [CrossRef]
- Aribi, I.; Chemat, S.; Van Puyvelde, L.; Luyten, W. Bioassay-guided fractionation of Calamintha baborensis Batt. herbal extracts reveals disaccharide glucuronide as a potent antistaphylococcal compound. S. Afr. J. Bot. 2022, 147, 35–41. [Google Scholar] [CrossRef]
- Cüce, M.; Bekircan, T.; Laghari, A.; Sokmen, M.; Sokmen, A.; Ucar, E.; Kılıç, A. Phenolic profiles, antimicrobial and cytotoxic properties of both micropropagated and naturally growing plantlets of Calamintha sylvatica subsp. sylvatica Bromf. Not. Bot. Horti Agrobo. 2019, 47, 1145–1152. [Google Scholar] [CrossRef]
- Benfreha, H.; Pereira, E.C.V.; Rolim, L.A.; Chelli, N.; Almeida, J.R.G.d.S.; Tirtouil, A.; Meddah, B. Additive effect of the probiotics Lactobacillus exopolysaccharides and the Satureja calamintha extracts on enteropathogenic Escherichia coli adhesion. Braz. J. Pharm. Sci. 2022, 58, e20015. [Google Scholar] [CrossRef]
- Georgieva, A.; Sulikovska, I.; Toshkova-Yotova, T.; Djeliova, V.; Amiri, S.; Tsonevski, N.; Petkova-Kirova, P.; Tasheva, K. Antitumor activity of whole-plant extracts from in vitro cultured and wild-growing Clinopodium vulgare plants on a panel of human tumor cell lines. Appl. Sci. 2025, 15, 925. [Google Scholar] [CrossRef]
- Kilinç, B.Ö.; Gödelek, D.; Süfer, Ö.; Saygideğer Demir, B.; Sezan, A.; Saygideğer, Y.; Bozok, F. Essential oils from some Lamiaceae plants: Antioxidant and anticancer potentials besides thermal properties. Chem. Biodivers. 2022, 19, e202200418. [Google Scholar] [CrossRef]
- Halmoune, A.; Salhi, N.; Hamza, E.L.F.; Abdessamad, A.B.; Khalid, K.; Abdelhamid, Z.; Ihoussaine, E.R. Phytochemical study, polyphenols determination and evaluation of antioxidant activity of Satureja calamintha from Morocco. Indian J. Pharm. Educ. Res. 2024, 58, 1167–1173. [Google Scholar] [CrossRef]
- Božović, M.; Ragno, R. Calamintha nepeta (L.) Savi and its main essential oil constituent pulegone: Biological activities and chemistry. Molecules 2017, 22, 290. [Google Scholar] [CrossRef] [PubMed]
- Salhi, N.; Deluyker, D.; Bito, V.; Zaid, A.; El Rhaffari, L. In vitro biological activities of Calamintha nepeta L. aqueous extracts. J. Appl. Biomed. 2024, 22, 155–163. [Google Scholar] [CrossRef]
- Eddouks, M.; Hebi, M.; Ajebli, M.; El Hidani, A.; Sulpice, T.; Burcelin, R. Study of antidiabetic effect of Capparis spinosa L. and Calamintha officinalis Moench in diabetic mice. Phytothérapie 2017, 1–9. [Google Scholar] [CrossRef]
- Lazarova, M.; Tsvetanova, E.; Georgieva, A.; Stefanova, M.; Uzunova, D.; Denev, P.; Vassileva, V.; Tasheva, K. Extracts of Sideritis scardica and Clinopodium vulgare alleviate cognitive impairments in scopolamine-induced rat dementia. Int. J. Mol. Sci. 2024, 25, 1480. [Google Scholar] [CrossRef]
- El Aatiaoui, A.; Daoudi, W.; El Badri, A.; Salhi, A.; El Massaoudi, M.; El Boutaybi, A.; Guo, L.; Loutou, M. Anticorrosive potential of essential oil extracted from the leaves of Calamintha plant for mild steel in 1 M HCl medium. J. Adhes. Sci. Technol. 2023, 37, 1191–1214. [Google Scholar] [CrossRef]
- Tian, J.-G.; Zhou, J.-J.; Ni, Q.; Feng, Y.-L. Establishment of HPLC specific chromatogram of Clinopodium herb and determination of the contents of 3 components. Northwest Pharm. J. 2022, 37, 12–17. [Google Scholar]
- Yang, Z.-Z.; Yang, Y.-H.; Zhong, Y.-M.; Jiang, M.-L.; Zhang, J.; Wang, L.-J.; Long, F.; Li, M. Study on quality standard for Miao medicine Clinopodium gracile. China Pharm. 2023, 34, 682–686. [Google Scholar]
- Gao, Y.; Wang, Y.; Wang, K.; Zhu, J.; Li, G.; Tian, J.; Li, C.; Wang, Z.; Li, J.; Lee, A.W.; et al. Acute and a 28-day repeated-dose toxicity study of total flavonoids from Clinopodium chinense (Benth.) O. Ktze in mice and rats. Regul. Toxicol. Pharmacol. 2017, 91, 117–123. [Google Scholar] [CrossRef]
- Li, L.-L.; Huang, Q.; Wang, M.-M.; Gan, J.-H.; Peng, D.-Y. Simultaneous determination of five compounds of Clinopodium chinense in rat plasma by LC-MS/MS and its application to a pharmacokinetic study. Lat. Am. J. Pharm. 2019, 38, 1143–1152. [Google Scholar]
- Althaher, A.R.; Jarrar, Y.; Al-Ibadah, M.A.; Balasmeh, R.; Jarrar, Q.; Abulebdah, D. Effects of Calamintha incana (Sm.) Helder ethanolic extract on the mRNA expression of drug-metabolizing cyp450s in the mouse livers. MicroRNA 2024, 13, 63–70. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).