The Synthesis and Biological Evaluation of a Novel Pleuromutilin Derivative Containing a 4-Fluorophenyl Group Targeting MRSA
Abstract
1. Introduction
2. Results
2.1. Synthesis Chemistry
2.2. In Vitro Antibacterial Activity
2.2.1. Minimum Inhibitory Concentration/Minimum Bactericidal Concentration
2.2.2. Time–Kill Kinetics
2.2.3. Post-Antibiotic Effect (PAE) and Post-Antibiotic Sub-MIC Effect

2.2.4. Scanning Electron Microscopy (SEM)
2.2.5. Biofilm Inhibition Assay
2.3. Cell Viability Assay

2.4. In Vivo Experiment
2.4.1. G. mellonella
2.4.2. MRSA-Induced Acute Pneumonia Model in Mice
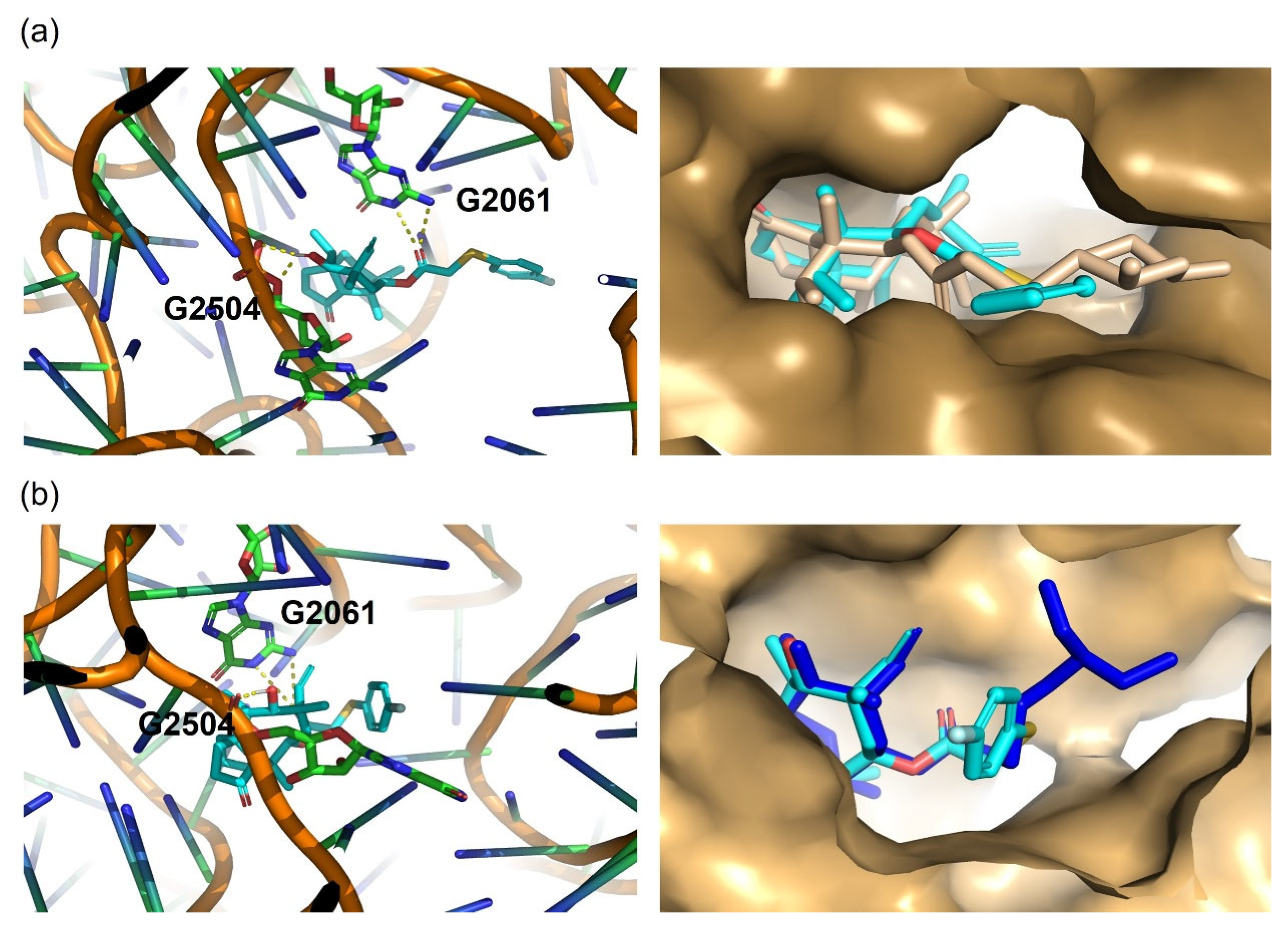
2.5. Molecular Docking Study
3. Experimental Section
3.1. Materials and Chemicals
3.2. Synthesis of Intermediate 2 and S-(4-fluorophenyl)-pleuromutilin
3.3. In Vitro Efficacy of PL-W
3.3.1. MIC/MBC Testing
3.3.2. Time–Kill Curves
3.3.3. PAE and PA-SME Assays
3.3.4. SEM Assays
3.3.5. Biofilm Influence Assays
3.3.6. Cell Viability Assay
3.4. In Vivo Assays
3.4.1. MRSA Infection Model in G. mellonella Larvae and Treatment
3.4.2. Determination of S. aureus Burden in G. mellonella Larvae
3.4.3. Mouse Model of MRSA-Induced Acute Pneumonia
3.5. Molecular Docking
3.6. Statistical Analysis
4. Discussion and Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abbas, A.; Barkhouse, A.; Hackenberger, D.; Wright, G.D. Antibiotic resistance: A key microbial survival mechanism that threatens public health. Cell Host Microbe 2024, 32, 837–851. [Google Scholar] [CrossRef]
- Terreni, M.; Taccani, M.; Pregnolato, M. New Antibiotics for Multidrug-Resistant Bacterial Strains: Latest Research Developments and Future Perspectives. Molecules 2021, 26, 2671. [Google Scholar] [CrossRef] [PubMed]
- Antimicrobial Resistance, C. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar]
- Liu, K.; Xia, J.; Li, Y.; Li, B.B.; Wang, M.Q.; Zhou, Q.; Ma, M.L.; He, Q.R.; Yang, W.Q.; Liu, D.F.; et al. Discovery of Novel Coumarin Pleuromutilin Derivatives as Potent Anti-MRSA Agents. J. Med. Chem. 2024, 67, 21030–21048. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Margarita, G.E.; Wu, D.; Yuan, W.; Yan, S.; Qi, S.; Xue, X.; Wang, K.; Wu, L. Antibacterial Activity of Chinese Red Propolis against Staphylococcus aureus and MRSA. Molecules 2022, 27, 1693. [Google Scholar] [CrossRef]
- Yi, Y.; Zhang, J.; Lin, S.; Wang, H.; Li, G.; Yang, S.; Shang, R.; Zhang, R.; Li, F. Design, synthesis, and biological evaluation of novel pleuromutilin derivatives with methicillin-resistant Staphylococcus aureus-targeting phenol linker groups. Eur. J. Med. Chem. 2025, 282, 117061. [Google Scholar] [CrossRef]
- Goethe, O.; Heuer, A.; Ma, X.; Wang, Z.; Herzon, S.B. Antibacterial properties and clinical potential of pleuromutilins. Nat. Prod. Rep. 2019, 36, 220–247. [Google Scholar] [CrossRef]
- Zhou, Y.; Yi, Y.; Wang, J.; Yang, Z.; Liu, Q.; Pu, W.; Shang, R. Discovery of novel pleuromutilin derivatives as potent antibacterial agents. Eur. J. Med. Chem. 2022, 237, 114403. [Google Scholar] [CrossRef]
- Watkins, R.R.; File, T.M. Lefamulin: A Novel Semisynthetic Pleuromutilin Antibiotic for Community-acquired Bacterial Pneumonia. Clin. Infect. Dis. 2020, 71, 2757–2762. [Google Scholar] [CrossRef]
- Li, B.; Zhang, Z.; Zhang, J.F.; Liu, J.; Zuo, X.Y.; Chen, F.; Zhang, G.Y.; Fang, H.Q.; Jin, Z.; Tang, Y.Z. Design, synthesis and biological evaluation of pleuromutilin-Schiff base hybrids as potent anti-MRSA agents in vitro and in vivo. Eur. J. Med. Chem. 2021, 223, 113624. [Google Scholar] [CrossRef]
- Sader, H.S.; Biedenbach, D.J.; Paukner, S.; Ivezic-Schoenfeld, Z.; Jones, R.N. Antimicrobial activity of the investigational pleuromutilin compound BC-3781 tested against Gram-positive organisms commonly associated with acute bacterial skin and skin structure infections. Antimicrob. Agents Chemother. 2012, 56, 1619–1623. [Google Scholar] [CrossRef] [PubMed]
- Williamson, D.A.; Carter, G.P.; Howden, B.P. Current and Emerging Topical Antibacterials and Antiseptics: Agents, Action, and Resistance Patterns. Clin. Microbiol. Rev. 2017, 30, 827–860. [Google Scholar] [CrossRef]
- Eraikhuemen, N.; Julien, D.; Kelly, A.; Lindsay, T.; Lazaridis, D. Treatment of Community-Acquired Pneumonia: A Focus on Lefamulin. Infect. Dis. Ther. 2021, 10, 149–163. [Google Scholar] [CrossRef]
- Yi, Y.; Zhang, J.; Zuo, J.; Zhang, M.; Yang, S.; Huang, Z.; Li, G.; Shang, R.; Lin, S. Novel pyridinium cationic pleuromutilin analogues overcoming bacterial multidrug resistance. Eur. J. Med. Chem. 2023, 251, 115269. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Xin, L.; Li, J.; Tian, L.; Wu, K.; Zhang, S.; Yan, W.; Li, H.; Zhao, Q.; Liang, C. Discovery of Quaternized Pyridine-Thiazole-Pleuromutilin Derivatives with Broad-Spectrum Antibacterial and Potent Anti-MRSA Activity. J. Med. Chem. 2023, 66, 5061–5078. [Google Scholar] [CrossRef]
- Sheikhi, N.; Bahraminejad, M.; Saeedi, M.; Mirfazli, S.S. A review: FDA-approved fluorine-containing small molecules from 2015 to 2022. Eur. J. Med. Chem. 2023, 260, 115758. [Google Scholar] [CrossRef]
- De Biasio, A.; Ibanez de Opakua, A.; Bostock, M.J.; Nietlispach, D.; Diercks, T.; Blanco, F.J. A generalized approach for NMR studies of lipid-protein interactions based on sparse fluorination of acyl chains. Chem. Commun. 2018, 54, 7306–7309. [Google Scholar] [CrossRef]
- Pettersson, M.; Hou, X.; Kuhn, M.; Wager, T.T.; Kauffman, G.W.; Verhoest, P.R. Quantitative Assessment of the Impact of Fluorine Substitution on P-Glycoprotein (P-gp) Mediated Efflux, Permeability, Lipophilicity, and Metabolic Stability. J. Med. Chem. 2016, 59, 5284–5296. [Google Scholar] [CrossRef]
- Guo, K.; van den Beucken, T. Advances in drug-induced liver injury research: In vitro models, mechanisms, omics and gene modulation techniques. Cell Biosci. 2024, 14, 134. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xie, C.; Liu, Y.; Shang, F.; Shao, R.; Yu, J.; Wu, C.; Yao, X.; Liu, D.; Wang, Z. Synthesis, biological activities and docking studies of pleuromutilin derivatives with piperazinyl urea linkage. J. Enzym. Inhib. Med. Chem. 2021, 36, 764–775. [Google Scholar] [CrossRef]
- Qi, X.L.; Zhang, H.C.; Xu, X.; Liu, X.W.; Yang, Y.J.; Li, Z.; Li, J.Y. Discovery of novel thiazole-pleuromutilin derivatives with potent antibacterial activity. Eur. J. Med. Chem. 2025, 287, 117374. [Google Scholar] [CrossRef] [PubMed]
- Eyal, Z.; Matzov, D.; Krupkin, M.; Paukner, S.; Riedl, R.; Rozenberg, H.; Zimmerman, E.; Bashan, A.; Yonath, A. A novel pleuromutilin antibacterial compound, its binding mode and selectivity mechanism. Sci. Rep. 2016, 6, 39004. [Google Scholar] [CrossRef]
- Koes, D.R.; Baumgartner, M.P.; Camacho, C.J. Lessons learned in empirical scoring with smina from the CSAR 2011 benchmarking exercise. J. Chem. Inf. Model. 2013, 53, 1893–1904. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef]
- Quiroga, R.; Villarreal, M.A. Vinardo: A Scoring Function Based on Autodock Vina Improves Scoring, Docking, and Virtual Screening. PLoS ONE 2016, 11, e0155183. [Google Scholar] [CrossRef]
- Matuschek, E.; Ahman, J.; Webster, C.; Kahlmeter, G. Antimicrobial susceptibility testing of colistin—Evaluation of seven commercial MIC products against standard broth microdilution for Escherichia coli, Klebsiella pneumoniae, Pseudomonas aeruginosa, and Acinetobacter spp. Clin. Microbiol. Infect. 2018, 24, 865–870. [Google Scholar] [CrossRef]
- Wang, J.; Hu, Y.H.; Zhou, K.X.; Wang, W.; Li, F.; Li, K.; Zhang, G.Y.; Tang, Y.Z. Design, Synthesis and Biological Evaluation of Novel Pleuromutilin Derivatives Containing 6-Chloro-1-R-1H-pyrazolo[3,4-d]pyrimidine-4-amino Side Chain. Molecules 2023, 28, 3975. [Google Scholar] [CrossRef]
- Gu, S.; Fan, B.; Wan, F.; Gao, T.; Qi, Y.; Zhou, J.; Zhang, Y.; Gu, D.; Xie, W. Antibacterial Activity and Mechanism of Canagliflozin against Methicillin-Resistant Staphylococcus aureus. Molecules 2023, 28, 5668. [Google Scholar] [CrossRef]
- Boyer, E.; Galan-Relano, A.; Romero-Salmoral, A.; Barraza, P.; Gomez-Gascon, L.; Tarradas, C.; Luque, I.; de Aguiar, F.C.; Huerta Lorenzo, B. Post-Antibiotic and Post-Antibiotic Sub-Minimum Inhibitory Concentration Effects of Carvacrol against Salmonella Typhimurium. Animals 2024, 14, 2631. [Google Scholar] [CrossRef]
- Phakatkar, A.H.; Firlar, E.; Alzate, L.; Song, B.; Narayanan, S.; Rojaee, R.; Foroozan, T.; Deivanayagam, R.; Banner, D.J.; Shahbazian-Yassar, R.; et al. TEM Studies on Antibacterial Mechanisms of Black Phosphorous Nanosheets. Int. J. Nanomed. 2020, 15, 3071–3085. [Google Scholar] [CrossRef]
- Xiao, Y.; Wan, C.; Wu, X.; Xu, Y.; Chen, Y.; Rao, L.; Wang, B.; Shen, L.; Han, W.; Zhao, H.; et al. Novel small-molecule compound YH7 inhibits the biofilm formation of Staphylococcus aureus in a sarX-dependent manner. mSphere 2024, 9, e0056423. [Google Scholar] [CrossRef]
- Mendogralo, E.Y.; Nesterova, L.Y.; Nasibullina, E.R.; Shcherbakov, R.O.; Tkachenko, A.G.; Sidorov, R.Y.; Sukonnikov, M.A.; Skvortsov, D.A.; Uchuskin, M.G. The Synthesis and Biological Evaluation of 2-(1H-Indol-3-yl)quinazolin-4(3H)-One Derivatives. Molecules 2023, 28, 5348. [Google Scholar] [CrossRef]
- Dzah, C.S.; Duan, Y.; Zhang, H.; Ma, H. Effects of pretreatment and type of hydrolysis on the composition, antioxidant potential and HepG2 cytotoxicity of bound polyphenols from Tartary buckwheat (Fagopyrum tataricum L. Gaerth) hulls. Food Res. Int. 2021, 142, 110187. [Google Scholar] [CrossRef]
- Todorovic, M.; Rivollier, P.; Wong, A.; Wang, Z.; Pryyma, A.; Nguyen, T.T.; Newell, K.C.; Froelich, J.; Perrin, D.M. Rationally Designed Amanitins Achieve Enhanced Cytotoxicity. J. Med. Chem. 2022, 65, 10357–10376. [Google Scholar] [CrossRef]
- Zhao, Y.; Mao, W.; Liu, B.; Wang, Y.F.; Zhang, S.Y.; Guo, L.L.; Qian, Y.H.; Gong, Z.G.; Zhao, J.M.; Yang, X.L.; et al. Preparation of ceftiofur-encapsulated hen-egg low-density lipoproteins and their antibacterial effects on intracellular Staphylococcus aureus. Int. J. Biol. Macromol. 2024, 278 Pt 4, 134840. [Google Scholar] [CrossRef]
- Pezzanite, L.; Chow, L.; Piquini, G.; Griffenhagen, G.; Ramirez, D.; Dow, S.; Goodrich, L. Use of in vitro assays to identify antibiotics that are cytotoxic to normal equine chondrocytes and synovial cells. Equine Vet. J. 2021, 53, 579–589. [Google Scholar] [CrossRef]
- Dong, C.L.; Li, L.X.; Cui, Z.H.; Chen, S.W.; Xiong, Y.Q.; Lu, J.Q.; Liao, X.P.; Gao, Y.; Sun, J.; Liu, Y.H. Synergistic Effect of Pleuromutilins with Other Antimicrobial Agents against Staphylococcus aureus In Vitro and in an Experimental Galleria mellonella Model. Front. Pharmacol. 2017, 8, 553. [Google Scholar] [CrossRef]
- Alnezary, F.S.; Almutairi, M.S.; Alhifany, A.A.; Almangour, T.A. Assessing Galleria mellonella as a preliminary model for systemic Staphylococcus aureus infection: Evaluating the efficacy and impact of vancomycin and Nigella sativa oil on gut microbiota. Saudi Pharm. J. 2023, 31, 101824. [Google Scholar] [CrossRef]
- Lazar, V.; Snitser, O.; Barkan, D.; Kishony, R. Antibiotic combinations reduce Staphylococcus aureus clearance. Nature 2022, 610, 540–546. [Google Scholar] [CrossRef]





| Test Compounds | MIC/MBC (µg/mL) | |
|---|---|---|
 |  | |
| S. aureus (ATCC29213) | 0.5/1 | 0.25/1 |
| MRSA (ATCC33591) | 0.5/2 | 0.03125/0.25 |
| MRSE(ATCC51625) | 0.5/1 | 0.125/0.5 |
| SA-R20220105 | 1/>2 | 0.125/0.5 |
| SA-R20220106 | 0.5/2 | 0.0625/0.5 |
| SA-R20220111 | >2/>2 | 0.125/0.5 |
| SA-R20230119 | 2/>2 | 0.25/1 |
| SA-R20240101 | 1/>2 | 0.125/0.5 |
| SA-R20240112 | 1/>2 | 0.25/0.5 |
| SA-R20240125 | 1/>2 | 0.25/2 |
| S. pneumoniaeATCC49619 | 0.5/1 | 0.125/0.5 |
| E. coliATCC25922 | >64 | >64 |
| B. subtilisATCC21216 | >64 | >64 |
| P. aeruginosaATCC14215 | >64 | >64 |
| Compounds | Concentrations | PAE (h) | PASME (h) |
|---|---|---|---|
| Tiamulin | 2 × MIC | 0.31 | 1.15 |
| 4 × MIC | 0.43 | 1.85 | |
| PL-W | 2 × MIC | 0.63 | 1.45 |
| 4 × MIC | 0.91 | 2.66 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Zhao, Y.; Wang, H.; Liu, B.; Zhang, S.; Liu, Y.; Li, R.; Zhang, T.; Hasi, S.; Mao, W. The Synthesis and Biological Evaluation of a Novel Pleuromutilin Derivative Containing a 4-Fluorophenyl Group Targeting MRSA. Molecules 2025, 30, 2366. https://doi.org/10.3390/molecules30112366
Wang Y, Zhao Y, Wang H, Liu B, Zhang S, Liu Y, Li R, Zhang T, Hasi S, Mao W. The Synthesis and Biological Evaluation of a Novel Pleuromutilin Derivative Containing a 4-Fluorophenyl Group Targeting MRSA. Molecules. 2025; 30(11):2366. https://doi.org/10.3390/molecules30112366
Chicago/Turabian StyleWang, Yongfei, Yi Zhao, Haiting Wang, Bo Liu, Shuangyi Zhang, Yuan Liu, Ruinan Li, Tao Zhang, Surong Hasi, and Wei Mao. 2025. "The Synthesis and Biological Evaluation of a Novel Pleuromutilin Derivative Containing a 4-Fluorophenyl Group Targeting MRSA" Molecules 30, no. 11: 2366. https://doi.org/10.3390/molecules30112366
APA StyleWang, Y., Zhao, Y., Wang, H., Liu, B., Zhang, S., Liu, Y., Li, R., Zhang, T., Hasi, S., & Mao, W. (2025). The Synthesis and Biological Evaluation of a Novel Pleuromutilin Derivative Containing a 4-Fluorophenyl Group Targeting MRSA. Molecules, 30(11), 2366. https://doi.org/10.3390/molecules30112366







