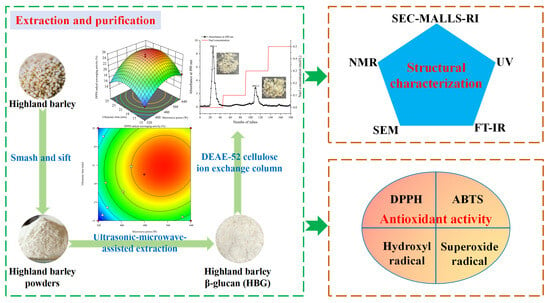
Structural Characterization and Antioxidant Activity of β-Glucans from Highland Barley Obtained with Ultrasonic–Microwave-Assisted Extraction
Abstract
1. Introduction
2. Results and Discussion
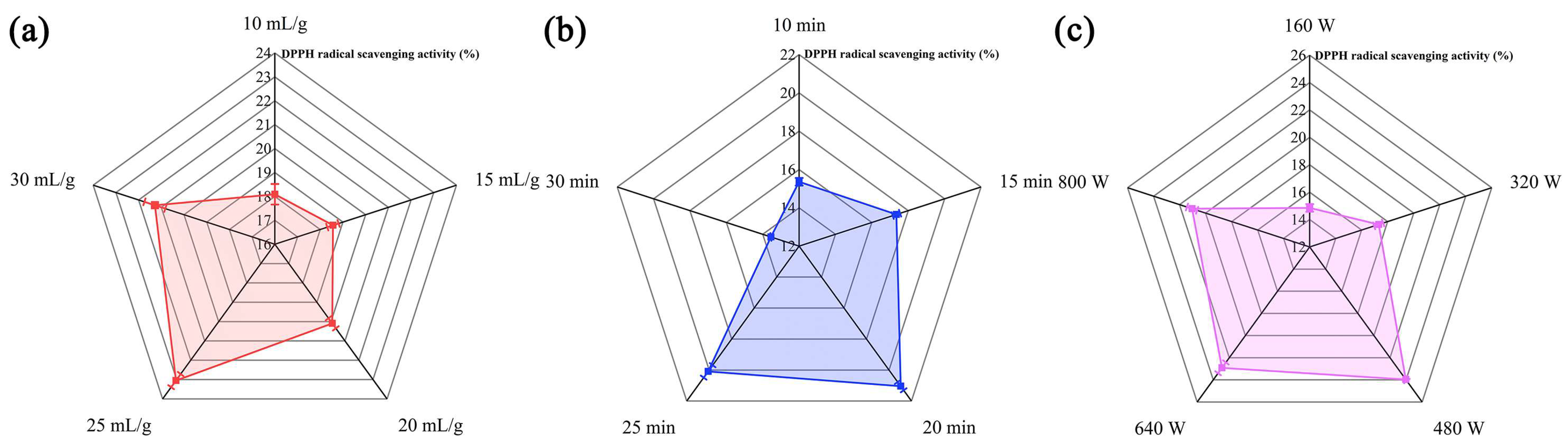
2.1. Single-Factor Test Results regarding Ultrasonic–Microwave-Assisted Extraction (UME)
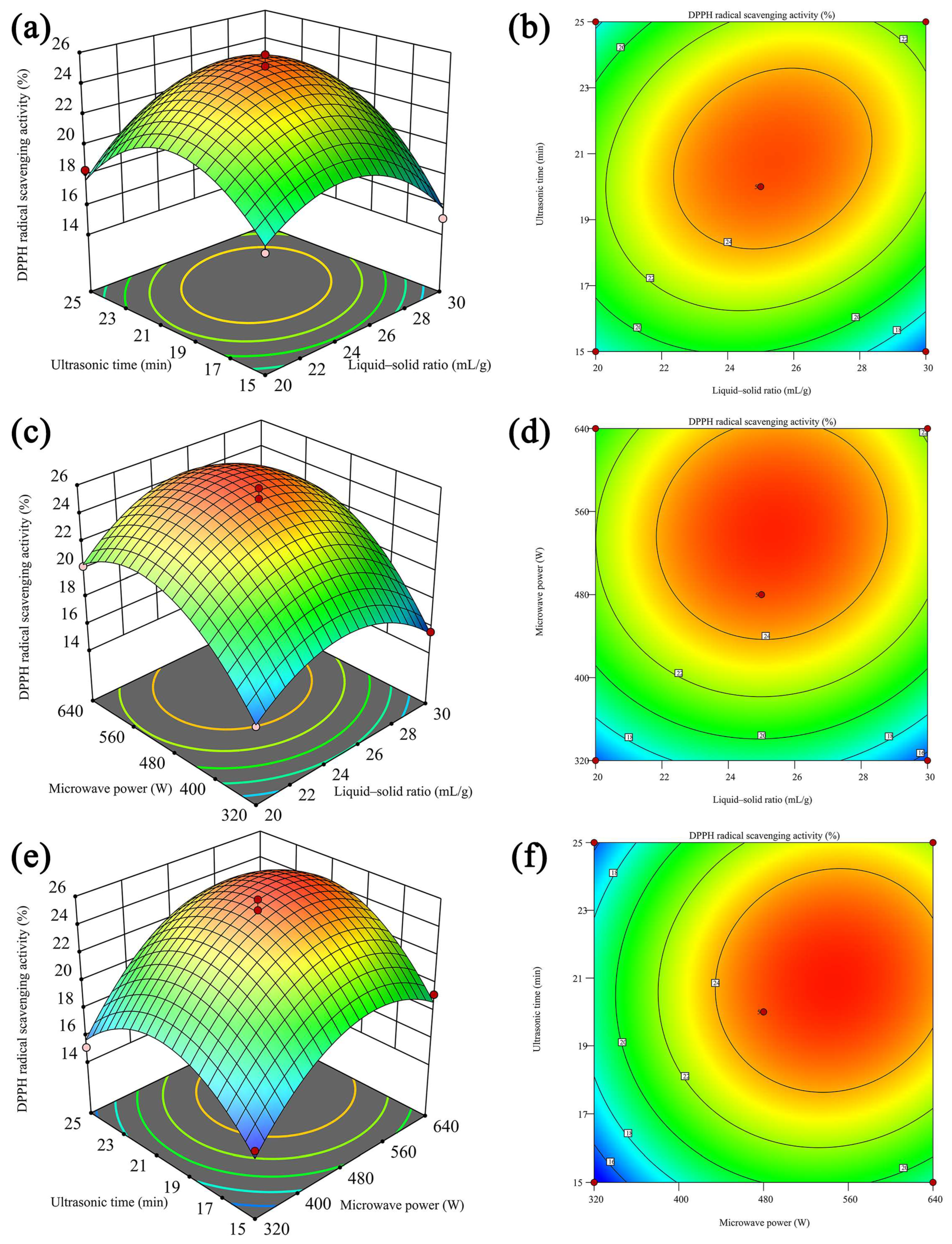
2.2. RSM Optimization of Extraction Conditions
2.2.1. Model Fitting and Statistical Analysis
2.2.2. Response Surface Analysis
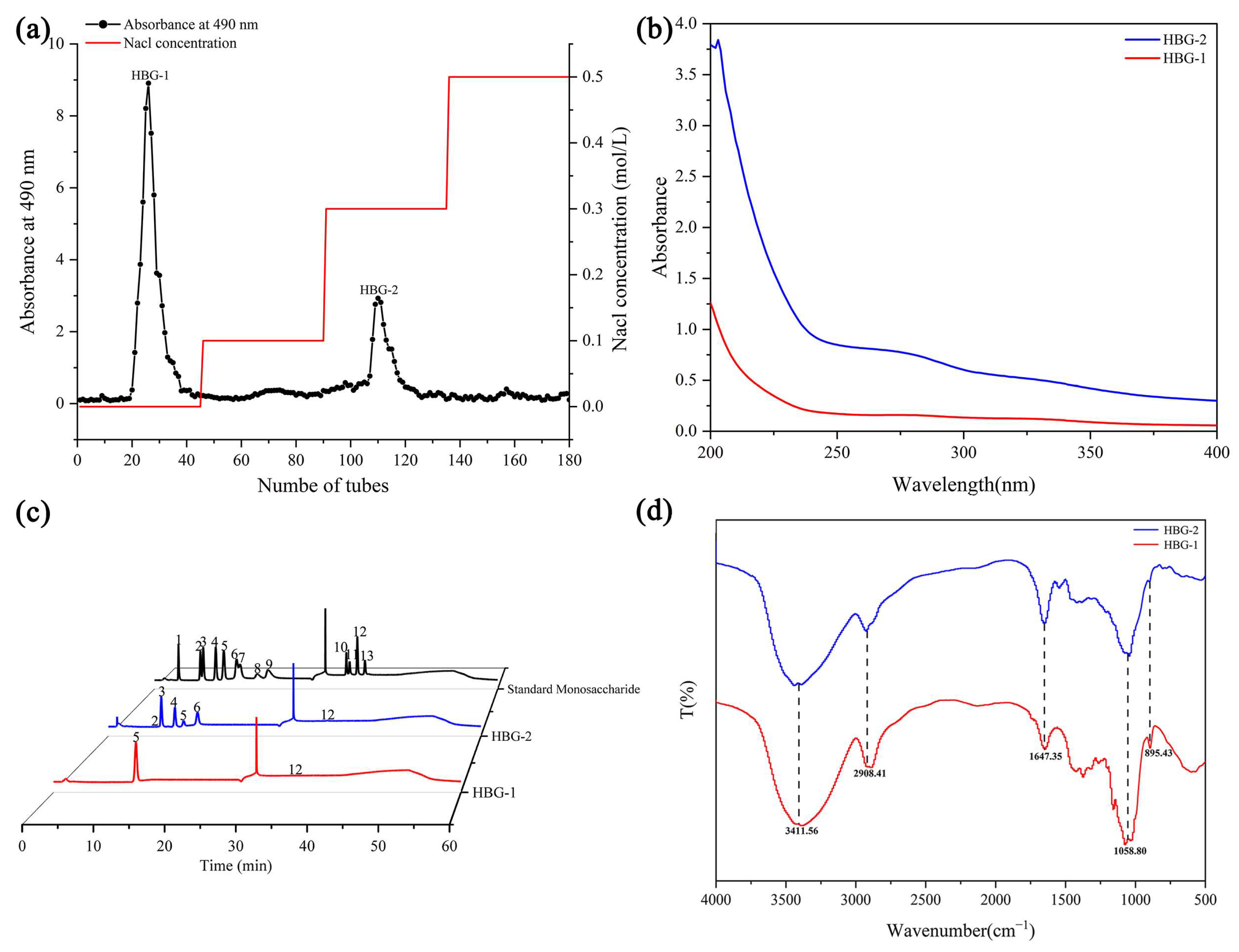
2.3. Separation and Purification Analysis of HBG
2.4. Structural Characterization
2.4.1. Monosaccharide Composition
2.4.2. Fourier-Transform Infrared Spectroscopy (FT-IR) Analysis
2.4.3. Molecular Weight and Chain Conformation
2.4.4. Microstructure
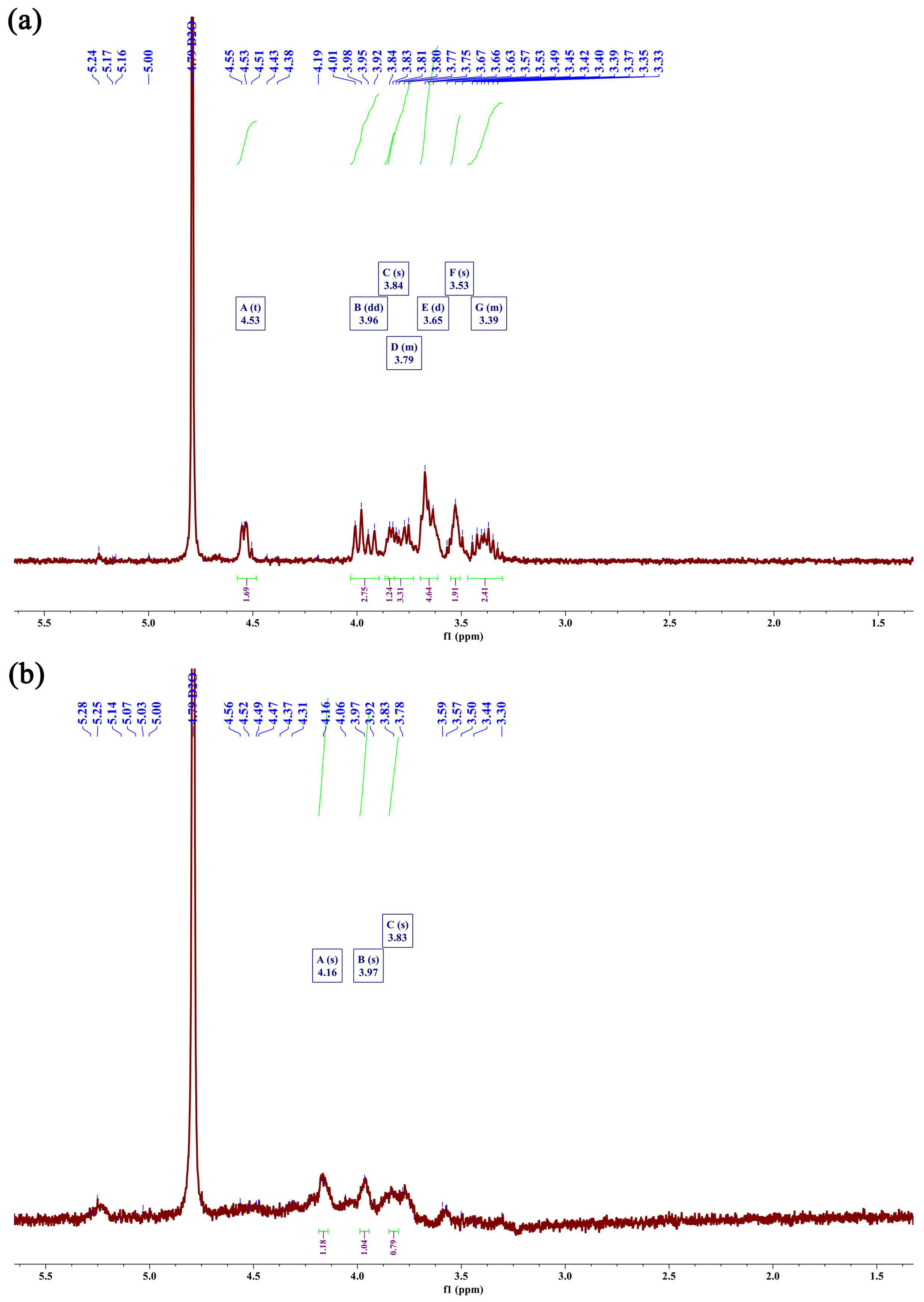
2.4.5. Analysis of the 1H Nuclear Magnetic Resonance (NMR) Spectra
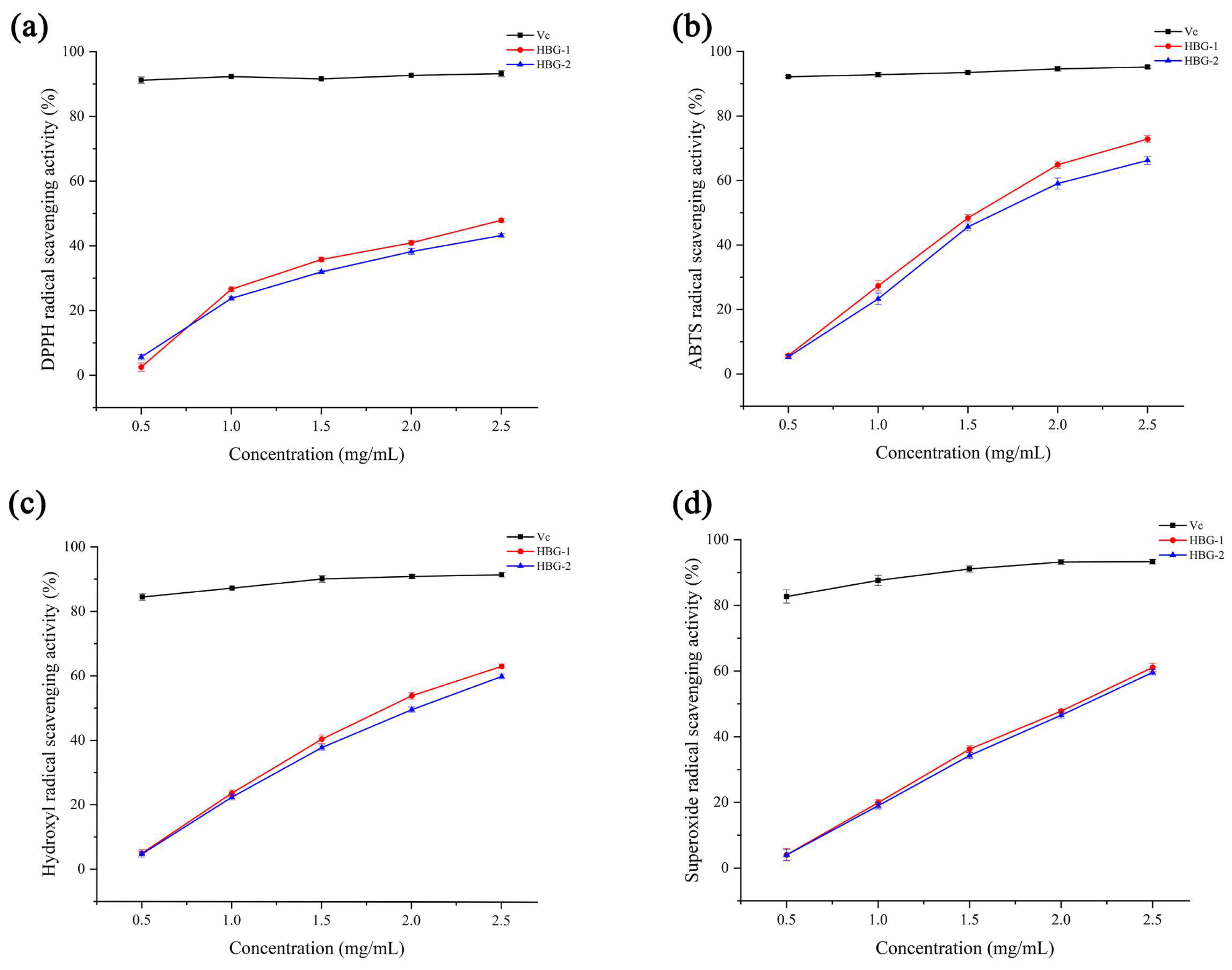
2.5. Antioxidant Activities of HBG-1 and HBG-2
2.5.1. DPPH Radical Scavenging Activity
2.5.2. ABTS Radical Scavenging Activity
2.5.3. Hydroxyl Radical Scavenging Activity
2.5.4. Superoxide Radical Scavenging Activity
3. Materials and Methods
3.1. Materials
3.2. UME of HBG
3.2.1. Extraction of Crude Polysaccharides
3.2.2. Single-Factor Experiments
3.2.3. Response Surface Optimization
3.3. Purification of HBG
3.4. Structural Characterization of HBG-1 and HBG-2
3.4.1. UV Spectroscopic
3.4.2. FT-IR
3.4.3. Monosaccharide Composition
3.4.4. SEC-MALLS-RI Measurement
3.4.5. SEM
3.4.6. NMR
3.5. In Vitro Antioxidant Activity Assays
3.6. Statistical Analyses
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Du, Y.; Liang, F.; Chen, Z.; Zhou, W.; Tu, Z.; Li, J. Effects of decolorization on aggregation behavior of highland barley proteins: Comparison with wheat proteins. Food Res. Int. 2022, 160, 111712. [Google Scholar] [CrossRef]
- Yao, Y.; Li, Z.; Qin, B.; Ju, X.; Wang, L. Evaluation of the intracellular lipid-lowering effect of polyphenols extract from highland barley in HepG2 cells. Food Sci. Hum. Wellness 2024, 13, 454–461. [Google Scholar] [CrossRef]
- Do, T.D.T.; Cozzolino, D.; Muhlhausler, B.; Box, A.; Able, A.J. Antioxidant capacity and vitamin E in barley: Effect of genotype and storage. Food Chem. 2015, 187, 65–74. [Google Scholar] [CrossRef]
- Guo, T.; Horvath, C.; Chen, L.; Chen, J.; Zheng, B. Understanding the nutrient composition and nutritional functions of highland barley (Qingke): A review. Trends Food Sci. Technol. 2020, 103, 109–117. [Google Scholar] [CrossRef]
- Yuan, C.; Hu, R.; He, L.; Hu, J.; Liu, H. Extraction and prebiotic potential of β-glucan from highland barley and its application in probiotic microcapsules. Food Hydrocoll. 2023, 139, 108520. [Google Scholar] [CrossRef]
- Zhang, C.; Xu, Z.; Liu, X.; Ma, M.; Hua, W.; Khalid, S.; Sui, Z.; Corke, H. Heat-moisture treated waxy highland barley starch: Roles of starch granule-associated surface lipids, temperature and moisture. Int. J. Biol. 2024, 254, 127991. [Google Scholar] [CrossRef]
- Ellefsen, C.F.; Lindstad, L.; Klau, L.J.; Aachmann, F.L.; Hiorth, M.; Samuelsen, A.B.C. Investigation of the structural and immunomodulatory properties of alkali-soluble β-glucans from Pleurotus eryngii fruiting bodies. Carbohydr. Polym. 2023, 322, 121367. [Google Scholar] [CrossRef]
- Chakraborty, S.; Beura, M.; Sharma, S.K.; Singh, A.; Dahuja, A.; Krishnan, V. Lentinan, β-glucan from Shiitake (Lentinula edodes): A review on structure, conformational transition, and gastro-intestinal interaction contributing towards its anti-diabetic potential. Trends Food Sci. Technol. 2023, 142, 104224. [Google Scholar] [CrossRef]
- Wouk, J.; Dekker, R.F.H.; Queiroz, E.A.I.F.; Barbosa-Dekker, A.M. β-Glucans as a panacea for a healthy heart? Their roles in preventing and treating cardiovascular diseases. Int. J. Biol. Macromol. 2021, 177, 176–203. [Google Scholar] [CrossRef]
- Wu, L.; Zhao, J.; Zhang, X.; Liu, S.; Zhao, C. Antitumor effect of soluble beta-glucan as an immune stimulant. Int. J. Biol. Macromol. 2021, 179, 116–124. [Google Scholar] [CrossRef]
- Li, Y.-C.; Luo, Y.; Meng, F.-B.; Li, J.; Chen, W.-J.; Liu, D.-Y.; Zou, L.-H.; Zhou, L. Preparation and characterization of feruloylated oat β-glucan with antioxidant activity and colon-targeted delivery. Carbohydr. Polym. 2022, 279, 119002. [Google Scholar] [CrossRef]
- He, S.; Yan, J.; Chen, L.; Chen, H.; Wang, W. Structure and in vitro antioxidant and immunomodulatory activity of a glucan from the leaves of Cyclocarya paliurus. J. Funct. Foods 2024, 113, 106016. [Google Scholar] [CrossRef]
- Ahmad, M.; Gani, A.; Shah, A.; Gani, A.; Masoodi, F.A. Germination and microwave processing of barley (Hordeum vulgare L) changes the structural and physicochemical properties of β- d -glucan & enhances its antioxidant potential. Carbohydr. Polym. 2016, 153, 696–702. [Google Scholar] [CrossRef]
- Wang, W.; Tan, J.; Nima, L.; Sang, Y.; Cai, X.; Xue, H. Polysaccharides from fungi: A review on their extraction, purification, structural features, and biological activities. Food Chem. X 2022, 15, 100414. [Google Scholar] [CrossRef]
- Xu, S.-Y.; Chen, X.-Q.; Liu, Y.; Cheong, K.-L. Ultrasonic/microwave-assisted extraction, simulated digestion, and fermentation in vitro by human intestinal flora of polysaccharides from Porphyra haitanensis. Int. J. Biol. Macromol. 2020, 152, 748–756. [Google Scholar] [CrossRef]
- Barbosa, J.R.; Freitas, M.M.S.; Oliveira, L.C.; Martins, L.H.S.; Almada-Vilhena, A.O.; Oliveira, R.M.; Pieczarka, J.C.; Brasil, D.D.S.B.; Carvalho Junior, R.N. Obtaining extracts rich in antioxidant polysaccharides from the edible mushroom Pleurotus ostreatus using binary system with hot water and supercritical CO2. Food Chem. 2020, 330, 127173. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Y.; Cai, Y.; Tian, Y.; Xu, L.; Zhang, A.; Zhang, C.; Zhang, S. Ultrasonic-assisted extraction brings high-yield polysaccharides from Kangxian flowers with cosmetic potential. Ultrason. Sonochem. 2023, 100, 106626. [Google Scholar] [CrossRef]
- Oprescu, E.-E.; Enascuta, C.-E.; Radu, E.; Ciltea-Udrescu, M.; Lavric, V. Does the ultrasonic field improve the extraction productivity compared to classical methods—Maceration and reflux distillation? Chem. Eng. Process. Process Intensif. 2022, 179, 109082. [Google Scholar] [CrossRef]
- Wang, C.; Li, J.; Cao, Y.; Huang, J.; Lin, H.; Zhao, T.; Liu, L.; Shen, P.; Julian McClements, D.; Chen, J.; et al. Extraction and characterization of pectic polysaccharides from Choerospondias axillaris peels: Comparison of hot water and ultrasound-assisted extraction methods. Food Chem. 2022, 401, 134156. [Google Scholar] [CrossRef]
- Hu, Z.; Zhou, H.; Zhao, J.; Sun, J.; Li, M.; Sun, X. Microwave-assisted extraction, characterization and immunomodulatory activity on RAW264.7 cells of polysaccharides from Trichosanthes kirilowii Maxim seeds. Int. J. Biol. Macromol. 2020, 164, 2861–2872. [Google Scholar] [CrossRef]
- Gharibzahedi, S.M.T.; Marti-Quijal, F.J.; Barba, F.J.; Altintas, Z. Current emerging trends in antitumor activities of polysaccharides extracted by microwave- and ultrasound-assisted methods. Int. J. Biol. Macromol. 2022, 202, 494–507. [Google Scholar] [CrossRef] [PubMed]
- Mirzadeh, M.; Arianejad, M.R.; Khedmat, L. Antioxidant, antiradical, and antimicrobial activities of polysaccharides obtained by microwave-assisted extraction method: A review. Carbohydr. Polym. 2020, 229, 115421. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Wang, Y.; Huang, G.; Huang, H. Ultrasound-assisted extraction, analysis and antioxidant activity of polysaccharide from the rinds of Garcinia mangostana L. Ultrason. Sonochem. 2023, 97, 106474. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Hu, D.; Shen, Y.; Zheng, Y.; Liang, Y. Optimization of ultrasonic-assisted polysaccharide extraction from Hyperici Perforati Herba using response surface methodology and assessment of its antioxidant activity. Int. J. Biol. Macromol. 2022, 225, 255–265. [Google Scholar] [CrossRef]
- Zhang, Y.; Lei, Y.; Qi, S.; Fan, M.; Zheng, S.; Huang, Q.; Lu, X. Ultrasonic-microwave-assisted extraction for enhancing antioxidant activity of Dictyophora indusiata polysaccharides: The difference mechanisms between single and combined assisted extraction. Ultrason. Sonochem. 2023, 95, 106356. [Google Scholar] [CrossRef]
- Yang, J.; Dong, S.; Zhou, X.; Zhang, W.; Gu, Y.; Zheng, L.; Yang, G.; Wang, J.; Zhang, Y. Polysaccharides from waste Zingiber mioga leaves: Ultrasonic-microwave-assisted extraction, characterization, antioxidant and anticoagulant potentials. Ultrason. Sonochem. 2023, 101, 106718. [Google Scholar] [CrossRef]
- Bai, Y.P.; Zhou, H.M.; Zhu, K.R.; Li, Q. Effect of thermal processing on the molecular, structural, and antioxidant characteristics of highland barley beta-glucan. Carbohydr. Polym. 2021, 271, 118416. [Google Scholar] [CrossRef]
- Zhu, Y.; Lv, J.; Gu, Y.; He, Y.; Chen, J.; Zhou, Z.; Ye, X. Polysaccharides of Chinese bayberry pomace wine: Structural characteristics, antioxidant activity and influence on the bayberry wine. Food Biosci. 2022, 50, 109082. [Google Scholar] [CrossRef]
- Yuan, L.; Qiu, Z.; Yang, Y.; Liu, C.; Zhang, R. Preparation, structural characterization and antioxidant activity of water-soluble polysaccharides and purified fractions from blackened jujube by an activity-oriented approach. Food Chem. 2022, 385, 132637. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Guo, J.; Cheng, J.; Zhao, X.; Ma, B.; Yang, X.; Shao, H. Ultrasound-assisted extraction of polysaccharide from spent Lentinus edodes substrate: Process optimization, precipitation, structural characterization and antioxidant activity. Int. J. Biol. Macromol. 2021, 191, 1038–1045. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhuang, S.; Liang, C.; He, J.; Brennan, C.S.; Brennan, M.A.; Ma, L.; Xiao, G.; Chen, H.; Wan, S. Effects of a polysaccharide extract from Amomum villosum Lour. on gastric mucosal injury and its potential underlying mechanism. Carbohydr. Polym. 2022, 294, 119822. [Google Scholar] [CrossRef]
- Yin, C.; Fan, X.; Fan, Z.; Shi, D.; Gao, H. Optimization of enzymes-microwave-ultrasound assisted extraction of Lentinus edodes polysaccharides and determination of its antioxidant activity. Int. J. Biol. Macromol. 2018, 111, 446–454. [Google Scholar] [CrossRef]
- Dong, J.L.; Yang, M.; Zhu, Y.Y.; Shen, R.L.; Zhang, K.Y. Comparative study of thermal processing on the physicochemical properties and prebiotic effects of the oat beta-glucan by in vitro human fecal microbiota fermentation. Food Res. Int. 2020, 138, 109818. [Google Scholar] [CrossRef]
- Hu, T.; Huang, Q.; Wong, K.; Yang, H. Structure, molecular conformation, and immunomodulatory activity of four polysaccharide fractions from Lignosus rhinocerotis sclerotia. Int. J. Biol. Macromol. 2017, 94, 423–430. [Google Scholar] [CrossRef]
- Zheng, Z.; Huang, Q.; Ling, C. Water-soluble yeast β-glucan fractions with different molecular weights: Extraction and separation by acidolysis assisted-size exclusion chromatography and their association with proliferative activity. Int. J. Biol. Macromol. 2019, 123, 269–279. [Google Scholar] [CrossRef]
- Xiao, X.; Tan, C.; Sun, X.; Zhao, Y.; Zhang, J.; Zhu, Y.; Bai, J.; Dong, Y.; Zhou, X. Effects of fermentation on structural characteristics and in vitro physiological activities of barley β-glucan. Carbohydr. Polym. 2020, 231, 115685. [Google Scholar] [CrossRef]
- Cui, Y.; Chen, Y.; Wang, S.; Wang, S.; Yang, J.; Ismael, M.; Wang, X.; Lu, X. Purification, structural characterization and antioxidant activities of two neutral polysaccharides from persimmon peel. Int. J. Biol. Macromol. 2023, 225, 241–254. [Google Scholar] [CrossRef]
- Zhou, S.; Huang, G.; Chen, G. Extraction, structural analysis, derivatization and antioxidant activity of polysaccharide from Chinese yam. Food Chem. 2021, 361, 130089. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.-Y.-Y.; Wang, J.-Q.; Yin, J.-Y.; Nie, S.-P.; Xie, M.-Y. A review of NMR analysis in polysaccharide structure and conformation: Progress, challenge and perspective. Food Res. Int. 2021, 143, 110290. [Google Scholar] [CrossRef] [PubMed]
- Shaheen, T.I.; Hussien, G.M.A.; Mekawey, A.A.; Ghalia, H.H.A.; Youssry, A.A.; El Mokadem, M.T. Facile extraction of nanosized β-glucans from edible mushrooms and their antitumor activities. J. Food Compos. Anal. 2022, 111, 104607. [Google Scholar] [CrossRef]
- Chen, F.; Huang, G. Extraction, derivatization and antioxidant activity of bitter gourd polysaccharide. Int. J. Biol. Macromol. 2019, 141, 14–20. [Google Scholar] [CrossRef]
- Wang, W.; Liu, X.; Wang, L.; Song, G.; Jiang, W.; Mu, L.; Li, J. Ficus carica polysaccharide extraction via ultrasound-assisted technique: Structure characterization, antioxidant, hypoglycemic and immunomodulatory activities. Ultrason. Sonochem. 2023, 101, 106680. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, P.; Tan, C.; Zhao, Y.; Zhu, Y.; Bai, J.; Xiao, X.; Zhang, L.; Teng, D.; Tian, J.; et al. Effects of L. plantarum dy-1 fermentation time on the characteristic structure and antioxidant activity of barley beta-glucan in vitro. Curr. Res. Food Sci. 2022, 5, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Golbargi, F.; Gharibzahedi, S.M.T.; Zoghi, A.; Mohammadi, M.; Hashemifesharaki, R. Microwave-assisted extraction of arabinan-rich pectic polysaccharides from melon peels: Optimization, purification, bioactivity, and techno-functionality. Carbohydr. Polym. 2021, 256, 117522. [Google Scholar] [CrossRef] [PubMed]
- Hashemifesharaki, R.; Xanthakis, E.; Altintas, Z.; Guo, Y.; Gharibzahedi, S.M.T. Microwave-assisted extraction of polysaccharides from the marshmallow roots: Optimization, purification, structure, and bioactivity. Carbohydr. Polym. 2020, 240, 116301. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Chen, J.; Ren, P.; Zhang, Y.; Omondi Onyango, S. Ultrasound irradiation alters the spatial structure and improves the antioxidant activity of the yellow tea polysaccharide. Ultrason. Sonochem. 2021, 70, 105355. [Google Scholar] [CrossRef] [PubMed]
- Wei, Q.; Zhang, Y.-h. Ultrasound-assisted polysaccharide extraction from Cercis chinensis and properites, antioxidant activity of polysaccharide. Ultrason. Sonochem. 2023, 96, 106422. [Google Scholar] [CrossRef] [PubMed]
- Zeng, F.; Chen, W.; He, P.; Zhan, Q.; Wang, Q.; Wu, H.; Zhang, M. Structural characterization of polysaccharides with potential antioxidant and immunomodulatory activities from Chinese water chestnut peels. Carbohydr. Polym. 2020, 246, 116551. [Google Scholar] [CrossRef]
- Liu, H.; Li, Y.; You, M.; Liu, X. Comparison of physicochemical properties of beta-glucans extracted from hull-less barley bran by different methods. Int. J. Biol. Macromol. 2021, 182, 1192–1199. [Google Scholar] [CrossRef]
- Chen, L.; Chen, S.; Rong, Y.; Zeng, W.; Hu, Z.; Ma, X.; Feng, S. Identification and evaluation of antioxidant peptides from highland barley distiller’s grains protein hydrolysate assisted by molecular docking. Food Chem. 2024, 434, 137441. [Google Scholar] [CrossRef]
- Li, C.; Zhang, Y.; Zhao, C.; Fu, X. Physicochemical characterization, antioxidative and immunoregulatory activity of polysaccharides from the flower of Hylocereus undatus (Haw.) Britton et Rose. Int. J. Biol. Macromol. 2023, 251, 126408. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; He, X.; Liu, G.; Wei, Z.; Sheng, J.; Sun, J.; Li, C.; Xin, M.; Li, L.; Yi, P. Effects of different extraction methods on the structural, antioxidant and hypoglycemic properties of red pitaya stem polysaccharide. Food Chem. 2023, 405, 134804. [Google Scholar] [CrossRef] [PubMed]
- Aćimović, M.; Šeregelj, V.; Šovljanski, O.; Tumbas Šaponjac, V.; Švarc Gajić, J.; Brezo-Borjan, T.; Pezo, L. In vitro antioxidant, antihyperglycemic, anti-inflammatory, and antimicrobial activity of Satureja kitaibelii Wierzb. ex Heuff. subcritical water extract. Ind. Crops Prod. 2021, 169. [Google Scholar] [CrossRef]
- He, Y.; Pan, X.; Chi, C.-F.; Sun, K.-L.; Wang, B. Ten new pentapeptides from protein hydrolysate of miiuy croaker (Miichthys miiuy) muscle: Preparation, identification, and antioxidant activity evaluation. LWT 2019, 105, 1–8. [Google Scholar] [CrossRef]
- Lin, Y.; Zeng, H.; Wang, K.; Lin, H.; Li, P.; Huang, Y.; Zhou, S.; Zhang, W.; Chen, C.; Fan, H. Microwave-assisted aqueous two-phase extraction of diverse polysaccharides from Lentinus edodes: Process optimization, structure characterization and antioxidant activity. Int. J. Biol. Macromol. 2019, 136, 305–315. [Google Scholar] [CrossRef]
- Zhu, Z.; Chen, J.; Chen, Y.; Ma, Y.; Yang, Q.; Fan, Y.; Fu, C.; Limsila, B.; Li, R.; Liao, W. Extraction, structural characterization and antioxidant activity of turmeric polysaccharides. LWT 2022, 154, 305–315. [Google Scholar] [CrossRef]


| Source | Sum of Squares | DF | Mean Square | F-Value | p-Value | p |
|---|---|---|---|---|---|---|
| Model | 249.33 | 9 | 27.70 | 52.29 | <0.0001 | significant |
| X1 | 0.4278 | 1 | 0.4278 | 0.8074 | 0.3987 | |
| X2 | 11.35 | 1 | 11.35 | 21.43 | 0.0024 | |
| X3 | 58.32 | 1 | 58.32 | 110.07 | <0.0001 | |
| X1X2 | 7.16 | 1 | 7.16 | 13.51 | 0.0079 | |
| X1X3 | 0.6724 | 1 | 0.6724 | 1.27 | 0.2971 | |
| X2X3 | 1.14 | 1 | 1.14 | 2.16 | 0.1850 | |
| X12 | 41.48 | 1 | 41.48 | 78.29 | <0.0001 | |
| X22 | 59.80 | 1 | 59.80 | 112.87 | <0.0001 | |
| X32 | 51.17 | 1 | 51.17 | 96.58 | <0.0001 | |
| Residual | 3.71 | 1 | 0.5298 | |||
| Lack of fit | 2.55 | 1 | 0.8541 | 2.95 | 0.1616 | Not significant |
| Pure Error | 1.15 | 4 | 0.2886 | |||
| Cor Total | 253.04 | 16 | ||||
| R2 | 0.9853 | |||||
| R2Adj | 0.9665 |
| Sample | HBG-1 | HBG-2 |
|---|---|---|
| Mw (kDa) | 5.24 | 172.31 |
| Mn (kDa) | 4.94 | 37.31 |
| Mw/Mn | 1.06 | 4.62 |
| Slope | 1.77 ± 0.38 | 0.19 ± 0.00 |
| Monosaccharide (mol%) | ||
| Rhamnose | - | 0.35 |
| Arabinose | - | 38.23 |
| Galactose | - | 22.01 |
| Glucose | 98.97 | 7.02 |
| Xylose | - | 31.60 |
| Glucuronic acid | 1.03 | 0.80 |
| Run | Liquid–Solid Ratio (X1, mL/g) | Ultrasonic Time (X2, min) | Microwave Power (X3, W) | DPPH Radical Scavenging Activity (Y, %) |
|---|---|---|---|---|
| 1 | 25 | 20 | 480 | 25.86 |
| 2 | 25 | 20 | 480 | 24.68 |
| 3 | 20 | 20 | 640 | 20.28 |
| 4 | 30 | 25 | 480 | 21.26 |
| 5 | 25 | 15 | 320 | 14.91 |
| 6 | 25 | 20 | 480 | 24.64 |
| 7 | 25 | 15 | 640 | 19.26 |
| 8 | 20 | 20 | 320 | 15.72 |
| 9 | 20 | 25 | 480 | 18.37 |
| 10 | 30 | 20 | 640 | 21.81 |
| 11 | 25 | 20 | 480 | 24.59 |
| 12 | 20 | 15 | 480 | 17.56 |
| 13 | 25 | 20 | 480 | 25.13 |
| 14 | 30 | 20 | 320 | 15.61 |
| 15 | 30 | 15 | 480 | 15.10 |
| 16 | 25 | 25 | 640 | 21.61 |
| 17 | 25 | 25 | 320 | 15.12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, L.; Cui, C.; Wang, Z.; Che, F.; Chen, Z.; Feng, S. Structural Characterization and Antioxidant Activity of β-Glucans from Highland Barley Obtained with Ultrasonic–Microwave-Assisted Extraction. Molecules 2024, 29, 684. https://doi.org/10.3390/molecules29030684
Chen L, Cui C, Wang Z, Che F, Chen Z, Feng S. Structural Characterization and Antioxidant Activity of β-Glucans from Highland Barley Obtained with Ultrasonic–Microwave-Assisted Extraction. Molecules. 2024; 29(3):684. https://doi.org/10.3390/molecules29030684
Chicago/Turabian StyleChen, Lihua, Chunfeng Cui, Zhiheng Wang, Fuhong Che, Zhanxiu Chen, and Shengbao Feng. 2024. "Structural Characterization and Antioxidant Activity of β-Glucans from Highland Barley Obtained with Ultrasonic–Microwave-Assisted Extraction" Molecules 29, no. 3: 684. https://doi.org/10.3390/molecules29030684
APA StyleChen, L., Cui, C., Wang, Z., Che, F., Chen, Z., & Feng, S. (2024). Structural Characterization and Antioxidant Activity of β-Glucans from Highland Barley Obtained with Ultrasonic–Microwave-Assisted Extraction. Molecules, 29(3), 684. https://doi.org/10.3390/molecules29030684