20 (S)-Protopanaxadiol Alleviates DRP1-Mediated Mitochondrial Dysfunction in a Depressive Model In Vitro and In Vivo via the SIRT1/PGC-1α Signaling Pathway
Abstract
1. Introduction
2. Results
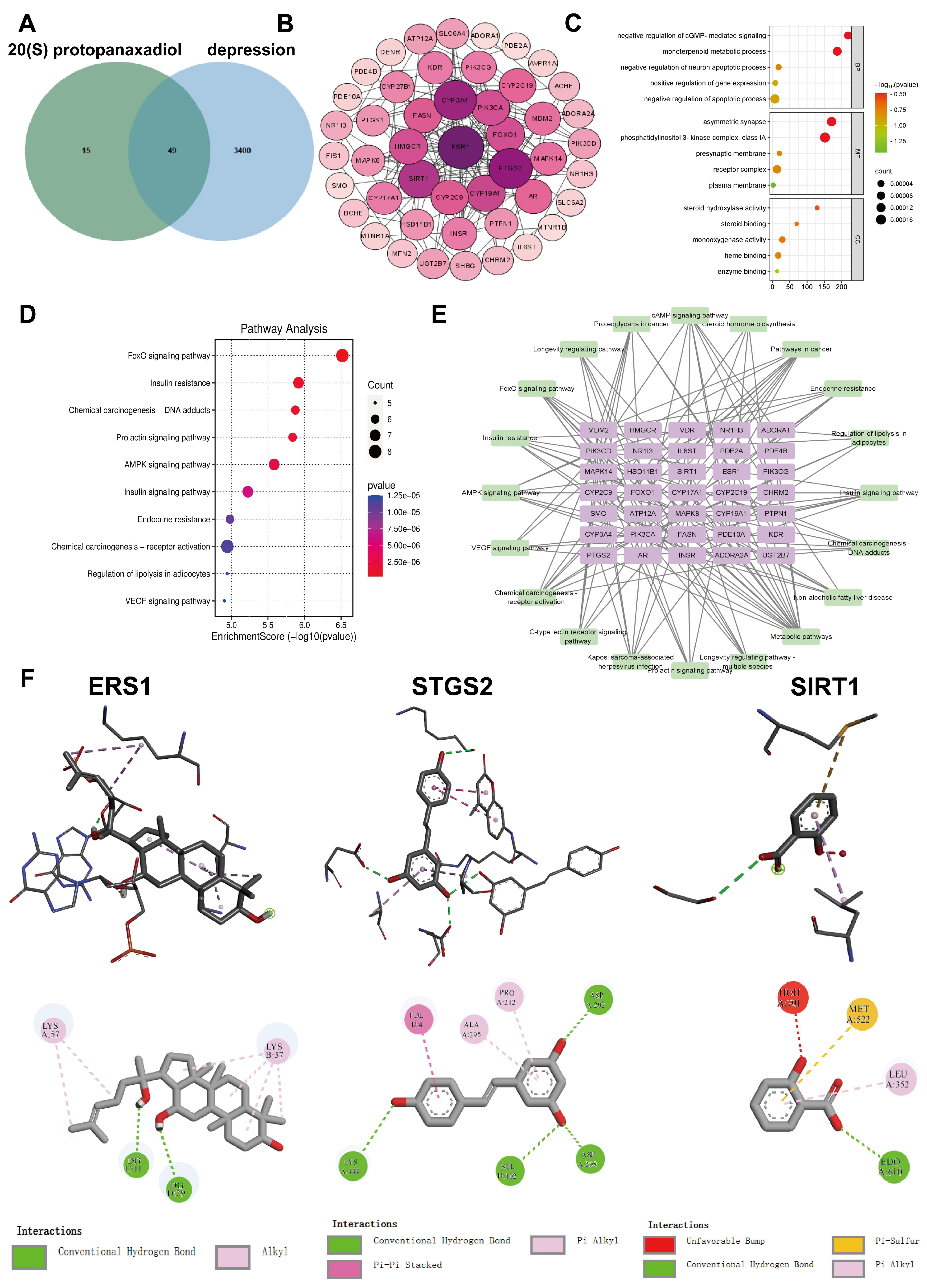
2.1. Network Pharmacology and Molecular Docking Analysis
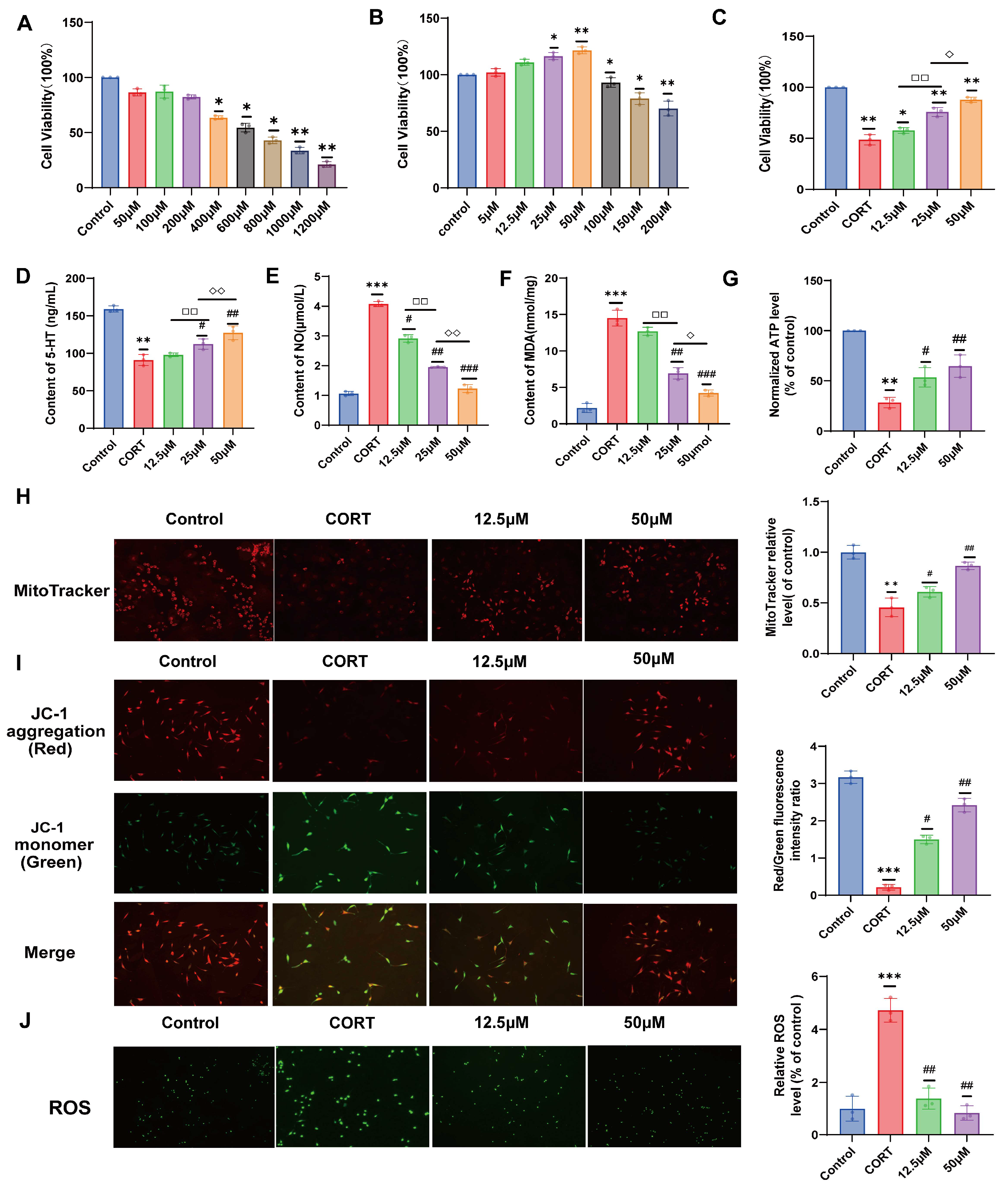
2.2. Effect of 20 (S)-Protopanaxadiol on CORT-Induced HT22 Cell Viability
2.3. Effects of 20 (S)-Protopanaxadiol on 5-HT, NO, and MDA Secretion in HT22 Cells Induced by CORT
2.4. Effect of 20 (S)-Protopanaxadiol on Mitochondrial Content and Function of HT22 Cells Induced by CORT
2.5. Effects of 20 (S)-Protopanaxadiol on Mitochondrial ROS Activation Induced by CORT in HT22 Cells
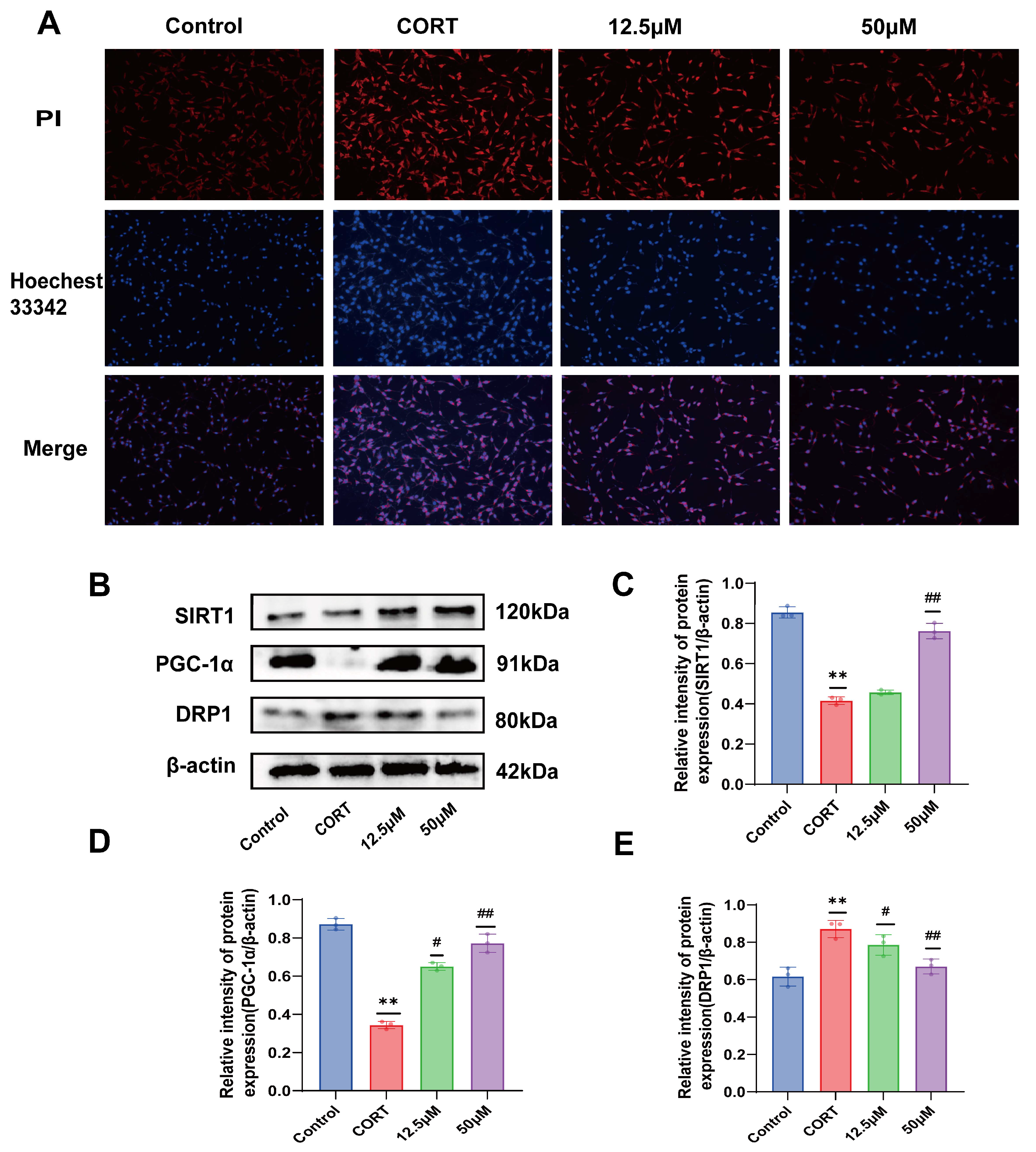
2.6. Effect of 20 (S)-Protopanaxadiol on CORT-Induced Apoptosis of HT22 Cells
2.7. Effect of 20 (S)-Protopanaxadiol on CORT-Induced Mitochondrial Dynamics-Related Proteins in HT22 Cells
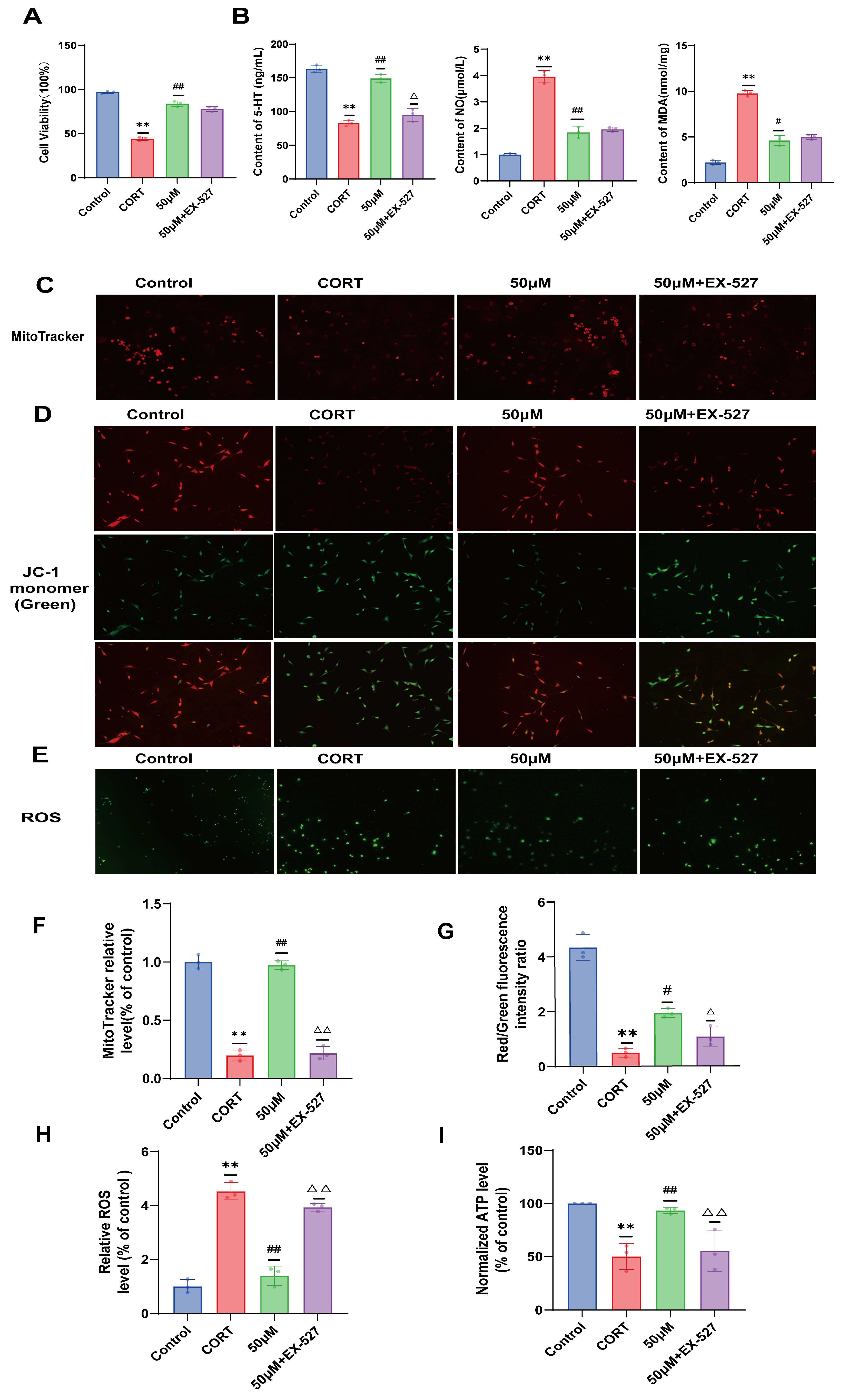
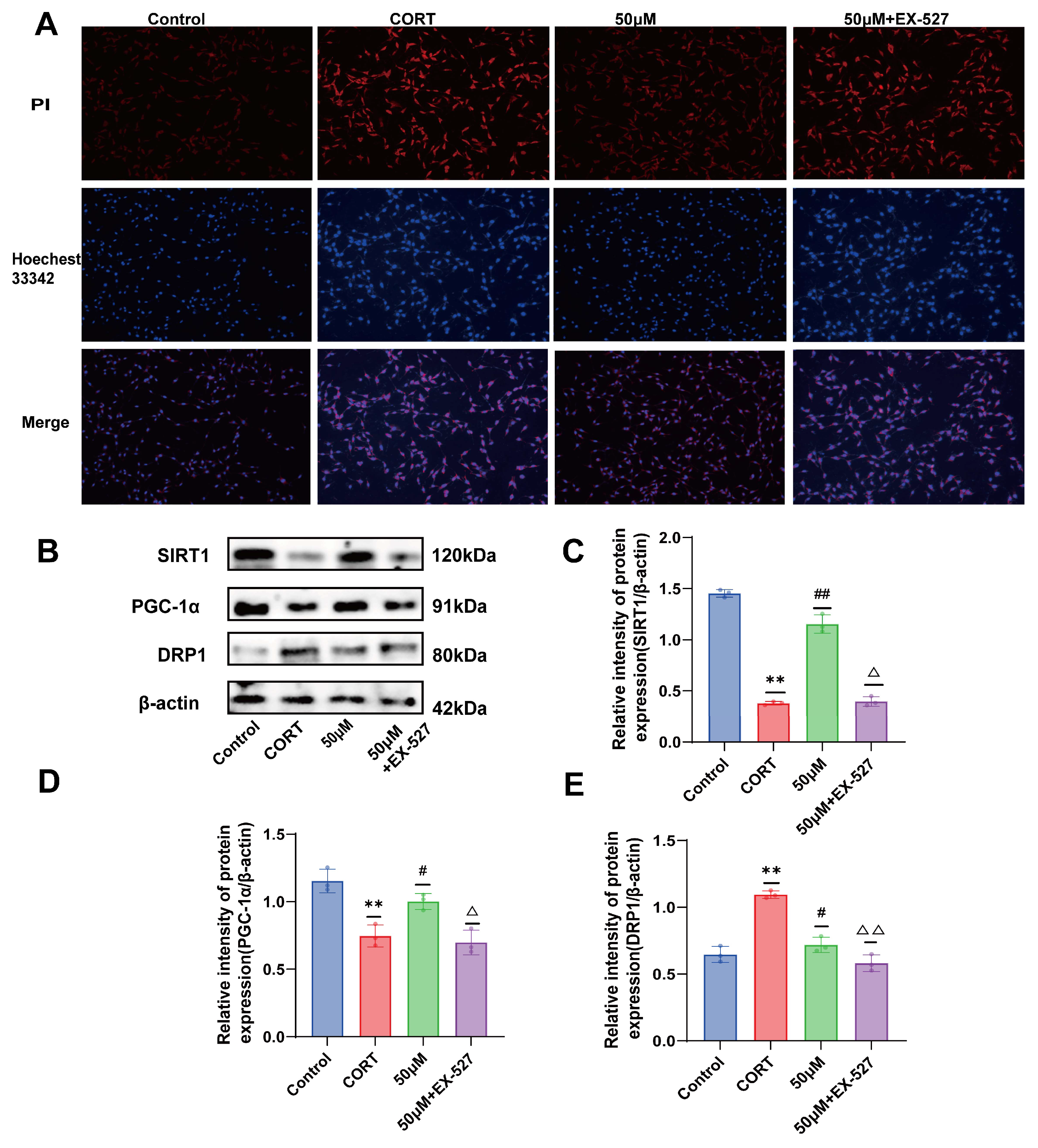
2.8. Verification of the Effect of 20 (S)-Protopanaxadiol on Cell Viability and Depression-Related Indicators Using SIRT1 Inhibitor EX-527
2.9. Effects of 20 (S)-Protopanaxadiol on Mitochondrial Content and Mitochondrial Function in Fine Cells Were Validated Using the SIRT1 Inhibitor EX-527
2.10. Verification of the Effect of 20 (S)-Protopanaxadiol on Apoptosis Using SIRT1 Inhibitor EX-527
2.11. Validation of the Effect of 20 (S)-Protopanaxadiol on Mitochondrial Dynamics-Related Proteins Using SIRT1 Inhibitor EX-527
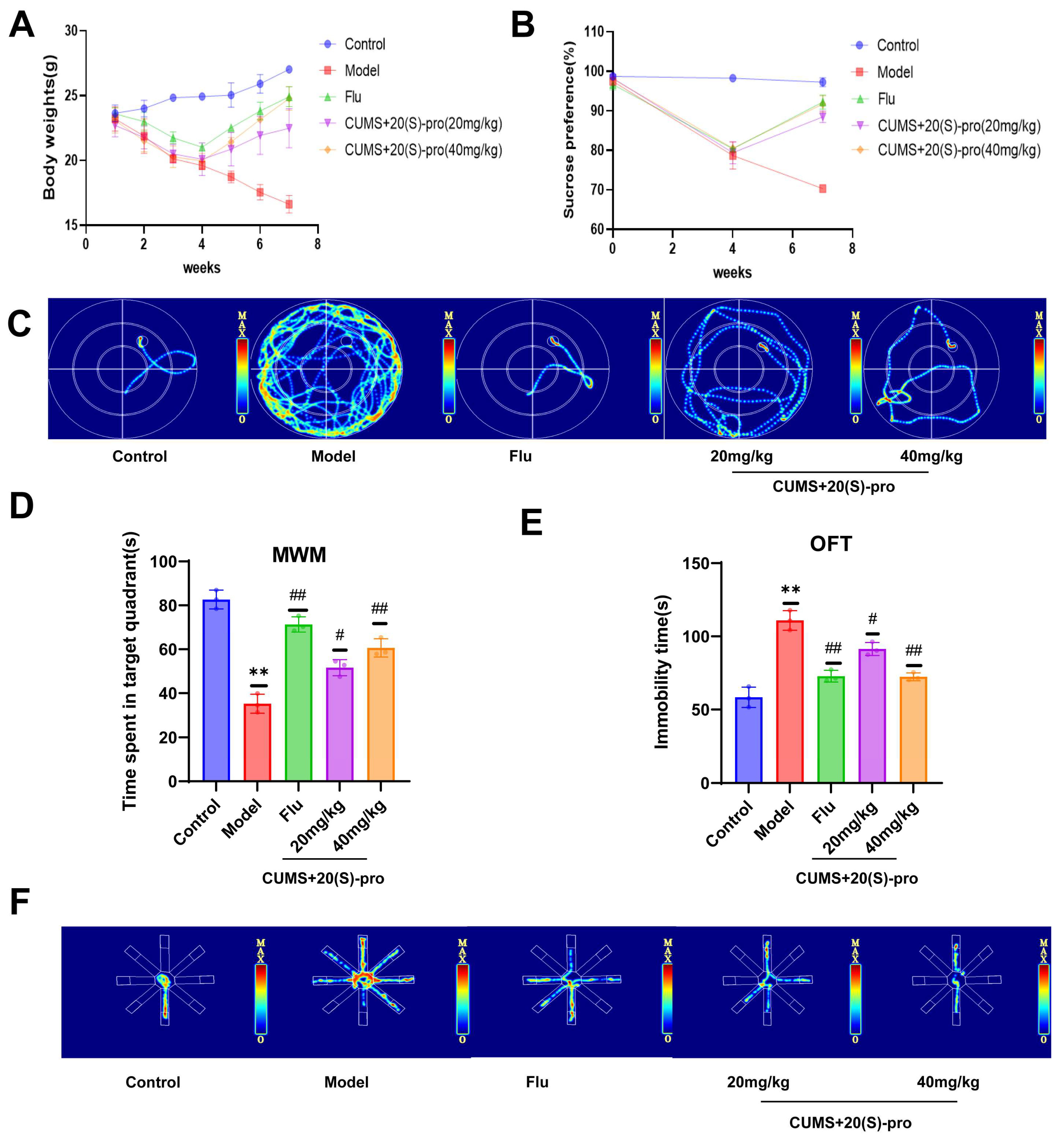
2.12. Behavioral Results
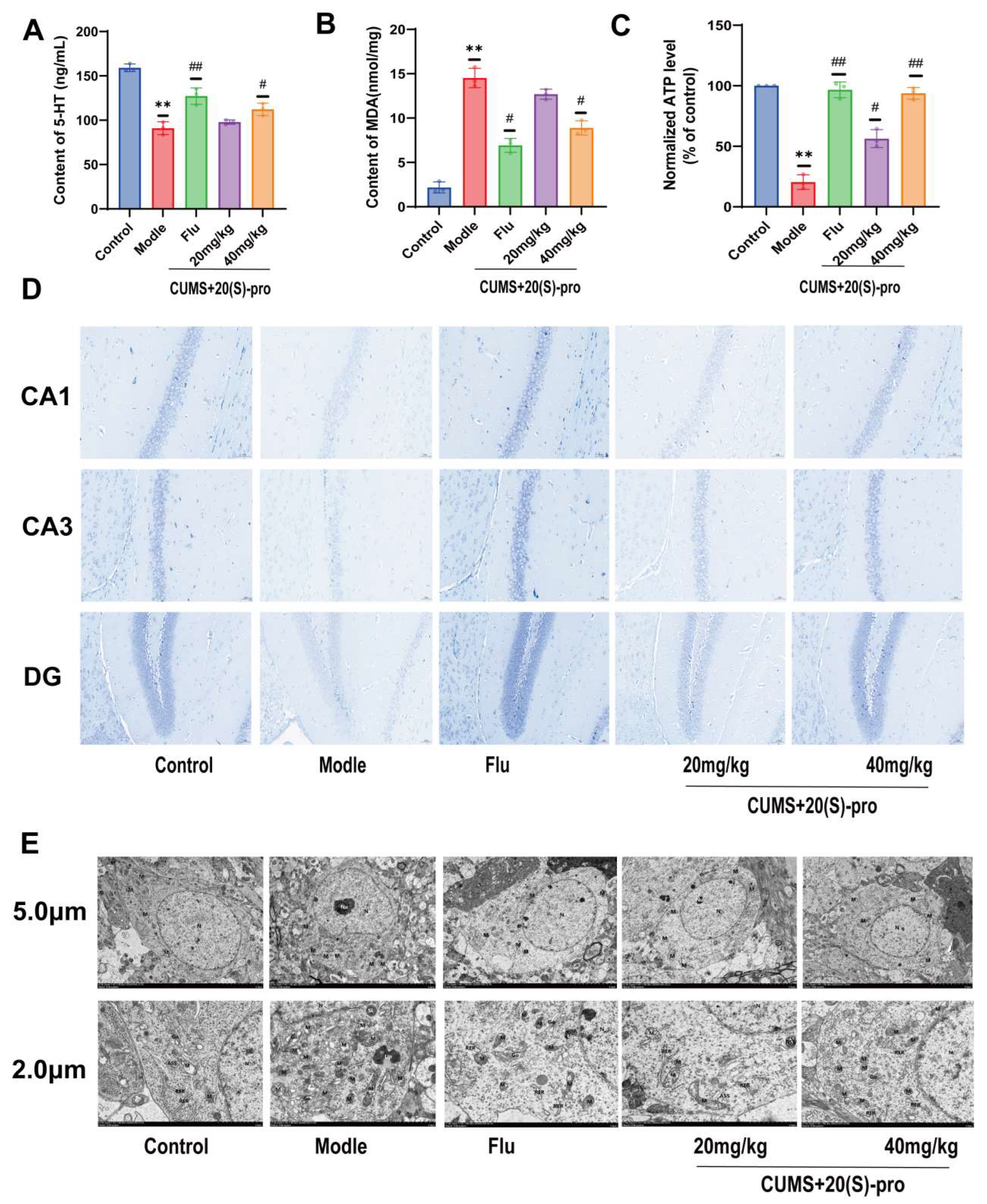
2.13. Expression of 5-HT, MDA, and ATP in Serum
2.14. Effect of 20 (S)-Protopanaxadiol on CUMS-Induced Hippocampal Neuronal Function in Mice
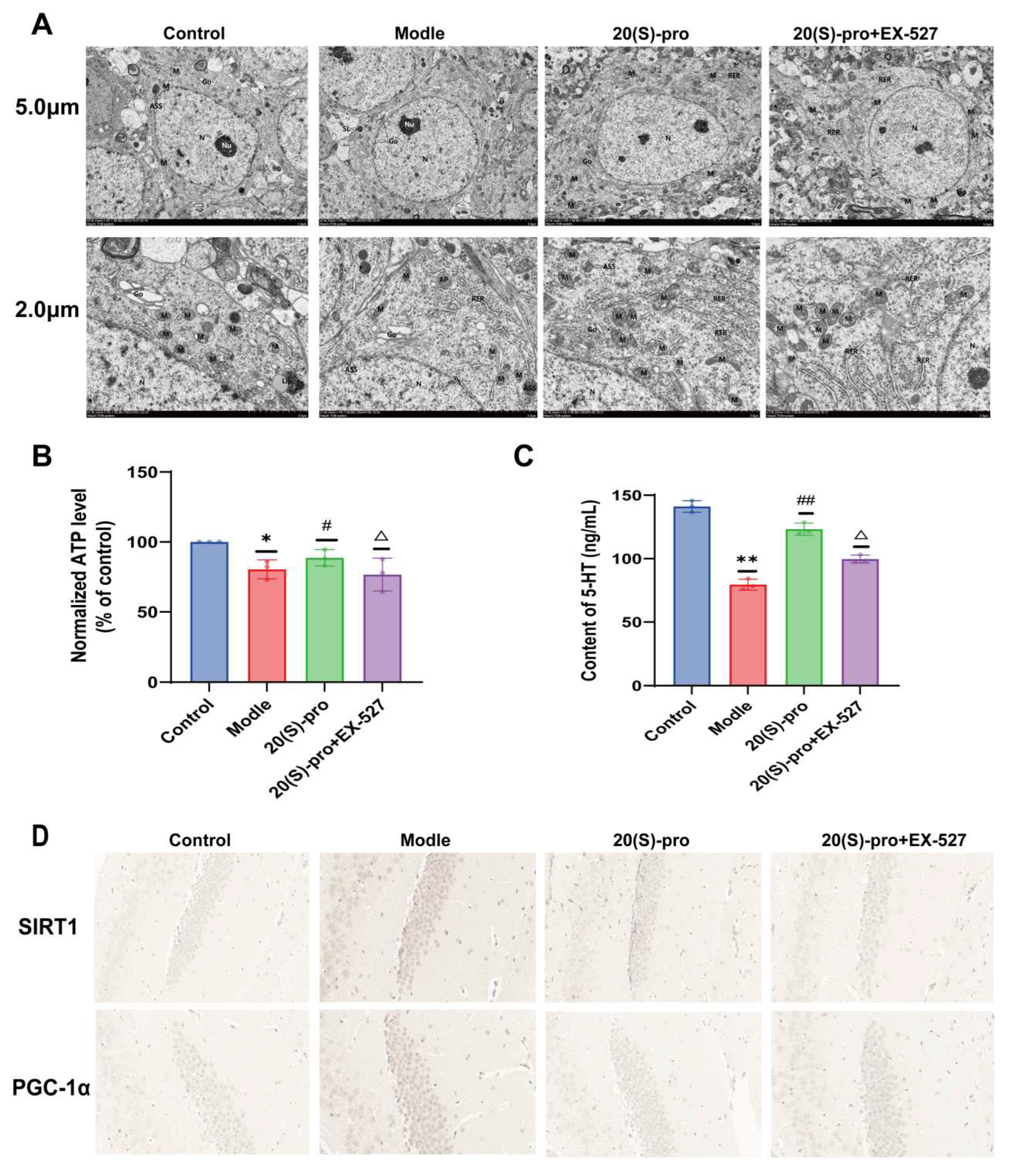
2.15. Effect of 20 (S)-Protopanaxadiol on Mitochondrial Morphology and Related Functions in the Hippocampus of Mice
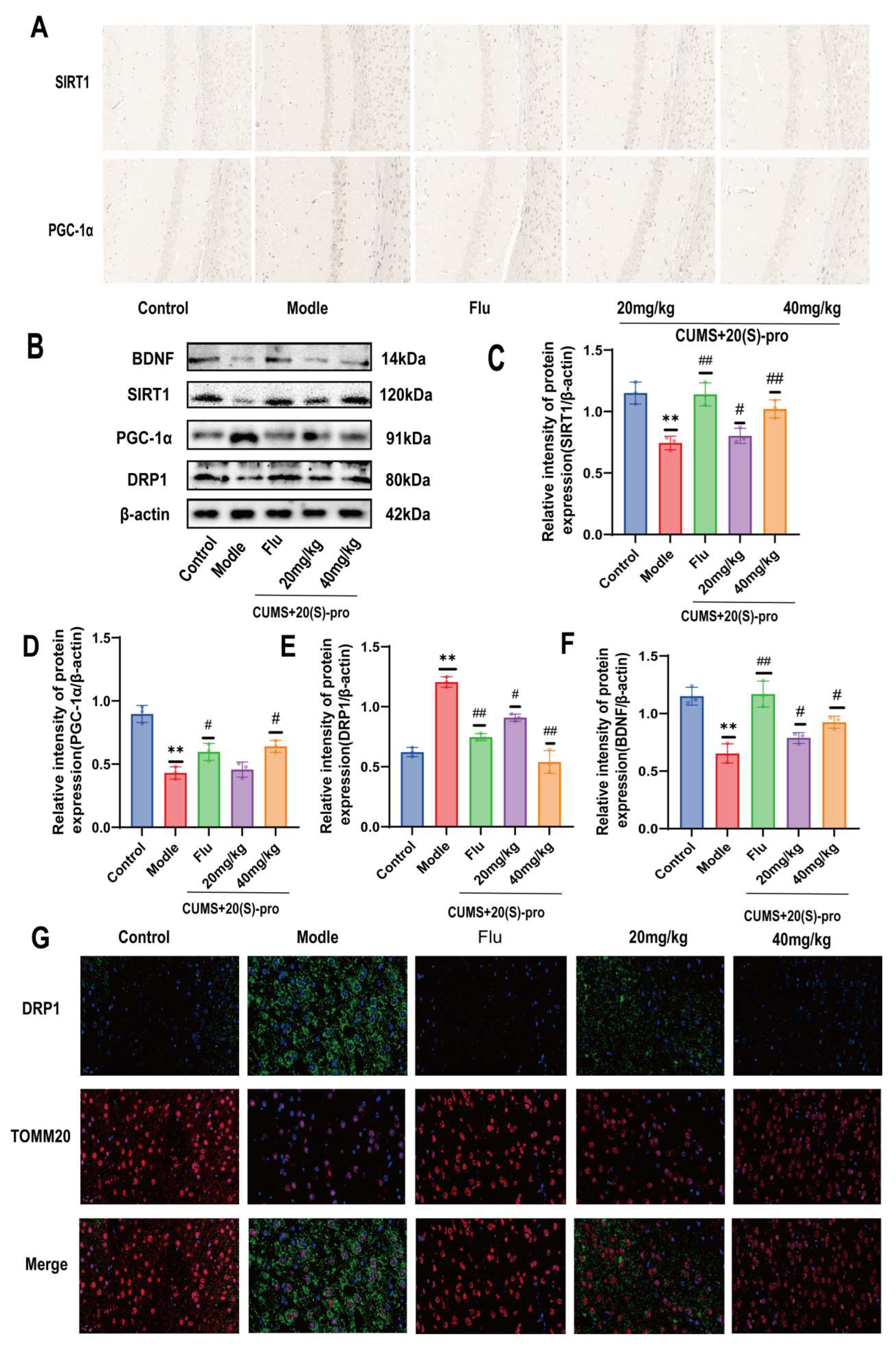
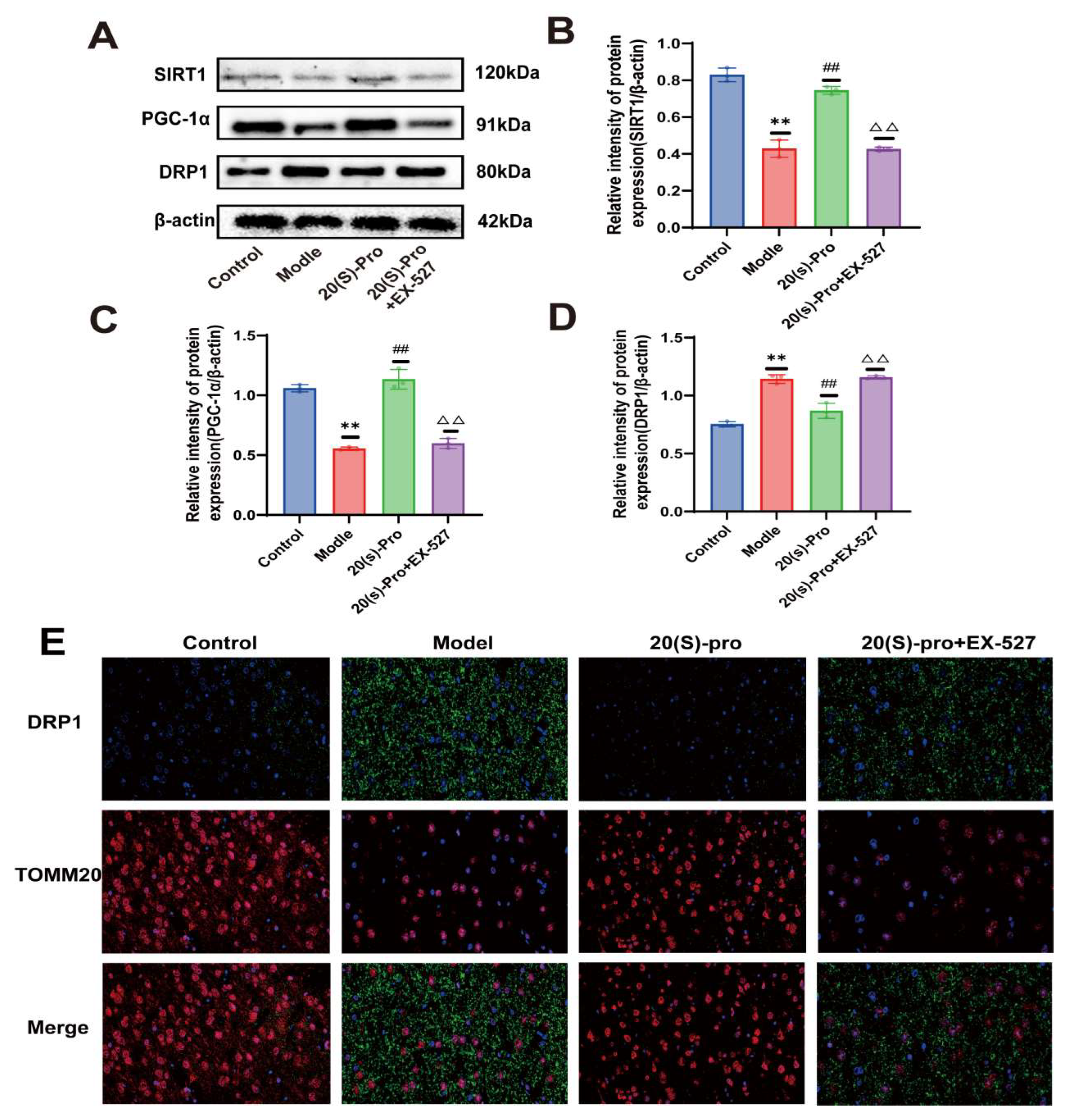
2.16. Effect of 20 (S)-Protopanaxadiol on Mitochondrial Dynamics-Related Protein Expression
2.17. SIRT1 Inhibitor EX-527 Verified the Effect of 20 (S)-Protopanaxadiol on Mitochondrial Ultrastructure and Function
2.18. SIRT1 Inhibitor EX-527 Verified the Effect of 20 (S)-Protopanaxadiol on the Expression of Mitochondrial Dynamics-Related Proteins
3. Materials and Methods
3.1. Materials
3.2. Target Acquisition of 20 (S)-Protopanaxadiol and Depression
3.3. Construction of the PPI Network
3.4. GO Functional Enrichment Analysis and KEGG Pathway Enrichment Analysis
3.5. Construction of the “Genes-Pathway” Network Map
3.6. Molecular Docking
3.7. Screening of Optimal CORT Molding Concentration and Optimal Administration of 20 (S)-Protopanaxadiol
3.8. Cell Modeling, Grouping, and Administration
3.9. Determination of 5-HT, NO, and MDA Expression in HT22 Cells
3.10. ATP Content Detection in Hippocampus and Cells
3.11. Determination of Mitochondrial Membrane Potential and ROS Production
3.12. MitoTracker Red CMXRos Labeling Mitochondria
3.13. Apoptosis Assays
3.14. Experimental Animals and Study Design
3.15. Sucrose Preference Test (SPT)
3.16. Morris Water Maze (MWM)
3.17. Open Field Test (OFT)
3.18. 8-Arm Maze
3.19. Nissl Staining
3.20. Transmission Electron Microscopy (TEM)
3.21. Immunohistochemical Staining
3.22. Immunofluorescence
3.23. Enzyme-Linked Immunosorbent Assay (ELISA)
3.24. Western Blot Analysis
3.25. Statistical Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yoon, S.; Iqbal, H.; Kim, S.M.; Jin, M. Phytochemicals That Act on Synaptic Plasticity as Potential Prophylaxis against Stress-Induced Depressive Disorder. Biomol. Ther. 2023, 31, 148–160. [Google Scholar] [CrossRef] [PubMed]
- Tokuda, T.; Yoshimoto, J.; Shimizu, Y.; Okada, G.; Takamura, M.; Okamoto, Y.; Yamawaki, S.; Doya, K. Identification of depression subtypes and relevant brain regions using a data-driven approach. Sci. Rep. 2018, 8, 14082. [Google Scholar] [CrossRef] [PubMed]
- Ménard, C.; Hodes, G.E.; Russo, S.J. Pathogenesis of depression: Insights from human and rodent studies. Neuroscience 2016, 321, 138–162. [Google Scholar] [CrossRef] [PubMed]
- Möller, H.J. Suicide, suicidality and suicide prevention in affective disorders. Acta Psychiatr. Scand. 2003, 108, 73–80. [Google Scholar] [CrossRef]
- Jiang, N.; Zhang, B.Y.; Dong, L.M.; Lv, J.W.; Lu, C.; Wang, Q.; Fan, L.X.; Zhang, H.X.; Pan, R.L.; Liu, X.M. Antidepressant effects of dammarane sapogenins in chronic unpredictable mild stress-induced depressive mice. Phytother. Res. PTR 2018, 32, 1023–1029. [Google Scholar] [CrossRef]
- Gong, W.; Xu, S.; Song, Y.; Zhou, Y.; Qin, X. Hepatic metabolomics combined with network pharmacology to reveal the correlation between the anti-depression effect and nourishing blood effect of Angelicae Sinensis Radix. Chin. J. Nat. Med. 2023, 21, 197–213. [Google Scholar] [CrossRef]
- Xia, C.Y.; Xu, J.K.; Li, L.; Lian, W.W.; Yan, Y.; Ma, B.Z.; He, J.; Zhang, W.-K. Identifying the mechanism underlying antidepressant-like effects of loganin by network pharmacology in combination with experimental validation. J. Ethnopharmacol. 2021, 281, 114526. [Google Scholar] [CrossRef]
- Bai, Y.; Niu, L.; Song, L.; Dai, G.; Zhang, W.; He, B.; San, W.; Li, S. Uncovering the effect and mechanism of Jiawei Xiaoyao Wan in treating breast cancer complicated with depression based on network pharmacology and experimental analysis. Phytomed. Int. J. Phytother. Phytopharm. 2024, 128, 155427. [Google Scholar] [CrossRef]
- Yeung, W.F.; Chung, K.F.; Ng, K.Y.; Yu, Y.M.; Ziea, E.T.; Ng, B.F. A systematic review on the efficacy, safety and types of Chinese herbal medicine for depression. J. Psychiatr. Res. 2014, 57, 165–175. [Google Scholar] [CrossRef]
- Kim, Y.C.; Kim, S.R.; Markelonis, G.J.; Oh, T.H. Ginsenosides Rb1 and Rg3 protect cultured rat cortical cells from glutamate-induced neurodegeneration. J. Neurosci. Res. 1998, 53, 426–432. [Google Scholar] [CrossRef]
- Wang, W.; Wang, L.; Wang, L.; Li, Y.; Lan, T.; Wang, C.; Chen, X.; Chen, S.; Yu, S. Ginsenoside-Rg1 synergized with voluntary running exercise protects against glial activation and dysregulation of neuronal plasticity in depression. Food Funct. 2023, 14, 7222–7239. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Cheng, Y.; Han, X.; Wang, T.; Zhang, H.; Yao, Q.; Chen, F.; Gu, L.; Yang, D.; Chen, L.; et al. 20 (S)-Protopanaxadiol Exerts Antidepressive Effects in Chronic Corticosterone-Induced Rodent Animal Models as an Activator of Brain-Type Creatine Kinase. J. Agric. Food Chem. 2024, 72, 10376–10390. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Teng, J.; Chen, W.; Ge, Q.; Yang, Z.; Yu, C.; Yang, Z.; Jia, W. 20 (S)-Protopanaxadiol, an active ginseng metabolite, exhibits strong antidepressant-like effects in animal tests. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2010, 34, 1402–1411. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Du, Z.; Lu, R.; Zhou, Q.; Shen, Y.; Jiang, Y. Investigating the Mechanism of Chufan Yishen Formula in Treating Depression through Network Pharmacology and Experimental Verification. ACS Omega 2024, 9, 12698–12710. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Jiang, M.; Yan, S.; Liang, M.; Wang, W.; Yuan, B.; Xu, Q. Network Pharmacology-Based and Experimental Identification of the Effects of Paeoniflorin on Major Depressive Disorder. Front. Pharmacol. 2021, 12, 793012. [Google Scholar] [CrossRef]
- Li, S.; Zhang, B. Traditional Chinese medicine network pharmacology: Theory, methodology and application. Chin. J. Nat. Med. 2013, 11, 110–120. [Google Scholar] [CrossRef]
- Shi, Y.; Chen, M.; Zhao, Z.; Pan, J.; Huang, S. Network Pharmacology and Molecular Docking Analyses of Mechanisms Underlying Effects of the Cyperi Rhizoma-Chuanxiong Rhizoma Herb Pair on Depression. Evid.-Based Complement. Altern. Med. Ecam 2021, 2021, 5704578. [Google Scholar] [CrossRef]
- Bansal, Y.; Kuhad, A. Mitochondrial Dysfunction in Depression. Curr. Neuropharmacol. 2016, 14, 610–618. [Google Scholar] [CrossRef]
- Khan, M.; Baussan, Y.; Hebert-Chatelain, E. Connecting Dots between Mitochondrial Dysfunction and Depression. Biomolecules 2023, 13, 695. [Google Scholar] [CrossRef]
- Hoppins, S.; Lackner, L.; Nunnari, J. The machines that divide and fuse mitochondria. Annu. Rev. Biochem. 2007, 76, 751–780. [Google Scholar] [CrossRef]
- Chen, H.; Wu, C.; Lv, Q.; Li, M.; Ren, L. Targeting Mitochondrial Homeostasis: The Role of Acupuncture in Depression Treatment. Neuropsychiatr. Dis. Treat. 2023, 19, 1741–1753. [Google Scholar] [CrossRef] [PubMed]
- Han, Q.; Han, L.; Tie, F.; Wang, Z.; Ma, C.; Li, J.; Wang, H.; Li, G. (20S)-Protopanaxadiol Ginsenosides Induced Cytotoxicity via Blockade of Autophagic Flux in HGC-27 Cells. Chem. Biodivers. 2020, 17, e2000187. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Wang, Y.; Zhou, X.; Pan, X.; Lü, J.; Sun, H.; Xie, Z.; Chen, S.; Gao, X. The anti-tumor efficacy of 20 (S)-Protopanaxadiol, an active metabolite of ginseng, according to fasting on hepatocellular carcinoma. J. Ginseng Res. 2022, 46, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Teng, B.; Jiang, J.; Zhao, L.; Gao, J.; Chen, J.; Liu, Z.; Wang, H.; Lu, B. Ginsenoside PPD’s Antitumor Effect via Down-Regulation of mTOR Revealed by Super-Resolution Imaging. Molecules 2017, 22, 486. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Liu, X.; Wang, T.; Yu, J.; Wang, M.; Sun, J.; Yu, X.; Niu, N.; Chen, L. HPLC Fingerprint Combined with Chemometrics and Network Pharmacology for Q-Markers Prediction Analysis of Saposhnikovia divaricata. J. Anal. Test. 2024, 8, 83–94. [Google Scholar] [CrossRef]
- Yu, Z.; Kong, D.; Liang, Y.; Zhao, X.; Du, G. Protective effects of VMY-2-95 on corticosterone-induced injuries in mice and cellular models. Acta Pharm. Sin. B 2021, 11, 1903–1913. [Google Scholar] [CrossRef] [PubMed]
- Ye, P.; Li, W.; Huang, X.; Zhao, S.; Chen, W.; Xia, Y.; Yu, W.; Rao, T.; Ning, J.; Zhou, X.; et al. BMAL1 regulates mitochondrial homeostasis in renal ischaemia-reperfusion injury by mediating the SIRT1/PGC-1α axis. J. Cell. Mol. Med. 2022, 26, 1994–2009. [Google Scholar] [CrossRef]
- Sun, X.; Zhang, T.; Zhao, Y.; Cai, E.; Zhu, H.; Liu, S. Panaxynol attenuates CUMS-induced anxiety and depressive-like behaviors via regulating neurotransmitters, synapses and the HPA axis in mice. Food Funct. 2020, 11, 1235–1244. [Google Scholar] [CrossRef]
- Du, R.H.; Tan, J.; Sun, X.Y.; Lu, M.; Ding, J.H.; Hu, G. Fluoxetine Inhibits NLRP3 Inflammasome Activation: Implication in Depression. Int. J. Neuropsychopharmacol. 2016, 19, pyw037. [Google Scholar] [CrossRef]
- Shu, X.; Sun, Y.; Sun, X.; Zhou, Y.; Bian, Y.; Shu, Z.; Ding, J.; Lu, M.; Hu, G. The effect of fluoxetine on astrocyte autophagy flux and injured mitochondria clearance in a mouse model of depression. Cell Death Dis. 2019, 10, 577. [Google Scholar] [CrossRef]
- Jiang, N.; Lv, J.; Wang, H.; Huang, H.; Wang, Q.; Zeng, G.; Li, S.; Liu, X. Ginsenoside 20 (S)-Protopanaxadiol attenuates depressive-like behaviour and neuroinflammation in chronic unpredictable mild stress-induced depressive rats. Behav. Brain Res. 2020, 393, 112710. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Yu, Q.; Sui, D.; Yu, X.; Xu, H.; Li, M. 20 (S)-Protopanaxadiol Alleviates Myocardial Ischemia/Reperfusion Injury in Rats Through Suppression of Oxidative Stress and Apoptosis. Nat. Prod. Commun. 2021, 16, 1934578X211029528. [Google Scholar] [CrossRef]
- Gao, J.L.; Lv, G.Y.; He, B.C.; Zhang, B.Q.; Zhang, H.; Wang, N.; Wang, C.-Z.; Du, W.; Yuan, C.-S.; He, T.-C. Ginseng saponin metabolite 20 (S)-Protopanaxadiol inhibits tumor growth by targeting multiple cancer signaling pathways. Oncol. Rep. 2013, 30, 292–298. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Dong, L.; Lv, J.; Wang, Y.; Fan, B.; Wang, F.; Liu, X. 20 (S)-Protopanaxadiol (PPD) alleviates scopolamine-induced memory impairment via regulation of cholinergic and antioxidant systems, and expression of Egr-1, c-Fos and c-Jun in mice. Chem.-Biol. Interact. 2018, 279, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Lei, M.Y.; Cong, L.; Liu, Z.Q.; Liu, Z.F.; Ma, Z.; Liu, K.; Li, J.; Deng, Y.; Liu, W.; Xu, B. Resveratrol reduces DRP1-mediated mitochondrial dysfunction via the SIRT1-PGC1α signaling pathway in manganese-induced nerve damage in mice. Environ. Toxicol. 2022, 37, 282–298. [Google Scholar] [CrossRef]
- Zhang, Z.; Cai, X.; Yao, Z.; Wen, F.; Fu, Z.; Zhang, J.; Zhong, Z.; Huang, Y.; Qu, S. EA Ameliorated Depressive Behaviors in CUMS Rats and Was Related to Its Suppressing Autophagy in the Hippocampus. Neural Plast. 2020, 2020, 8860968. [Google Scholar] [CrossRef]
- Lan, T.; Li, Y.; Fan, C.; Wang, L.; Wang, W.; Chen, S.; Yu, S.Y. MicroRNA-204-5p reduction in rat hippocampus contributes to stress-induced pathology via targeting RGS12 signaling pathway. J. Neuroinflamm. 2021, 18, 243. [Google Scholar] [CrossRef]
- Floyd, R.A. Antioxidants, oxidative stress, and degenerative neurological disorders. Proc. Soc. Exp. Biol. Med. 1999, 222, 236–245. [Google Scholar] [CrossRef]
- Tarnopolsky, M.A.; Beal, M.F. Potential for creatine and other therapies targeting cellular energy dysfunction in neurological disorders. Ann. Neurol. 2001, 49, 561–574. [Google Scholar] [CrossRef]
- Minagar, A.; Shapshak, P.; Fujimura, R.; Ownby, R.; Heyes, M.; Eisdorfer, C. The role of macrophage/microglia and astrocytes in the pathogenesis of three neurologic disorders: HIV-associated dementia, Alzheimer disease, and multiple sclerosis. J. Neurol. Sci. 2002, 202, 13–23. [Google Scholar] [CrossRef]
- Bak, D.H.; Kim, H.D.; Kim, Y.O.; Park, C.G.; Han, S.Y.; Kim, J.J. Neuroprotective effects of 20 (S)-Protopanaxadiol against glutamate-induced mitochondrial dysfunction in PC12 cells. Int. J. Mol. Med. 2016, 37, 378–386. [Google Scholar] [CrossRef] [PubMed]
- Badr, A.M.; Attia, H.A.; Al-Rasheed, N. Oleuropein Reverses Repeated Corticosterone-Induced Depressive-like Behavior in mice: Evidence of Modulating Effect on Biogenic Amines. Sci. Rep. 2020, 10, 3336. [Google Scholar] [CrossRef] [PubMed]
- Ren, H.C.; Sun, J.G.; Wang, G.J.; A, J.Y.; Xie, H.T.; Zha, W.B.; Yan, B.; Sun, F.-Z.; Hao, H.-P.; Gu, S.-H.; et al. Sensitive determination of 20 (S)-Protopanaxadiol in rat plasma using HPLC-APCI-MS: Application of pharmacokinetic study in rats. J. Pharm. Biomed. Anal. 2008, 48, 1476–1480. [Google Scholar] [CrossRef] [PubMed]
- Jiang, N.; Lv, J.W.; Wang, H.X.; Wang, Q.; Lu, C.; Yang, Y.J.; Huang, H.; Xia, T.; Lv, G.; Liu, X. Antidepressant-like effects of 20 (S)-Protopanaxadiol in a mouse model of chronic social defeat stress and the related mechanisms. Phytother. Res. PTR 2019, 33, 2726–2736. [Google Scholar] [CrossRef]
- Rahman, S.; Islam, R. Mammalian Sirt1: Insights on its biological functions. Cell Commun. Signal. CCS 2011, 9, 11. [Google Scholar] [CrossRef]
- Shutt, T.E.; McBride, H.M. Staying cool in difficult times: Mitochondrial dynamics, quality control and the stress response. Biochim. Biophys. Acta 2013, 1833, 417–424. [Google Scholar] [CrossRef]
- Meyer, J.N.; Leuthner, T.C.; Luz, A.L. Mitochondrial fusion, fission, and mitochondrial toxicity. Toxicology 2017, 391, 42–53. [Google Scholar] [CrossRef]
- Głombik, K.; Budziszewska, B.; Basta-Kaim, A. Mitochondria-targeting therapeutic strategies in the treatment of depression. Mitochondrion 2021, 58, 169–178. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, P.; Wang, Z.; Sun, L.; He, Z.; Li, J.; Geng, J.; Zong, Y.; Chen, W.; Du, R. 20 (S)-Protopanaxadiol Alleviates DRP1-Mediated Mitochondrial Dysfunction in a Depressive Model In Vitro and In Vivo via the SIRT1/PGC-1α Signaling Pathway. Molecules 2024, 29, 5085. https://doi.org/10.3390/molecules29215085
Guo P, Wang Z, Sun L, He Z, Li J, Geng J, Zong Y, Chen W, Du R. 20 (S)-Protopanaxadiol Alleviates DRP1-Mediated Mitochondrial Dysfunction in a Depressive Model In Vitro and In Vivo via the SIRT1/PGC-1α Signaling Pathway. Molecules. 2024; 29(21):5085. https://doi.org/10.3390/molecules29215085
Chicago/Turabian StyleGuo, Pengli, Zixian Wang, Li Sun, Zhongmei He, Jianming Li, Jianan Geng, Ying Zong, Weijia Chen, and Rui Du. 2024. "20 (S)-Protopanaxadiol Alleviates DRP1-Mediated Mitochondrial Dysfunction in a Depressive Model In Vitro and In Vivo via the SIRT1/PGC-1α Signaling Pathway" Molecules 29, no. 21: 5085. https://doi.org/10.3390/molecules29215085
APA StyleGuo, P., Wang, Z., Sun, L., He, Z., Li, J., Geng, J., Zong, Y., Chen, W., & Du, R. (2024). 20 (S)-Protopanaxadiol Alleviates DRP1-Mediated Mitochondrial Dysfunction in a Depressive Model In Vitro and In Vivo via the SIRT1/PGC-1α Signaling Pathway. Molecules, 29(21), 5085. https://doi.org/10.3390/molecules29215085






