Abstract
Drawing inspiration from the structural resemblance between a natural product N-(3-carboxypropyl)-2-acetylpyrrole and phenylbutyric acid, a pioneer HDAC inhibitor evaluated in clinical trials, we embarked on the design and synthesis of a novel array of HDAC inhibitors containing an N-linked 2-acetylpyrrole cap by utilizing the pharmacophore fusion strategy. Among them, compound 20 exhibited potential inhibitory activity on HDAC1, and demonstrated notable potency against RPMI-8226 cells with an IC50 value of 2.89 ± 0.43 μM, which was better than chidamide (IC50 = 10.23 ± 1.02 μM). Western blot analysis and Annexin V-FTIC/propidium iodide (PI) staining showed that 20 could enhance the acetylation of histone H3, as well as remarkably induce apoptosis of RPMI-8226 cancer cells. The docking study highlighted the presence of a hydrogen bond between the carbonyl oxygen of the 2-acetylpyrrole cap group and Phe198 of the HDAC1 enzyme in 20, emphasizing the crucial role of introducing this natural product-inspired cap group. Molecular dynamics simulations showed that the docked complex had good conformational stability. The ADME parameters calculation showed that 20 possesses remarkable theoretical drug-likeness properties. Taken together, these results suggested that 20 is worthy of further exploration as a potential HDAC-targeted anticancer drug candidate.
1. Introduction
As one of the most serious threats to human health, cancer has become the leading cause of death worldwide after cardiovascular and cerebrovascular diseases [1,2]. Although great progress of chemotherapeutic agents has been made in cancer treatment, cancer-related deaths continue to dominate mortality due to drug resistance, which is the foremost reason for failure of cancer treatment [3,4,5,6,7]. Among the multiple contributing factors in cancer drug resistance, epigenetic dysregulation is one of the most important determinants [8]. Modification of epigenetic processes is considered to be an innovative strategy for cancer therapy [9,10]. Deacetylation of histone or non-histone catalyzed by histone deacetylases (HDACs) is one of the most intensively investigated epigenetic modifications [11,12].
HDAC inhibitors (HDACis) have proved to be effective anticancer agents, owing to the potential capacity to inhibit cancer cell invasion, sensitize the cancer cells to chemotherapy, induce apoptosis, and boost immunogenicity [13]. To date, four HDACis, vorinostat (SAHA), belinostat, panobinostat, and romidepsin, have gained US Food and Drug Administration (FDA) approval for the treatment of refractory or relapsed cutaneous and peripheral T-cell lymphomas or multiple myeloma. Chidamide is another HDACi approved by the China FDA for the treatment of peripheral T-cell lymphoma (Figure 1) [14,15,16]. However, most HDACis are associated with adverse side effects, such as fatigue, nausea, thrombocytopenia, and neutropenia, prompting the search for new HDACis [4,17,18].
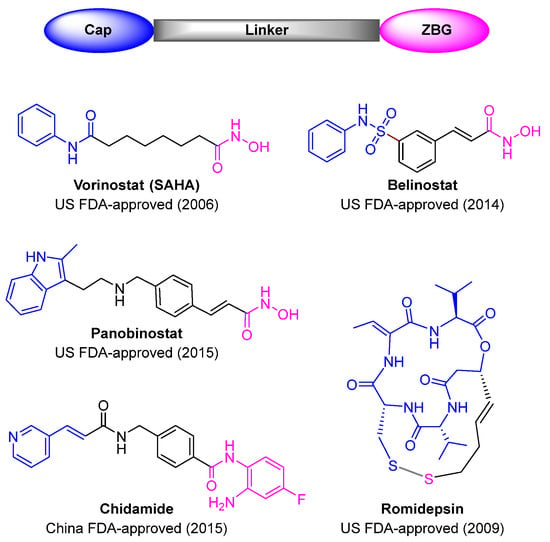
Figure 1.
Chemical structures of currently approved HDAC inhibitors.
Natural products are an important source of inspiration in drug design and development [19,20]. A short-chain aliphatic acid, N-(3-carboxypropyl)-2-acetyl pyrrole, isolated from Sauropus spatulifolius [21,22], captured our attention due to its highly similar structure to that of phenylbutyric acid (Figure 2), the first HDACi evaluated in clinical trials [23]. Pyrroles are among the most prevalent natural pharmacophores embedded in a number of drug molecules, such as ketorolac, atorvastatin, sunitinib, etc. [24,25], and they play a crucial role in the development of HDACis [26,27,28]. However, the utilization of a pyrrole motif as the cap group remains uncommon in existing works, where the linker chain is typically connected at the C-2 position [28,29,30]. Despite several reports utilizing indole as the cap group, only a limited number of HDACis possess the linker connection point on the nitrogen atom [4,11,31,32,33]. In the present work, we envisioned a new design strategy that integrates the natural product-derived 2-acetylpyrrole cap group with established HDACi pharmacophores. Leveraging this molecular hybridization strategy, two novel series of HDACis with hydroxamic acid or o-phenylenediamine as a ZBG have been successfully synthesized (Figure 2). Biological screening revealed that most of them exhibited nanomolar inhibitory activity against three HDAC isomers. Among them, compound 20 demonstrated the strongest anti-proliferative effects targeting RPMI-8226 cells. Herein, we report the discovery, biological evaluation, kinetic binding analysis, and ADME prediction of the synthesized HDACis.
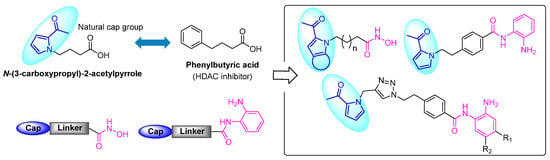
Figure 2.
Design strategy and modification of novel HDAC inhibitors containing a natural product-inspired N-linked 2-acetylpyrrole cap.
2. Results and Discussion
2.1. Chemistry
Two series of target compounds were designed and prepared. One is with hydroxamic acid as a ZBG (Scheme 1, Scheme 2 and Scheme 3), and the other is with o-phenylenediamine (Scheme 4).
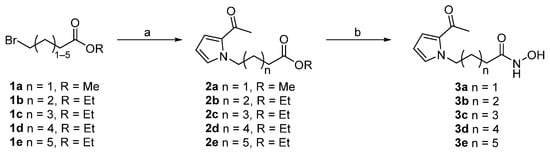
Scheme 1.
Reagents and conditions: (a) 2-acetyl pyrrole, NaH, DMF, 0 °C to rt; (b) NH2OH·HCl, KOH, MeOH, 0 °C to 40 °C.
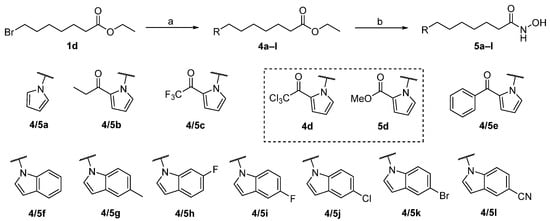
Scheme 2.
Reagents and conditions: (a) pyrrole or indole (RH), NaH, DMF, 0 °C to rt; (b) NH2OH·HCl, KOH, MeOH, 0 °C to 40 °C.
Scheme 3.
Reagents and conditions: (a) SOCl2, MeOH, 0 °C to reflux; (b) 2-acetylpyrrole, KOH, 18-crown-6, toluene, reflux; (c) LiOH, H2O/MeOH (1:4), 40 °C; (d) O-(tetrahydro-2H-pyran-2-yl)hydroxylamine, DIPEA, HATU, DCM, rt; (e) TsOH, MeOH, rt; (f) 2-acetylpyrrole, NaH, DMF, 0 °C to rt.
Scheme 4.
Reagents and conditions: (a) 2-acetylpyrrole, NaH, DMF, 0 °C to rt; (b) TBAF, TMSN3, THF, rt; (c) CuSO4·5H2O, sodium l-ascorbate, DMF/H2O (3:1), rt; (d) EDCI, DMAP, DCM, rt; (e) 1,2-diaminobenzene, DIPEA, HATU, DCM, rt.
Firstly, we synthesized hydroxamic acid-based SAHA-like targeting products 3a–e comprising aliphatic linkers of various lengths (Scheme 1). The reactions of commercially available bromo esters 1a–e with 2-acetylpyrrole under basic conditions successfully provided intermediates 2a–e. Next, compounds 2a–e were converted into the target compounds 3a–e in a single step by using hydroxyamine in the presence of potassium hydroxide. Through a similar two-step procedure, the target compounds 5a–l were successfully synthesized from ethyl 7-bromoheptanoate (1d) (Scheme 2). Particularly, the 2-trichloroacetyl pyrrole derivative 4d yielded the methyl ester 5d. Two more target compounds 11 and 14 were synthesized by similar procedures from brominated starting materials 4-(2-bromoethyl)benzoic acid (6) and methyl (E)-3-(4-(bromomethyl)phenyl)acrylate (12), respectively (Scheme 3).
Next, we prepared a series of o-phenylenediamine-based targeting products (Scheme 4). Starting from propargyl bromide (15) and 4-(2-bromoethyl)benzoic acid (6), alkyne 16 and azide 17 were synthesized in a single step, respectively. Subsequent copper(II)-catalyzed click reaction afforded triazole intermediate 18, which underwent amidation with various o-phenylenediamines to furnish target compounds 19a–f. The advanced intermediate 9 was coupled with o-phenylenediamine to result in an additional target compound 20.
2.2. In Vitro HDAC Inhibitory Activity and Structure-Activity Relationship
Upon completion of the synthesis, all target compounds 3a–e, 5a–l, 11, 14, 19a–f, and 20 were evaluated against three HDAC isomers (HDAC1/3/6) (Table 1, Table 2 and Table 3). Among the hydroxamic acid-based targeting products 3a–e, compound 3d exhibited the best inhibitory activity, indicating that a six-methylene linker is optimal for the activity. Introduction of phenyl groups into the linker chain, as exemplified by 11 and 14, resulted in a slight diminution of inhibitory activities (Table 1).

Table 1.
Structures of compounds 3a–e, 11, and 14, and their inhibitory activities against HDACs.

Table 2.
Structures of compounds 5a–l and their inhibitory activities against HDACs.

Table 3.
Structures of compounds 19a–f, and 20, and their inhibitory activities against HDACs.
Based on these results, we preferentially introduced hydroxamic acid into the N-1 position of the pyrrole cap with a 6-carbon aliphatic linker (5a–e). Compound 5a without any substituent on the pyrrole cap showed diminished inhibitory activity, implying that the 2-acyl group is pivotal in maintaining the activity (Table 2). Compound 5e incorporating a 2-benzoylpyrrole cap exhibited comparable inhibitory effects to SAHA. Most of these compounds showed good selectivity towards the HDAC6 isomer. However, compound 5e stood out as it displayed a slight preferential inhibition towards HDAC3 compared to HDAC1 and HDAC6. Replacing the 2-acyl pyrrole cap with an indole cap (5f–l) basically led to better inhibitory effects (Table 2). Both compounds 5i and 5l possessing a 5-fluoro and 5-cyano indole cap group, respectively, showed even stronger HDAC inhibition potency than SAHA.
Among the target compounds (19a–f and 20) with an o-phenylenediamine cap group, only compounds 19a and 20 showed certain inhibitory activity against HDAC1 and HDAC3 isoforms (Table 3). Substitution with the fluorine (19b and 19d), chloro (19c), methoxy (19e), or ester group (19f) in the o-phenylenediamine ZBG leads to a loss of activity.
2.3. In Vitro Anti-Proliferation Against Cancer Cells
To assess the potential, those compounds with good HDAC inhibition (3d, 5a–l, 11, 14, 19a, and 20) were evaluated for their anti-proliferation against three different tumor cell lines, HCT-116 (human colon cancer cell line), HL-60 (human leukemia cell line), and RPMI-8226 (human myeloma cell line) (Table 4). Surprisingly, among the hydroxamic acid-based HDACis, a decrease in antiproliferative activity of the indole derivatives (5f–l) was observed in contrast to their displayed potency against HDACs, and most pyrrole derivatives (5a, 5b, 5d, 5e, and 11) also exhibited weaker antiproliferative activity. On one hand, it may be attributed to off-target effects, as hydroxamic acid is known for its non-specific metal binding capability [34]. On the other hand, the hydroxamic acid group is susceptible to hydrolysis by esterases, as exemplified in trichostatin A [35,36]. In addition, hydroxamic acid is prone to form isocyanates by Lossen rearrangement, which consequently leads to the loss of its inhibitory activity [37,38]. In contrast, compounds 5c and 14, and two o-phenylenediamine-based HDACis, 19a and 20, showed relatively good inhibitory effects, and the anti-proliferative activity of 20 on RPMI-8226 cells was even better than that of the positive control chidamide. After a thorough analysis of the aforementioned information, we chose compounds 5c, 14, 19a, and 20 for in-depth evaluation to elucidate their efficacy and mechanism on the cellular level.

Table 4.
Proliferation inhibition of compounds 3d, 5a–l, 11, 14, 19a, and 20 against cancer cells.
2.4. Analysis of Ac-Tubulin and Ac-Histone H3 Levels in RPMI-8226 Cells by Western Blotting
To firmly establish the inhibitory effects of compounds 5c, 14, 19a, and 20 on HDAC deacetylation activity, we performed a meticulous assessment of their impact on tubulin and histone H3 acetylation levels in RPMI-8226 cells.
As shown in Figure 3a, compound 14 induced more acetylated tubulin and acetylated histone H3 at the same concentration compared to compound 5c, aligning with its stronger anti-proliferation effects on cancer cells (Table 4). At a concentration of 10 μM, compounds 20 and 19a increased histone H3 acetylation levels comparable to MS-275. HDAC6 is primarily located in the cytoplasm and deacetylates cytoplasmic non-histone substrates, such as α-tubulin, to regulate tumor cell proliferation and apoptosis [38]. In Figure 3b, compounds 20, 19a, MS-275, and chidamide at tested concentrations had no discernible effect on Ac-α-tubulin protein expression associated with HDAC6 activity. This result confirms the selectivity of compounds 20 and 19a observed in the isoform profiling (Table 3).
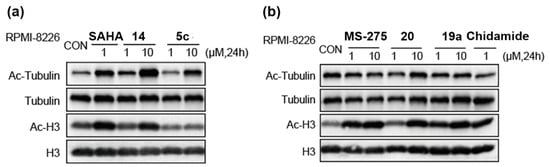
Figure 3.
Western blot analysis of Ac-tubulin and Ac-histone H3 from RPMI 8226 cells cultured for 24 h with DMSO control and selected compounds. (a) Effect of compounds 5c, 14, and SAHA on tubulin and histone H3 acetylation; (b) effect of compounds 19a, 20, MS-275, and chidamide on tubulin and histone H3 acetylation.
2.5. Effects of Compounds 5c, 14, 19a, and 20 on Cancer Cell Apoptosis
To investigate whether the antiproliferative activities of compounds 5c, 14, 19a, and 20 are accompanied by enhanced cancer cell apoptosis, a flow cytometry assay was implemented. RPMI-8226 cells were treated with selected compounds at concentrations of 1, 3, and 10 μM for 48 h, respectively, then stained with Annexin V-FTIC/propidium iodide (PI), and finally analyzed by flow cytometry. The flow cytometry data are shown in Figure 4 and Figure 5. Four tested compounds (20, 19a, 14, and 5c) could promote RPMI-8226 cell apoptosis in a dose-dependent manner. Remarkably, compared with chidamide, compound 20 at 1 μM and 3 μM induced apoptosis in RPMI-8226 cells more effectively.
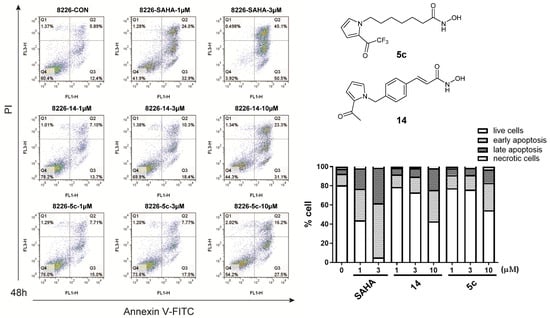
Figure 4.
RPMI 8226 cells were treated with compounds 14 (1, 3, 10 μM), 5c (1, 3, 10 μM), and SAHA (1, 3 μM) and DMSO (0.1%) for 48 h, and then stained with Annexin-V-FITC and propidium iodide (PI) and analyzed by flow cytometry.
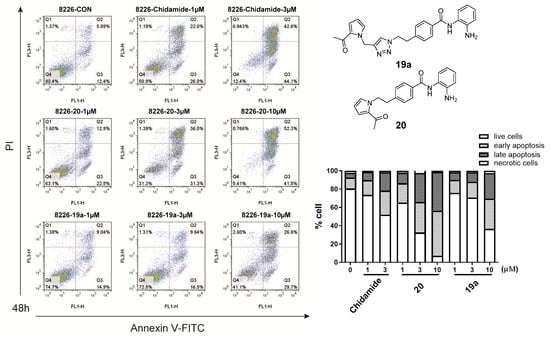
Figure 5.
RPMI 8226 cells were treated with compounds 20 (1, 3, 10 μM), 19a (1, 3, 10 μM), and chidamide (1, 3 μM) and DMSO (0.1%) for 48 h, and then stained with Annexin-V-FITC and propidium iodide (PI) and analyzed by flow cytometry.
2.6. Molecular Docking of Compounds 19a and 20 with HDAC1
Based on the protein crystal structure (PDB code: 1C3S), an in silico docking study was carried out to investigate the interaction of compounds 19a and 20 with the active site of HDAC1 [39].
The docking results revealed that compound 20 could effectively penetrate the active pocket of the protein (Figure 6). The N-(2-aminophenyl)benzamide group of compound 20 chelated the Zn2+ ion well in a bidentate fashion, maintaining a distance of 3.0 Å to the oxygen atom in the carbonyl group and 2.1 Å to the nitrogen atom in aniline, respectively. In addition, the N-(2-aminophenyl)benzamide group also formed hydrogen bonding interactions with His132, Gly140, and Tyr297, respectively. The carbonyl oxygen of the 2-acetylpyrrole cap group formed a hydrogen bond with Phe198. The phenyl group within the linker fit well into the hydrophobic channel, engaging in π–π interactions with Phe198 and Phe141 in a face-to-face configuration, and with His170 in an edge-to-face orientation (Figure 6 and Figure 7a). For comparison, in the binding model of compound 19a with HDAC1, the introduction of triazole resulted in a displacement and flip of the 2-acetylpyrrole group towards the outer solvent region, thereby abolishing the hydrogen bonding interaction with Phe198 and subsequently reducing the affinity (Figure 7b). In summary, molecular docking revealed that compound 20 interacts more closely with HDAC1, thus further validating the experimental findings.
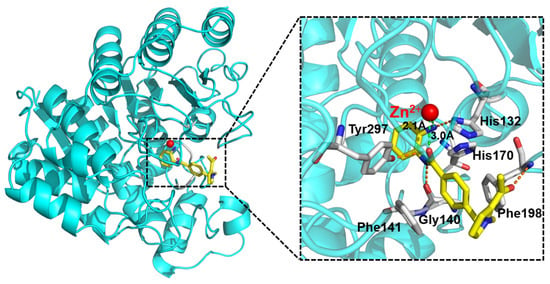
Figure 6.
Binding pose of 20 with HDAC1 predicted by molecular docking (PDB entry: 1C3S). The figure was generated using PyMol (http://www.pymol.org/, accessed on 7 May 2024). Dash green lines represent the chelation of ZBG and zinc ions; Dash orange lines represent hydrogen bonding.
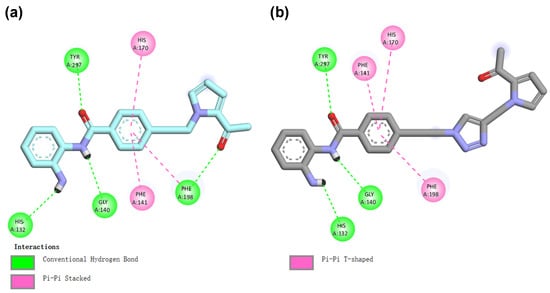
Figure 7.
Predicted binding modes of compounds 20 (a) and 19a (b) with HDAC1. The 2D figure was generated using Discovery Studio 4.5.
2.7. Molecular Dynamic Simulation
Molecular dynamics (MD) simulations have been performed to elucidate the behavior of the HDAC1 enzyme upon inhibitor binding, and the stability and interactions of the structure throughout the simulations. Root mean square deviation (RMSD) represents the sum of all atomic deviations between the conformation and the target conformation at a certain time, and serves as a crucial benchmark to measure the stability of the system [40]. The root mean square fluctuation (RMSF) is utilized to assess the macromolecular flexibility, highlighting localized structural changes within the protein [41].
Figure 8a depicts the RMSD values of the protein backbone atoms during the MD simulation, revealing that the 20-HDAC1 complex attains a stable RMSD value after approximately 30 ns of simulation. As shown in Figure 8b, the RMSF data suggested a flexibility ranging from 0.4 to 2.4 Å, indicating that the residues within the binding pocket are relatively stable. Subsequently, protein interactions with the ligand are monitored throughout the simulation (Figure 8c). Zn2+ ion was penta-coordinated with Asp168, His170, and Asp258 as well as the carbonyl and aniline of benzamide of compound 20. Hydrogen bonds are formed with His132, Gly140, Asp168, and Phe198. Moreover, hydrophobic contacts were observed with His132, Phe141, His170, Phe198, and Leu265, during the entire MD simulation process.
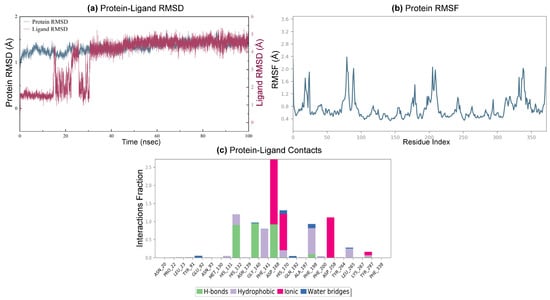
Figure 8.
Molecular dynamics simulation of the docked complex of 20-HDAC1. (a) RMSD of HDAC1 protein backbone atoms (blue line) and 20 (red line) monitored throughout the 100 ns; (b) RMSF of HDAC1 residue; (c) histogram showing interaction fractions.
2.8. ADME Analysis
The ADME parameters (physicochemical properties, lipophilicity, pharmacokinetics, drug-likeness, and medicinal chemistry) of compound 20 and chidamide were measured using the SwissADME free web tool (http://www.swissadme.ch, accessed on 10 March 2024) [42,43,44]. The results are summarized in Table 5. Regarding the druglike properties, it was observed that 20 adheres to Lipinski’s rule of five and does not violate any Ghose, Muegge, Egan, or Veber filters. Besides, compound 20 exhibited a topological polar surface area (TPSA) of 77.12 Å2, suggesting that it could effectively permeate cell membranes (See Supplementary Materials Figure S1 for details).

Table 5.
Prediction of physicochemical properties and pharmacokinetic properties for “drug-likeness” for 20 and chidamide.
The Boiled-Egg model of 20 shows high gastrointestinal (GI) absorption, indicating potentially good overall oral bioavailability (Figure 9a). Furthermore, 20 is predicted as passively traverse the brain penetration (BBB, at the yolk edge), but it can be pumped out of the brain by P-glycoprotein (P-gp, blue dot), thereby reducing its absorption and penetration into the brain [42,45]. As depicted in Figure 9b, the bioavailability radar of compound 20 also highly resembles that of chidamide.
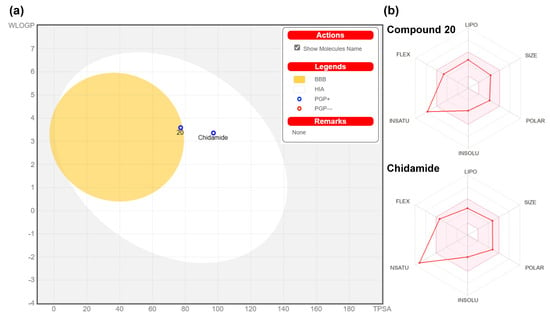
Figure 9.
Boiled-Egg predictive model (a) and bioavailability radar (b) for 20 and chidamide. The white area is a high-probability area for passive absorption (HIA) in the gastrointestinal tract, and the yellow area (yolk) is a high-probability area for the blood-brain barrier (BBB). The yolk and white zone are not mutually exclusive. Points are shown in blue if predicted to be an active P-gp efflux (PGP+) and in red if predicted to be a non-P-gp substrate (PGP−).
2.9. Preliminary Pharmacokinetic Profiling
Subsequently, a pharmacokinetic study of compound 20 was conducted in male ICR mice. The pharmacokinetic profiles are summarized in Table 6. The results reveal that it has a high clearance of 102 mL/min/kg and a terminal half-life (t1/2) of 0.192 h after intravenous (iv) route at 1 mg/kg dose. Although the oral bioavailability was lower than expected due to lower plasma exposure (F% = 5.00%), it was better than panobinostat (F% = 4.62%) and comparable to SAHA (F% = 8.33%) [46,47]. This preliminary pharmacokinetic assessment highlights the need to refine its pharmacokinetic parameters as a crucial step in further development and optimization, aiming to enhance in vivo stability, extend the duration of action, and elevate oral bioavailability.

Table 6.
Pharmacokinetic parameters of compound 20 in vivo a.
3. Materials and Methods
3.1. Chemistry
All solvents used were commercially available and of analytical grade unless stated otherwise. Chemical shifts are given in ppm with the solvent as internal standard or TMS, if added. Protons on heteroatoms such as COOH, NH, and OH protons are only reported when detected in NMR and may therefore be missing. NMR spectra were acquired on Varian Mercury 400, Bruker AM-400, and AM-500 spectrometers using CDCl3 or CD3OD as solvent (Bruker, Billerica, MA, USA). Unless otherwise noted, all reagents were commercially available (Sinopharm (Beijing, China), Energy, Bide, Adamas-beta (Shanghai, China), TCI (Qingdao, China), J&K (Beijing, China), etc.) and used without further purification. Purifications were performed by flash column chromatography using a Biotage Isolera (Shanghai, China) one-flash purification system. The chromatographic columns used with the Biotage Isolera system were purchased from Changzhou Santai Technology Co., Ltd. (Changzhou, China).
3.1.1. Methyl 4-(2-acetyl-1H-pyrrol-1-yl)butanoate (2a)
To a solution of 2-acetyl pyrrole (109 mg, 1.00 mmol) in dry DMF (10 mL) was added NaH (44 mg, 60%, dispersion in paraffin liquid, 1.10 mmol) at 0 °C. The resulting mixture was stirred for 15 min followed by the addition of methyl 4-bromobutyrate (1a) (217 mg, 1.20 mmol). Then, the mixture was stirred at room temperature and monitored by TLC. After 12 h, H2O (20 mL) was added, and the mixture was extracted with ethyl acetate (3 × 10 mL). The combined organic layers were washed with brine, dried by anhydrous Na2SO4, filtered, and then concentrated under reduced pressure to give the crude product, which was further purified by column chromatography eluting with 10% ethyl acetate in petroleum ether to give 2a as a colorless oil (128 mg, 61%). 1H NMR (400 MHz, CDCl3) δ 6.93 (dd, J = 4.1, 1.7 Hz, 1H), 6.82 (t, J = 2.1 Hz, 1H), 6.10 (dd, J = 4.1, 2.5 Hz, 1H), 4.34 (t, J = 6.9 Hz, 2H), 3.63 (s, 3H), 2.39 (s, 3H), 2.26 (t, J = 7.3 Hz, 2H), 2.03 (p, J = 7.1 Hz, 2H). 13C NMR (100 MHz, CDCl3) δ 188.3, 173.4, 130.4, 130.1, 120.5, 108.2, 51.7, 48.6, 30.7, 27.3, 26.5.
3.1.2. Ethyl 5-(2-acetyl-1H-pyrrol-1-yl)pentanoate (2b)
Starting from ethyl 5-bromovalerate (1b) (500 mg, 2.39 mmol) and 2-acetyl pyrrole (217 mg, 1.99 mmol), the title compound was obtained following the procedure previously described for compound 2a. The crude product was purified by silica gel chromatography to give the colorless oil 2b (388 mg, 82%). 1H NMR (400 MHz, CDCl3) δ 6.95 (dd, J = 4.1, 1.7 Hz, 1H), 6.84 (t, J = 2.2 Hz, 1H), 6.11 (dd, J = 4.1, 2.5 Hz, 1H), 4.31 (t, J = 7.1 Hz, 2H), 4.10 (q, J = 7.1 Hz, 2H), 2.42 (s, 3H), 2.30 (t, J = 7.4 Hz, 2H), 1.79–1.71 (m, 2H), 1.62–1.55 (m, 2H), 1.23 (t, J = 7.1 Hz, 3H). 13C NMR (100 MHz, CDCl3) δ 188.4, 173.5, 130.3, 130.1, 120.5, 108.1, 60.4, 49.5, 33.9, 30.9, 27.4, 22.1, 14.3.
3.1.3. Ethyl 6-(2-acetyl-1H-pyrrol-1-yl)hexanoate (2c)
Starting from ethyl 6-bromohexanoate (1c) (533 mg, 2.39 mmol) and 2-acetyl pyrrole (217 mg, 1.99 mmol), the title compound was obtained following the procedure previously described for compound 2a. The crude product was purified by silica gel chromatography to give the colorless oil 2c (386 mg, 77%). 1H NMR (400 MHz, CDCl3) δ 6.95 (dd, J = 4.0, 1.7 Hz, 1H), 6.84 (dd, J = 2.5, 1.8 Hz, 1H), 6.11 (dd, J = 4.1, 2.5 Hz, 1H), 4.29 (t, J = 7.2 Hz, 2H), 4.10 (q, J = 7.1 Hz, 2H), 2.42 (s, 3H), 2.28 (t, J = 7.5 Hz, 2H), 1.78–1.70 (m, 2H), 1.67–1.58 (m, 2H), 1.34–1.27 (m, 2H), 1.24 (t, J = 7.1 Hz, 3H). 13C NMR (100 MHz, CDCl3) δ 188.4, 173.8, 130.3, 130.1, 120.4, 108.0, 60.4, 49.7, 34.3, 31.2, 27.5, 26.2, 24.7, 14.4.
3.1.4. Ethyl 7-(2-acetyl-1H-pyrrol-1-yl)heptanoate (2d)
Starting from ethyl 7-bromoheptanoate (1d) (566 mg, 2.39 mmol) and 2-acetyl pyrrole (217 mg, 1.99 mmol), the title compound was obtained following the procedure previously described for compound 2a. The crude product was purified by silica gel chromatography to give the colorless oil 2d (498 mg, 94%). 1H NMR (400 MHz, CDCl3) δ 6.94 (dd, J = 4.1, 1.7 Hz, 1H), 6.83 (t, J = 2.1 Hz, 1H), 6.10 (dd, J = 4.0, 2.5 Hz, 1H), 4.28 (t, J = 7.4 Hz, 2H), 4.10 (q, J = 7.1 Hz, 2H), 2.41 (s, 3H), 2.26 (t, J = 7.5 Hz, 2H), 1.74–1.66 (m, 2H), 1.63–1.53 (m, 2H), 1.36–1.26 (overlap, 4H), 1.23 (t, J = 7.1 Hz, 3H). 13C NMR (100 MHz, CDCl3) δ 188.3, 173.9, 130.3, 130.1, 120.4, 108.0, 60.3, 49.8, 34.3, 31.4, 28.8, 27.5, 26.4, 24.9, 14.4.
3.1.5. Ethyl 8-(2-acetyl-1H-pyrrol-1-yl)octanoate (2e)
Starting from ethyl 8-bromooctanoate (1e) (379 mg, 1.51 mmol) and 2-acetyl pyrrole (137 mg, 1.26 mmol), the title compound was obtained following the procedure previously described for compound 2a. The crude product was purified by silica gel chromatography to give the colorless oil 2e (300 mg, 85%). 1H NMR (400 MHz, CDCl3) δ 6.95 (dd, J = 4.1, 1.8 Hz, 1H), 6.83 (t, J = 2.1 Hz, 1H), 6.11 (dd, J = 4.0, 2.5 Hz, 1H), 4.28 (t, J = 7.3 Hz, 2H), 4.11 (q, J = 7.1 Hz, 2H), 2.42 (s, 3H), 2.26 (t, J = 7.5 Hz, 2H), 1.72–1.67 (m, 2H), 1.62–1.55 (m, 2H), 1.32–1.26 (overlap, 6H), 1.24 (t, J = 7.1 Hz, 3H). 13C NMR (125 MHz, CDCl3) δ 188.3, 174.0, 130.3, 130.2, 120.4, 108.0, 60.3, 49.9, 34.5, 31.5, 29.2, 29.0, 27.5, 26.6, 25.0, 14.4.
3.1.6. 4-(2-Acetyl-1H-pyrrol-1-yl)-N-hydroxybutanamide (3a)
Potassium hydroxide (134 mg, 2.39 mmol) was added to a solution of hydroxylamine hydrochloride (147 mg, 2.13 mmol) in MeOH (15 mL) at 0 °C. The mixture was stirred for 30 min at 0 °C. Then compound 2a (128 mg, 0.61 mmol) was added, and the mixture was stirred at 40 °C overnight. The reaction was complete detected by TLC. After cooling to room temperature, the solvent was removed under reduced pressure to give a yellow oil. H2O (15 mL) was added, and the mixture was adjusted to pH 7 with acetic acid and extracted with ethyl acetate (3 × 10 mL). The combined organic layers were washed with brine, dried by anhydrous Na2SO4, filtered, and concentrated under reduced pressure to give a crude product, which was purified by flash silica gel column (1%–10% MeOH/DCM) to afford compound 3a as a light brown solid (86 mg, 67%). 1H NMR (400 MHz, CDCl3) δ 6.91–6.84 (overlap, 2H), 6.03 (t, J = 3.3 Hz, 1H), 4.20 (t, J = 7.2 Hz, 2H), 2.32 (s, 3H), 2.12 (t, J = 7.4 Hz, 2H), 1.99–1.89 (m, 2H). 13C NMR (100 MHz, CDCl3) δ 188.9, 171.0, 131.4, 129.7, 121.4, 108.5, 48.8, 29.7, 27.2, 27.1. HRMS (ESI), m/z: calcd for C10H13N2O3−: 209.0932 [M − H]−; found: 209.0931.
3.1.7. 5-(2-Acetyl-1H-pyrrol-1-yl)-N-hydroxypentanamide (3b)
Compound 3b was prepared using similar procedures as for 3a from compound 2b (388 mg, 1.64 mmol). The crude product was purified by silica gel chromatography to give the light brown solid (106 mg, 29%). 1H NMR (400 MHz, CDCl3) δ 6.94 (dd, J = 4.1, 1.5 Hz, 1H), 6.86 (d, J = 2.0 Hz, 1H), 6.08 (dd, J = 4.1, 2.4 Hz, 1H), 4.20 (t, J = 7.1 Hz, 2H), 2.38 (s, 3H), 2.16 (t, J = 7.2 Hz, 2H), 1.72–1.61 (m, 2H), 1.60–1.51 (m, 2H). 13C NMR (100 MHz, CDCl3) δ 189.1, 171.7, 131.2, 129.8, 121.4, 108.5, 49.2, 32.1, 30.7, 27.3, 22.4. HRMS (ESI), m/z: calcd for C11H15N2O3−: 223.1088 [M − H]−; found: 223.1085.
3.1.8. 6-(2-Acetyl-1H-pyrrol-1-yl)-N-hydroxyhexanamide (3c)
Compound 3c was prepared using similar procedures as for 3a from compound 2c (386 mg, 1.54 mmol). The crude product was purified by silica gel chromatography to give the light brown solid (154 mg, 42%). 1H NMR (400 MHz, CDCl3) δ 6.92 (dd, J = 4.2, 1.7 Hz, 1H), 6.83 (t, J = 2.1 Hz, 1H), 6.06 (dd, J = 4.1, 2.5 Hz, 1H), 4.19 (t, J = 7.4 Hz, 2H), 2.37 (s, 3H), 2.13 (t, J = 7.5 Hz, 2H), 1.69–1.55 (overlap, 4H), 1.32–1.22 (m, 2H). 13C NMR (100 MHz, CDCl3) δ 188.7, 171.7, 130.8, 129.7, 121.0, 108.2, 49.5, 32.5, 30.9, 27.2, 25.8, 24.8. HRMS (ESI), m/z: calcd for C12H19N2O3+: 239.1390 [M + H]+; found: 239.1389.
3.1.9. 7-(2-Acetyl-1H-pyrrol-1-yl)-N-hydroxyheptanamide (3d)
Compound 3d was prepared using similar procedures as for 3a from compound 2d (498 mg, 1.88 mmol). The crude product was purified by silica gel chromatography to give the light brown solid (166 mg, 35%). 1H NMR (400 MHz, CDCl3) δ 6.94 (dd, J = 4.1, 1.7 Hz, 1H), 6.83 (t, J = 2.1 Hz, 1H), 6.08 (dd, J = 4.1, 2.5 Hz, 1H), 4.21 (t, J = 7.4 Hz, 2H), 2.40 (s, 3H), 2.12 (t, J = 7.4 Hz, 2H), 1.69–1.60 (m, 2H), 1.59–1.50 (m, 2H), 1.32–1.20 (overlap, 4H). 13C NMR (100 MHz, CDCl3) δ 188.7, 171.8, 130.7, 129.8, 121.0, 108.2, 49.7, 32.7, 31.1, 28.4, 27.3, 26.0, 25.2. HRMS (ESI), m/z: calcd for C13H19N2O3−: 251.1401 [M − H]−; found: 251.1399.
3.1.10. 8-(2-Acetyl-1H-pyrrol-1-yl)-N-hydroxyoctanamide (3e)
Compound 3e was prepared using similar procedures as for 3a from compound 2e (300 mg, 1.08 mmol). The crude product was purified by silica gel chromatography to give the light brown solid (121 mg, 42%). 1H NMR (400 MHz, CDCl3) δ 6.98 (dd, J = 4.1, 1.7 Hz, 1H), 6.86 (t, J = 2.1 Hz, 1H), 6.13 (dd, J = 4.1, 2.5 Hz, 1H), 4.27 (t, J = 7.3 Hz, 2H), 2.44 (s, 3H), 2.16 (t, J = 7.3 Hz, 2H), 1.72–1.60 (overlap, 4H), 1.33–1.26 (overlap, 6H). 13C NMR (125 MHz, CDCl3) δ 188.9, 171.5, 130.7, 130.1, 120.9, 108.3, 49.8, 32.9, 31.3, 28.6, 28.4, 27.4, 26.2, 25.2. HRMS (ESI), m/z: calcd for C14H21N2O3−: 265.1558 [M − H]−; found: 265.1558.
3.1.11. Ethyl 7-(1H-pyrrol-1-yl)heptanoate (4a)
Starting from ethyl 7-bromoheptanoate (1d) (1.55 g, 6.53 mmol) and pyrrole (364 mg, 5.44 mmol), the title compound was obtained following the procedure previously described for compound 2a. The crude product was purified by silica gel chromatography to give the colorless oil 4a (546 mg, 45%). 1H NMR (400 MHz, CDCl3) δ 6.63 (t, J = 2.1 Hz, 2H), 6.12 (t, J = 2.1 Hz, 2H), 4.12 (q, J = 7.1 Hz, 2H), 3.86 (t, J = 7.1 Hz, 2H), 2.27 (t, J = 7.5 Hz, 2H), 1.76 (p, J = 7.3 Hz, 2H), 1.64–1.57 (m, 2H), 1.37–1.28 (overlap, 4H), 1.25 (t, J = 7.1 Hz, 3H). 13C NMR (100 MHz, CDCl3) δ 173.8, 120.5 × 2, 107.9 × 2, 60.3, 49.6, 34.3, 31.5, 28.8, 26.5, 24.9, 14.4.
3.1.12. Ethyl 7-(2-propionyl-1H-pyrrol-1-yl)heptanoate (4b)
Starting from ethyl 7-bromoheptanoate (1d) (927 mg, 3.91 mmol) and 2-propionylpyrrole (401 mg, 3.26 mmol), the title compound was obtained following the procedure previously described for compound 2a. The crude product was purified by silica gel chromatography to give the colorless oil 4b (791 mg, 87%). 1H NMR (600 MHz, CDCl3) δ 6.96 (dd, J = 4.1, 1.7 Hz, 1H), 6.83 (t, J = 2.1 Hz, 1H), 6.10 (dd, J = 4.1, 2.5 Hz, 1H), 4.29 (t, J = 7.3 Hz, 2H), 4.11 (q, J = 7.1 Hz, 2H), 2.81 (q, J = 7.4 Hz, 2H), 2.26 (t, J = 7.5 Hz, 2H), 1.72 (p, J = 7.4 Hz, 2H), 1.60 (p, J = 7.5 Hz, 2H), 1.36–1.27 (overlap, 4H), 1.24 (t, J = 7.1 Hz, 3H), 1.17 (t, J = 7.4 Hz, 3H). 13C NMR (150 MHz, CDCl3) δ 191.8, 173.9, 130.0, 129.8, 119.3, 107.9, 60.3, 49.9, 34.4, 32.4, 31.5, 28.9, 26.4, 25.0, 14.4, 9.1.
3.1.13. Ethyl 7-(2-(2,2,2-trifluoroacetyl)-1H-pyrrol-1-yl)heptanoate (4c)
Starting from ethyl 7-bromoheptanoate (1d) (870 mg, 3.67 mmol) and 2-(trifluoroacetyl)pyrrole (499 mg, 3.06 mmol), the title compound was obtained following the procedure previously described for compound 2a. The crude product was purified by silica gel chromatography to give the colorless oil 4c (810 mg, 83%). 1H NMR (600 MHz, CDCl3) δ 7.24–7.21 (m, 1H), 7.08 (t, J = 2.0 Hz, 1H), 6.26 (dd, J = 4.4, 2.4 Hz, 1H), 4.31 (t, J = 7.3 Hz, 2H), 4.12 (q, J = 7.1 Hz, 2H), 2.28 (t, J = 7.5 Hz, 2H), 1.77–1.71 (m, 2H), 1.62–1.60 (m, 2H), 1.38–1.29 (overlap, 4H), 1.25 (t, J = 7.1 Hz, 3H). 13C NMR (150 MHz, CDCl3) δ 173.8, (169.9, 169.7), 134.2, (124.6, 124.5), 124.1, (118.2, 116.3), 110.3, 60.4, 50.3, 34.3, 31.1, 28.7, 26.3, 24.9, 14.4.
3.1.14. Ethyl 7-(2-(2,2,2-trichloroacetyl)-1H-pyrrol-1-yl)heptanoate (4d)
Starting from ethyl 7-bromoheptanoate (1d) (668 mg, 2.82 mmol) and 2-(trichloroacetyl)pyrrole (498 mg, 2.35 mmol), the title compound was obtained following the procedure previously described for compound 2a. The crude product was purified by silica gel chromatography to give the colorless oil 4d (675 mg, 78%). 1H NMR (600 MHz, CDCl3) δ 7.52 (dd, J = 4.4, 1.6 Hz, 1H), 7.00 (t, J = 2.0 Hz, 1H), 6.21 (dd, J = 4.4, 2.4 Hz, 1H), 4.29 (t, J = 7.3 Hz, 2H 2H), 4.11 (q, J = 7.2 Hz, 2H), 2.27 (t, J = 7.5 Hz, 2H), 1.75 (p, J = 7.2 Hz, 2H), 1.61 (p, J = 7.4 Hz, 2H), 1.37–1.30 (overlap, 4H), 1.24 (t, J = 7.2 Hz, 3H). 13C NMR (150 MHz, CDCl3) δ 173.8, 172.6, 133.1, 124.7, 121.1, 109.1, 96.6, 60.3, 50.7, 34.3, 31.1, 28.8, 26.3, 24.9, 14.4.
3.1.15. Ethyl 7-(2-benzoyl-1H-pyrrol-1-yl)heptanoate (4e)
Starting from ethyl 7-bromoheptanoate (1d) (827 mg, 3.49 mmol) and 2-benzoylpyrrole (498 mg, 2.91 mmol), the title compound was obtained following the procedure previously described for compound 2a. The crude product was purified by silica gel chromatography to give the colorless oil 4e (752 mg, 79%). 1H NMR (400 MHz, CDCl3) δ 7.79–7.75 (overlap, 2H), 7.53–749 (m, 1H), 7.45–7.40 (overlap, 2H), 6.96 (dd, J = 2.5, 1.7 Hz, 1H), 6.72 (dd, J = 4.0, 1.7 Hz, 1H), 6.14 (dd, J = 4.1, 2.5 Hz, 1H), 4.39 (t, J = 7.4 Hz, 2H), 4.11 (q, J = 7.1 Hz, 2H), 2.27 (t, J = 7.5 Hz, 2H), 1.86–1.76 (m, 2H), 1.67–1.56 (m, 2H), 1.38–1.30 (overlap, 4H), 1.24 (t, J = 7.1 Hz, 3H). 13C NMR (100 MHz, CDCl3) δ 186.2, 173.8, 140.3, 131.4, 130.7, 129.9, 129.3 × 2, 128.1 × 2, 123.6, 108.2, 60.3, 49.6, 34.3, 31.7, 28.8, 26.5, 25.0, 14.4.
3.1.16. Ethyl 7-(1H-indol-1-yl)heptanoate (4f)
Starting from ethyl 7-bromoheptanoate (1d) (1.21 g, 5.10 mmol) and indole (497 mg, 4.25 mmol), the title compound was obtained following the procedure previously described for compound 2a. The crude product was purified by silica gel chromatography to give the colorless oil 4f (963 mg, 83%). 1H NMR (600 MHz, CDCl3) δ 7.62 (d, J = 7.9 Hz, 1H), 7.32 (d, J = 8.3 Hz, 1H), 7.19 (t, J = 7.6 Hz, 1H), 7.11–7.04 (overlap, 2H), 6.47(d, J = 3.1 Hz, 1H), 4.13–4.06 (overlap, 4H), 2.25 (t, J = 7.5 Hz, 2H), 1.82 (p, J = 7.1 Hz, 2H), 1.59 (p, J = 7.5 Hz, 2H), 1.35–1.28 (overlap, 4H), 1.23 (t, J = 7.2 Hz, 3H). 13C NMR (150 MHz, CDCl3) δ 173.7, 136.0, 128.7, 127.8, 121.4, 121.0, 119.3, 109.4, 101.0, 60.3, 46.3, 34.3, 30.1, 28.8, 26.7, 24.9, 14.3.
3.1.17. Ethyl 7-(5-methyl-1H-indol-1-yl)heptanoate (4g)
Starting from ethyl 7-bromoheptanoate (1d) (870 mg, 3.67 mmol) and 5-methylindole (401 mg, 3.06 mmol), the title compound was obtained following the procedure previously described for compound 2a. The crude product was purified by silica gel chromatography to give the colorless oil 4g (677 mg, 77%). 1H NMR (600 MHz, CDCl3) δ 7.42–7.39 (m, 1H), 7.22 (d, J = 8.3 Hz, 1H), 7.05–7.01 (overlap, 2H), 6.39 (dd, J = 3.1, 0.9 Hz, 1H), 4.13–4.09 (m, 2H), 4.08 (t, J = 7.1 Hz, 2H), 2.45 (s, 3H), 2.26 (t, J = 7.5 Hz, 2H), 1.83 (p, J = 7.1 Hz, 2H), 1.62–1.57 (m, 2H), 1.40–1.29 (overlap, 4H), 1.25 (t, J = 7.1 Hz, 3H). 13C NMR (150 MHz, CDCl3) δ 173.8, 134.5, 129.0, 128.5, 128.0, 123.1, 120.7, 109.2, 100.4, 60.4, 46.5, 34.4, 30.2, 28.9, 26.8, 24.9, 21.5, 14.4.
3.1.18. Ethyl 7-(6-fluoro-1H-indol-1-yl)heptanoate (4h)
Starting from ethyl 7-bromoheptanoate (1d) (1.39 g, 5.87 mmol) and 6-fluoroindole (660 mg, 4.89 mmol), the title compound was obtained following the procedure previously described for compound 2a. The crude product was purified by silica gel chromatography to give the colorless oil 4h (711 mg, 50%). 1H NMR (400 MHz, CDCl3) δ 7.52 (dd, J = 8.6, 5.4 Hz, 1H), 7.06 (d, J = 3.2 Hz, 1H), 7.00 (dd, J = 10.0, 2.3 Hz, 1H), 6.86 (ddd, J = 9.6, 8.6, 2.3 Hz, 1H), 6.46 (dd, J = 3.1, 0.9 Hz, 1H), 4.12 (q, J = 7.1 Hz, 2H), 4.04 (t, J = 7.1 Hz, 2H), 2.27 (t, J = 7.5 Hz, 2H), 1.82 (p, J = 7.3 Hz, 2H), 1.64–1.58 (m, 2H), 1.40–1.29 (overlap, 4H), 1.25 (t, J = 7.1 Hz, 3H). 13C NMR (100 MHz, CDCl3) δ 173.8, (161.0 158.6), (136.1, 136.0), (128.3, 128.2), 125.1, (121.8, 121.7), (108.2, 107.9), 101.3, (96.0, 95.7), 60.4, 46.5, 34.3, 30.0, 28.8, 26.7, 24.9, 14.4.
3.1.19. Ethyl 7-(5-fluoro-1H-indol-1-yl)heptanoate (4i)
Starting from ethyl 7-bromoheptanoate (1d) (1.05 g, 4.44 mmol) and 5-fluoroindole (500 mg, 3.70 mmol), the title compound was obtained following the procedure previously described for compound 2a. The crude product was purified by silica gel chromatography to give the colorless oil 4i (829 mg, 77%). 1H NMR (400 MHz, CDCl3) δ 7.27–7.23 (m, 1H), 7.22–7.19 (m, 1H), 7.10 (d, J = 3.1 Hz, 1H), 6.93 (td, J = 9.1, 2.5 Hz, 1H), 6.42 (dd, J = 3.1, 0.8 Hz, 1H), 4.13–4.04 (overlap, 4H), 2.26 (t, J = 7.4 Hz, 2H), 1.81 (p, J = 7.2 Hz, 2H), 1.63–1.55 (m, 2H), 1.37–1.27 (overlap, 4H), 1.24 (t, J = 7.1 Hz, 3H). 13C NMR (100 MHz, CDCl3) δ 173.7, (159.0, 156.7), 132.7, 129.4, (128.9, 128.8), (110.0, 109.7), (109.96, 109.94), (105.8, 105.5), (101.0, 100.9), 60.3, 46.6, 34.3, 30.1, 28.8, 26.7, 24.9, 14.4.
3.1.20. Ethyl 7-(5-chloro-1H-indol-1-yl)heptanoate (4j)
Starting from ethyl 7-bromoheptanoate (1d) (754 mg, 3.18 mmol) and 5-chloroindole (400 mg, 2.65 mmol), the title compound was obtained following the procedure previously described for compound 2a. The crude product was purified by silica gel chromatography to give the colorless oil 4j (724 mg, 89%). 1H NMR (600 MHz, CDCl3) δ 7.58 (d, J = 2.0 Hz, 1H), 7.24 (d, J = 8.7 Hz, 1H), 7.14 (dd, J = 8.7, 2.0 Hz, 1H), 7.10 (d, J = 3.1 Hz, 1H), 6.42 (d, J = 3.0 Hz, 1H), 4.19–4.02 (overlap, 4H), 2.26 (t, J = 7.5 Hz, 2H), 1.82 (p, J = 7.2 Hz, 2H), 1.62–1.57 (m, 2H), 1.36–1.30 (overlap, 4H), 1.24 (t, J = 7.1 Hz, 3H). 13C NMR (150 MHz, CDCl3) δ 173.8, 134.5, 129.7, 129.2, 125.1, 121.8, 120.4, 110.5, 100.8, 60.4, 46.6, 34.3, 30.2 28.8, 26.7, 24.9, 14.4.
3.1.21. Ethyl 7-(5-bromo-1H-indol-1-yl)heptanoate (4k)
Starting from ethyl 7-bromoheptanoate (1d) (723 mg, 3.05 mmol) and 5-bromoindole (498 mg, 2.54 mmol), the title compound was obtained following the procedure previously described for compound 2a. The crude product was purified by silica gel chromatography to give the colorless oil 4k (715 mg, 80%). 1H NMR (600 MHz, CDCl3) δ 7.73 (d, J = 1.9 Hz, 1H), 7.26 (dd, J = 8.8, 1.8 Hz, 1H), 7.18 (d, J = 8.7 Hz, 1H), 7.07 (d, J = 3.1 Hz, 1H), 6.41 (d, J = 3.1 Hz, 1H), 4.11 (q, J = 7.1 Hz, 2H), 4.06 (t, J = 7.1 Hz, 2H), 2.25 (t, J = 7.5 Hz, 2H), 1.80 (p, J = 7.2 Hz, 2H), 1.59 (p, J = 7.5 Hz, 2H), 1.35–1.27 (overlap, 4H), 1.24 (t, J = 7.2 Hz, 3H). 13C NMR (150 MHz, CDCl3) δ 173.7, 134.7, 130.3, 129.0, 124.3, 123.5, 112.6, 110.9, 100.7, 60.4, 46.6, 34.3, 30.1, 28.8, 26.7, 24.8, 14.4.
3.1.22. Ethyl 7-(5-cyano-1H-indol-1-yl)heptanoate (4l)
Starting from ethyl 7-bromoheptanoate (1d) (1.41 g, 5.94 mmol) and 5-cyanoindole (703 mg, 4.95 mmol), the title compound was obtained following the procedure previously described for compound 2a. The crude product was purified by silica gel chromatography to give the colorless oil 4l (1.33 g, 90%). 1H NMR (400 MHz, CDCl3) δ 7.95–7.92 (m, 1H), 7.45–7.30 (overlap, 2H), 7.20 (d, J = 3.2 Hz, 1H), 6.55 (dd, J = 3.2, 0.8 Hz, 1H), 4.15–4.06 (overlap, 4H), 2.25 (t, J = 7.4 Hz, 2H), 1.83 (p, J = 7.2 Hz, 2H), 1.59 (p, J = 7.5 Hz, 2H), 1.37–1.27 (overlap, 4H), 1.23 (t, J = 7.1 Hz, 3H). 13C NMR (100 MHz, CDCl3) δ 173.6, 137.5, 130.2, 128.3, 126.6, 124.4, 120.9, 110.3, 102.3 × 2, 60.3, 46.6, 34.2, 30.1, 28.7, 26.6, 24.7, 14.3.
3.1.23. N-hydroxy-7-(1H-pyrrol-1-yl)heptanamide (5a)
Compound 5a was prepared using similar procedures as for 3a from compound 4a (546 mg, 2.45 mmol). The crude product was purified by silica gel chromatography to give the dark brown solid (360 mg, 70%). 1H NMR (600 MHz, CDCl3) δ 6.65 (t, J = 2.1 Hz, 2H), 6.15 (t, J = 2.1 Hz, 2H), 3.86 (t, J = 7.1 Hz, 2H), 2.09 (t, J = 7.5 Hz, 2H), 1.74 (p, J = 7.1 Hz, 2H), 1.59 (p, J = 7.5 Hz, 2H), 1.34–1.24 (overlap, 4H). 13C NMR (150 MHz, CDCl3) δ 172.0, 120.6 × 2, 107.9 × 2, 49.5, 32.7, 31.3, 28.6, 26.3, 25.2. HRMS (ESI), m/z: calcd for C11H17N2O2−: 209.1296 [M − H]−; found: 209.1298.
3.1.24. N-hydroxy-7-(2-propionyl-1H-pyrrol-1-yl)heptanamide (5b)
Compound 5b was prepared using similar procedures as for 3a from compound 4b (399 mg, 1.43 mmol). The crude product was purified by silica gel chromatography to give the white solid (228 mg, 60%). 1H NMR (600 MHz, CDCl3) δ 6.97 (dd, J = 4.1, 1.7 Hz, 1H), 6.84 (t, J = 2.1 Hz, 1H), 6.10 (dd, J = 4.1, 2.4 Hz, 1H), 4.25 (t, J = 7.5 Hz, 2H), 2.81 (q, J = 7.4 Hz, 2H), 2.15 (t, J = 7.5 Hz, 2H), 1.69 (p, J = 7.4 Hz, 2H), 1.61 (p, J = 7.4 Hz, 2H), 1.36–1.26 (overlap, 4H), 1.16 (t, J = 7.4 Hz, 3H). 13C NMR (150 MHz, CDCl3) δ 192.4, 171.7, 130.4, 129.7, 119.9, 108.1, 49.8, 32.8, 32.4, 31.1, 28.3, 26.0, 25.1, 9.3. HRMS (ESI), m/z: calcd for C14H21N2O3−: 265.1558 [M − H]−; found: 265.1557.
3.1.25. N-hydroxy-7-(2-(2,2,2-trifluoroacetyl)-1H-pyrrol-1-yl)heptanamide (5c)
Compound 5c was prepared using similar procedures as for 3a from compound 4c (393 mg, 1.23 mmol). The crude product was purified by silica gel chromatography to give the light brown solid (53 mg, 14%). 1H NMR (600 MHz, CDCl3 (CD3OD)) δ 7.22–7.18 (m, 1H), 7.09–7.06 (m, 1H), 6.24 (t, J = 3.4 Hz, 1H), 4.26 (t, J = 7.4 Hz, 2H), 2.08 (t, J = 7.5 Hz, 2H), 1.68 (p, J = 7.4 Hz, 2H), 1.57 (p, J = 7.4 Hz, 2H), 1.32–1.25 (overlap, 4H). 13C NMR (151 MHz, CDCl3 (CD3OD)) δ 171.6, (169.8, 169.6), 134.6, (124.86, 124.84, 124.81), 123.9, (118.1, 116.2), 110.4, 50.2, 32.7, 30.9, 28.5, 26.0, 25.2. HRMS (ESI), m/z: calcd for C13H16F3N2O3−: 305.1119 [M − H]−; found: 305.1119.
3.1.26. Methyl 1-(7-(hydroxyamino)-7-oxoheptyl)-1H-pyrrole-2-carboxylate (5d)
Compound 5d was prepared using similar procedures as for 3a from compound 4d (412 mg, 1.12 mmol). The crude product was purified by silica gel chromatography to give the black solid (48 mg, 16%). 1H NMR (600 MHz, CDCl3) δ 6.93 (dd, J = 3.9, 1.8 Hz, 1H), 6.82 (t, J = 2.2 Hz, 1H), 6.09 (dd, J = 4.0, 2.5 Hz, 1H), 4.24 (t, J = 7.4 Hz, 2H), 3.78 (s, 3H), 2.11 (t, J = 7.6 Hz, 2H), 1.72 (p, J = 7.4 Hz, 2H), 1.59 (p, J = 7.4 Hz, 2H), 1.32–1.24 (overlap, 4H). 13C NMR (150 MHz, CDCl3) δ 171.8, 161.8, 129.0, 121.4, 118.5, 108.1, 51.2, 49.1, 32.8, 31.3, 28.4, 26.1, 25.2. HRMS (ESI), m/z: calcd for C13H19N2O4−: 267.1350 [M − H]−; found: 267.1350.
3.1.27. 7-(2-Benzoyl-1H-pyrrol-1-yl)-N-hydroxyheptanamide (5e)
Compound 5e was prepared using similar procedures as for 3a from compound 4e (396 mg, 1.21 mmol). The crude product was purified by silica gel chromatography to give the gray solid (114 mg, 30%). 1H NMR (600 MHz, CDCl3) δ 7.77–7.70 (overlap, 2H), 7.50 (t, J = 7.5 Hz, 1H), 7.43–7.38 (overlap, 2H), 6.96–6.93 (m, 1H), 6.71 (d, J = 4.1 Hz, 1H), 6.13 (t, J = 3.2 Hz, 1H), 4.32 (t, J = 7.5 Hz, 2H), 2.09 (t, J = 7.5 Hz, 2H), 1.76 (p, J = 7.1 Hz, 2H), 1.57 (t, J = 7.3 Hz, 2H), 1.33–1.25 (overlap, 4H). 13C NMR (150 MHz, CDCl3) δ 186.5, 171.8, 140.1, 131.5, 131.2, 129.7, 129.2 × 2, 128.1 × 2, 124.1, 108.4, 49.5, 32.7, 31.4, 28.4, 26.1, 25.2. HRMS (ESI), m/z: calcd for C18H21N2O3−: 313.1558 [M − H]−; found: 313.1557.
3.1.28. N-hydroxy-7-(1H-indol-1-yl)heptanamide (5f)
Compound 5f was prepared using similar procedures as for 3a from compound 4f (399 mg, 1.46 mmol). The crude product was purified by silica gel chromatography to give the brown solid (205 mg, 54%). 1H NMR (600 MHz, CDCl3) δ 7.62 (d, J = 7.9 Hz, 1H), 7.32 (d, J = 8.2 Hz, 1H), 7.20 (t, J = 7.6 Hz, 1H), 7.09 (t, J = 7.5 Hz, 1H), 7.06 (d, J = 3.1 Hz, 1H), 6.47 (d, J = 3.1 Hz, 1H), 4.09 (t, J = 7.0 Hz, 2H), 1.99 (t, J = 7.5 Hz, 2H), 1.80 (p, J = 7.1 Hz, 2H), 1.55 (q, J = 7.4 Hz, 2H), 1.30–1.23 (overlap, 4H). 13C NMR (150 MHz, CDCl3) δ 171.7, 136.1, 128.7, 128.0, 121.5, 121.1, 119.4, 109.5, 101.1, 46.4, 32.7, 30.0, 28.6, 26.6, 25.1. HRMS (ESI), m/z: calcd for C15H19N2O2−: 259.1452 [M − H]−; found: 259.1452.
3.1.29. N-hydroxy-7-(5-methyl-1H-indol-1-yl)heptanamide (5g)
Compound 5g was prepared using similar procedures as for 3a from compound 4g (100 mg, 0.35 mmol). The crude product was purified by silica gel chromatography to give the brown solid (53 mg, 55%). 1H NMR (400 MHz, CDCl3) δ 7.42 (s, 1H), 7.22 (d, J = 8.3 Hz, 1H), 7.05–7.00 (overlap, 2H), 6.39 (d, J = 3.0 Hz, 1H), 4.03 (t, J = 7.0 Hz, 2H), 2.46 (s, 3H), 1.96 (t, J = 7.5 Hz, 2H), 1.80–1.70 (m, 2H), 1.56–1.46 (m, 2H), 1.28–1.20 (overlap, 4H). 13C NMR (100 MHz, CDCl3) δ 171.9, 134.4, 128.9, 128.5, 128.0, 123.1, 120.7, 109.2, 100.4, 46.3, 32.7, 30.0, 28.6, 26.5, 25.2, 21.5. HRMS (ESI), m/z: calcd for C16H21N2O2−: 273.1609 [M − H]−; found: 273.1608.
3.1.30. 7-(6-Fluoro-1H-indol-1-yl)-N-hydroxyheptanamide (5h)
Compound 5h was prepared using similar procedures as for 3a from compound 4h (296 mg, 1.02 mmol). The crude product was purified by silica gel chromatography to give the white solid (119 mg, 42%). 1H NMR (600 MHz, CDCl3) δ 7.50 (dd, J = 8.6, 5.4 Hz, 1H), 7.03 (d, J = 3.1 Hz, 1H), 6.98 (dd, J = 10.0, 2.3 Hz, 1H), 6.85 (td, J = 9.1, 2.2 Hz, 1H), 6.44 (d, J = 3.1 Hz, 1H), 4.00 (t, J = 7.1 Hz, 2H), 2.04 (t, J = 7.4 Hz, 2H), 1.76 (p, J = 7.0 Hz, 2H), 1.55 (p, J = 7.1 Hz, 2H), 1.30–1.20 (overlap, 4H). 13C NMR (150 MHz, CDCl3) δ 171.7, (160.6, 159.0), (136.1, 136.0), (128.4, 128.3), 125.1, (121.8, 121.7), (108.2, 108.0), 101.30, (95.9, 95.8), 46.5, 32.8, 29.9, 28.6, 26.6, 25.2. HRMS (ESI), m/z: calcd for C15H18FN2O2−: 277.1358 [M − H]−; found: 277.1356.
3.1.31. 7-(5-Fluoro-1H-indol-1-yl)-N-hydroxyheptanamide (5i)
Compound 5i was prepared using similar procedures as for 3a from compound 4i (499 mg, 1.71 mmol). The crude product was purified by silica gel chromatography to give the light brown solid (243 mg, 51%). 1H NMR (600 MHz, CDCl3) δ 7.25–7.23 (m, 1H), 7.21 (dd, J = 8.9, 4.3 Hz, 1H), 7.09 (d, J = 3.1 Hz, 1H), 6.94 (td, J = 9.1, 2.5 Hz, 1H), 6.42 (d, J = 3.0 Hz, 1H), 4.06 (t, J = 7.0 Hz, 2H), 2.04 (t, J = 7.5 Hz, 2H), 1.79 (p, J = 7.1 Hz, 2H), 1.57 (p, J = 7.4 Hz, 2H), 1.30–1.23 (overlap, 4H). 13C NMR (150 MHz, CDCl3) δ 171.5, (158.7 157.1), 132.7, 129.5, (128.9, 128.8), (110.1, 109.8), 110.0, (105.8, 105.7), (101.1, 101.0), 46.6, 32.8, 30.0, 28.7, 26.6, 25.1. HRMS (ESI), m/z: calcd for C15H18FN2O2−: 277.1358 [M − H]−; found: 277.1356.
3.1.32. 7-(5-Chloro-1H-indol-1-yl)-N-hydroxyheptanamide (5j)
Compound 5j was prepared using similar procedures as for 3a from compound 4j (305 mg, 0.99 mmol). The crude product was purified by silica gel chromatography to give the brown solid (70 mg, 24%). 1H NMR (600 MHz, CDCl3) δ 7.56 (d, J = 2.0 Hz, 1H), 7.20 (d, J = 8.6 Hz, 1H), 7.12 (dd, J = 8.6, 2.0 Hz, 1H), 7.05 (d, J = 3.0 Hz, 1H), 6.39 (d, J = 2.9 Hz, 1H), 3.98 (t, J = 7.0 Hz, 2H), 2.06–1.90 (m, 2H), 1.73–1.64 (m, 2H), 1.52–1.42 (m, 2H), 1.23–1.12 (overlap, 4H). 13C NMR (150 MHz, CDCl3) δ 172.0, 134.4, 129.6, 129.3, 125.0, 121.7, 120.3, 110.5, 100.7, 46.4, 32.7, 30.0, 28.5, 26.5, 25.2. HRMS (ESI), m/z: calcd for C15H18ClN2O2−: 293.1062 [M − H]−; found: 293.1062.
3.1.33. 7-(5-Bromo-1H-indol-1-yl)-N-hydroxyheptanamide (5k)
Compound 5k was prepared using similar procedures as for 3a from compound 4k (398 mg, 1.13 mmol). The crude product was purified by silica gel chromatography to give the brown solid (77 mg, 20%). 1H NMR (600 MHz, CDCl3) δ 7.73–7.68 (m, 1H), 7.26–7.19 (m, 1H), 7.15–7.11 (m, 1H), 7.01 (d, J = 2.8 Hz, 1H), 6.37 (d, J = 3.0 Hz, 1H), 3.97 (t, J = 7.1 Hz, 2H), 1.97 (t, J = 7.5 Hz, 2H), 1.68 (t, J = 7.3 Hz, 2H), 1.47 (p, J = 7.1 Hz, 2H), 1.21–1.11 (overlap, 4H). 13C NMR (150 MHz, CDCl3) δ 171.9, 134.7, 130.3, 129.1, 124.2, 123.4, 112.6, 111.0, 100.7, 46.4, 32.7, 30.0, 28.5, 26.5, 25.2. HRMS (ESI), m/z: calcd for C15H18BrN2O2−: 337.0557 [M − H]−; found: 337.0556.
3.1.34. 7-(5-Cyano-1H-indol-1-yl)-N-hydroxyheptanamide (5l)
Compound 5l was prepared using similar procedures as for 3a from compound 4l (409 mg, 1.37 mmol). The crude product was purified by silica gel chromatography to give the gray solid (43 mg, 11%). 1H NMR (600 MHz, CD3OD) δ 7.93–790 (m, 1H), 7.42 (d, J = 8.6 Hz, 1H), 7.37 (d, J = 8.6 Hz, 1H), 7.27 (d, J = 3.3 Hz, 1H), 6.54 (d, J = 3.2 Hz, 1H), 4.14 (t, J = 7.1 Hz, 2H), 2.05–2.00 (m, 2H), 1.83–1.76 (m, 2H), 1.56 (p, J = 7.4 Hz, 2H), 1.34–1.24 (overlap, 4H). 13C NMR (150 MHz, CD3OD) δ 171.0, 137.3, 130.0, 127.9, 125.9, 123.5, 120.3, 109.9, 101.5, 100.9, 45.8, 32.1, 29.5, 28.0, 25.9, 24.8. HRMS (ESI), m/z: calcd for C16H18N3O2−: 284.1405 [M − H]−; found: 284.1403.
3.1.35. Methyl 4-(2-bromoethyl)benzoate (7)
To a solution of 4-(2-bromoethyl)benzoic acid (6) (500 mg, 2.19 mmol) in MeOH (10 mL) was added thionyl chloride (0.3 mL), and the resulting mixture was stirred under refluxing condition for 2 h. The reaction was complete detected by TLC. The solvent was evaporated under reduced pressure and diluted with H2O (20 mL). The mixture was extracted with ethyl acetate (3 × 10 mL). The combined organic layers were washed with brine, dried by anhydrous Na2SO4, filtered, and evaporated under reduced pressure to afford the crude product, which was purified by column chromatography eluting with petroleum ether/ethyl acetate (10:1) to give 7 as a colorless oil (513 mg, 97%). 1H NMR (400 MHz, CDCl3) δ 8.02–7.98 (overlap, 2H), 7.32–7.27 (overlap, 2H), 3.91 (s, 3H), 3.59 (t, J = 7.4 Hz, 2H), 3.23 (t, J = 7.4 Hz, 2H). 13C NMR (125 MHz, CDCl3) δ 167.0, 144.1, 130.0 × 2, 128.8 × 2, 128.5, 52.2, 39.2, 32.2.
3.1.36. Methyl 4-(2-(2-acetyl-1H-pyrrol-1-yl)ethyl)benzoate (8)
To a solution of 2-acetyl pyrrole (196 mg, 1.80 mmol) in dry toluene (15 mL) was added potassium hydroxide (61 mg, 1.09 mmol) and 18-crown-6 (79 mg, 0.30 mmol). The reaction solution was refluxed and stirred for 1 h. Compound 7 (300 mg, 1.24 mmol) was added, and the mixture was stirred overnight. The reaction was complete detected by TLC. The solvent was evaporated under reduced pressure and diluted with H2O (20 mL). The mixture was extracted with ethyl acetate (3 × 15 mL). The combined organic layers were washed with brine, dried by anhydrous Na2SO4, filtered, and the solvent was evaporated under reduced pressure to afford the crude product, which was purified by column chromatography eluting with petroleum ether/ethyl acetate (10:1) to give 8 as a colorless oil (130 mg, 39%). 1H NMR (400 MHz, CDCl3) δ 7.96–7.87 (overlap, 2H), 7.21–7.12 (overlap, 2H), 6.98 (dd, J = 4.1, 1.7 Hz, 1H), 6.58 (t, J = 2.2 Hz, 1H), 6.05 (dd, J = 4.1, 2.5 Hz, 1H), 4.51 (t, J = 7.2 Hz, 2H), 3.90 (s, 3H), 3.07 (t, J = 7.3 Hz, 2H), 2.46 (s, 3H). 13C NMR (125 MHz, CDCl3) δ 188.5, 167.2, 144.1, 130.6, 130.0, 129.9 × 2, 129.2 × 2, 128.6, 120.8, 108.1, 52.2, 51.3, 38.2, 27.4.
3.1.37. 4-(2-(2-Acetyl-1H-pyrrol-1-yl)ethyl)benzoic Acid (9)
To a solution of compound 8 (130 mg, 0.48 mmol) in H2O (2 mL) and MeOH (8 mL) was added lithium hydroxide (23 mg, 0.96 mmol). The reaction mixture was stirred at 40 °C until complete as indicated by TLC. Then the mixture was acidified by adding 1 M HCl solution and extracted with ethyl acetate (3 × 10 mL). The combined organic layers were washed with brine, dried by anhydrous Na2SO4, filtered, and concentrated under reduced pressure to give the crude product, which was further purified by column chromatography eluting with 2%–10% MeOH/DCM to give 9 as a white solid (100 mg, 81%). 1H NMR (400 MHz, CDCl3) δ 8.03–7.98 (overlap, 2H), 7.24–7.18 (overlap, 2H), 7.00 (dd, J = 4.0, 1.7 Hz, 1H), 6.59 (m, 1H), 6.06 (dd, J = 4.1, 2.5 Hz, 1H), 4.53 (t, J = 7.2 Hz, 2H), 3.10 (t, J = 7.2 Hz, 2H), 2.48 (s, 3H). 13C NMR (125 MHz, CDCl3) δ 188.6, 171.7, 145.1, 130.7, 130.6 × 2, 130.0, 129.3 × 2, 127.8, 120.9, 108.2, 51.2, 38.2, 27.4.
3.1.38. 4-(2-(2-Acetyl-1H-pyrrol-1-yl)ethyl)-N-((tetrahydro-2H-pyran-2-yl)oxy) Benzamide (10)
To a solution of 9 (100 mg, 0.39 mmol) and O-(tetrahydro-2H-pyran-2-yl)hydroxylamine (91 mg, 0.78 mmol) in dichloromethane (10 mL) was added HATU (372 mg, 0.98 mmol) and DIPEA (126 mg, 0.98 mmol). The resulting mixture was stirred at room temperature overnight. The reaction was complete detected by TLC. The reaction mixture was then quenched with H2O (20 mL). The mixture was extracted with ethyl acetate (3 × 15 mL). The combined organic layers were washed with brine, dried by anhydrous Na2SO4, filtered, and concentrated under reduced pressure to afford the crude product, which was purified by column chromatography eluting with 1%–5% DCM/MeOH to give 10 as a colorless oil (50 mg, 36%). 1H NMR (400 MHz, CDCl3) δ 9.09 (s, 1H), 7.68–7.62 (overlap, 2H), 7.18–7.13 (overlap, 2H), 6.97 (d, J = 4.1 Hz, 1H), 6.57 (t, J = 2.1 Hz, 1H), 6.03 (t, J = 3.3 Hz, 1H), 5.09–5.02 (m, 1H), 4.48 (t, J = 7.2 Hz, 2H), 4.03–3.95 (m, 1H), 3.65–3.59 (m, 1H), 3.03 (t, J = 7.2 Hz, 2H), 2.45 (s, 3H), 1.91–1.81 (overlap, 3H), 1.68–1.55 (overlap, 3H). 13C NMR (150 MHz, CDCl3) δ 188.6, 166.0, 143.1, 130.7 × 2, 130.2, 129.9, 129.4 × 2, 127.5, 120.9, 108.2, 102.8, 62.9, 51.3, 38.0, 28.2, 27.4, 25.1, 18.8.
3.1.39. 4-(2-(2-Acetyl-1H-pyrrol-1-yl)ethyl)-N-hydroxybenzamide (11)
To a solution of compound 10 (50 mg, 0.14 mmol) in MeOH (6 mL) was added p-toluenesulfonic acid (2.5 mg, 0.014 mmol). The reaction mixture was stirred at room temperature for 3 h. The reaction was complete detected by TLC. The solvent was evaporated under reduced pressure. The residue was pulped with dichloromethane and ethyl acetate (1:1) and filtered to give 11 as a brown solid (12 mg, 32%). 1H NMR (400 MHz, CD3OD) δ 7.68–7.58 (overlap, 2H), 7.25–7.17 (overlap, 2H), 7.11 (d, J = 4.2 Hz, 1H), 6.85–6.78 (m, 1H), 6.08–6.03 (m, 1H), 4.54 (t, J = 7.2 Hz, 2H), 3.03 (t, J = 7.2 Hz, 2H), 2.43 (s, 3H). 13C NMR (125 MHz, CD3OD) δ 190.5, 144.1, 132.7, 131.7, 131.0, 130.3 × 2, 128.2 × 2, 122.9, 109.3, 51.9, 38.8, 27.1. HRMS (ESI), m/z: calcd for C15H15N2O−: 271.1088 [M − H]−; found: 271.1087.
3.1.40. Methyl (E)-3-(4-((2-acetyl-1H-pyrrol-1-yl)methyl)phenyl)acrylate (13)
Starting from methyl (E)-3-(4-(bromomethyl)phenyl)acrylate (12) (278 mg, 1.09 mmol) and 2-acetyl pyrrole (99 mg, 0.91 mmol), the title compound was obtained following the procedure previously described for compound 2a. The crude product was purified by silica gel chromatography to give the colorless oil 13 (206 mg, 80%). 1H NMR (600 MHz, CDCl3) δ 7.64 (d, J = 16.0 Hz, 1H), 7.45–7.42 (overlap, 2H), 7.11–7.07 (overlap, 2H), 7.02 (dd, J = 4.1, 1.7 Hz, 1H), 6.92 (dd, J = 2.6, 1.7 Hz, 1H), 6.39 (d, J = 16.0 Hz, 1H), 6.21 (dd, J = 4.1, 2.6 Hz, 1H), 5.58 (s, 2H), 3.79 (s, 3H), 2.40 (s, 3H). 13C NMR (150 MHz, CDCl3) δ 188.5, 167.5, 144.5, 140.9, 133.7, 130.6, 130.4, 128.5 × 2, 127.5 × 2, 120.6, 117.8, 108.9, 52.5, 51.8, 27.4.
3.1.41. (E)-3-(4-((2-acetyl-1H-pyrrol-1-yl)methyl)phenyl)-N-hydroxyacrylamide (14)
Starting from 13 (43 mg, 0.15 mmol), the title compound was obtained following the procedure previously described for compound 11. All the intermediates were only extracted and dried without further purification. The final crude product was purified by silica gel chromatography to give the white solid 14 (15 mg, 35% yield over three steps). 1H NMR (500 MHz, CD3OD) δ 7.52 (d, J = 15.8 Hz, 1H), 7.48–7.43 (overlap, 2H), 7.18–7.14 (overlap, 2H), 7.08–7.05 (overlap, 2H), 6.42 (d, J = 15.8 Hz, 1H), 6.24 (dd, J = 4.0, 2.7 Hz, 1H), 5.59 (s, 2H), 2.37 (s, 3H). 13C NMR (125 MHz, CD3OD) δ 190.4, 166.3, 142.2, 141.2, 135.2, 133.0, 131.3, 128.9 × 2, 128.3 × 2, 122.8, 118.3, 109.9, 53.2, 27.0. HRMS (ESI), m/z: calcd for C16H15N2O−: 283.1088 [M − H]−; found: 283.1086.
3.1.42. 1-(1-(Prop-2-yn-1-yl)-1H-pyrrol-2-yl)ethanone (16)
Starting from propargyl bromide (15) (577 mg, 4.85 mmol) and 2-acetyl pyrrole (440 mg, 4.04 mmol), the title compound was obtained following the procedure previously described for compound 2a. The crude product was purified by silica gel chromatography to give the colorless oil 16 (529 mg, 89%). 1H NMR (400 MHz, CDCl3) δ 7.17 (t, J = 2.2 Hz, 1H), 6.97 (dd, J = 4.1, 1.7 Hz, 1H), 6.18 (dd, J = 4.1, 2.6 Hz, 1H), 5.19 (d, J = 2.5 Hz, 2H), 2.43–2.40 (overlap, 4H). 13C NMR (100 MHz, CDCl3) δ 188.7, 130.0, 129.3, 120.6, 108.7, 78.3, 74.0, 38.9, 27.2.
3.1.43. 4-(2-Azidoethyl)benzoic Acid (17)
To a solution of 4-(2-bromoethyl)benzoic acid (6) (684 mg, 3.00 mmol) in THF (25 mL) was added azido trimethyl silane (518 mg, 4.50 mmol) and tetrabutylammonium fluoride trihydrate (1.26 g, 4.50 mmol). The mixture was stirred at room temperature overnight. Upon completion of the reaction, acetic acid (1 mL) and H2O (15 mL) were added. The mixture was extracted with ethyl acetate (3 × 15 mL). The combined organic layers were washed with brine, dried by anhydrous Na2SO4, filtered, and concentrated in vacuo. The residue was purified by column chromatography eluting with petroleum ether/ethyl acetate (5:1) to give 17 as a yellow oil (558 mg, 97%). 1H NMR (400 MHz, CDCl3) δ 8.11–8.05 (overlap, 2H), 7.36–7.30 (overlap, 2H), 3.56 (t, J = 7.1 Hz, 2H), 2.97 (t, J = 7.1 Hz, 2H). 13C NMR (100 MHz, CDCl3) δ 172.4, 144.6, 130.7 × 2, 129.1 × 2, 128.0, 52.0, 35.5.
3.1.44. 4-(2-(4-((2-Acetyl-1H-pyrrol-1-yl)methyl)-1H-1,2,3-triazol-1-yl)ethyl)benzoic Acid (18)
To a solution of compounds 16 (441 mg, 3.00 mmol) and 17 (478 mg, 2.50 mmol) in DMF (30 mL) was added copper (II) sulfate pentahydrate (31 mg, 0.125 mmol) and sodium L-ascorbate (50 mg, 0.25 mmol) dissolved in H2O (10 mL). The reaction mixture was stirred at room temperature overnight. The reaction was complete detected by TLC. Then, H2O (30 mL) was added, and the mixture was extracted with ethyl acetate (3 × 15 mL). The combined organic layers were washed with brine, dried by anhydrous Na2SO4, filtered, and concentrated in vacuo, affording the crude product, which was purified by column chromatography eluting with 1%–5% DCM/MeOH to give 18 as a yellow oil (740 mg, 88%). 1H NMR (400 MHz, CDCl3) δ 8.00–7.94 (overlap, 2H), 7.49 (s, 1H), 7.19–7.15 (m, 1H), 7.14–7.09 (overlap, 2H), 6.98 (dd, J = 4.1, 1.7 Hz, 1H), 6.16 (dd, J = 4.1, 2.6 Hz, 1H), 5.59 (s, 2H), 4.56 (t, J = 7.2 Hz, 2H), 3.25 (t, J = 7.2 Hz, 2H), 2.42 (s, 3H). 13C NMR (100 MHz, CDCl3) δ 189.0, 170.2, 144.4, 142.7, 131.2, 130.7 × 2, 129.5, 128.9, 128.8 × 2, 123.8, 121.2, 109.0, 51.3, 44.1, 36.8, 27.2.
3.1.45. 4-(2-(4-((2-Acetyl-1H-pyrrol-1-yl)methyl)-1H-1,2,3-triazol-1-yl)ethyl)-N-(2-aminophenyl)benzamide (19a)
To a solution of 18 (34 mg, 0.10 mmol) and 1,2-diaminobenzene (11 mg, 0.10 mmol) in dichloromethane (10 mL) was added EDCI (23 mg, 0.12 mmol) and DMAP (1.22 mg, 0.01 mmol). The reaction mixture was stirred at room temperature until complete as indicated by TLC. Then, the mixture was quenched with H2O (15 mL) and extracted with dichloromethane (3 × 10 mL). The combined organic layers were washed with brine, dried by Na2SO4, filtered, and then concentrated under reduced pressure to give the crude product, which was purified by column chromatography eluting with petroleum ether/ethyl acetate (3:1) to give 19a as a light brown solid (15 mg, 35%). 1H NMR (400 MHz, CDCl3) δ 8.18 (s, 1H), 7.79–7.72 (overlap, 2H), 7.31 (d, J = 7.9 Hz, 1H), 7.27 (s, 1H), 7.12–7.09 (overlap, 4H), 6.96 (dd, J = 4.1, 1.7 Hz, 1H), 6.87–6.80 (overlap, 2H), 6.16 (dd, J = 4.1, 2.6 Hz, 1H), 5.54 (s, 2H), 4.53 (t, J = 7.0 Hz, 2H), 3.22 (t, J = 7.0 Hz, 2H), 2.39 (s, 3H). 13C NMR (125 MHz, CDCl3) δ 188.9, 165.6, 144.5, 141.2, 140.7, 133.3, 131.1, 129.7, 129.1 × 2, 128.0 × 2, 127.4, 125.3, 124.7, 123.5, 121.0, 119.9, 118.6, 109.0, 51.4, 44.3, 36.6, 27.3. HRMS (ESI), m/z: calcd for C24H23N6O2−: 427.1888 [M − H]−; found: 427.1888.
3.1.46. 4-(2-(4-((2-Acetyl-1H-pyrrol-1-yl)methyl)-1H-1,2,3-triazol-1-yl)ethyl)-N-(2-amino-4-fluorophenyl)benzamide (19b)
Compound 19b was prepared using similar procedures as for 19a from compound 18 (34 mg, 0.10 mmol) and 3,4-diaminofluorobenzene (12.6 mg, 0.10 mmol). The crude product was purified by silica gel chromatography to give the brown solid (23 mg, 51%). 1H NMR (400 MHz, CDCl3) δ 8.07 (s, 1H), 7.77–7.71 (overlap, 2H), 7.26 (s, 1H), 7.16 (dd, J = 8.6, 5.8 Hz, 1H), 7.11–7.05 (overlap, 3H), 6.96 (dd, J = 4.0, 1.7 Hz, 1H), 6.54–6.47 (overlap, 2H), 6.16 (dd, J = 4.1, 2.6 Hz, 1H), 5.54 (s, 2H), 4.54 (t, J = 7.0 Hz, 2H), 3.22 (t, J = 7.0 Hz, 2H), 2.39 (s, 3H). 13C NMR (125 MHz, CDCl3) δ 189.0, 166.0, (163.0, 161.1), 144.6, (143.1, 143.0), 141.4, 133.0, 131.2, 129.7, 129.2 × 2, 128.0 × 2, (127.4, 127.3), 123.5, 121.1, 120.1, 109.0, (106.3, 106.1), (104.7, 104.5), 51.4, 44.3, 36.6, 27.3. HRMS (ESI), m/z: calcd for C24H22FN6O2−: 445.1794 [M − H]−; found: 445.1794.
3.1.47. 4-(2-(4-((2-Acetyl-1H-pyrrol-1-yl)methyl)-1H-1,2,3-triazol-1-yl)ethyl)-N-(2-amino-4-chlorophenyl)benzamide (19c)
Compound 19c was prepared using similar procedures as for 19a from compound 18 (34 mg, 0.10 mmol) and 4-chloro-o-phenylenediamine (14.2 mg, 0.10 mmol). The crude product was purified by silica gel chromatography to give the brown solid (12 mg, 26%). 1H NMR (400 MHz, CDCl3) δ 8.16 (s, 1H), 7.77–7.72 (overlap, 2H), 7.24 (s, 1H), 7.21 (d, J = 8.4 Hz, 1H), 7.11–7.09 (m, 1H), 7.09–7.05 (overlap, 2H), 6.97 (dd, J = 4.1, 1.7 Hz, 1H), 6.83 (d, J = 2.3 Hz, 1H), 6.78 (d, J = 8.3, 2.2 Hz, 1H), 6.16 (dd, J = 4.1, 2.5 Hz, 1H), 5.54 (s, 2H), 4.54 (t, J = 7.0 Hz, 2H), 3.23 (t, J = 7.0 Hz, 2H), 2.39 (s, 3H). 13C NMR (150 MHz, CDCl3) δ 189.0, 166.0, 144.5, 142.1, 141.4, 132.9, 132.5, 131.2, 129.6, 129.1 × 2, 128.0 × 2, 126.7, 123.5, 123.1, 121.1, 119.6, 117.9, 109.0, 51.3, 44.3, 36.6, 27.3. HRMS (ESI), m/z: calcd for C24H22ClN6O2−: 461.1498 [M − H]−; found: 461.1500.
3.1.48. 4-(2-(4-((2-Acetyl-1H-pyrrol-1-yl)methyl)-1H-1,2,3-triazol-1-yl)ethyl)-N-(2-amino-4,5-difluorophenyl)benzamide (19d)
Compound 19d was prepared using similar procedures as for 19a from compound 18 (34 mg, 0.10 mmol) and 4,5-difluorophenylene-1,2-diamine (14.4 mg, 0.10 mmol). The crude product was purified by silica gel chromatography to give the brown solid (16 mg, 35%). 1H NMR (400 MHz, CDCl3) δ 8.32 (s, 1H), 7.78–7.70 (overlap, 2H), 7.27 (s, 1H), 7.11–7.05 (overlap, 3H), 6.97 (dd, J = 4.1, 1.6 Hz, 1H), 6.68–6.60 (m, 1H), 6.15 (dd, J = 4.1, 2.5 Hz, 1H), 5.53 (s, 2H), 4.53 (t, J = 6.9 Hz, 2H), 3.22 (t, J = 7.0 Hz, 2H), 2.39 (s, 3H). 13C NMR (150 MHz, CDCl3) δ 189.0, 165.9, 147.6, 144.6, 142.7, 141.5, 137.0, 132.8, 131.2, 129.6, 129.2 × 2, 128.0 × 2, 123.4, 121.2, (120.7, 120.6), (114.2, 114.0), 109.0, (106.8, 106.6), 51.3, 44.3, 36.6, 27.3. HRMS (ESI), m/z: calcd for C24H21F2N6O2−: 463.1700 [M − H]−; found: 463.1700.
3.1.49. 4-(2-(4-((2-Acetyl-1H-pyrrol-1-yl)methyl)-1H-1,2,3-triazol-1-yl)ethyl)-N-(2-amino-4-methoxyphenyl)benzamide (19e)
Compound 19e was prepared using similar procedures as for 19a from compound 18 (34 mg, 0.10 mmol) and 4-methoxy-o-phenylenediamine (13.8 mg, 0.10 mmol). The crude product was purified by silica gel chromatography to give the brown solid (28 mg, 62%). 1H NMR (400 MHz, CDCl3) δ 8.13 (s, 1H), 7.77–7.71 (overlap, 2H), 7.29 (s, 1H), 7.10–7.03 (overlap, 4H), 6.96 (dd, J = 4.1, 1.7 Hz, 1H), 6.39–6.31 (m, 2H), 6.15 (dd, J = 4.1, 2.6 Hz, 1H), 5.53 (s, 2H), 4.52 (t, J = 7.0 Hz, 2H), 3.75 (s, 3H), 3.20 (t, J = 7.0 Hz, 2H), 2.39 (s, 3H). 13C NMR (100 MHz, CDCl3) δ 188.9, 166.1, 159.2, 144.5, 143.0, 141.0, 133.1, 131.1, 129.6, 129.0 × 2, 128.0 × 2, 127.2, 123.5, 121.0, 117.2, 108.9, 104.9, 103.0, 55.5, 51.3, 44.2, 36.6, 27.3. HRMS (ESI), m/z: calcd for C25H25N6O3−: 457.1994 [M − H]−; found: 457.1992.
3.1.50. Methyl 4-(4-(2-(4-((2-acetyl-1H-pyrrol-1-yl)methyl)-1H-1,2,3-triazol-1-yl)ethyl)benzamido)-3-aminobenzoate (19f)
Compound 19f was prepared using similar procedures as for 19a from compound 18 (34 mg, 0.10 mmol) and methyl 3,4-diaminobenzoate (16.6 mg, 0.10 mmol). The crude product was purified by silica gel chromatography to give the brown solid (12 mg, 25%). 1H NMR (400 MHz, CD3OD) δ 7.85–7.82 (overlap, 3H), 7.72 (dd, J = 8.5, 2.0 Hz, 1H), 7.53 (s, 1H), 7.18–7.14 (overlap, 2H), 7.13–7.11 (m, 1H), 7.11–7.08 (m 1H), 6.85 (d, J = 8.5 Hz, 1H), 6.18 (dd, J = 4.2, 2.5 Hz, 1H), 5.55 (s, 2H), 4.65 (t, J = 6.8 Hz, 2H), 3.83 (s, 3H), 3.25 (t, J = 6.8 Hz, 2H), 2.39 (s, 3H). 13C NMR (125 MHz, CD3OD) δ 190.6, 169.0, 168.7, 149.8, 143.1, 133.9, 133.6, 132.4, 130.9, 130.5 × 2, 130.0 × 2, 129.2 × 2, 125.0, 123.1, 122.7, 119.4, 116.5, 110.0, 52.2 × 2, 44.9, 37.2, 27.1. HRMS (ESI), m/z: calcd for C26H25N6O4−: 485.1943 [M − H]−; found: 485.1945.
3.1.51. 4-(2-(2-Acetyl-1H-pyrrol-1-yl)ethyl)-N-(2-aminophenyl)benzamide (20)
Compound 20 was prepared using similar procedures as for 10 from compound 9 (32 mg, 0.12 mmol) and 1,2-diaminobenzene (26 mg, 0.24 mmol). The crude product was purified by silica gel chromatography to give the light brown solid (27 mg, 65%). 1H NMR (400 MHz, CDCl3) δ 7.85–7.77 (overlap, 2H), 7.32 (d, J = 7.8 Hz, 1H), 7.23–7.18 (overlap, 2H), 7.11–7.06 (m, 1H), 6.99 (dd, J = 4.0, 1.7 Hz, 1H), 6.92–6.84 (overlap, 2H), 6.61 (t, J = 2.2 Hz, 1H), 6.06 (dd, J = 4.1, 2.5 Hz, 1H), 4.51 (t, J = 7.3 Hz, 2H), 3.07 (t, J = 7.2 Hz, 2H), 2.47 (s, 3H). 13C NMR (150 MHz, CDCl3) δ 188.6, 166.0, 143.1, 139.4, 132.3, 130.7 × 2, 130.0, 129.5 × 2, 127.7, 127.4, 125.5, 125.3, 120.9, 120.8, 119.1, 108.2, 51.3, 38.0, 27.4. HRMS (ESI), m/z: calcd for C21H20N3O2−: 346.1561 [M − H]−; found: 346.1560.
3.2. Biological Evaluations
3.2.1. HDAC Inhibition Assay
HDAC1 (#50001, BPS, San Diego, USA), HDAC3 (#50003, BPS, San Diego, CA, USA) or HDAC6 (#50006, BPS, San Diego, USA), H3(1-21)K9Ac (#AS-64361, Anaspec, Fremont, CA, USA) substrate and test compounds were added into each well (1 × Enzymatic buffer preparation: 50 mM Tris-HCl pH 8.0, 137 mM NaCl, 2.7 mM KCl, 1 mM MgCl2, 0.01% Tween20), incubated at room temperature for 1 h in the dark. Then, SA-XL665 (#610SAXLG, Cisbio, Bedford, MA, USA) and anti-H3K9me0-Eu(K) (#61KB0KAD, Cisbio, USA) were added. A multi-label microplate analysis system (PerkinElmer Envision, Waltham, MA, USA) detected the fluorescence values at 620 nm and 665 nm, and calculated the HTRF signal ratio (665 nm/620 nm) of each well. The IC50 value of the compounds was calculated using GraphPad 7.0 software. Each set of experiments was independently repeated twice, with two replicate wells per concentration each time. The results were displayed as Mean ± SD.
3.2.2. Cell Viability Assay
The CCK8 (#AC11L054, Life ilab, Shanghai, China) method was used to detect the cells’ proliferation. Cells were seeded into a 96-well culture plate at an appropriate density. After treatment with different concentrations of agents for 72 h, 10 μL of CCK8 was added to each well, and their optical density (OD value) was measured at a wavelength of 450 nm using SpectraMax 190 (Sunnyvale, CA, USA) microplate reader. IC50 values were estimated using the four-parameter method. Each set of experiments was repeated independently three times with three replicate wells per concentration each time. The results were displayed as Mean ± SD.
3.2.3. Western Immunoblotting
RPMI-8226 cells were seeded in six-well plates and treated with compounds for 24 h. Cells were lysed with 1× SDS loading buffer (50 mM Tris pH 6.8, 100 mM DTT, 2% SDS, 0.1% bromophenol blue, 10% glycerol), and then centrifuged at 12,000 rpm for 5 min at 4 °C. Cell extracts were probed with antibodies Ac-Tubulin (#5335T, CST, Kansas City, MO, USA), α-Tubulin (#2144S, CST, Kansas City, MO, USA), AcH3 (#ab39139, active motif, Carlsbad, CA, USA), Histone H3 (#17168-1-AP, Proteintech, Chicago, IL, USA), GAPDH (#60004-1-1g, Proteintech, Chicago, IL, USA), Goat Anti-Mouse IgG, H&L Chain Specific Peroxidase Conjugate (#401215, Calbiochem, Darmstadt, Germany), Goat Anti-Rabbit IgG, or H&L Chain Specific Peroxidase Conjugate (#401353, Calbiochem, Germany), then detected using Bio-RAD power pal Basic (#041BR66615, BioRad Laboratories, Hercules, CA, USA), Bio-RAD Mini-PROTEAN Tetrade System (#IEC/EN 61010-1, BioRad Laboratories, Hercules, CA, USA), or Bio-RAD Chemi Touch Imagin (BioRad Laboratories, Hercules, CA, USA).
3.2.4. Apoptosis Assay
RPMI-8226 cells were treated with indicated compounds for 48 h. Annexin V-FITC and PI double-staining detection kits were used to determine apoptotic cells. All stained cells analyzed using BD FlowJo 10.8 software and Annexin V+/PI− is regarded as early apoptotic cells, while Annexin V+/PI+ (A211-02, Vazyme, Nanjing, China) is regarded as late apoptotic cells. Data results were obtained from three independent experiments.
3.3. Molecular Docking and Molecular Dynamics Simulation Studies
The docking was conducted in AutoDock4.2. The PDB code 1C3S was downloaded from the Protein Data Bank for molecular docking. In the Protein Preparation Wizard, HDAC1 protein was prepared by removing H2O and adding hydrogen. Results analysis was presented using PyMol (http://www.pymol.org/, accessed on 7 May 2024) and Discovery Studio 4.5. Molecular dynamics simulations were performed using Desmond.
3.4. Pharmacokinetic Analysis
Specific procedures were referred to the reported method [48]. ICR mice (n = 6) were fasted for 12 h before administration and remained fasting for 2 h. The animal room environment was controlled (target conditions: temperature 18 to 29 °C, relative humidity 30 to 70%). Temperature and relative humidity were monitored daily. An electronic time-controlled lighting system was used to provide a 12 h light/12 h dark cycle. Compound 20 was administered at doses of 5 mg/kg (po) and 1 mg/kg (iv). The time points for blood sample collection were 0.25, 0.50, 1.00, 2.00, 4.00, 8.00, and 24 h post dose for the po-administrated group, and the time points were 0.05, 0.25, 0.75, 2.00, 4.00, 8.00, and 24 h for the iv group. For each point, 20 μL of plasma were transferred into a clean tube, 200 μL of MeOH/ACN (50/50, v/v) containing IS were added, and they were thoroughly vortexed by a vortex mixer for 1 min. The mixture was centrifuged at 15000 rpm for 5 min, and then 20 μL of supernatant was mixed with 20 μL of water for the analysis by LC-MS.
4. Conclusions
In this study, a new class of HDACis bearing the natural product-inspired N-linked 2-acetylpyrrole cap group were designed, synthesized, and biologically evaluated leveraging the molecular hybridization strategy. Most of the synthetic compounds exhibited significant HDAC inhibitory effects and potent anti-proliferation against cancer cells. Among them, compound 20 showed high potency against RPMI-8226 cells with an IC50 value of 2.89 ± 0.43 μM, which was better than that of chidamide (IC50 = 10.23 ± 1.02 μM). Compound 20 promoted apoptosis in RPMI-8226 cells in a dose-dependent manner, exhibiting a superior inhibitory effect compared to the identical concentration of chidamide. The molecular docking study revealed that 20 could bind tightly to the active site of HDAC1, and the hydrogen bond connecting the carbonyl oxygen of the 2-acetylpyrrole cap group with Phe198 underscores the significance of incorporating this natural product-inspired cap group. The results of molecular dynamics simulations indicated a robust conformational stability in the docked complex. Additionally, 20 possesses drug similarity and bioavailability scores suitable for in vivo experiments, as determined by the SwissADME algorithm. Similar to entinostat (MS-275) [48], preliminary pharmacokinetic evaluations showed that compound 20’s properties did not fully meet expectations, thereby limiting its further in vivo efficacy studies. Nevertheless, 20 can serve as a promising lead compound for further optimization in the development of novel HDAC-targeted anticancer agents.
Most medicinal chemistry modifications of HDACis centered on the cap and linker parts. The cap group extending into the solvent region offers versatility for substitution with diverse structures aimed at enhancing the efficacy and pharmacokinetic properties. Meanwhile, the linker’s design can modulate molecular flexibility and accessibility to the zinc ion within the enzyme active site [39]. With this understanding, we will use 20 as the lead compound and prioritize modifications to both the cap group and linker to develop new HDACis that widen the therapeutic window and optimize pharmacokinetic properties.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules29194653/s1, Figure S1: Prediction of the physicochemical properties of compound 20 and chidamide, Figures S2–S79: NMR and HR-MS spectrum of all target compounds. Figure S80. The whole un-cropped images of the original western blots in Figure 3. Figure S81. The STR identification results of the three cells were correct. Table S1. General information about the cells used in the experiments.
Author Contributions
H.Z.: Writing—original draft; Software; Resources; Data curation. Q.S.: Bioactivity test; Writing—review and editing. Z.H.: Resources; Investigation; Data curation. P.-Q.W.: Writing—review and editing; Resources. Y.C.: Supervision; Project administration. J.-X.Z.: Writing—review and editing; Visualization; Supervision; Funding acquisition; Data curation. J.-M.Y.: Writing—review and editing; Supervision; Project administration; Funding acquisition; Conceptualization. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (Nos. 22237007 and T2192972), the Youth Innovation Promotion Association of Chinese Academy of Sciences (CAS) (No. 2022282), the Shandong Laboratory Program (No. SYS202205), and the Shanghai Institute of Materia Medica of CAS (No. SIMM0120231002).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article and Supplementary Materials.
Conflicts of Interest
The authors declare no competing financial interest.
References
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Zhuang, Y.; Chen, Q. Current scenario of pyrazole hybrids with in vivo therapeutic potential against cancers. Eur. J. Med. Chem. 2023, 257, 115495. [Google Scholar] [CrossRef]
- Bass, A.K.A.; El-Zoghbi, M.S.; Nageeb, E.-S.M.; Mohamed, M.F.A.; Badr, M.; Abuo-Rahma, G.E.-D.A. Comprehensive review for anticancer hybridized multitargeting HDAC inhibitors. Eur. J. Med. Chem. 2021, 209, 112904. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Hou, H.; Zhou, B.; Gao, J.; Gao, F. Hydroxamic acid hybrids: Histone deacetylase inhibitors with anticancer therapeutic potency. Eur. J. Med. Chem. 2023, 262, 115879. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.-Z.; Wang, L.; Gao, K.; Zhu, X.; Lou, L.-G.; Yue, J.-M. Design and synthesis of cyclolipopeptide mimics of dysoxylactam A and evaluation of the reversing potencies against P-glycoprotein-mediated multidrug resistance. J. Med. Chem. 2024, 67, 4560–4582. [Google Scholar] [CrossRef]
- Liu, C.-P.; Xie, C.-Y.; Zhao, J.-X.; Ji, K.-L.; Lei, X.-X.; Sun, H.; Lou, L.-G.; Yue, J.-M. Dysoxylactam A: A macrocyclolipopeptide reverses P-glycoprotein-mediated multidrug resistance in cancer cells. J. Am. Chem. Soc. 2019, 141, 6812–6816. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.-Z.; Wang, L.; Fan, Y.-Y.; Lai, Z.-W.; Yu, X.-N.; Lou, L.-G.; Gao, K.; Yue, J.-M. Concise total synthesis of dysoxylactam A and a simplified analog. Chin. J. Chem. 2022, 40, 2027–2034. [Google Scholar] [CrossRef]
- Garcia-Martinez, L.; Zhang, Y.; Nakata, Y.; Chan, H.L.; Morey, L. Epigenetic mechanisms in breast cancer therapy and resistance. Nat. Commun. 2021, 12, 1786. [Google Scholar] [CrossRef] [PubMed]
- Shirbhate, E.; Singh, V.; Jahoriya, V.; Mishra, A.; Veerasamy, R.; Tiwari, A.K.; Rajak, H. Dual inhibitors of HDAC and other epigenetic regulators: A novel strategy for cancer treatment. Eur. J. Med. Chem. 2024, 263, 115938. [Google Scholar] [CrossRef]
- Wang, N.; Ma, T.; Yu, B. Targeting epigenetic regulators to overcome drug resistance in cancers. Signal Transduct. Target. Ther. 2023, 8, 69. [Google Scholar] [CrossRef] [PubMed]
- Ru, J.; Wang, Y.; Li, Z.; Wang, J.; Ren, C.; Zhang, J. Technologies of targeting histone deacetylase in drug discovery: Current progress and emerging prospects. Eur. J. Med. Chem. 2023, 261, 115800. [Google Scholar] [CrossRef] [PubMed]
- Nepali, K.; Wu, A.C.; Lo, W.L.; Chopra, B.; Lai, M.J.; Chuang, J.Y.; Liou, J.P. Rationally designed donepezil-based hydroxamates modulate Sig-1R and HDAC isoforms to exert anti-glioblastoma effects. Eur. J. Med. Chem. 2023, 248, 115054. [Google Scholar] [CrossRef]
- Ramaiah, M.J.; Tangutur, A.D.; Manyam, R.R. Epigenetic modulation and understanding of HDAC inhibitors in cancer therapy. Life Sci. 2021, 277, 119504. [Google Scholar] [CrossRef] [PubMed]
- Ho, T.C.S.; Chan, A.H.Y.; Ganesan, A. Thirty years of HDAC inhibitors: 2020 insight and hindsight. J. Med. Chem. 2020, 63, 12460–12484. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.H.; Qin, M.; Wu, H.P.; Khamis, M.Y.; Li, Y.H.; Ma, L.Y.; Liu, H.M. A review of progress in histone deacetylase 6 inhibitors research: Structural specificity and functional diversity. J. Med. Chem. 2021, 64, 1362–1391. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Li, Y.; Zhang, J.; Zhang, M.; Wei, A.; Liu, H.; Xie, Z.; Ren, W.; Duan, W.; Zhang, Z.; et al. Discovery of selective HDAC/BRD4 dual inhibitors as epigenetic probes. Eur. J. Med. Chem. 2021, 209, 112868. [Google Scholar] [CrossRef]
- Liang, T.; Wang, F.; Elhassan, R.M.; Cheng, Y.; Tang, X.; Chen, W.; Fang, H.; Hou, X. Targeting histone deacetylases for cancer therapy: Trends and challenges. Acta Pharm. Sin. B 2023, 13, 2425–2463. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.-W.; Gong, C.-J.; Su, M.-B.; Chen, F.; He, T.; Zhang, Y.-M.; Shen, Q.-Q.; Su, Y.; Ding, J.; Li, J.; et al. Synthesis and in vitro and in vivo biological evaluation of tissue-specific bisthiazole histone deacetylase (HDAC) inhibitors. J. Med. Chem. 2019, 63, 804–815. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef]
- Zhao, J.-X.; Yue, J.-M. Frontier studies on natural products: Moving toward paradigm shifts. Sci. Chi. Chem. 2023, 66, 928–942. [Google Scholar] [CrossRef]
- Zou, Y.-S.; Foubert, K.; Tuenter, E.; Lemière, F.; Cos, P.; Maes, L.; Smits, J.M.M.; de Gelder, R.; Apers, S.; Pieters, L. Antiplasmodial and cytotoxic activities of Striga asiatica and Sauropus spatulifolius extracts, and their isolated constituents. Phytochem. Lett. 2013, 6, 53–58. [Google Scholar] [CrossRef]
- Wu, P.-Q.; Cui, Y.-S.; Han, X.-Y.; Wang, C.; An, P.-P.; Zhou, J.-S.; Ren, Y.-H.; Liu, Z.-L.; Lin, R.-T.; Zhou, B.; et al. Diterpenoids from sauropus spatulifolius leaves with antimicrobial activities. J. Nat. Prod. 2022, 85, 1304–1314. [Google Scholar] [CrossRef] [PubMed]
- Rodriquez, M.; Aquino, M.; Bruno, I.; Martino, D.G.; Taddei, M.; Gomez-Paloma, L. Chemistry and biology of chromatin remodeling agents: State of art and future perspectives of HDAC inhibitors. Curr. Med. Chem. 2006, 13, 1119–1139. [Google Scholar] [CrossRef] [PubMed]
- Domagala, A.; Jarosz, T.; Lapkowski, M. Living on pyrrolic foundations—Advances in natural and artificial bioactive pyrrole derivatives. Eur. J. Med. Chem. 2015, 100, 176–187. [Google Scholar] [CrossRef] [PubMed]
- Li Petri, G.; Spanò, V.; Spatola, R.; Holl, R.; Raimondi, M.V.; Barraja, P.; Montalbano, A. Bioactive pyrrole-based compounds with target selectivity. Eur. J. Med. Chem. 2020, 208, 112783. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.; Alam, O.; Naim, M.J.; Shaquiquzzaman, M.; Alam, M.M.; Iqbal, M. Pyrrole: An insight into recent pharmacological advances with structure activity relationship. Eur. J. Med. Chem. 2018, 157, 527–561. [Google Scholar] [CrossRef] [PubMed]
- Fleming, C.L.; Ashton, T.D.; Gaur, V.; McGee, S.L.; Pfeffer, F.M. Improved synthesis and structural reassignment of MC1568: A Class IIa selective HDAC inhibitor. J. Med. Chem. 2014, 57, 1132–1135. [Google Scholar] [CrossRef] [PubMed]
- Schäker-Hübner, L.; Warstat, R.; Ahlert, H.; Mishra, P.; Kraft, F.B.; Schliehe-Diecks, J.; Schöler, A.; Borkhardt, A.; Breit, B.; Bhatia, S.; et al. 4-Acyl pyrrole capped HDAC inhibitors: A new scaffold for hybrid inhibitors of BET proteins and histone deacetylases as antileukemia drug leads. J. Med. Chem. 2021, 64, 14620–14646. [Google Scholar] [CrossRef] [PubMed]
- Zubia, A.; Ropero, S.; Otaegui, D.; Ballestar, E.; Fraga, M.F.; Boix-Chornet, M.; Berdasco, M.; Martinez, A.; Coll-Mulet, L.; Gil, J.; et al. Identification of (1H)-pyrroles as histone deacetylase inhibitors with antitumoral activity. Oncogene 2009, 28, 1477–1484. [Google Scholar] [CrossRef]
- Singh, A.; Patel, V.K.; Rajak, H. Appraisal of pyrrole as connecting unit in hydroxamic acid based histone deacetylase inhibitors: Synthesis, anticancer evaluation and molecular docking studies. J. Mol. Struct. 2021, 1240, 130590. [Google Scholar] [CrossRef]
- Zhang, W.-X.; Huang, J.; Tian, X.-Y.; Liu, Y.-H.; Jia, M.-Q.; Wang, W.; Jin, C.-Y.; Song, J.; Zhang, S.-Y. A review of progress in o-aminobenzamide-based HDAC inhibitors with dual targeting capabilities for cancer therapy. Eur. J. Med. Chem. 2023, 259, 115673. [Google Scholar] [CrossRef] [PubMed]
- Han, B.; Wang, M.; Li, J.; Chen, Q.; Sun, N.; Yang, X.; Zhang, Q. Perspectives and new aspects of histone deacetylase inhibitors in the therapy of CNS diseases. Eur. J. Med. Chem. 2023, 258, 115613. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.; Li, Y.; Yan, C.; Yan, M.; Tang, Z. Indole: A privileged scaffold for the design of anti-cancer agents. Eur. J. Med. Chem. 2019, 183, 111691. [Google Scholar] [CrossRef] [PubMed]
- Gong, C.-J.; Gao, A.-H.; Zhang, Y.-M.; Su, M.-B.; Chen, F.; Sheng, L.; Zhou, Y.-B.; Li, J.-Y.; Li, J.; Nan, F.-J. Design, synthesis and biological evaluation of bisthiazole-based trifluoromethyl ketone derivatives as potent HDAC inhibitors with improved cellular efficacy. Eur. J. Med. Chem. 2016, 112, 81–90. [Google Scholar] [CrossRef]
- Hermant, P.; Bosc, D.; Piveteau, C.; Gealageas, R.; Lam, B.; Ronco, C.; Roignant, M.; Tolojanahary, H.; Jean, L.; Renard, P.-Y.; et al. Controlling plasma stability of hydroxamic acids: A MedChem Toolbox. J. Med. Chem. 2017, 60, 9067–9089. [Google Scholar] [CrossRef] [PubMed]
- Qiu, X.; Zhu, L.; Wang, H.; Tan, Y.; Yang, Z.; Yang, L.; Wan, L. From natural products to HDAC inhibitors: An overview of drug discovery and design strategy. Bioorg. Med. Chem. 2021, 52, 116510. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.; Kozikowski, A.P. Why hydroxamates may not be the best histone deacetylase inhibitors—What some may have forgotten or would rather forget? Chem. Med. Chem. 2016, 11, 15–21. [Google Scholar] [CrossRef]
- Ripa, L.; Sandmark, J.; Hughes, G.; Shamovsky, I.; Gunnarsson, A.; Johansson, J.; Llinas, A.; Collins, M.; Jung, B.; Novén, A.; et al. Selective and bioavailable HDAC6 2-(difluoromethyl)-1,3,4-oxadiazole substrate inhibitors and modeling of their bioactivation mechanism. J. Med. Chem. 2023, 66, 14188–14207. [Google Scholar] [CrossRef]
- Sun, N.; Yang, K.; Yan, W.; Yao, M.; Yu, C.; Duan, W.; Gu, X.; Guo, D.; Jiang, H.; Xie, C.; et al. Design and synthesis of triazole-containing HDAC inhibitors that induce antitumor effects and immune response. J. Med. Chem. 2023, 66, 4802–4826. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Chu, H.R.; Wang, A.Y.; Yin, H.H.; Gao, Y.Q.; Liu, S.H.; Li, W.; Han, L.Q. Discovery of 2,5-diphenyl-1,3,4-thiadiazole derivatives as HDAC inhibitors with DNA binding affinity. Eur. J. Med. Chem. 2022, 241, 114634. [Google Scholar] [CrossRef] [PubMed]
- Abdizadeh, T.; Kalani, M.R.; Abnous, K.; Tayarani-Najaran, Z.; Khashyarmanesh, B.Z.; Abdizadeh, R.; Ghodsi, R.; Hadizadeh, F. Design, synthesis and biological evaluation of novel coumarin-based benzamides as potent histone deacetylase inhibitors and anticancer agents. Eur. J. Med. Chem. 2017, 132, 42–62. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef]
- McCoy, M.A.; Spicer, D.; Wells, N.; Hoogewijs, K.; Fiedler, M.; Baud, M.G.J. Biophysical survey of small-molecule β-catenin inhibitors: A cautionary tale. J. Med. Chem. 2022, 65, 7246–7261. [Google Scholar] [CrossRef]
- Brandão, P.; Marques, C.; Burke, A.J.; Pineiro, M. The application of isatin-based multicomponent-reactions in the quest for new bioactive and druglike molecules. Eur. J. Med. Chem. 2021, 211, 113102. [Google Scholar] [CrossRef] [PubMed]
- Sicak, Y. Design and antiproliferative and antioxidant activities of furan-based thiosemicarbazides and 1,2,4-triazoles: Their structure-activity relationship and SwissADME predictions. Med. Chem. Res. 2021, 30, 1557–1568. [Google Scholar] [CrossRef]
- Jiang, Y.Q.; Xu, J.; Yue, K.R.; Huang, C.; Qin, M.T.; Chi, D.Y.; Yu, Q.X.; Zhu, Y.; Hou, X.H.; Xu, T.Q.; et al. Potent hydrazide-based HDAC inhibitors with a superior pharmacokinetic profile for efficient treatment of acute myeloid leukemia in vivo. J. Med. Chem. 2022, 65, 285–302. [Google Scholar] [CrossRef]
- Yeo, P.; Xin, L.; Goh, E.; New, L.S.; Zeng, P.; Wu, X.; Venkatesh, P.; Kantharaj, E. Development and validation of high-performance liquid chromatography-tandem mass spectrometry assay for 6-(3-benzoyl-ureido)-hexanoic acid hydroxyamide, a novel HDAC inhibitor, in mouse plasma for pharmacokinetic studies. Biomed. Chromatogr. 2007, 21, 184–189. [Google Scholar] [CrossRef]
- Li, D.Q.; Zhang, Z.; Li, Y.L.; Wang, X.Y.; Zhong, H.Y.; Yang, H.J.; Xi, Y.; Liu, H.C.; Shen, A.J.; Hu, Y.H. Discovery of (S)-N-(2-Amino-4-fluorophenyl)-4-(1-(3-(4-((dimethylamino)methyl)phenyl)-6-oxopyridazin-1(6H)-yl)ethyl)benzamide as potent class I selective HDAC inhibitor for oral anticancer drug candidate. J. Med. Chem. 2023, 66, 7016–7037. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).