Psilocin, the Psychoactive Metabolite of Psilocybin, Modulates Select Neuroimmune Functions of Microglial Cells in a 5-HT2 Receptor-Dependent Manner
Abstract
1. Introduction
2. Results
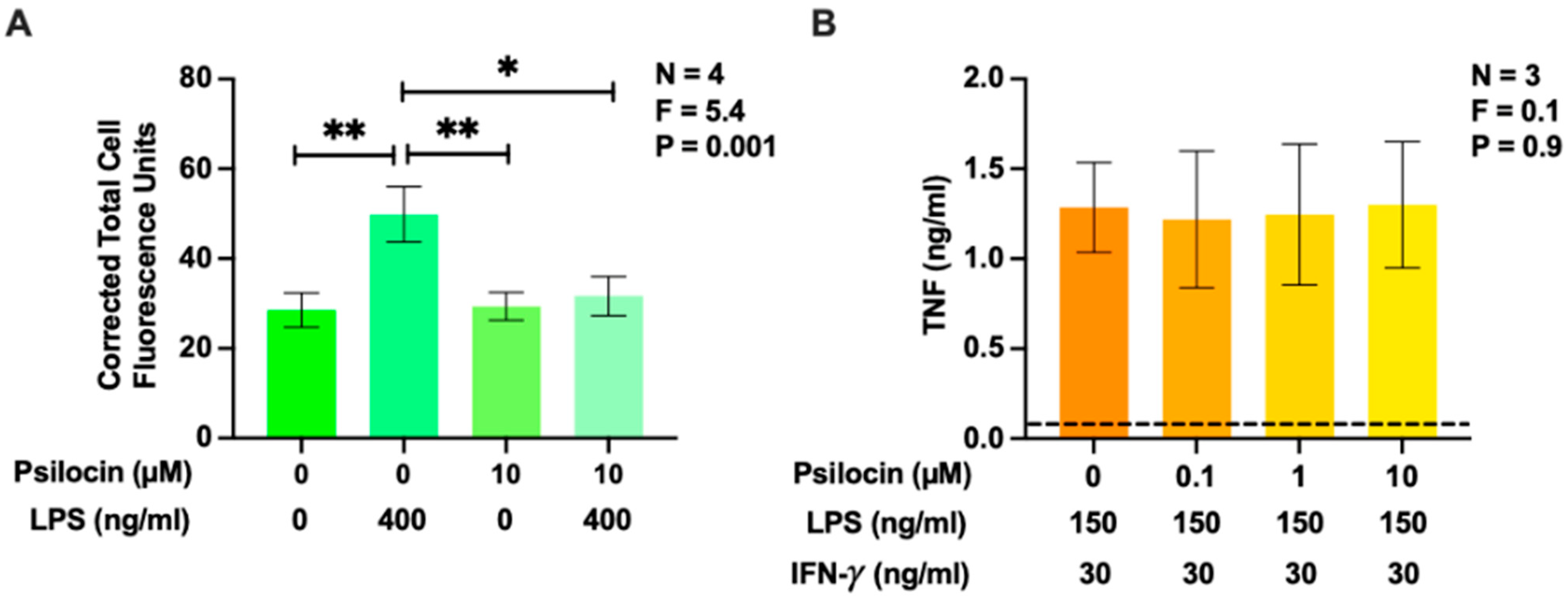
2.1. Effects of Psilocin on Phagocytosis of Latex Beads and Secretion of Tumor Necrosis Factor by BV-2 Murine Microglia
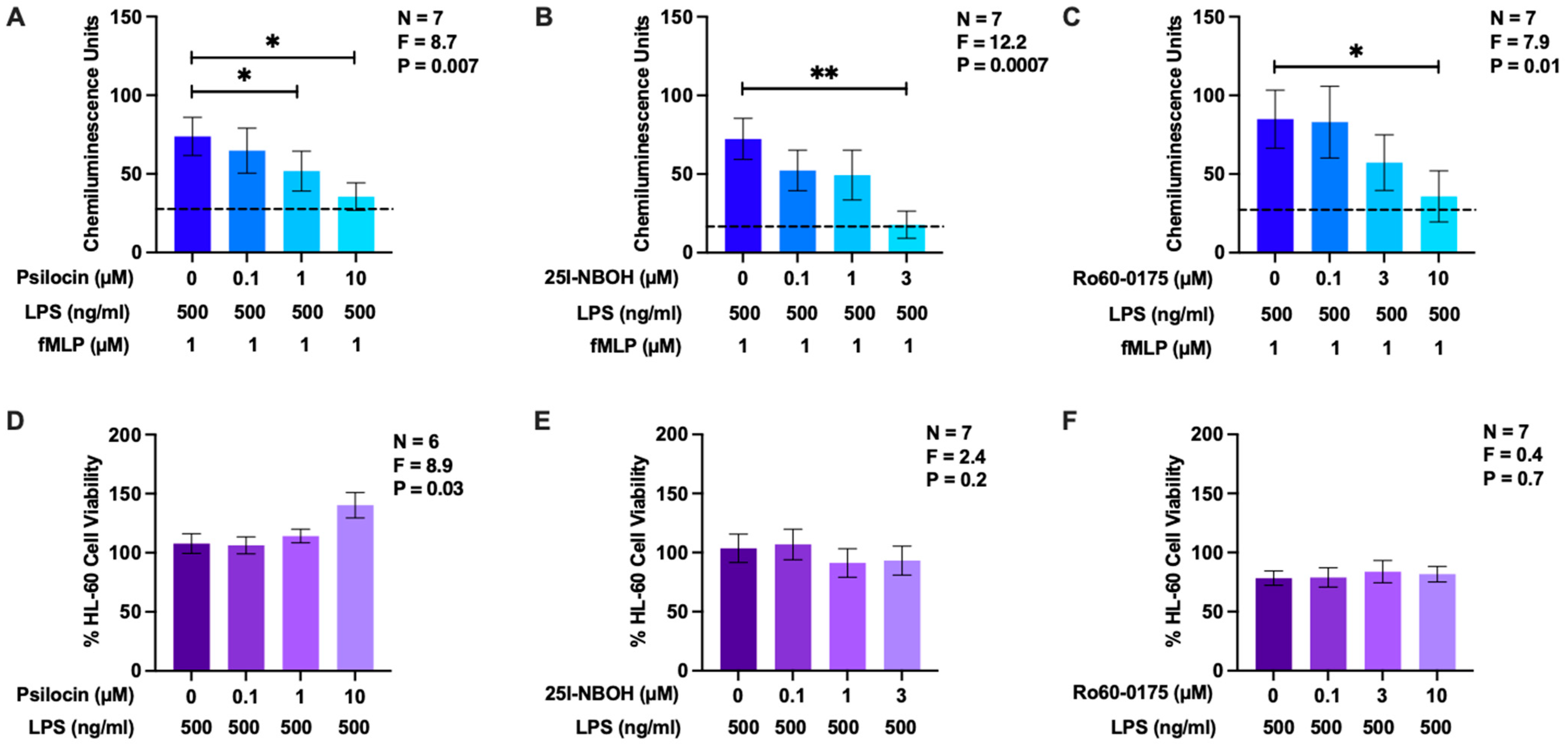
2.2. Effects of Psilocin, 25I-NBOH, and Ro60-0175 on Reactive Oxygen Species Generation by HL-60 Human Microglia-Like Cells
2.3. Effects of Psilocin, 25I-NBOH, and Ro60-0175 on Nitric Oxide Production by BV-2 Murine Microglia Cells
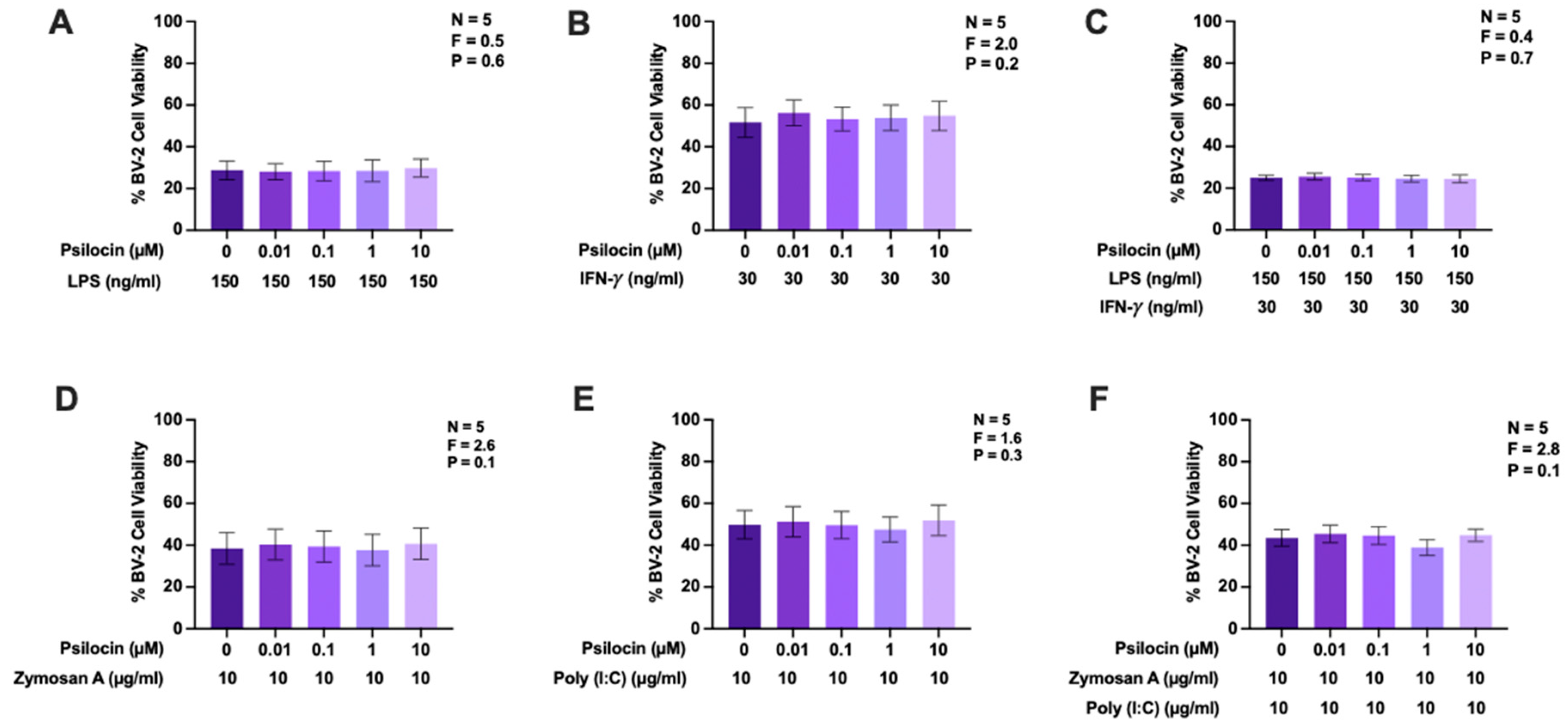
2.4. Effects of Psilocin on Nitric Oxide Production by BV-2 Murine Microglia Exposed to Diverse Stimuli
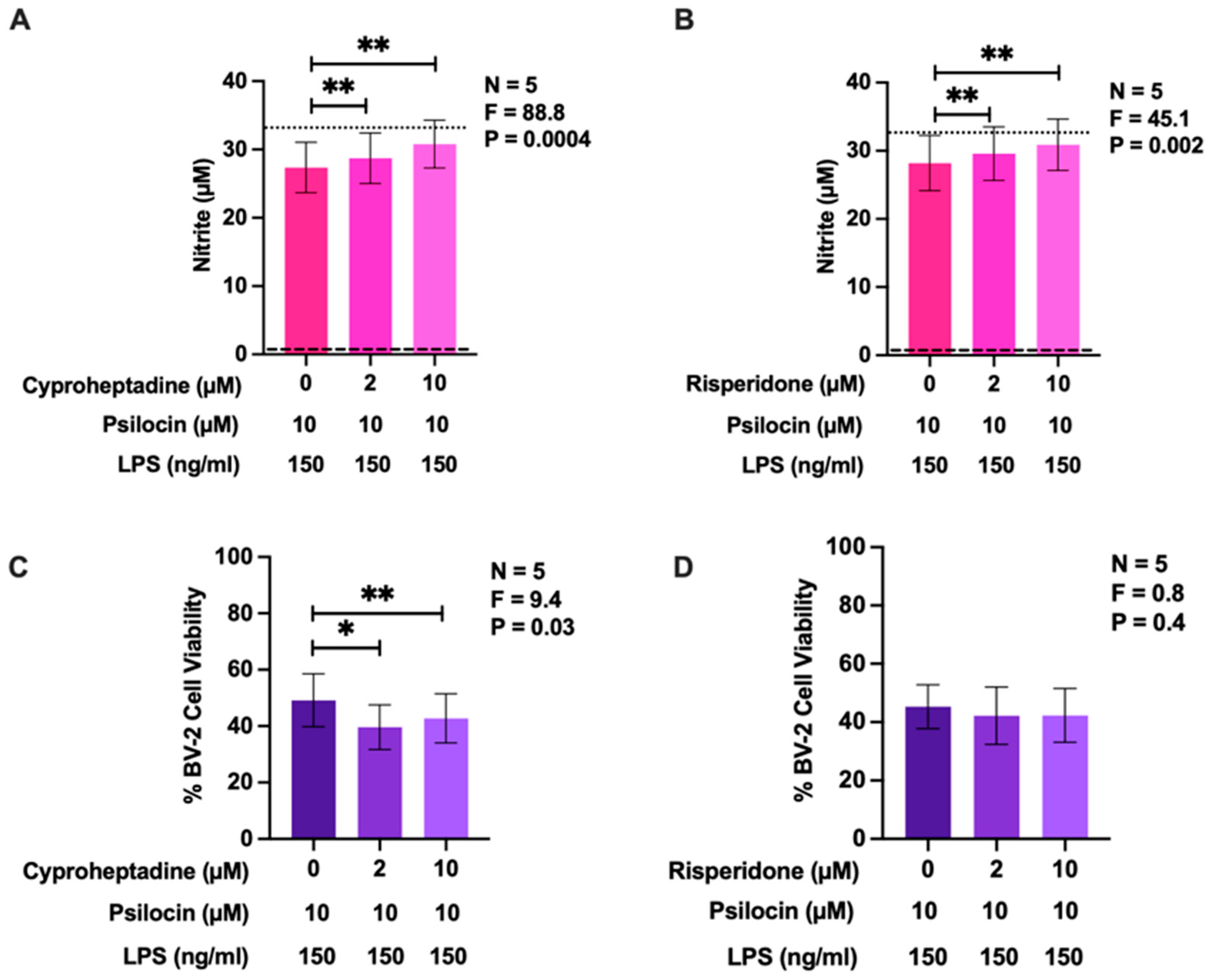
2.5. Effects of 5-HT2 Receptor Antagonists on the Inhibitory Activity of Psilocin
3. Materials and Methods
3.1. Reagents
3.2. Cell Culture
3.3. Measurement of Phagocytic Activity
3.4. Measurement of Nitric Oxide and Tumor Necrosis Factor
3.5. Measurement of Reactive Oxygen Species
3.6. Measurement of Cell Viability
3.7. Statistical Analyses
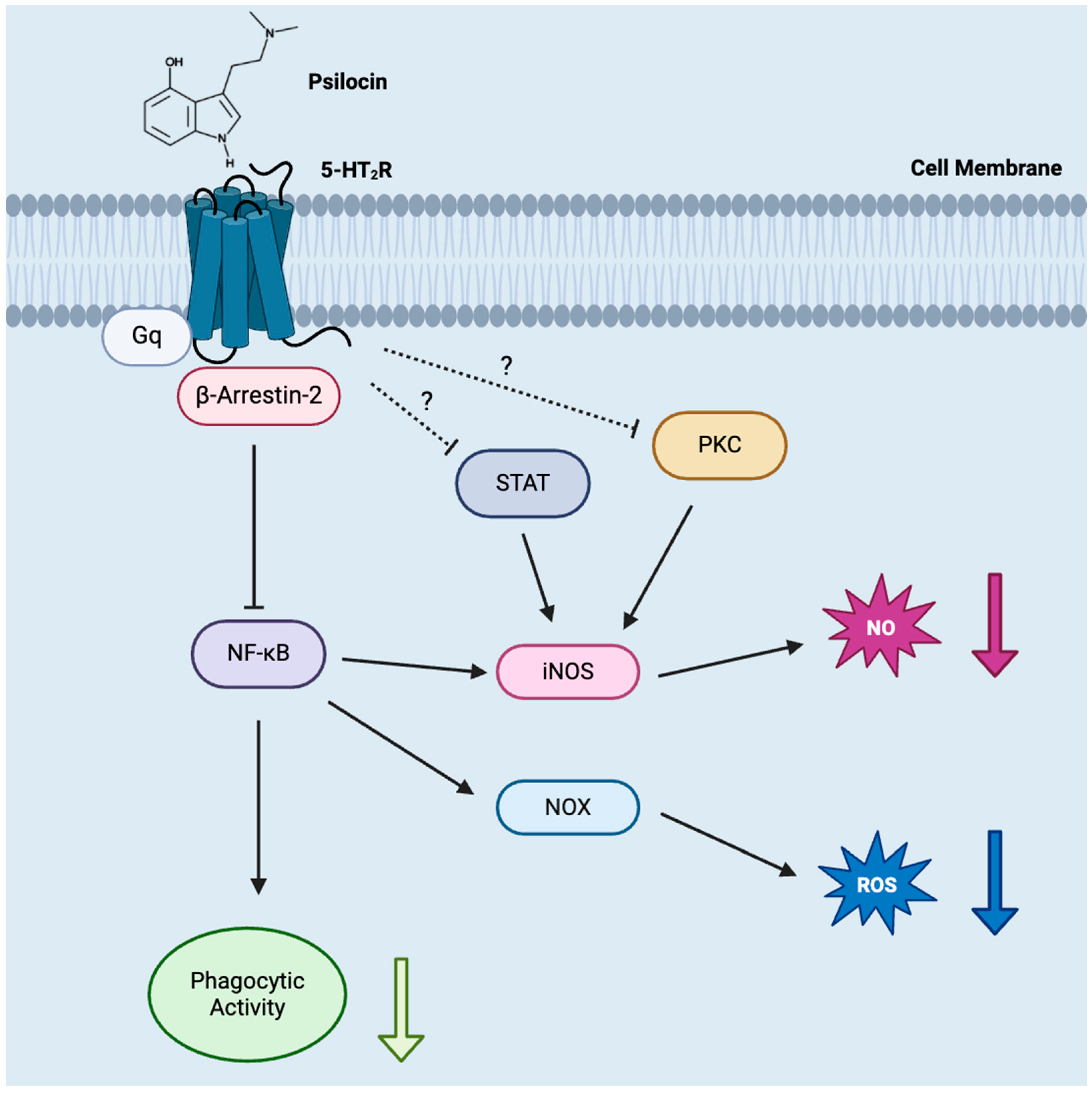
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
Appendix A
References
- Busche, M.A.; Hyman, B.T. Synergy between amyloid-β and tau in Alzheimer’s disease. Nat. Neurosci. 2020, 23, 1183–1193. [Google Scholar] [CrossRef] [PubMed]
- Heneka, M.T.; Carson, M.J.; El Khoury, J.; Landreth, G.E.; Brosseron, F.; Feinstein, D.L.; Jacobs, A.H.; Wyss-Coray, T.; Vitorica, J.; Ransohoff, R.M.; et al. Neuroinflammation in Alzheimer’s disease. Lancet Neurol. 2015, 14, 388–405. [Google Scholar] [CrossRef] [PubMed]
- DeTure, M.A.; Dickson, D.W. The neuropathological diagnosis of Alzheimer’s disease. Mol. Neurodegener. 2019, 14, 32. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Gomez, J.A.; Kavanagh, E.; Engskog-Vlachos, P.; Engskog, M.K.R.; Herrera, A.J.; Espinosa-Oliva, A.M.; Joseph, B.; Hajji, N.; Venero, J.L.; Burguillos, M.A. Microglia: Agents of the CNS pro-inflammatory response. Cells 2020, 9, 1717. [Google Scholar] [CrossRef] [PubMed]
- Butler, C.A.; Popescu, A.S.; Kitchener, E.J.A.; Allendorf, D.H.; Puigdellívol, M.; Brown, G.C. Microglial phagocytosis of neurons in neurodegeneration, and its regulation. J. Neurochem. 2021, 158, 621–639. [Google Scholar] [CrossRef]
- Ganguly, U.; Kaur, U.; Chakrabarti, S.S.; Sharma, P.; Agrawal, B.K.; Saso, L.; Chakrabarti, S. Oxidative stress, neuroinflammation, and NADPH oxidase: Implications in the pathogenesis and treatment of Alzheimer’s disease. Oxid. Med. Cell. Longev. 2021, 2021, 7086512. [Google Scholar] [CrossRef]
- Muzio, L.; Viotti, A.; Martino, G. Microglia in neuroinflammation and neurodegeneration: From understanding to therapy. Front. Neurosci. 2021, 15, 742065. [Google Scholar] [CrossRef]
- Ozben, T.; Ozben, S. Neuro-inflammation and anti-inflammatory treatment options for Alzheimer’s disease. Clin. Biochem. 2019, 72, 87–89. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, Y.; Wang, D.; Zhang, J.; Zhang, F. NSAID exposure and risk of Alzheimer’s disease: An updated meta-analysis from cohort studies. Front. Aging Neurosci. 2018, 10, 83. [Google Scholar] [CrossRef]
- Pasqualetti, P.; Bonomini, C.; Dal Forno, G.; Paulon, L.; Sinforiani, E.; Marra, C.; Zanetti, O.; Maria Rossini, P. A randomized controlled study on effects of ibuprofen on cognitive progression of Alzheimer’s disease. Aging Clin. Exp. Res. 2009, 21, 102–110. [Google Scholar] [CrossRef]
- Wang, J.; Tan, L.; Wang, H.-F.; Tan, C.-C.; Meng, X.-F.; Wang, C.; Tang, S.-W.; Yu, J.-T. Anti-inflammatory drugs and risk of Alzheimer’s disease: An updated systematic review and meta-analysis. J. Alzheimer’s Dis. 2015, 44, 385–396. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Yau, Y.F.; Leung, K.S.; El-Nezami, H.; Lee, J.C.-Y. Interaction of polyphenols as antioxidant and anti-inflammatory compounds in brain–liver–gut axis. Antioxidants 2020, 9, 669. [Google Scholar] [CrossRef] [PubMed]
- Koppula, S.; Kumar, H.; Kim, I.S.; Choi, D.-K. Reactive oxygen species and inhibitors of inflammatory enzymes, NADPH oxidase, and iNOS in experimental models of Parkinson’s disease. Mediat. Inflamm. 2012, 2012, 823902. [Google Scholar] [CrossRef] [PubMed]
- Yagami, T.; Koma, H.; Yamamoto, Y. Pathophysiological roles of cyclooxygenases and prostaglandins in the central nervous system. Mol. Neurobiol. 2016, 53, 4754–4771. [Google Scholar] [CrossRef] [PubMed]
- Crinelli, R.; Antonelli, A.; Bianchi, M.; Gentilini, L.; Scaramucci, S.; Magnani, M. Selective inhibition of NF-KB activation and TNF-α production in macrophages by red blood cell-mediated delivery of dexamethasone. Blood Cells Mol. Dis. 2000, 26, 211–222. [Google Scholar] [CrossRef]
- Flanagan, T.W.; Nichols, C.D. Psychedelics as anti-inflammatory agents. Int. Rev. Psychiatry 2018, 30, 363–375. [Google Scholar] [CrossRef]
- Kozlowska, U.; Nichols, C.; Wiatr, K.; Figiel, M. From Psychiatry to neurology: Psychedelics as prospective sherapeutics for neurodegenerative disorders. J. Neurochem. 2022, 162, 89–108. [Google Scholar] [CrossRef]
- Galecki, P.; Mossakowska-Wojcik, J.; Talarowska, M. The anti-inflammatory mechanism of antidepressants—SSRIs, SNRIs. Prog. Neuropsychopharmacol. Biol. Psychiatry 2018, 80, 291–294. [Google Scholar] [CrossRef]
- Troubat, R.; Barone, P.; Leman, S.; Desmidt, T.; Cressant, A.; Atanasova, B.; Brizard, B.; El Hage, W.; Surget, A.; Belzung, C.; et al. Neuroinflammation and depression: A review. Eur. J. Neurosci. 2021, 53, 151–171. [Google Scholar] [CrossRef]
- Strawbridge, R.; Arnone, D.; Danese, A.; Papadopoulos, A.; Herane Vives, A.; Cleare, A.J. Inflammation and clinical response to treatment in depression: A meta-analysis. Eur. Neuropsychopharmacol. 2015, 25, 1532–1543. [Google Scholar] [CrossRef]
- Kim, Y.-K.; Paik, J.-W.; Lee, S.-W.; Yoon, D.; Han, C.; Lee, B.-H. Increased plasma nitric oxide level associated with suicide attempt in depressive patients. Prog. Neuropsychopharmacol. Biol. Psychiatry 2006, 30, 1091–1096. [Google Scholar] [CrossRef] [PubMed]
- Rybka, J.; Kedziora-Kornatowska, K.; Banas-Lezanska, P.; Majsterek, I.; Carvalho, L.A.; Cattaneo, A.; Anacker, C.; Kedziora, J. Interplay between the pro-oxidant and antioxidant systems and proinflammatory cytokine levels, in relation to iron metabolism and the erythron in depression. Free Radic. Biol. Med. 2013, 63, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Keating, B.A.; Dale, R.C. Anti-inflammatory properties of commonly used psychiatric drugs. Front. Neurosci. 2023, 16, 1039379. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, I.L.; Gonzalez-Prieto, M.; Caso, J.R.; Garcia-Bueno, B.; Leza, J.C.; Madrigal, J.L.M. Reboxetine treatment reduces neuroinflammation and neurodegeneration in the 5xFAD mouse model of Alzheimer’s disease: Role of CCL2. Mol. Neurobiol. 2019, 56, 8628–8642. [Google Scholar] [CrossRef]
- Nykamp, M.J.; Zorumski, C.F.; Reiersen, A.M.; Nicol, G.E.; Cirrito, J.; Lenze, E.J. Opportunities for drug repurposing of serotonin reuptake inhibitors: Potential uses in inflammation, infection, cancer, neuroprotection, and Alzheimer’s disease prevention. Pharmacopsychiatry 2022, 55, 24–29. [Google Scholar] [CrossRef]
- Strauss, D.; Ghosh, S.; Murray, Z.; Gryzenhout, M. An overview on the taxonomy, phylogenetics and ecology of the psychedelic genera Psilocybe, Panaeolus, Pluteus and Gymnopilus. Front. For. Glob. Chang. 2022, 5, 813998. [Google Scholar] [CrossRef]
- Dinis-Oliveira, R.J. Metabolism of psilocybin and psilocin: Clinical and forensic toxicological relevance. Drug Metab. Rev. 2017, 49, 84–91. [Google Scholar] [CrossRef]
- Madsen, M.K.; Fisher, P.M.; Burmester, D.; Dyssegaard, A.; Stenbæk, D.S.; Kristiansen, S.; Johansen, S.S.; Lehel, S.; Linnet, K.; Svarer, C.; et al. Psychedelic effects of psilocybin correlate with serotonin 2A receptor occupancy and plasma psilocin levels. Neuropsychopharmacology 2019, 44, 1328–1334. [Google Scholar] [CrossRef]
- Gonzalez-Maeso, J.; Weisstaub, N.V.; Zhou, M.; Chan, P.; Ivic, L.; Ang, R.; Lira, A.; Bradley-Moore, M.; Ge, Y.; Zhou, Q.; et al. Hallucinogens recruit specific cortical 5-HT2A receptor-mediated signaling pathways to affect behavior. Neuron 2007, 53, 439–452. [Google Scholar] [CrossRef]
- Carhart-Harris, R.; Giribaldi, B.; Watts, R.; Baker-Jones, M.; Murphy-Beiner, A.; Murphy, R.; Martell, J.; Blemings, A.; Erritzoe, D.; Nutt, D.J. Trial of psilocybin versus escitalopram for depression. N. Engl. J. Med. 2021, 384, 1402–1411. [Google Scholar] [CrossRef]
- Goodwin, G.M.; Aaronson, S.T.; Alvarez, O.; Arden, P.C.; Baker, A.; Bennett, J.C.; Bird, C.; Blom, R.E.; Brennan, C.; Brusch, D.; et al. Single-dose psilocybin for a treatment-resistant episode of major depression. N. Engl. J. Med. 2022, 387, 1637–1648. [Google Scholar] [CrossRef] [PubMed]
- Vargas, A.S.; Luis, A.; Barroso, M.; Gallardo, E.; Pereira, L. Psilocybin as a new approach to treat depression and anxiety in the context of life-threatening diseases—A systematic review and meta-analysis of clinical trials. Biomedicines 2020, 8, 331. [Google Scholar] [CrossRef] [PubMed]
- Zanikov, T.; Gerasymchuk, M.; Ghasemi Gojani, E.; Robinson, G.I.; Asghari, S.; Groves, A.; Haselhorst, L.; Nandakumar, S.; Stahl, C.; Cameron, M.; et al. The effect of combined treatment of psilocybin and eugenol on lipopolysaccharide-induced brain inflammation in mice. Molecules 2023, 28, 2624. [Google Scholar] [CrossRef] [PubMed]
- Bos, M.; Jenck, F.; Martin, J.R.; Moreau, J.-L.; Sleight, A.J.; Wichmann, J.; Widmer, U. Novel agonists of 5HT2C receptors. Synthesis and biological evaluation of substituted 2-(Indol-1-Yl)-1-methylethylamines and 2-(indeno[1,2-b]pyrrol-1-Yl)-1-methylethylamines: Improved therapeutics for obsessive compulsive disorder. J. Med. Chem. 1997, 40, 2762–2769. [Google Scholar] [CrossRef]
- Braden, M.R.; Parrish, J.C.; Naylor, J.C.; Nichols, D.E. Molecular interaction of serotonin 5-HT2A receptor residues Phe339(6.51) and Phe340(6.52) with superpotent N-benzyl phenethylamine agonists. Mol. Pharmacol. 2006, 70, 1956–1964. [Google Scholar] [CrossRef]
- Rickli, A.; Moning, O.D.; Hoener, M.C.; Liechti, M.E. Receptor interaction profiles of novel psychoactive tryptamines compared with classic hallucinogens. Eur. Neuropsychopharmacol. 2016, 26, 1327–1337. [Google Scholar] [CrossRef]
- Blair, J.B.; Kurrasch-Orbaugh, D.; Marona-Lewicka, D.; Cumbay, M.G.; Watts, V.J.; Barker, E.L.; Nichols, D.E. Effect of ring fluorination on the pharmacology of hallucinogenic tryptamines. J. Med. Chem. 2000, 43, 4701–4710. [Google Scholar] [CrossRef]
- Arantes, L.C.; Junior, E.F.; de Souza, L.F.; Cardoso, A.C.; Alcantara, T.L.F.; Liao, L.M.; Machado, Y.; Lordeiro, R.A.; Neto, J.C.; Andrade, A.F.B. 25I-NBOH: A new potent serotonin 5-HT2A receptor agonist identified in blotter paper seizures in Brazil. Forensic Toxicol. 2017, 35, 408–414. [Google Scholar] [CrossRef]
- Martin, J.R.; Bos, M.; Jenck, F.; Moreau, J.; Mutel, V.; Sleight, A.J.; Wichmann, J.; Andrews, J.S.; Berendsen, H.H.; Broekkamp, C.L.; et al. 5-HT2C receptor agonists: Pharmacological characteristics and therapeutic potential. J. Pharmacol. Exp. Ther. 1998, 286, 913–924. [Google Scholar]
- Krabbe, G.; Matyash, V.; Pannasch, U.; Mamer, L.; Boddeke, H.W.G.M.; Kettenmann, H. Activation of serotonin receptors promotes microglial injury-induced motility but attenuates phagocytic activity. Brain Behav. Immun. 2012, 26, 419–428. [Google Scholar] [CrossRef]
- Kozlowska, U.; Klimczak, A.; Wiatr, K.; Figiel, M. The DMT and psilocin treatment changes CD11b+ activated microglia immunological phenotype. BioRxiv 2021. [Google Scholar] [CrossRef]
- Laabi, S.; LeMmon, C.; Vogel, C.; Chacon, M.; Jimenez, V.M. Deciphering psilocybin: Cytotoxicity, anti-inflammatory effects, and mechanistic insights. Int. Immunopharmacol. 2024, 130, 111753. [Google Scholar] [CrossRef] [PubMed]
- Mason, N.L.; Szabo, A.; Kuypers, K.P.C.; Mallaroni, P.A.; de la Torre Fornell, R.; Reckweg, J.T.; Tse, D.H.Y.; Hutten, N.R.P.W.; Feilding, A.; Ramaekers, J.G. Psilocybin induces acute and persisting alterations in immune status in healthy volunteers: An experimental, placebo-controlled study. Brain Behav. Immun. 2023, 114, 299–310. [Google Scholar] [CrossRef] [PubMed]
- Bove, G.M.; Mokler, D.J. Effects of a single dose of psilocybin on cytokines, chemokines and leptin in rat serum. J. Psychedelic Stud. 2023, 6, 171–175. [Google Scholar] [CrossRef]
- Bernath, A.K.; Murray, T.E.; Yang, S.; Gibon, J.; Klegeris, A. Microglia secrete distinct sets of neurotoxins in a stimulus-dependent manner. Brain Res. 2023, 1807, 148315. [Google Scholar] [CrossRef]
- Wenzel, T.J.; Gates, E.J.; Ranger, A.L.; Klegeris, A. Short-chain fatty acids (SCFAs) alone or in combination regulate select immune functions of microglia-like cells. Mol. Cell. Neurosci. 2020, 105, 103493. [Google Scholar] [CrossRef]
- Nkadimeng, S.M.; Steinmann, C.M.L.; Eloff, J.N. Effects and safety of Psilocybe Cubensis and Panaeolus Cyanescens magic mushroom extracts on endothelin-1-induced hypertrophy and cell injury in cardiomyocytes. Sci. Rep. 2020, 10, 22314. [Google Scholar] [CrossRef]
- Greuel, B.K.; Da Silva, D.E.; Robert-Gostlin, V.N.; Klegeris, A. Natural compounds oridonin and shikonin exhibit potentially beneficial regulatory effects on select functions of microglia. Brain Sci. 2024, 14, 328. [Google Scholar] [CrossRef]
- Hughes, J.E.; Stewart, J.; Barclay, G.R.; Govan, J.R. Priming of neutrophil respiratory burst activity by lipopolysaccharide from Burkholderia Cepacia. Infect. Immun. 1997, 65, 4281–4287. [Google Scholar] [CrossRef]
- Levy, R.; Rotrosen, D.; Nagauker, O.; Leto, T.L.; Malech, H.L. Induction of the respiratory burst in HL-60 cells. Correlation of Function and Protein Expression. J. Immunol. 1990, 145, 2595–2601. [Google Scholar] [CrossRef]
- Li, C.; Wu, Y.; Huang, M.-Y.; Song, X.-J. Characterization of inflammatory signals in BV-2 microglia in response to Wnt3a. Biomedicines 2023, 11, 1121. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Wu, Z.; Cao, X.; Ding, L.; Wen, Z.; Bian, J.-S. HNO suppresses LPS-induced inflammation in BV-2 microglial cells via inhibition of NF-ΚB and P38 MAPK pathways. Pharmacol. Res. 2016, 111, 885–895. [Google Scholar] [CrossRef] [PubMed]
- Dakik, H.; El Dor, M.; Leclerc, J.; Kouzi, F.; Nehme, A.; Deynoux, M.; Debeissat, C.; Khamis, G.; Ducrocq, E.; Ibrik, A.; et al. Characterization of NADPH oxidase expression and activity in acute myeloid leukemia cell lines: A correlation with the differentiation status. Antioxidants 2021, 10, 498. [Google Scholar] [CrossRef]
- Gouveia, A.; Bajwa, E.; Klegeris, A. Extracellular cytochrome c as an intercellular signaling molecule regulating microglial functions. Biochim. Biophys. Acta—Gen. Subj. 2017, 1861, 2274–2281. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Dixon, B.; Jones, L.; Gorbet, M. The differential reactive oxygen species production of tear neutrophils in response to various stimuli in vitro. Int. J. Mol. Sci. 2021, 22, 12899. [Google Scholar] [CrossRef]
- Kumar, P.; Nagarajan, A.; Uchil, P.D. Analysis of cell viability by the MTT assay. Cold Spring Harb. Protoc. 2018, 2018, pdb.prot095505. [Google Scholar] [CrossRef]
- Nichols, D.E. Psilocybin: From ancient magic to modern medicine. J. Antibiot. 2020, 73, 679–686. [Google Scholar] [CrossRef]
- Watford, T.; Masood, N. Psilocybin, an effective treatment for major depressive disorder in adults—A systematic review. Clin. Psychopharmacol. Neurosci. 2024, 22, 2–12. [Google Scholar] [CrossRef]
- Glebov, K.; Lochner, M.; Jabs, R.; Lau, T.; Merkel, O.; Schloss, P.; Steinhauser, C.; Walter, J. Serotonin stimulates secretion of exosomes from microglia cells. Glia 2015, 63, 626–634. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, K.; Sloan, S.A.; Bennett, M.L.; Scholze, A.R.; O’Keeffe, S.; Phatnani, H.P.; Guarnieri, P.; Caneda, C.; Ruderisch, N.; et al. An RNA-sequencing transcriptome and splicing database of glia, neurons, and vascular vells of the cerebral cortex. J. Neurosci. 2014, 34, 11929–11947. [Google Scholar] [CrossRef]
- Chen, M.-L.; Wu, S.; Tsai, T.-C.; Wang, L.-K.; Tsai, F.-M. Regulation of neutrophil phagocytosis of Escherichia Coli by antipsychotic drugs. Int. Immunopharmacol. 2014, 23, 550–557. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, L.; Paolicelli, R.C. Microglia-mediated synapse loss in Alzheimer’s disease. J. Neurosci. 2018, 38, 2911–2919. [Google Scholar] [CrossRef] [PubMed]
- Brucato, F.H.; Benjamin, D.E. Synaptic pruning in Alzheimer’s disease: Role of the complement system. Glob. J. Med. Res. 2020, 20, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Fu, R.; Shen, Q.; Xu, P.; Luo, J.J.; Tang, Y. Phagocytosis of microglia in the central nervous system diseases. Mol. Neurobiol. 2014, 49, 1422–1434. [Google Scholar] [CrossRef] [PubMed]
- Simoncicova, E.; Goncalves de Andrade, E.; Vecchiarelli, H.A.; Awogbindin, I.O.; Delage, C.I.; Tremblay, M.-E. Present and future of microglial pharmacology. Trends Pharmacol. Sci. 2022, 43, 669–685. [Google Scholar] [CrossRef]
- Brown, G.C. Cell death by phagocytosis. Nat. Rev. Immunol. 2024, 24, 91–102. [Google Scholar] [CrossRef]
- Rudin, D.; Areesanan, A.; Liechti, M.E.; Grundemann, C. Classic psychedelics do not affect T cell and monocyte immune responses. Front. Psychiatry 2023, 14, 1042440. [Google Scholar] [CrossRef]
- Buccellato, F.R.; D’Anca, M.; Fenoglio, C.; Scarpini, E.; Galimberti, D. Role of oxidative damage in Alzheimer’s disease and neurodegeneration: From pathogenic mechanisms to biomarker discovery. Antioxidants 2021, 10, 1353. [Google Scholar] [CrossRef]
- Yu, B.; Becnel, J.; Zerfaoui, M.; Rohatgi, R.; Boulares, A.H.; Nichols, C.D. Serotonin 5-hydroxytryptamine2A receptor activation suppresses tumor necrosis factor-α-induced inflammation with extraordinary potency. J. Pharmacol. Exp. Ther. 2008, 327, 316–323. [Google Scholar] [CrossRef]
- Maura, G.; Marcoli, M.; Pepicelli, O.; Rosu, C.; Viola, C.; Raiteri, M. Serotonin inhibition of the NMDA receptor/nitric oxide/cyclic GMP pathway in human neocortex slices: Involvement of 5-HT 2C and 5-HT 1A receptors. Br. J. Pharmacol. 2000, 130, 1853–1858. [Google Scholar] [CrossRef]
- Aghili-Mehrizi, S.; Williams, E.; Yan, S.; Willman, M.; Willman, J.; Lucke-Wold, B. Secondary mechanisms of neurotrauma: A closer look at the evidence. Diseases 2022, 10, 30. [Google Scholar] [CrossRef] [PubMed]
- Olufunmilayo, E.O.; Gerke-Duncan, M.B.; Holsinger, R.M.D. Oxidative stress and antioxidants in neurodegenerative disorders. Antioxidants 2023, 12, 517. [Google Scholar] [CrossRef] [PubMed]
- Leitner, G.R.; Wenzel, T.J.; Marshall, N.; Gates, E.J.; Klegeris, A. Targeting toll-like receptor 4 to modulate neuroinflammation in central nervous system disorders. Expert Opin. Ther. Targets 2019, 23, 865–882. [Google Scholar] [CrossRef] [PubMed]
- Faure, E.; Equils, O.; Sieling, P.A.; Thomas, L.; Zhang, F.X.; Kirschning, C.J.; Polentarutti, N.; Muzio, M.; Arditi, M. Bacterial lipopolysaccharide activates NF-ΚB through toll-like receptor 4 (TLR-4) in cultured human dermal endothelial cells. J. Biol. Chem. 2000, 275, 11058–11063. [Google Scholar] [CrossRef] [PubMed]
- Sato, M.; Sano, H.; Iwaki, D.; Kudo, K.; Konishi, M.; Takahashi, H.; Takahashi, T.; Imaizumi, H.; Asai, Y.; Kuroki, Y. Direct binding of toll-like receptor 2 to zymosan, and zymosan-induced NF-κB zctivation and TNF-α secretion are down-regulated by lung collectin surfactant protein A. J. Immunol. 2003, 171, 417–425. [Google Scholar] [CrossRef]
- Matsumoto, M.; Seya, T. TLR3: Interferon induction by double-stranded RNA including Poly(I:C). Adv. Drug Deliv. Rev. 2008, 60, 805–812. [Google Scholar] [CrossRef]
- Larkin, J.; Johnson, H.M.; Subramaniam, P.S. Differential nuclear localization of the IFNGR-1 and IFNGR-2 subunits of the IFN-γ receptor complex following activation by IFN-γ. J. Interferon Cytokine Res. 2000, 20, 565–576. [Google Scholar] [CrossRef]
- Belardelli, F. Role of interferons and other cytokines in the regulation of the immune response. APMIS 1995, 103, 161–179. [Google Scholar] [CrossRef]
- Kapur, S.; Zipursky, R.; Jones, C.; Wilson, A.; DaSilva, J.; Houle, S. Cyproheptadine: A potent in vivo serotonin antagonist. Am. J. Psychiatry 1997, 154, 884. [Google Scholar] [CrossRef]
- Schotte, A.; Janssen, P.F.M.; Gommeren, W.; Luyten, W.H.M.L.; Van Gompel, P.; Lesage, A.S.; De Loore, K.; Leysen, J.E. Risperidone compared with new and reference antipsychotic drugs: In vitro and in vivo receptor binding. Psychopharmacology 1996, 124, 57–73. [Google Scholar] [CrossRef]
- Shah, U.H.; Gaitonde, S.A.; Moreno, J.L.; Glennon, R.A.; Dukat, M.; González-Maeso, J. Revised pharmacophore model for 5-HT2A receptor antagonists derived from the atypical antipsychotic agent risperidone. ACS Chem. Neurosci. 2019, 10, 2318–2331. [Google Scholar] [CrossRef] [PubMed]
- Barnes, N.M.; Sharp, T. A review of central 5-HT receptors and their function. Neuropharmacology 1999, 38, 1083–1152. [Google Scholar] [CrossRef] [PubMed]
- Wallach, J.; Cao, A.B.; Calkins, M.M.; Heim, A.J.; Lanham, J.K.; Bonniwell, E.M.; Hennessey, J.J.; Bock, H.A.; Anderson, E.I.; Sherwood, A.M.; et al. Identification of 5-HT2A receptor signaling pathways associated with psychedelic potential. Nat. Commun. 2023, 14, 8221. [Google Scholar] [CrossRef] [PubMed]
- Cheshmehkani, A.; Senatorov, I.S.; Dhuguru, J.; Ghoneim, O.; Moniri, N.H. Free-fatty acid receptor-4 (FFA4) modulates ROS generation and COX-2 expression via the C-terminal β-arrestin phosphosensor in RAW 264.7 macrophages. Biochem. Pharmacol. 2017, 146, 139–150. [Google Scholar] [CrossRef] [PubMed]
- Anrather, J.; Racchumi, G.; Iadecola, C. NF-ΚB regulates phagocytic NADPH oxidase by inducing the expression of Gp91. J. Biol. Chem. 2006, 281, 5657–5667. [Google Scholar] [CrossRef]
- Kolyada, A.Y.; Savikovsky, N.; Madias, N.E. Transcriptional regulation of the human iNOS gene in vascular-smooth-muscle cells and macrophages: Evidence for tissue specificity. Biochem. Biophys. Res. Commun. 1996, 220, 600–605. [Google Scholar] [CrossRef]
- Zhu, F.; Yue, W.; Wang, Y. The nuclear factor kappa B (NF-κB) activation is required for phagocytosis of Staphylococcus Aureus by RAW 264.7 cells. Exp. Cell Res. 2014, 327, 256–263. [Google Scholar] [CrossRef]
- Gojani, E.; Wang, B.; Li, D.; Kovalchuk, O.; Kovalchuk, I. The effects of psilocybin on lipopolysaccharide-induced inflammation in THP-1 human macrophages. Psychoactives 2024, 3, 48–64. [Google Scholar] [CrossRef]
- Stempelj, M.; Kedinger, M.; Augenlicht, L.; Klampfer, L. Essential role of the JAK/STAT1 signaling pathway in the expression of inducible nitric-oxide synthase in intestinal epithelial cells and its regulation by butyrate. J. Biol. Chem. 2007, 282, 9797–9804. [Google Scholar] [CrossRef]
- Hecker, M.; Preib, C.; Klemm, P.; Busse, R. Inhibition by antioxidants of nitric oxide synthase expression in murine macrophages: Role of nuclear factor kB and interferon regulatory factor 1. Br. J. Pharmacol. 1996, 118, 2178–2184. [Google Scholar] [CrossRef]
- Butzlaff, M.; Ponimaskin, E. The role of serotonin receptors in Alzheimer’s disease. Opera Med. Physiol. 2016, 2, 77–86. [Google Scholar]


| Parameter Studied and the Cell Type Used | Assay | Drugs Added 20 min Before Stimulants (Solvent Used in Controls) | Concentrations of Drugs | Stimulants (Solvent Used in Controls) | Concentrations of Stimulants | Duration of Exposure to Stimulants |
|---|---|---|---|---|---|---|
| Phagocytic activity of BV-2 murine microglia | Engulfment of fluorescent latex beads | Psilocin (20% v/v acetonitrile) | 10 µM | LPS (PBS) | 400 ng/mL | 24 h |
| TNF secretion by BV-2 murine microglia | ELISA | Psilocin (20% v/v acetonitrile) | 0.1–10 µM | LPS (PBS) | 150 ng/mL | 24 h |
| LPS + IFN (PBS) | 150 ng/mL + 30 ng/mL | |||||
| Production of NO by BV-2 murine microglia | Griess assay | Psilocin (20% v/v acetonitrile) | 0.01–10 µM | LPS (PBS) | 150 ng/mL | 24 h |
| IFN (PBS) | 30 ng/mL | |||||
| 25I-NBOH (DMSO) | 0.1–3 µM | LPS + IFN (PBS) | 150 ng/mL + 30 ng/mL | |||
| Zymosan A (PBS) | 10 µg/mL | |||||
| Ro60-0175 (DMSO) | 0.1–10 µM | Poly (I:C) (deionized H2O) | 10 µg/mL | |||
| Zymosan (PBS) + Poly (I:C) (deionized H2O) | 10 µg/mL + 10 µg/mL | |||||
| Generation of ROS by DMSO-differentiated human HL-60 cells | Luminol-dependent chemiluminescence | Psilocin (20% v/v acetonitrile) | 0.01–10 µM | Priming: LPS (PBS) Stimulation: fMLP (PBS) | 500 ng/mL 1 µM | 24 h 25 min |
| 25I-NBOH (DMSO) | 0.1–3 µM | |||||
| Ro60-0175 (DMSO) | 0.1–10 µM | |||||
| Viability of all cell types | MTT assay | Psilocin (20% v/v acetonitrile) 25I-NBOH (DMSO) Ro60-0175 (DMSO) | Correspond to the functional assays listed above | Correspond to the functional assays listed above | Correspond to the functional assays listed above | 24 h |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wiens, K.R.; Brooks, N.A.H.; Riar, I.; Greuel, B.K.; Lindhout, I.A.; Klegeris, A. Psilocin, the Psychoactive Metabolite of Psilocybin, Modulates Select Neuroimmune Functions of Microglial Cells in a 5-HT2 Receptor-Dependent Manner. Molecules 2024, 29, 5084. https://doi.org/10.3390/molecules29215084
Wiens KR, Brooks NAH, Riar I, Greuel BK, Lindhout IA, Klegeris A. Psilocin, the Psychoactive Metabolite of Psilocybin, Modulates Select Neuroimmune Functions of Microglial Cells in a 5-HT2 Receptor-Dependent Manner. Molecules. 2024; 29(21):5084. https://doi.org/10.3390/molecules29215084
Chicago/Turabian StyleWiens, Kennedy R., Noah A. H. Brooks, Ishvin Riar, Bridget K. Greuel, Ivan A. Lindhout, and Andis Klegeris. 2024. "Psilocin, the Psychoactive Metabolite of Psilocybin, Modulates Select Neuroimmune Functions of Microglial Cells in a 5-HT2 Receptor-Dependent Manner" Molecules 29, no. 21: 5084. https://doi.org/10.3390/molecules29215084
APA StyleWiens, K. R., Brooks, N. A. H., Riar, I., Greuel, B. K., Lindhout, I. A., & Klegeris, A. (2024). Psilocin, the Psychoactive Metabolite of Psilocybin, Modulates Select Neuroimmune Functions of Microglial Cells in a 5-HT2 Receptor-Dependent Manner. Molecules, 29(21), 5084. https://doi.org/10.3390/molecules29215084






