Cellulose-Based Sorbents: A Comprehensive Review of Current Advances in Water Remediation and Future Prospects
Abstract
1. Introduction
2. Cellulose as a Basis for Creating Sorbents
3. Methods of Pre-Treatment of Cellulose
4. Methods for Obtaining Cellulose-Based Sorbents
4.1. Chemical Modification of Cellulose
4.1.1. Carboxylation
4.1.2. Amination
4.1.3. Esterification
4.1.4. Graphene Modification
4.2. Physical Treatment of Cellulose Materials
4.2.1. Mechanical Activation
4.2.2. Thermal Treatment
4.2.3. Plasma Treatment
4.2.4. Radiation-Induced Modification
4.3. Advantages and Disadvantages of the Methods of Cellulose Modification
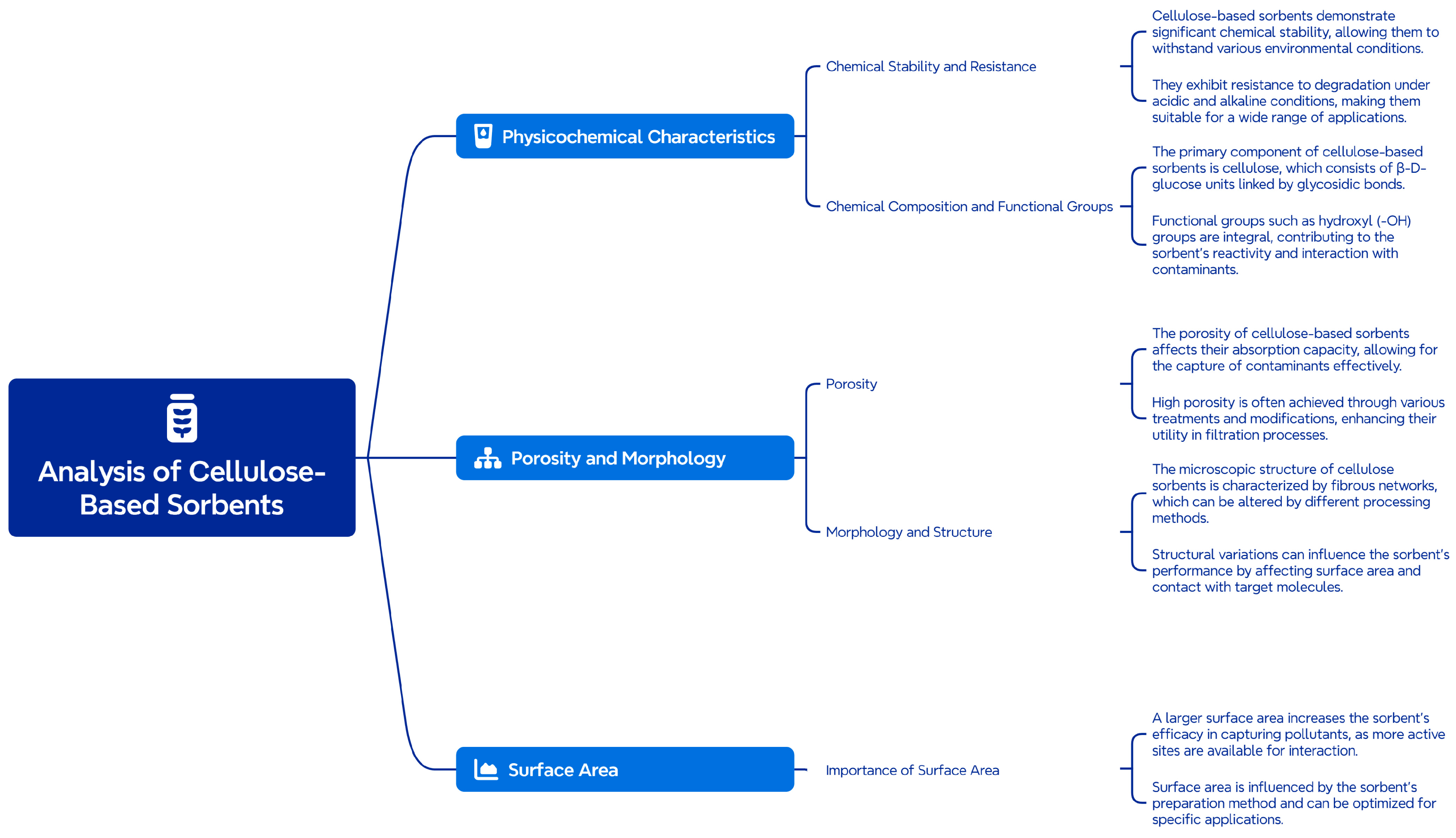
5. Physicochemical Properties of Cellulose-Based Sorbents
5.1. Morphology and Structure
5.2. Surface and Porous Characteristics
5.3. Chemical Stability and Resistance
6. Sorption Properties of Cellulose Sorbents
- Physical sorption (electrostatic interaction):
- Ion exchange:
- Complexation (on modified cellulose):
- Modification of cellulose using phosphate groups:
- Chemisorption (coordination of metal ions with functional groups):
7. Prospects for the Use of Sorbents Based on Cellulose
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Seidi, F.; Yazdi, M.K.; Jouyandeh, M.; Habibzadeh, S.; Munir, M.T.; Vahabi, H.; Bagheri, B.; Rabiee, N.; Zarrintaj, P.; Saeb, M.R. Crystalline Polysaccharides: A Review. Carbohydr. Polym. 2022, 275, 118624. [Google Scholar] [CrossRef]
- Gopinath, V.; Kamath, S.M.; Priyadarshini, S.; Chik, Z.; Alarfaj, A.A.; Hirad, A.H. Multifunctional Applications of Natural Polysaccharide Starch and Cellulose: An Update on Recent Advances. Biomed. Pharmacother. 2022, 146, 112492. [Google Scholar] [CrossRef] [PubMed]
- French, A.D.; Pérez, S.; Bulone, V.; Rosenau, T.; Gray, D. Cellulose. In Encyclopedia of Polymer Science and Technology; Mark, H.F., Ed.; Wiley: Hoboken, NJ, USA, 2018; pp. 1–69. ISBN 978-1-118-63389-2. [Google Scholar]
- Klemm, D.; Heublein, B.; Fink, H.; Bohn, A. Cellulose: Fascinating Biopolymer and Sustainable Raw Material. Angew. Chem. Int. Ed. 2005, 44, 3358–3393. [Google Scholar] [CrossRef] [PubMed]
- Moon, R.J.; Martini, A.; Nairn, J.; Simonsen, J.; Youngblood, J. Cellulose Nanomaterials Review: Structure, Properties and Nanocomposites. Chem. Soc. Rev. 2011, 40, 3941. [Google Scholar] [CrossRef] [PubMed]
- Baumann, H.; Richter, A.; Klemm, D.; Faust, V. Concepts for Preparation of Novel Regioselective Modified Cellulose Derivatives Sulfated, Aminated, Carboxylated and Acetylated for Hemocompatible Ultrathin Coatings on Biomaterials. Macromol. Chem. Phys. 2000, 201, 1950–1962. [Google Scholar] [CrossRef]
- Kassab, Z.; Syafri, E.; Tamraoui, Y.; Hannache, H.; Qaiss, A.E.K.; El Achaby, M. Characteristics of Sulfated and Carboxylated Cellulose Nanocrystals Extracted from Juncus Plant Stems. Int. J. Biol. Macromol. 2020, 154, 1419–1425. [Google Scholar] [CrossRef] [PubMed]
- Lam, E.; Hemraz, U.D. Preparation and Surface Functionalization of Carboxylated Cellulose Nanocrystals. Nanomaterials 2021, 11, 1641. [Google Scholar] [CrossRef]
- O’Connell, D.W.; Birkinshaw, C.; O’Dwyer, T.F. Heavy Metal Adsorbents Prepared from the Modification of Cellulose: A Review. Bioresour. Technol. 2008, 99, 6709–6724. [Google Scholar] [CrossRef] [PubMed]
- Lu, P.; Hsieh, Y.-L. Cellulose Nanocrystal-Filled Poly(Acrylic Acid) Nanocomposite Fibrous Membranes. Nanotechnology 2009, 20, 415604. [Google Scholar] [CrossRef]
- Peng, B.L.; Dhar, N.; Liu, H.L.; Tam, K.C. Chemistry and Applications of Nanocrystalline Cellulose and Its Derivatives: A Nanotechnology Perspective. Can. J. Chem. Eng. 2011, 89, 1191–1206. [Google Scholar] [CrossRef]
- Zaman, M.; Xiao, H.; Chibante, F.; Ni, Y. Synthesis and Characterization of Cationically Modified Nanocrystalline Cellulose. Carbohydr. Polym. 2012, 89, 163–170. [Google Scholar] [CrossRef]
- Azizi Samir, M.A.S.; Alloin, F.; Dufresne, A. Review of Recent Research into Cellulosic Whiskers, Their Properties and Their Application in Nanocomposite Field. Biomacromolecules 2005, 6, 612–626. [Google Scholar] [CrossRef] [PubMed]
- Siró, I.; Plackett, D. Microfibrillated Cellulose and New Nanocomposite Materials: A Review. Cellulose 2010, 17, 459–494. [Google Scholar] [CrossRef]
- Habibi, Y.; Lucia, L.A.; Rojas, O.J. Cellulose Nanocrystals: Chemistry, Self-Assembly, and Applications. Chem. Rev. 2010, 110, 3479–3500. [Google Scholar] [CrossRef] [PubMed]
- Yi, T.; Zhao, H.; Mo, Q.; Pan, D.; Liu, Y.; Huang, L.; Xu, H.; Hu, B.; Song, H. From Cellulose to Cellulose Nanofibrils—A Comprehensive Review of the Preparation and Modification of Cellulose Nanofibrils. Materials 2020, 13, 5062. [Google Scholar] [CrossRef]
- Niinivaara, E.; Vanderfleet, O.M.; Kontturi, E.; Cranston, E.D. Tuning the Physicochemical Properties of Cellulose Nanocrystals through an In Situ Oligosaccharide Surface Modification Method. Biomacromolecules 2021, 22, 3284–3296. [Google Scholar] [CrossRef]
- Chauhan, N.P.S. (Ed.) Functionalized Polymers: Synthesis, Characterization and Applications; CRC Press: Boca Raton, FL, USA, 2021; ISBN 978-0-367-82191-3. [Google Scholar]
- Qiao, L.; Lei, S.; Du, K. High-Surface-Area Interconnected Macroporous Nanofibrous Cellulose Microspheres: A Versatile Platform for Large Capacity and High-Throughput Protein Separation. Cellulose 2021, 28, 2125–2136. [Google Scholar] [CrossRef]
- Aziz, T.; Farid, A.; Haq, F.; Kiran, M.; Ullah, A.; Zhang, K.; Li, C.; Ghazanfar, S.; Sun, H.; Ullah, R.; et al. A Review on the Modification of Cellulose and Its Applications. Polymers 2022, 14, 3206. [Google Scholar] [CrossRef]
- Kumari, S.V.G.; Pakshirajan, K.; Pugazhenthi, G. Recent Advances and Future Prospects of Cellulose, Starch, Chitosan, Polylactic Acid and Polyhydroxyalkanoates for Sustainable Food Packaging Applications. Int. J. Biol. Macromol. 2022, 221, 163–182. [Google Scholar] [CrossRef]
- Tupciauskas, R.; Rizhikovs, J.; Brazdausks, P.; Fridrihsone, V.; Andzs, M. Influence of Steam Explosion Pre-Treatment Conditions on Binder-Less Boards from Hemp Shives and Wheat Straw. Ind. Crops Prod. 2021, 170, 113717. [Google Scholar] [CrossRef]
- Lorenzo-Hernando, A.; Martín-Juárez, J.; Bolado-Rodríguez, S. Study of Steam Explosion Pretreatment and Preservation Methods of Commercial Cellulose. Carbohydr. Polym. 2018, 191, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Hiltunen, S.; Sapkota, J.; Ioannou, E.; Haddad Momeni, M.; Master, E.; Ristolainen, M. Comparative Assessment of Chemical and Biochemical Approaches for the Activation of Lignocellulosic Materials and Emerging Opportunities for Expansin-Related Proteins. Cellulose 2024, 31, 147–168. [Google Scholar] [CrossRef]
- Levanič, J.; Svedström, K.; Liljeström, V.; Šernek, M.; Osojnik Črnivec, I.G.; Poklar Ulrih, N.; Haapala, A. Cellulose Fiber and Nanofibril Characteristics in a Continuous Sono-Assisted Process for Production of TEMPO-Oxidized Nanofibrillated Cellulose. Cellulose 2022, 29, 9121–9142. [Google Scholar] [CrossRef]
- Freitas, P.A.V.; González-Martínez, C.; Chiralt, A. Applying Ultrasound-Assisted Processing to Obtain Cellulose Fibres from Rice Straw to Be Used as Reinforcing Agents. Innov. Food Sci. Emerg. Technol. 2022, 76, 102932. [Google Scholar] [CrossRef]
- Huerta, R.R.; Silva, E.K.; Ekaette, I.; El-Bialy, T.; Saldaña, M.D.A. High-Intensity Ultrasound-Assisted Formation of Cellulose Nanofiber Scaffold with Low and High Lignin Content and Their Cytocompatibility with Gingival Fibroblast Cells. Ultrason. Sonochem. 2020, 64, 104759. [Google Scholar] [CrossRef]
- Mazela, B.; Perdoch, W.; Peplińska, B.; Zieliński, M. Influence of Chemical Pre-Treatments and Ultrasonication on the Dimensions and Appearance of Cellulose Fibers. Materials 2020, 13, 5274. [Google Scholar] [CrossRef] [PubMed]
- Ramos, A.; Monteiro, E.; Rouboa, A. Biomass Pre-Treatment Techniques for the Production of Biofuels Using Thermal Conversion Methods—A Review. Energy Convers. Manag. 2022, 270, 116271. [Google Scholar] [CrossRef]
- Chen, S.-Q.; Meldrum, O.W.; Liao, Q.; Li, Z.; Cao, X.; Guo, L.; Zhang, S.; Zhu, J.; Li, L. The Influence of Alkaline Treatment on the Mechanical and Structural Properties of Bacterial Cellulose. Carbohydr. Polym. 2021, 271, 118431. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Allardyce, B.J.; Rajkhowa, R.; Agrawal, R. Rice Straw-Derived Cellulose: A Comparative Study of Various Pre-Treatment Technologies and Its Conversion to Nanofibres. Sci. Rep. 2023, 13, 16327. [Google Scholar] [CrossRef] [PubMed]
- Jankovičová, B.; Hutňan, M.; Nagy Czölderová, M.; Hencelová, K.; Imreová, Z. Comparison of Acid and Alkaline Pre-Treatment of Lignocellulosic Materials for Biogas Production. Plant Soil Environ. 2022, 68, 195–204. [Google Scholar] [CrossRef]
- Mnasri, A.; Khiari, R.; Dhaouadi, H.; Halila, S.; Mauret, E. Acidic and Alkaline Deep Eutectic Solvents Pre-Treatment to Produce High Aspect Ratio Microfibrillated Cellulose. Bioresour. Technol. 2023, 368, 128312. [Google Scholar] [CrossRef]
- Özbek, H.N.; Koçak Yanık, D.; Fadıloğlu, S.; Göğüş, F. Effect of Microwave-assisted Alkali Pre-treatment on Fractionation of Pistachio Shell and Enzymatic Hydrolysis of Cellulose-rich Residues. J. Chem. Technol. Biotechnol. 2021, 96, 521–531. [Google Scholar] [CrossRef]
- Singh, S.S.; Lim, L.-T.; Manickavasagan, A. Ultrasound-Assisted Alkali-Urea Pre-Treatment of Miscanthus × Giganteus for Enhanced Extraction of Cellulose Fiber. Carbohydr. Polym. 2020, 247, 116758. [Google Scholar] [CrossRef] [PubMed]
- Riva, L.; Pastori, N.; Panozzo, A.; Antonelli, M.; Punta, C. Nanostructured Cellulose-Based Sorbent Materials for Water Decontamination from Organic Dyes. Nanomaterials 2020, 10, 1570. [Google Scholar] [CrossRef]
- El Mahdaoui, A.; Radi, S.; Elidrissi, A.; Faustino, M.A.F.; Neves, M.G.P.M.S.; Moura, N.M.M. Progress in the Modification of Cellulose-Based Adsorbents for the Removal of Toxic Heavy Metal Ions. J. Environ. Chem. Eng. 2024, 12, 113870. [Google Scholar] [CrossRef]
- Wu, J.-H.; He, C.-Y. Advances in Cellulose-Based Sorbents for Extraction of Pollutants in Environmental Samples. Chromatographia 2019, 82, 1151–1169. [Google Scholar] [CrossRef]
- Sumit; Sharma, K.; Tewatia, P.; Samota, S.; Kaur, M.; Paulik, C.; Sharma, M.; Kaushik, A. Efficient Mercury Ion Abatement through Highly Porous Cellulose Nanofibrils Combined with Microporous Organic Polymer Enhancements. Int. J. Biol. Macromol. 2024, 280, 136136. [Google Scholar] [CrossRef] [PubMed]
- Orasugh, J.T.; Ray, S.S. Nanocellulose-Graphene Oxide-Based Nanocomposite for Adsorptive Water Treatment. In Functional Polymer Nanocomposites for Wastewater Treatment; Hato, M.J., Sinha Ray, S., Eds.; Springer Series in Materials Science; Springer International Publishing: Cham, Switzerland, 2022; Volume 323, pp. 1–53. ISBN 978-3-030-94994-5. [Google Scholar]
- Rahman, U.U.; Humayun, M.; Khan, A.; Farooq, S.; Sadiq, M.; Bououdina, M.; Shah, N. Thermo-Chemical Modification of Cellulose for the Adsorptive Removal of Titan Yellow from Wastewater. Molecules 2023, 28, 3955. [Google Scholar] [CrossRef]
- Gerullis, S.; Pfuch, A.; Beier, O.; Kretzschmar, B.-S.-M.; Beyer, M.; Fischer, S. Plasma Treatment of Cellulose: Investigation on Molecular Changes Using Spectroscopic Methods and Chemical Derivatization. Cellulose 2022, 29, 7163–7176. [Google Scholar] [CrossRef]
- Li, S.-S.; Song, Y.-L.; Yang, H.-R.; An, Q.-D.; Xiao, Z.-Y.; Zhai, S.-R. Carboxymethyl Cellulose-Based Cryogels for Efficient Heavy Metal Capture: Aluminum-Mediated Assembly Process and Sorption Mechanism. Int. J. Biol. Macromol. 2020, 164, 3275–3286. [Google Scholar] [CrossRef]
- Yu, X.; Tong, S.; Ge, M.; Wu, L.; Zuo, J.; Cao, C.; Song, W. Adsorption of Heavy Metal Ions from Aqueous Solution by Carboxylated Cellulose Nanocrystals. J. Environ. Sci. 2013, 25, 933–943. [Google Scholar] [CrossRef] [PubMed]
- Siqueira, G.; Bras, J.; Dufresne, A. Cellulosic Bionanocomposites: A Review of Preparation, Properties and Applications. Polymers 2010, 2, 728–765. [Google Scholar] [CrossRef]
- Sjahro, N.; Yunus, R.; Abdullah, L.C.; Rashid, S.A.; Asis, A.J.; Akhlisah, Z.N. Recent Advances in the Application of Cellulose Derivatives for Removal of Contaminants from Aquatic Environments. Cellulose 2021, 28, 7521–7557. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, L.; Yu, J.; Fan, Y. A Review of Cellulose Amination in Homogeneous and Heterogeneous Systems and Their Applications. Ind. Crops Prod. 2024, 222, 119500. [Google Scholar] [CrossRef]
- Bhatnagar, A.; Hogland, W.; Marques, M.; Sillanpää, M. An Overview of the Modification Methods of Activated Carbon for Its Water Treatment Applications. Chem. Eng. J. 2013, 219, 499–511. [Google Scholar] [CrossRef]
- Yu, T.; Liu, S.; Xu, M.; Peng, J.; Li, J.; Zhai, M. Synthesis of Novel Aminated Cellulose Microsphere Adsorbent for Efficient Cr(VI) Removal. Radiat. Phys. Chem. 2016, 125, 94–101. [Google Scholar] [CrossRef]
- Wang, J.; Liu, M.; Duan, C.; Sun, J.; Xu, Y. Preparation and Characterization of Cellulose-Based Adsorbent and Its Application in Heavy Metal Ions Removal. Carbohydr. Polym. 2019, 206, 837–843. [Google Scholar] [CrossRef] [PubMed]
- Pereira, A.R.; Soares, L.C.; Teodoro, F.S.; Elias, M.M.C.; Ferreira, G.M.D.; Savedra, R.M.L.; Siqueira, M.F.; Martineau-Corcos, C.; Da Silva, L.H.M.; Prim, D.; et al. Aminated Cellulose as a Versatile Adsorbent for Batch Removal of As(V) and Cu(II) from Mono- and Multicomponent Aqueous Solutions. J. Colloid Interface Sci. 2020, 576, 158–175. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, X.; Xie, Y.; Zhang, K. Functional Nanomaterials through Esterification of Cellulose: A Review of Chemistry and Application. Cellulose 2018, 25, 3703–3731. [Google Scholar] [CrossRef]
- Geim, A.K.; Novoselov, K.S. The Rise of Graphene. Nat. Mater. 2007, 6, 183–191. [Google Scholar] [CrossRef]
- Safarpour, M.; Khataee, A. Graphene-Based Materials for Water Purification. In Nanoscale Materials in Water Purification; Elsevier: Amsterdam, The Netherlands, 2019; pp. 383–430. ISBN 978-0-12-813926-4. [Google Scholar]
- Ahmed, M.; Giwa, A.; Hasan, S.W. Challenges and Opportunities of Graphene-Based Materials in Current Desalination and Water Purification Technologies. In Nanoscale Materials in Water Purification; Elsevier: Amsterdam, The Netherlands, 2019; pp. 735–758. ISBN 978-0-12-813926-4. [Google Scholar]
- Zubair, M.; Roopesh, M.S.; Ullah, A. Challenges and Prospects: Graphene Oxide-Based Materials for Water Remediation Including Metal Ions and Organic Pollutants. Environ. Sci. Nano 2024, 11, 3693–3720. [Google Scholar] [CrossRef]
- Pestunov, A.V.; Kuzmin, A.O.; Yatsenko, D.À.; Pravdina, M.K.; Taran, O.P. The Mechanical Activation of Crystal and Wooden Sawdust Cellulose in Various Fine-Grinding Mills. J. Sib. Fed. Univ. Chem. 2015, 8, 386–400. [Google Scholar] [CrossRef]
- Huang, L.; Wu, Q.; Wang, Q.; Wolcott, M. Mechanical Activation and Characterization of Micronized Cellulose Particles from Pulp Fiber. Ind. Crops Prod. 2019, 141, 111750. [Google Scholar] [CrossRef]
- Dejene, D.; Tilahun, E. Characterization of Biochar Produced from Different Feed Stocks. Asian J. Environ. Ecol. 2020, 12, 1–6. [Google Scholar] [CrossRef]
- Zhang, S.; Zhu, S.; Zhang, H.; Liu, X.; Xiong, Y. Synthesis and Characterization of Rice Husk-Based Magnetic Porous Carbon by Pyrolysis of Pretreated Rice Husk with FeCl3 and ZnCl2. J. Anal. Appl. Pyrolysis 2020, 147, 104806. [Google Scholar] [CrossRef]
- Pérez, J.; Muñoz-Dorado, J.; De La Rubia, T.; Martínez, J. Biodegradation and Biological Treatments of Cellulose, Hemicellulose and Lignin: An Overview. Int. Microbiol. 2002, 5, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Fazeli, M.; Florez, J.P.; Simão, R.A. Improvement in Adhesion of Cellulose Fibers to the Thermoplastic Starch Matrix by Plasma Treatment Modification. Compos. Part B Eng. 2019, 163, 207–216. [Google Scholar] [CrossRef]
- Kolářová, K.; Vosmanská, V.; Rimpelová, S.; Švorčík, V. Effect of Plasma Treatment on Cellulose Fiber. Cellulose 2013, 20, 953–961. [Google Scholar] [CrossRef]
- Bhanthumnavin, W.; Wanichapichart, P.; Taweepreeda, W.; Sirijarukula, S.; Paosawatyanyong, B. Surface Modification of Bacterial Cellulose Membrane by Oxygen Plasma Treatment. Surf. Coat. Technol. 2016, 306, 272–278. [Google Scholar] [CrossRef]
- Barsbay, M.; Güven, O. Surface Modification of Cellulose via Conventional and Controlled Radiation-Induced Grafting. Radiat. Phys. Chem. 2019, 160, 1–8. [Google Scholar] [CrossRef]
- Wojnárovits, L.; Földváry, C.M.; Takács, E. Radiation-Induced Grafting of Cellulose for Adsorption of Hazardous Water Pollutants: A Review. Radiat. Phys. Chem. 2010, 79, 848–862. [Google Scholar] [CrossRef]
- Zhang, N.; Li, J.; Tian, B.; Li, T.; Zhang, J.; Wang, Q.; Zhao, H. RIGP-Induced Surface Modification of Cellulose for the Preparation of Amidoxime-Modified Cellulose/Graphite Oxide Composites with Enhanced Uranium Adsorption. Ind. Eng. Chem. Res. 2024, 63, 2337–2346. [Google Scholar] [CrossRef]
- Li, C.; Ma, H.; Venkateswaran, S.; Hsiao, B.S. Highly Efficient and Sustainable Carboxylated Cellulose Filters for Removal of Cationic Dyes/Heavy Metals Ions. Chem. Eng. J. 2020, 389, 123458. [Google Scholar] [CrossRef]
- Zhang, C.; Su, J.; Zhu, H.; Xiong, J.; Liu, X.; Li, D.; Chen, Y.; Li, Y. The Removal of Heavy Metal Ions from Aqueous Solutions by Amine Functionalized Cellulose Pretreated with Microwave-H2O2. RSC Adv. 2017, 7, 34182–34191. [Google Scholar] [CrossRef]
- Fryczkowska, B.; Biniaś, D.; Ślusarczyk, C.; Fabia, J.; Janicki, J. Properties and Application of Cellulose Membranes with Graphene Oxide Addition for Removal of Heavy Metals from Aqueous Solutions. Desalin. Water Treat. 2018, 117, 66–77. [Google Scholar] [CrossRef]
- Hu, H.; Zhang, Y.; Liu, X.; Huang, Z.; Chen, Y.; Yang, M.; Qin, X.; Feng, Z. Structural Changes and Enhanced Accessibility of Natural Cellulose Pretreated by Mechanical Activation. Polym. Bull. 2014, 71, 453–464. [Google Scholar] [CrossRef]
- Mubark, A.E.; Falila, N.I.; Salem, H.M. Use of Modified Cellulose Sorbents for the Extraction of Th(IV) Ions from Chloride Solutions. Radiochemistry 2021, 63, 484–497. [Google Scholar] [CrossRef]
- Morales, J.; Olayo, M.G.; Cruz, G.J.; Herrera-Franco, P.; Olayo, R. Plasma Modification of Cellulose Fibers for Composite Materials. J. Appl. Polym. Sci. 2006, 101, 3821–3828. [Google Scholar] [CrossRef]
- Kim, A.; Kim, N.-H. Effect of Heat Treatment and Particle Size on the Crystalline Properties of Wood Cellulose. J. Korean Wood Sci. Technol. 2019, 47, 299–310. [Google Scholar] [CrossRef]
- Abdelhamid, H.N.; Mathew, A.P. Cellulose-Based Materials for Water Remediation: Adsorption, Catalysis, and Antifouling. Front. Chem. Eng. 2021, 3, 790314. [Google Scholar] [CrossRef]
- Podgorbunskikh, E.M.; Bychkov, A.L.; Bulina, N.V.; Lomovskii, O.I. Disordering of the Crystal Structure of Cellulose Under Mechanical Activation. J. Struct. Chem. 2018, 59, 201–208. [Google Scholar] [CrossRef]
- He, X.; Zhang, T.; Xue, Q.; Zhou, Y.; Wang, H.; Bolan, N.S.; Jiang, R.; Tsang, D.C.W. Enhanced Adsorption of Cu(II) and Zn(II) from Aqueous Solution by Polyethyleneimine Modified Straw Hydrochar. Sci. Total Environ. 2021, 778, 146116. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Li, Z.; Kang, W.; Deng, N.; Yan, J.; Ju, J.; Liu, Y.; Cheng, B. Preparation and Characterization of Tree-like Cellulose Nanofiber Membranes via the Electrospinning Method. Carbohydr. Polym. 2018, 183, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Hamad, A.A.; Hassouna, M.S.; Shalaby, T.I.; Elkady, M.F.; Abd Elkawi, M.A.; Hamad, H.A. Electrospun Cellulose Acetate Nanofiber Incorporated with Hydroxyapatite for Removal of Heavy Metals. Int. J. Biol. Macromol. 2020, 151, 1299–1313. [Google Scholar] [CrossRef]
- Toledo, P.V.O.; Martins, B.F.; Pirich, C.L.; Sierakowski, M.R.; Neto, E.T.; Petri, D.F.S. Cellulose Based Cryogels as Adsorbents for Organic Pollutants. Macromol. Symp. 2019, 383, 1800013. [Google Scholar] [CrossRef]
- Sun, B.; Yuan, Y.; Li, H.; Li, X.; Zhang, C.; Guo, F.; Liu, X.; Wang, K.; Zhao, X.S. Waste-Cellulose-Derived Porous Carbon Adsorbents for Methyl Orange Removal. Chem. Eng. J. 2019, 371, 55–63. [Google Scholar] [CrossRef]
- Ibrahim, N.A.; Eid, B.M. Plasma Treatment Technology for Surface Modification and Functionalization of Cellulosic Fabrics. In Advances in Functional Finishing of Textiles; Shahid, M., Adivarekar, R., Eds.; Textile Science and Clothing Technology; Springer Singapore: Singapore, 2020; pp. 275–287. ISBN 9789811536687. [Google Scholar]
- Tahmasebi, E.; Ebadollahi, R. Electrospun Nanofibers Adsorbent for Water Purification. In Electrospun Nanofibrous Technology for Clean Water Production; Das, R., Ed.; Nanostructure Science and Technology; Springer Nature Singapore: Singapore, 2023; pp. 75–121. ISBN 978-981-9954-82-7. [Google Scholar]
- Paulauskiene, T.; Uebe, J.; Karasu, A.U.; Anne, O. Investigation of Cellulose-Based Aerogels for Oil Spill Removal. Water. Air. Soil Pollut. 2020, 231, 424. [Google Scholar] [CrossRef]
- Chalupa, J.; Pocik, O.; Halecky, M.; Kozliak, E. Thermophilic Waste Air Treatment of an Airborne Ethyl Acetate/Toluene Mixture in a Bubble Column Reactor: Stability towards Temperature Changes. J. Hazard. Mater. 2020, 384, 120744. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Yang, L.; Li, Q.; Zhao, J.; Li, X.; Wang, X.; Liu, H. Amino-Functionalized Magnetic Cellulose Nanocomposite as Adsorbent for Removal of Cr(VI): Synthesis and Adsorption Studies. Chem. Eng. J. 2014, 241, 175–183. [Google Scholar] [CrossRef]
- Abd-Elhamid, A.I.; Abu Elgoud, E.M.; Aly, H.F. Graphene Oxide Modified with Carboxymethyl Cellulose for High Adsorption Capacities towards Nd(III) and Ce(III) from Aqueous Solutions. Cellulose 2022, 29, 9831–9846. [Google Scholar] [CrossRef]
- Mishra, K.; Siwal, S.S.; Sithole, T.; Singh, N.; Hart, P.; Thakur, V.K. Biorenewable Materials for Water Remediation: The Central Role of Cellulose in Achieving Sustainability. J. Bioresour. Bioprod. 2024, 9, 253–282. [Google Scholar] [CrossRef]
- Aslam, A.A.; Hassan, S.U.; Saeed, M.H.; Kokab, O.; Ali, Z.; Nazir, M.S.; Siddiqi, W.; Aslam, A.A. Cellulose-Based Adsorbent Materials for Water Remediation: Harnessing Their Potential in Heavy Metals and Dyes Removal. J. Clean. Prod. 2023, 421, 138555. [Google Scholar] [CrossRef]
- Sharma, R.; Nath, P.C.; Mohanta, Y.K.; Bhunia, B.; Mishra, B.; Sharma, M.; Suri, S.; Bhaswant, M.; Nayak, P.K.; Sridhar, K. Recent Advances in Cellulose-Based Sustainable Materials for Wastewater Treatment: An Overview. Int. J. Biol. Macromol. 2024, 256, 128517. [Google Scholar] [CrossRef] [PubMed]
- Salama, A. Novel Cellulose Derivative Containing Aminophenylacetic Acid as Sustainable Adsorbent for Removal of Cationic and Anionic Dyes. Int. J. Biol. Macromol. 2023, 253, 126687. [Google Scholar] [CrossRef] [PubMed]
- Xiao, W.; Sun, R.; Hu, S.; Meng, C.; Xie, B.; Yi, M.; Wu, Y. Recent Advances and Future Perspective on Lignocellulose-Based Materials as Adsorbents in Diverse Water Treatment Applications. Int. J. Biol. Macromol. 2023, 253, 126984. [Google Scholar] [CrossRef] [PubMed]
- Nag, S.; Biswas, S. Cellulose-Based Adsorbents for Heavy Metal Removal. In Green Adsorbents to Remove Metals, Dyes and Boron from Polluted Water; Inamuddin, Ahamed, M.I., Lichtfouse, E., Asiri, A.M., Eds.; Environmental Chemistry for a Sustainable World; Springer International Publishing: Cham, Switzerland, 2021; Volume 49, pp. 113–142. ISBN 978-3-030-47399-0. [Google Scholar]
- Varghese, A.G.; Paul, S.A.; Latha, M.S. Remediation of Heavy Metals and Dyes from Wastewater Using Cellulose-Based Adsorbents. Environ. Chem. Lett. 2019, 17, 867–877. [Google Scholar] [CrossRef]
- Ji, S.; Park, C.; Lee, Y.B.; Kim, S.K.; An, K.-S.; Lee, S.S. Sorption of Hazardous Industrial Organic Liquids with Environmentally Friendly Functionalized Cellulosic Sorbents. J. Polym. Eng. 2023, 43, 243–253. [Google Scholar] [CrossRef]
- Nikiforova, T.E.; Kozlov, V.A.; Karaseva, E.N. The Influence of Chemical Modification of Cellulose with 4-Aminobenzoic Acid on Sorption of Cu(II) Ions. Prot. Met. Phys. Chem. Surf. 2021, 57, 680–686. [Google Scholar] [CrossRef]
- Kumar, R.; Sharma, R.K. Synthesis and Characterization of Cellulose Based Adsorbents for Removal of Ni(II), Cu(II) and Pb(II) Ions from Aqueous Solutions. React. Funct. Polym. 2019, 140, 82–92. [Google Scholar] [CrossRef]
- Kaur, J.; Sengupta, P.; Mukhopadhyay, S. Critical Review of Bioadsorption on Modified Cellulose and Removal of Divalent Heavy Metals (Cd, Pb, and Cu). Ind. Eng. Chem. Res. 2022, 61, 1921–1954. [Google Scholar] [CrossRef]
- Guleria, A.; Kumari, G.; Lima, E.C.; Ashish, D.K.; Thakur, V.; Singh, K. Removal of Inorganic Toxic Contaminants from Wastewater Using Sustainable Biomass: A Review. Sci. Total Environ. 2022, 823, 153689. [Google Scholar] [CrossRef]
- Vokurova, D.A.; Nikiforova, T.E. Influence of Cellulose-Containing Sorbent Preparation Method Based on Linen Fiber on Its Functional Properties. Vestn. MGTU 2022, 25, 153–167. [Google Scholar] [CrossRef]
- Li, H.; Gao, Y.; Zheng, Y. Study on Adsorption Properties of Cellulose Based Hydrogels. J. Phys. Conf. Ser. 2023, 2610, 012062. [Google Scholar] [CrossRef]
- Thakur, S.; Verma, A.; Kumar, V.; Jin Yang, X.; Krishnamurthy, S.; Coulon, F.; Thakur, V.K. Cellulosic Biomass-Based Sustainable Hydrogels for Wastewater Remediation: Chemistry and Prospective. Fuel 2022, 309, 122114. [Google Scholar] [CrossRef]
- Poulose, A.; Mathew, A.; Gopakumar, D.A.; Pasquini, D.; Mathiazhagan, A.; George, J.J. Cellulose Nanofibers-Based Porous Materials, a Green Platform to Construct Advanced Sorbents for Oil/Spill Cleanup. In Nanomaterials for Air and Water Purification; Parameswaranpillai, J., Gopakumar, D.A., George, J.J., Dominic, M., Eds.; Wiley: Hoboken, NJ, USA, 2024; pp. 305–328. ISBN 978-3-527-35052-0. [Google Scholar]
- Grunin, Y.B.; Ivanova, M.S.; Masas, D.S.; Grunin, L.Y. The Nature of the Supramolecular Structural Variation and Hydrophilic Properties of Cellulose during Water Sorption. Biophysics 2019, 64, 866–869. [Google Scholar] [CrossRef]
- Köse, K.; Mavlan, M.; Youngblood, J.P. Applications and Impact of Nanocellulose Based Adsorbents. Cellulose 2020, 27, 2967–2990. [Google Scholar] [CrossRef]
- Mahfoudhi, N.; Boufi, S. Nanocellulose as a Novel Nanostructured Adsorbent for Environmental Remediation: A Review. Cellulose 2017, 24, 1171–1197. [Google Scholar] [CrossRef]
- Abou-Zeid, R.E.; Ali, K.A.; Gawad, R.M.A.; Kamal, K.H.; Kamel, S.; Khiari, R. Removal of Cu(II), Pb(II), Mg(II), and Fe(II) by Adsorption onto Alginate/Nanocellulose Beads as Bio-Sorbent. J. Renew. Mater. 2021, 9, 601–613. [Google Scholar] [CrossRef]
- Basheer, J.; Uthaman, A.; Lal, H.M.; Thomas, S.; Gopakumar, D.A.; George, J.J. Nanocellulose: A Sustainable Functional Construct for the Remediation of Heavy Metal Ions from Water. J. Thermoplast. Compos. Mater. 2024, 08927057241249731. [Google Scholar] [CrossRef]
- Abdelhamid, H.N. Nanocellulose-Based Materials for Water Pollutants Removal: A Review. Int. J. Mol. Sci. 2024, 25, 8529. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Li, Y.; Yang, W.; Cui, X.; Lei, T. Preparation and Surface Modification of Nanocellulose and Its Adsorption Performance on Cu2+. J. Biobased Mater. Bioenergy 2024, 18, 157–162. [Google Scholar] [CrossRef]
- Rana, H.; Anamika; Sareen, D.; Goswami, S. Nanocellulose-Based Ecofriendly Nanocomposite for Effective Wastewater Remediation: A Study on Its Process Optimization, Improved Swelling, Adsorption, and Thermal and Mechanical Behavior. ACS Omega 2024, 9, 8904–8922. [Google Scholar] [CrossRef]
- Hammouda, S.B.; Chen, Z.; An, C.; Lee, K. Recent Advances in Developing Cellulosic Sorbent Materials for Oil Spill Cleanup: A State-of-the-Art Review. J. Clean. Prod. 2021, 311, 127630. [Google Scholar] [CrossRef]
- Thai, Q.B.; Nguyen, S.T.; Ho, D.K.; Tran, T.D.; Huynh, D.M.; Do, N.H.N.; Luu, T.P.; Le, P.K.; Le, D.K.; Phan-Thien, N.; et al. Cellulose-Based Aerogels from Sugarcane Bagasse for Oil Spill-Cleaning and Heat Insulation Applications. Carbohydr. Polym. 2020, 228, 115365. [Google Scholar] [CrossRef] [PubMed]
- Chhajed, M.; Yadav, C.; Agrawal, A.K.; Maji, P.K. Esterified Superhydrophobic Nanofibrillated Cellulose Based Aerogel for Oil Spill Treatment. Carbohydr. Polym. 2019, 226, 115286. [Google Scholar] [CrossRef]
- Rafieian, F.; Hosseini, M.; Jonoobi, M.; Yu, Q. Development of Hydrophobic Nanocellulose-Based Aerogel via Chemical Vapor Deposition for Oil Separation for Water Treatment. Cellulose 2018, 25, 4695–4710. [Google Scholar] [CrossRef]
- Zhuang, J.; Rong, N.; Wang, X.; Chen, C.; Xu, Z. Adsorption of Small Size Microplastics Based on Cellulose Nanofiber Aerogel Modified by Quaternary Ammonium Salt in Water. Sep. Purif. Technol. 2022, 293, 121133. [Google Scholar] [CrossRef]
- Olorunnisola, D.; Olorunnisola, C.G.; Otitoju, O.B.; Okoli, C.P.; Rawel, H.M.; Taubert, A.; Easun, T.L.; Unuabonah, E.I. Cellulose-Based Adsorbents for Solid Phase Extraction and Recovery of Pharmaceutical Residues from Water. Carbohydr. Polym. 2023, 318, 121097. [Google Scholar] [CrossRef] [PubMed]
- Taherpoor, P.; Farzad, F.; Zaboli, A. Performance Evaluation and Efficiency of Functionalized Cellulose Nanocomposites for the Removal of Pharmaceutical Contaminants from the Aqueous Environment. J. Mol. Liq. 2023, 392, 123414. [Google Scholar] [CrossRef]
- Kausar, A.; Zohra, S.T.; Ijaz, S.; Iqbal, M.; Iqbal, J.; Bibi, I.; Nouren, S.; El Messaoudi, N.; Nazir, A. Cellulose-Based Materials and Their Adsorptive Removal Efficiency for Dyes: A Review. Int. J. Biol. Macromol. 2023, 224, 1337–1355. [Google Scholar] [CrossRef]
- Suteu, D.; Coseri, S.; Zaharia, C.; Biliuta, G.; Nebunu, I. Modified Cellulose Fibers as Adsorbent for Dye Removal from Aqueous Environment. Desalin. Water Treat. 2017, 90, 341–349. [Google Scholar] [CrossRef]
- Samuel, M.S.; John, J.A.; Ravikumar, M.; Raizada, P.; Wan Azelee, N.I.; Selvarajan, E.; Selvasembian, R. Recent Progress on the Remediation of Dyes in Wastewater Using Cellulose-Based Adsorbents. Ind. Crops Prod. 2023, 206, 117590. [Google Scholar] [CrossRef]
- Aziz, T.; Li, W.; Zhu, J.; Chen, B. Developing Multifunctional Cellulose Derivatives for Environmental and Biomedical Applications: Insights into Modification Processes and Advanced Material Properties. Int. J. Biol. Macromol. 2024, 278, 134695. [Google Scholar] [CrossRef] [PubMed]
- Joshi, N.C.; Joshi, A.; Mitra, D.; Gururani, P.; Kumar, N.; Joshi, H.K. Removal of Heavy Metals Using Cellulose-Based Materials: A Mini-Review. Environ. Nanotechnol. Monit. Manag. 2024, 21, 100942. [Google Scholar] [CrossRef]
- Zhang, M.; Xu, T.; Zhao, Q.; Liu, K.; Liang, D.; Si, C. Cellulose-Based Materials for Carbon Capture and Conversion. Carbon Capture Sci. Technol. 2024, 10, 100157. [Google Scholar] [CrossRef]

| Property | Description | Value for Sorption | Sources |
|---|---|---|---|
| Chemical structure | A linear polysaccharide consisting of β-d-glucose linked by β-(1→4)-glycosidic bonds. | It forms crystalline and amorphous areas. Amorphous regions provide better accessibility of hydroxyl groups, crystalline zones provide mechanical stability. | [4] |
| Crystallinity | Cellulose has both crystalline and amorphous zones. The degree of crystallinity varies depending on the source of cellulose. | Crystalline zones improve mechanical strength but reduce reactivity, while amorphous zones promote adsorption. | [5] |
| Porous structure | Depending on the treatment, cellulose can form porous structures. | Porosity increases the specific surface area, which improves sorption properties by increasing contact with pollutants. | [13] |
| Mechanical strength and stability | High strength and resistance to mechanical damage, due to crystalline zones and hydrogen bonds. | Mechanical strength is important for the stability of sorbents during operation, especially during filtration and dynamic sorption processes. | [14] |
| Presence of hydroxyl groups (-OH) | Cellulose contains a large number of active hydroxyl groups on the surface. | Hydroxyl groups provide the possibility of chemical modification, which increases the sorption capacity. | [15] |
| Hydrophilicity | High affinity for water due to hydroxyl groups. | Hydrophilicity promotes adsorption of polar pollutants, such as heavy metals and some organic compounds. | [15] |
| Stability in aggressive environments | Cellulose is resistant to weak acids and alkalis, but sensitive to strong acids and alkalis. | Resistance to aggressive environments allows for the use of cellulose sorbents in industrial water purification processes. | [16] |
| Modifiability | Easily modified through esterification, carboxylation, acetylation, and other chemical reactions. | Chemical modification allows for the creation of specialized sorbents for target pollutants, such as heavy metals or organic compounds. | [17,18] |
| Specific surface area | Increases with the transition to nano-sized forms, such as nanocrystals and nanofibrils. | High specific surface area contributes to an increase in the number of available sorption centers. | [19] |
| Environmental safety | Cellulose is a renewable natural polymer, biodegradable, and environmentally friendly. | Environmental safety makes cellulose an excellent basis for the development of “green” technologies for water purification and cleaning contaminated soils. | [20,21] |
| Modification Method | Advantages | Disadvantages | Process Conditions | Sorbent Yield | Results | Sources |
|---|---|---|---|---|---|---|
| Carboxylation of cellulose | 1. High adsorption capacity for metal ions. 2. Simplicity of the process. | 1. The use of acids may lead to corrosion of equipment. 2. Possibility of formation of by-products. | Temperature: 60–80 °C, pH: 3–5, time: 2–4 h | 80% of the initial material mass | High adsorption capacity for metal ions (e.g., Pb2+)—81.3 mg/g | [68] |
| Amination of cellulose | 1. High efficiency for heavy metal removal. 2. Modified sorbent is resistant to repeated use. | 1. The process requires the use of toxic reagents. 2. Expensive reagents for amination. | Temperature: 90 °C, pH: 8, time: 5 h | 85% of the initial material mass | Heavy metal removal efficiency—98% for Cd2+ | [69] |
| Graphene modification of cellulose | 1. Increased porosity and extended diffusion path. 2. Improved sorption capacity for organic pollutants. | 1. Complexity of synthesizing graphene materials. 2. High cost of graphene additives. | Temperature: 100 °C, time: 4 h | 70% of the initial material mass | ~100% removal of heavy metals | [70] |
| Mechanical activation of cellulose | 1. Fast processing. 2. Increased material stability during operation. | 1. Reduction in porosity can lead to a decrease in sorption capacity. 2. Need for special equipment | Temperature: 50 °C, time: 1 h | 90% of the initial material mass | Reduction in porosity, but increase in sorbent stability | [71] |
| Radiation-induced grafting | 1. High adsorption capacities 2. Good mechanical and thermal stability | 1. High cost of equipment and energy consumption. 2. Possibility of uncontrolled destruction of cellulose with excessive radiation dose. 3. Further optimization of methods is required for large-scale application. | Processing is usually carried out at room temperature. Gamma rays or electron beams with energies of 2–10 MeV are used. | About 70–90%, depending on the radiation dose and the type of associated reactions | Adsorption of organic dyes increases by 30–50% compared to unmodified cellulose. | [72] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Darmenbayeva, A.; Rajasekharan, R.; Massalimova, B.; Bektenov, N.; Taubayeva, R.; Bazarbaeva, K.; Kurmanaliev, M.; Mukazhanova, Z.; Nurlybayeva, A.; Bulekbayeva, K.; et al. Cellulose-Based Sorbents: A Comprehensive Review of Current Advances in Water Remediation and Future Prospects. Molecules 2024, 29, 5969. https://doi.org/10.3390/molecules29245969
Darmenbayeva A, Rajasekharan R, Massalimova B, Bektenov N, Taubayeva R, Bazarbaeva K, Kurmanaliev M, Mukazhanova Z, Nurlybayeva A, Bulekbayeva K, et al. Cellulose-Based Sorbents: A Comprehensive Review of Current Advances in Water Remediation and Future Prospects. Molecules. 2024; 29(24):5969. https://doi.org/10.3390/molecules29245969
Chicago/Turabian StyleDarmenbayeva, Akmaral, Reshmy Rajasekharan, Bakytgul Massalimova, Nessipkhan Bektenov, Raushan Taubayeva, Karlygash Bazarbaeva, Musrepbek Kurmanaliev, Zhazira Mukazhanova, Aisha Nurlybayeva, Kamila Bulekbayeva, and et al. 2024. "Cellulose-Based Sorbents: A Comprehensive Review of Current Advances in Water Remediation and Future Prospects" Molecules 29, no. 24: 5969. https://doi.org/10.3390/molecules29245969
APA StyleDarmenbayeva, A., Rajasekharan, R., Massalimova, B., Bektenov, N., Taubayeva, R., Bazarbaeva, K., Kurmanaliev, M., Mukazhanova, Z., Nurlybayeva, A., Bulekbayeva, K., Kabylbekova, A., & Ungarbayeva, A. (2024). Cellulose-Based Sorbents: A Comprehensive Review of Current Advances in Water Remediation and Future Prospects. Molecules, 29(24), 5969. https://doi.org/10.3390/molecules29245969






