Polyethyleneimine Modified Two-Dimensional GO/MXene Composite Membranes with Enhanced Mg2+/Li+ Separation Performance for Salt Lake Brine
Abstract
1. Introduction
2. Results and Discussion
2.1. Characterization of Materials and Composite Membranes
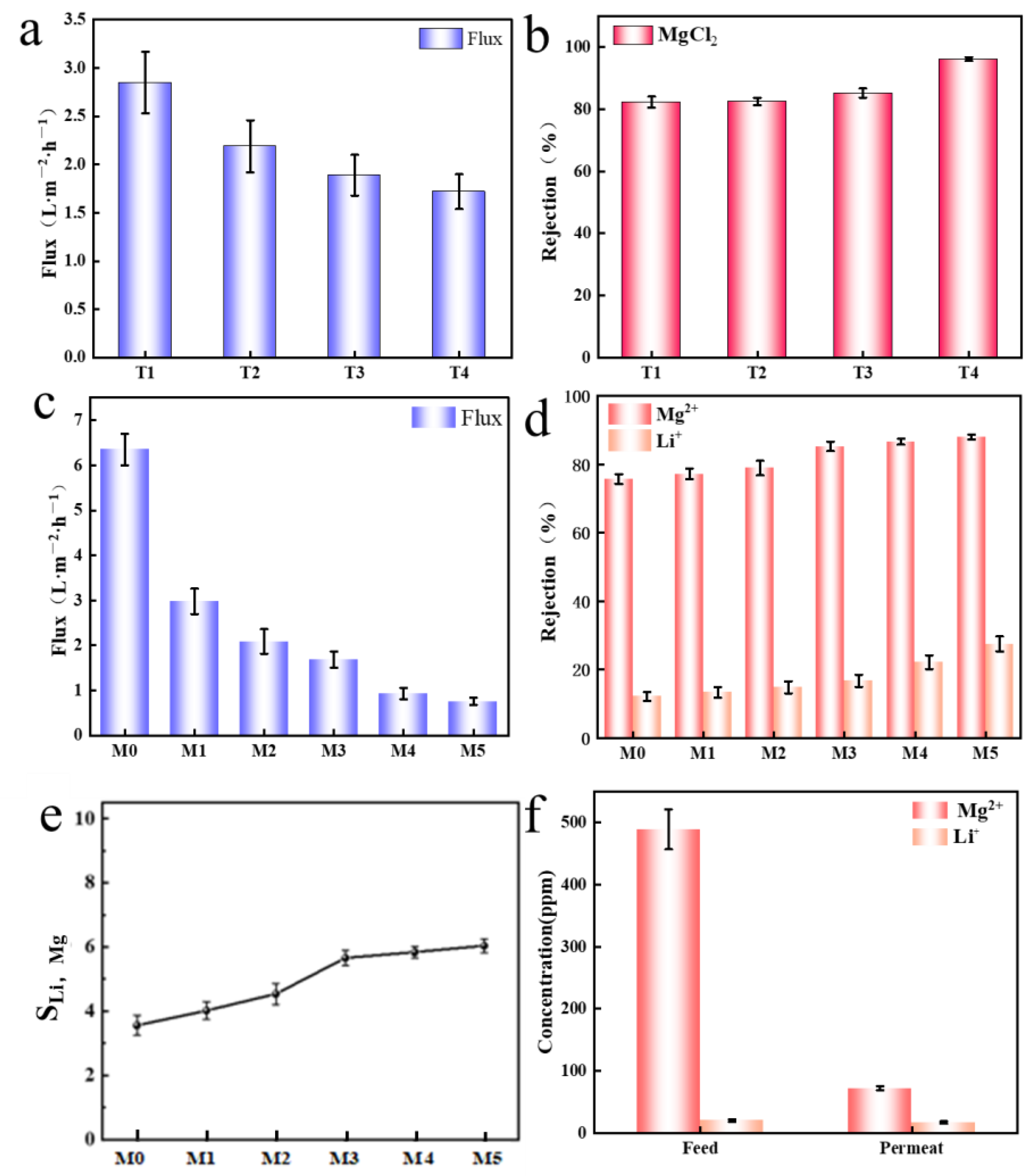
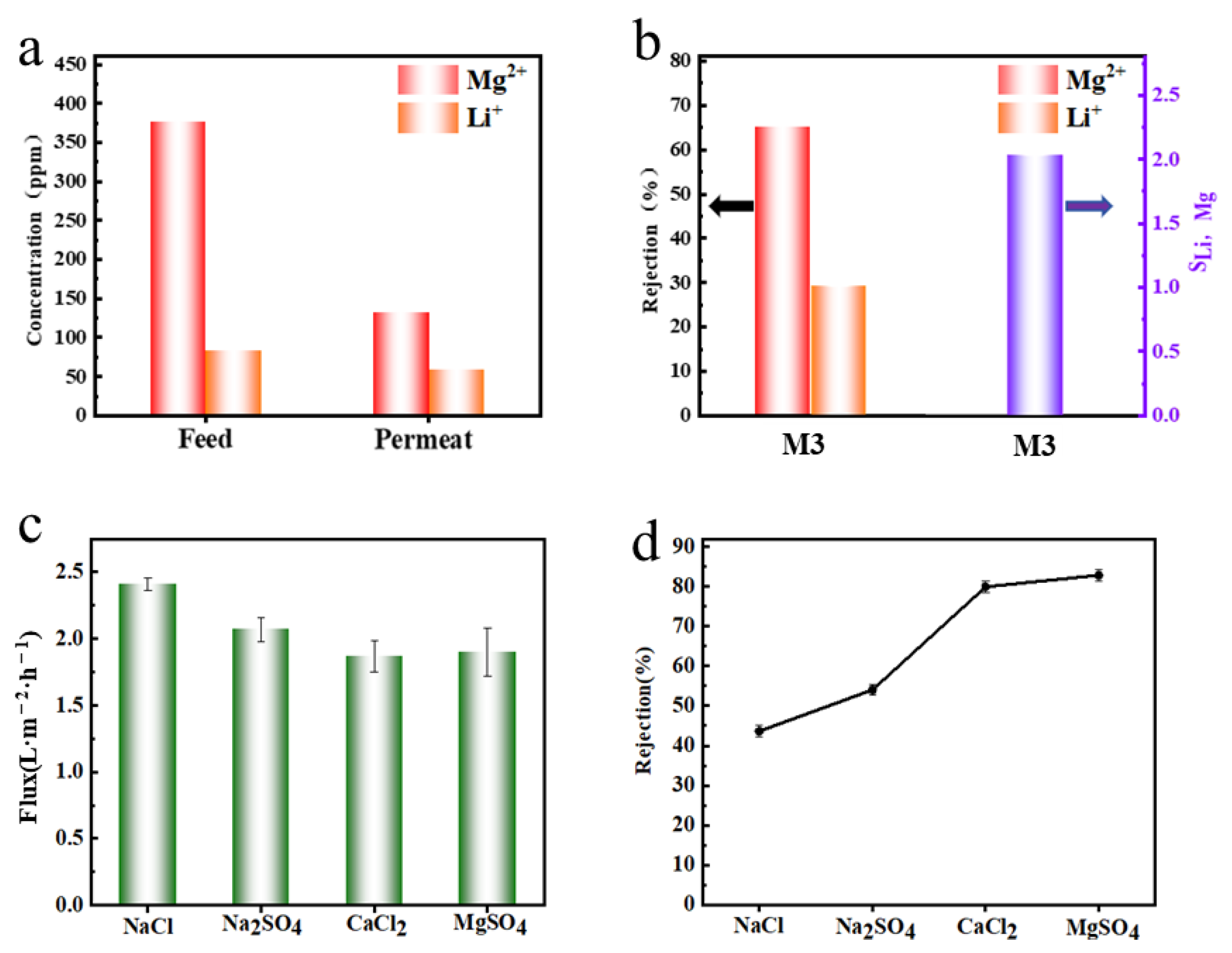
2.2. Performance Testing of Composite Membranes
3. Experiments
3.1. The Synthesis of MXene Nanosheets
3.2. The Fabrication of a PEI-GO/MXene Composite Membrane
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xu, P.; Hong, J.; Qian, X.; Xu, Z.; Xia, H.; Tao, X.; Xu, Z.; Ni, Q.-Q. Materials for lithium recovery from salt lake brine. J. Mater. Sci. 2021, 56, 16–23. [Google Scholar] [CrossRef]
- Lebedeva, N.P.; Boon-Brett, L. Considerations on the Chemical Toxicity of Contemporary Li-Ion Battery Electrolytes and Their Components. J. Electrochem. Soc. 2016, 163, A821–A830. [Google Scholar] [CrossRef]
- Meshram, P.; Pandey, B.D.; Mankhand, T.R. Extraction of lithium from primary and secondary sources by pre-treatment, leaching and separation: A comprehensive review. Hydrometallurgy 2014, 150, 192–208. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, Q.; Wang, Y.; Yun, R.; Xiang, X. Recent advances in magnesium/lithium separation and lithium extraction technologies from salt lake brine. Sep. Purif. Technol. 2021, 256, 117807. [Google Scholar] [CrossRef]
- Swain, B. Recovery and recycling of lithium: A review. Sep. Purif. Technol. 2017, 172, 388–403. [Google Scholar] [CrossRef]
- Xu, S.; Song, J.; Bi, Q.; Chen, Q.; Zhang, W.-M.; Qian, Z.; Zhang, L.; Xu, S.; Tang, N.; He, T. Extraction of lithium from Chinese salt-lake brines by membranes: Design and practice. J. Membr. Sci. 2021, 635, 119441. [Google Scholar] [CrossRef]
- Lu, Z.; Wu, Y.; Ding, L.; Wei, Y.; Wang, H. A Lamellar MXene (Ti3C2Tx)/PSS Composite Membrane for Fast and Selective Lithium-Ion Separation. Angew. Chem. Int. Ed. 2021, 60, 22265–22269. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, L.; Sun, W.; Hu, Y.; Tang, H. Membrane technologies for Li+/Mg2+ separation from salt-lake brines and seawater: A comprehensive review. J. Ind. Eng. Chem. 2020, 81, 7–23. [Google Scholar] [CrossRef]
- Foo, Z.H.; Thomas, J.B.; Heath, S.M.; Garcia, J.A.; Lienhard, J.H. Sustainable Lithium Recovery from Hypersaline Salt-Lakes by Selective Electrodialysis: Transport and Thermodynamics. Environ. Sci. Technol. 2023, 57, 14747–14759. [Google Scholar] [CrossRef]
- Guo, Z.-Y.; Ji, Z.-Y.; Chen, Q.-B.; Liu, J.; Zhao, Y.-Y.; Li, F.; Liu, Z.-Y.; Yuan, J.-S. Prefractionation of LiCl from concentrated seawater/salt lake brines by electrodialysis with monovalent selective ion exchange membranes. J. Clean. Prod. 2018, 193, 338–350. [Google Scholar] [CrossRef]
- Ren, P.; Yin, Z.; Wang, G.; Zhao, H.; Ji, P. The sustainable supply of lithium resources from the Qinghai-Tibet plateau salt lakes group: The selection of extraction methods and the assessment of adsorbent application prospects. Desalination 2024, 583, 117659. [Google Scholar] [CrossRef]
- Yu, J.; Zhu, J.; Luo, G.; Chen, L.; Li, X.; Cui, P.; Wu, P.; Chao, Y.; Zhu, W.; Liu, Z. 3D-printed titanium-based ionic sieve monolithic adsorbent for selective lithium recovery from salt lakes. Desalination 2023, 560, 116651. [Google Scholar] [CrossRef]
- Vera, M.L.; Torres, W.R.; Galli, C.I.; Chagnes, A.; Flexer, V. Environmental impact of direct lithium extraction from brines, Nature Reviews Earth & Environment. Nat. Rev. Earth Environ. 2023, 4, 149–165. [Google Scholar] [CrossRef]
- Shahzad, A.; Oh, J.-M.; Azam, M.; Iqbal, J.; Hussain, S.; Miran, W.; Rasool, K. Advances in the Synthesis and Application of Anti-Fouling Membranes Using Two-Dimensional Nanomaterials. Membranes 2021, 11, 605. [Google Scholar] [CrossRef] [PubMed]
- Werber, J.R.; Osuji, C.O.; Elimelech, M. Materials for next-generation desalination and water purification membranes. Nat. Rev. Mater. 2016, 1, 16018. [Google Scholar] [CrossRef]
- Lu, Z.; Wei, Y.; Deng, J.; Ding, L.; Li, Z.-K.; Wang, H. Self-Crosslinked MXene (Ti3C2Tx) Membranes with Good Antiswelling Property for Monovalent Metal Ion Exclusion. Angew. Chem. Int. Ed. 2019, 13, 10535–10544. [Google Scholar] [CrossRef]
- Ang, E.Y.M.; Ng, T.Y.; Yeo, J.; Lin, R.; Liu, Z.; Geethalakshmi, K.R. Investigations on different two-dimensional materials as slit membranes for enhanced desalination. J. Membr. Sci. 2020, 598, 117653. [Google Scholar] [CrossRef]
- Shen, J.; Wu, J.; Wang, M.; Dong, P.; Xu, J.; Li, X.; Zhang, X.; Yuan, J.; Wang, X.; Ye, M.; et al. Surface Tension Components Based Selection of Cosolvents for Efficient Liquid Phase Exfoliation of 2D Materials. Small 2016, 12, 2741–2749. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.-H.; Liu, Z.; Hu, J.-Q.; Cai, Q.-W.; Li, X.-Y.; Wang, W.; Faraj, Y.; Ju, X.-J.; Xie, R.; Chu, L.-Y. β-Cyclodextrin-modified graphene oxide membranes with large adsorption capacity and high flux for efficient removal of bisphenol A from water. J. Membr. Sci. 2020, 595, 117510. [Google Scholar] [CrossRef]
- Compton, O.C.; Cranford, S.W.; Putz, K.W.; An, Z.; Brinson, L.C.; Buehler, M.J.; Nguyen, S.T. Tuning the Mechanical Properties of Graphene Oxide Paper and Its Associated Polymer Nanocomposites by Controlling Cooperative Intersheet Hydrogen Bonding. Angew. Chem. Int. Ed. 2012, 6, 2008–2019. [Google Scholar] [CrossRef]
- Putz, K.W.; Compton, O.C.; Segar, C.; An, Z.; Nguyen, S.T.; Brinson, L.C. Evolution of Order During Vacuum-Assisted Self-Assembly of Graphene Oxide Paper and Associated Polymer Nanocomposites. Angew. Chem. Int. Ed. 2011, 5, 6601–6609. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Hu, R.; Zhao, X.; He, Y.; Zhu, H. High flux nanofiltration membranes prepared with a graphene oxide homo-structure. J. Membr. Sci. 2019, 585, 29–37. [Google Scholar] [CrossRef]
- Zhan, X.; Gao, Z.; Ge, R.; Lu, J.; Li, J.; Wan, X. Rigid POSS intercalated graphene oxide membranes with hydrophilic/hydrophobic heterostructure for efficient pervaporation desalination. Desalination 2022, 543, 116106. [Google Scholar] [CrossRef]
- Ihsanullah, I. Potential of MXenes in Water Desalination: Current Status and Perspectives. Nano-Micro Lett. 2020, 12, 72. [Google Scholar] [CrossRef]
- Karahan, H.E.; Goh, K.; Zhang, C.J.; Yang, E.; Yildirim, C.; Chuah, C.Y.; Ahunbay, M.G.; Lee, J.; Tantekin-Ersolmaz, S.B.; Chen, Y.; et al. MXene Materials for Designing Advanced Separation Membranes. Adv. Mater. 2020, 32, 1906697. [Google Scholar] [CrossRef]
- Ding, L.; Li, L.; Liu, Y.; Wu, Y.; Lu, Z.; Deng, J.; Wei, Y.; Caro, J.; Wang, H. Effective ion sieving with Ti3C2Tx MXene membranes for production of drinking water from seawater. Nat. Sustain. 2020, 3, 296–302. [Google Scholar] [CrossRef]
- Ren, C.E.; Hatzell, K.B.; Alhabeb, M.; Ling, Z.; Mahmoud, K.A.; Gogotsi, Y. Charge- and Size-Selective Ion Sieving through Ti3C2Tx MXene Membranes. J. Phys. Chem. Lett. 2015, 6, 4026–4031. [Google Scholar] [CrossRef]
- Ding, M.; Xu, H.; Chen, W.; Yang, G.; Kong, Q.; Ng, D.; Lin, T.; Xie, Z. 2D laminar maleic acid-crosslinked MXene membrane with tunable nanochannels for efficient and stable pervaporation desalination. J. Membr. Sci. 2020, 600, 117871. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, H.; Wang, X.; Chen, S.; Yu, H.; Quan, X. Electroconductive RGO-MXene membranes with wettability-regulated channels: Improved water permeability and electro-enhanced rejection performance. Front. Environ. Sci. Eng. 2022, 17, 1. [Google Scholar] [CrossRef]
- Yang, G.; Shi, H.; Liu, W.; Xing, W.; Xu, N. Investigation of Mg2+/Li+ Separation by Nanofiltration. Chin. J. Chem. Eng. 2011, 19, 586–591. [Google Scholar] [CrossRef]
- Meng, B.; Liu, G.; Mao, Y.; Liang, F.; Liu, G.; Jin, W. Fabrication of surface-charged MXene membrane and its application for water desalination. J. Membr. Sci. 2021, 623, 119076. [Google Scholar] [CrossRef]
- Chen, K.; Zhao, S.; Lan, H.; Xie, T.; Wang, H.; Chen, Y.; Li, P.; Sun, H.; Niu, Q.J.; Yang, C. Dual-electric layer nanofiltration membranes based on polyphenol/PEI interlayer for highly efficient Mg2+/Li+ separation. J. Membr. Sci. 2022, 660, 120860. [Google Scholar] [CrossRef]
- Guo, C.; Qian, X.; Tian, F.; Li, N.; Wang, W.; Xu, Z.; Zhang, S. Amino-rich carbon quantum dots ultrathin nanofiltration membranes by double “one-step” methods: Breaking through trade-off among separation, permeation and stability. Chem. Eng. J. 2021, 404, 127144. [Google Scholar] [CrossRef]
- He, J.-H.; Elgazery, N.; Elagamy, K.; Abd Elazem, N. Efficacy of a Modulated Viscosity-dependent Temperature/nanoparticles Concentration Parameter on a Nonlinear Radiative Electromagneto-nanofluid Flow along an Elongated Stretching Sheet. J. Appl. Comput. Mech. 2023, 9, 848–860. [Google Scholar] [CrossRef]
- Zhang, P.; Gong, J.-L.; Zeng, G.-M.; Song, B.; Cao, W.; Liu, H.-Y.; Huan, S.-Y.; Peng, P. Novel “loose” GO/MoS2 composites membranes with enhanced permeability for effective salts and dyes rejection at low pressure. J. Membr. Sci. 2019, 574, 112–123. [Google Scholar] [CrossRef]
- Zhao, X.; Che, Y.; Mo, Y.; Huang, W.; Wang, C. Fabrication of PEI modified GO/MXene composite membrane and its application in removing metal cations from water. J. Membr. Sci. 2021, 640, 119847. [Google Scholar] [CrossRef]
- Zhang, M.; Guan, K.; Ji, Y.; Liu, G.; Jin, W.; Xu, N. Controllable ion transport by surface-charged graphene oxide membrane. Nat. Commun. 2019, 10, 1253. [Google Scholar] [CrossRef]
- Yadav, S.; Ibrar, I.; Altaee, A.; Samal, A.K.; Ghobadi, R.; Zhou, J. Feasibility of brackish water and landfill leachate treatment by GO/MoS2-PVA composite membranes. Sci. Total Environ. 2020, 745, 141088. [Google Scholar] [CrossRef]
- Cheng, P.; Chen, Y.; Gu, Y.-H.; Yan, X.; Lang, W.-Z. Hybrid 2D WS2/GO nanofiltration membranes for finely molecular sieving. J. Membr. Sci. 2019, 591, 117308. [Google Scholar] [CrossRef]
- Han, S.; Li, W.; Xi, H.; Yuan, R.; Long, J.; Xu, C. Plasma-assisted in-situ preparation of graphene-Ag nanofiltration membranes for efficient removal of heavy metal ions. J. Hazard. Mater. 2022, 423, 127012. [Google Scholar] [CrossRef]
- Liu, T.; Liu, X.; Graham, N.; Yu, W.; Sun, K. Two-dimensional MXene incorporated graphene oxide composite membrane with enhanced water purification performance. J. Membr. Sci. 2020, 593, 117431. [Google Scholar] [CrossRef]
- Yan, M.; Huang, W.; Li, Z. Chitosan cross-linked graphene oxide/lignosulfonate composite aerogel for enhanced adsorption of methylene blue in water. Int. J. Biol. Macromol. 2019, 136, 927–935. [Google Scholar] [CrossRef]
- Ma, J.; Tang, X.; He, Y.; Fan, Y.; Chen, J.; Yu, H. Robust stable MoS2/GO filtration membrane for effective removal of dyes and salts from water with enhanced permeability. Desalination 2020, 480, 114328. [Google Scholar] [CrossRef]
- Liu, X.; Feng, Y.; Ni, Y.; Peng, H.; Li, S.; Zhao, Q. High-permeance Mg2+/Li+ separation nanofiltration membranes intensified by quadruple imidazolium salts. J. Membr. Sci. 2023, 667, 121178. [Google Scholar] [CrossRef]
- Ding, J.; Zhao, H.; Xu, B.; Yu, H. Biomimetic Sustainable Graphene Ultrafast-Selective Nanofiltration Membranes. ACS Sustain. Chem. Eng. 2020, 8, 8986–8993. [Google Scholar] [CrossRef]
- Wang, H.; Zeng, G.; Yang, Z.; Chen, X.; Wang, L.; Xiang, Y.; Zeng, X.; Feng, Z.; Tang, B.; Yu, X.; et al. Nanofiltration membrane based on a dual-reinforcement strategy of support and selective layers for efficient Mg2+/Li+ separation. Sep. Purif. Technol. 2024, 330, 125254. [Google Scholar] [CrossRef]
- Dixit, F.; Zimmermann, K.; Dutta, R.; Prakash, N.J.; Barbeau, B.; Mohseni, M.; Kandasubramanian, B. Application of MXenes for water treatment and energy-efficient desalination: A review. J. Hazard. Mater. 2022, 423, 127050. [Google Scholar] [CrossRef]
- Mozafari, M.; Shamsabadi, A.A.; Rahimpour, A.; Soroush, M. Ion-Selective MXene-Based Membranes: Current Status and Prospects. Adv. Mater. Technol. 2021, 6, 2001189. [Google Scholar] [CrossRef]
- Peng, H.; Zhao, Q. A Nano-Heterogeneous Membrane for Efficient Separation of Lithium from High Magnesium/Lithium Ratio Brine. Adv. Funct. Mater. 2021, 31, 2009430. [Google Scholar] [CrossRef]
- Sun, S.-Y.; Cai, L.-J.; Nie, X.-Y.; Song, X.; Yu, J.-G. Separation of magnesium and lithium from brine using a Desal nanofiltration membrane. J. Water Process Eng. 2015, 7, 10–217. [Google Scholar] [CrossRef]
- Wen, X.; Ma, P.; Zhu, C.; He, Q.; Deng, X. Preliminary study on recovering lithium chloride from lithium-containing waters by nanofiltration. Sep. Purif. Technol. 2006, 49, 230–236. [Google Scholar] [CrossRef]
- Gao, J.; Sun, S.-P.; Zhu, W.-P.; Chung, T.-S. Polyethyleneimine (PEI) cross-linked P84 nanofiltration (NF) hollow fiber membranes for Pb2+ removal. J. Membr. Sci. 2014, 452, 300–310. [Google Scholar] [CrossRef]
- Gumbi, N.N.; Li, J.; Mamba, B.B.; Nxumalo, E.N. Relating the performance of sulfonated thin-film composite nanofiltration membranes to structural properties of macrovoid-free polyethersulfone/sulfonated polysulfone/O-MWCNT supports. Desalination 2020, 474, 11476. [Google Scholar] [CrossRef]
- Zeng, G.; Liu, Y.; Lin, Q.; Pu, S.; Zheng, S.; Ang, M.B.M.Y.; Chiao, Y.-H. Constructing composite membranes from functionalized metal organic frameworks integrated MXene intended for ultrafast oil/water emulsion separation. Sep. Purif. Technol. 2022, 293, 121052. [Google Scholar] [CrossRef]
- Yang, Z.; Lin, Q.; Zeng, G.; Zhao, S.; Yan, G.; Ang, M.B.M.Y.; Chiao, Y.-H.; Pu, S. Ternary hetero-structured BiOBr/Bi2MoO6@MXene composite membrane: Construction and enhanced removal of antibiotics and dyes from water. J. Membr. Sci. 2023, 669, 121329. [Google Scholar] [CrossRef]
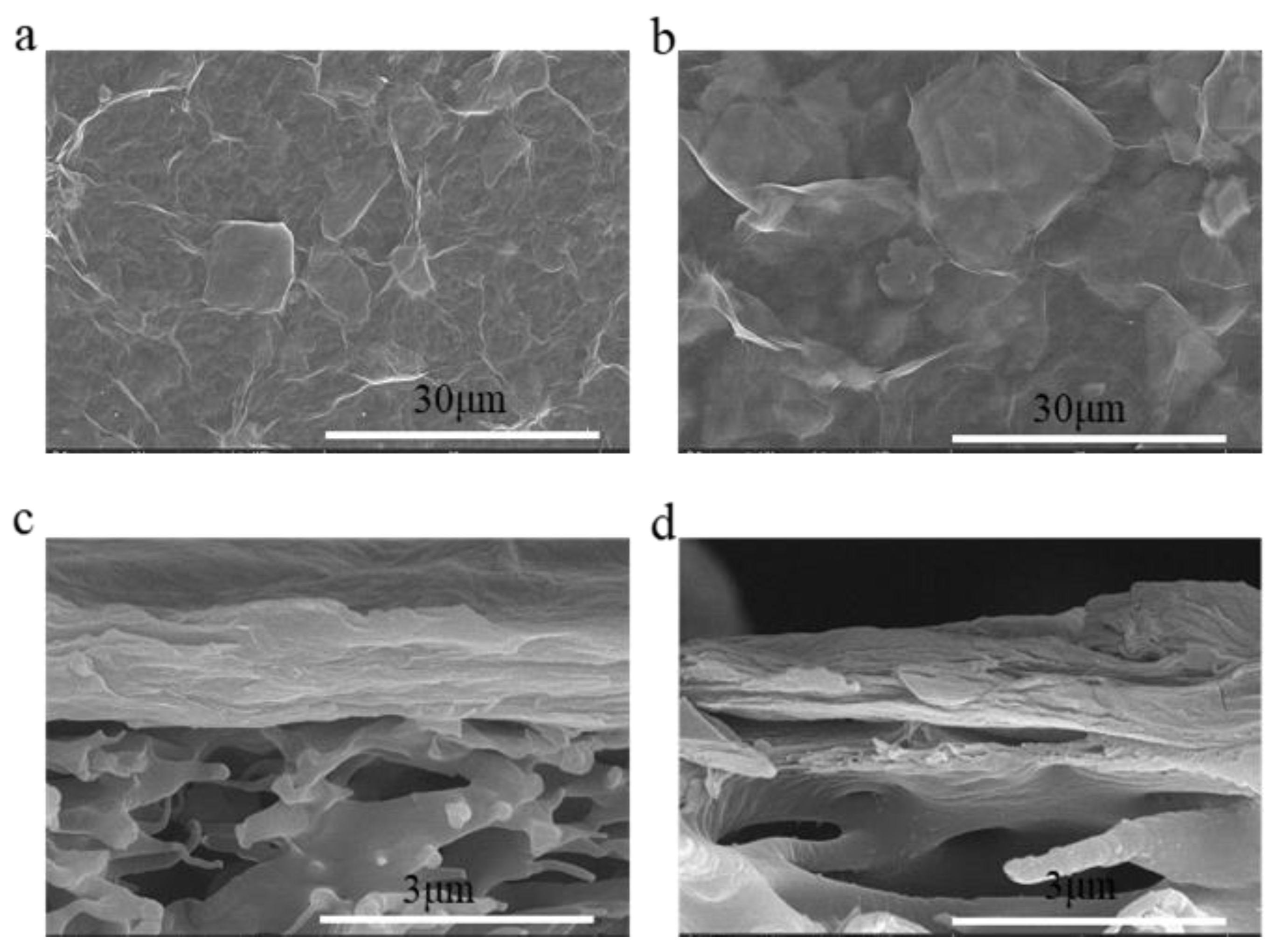

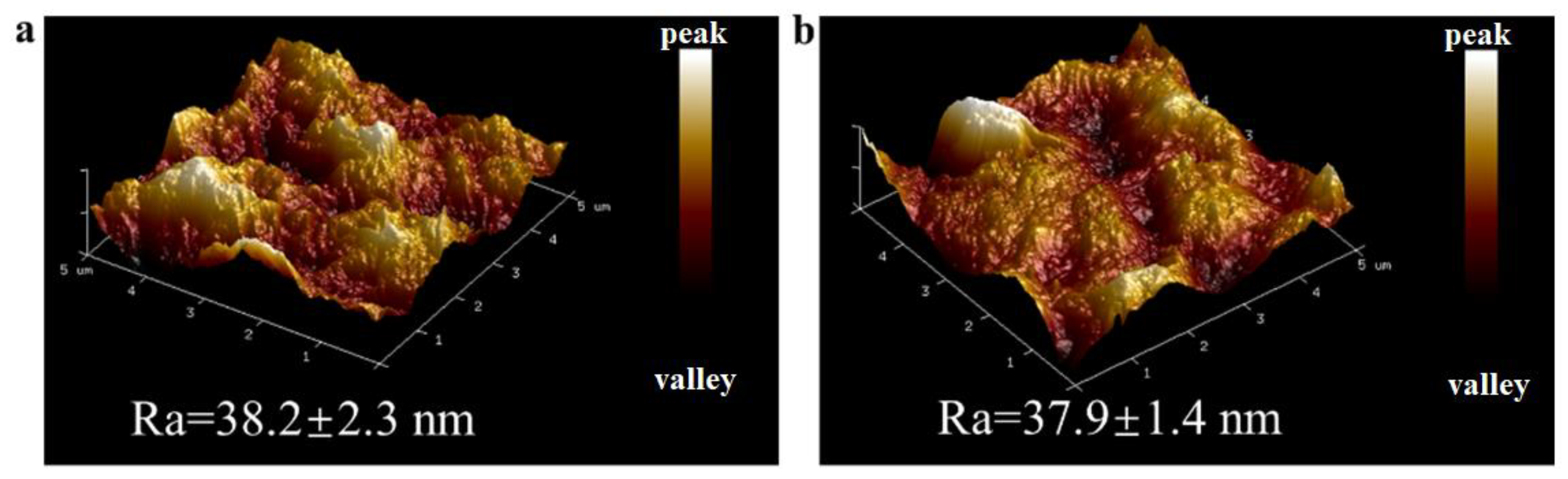
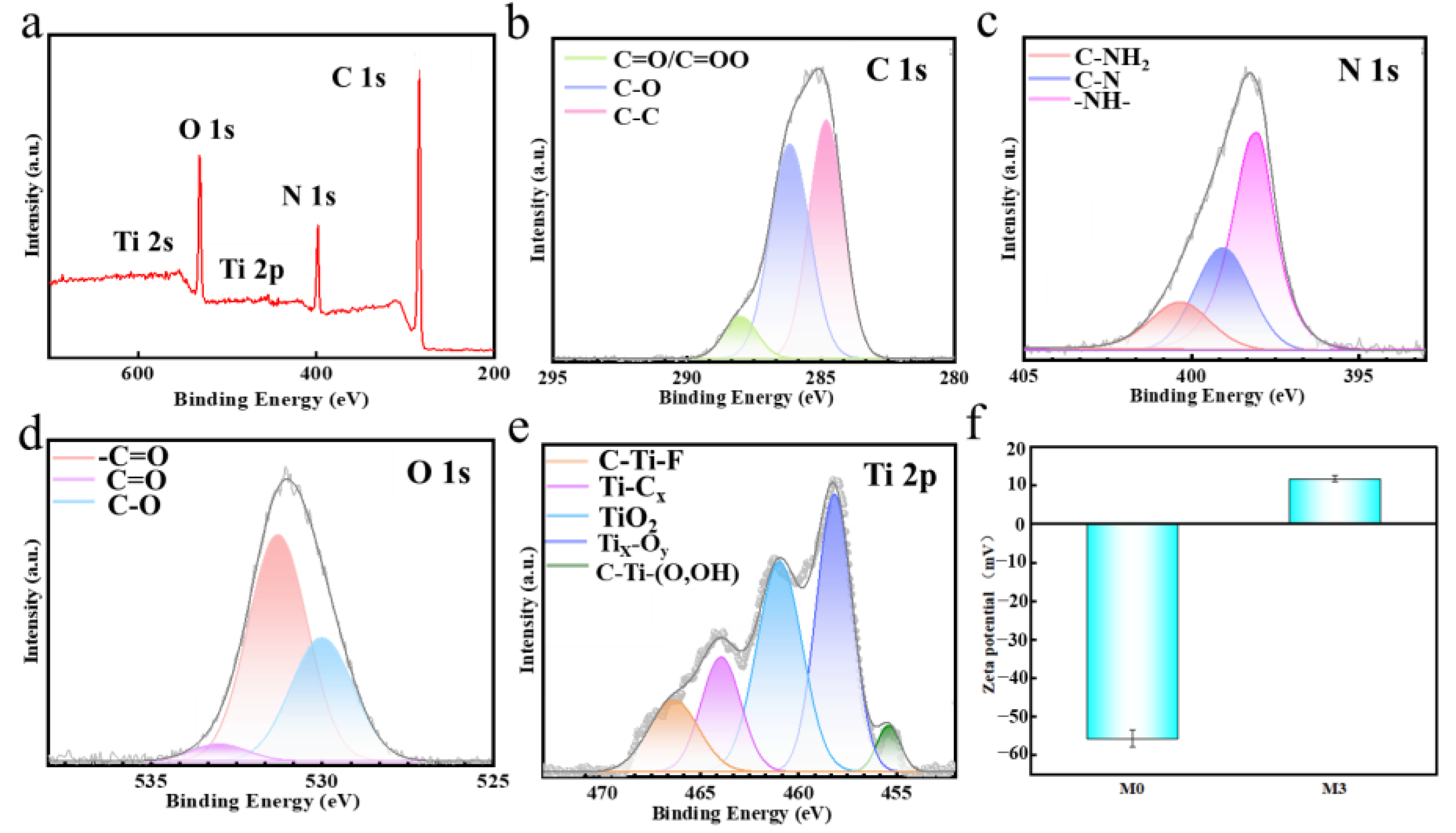
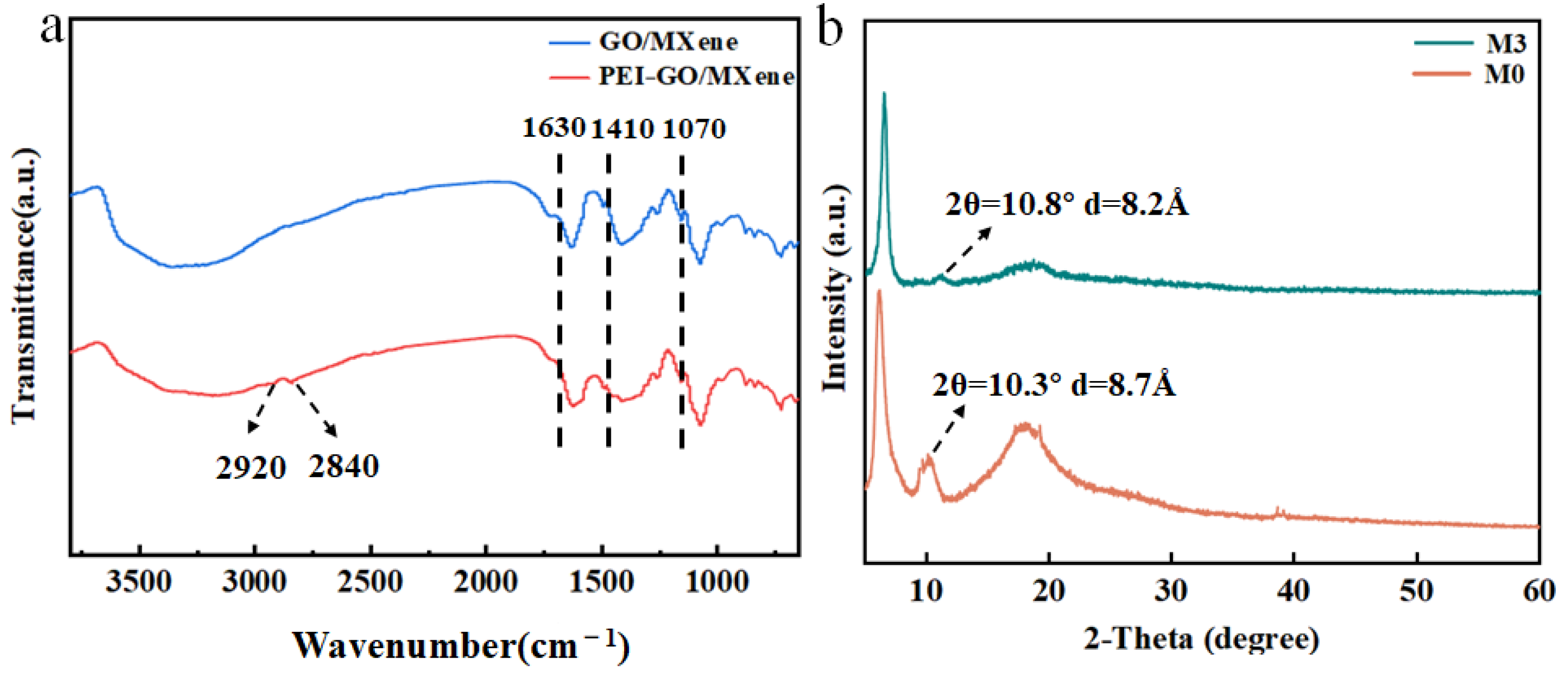


| Type of Membrane | C (%) | O (%) | Ti (%) | N (%) |
|---|---|---|---|---|
| M3 | 70.81 | 15.87 | 0.15 | 13.16 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Wang, A.; Liu, J.; Niu, Q.; Zhang, Y.; Liu, P.; Liu, C.; Wang, H.; Zeng, X.; Zeng, G. Polyethyleneimine Modified Two-Dimensional GO/MXene Composite Membranes with Enhanced Mg2+/Li+ Separation Performance for Salt Lake Brine. Molecules 2024, 29, 4326. https://doi.org/10.3390/molecules29184326
Wang J, Wang A, Liu J, Niu Q, Zhang Y, Liu P, Liu C, Wang H, Zeng X, Zeng G. Polyethyleneimine Modified Two-Dimensional GO/MXene Composite Membranes with Enhanced Mg2+/Li+ Separation Performance for Salt Lake Brine. Molecules. 2024; 29(18):4326. https://doi.org/10.3390/molecules29184326
Chicago/Turabian StyleWang, Jun, Andong Wang, Jiayuan Liu, Qiang Niu, Yijia Zhang, Ping Liu, Chengwen Liu, Hongshan Wang, Xiangdong Zeng, and Guangyong Zeng. 2024. "Polyethyleneimine Modified Two-Dimensional GO/MXene Composite Membranes with Enhanced Mg2+/Li+ Separation Performance for Salt Lake Brine" Molecules 29, no. 18: 4326. https://doi.org/10.3390/molecules29184326
APA StyleWang, J., Wang, A., Liu, J., Niu, Q., Zhang, Y., Liu, P., Liu, C., Wang, H., Zeng, X., & Zeng, G. (2024). Polyethyleneimine Modified Two-Dimensional GO/MXene Composite Membranes with Enhanced Mg2+/Li+ Separation Performance for Salt Lake Brine. Molecules, 29(18), 4326. https://doi.org/10.3390/molecules29184326










