Influence of Nε-Lysine Acetylation on the Formation of Protein Aggregates and Antibiotic Persistence in E. coli
Abstract
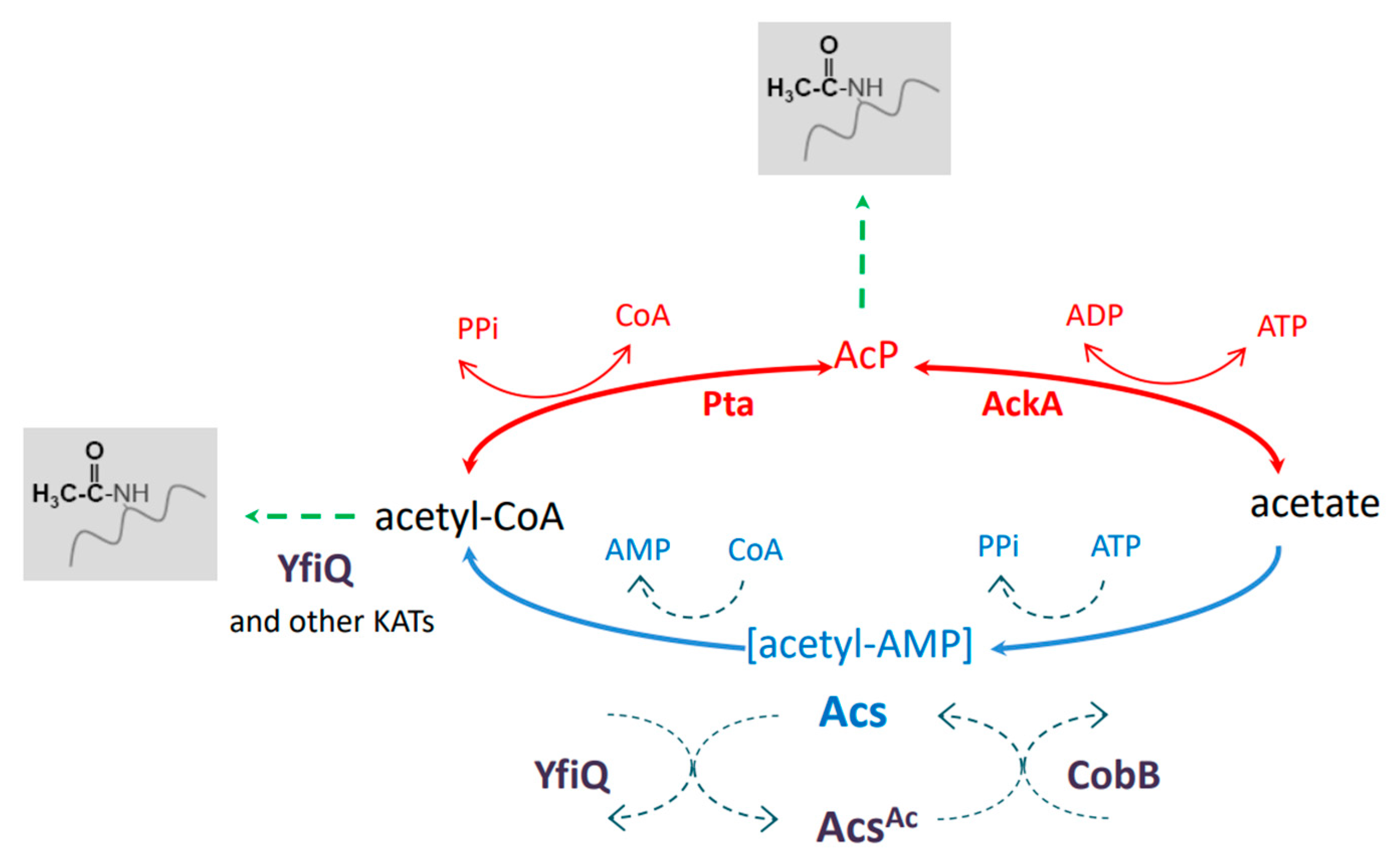
1. Introduction
2. Results
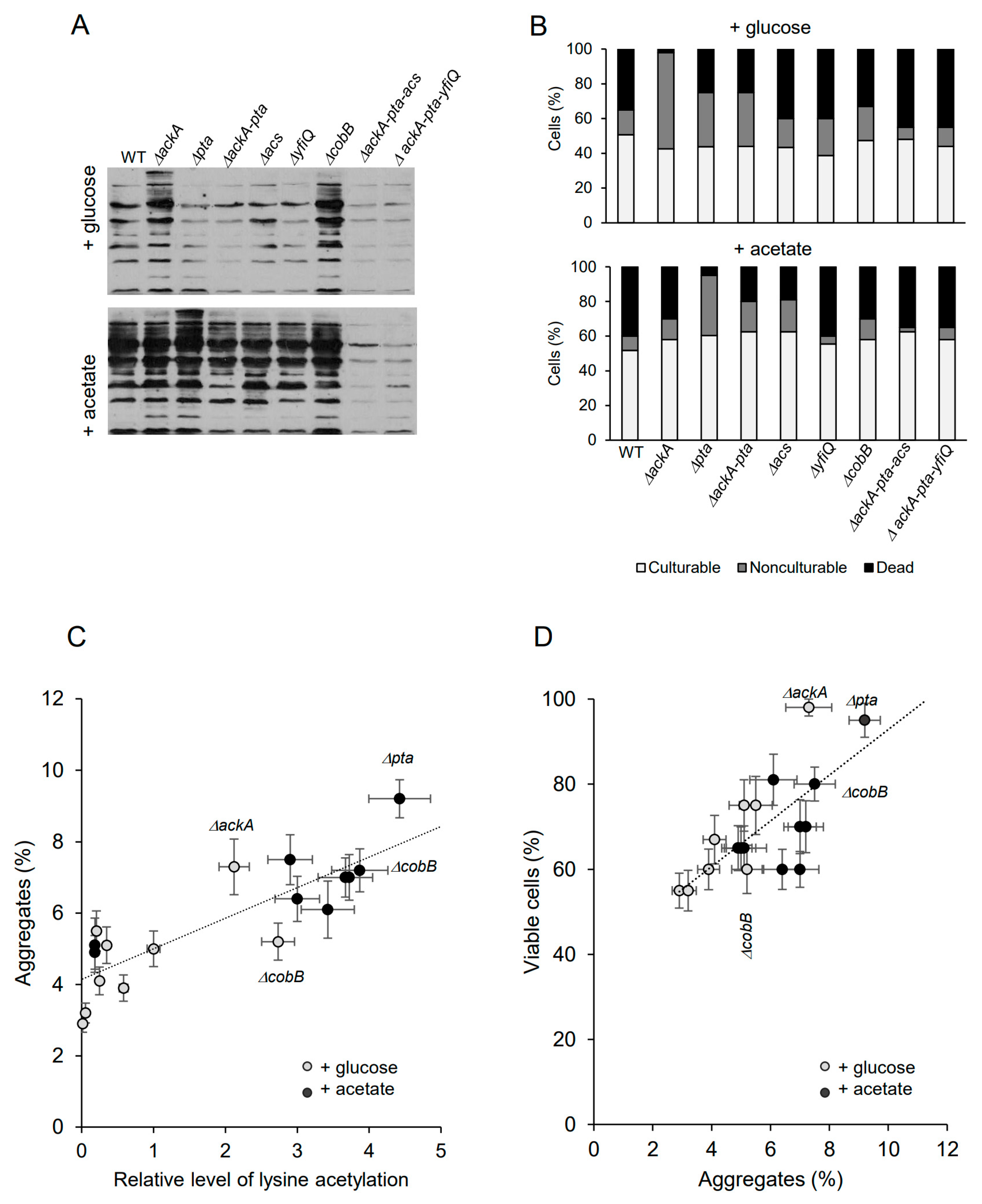
2.1. Lysine Acetylation Enhances Protein Aggregation and E. coli Viability under Late Stationary Phase
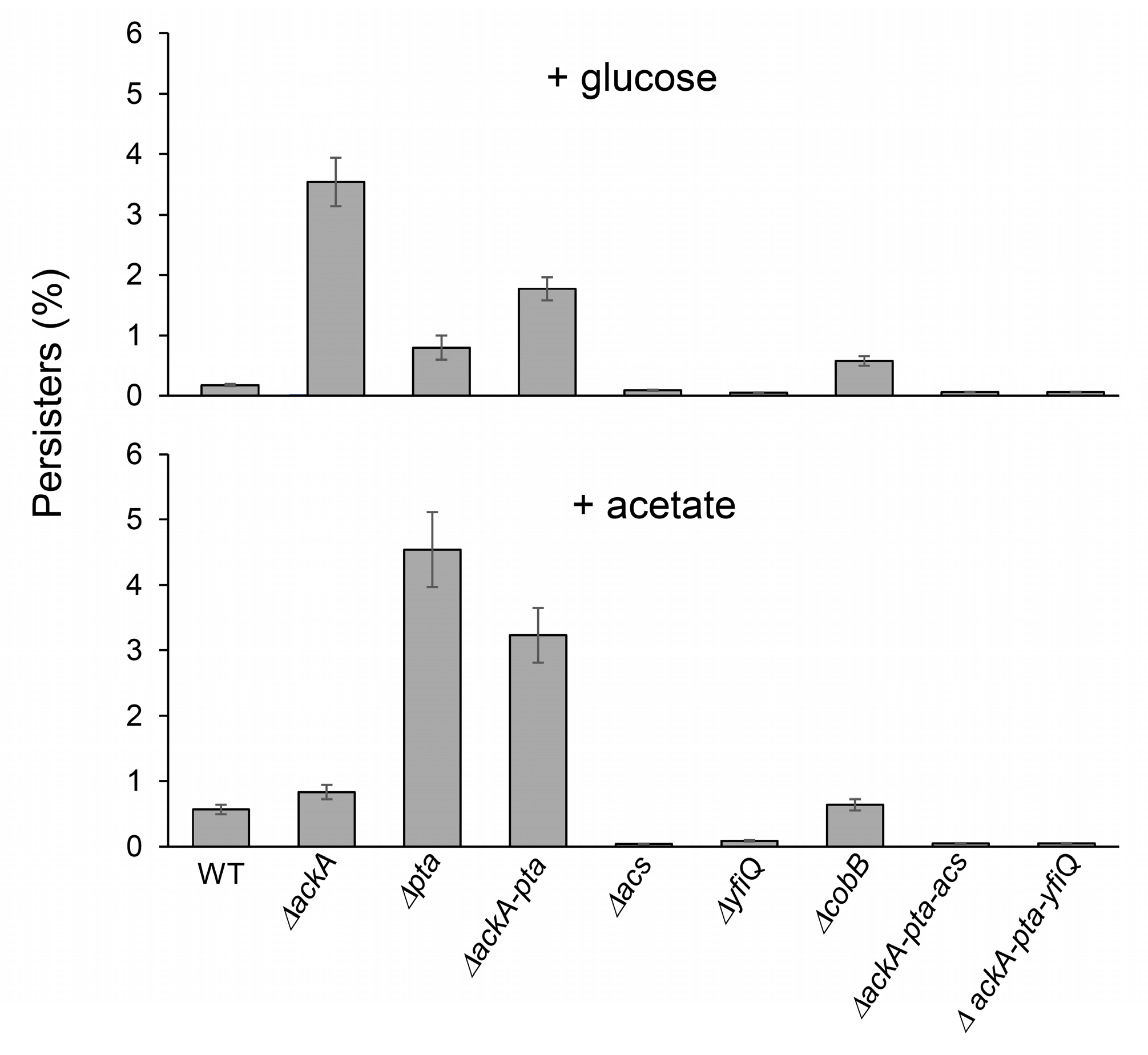
2.2. Lysine Acetylation Stimulates the Formation of Persisters in E. coli
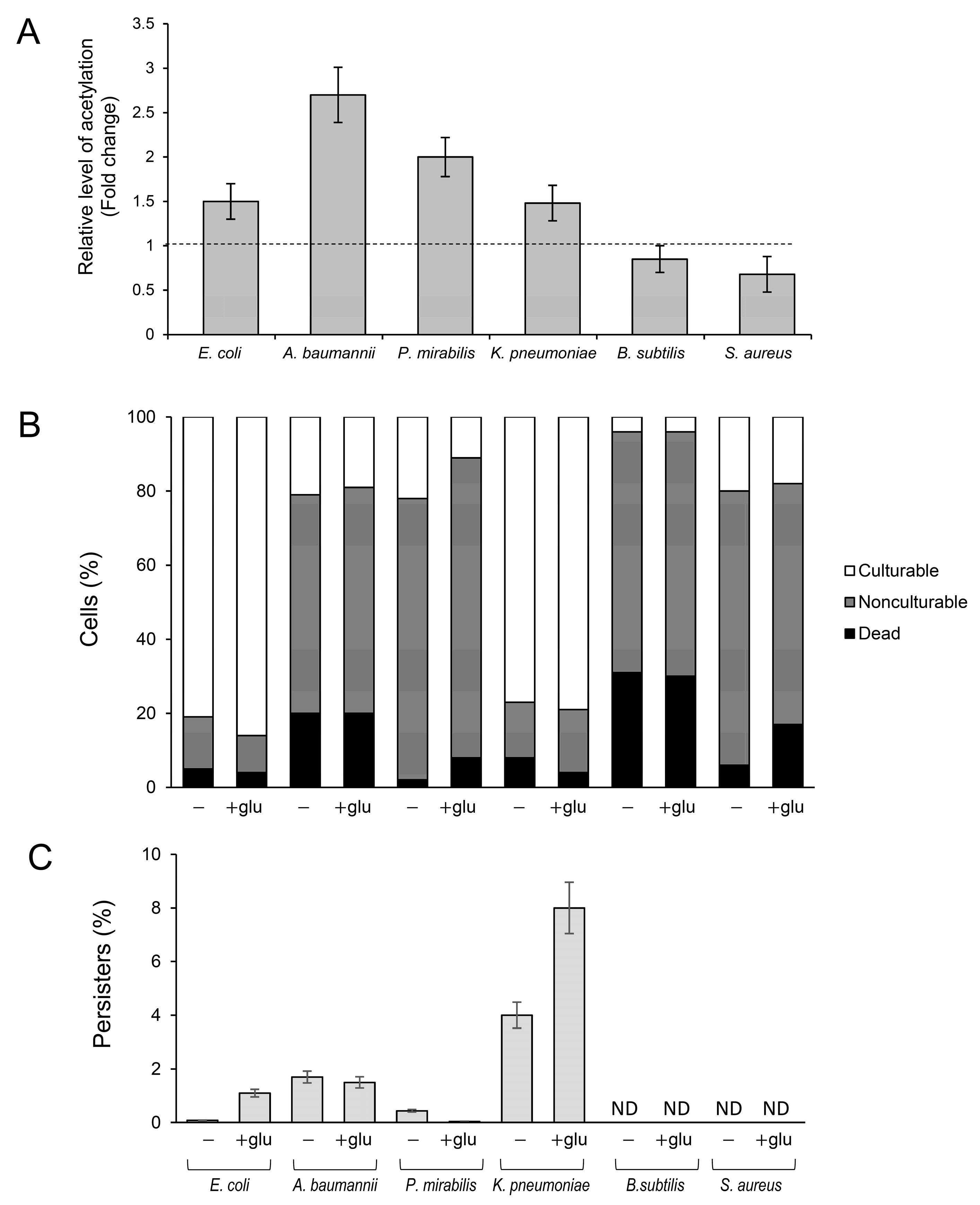
2.3. Protein Acetylation and Persister Formation in Clinical Isolates of Gram-Negative and Gram-Positive Bacteria
3. Discussion
4. Materials and Methods
4.1. Strains and Growth Conditions
4.2. Isolation and Analysis of Protein Aggregates
4.3. Immunodetection of Acetyl-Lysine
4.4. Determination of the Number of Persister, Nonculturable, and Dead Cells
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Luu, J.; Carabetta, V.J. Contribution of Nε-lysine acetylation towards regulation of bacterial pathogenesis. mSystems 2021, 6, e0042221. [Google Scholar] [CrossRef]
- Liu, M.; Huo, M.; Liu, C.; Guo, L.; Ding, Y.; Ma, Q.; Qi, Q.; Xian, M.; Zhao, G. Lysine acetylation of Escherichia coli lactate dehydrogenase regulates enzyme activity and lactate synthesis. Front. Bioeng. Biotechnol. 2022, 10, 966062. [Google Scholar] [CrossRef]
- Cai, S.S.; Zhang, L.Q.; Zhang, Q.; Ye, B.C.; Zhou, Y. Acetylation of NarL K188 and K192 Is Involved in Regulating Escherichia coli Anaerobic Nitrate Respiration. Appl. Microbiol. Biotechnol. 2022, 106, 7209–7221. [Google Scholar] [CrossRef]
- Tan, Y.; Liu, W.; Chen, Y.; Zhou, Y.; Song, K.; Cao, S.; Zhang, Y.; Song, Y.; Deng, H.; Yang, R.; et al. Comparative lysine acetylome analysis of Y. pestis YfiQ/CobB mutants reveals that acetylation of SlyA Lys73 significantly promotes biofilm formation of Y. pestis. Microbio. Spectr. 2023, 11, e0046023. [Google Scholar] [CrossRef]
- Liu, W.; Tan, Y.; Cao, S.; Zhao, H.; Fang, H.; Yang, X.; Wang, T.; Zhou, Y.; Yan, Y.; Han, Y.; et al. Protein acetylation mediated by YfiQ and CobB is involved in the virulence and stress response of Yersinia pestis. Infect. Immun. 2018, 86, e00224-18. [Google Scholar] [CrossRef]
- Ren, J.; Sang, Y.; Qin, R.; Su, Y.; Cui, Z.; Mang, Z.; Li, H.; Lu, S.; Zhang, J.; Cheng, S.; et al. Metabolic Intermediate Acetyl Phosphate Modulates Bacterial Virulence via Acetylation. Emerg. Microbes Infect. 2019, 8, 55–69. [Google Scholar] [CrossRef]
- Zhang, B.-Q.; Bu, H.-L.; You, D.; Ye, B.-C. Acetylation of translation machinery affected protein translation in E. coli. Appl. Microbiol. Biotechnol. 2020, 104, 10697–11070. [Google Scholar] [CrossRef]
- Feid, S.C.; Walukiewicz, H.E.; Wang, X.; Nakayasu, E.S.; Rao, C.V.; Wolfe, A.J. Regulation of translation by lysine acetylation in Escherichia coli. MBio 2022, 13, e0122422. [Google Scholar] [CrossRef]
- Christensen, D.G.; Xie, X.; Basisty, N.; Byrnes, J.; McSweeney, S.; Schilling, B.; Wolfe, A.J. Post-translational protein acetylation: An elegant mechanism for bacteria to dynamically regulate metabolic functions. Front. Microbiol. 2019, 10, 1604. [Google Scholar] [CrossRef] [PubMed]
- Lammers, M. Post-translational lysine ac(et)ylation in bacteria: A biochemical, structural, and synthetic biological perspective. Front. Microbiol. 2021, 12, 757179. [Google Scholar] [CrossRef] [PubMed]
- Dash, A.; Modak, R. Protein acetyltransferases mediate bacterial adaptation to a diverse environment. J. Bacteriol. 2021, 203, e0023121. [Google Scholar] [CrossRef] [PubMed]
- Christensen, D.G.; Meyer, J.G.; Baumgartner, J.T.; D’souza, A.K.; Nelson, W.C.; Payne, S.H.; Kuhn, M.L.; Schilling, B.; Wolfe, A.J.; Freitag, N.E.; et al. Identification of novel protein lysine acetyltransferases in Escherichia coli. MBio 2019, 10, e00592-19. [Google Scholar] [CrossRef]
- Gallego-Jara, J.; Conesa, A.É.; de Diego Puente, T.; Terol, G.L.; Díaz, M.C. Characterization of CobB kinetics and inhibition by nicotinamide. PLoS ONE 2017, 12, e0189689. [Google Scholar] [CrossRef]
- Abouelfetouh, A.; Kuhn, M.L.; Hu, L.I.; Scholle, M.D.; Sorensen, D.J.; Sahu, A.K.; Becher, D.; Antelmann, H.; Mrksich, M.; Anderson, W.F.; et al. The E. coli sirtuin CobB shows no preference for enzymatic and nonenzymatic lysine acetylation substrate sites. Microbiol. Open 2015, 4, 66–83. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Zhai, G.; Chen, C.; Bai, X.; Tian, S.; Hu, D.; Fan, E.; Zhang, K. Protein lysine de-2-hydroxyisobutyrylation by CobB in Prokaryotes. Sci. Adv. 2019, 5, eaaw6703. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.; Yang, Y.; Shang, W.; Hu, Z.; Peng, H.; Li, S.; Hu, X.; Rao, X. Identification of lysine succinylome and acetylome in the vancomycin-intermediate Staphylococcus aureus XN108. Microbiol. Spectr. 2022, 10, e0348122. [Google Scholar] [CrossRef]
- Tu, S.; Guo, S.-J.; Chen, C.-S.; Liu, C.-X.; Jiang, H.-W.; Ge, F.; Deng, J.-Y.; Zhou, Y.-M.; Czajkowsky, D.M.; Li, Y.; et al. YcgC represents a new protein deacetylase family in prokaryotes. Elife 2015, 4, e05322. [Google Scholar] [CrossRef]
- Kremer, M.; Kuhlmann, N.; Lechner, M.; Baldus, L.; Lammers, M. Comment on “YcgC represents a new protein deacetylase family in prokaryotes”. eLife 2018, 7, e37798. [Google Scholar] [CrossRef]
- Starai, V.J.; Escalante-Semerena, J.C. Identification of the protein acetyltransferase (Pat) enzyme that acetylates acetyl-CoA synthetase in Salmonella enterica. J. Mol. Biol. 2004, 340, 1005–1012. [Google Scholar] [CrossRef]
- Gardner, J.G.; Grundy, F.J.; Henkin, T.M.; Escalante-Semerena, J.C. Control of acetyl-Coenzyme A synthetase (AcsA) activity by acetylation/deacetylation without NAD+ involvement in Bacillus subtilis. J. Bacteriol. 2006, 188, 5460–5468. [Google Scholar] [CrossRef]
- Xu, H.; Hegde, S.S.; Blanchard, J.S. Reversible acetylation and inactivation of Mycobacterium tuberculosis acetyl-CoA synthetase is dependent on cAMP. Biochemistry 2011, 50, 5883–5892. [Google Scholar] [CrossRef]
- Castaño-Cerezo, S.; Bernal, V.; Blanco-Catalá, J.; Iborra, J.L.; Cánovas, M. CAMP-CRP Co-ordinates the expression of the protein acetylation pathway with central metabolism in Escherichia coli. Mol. Microbiol. 2011, 82, 1110–1128. [Google Scholar] [CrossRef]
- Schilling, B.; Basisty, N.; Christensen, D.G.; Sorensen, D.; Orr, J.S.; Wolfe, A.J.; Rao, C.V. Global lysine acetylation in Escherichia coli results from growth conditions that favor acetate fermentation. J. Bacteriol. 2019, 201, e00768-18. [Google Scholar] [CrossRef]
- Kuczyńska-Wiśnik, D.; Moruno-Algara, M.; Stojowska-Swȩdrzyńska, K.; Laskowska, E. The effect of protein acetylation on the formation and processing of inclusion bodies and endogenous protein aggregates in Escherichia coli cells. Microb. Cell. Fact. 2016, 15, 189. [Google Scholar] [CrossRef]
- Cohen, T.J.; Hwang, A.W.; Restrepo, C.R.; Yuan, C.X.; Trojanowski, J.Q.; Lee, V.M.Y. An acetylation switch controls TDP-43 function and aggregation propensity. Nat. Commun. 2015, 6, 5845. [Google Scholar] [CrossRef]
- Ferreon, J.C.; Jain, A.; Choi, K.J.; Tsoi, P.S.; Mackenzie, K.R.; Jung, S.Y.; Ferreon, A.C. Acetylation disfavors Tau phase separation. Int. J. Mol. Sci. 2018, 19, 1360. [Google Scholar] [CrossRef]
- Olzscha, H.; Fedorov, O.; Kessler, B.M.; Knapp, S.; La Thangue, N.B. CBP/P300 bromodomains regulate amyloid-like protein aggregation upon aberrant lysine acetylation. Cell. Chem. Biol. 2017, 24, 9–23. [Google Scholar] [CrossRef]
- Bollen, C.; Dewachter, L.; Michiels, J. Protein aggregation as a bacterial strategy to survive antibiotic treatment. Front. Mol. Biosci. 2021, 8, 669664. [Google Scholar] [CrossRef]
- Gollan, B.; Grabe, G.; Michaux, C.; Helaine, S.; ARjatscls, H. Annual review of microbiology bacterial persisters and infection: Past, present, and progressing. Annu. Rev. Microbiol. 2019, 73, 359–385. [Google Scholar] [CrossRef]
- Jin, X.; Lee, J.-E.; Schaefer, C.; Luo, X.; M Wollman, A.J.; Payne-Dwyer, A.L.; Tian, T.; Zhang, X.; Chen, X.; Li, Y.; et al. Membraneless organelles formed by liquid-liquid phase separation increase bacterial fitness. Sci. Adv. 2021, 7, eabh2929. [Google Scholar] [CrossRef]
- Goode, O.; Smith, A.; Łapińska, U.; Bamford, R.; Kahveci, Z.; Glover, G.; Attrill, E.; Carr, A.; Metz, J.; Pagliara, S. Heterologous protein expression favors the formation of protein aggregates in persister and viable but nonculturable bacteria. ACS Infect. Dis. 2021, 7, 1848–1858. [Google Scholar] [CrossRef]
- Schramm, F.D.; Schroeder, K.; Jonas, K. Protein Aggregation in Bacteria. FEMS Microbiol. Rev. 2019, 44, 54–72. [Google Scholar] [CrossRef]
- Wang, X.; Cole, C.G.; Dupai, C.D.; Davies, B.W. Protein aggregation is associated with Acinetobacter baumannii desiccation tolerance. Microorganisms 2020, 8, 343. [Google Scholar] [CrossRef]
- Kuczyńska-Wiśnik, D.; Stojowska-Swędrzyńska, K.; Laskowska, E. Liquid–Liquid Phase Separation and protective protein aggregates in bacteria. Molecules 2023, 28, 6582. [Google Scholar] [CrossRef]
- Gao, Z.; Zhang, W.; Chang, R.; Zhang, S.; Yang, G.; Zhao, G. Liquid-Liquid Phase Separation: Unraveling the enigma of biomolecular condensates in microbial cells. Front. Microbiol. 2021, 12, 751880. [Google Scholar] [CrossRef]
- Wei, S.P.; Qian, Z.G.; Hu, C.F.; Pan, F.; Chen, M.T.; Lee, S.Y.; Xia, X.X. Formation and functionalization of membraneless compartments in Escherichia coli. Nat. Chem. Biol. 2020, 16, 1143–1148. [Google Scholar] [CrossRef]
- Jalal, A.S.B.; Le, T.B.K. Bacterial chromosome segregation by the ParABS system. Open Biol. 2020, 10, 200097. [Google Scholar] [CrossRef]
- Ladouceur, A.-M.; Singh Parmar, B.; Biedzinski, S.; Wall, J.; Graydon Tope, S.; Cohn, D.; Kim, A.; Soubry, N.; Reyes-Lamothe, R.; Weber, S.C.; et al. Clusters of bacterial RNA polymerase are biomolecular condensates that assemble through liquid-liquid phase separation. Proc. Natl. Acad. Sci. USA 2020, 117, 18540–18549. [Google Scholar] [CrossRef]
- Gupta, A.; Joshi, A.; Arora, K.; Mukhopadhyay, S.; Guptasarma, P. The bacterial nucleoid-associated proteins, HU and Dps, condense DNA into context-dependent biphasic or multiphasic complex coacervates. J. Biol. Chem. 2023, 299, 104637. [Google Scholar] [CrossRef]
- Leszczynska, D.; Matuszewska, E.; Kuczynska-Wisnik, D.; Furmanek-Blaszk, B.; Laskowska, E. The formation of persister cells in stationary-phase cultures of Escherichia coli is associated with the aggregation of endogenous proteins. PLoS ONE 2013, 8, e54737. [Google Scholar] [CrossRef]
- Pu, Y.; Li, Y.; Jin, X.; Tian, T.; Ma, Q.; Zhao, Z.; Lin, S.-Y.; Chen, Z.; Li, B.; Yao, G.; et al. ATP-dependent dynamic protein aggregation regulates bacterial dormancy depth critical for antibiotic tolerance. Mol. Cell 2019, 73, 143–156.e4. [Google Scholar] [CrossRef]
- Fang, Z.; Lai, F.; Cao, K.; Zhang, Z.; Cao, L.; Liu, S.; Duan, Y.; Yin, X.; Ge, R.; He, Q.-Y.; et al. Potential role of lysine acetylation in antibiotic resistance of Escherichia coli. Msystems 2022, 7, e0064922. [Google Scholar] [CrossRef]
- Balaban, N.Q.; Helaine, S.; Lewis, K.; Ackermann, M.; Aldridge, B.; Andersson, D.I.; Brynildsen, M.P.; Bumann, D.; Camilli, A.; Collins, J.J.; et al. Definitions and Guidelines for Research on Antibiotic Persistence. Nat. Rev. Microbio. 2019, 17, 441–448. [Google Scholar] [CrossRef]
- Bollen, C.; Louwagie, E.; Verstraeten, N.; Michiels, J.; Ruelens, P. Environmental, mechanistic and evolutionary landscape of antibiotic persistence. EMBO Rep. 2023, 24, e57309. [Google Scholar] [CrossRef]
- Jurenas, D.; Chatterjee, S.; Konijnenberg, A.; Sobott, F.; Droogmans, L.; Garcia-Pino, A.; Van Melderen, L. AtaT blocks translation initiation by n-acetylation of the initiator tRNAfMet. Nat. Chem. Biol. 2017, 13, 640–646. [Google Scholar] [CrossRef]
- Qian, H.; Yao, Q.; Tai, C.; Deng, Z.; Gan, J.; Ou, H.Y. Identification and characterization of acetyltransferase-type toxin-antitoxin locus in Klebsiella pneumoniae. Mol. Microbiol. 2018, 108, 336–349. [Google Scholar] [CrossRef]
- Cheverton, A.M.; Gollan, B.; Przydacz, M.; Wong, C.T.; Mylona, A.; Hare, S.A.; Helaine, S. A Salmonella toxin promotes persister formation through acetylation of tRNA. Mol. Cell 2016, 63, 86–96. [Google Scholar] [CrossRef]
- Moruno Algara, M.; Kuczyńska-Wiśnik, D.; Dębski, J.; Stojowska-Swędrzyńska, K.; Sominka, H.; Bukrejewska, M.; Laskowska, E. Trehalose protects Escherichia coli against carbon stress manifested by protein acetylation and aggregation. Mol. Microbiol. 2019, 112, 866–880. [Google Scholar] [CrossRef]
- Kuhn, M.L.; Zemaitaitis, B.; Hu, L.I.; Sahu, A.; Sorensen, D.; Minasov, G.; Lima, B.P.; Scholle, M.; Mrksich, M.; Anderson, W.F.; et al. Structural, kinetic and proteomic characterization of acetyl phosphate-dependent bacterial protein acetylation. PLoS ONE 2014, 9, e94816. [Google Scholar] [CrossRef]
- Schütze, A.; Benndorf, D.; Püttker, S.; Kohrs, F.; Bettenbrock, K. The impact of ackA, pta, and ackA-pta mutations on growth, gene expression and protein acetylation in Escherichia coli K-12. Front. Microbiol. 2020, 11, 233. [Google Scholar] [CrossRef]
- Kaldalu, N.; Hauryliuk, V.; Tenson, T. Persisters—As elusive as Ever. Appl. Microbiol. Biotechnol. 2016, 100, 6545–6553. [Google Scholar] [CrossRef]
- Jõers, A.; Kaldalu, N.; Tenson, T. The Frequency of persisters in Escherichia coli reflects the kinetics of awakening from dormancy. J. Bacteriol. 2010, 192, 3379–3384. [Google Scholar] [CrossRef]
- Zhang, Y. Persisters, persistent infections and the yin-yang model. Emerg. Microbes Infect. 2014, 3, e3. [Google Scholar] [CrossRef]
- Ahn, D.; Bhushan, G.; McConville, T.H.; Annavajhala, M.K.; Soni, R.K.; Wong Fok Lung, T.; Hofstaedter, C.E.; Shah, S.S.; Chong, A.M.; Castano, V.G.; et al. An acquired acyltransferase promotes Klebsiella pneumoniae ST258 respiratory infection. Cell Rep. 2021, 35, 109196. [Google Scholar] [CrossRef]
- Kentache, T.; Jouenne, T.; Dé, E.; Hardouin, J. Proteomic characterization of Nα- and Nε-Acetylation in Acinetobacter baumannii. J. Proteom. 2016, 144, 148–158. [Google Scholar] [CrossRef]
- Watson, P.R.; Christianson, D.W. Structure and Function of Kdac1, a class ii deacetylase from the multidrug-resistant pathogen Acinetobacter baumannii. Biochemistry 2023, 62, 2689–2699. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, Z.X.; Wan, X.L.; Liu, P.; Zhang, J.B.; Ye, Y.; Zhao, Y.M.; Tan, M.J. Comprehensive profiling of lysine acetylome in Staphylococcus aureus. Sci. China Chem. 2014, 57, 732–738. [Google Scholar] [CrossRef]
- Ma, Q.; Wood, T.K. Protein acetylation in prokaryotes increases stress resistance. Biochem. Biophys. Res. Commun. 2011, 410, 846–851. [Google Scholar] [CrossRef]
- Laskowska, E.; Kuczyńska-Wiśnik, D. New insight into the mechanisms protecting bacteria during desiccation. Curr. Genet. 2020, 66, 313–318. [Google Scholar] [CrossRef]
- Zheng, Q.; Omans, N.D.; Leicher, R.; Osunsade, A.; Agustinus, A.S.; Finkin-Groner, E.; D’Ambrosio, H.; Liu, B.; Chandarlapaty, S.; Liu, S.; et al. Reversible histone glycation is associated with disease-related changes in chromatin architecture. Nat. Commun. 2019, 10, 1289. [Google Scholar] [CrossRef]
- Nahomi, R.B.; Oya-Ito, T.; Nagaraj, R.H. The combined effect of acetylation and glycation on the chaperone and anti-apoptotic functions of human α-crystallin. Biochim. Biophys. Acta Mol. Basis Dis. 2013, 1832, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Reverdy, A.; Chen, Y.; Hunter, E.; Gozzi, K.; Chai, Y. Protein lysine acetylation plays a regulatory role in Bacillus subtilis multicellularity. PLoS ONE 2018, 13, e0204687. [Google Scholar] [CrossRef] [PubMed]
- Carabetta, V.J.; Greco, T.M.; Cristea, I.M.; Dubnau, D. YfmK Is an Nε-Lysine acetyltransferase that directly acetylates the histone-like protein HBsu in Bacillus subtilis. Proc. Natl. Acad. Sci. USA 2019, 116, 3752–3757. [Google Scholar] [CrossRef] [PubMed]
- Carabetta, V.J.; Greco, T.M.; Tanner, A.W.; Cristea, I.M.; Dubnau, D. Temporal regulation of the Bacillus subtilis acetylome and evidence for a role of MreB acetylation in cell wall growth. mSystems 2016, 1, e00005–e00016. [Google Scholar] [CrossRef]
- Song, S.; Wood, T.K. ‘Viable but Nonculturable Cells’ are dead. Environ. Microbiol. 2021, 23, 2335–2338. [Google Scholar] [CrossRef]
| Strain | Lack of |
|---|---|
| ΔackA | Acetate kinase (EC 2.7.2.1) |
| Δpta | Phosphate acetyltransferase (EC 2.3.1.8) |
| ΔackA-pta | Acetate kinase (EC 2.7.2.1) and phosphate acetyltransferase (EC 2.3.1.8) |
| Δacs | Acetate–CoA ligase (EC 6.2.1.1) |
| ΔyfiQ | Protein lysine acetyltransferase (EC 2.3.1.48) |
| ΔcobB | Protein lysine deacetylase (EC 2.3.1.286) |
| ΔackA-pta-acs | Acetate kinase (EC 2.7.2.1), phosphate acetyltransferase (EC 2.3.1.8), and acetate–CoA ligase (EC 6.2.1.1) |
| ΔackA-pta-yfiQ | Acetate kinase (EC 2.7.2.1), phosphate acetyltransferase (EC 2.3.1.8), and protein lysine deacetylase (EC 2.3.1.286) |
| WT | ΔackA | Δpta | ΔackA-pta | Δacs | ΔyfiQ | ΔcobB | ΔackA-pta-acs | ΔackA-pta-yfiQ | |
|---|---|---|---|---|---|---|---|---|---|
| +glucose | |||||||||
| Persisters (culturable) | 0.1 ± 0.01 | 2.7 ± 0.16 | 0.8 ± 0.16 | 1.3 ± 0.25 | 0.1 ± 0.04 | 0.03 ± 0.01 | 0.4 ± 0.14 | 0.03 ± 0.01 | 0.03 ± 0.01 |
| Nonculturable | 60 ± 3.3 | 82 ± 5.4 | 64 ± 2.4 | 62 ± 5.4 | 60 ± 6.2 | 48 ± 5 | 55 ± 3.7 | 35 ± 7 | 33 ± 4 |
| Dead | 40 ± 3.3 | 15 ± 5.5 | 35 ± 2.4 | 37± 5.5 | 40 ± 6.2 | 52 ± 5 | 45 ± 3.8 | 65 ± 7 | 67 ± 4 |
| +acetate | |||||||||
| Persisters (culturable) | 0.5 ± 0.14 | 0.8 ± 0.37 | 6.0 ± 0.44 | 3.9 ± 0.19 | 0.1 ± 0.02 | 0.1 ± 0.01 | 0.6 ± 0.1 | 0.06 ± 0.01 | 0.04 ± 0.01 |
| Nonculturable | 56 ± 3.7 | 49 ± 3.6 | 86 ± 0.5 | 66 ± 3.7 | 63 ± 4.2 | 50 ± 5 | 55 ± 6.3 | 33 ± 4.4 | 36 ± 5 |
| Dead | 44 ± 3.8 | 50 ± 2.2 | 8 ± 0.44 | 30 ± 3.6 | 37 ± 4.1 | 50 ± 5 | 44 ± 6.2 | 67 ± 4.4 | 64 ± 5 |
| Strain | Source * | Mutation |
|---|---|---|
| ΔackA | CGSC#:9834 (JW2293-1) | ackA-778(del)::kan |
| Δpta | CGSC#:9844 (JW2294-1) | pta-779(del)::kan |
| ΔackA-pta | [24] | ackA-pta (del)::cat |
| Δacs | CGSC#:10898 (JW4030-1) | acs-763(del)::kan |
| ΔyfiQ | CGSC#:10042 (JW2568-1) | yfiQ-752(del)::kan |
| ΔcobB | CGSC#:9039 (JW1106-1) | cobB-779(del)::kan |
| ΔackA-pta-acs | This study | ackA-pta (del)::cat, acs-763(del)::kan |
| ΔackA-pta-yfiQ | This study | ackA-pta (del)::cat, yfiQ-752(del)::kan |
| Primer | Sequence 5′ → 3′ | |
|---|---|---|
| pta-F | GCTCAGCTGGCGGTGCTGTTTTGTAAC | Amplification of the pta gene |
| pta-R | CAAAGCTGCGGATGATGACGAGATTAC | |
| ack-F | CATCATGCGCTACGCTCTATGGCTCC | Amplification of the ackA gene |
| ack-R | AGCTGAGCTGGCGGTGTGAAATCAG | |
| acs-F | AAACCGTTACCGACTCGCAT | Amplification of the acs gene fragment |
| acs-R | ACCCTGCCGTTTATTTGCAC | |
| yfiQ-F | TCTGGCAGGAAAAACGCAAC | Amplification of the yfiQ gene fragment |
| yfiQ-R | ATGCAGACGACATAAGCGGG | |
| cob-F | GTGCGGCCTTCCTACATCTAA | Amplification of the cobB gene fragment |
| cob-R | TGCCGCATTGTTATTGACGAG | |
| ack-F | CATCATGCGCTACGCTCTATGGCTCC | Amplification of the ackA-pta DNA fragment |
| pta-R | CAAAGCTGCGGATGATGACGAGATTAC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stojowska-Swędrzyńska, K.; Kuczyńska-Wiśnik, D.; Laskowska, E. Influence of Nε-Lysine Acetylation on the Formation of Protein Aggregates and Antibiotic Persistence in E. coli. Molecules 2024, 29, 383. https://doi.org/10.3390/molecules29020383
Stojowska-Swędrzyńska K, Kuczyńska-Wiśnik D, Laskowska E. Influence of Nε-Lysine Acetylation on the Formation of Protein Aggregates and Antibiotic Persistence in E. coli. Molecules. 2024; 29(2):383. https://doi.org/10.3390/molecules29020383
Chicago/Turabian StyleStojowska-Swędrzyńska, Karolina, Dorota Kuczyńska-Wiśnik, and Ewa Laskowska. 2024. "Influence of Nε-Lysine Acetylation on the Formation of Protein Aggregates and Antibiotic Persistence in E. coli" Molecules 29, no. 2: 383. https://doi.org/10.3390/molecules29020383
APA StyleStojowska-Swędrzyńska, K., Kuczyńska-Wiśnik, D., & Laskowska, E. (2024). Influence of Nε-Lysine Acetylation on the Formation of Protein Aggregates and Antibiotic Persistence in E. coli. Molecules, 29(2), 383. https://doi.org/10.3390/molecules29020383







