Solvent-Exchange Triggered Solidification of Peptide/POM Coacervates for Enhancing the On-Site Underwater Adhesion
Abstract
1. Introduction
2. Results and Discussion
2.1. Preparation and Characterization of Peptide/SiW Coacervates
2.1.1. Design of Peptide Sequences and Preparation of Peptide/SiW Coacervates
2.1.2. Characterization of L1/SiW Coacervate
2.2. Solvent-Exchange Triggered Solidification of Peptide/SiW Coacervates
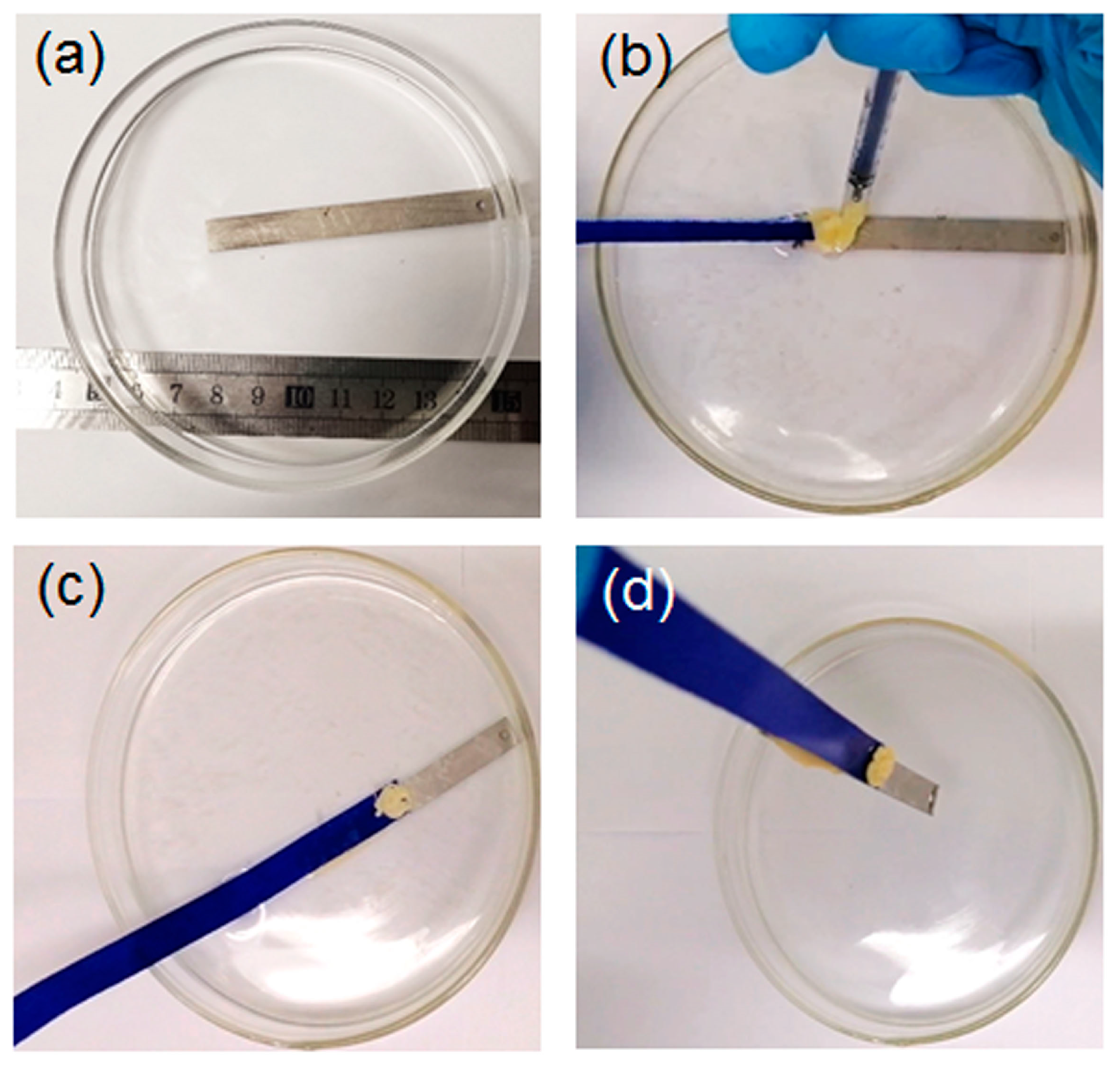
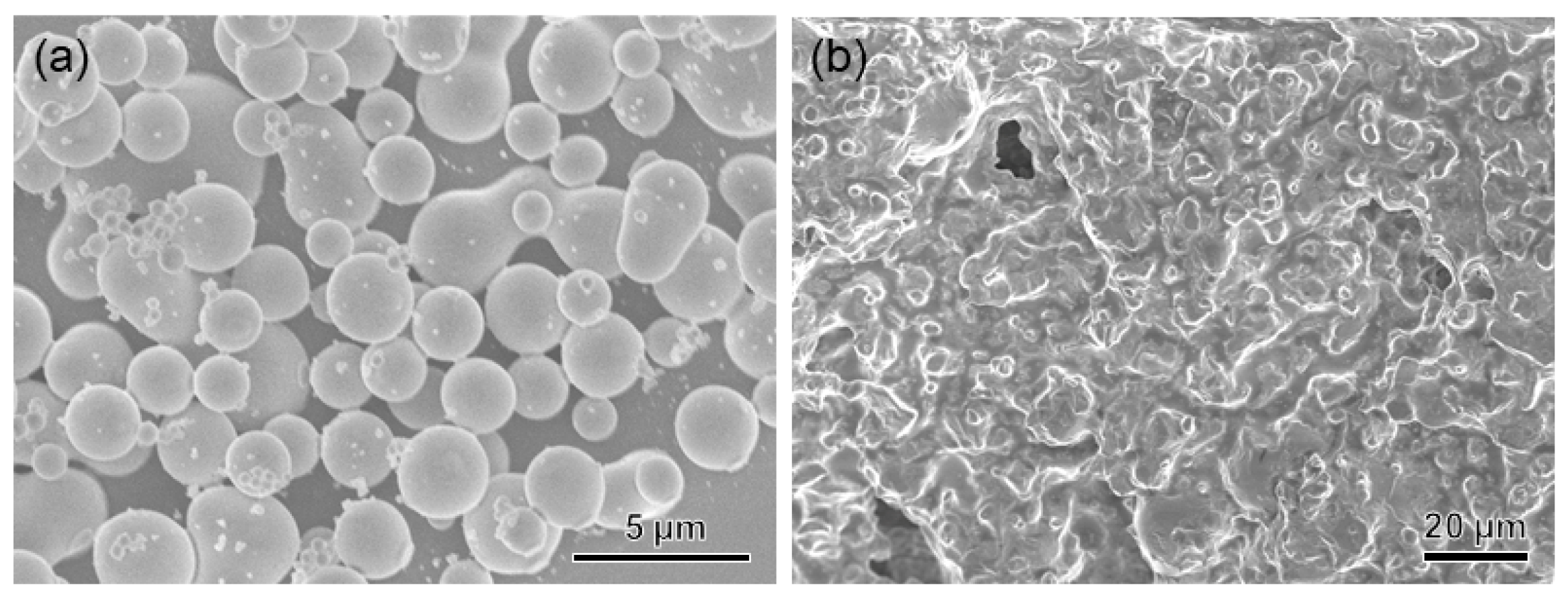
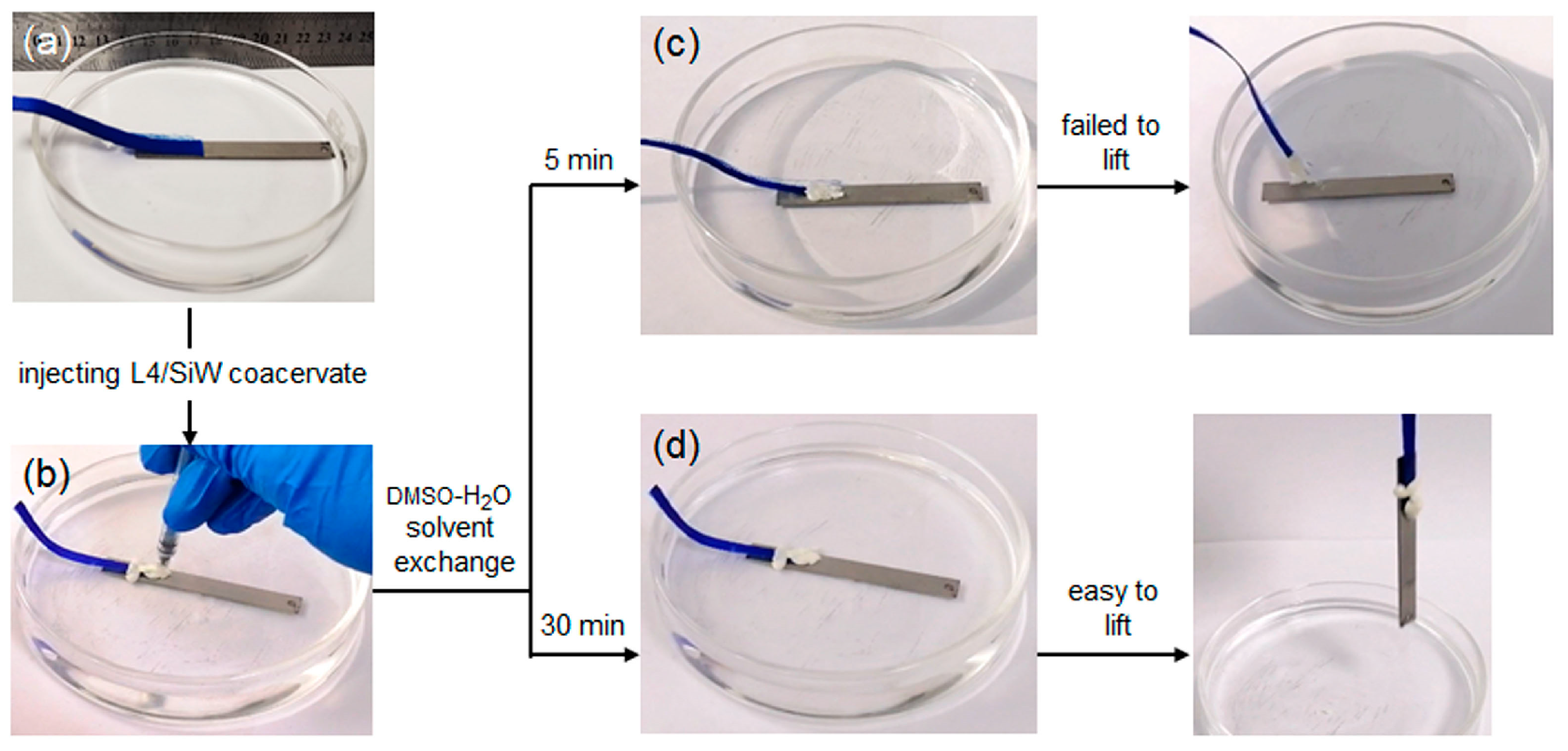
2.2.1. Solvent-Exchange Triggered Phase Transition of L1/SiW and Its Enhanced Adhesion Performance
2.2.2. Effect of Single Amino Acid Residue on Adhesion Performance of Peptide/SiW Adhesives
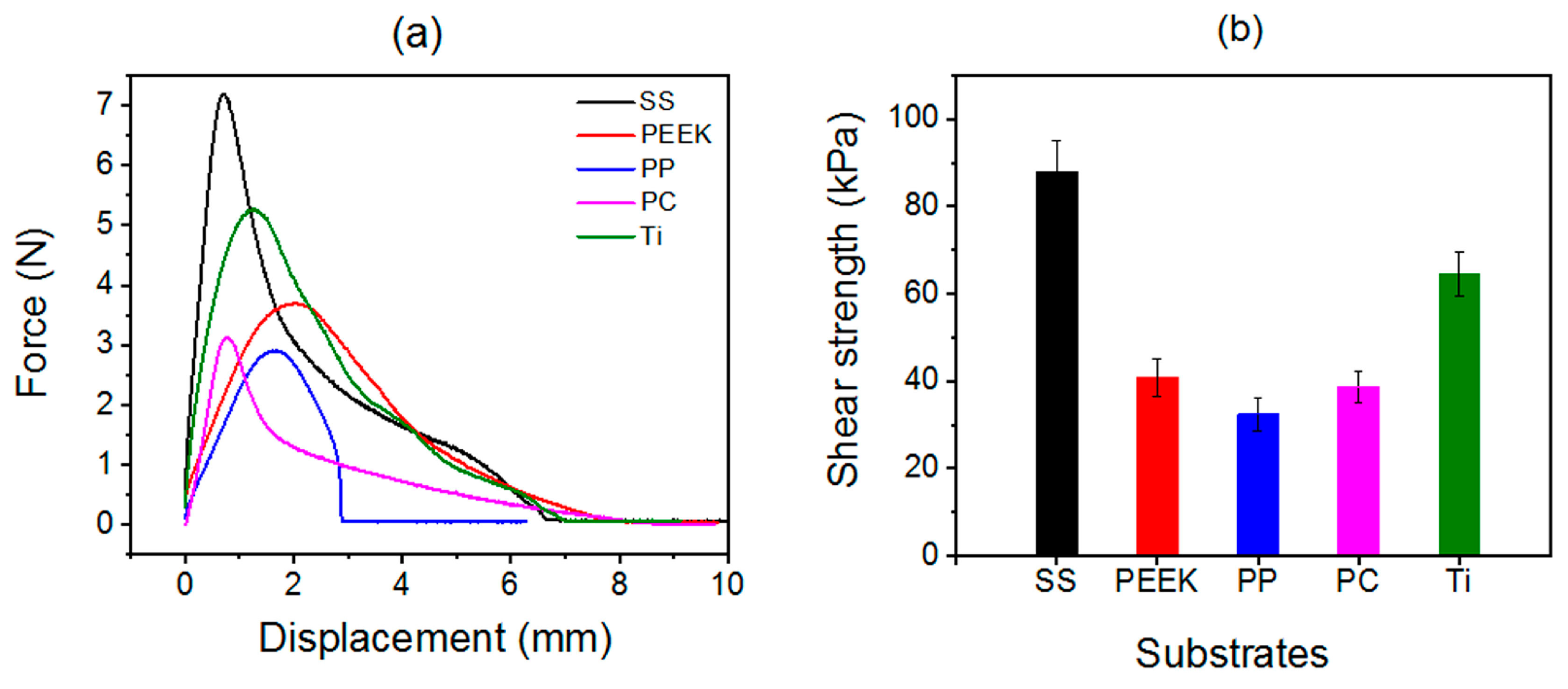
2.3. Universality of Solvent-Exchange Strategy
3. Materials and Methods
3.1. Materials
3.2. Preparation of the Peptide/SiW Complex Coacervates
3.3. Description of the Characteristics of the Peptide/SiW Complex Coacervates
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Waite, J.H. Mussel Adhesion–Essential Footwork. Exp. Biol. 2017, 220, 517–530. [Google Scholar] [CrossRef]
- Tibabuzo, P.A.M.; Alberts, E.M.; Taylor, S.D.; Sherman, D.M.; Huang, C.P.; Wilker, J.J. Changes in Cementation of Reef Building Oysters Transitioning from Larvae to Adults. ACS Appl. Mater. Interfaces 2018, 10, 14248–14253. [Google Scholar] [CrossRef]
- Stewart, R.J.; Wang, C.S.; Song, I.T.; Jones, J.P. The Role of Coacervation and Phase Transitions in the Sandcastle Worm Adhesive System. Adv. Colloid Interface Sci. 2017, 239, 88–96. [Google Scholar] [CrossRef]
- Ryu, J.H.; Kim, H.J.; Kim, K.; Yoon, G.; Wang, Y.; Choi, G.S.; Lee, H.; Park, J.S. Multipurpose Intraperitoneal Adhesive Patches. Adv. Funct. Mater. 2019, 29, 1900495. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, R.; Sun, Z.; Zhu, X.; Zhao, Q.; Zhang, T.; Cholewinski, A.; Yang, F.; Zhao, B.X.; Pinnaratip, R.; et al. Catechol-Functionalized Hydrogels: Biomimetic Design, Adhesion Mechanism, and Biomedical Applications. Chem. Soc. Rev. 2020, 49, 433–464. [Google Scholar] [CrossRef]
- Zhu, H.; Mei, X.; He, Y.; Mao, H.; Tang, W.; Liu, R.; Yang, J.; Luo, K.; Gu, Z.; Zhou, L. Fast and High Strength Soft Tissue Bioadhesives Based on a Peptide Dendrimer with Antimicrobial Properties and Hemostatic Ability. ACS Appl. Mater. Interfaces 2020, 12, 4241–4253. [Google Scholar] [CrossRef]
- Fan, H.L.; Gong, J.P. Bioinspired Underwater Adhesives. Adv. Mater. 2021, 33, 2102983. [Google Scholar] [CrossRef]
- Narayanan, A.; Dhinojwala, A.; Joy, A. Design Principles for Creating Synthetic Underwater Adhesives. Chem. Soc. Rev. 2021, 50, 13321–13345. [Google Scholar] [CrossRef]
- Vahdati, M.; Hourdet, M.; Creton, C. Soft Underwater Adhesives based on Weak Molecular Interactions. Prog. Polym. Sci. 2023, 139, 101649. [Google Scholar] [CrossRef]
- Akram Bhuiyan, M.S.; Roland, J.D.; Liu, B.; Reaume, M.; Zhang, Z.; Kelley, J.D.; Lee, B.P. In Situ Deactivation of Catechol-Containing Adhesive Using Electrochemistry. J. Am. Chem. Soc. 2020, 142, 4631–4638. [Google Scholar] [CrossRef]
- Mazzotta, M.G.; Putnam, A.A.; North, M.A.; Wilker, J.J. Weak Bonds in a Biomimetic Adhesive Enhance Toughness and Performance. J. Am. Chem. Soc. 2020, 142, 4762–4768. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Hwang, H.; Kim, J.S.; Park, J.; Youn, D.; Kim, D.; Hahn, J.; Seo, M.; Lee, H. VATA: A Poly(vinyl alcohol)- and Tannic Acid-Based Nontoxic Underwater Adhesive. ACS Appl. Mater. Interfaces 2020, 12, 20933–20941. [Google Scholar] [CrossRef]
- Peng, X.; Xia, X.; Xu, X.; Yang, X.; Yang, B.; Zhao, P.; Yuan, W.; Chiu, P.W.Y.; Bian, L. Ultrafast Self-Gelling Powder Mediates Robust Wet Adhesion to Promote Healing of Gastrointestinal Perforations. Sci. Adv. 2021, 7, 8739. [Google Scholar] [CrossRef]
- Cui, C.; Liu, W.G. Recent Advances in Wet Adhesives: Adhesion Mechanism, Design Principle and Applications. Prog. Polym. Sci. 2021, 116, 101388. [Google Scholar] [CrossRef]
- Xiao, L.; Qang, Z.; Sun, Y.; Li, B.; Wu, B.; Ma, C.; Petrovskii, V.S.; Gu, X.; Chen, D.; Potemkin, I.I.; et al. An Artificial Phase-Transitional Underwater Bioglue with Robust and Switchable Adhesion Performance. Angew. Chem. Int. Ed. 2021, 60, 12082–12089. [Google Scholar] [CrossRef]
- Nitin, A.P.; John, A.K.; John, D.W.; Mohammed, A.H.; Julien, T. Rapid Photolysis-Mediated Folding of Disulfide-Rich Peptides. Chem. Eur. J. 2019, 25, 8559–8603. [Google Scholar]
- Nhan Dai, T.T.; Xu, J.; Devika, M.; Antonio, E.O.; Venkatesh, M.; Vanitha, S.; Zhu, X.; Teo, J.; Veluchamy, A.B.; Rajamani, L.; et al. Bacteria-Responsive Self-Assembly of Antimicrobial Peptide Nanonets for Trap-and-Kill of Antibiotic-Resistant Strains. Adv. Func. Mater. 2023, 33, 2210858. [Google Scholar]
- Zhu, H.; Xu, G.; He, Y.; Mao, H.; Kong, D.; Luo, K.; Tang, W.; Liu, R.; Gu, Z. A Dual-Bioinspired Tissue Adhesive Based on Peptide Dendrimer with Fast and Strong Wet Adhesion. Adv. Healthc. Mater. 2022, 11, 22008. [Google Scholar] [CrossRef]
- Liu, X.H.; Cheng, X.L.; Sun, Y.C.; Nie, J.L.; Cheng, M.; Li, W.; Zhao, J.W. Peptide/glycyrrhizic acid supramolecular polymer: An emerging medical adhesive for dural sealing and repairing. Biomaterials 2023, 301, 122239. [Google Scholar] [CrossRef]
- Sato, K.; Hendricks, M.P.; Palmer, L.C.; Stupp, S.I. Peptide Supramolecular Materials for Therapeutics. Chem. Soc. Rev. 2018, 47, 7539–7551. [Google Scholar] [CrossRef]
- Ren, P.; Li, J.; Zhao, L.; Wang, A.; Wang, M.; Li, J.; Jian, H.; Li, X.; Yan, X.; Bai, S. Dipeptide Self-assembled Hydrogels with Shear-Thinning and Instantaneous Self-healing Properties Determined by Peptide Sequences. ACS Appl. Mater. Interfaces 2020, 12, 21433–21440. [Google Scholar] [CrossRef]
- Zhang, Q.; Shi, C.Y.; Qu, D.H.; Long, Y.T.; Feringa, B.L.; Tian, H. Exploring a Naturally Tailored Small Molecule for Stretchable, Self-Healing, and Adhesive Supramolecular Polymers. Sci. Adv. 2018, 4, 8192. [Google Scholar] [CrossRef]
- Cui, M.; Wang, X.; An, B.; Zhang, C.; Gui, X.; Li, K.; Li, Y.; Ge, P.; Zhang, J.; Liu, C.; et al. Exploiting Mammalian Low-Complexity Domains for Liquid-Liquid Phase Separation-Driven Underwater Adhesive Coatings. Sci. Adv. 2019, 5, 3155. [Google Scholar] [CrossRef]
- Kaminker, I.; Wei, W.; Schrader, A.M.; Talmon, Y.; Valentine, M.T.; Israelachvili, J.N.; Waite, J.H.; Han, S. Simple Peptide Coacervates Adapted for Rapid Pressure-Sensitive Wet Adhesion. Soft Matter. 2017, 13, 9122–9131. [Google Scholar] [CrossRef]
- Huang, Y.; Liu, L.X.; Luo, C.H.; Liu, W.; Lou, X.D.; Jiang, L.; Xia, F. Solid-State Nanochannels for Bio-marker Analysis. Chem. Soc. Rev. 2023, 52, 6270–6293. [Google Scholar] [CrossRef]
- Yang, X.; Wang, J.H.; Gao, Z.F.; Zhang, W.Q.; Zhu, H.; Song, Y.J.; Wang, Q.; Liu, M.J.; Jiang, L.; Huang, Y.; et al. An Orthogonal Dual-Regulation Strategy for Sensitive Biosensing Applications. Natl. Sci. Rev. 2022, 9, nwac048. [Google Scholar] [CrossRef]
- Sun, J.; Xiao, L.; Li, B.; Zhao, K.; Wang, Z.; Zhou, Y.; Ma, C.; Li, J.J.; Zhang, H.J.; Herrmann, A.; et al. Genetically Engineered Polypeptide Adhesive Coacervates for Surgical Applications. Angew. Chem. Int. Ed. 2021, 60, 23687–23694. [Google Scholar] [CrossRef]
- Makam, P.; Gazit, E. Minimalistic Peptide Supramolecular Co-Assembly: Expanding the Conformational Space for Nanotechnology. Chem. Soc. Rev. 2018, 47, 3406–3420. [Google Scholar] [CrossRef] [PubMed]
- Shin, M.; Shin, J.Y.; Kim, K.; Yang, B.; Han, J.W.; Kim, N.K.; Cha, H.J. The Position of Lysine Controls the Catechol-Mediated Surface Adhesion and Cohesion in Underwater Mussel Adhesion. Colloid Interface Sci. 2020, 563, 168–176. [Google Scholar] [CrossRef]
- Wei, W.; Petrone, L.; Tan, Y.P.; Cai, H.; Israelachvili, J.N.; Miserez, A.; Waite, J.H. An Underwater Surface-Drying Peptide Inspired by a Mussel Adhesive Protein. Adv. Funct. Mater. 2016, 26, 3496–3507. [Google Scholar] [CrossRef]
- Matthew, A.G.; Wei, W.; Alex, M.; Schrader, T.R.; Cristiani, H.A.; Dobbs, M.I.; Bradley, F.; Chmelka, J.; Herbert, W.; Jacob, N.I. Tuning underwater adhesion with cation–π interactions. Nat. Chem. 2017, 9, 473–479. [Google Scholar]
- Sangsilk, K.; Ali, F. Cation–π interaction in DOPA-deficient mussel adhesive protein mfp-1. J. Mater. Chem. B 2015, 3, 738–743. [Google Scholar]
- Xu, J.; Li, X.Y.; Li, X.D.; Li, B.; Wu, L.X.; Li, W.; Xie, X.M.; Xue, R. Supramolecular Copolymerization of Short Peptides and Polyoxometalates: Toward the Fabrication of Underwater Adhesives. Biomacromolecules 2017, 18, 3524–3530. [Google Scholar] [CrossRef]
- Liu, X.H.; Xu, J.; Xie, X.M.; Ma, Z.Y.; Zheng, T.T.; Wu, L.X.; Li, B.; Li, W. Heteropoly acid-driven assembly of glutathione into redox-responsive underwater adhesive. Chem. Commun. 2020, 56, 11034–11037. [Google Scholar] [CrossRef]
- Anton, H.H.; Ilse, A.; Van, H.; Yang, J.; Marleen, K. Bioinspired Underwater Adhesives by Using the Supramolecular Toolbox. Adv. Mater. 2018, 30, 170464. [Google Scholar]
- Saad, M. Recent Developments in Catecholic Polymers: Polymerization and Applications. Curr. Mater. Sci. 2023, 16, 262–315. [Google Scholar]
- Yu, Y.; Lv, B.; Wu, J.; Chen, W. Mussel-Based Biomimetic Strategies in Musculoskeletal Disorder Treatment: From Synthesis Principles to Diverse Applications. Int. J. Nanomed. 2023, 18, 455–472. [Google Scholar] [CrossRef]
- Li, X.Y.; Zheng, T.T.; Liu, X.H.; Du, Z.L.; Xie, X.M.; Li, B.; Wu, L.X.; Li, W. Coassembly of Short Peptide and Polyoxometalate into Complex Coacervate Adapted for pH and Metal Ion-Triggered Underwater Adhesion. Langmuir 2019, 35, 4995–5003. [Google Scholar] [CrossRef]
- Liu, X.H.; Ma, Z.Y.; Nie, J.L.; Fang, J.; Li, W. Exploiting Redox-Complementary Peptide/Polyoxometalate Coacervates for Spontaneously Curing into Antimicrobial Adhesives. Biomacromolecules 2022, 23, 1009–1019. [Google Scholar] [CrossRef]
- Xu, L.; Gao, S.; Guo, Q.; Wang, C.; Qiao, Y.; Qiu, D. A Solvent-Exchange Strategy to Regulate Noncovalent Interactions for Strong and Antiswelling Hydrogels. Adv. Mater. 2020, 32, 2004579. [Google Scholar] [CrossRef]
- Ren, J.; Kong, R.; Wang, H.; Du, S.; Liu, P.; Wang, H.; Chen, Y.; Xie, G.; Zhang, L.; Zhu, J. Robust Underwater Adhesion of Catechol-Functionalized Polymer Triggered by Water Exchange. Small. Methods 2023, 7, 2201235. [Google Scholar] [CrossRef]
- Cai, C.; Wu, S.; Tan, Z.; Li, F.; Dong, S. On-Site Supramolecular Adhesion to Wet and Soft Surfaces via Solvent Exchange. ACS Appl. Mater. Interfaces 2021, 13, 53083–53090. [Google Scholar] [CrossRef]
- Song, Q.; Chen, Y.; Huang, Y.; Dan, W.; Wang, M.; Dan, N. Adhesive Capable of Underwater Adhesion and Wound Hemostasis Prepared Based on Solvent Exchange. Adv. Mater. Technol. 2022, 7, 2100852. [Google Scholar] [CrossRef]
- Li, B.; Li, H.; Chen, H.; Liu, Y.; Chen, J.; Feng, Q.; Cao, X.; Dong, H. Microgel Assembly Powder Improves Acute Hemostasis, Antibacterial, and Wound Healing via In Situ Co-Assembly of Erythrocyte and Microgel. Adv. Funct. Mater. 2023, 33, 23027. [Google Scholar] [CrossRef]
- Mohammed, H.A.M.; Claudio, C.; John, P.B.; Martin, K.S.; Scarsdale, J.N.; Glen, E.K. 3D Interaction Homology: Hydropathic Analyses of the “π–Cation” and “π–π” Interaction Motifs in Phenylalanine, Tyrosine, and Tryptophan Residues. J. Chem. Inf. Model. 2021, 61, 2937–2956. [Google Scholar]
- Niu, J.J.; You, X.X.; Duan, C.C. A Novel Optical Complex between an Organic Substrate and a Polyoxometalate. Crystal and Molecular Structure of α-H4SiW12O40.4HMPA.2H2O (HMPA=Hexamethylphosphoramide). Inorg. Chem. 1996, 35, 4211–4217. [Google Scholar] [CrossRef]
- Chi, Y.N.; Shen, P.P.; Cui, F.Y.; Lin, Z.G.; Chen, S.L.; Hu, C.W. Symmetry Breaking of α-[H2W12O40](6-) Depends on the Transformation of Isopolyoxotungstates. Inorg. Chem. 2014, 53, 5029–5036. [Google Scholar] [CrossRef]
- Wei, X.R.; Ma, K.; Cheng, Y.B.; Sun, L.Y.; Chen, D.J.; Zhao, X.L.; Lu, H.; Song, B.T.; Yang, K.W.; Jia, P.X. Adhesive, Conductive, Self-Healing, and Antibacterial Hydrogel Based on Chitosan–Polyoxometalate Complexes for Wearable Strain Sensor. ACS Appl. Polym. Mater. 2020, 2, 2541–2549. [Google Scholar] [CrossRef]
- Nie, J.; Sun, Y.; Cheng, X.; Wen, G.; Liu, X.; Cheng, M.; Zhao, J.; Li, W. Plant Protein-Peptide Supramolecular Polymers with Reliable Tissue Adhesion for Surgical Sealing. Adv. Healthc. Mater. 2023, 20, 2203301. [Google Scholar] [CrossRef]
- Payra, D.; Fu, Y.; Das, S. Rational design of a biomimetic glue with tunable strength and ductility. Polym. Chem. 2017, 8, 1654–1663. [Google Scholar] [CrossRef]
- Li, X.; Li, S.; Huang, X. Protein-mediated bioadhesion in marine organisms: A review. Mar. Environ. Res. 2021, 170, 105409. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ji, F.; Li, Y.; Zhao, H.; Wang, X.; Li, W. Solvent-Exchange Triggered Solidification of Peptide/POM Coacervates for Enhancing the On-Site Underwater Adhesion. Molecules 2024, 29, 681. https://doi.org/10.3390/molecules29030681
Ji F, Li Y, Zhao H, Wang X, Li W. Solvent-Exchange Triggered Solidification of Peptide/POM Coacervates for Enhancing the On-Site Underwater Adhesion. Molecules. 2024; 29(3):681. https://doi.org/10.3390/molecules29030681
Chicago/Turabian StyleJi, Fangyan, Yiwen Li, He Zhao, Xinyan Wang, and Wen Li. 2024. "Solvent-Exchange Triggered Solidification of Peptide/POM Coacervates for Enhancing the On-Site Underwater Adhesion" Molecules 29, no. 3: 681. https://doi.org/10.3390/molecules29030681
APA StyleJi, F., Li, Y., Zhao, H., Wang, X., & Li, W. (2024). Solvent-Exchange Triggered Solidification of Peptide/POM Coacervates for Enhancing the On-Site Underwater Adhesion. Molecules, 29(3), 681. https://doi.org/10.3390/molecules29030681






