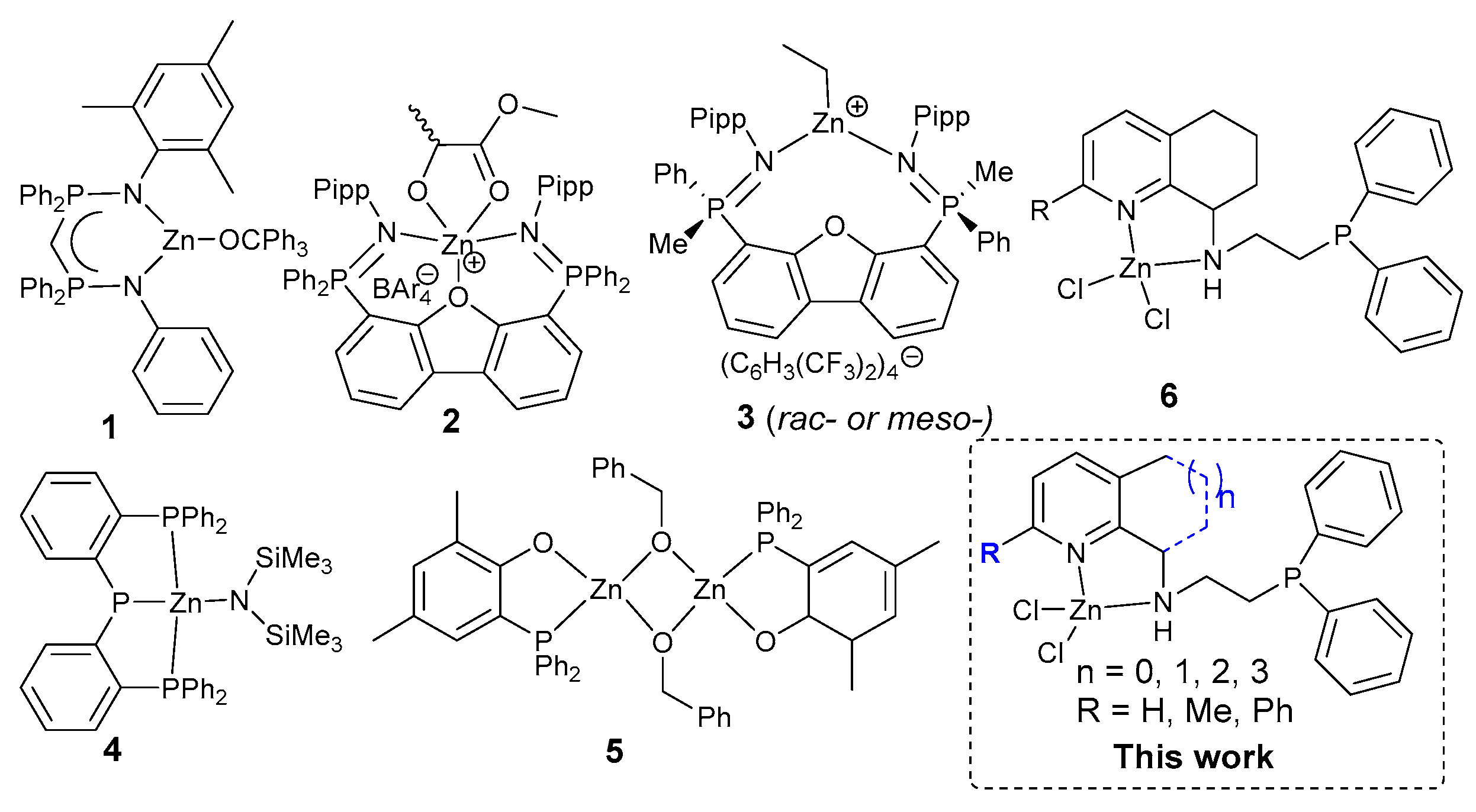
Intensive Cycloalkyl-Fused Pyridines for Aminopyridyl–Zinc–Heteroimidazoles Achieving High Efficiency toward the Ring-Opening Polymerization of Lactides
Abstract
1. Introduction
2. Results and Discussion
2.1. Synthesis and Characterization of L1–L8 and Their Zn (II) Complexes Zn1–Zn8
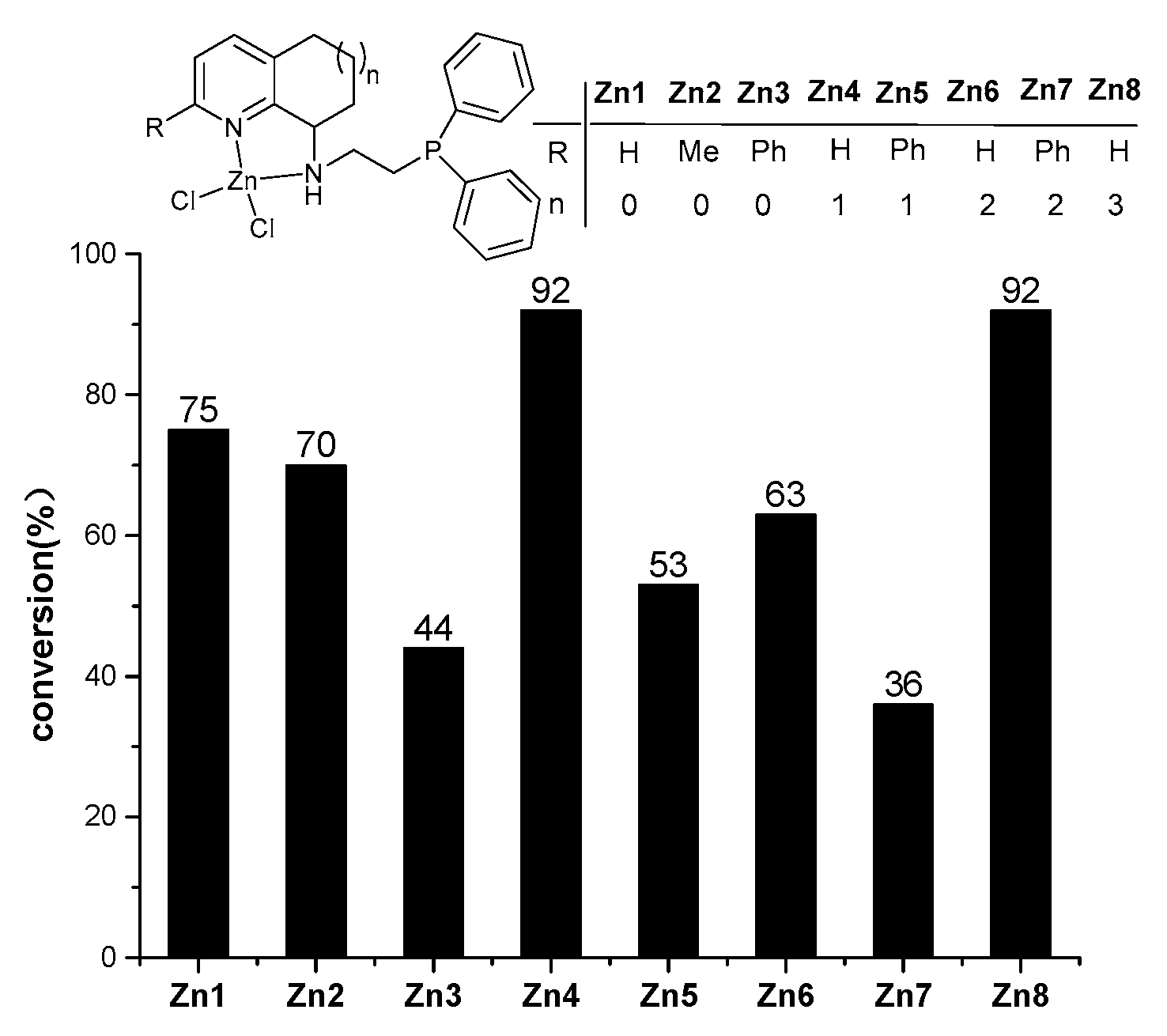
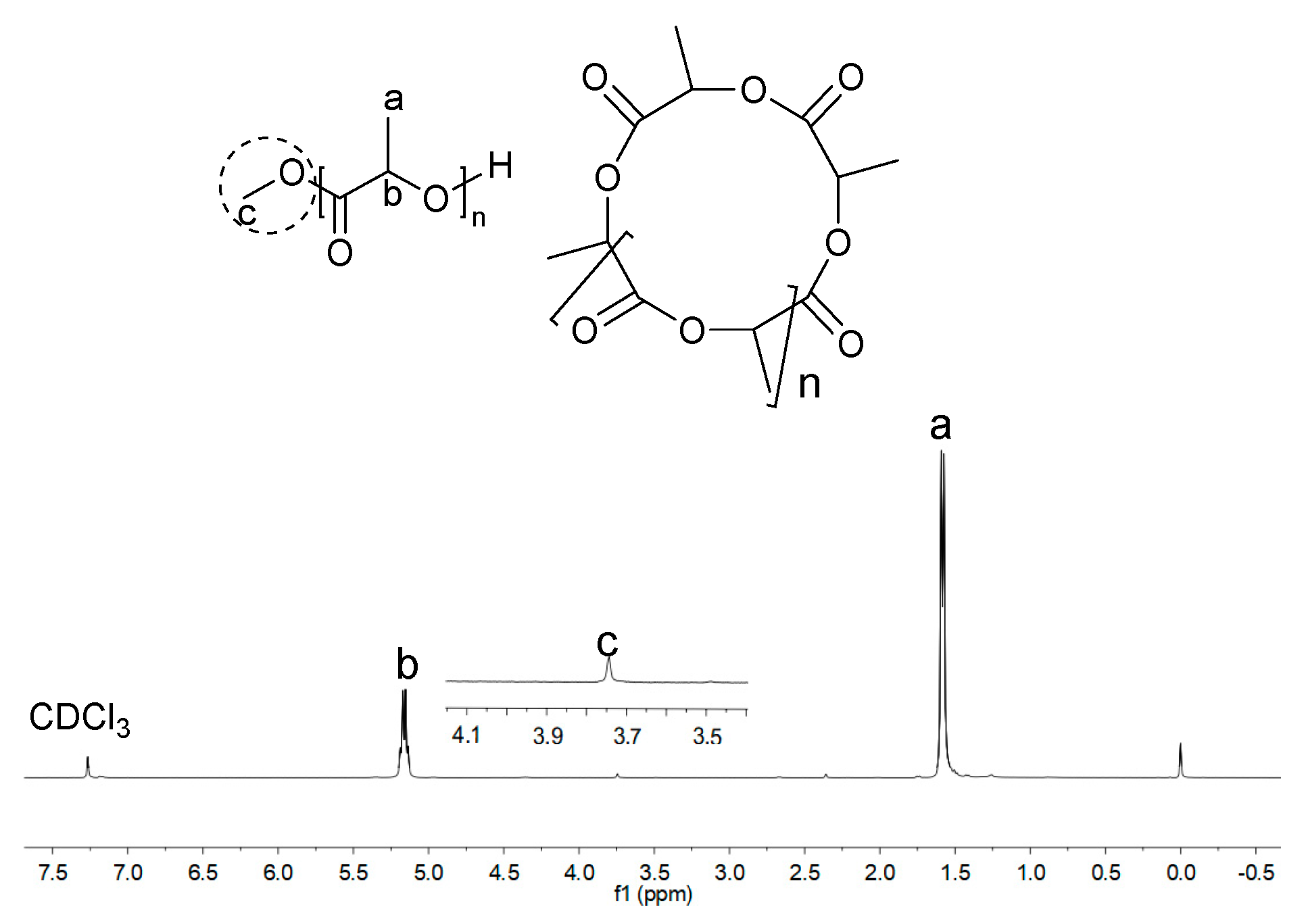
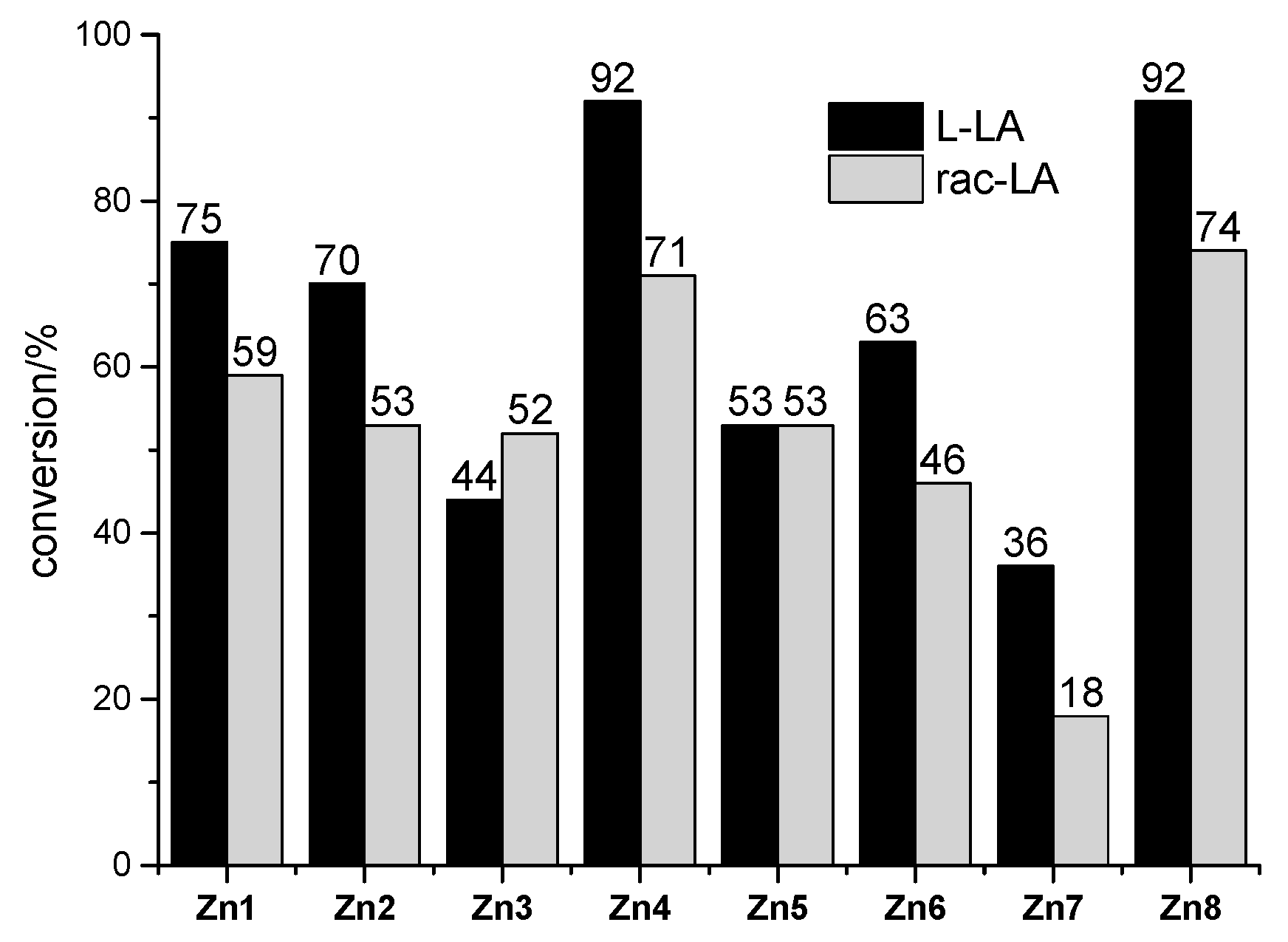
2.2. Ring-Opening Polymerization (ROP) of L-LA by Zn1-Zn8/2LiN(SiMe3)2
2.3. Ring-Opening Polymerization of rac-LA by Zn1–Zn8/2LiN(SiMe3)2
3. Materials and Methods
3.1. General Considerations
3.2. Synthesis and Characterization of Ligands and Zinc Complexes
3.2.1. 2-(Diphenylphosphino)ethanamine, Ligands (L1–L8) and Zinc Complexes (Zn4, Zn5) Were Synthesized as Previously Reported [51,53,54,55]
3.2.2. General Procedure for Ring-Opening Polymerization of L-lactide or rac-LA
3.2.3. X-ray Crystallographic Analyses
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, use, and fate of all plastics ever made. Sci. Adv. 2017, 3, e1700782. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Sun, Z.; Duan, R.; Li, L.; Pang, X.; Chen, X. Copolymer of lactide and ε-caprolactone catalyzed by bimetallic Schiff base aluminum complexes. Sci. China Chem. 2016, 59, 1384–1389. [Google Scholar] [CrossRef]
- Hartmann, M.H. Biopolymers from Renewable Resources; Kaplan, D.H., Ed.; Springer: Berlin, Germany, 1998; pp. 367–411. [Google Scholar]
- Reddy, M.M.; Vivekanandhan, S.; Misra, M.; Bhatia, S.K.; Mohanty, A.K. Biobased plastics and bionanocomposites: Current status and future opportunities. Prog. Polym. Sci. 2013, 38, 1653–1689. [Google Scholar]
- Duan, R.; Hu, C.; Li, X.; Pang, X.; Sun, Z.; Wang, X. Air-stable salen–iron complexes: Stereoselective catalysts for lactide and ε-caprolactone polymerization through in situ initiation. Macromolecules 2017, 50, 9188–9195. [Google Scholar] [CrossRef]
- Zhang, W.; Sun, W.-H. Chapter 39. In Synthesis and Applications in Chemistry and Materials; Pombeiro, A.J.L., Mahmudov, K.T., Da Silva, M.D.F.C.G., Eds.; World Scientific: Singapore, 2024; pp. 217–313. [Google Scholar]
- Thomas, C.M. Stereocontrolled ring-opening polymerization of cyclic esters: Synthesis of new polyester microstructures. Chem. Soc. Rev. 2010, 39, 165–173. [Google Scholar] [CrossRef]
- Parwe, S.P.; Warkad, S.D.; Mane, M.V.; Shedage, P.S.; Garnaik, B. Investigation of the biocompatibility and cytotoxicity associated with ROP initiator and its role in bulk polymerization of l-lactide. Polymer 2017, 111, 244–251. [Google Scholar]
- Conn, R.E.; Kolstad, J.J.; Borzelleca, J.F.; Dixler, D.S.; Filer, L.J.; Ladu, B.N.; Pariza, M.W. Safety assessment of polylactide (PLA) for use as a food-contact polymer. Food Chem. Toxicol. 1995, 33, 273–283. [Google Scholar] [CrossRef]
- Tanzi, M.C.; Verderio, P.; Lampugnani, M.G.; Resnati, M.; Dejana, E.; Sturani, E. Cytotoxicity of some catalysts commonly used in the synthesis of copolymers for biomedical use. J. Mater. Sci. Mater. Med. 1994, 5, 393–396. [Google Scholar] [CrossRef]
- Gao, J.; Zhang, W.; Wang, X.; Wang, R.; Han, M.; Cao, F.; Hao, X. Isoselective Ring-Opening Polymerization of rac-Lactide Catalyzed by Simple Potassium Amidate Complexes Containing Polycyclic Aryl Group. Catalysts 2023, 13, 770. [Google Scholar] [CrossRef]
- Gao, J.; Zhang, W.; Cao, F.; Solan, G.A.; Zhang, X.; Jiang, Y.; Hao, X.; Sun, W.-H. Potassium N-arylbenzimidates as readily accessible and benign (pre) catalysts for the ring opening polymerization of ε-CL and L-LA. Mol. Catal. 2020, 498, 111280. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, X.; Zhang, W.; Sun, W.-H. Progress of ring-opening polymerization of cyclic esters catalyzed by iron compounds. Organometallics 2023, 42, 1680–1692. [Google Scholar] [CrossRef]
- Gao, J.; Zhu, D.; Zhang, W.; Solan, G.A.; Ma, Y.; Sun, W.-H. Recent progress in the application of group 1, 2 & 13 metal complexes as catalysts for the ring opening polymerization of cyclic esters. Inorg. Chem. Front. 2019, 6, 2619–2652. [Google Scholar]
- Porchia, M.; Pellei, M.; Del Bello, F.; Santini, C. Zinc complexes with nitrogen donor ligands as anticancer agents. Molecules 2020, 25, 5814. [Google Scholar] [CrossRef] [PubMed]
- Platel, R.H.; Hodgson, L.M.; Williams, C.K. Biocompatible initiators for lactide polymerization. Polym. Rev. 2008, 48, 11–63. [Google Scholar] [CrossRef]
- Cheng, M.; Attygalle, A.B.; Lobkovsky, E.B.; Coates, G.W. Single-site catalysts for ring-opening polymerization: Synthesis of heterotactic poly (lactic acid) from rac-lactide. Chem. Soc. 1999, 121, 11583–11584. [Google Scholar] [CrossRef]
- Chisholm, M.H.; Gallucci, J.; Phomphrai, K. Coordination chemistry and reactivity of monomeric alkoxides and amides of magnesium and zinc supported by the diiminato ligand CH (CMeNC6H3-2, 6-iPr2)2. A comparative study. Inorg. Chem. 2002, 41, 2785–2794. [Google Scholar] [CrossRef]
- Keram, M.; Ma, H. Ring-opening polymerization of lactide, ε-caprolactone and their copolymerization catalyzed by β-diketiminate zinc complexes. Appl. Organomet. Chem. 2017, 31, e3893. [Google Scholar] [CrossRef]
- Köhler, M.; Rinke, P.; Fiederling, K.; Görls, H.; Ueberschaar, N.; Schacher, F.H.; Kretschmer, R. Catalytic Activity of Various β-Diketiminate Zinc Complexes toward the Ring-Opening Polymerization of Caprolactone and Derivatives. Macromol. Chem. Phys. 2021, 222, 2100187. [Google Scholar] [CrossRef]
- Huang, M.; Pan, C.; Ma, H. Ring-opening polymerization of rac-lactide and α-methyltrimethylene carbonate catalyzed by magnesium and zinc complexes derived from binaphthyl-based iminophenolate ligands. Dalton Trans. 2015, 44, 12420–12431. [Google Scholar] [CrossRef]
- Gong, Y.; Ma, H. High performance benzoimidazolyl-based aminophenolate zinc complexes for isoselective polymerization of rac-lactide. Chem. Commun. 2019, 55, 10112–10115. [Google Scholar] [CrossRef]
- Jędrzkiewicz, D.; Ejfler, J.; Gulia, N.; Szafert, S. Designing ancillary ligands for heteroleptic/homoleptic zinc complex formation: Synthesis, structures and application in ROP of lactides. Dalton Trans. 2015, 44, 13700–13715. [Google Scholar] [CrossRef] [PubMed]
- Kan, C.; Hu, J.; Huang, Y.; Wang, H.; Ma, H. Highly isoselective and active zinc catalysts for rac-lactide polymerization: Effect of pendant groups of aminophenolate ligands. Macromolecules 2017, 50, 7911–7919. [Google Scholar] [CrossRef]
- Chotard, F.; Lapenta, R.; Bolley, A.; Trommenschlager, A.; Balan, C.; Bayardon, J.; Malacea-Kabbara, R.; Bonnin, Q.; Bodio, E.; Cattey, H.; et al. Phenoxyamidine Zn and Al complexes: Synthesis, characterization, and use in the ring-opening polymerization of lactide. Organometallics 2019, 38, 4147–4157. [Google Scholar] [CrossRef]
- Li, M.; Behzadi, S.; Chen, M.; Pang, W.; Wang, F.; Tan, C. Phenoxyimine ligands bearing nitrogen-containing second coordination spheres for zinc catalyzed stereoselective ring-opening polymerization of rac-lactide. Organometallics 2019, 38, 461–468. [Google Scholar] [CrossRef]
- Fuchs, M.; Schmitz, S.; Schäfer, P.M.; Secker, T.; Metz, A.; Ksiazkiewicz, A.N.; Pich, A.; Kögerler, P.; Monakhov, K.Y.; Herres-Pawlis, S. Mononuclear zinc (II) Schiff base complexes as catalysts for the ring-opening polymerization of lactide. Eur. Polym. J. 2020, 122, 109302. [Google Scholar] [CrossRef]
- Soobrattee, S.; Zhai, X.; Nyamayaro, K.; Diaz, C.; Kelley, P.; Ebrahimi, T.; Mehrkhodavandi, P. Dinucleating amino-phenolate platform for zinc catalysts: Impact on lactide polymerization. Inorg. Chem. 2020, 59, 5546–5557. [Google Scholar] [CrossRef] [PubMed]
- Lai, F.J.; Chiu, L.L.; Lee, C.L.; Lu, W.Y.; Lai, Y.C.; Ding, S.; Chen, H.Y.; Wu, K.H. Improvement in zinc complexes bearing Schiff base in ring-opening polymerization of ε-caprolactone: A five-membered ring system. Polymer 2019, 182, 121812. [Google Scholar]
- Santulli, F.; Bruno, F.; Mazzeo, M.; Lamberti, M. Zinc Complexes Bearing Dinucleating Bis (imino-pyridine) binaphthol Ligands: Highly Active and Robust Catalysts for the Lactide Polymerization. ChemCatChem 2023, 15, e202300498. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, K.; Chicoma, M.; Goins, K.; Prior, T.J.; Nile, T.A.; Redshaw, C. Alkoxy-Functionalized Schiff-Base Ligation at Aluminum and Zinc: Synthesis, Structures and ROP Capability. Catalysts 2021, 11, 1090. [Google Scholar] [CrossRef]
- Lian, B.; Thomas, C.M.; Casagrande, O.L.; Lehmann, C.W.; Roisnel, T.; Carpentier, J.F. Aluminum and zinc complexes based on an amino-bis (pyrazolyl) ligand: Synthesis, structures, and use in MMA and lactide polymerization. Inorg. Chem. 2007, 46, 328–340. [Google Scholar] [CrossRef]
- Zikode, M.; Ojwach, S.O.; Akerman, M.P. Structurally rigid bis (pyrazolyl) pyridine Zn (II) and Cu (II) complexes: Structures and kinetic studies in ring-opening polymerization of ε-caprolactone. Appl. Organomet. Chem. 2017, 31, e3556. [Google Scholar] [CrossRef]
- Fliedel, C.; Mameri, S.; Dagorne, S.; Avilés, T. Controlled ring-opening polymerization of trimethylene carbonate and access to PTMC-PLA block copolymers mediated by well-defined N-heterocyclic carbene zinc alkoxides. Appl. Organomet. Chem. 2014, 28, 504–511. [Google Scholar] [CrossRef]
- Schnee, G.; Fliedel, C.; Avilés, T.; Dagorne, S. Neutral and Cationic N-Heterocyclic Carbene Zinc Adducts and the BnOH/Zn (C6F5)2 Binary Mixture–Characterization and Use in the Ring-Opening Polymerization of β-Butyrolactone, Lactide, and Trimethylene Carbonate. Eur. J. Inorg. Chem. 2013, 21, 3699–3709. [Google Scholar] [CrossRef]
- Tufano, F.; Santulli, F.; Grisi, F.; Lamberti, M. N-Heterocyclic Carbene-Based Zinc Complexes: Same Precursors for Different Lactide Ring-Opening Polymerization Mechanisms. ChemCatChem 2022, 14, e202200962. [Google Scholar] [CrossRef]
- Hermann, A.; Hill, S.; Metz, A.; Heck, J.; Hoffmann, A.; Hartmann, L.; Herres-Pawlis, S. Next generation of zinc bisguanidine polymerization catalysts towards highly crystalline, biodegradable polyesters. Angew. Chem. Int. Ed. 2020, 59, 21778–21784. [Google Scholar] [CrossRef] [PubMed]
- Schäfer, P.M.; Fuchs, M.; Ohligschläger, A.; Rittinghaus, R.; McKeown, P.; Akin, E.; Schmidt, M.; Hoffmann, A.; Liauw, M.A.; Jones, M.D.; et al. Highly Active N, O Zinc Guanidine Catalysts for the Ring-Opening Polymerization of Lactide. ChemSusChem 2017, 10, 3547–3556. [Google Scholar] [CrossRef] [PubMed]
- Hermann, A.; Becker, T.; Schäfer, M.A.; Hoffmann, A.; Herres-Pawlis, S. Effective Ligand Design: Zinc Complexes with Guanidine Hydroquinoline Ligands for Fast Lactide Polymerization and Chemical Recycling. ChemSusChem 2022, 15, e202201075. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Schäfer, P.M.; Dittrich, D.; Scheiper, C.; Steiniger, P.; Fink, G.; Ksiazkiewicz, A.N.; Tjaberings, A.; Wölper, C.; Gröschel, A.H.; et al. Heterolepic β-Ketoiminate Zinc Phenoxide Complexes as Efficient Catalysts for the Ring Opening Polymerization of Lactide. ChemistryOpen 2019, 8, 951–960. [Google Scholar] [CrossRef]
- Hill, M.S.; Hitchcock, P.B. Synthesis of C2 and Cs symmetric zinc complexes supported by bis(phosphinimino)methyl ligands and their use in ring opening polymerisation catalysis. J. Chem. Soc. Dalton Trans. 2002, 4694–4702. [Google Scholar] [CrossRef]
- Wheaton, C.A.; Hayes, P.G. Cationic zinc complexes: A new class of catalyst for living lactide polymerization at ambient temperature. Chem. Commun. 2010, 46, 8404–8406. [Google Scholar] [CrossRef]
- Wheaton, C.A.; Hayes, P.G. Cationic organozinc complexes of a bis (phosphinimine) pincer ligand: Synthesis, structural and polymerization studies. Dalton Trans. 2010, 39, 3861–3869. [Google Scholar] [CrossRef] [PubMed]
- Wheaton, C.A.; Hayes, P.G. Exploring the versatility of a bis (phosphinimine) pincer ligand: Effect of sterics on structure and lactide polymerization activity of cationic zinc complexes. Catal. Sci. Technol. 2012, 2, 125–138. [Google Scholar] [CrossRef]
- Sun, H.; Ritch, J.S.; Hayes, P.G. Ring-opening polymerisation of rac-lactide mediated by cationic zinc complexes featuring P-stereogenic bisphosphinimine ligands. Dalton Trans. 2012, 41, 3701–3713. [Google Scholar] [CrossRef]
- D’Auria, I.; Lamberti, M.; Mazzeo, M.; Milione, S.; Roviello, G.; Pellecchia, C. Coordination chemistry and reactivity of zinc complexes supported by a phosphido pincer ligand. Chem. Eur. J. 2012, 18, 2349–2360. [Google Scholar] [CrossRef] [PubMed]
- D’Auria, I.; Lamberti, M.; Rescigno, R.; Venditto, V.; Mazzeo, M. Copolymerization of L-Lactide and ε-Caprolactone promoted by zinc complexes with phosphorus based ligands. Heliyon 2021, 7, e07630. [Google Scholar] [CrossRef] [PubMed]
- Fliedel, C.; Rosa, V.; Alves, F.M.; Martins, A.M.; Avilés, T.; Dagorne, S. P, O-Phosphinophenolate zinc (II) species: Synthesis, structure and use in the ring-opening polymerization (ROP) of lactide, ε-caprolactone and trimethylene carbonate. Dalton Trans. 2015, 44, 12376–12387. [Google Scholar] [CrossRef]
- Goel, R.G.; Henry, W.P.; Jha, N.K. Reinvestigation of tricyclohexylphosphine complexes of zinc (II) and cadmium (II) halides. Preparation, characterization, and phosphorus-31 nuclear magnetic resonance and vibrational spectroscopic studies. Inorg. Chem. 1982, 21, 2551–2555. [Google Scholar] [CrossRef]
- Goel, R.G.; Ogini, W.O. Preparation, characterization, and spectral studies of neutral tri-tert-butylphosphine complexes of zinc (II) and cadmium (II). Inorg. Chem. 1977, 16, 1968–1972. [Google Scholar] [CrossRef]
- Cao, F.; Wang, Y.; Wang, X.; Zhang, W.; Solan, G.A.; Wang, R.; Ma, Y.; Hao, X.; Sun, W.-H. Zinc 8-aminotrihydroquinolines appended with pendant N-diphenylphosphinoethyl arms as exceptionally active catalysts for the ROP of ε-CL. Catal. Sci. Technol. 2022, 12, 6687–6703. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, W.; Wang, X.; Zuo, W.; Xue, X.; Ma, Y.; Sun, W.-H. N-(2-(Diphenylphosphino) ethyl)-2-alkyl-5, 6, 7, 8-tetrahydro-quinolin-8-amines iron (ii) complexes: Structural diversity and the ring opening polymerization of ε-caprolactone. RSC Adv. 2023, 13, 29866–29878. [Google Scholar] [CrossRef]
- Hayashi, T.; Konishi, M.; Fukushima, M.; Kanehira, K.; Hioki, T.; Kumada, M. Chiral (beta-aminoalkyl) phosphines. Highly efficient phosphine ligands for catalytic asymmetric Grignard cross-coupling. J. Org. Chem. 1983, 48, 2195–2202. [Google Scholar] [CrossRef]
- Wang, Z.; Pan, B.; Liu, Q.; Yue, E.; Solan, G.A.; Ma, Y.; Sun, W.-H. Efficient acceptorless dehydrogenation of secondary alcohols to ketones mediated by a PNN-Ru (II) catalyst. Catal. Sci. Technol. 2017, 7, 1654–1661. [Google Scholar] [CrossRef]
- Wang, Z.; Li, Y.; Liu, Q.B.; Solan, G.A.; Ma, Y.; Sun, W.-H. Direct Hydrogenation of a Broad Range of Amides under Base-free Conditions using an Efficient and Selective Ruthenium (II) Pincer Catalyst. ChemCatChem 2017, 9, 4275–4281. [Google Scholar] [CrossRef]
- Kang, M.S.; Cho, J.; Nayab, S.; Jeong, J.H. Synthesis and characterization of Zn (II) and Cu (II) complexes bearing (chiral substituent)(diethyl)-ethanediamine derivatives as precatalysts for rac-lactide polymerisation. Polyhedron 2019, 158, 135–143. [Google Scholar] [CrossRef]
- Kwon, K.S.; Nayab, S.; Lee, H.; Jeong, J.H. Synthesis and structural characterisation of zinc complexes bearing furanylmethyl and thiophenylmethyl derivatives of (R, R)-1, 2-diaminocyclohexanes for stereoselective polymerisation of poly (rac-lactide). Polyhedron 2014, 77, 32–38. [Google Scholar] [CrossRef]
- Save, M.; Schappacher, M.; Soum, A. Controlled ring-opening polymerization of lactones and lactides initiated by lanthanum isopropoxide, 1. General aspects and kinetics. Macromol. Chem. Phys. 2002, 203, 889–899. [Google Scholar] [CrossRef]
- Zhu, D.; Guo, L.; Zhang, W.; Hu, X.; Nomura, K.; Vignesh, A.; Hao, X.; Zhang, Q.; Sun, W.-H. Dialkylaluminum 2-substituted 6, 6-dimethylcyclopentylpyridin-7-oxylates toward structural-differentia-tion of the ring-opening polymerization of ε-caprolactone and L-lactides. Dalton Trans. 2019, 48, 4157–4167. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhang, W.; Han, M.; Wang, X.; Solan, G.A.; Wang, R.; Ma, Y.; Sun, W.-H. Phenoxy-imine/-amide aluminum complexes with pendant or coordinated pyridine moieties: Solvent effects on structural type and catalytic capability for the ROP of cyclic esters. Polymer 2022, 242, 124602. [Google Scholar] [CrossRef]
- Duda, A.; Penczek, S. Polymerization of epsilon-Caprolactone Initiated by aluminum isopropoxide trimer and/or tetramer. Macromolecules 1995, 28, 5981–5992. [Google Scholar] [CrossRef]
- Cayuela, J.; Bounor-Legaré, V.; Cassagnau, P.; Michel, A. Ring-opening polymerization of ε-caprolactone initiated with titanium n-propoxide or titanium phenoxide. Macromolecules 2006, 39, 1338–1346. [Google Scholar] [CrossRef]
- Jhurry, D.; Bhaw-Luximon, A.; Spassky, N. October. Synthesis of polylactides by new aluminium Schoff’s base complexes. Macromol. Symp. 2001, 175, 67–79. [Google Scholar] [CrossRef]
- Ropson, N.; Dubois, P.; Jérôme, R.; Teyssié, P. Macromolecular engineering of polylactones and polylactides. 20. Effect of monomer, solvent, and initiator on the ring-opening polymerization as initiated with aluminum alkoxides. Macromolecules 1995, 28, 7589–7598. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, W.; Wang, S.; Solan, G.A.; Liang, T.; Rajendran, N.M.; Sun, W.-H. Sodium iminoquinolates with cubic and hexagonal prismatic motifs: Synthesis, characterization and their catalytic behavior toward the ROP of rac-lactide. Inorg. Chem. Front. 2016, 3, 1178–1189. [Google Scholar] [CrossRef]
- Dou, J.; Zhu, D.; Zhang, W.; Wang, R.; Wang, S.; Zhang, Q.; Zhang, X.; Sun, W.-H. Highly efficient iron (II) catalysts toward ring opening polymerization of ε-caprolactone through in situ initiation. Inorg. Chim. Acta. 2019, 488, 299–303. [Google Scholar] [CrossRef]
- Montaudo, G.; Montaudo, M.S.; Puglisi, C.; Samperi, F.; Spassky, N.; LeBorgne, A.; Wisniewski, M. Evidence for ester-exchange reactions and cyclic oligomer formation in the ring-opening polymerization of lactide with aluminum complex initiators. Macromolecules 1996, 29, 6461–6465. [Google Scholar] [CrossRef]
- Spassky, N.; Simic, V.; Montaudo, M.S.; Hubert-Pfalzgraf, L.G. Inter-and intramolecular ester exchange reactions in the ring-opening polymerization of (D, L)-lactide using lanthanide alkoxide initiators. Macromol. Chem. Phys. 2000, 201, 2432–2440. [Google Scholar] [CrossRef]
- Castro-Osma, J.A.; Alonso-Moreno, C.; García-Martinez, J.C.; Fernandez-Baeza, J.; Sanchez-Barba, L.F.; Lara-Sanchez, A.; Otero, A. Ring-opening (ROP) versus ring-expansion (REP) polymerization of ε-caprolactone to give linear or cyclic polycaprolactones. Macromolecules 2013, 46, 6388–6394. [Google Scholar] [CrossRef]
- Shaik, M.; Peterson, J.; Du, G. Cyclic and linear polyhydroxylbutyrates from ring-opening polymerization of β-butyrolactone with amido-oxazolinate zinc catalysts. Macromolecules 2019, 52, 157–166. [Google Scholar] [CrossRef]
- Bhunia, M.; Vijaykumar, G.; Adhikari, D.; Mandal, S.K. Highly active carbene potassium complexes for the ring-opening polymerization of ε-caprolactone. Inorg. Chem. 2017, 56, 14459–14466. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. 2015, C71, 3–8. [Google Scholar]
- Sheldrick, G.M. SHELXT-Integrated space-group and crystal-structure determination. Acta Cryst. 2015, A71, 3–8. [Google Scholar] [CrossRef] [PubMed]
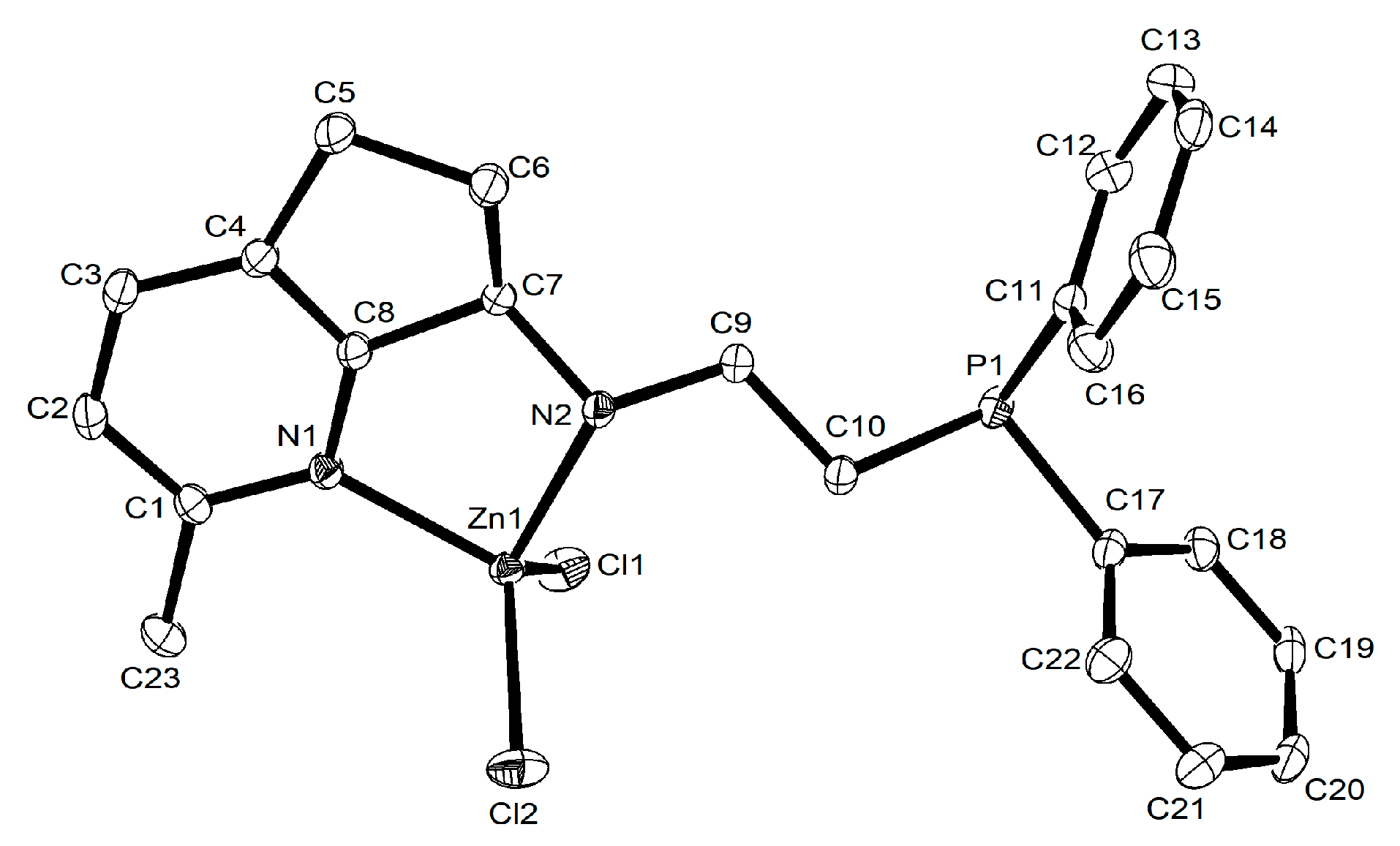
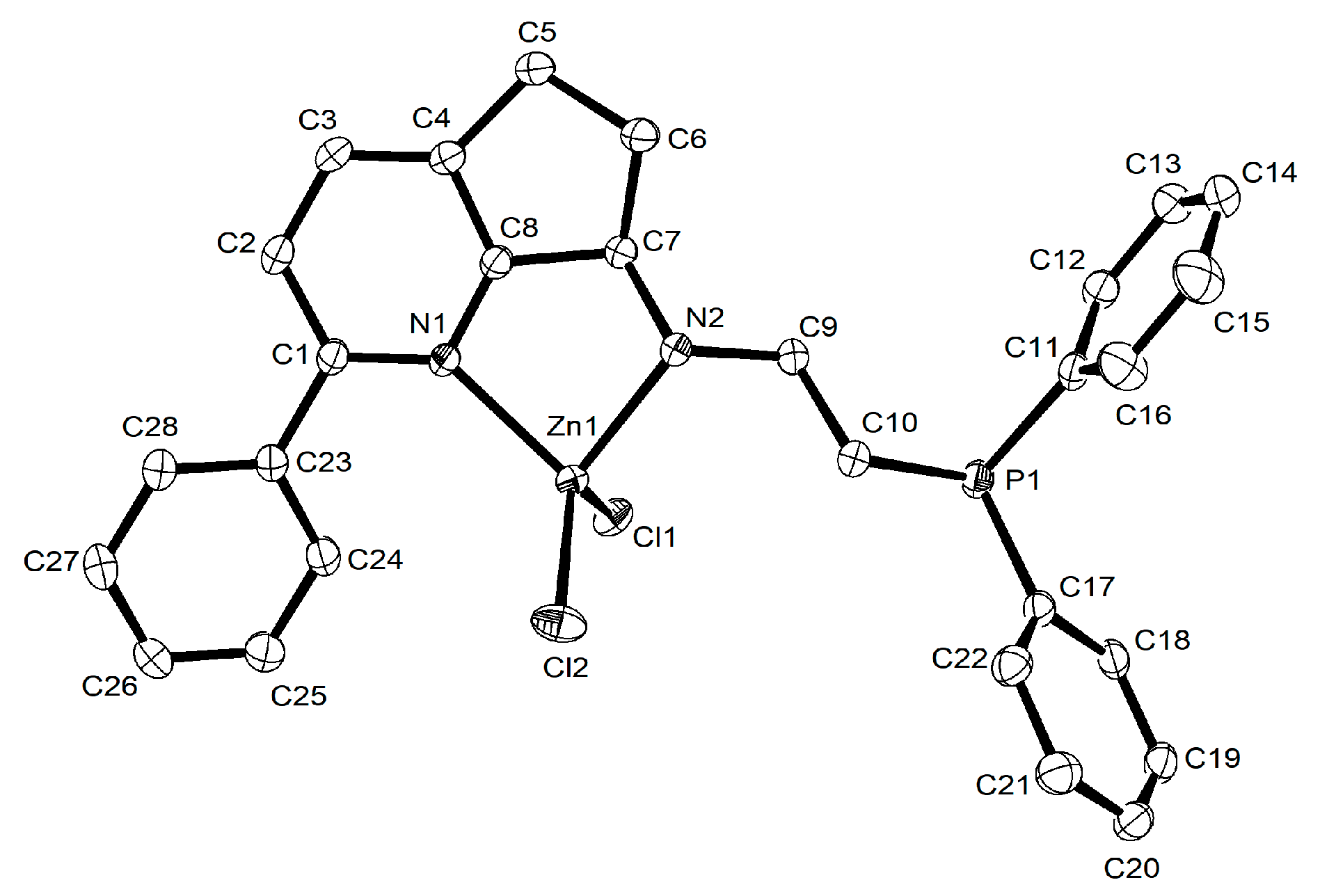
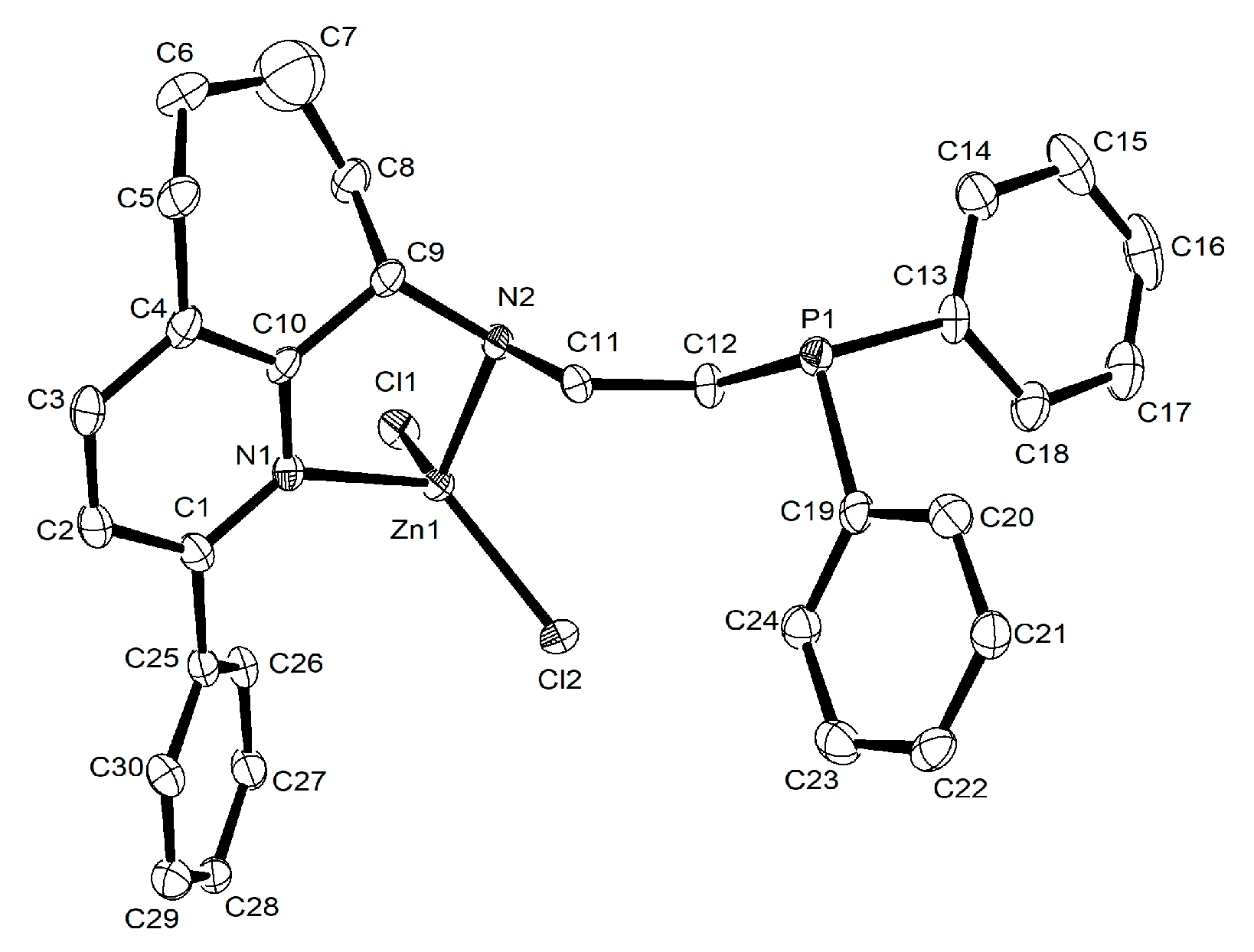
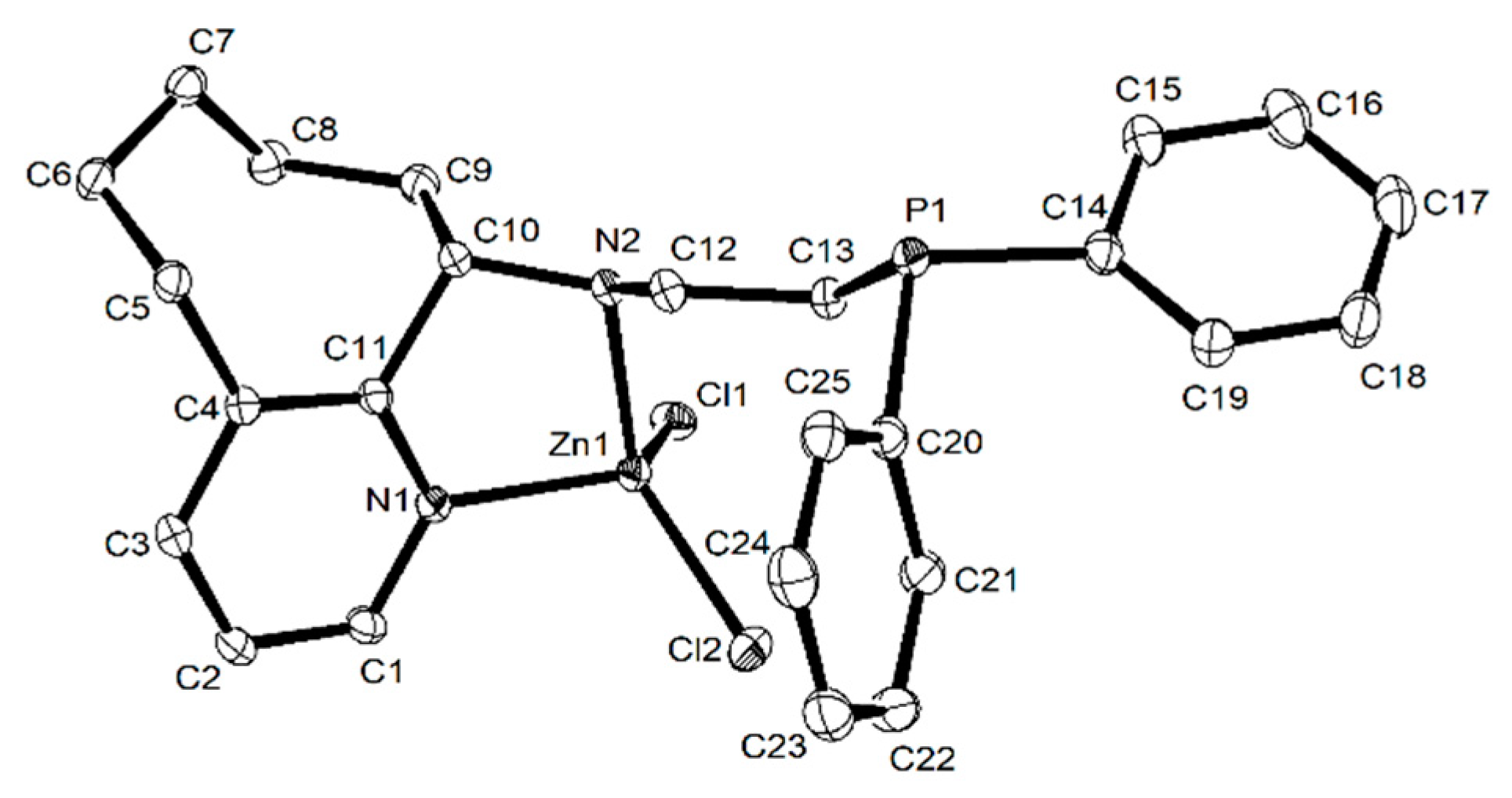

| Zn2 | Zn3 | Zn7 | Zn8 | |
|---|---|---|---|---|
| Bond lengths (Å) | ||||
| Zn1–Cl1 | 2.2018(5) | 2.2220(6) | 2.230(2) | 2.2214(4) |
| Zn1–Cl2 | 2.2287(5) | 2.2053(6) | 2.220(2) | 2.2081(4) |
| Zn1–N1 | 2.0777(15) | 2.1162(16) | 2.085(7) | 2.0442(13) |
| Zn1–N2 | 2.1091(15) | 2.0846(17) | 2.080(7) | 2.0920(12) |
| Bond angles (°) | ||||
| Cl2–Zn1–Cl1 | 115.75(2) | 118.29(2) | 116.84(10) | 117.623(18) |
| N1–Zn1–Cl1 | 114.14(5) | 106.87(5) | 111.9(2) | 117.20(4) |
| N1–Zn1–Cl2 | 111.37(5) | 118.83(5) | 118.9(2) | 105.86(4) |
| N1–Zn1–N2 | 85.08(6) | 85.86(7) | 82.4(3) | 82.74(5) |
| N2–Zn1–Cl1 | 113.51(5) | 107.26(5) | 112.7(2) | 109.14(4) |
| N2–Zn1–Cl2 | 113.23(5) | 114.83(5) | 109.0(2) | 119.54(4) |
| Run | Cat | L-LA:Zn | Solvent | T/°C | t/min | Conv./% b | TOF/h−1 | Mn(calcd) c | Mn d | Mw/Mn d |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Zn6 | 250:1 | toluene | 30 | 10 | 36 | 540 | 1.30 | 3.99 | 1.74 |
| 2 e | Zn6 | 250:1 | toluene | 30 | 10 | 11 | 165 | 0.39 | 0.59 | 1.17 |
| 3 f | Zn6 | 250:1 | toluene | 30 | 10 | 8 | 120 | 0.28 | 0.51 | 1.15 |
| 4 | Zn6 | 250:1 | toluene | 30 | 20 | 48 | 360 | 1.73 | 4.19 | 1.62 |
| 5 | Zn6 | 250:1 | toluene | 30 | 40 | 65 | 244 | 2.34 | 3.06 | 1.72 |
| 6 | Zn6 | 250:1 | toluene | 30 | 60 | 86 | 215 | 3.10 | 1.65 | 2.03 |
| 7 | Zn6 | 250:1 | toluene | 50 | 10 | 68 | 1020 | 2.45 | 2.72 | 1.87 |
| 8 | Zn6 | 250:1 | toluene | 60 | 10 | 85 | 1275 | 3.06 | 2.83 | 2.06 |
| 9 | Zn6 | 250:1 | toluene | 70 | 10 | 96 | 1440 | 3.46 | 2.77 | 1.87 |
| 10 | Zn6 | 250:1 | toluene | 80 | 10 | 100 | 1500 | 3.60 | 2.66 | 2.08 |
| 11 | Zn6 | 250:1 | CH2Cl2 | 30 | 10 | 0 | 0 | – | – | – |
| 12 | Zn6 | 250:1 | THF | 60 | 10 | 92 | 1380 | 3.31 | 1.58 | 2.12 |
| 13 | Zn6 | 250:1 | hexane | 60 | 10 | 8 | 120 | 0.29 | 2.52 | 1.97 |
| 14 | Zn6 | 500:1 | toluene | 80 | 10 | 96 | 2880 | 6.91 | 2.49 | 2.13 |
| 15 | Zn6 | 1000:1 | toluene | 80 | 10 | 89 | 5340 | 12.81 | 2.61 | 2.13 |
| 16 g | Zn6 | 1000:1 | toluene | 80 | 10 | 79 | 4740 | 11.38 | 2.38 | 1.79 |
| 17 h | Zn6 | 1000:1 | toluene | 80 | 10 | 65 | 3900 | 9.36 | 2.02 | 2.33 |
| 18 | Zn6 | 500:1 | toluene | 80 | 5 | 63 | 3780 | 4.54 | 1.86 | 1.97 |
| 19 | Zn1 | 500:1 | toluene | 80 | 5 | 75 | 4500 | 5.40 | 1.96 | 1.90 |
| 20 | Zn2 | 500:1 | toluene | 80 | 5 | 70 | 4200 | 5.04 | 1.68 | 2.04 |
| 21 | Zn3 | 500:1 | toluene | 80 | 5 | 44 | 2640 | 3.17 | 2.68 | 2.01 |
| 22 | Zn4 | 500:1 | toluene | 80 | 5 | 92 | 5520 | 6.62 | 3.90 | 1.75 |
| 23 | Zn5 | 500:1 | toluene | 80 | 5 | 53 | 3180 | 3.82 | 1.51 | 2.17 |
| 24 | Zn7 | 500:1 | toluene | 80 | 5 | 36 | 2160 | 2.59 | 1.92 | 1.99 |
| 25 | Zn8 | 500:1 | toluene | 80 | 5 | 92 | 5520 | 6.62 | 1.84 | 2.27 |
| Run | Cat | rac-LA: Zn | T/°C | t/min | Conv./% b | TOF/h−1 |
|---|---|---|---|---|---|---|
| 1 | Zn1 | 500:1 | 80 | 5 | 59 | 3540 |
| 2 | Zn2 | 500:1 | 80 | 5 | 53 | 3180 |
| 3 | Zn3 | 500:1 | 80 | 5 | 52 | 3120 |
| 4 | Zn4 | 500:1 | 80 | 5 | 71 | 4260 |
| 5 | Zn5 | 500:1 | 80 | 5 | 53 | 3180 |
| 6 | Zn6 | 500:1 | 80 | 5 | 46 | 2760 |
| 7 | Zn7 | 500:1 | 80 | 5 | 18 | 1080 |
| 8 | Zn8 | 500:1 | 80 | 5 | 74 | 4440 |
| Zn2 | Zn3 | Zn7 | Zn8 | |
|---|---|---|---|---|
| empirical formula | C23H25Cl2N2PZn | C28H27Cl2N2PZn | C30H31Cl2N2PZn | C25H29Cl2N2PZn |
| formula weight | 496.69 | 558.75 | 586.81 | 524.74 |
| temperature/K | 170.00(10) | 169.98(10) | 169.98(10) | 169.98(10) |
| crystal system | triclinic | triclinic | monoclinic | triclinic |
| space group | P-1 | P-1 | P21/c | P-1 |
| a/Å | 9.2670(2) | 8.8906(3) | 12.9499(5) | 9.5988(3) |
| b/Å | 10.2665(3) | 10.7637(3) | 16.8402(6) | 10.9347(4) |
| c/Å | 12.7846(3) | 15.1833(5) | 13.0719(5) | 13.0072(4) |
| α/˚ | 84.143(2) | 108.512(3) | 90 | 84.306(3) |
| β/˚ | 75.169(2) | 105.259(3) | 106.844(4) | 75.326(3) |
| γ/˚ | 87.433(2) | 94.871(2) | 90 | 74.958(3) |
| volume/Å3 | 1169.44(5) | 1306.49(8) | 2728.40(19) | 1274.64(8) |
| Z | 2 | 2 | 4 | 2 |
| ρcalc/g cm3 | 1.411 | 1.420 | 1.429 | 1.367 |
| μ/mm−1 | 4.289 | 3.908 | 3.770 | 3.964 |
| F (000) | 512.0 | 576.0 | 1216.0 | 544.0 |
| crystal size/mm3 | 0.25 × 0.25 × 0.2 | 0.3 × 0.3 × 0.25 | 0.15 × 0.1 × 0.02 | 0.33 × 0.3 × 0.28 |
| radiation | CuKα (λ = 1.54184) | CuKα (λ = 1.54184) | CuKα (λ = 1.54184) | CuKα (λ = 1.54184) |
| 2θ range for data collection/° | 7.184 to 154.478 | 6.45 to 153.556 | 7.132 to 154.446 | 8.378 to 154.348 |
| index ranges | −11 ≤ h ≤ 11, −12 ≤ k ≤ 12, −16 ≤ l ≤ 14 | −11 ≤ h ≤ 11, −13 ≤ k ≤ 13, −18 ≤ l ≤ 19 | −16 ≤ h ≤ 16, −20 ≤ k ≤ 21, −16 ≤ l ≤ 16 | −12 ≤ h ≤ 12, −13 ≤ k ≤ 13, −16 ≤ l ≤ 14 |
| reflections collected | 13,881 | 15,346 | 19,546 | 15,774 |
| independent reflections | 4716 [Rint = 0.0236, Rsigma = 0.0229] | 5274 [Rint = 0.0245, Rsigma = 0.0314] | 5574 [Rint = 0.0669, Rsigma = 0.0560] | 5156 [Rint = 0.0218, Rsigma = 0.0196] |
| data/restraints/parameters | 4716/0/263 | 5274/0/307 | 5574/6/325 | 5156/0/281 |
| goodness-of-fit on F2 | 1.050 | 1.032 | 1.100 | 1.024 |
| final R indices [I ≥ 2σ (I)] | R1 = 0.0319, wR2 = 0.0838 | R1 = 0.0367, wR2 = 0.0969 | R1 = 0.1142, wR2 = 0.3324 | R1 = 0.0272, wR2 = 0.0722 |
| final R indices [all data] | R1 = 0.0333, wR2 = 0.0849 | R1 = 0.0388, wR2 = 0.0989 | R1 = 0.1221, wR2 = 0.3364 | R1 = 0.0281, wR2 = 0.0730 |
| largest diff. peak/hole/e Å−3 | 1.13/−0.43 | 1.53/−0.39 | 2.67/−0.93 | 1.04/−0.35 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Zhang, W.; Zhu, P.; You, W.; Xue, X.; Wang, R.; Ma, Y.; Sun, W.-H. Intensive Cycloalkyl-Fused Pyridines for Aminopyridyl–Zinc–Heteroimidazoles Achieving High Efficiency toward the Ring-Opening Polymerization of Lactides. Molecules 2024, 29, 4150. https://doi.org/10.3390/molecules29174150
Wang Y, Zhang W, Zhu P, You W, Xue X, Wang R, Ma Y, Sun W-H. Intensive Cycloalkyl-Fused Pyridines for Aminopyridyl–Zinc–Heteroimidazoles Achieving High Efficiency toward the Ring-Opening Polymerization of Lactides. Molecules. 2024; 29(17):4150. https://doi.org/10.3390/molecules29174150
Chicago/Turabian StyleWang, Yun, Wenjuan Zhang, Pengjiang Zhu, Wei You, Xiaopan Xue, Rui Wang, Yanping Ma, and Wen-Hua Sun. 2024. "Intensive Cycloalkyl-Fused Pyridines for Aminopyridyl–Zinc–Heteroimidazoles Achieving High Efficiency toward the Ring-Opening Polymerization of Lactides" Molecules 29, no. 17: 4150. https://doi.org/10.3390/molecules29174150
APA StyleWang, Y., Zhang, W., Zhu, P., You, W., Xue, X., Wang, R., Ma, Y., & Sun, W.-H. (2024). Intensive Cycloalkyl-Fused Pyridines for Aminopyridyl–Zinc–Heteroimidazoles Achieving High Efficiency toward the Ring-Opening Polymerization of Lactides. Molecules, 29(17), 4150. https://doi.org/10.3390/molecules29174150








