Ceratocarpus arenarius: Botanical Characteristics, Proximate, Mineral Composition, and Cytotoxic Activity
Abstract
1. Introduction
2. Results and Discussion
2.1. Morphological Characteristics
2.2. Anatomical Characteristics
2.2.1. Microscopic Structure of the Leaf
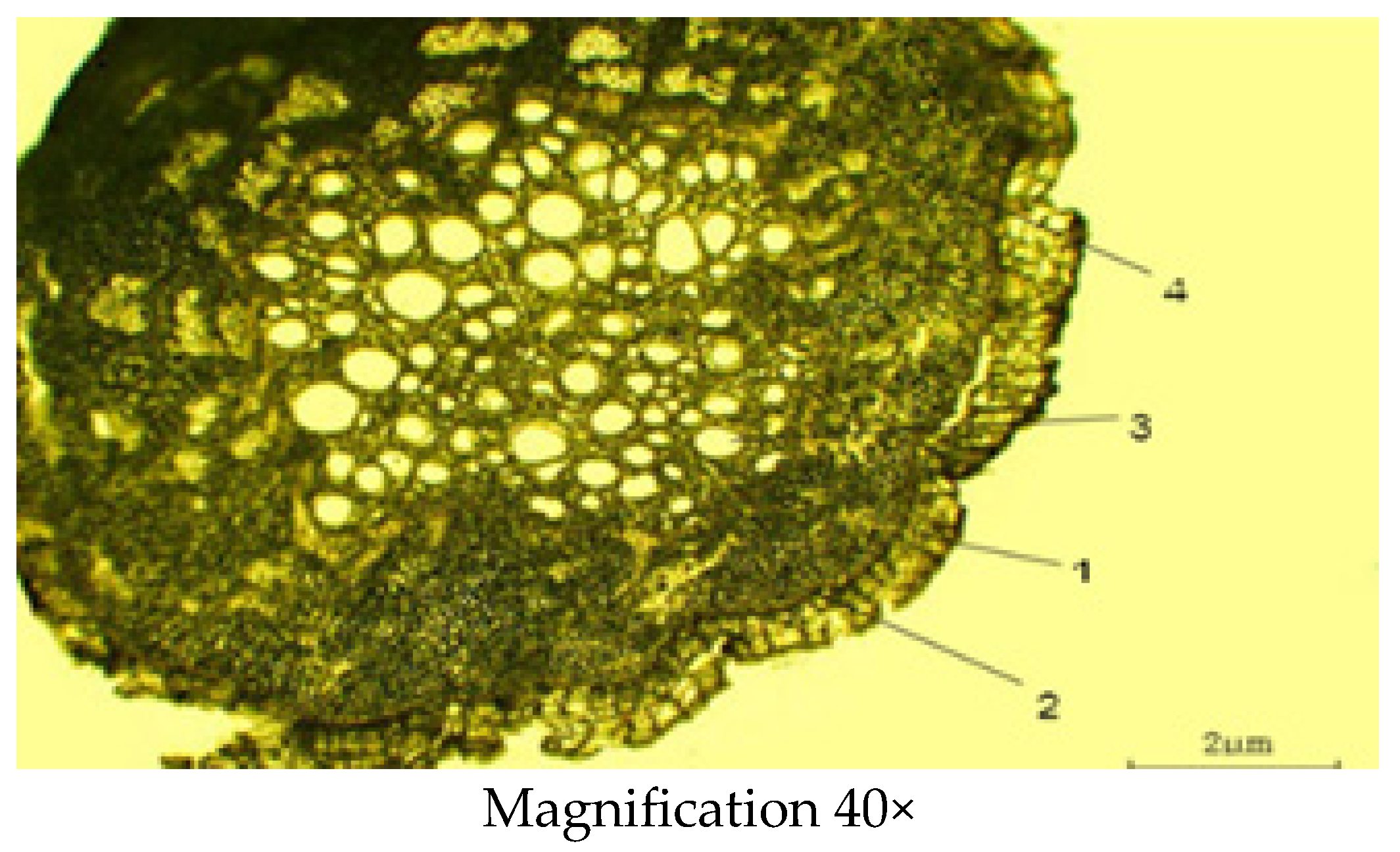
2.2.2. Microscopic Structure of the Stem
2.2.3. Microscopic Structure of the Root
2.3. Determination of Proximate Parameters
2.4. Cytotoxic Activity
3. Materials and Methods
3.1. Plant Material
3.2. Morphological Studies
Anatomical Studies
3.3. Determination of Proximate Parameters
3.3.1. Determination of Moisture
- W1 = weight (g) of sample before drying;
- W2 = weight (g) of sample after drying.
3.3.2. Determination of Ash
- W1 = weight of empty crucible;
- W2 = weight of crucible + sample;
- W3 = weight of crucible + ash.
3.3.3. Determination of Fatty Acid Composition
3.3.4. Determination of Amino Acid Composition
3.3.5. Determination of Mineral Content
3.4. Extraction
3.5. Cytotoxic Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Grigore, M.; Toma, C. Histo-anatomical strategies of Chenopodiaceae halophytes: Adaptive, ecological and evolutionary implications. WSEAS Trans. Biol. Biomed. 2007, 12, 4. [Google Scholar] [CrossRef]
- Heklau, H.; Gasson, P.; Schweingruber, F.; Baas, P. Wood anatomy of Chenopodiaceae (Amaranthaceae s.l.). IAWA J. 2012, 33, 205–232. [Google Scholar] [CrossRef]
- Lomonosova, M.N.; An’kova, T.V.; Voronkova, M.S.; Korolyuk, E.A.; Banaev, E.V.; Skaptsov, M.V. Ploidy level of the representatives of Chenopodiaceae based on genome size and chromosome numbers. Turczaninowia 2020, 23, 24–31. Available online: http://turczaninowia.asu.ru (accessed on 15 February 2023).
- Zhang, T.; Shi, N.; Bai, D.; Chen, Y.; Feng, G. Arbuscular Mycorrhizal Fungi Promote the Growth of Ceratocarpus arenarius (Chenopodiaceae) with No Enhancement of Phosphorus Nutrition. PLoS ONE 2012, 7, e41151. [Google Scholar] [CrossRef]
- Zhaglovskaya, A.; Aidosova, S.; Akhtayeva, N.; Mamurova, A.; Yesimova, D. Anatomical and morphological stem features of two Haloxylon Species (Chenopodiaceae Vent.) of Drought Stress, Kazakhstan. Biosci. Biotechnol. Res. Asia 2015, 12, 1965–1974. [Google Scholar] [CrossRef]
- Gemejiyeva, N.G.; Grudzinskaya, L.M. Current State and Prospects for Studies on the Diversity of Medicinal Flora in Kazakhstan. In Book Vegetation of Central Asia and Environs; Egamberdieva, D., Öztürk, M., Eds.; Springer: Almaty, Kazakhstan, 2018; pp. 239–262. [Google Scholar]
- Gan, L.; Lu, J.; Jerry, M.B.; Baskin, C.C.; Tan, D. Phenotypic plasticity in diaspore production of a amphi basicarpic cold desert annual that produces polymorphic diaspores. Sci. Rep. 2020, 10, 11142. [Google Scholar] [CrossRef]
- Ebrahimi, E.; Eslami, S.V. Effect of environmental factors on seed germination and seedling emergence of invasive Ceratocarpus arenarius. Eur. Weed Res. Soc. Weed Res. 2011, 52, 50–59. [Google Scholar] [CrossRef]
- Grudzinskaya, L.M.; Gemedzhieva, N.G.; Nelina, N.V.; Karzhaubekov, Z.Z. (Eds.) Annotated List of Medicinal Plants of Kazakhstan; KazNMU: Almaty, Kazakhstan, 2014; Volume 20, p. 200. [Google Scholar]
- Pan, L.; Li, L.; Xu, L.; Zhang, J.; Li, J.; Gao, M.; Yu, J.; Jin, J.; Lei, D. UHPLC-QTOF-MS/MS based characterization of anti-tumor constituents in Ceratocarpus arenarius L. and identification of EGFR-TK inhibitors by virtual screening. Nat. Prod. Prod. 2022, 36, 6111–6115. [Google Scholar] [CrossRef]
- Ullah, Z.; Baloch, M.K.; Khader, J.A.; AbdEIslam, N.M.; Noor, S. Proximate and nutrient analysis of selected medicinal plants of Tank and South Waziristan area of Pakistan. Afr. J. Pharm. Pharmacol. 2013, 7, 179–184. [Google Scholar] [CrossRef]
- Radha, M.K.; Sunil, P.; Ashok, P.; Sneh, P.B.; Sushil, C.; Poonam, C.; Parameswari, E.; Ahmad, A.; Mahesh, K.S.; Rahul, D.D.; et al. Evaluation of Nutritional, Phytochemical, and Mineral Composition of Selected Medicinal Plants for Therapeutic Uses from Cold Desert of Western Himalaya. Plants 2021, 10, 1429. [Google Scholar] [CrossRef]
- Princewill-Ogbonna, I.L.; Ogbonna, P.C.; Ogujiofor, I.B. Proximate Composition, Vitamin, Mineral and biologically Active Compounds Levels in Leaves of Mangifera indica (Mango), Persea americana (Avocado pea), and Annona muricata (Sour sop). J. Appl. Sci. Environ. Manag. 2019, 23, 65–74. [Google Scholar]
- Grundy, S.M. Monounsaturated fatty acids and cholesterol metabolism: Implication for dietary recommendations. J. Nutr. 1989, 4, 529–533. [Google Scholar] [CrossRef]
- Ramsden, C.E.; Ringel, A.; Feldstein, A.E.; Taha, A.Y.; MacIntosh, B.A.; Hibbeln, J.R.; Majchrzak-Hong, S.F.; Faurot, K.R.; Rapoport, S.I.; Cheon, Y.; et al. Lowering dietary linoleic acid reduces bioactive oxidized linoleic acid metabolites in humans. Prostagland. Leukot. Essent. Fat. Acids 2012, 87, 135–141. [Google Scholar] [CrossRef]
- Karaca, E.; Aytac, S. The factors affecting on fatty acid composition of oil crops. J. Agric. Fac. Ondokuz Mayıs Univ. 2007, 22, 123–131. [Google Scholar]
- Nas, S.; Gokalp, Y.H.; Unsal, M. Vegetable Oil Technology; Pamukkale University Faculty of Architecture Printing House: Denizli, Turkey, 2001; p. 322. [Google Scholar]
- Hunter, J.E. Studies on effects of dietary fatty acids as related to their position on triglycerides. Lipids 2001, 36, 655–668. [Google Scholar] [CrossRef]
- Sakthivel, R.; Devi, K.P. Evaluation of physicochemical properties, proximate and nutritional composition of Gracilaria edulis collected from Palk Bay. Food Chem. 2015, 174, 68–74. [Google Scholar] [CrossRef]
- Francavilla, M.; Franchi, M.; Monteone, M.; Caroppo, C. The red seaweed Gracilaria gracilis as a multi products source. Mar. Drugs 2013, 11, 3754–3776. [Google Scholar] [CrossRef]
- Woods, P.W. Herbal healing. Essence 1999, 30, 42–46. [Google Scholar]
- Hussain, J.; Rehman, N.; Al-Harrasi, A.; Ali, L.; Ullah, R.; Mabood, F.; Hussain, H.; Ismail, M. Nutritional prospects and mineral compositions of selected vegetables from Dhoda sharif Kohat. J. Med. Plants Res. 2011, 5, 6509–6514. [Google Scholar]
- Saupi, N.; Zakaria, M.H.; Bujang, J.S. Analytic chemical composition and mineral content of yellow velvet leaf (Limnocharis flava L. Buchenau)’s edible parts. J. Appl. Sci. 2009, 9, 2969–2974. [Google Scholar] [CrossRef]
- Chaturvedi, V.C.; Shrivastava, R.; Upreti, R.K. Viral infections and trace elements: A complex trace element. Curr. Sci. 2004, 87, 1536–1554. Available online: http://www.jstor.org/stable/24109032 (accessed on 20 May 2023).
- Saikia, P.; Deka, D.C. Mineral content of some wild green leafy vegetables of North-East India. J. Chem. Pharm. Res. 2013, 5, 117–121. [Google Scholar] [CrossRef]
- Indrayan, A.K.; Sharma, S.; Durgapal, D.; Kumar, N.; Kumar, M. Determination of nutritive value and analysis of mineral elements for some medicinally valued plants from Uttaranchal. Curr. Sci. 2005, 89, 1252–1255. [Google Scholar]
- Kruczek, A. Effect of row fertilization with different kinds of fertilizers on the maize yield. Acta Sci. Pol. Agric. 2005, 4, 37–46. [Google Scholar]
- Léchaudel, M.; Joas, J.; Caro, Y.; Génard, M.; Jannoyer, M. Leaf: Fruit ratio and irrigation supply affect seasonal changes in minerals, organic acids and sugars of mango fruit. J. Sci. Food Agric. 2004, 85, 251–260. [Google Scholar] [CrossRef]
- Lokhande, R.; Singare, P.; Andhale, M. Study on Mineral content of some ayurvedic indian medicinal plants by instrumental Neutron Activation analysis and AAS techniques. Health Sci. J. 2010, 4, 157–168. [Google Scholar]
- Wanyoike, G.N.; Chhabra, S.C.; Lang’at-Thoruwa, C.C.; Omar, S.A. Brine shrimp toxicity and antiplasmodial activity of five Kenyan medicinal plants. J. Ethnopharmacol. 2004, 90, 129–133. [Google Scholar] [CrossRef]
- Anderson, J.E.; Goetz, C.M.; McLaughlin, J.L.; Suffness, M.A. Blind Comparison of Simple Bench-top Bioassays and Human Tumour Cell Cytotoxicities as Antitumor Prescreens. Phytochem. Anal. 1991, 2, 107–111. [Google Scholar] [CrossRef]
- Arzu, K. Cytotoxic Activities on Selected Lamiaceae Species from Turkey by Brine Shrimp Lethality Bioassay. Ordu Univ. J. Sci. Tech. 2019, 9, 105–111. Available online: https://dergipark.org.tr/en/pub/ordubtd/issue/51531/579909 (accessed on 12 June 2023).
- State Pharmacopoeia of the Republic of Kazakhstan; Zhibek-Zholy: Almaty, Kazakhstan, 2008; Volume 1, 592p.
- Vekhov, V.N.; Lotova, L.I.; Filin, V.R. Practicum on Anatomy and Morphology of Higher Plants; MSU: Moscow, Russia, 1980; 560p. [Google Scholar]
- Barykina, R.P.; Veselova, T.D.; Devyatov, A.G.; Jalilova, H.H.; Ilyina, G.M.; Chubatova, N.V. Handbook of Botanical Microtechnology (Fundamentals and Methods); Publishing House, Moscow State University: Moscow, Russia, 2004; p. 312. [Google Scholar]
- Dospekhov, B.A. Book Methodology of Field Experience (with the Basics of Statistical Processing of Research Results); Agropromizdat: Moscow, Russia, 1985; p. 157. Available online: https://www.scirp.org/(S(i43dyn45teexjx455qlt3d2q))/reference/ReferencesPapers.aspx?ReferenceID=1877236 (accessed on 2 March 2023).
- Association of Official Analytical Chemist (AOAC). Official Methods of Analysis of AOAC International; AOAC: Washington, DC, USA, 2016; ISBN 0935584870. Available online: https://www.techstreet.com/standards/official-methods-of-analysis-of-aoac-international-20th-edition-2016?product_id=1937367 (accessed on 25 June 2023).
- Moldabergenova, A.K.; Litvinenko, Y.A.; Akhtayeva, N.Z.; Kiekbayeva, I.N.; Ross, S.A. Amino and fatty acid composition of the aerial parts of Echinops albicaulis, growing in Kazakhstan. Int. J. Biol. Chem. 2016, 9, 1–4. Available online: https://elibrary.kaznu.kz/wp-content/uploads/2021/06/international-journal-of-biology-and-chemistry_2016-2.pdf (accessed on 30 June 2023). [CrossRef]
- Idris, O.A.; Wintola, O.A.; Afolayan, A.J. Comparison of the Proximate Composition, Vitamins (Ascorbic Acid, α-Tocopherol and Retinol), Anti-Nutrients (Phytate and Oxalate) and the GC-MS Analysis of the Essential Oil of the Root and Leaf of Rumex crispus L. Plants 2019, 8, 51. [Google Scholar] [CrossRef]







| Sample | Indicator/Mean ± SD |
|---|---|
| Herb color | grayish-green |
| Odor | Aromatic |
| Plant height (cm) | 9.5 ± 1.16 |
| Phyllotaxis | Alternate |
| Flowers | single, pale yellow |
| Leaf length (cm) | 3.5 ± 0.07 |
| Number of leaves per plant | 37.2 ± 1.1 |
| Stem length (cm) | 11.4 ± 0.76 |
| Seed length (mm) | 6.27 ± 0.12 |
| Indicator | Mean ± SD (µm) |
|---|---|
| Thickness of the leaf | 1.99 ± 0.18 |
| Thickness of the upper epidermis | 0.03 ± 0.02 |
| Thickness of the lower epidermis | 0.25 ± 0.05 |
| Thickness of the spongy mesophyll | 0.49 ± 0.13 |
| Thickness of the palisade mesophyll | 1.98 ± 0.02 |
| Diameter of the vascular bundle | 0.148 ± 0.13 |
| Indicator | Mean ± SD (µm) |
|---|---|
| Thickness of the epidermis | 0.02 ± 0.003 |
| Thickness of the primary parenchyma | 0.27 ± 0.02 |
| Thickness of collenchyma | 0.38 ± 0.14 |
| Diameter of xylem | 0.22 ± 0.04 |
| Diameter of phloem | 0.38 ± 0.09 |
| Thickness of conducting beam | 1.06 ± 0.01 |
| Diameter of core parenchyma zone | 3.27 ± 0.003 |
| Indicator | Mean ± SD (µm) |
|---|---|
| Thickness of the periderm | 0.16 ± 0.02 |
| Thickness of the primary cortex or cortex parenchyma | 0.48 ± 0.03 |
| Diameter of the central cylinder | 2.89 ± 0.25 |
| Diameter of xylem | 0.24 ± 0.01 |
| Diameter of phloem | 0.32 ± 0.01 |
| Parameter | Value |
|---|---|
| Moisture (%) | 6.8 ± 0.28 |
| Protein (mg/100 g) | 392.85 ± 25.50 |
| Ash (%) | 5.9 ± 0.40 |
| Fat (%) | 12.5 ± 21.28 |
| №. | Parameter | C Number: Number of Double Bonds | Class of Compound | Content, % |
|---|---|---|---|---|
| 1 | Myristic acid | 14:0 | Saturated | 0.8 |
| 2 | Pentadecanoic acid | 15:0 | Saturated | 0.5 |
| 3 | Palmitic acid | 16:0 | Saturated | 9.3 |
| 4 | Palmitoleic acid | 16:1 | Monounsaturated | 0.3 |
| 5 | Stearic acid | 18:0 | Saturated | 5.6 |
| 6 | Oleic acid | 18:1 | Monounsaturated | 62.2 |
| 7 | Linoleic acid | 18:2 | Polyunsaturated | 20.8 |
| 8 | Linolenic acid | 18:3 | Polyunsaturated | 0.5 |
| Amino Acid | Content mg/100 g |
|---|---|
| Alanine | 680 |
| Glycine | 222 |
| Leucine | 374 |
| Isoleucine | 345 |
| Valine | 236 |
| Glutamic acid | 2298 |
| Threonine | 218 |
| Proline | 435 |
| Methionine | 56 |
| Serine | 370 |
| Aspartic acid | 1204 |
| Cysteine | 28 |
| Oxyproline | 1 |
| Phenylalanine | 352 |
| Tyrosine | 384 |
| Histidine | 183 |
| Ornithine | 1 |
| Arginine | 256 |
| Lysine | 16 2 |
| Tryptophan | 52 |
| Mineral (mg/100 g Dry Weight) | |||||||
|---|---|---|---|---|---|---|---|
| K | Ca | Mg | Fe | Na | Mn | Zn | Cu |
| 302.73 ± 1.15 | 131.23 ± 0.09 | 60.69 ± 0.72 | 1.18 ± 0.03 | 20.48 ± 0.29 | 0.76 ± 0.01 | 4.45 ± 0.35 | 0.11 ± 0.02 |
| Sample | Concentration mg/mL | % of Surviving Nauplii in the Control | % of Surviving Larvae in the Sample | % Mortality |
|---|---|---|---|---|
| Actinomycin D | 10 | 96 | 0 | 96 |
| 5 | 96 | 4 | 92 | |
| 1 | 96 | 33 | 63 | |
| Extract | 10 | 96 | 96 | 0 |
| 5 | 96 | 96 | 0 | |
| 1 | 96 | 96 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kantureyeva, A.; Ustenova, G.; Zvonar Pobirk, A.; Mombekov, S.; Koilybayeva, M.; Amirkhanova, A.; Gemejiyeva, N.; Mamurova, A.; Kočevar Glavač, N. Ceratocarpus arenarius: Botanical Characteristics, Proximate, Mineral Composition, and Cytotoxic Activity. Molecules 2024, 29, 384. https://doi.org/10.3390/molecules29020384
Kantureyeva A, Ustenova G, Zvonar Pobirk A, Mombekov S, Koilybayeva M, Amirkhanova A, Gemejiyeva N, Mamurova A, Kočevar Glavač N. Ceratocarpus arenarius: Botanical Characteristics, Proximate, Mineral Composition, and Cytotoxic Activity. Molecules. 2024; 29(2):384. https://doi.org/10.3390/molecules29020384
Chicago/Turabian StyleKantureyeva, Aigerim, Gulbaram Ustenova, Alenka Zvonar Pobirk, Serzhan Mombekov, Moldir Koilybayeva, Akerke Amirkhanova, Nadezhda Gemejiyeva, Assem Mamurova, and Nina Kočevar Glavač. 2024. "Ceratocarpus arenarius: Botanical Characteristics, Proximate, Mineral Composition, and Cytotoxic Activity" Molecules 29, no. 2: 384. https://doi.org/10.3390/molecules29020384
APA StyleKantureyeva, A., Ustenova, G., Zvonar Pobirk, A., Mombekov, S., Koilybayeva, M., Amirkhanova, A., Gemejiyeva, N., Mamurova, A., & Kočevar Glavač, N. (2024). Ceratocarpus arenarius: Botanical Characteristics, Proximate, Mineral Composition, and Cytotoxic Activity. Molecules, 29(2), 384. https://doi.org/10.3390/molecules29020384






