Antifouling Properties of N,N′-Dialkylated Tetraazamacrocyclic Polyamines and Their Metal Complexes
Abstract
1. Introduction
2. Results
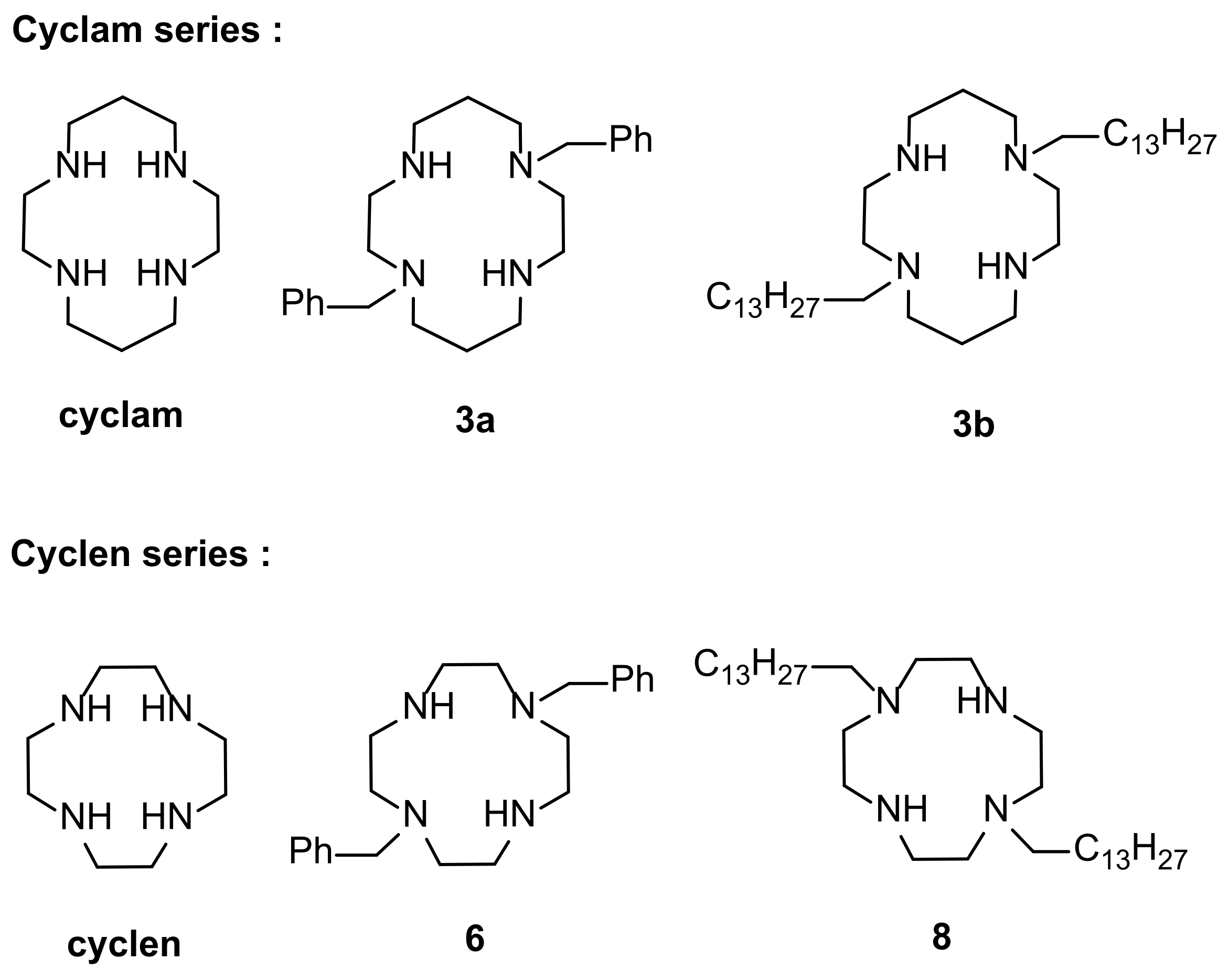
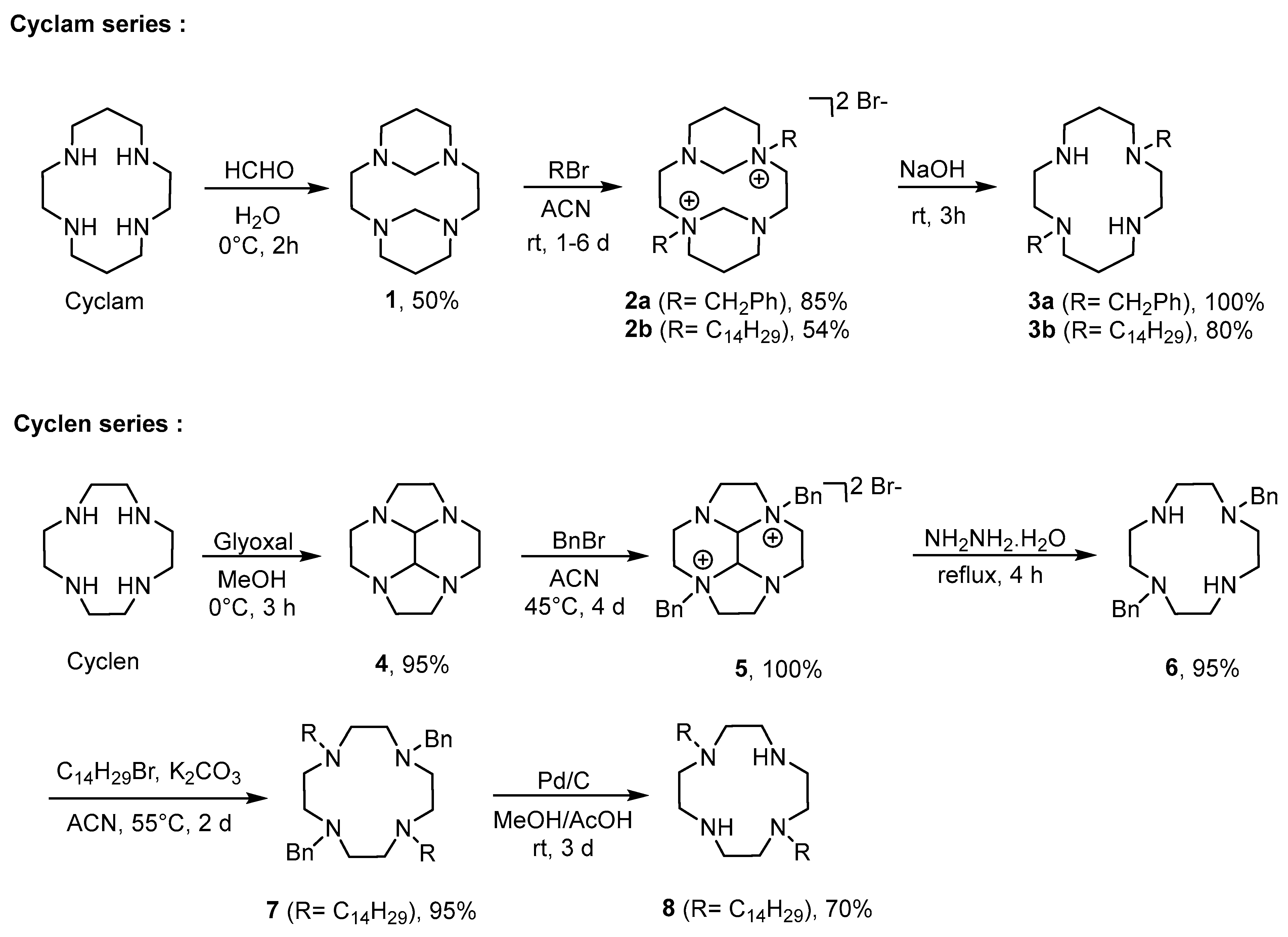
2.1. Synthesis of Polyazamacrocyclic Compounds
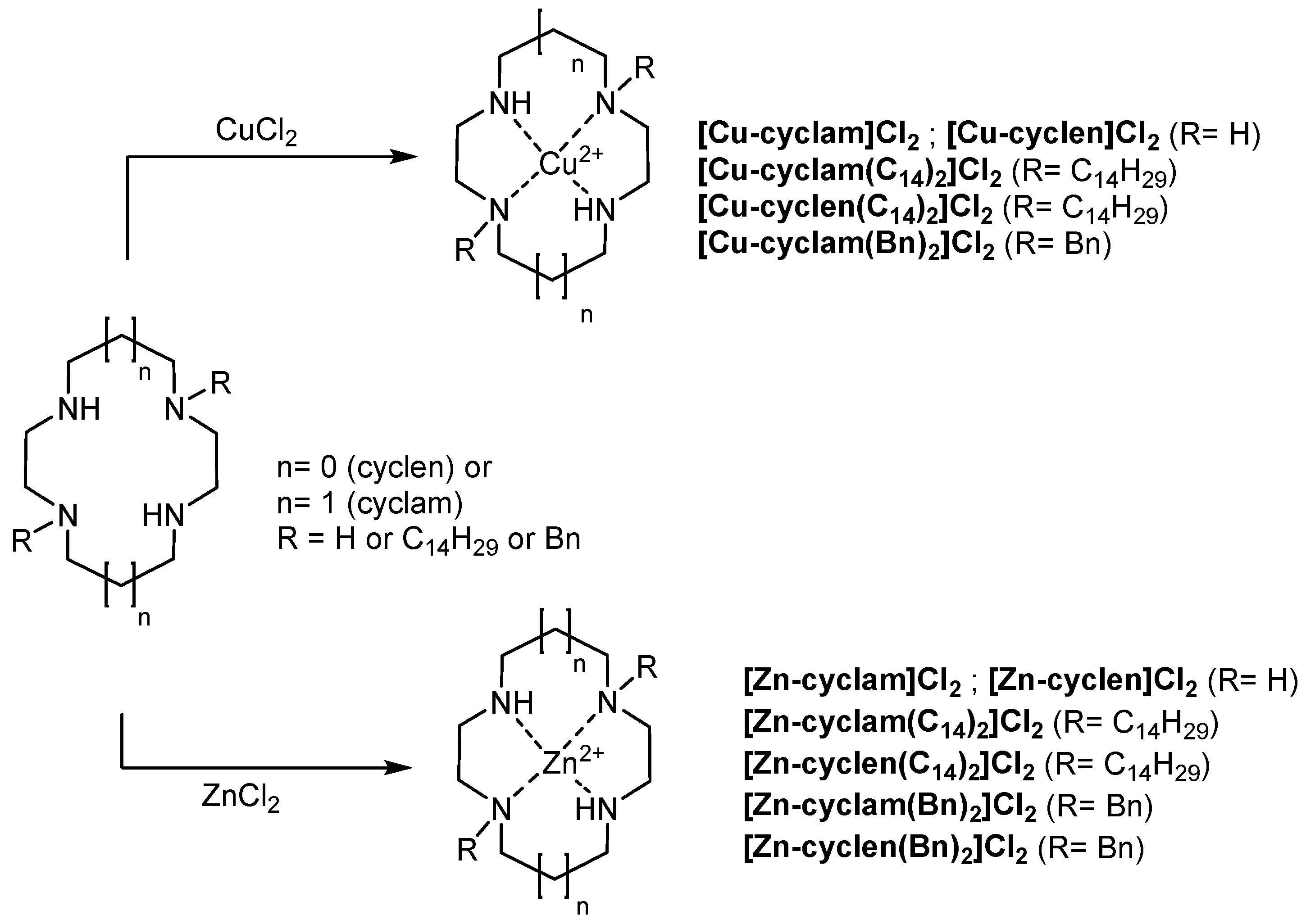
2.2. Synthesis of Copper and Zinc Complexes of Polyazamacrocyclic Compounds
2.3. Effect of CuCl2 and ZnCl2 on Bacterial Growth and Adhesion
2.4. Effect of Free Base Ligands on Bacterial Growth and Adhesion
2.5. Effect of Copper and Zinc Complexes on Bacterial Growth and Adhesion
2.6. Effect of Copper and Zinc Complexes on the Microalgae S. costatum
3. Discussion
4. Materials and Methods
4.1. Preparation of Compound 3b
4.2. Preparation of Compound 7
4.3. Preparation of Compound 8
4.4. General Procedure for Synthesis of Cu and Zn Complexes from Free Base Ligands
4.5. General Procedure for Synthesis of Cu and Zn Complexes from 3b
4.6. Synthesis of [Zn-cyclen(C14)2]Cl2
4.7. General Procedure for Synthesis of Zn Complexes from Cyclam(Bn)2 and Cyclen(Bn)2
4.8. Marine Bacteria
4.9. Bacterial Growth and Adhesion Assays
4.10. Marine Microalga Culture and Growth
4.11. Microalga Inoculation
4.12. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yebra, D.M.; Kiil, S.; Dam-Johansen, K. Antifouling technology—Past, present and future steps towards efficient and environmentally friendly antifouling coatings. Prog. Org. Coatings 2004, 50, 75–104. [Google Scholar] [CrossRef]
- Dong-Ho, K.; Alayande, A.B.; Jung-Min, L.; Jin-Hyeok, J.; Su-Min, J.; Mi-Ri, J.; Yang, E.; Chae, K.J. Emerging marine environmental pollution and ecosystem disturbance in ship hull cleaning for biofouling removal. Sci. Total Environ. 2024, 906, 167459–167472. [Google Scholar] [CrossRef]
- Qian, P.Y.; Cheng, A.; Wang, R.; Zhang, R. Marine biofilms: Diversity, interactions and biofouling. Nat. Rev. Microbiol. 2022, 20, 671–684. [Google Scholar] [CrossRef] [PubMed]
- Conlan, K.E. Amphipod crustaceans and environmental disturbance: A review. J. Nat. Hist. 1994, 28, 519–554. [Google Scholar] [CrossRef]
- Chambers, L.D.; Stokes, K.R.; Walsh, F.C.; Wood, R.J.K. Modern approaches to marine antifouling coatings. Surf. Coat. Technol. 2006, 201, 3642–3652. [Google Scholar] [CrossRef]
- Hoch, M. Organotin compounds in the environment—An overview. Appl. Geochem. 2001, 16, 719–743. [Google Scholar] [CrossRef]
- Beyer, J.; Song, Y.; Tollefsen, K.E.; Berge, J.A.; Tveiten, L.; Helland, A.; Øxnevad, S.; Schøyen, M. The ecotoxicology of marine tributyltin (TBT) hotspots: A review. Mar. Environ. Res. 2022, 179, 105689–105702. [Google Scholar] [CrossRef] [PubMed]
- Brooks, S.; Waldock, M. The use of copper as a biocide in marine antifouling paints. In Marine Antifouling Coatings and Technologies; Elsevier: Amsterdam, The Netherlands, 2009; pp. 492–521. [Google Scholar] [CrossRef]
- Voulvoulis, N.; Scrimshaw, D.; Lester, J. Comparative environmental assessment of biocides used in antifouling paints. Chemosphere 2002, 47, 789–795. [Google Scholar] [CrossRef]
- Dafforn, K.A.; Lewis, A.; Johnston, E.L. Antifouling strategies: History and regulation, ecological impacts and mitigation. Mar. Pollut. Bull. 2011, 62, 453–465. [Google Scholar] [CrossRef]
- Turner, A. Marine pollution from antifouling paint particles. Mar. Pollut. Bull. 2010, 60, 159–171. [Google Scholar] [CrossRef]
- Flach, C.F.; Svensson, C.J.; Kristiansson, E.; Östman, M.; Bengtsson-Palme, J.; Tysklind, M.; Larsson, D.G.J. Does antifouling paint select for antibiotic resistance? Sci. Total Environ. 2017, 590, 461–468. [Google Scholar] [CrossRef] [PubMed]
- SCENIHR—Scientific Committee on Emerging and Newly Identified Health Risks. SCENIHR Assessment of the Antibiotic Resistance Effects of Biocides; European Commission: Brussels, Belgium, 2009. [Google Scholar]
- Claudel, M.; Schwarte, J.V.; Fromm, K.M. New Antimicrobial Strategies Based on Metal Complexes. Chemistry 2020, 2, 849–899. [Google Scholar] [CrossRef]
- Elshaarawy, R.F.M.; Janiak, C. Antibacterial susceptibility of new copper(II) Npyruvoyl anthranilate complexes against marine bacterial strains—In search of new antibiofouling candidate. Arab. J. Chem. 2016, 9, 825–834. [Google Scholar] [CrossRef]
- Turley, P.A.; Fenn, R.J.; Ritter, J.C.; Callow, M.E. Pyrithiones as antifoulants: Environmental fate and loss of toxicity. Biofouling J. 2005, 21, 31–40. [Google Scholar] [CrossRef]
- Soon, Z.Y.; Jung, J.H.; Jang, M.; Kang, J.H.; Jang, M.C.; Lee, J.S.; Kim, M. Zinc Pyrithione (ZnPT) as an Antifouling Biocide in the Marine Environment—A Literature Review of Its Toxicity, Environmental Fates, and Analytical Methods. Water Air Soil Pollut. 2019, 230, 310–318. [Google Scholar] [CrossRef]
- Martins, S.E.; Fillmann, G.; Lillicrap, A.; Thomas, K.V. Review: Ecotoxicity of organic and organo-metallic antifouling co-biocides and implications for environmental hazard and risk assessments in aquatic ecosystems. Biofouling J. 2018, 34, 34–52. [Google Scholar] [CrossRef]
- Hemaida, H.A.E.; El-Dissouky, A.A.; Sadek, S.M.M. Potential anti-fouling agents: Metal complexes of 3-(2-furylidene)hydrazino-5,6-diphenyl-1,2,4-triazine. Pigm. Resin Technol. 2008, 37, 243–249. [Google Scholar] [CrossRef]
- Bayer, M.; Hellio, C.; Maréchal, J.P.; Frank, W.; Lin, W.; Weber, H.; Proksch, P. Antifouling bastadin congeners target mussel phenoloxidase and complex copper(II) ions. Mar. Biotechnol. 2011, 13, 1148–1158. [Google Scholar] [CrossRef]
- Robinson, H. Selective Metal Coordination in Antifouling Coatings. Ph.D. Thesis, Victoria University of Wellington, Wellington, New Zealand, 2020. [Google Scholar]
- Soto-Aguilera, S.; Modak, B.; Aldabaldetrecu, M.; Lozano, C.P.; Guerrero, J.; Lefimil, C.; Parra, M. In Vitro Effect of Copper (I) Complex [Cu(NN1)2](ClO4) on Vibrio harveyi BB170 Biofilm Formation. Microorganisms 2021, 9, 2273. [Google Scholar] [CrossRef]
- Ross, A.; Choi, J.H.; Hunter, T.M.; Pannecouque, C.; Moggach, S.A.; Parsons, S.; De Clercq, E.; Sadler, P.J. Zinc(II) complexes of constrained antiviral macrocycles. Dalton Trans. 2012, 41, 6408–6418. [Google Scholar] [CrossRef]
- Konai, M.M.; Pakrudheen, I.; Barman, S.; Sharma, N.; Tabbasum, K.; Garg, P.; Haldar, J. Cyclam-based antibacterial molecules eradicate Gram-negative superbugs with potent efficacy against human corneal infection. Chem. Commun. 2020, 56, 2147–2150. [Google Scholar] [CrossRef] [PubMed]
- Almada, S.; Maia, L.B.; Waerenborgh, J.C.; Vieira, B.J.C.; Mira, N.P.; Silva, E.; Cerqueira, F.; Pinto, E.; Alves, L.G. Cyclam-based iron(iii) and copper(ii) complexes. New J. Chem. 2022, 46, 16764–16770. [Google Scholar] [CrossRef]
- Pilon, A.; Lorenzo, J.; Rodriguez-Calado, S.; Adão, P.; Martins, A.M.; Valente, A.; Alves, L.G. New Cyclams and Their Copper(II) and Iron(III) Complexes: Synthesis and Potential Application as Anticancer Agents. ChemMedChem 2019, 14, 770–778. [Google Scholar] [CrossRef] [PubMed]
- Hancock, R.D.; Martell, A.E. The Chelate, Cryptate and Macrocyclic Effects. Inorg. Chem. 1988, 6, 237–284. [Google Scholar] [CrossRef]
- Hancock, R.D.; Dobson, S.M.; Evers, A.; Wade, P.W.; Ngwenya, M.P.; Boeyens, C.A.; Wainwright, K.P. More rigid macrocyclic ligands that show metal ion size-based selectivity. Crystallographic, molecular mechanics, and formation constant study of the complexes of bridged cyclen. J. Am. Chem. Soc. 1988, 110, 2788–2794. [Google Scholar] [CrossRef]
- Shakir, M.; Azim, Y.; Chishti, H.T.N.; Parveen, S. Characterization of complexes of Co(II), Ni(II), Cu(II) and Zn(II) with 12-membered Schiff base tetraazamacrocyclic ligand and the study of their antimicrobial and reducing power. Spectrochimica Acta Part. A Mol. Biomol. Spectros. 2006, 65, 490–496. [Google Scholar] [CrossRef]
- Firdaus, F.; Fatma, K.; Azam, M.; Shakir, M. Synthesis, spectroscopic, thermal, and antimicrobial studies of tetradentate 12 and 14 member Schiff bases and their complexes with Fe(III), Co(II), and Cu(II). J. Coord. Chem. 2010, 63, 3956–3968. [Google Scholar] [CrossRef]
- Li, S.; Chen, J.X.; Xiang, Q.X.; Zhang, L.Q.; Zhou, C.H.; Xie, J.Q.; Yu, L.; Li, F.Z. 2014. The synthesis and activities of novel mononuclear or dinuclear cyclen complexes bearing azole pendants as antibacterial and antifungal agents. Eur. J. Med. Chem. 2014, 84, 677–686. [Google Scholar] [CrossRef]
- Hubin, T.J.; Walker, A.N.; Davilla, D.J.; Carder Freeman, T.N.; Epley, B.M.; Hasley, T.R.; Amoyaw, P.N.A.; Jain, S.; Archibald, S.J.; Prior, T.J.; et al. Tetraazamacrocyclic derivatives and their metal complexes as antileishmanial leads. Polyhedron 2019, 163, 42–53. [Google Scholar] [CrossRef]
- Hubin, T.J.; Amoyaw, P.N.A.; Roewe, K.D.; Simpson, N.C.; Maples, R.D.; Carder Freeman, T.N.; Cain, A.N.; Le, J.G.; Archibald, S.J.; Khan, S.I.; et al. Synthesis and antimalarial activity of metal complexes of cross-bridged tetraazamacrocyclic ligands. Bioorg. Med. Chem. 2014, 22, 3239–3244. [Google Scholar] [CrossRef]
- Labreuche, Y. Caractérisation de La Virulence d’une Souche de Vibrio aestuarianus, Pathogène de l’huître Crassostrea gigas. Ph.D. Thesis, Université de Bretagne Occidentale, Brest, France, 2006. [Google Scholar]
- Arunkumar, M.; LewisOscar, F.; Thajuddin, N.; Pugazhendhi, A.; Nithya, C. In vitro and in vivo biofilm forming Vibrio spp: A significant threat in aquaculture. Process Biochem. 2020, 94, 213–223. [Google Scholar] [CrossRef]
- Royal, G.; Dahaoui-Gindrey, V.; Dahaoui, S.; Tabard, A.; Guilard, R.; Pullumbi, P.; Lecomte, C. New Synthesis of trans-Disubstituted Cyclam Macrocycles-Elucidation of the Disubstitution Mechanism on the Basis of X-ray Data and Molecular Modeling. Eur. J. Org. Chem. 1998, 1998, 1971–1975. [Google Scholar] [CrossRef]
- Le Baccon, M.; Chuburu, F.; Toupet, L.; Handel, H.; Soibinet, M.; Déchamps-Olivier, I.; Barbier, J.P.; Aplincourt, M. Bis-aminals: Efficient tools for bis-macrocycle synthesis. New J. Chem. 2001, 25, 1168–1174. [Google Scholar] [CrossRef]
- Lejault, P.; Duskova, K.; Bernhard, C.; Valverde, I.E.; Romieu, A.; Monchaud, D. The Scope of Application of Macrocyclic Polyamines Beyond Metal Chelation. Eur. J. Org. Chem. 2019, 2019, 6146–6157. [Google Scholar] [CrossRef]
- Liang, F.; Wan, S.; Li, Z.; Xiong, X.; Yang, L.; Zhou, X.; Wu, C. Medical applications of macrocyclic polyamines. Curr. Med. Chem. 2006, 13, 711–727. [Google Scholar] [CrossRef]
- Wahsner, J.; Gale, E.M.; Rodríguez-Rodríguez, A.; Caravan, P. Chemistry of MRI Contrast Agents: Current Challenges and New Frontiers. Chem. Rev. 2019, 119, 957–1057. [Google Scholar] [CrossRef] [PubMed]
- Motekaitis, R.J.; Rogers, B.E.; Reichert, D.E.; Martell, A.E.; Welch, M.J. Stability and Structure of Activated Macrocycles. Ligands with Biological Applications. J. Inorg. Chem. 1996, 35, 3821–3827. [Google Scholar] [CrossRef]
- Vincent, M.; Duval, R.E.; Hartemann, P.; Engels-Deutsch, M. Contact killing and antimicrobial properties of copper. J. Appl. Microbiol. 2018, 124, 1032–1046. [Google Scholar] [CrossRef]
- Grass, G.; Rensing, C.; Solioz, M. Metallic copper as an antimicrobial surface. Appl. Environ. Microbiol. 2011, 77, 1541–1547. [Google Scholar] [CrossRef]
- Alves, L.G.; Souto, M.; Madeira, F.; Adão, P.; Munhá, R.F.; Martins, A.M. Syntheses and solid-state structures of cyclam-based copper and zinc compounds. J. Organomet. Chem. 2014, 760, 130–137. [Google Scholar] [CrossRef]
- Bovio, E.; Fauchon, M.; Toueix, Y.; Mehiri, M.; Varese, G.C.; Hellio, C. The Sponge-Associated Fungus Eurotium chevalieri MUT 2316 and its Bioactive Molecules: Potential Applications in the Field of Antifouling. Mar. Biotechnol. 2019, 21, 743–752. [Google Scholar] [CrossRef] [PubMed]
- Guillard, R.R.L.; Ryther, J.H. Studies on Marine Planktonic Diatoms I. Cyclotella nana Hustedt and Detonula confervacea (Cleve) Gran. Can. J. Microbiol. 1962, 8, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Dupraz, V.; Stachowski-Haberkorn, S.; Ménard, D.; Limon, G.; Akcha, F.; Budzinski, H.; Cedergreen, N. Combined effects of antifouling biocides on the growth of three marine microalgal species. Chemosphere 2018, 209, 801–814. [Google Scholar] [CrossRef] [PubMed]
- Vindimian, E.; Robaut, C.; Fillion, G. A method for cooperative and non comparative binding studies using non regression analysis on a microcomputer. J. Appl. Biochem. 1983, 5, 261–268. [Google Scholar]
| Compounds | Growth Inhibition Percentage at 200 µM | Adhesion Inhibition Percentage at 200 µM | |||
|---|---|---|---|---|---|
| V. natriegens | V. aestuarianus | V. natriegens | V. aestuarianus | ||
| Salts | CuCl2 | 37.5 ± 9.5 | 56.2 ± 0.2 | −11.3 ± 7.1 | −10.4 ± 5.0 |
| ZnCl2 | 60.9 ± 8.5 | −12.1 ± 4.8 | 60.6 ± 21.8 | 20.1 ± 4.7 | |
| Ligands | Cyclam | −0.2 ± 8.3 | 12.2 ± 20.2 | −2.0 ± 11.4 | 0.7 ± 31.2 |
| Cyclam(Bn)2 | 52.9 ± 1.8 | 21.0 ± 3.7 | 32.3 ± 2.9 | −20.9 ± 0.5 | |
| Cyclam(C14)2 | 6.3 ± 8.5 | −21.7 ± 33.9 | −54.9 ± 40.9 | −19.9 ± 7.4 | |
| Cyclen | −0.6 ± 7.5 | −1.4 ± 0.1 | −5.7 ± 2.4 | 14.9 ± 2.5 | |
| Cyclen(Bn)2 | 40.0 ± 3.4 | 11.6 ± 22.2 | −7.5 ± 9.0 | 1.9 ± 3.7 | |
| Cyclen(C14)2 | −12.9 ± 4.0 | 20.6 ± 23.2 | −124.7 ± 1.1 | 13.8 ± 12.8 | |
| Cu complexes | [Cu(cyclam)]Cl2 | 11.7 ± 7.1 | −3.5 ± 11.5 | −72.3 ± 22.2 | −51.1 ± 23.6 |
| [Cu-cyclam-(C14)2]Cl2 | −8.9 ± 17.2 | 34.5 ± 7.8 | −25.5 ± 9.9 | 8.2 ± 29.9 | |
| [Cu-cyclen]Cl2 | 9.2 ± 10.7 | 29.7 ± 0.9 | −30.0 ± 13.1 | −10.8 ± 3.0 | |
| Zn complexes | [Zn-cyclam]Cl2 | 10.6 ± 11.5 | −0.2 ± 7.3 | −33.9 ± 12.9 | 4.3 ± 1.9 |
| [Zn-cyclam(C14)2]Cl2 | 99.0 ± 0.3 | 14.3 ± 11.2 | 82.8 ± 6.9 | 51.1 ± 19.9 | |
| [Zn-cyclam(Bn)2]Cl2 | 99.6 ± 3.3 | 74.0 ± 5.7 | 92.0 ± 7.3 | −28.0 ± 21.8 | |
| [Zn-cyclen)]Cl2 | −22,1 ± 7.2 | 17.5 ± 0.5 | −7.4 ± 23.5 | 41.8 ± 14.8 | |
| [Zn-cyclen(C14)2]Cl2 | −11.1 ± 7.1 | −13.3 ± 17.6 | −3.6 ± 10.8 | 61.3 ± 14.6 | |
| [Zn-cyclen(Bn)2]Cl2 | 25.1 ± 3.4 | 20.9 ± 4.1 | −15.4 ± 5.5 | −3.6 ± 8.8 | |
| Compound | 2 µM | 20 µM | 200 µM |
|---|---|---|---|
| CuCl2 | 100.7 ± 3.0 | 101.0 ± 3.9 | 0 ± 0 |
| ZnCl2 | 101.7 ± 1.9 | 100.6 ± 0.7 | 94.3 ± 2.1 |
| Cyclam(C14)2 | 105.23 ± 0.6 | 98.9 ± 0.8 | 0 ± 0 |
| Cyclam(Bn)2 | 99.0 ± 0.1 | 96 ± 1.0 | 0 ± 0 |
| [Zn-cyclen)]Cl2 | 105.3 ± 1.2 | 95.5 ± 2.1 | 91.4 ± 5.2 |
| [Zn-cyclam(C14)2]Cl2 | 104.7 ± 1.5 | 101.9 ± 0.3 | 0 ± 0 |
| [Zn-cyclam(Bn)2]Cl2 | 99.8 ± 3.1 | 94.9 ± 3.8 | 0 ± 0 |
| [Zn-cyclen(C14)2]Cl2 | 100.7 ± 2.7 | 93.8 ± 1.6 | 0 ± 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Berchel, M.; Malouch, D.; Beyler, M.; Fauchon, M.; Toueix, Y.; Hellio, C.; Jaffrès, P.-A. Antifouling Properties of N,N′-Dialkylated Tetraazamacrocyclic Polyamines and Their Metal Complexes. Molecules 2025, 30, 2368. https://doi.org/10.3390/molecules30112368
Berchel M, Malouch D, Beyler M, Fauchon M, Toueix Y, Hellio C, Jaffrès P-A. Antifouling Properties of N,N′-Dialkylated Tetraazamacrocyclic Polyamines and Their Metal Complexes. Molecules. 2025; 30(11):2368. https://doi.org/10.3390/molecules30112368
Chicago/Turabian StyleBerchel, Mathieu, Dorsaf Malouch, Maryline Beyler, Maryline Fauchon, Yannick Toueix, Claire Hellio, and Paul-Alain Jaffrès. 2025. "Antifouling Properties of N,N′-Dialkylated Tetraazamacrocyclic Polyamines and Their Metal Complexes" Molecules 30, no. 11: 2368. https://doi.org/10.3390/molecules30112368
APA StyleBerchel, M., Malouch, D., Beyler, M., Fauchon, M., Toueix, Y., Hellio, C., & Jaffrès, P.-A. (2025). Antifouling Properties of N,N′-Dialkylated Tetraazamacrocyclic Polyamines and Their Metal Complexes. Molecules, 30(11), 2368. https://doi.org/10.3390/molecules30112368








