Clearance of Intracellular Pathogens with Hyaluronic Acid Nanomicelles Responsive to H2S and pH
Abstract
1. Introduction
2. Results
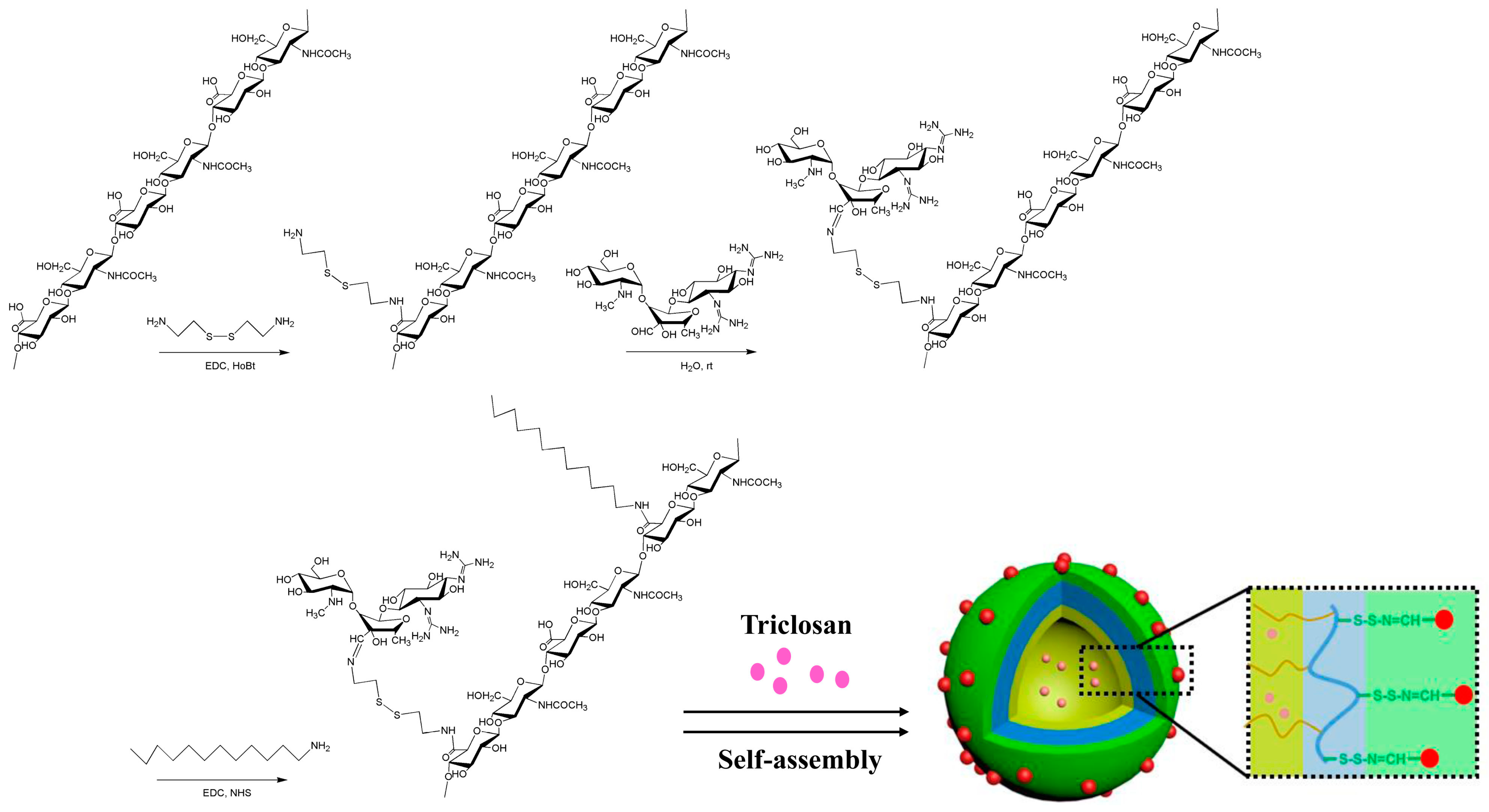
2.1. Synthesis and Identification of Micellar HASS
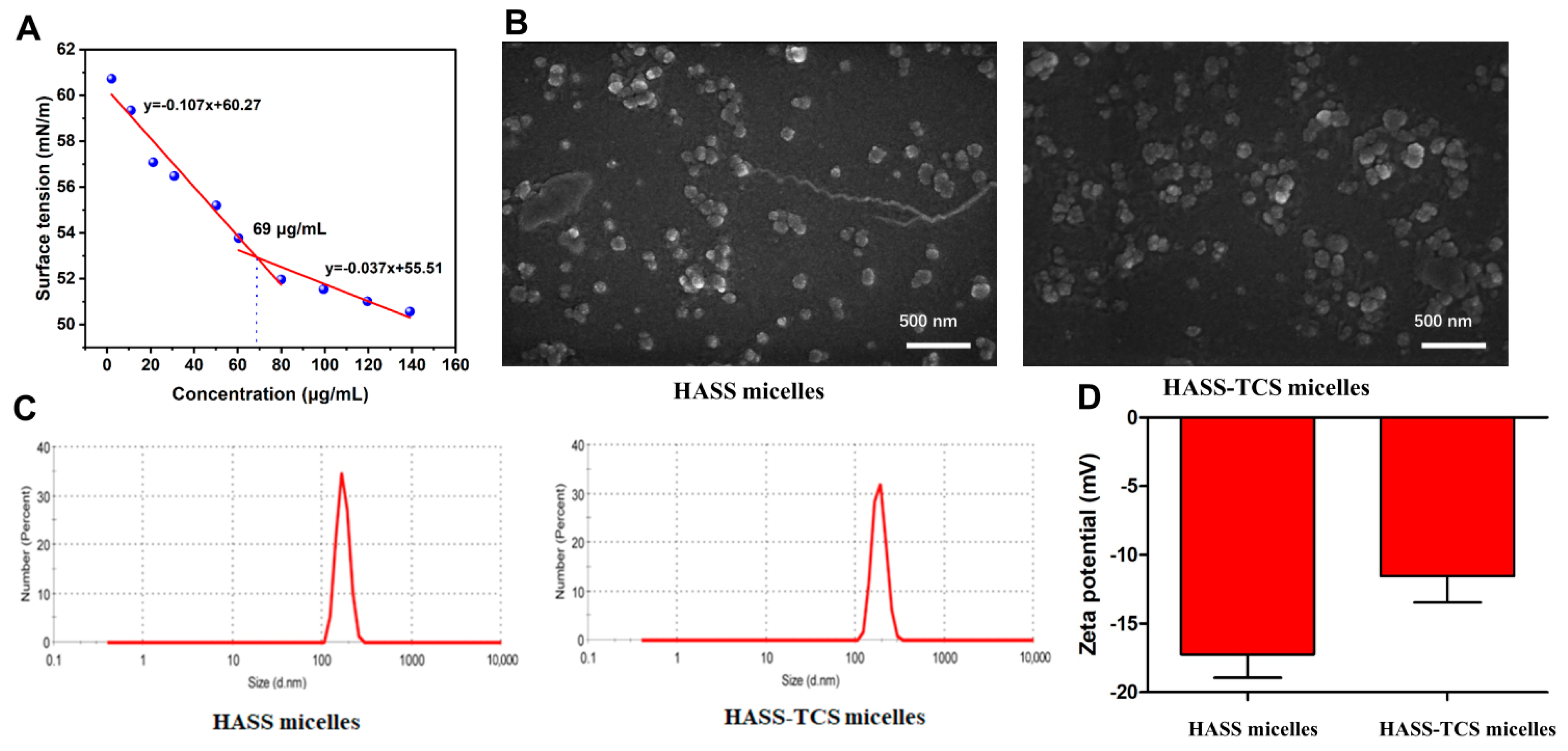
2.2. Characterization of HASS and HASS-TCS Nanomicelles
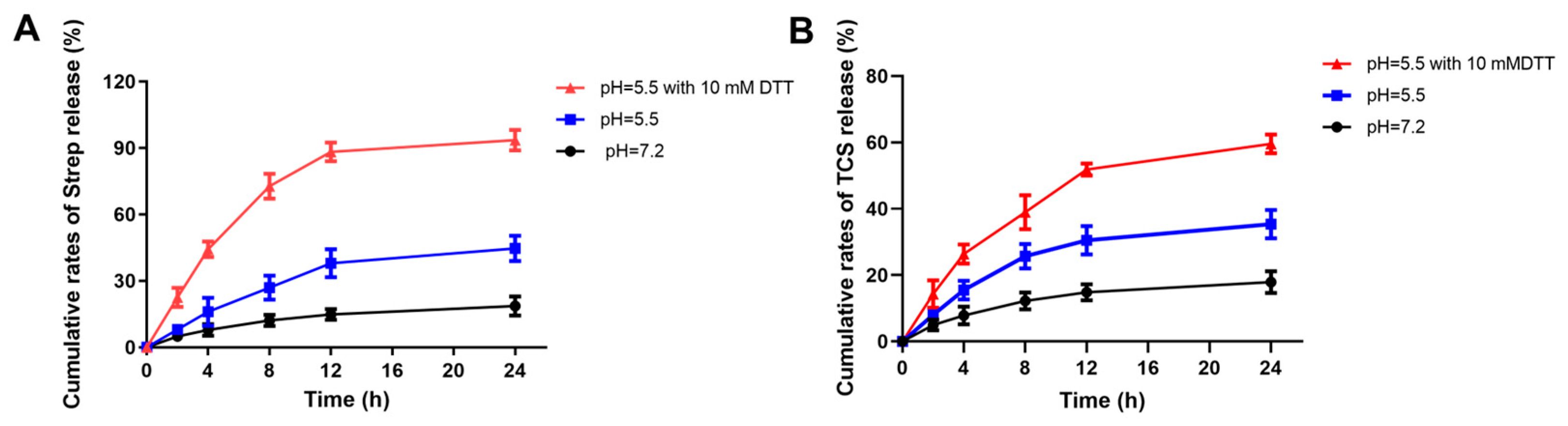
2.3. The Effect of Different pH on the Release of Streptomycin and TCS
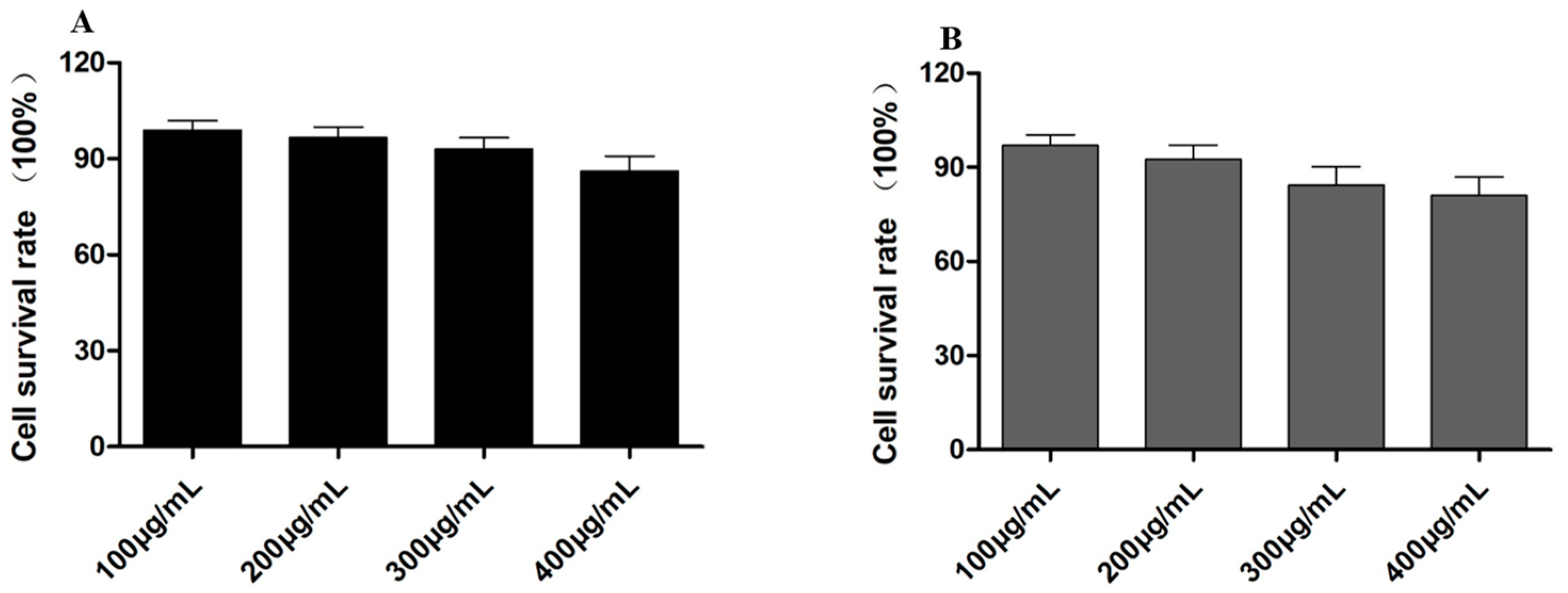
2.4. Cell Toxicity Detection of HASS and HASS-TCS
2.5. HASS-TCS Effectively Removes Biofilms and Intracellular Bacteria
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Methods
4.2.1. Coupling of Hyaluronic Acid and Cysteine
4.2.2. Coupling of Streptomycin with Hyaluronic Acid and Cysteine Complex
4.2.3. Synthesis of HASS
4.2.4. Preparation of HASS Nanomicelles Loaded with TCS
4.2.5. Characterization of HASS-TCS Micellar Complex
4.2.6. Critical Micelle Concentration
4.2.7. Drug Release of HASS-TCS Nanomicelles Loaded with TCS
4.2.8. Cell Viability Assay
4.2.9. Biofilm Removal Experiment
4.2.10. Anti Intracellular Bacterial Activity
4.2.11. CD44 Closed Experiment
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mak, T.W.; Saunders, M.E. The Immune Response: Basic and Clinical Principles, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2005; pp. 656–657. [Google Scholar]
- Finlay, B.B.; Cossart, P. Exploitation of mammalian host cell functions by bacterial pathogens. Science 1997, 276, 718–725. [Google Scholar] [CrossRef] [PubMed]
- Laskay, T.; Van Zandbergen, G.; Solbach, W. Neutrophil granulocytes–Trojan horses for Leishmania major and other intracellular microbes? Trends Microbiol. 2003, 11, 210–214. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, G.; Chen, C.; Portnoy, D.A. Strategies used by bacteria to grow in macrophages. In Myeloid Cells in Health and Disease: A Synthesis, 1st ed.; Siamon Gordon, A., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 2017; pp. 701–725. [Google Scholar]
- Farouk, F.; Azzazy, H.M.E.; Niessen, W.M.A. Challenges in the determination of aminoglycoside antibiotics, a review. Anal. Chim. Acta 2015, 890, 21–43. [Google Scholar] [CrossRef] [PubMed]
- Palvannan, T.; Boopathy, R. Interaction of aminoglycoside antibiotics with surface Asp and Glu residues of phosphatidylinositol-specific phospholipase C. Enzyme Microb. Technol. 2006, 38, 899–904. [Google Scholar] [CrossRef]
- Weiss, G.; Schaible, U.E. Macrophage defense mechanisms against intracellular bacteria. Immunol. Rev. 2015, 264, 182–203. [Google Scholar] [CrossRef]
- Subramaniam, S.; Joyce, P. Bioinspired drug delivery strategies for repurposing conventional antibiotics against intracellular infections. Adv. Drug Delivery Rev. 2021, 177, 113948. [Google Scholar] [CrossRef]
- Petit, T.J.P.; Lebreton, A. Adaptations of intracellular bacteria to vacuolar or cytosolic niches. Trends Microbiol. 2022, 30, 736–748. [Google Scholar] [CrossRef] [PubMed]
- Van Bambeke, F.; Michot, J.M. Antibiotic efflux pumps in eukaryotic cells: Occurrence and impact on antibiotic cellular pharmacokinetics, pharmacodynamics and toxicodynamics. J. Antimicrob. Chemother. 2003, 51, 1067–1077. [Google Scholar] [CrossRef] [PubMed]
- Bag, B.G.; Das, S. Nanoarchitectures by hierarchical self-assembly of ursolic acid: Entrapment and release of fluorophores including anticancer drug doxorubicin. RSC Adv. 2017, 7, 18136–18143. [Google Scholar] [CrossRef]
- Lu, J.; Hu, J. A new dual-responsive organogel based on uracil-appended glycyrrhetinic acid. Org. Lett. 2011, 13, 3372–3375. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.; Guo, J.; Tian, J.; Ma, K.; Liu, Y. Research progress on degradation methods and product properties of plant polysaccharides. J. Light Ind. 2023, 38, 55–62. [Google Scholar]
- Ji, X.; Cheng, Y.; Tian, J.; Zhang, S.; Jing, Y.; Shi, M. Structural characterization of polysaccharide from jujube (Ziziphus jujuba Mill.) fruit. Chem. Biol. Technol. Agric. 2021, 8, 54. [Google Scholar] [CrossRef]
- Alaniz, L.; Cabrera, P.V. Interaction of CD44 with different forms of hyaluronic acid. Its role in adhesion and migration of tumor cells. Cell Commun. Adhes. 2002, 9, 117–130. [Google Scholar] [CrossRef] [PubMed]
- Ossipov, D.A. Nanostructured hyaluronic acid-based materials for active delivery to cancer. Expert Opin. Drug Delivery 2010, 7, 681–703. [Google Scholar] [CrossRef]
- Chen, W.H.; Luo, G.F. Mesoporous silica-based versatile theranostic nanoplatform constructed by layer-by-layer assembly for excellent photodynamic/chemo therapy. Biomaterials 2017, 117, 54–65. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.J.; Yoon, H.Y. Self-assembled nanoparticles based on hyaluronic acid-ceramide (HA-CE) and Pluronic® for tumor-targeted delivery of docetaxel. Biomaterials 2011, 32, 7181–7190. [Google Scholar] [CrossRef] [PubMed]
- Munar-Bestard, M.; Vargas-Alfredo, N. Mangostanin hyaluronic acid hydrogel as an effective biocompatible alternative to chlorhexidine. Int. J. Biol. Macromol. 2024, 279, 135187. [Google Scholar] [CrossRef]
- Choi, K.Y.; Min, K.H. Self-assembled hyaluronic acid nanoparticles as a potential drug carrier for cancer therapy: Synthesis, characterization, and in vivo bio-distribution. J. Mater. Chem. 2009, 19, 4102–4107. [Google Scholar] [CrossRef]
- Yang, X.; Li, Y. Hyaluronic acid-coated nanostructured lipid carriers for targeting paclitaxel to cancer. Cancer Lett. 2013, 334, 338–345. [Google Scholar] [CrossRef]
- Li, X.; Ma, L.; Zhou, Y.; Lu, X.; Jing, L.; Jing, D. Rheological behavior and solution pH response properties of nanoparticle-regulated low surface tension systems. J. Chem. Phys. 2024, 161, 054505. [Google Scholar] [CrossRef] [PubMed]
- Glavas, L.; Olsén, P.; Odelius, K.; Albertsson, A.C. Achieving micelle control through core crystallinity. Biomacromolecules 2013, 14, 4150–4156. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Lu, C. Synergistic clearance of intracellular pathogens by hyaluronan-streptomycin nanomicelles encapsulated with rapamycin. Carbohydr. Polym. 2019, 210, 364–371. [Google Scholar] [CrossRef]
- Kim, D.Y.; Sharma, S.K. Development of novel peptide-modified silver nanoparticle-based rapid biosensors for detecting aminoglycoside antibiotics. J. Agric. Food Chem. 2023, 71, 12883–12898. [Google Scholar] [CrossRef] [PubMed]
- McClure, E.E.; Chávez, A.S.O. Engineering of obligate intracellular bacteria: Progress, challenges and paradigms. Nat. Rev. Microbiol. 2017, 15, 544–558. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Sun, J. Drug delivery systems based on CD44-targeted glycosaminoglycans for cancer therapy. Carbohydr. Polym. 2021, 251, 117103. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.; Zheng, H. Tumor-targeting and pH-responsive nanoparticles from hyaluronic acid for the enhanced delivery of doxorubicin. Int. J. Biol. Macromol. 2018, 113, 737–747. [Google Scholar] [CrossRef]
- Abatangelo, G.; Vindigni, V. Hyaluronic acid: Redefining its role. Cells 2020, 9, 1743. [Google Scholar] [CrossRef] [PubMed]
- Stern, R. Hyaluronan catabolism: A new metabolic pathway. Eur. J. Cell Biol. 2004, 83, 317–325. [Google Scholar] [CrossRef]
- Vasvani, S.; Kulkarni, P. Hyaluronic acid: A review on its biology, aspects of drug delivery, route of administrations and a special emphasis on its approved marketed products and recent clinical studies. Int. J. Biol. Macromol. 2020, 151, 1012–1029. [Google Scholar] [CrossRef]
- Fu, C.; Li, H. Conjugating an anticancer drug onto thiolated hyaluronic acid by acid liable hydrazone linkage for its gelation and dual stimuli-response release. Carbohydr. Polym. 2015, 128, 163–170. [Google Scholar] [CrossRef]
- Vafaei, S.Y.; Esmaeili, M. Self assembled hyaluronic acid nanoparticles as a potential carrier for targeting the inflamed intestinal mucosa. Carbohydr. Polym. 2016, 144, 371–381. [Google Scholar] [CrossRef] [PubMed]
- Tyrrell, Z.L.; Shen, Y. Fabrication of micellar nanoparticles for drug delivery through the self-assembly of block copolymers. Prog. Polym. Sci. 2010, 35, 1128–1143. [Google Scholar] [CrossRef]
- Pretorius, D.C.; Van Staden, J.F.; Botha, A.D.P. An automated colorimetric method for the determination of cyanoguanidine in water. Water SA 1991, 17, 273–280. [Google Scholar]
- Peris-Camarasa, B.; Dualde, P. Fast and eco-friendly analytical method to determine bisphenols, parabens, benzophenone-3 and triclosan in human urine by ultra-performance liquid chromatography coupled to mass spectrometry. Microchem. J. 2024, 203, 110972. [Google Scholar] [CrossRef]
- Sun, B.; Sun, M. Magnetic hydrogel micromachines with active release of antibacterial agent for biofilm eradication. Adv. Intell. Syst. 2024, 6, 2300092. [Google Scholar] [CrossRef]
- Ferriol-González, C.; Domingo-Calap, P. Phages for biofilm removal. Antibiotics 2020, 9, 268. [Google Scholar] [CrossRef]
- Song, M.; Wang, J. Isolation, structural characterization and immunomodulatory activity on RAW264. 7 cells of a novel exopolysaccharide of Dictyophora rubrovalvata. Int. J. Biol. Macromol. 2024, 270, 132222. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, J.; Huang, H.; Jiang, J.; Zheng, W.; Chen, P.; Bai, H. Clearance of Intracellular Pathogens with Hyaluronic Acid Nanomicelles Responsive to H2S and pH. Molecules 2024, 29, 5971. https://doi.org/10.3390/molecules29245971
Luo J, Huang H, Jiang J, Zheng W, Chen P, Bai H. Clearance of Intracellular Pathogens with Hyaluronic Acid Nanomicelles Responsive to H2S and pH. Molecules. 2024; 29(24):5971. https://doi.org/10.3390/molecules29245971
Chicago/Turabian StyleLuo, Jun, Hui Huang, Junfeng Jiang, Wenyu Zheng, Peng Chen, and Hongjin Bai. 2024. "Clearance of Intracellular Pathogens with Hyaluronic Acid Nanomicelles Responsive to H2S and pH" Molecules 29, no. 24: 5971. https://doi.org/10.3390/molecules29245971
APA StyleLuo, J., Huang, H., Jiang, J., Zheng, W., Chen, P., & Bai, H. (2024). Clearance of Intracellular Pathogens with Hyaluronic Acid Nanomicelles Responsive to H2S and pH. Molecules, 29(24), 5971. https://doi.org/10.3390/molecules29245971






