Quinoxaline 1,4-di-N-oxide Derivatives as New Antinocardial Agents
Abstract
1. Introduction
2. Results
2.1. Antinocardial Activity
2.2. Cytotoxic Activity
3. Discussion
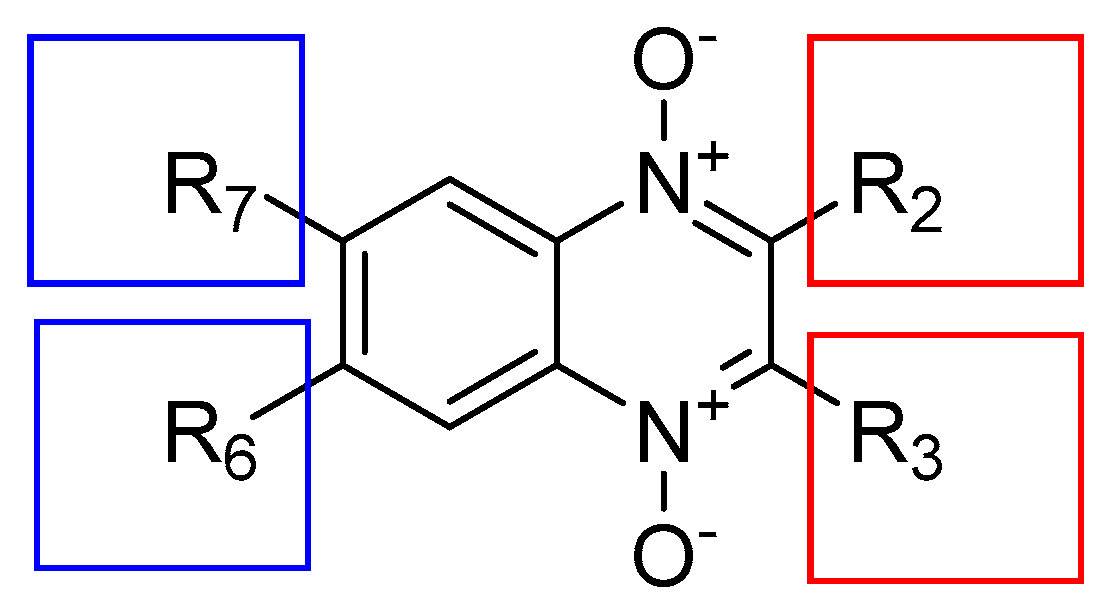
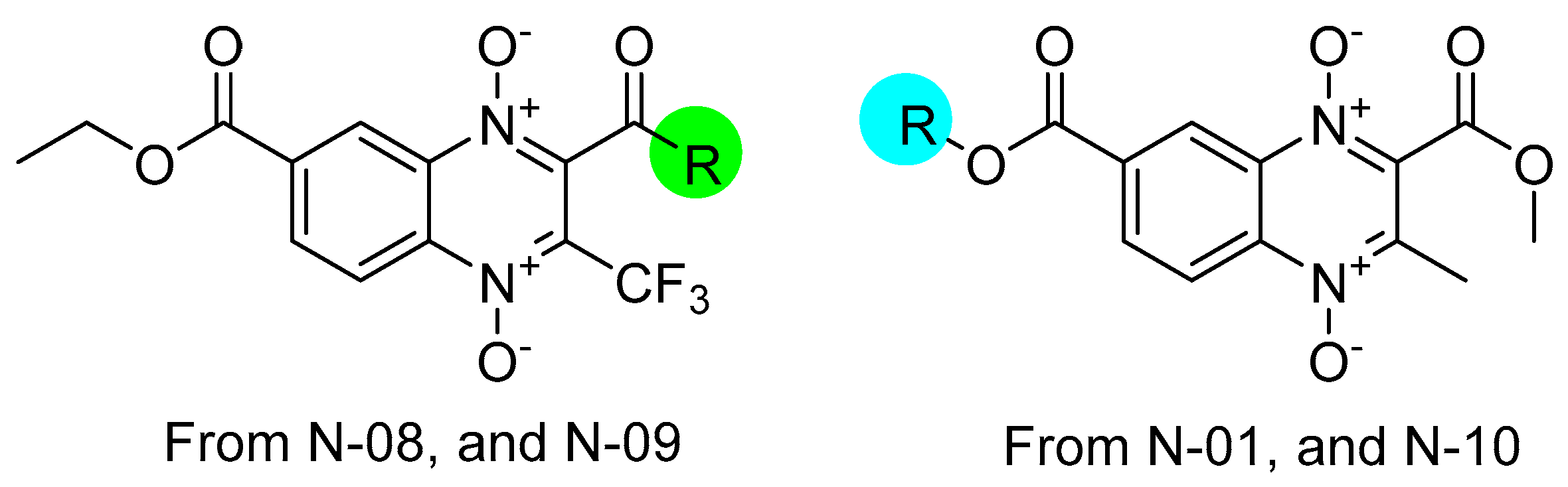
3.1. Structure–Activity Relationship
3.2. Selectivity of Antinocardial Activity
4. Materials and Methods
4.1. Quinoxaline 1,4-di-N-oxide Derivatives
4.2. Biological Material
4.3. Biological Evaluation In Vitro against N. brasiliensis
4.4. Cytotoxic Activity and Selectivity Index Determination
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Barka, E.A.; Vatsa, P.; Sanchez, L.; Gaveau-Vaillant, N.; Jacquard, C.; Klenk, H.-P.; Clément, C.; Ouhdouch, Y.; van Wezel, G.P. Taxonomy, Physiology, and Natural Products of Actinobacteria. Microbiol. Mol. Biol. Rev. 2016, 80, 1–43. [Google Scholar] [CrossRef] [PubMed]
- Brown-Elliott, B.A.; Brown, J.M.; Conville, P.S.; Wallace, R.J. Clinical and Laboratory Features of the Nocardia Spp. Based on Current Molecular Taxonomy. Clin. Microbiol. Rev. 2006, 19, 259–282. [Google Scholar] [CrossRef]
- McNeil, M.M.; Brown, J.M. The Medically Important Aerobic Actinomycetes: Epidemiology and Microbiology. Clin. Microbiol. Rev. 1994, 7, 357–417. [Google Scholar] [CrossRef] [PubMed]
- Schlaberg, R.; Fisher, M.A.; Hanson, K.E. Susceptibility Profiles of Nocardia Isolates Based on Current Taxonomy. Antimicrob. Agents Chemother. 2014, 58, 795–800. [Google Scholar] [CrossRef]
- Traxler, R.M.; Bell, M.E.; Lasker, B.; Headd, B.; Shieh, W.-J.; McQuiston, J.R. Updated Review on Nocardia Species: 2006–2021. Clin. Microbiol. Rev. 2022, 35, e0002721. [Google Scholar] [CrossRef] [PubMed]
- Vicente, E.; Pérez-Silanes, S.; Lima, L.M.; Ancizu, S.; Burguete, A.; Solano, B.; Villar, R.; Aldana, I.; Monge, A. Selective Activity against Mycobacterium Tuberculosis of New Quinoxaline 1,4-Di-N-Oxides. Bioorg. Med. Chem. 2009, 17, 385–389. [Google Scholar] [CrossRef] [PubMed]
- Patel, N.B.; Patel, J.N.; Purohit, A.C.; Patel, V.M.; Rajani, D.P.; Moo-Puc, R.; Lopez-Cedillo, J.C.; Nogueda-Torres, B.; Rivera, G. In Vitro and in Vivo Assessment of Newer Quinoxaline–Oxadiazole Hybrids as Antimicrobial and Antiprotozoal Agents. Int. J. Antimicrob. Agents 2017, 50, 413–418. [Google Scholar] [CrossRef]
- Palos, I.; Luna-Herrera, J.; Lara-Ramírez, E.; Loera-Piedra, A.; Fernández-Ramírez, E.; Aguilera-Arreola, M.; Paz-González, A.; Monge, A.; Wan, B.; Franzblau, S.; et al. Anti-Mycobacterium Tuberculosis Activity of Esters of Quinoxaline 1,4-Di-N-Oxide. Molecules 2018, 23, 1453. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Tang, Y.; Yang, Y.; An, Q.; Sang, Z.; Yang, T.; Liu, P.; Zhang, T.; Deng, Y.; Luo, Y. Discovery of Novel Anti-Tuberculosis Agents with Pyrrolo[1,2-a]Quinoxaline-Based Scaffold. Bioorg. Med. Chem. Lett. 2018, 28, 2084–2090. [Google Scholar] [CrossRef]
- Monge, A.; Martinez-Crespo, F.J.; Lopez de Cerain, A.; Palop, J.A.; Narro, S.; Senador, V.; Marin, A.; Sainz, Y.; Gonzalez, M. Hypoxia-Selective Agents Derived from 2-Quinoxalinecarbonitrile 1,4-Di-N-Oxides. 2. J. Med. Chem. 1995, 38, 4488–4494. [Google Scholar] [CrossRef] [PubMed]
- Carta, A.; Paglietti, G.; Rahbar Nikookar, M.E.; Sanna, P.; Sechi, L.; Zanetti, S. Novel Substituted Quinoxaline 1,4-Dioxides with in Vitro Antimycobacterial and Anticandida Activity. Eur. J. Med. Chem. 2002, 37, 355–366. [Google Scholar] [CrossRef] [PubMed]
- Zarranz, B.; Jaso, A.; Aldana, I.; Monge, A. Synthesis and Antimycobacterial Activity of New Quinoxaline-2-Carboxamide 1,4-Di-N-Oxide Derivatives. Bioorg. Med. Chem. 2003, 11, 2149–2156. [Google Scholar] [CrossRef] [PubMed]
- Jaso, A.; Zarranz, B.; Aldana, I.; Monge, A. Synthesis of New 2-Acetyl and 2-Benzoyl Quinoxaline 1,4-Di-N-Oxide Derivatives as Anti-Mycobacterium Tuberculosis Agents. Eur. J. Med. Chem. 2003, 38, 791–800. [Google Scholar] [CrossRef] [PubMed]
- Jaso, A.; Zarranz, B.; Aldana, I.; Monge, A. Synthesis of New Quinoxaline-2-Carboxylate 1,4-Dioxide Derivatives as Anti- Mycobacterium t Uberculosis Agents. J. Med. Chem. 2005, 48, 2019–2025. [Google Scholar] [CrossRef]
- Vicente, E.; Villar, R.; Burguete, A.; Solano, B.; Pérez-Silanes, S.; Aldana, I.; Maddry, J.A.; Lenaerts, A.J.; Franzblau, S.G.; Cho, S.; et al. Efficacy of Quinoxaline-2-Carboxylate 1,4-Di- N. -Oxide Derivatives in Experimental Tuberculosis. Antimicrob. Agents Chemother. 2008, 52, 3321–3326. [Google Scholar] [CrossRef] [PubMed]
- Villar, R.; Vicente, E.; Solano, B.; Perez-Silanes, S.; Aldana, I.; Maddry, J.A.; Lenaerts, A.J.; Franzblau, S.G.; Cho, S.-H.; Monge, A.; et al. In Vitro and in Vivo Antimycobacterial Activities of Ketone and Amide Derivatives of Quinoxaline 1,4-Di-N-Oxide. J. Antimicrob. Chemother. 2008, 62, 547–554. [Google Scholar] [CrossRef]
- Ancizu, S.; Moreno, E.; Solano, B.; Villar, R.; Burguete, A.; Torres, E.; Pérez-Silanes, S.; Aldana, I.; Monge, A. New 3-Methylquinoxaline-2-Carboxamide 1,4-Di-N-Oxide Derivatives as Anti-Mycobacterium Tuberculosis Agents. Bioorg. Med. Chem. 2010, 18, 2713–2719. [Google Scholar] [CrossRef] [PubMed]
- Das, U.; Das, S.; Bandy, B.; Gorecki, D.K.J.; Dimmock, J.R. E-2-[3-(3,4-Dichlorophenyl)-1-Oxo-2-Propenyl]-3-Methylquinoxaline-1,4-Dioxide: A Lead Antitubercular Agent Which Alters Mitochondrial Respiration in Rat Liver. Eur. J. Med. Chem. 2010, 45, 4682–4686. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Torres, E.; Moreno, E.; Ancizu, S.; Barea, C.; Galiano, S.; Aldana, I.; Monge, A.; Pérez-Silanes, S. New 1,4-Di-N-Oxide-Quinoxaline-2-Ylmethylene Isonicotinic Acid Hydrazide Derivatives as Anti-Mycobacterium Tuberculosis Agents. Bioorg. Med. Chem. Lett. 2011, 21, 3699–3703. [Google Scholar] [CrossRef]
- Puratchikody, A.; Natarajan, R.; Jayapal, M.; Doble, M. Synthesis, In Vitro Antitubercular Activity and 3D-QSAR of Novel Quinoxaline Derivatives. Chem. Biol. Drug Des. 2011, 78, 988–998. [Google Scholar] [CrossRef]
- Cheng, G.; Sa, W.; Cao, C.; Guo, L.; Hao, H.; Liu, Z.; Wang, X.; Yuan, Z. Quinoxaline 1,4-Di-N-Oxides: Biological Activities and Mechanisms of Actions. Front. Pharmacol. 2016, 7, 64. [Google Scholar] [CrossRef] [PubMed]
- Vicente, E.; Villar, R.; Perez-Silanes, S.; Aldana, I.; Goldman, R.C.; Monge, A. Quinoxaline 1,4-Di-N-Oxide and the Potential for Treating Tuberculosis. Infect. Disord. Drug Targets 2011, 11, 196–204. [Google Scholar] [CrossRef]
- Gómez-Caro, L.C.; Sánchez-Sánchez, M.; Bocanegra-García, V.; Rivera, G.; Monge, A. Synthesis of Quinoxaline 1,4-Di-n-Oxide Derivatives on Solid Support Using Room Temperature and Microwave-Assisted Solvent-Free Procedures. Quim. Nova 2011, 34, 1147–1151. [Google Scholar] [CrossRef]
- Duque-Montaño, B.E.; Gómez-Caro, L.C.; Sanchez-Sanchez, M.; Monge, A.; Hernández-Baltazar, E.; Rivera, G.; Torres-Angeles, O. Synthesis and in Vitro Evaluation of New Ethyl and Methyl Quinoxaline-7-Carboxylate 1,4-Di-N-Oxide against Entamoeba Histolytica. Bioorg. Med. Chem. 2013, 21, 4550–4558. [Google Scholar] [CrossRef] [PubMed]
- Villalobos-Rocha, J.C.; Sánchez-Torres, L.; Nogueda-Torres, B.; Segura-Cabrera, A.; García-Pérez, C.A.; Bocanegra-García, V.; Palos, I.; Monge, A.; Rivera, G. Anti-Trypanosoma Cruzi and Anti-Leishmanial Activity by Quinoxaline-7-Carboxylate 1,4-Di-N-Oxide Derivatives. Parasitol. Res. 2014, 113, 2027–2035. [Google Scholar] [CrossRef] [PubMed]
- Rivera, G.; Andrade-Ochoa, S.; Ortega Romero, M.S.; Palos, I.; Monge, A.; Sanchez-Torres, L.E. Ester of Quinoxaline-7-Carboxylate 1,4-Di-N-Oxide as Apoptosis Inductors in K-562 Cell Line: An in Vitro, QSAR and DFT Study. Anticancer Agents Med. Chem. 2017, 17, 682–691. [Google Scholar] [CrossRef] [PubMed]
- Chacón-Vargas, K.F.; Nogueda-Torres, B.; Sánchez-Torres, L.E.; Suarez-Contreras, E.; Villalobos-Rocha, J.C.; Torres-Martinez, Y.; Lara-Ramirez, E.E.; Fiorani, G.; Krauth-Siegel, R.L.; Bolognesi, M.L.; et al. Trypanocidal Activity of Quinoxaline 1,4 Di-N-Oxide Derivatives as Trypanothione Reductase Inhibitors. Molecules 2017, 22, 220. [Google Scholar] [CrossRef] [PubMed]
- Rampersad, S.N. Multiple Applications of Alamar Blue as an Indicator of Metabolic Function and Cellular Health in Cell Viability Bioassays. Sensors 2012, 12, 12347–12360. [Google Scholar] [CrossRef]

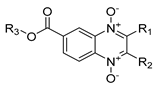 | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Code | R1 | R2 | R3 | J774A.1 * | CECT3052 | SI | N100 | SI | N200 | SI | N300 | SI | N400 | SI | N600 | SI | N700 | SI |
| N-01 | COOCH3 | CH3 | CH3 | 360 ± 4.5 | 0.5 | 720 | 0.5 | 720 | 2 | 180 | 3.12 | 115 | 1.56 | 231 | 6.25 | 57 | 1.56 | 231 |
| N-02 | COOCH2CH3 | CH3 | CH3 | 214 ± 1 | ND | ND | 3.12 | 68 | 6.25 | 34 | 25 | 8 | 6.25 | 34 | 50 | 4 | 6.25 | 34 |
| N-03 | COC6H5 | CH3 | CH3 | 179 ± 3.8 | 6.25 | 28 | 6.25 | 28 | 50 | 3 | 25 | 7 | 25 | 7 | 25 | 7 | 12.5 | 14 |
| N-04 | COCH(CH3)2 | CF3 | CH3 | 36 ± 5.9 | 0.5 | 71 | 0.5 | 71 | 1.56 | 22 | 1.56 | 22 | 1.56 | 22 | 2 | 17 | 2 | 17 |
| N-05 | COC6H5 | CF3 | CH3 | 47 ± 4.1 | 0.25 | 188 | 0.25 | 188 | 0.5 | 94 | 0.5 | 94 | 1 | 47 | 0.5 | 94 | 0.5 | 94 |
| N-06 | COC4H3S | CF3 | CH3 | 36 ± 7.2 | 0.25 | 142 | 0.25 | 142 | 2 | 17 | 2 | 17 | 1.56 | 22 | 0.5 | 71 | 1 | 35 |
| N-07 | COOCH2CH3 | C6H5 | CH3 | 75 ± 12.6 | <1.56 | 48 | 6.25 | 12 | 12.5 | 6 | 6.25 | 12 | 3.12 | 24 | 12.5 | 6 | 3.12 | 24 |
| N-08 | COCH3 | CF3 | CH3CH2 | 260 ± 2 | ND | ND | 0.5 | 519 | 0.5 | 519 | 1 | 259 | 2 | 129 | 1 | 259 | 2 | 129 |
| N-09 | COC4H3S | CF3 | CH3CH2 | 28 ± 3.5 | <0.06 | 46 | 0.06 | 459 | 0.25 | 114 | 0.25 | 114 | 0.25 | 114 | 0.25 | 114 | 0.12 | 237 |
| N-10 | COOCH3 | CH3 | CH3CH2CH2 | 301 ± 4.4 | 1 | 300 | 1 | 300 | 3.12 | 96 | 0.25 | 1203 | 3.12 | 96 | 3.12 | 96 | 3.12 | 96 |
| N-11 | COC4H3S | CF3 | CH3CH2CH2 | 41 ± 6.5 | 0.25 | 164 | 0.25 | 164 | 0.5 | 82 | 0.5 | 82 | 0.5 | 82 | 0.25 | 164 | 2 | 20 |
| N-12 | COCH(CH3)2 | CF3 | (CH3)2CH | 50 ± 6.1 | 0.25 | 201 | 0.25 | 201 | 1.56 | 32 | 1 | 50 | 2 | 25 | 1.56 | 32 | 2 | 25 |
| N-13 | COC4H3S | CF3 | (CH3)2CH | 50 ± 3.5 | 0.25 | 200 | 0.25 | 200 | 0.5 | 100 | 0.25 | 200 | 0.25 | 200 | 1 | 50 | 0.5 | 100 |
| TR1/SUL | 0.25/4.75 | 0.5/9.5 | 0.25/4.75 | 1/19 | 0.25/4.75 | 0.25/4.75 | 0.25/4.75 | |||||||||||
| AMI | 0.12 | 0.25 | 0.5 | 0.25 | 0.25 | 0.5 | 0.25 | |||||||||||
| AMO | 2 | 64 | 4 | 2 | 32 | 4 | 8 | |||||||||||
| CIP | 1 | 2 | 2 | 2 | 2 | 1 | 1 | |||||||||||
| CLA | 1 | 16 | 2 | 1 | 8 | 2 | 4 | |||||||||||
| DOX | 1 | 1 | 0.25 | 1 | 1 | 0.5 | 1 | |||||||||||
| LIN | 2 | 1 | 1 | 1 | 1 | 1 | 1 | |||||||||||
| RIF | 8 | 32 | 0.25 | 4 | 0.25 | 0.25 | 64 | |||||||||||
| TOB | 0.12 | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 | |||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palos, I.; González-González, A.; Paz-González, A.D.; Espinoza-Hicks, J.C.; Bandyopadhyay, D.; Paniagua-Castro, N.; Galeana-Salazar, M.S.; Castañeda-Sánchez, J.I.; Luna-Herrera, J.; Rivera, G. Quinoxaline 1,4-di-N-oxide Derivatives as New Antinocardial Agents. Molecules 2024, 29, 4652. https://doi.org/10.3390/molecules29194652
Palos I, González-González A, Paz-González AD, Espinoza-Hicks JC, Bandyopadhyay D, Paniagua-Castro N, Galeana-Salazar MS, Castañeda-Sánchez JI, Luna-Herrera J, Rivera G. Quinoxaline 1,4-di-N-oxide Derivatives as New Antinocardial Agents. Molecules. 2024; 29(19):4652. https://doi.org/10.3390/molecules29194652
Chicago/Turabian StylePalos, Isidro, Alonzo González-González, Alma D. Paz-González, José C. Espinoza-Hicks, Debasish Bandyopadhyay, Norma Paniagua-Castro, Marlene S. Galeana-Salazar, Jorge Ismael Castañeda-Sánchez, Julieta Luna-Herrera, and Gildardo Rivera. 2024. "Quinoxaline 1,4-di-N-oxide Derivatives as New Antinocardial Agents" Molecules 29, no. 19: 4652. https://doi.org/10.3390/molecules29194652
APA StylePalos, I., González-González, A., Paz-González, A. D., Espinoza-Hicks, J. C., Bandyopadhyay, D., Paniagua-Castro, N., Galeana-Salazar, M. S., Castañeda-Sánchez, J. I., Luna-Herrera, J., & Rivera, G. (2024). Quinoxaline 1,4-di-N-oxide Derivatives as New Antinocardial Agents. Molecules, 29(19), 4652. https://doi.org/10.3390/molecules29194652










