Design, Synthesis, and Acaricidal Activity of 2,5-Diphenyl-1,3-oxazoline Compounds
Abstract
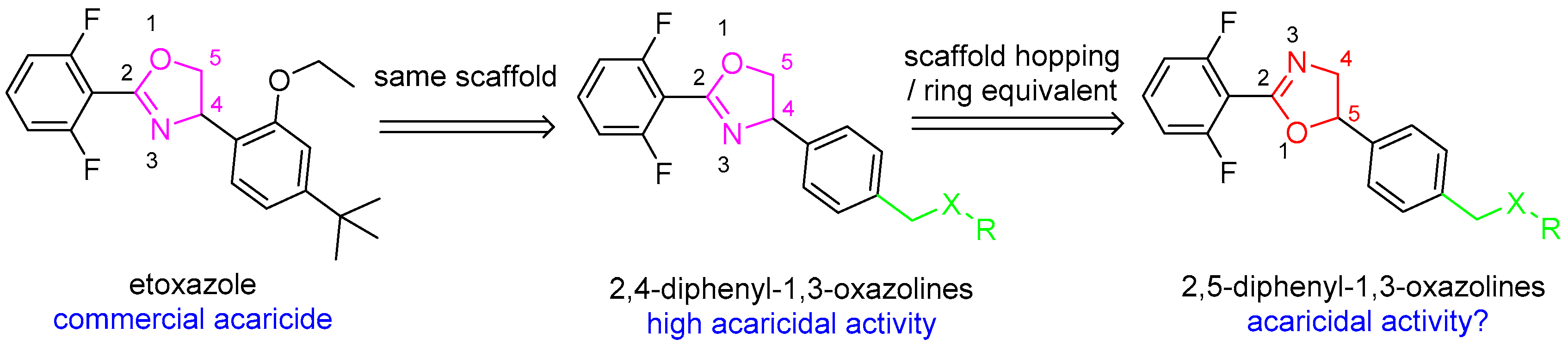
1. Introduction
2. Result and Discussion
2.1. Synthesis
2.2. Acaricidal Activity
3. Materials and Methods
3.1. Preparation of Test Compounds
3.1.1. (4-Vinylphenyl)methanol (2)
3.1.2. 2-Bromo-1-(4-(hydroxymethyl)phenyl)ethan-1-ol (3)
3.1.3. 2-(2-Hydroxy-2-(4-(hydroxymethyl)phenyl)ethyl)isoindoline-1,3-dione (4)
3.1.4. 2-(2-((tert-Butyldimethylsilyl)oxy)-2-(4-(((tert-butyldimethylsilyl)oxy)methyl)phenyl)ethyl)isoindoline-1,3-dione (5)
3.1.5. 2-((tert-Butyldimethylsilyl)oxy)-2-(4-(((tert-butyldimethylsilyl)oxy)methyl)phenyl)ethan-1-amine (6)
3.1.6. N-(2-((tert-Butyldimethylsilyl)oxy)-2-(4-(((tert-butyldimethylsilyl)oxy)methyl)phenyl)ethyl)-2,6-difluorobenzamide (7)
3.1.7. 2,6-Difluoro-N-(2-hydroxy-2-(4-(hydroxymethyl)phenyl)ethyl)benzamide (8)
3.1.8. N-(2-Chloro-2-(4-(chloromethyl)phenyl)ethyl)-2,6-difluorobenzamide (9)
3.1.9. 5-(4-(Chloromethyl)phenyl)-2-(2,6-difluorophenyl)-4,5-dihydrooxazole (10)
3.1.10. Preparation of Target Compound 2-(4-(2-(2,6-difluorophenyl)-4,5-dihydrooxazol-5-yl)benzyl)isoindoline-1,3-dione (11a)
3.1.11. Preparation of Target Compounds 11b–11g and 11t–11x
3.1.12. Preparation of Target Compounds 11h–11m
3.1.13. Preparation of Target Compounds 11n–11s
3.1.14. Preparation of Target Compound 11y
3.2. Acaricidal Activity Evaluation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jeschke, P. Status and outlook for acaricide and insecticide discovery. Pest Manag. Sci. 2021, 77, 64–76. [Google Scholar] [CrossRef]
- Suzuki, J.; Ishida, T.; Shibuya, I.; Toda, K. Development of a new acaricide, etoxazole. J. Pestic. Sci. 2001, 26, 215–223. [Google Scholar] [CrossRef]
- Suzuki, J.; Ishida, T.; Kikuchi, Y.; Morikawa, C.; Tsukidate, Y.; Tanji, I.; Ota, Y.; Toda, K. Synthesis and activity of novel acaricidal/insecticidal 2,4-diphenyl-1,3-oxazolines. J. Pestic. Sci. 2002, 27, 1–8. [Google Scholar] [CrossRef]
- Demaeght, P.; Osborne, E.J.; Odman-Naresh, J.; Grbic, M.; Nauen, R.; Merzendorfer, H.; Clark, R.M.; Van Leeuwen, T. High resolution genetic mapping uncovers chitin synthase-1 as the target-site of the structurally diverse mite growth inhibitors clofentezine, hexythiazox and etoxazole in Tetranychus urticae. Insect Biochem. Mol. Biol. 2014, 51, 52–61. [Google Scholar] [CrossRef]
- Douris, V.; Steinbach, D.; Panteleri, R.; Livadaras, I.; Pickett, J.A.; Van Leeuwen, T.; Nauen, R.; Vontas, J. Resistance mutation conserved between insects and mites unravels the benzoylurea insecticide mode of action on chitin biosynthesis. Proc. Natl. Acad. Sci. USA 2016, 113, 14692–14697. [Google Scholar] [CrossRef]
- Xin, T.; Li, Z.; Chen, J.; Wang, J.; Zou, Z.; Xia, B. Molecular characterization of chitin synthase gene in Tetranychus cinnabarinus (Boisduval) and its response to sublethal concentrations of an insecticide. Insects 2021, 12, 501. [Google Scholar] [CrossRef]
- Chen, W.; Yang, Q. Development of Novel Pesticides Targeting Insect Chitinases: A Minireview and Perspective. J. Agric. Food Chem. 2020, 68, 4559–4565. [Google Scholar] [CrossRef]
- Chen, W.; Cao, P.; Liu, Y.; Yu, A.; Wang, D.; Chen, L.; Sundarraj, R.; Yuchi, Z.; Gong, Y.; Merzendorfer, H.; et al. Structural basis for directional chitin biosynthesis. Nature 2022, 610, 402–408. [Google Scholar] [CrossRef]
- Guan, A.; Liu, C.; Yang, X.; Dekeyser, M. Application of the intermediate derivatization approach in agrochemical discovery. Chem. Rev. 2014, 114, 7079–7107. [Google Scholar] [CrossRef]
- Li, Y.; Li, C.; Zheng, Y.; Wei, X.; Ma, Q.; Wei, P.; Liu, Y.; Qin, Y.; Yang, N.; Sun, Y.; et al. Design, synthesis, acaricidal activity, and mechanism of oxazoline derivatives containing an oxime ether moiety. J. Agric. Food Chem. 2014, 62, 3064–3072. [Google Scholar] [CrossRef]
- Yu, X.; Liu, Y.; Li, Y.; Wang, Q. Design, synthesis, and acaricidal/insecticidal activities of oxazoline derivatives containing a sulfur ether moiety. J. Agric. Food Chem. 2015, 63, 9690–9695. [Google Scholar] [CrossRef]
- Chen, S.; Zhang, Y.; Liu, Y.; Wang, Q. Highly efficient synthesis and acaricidal and insecticidal activities of novel oxazolines with N-heterocyclic substituents. J. Agric. Food Chem. 2021, 69, 3601–3606. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, Y.; Xun, X.; Chen, S.; Liu, Y.; Wang, Q. Design, Synthesis, Acaricidal Activities, and Structure−Activity Relationship Studies of Oxazolines Containing Ether Moieties. J. Agric. Food Chem. 2022, 70, 13538–13544. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, Y.; Xun, X.; Ma, Y.; Liu, Y.; Wang, Q. Homologous design and three-dimensional quantitative structure-activity relationship study of acaricidal 2,4-diphenyloxazolines containing different heteroatoms and alkyl chains. J. Agric. Food Chem. 2024, 72, 13431–13438. [Google Scholar] [CrossRef]
- Langdon, S.R.; Ertl, P.; Brown, N. Bioisosteric Replacement and Scaffold Hopping in Lead Generation and Optimization. Mol. Inf. 2010, 29, 366–385. [Google Scholar] [CrossRef]
- Lamberth, C. Agrochemical lead optimization by scaffold hopping. Pest Manag. Sci. 2018, 74, 282–292. [Google Scholar] [CrossRef]
- Patani, G.A.; LaVoie, E.J. Bioisosterism: A rational approach in drug design. Chem. Rev. 1996, 96, 3147–3176. [Google Scholar] [CrossRef]
- Cao, X.; Yang, H.; Liu, C.; Zhang, R.; Maienfisch, P.; Xu, X. Bioisosterism and Scaffold Hopping in Modern Nematicide Research. J. Agric. Food Chem. 2022, 70, 11042–11055. [Google Scholar] [CrossRef]
- Lamberth, C. Heterocyclic chemistry in crop protection. Pest Manage. Sci. 2013, 69, 1106–1114. [Google Scholar] [CrossRef]
- Takahashi, J.; Kato, T.; Iwasa, T. Preparation of N-Heteroaryl Sulfonamide Compounds and Pest Control Agents. WO Patent 2019167863 A1, 6 September 2019. [Google Scholar]
- Mao, G.; Tian, Y.; Shi, J.; Liao, C.; Huang, W.; Wu, Y.; Wen, Z.; Yu, L.; Zhu, X.; Li, J. Design, Synthesis, Antibacterial, and Antifungal Evaluation of Phenylthiazole Derivatives Containing a 1,3,4-Thiadiazole Thione Moiety. Molecules 2024, 29, 285. [Google Scholar] [CrossRef]
- Yang, H.; Zhang, R.; Li, Z.; Maienfisch, P.; Xu, X. Design, synthesis and nematicidal activitives of trifluorobutene amide derivatives against Meloidogyne incognita. Bioorg. Med. Chem. Lett. 2021, 40, 127917. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Yang, H.; Zhang, R.; Li, Z.; Maienfisch, P.; Xu, X. Synthesis and nematicidal activity of 4,5,5-trifluoro-N- (heteroarylmethyl)pent-4-enamide. Chin. J. Pestic. Sci. 2022, 24, 1–13. [Google Scholar]
- Liu, C.; Zhang, L.; Cao, X.; Chen, Y.; Li, Z.; Maienfisch, P.; Xu, X. Discovery of Trifluorobutene Amide Derivatives as Potential Nematicides: Design, Synthesis, Nematicidal Activity Evaluation, SAR, and Mode of Action Study. J. Agric. Food Chem. 2024, 72, 1429–1443. [Google Scholar] [CrossRef]
- Schwarz, H.; Trautwein, A.; Willms, L.; Forstner, M.; Luemmen, P.; Goergens, U.; Coqueron, P.-Y.; Harder, A.; Welz, C. (Bayer Intellectual Property GmbH) Use of Aryl- and Heteroarylcarboxamides as Endoparasiticides and Their Preparation. WO Patent 2013076230 A1, 30 May 2013. Available online: https://worldwide.espacenet.com/patent/search/family/047216313/publication/WO2013076230A1?q=WO2013076230A1&queryLang=en%3Ade%3Afr (accessed on 27 August 2024).
- Oda, M.; Matsuzaki, Y.; Tanaka, K.; Takizawa, E.; Hasebe, M.; Kuroki, N.; Suwa, A.; Oshima, K. (Nihon Nohyaku Co., Ltd.) Preparation of N-2-(hetero)arylethylcarboxamide Derivatives as Pest-Controlling Agents. WO Patent 2007108483 A1, 27 September 2007. Available online: https://worldwide.espacenet.com/patent/search/family/038522511/publication/WO2007108483A1?q=WO2007108483&queryLang=en%3Ade%3Afr (accessed on 27 August 2024).
- Coqueron, P.-Y.; Schwarz, H.-G.; Heilmann, E.K.; Portz, D.; Ilg, K.; Goergens, U.; Greul, J.; Decor, A.; Malsam, O.; Luemmen, P.; et al. (Bayer CropScience AG) N-(2-fluoro-2-phenethyl)carboxamides as Nematicides and Endoparasiticides and Their Preparation. WO Patent 2014177582 A1, 6 November 2014. Available online: https://worldwide.espacenet.com/patent/search/family/048190363/publication/WO2014177582A1?q=WO2014177582&queryLang=en%3Ade%3Afr (accessed on 27 August 2024).
- Decor, A.; Schwarz, H.-G.; Greul, J.; Coqueron, P.-Y.; Koehler, A.; Toquin, V.; Rinolfi, P.; Wachendorff-Neumann, U.; Dahmen, P. (Bayer CropScience AG) N-(2-halogen-2-phenethyl)carboxamides as Fungicides for Control of Phytopathogenic Microorganisms in Agriculture. WO Patent 2016066636 A1, 6 May 2016. Available online: https://worldwide.espacenet.com/patent/search/family/051795581/publication/WO2016066636A1?q=WO2016066636&queryLang=en%3Ade%3Afr (accessed on 27 August 2024).
- Zhang, Y.; Chen, S.; Liu, Y.; Wang, Q. Route evaluation and Ritter reaction-based synthesis of oxazoline acaricide candidates FET-II-L and NK-12. Org. Process Res. Dev. 2020, 24, 216–227. [Google Scholar] [CrossRef]
- Denmark, S.E.; Butler, C.R. Vinylation of Aryl Bromides Using an Inexpensive Vinylpolysiloxane. Org. Lett. 2006, 8, 63–66. [Google Scholar] [CrossRef]
- Song, S.; Huang, X.; Liang, Y.-F.; Tang, C.; Li, X.; Jiao, N. From simple organobromides or olefins to highly value-added bromohydrins: A versatile performance of dimethyl sulfoxide. Green Chem. 2015, 17, 2727–2731. [Google Scholar] [CrossRef]

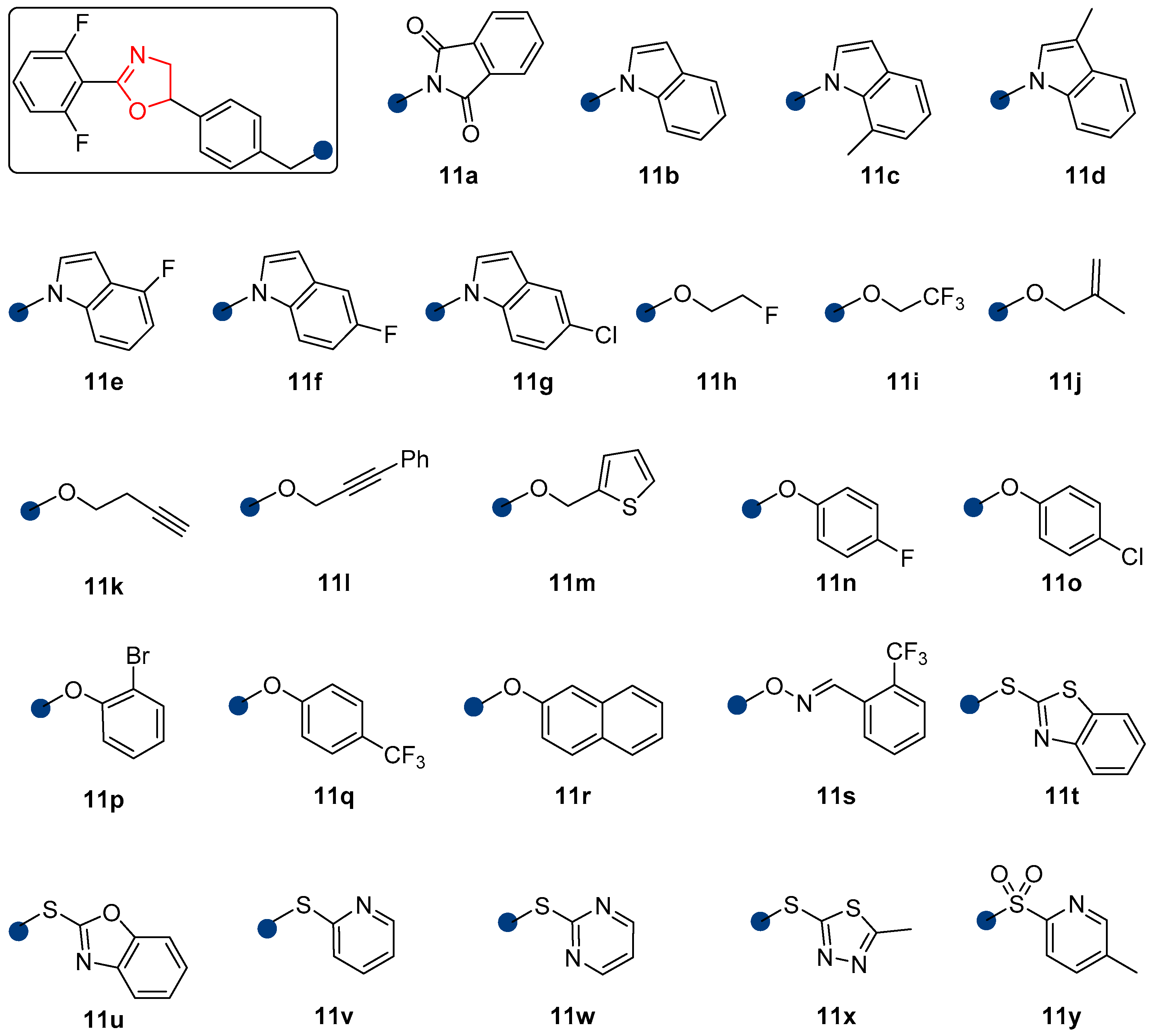
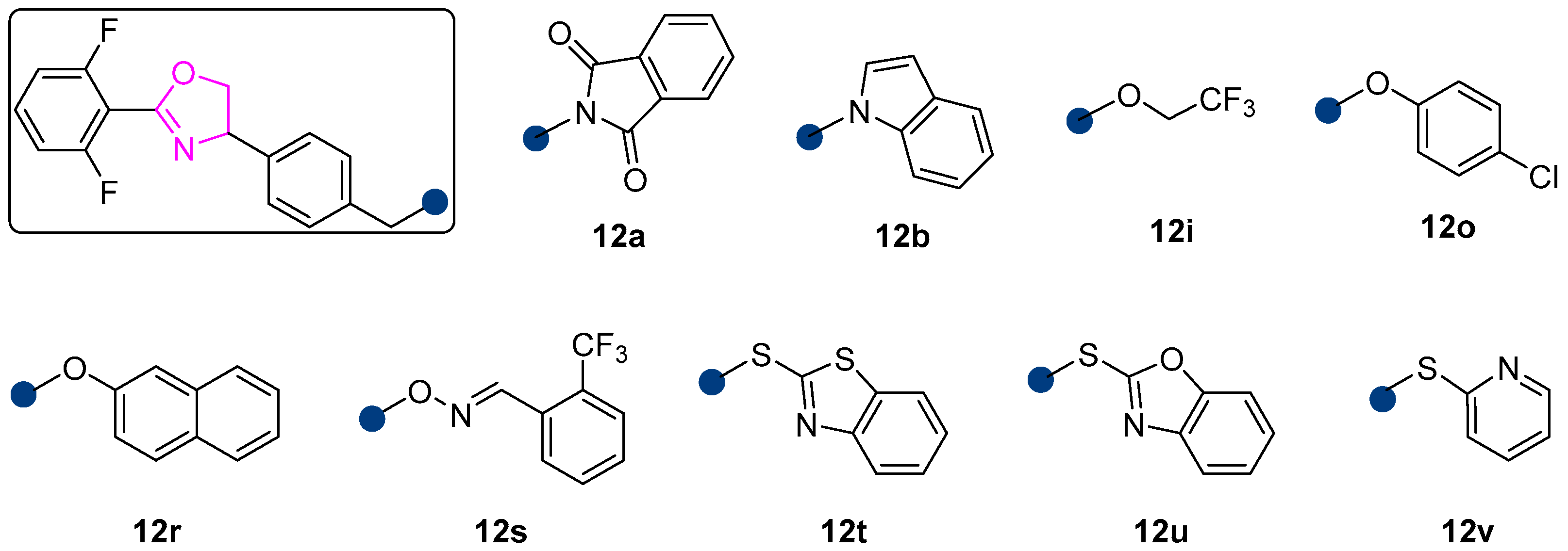
| Mortality (%) against Eggs | Mortality (%) against Larvae | |||||
|---|---|---|---|---|---|---|
| Compound | 100 mg/L | 10 mg/L | 5 mg/L | 100 mg/L | 10 mg/L | 5 mg/L |
| 11a | 100 | 68.7±9.8 | 0 | 100 | 61.7 ± 12.8 | 33.7 ± 10.2 |
| 11b | 100 | 20.0 ± 2.2 | - | 100 | 54.3 ± 7.2 | 31.0 ± 6.2 |
| 11c | 100 | 76.8 ± 4.9 | 14.3 ± 3.4 | 100 | 78.4 ± 6.0 | 55.4 ± 2.6 |
| 11d | 100 | 91.5 ± 2.9 | 6.6 ± 2.5 | 68.4 ± 7.3 | 30.4 ± 9.4 | - |
| 11e | 100 | 86.8 ± 6.5 | 0 | 61.7 ± 12.2 | 23.7 ± 1.8 | - |
| 11f | 100 | 28.6 ± 13 | - | 100 | 54.7 ± 5.0 | 26.7 ± 5.7 |
| 11g | 100 | 20.4 ± 0.3 | - | 100 | 78.3 ± 18.2 | 42.5 ± 15.7 |
| 11i | 100 | 49.5 ± 0.7 | - | 100 | 72.0 ± 3.6 | 41.4 ± 9.7 |
| 11j | 100 | 0.0 | - | 100 | 83.1 ± 9.2 | 59.7 ± 15.7 |
| 11k | 100 | 49.6 ± 3.5 | - | 100 | 74.2 ± 7.4 | 52.0 ± 2.0 |
| 11l | 100 | 100 | 15.3 ± 5.0 | 100 | 52.3 ± 3.4 | 38.6 ± 9.2 |
| 11n | 100 | 20 ± 0.5 | - | 100 | 63.0 ± 8.3 | 47.0 ± 1.5 |
| 11o | 100 | 100 | 16.3 ± 5.3 | 100 | 61.1 ± 12.1 | 44.1 ± 23.3 |
| 11p | 0 | 0 | - | 91.8 ± 6.8 | 36.8 ± 3.5 | - |
| 11q | 100 | 100 | 22.3 ± 11.6 | 100 | 56.6 ± 7.7 | 42.6 ± 6.9 |
| 11r | 100 | 50 ± 4.2 | - | 78.5 ± 2.0 | 36.4 ± 10.2 | - |
| 11s | 0 | 0 | - | 73.2 ± 1.8 | 44.8 ± 10.6 | - |
| 11t | 100 | 100 | 0 | 88.2 ± 6.8 | 54.7 ± 5.0 | 20.6 ± 5.4 |
| 11u | 100 | 90.6 ± 8.3 | 0 | 94.2 ± 5.5 | 52.9 ± 9.0 | 36.7 ± 2.3 |
| 11v | 100 | 59.0 ± 0.9 | 0 | 91.6 ± 8.4 | 36.2 ± 2.1 | - |
| 11w | 0 | 0 | - | 83.7 ± 1.8 | 39.4 ± 6.1 | - |
| 11x | 91.4 ± 8.8 | 74.8 ± 5.6 | 0 | 97.0 ± 4.4 | 45.9 ± 15.9 | - |
| 11y | 100 | 100 | 0 | 100 | 81.8 ± 10.5 | 58.9 ± 2.5 |
| 8 | 100 | 79.8 ± 12.1 | 0 | 100 | 79.5 ± 6.4 | 59.8 ± 5.2 |
| 9 | 100 | 100 | 0 | 100 | 100 | 100 |
| 10 | 100 | 77.4 ± 5.0 | 0 | 100 | 100 | 76.2 ± 12.6 |
| 12a | 100 | 100 | 100 | 100 | 96.6 ± 3.3 | 65.4 ± 3.9 |
| 12b | 100 | 100 | 100 | 100 | 75.3 ± 2.1 | 49.6 ± 2.1 |
| 12i | 100 | 100 | 100 | 100 | 60.8 ± 1.7 | 36.9 ± 3.1 |
| 12o | 100 | 100 | 100 | 100 | 74.4 ± 9.2 | 50.0 ± 3.4 |
| 12r | 100 | 100 | 100 | 100 | 94.7 ± 5.2 | 73.6 ± 11.6 |
| 12s | 100 | 100 | 100 | 100 | 93.8 ± 5.8 | 77.8 ± 9.9 |
| 12t | 100 | 100 | 100 | 95.3 ± 4.3 | 77.6 ± 2.3 | 47.1 ± 2.4 |
| 12u | 100 | 100 | 100 | 100 | 73.5 ± 7.9 | 45.8 ± 8.9 |
| 12v | 100 | 100 | 100 | 100 | 69.0 ± 13.5 | 52.6 ± 3.2 |
| etoxazole | 100 | 100 | 100 | 100 | 100 | 92.0 ± 7.7 |
| Compound | 5 mg/L | 2.5 mg/L | 1.25 mg/L | 0.63 mg/L | 0.31 mg/L | 0.16 mg/L |
|---|---|---|---|---|---|---|
| 9 | 100 | 100 | 100 | 35.6 ± 10.4 | 17.2 ± 13.5 | - |
| etoxazole | 92.0 | 86.3 ± 5.8 | 85.7 ± 5.0 | 61.2 ± 12.8 | 53.9 ± 19.3 | 37.1 ± 10.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Tian, J.; Tan, Y.; Liu, Y.; Wang, Q. Design, Synthesis, and Acaricidal Activity of 2,5-Diphenyl-1,3-oxazoline Compounds. Molecules 2024, 29, 4149. https://doi.org/10.3390/molecules29174149
Chen Y, Tian J, Tan Y, Liu Y, Wang Q. Design, Synthesis, and Acaricidal Activity of 2,5-Diphenyl-1,3-oxazoline Compounds. Molecules. 2024; 29(17):4149. https://doi.org/10.3390/molecules29174149
Chicago/Turabian StyleChen, Yuming, Jiarui Tian, Yuhao Tan, Yuxiu Liu, and Qingmin Wang. 2024. "Design, Synthesis, and Acaricidal Activity of 2,5-Diphenyl-1,3-oxazoline Compounds" Molecules 29, no. 17: 4149. https://doi.org/10.3390/molecules29174149
APA StyleChen, Y., Tian, J., Tan, Y., Liu, Y., & Wang, Q. (2024). Design, Synthesis, and Acaricidal Activity of 2,5-Diphenyl-1,3-oxazoline Compounds. Molecules, 29(17), 4149. https://doi.org/10.3390/molecules29174149






