The Chemical Composition, Pharmacological Activity, Quality Control, Toxicity, and Pharmacokinetics of the Genus Clinopodium L.
Abstract
1. Introduction
2. Phytochemistry
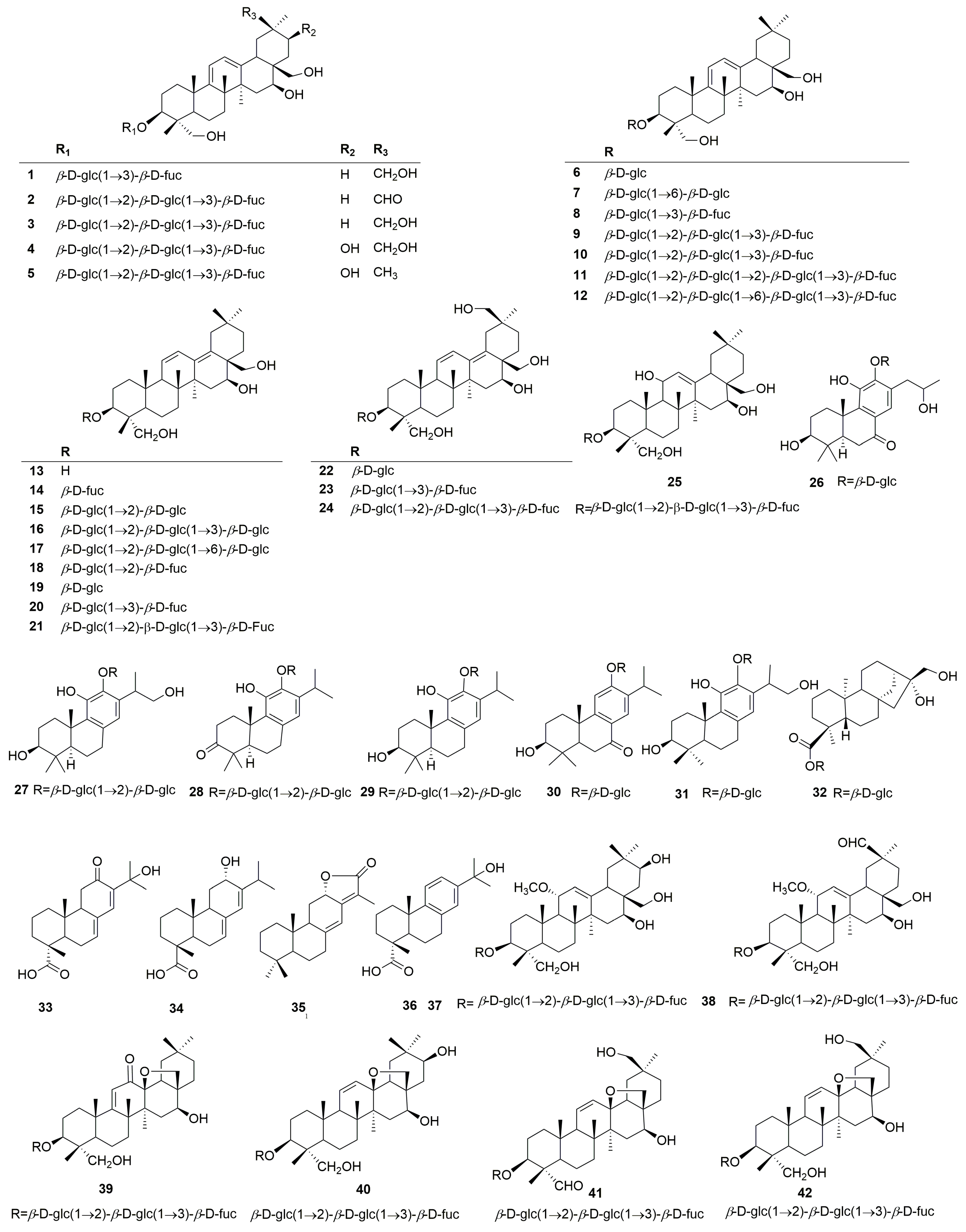
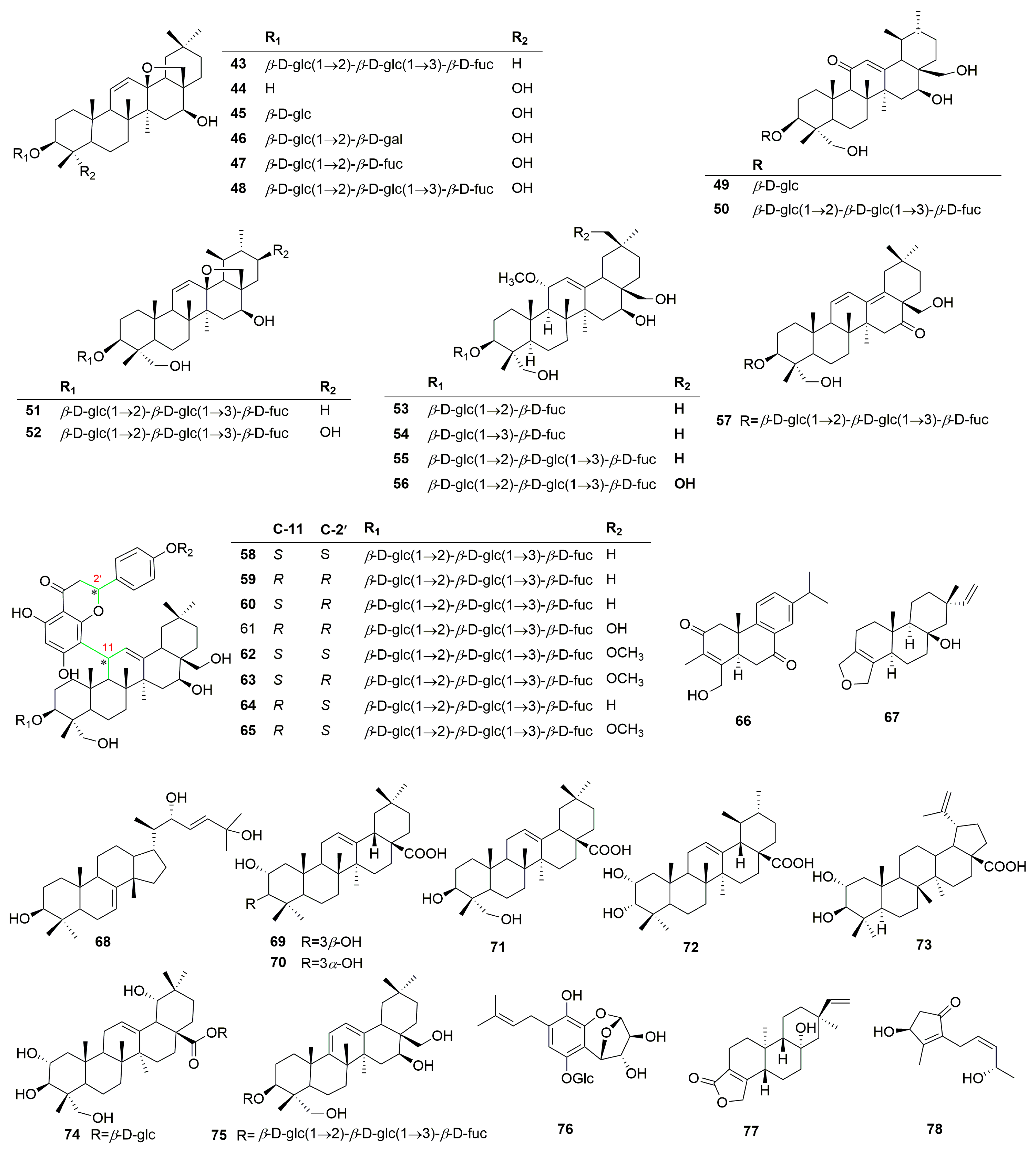
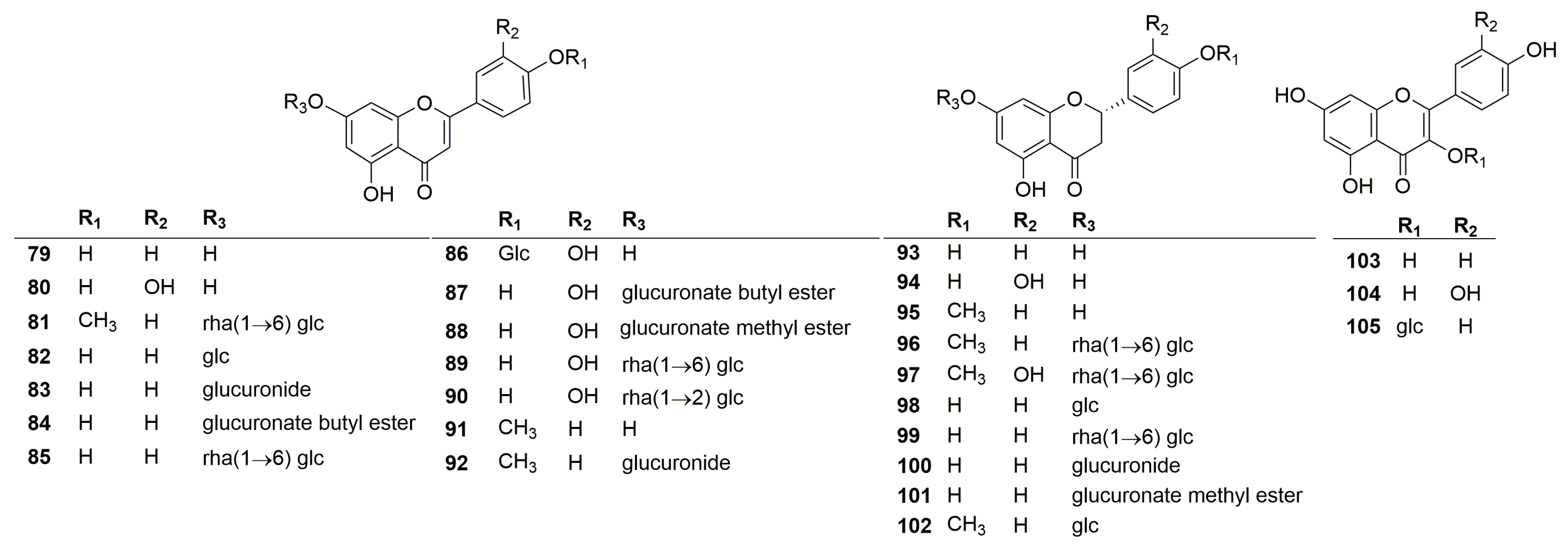
2.1. Compounds Isolated and Identified from the Genus Clinopodium
2.1.1. Terpenoids
2.1.2. Flavonoids
2.1.3. Phenylpropanoids
2.1.4. Other Compounds
2.2. Compounds Identified by GC-MS
| No. | Name | Collected Areas | Identified Compounds and Major Components | Ref. |
|---|---|---|---|---|
| 1 | C. axillare | Quiriría, San José, Province of Esteban Arce | Seventy-five compounds were identified. The major constituents were piperidone oxide (20–30%), piperidone epoxide (15–19%), piperidone (13%), pulerone (3–5%), and piperidone (4–5%), as well as limonene (8–12%) and -pinene (1–5%). | [37] |
| 2 | C. brevicalyx | The southern high Andean regions of Peru | The most abundant compounds were isomaltone (44.25%), menthol (22.22%), and prunone (8.23%). | [44] |
| 3 | C. brownei | Amazonian region of Ecuador | Non-polar constituents: ethyl cinnamate (21.4%), pridon (20.76%), methyl cinnamate (16.68%), caryophyllene (8.17%), β-chromoselenene (7.92%), and menthone (7.51%).Polar constituents: pridon (29.90%), ethyl cinnamate (18.75%), methyl cinnamate (13.82%), caryophyllene (10.0%), and menthone (8.04%). | [45] |
| 4 | Calamintha baborensis | Jijel eastern region of Algeria | The major constituent is eugenol (27.04%), followed by 3-methoxy acetophenone (26.4%) and phenyl ethyl alcohol (6.58%). | [46] |
| 5 | C. candidissimum | Region of Djebel Murdjadjo, Oran, Northwestern Algeria | Thirty-eight compounds were identified, including oxygenated monoterpenes pulegone (44.8%), piperitenone 1, * (6.6%), isopulegone (5.8%), and neo-menthol (3.8%). Among them, the sesquiterpene hydrocarbons germacrene D (16.2%) and bicyclogermacrene (3.0%) were the most abundant. | [47] |
| 6 | C. chinense | Lishui, Zhejiang Province, China | Thirty-five compounds were identified, accounting for 99.18% of the total oil. The major components were phorbol (18.54%), piperitone (18.9%), caryophyllene (12.04%), and bornyl acetate (8.14%), followed by caryophyllene oxide (4.19%), piperitone (4.09%), and carvacrol (4.01%). | [48] |
| 7 | Satureja calamintha | Faculty of Sciences Semlalia, Marrakech, Morocco | Fifteen compounds accounted for 99.88% and 98.14% of the total oils obtained from wild and cultivated plants, respectively. Pulegone (72.93–68.58%), menthone (12.07–10.15%), and menthol (6.31–9.83%) were found as the main constituents. | [38] |
| 8 | Satureja calamintha | Taounate, Morocco | Twenty-four compounds were identified. The main constituents were pulegone (21.48%), piperitenone * oxide (17.71%), and eucalyptol (11.99%). | [49] |
| 9 | Satureja calamintha | Jijel region of Algeria | Three most abundant compounds identified were l-menthone (32.10%), neo-menthol (32.07%), and pulegone (22.35%). | [50] |
| 10 | Calamintha fenzlii | Nablus region of Palestine | The chemical constituents were dominated by oxygenated monoterpenoid (96.91%). The major chemical components were represented by menthone 68.93% and pulegone 23.1%. | [51] |
| 11 | Calamintha glandulosa | Luštica in Stari Krašići (Montenegro) | Seventeen compounds were identified. The major compounds were pulegone (35.1%), piperitenone * (23.4%), menthone (15.7%) and piperitone (11.5%). | [30] |
| 12 | Calamintha incana (Sm.) Boiss. | Kestel, Bursa, Turkey | The oxygenated monoterpenes trans-piperitone oxide (41.37%), piperitenone oxide (34.47%), piperitenone * (6.67%), and monoterpene phenol thymol (3.37%) were found to be the major constituents. | [52] |
| 13 | Calamintha incana | Ajloun county in Jordan | The main constituents were benzenamine-4-methyl-3-nitro-(34.11%) and (2S,4R)-p-mentha-6,8-diene 2-hydroperoxide (31.48%). | [53] |
| 14 | C. macrostemum | San Andrés, Paxtlán, Oaxaca, México | Twenty-six compounds were identified, including menthone (approximately 35%) and piperitone oxide (approximately 30%). | [54] |
| 15 | C. menthifolium | AinDraham, Babouch, and Tabarka, Tunisia | Sixty-three different compounds were identified: piperitone (34.5%), cis-piperitone oxide (26.1%), and piperitone (47.9%). | [55] |
| 16 | Calamintha nepeta | Vratarnica near Zaječar (Serbia) | Fourteen compounds were identified. The major compounds were pulegone (58.0%) and piperitenone * (27.4%). | [30] |
| 17 | Calamintha nepeta | Morano Calabro, Cosenza, Italy | Thirty-four compounds were identified. The major components were 1,8-cineole (34.09%), eugenol (14.66%) and linalool acetate (11.25%), followed by sabinene (6.97%) and linalool (6.64%). | [39] |
| 18 | Calamintha nepeta | Beni-Saf region in the northwest of Algeria | The primary components included oxygenated monoterpenes, notably pulegone (58.36%), isoborneol (10.40%), menthone (8.91%), and piperitenone * (3.86%). | [56] |
| 19 | Calamintha nepeta | Basilicata region, Southeastern Italy | Twenty-four compounds were identified, accounting for 90.17% of total oil composition. Pulegone (44.7%), menthone (16.4%), piperitenone * (13.3%), and piperitone (6.01%) were the major constituents. | [57] |
| 20 | Calamintha nepeta | Tarquinia, Viterbo, Italy | Thirty-nine different chemical constituents have different concentrations in various fractions. Pulegone (37.7–77.7%) and crysanthenone (14.4–27.3%) were the most abundant components. | [32] |
| 21 | Calamintha nepeta | Alentejo region, Herdade da Mitra, Évora | Twenty-nine compounds were identified, representing 91% of oxygenated monoterpenes, 7% of hydrocarbon monoterpenes, and 1% of sesquiterpenes. The major components were 1,8-cineole (28%), menthone (22%), menthol (16%), and pulegone (5%). | [58] |
| 22 | Calamintha nepeta | Tengalti village and the region near the Velvelechay river of Quba | Seventy-eight compounds were identified; the major components were thymol (19.81%), cyclopropane, 1,1-diethyl-(19.77%), cyclohexanone, 3-vinyl3-methyl-(18.66%), D-limonene (7.45%), and caryophyllene (6.16%). | [59] |
| 23 | Satureja calamintha subsp. nepeta Briq. | Medea region, South Algiers and Chlef region, western Algiers | Seventy compounds were identified, representing 97.4% of the oil. 1,8-cineole (28.4%), pulegone (10.2%), menthone (9.7%), and isomenthone (9.6%) were the most important constituents. | [60] |
| 24 | S. calamintha nepeta | Mountains of the Skikda region located in northeastern Algeria | One hundred and ten compounds were identified. Piperitenone oxide, trans-piperitenone oxide, caryophyllene oxide, 3-methyldiphenyl ether, (E)-caryophyllene, gensmin, germacrene D, (Z)-jasmone, trans-calamenene, γ-gurjunene, and pulegone are the main constituents. | [61] |
| 25 | S. calamintha nepeta | Mountainous terrain of the Moroccan province of Ouazzane | Twenty-seven compounds were identified, making up 99.2% of the essential oil, with 1,8-cineole (34.34%) and cis-pinocamphone (11.87%) being the most significant. | [35] |
| 26 | C. nepeta | Béni-Mtir (Aîn Draham, Jendouba), North-western Tunisia | Forty-seven compounds were identified: the main components were piperitone oxide (16.3–51.7%) and piperitenone oxide (23.4–39.3%). | [33] |
| 27 | C. nepeta | Bilecik, Turkey | Forty-four compounds were identified. The main components were piperitenone oxide (47.8%), limonene (18.6%), and piperitone oxide II (13.6%). | [62] |
| 28 | C. nepeta | Antalya-Finike, in southwestern Turkey | Thirty-five compounds were identified and quantified. The major compounds were sabine (34.2%), β-pinene (25.9%), α-pinene (13.8%), and caryophyllene oxide (3.7%). | [63] |
| 29 | C. nepeta | sub-Mediterranean area of Bosnia and Herzegovina | The EOs contained 42 compounds, including pulegone (44.8%), piperitenone * (48.8%), and piperitenone oxide (60.2%) as the major compounds. | [64] |
| 30 | C. nubigenum | Mountains near Hacienda Zuleta, Imbaburra, Ecuador | Thirty-three compounds were identified. The major chemical constituents were carvacrol (32.9%), followed by pulegone (25.4%). Other important volatiles were p-cymene (9.1%) and iso-menthone (6.4%). Monoterpenes, both in their oxygenated and hydrocarbon forms (74 and 19.7%, respectively), were the major chemical class. | [40] |
| 31 | Calamintha officinalis | Northern Iran (Guilan, Lahijan) | Forty-one components were isolated, constituting 23.09% of the total oil. The major constituents were trans-caryophyllene (8.55%), isomenthol (2.98%), tetrahydrolinalyl acetate (2.96%), and pinene (2.24%). | [65] |
| 32 | C. pulegium | Svrljiški Timok gorge, Serbia | Nineteen previously described mono- and sesquiterpenes were found. The major compound was menthone (47.1%), followed by β-pinene (19.8%), isomenthone (12.3%), and pulegone (12, 8.5%). | [66] |
| 33 | C. rouyanum | Mountains of the island of Majorca, Spain | Twenty-seven compounds were identified from five samples of C. rouyanum, among which pulegone (73.0–82.2%), menthone (6.5–11.8%), and limonene (3.5–6.0%) were the major compounds. | [41] |
| 34 | C. serpyllifolium | BERC Experimental Station, Til, Nablus, Palestine | Twenty-three compounds were identified. Pulegone (50.22–81.51%), menthol (1.91–15.68%), and p-menth-3-en-8-ol (1.64–11.94%) were the major compounds. | [67] |
| 35 | C. serpyllifolium | The Newe Ya’ar living germplasm, Israel | The major constituents were oxygenated monoterpenes pulegone (10.4–50.6%), piperitenone oxide (3.2–28.6%), piperitenone * (0.9–14.6%), trans-piperitone oxide (0.3–11.2%), iso-menthol (0.3–8.8%), and sesquiterpene β-caryophyllene (7.4–13.7%). | [68] |
| 36 | C. sericeum | Region of Cajamarca (Perú) | Seventy-three compounds were identified. The major compounds were β-germacrene D (15%), β-caryophyllene (13.8%), and sabinene (11.2%). | [69] |
| 37 | Calamintha sylvatica | Morano Calabro, Cosenza, Italy | Twenty compounds were identified. The major compounds were piperitone oxide (37.70%), pulegone (20.91%), and piperitenone oxide (18.26%), iso-menthone (7.5%), and limonene (6.58%). | [39] |
| 38 | Calamintha sylvatica | The edge of a beech and hornbeam forest, under Mt. Rudnik (Serbia) | Twenty-eight compounds were identified. The major compounds were cis-piperitone epoxide (63.3%) and menthone (10.8%). | [30] |
| 39 | C. taxifolium | Province of Loja, Mount Villonaco | Thirty-seven compounds were identified, mainly including (E)-β-caryophyllene (17.8%), α-copperene (10.5%), β-bourbonene (9.9%), δ-carpentene (6.6%), cis-cadina-1(6),4-diene (6.4%), and myricene D (4.9%). | [70] |
| 40 | C. thymifolium | Limestone habitat near Tutin, SW Serbia | Fifty-six compounds were identified, mainly including pulegone (75.9% in vegetative stage and 50.4% in late flowering stage), piperitenone * (6.2% in vegetative stage and 10.4% in late flowering stage), isomenthone (3.1% in vegetative stage and 17.8% in late flowering stage), and limonene (vegetative stage). | [42] |
| 41 | C. umbrosum | Kheyroud forest near Noshahr, Mazandaran, Iran | Sixteen compounds were identified. The major compounds were tolualdehyde (29.16%), palmitic acid (17.57%), and acetophenone (13.44%). | [18] |
| 42 | Calamintha vardarensis | The underbrush in Radika Canyon (FYR Macedonia) | Twenty-five compounds were identified. The major compounds were pulegone (51.6%) and menthone (19.9%) | [30] |
2.3. Compounds Identified by LC-MS
3. Pharmacology
3.1. Hemostatic Activity
3.2. Anti-Cardiomyocyte Damage and Cardiovascular Protection
3.3. Anti-Inflammatory Activity
3.4. Antimicrobial Activity
3.5. Antitumor Activity
3.6. Antioxidant Activity
3.7. Insecticidal Effect
3.8. Others
4. Quality Evaluation
5. Toxicity
6. Pharmacokinetics
7. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kaya, A. Comparative root and stem anatomy of six Clinopodium (Lamiaceae) taxa. Biologia 2016, 71, 1330–1337. [Google Scholar] [CrossRef]
- Bräuchler, C. And now for something completely different-new names in Clinopodium with comments on some types. Phytotaxa 2018, 356, 71–80. [Google Scholar] [CrossRef]
- Clinopodium, L. Plants of the World Online|Kew Science. Available online: https://powo.science.kew.org/taxon/urn:lsid:ipni.org:names:30008690-2 (accessed on 15 May 2025).
- Qureshi, K.A.; Parvez, A.; Uzzaman Khan, M.M.; Aspatwar, A.; Atiya, A.; Elhassan, G.O.; Khan, R.A.; Erattil Ahammed, S.Y.; Khan, W.U.; Jaremko, M. Exploring nature’s hidden treasure: Unraveling the untapped phytochemical and pharmacological potentials of Clinopodium vulgare L.—A hidden gem in the Lamiaceae family. Heliyon 2024, 10, e24781. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.; Li-Min, L.; Yuan-Gen, X.; Xue-Bin, S.; Huan, L.; Si-Hui, N. Research progress on genus Clinopodium. China J. Chin. Mater. Med. 2020, 45, 4349–4357. [Google Scholar]
- Dobrev, H.P. Treatment of numerous hand warts with Clinopodium vulgare tea. Wien. Med. Wochenschr. 2021, 171, 82–83. [Google Scholar] [CrossRef]
- Zhong, M.; Sun, G.; Zhang, X.; Sun, G.; Xu, X.; Yu, S. A new prenylated naphthoquinoid from the aerial parts of Clinopodium chinense (Benth.) O. Kuntze. Molecules 2012, 17, 13910–13916. [Google Scholar] [CrossRef]
- Zhu, Y.-D.; Hong, J.-Y.; Bao, F.-D.; Xing, N.; Wang, L.-T.; Sun, Z.-H.; Luo, Y.; Jiang, H.; Xu, X.-D.; Zhu, N.-L.; et al. Triterpenoid saponins from Clinopodium chinense (Benth.) O. Kuntze and their biological activity. Arch. Pharmacal Res. 2018, 41, 1117–1130. [Google Scholar] [CrossRef]
- Hu, Y.-X.; Zhang, W.; Zhang, W.; Zhu, Y.-D.; Ma, G.-X.; Zhu, N.-L.; Sun, W.; Ma, Z.-X.; Yu, S.-C.; Xu, X.-D. Oleanane triterpene saponins with cardioprotective activity from Clinopodium polycephalum. J. Asian Nat. Prod. Res. 2017, 19, 697–703. [Google Scholar] [CrossRef]
- Zhao, T.; Liu, Y.N.; Sun, X.Y.; Li, J.Y.; Lei, C.; Hou, A.J. Two new oleanane-triterpenoid saponins from Clinopodium gracile. Chem. Biodivers. 2021, 18, e2100672. [Google Scholar] [CrossRef]
- Zeng, B.; Liu, G.-D.; Zhang, B.-B.; Wang, S.-S.; Ma, R.; Zhong, B.-S.; He, B.-Q.; Liang, Y.; Wu, F.-H. A new triterpenoid saponin from Clinopodium chinense (Benth.) O. Kuntze. Nat. Prod. Res. 2016, 30, 1001–1008. [Google Scholar] [CrossRef]
- Xu, M.-J.; Wu, Y.-W.; Zhao, D.; Xu, Y.-Y.; Pan, Q.-L.; Zhao, T.-T.; Zhou, W.-S.; Yuan, Y.-Y.; Xu, T.-H.; Zhu, Y.-D. Two new oleanane-type triterpenoid saponins from aerial part of Clinopodium chinense. Mod. Chin. Med. 2022, 24, 1447–1455. [Google Scholar]
- Qu, L.-X.; Liu, Y.-Q.; Wang, Y.; Wang, H.; Huang, X.-L.; Zhang, M.-L.; Mou, Y.-X.; Xu, T.-H.; Zhu, Y.-D. Diterpenoid and triterpenoid glycosides from Clinopodium chinense. Nat. Prod. Res. 2021, 35, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.-D.; Zhang, J.-Y.; Li, P.-F.; Wu, H.-F.; Zhu, N.-L.; Jiang, H.; Lv, C.-Y.; Wu, L.-L.; Ma, Z.-X.; Xu, X.-D.; et al. Two new abietane diterpenoid glycosides from Clinopodium chinense. Nat. Prod. Res. 2016, 30, 1075–1080. [Google Scholar] [CrossRef]
- Ticona, L.A.; Noguerón, A.M.; Sánchez-Corral, J.S.; Lozano, N.M.; Domenech, M.O. Anti-inflammatory, antibacterial, anti-biofilm, and anti-quorum sensing activities of the diterpenes isolated from Clinopodium bolivianum. Pharmaceutics 2024, 16, 1094. [Google Scholar] [CrossRef]
- Wang, Y.; He, L.; Li, L.-Y.; Xu, M.-J.; Wu, Y.-W.; Li, Y.-S.; Chu, X.-Y.; Pan, Q.; Zhao, T.-T.; Ye, X.-X.; et al. A new triterpenoid saponin from the aerial parts of Clinopodium chinense. Mod. Chin. Med. 2020, 22, 1596–1606. [Google Scholar]
- Kim, D.; Lee, S.K.; Park, K.-S.; Park, H.-J. Isolation of the constituents from Clinopodium chinense var. shibetchense and inhibition activity on cancer cell growth and nitric oxide production. Korean J. Pharmacogn. 2020, 51, 93–99. [Google Scholar]
- Fathiazad, F.; Kaboudi, N.; Esfahanizadeh, M.; Hamedeyazdan, S. Oleanane-type triterpenoid saponins and rosmarinic acid from Clinopodium umbrosum. Iran. J. Chem. Chem. Eng. 2023, 42, 2211–2220. [Google Scholar]
- Tao, M.K.; Liu, Y.X.; Zhou, X.M.; Yu, X.L.; Zhang, Y.; Guo, L.; Meng, D.L. Polycephalums A and B, New anticoagulant constituents isolated from Clinopodium polycephalum. Chem. Biodivers. 2023, 20, e202300448. [Google Scholar] [CrossRef]
- Zhu, Y.D.; Wu, H.F.; Ma, G.X.; Chen, R.C.; Long, H.L.; Zuo, Z.L.; Luo, Y.; Zhu, N.L.; Hou, B.; Xu, X.D.; et al. Clinoposides A-F: Meroterpenoids with protective effects on H9c2 cardiomyocyte from Clinopodium chinense. RSC Adv. 2016, 6, 7260–7266. [Google Scholar] [CrossRef]
- Zhu, Y.-D.; Chen, R.-C.; Wang, H.; Jiang, H.; Huang, X.-L.; Zhang, M.-L.; Li, L.-Y.; Hu, Z.; Xu, X.-D.; Wang, C.-J. Two new flavonoid–triterpene saponin meroterpenoids from Clinopodium chinense and their protective effects against anoxia/reoxygenation-induced apoptosis in H9c2 cells. Fitoterapia 2018, 128, 180–186. [Google Scholar] [CrossRef]
- Liu, Y.; Song, H.; Xu, J.; Bi, G.; Meng, D. Anti-inflammatory abietanes diterpenes and triterpenoids isolated from Clinopodium polycephalum. Fitoterapia 2022, 161, 105244. [Google Scholar] [CrossRef] [PubMed]
- Shen, M.-Y.; Zheng, C.-W.; Hao, Z.-J.; Song, H.-J.; Wang, Y.-M.; Meng, D.-L. Undescribed compounds from Clinopodium chinense (Benth.) O. Kuntze and their coagulation activity studies. Fitoterapia 2024, 172, 105736. [Google Scholar] [CrossRef]
- Zhao, D.; Wu, Y.-W.; Xu, Y.-Y.; Fan, J.-P.; Zhang, Y.-H.; Yang, R.-Y.; Wang, H.; Wu, L.-L.; Xu, T.-H.; Zhu, Y.-D. A new monoterpenoid from Clinopodium chinense (Benth.) O. Kuntze. Mod. Chin. Med. 2023, 25, 1918–1924. [Google Scholar]
- Zeng, B.; Chen, K.; Du, P.; Wang, S.S.; Ren, B.; Ren, Y.L.; Yan, H.S.; Liang, Y.; Wu, F.H. Phenolic compounds from Clinopodium chinense (Benth.) O. Kuntze and their inhibitory effects on α-glucosidase and vascular endothelial cells injury. Chem. Biodivers. 2016, 13, 596–601. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.-T.; Sun, Z.-H.; Zhong, M.-L.; Wu, H.-F.; Zhang, H.-J.; Zhu, N.-L.; Sun, G.-B.; Ye, X.-X.; Xu, X.-D.; Zhu, Y.-D.; et al. Studies on chemical constituents of Clinopodium chinense. China J. Chin. Mater. Med. 2017, 42, 2510–2517. [Google Scholar]
- Yu, Y.; Xing, N.; Xu, X.; Zhu, Y.; Wang, S.; Sun, G.; Sun, X. Tournefolic acid B, derived from Clinopodium chinense (Benth.) Kuntze, protects against myocardial ischemia/reperfusion injury by inhibiting endoplasmic reticulum stress-regulated apoptosis via PI3K/AKT pathways. Phytomedicine 2019, 52, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Gezici, S.; Turkmen, M.; Karahan, F. Exploring the anti-cancer properties of essential oils from some Lamiaceae species against human cancer cells with multivariate analysis. S. Afr. J. Bot. 2024, 166, 287–296. [Google Scholar] [CrossRef]
- Khodja, N.K.; Boulekbache, L.; Chegdani, F.; Dahmani, K.; Bennis, F.; Madani, K. Chemical composition and antioxidant activity of phenolic compounds and essential oils from Calamintha nepeta L. J. Complement. Integr. Med. 2018, 15, 20170080. [Google Scholar] [CrossRef]
- Milenković, M.; Stošović, J.; Slavkovska, V. Synergy between essential oils of Calamintha species (Lamiaceae) and antibiotics. Nat. Prod. Commun. 2018, 13, 371–374. [Google Scholar] [CrossRef]
- Kremer, D.; Dunkic, V.; Radosavljevic, I.; Bogunic, F.; Ivanova, D.; Ballian, D.; Stesevic, D.; Matevski, V.; Randelovic, V.; Eleftheriadou, E.; et al. Phytochemicals and their correlation with Molecular Data in Micromeria and Clinopodium (Lamiaceae) Taxa. Plants 2022, 11, 3407. [Google Scholar] [CrossRef]
- Božović, M.; Garzoli, S.; Sabatino, M.; Pepi, F.; Baldisserotto, A.; Andreotti, E.; Romagnoli, C.; Mai, A.; Manfredini, S.; Ragno, R. Essential oil extraction, chemical analysis and anti-candida activity of Calamintha nepeta (L.) Savi subsp. glandulosa (Req.) Ball—New approaches. Molecules 2017, 22, 203. [Google Scholar]
- Debbabi, H.; Mokni, R.E.; Chaieb, I.; Nardoni, S.; Maggi, F.; Caprioli, G.; Hammami, S. Chemical composition, antifungal and insecticidal activities of the essential oils from Tunisian Clinopodium nepeta Subsp. nepeta and Clinopodium nepeta Subsp. glandulosum. Molecules 2020, 25, 2137. [Google Scholar] [PubMed]
- Vlachou, G.; Papafotiou, M.; Daferera, D.J.; Tarantilis, P.A. Yield and composition of the essential oil of Clinopodium nepeta subsp. spruneri as affected by harvest season and cultivation method, i.e., outdoor, greenhouse and in vitro culture. Plants 2023, 12, 4098. [Google Scholar]
- Hayani, M.; Benabbouha, T.; Nachit, W.; Byadi, S.; Chefira, K.; Aboulmouhajir, A.; Tounsi, A.; Zair, T. Unveiling the potential of Satureja calamintha nepeta: A study on its phytochemical composition and antibacterial activity. Vietnam J. Chem. 2024, 62, 772–779. [Google Scholar] [CrossRef]
- Debbabi, H.; El Mokni, R.; Majdoub, S.; Aliev, A.; Hammami, S. The effect of pressure on the characteristics of supercritical carbon dioxide extracts from Calamintha nepeta subsp. nepeta. Biomed. Chromatogr. 2020, 34, e4871. [Google Scholar] [CrossRef] [PubMed]
- Arze, J.B.L.; Collin, G.; Jean, F.-I.; Gagnon, H. Essential oils from Bolivia. xiv. Lamiaceae: Clinopodium axillare. Am. J. Essent. Oils Nat. Prod. 2019, 7, 1–5. [Google Scholar]
- Abbad, I.; Soulaimani, B.; Abbad, A. Chemical composition, insecticidal and allelopathic properties of essential oils obtained from wild and cultivated Moroccan Satureja calamintha (L.). J. Nat. Pestic. Res. 2023, 3, 100021. [Google Scholar] [CrossRef]
- Bava, R.; Castagna, F.; Lupia, C.; Ruga, S.; Conforti, F.; Marrelli, M.; Argentieri, M.P.; Musella, V.; Britti, D.; Statti, G.; et al. Phytochemical Composition and Pharmacological Efficacy Evaluation of Calamintha nepeta, Calamintha sylvatica, Lavandula austroapennina and Mentha piperita Essential Oils for the Control of Honeybee (Apis mellifera) Varroosis. Animals 2024, 14, 69. [Google Scholar] [CrossRef]
- Bedini, S.; Flamini, G.; Cosci, F.; Ascrizzi, R.; Echeverria, M.C.; Gomez, E.V.; Guidi, L.; Landi, M.; Lucchi, A.; Conti, B. Toxicity and oviposition deterrence of essential oils of Clinopodium nubigenum and Lavandula angustifolia against the myiasis-inducing blowfly Lucilia sericata. PLoS ONE 2019, 14, e0212576. [Google Scholar] [CrossRef]
- Tomas, J.; Gil, L.; Llorens-Molina, J.; Cardona, C.; García, M.; Llorens, L. Biogenic volatiles of rupicolous plants act as direct defenses against molluscs: The case of the endangered Clinopodium rouyanum. Flora 2019, 258, 151428. [Google Scholar] [CrossRef]
- Dekić, M.; Radulović, N.; Antonijević, M.; Dekić, D.; Ličina, B. The essential oil of the condiment species Clinopodium thymifolium (Scop.) Kuntze: New natural products and seasonal variation. J. Sci. Food Agric. 2022, 102, 2437–2444. [Google Scholar] [CrossRef]
- Novakovic, M.; Bukvicki, D.; Vajs, V.; Tesevic, V.; Milosavljevic, S.; Marin, P.; Asakawa, Y. Microbial transformation of Calamintha glandulosa essential oil by Aspergillus Niger. Nat. Prod. Commun. 2018, 13, 479–482. [Google Scholar] [CrossRef]
- Merma Ccana, C.; Tomaylla Cruz, C.; del Carpio Jiménez, C. Anti-Trichophyton rubrum activity of the essential oil of Clinopodium brevicalyx and elaboration of a topical emulsion. J. High Andean Res. 2020, 22, 182–190. [Google Scholar]
- Noriega, P.; Calderón, L.; Ojeda, A.; Paredes, E. Chemical composition, antimicrobial and antioxidant bioautography activity of essential oil from leaves of Amazon plant Clinopodium brownei (Sw.). Molecules 2023, 28, 1741. [Google Scholar] [CrossRef]
- Seraoui, R.; Benkiniouar, R.; Akkal, S.; Ros, G.; Nieto, G. Phytochemical investigation, antioxidant and antimicrobial assays of Algerian Plant Calamintha baborensis Batt. Pharm. Chem. J. 2018, 52, 347–356. [Google Scholar] [CrossRef]
- Hamdi, B.; Peron, G.; Miara, M.D.; Bouriah, N.; Flamini, G.; Maggi, F.; Sut, S.; Dall’Acqua, S. Phytochemical analysis of Clinopodium candidissimum (Munby) Kuntze growing in Algeria by an integrated HS-SPME-GC-MS, NMR and HPLC-DAD-MSn approach: Valorisation of an endemic natural source of bioactive compounds. Nat. Prod. Res. 2024, 38, 140–145. [Google Scholar] [CrossRef] [PubMed]
- Li, H.-Y.; Liu, X.-C.; Chen, X.-B.; Liu, Q.-Z.; Liu, Z.-L. Chemical composition and insecticidal activities of the essential oil of Clinopodium chinense (Benth.) Kuntze aerial parts against Liposcelis bostrychophila Badonnel. J. Food Prot. 2015, 78, 1870–1874. [Google Scholar] [CrossRef]
- Baghouz, A.; Bouchelta, Y.; Es-safi, I.; El Brahimi, R.; Imtara, H.; AlZain, M.N.; Noman, O.M.; Shahat, A.A.; Guemmouh, R. Biocidal activity of Ziziphora hispanica L and Satureja calamintha Scheele L essential oils against the Callosobruchus maculatus (Fabricius) pest on cowpea seeds during storage. Front. Sustain. Food Sys. 2024, 8, 1329100. [Google Scholar] [CrossRef]
- Boudjema, K.; Bouanane, A.; Gamgani, S.; Djeziri, M.; Abou Mustapha, M.; Fazouane, F. Phytochemical profile and antimicrobial properties of volatile compounds of Satureja calamintha (L) Scheel from northern Algeria. Trop. J. Pharm. Res. 2018, 17, 857–864. [Google Scholar] [CrossRef]
- Jaradat, N.; Al-lahham, S.; Abualhasan, M.N.; Ghannam, D.; Mousa, K.; Kolayb, H.; Hussein, F.; Issa, L.; Mousa, A. Chemical fingerprinting, anticancer, anti-inflammatory and free radical scavenging properties of Calamintha fenzlii Vis. volatile oil from Palestine. Arab. J. Sci. Eng. 2020, 45, 63–70. [Google Scholar] [CrossRef]
- Popović-Djordjević, J.; Cengiz, M.; Ozer, M.S.; Sarikurkcu, C. Calamintha incana: Essential oil composition and biological activity. Ind. Crop. Prod. 2019, 128, 162–166. [Google Scholar] [CrossRef]
- Althaher, A.R.; Oran, S.A.; Bustanji, Y.K. Chemical composition, in vitro evaluation of antioxidant properties and cytotoxic activity of the essential oil from Calamintha incana (Sm.) Helder (Lamiaceae). Trop. J. Nat. Prod. Res. 2021, 5, 1333–1339. [Google Scholar]
- Villa-Ruano, N.; Pacheco-Hernández, Y.; Cruz-Durán, R.; Lozoya-Gloria, E. Volatiles and seasonal variation of the essential oil composition from the leaves of Clinopodium macrostemum var. laevigatum and its biological activities. Ind. Crop. Prod. 2015, 77, 741–747. [Google Scholar] [CrossRef]
- Debbabi, H.; El Mokni, R.; Nardoni, S.; Chaieb, I.; Maggi, F.; Nzekoue, F.K.; Caprioli, G.; Hammami, S. Chemical diversity and biological activities of essential oils from native populations of Clinopodium menthifolium subsp. ascendens (Jord.) Govaerts. Environ. Sci. Pollut. Res. 2021, 28, 13624–13633. [Google Scholar] [CrossRef] [PubMed]
- Bahri, F.; Benhassaini, H.; Szumny, A.; Bentaiba, K. Chemical composition and pharmacological activities of Calamintha nepeta essential oil. Trop. J. Nat. Prod. Res. 2024, 8, 7097–7105. [Google Scholar]
- Ambrico, A.; Trupo, M.; Martino, M.; Sharma, N. Essential oil of Calamintha nepeta (L.) Savi subsp. nepeta is a potential control agent for some postharvest fruit diseases. Org. Agric. 2020, 10, 35–48. [Google Scholar]
- Arantes, S.M.; Piçarra, A.; Guerreiro, M.; Salvador, C.; Candeias, F.; Caldeira, A.T.; Martins, M.R. Toxicological and pharmacological properties of essential oils of Calamintha nepeta, Origanum virens and Thymus mastichina of Alentejo (Portugal). Food Chem. Toxicol. 2019, 133, 110747. [Google Scholar] [CrossRef]
- Zeynalova, S.A. Chemical composition of the essential oil from Calamintha nepeta (L.) Savi plants growing in the flora Azerbaijan. Plant Fungal Res. 2018, 1, 62–68. [Google Scholar] [CrossRef]
- Benchaa, S.; Hazzit, M.; Zermane, N.; Abdelkrim, H. Chemical composition and herbicidal activity of essential oils from two Labiatae species from Algeria. J. Essent. Oil Res. 2019, 31, 335–346. [Google Scholar] [CrossRef]
- Medjdoub, A.R.; Benmehdi, H.; Oukali, Z. Chemical composition and antifungal activity of essential oil of Satureja calamintha spp. nepeta (L.) Briq against some toxinogenous mold. Nat. Volatiles Essent. Oils 2022, 9, 1981–2000. [Google Scholar]
- Öztürk, G.; Yilmaz, G.; Gülnur, E.; Demirci, B. Chemical composition and antibacterial activity of Clinopodium nepeta subsp. glandulosum (Req.) Govaerts essential oil. Nat. Volatiles Essent. Oils 2021, 8, 75–80. [Google Scholar]
- Çelik, G.; Kılıç, G.; Kanbolat, Ş.; Özlem Şener, S.; Karaköse, M.; Yaylı, N.; Karaoğlu, Ş.A. Biological activity, and volatile and phenolic compounds from five Lamiaceae species. Flavour Frag. J. 2021, 36, 223–232. [Google Scholar] [CrossRef]
- Boskailo, E.; Dzudzevic-Cancar, H.; Dedic, A.; Marijanovic, Z.; Alispahic, A.; Cancar, I.F.; Vidic, D.; Jerkovic, I. Clinopodium nepeta (L.) Kuntze from Bosnia and Herzegovina: Chemical characterisation of headspace and essential oil of fresh and dried samples. Rec. Nat. Prod. 2023, 17, 300–311. [Google Scholar]
- Shams Moattar, F.; Sariri, R.; Giahi, M.; Yaghmaee, P. Essential oil composition and antioxidant activity of Calamintha officinalis Moench. J. Appl. Biotechnol. Rep. 2018, 5, 55–58. [Google Scholar] [CrossRef]
- Stojičić, D.; Tošić, S.; Stojanović, G.; Zlatković, B.; Jovanović, S.; Budimir, S.; Uzelac, B. Volatile organic compound composition and glandular trichome characteristics of in vitro propagated Clinopodium pulegium (Rochel) Bräuchler: Effect of carbon source. Plants 2022, 11, 198. [Google Scholar] [CrossRef] [PubMed]
- Ali-Shtayeh, M.S.; Jamous, R.M.; Abu-Zaitoun, S.Y.; Akkawi, R.J.; Kalbouneh, S.R.; Bernstein, N.; Dudai, N. Chemical profile and bioactive properties of the essential oil isolated from Clinopodium serpyllifolium (M.Bieb.) Kuntze growing in Palestine. Ind. Crop. Prod. 2018, 124, 617–625. [Google Scholar] [CrossRef]
- Sharma, R.; Shachter, A.; Almog, L.; Oren, G.; Roynik-Toshner, H.; Dudai, N. Genetic chemical variability of the volatiles and phenolic compounds in Clinopodium serpyllifolium subsp. fruticosum (L.) Bräuchler syn. Micromeria fruticosa (L.) Druce grown in Israel. Israel J. Plant Sci. 2021, 68, 142–150. [Google Scholar]
- Benites, J.; Ríos, D.; Guerrero-Castilla, A.; Enríquez, C.; Zavala, E.; Ybañez-Julca, R.O.; Quispe-Díaz, I.; Jara-Aguilar, R.; Calderon, P.B. Chemical composition and assessment of antimicrobial, antioxidant and antiproliferative activities of essential oil from Clinopodium sericeum, a Peruvian medicinal plant. Rec. Nat. Prod. 2021, 15, 175–186. [Google Scholar]
- Espinosa, S.; Bec, N.; Larroque, C.; Ramírez, J.; Sgorbini, B.; Bicchi, C.; Cumbicus, N.; Gilardoni, G. A novel chemical profile of a selective In vitro cholinergic essential oil from Clinopodium taxifolium (Kunth) Govaerts (Lamiaceae), a native Andean species of Ecuador. Molecules 2021, 26, 45. [Google Scholar] [CrossRef]
- Qi, J.-J.; Zhang, Q.-Q.; Li, L.-L.; Huang, Q.; Yao, M.; Wang, N.; Peng, D.-Y. Spectrum-effect relationship between UPLC-Q-TOF-MS fingerprint and anti-AUB effect of Clinopodium chinense (Benth.) O. Kuntze. J. Pharm. Biomed. Anal. 2022, 217, 114828. [Google Scholar] [CrossRef]
- Althaher, A.R.; Mastinu, A. Calamintha incana (Sm.) Helder: A New Phytoextract with In Vitro Antioxidant and Antidiabetic Action. Appl. Sci. 2023, 13, 3966. [Google Scholar] [CrossRef]
- Althaher, A.R.; Shehabi, R.F.; Ameen, H.H.; Awadallah, M.W.; Mastinu, A.; Proestos, C. Calamintha incana methanolic extract: Investigation of phytochemical composition and antioxidant and antibacterial activities. J. Food Biochem. 2024, 2024, 6634969. [Google Scholar] [CrossRef]
- Bardarov, K.; Todorova, T.; Miteva, D.; Bardarov, V.; Atanassov, A.; Chankova, S. Preliminary screening for study of the chemical composition of Clinopodium vulgare L. water extract. Comptes Rendus Acad. Bulg. Sci. 2016, 69, 717–724. [Google Scholar]
- Zheleva-Dimitrova, D.; Simeonova, R.; Gevrenova, R.; Savov, Y.; Balabanova, V.; Nasar-Eddin, G.; Bardarov, K.; Danchev, N. In vivo toxicity assessment of Clinopodium vulgare L. water extract characterized by UHPLC-HRMS. Food Chem. Toxicol. 2019, 134, 110841. [Google Scholar] [CrossRef]
- Yumruta, N.; Pehlivan, M.; Yumruta, P.; Kahraman, D. Possible induction of apoptotic and necrotic death pathways in lung cancer cells by Clinopodium vulgare L. and phenolic acids and flavonoids detection by LC-MS-MS. J. Boletín Latinoam. Caribe Plantas Med. Aromáticas 2024, 23, 740. [Google Scholar] [CrossRef]
- Pacifico, S.; Galasso, S.; Piccolella, S.; Kretschmer, N.; Pan, S.-P.; Marciano, S.; Bauer, R.; Monaco, P. Seasonal variation in phenolic composition and antioxidant and anti-inflammatory activities of Calamintha nepeta (L.) Savi. Food Res. Int. 2015, 69, 121–132. [Google Scholar] [CrossRef]
- Bai, S.-Y.; Li, M.-T.; Cao, Z.-Q.; Shan, S.; Wu, J.-B.; He, N. Evaluation in vitro and vivo of hemostatic properties of extract from Chinese Clinopodium herb. J. Shanxi Med. Univ. 2022, 53, 1590–1593. [Google Scholar]
- Li, L.-L.; Huang, Q.; Duan, X.-C.; Han, L.; Peng, D.-Y. Protective effect of Clinopodium chinense (Benth.) O. Kuntze against abnormal uterine bleeding in female rats. J. Pharmacol. Sci. 2020, 143, 1–8. [Google Scholar] [CrossRef]
- Li, L.-L.; Huang, Q.; Peng, D.-Y.; Qi, J.-J.; Yao, M. Anti-inflammatory and hemostatic effects of total extract, saponins, and flavonoids of Clinopodium chinense in female rats with abnormal uterine bleeding and the mechanism. China J. Chin. Mater. Med. 2022, 47, 3372–3379. [Google Scholar]
- Xing, N.; Zhang, H.-J.; Shu, Z.-P.; Xu, X.-D.; Zhu, Y.-D.; Sun, G.-B.; Wang, Q.-H.; Sun, X.-B. Antioxidant effect of different isolated polar fractions in total flavones of Clinopodium chinense. World Chin. Med. 2015, 10, 1169–1172. [Google Scholar]
- Chen, R.-C.; Xu, X.-D.; Liu, X.-Z.; Sun, G.-B.; Zhu, Y.-D.; Dong, X.; Wang, J.; Zhang, H.-J.; Zhang, Q.; Sun, X.-B. Total flavonoids from Clinopodium chinense (Benth.) O. Ktze protect against doxorubicin-induced cardiotoxicity in vitro and in vivo. Evid. Based Complement. Altern. Med. 2015, 2015, 472565. [Google Scholar]
- Zhang, H.-J.; Chen, R.-C.; Sun, G.-B.; Yang, L.-P.; Zhu, Y.-D.; Xu, X.-D.; Sun, X.-B. Protective effects of total flavonoids from Clinopodium chinense (Benth.) O. Ktze on myocardial injury in vivo and in vitro via regulation of Akt/Nrf2/HO-1 pathway. Phytomedicine 2018, 40, 88–97. [Google Scholar] [CrossRef]
- Zhang, N.; Ma, J.; Wu, G.-L. Effects of total flavonoids of Clinopodium chinense combined with miR-702-5p in-hibitor on myocardial cell injury induced by hypoxia / reoxygenation. Pract. Pharm. Clin. Remedies 2021, 24, 23–28. [Google Scholar]
- Tubon, I.; Bernardini, C.; Antognoni, F.; Mandrioli, R.; Potente, G.; Bertocchi, M.; Vaca, G.; Zannoni, A.; Salaroli, R.; Forni, M. Clinopodium tomentosum (Kunth) Govaerts leaf extract influences in vitro cell proliferation and angiogenesis on primary cultures of porcine aortic endothelial cells. Oxidative Med. Cell. Longev. 2020, 2020, 2984613. [Google Scholar] [CrossRef] [PubMed]
- Nasar-Eddin, G.; Simeonova, R.; Zheleva-Dimitrova, D.; Gevrenova, R.; Savov, I.; Bardarov, K.; Danchev, N. Beneficial effects of Clinopodium vulgare water extract on spontaneously hypertensive rats. Bulg. Chem. Commun 2019, 51, 156–160. [Google Scholar]
- Shi, X.-J.; Wang, S.-S.; Luan, H.-L.; Tuerhong, D.; Lin, Y.-N.; Liang, J.-Y.; Xiong, Y.; Rui, L.-Y.; Wu, F.-H. Clinopodium chinense attenuates palmitic acid-induced vascular endothelial inflammation and insulin resistance through TLR4-mediated NF-κ B and MAPK pathways. Am. J. Chin. Med. 2019, 47, 97–117. [Google Scholar] [CrossRef]
- Wang, Y.; Shao, Z.; Song, C.; Zhou, H.; Zhao, J.; Zong, K.; Zhou, G.; Meng, D. Clinopodium chinense Kuntze ameliorates dextran sulfate sodium-induced ulcerative colitis in mice by reducing systematic inflammation and regulating metabolism. J Ethnopharmacol. 2023, 309, 116330. [Google Scholar] [CrossRef]
- Wang, S.-L.; Chen, L.-M.; Jin, T.; Lin, J.-M.; Shi, Y. The effect and mechanism of polyphenolic extract from Lonicera japonica on apoptosis promotion of lung cancer cells in vitro. Pharm. Clin. Chin. Mater. Med. 2018, 34, 93–98. [Google Scholar]
- Mohanty, S.; Kamolvit, W.; Zambrana, S.; Sandström, C.; Gonzales, E.; Östenson, C.-G.; Brauner, A. Extract of Clinopodium bolivianum protects against E. coli invasion of uroepithelial cells. J. Ethnopharmacol. 2017, 198, 214–220. [Google Scholar] [CrossRef]
- D’Aquila, P.; Paparazzo, E.; Crudo, M.; Bonacci, S.; Procopio, A.; Passarino, G.; Bellizzi, D. Antibacterial activity and epigenetic remodeling of essential oils from Calabrian aromatic plants. Nutrients 2022, 14, 391. [Google Scholar] [CrossRef]
- Beddiar, H.; Boudiba, S.; Benahmed, M.; Tamfu, A.N.; Ceylan, O.; Hanini, K.; Kucukaydin, S.; Elomri, A.; Bensouici, C.; Laouer, H.; et al. Chemical composition, anti-quorum sensing, enzyme inhibitory, and antioxidant properties of phenolic extracts of Clinopodium nepeta L. Kuntze. Plants 2021, 10, 1955. [Google Scholar] [CrossRef] [PubMed]
- Bouzidi, N.; Kemieg, M. Antioxidant and antimicrobial activities of essential oil of Satureja calamintha ssp. nepeta (L.) Briq. from the northwest of Algeria. Agric. Conspec. Sci. 2021, 86, 349–356. [Google Scholar]
- Sharifi, S.; Naseri, N.; Fathiazad, F.; Asnaashari, S.; Hamedeyazdan, S. Anticancer effect of buddlejasaponin IV and buddlejasaponin IVa from Clinopodium umbrosum on oral cancer cells (HN-5). Toxicon 2022, 220, 106939. [Google Scholar] [CrossRef]
- Campos, D.; García-Ríos, D.; Aguilar-Galvez, A.; Chirinos, R.; Pedreschi, R. Comparison of conventional and ultrasound-assisted extractions of polyphenols from Inca muña (Clinopodium bolivianum) and their characterization using UPLC–PDA-ESI–Q/TOF–MSn technique. J. Food Process. Preserv. 2022, 46, e16310. [Google Scholar] [CrossRef]
- Hodaj-Çeliku, E.; Tsiftsoglou, O.; Shuka, L.; Abazi, S.; Hadjipavlou-Litina, D.; Lazari, D. Antioxidant activity and chemical composition of essential oils of some aromatic and medicinal plants from Albania. Nat. Prod. Commun. 2017, 12, 785–790. [Google Scholar] [CrossRef] [PubMed]
- El Brahimi, R.; El Barnossi, A.; El Moussaoui, A.; Chebaibi, M.; Kachkoul, R.; Baghouz, A.; Nafidi, H.-A.; Salamatullah, A.M.; Bourhia, M.; Bari, A. Phytochemistry and biological activities of essential oils from Satureja calamintha nepeta. Separations 2023, 10, 344. [Google Scholar] [CrossRef]
- Gezici, S.; Koçum, D.; Yayla, F.; Sekeroglu, N.; Khan, A.A. Screening for in vitro antioxidant activities, polyphenolic contents and neuroprotective potentials of Clinopodium serpyllifolium subsp. serpyllifolium endemic to Turkey. . Ann. Phytomedicine Int. J. 2020, 9, 181–186. [Google Scholar]
- Sarikurkcu, C.; Ozer, M.S.; Tepe, B.; Dilek, E.; Ceylan, O. Phenolic composition, antioxidant and enzyme inhibitory activities of acetone, methanol and water extracts of Clinopodium vulgare L. subsp. vulgare L. Ind. Crop. Prod. 2015, 76, 961–966. [Google Scholar] [CrossRef]
- Nassar-Eddin, G.; Zheleva-Dimitrova, D.; Danchev, N.; Vitanska-Simeonova, R. Antioxidant and enzyme-inhibiting activity of lyophilized extract from Clinopodium vulgare L. (Lamiaceae). Pharmacia 2021, 68, 259–263. [Google Scholar] [CrossRef]
- Bektašević, M.; Politeo, O.; Roje, M.; Jurin, M. Polyphenol composition, anticholinesterase and antioxidant potential of the extracts of Clinopodium vulgare L. Chem. Biodivers. 2022, 19, e202101002. [Google Scholar] [CrossRef]
- Khan, S.; Khan, T.; Shah, A.J. Total phenolic and flavonoid contents and antihypertensive effect of the crude extract and fractions of Calamintha vulgaris. Phytomedicine 2018, 47, 174–183. [Google Scholar] [CrossRef] [PubMed]
- Azzane, A.; Azzaoui, B.; Akdad, M.; Bouadid, I.; Eddouks, M. Effect of calamintha officinalis on vascular contractility and angiotensinconverting enzyme-2. Cardiovasc. Hematol. Agents Med.Chem. 2022, 20, 219–236. [Google Scholar] [PubMed]
- Arantes, S.; Piçarra, A.; Candeias, F.; Teixeira, D.; Caldeira, A.T.; Martins, M.R. Antioxidant activity and cholinesterase inhibition studies of four flavouring herbs from Alentejo. Nat. Prod. Res. 2017, 31, 2183–2187. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-M.; Du, K.-C.; Wang, Q.-Y.; Yang, X.-Y.; Meng, D.-L. A multidimensional strategy for characterization, distinction, and quality control of two Clinopodium medicinal plants. J. Ethnopharmacol. 2024, 327, 118019. [Google Scholar] [CrossRef]
- Zoheir, K.; Bnouham, M.; Legssyer, A.; Ziyyat, A.; Berrabah, M.; Aziz, M.; Bensaid, M.; Mekhfi, H. Antithrombotic, antiaggregant and anticoagulant effect of methanolic fraction of Calamintha officinalis: In vitro and ex vivo experiments. J. Nat. Remedies 2018, 18, 131–142. [Google Scholar] [CrossRef]
- Ciumarnean, L.; Milaciu, M.V.; Runcan, O.; Vesa, S.C.; Rachisan, A.L.; Negrean, V.; Perne, M.G.; Donca, V.I.; Alexescu, T.G.; Para, I.; et al. The effects of flavonoids in cardiovascular diseases. Molecules 2020, 25, 4320. [Google Scholar] [CrossRef]
- Sánchez, M.; Romero, M.; Gómez-Guzmán, M.; Tamargo, J.; Pérez-Vizcaino, F.; Duarte, J. Cardiovascular effects of flavonoids. Curr. Med. Chem. 2019, 26, 6991–7034. [Google Scholar] [CrossRef]
- Taibi, M.; Elbouzidi, A.; Haddou, M.; Baraich, A.; Gharsallaoui, A.; Mothana, R.A.; Alqahtani, A.M.; Asehraou, A.; Bellaouchi, R.; Addi, M.; et al. Evaluation of the interaction between menthol and camphor, major compounds of Clinopodium nepeta essential oil: Antioxidant, anti-inflammatory and anticancer activities against breast cancer cell lines. Chem. Biodivers. 2025, 22, e202403098. [Google Scholar] [CrossRef]
- Amirova, K.M.; Dimitrova, P.; Marchev, A.S.; Aneva, I.Y.; Georgiev, M.I. Clinopodium vulgare L. (wild basil) extract and its active constituents modulate cyclooxygenase-2 expression in neutrophils. Food Chem. Toxicol. 2019, 124, 1–9. [Google Scholar] [CrossRef]
- Medjdoub, A.R.; Moussaoui, A.; Benzina, F.T.; Benmehdi, H. Qualitative and quantitative analysis and antifungal effects of Satureja calamintha spp. (nepeta) Briq from eastern Algeria. Adv. Food Sci. 2021, 43, 25–35. [Google Scholar]
- Gao, J.-Y.; Cai, G.; Liao, J.; Pan, F.-L.; Peng, X.-L.; Liu, S.-B.; Zhang, L. Bacteriostasis effects of extract from Clinopodium chinense and Leptopus chinensis, two folk medicinal herbs. Hunan Agric. Sci. 2019, 2, 7–10. [Google Scholar] [CrossRef]
- Vorobets, N.; Yavorska, H.; Svydenko, L. Antibacterial activity of Calamintha mentifolia Host. essential oils. Planta Medica 2022, 88, 1530. [Google Scholar]
- Jaramillo, L.I.; Bouajila, J.; Vera, E.; Camy, S. Influence of process parameters on yield, chemical composition and biological activities of Clinopodium nubigenum (Kunth) Kuntze (sunfo) extracts obtained by supercritical CO2 extraction and other techniques. J. Supercrit. Fluids 2025, 218, 106492. [Google Scholar] [CrossRef]
- Hernández, E.; Bermejo, P.; Abad, M.J.; Beltrán, M.; Alcamí, J.; Prieto, A.; Guerra, J.A.; Bedoya, L.M. Characterization of heterogeneous polysaccharides from the aerial parts of Clinopodium bolivianum (Benth.) with neutralizing activity against HIV-1 infection. Polysaccharides 2025, 6, 18. [Google Scholar] [CrossRef]
- Aribi, I.; Chemat, S.; Van Puyvelde, L.; Luyten, W. Bioassay-guided fractionation of Calamintha baborensis Batt. herbal extracts reveals disaccharide glucuronide as a potent antistaphylococcal compound. S. Afr. J. Bot. 2022, 147, 35–41. [Google Scholar] [CrossRef]
- Cüce, M.; Bekircan, T.; Laghari, A.; Sokmen, M.; Sokmen, A.; Ucar, E.; Kılıç, A. Phenolic profiles, antimicrobial and cytotoxic properties of both micropropagated and naturally growing plantlets of Calamintha sylvatica subsp. sylvatica Bromf. Not. Bot. Horti Agrobo. 2019, 47, 1145–1152. [Google Scholar] [CrossRef]
- Benfreha, H.; Pereira, E.C.V.; Rolim, L.A.; Chelli, N.; Almeida, J.R.G.d.S.; Tirtouil, A.; Meddah, B. Additive effect of the probiotics Lactobacillus exopolysaccharides and the Satureja calamintha extracts on enteropathogenic Escherichia coli adhesion. Braz. J. Pharm. Sci. 2022, 58, e20015. [Google Scholar] [CrossRef]
- Georgieva, A.; Sulikovska, I.; Toshkova-Yotova, T.; Djeliova, V.; Amiri, S.; Tsonevski, N.; Petkova-Kirova, P.; Tasheva, K. Antitumor activity of whole-plant extracts from in vitro cultured and wild-growing Clinopodium vulgare plants on a panel of human tumor cell lines. Appl. Sci. 2025, 15, 925. [Google Scholar] [CrossRef]
- Kilinç, B.Ö.; Gödelek, D.; Süfer, Ö.; Saygideğer Demir, B.; Sezan, A.; Saygideğer, Y.; Bozok, F. Essential oils from some Lamiaceae plants: Antioxidant and anticancer potentials besides thermal properties. Chem. Biodivers. 2022, 19, e202200418. [Google Scholar] [CrossRef]
- Halmoune, A.; Salhi, N.; Hamza, E.L.F.; Abdessamad, A.B.; Khalid, K.; Abdelhamid, Z.; Ihoussaine, E.R. Phytochemical study, polyphenols determination and evaluation of antioxidant activity of Satureja calamintha from Morocco. Indian J. Pharm. Educ. Res. 2024, 58, 1167–1173. [Google Scholar] [CrossRef]
- Božović, M.; Ragno, R. Calamintha nepeta (L.) Savi and its main essential oil constituent pulegone: Biological activities and chemistry. Molecules 2017, 22, 290. [Google Scholar] [CrossRef] [PubMed]
- Salhi, N.; Deluyker, D.; Bito, V.; Zaid, A.; El Rhaffari, L. In vitro biological activities of Calamintha nepeta L. aqueous extracts. J. Appl. Biomed. 2024, 22, 155–163. [Google Scholar] [CrossRef]
- Eddouks, M.; Hebi, M.; Ajebli, M.; El Hidani, A.; Sulpice, T.; Burcelin, R. Study of antidiabetic effect of Capparis spinosa L. and Calamintha officinalis Moench in diabetic mice. Phytothérapie 2017, 1–9. [Google Scholar] [CrossRef]
- Lazarova, M.; Tsvetanova, E.; Georgieva, A.; Stefanova, M.; Uzunova, D.; Denev, P.; Vassileva, V.; Tasheva, K. Extracts of Sideritis scardica and Clinopodium vulgare alleviate cognitive impairments in scopolamine-induced rat dementia. Int. J. Mol. Sci. 2024, 25, 1480. [Google Scholar] [CrossRef]
- El Aatiaoui, A.; Daoudi, W.; El Badri, A.; Salhi, A.; El Massaoudi, M.; El Boutaybi, A.; Guo, L.; Loutou, M. Anticorrosive potential of essential oil extracted from the leaves of Calamintha plant for mild steel in 1 M HCl medium. J. Adhes. Sci. Technol. 2023, 37, 1191–1214. [Google Scholar] [CrossRef]
- Tian, J.-G.; Zhou, J.-J.; Ni, Q.; Feng, Y.-L. Establishment of HPLC specific chromatogram of Clinopodium herb and determination of the contents of 3 components. Northwest Pharm. J. 2022, 37, 12–17. [Google Scholar]
- Yang, Z.-Z.; Yang, Y.-H.; Zhong, Y.-M.; Jiang, M.-L.; Zhang, J.; Wang, L.-J.; Long, F.; Li, M. Study on quality standard for Miao medicine Clinopodium gracile. China Pharm. 2023, 34, 682–686. [Google Scholar]
- Gao, Y.; Wang, Y.; Wang, K.; Zhu, J.; Li, G.; Tian, J.; Li, C.; Wang, Z.; Li, J.; Lee, A.W.; et al. Acute and a 28-day repeated-dose toxicity study of total flavonoids from Clinopodium chinense (Benth.) O. Ktze in mice and rats. Regul. Toxicol. Pharmacol. 2017, 91, 117–123. [Google Scholar] [CrossRef]
- Li, L.-L.; Huang, Q.; Wang, M.-M.; Gan, J.-H.; Peng, D.-Y. Simultaneous determination of five compounds of Clinopodium chinense in rat plasma by LC-MS/MS and its application to a pharmacokinetic study. Lat. Am. J. Pharm. 2019, 38, 1143–1152. [Google Scholar]
- Althaher, A.R.; Jarrar, Y.; Al-Ibadah, M.A.; Balasmeh, R.; Jarrar, Q.; Abulebdah, D. Effects of Calamintha incana (Sm.) Helder ethanolic extract on the mRNA expression of drug-metabolizing cyp450s in the mouse livers. MicroRNA 2024, 13, 63–70. [Google Scholar] [CrossRef]
| No. | Name | Sources | Organs | Extraction Solvent | Collection Areas | Ref. |
|---|---|---|---|---|---|---|
| Triterpenoids | ||||||
| 1 *, 1 | clinopodiside VIII | C. chinense | Aerial parts | 70% ethanol | Bozhou, Anhui Province, China | [8] |
| 2 * | clinopodiside IX | C. chinense | Aerial parts | 70% ethanol | Bozhou, Anhui Province, China | [8] |
| 3 * | clinopodiside X | C. chinense | Aerial parts | 70% ethanol | Bozhou, Anhui Province, China | [8] |
| 4 * | clinopodiside XI | C. chinense | Aerial parts | 70% ethanol | Bozhou, Anhui Province, China | [8] |
| 5 | 16β,21β,23,28-tetrahydroxyoleana-9(11),12(13)-diene-3-yl-[β-D-glucopyranosyl-(1→2)]-[β-D-glucopyranosyl-(1→3)]-β-D-fucopyranoside | C. chinense | Aerial parts | 70% ethanol | Bozhou, Anhui Province, China | [8] |
| 6 * | clinopodiside VI | C. polycephalum | Aerial parts | 70% aqueous EtOH | Anhui Province, China | [9] |
| 7 * | clinopodiside VII | C. chinense | Aerial parts | 70% ethanol | Bozhou, Anhui Province, China | [8] |
| 8 | saikosaponin g | C. chinense | Aerial parts | 70% ethanol | Bozhou, Anhui Province, China | [8] |
| 9 | pleurosaponin I | C. gracile | Whole herb | 70% aqueous EtOH | Shaanxi province, China | [10] |
| 10 | 16β,23,28-trihydroxyoleana-9(11),12(13)-dien-3-yl-[β-D-glucopyranosyl-(1→2)]-[β-D-glucopyranosyl (1→3)]-β-D-fucopyranoside | C. chinense | Aerial parts | 70% ethanol | Bozhou, Anhui Province, China | [8] |
| 11 * | clinopodiside XII | C. chinense | Aerial parts | 70% ethanol | Bozhou, Anhui Province, China | [8] |
| 12 | 16β,23,28-trihydroxyoleana-9(11),12(13)-diene-3-yl-[β-D-glucopyranosyl-(1→4)-β-D-glucopyranosyl-(1→6)-β-D-glucopyranosyl-(1→3)]-[β-D-glucopyranosyl-(1→2)]-β-D-fucopyranoside | C. chinense | Aerial parts | 70% ethanol | Bozhou, Anhui Province, China | [8] |
| 13 | saikogenin A | C. chinense | Aerial parts | 80% EtOH | Putian, Fujian province, China | [11] |
| 14 | prosaikogenin A | C. chinense | Aerial parts | 80% EtOH | Putian, Fujian province, China | [11] |
| 15 * | clinoposaponin F | C. chinense | Aerial parts | 70% ethanol | Bozhou, Anhui province, China | [12] |
| 16 * | clinoposaponin G | C. chinense | Aerial parts | 70% ethanol | Bozhou, Anhui province, China | [12] |
| 17 * | clinoposaponin E | C. chinense | Aerial parts | 70% EtOH | Bozhou, Anhui Province, China | [13] |
| 18 * | saikosaponin c | C. polycephalum | Aerial parts | 70% aqueous EtOH | Anhui Province, China | [9] |
| C. gracile | Whole herb | 70% aqueous EtOH | Shaanxi province, China | [10] | ||
| 19 | clinopodiside I | C. polycephalum | Aerial parts | 70% aqueous EtOH | Anhui Province, China | [9] |
| 20 | saikosaponin b1 | C. polycephalum | Aerial parts | 70% aqueous EtOH | Anhui Province, China | [9] |
| 21 | buddlejasaponin IVb | C. polycephalum | Aerial parts | 70% aqueous EtOH | Anhui Province, China | [9] |
| C. gracile | Whole herb | 70% aqueous EtOH | Shaanxi province, China | [10] | ||
| C. chinense | Aerial parts | 80% EtOH | Putian, Fujian province, China | [11] | ||
| 22 * | clinograsaponin B | C. gracile | Whole herb | 70% aqueous EtOH | Shaanxi province, China | [10] |
| 23 | tibesaikosaponin IV | C. gracile | Whole herb | 70% aqueous EtOH | Shaanxi province, China | [10] |
| 24 * | clinoposaponin D | C. gracile | Whole herb | 70% aqueous EtOH | Shaanxi province, China | [10] |
| C. chinense | Aerial parts | 80% EtOH | Putian, Fujian province, China | [11] | ||
| 25 | 11α,16β,23,28-tetrahydroxyolean-12-en-3β-yl-[β-D-glucopyranosyl-(1→2)]-[β-D-glucopyranosyl-(1→3)]-β-D-fucopyranoside | C. chinense | Aerial parts | 80% EtOH | Putian, Fujian province, China | [11] |
| 26 * | clinopoditerpene B | C. chinense | Aerial parts | 70% ethanol | Bozhou, Anhui Province, China | [14] |
| 27 * | clinopoditerpene C | C. chinense | Aerial parts | 70% ethanol | Bozhou, Anhui Province, China | [14] |
| 28 * | clinopoditerpene D | C. chinense | Aerial parts | 70% EtOH | Bozhou, Anhui Province, China | [13] |
| 29 | perovskiaditerpenoside B | C. chinense | Aerial parts | 70% EtOH | Bozhou, Anhui Province, China | [13] |
| 30 | 3β-hydroxy-12-O-β-D-glucopyranosyl-8,11,13-abietatrien-7-one | C. chinense | Aerial parts | 70% EtOH | Bozhou, Anhui Province, China | [13] |
| 31 | 12-O-β-D-glucopyranosyl-3,11,16-trihydroxyabieta-8,11,13-triene | C. chinense | Aerial parts | 70% EtOH | Bozhou, Anhui Province, China | [13] |
| 32 | cussoracoside A | C. chinense | Aerial parts | 70% EtOH | Bozhou, Anhui Province, China | [13] |
| 33 | 15-hydroxy-12-oxo-abietic acid | C. bolivianum | Aerial parts | Aqueous extract | Ingavi province, La Paz department, Bolivia | [15] |
| 34 | 12α-hydroxy-abietic acid | C. bolivianum | Aerial parts | Aqueous extract | Ingavi province, La Paz department, Bolivia | [15] |
| 35 | (−)-jolkinolide E | C. bolivianum | Aerial parts | Aqueous extract | Ingavi province, La Paz department, Bolivia | [15] |
| 36 | 15-hydroxy-dehydroabietic acid | C. bolivianum | Aerial parts | Aqueous extract | Ingavi province, La Paz department, Bolivia | [15] |
| 37 | clinopodiside G | C. chinense | Aerial parts | 70% EtOH | Bozhou, Anhui Province, China | [13] |
| 38 * | clinopodiside H | C. chinense | Aerial parts | 70% EtOH | Bozhou, Anhui Province, China | [16] |
| 39 | clinopodiside D | C. chinense | Aerial parts | 80% EtOH | Putian, Fujian province, China | [11] |
| 40 | clinoposaponin XVI | C. chinense | Aerial parts | 70% ethanol | Bozhou, Anhui Province, China | [8] |
| 41 | clinoposaponin XX | C. chinense | Aerial parts | 70% ethanol | Bozhou, Anhui Province, China | [8] |
| 42 | clinoposaponin XIX | C. chinense | Aerial parts | 70% ethanol | Bozhou, Anhui Province, China | [8] |
| 43 | clinoposaponin XI | C. gracile | Whole herb | 70% aqueous EtOH | Shaanxi province, China | [10] |
| 44 | clinoposaponin IV | C. gracile | Whole herb | 70% aqueous EtOH | Shaanxi province, China | [10] |
| 45 | saikogenin F | C. chinense | Aerial parts | 80% EtOH | Putian, Fujian province, China | [11] |
| 46 | saikosaponin a | C. chinense | Aerial parts | 70% ethanol | Bozhou, Anhui province, China | [12] |
| 47 | clinoposaponin XV | C. gracile | Whole herb | 70% aqueous EtOH | Shaanxi province, China | [10] |
| C. chinense | Whole grass | MeOH | Wonju, Gangwon, Korea | [17] | ||
| 48 | buddlejasaponin IV | C. chinense | Aerial parts | 70% ethanol | Bozhou, Anhui Province, China | [8] |
| C. polycephalum | Aerial parts | 70% aqueous EtOH | Anhui Province, China | [9] | ||
| C. gracile | Whole herb | 70% aqueous EtOH | Shaanxi province, China | [10] | ||
| C. chinense | Aerial parts | 80% EtOH | Putian, Fujian province, China | [11] | ||
| C. chinense | Whole grass | MeOH | Wonju, Gangwon, Korea | [17] | ||
| C. umbrosum | Aerial parts | Extraction using methanol | Noshahr, Mazandaran, Iran | [18] | ||
| 49 * | clinopoursaponin A | C. chinense | Aerial parts | 70% ethanol | Bozhou, Anhui Province, China | [8] |
| 50 * | clinopoursaponin B | C. chinense | Aerial parts | 70% ethanol | Bozhou, Anhui Province, China | [8] |
| 51 * | clinopoursaponin C | C. chinense | Aerial parts | 70% ethanol | Bozhou, Anhui Province, China | [8] |
| 52 * | clinopoursaponin D | C. chinense | Aerial parts | 70% ethanol | Bozhou, Anhui Province, China | [8] |
| 53 | comastomasaponin E | C. gracile | Whole herb | 70% aqueous EtOH | Shaanxi province, China | [10] |
| 54 | saikosaponin b3 | C. gracile | Whole herb | 70% aqueous EtOH | Shaanxi province, China | [10] |
| 55 | buddlejasaponin IVa | C. gracile | Whole herb | 70% aqueous EtOH | Shaanxi province, China | [10] |
| C. chinense | Aerial parts | 80% EtOH | Putian, Fujian province, China | [11] | ||
| C. chinense | Aerial parts | 70% EtOH | Bozhou, Anhui Province, China | [13] | ||
| C. umbrosum | Aerial parts | Extraction using methanol | Noshahr, Mazandaran, Iran | [18] | ||
| 56 * | Polycephalum A | C. polycephalum | Whole grass | 70% ethanol | Liangwang mountain, Kunming, Yunnan, China | [19] |
| 57 * | clinograsaponin A | C. gracile | Whole herb | 70% aqueous EtOH | Shaanxi province, China | [10] |
| 58 * | clinoposide A | C. chinense | Aerial parts | 70% ethanol | Bozhou, Anhui Province, China | [20] |
| 59 | clinoposide B | C. chinense | Aerial parts | 70% ethanol | Bozhou, Anhui Province, China | [20] |
| 60 * | clinoposide C | C. chinense | Aerial parts | 70% ethanol | Bozhou, Anhui Province, China | [20] |
| 61 * | clinoposide D | C. chinense | Aerial parts | 70% ethanol | Bozhou, Anhui Province, China | [20] |
| 62 * | clinoposide E | C. chinense | Aerial parts | 70% ethanol | Bozhou, Anhui Province, China | [20] |
| 63 * | clinoposide F | C. chinense | Aerial parts | 70% ethanol | Bozhou, Anhui Province, China | [20] |
| 64 * | clinoposide G | C. chinense | Aerial parts | 70% ethanol | Bozhou, Anhui Province, China | [21] |
| 65 * | clinoposide H | C. chinense | Aerial parts | 70% ethanol | Bozhou, Anhui Province, China | [21] |
| 66 * | imbricatusol I | C. polycephalum | Whole grass | 70% ethanol | Kunming, Yunnan province, China | [22] |
| 67 * | saturol I | C. polycephalum | Whole grass | 70% ethanol | Kunming, Yunnan province, China | [22] |
| 68 * | 3β-22,25-dihydroxy-tirucalla-7,23-diene | C. polycephalum | Whole grass | 70% ethanol | Kunming, Yunnan province, China | [22] |
| 69 | maslinic acid | C. polycephalum | Whole grass | 70% ethanol | Kunming, Yunnan province, China | [22] |
| 70 | 2α,3α-dihydroxyolean-12-en-28-oic acid | C. polycephalum | Whole grass | 70% ethanol | Kunming, Yunnan province, China | [22] |
| 71 | hederagenin | C. polycephalum | Whole grass | 70% ethanol | Kunming, Yunnan province, China | [22] |
| 72 | 2α,3α-dihydroxyursolic acid | C. polycephalum | Whole grass | 70% ethanol | Kunming, Yunnan province, China | [22] |
| 73 | alphitolic acid | C. polycephalum | Whole grass | 70% ethanol | Kunming, Yunnan province, China | [22] |
| 74 | arjunglucoside I | C. polycephalum | Aerial parts | 70% aqueous EtOH | Anhui Province, China | [9] |
| 75 | clinopodiside II | C. polycephalum | Aerial parts | 70% aqueous EtOH | Anhui Province, China | [9] |
| 76 * | chinense A | C. chinense | Whole herb | 70% EtOH | Guilin, Guangxi, China | [23] |
| 77 * | clinopoditerpene E | C. chinense | Whole herb | 70% EtOH | Guilin, Guangxi, China | [23] |
| 78 * | (4S,9S)-9-hydroxyjasmololone | C. chinense | Aerial parts | 70% EtOH | Bozhou, Anhui province, China | [24] |
| Flavonoids | ||||||
| 79 | apigenin | C. chinense | Whole plant | 80% EtOH | Putian, Fujian province, China | [25] |
| Aerial parts | 70% ethanol | Anhui province, China | [26] | |||
| 80 | luteolin | C. chinense | Whole plant | 80% EtOH | Putian, Fujian province, China | [25] |
| Aerial parts | 70% ethanol | Anhui province, China | [26] | |||
| 81 | buddleoside | C. chinense | Whole plant | 80% EtOH | Putian, Fujian province, China | [25] |
| Aerial parts | 70% ethanol | Anhui province, China | [26] | |||
| 82 | apigenin-7-O-β-D-glucuronide | C. chinense | Aerial parts | 70% ethanol | Anhui province, China | [26] |
| 83 | apigenin-7-O-β-D-glucuronopyranoside | C. chinense | Aerial parts | 70% ethanol | Anhui province, China | [26] |
| 84 | apigenin 7-O-β-D-pyranglycuronate butyl ester | C. chinense | Aerial parts | 70% ethanol | Anhui province, China | [26] |
| 85 | apigenin-7-O-α-L-rhamnopyranosyl (1→6)-β-D-glucopyranoside | C. chinense | Aerial parts | 70% ethanol | Anhui province, China | [26] |
| 86 | luteolin-4′-O-β-D-glucopyranoside | C. chinense | Aerial parts | 70% ethanol | Anhui province, China | [26] |
| 87 | luteolin-7-O-β-D-pyranglycuronate butyl ester | C. chinense | Aerial parts | 70% ethanol | Anhui province, China | [26] |
| 88 | luteolin-7-O-β-D-glucuronide methyl ester | C. chinense | Aerial parts | 70% EtOH | Bozhou, Anhui province, China | [24] |
| 89 | luteolin-7-O-rutinoside | C. chinense | Aerial parts | 70% ethanol | Anhui province, China | [26] |
| 90 | luteolin-7-O-neohesperidoside | C. chinense | Aerial parts | 70% ethanol | Anhui province, China | [26] |
| 91 | acacetin | C. chinense | Aerial parts | 70% ethanol | Anhui province, China | [26] |
| 92 | acacetin 7-O-glucuronide | C. chinense | Aerial parts | 70% ethanol | Anhui province, China | [26] |
| 93 | naringenin | C. chinense | Whole plant | 80% EtOH | Putian, Fujian province, China | [25] |
| Aerial parts | 70% ethanol | Anhui province, China | [26] | |||
| 94 | eriodictyol | C. chinense | Whole plant | 80% EtOH | Putian, Fujian province, China | [25] |
| 95 | isosakuranetin | C. chinense | Whole plant | 80% EtOH | Putian, Fujian province, China | [25] |
| Aerial parts | 70% ethanol | Anhui province, China | [26] | |||
| 96 | didymin/neoponcirin | C. chinense var. shibetchense (H. Lev) Koidz | Whole grass | MeOH | Wonju, Gangwon, Korea | [17] |
| C. chinense | Whole plant | 80% EtOH | Putian, Fujian province, China | [25] | ||
| Aerial parts | 70% ethanol | Anhui province, China | [26] | |||
| 97 | hesperidin | C. chinense | Whole plant | 80% EtOH | Putian, Fujian province, China | [25] |
| Aerial parts | 70% ethanol | Anhui province, China | [26] | |||
| 98 | prunin | C. chinense | Aerial parts | 70% ethanol | Anhui province, China | [26] |
| 99 | naringenin 7-O-rutinoside/isonaringin | C. chinense var. shibetchense (H. Lev) Koidz | Whole grass | MeOH | Wonju, Gangwon, Korea | [17] |
| C. chinense | Aerial parts | 70% ethanol | Anhui province, China | [26] | ||
| 100 | naringenin-7-O-β-D-glucuronide | C. chinense | Aerial parts | 70% EtOH | Bozhou, Anhui province, China | [24] |
| 101 * | polycephalum B | C. polycephalum | Whole grass | 70% ethanol | Liangwang mountain, Kunming, Yunnan, China | [19] |
| 102 | isosakuranin | C. chinense | Aerial parts | 70% ethanol | Anhui province, China | [26] |
| 103 | kaempferol | C. chinense | Aerial parts | 70% ethanol | Anhui province, China | [26] |
| 104 | quercetin | C. chinense | Aerial parts | 70% ethanol | Anhui province, China | [26] |
| 105 | kaempferol-3-O-glucorhamnoside | C. chinense | Aerial parts | 70% ethanol | Anhui province, China | [26] |
| Phenylpropanoids | ||||||
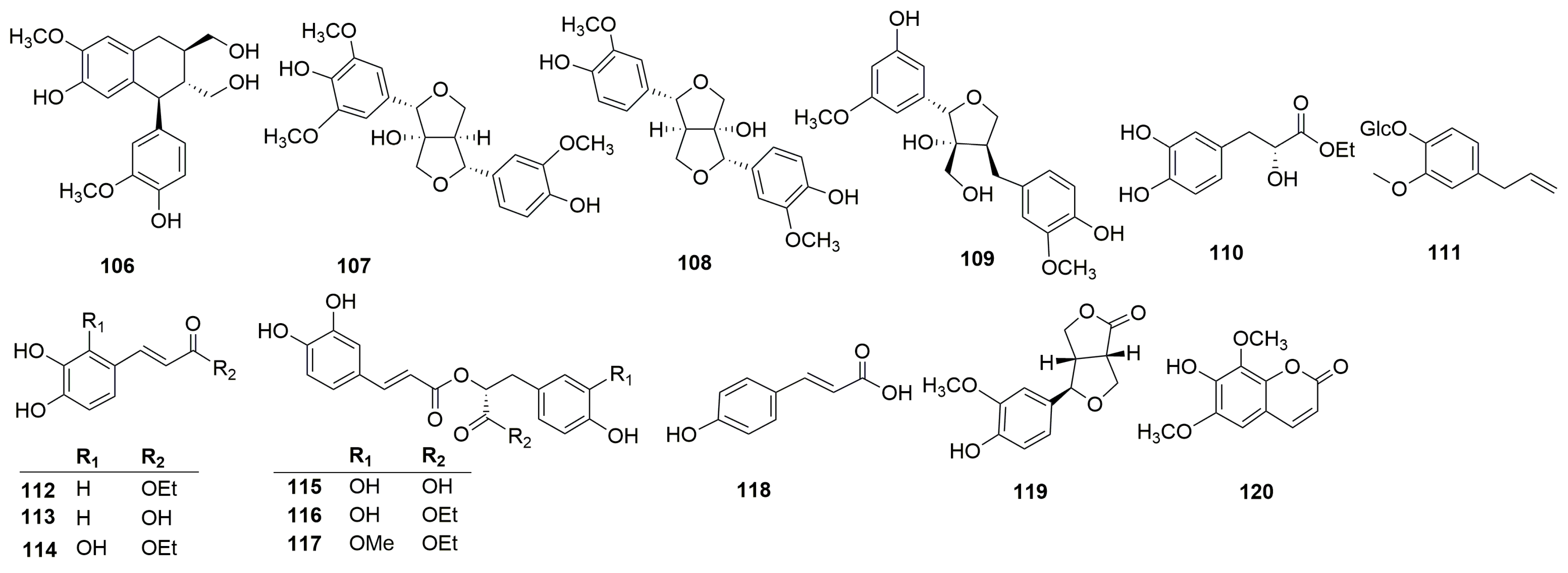
| 106 | (+)-isolariciresinol | C. chinense | Aerial parts | 70% ethanol | Bozhou, Anhui province, China | [12] |
| 107 | fraxiresinol | C. chinense | Aerial parts | 70% ethanol | Bozhou, Anhui province, China | [12] |
| 108 | 8-hydroxy-7′-epipinoresinol | C. chinense | Aerial parts | 70% ethanol | Bozhou, Anhui province, China | [12] |
| 109 | deltoignan A | C. chinense | Aerial parts | 70% ethanol | Bozhou, Anhui province, China | [12] |
| 110 | ethyl (2R)-3-(3,4-dihydroxyphenyl)-2-hydroxypropanoate | C. chinense | Whole plant | 80% EtOH | Putian, Fujian province, China | [25] |
| 111 | 2-methoxy-4-(2-propenyl)-phenyl-β-D-glucopyranoside | C. chinense | Aerial parts | 70% EtOH | Bozhou, Anhui province, China | [24] |
| 112 | ethyl (2E)-3-(3,4-dihydroxyphenyl) prop-2-enoate | C. chinense | Whole plant | 80% EtOH | Putian, Fujian province, China | [25] |
| 113 | caffeic acid | C. chinense | Whole plant | 80% EtOH | Putian, Fujian province, China | [25] |
| Aerial parts | 70% ethanol | Anhui province, China | [26] | |||
| 114 | ethyl (2E)-3-(2,3,4-trihydroxyphenyl) prop-2-enoate | C. chinense | Whole plant | 80% EtOH | Putian, Fujian province, China | [25] |
| 115 | rosmarinic acid | C. umbrosum | Aerial parts | Methanol | Noshahr, Mazandaran, Iran | [18] |
| 116 | ethyl rosmarinate | C. chinense | Whole plant | 80% EtOH | Putian, Fujian province, China | [25] |
| 117 | clinopodic acid B | C. chinense | Whole plant | 80% EtOH | Putian, Fujian province, China | [25] |
| 118 | p-hydroxycinnamic acid | C. chinense | Aerial parts | 70% ethanol | Anhui province, China | [26] |
| 119 | salicifoliol | C. chinense | Aerial parts | 70% ethanol | Bozhou, Anhui Province, China | [12] |
| 120 | isofraxidin | C. chinense | Aerial parts | 70% ethanol | Bozhou, Anhui province, China | [12] |
| Others | ||||||
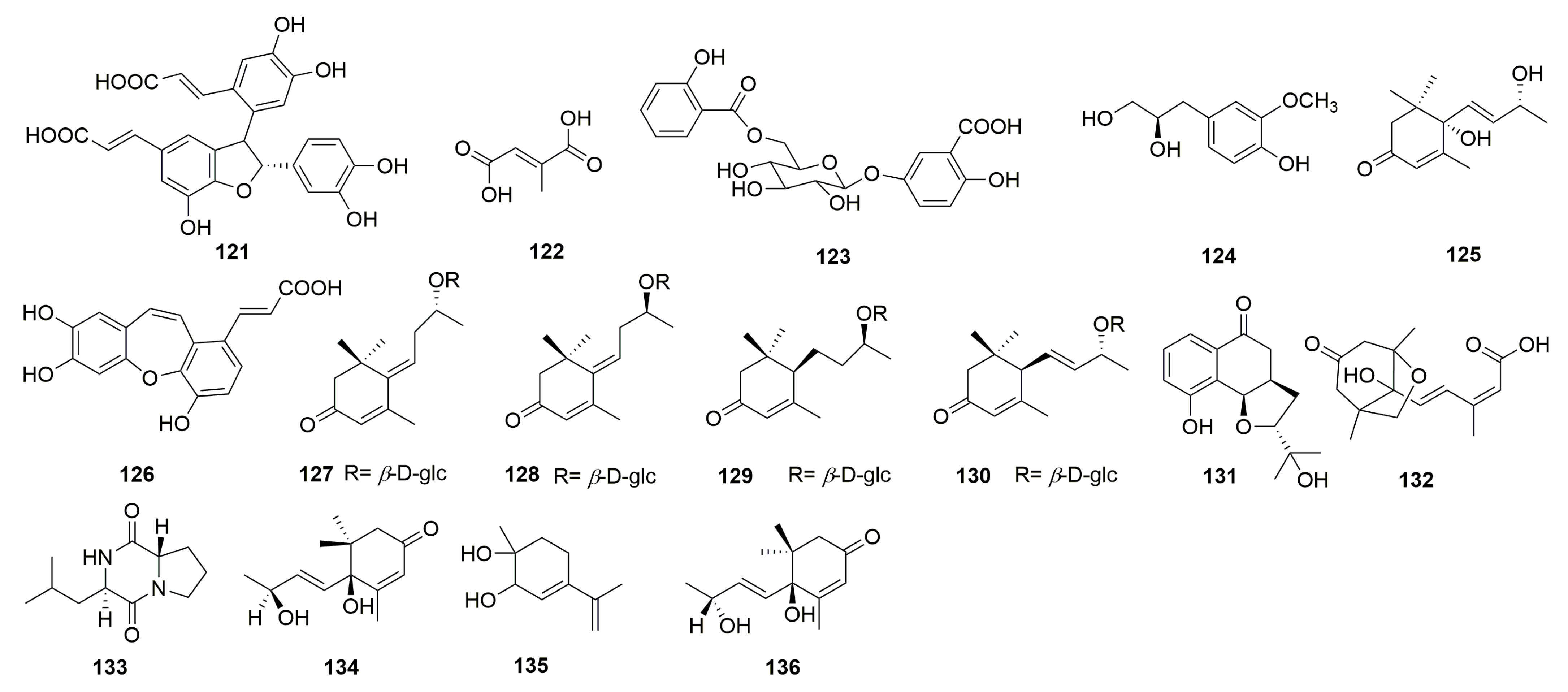
| 121 | cis-3-[2-[1-(3,4-dihydroxy-phenyl)-1-hydroxymethyl]-1,3-ben-zodioxol-5-yl]-(E)-2-propenoic acid | C. chinense | Aerial parts | 70% ethanol | Anhui province, China | [26] |
| 122 | mesaconic acid | C. chinense | Aerial parts | 70% ethanol | Anhui province, China | [26] |
| 123 | gentisic acid 5-O-β-D-(6′-salicylyl)-glucopyranoside | C. chinense | Aerial parts | 70% ethanol | Anhui province, China | [26] |
| 124 | 4-hydroxyl-3-methoxyphenyl-1-propane-1,2-diol | C. chinense | Aerial parts | 70% ethanol | Bozhou, Anhui province, China | [12] |
| 125 | blumenol A | C. chinense | Aerial parts | 70% ethanol | Bozhou, Anhui province, China | [12] |
| 126 | tournefolic acid B | C. chinense | / | / | / | [27] |
| 127 * | (E)-6-[9R-(β-D-glucopyranosyloxy) butylidene]-1,1,5-trimethyl-4-cyclohexen-3-one | C. chinense | Whole herb | 70% EtOH | Guilin, Guangxi, China | [23] |
| 128 | (E)-6-[9S-(β-D-glucopyranosyloxy) butylidene]-1,1,5-trimethyl-4-cyclohexen-3-one | C. chinense | Whole herb | 70% EtOH | Guilin, Guangxi, China | [23] |
| 129 | blumenol C 9-O-β-D-glucopyranoside | C. chinense | Whole herb | 70% EtOH | Guilin, Guangxi, China | [23] |
| 130 | (6R,9R)-3-oxo-α-ionol-9-O-β-D-glucopyranoside | C. chinense | Whole herb | 70% EtOH | Guilin, Guangxi, China | [23] |
| 131 * | chinense B | C. chinense | Whole herb | 70% EtOH | Guilin, Guangxi, China | [23] |
| 132 | phaseic acid | C. chinense | Aerial parts | 70% EtOH | Bozhou, Anhui province, China | [24] |
| 133 | cyclo-(S-Pro-R-Leu) | C. chinense | Aerial parts | 70% EtOH | Bozhou, Anhui province, China | [24] |
| 134 | vomifoliol | C. chinense | Aerial parts | 70% EtOH | Bozhou, Anhui province, China | [24] |
| 135 | p-mentha-3,8-dien-1,2-diol | C. chinense | Aerial parts | 70% EtOH | Bozhou, Anhui province, China | [24] |
| 136 | corchoionol C | C. chinense | Aerial parts | 70% EtOH | Bozhou, Anhui province, China | [24] |
| Activity | Source | Extract, Fraction, or Compounds | Pharmacological Effects | Ref. |
|---|---|---|---|---|
| Hemostatic activity | C. chinense | Buddlejasaponin IVa (55), clinopodiside D (39), saikogenin A (13), saikogenin F (45), prosaikogenin A (14), buddlejasaponin IVb (21), clinoposaponin D (24), 11α,16β,23,28-tetrahydroxyolean-12-en-3β-yl-[β-D-glucopyranosyl-(1→2)]-[β-D-glucopyranosyl-(1→3)]-β-D-fucopyranoside (25), and buddlejasaponin IV (48) | (1) Platelet aggregation (%): 27.2 ±1.7 (24), 8.6 ± 1.2 (21), 5.0 ± 0.7 (55), 69.9 ± 1.8 (48), 2.0 ± 0.5 (39), 11.4 ± 1.2 (25), 74.1 ± 2.6 (14), 5.0 ± 0.7 (13), and 9.0 ± 1.1 (45) at 100 μM; ADP at 10 µM: 69.88±1.65 (positive control), polyphyllins II at 100 μM: 44.5 ± 5.5 (positive control). (2) EC50 of platelet aggregation activity: 53.4 μM (48), 12.2 μM (14), and other compounds (>100 μM). (3) Compounds 21 and 13 remarkably shortened TT by 20.6 and 25.1% at 200 μM, respectively. (4) Promoting effects on platelet aggregation and shortened TT. | [11] |
| C. chinense | Ethanol extract | (1) Platelet adhesion rate 54.7 ± 10.7%. (2) Plasma calcium rehydration time 77.5 ± 9.6 s. (3) Hemostasis time of rats with femoral vein bleeding mode: 56.7 ± 5.8 s; hemostasis time in rabbit ear artery bleeding mode: 160.0 ± 17.3 s. (4) Improve platelet adhesion rate, shorten plasma recovery–calcium time. | [78] | |
| C. chinense | Total extract of C. chinense (TEC) obtained by water extraction and alcohol precipitation | Reduce metrorrhagia volume, alleviate pathological injury and increase MVD to promote recovery of the endometrium; TEC could also increase the levels of TXB2 and the expression of VEGF, TGF-b, and decrease the levels of IL-6, TNF-a and the expression of MMP-2/9. | [79] | |
| C. chinense | Total extract of C. chinense (TEC), total saponins of C. chinense (TSC), and total flavonoids of C. chinense (TFC). | TEC, TSC, and TFC all show therapeutic effects on AUB, particularly TEC. TSC exerts the effects by enhancing the coagulation function and promoting endometrial repair, and TFC by regulating estrogen levels and reducing inflammatory response. | [80] | |
| C. chinense | Total extract, buddlejasaponin IVb (21), hesperidin (97), naringenin (93), apigenin (79), and saikosaponin a (46) from C. chinense | (1) Hesperidin (97) and buddlejasaponin IVb (21) exerted a hemostatic effect. (2) Hesperidin (97), apigenin (79), naringenin (93), and saikosaponin a (46) promoted the proliferation of HEECs damaged by LPS, while buddlejasaponin IVb (21) did not significantly affect HEEC proliferation. (3) Significantly reduced the uterine bleeding volume, alleviated endometrial injury, increased plasma TXB2 level, and decreased plasma IL-6 and TNF-α levels. | [71] | |
| Anti-cardiomyocyte damage and cardiovascular protection | C. chinense | Different polar fractions in total flavonoids | Inhibitory effect on the decrease in H9c2 cardiomyocyte viability.Increase SOD, GSH-Px, CAT activity, reduce LDH, MDA content. | [81] |
| C. chinense | 40% and 70% ethanol fractions of the total flavones | TFCC suppressed DOX-induced overexpression of p53 and phosphorylation of JNK, p38, and ERK. Studies with LY294002 (a PI3K/AKT inhibitor) demonstrated that the mechanism of TFCC-induced cardioprotection also involves activation of PI3K/AKT. | [82] | |
| C. chinense | Total flavonoids | (1) Prevented ISO-induced myocardial damage, including the decrease in serum cardiac enzymes and cardiomyocyte apoptotic index and improvement in the heart rate and vacuolation. TFCC also improved the free radical scavenging and antioxidant potential, thereby suggesting that one possible mechanism of TFCC-induced cardio protection is mediated by blocking oxidative stress. (2) TFCC pretreatment prevented apoptosis, increased the expression of HO-1, and enhanced the nuclear translocation of Nrf2. TFCC also activated phosphorylation of AKT, whereas the addition of LY294002, which is the pharmacologic inhibitor of PI3K, blocked the TFCC-induced Nrf2/HO-1 activation and cytoprotective effect. | [83] | |
| C. chinense | Total flavonoids | The expression levels of P21 and caspase-3 were reduced and the cell survival rate was increased, while the apoptosis rate, the MDA content, and the LDH activity were reduced. The CAT and SOD activities were increased. Inhibition of miR-702-5p inhibited hypoxia/reoxygenation-induced cardiomyocyte injury. | [84] | |
| C. chinense | Apigenin (79), luteolin (80), buddleoside (81), naringenin (93), eriodictyol (94), isosakuranetin (95), didymin (96), hesperidin (97), ethyl (2R)-3-(3,4-dihydroxyphenyl)-2-hydroxypropanoate (110), ethyl (2E)-3-(3,4-dihydroxyphenyl) prop-2-enoate (112), caffeic acid (113), ethyl (2E)-3-(2,3,4-trihydroxyphenyl) prop-2-enoate (114), ethyl rosmarinate (116), and clinopodic acid B (117) | Approximate EC50 of cell viability in high glucose-treated HUVECs: 11 μM (80), 8 μM (93), 19 μM (94), 3 μM (110), 36 μM (113), 4 μM (116), and 17 μM (117), 47 (Vit C, model). Other compounds (79, 81, 95, 96, 97, 110, 112, and 114) showed weak protective effects (>60 μM). | [25] | |
| C. chinense | Clinopoditerpenes B (26) and C (27) | Cell viability: 73.7 ± 3.9% (26) at 12.5 μg mL−1, 64.6 ± 3.0% (H2O2-treated, model). | [14] | |
| C. chinense | Prunin (98) | Cell viability: exhibited viabilities of 84.25±7.36% (98) at 25.0 mg·mL−1, 62.12 ± 6.18% (model). | [26] | |
| C. chinense | Clinopodiside X (3); clinopodiside XI (4): clinoposaponin XIX (42) | Cell viability: 78.46 ± 1.47 (3), 80.77 ± 2.30 (4), 79.55 ± 1.85% (42), 64.19 ± 2.01% (model) at 50.0 μg·mL−1. | [8] | |
| C. chinense | Clinoposides G (64) and H (65) | (1) Cell viability of clinoposide G (64): 76.44 ± 2.75% (5 μg·mL−1), 81.25 ± 4.29% (10 μg·mL−1), and 87.66 ± 4.13% (20 μg·mL−1). (2) Cell viability of clinoposide H (65): 72.62 ± 3.51% (5 μg·mL−1), 77.89 ± 2.58% (10 μg·mL−1), and 85.62 ± 5.37 (20 μg·mL−1). | [21] | |
| C. chinense | Tournefolic acid B (TAB, 126) | Tournefolic acid B (126) significantly improved the hemodynamic parameters (LVeDP, LVSP, +dP/dtmax, −dP/dtmin, and HR) of isolated rat hearts, and depressed the cardiomyocyte apoptosis. Furthermore, TAB inhibited the oxidative stress by adjusting the activities of antioxidant enzymes (SOD, CAT, and GSH-Px). The I/ R injury triggered endoplasmic reticulum (ER) stress by activating the ER proteins, such as Grp78, ATF6, PERK, and eIf2α, which are all refrained by TAB. TAB also enhanced the phosphorylation of PI3K and AKT, inhibited the expression of CHOP and Caspase-12, reduced the phosphorylation of JNK, and increased the Bcl-2/Bax ratio. | [27] | |
| C. polycephalum | Clinopodiside VI (6), saikosaponin c (18), arjunglucoside I (74) | Cell viability: 77.8 ± 2.6% (6), 80.9 ± 4.4% (18), 79.8 ± 2.7% (74), 63.3 ± 2.4% (model) at 100.0 μg·mL−1. | [9] | |
| C. tomentosum | Ethanolic extract | A significant proliferative effect of pAEC was observed at the highest dose. cTEE treatment was able to rescue LPS-induced injury. cTEE resulted in a significant increase in the migration and test tube formation capacity of pAEC. Quantitative PCR data showed a significant increase in FLK-1 mRNA expression. | [85] | |
| C. vulgare | Aqueous extract | Reduced the biomarkers of oxidative stress, including glutathione (GSH), malonedialdehyde (MDA), superoxide dismutase (SOD), glutathione peroxidase (GPx) and catalase (CAT); slightly decreased the systolic blood pressure by 20%. | [86] | |
| Anti-inflammatory effect | C. chinense | Ethyl acetate fractions partitioned from 80% ethanol (CCE) | CCE suppresses PA-induced TLR4 expression in HUVECs, inhibiting downstream adaptor proteins (MyD88, TRIF, TRAF6) and blocking phosphorylation of IKKβ, NF-κB, JNK, ERK, and p38 MAPK, thereby reducing TNF-α, IL-1β, and IL-6 release. CCE also improves insulin signaling by reducing IRS-1 serine phosphorylation and enhancing tyrosine phosphorylation, restoring Akt/eNOS activation, and increasing NO production in PA-treated HUVECs. Additionally, CCE reverses impaired insulin-mediated vasodilation and eNOS function in rat aortas, suggesting CCE mitigates inflammation and insulin resistance by targeting TLR4-mediated NF-κB/MAPK pathways. | [87] |
| C. chinense | Ethanol extract | (1) Inhibited inflammation by LPS-TLR4-NF-κB-iNOS/COX-2 signaling pathway in RAW264.7 cells. (2) Significantly alleviated pathological features with increased body weight and colonic length, decreased DAI and oxidative damage, and mediated inflammatory factors like NO, PGE2, IL-6, IL-10, and TNF-α. | [88] | |
| C. gracile | Aqueous extract and 95% ethanol extract | (1) Effectively reduce the twisting caused by acetic acid, raise the pain threshold of hot-plate mice, and inhibit the phase I and phase II reaction of mice treated by formalin. (2) Inhibit the significant degree of swelling of the ear caused by xylene in mice and inhibit the increase in the permeability of capillary wall caused by acetic acid. Significantly reduce the levels of NO, MDA, PGE2, IL-6, and TNF-α in the brain tissue and serum of mice. | [89] | |
| C. polycephalum | Imbricatusol I (66), saturol I (67), 3β-22, 25-dihydroxy-tirucalla-7,23-diene (68), maslinic acid (69), 2α, 3α-Dihydroxyolean-12-en-28-oic acid (70), hederagenin (71), 2α, 3α-dihydroxyursolic acid (72), alphitolic acid (73) | Inhibit the productions of NO in LPS-induced RAW 264.7 cells. | [22] | |
| Antimicrobial and antibacterial activity | C. bolivianum | Ethanol extract | Decreased the uroplakin 1a expression and E. coli adhesion and invasion of uroepithelial cells while up-regulating caveolin-1. | [90] |
| Calamintha baborensis | Hexanoic and chloroformic fractions from hydroalcoholic extract | The antibacterial activity of extracts showed good results with hexanoic and chloroformic fractions against E. coli (19 mm and 19.2 mm diameter of inhibition zone and MIC values about 43 and 43.4 μg·mL−1, respectively). | [46] | |
| C. brevicalyx | Essential oil | MIC: 125 μL·mL−1. | [44] | |
| C. brownei | Essential oil | Inhibitory concentrations ranging from 13.6 mg·mL−1 for Staphylococcus epidermidis ATCC 14990 to 3.1 mg·mL−1 for Candida albicans ATCC 10231. | [45] | |
| C. menthifolium | Essential oils from three Tunisian regions | Exhibited the highest fungitoxic properties toward A. terreus mold, M. canis dermatophyte, and C. albicans yeast (MIC values ranged from 40 to 400 μg mL−1). | [55] | |
| C. macrostemum | Essential oil | A remarkable antimicrobial activity on Erwinia carotovora (0.145), Agrobacterium tumefaciens (0.149), Clavibacter michiganensis (0.184), Pseudomonas syringae pv. phaseolitica (0.381), Pseudomonas syringae pv. Glycinea (0.437), Escherichia coli strain DH5a (0.515), Fusarium oxysporum (2.3), Aspergillus niger (2.9) and Rhizopus stolonifer (3.6). | [54] | |
| C. nepeta | Essential oil | The highest activity was found against S. typhimurium (1250 µg·mL−1). The essential oil is more effective against B. cereus (2500 µg·mL−1) and S. sanguinis (2500 µg·mL−1). The lowest activities were determined against E. coli (5000 µg·mL−1) and P. aeruginosa (10,000 µg·mL−1). | [62] | |
| C. nepeta | Essential oil | 0.966 ± 0.057 µL·mL−1. | [91] | |
| C. nepeta | Ethyl acetate (AcOEt) extract, n-butanol (BuOH) extract, and dichloromethane (DCM) extract | P. aeruginosa: 100 14.89 ± 0.40 µg·mL−1 (DCM extract), 35.42 ± 1.00 µg·mL−1 (AcOEt extract), and 08.27 ± 3.11 µg·mL−1 (BuOH extract). | [92] | |
| S. Calamintha Spp. Nepeta | Essential oil | All tested molds were inhibited with 1/100 and 1/250 (v/v) concentrations after seven days of incubation. The minimum inhibitory and fungicidal concentrations of EO were in the orders of 0.666–2.666 μL·mL−1 and 2.666–5.333 μL·mL−1, respectively. | [61] | |
| S. calamintha ssp. nepeta | Essential oil | MIC ranged from 0.09 to 1.56 µL·mL−1. | [93] | |
| C. sericeum | Essential oil | Displays antibacterial activity against Gram-negative and Gram-positive bacterial strains (MIC 50–200 µg·mL−1) in a dose range close to standard antibiotics: 6–7 mm in Gram-positive bacterial strains, while Gram-negative bacteria have an inhibitory diameter of 6–8 mm. | [69] | |
| S. calamintha (L.) Scheel. | Essential oil | (1) MIC for bacteria was 0.007% (v/v) against Enterococcus faecalis and Klebsiella pneumoniae, whereas for fungi, it was 0.500% (v/v) against Candida albicans. (2) Enterococcus faecalis and Listeria innocua had the lowest minimum bactericidal concentration (MBC) at 0.125% (v/v), in contrast to the lowest fungicidal concentration (MFC) for Candida albicans at 0.500% (v/v). | [50] | |
| Anti-cancer effect | C. chinense | Clinopoditerpene D (28), perovskiaditerpenoside B (29), 3β-hydroxy-12-O-β-D-glucopyranosyl-8,11,13-abietatrien-7-one (30), 12-O-β-D-glucopyranosyl-3,11,16-trihydroxyabieta-8,11,13-triene (31), cussoracoside A (32), clinoposaponin E (17), buddlejasaponin IVa (55), and clinopodiside G (37) | None of the compounds were cytotoxic (IC50 > 100 μM) against the A549 and HepG2 cancer cell lines. | [13] |
| C. chinense | Clinopoursaponins A–D (49–52), clinopodisides VII–XII (7, 1–4, 11), saikosaponin g (8), 16β,23,28-trihydroxyoleana-9(11),12(13)-dien-3-yl-[β-D-glucopyranosyl-(1→2)]-[β-D-glucopyranosyl (1→3)]-β-D-fucopyranoside (10), 16β,21β,23,28-tetrahydroxyoleana-9(11),12(13)-diene-3-yl-[β-D-glucopyranosyl-(1→2)]-[β-D-glucopyranosyl-(1→3)]-β-D-fucopyranoside (5), 16β,23,28-trihydroxyoleana-9(11),12(13)-diene-3-yl-[β-D-glucopyranosyl-(1→4)-β-D-glucopyranosyl-(1→6)-β-D-glucopyranosyl-(1→3)]-[β-D-glucopyranosyl-(1→2)]-β-D-fucopyranoside (12), buddlejasaponin IV (48), clinoposaponin XVI (40), clinoposaponin XX (41), clinoposaponin XIX (42) | IC50 values (μM): 7.4 (49), 43.4 (50), 96.4 (51), 102.7 (52), 55.4 (7), 78.4 (compound 1), 36.3 (2), 88.6 (3), 65.9 (4), 57.7 (11), >150 (8), 73.7 (10), 46.5 (5), 51.4 (12), 86.5 (48), 117.2 (40), 21.6 (41), 69.1 μM (42) and 7.6 μM (positive control, 10-hydroxycamptothecin) on 4T1 cells. | [8] | |
| C. sericeum | Essential oil | IC50 values: 213.40 ± 4.14 μM on T24, 202.50 ± 0.18 μM on DU-145, 197.80 ± 5.19 μM on MCF-7, and 195.90 ± 7.46 μM on HEK-293. | [69] | |
| Calamintha incana | Ethanolic extract | IC50 values: 260.20 ± 0.1 mg·mL−1 (Ethanolic extract), 0.23 ± 0.2 mg·mL−1 (Doxorubicin) on HepG2 cells. | [72] | |
| C. umbrosum | Petroleum ether, chloroform, and methanol extracts, buddlejasaponin IVa (55), and buddlejasaponin IV (48) | (1) IC50 values: >250 (petroleum ether extract), >167 (chloroform extract), and 239.5 μg·mL−1 (methanol extract) on the HN-5 cell line. (2) IC50 values: 472.7 (55), 19.1 μg·mL−1 (48) on HN-5 cells. (3) IC50 values: >500 (55), 18.6 μg·mL−1 (48) on HUVEC cells. | [94] | |
| Antioxidant effect | C. bolivianum | Water, 65% ethanol, and 65% methanol | Antioxidant capacity: 725.9 ± 60.1 µmol TE/g (water extract), 908.2 ± 53.6 µmol TE/g (65% ethanol extract), 891.2 ± 41.5 µmol TE/g (65% methanol extract). | [95] |
| Calamintha baborensis | EtOAc and n-BuOH extracts | (1) ABTS inhibition rate: 68.9% (EtOAc extract), 81.7% (n-BuOH extract). (2) DPPH: 27.6% (EtOAc extract), 80.99% (n-BuOH extract). (3) ORAC: 37.28% (EtOAc extract), 28.47 (n-BuOH extract). (4) FRAP: 21.73% (EtOAc extract), 19.52 μM·mL−1 (n-BuOH extract). (5) IC50 values: 23 ppm (EtOAc extract), 53.5 ppm (n-BuOH extract). | [46] | |
| C. brownei | Essential oil | IC50 (DPPH) 1.77 mg·mL−1, IC50 (ABTS) 0.06 mg·mL−1. | [45] | |
| C. incana | Essential oil | (1) ABTS: 129.58 ± 2.21 mg TEs/g oil. (2) CUPRAC: 51.14 ± 0.05 mg TEs/g oil. (3) FRAP: 53.63 ± 0.10 mg TEs/g oil. | [52] | |
| Calamintha nepeta | Essential oil | Interaction with the stable free radical of DPPH: 17.1% (20 min), 54.7% (60 min). | [96] | |
| S. calamintha nepeta | Essential oils extracted from wild S. calamintha (EOSS) and domesticated S. calamintha (EOSD) | (1) IC50 values of DPPH assay: 23.03 ± 4.30 (EOSS), 24.09 ± 4.38 µg/mL (EOSD). (2) EC50 values of FRAP assay: 55.38 ± 2.16 (EOSS), 60.72 ± 7.71 µg·mL−1 (EOSD). | [97] | |
| C. nepeta | AcOEt extract, n-butanol (BuOH) extract, and dichloromethane (DCM) extract | (1) IC50 value of DPPH: 8.12 ± 0.11 µg·mL−1 (BuOH extract). (2) IC50 value of ABTS•+ assay: 9.56 ± 1.12 µg·mL−1 (DCM extract). (3) IC50 value of GOR assay: 10.07 ± 0.40 µg·mL−1 (BuOH extract). (4) A0.50 of CUPRAC assay: 29.44 ± 0.65 µg·mL−1 (BuOH extract). (5) A0.50 of the phenanthroline assay: 9.85 ± 0.07 µg·mL−1 (BuOH extract). (6) A0.50 of FRAP: 17.42 ± 0.25 µg·mL−1 (BuOH extract). | [92] | |
| C. serpyllifolium | EtOH and dH2O extracts of stem and flower parts | (1) DPPH free radical scavenging activity: 92.14 ± 2.03% (EtOH extract of flower).ABTS cation scavenging assay: 89.2% (dH2O extract of flower). (2) FRAP value: 3.138 ± 0.08 (EtOH extract of flower). (3) CUPRAC value: 2.207 ± 0.92 (EtOH extract of stem), 2.061 ± 0.43 (dH2O extract of stem). | [98] | |
| Calamintha incana | Ethanolic extract | (1) IC50 values of DPPH: 35.9 ± 0.1 (ethanolic extract), 19.6 ± 0.2 µg·mL−1 (ascorbic acid, positive control). (2) IC50 of Reducing Power Assay: 90.3 ± 0.5 µg·mL−1 (ethanolic extract), 26.4 ± 0.2 µg·mL−1 (ascorbic acid, positive control). | [72] | |
| C. sericeum | Essential oil | (1) FRAP: 1.40 ± 0.05 mg TEAC/mL. (2) CUPRAC: 30.17 ± 1.60 mg TEAC/mL. (3) IC50 (ABTS): 106.06 ± 7.92. (4) IC50 (DPPH): 473.03 ± 14.11. | [69] | |
| C. vulgare | Acetone, methanol, and water extracts | (1) DPPH free radical scavenging activity: 81.72 mg TEs/g extract (water extract). (2) ABTS cation scavenging assay: 51.45 mg TEs/g extract (methanol extract). (3) CUPRAC assay: 44.32 (methanol extract). (4) FRAP assay: 87.25 (methanol extract). | [99] | |
| C. vulgare | CV3: fraction from C. vulgare extract by an RP 18 reversed-phase column | IC50 values of Fraction CV3: 0.02 mg·mL−1 (DPPH) and 0.0002 mg·mL−1 (ABTS), as well as the strongest ferric reducing potential (FRAP) of 0.89 mM TE/mg dw. | [100] | |
| C. vulgare | Aqueous and methanolic extract | (1) DPPH: 32.4 (aqueous extract), 25.7 μg·mL−1 (methanolic extract). (2) FRAP: 976.6 ± 17.1 μmol Fe2+/g of extract (aqueous extract), 2115.6 ± 99.4 μmol Fe2+/g of extract (methanolic extract). | [101] | |
| Antihypertensive effect | Calamintha vulgaris | Crude extract and n-Hexane, chloroform, ethylacetate, and aqueous fractions | (1) Crude extract and fractions induced a fall in MAP in normotensive and high salt-induced hypertensive rats at different doses. The effect was more significant in the hypertensive rats (max. fall, 38.67 ± 2.17 vs 44.16 ± 4.67 mmHg). Among the fractions, chloroform was more effective (max. fall, 53.20 ± 1.23 mmHg) and aqueous the least (max. fall, 38.66 ± 1.12 mmHg); (2) The antihypertensive effect of C. vulgaris is the outcome of vasodilation, which is mediated through a combination of muscarinic receptor-linked NO, activation of TEA-sensitive K+ channels, prostacyclin and Ca2+ antagonism. | [102] |
| Calamintha officinalis | Aqueous extract | (1) Reduced the systolic, diastolic, and mean arterial blood pressure in hypertensive rats. (2) Exerts a vasorelaxant ability through the sGC-cGMP induction pathway, vascular cyclooxygenase pathway, and the opening of K+ channels. | [103] | |
| Enzyme inhibitory activity | Calamintha incana | Ethanolic extract | (1) α-Amylase Inhibition Assay: At the highest concentration investigated (100 μg·mL−1), the extract exhibited a noticeable effect on α-amylase by 88.5%. IC50 33.5 ± 0.1 mg·mL−1 (acarbose, positive control), IC50 46.3 ± 0.2 mg·mL−1 (ethanolic extract); (2) α-Glucosidase Inhibition Assay: The extract showed a noticeable effect on α-glucosidase activity at the highest concentration (100 μg·mL−1) of 70.5%. IC50 37.1 ± 0.2 mg·mL−1 (epigallocatechin gallate, positive control), 56.8 ± 0.1 mg·mL−1 (ethanolic extract). (3) Pancreatic Lipase Inhibition Assay: The greatest levels of inhibition were 8.1% (ethanolic extract) and 92.2% (orlistat, positive control) at the same dose (100 mg·mL−1). (4) Dipeptidyl Peptidase-IV (DPP-IV) Inhibition Assay: The ethanolic extract of C. incana did not exert any considerable inhibition at any of the assessed concentrations compared to the positive control (sitagliptin), which displayed 95.2% inhibition of DPP-IV activity at the highest concentration (100 mg·mL−1). | [72] |
| C. chinense | Apigenin (79), luteolin (80), buddleoside (81), naringenin (93), eriodictyol (94), isosakuranetin (95), didymin (96), hesperidin (97), ethyl (2R)-3-(3,4-dihydroxyphenyl)-2-hydroxypropanoate (110), ethyl (2E)-3-(3,4-dihydroxyphenyl) prop-2-enoate (112), caffeic acid (113), ethyl (2E)-3-(2,3,4-trihydroxyphenyl) prop-2-enoate (114), ethyl rosmarinate (116), and clinopodic acid B (117) | IC50 value of α-Glucosidase activity: 15.4 ± 2.3 μM (79), 2.0 ± 1.8 μM (80), 14.6 ± 2.2 μM (81), 57.1 ± 2.1 μM (93), 1.4 ± 3.4 μM (94), 31.2 ± 2.8 μM (95), > 60 μM (96, 97, and 113), 18.5 ± 2.9 μM (110), 17.8 ± 1.8 μM (112), 12.0 ± 2.3 μM (114), 1.2 ± 4.8 μM (116), and 0.6 ± 2.8 μM (117). | [25] | |
| C. nepeta | AcOEt extract, n-butanol (BuOH) extract, and dichloromethane (DCM) extract | (1) IC50 value of AChE assay: 170.1 ± 1.58 µg·mL−1 (DCM extract). (2) IC50 values of BChE assay: 73.06 ± 0.83 µg·mL−1 (DCM extract), 187.8 ± 1.57 µg·mL−1 (AcOEt extract). | [92] | |
| C. nepeta | EO and aqueous extracts | (1) AChE IC50 (μg·mL−1): 205.6 ± 10.3 (EO); 983.9 ± 49.2 (aqueous extracts). (2) BChE IC50 (μg·mL−1): 88.3 ± 4.4 (EO); 1669.9 ± 83.5 (aqueous extracts). | [104] | |
| C. serpyllifolium | EtOH extract of flower | AChE inhibition percentage: 60.18 ± 1.37%, BChE: 72.15 ± 0.98%, and TYR 55.04 ± 2.04% at 1000 μg·mL−1. | [98] | |
| C. vulgare | Acetone extract | (1) Acetylcholinesterase: 1.34 ± 0.01 mg GALAEs/g extract. (2) Butyrylcholinesterase: 0.93 ± 0.23 mg KAEs/g extract. (3) Tyrosinase: 1.85 ± 0.40 mg KAEs/g extract. | [99] | |
| C. vulgare | Methanol and water extracts | (1) α-Amylase: 0.70 ± 0.03 (methanol extract), 0.10 ± 0.01 (water extract); (2) α-Glucosidase: 1.82 ± 0.27 (methanol extract), 3.54 ± 0.01 (water extract). | [99] | |
| C. vulgare | CV3: fractions from C. vulgare extract by an RP 18 reversed-phase column | CV3 showed moderate α-glucosidase and α-amylase inhibitory potential. | [100] | |
| C. vulgare | Aqueous extract and methanolic extract | AChE enzyme: 14.6 ± 2.1% (aqueous extract), 6.3 ± 1.1% (methanolic extract). | [101] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, W.; Pan, J.; Chen, X.; Guo, S.; Ouyang, X. The Chemical Composition, Pharmacological Activity, Quality Control, Toxicity, and Pharmacokinetics of the Genus Clinopodium L. Molecules 2025, 30, 2425. https://doi.org/10.3390/molecules30112425
Li W, Pan J, Chen X, Guo S, Ouyang X. The Chemical Composition, Pharmacological Activity, Quality Control, Toxicity, and Pharmacokinetics of the Genus Clinopodium L. Molecules. 2025; 30(11):2425. https://doi.org/10.3390/molecules30112425
Chicago/Turabian StyleLi, Wen, Jianping Pan, Xiaobing Chen, Senhui Guo, and Xilin Ouyang. 2025. "The Chemical Composition, Pharmacological Activity, Quality Control, Toxicity, and Pharmacokinetics of the Genus Clinopodium L." Molecules 30, no. 11: 2425. https://doi.org/10.3390/molecules30112425
APA StyleLi, W., Pan, J., Chen, X., Guo, S., & Ouyang, X. (2025). The Chemical Composition, Pharmacological Activity, Quality Control, Toxicity, and Pharmacokinetics of the Genus Clinopodium L. Molecules, 30(11), 2425. https://doi.org/10.3390/molecules30112425







