Abstract
Transition-metal-catalyzed directed C–H functionalization with various carbene precursors has been widely employed for constructing a wide range of complex and diverse active molecules through metal carbene migratory insertion processes. Among various carbene precursors, iodonium ylides serve as a novel and emerging carbene precursor with features including easy accessibility, thermal stability and high activity, which have attracted great attention from organic chemists and have achieved tremendous success in organic transformation. In this review, recent progress on the application of iodonium ylides with multifunctional coupling characteristics in C–H bond activation reactions is summarized, and the potential of iodonium ylides is discussed.
1. Introduction
Metal carbenoid species resulting from carbene precursors and different metals have been widely recognized as an important and versatile active intermediate to build complex and diverse skeletons in organic synthesis [1,2,3]. Carbene precursors, such as diazo compounds, enynones, hydrazones, vinylene carbonates, sulfoxonium ylides, and others, have been extensively explored as coupling partners in various organic reactions [4,5]. Despite this great progress, it is greatly demanding and challenging to continuously seek an efficient and novel carbene precursor as a substitute. Iodonium ylides, which exhibit good thermal stability, easy accessibility and high reactivity, are known to construct various structures, especially cyclic ones [6,7]. In the past decade, iodonium ylides have been mainly used as a carbene-transfer reagent and as a carbenoid insertion into X–H (X = O, S, N) bonds of nucleophiles for constructing the C–X bond [8,9].
Transition-metal-catalyzed directed C–H activation has emerged as an atom/step-economical method for the rapid synthesis of various useful molecules [10,11,12,13]. In contrast to the traditional cross-coupling of iodonium ylides with nucleophiles, directly using iodonium ylides as a significant coupling partner in transition-metal-catalyzed C–H activation reactions are especially appealing. In 2020, the Li group first applied iodonium ylides as carbene precursors in Rh(III)-catalyzed directed C–H activation reactions under mild and redox-neutral conditions [14]. Since then, a large number of studies have reported employing iodonium ylides as a novel and emerging carbene precursor in transition-metal-catalyzed chelation-assisted C–H functionalization [15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54]. To the best of our knowledge, only one simple review of 21 examples using iodonium ylides in transition-metal-catalyzed C–H functionalization was reported by the Kanchupalli group in 2022 [55]. However, after the publication of Kanchupalli’s review, there are many studies (about 20 references) on the C–H functionalization of iodonium ylides, which was intensively reported in 2023 and 2024. Herein, we aim to provide a comprehensive overview of transition-metal-catalyzed C–H functionalization utilizing iodonium ylides with multifunctional coupling characteristics over the last 5 years. It should be noted that most of the carbene precursors were limited to the cyclic 1,3-diketones-derived iodonium ylides, because this type of transformation may be sensitive to the steric hindrance of iodonium ylides. Based on the different forms of iodonium ylides in C–H transformations, this review article is organized into three sections: (1) those serving as C2 synthon in C–H annulation, (2) serving as C3 synthon in C–H annulation, or (3) serving as an alkenylating reagent in C–H functionalization (Scheme 1).
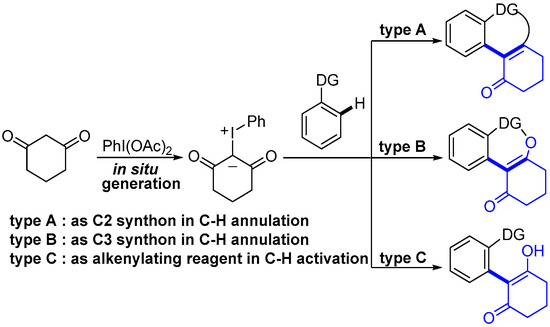
Scheme 1.
Transition–metal–catalyzed C–H bond activation reaction with iodonium ylides.
2. Iodonium Ylides Serve as C2 Synthon in C–H Annulation
C2 synthon is one of the most common synthetic forms in the C–H activation reaction, which could be readily converted into the valuable heteroaromatic moieties by undergoing [n+2] annulation with the C–H bond. The utilization of iodonium ylides as C2 synthon are extensively studied in transition-metal-catalyzed C–H activation reactions.
2.1. Amide Group-Directed C–H Annulation
In 2020, Mayakrishnan and co-workers [15] reported Rh(III)-catalyzed arene C–H annulation of N-methoxybenzamides 1 with iodonium ylide 2 as a carbene precursor for the assembly of dihydrophenanthridines 3 (Scheme 2a). A wide range of substrates were compatible with this catalytic system. Importantly, the potential synthetic utility of the coupling product 3 was demonstrated by synthesizing pyranoisocoumarin 5. The optoelectronic properties of product 5 were tested by UV/vis and fluorescence spectrometers. The results showed excellent optical properties with emission maxima, with the potential to be used in the photoelectric and biomedical imaging fields. Very shortly after Mayakrishnan’s publication, Ji and co-workers [16] also developed a similar procedure (Scheme 2b). They disclosed a Rh(III)-catalyzed cross-coupling reaction of N-carboxamide indole 6 with iodonium ylide 2 to deliver indoloquinazolinone 7. Various substrates with different substituents were well tolerated in this transformation.
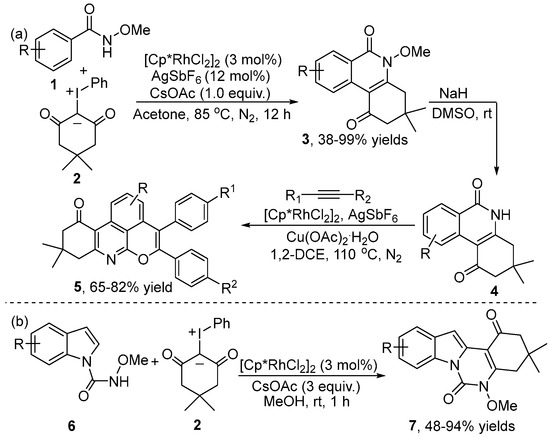
Scheme 2.
Rh(III)–catalyzed [4+2] annulation of (a) N-methoxyarylzamides and (b) N-carboxamide indole with iodonium ylides.
A plausible mechanism is provided in Scheme 3. After the coordination of N-methoxybenzamide 1 to a cationic Rh(III) catalyst, C–H bond activation occurred and gave five-membered cyclometalated species A. Subsequently, the coordination of iodonium ylide 2 and the elimination of PhI led to the formation of Rh(III) intermediate B, which underwent the migratory insertion and protonation process to yield intermediate E. Finally, the intramolecular nucleophilic addition and dehydration annulation process generated the desired product 3a.
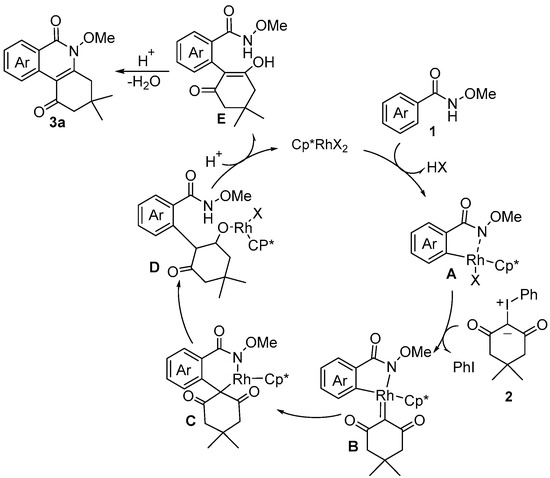
Scheme 3.
Proposed reaction mechanism for the [4+2] annulation of N-methoxyarylzamides with iodonium ylides.
In 2023, Asish and co-workers [17] also demonstrated the assembly of coumarin-3-carboxamide 9 via [4+2] cyclization of coumarin 8 with iodonium ylide 2 using an N-methoxy carboxamide unit as the chelating fragment (Scheme 4). Moreover, when oxime-derivatized tetralone 10 was used as an arene substrate under similar reaction conditions, the corresponding tetracyclic pyridine-N-oxide product 11 was obtained in good to excellent yields. A variety of substrates bearing various functional groups were well tolerated under mild and redox-neutral conditions, in which 30 mol% KPF6 could absolutely substitute the more expensive silver salts. Significantly, pyridine-N-oxide product 11 showed excellent fluorescence quenching activity, which could be applied to a fluorescence sensing probe.
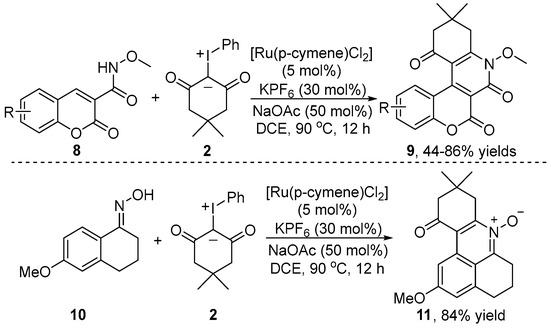
Scheme 4.
Ru(II)–catalyzed C–H [4+2] annulation of coumarins and oxime-derivatized tetralone with iodonium ylides.
Subsequently, the Wu group [18] also accomplished the Rh(III)-catalyzed olefinic [4+2] cyclization of α, β-unsaturated amide 12 with iodonium ylide 13 for the synthesis of dihydroquinoline-2,5-dione 14 in moderate to high yields (Scheme 5). The transformation featured water and air compatibility and excellent functional group tolerance. Interestingly, the authors applied this protocol to a large-scale reaction with a low catalyst load (0.25–1.5 mol%) and extracted the Rh catalyst three times during the [4+2] cyclization at a 65% yield.
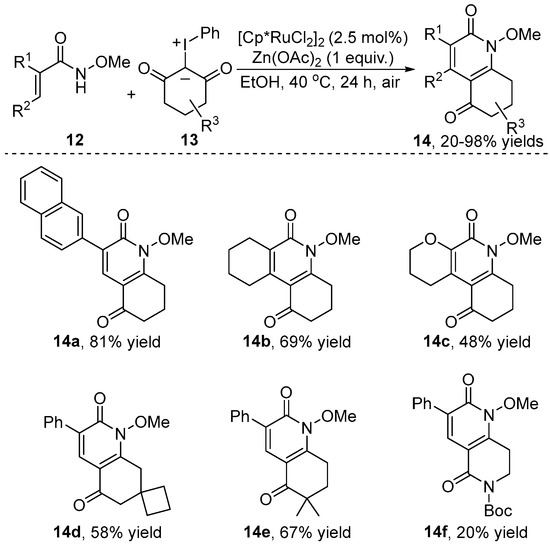
Scheme 5.
Rh(III)–catalyzed [4+2] annulation of N-arylpyrazolidinones with iodonium ylides.
2.2. Hydrazine Group-Directed C–H Annulation
In 2022, Li and co-workers [19] described a Rh(III)-catalyzed C–H coupling/cyclization of pyrazolidinone 15 with iodonium ylide 13 for the synthesis of pyrazolo[1,2-a]cinnoline 16 (Scheme 6). This transformation proceeded under oxidant-free conditions and resulted in a decent to high yield. An excellent functional group compatibility was observed in this reaction. The H/D exchange experiment suggested a reversible C–H activation process.
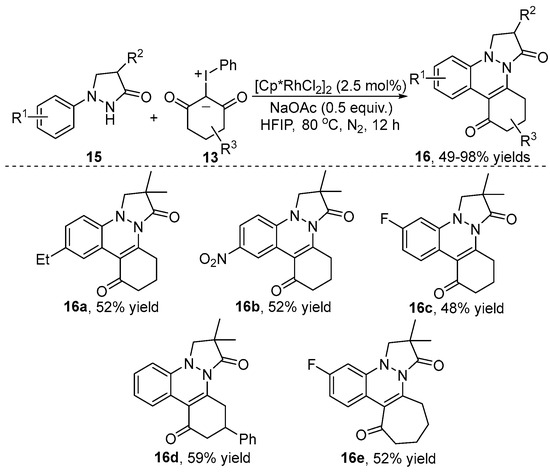
Scheme 6.
Rh(III)–catalyzed [4+2] annulation between pyrazolidinones and iodonium ylides.
Owing to the valuable applications of cinnoline in the medicinal and organic areas, a Rh(III)-catalyzed [4+2] annulation for the synthesis of cinnoline 17 had been realized by Hu’s group [20] in 2022 (Scheme 7a). Compared with the above-mentioned mechanism using the same substrates, the [4+2] cyclization intermediate C was further aromatized under HFIP to give annelated cinnoline 17. In this same time, Yu and co-workers [21] also explored the construction of cinnoline 17 from N-methyl arylhydrazine 18 and iodonium ylide 13 (Scheme 7b). A large number of functional groups were compatible in this Rh(III)-catalyzed C–H activation reaction. Similarly, product 17 was obtained through a C–H activation, dehydration and demethylative aromatization process.
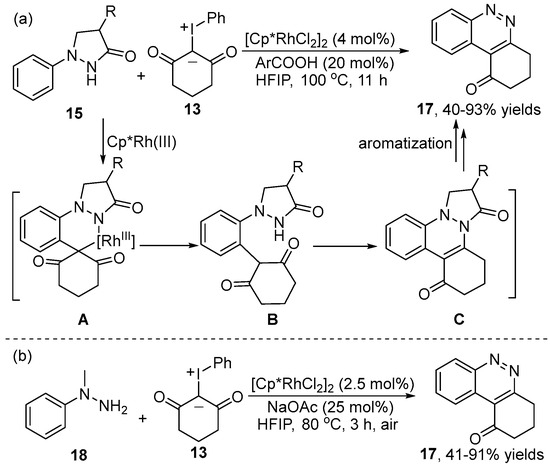
Scheme 7.
Rh(III)–catalyzed [4+2] annulation for the synthesis of cinnoline.
Tetrahydrocarbazol-4-ones are a privileged scaffold found in numerous bioactive molecules and drugs, such as ondansetron and heat shock protein inhibitor. In this regard, Liu et al. [22] independently reported an approach to produce tetrahydrocarbazol-4-one 20 via the [4+2] annulation of readily available arylhydrazine 19 with iodonium ylide 2 by using [Cp*RhCl2]2 as a catalyst and AgOAc as an additive (Scheme 8). A variety of substrates with different substituents were well tolerated in this transformation. It should be noted that the authors demonstrated the utility of this strategy by performing a gram-scale experiment of product 20. The tetrahydrocarbazol-4-one 20a was further derived to produce the corresponding compounds 21, 22 and 23 via substitution, hydrolysis and reductive hydrogenation reactions, respectively.
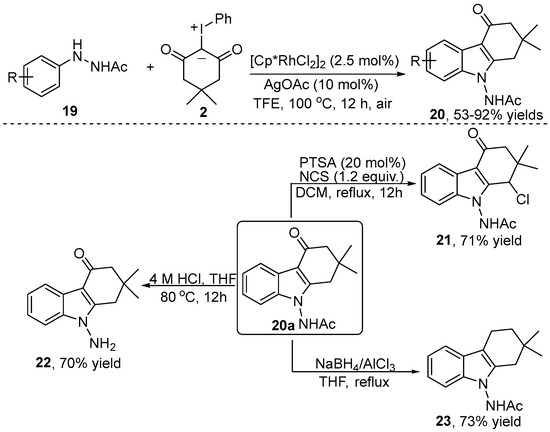
Scheme 8.
Rh(III)–catalyzed [3+2] annulation of arylhydrazines with iodonium ylides.
2.3. Sulfamide Group-Directed C–H Annulation
In 2021, Pan’s group [23] demonstrated a Rh(III)-catalyzed C–H and N–H bond functionalization of S-aryl-sulfoximine 24 with iodonium ylide 13 by using the sulfoximine moiety as a directing group (Scheme 9). This transformation provided a new way to access multifariously switched tricyclic and tetracyclic 1,2-benzothiazines 25 in moderate to good yields. Various substrates with different substituents were well tolerated in this transformation. In the case of S-2-naphthalenyl sulfoximine, the reaction predominantly occurred at the C–H bond with less steric hindrance to generate the single product 25i. To explore the reaction mechanism, a stable cyclometalated Rh(III) complex A was isolated, and it was found to efficiently catalyze the reaction to provide the desired product.
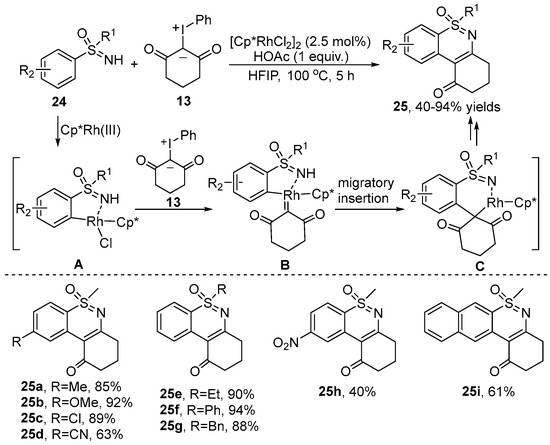
Scheme 9.
Rh(III)–catalyzed [4+2] annulation of S-aryl-sulfoximines with iodonium ylides.
More recently, similar work was reported independently by Chen and co-workers [24] (Scheme 10). In contrast with Pan’s work, this transformation was performed under low temperature conditions. In addition, this reaction was characterized by using EtOH as a green solvent under oxygen/water-insensitive conditions and with the requirement for only a low catalyst load. To demonstrate the potential application of this annulation reaction, the authors used this methodology to rapidly synthesize a complex pharmaceutical Folliculin analog.
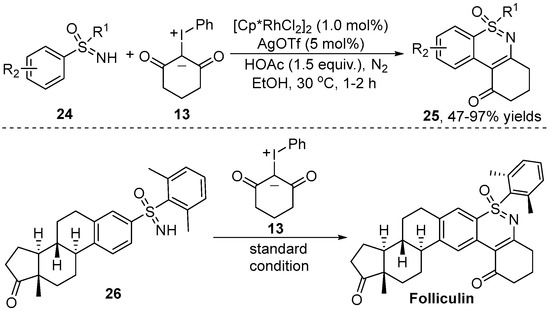
Scheme 10.
Rh(III)–catalyzed [4+2] annulation for the synthesis of a Folliculin analog.
2.4. Nitroso Group-Directed C–H Annulation
The nitroso group has been recognized as an efficient directing group and an internal oxidant in various C–H bond redox-neutral functionalization/annulation reactions. In recent years, a Rh(III)-catalyzed C–H bond [3+2] cyclization approach was applied to form tetrahydrocarbazol-4-one 20 using a nitroso motif as the directing group, by the Yang group [25] (Scheme 11a). The reaction conditions were mild without any additional oxidant, and a variety of N-nitrosoanilines and iodonium ylides were compatible. At almost the same time, a similar Rh(III)-catalyzed synthesis of polysubstituted tetrahydrocarbazol-4-one 20 employing the same substrates was developed by Liu and co-workers [26] (Scheme 11b). The authors further demonstrated metal-catalyzed C–H bond activation reactions at the C5-position of tetralydrocarbzol-4-one 20 using different coupling partners under mild conditions, including alkylation, alkenylation, amidation and (hetero)arylation.
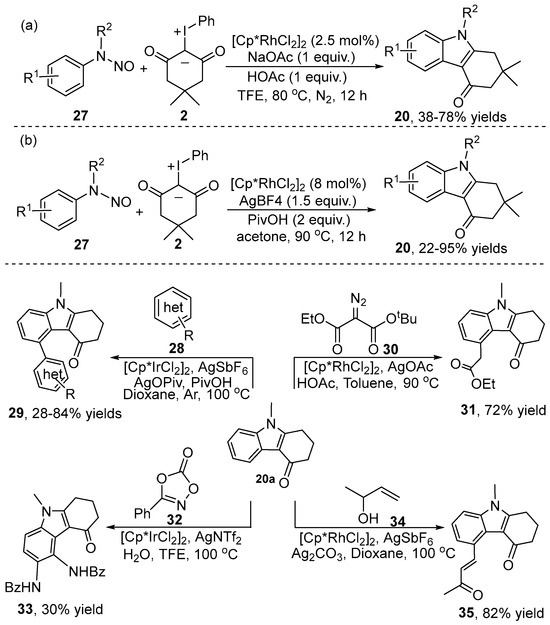
Scheme 11.
Rh(III)–catalyzed [3+2] cyclization of N-nitrosoanilines with iodonium ylides.
Very shortly after Liu’s publication, the Fan group [27] also realized a condition-controlled C–H bond functionalization of N-nitrosoaniline 27 with iodonium ylide 2 (Scheme 12). This reaction was conducted in HFIP using [Cp*RhCl2]2 as the catalyst in the presence of Na2CO3 at 60 °C to produce tetrahydrocarbazol-4-one 20 by selectively controlling the [3+2] annulation. In contrast, switching the catalyst to [RhCp*(MeCN)3](SbF6)2 and the additive to Ag2O resulted in the formation of pyranone-tethered indazole 36 via [4+1] annulation. The compound 37 and the γ-butyrolactone derivative 38 were produced by a regioselective C–H alkylation and a ring contraction reaction, respectively, which proved the successful application of the catalytic system.
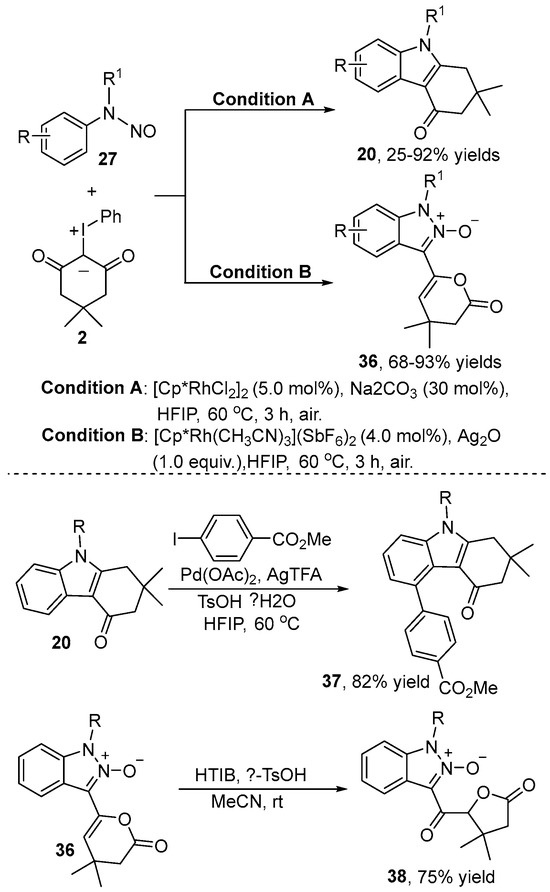
Scheme 12.
Condition-controlled Rh(III)–catalyzed C–H annulation of N-nitrosoanilines and iodonium ylides.
This transformation underwent C–H bond activation, carbene migratory insertion and protonation processes to deliver alkylation intermediate A. In pathway A, the intramolecular cyclization and denitrosation of intermediate A provided the desired product 20. However, in pathway B, the intermediate D formed via intramolecular C-nucleophilic addition underwent ring opening of cyclohexanedione to generate the intermediate E. Intermediate E underwent tautomerization and intramolecular transesterification/cyclization to furnish intermediate G, which finally delivered product 36 via Ag(I)/O2-mediated aromatization-driven oxidative dehydrogenation (Scheme 13).
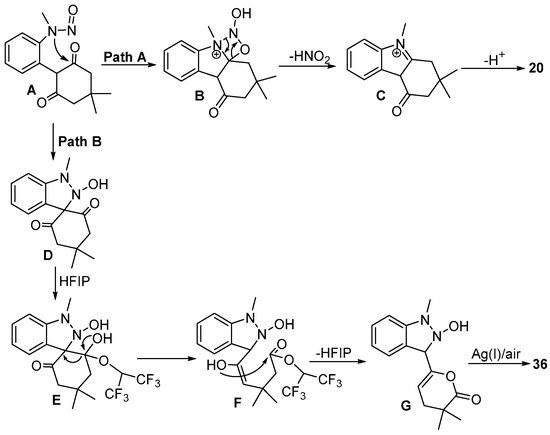
Scheme 13.
Proposed reaction mechanism for condition-controlled Rh(III)–catalyzed C–H annulation of N-nitrosoanilines and iodonium ylides.
From the perspective of step economy, the in situ generation of iodonium ylides as carbene precursors in a one-pot manner is very attractive. At almost the same time, our group [28] explored a Rh(III)-catalyzed direct C–H bond tandem [4+2] annulation of N-nitrosoaniline 27 by using simple cyclohexane-1,3-dione 39 as a coupling partner through two C–H bond cleavages, producing tetrahydrocarbazol-4-one 20, and in which the in situ generation of iodonium ylides was achieved (Scheme 14). Subsequently, the biological application of these target coupling products was investigated. The results showed that they displayed significant cytotoxicity against HepG2 cells. Furthermore, product 20d could be further functionalized to assemble ondansetron using the Mannich reaction.
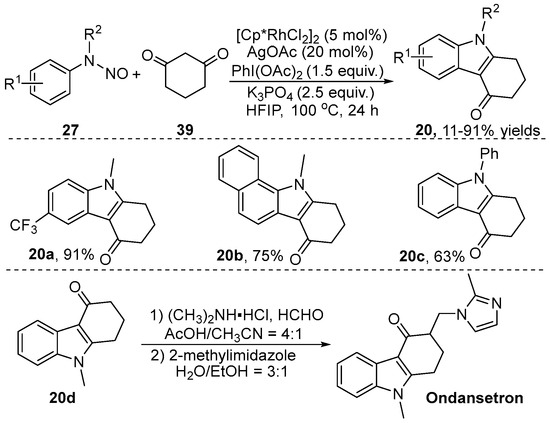
Scheme 14.
Rh(III)–catalyzed [3+2] annulation of N-nitrosoanilines with cyclohexane-1,3-dione.
2.5. Amino Group-Directed C–H Annulation
In 2023, Huang’s group [29] accomplished a Rh(III)-catalyzed selective mono- and dual-C–H bond unsymmetrical functionalization/cyclization of 1-aryl-5-aminopyrazole 40 and iodonium ylide 2 using the aromatic NH2 moiety as the directing group, leading to fused benzodiazepine skeletons 41 and 42 (Scheme 15). The amount of iodonium ylides played an important role in chemo-selectivity. In particular, dual-C–H functionalization proceeded via the utilization of three equivalence of iodonium ylides to give product 42. To elucidate the reaction mechanism, the reaction between product 41 and iodonium ylides was performed under optimal reaction conditions to obtain an 87% yield of product 42. This result indicated that product 41 was a possible intermediate in the dual C–H functionalization process.
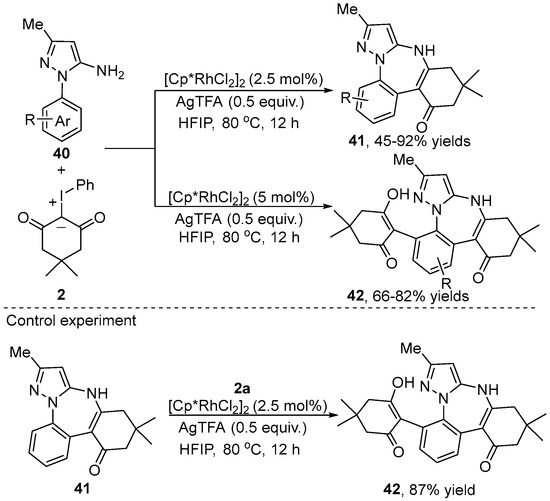
Scheme 15.
Rh(III)–catalyzed aromatic NH2 moiety-directed [5+2] annulation of 1-aryl-5-aminopyrazoles with iodonium ylides.
Compared with the aromatic NH2 group, the aliphatic NH2 group-directed C–H functionalization has attracted great attention because the aliphatic NH2 unit has poor coordination abilities and is easily oxidized by oxidants. In this aspect, our group [30] revealed a Rh(III)-catalyzed free aliphatic amines-assisted arene C–H bond coupling/annulation of primary benzylamine 43 with iodonium ylide 13 using air as a green oxidant (Scheme 16). This reaction provided a general, green and step-economic approach to construct a wide range of dihydrophenanthridin-1-one 44, which are bioactive scaffolds found in numerous natural products. Excellent functional group tolerance and regioselectivity of this catalytic system were observed, except for the furanylmethanamine (44m).
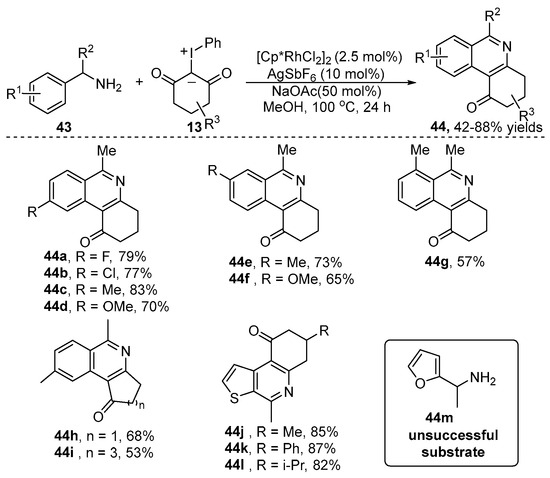
Scheme 16.
Rh(III)–catalyzed aliphatic NH2 group-directed [4+2] annulation of primary benzylamines with iodonium ylides.
Except for the aromatic C–H bond, the olefinic C–H bond functionalization/cyclization using iodonium ylides as carbene precursors was recently reported by the Yu group [31,32] (Scheme 17). This protocol involved a novel Rh(III)-catalyzed cascade alkenyl C–H activation and a subsequent pinacol rearrangement reaction of enaminone 45 with iodonium ylide 13, producing a series of 2-spirocyclo-pyrrol-3-ones 46 in good to high yields (Scheme 17a) [31]. However, a range of dihydroxy hexahydro-4H-indol-4-ones 47 were obtained when using Ru(II) as a catalyst (Scheme 17b) [32]. Moreover, these N-heterocyclic products could all be further functionalized to product 48 via the open-ring/hydrolysis of the spiro ring under H2SO4-mediated conditions. Notably, an 18O labeling experiment indicated that water served as the oxygen source for the newly generated carbonyl group in this reaction system.
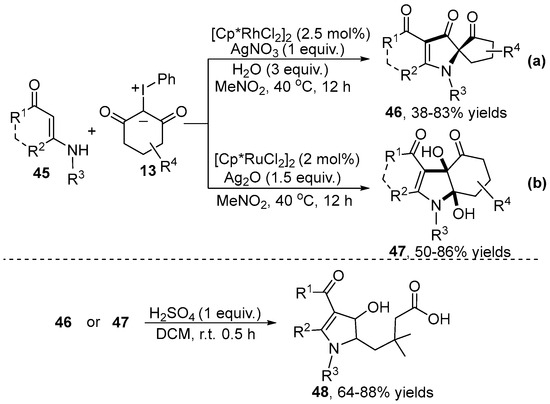
Scheme 17.
Transition–meta–catalyzed C–H cyclization of enaminones with iodonium ylides.
A plausible mechanism was proposed (Scheme 18). After the coordination of enaminone 45 to a cationic metal catalyst, direct alkenyl C–H bond activation occurred and gave four-membered cyclometalated species A. Subsequently, coordination of iodonium ylide 13 and the elimination of PhI led to the formation of metal intermediate B, which was followed by the migratory insertion and protonation processes to generate intermediate D. Then, the intermediate E obtained through the oxidative dehydrogenation of intermediate D underwent intermolecular nucleophilic addition and intramolecular nucleophilic cyclization to generate product 47. Product 47 could be further converted to 46 by the dehydration, annulation and pinacol rearrangement.
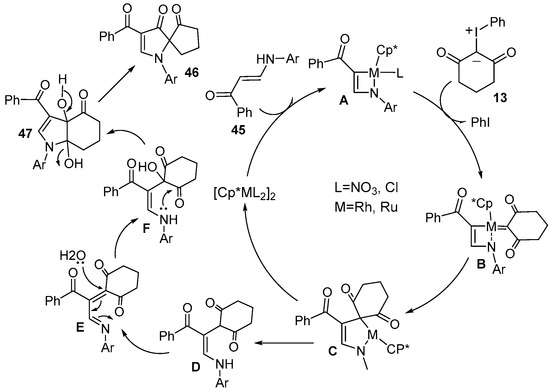
Scheme 18.
Proposed reaction mechanism for the cyclization of enaminone with iodonium ylides.
Apart from the C–H bonds with arenes and olefins, the NH2-directed aldehydic C–H functionalization with iodonium ylides was explored by the Song group [33] in 2022 (Scheme 19). They selected 2-aminobenzaldehyde 49 as a substrate to react with iodonium ylide 13 in the presence of the inexpensive Ru(II) catalyst, giving NH-free carbazolone 50 in moderate to excellent yields via decarbonylative alkylation and annulation processes. Interesting, hydroxy group-directed C–H functionalization with iodonium ylides is also compatible with this catalytic system. The mechanism was proposed to involve aldehydic C–H activation, decarbonylation, coordination, migratory insertion, protonolysis, nucleophilic attack and dehydration processes.
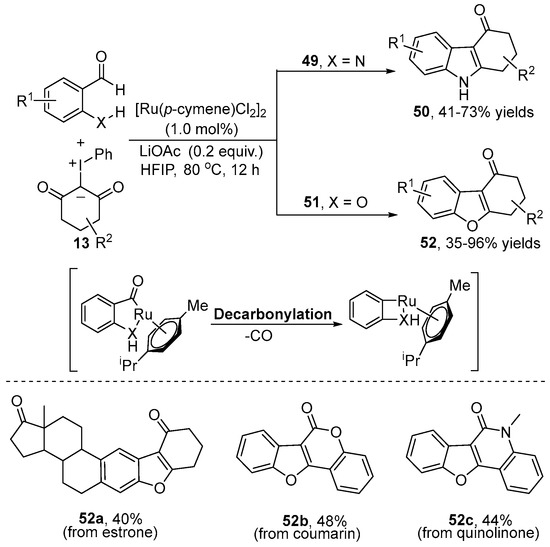
Scheme 19.
Ru(II)–catalyzed aldehydic C–H [3+2] annulation with iodonium ylides.
2.6. Heterocyclic Group-Directed C–H Annulation
In view of the biological and pharmacological activities of the indolo [2,1-a]isoquinoline derivatives and benzo[a]carbazoles, Kanchupalli [34] developed a condition-controlled Rh(III)-catalyzed regioselective C–H activation of 2-arylindole 53 with iodonium ylide 13, leading to the generation of indolo [2,1-a]isoquinolines derivative 54 and benzo[a]carbazole 55 (Scheme 20). This protocol was conducted in DCM using NaHCO3 as a base to merely produce the indolo [2,1-a]isoquinolines derivative 54. In contrast, switching DCM to HFIP without any base led to benzo[a]carbazole 55 with an excellent chemoselectivity. The H/D exchange experiment and KIE experiment revealed that the C–H activation process was reversible and was not involved in the rate-determining step.
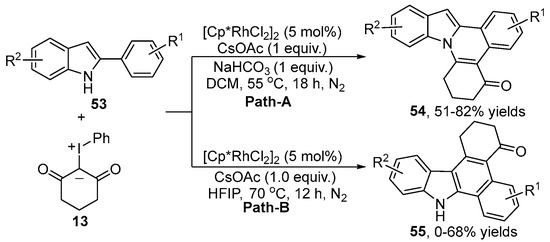
Scheme 20.
Condition-controlled Rh(III)–catalyzed [4+2] annulation of 2-arylindoles with iodonium ylides.
More recently, a similar strategy was reported independently by Cui and co-workers [35] (Scheme 21). They employed the imidazole group as a directing group to realize a Rh(III)-catalyzed C–H alkylation and a subsequent intramolecular [4+2] annulation of 3-aryl-1H-indazole 56 with iodonium ylide 13. This protocol offered a rapid route to a range of tetracyclic and pentacyclic aza-heterocyclics 57, which could be transformed into a fluorescent dye and biofluorescent probes. Additionally, the H/D exchange experiment suggested a reversible C–H activation process.
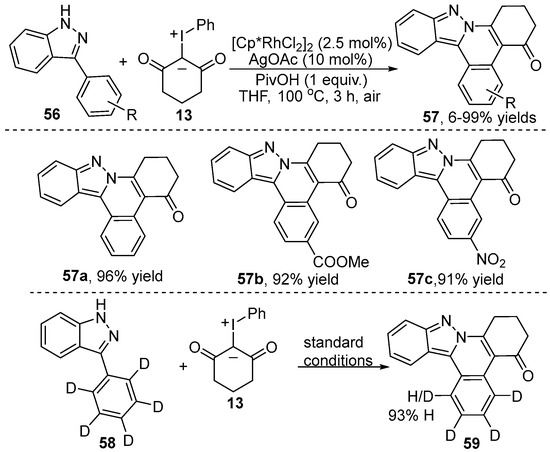
Scheme 21.
Rh(III)–catalyzed [4+2] annulation of 3-aryl-1H-indazoles and iodonium ylides.
Recently, Ru(II) catalyst has received great attention in C–H functionalization due to advantages like higher catalytic activity, excellent regioselectivity and low cost. In this regard, the group of Kanchupalli developed a Ru(II)-catalyzed [4+2] annulation of 2-arylbenzimidazole 60 with iodonium ylide 13 (Scheme 22a) [36]. This protocol provided easy access to a wide variety of useful substituted tetracyclic and pentacyclic bridgehead N-heterocycles 61, which had been utilized to synthesize the core moiety of the natural product zephycandidine A with significant anti-tumor and anti-acetylcholinesterase activities. At almost the same time, Wu and co-workers [37] also reported similar work (Scheme 22b). In contrast to Kanchupalli’s work, this reaction underwent LED-irradiated C–H coupling of 2-arylbenzimidazole 60 with iodonium ylide 13 in the presence of Rh(III) and an EosinY co-catalyst at room temperature, leading to a variety of N-fused polycyclic compounds 61 in high yields. Excellent functional group tolerance and regioselectivity of this catalytic system were observed.
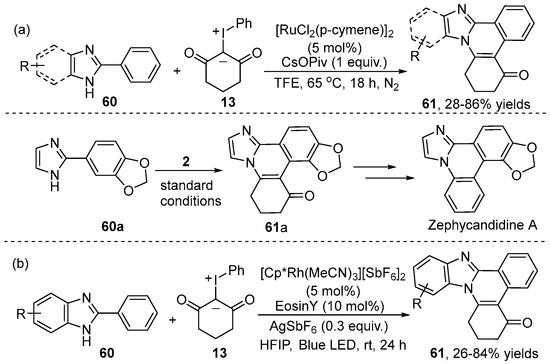
Scheme 22.
Ru(II)–catalyzed [4+2] cyclization of 2-arylbenzimidazoles with iodonium ylides.
A Ru(II)-catalyzed C–H bond activation and tandem cyclization of 2-arylimidazo[1,2-a]pyridine 62 with iodonium ylide 2 to synthesize pyrido[1,2-a]benzimidazole derivative 63 was revealed by Wang and co-workers [38] (Scheme 23). Furthermore, product 63 reacted with 1,2-diphenylethyne and methyl acrylate to form the corresponding coupling compounds 65 and 67 through C–H activation in the present of an Rh(III) catalyst. In addition, a gram-scale (5 mmol) synthesis of pyrido[1,2-a]benzimidazole 63 was carried out, achieving a good yield of 67%.
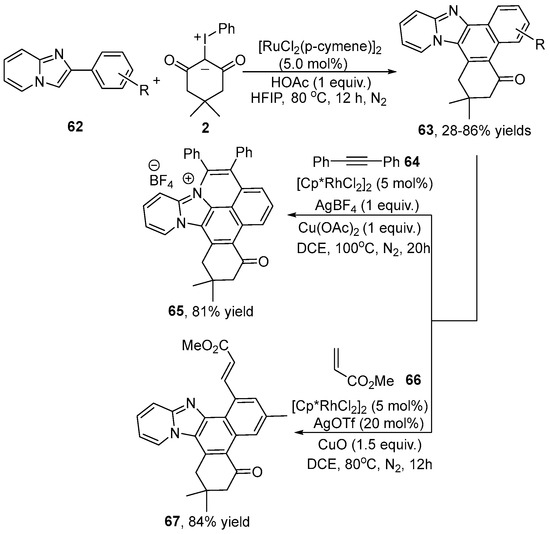
Scheme 23.
Ru(II)–catalyzed [4+2] cyclization of 2-arylimidazo[1,2-a]pyridines with iodonium ylides.
Arylimidazoles are potential substrates because the imidazole moiety has a strong ability to coordinate with the transition-metal catalyst and serves as a nucleophilic group for intramolecular cyclization. In this respect, Tao and co-workers [39] demonstrated a Rh(III)-catalyzed C–H coupling/cyclization of alkenyl- or arylimidazoles 68 with cyclic 1,3-dicarbonyl compound 39 for the assembly of imidazo-fused polycyclic compound 69 by selectively cleaving two different C–H bonds in a single step (Scheme 24). In this transformation, iodonium ylides were generated in situ from cyclic 1,3-dicarbonyl compound 39 and PhI(OAc)2 in one pot. Notably, the imidazole moiety served as a major directing group that played a significant role in controlling the regioselectivity. Excellent functional group compatibility, readily available starting materials and easy operation were observed in this reaction. Importantly, the potential synthetic utility of the coupling product was demonstrated by synthesizing the highly step-economic synthesis of a Janus kinase inhibitor, which was difficult to efficiently achieve by previous methods, in only three steps.
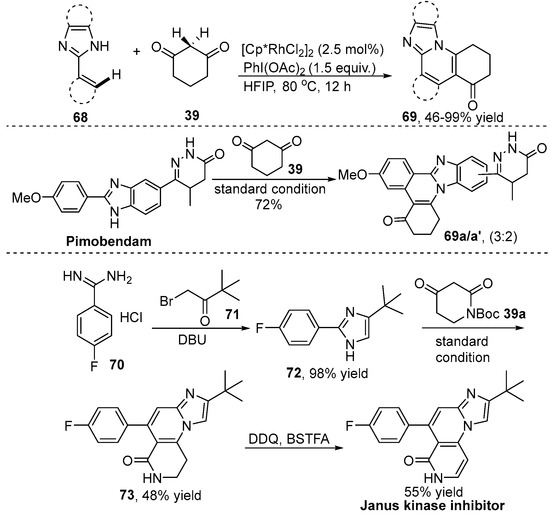
Scheme 24.
Rh(III)–catalyzed C–H [4+2] cyclization of imidazole with 1,3-dicarbonyl compounds and the total synthesis of a Janus kinase inhibitor.
2.7. Switchable Group-Directed C–H Annulation
Switchable group-directed C–H functionalization is of great significance for the synthesis of valuable skeleton structures due to the facile transformations from the switchable group. More recently, Liu and co-workers [40] realized oxazoline-directed Rh(III)-catalyzed C–H functionalization of oxazoline 74 with iodonium ylide 13 to give isoquinolone derivative 75 in a relatively high yield (Scheme 25). This reaction was compatible with a range of oxazolines and iodonium ylides. Moreover, the product could be further functionalized for the construction of the corresponding morpholine derivative 77, which is useful for diverse chemical transformations in organic synthesis.
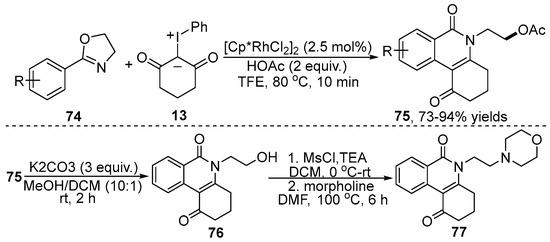
Scheme 25.
Rh(III)–catalyzed [4+2] annulation of 2-oxazolines with iodonium ylides.
A possible reaction mechanism is provided in Scheme 26. The reaction was initiated by oxazoline-assisted C–H bond activation to give the five-membered rhodacyclic intermediate A. Then, iodonium ylide 13 coordinated with the metal center of intermediate A to deliver the active carbene species B followed by the elimination of PhI. With subsequent migratory insertion and protonation, the intermediate E was generated. The intermediate E underwent intramolecular nucleophilic cyclization/dehydration to produce the oxazolinium salt F. Finally, acetate attacked the oxazolinium salt F to give the expected isoquinolone 75.
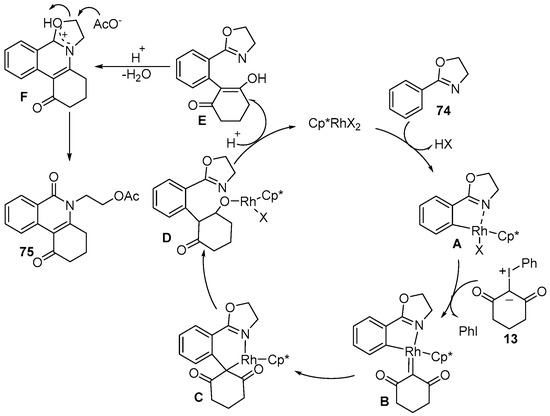
Scheme 26.
Proposed reaction mechanism for Rh(III)–catalyzed C–H annulation of 2-oxazolines with iodonium ylides.
A Rh(III)-catalyzed C–H/N–H [4+2] annulation between oxadiazolone 78 and iodonium ylide 13 was accomplished by the Shu group [41], which enabled the assembly of fused-isoquinoline 79 (Scheme 27). This protocol showed outstanding functional group tolerance, a wide range of substrates and high atom economy. Furthermore, the authors proved that product 79a containing oxadiazolones as a switchable directing group could be easily further transformed into the other compounds 80 and 81. In mechanistic studies, a primary KIE value of 1.7 indicated that the C–H bond cleavage was not involved in the rate-determining step.
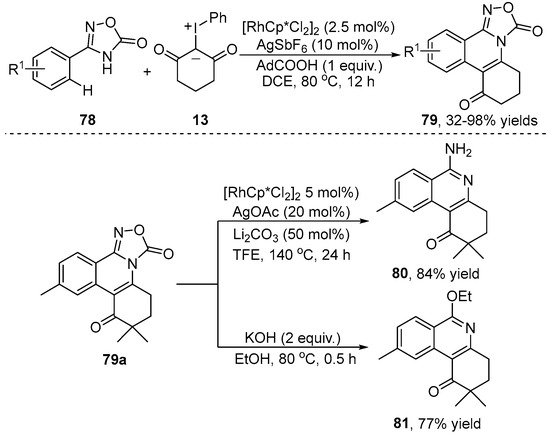
Scheme 27.
Rh(III)–catalyzed C–H/N–H [4+2] annulation of 3-aryl-1H-indazoles and iodonium ylides.
The Wang group [42] established that transmetalation triggered Rh(III)-catalyzed C–H bond activation and the tandem annulation of 2-biphenylboronic acid 82 with iodonium ylide 13 for the assembly of phenanthrene 83 without any directing group (Scheme 28). The merit of this methodology encompassed stable and easily available substrates, simple operation and redox-neutral conditions. A plausible reaction mechanism was proposed. The reaction was initiated by the transmetalation of substrate 82 with the active catalyst Cp*RhX2 to provide intermediate A. Subsequently, intermediate A underwent intramolecular C–H bond activation, coordination with iodonium ylide 13, migratory insertion and hydrolysis, to synthesize intermediate E. Ultimately, product 83 was obtained by intramolecular nucleophilic cyclization, protonation and intramolecular dehydration.
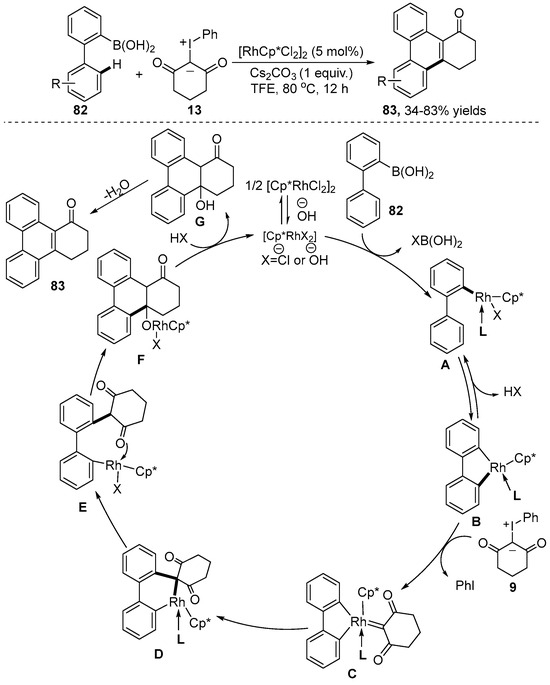
Scheme 28.
Rh(III)–catalyzed [4+2] annulation of 2-biphenylboronic acid with iodonium ylides.
3. Iodonium Ylides Serve as C3 Synthon in C–H Annulation
In comparison to the [n+2] annulation induced by the nucleophilic attack of directing groups against the carbonyl group of iodonium ylides, the [n+3] annulation reaction between the electrophilic directing groups and the nucleophilic enol hydroxyl moiety of iodonium ylides have aroused widespread interest. This method provides a facile and convenient approach to generate multifarious fused heterocyclic compounds.
The first example of using iodonium ylides as carbene precursors in C–H functionalization was reported in 2020 by Li and co-workers [14] (Scheme 29). They revealed [Cp*RhCl2]2-catalyzed cross-coupling [3+3] annulation reactions of 2-benzylacrylic acid 84 with iodonium ylide 13 to yield dihydro-2H-chromene derivative 85 with high anti-tumor activities. Meanwhile, the catalyst load could be decreased to 0.5 mol% in a gram-scale synthesis, which has great prospects for industrial applications. In this mechanism (Scheme 29), intermediate A is generated through C–H activation and coordination with iodonium ylides, which undergoes migratory insertion and protonation to produce intermediate C. Then, intermediate C undergoes intramolecular nucleophilic cyclization/dehydration to deliver the target product 85.
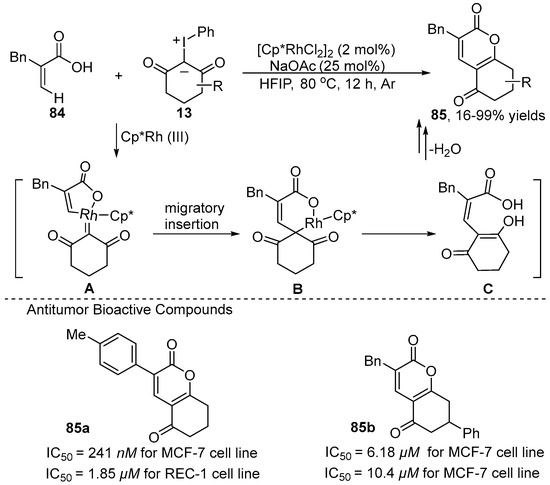
Scheme 29.
Rh(III)–catalyzed olefinic C–H [3+3] annulation of 2-benzylacrylic acid with iodonium ylides.
Subsequently, a condition-controlled chemodivergent cyclization of N-carboxamide indole 86 with iodonium ylide 13 employing [RhCp*Cl2]2 as a catalyst was reported by the Kanchupalli group [43] (Scheme 30). This methodology provided rapid access to functionalized 1H-[1,3]oxazino[3,4-a]indol-1-one derivative 87 and 3,4-dihydroindolo[1,2-c]quinazoline-1,6(2H,5H)-dione 88 under redox-neutral and mild conditions. Note that the acid additives played an important role in the chemoselectivity of this reaction system. The AcOH could activate the amide group that could be attacked by the enol oxygen for the synthesis of the [3+3] annulation product 87 with the elimination of NH2OR. On the contrary, the amide NH group could nucleophilically attack the ketone carbonyl of the iodonium ylides to generate the [4+2] annulation product 88 in the presence of AgOAc and DCE.
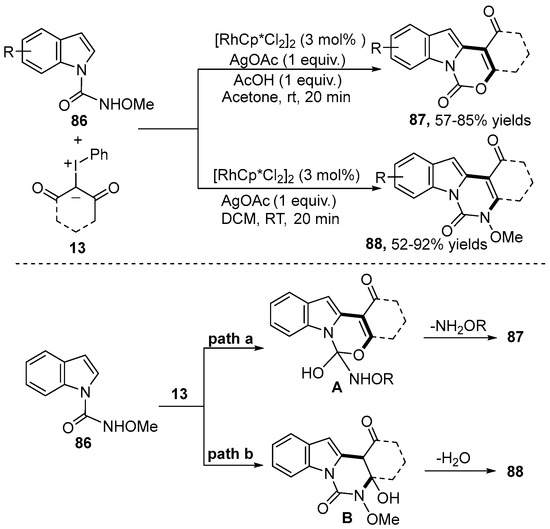
Scheme 30.
Rh(III)–catalyzed C–H annulation of N-carboxamide indoles with iodonium ylides.
1H-isochromene frameworks are ubiquitous in numerous natural products and drugs due to their significant biological activity. A straightforward strategy for the synthesis of 1H-isochromene framework 90 without extra additives via a Rh(III)-catalyzed C–H activation/annulation cascade reaction of pyridotriazole 89 with iodonium ylide 13 was disclosed by Wu and co-workers [44] (Scheme 31). In this reaction, a wide range of pyridotriazole 89 and iodonium ylide 13 reacted smoothly to produce the corresponding cyclization products in decent to good yields. However, the meta-substituted substrates with electron-withdrawing groups resulted in mixed products, which indicated the poor regioselectivity in this transformation.
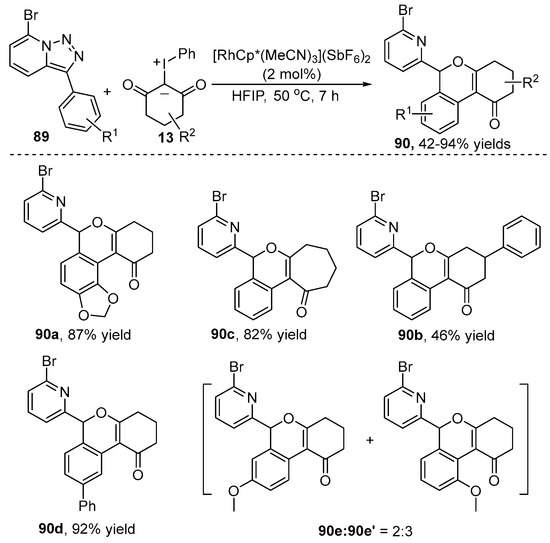
Scheme 31.
Rh(III)–catalyzed C–H [3+3] annulation of pyridotriazoles with iodonium ylides.
A possible mechanism of this reaction is shown in Scheme 32. Firstly, 7-Bromopyrido-triazole 89 coordinated with a [RhCp*(MeCN)3](SbF6)2 catalyst via the C–H activation to generate the five-membered rhodacyclic intermediate A, which further coordinated with iodonium ylide 13 to form the rhodium carbene species B. Then, intermediate B underwent an intramolecular migratory insertion to produce intermediate C. Then, a 1,3-shift of intermediate C gave the alkoxyrhodium intermediate D, which was further converted to the Rh-carbene E by denitrogenation. Finally, the intermediate F underwent a second migratory insertion and protonation to generate the desired product 90, followed by the regeneration of the Rh catalyst.
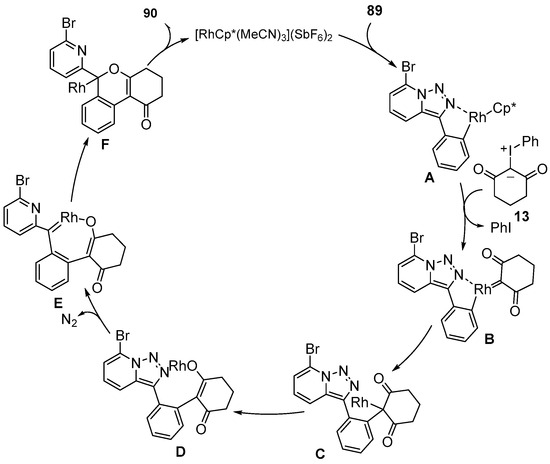
Scheme 32.
Proposed reaction mechanism for Rh(III)–catalyzed C–H [3+3] annulation of pyridotriazole with iodonium ylides.
Isocoumarin derivatives represent a class of privileged structural motifs with a variety of biological and pharmacological activities, such as anti-fungal, anti-allergic and anticoagulant activities. In 2021, Kanchupalli’s group [45] unveiled a direct strategy to prepare isocoumarin skeleton 92 via the [Cp*RhCl2]2-catalyzed [3+3] cyclization of sulfoxonium ylide 91 with iodonium ylide 13 using sulfoxonium ylide functionality as the traceless directing group (Scheme 33a). Importantly, to demonstrate the potential application of this annulation reaction, the natural products cannabinol 93, urolithin core structure 94 and isoquinolone scaffold 95 were rapidly synthesized. Subsequently, a similar Rh(III)-catalyzed synthesis of an isocoumarin skeleton employing the same substrates was developed by Yu and co-workers [46] (Scheme 33b). Excellent functional group tolerance and regioselectivity in this catalytic system were observed.
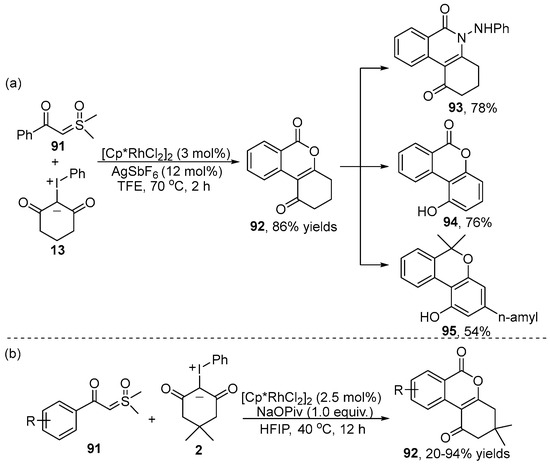
Scheme 33.
Rh(III)–catalyzed C–H [3+3] annulation of sulfoxonium ylides with iodonium ylides.
Recently, the efficient synthesis of diverse substituted isocoumarin 92 had also been realized by Yu’s group [47] via the Rh(III)-catalyzed cascade annulation reaction of N,N-dimethyl enaminone 96 with iodonium ylide 2 (Scheme 34a). Additionally, Liu and Li [48] developed a highly simple, efficient one-pot synthesis of isocoumarin 92 through C–H bond activation and intramolecular C–C cascade annulation of enaminone 96 and cyclic 1,3-dicarbonyl 39 by employing [Cp*RhCl2]2/AgSbF6 as co-catalysts, in which the in situ generation of iodonium ylides was perfectly achievable (Scheme 34b). It is worth mentioning that this protocol provided a rapid and efficient method to synthesize the key intermediates of nonsteroidal selective glucocorticoid receptor modulator (SEGRM) derivatives with anti-inflammatory properties.
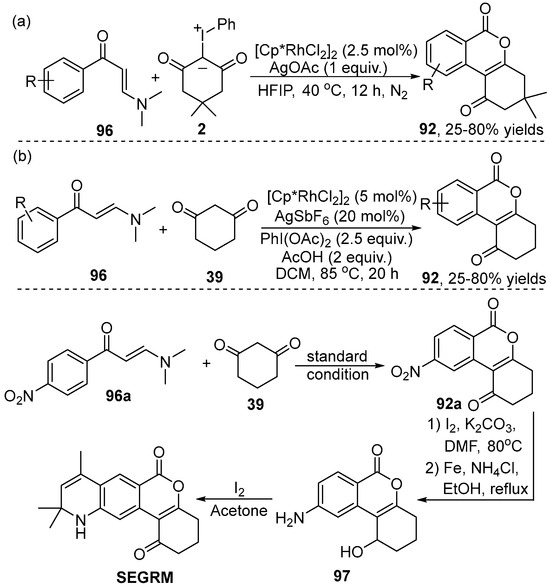
Scheme 34.
Rh(III)–catalyzed C–H [3+3] cyclization of enaminones and the total synthesis of the nonsteroidal selective glucocorticoid receptor modulator.
A plausible mechanism is proposed in Scheme 35. Initially, C–H bond activation of enaminone 96 occurred to produce the five-membered rhodacyclic intermediate A. Meanwhile, iodonium ylide 13 was formed in situ by the reaction of cyclic 1, 3-dicarbonyl compound 39 with PhI (OAc)2. Then, coordination of the iodonium ylide 13 to Rh(III) complex A generated a Rh(III) intermediate B, which further produced a reactive Rh(III) carbene species C via the elimination of PhI. Subsequently, the migratory insertion and protonolysis gave the open-chain alkylation intermediate D with the release of the Rh(III) catalyst. Finally, the intermediate D was tautomerized to enol E, which underwent intramolecular nucleophilic addition and elimination to give the target product 92.
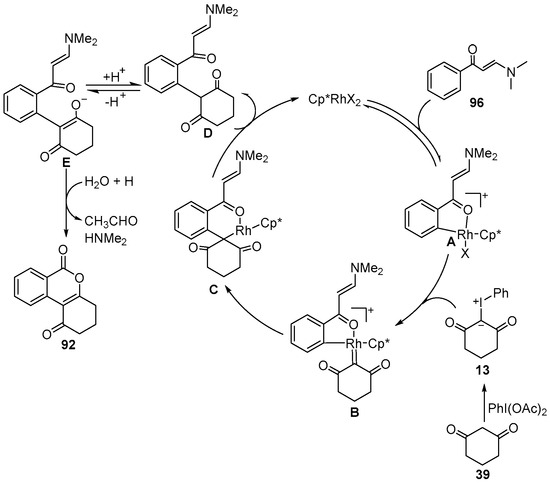
Scheme 35.
Proposed reaction mechanism for Rh(III)–catalyzed C–H [3+3] cyclization of enaminones with iodonium ylides.
In 2021, Liu [49] also described the synthesis of various isocoumarins 92 by annulation of benzoic acid 98 with the 1,3-dicarbonyl compound 39 through an in situ-generated iodonium ylides process in the presence of PhI(OAc)2 (Scheme 36). This reaction was conducted in HFIP using air as an oxidant in the presence of K3PO4 as a base at 80℃ to generate the products. To elucidate the reaction mechanism, control experiments with benzoic acid 98 and cyclic 1,3-dicarbonyl 39 were performed, and the results demonstrated that iodonium ylides were the key intermediates in the cyclization reaction.
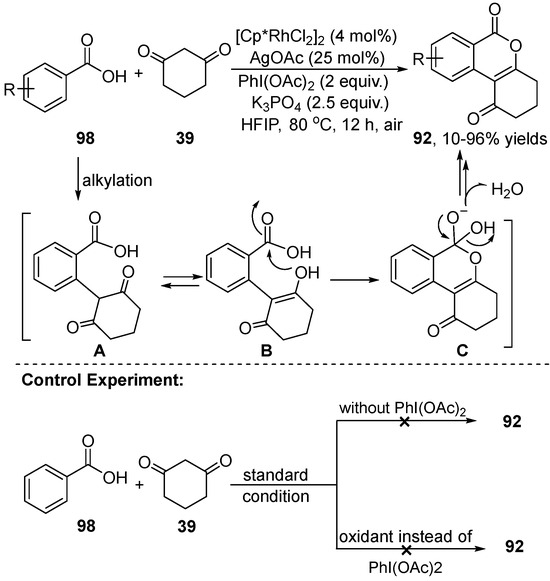
Scheme 36.
Rh(III)–catalyzed C–H activation of benzoic acids and 1,3-dicarbonyl compounds.
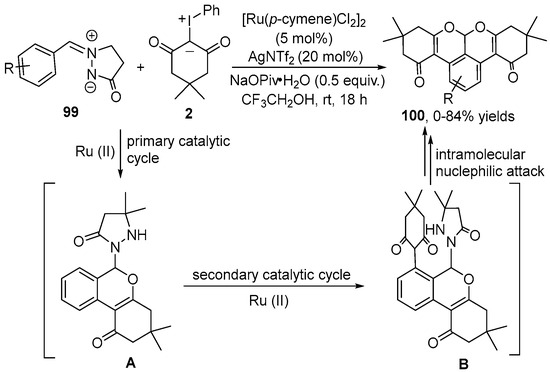
Given that the Ru(II) catalyst has been shown to efficiently catalyze C–H bond activation reactions with outstanding efficiency, the Wu group [50] described the selective [3+3] cycloaddition of azomethine imine 99 with iodonium ylide 2 using relatively cheap [Ru(p-cymene)Cl2]2 as the catalyst and the azomethine imine group as a switchable and transient directing group (Scheme 37). This reaction underwent dual C–H bond activation and dual intramolecular nucleophilic attack, leading to a variety of the pyrano[de]isochromene 100. Furthermore, the H/D exchange experiment indicated that the C–H cleavage was reversible and was faster in the overall reaction. The reaction mechanism was similar to that described above, except for the rearrangement pathway of the directing group in the secondary catalytic cycle.
Scheme 37.
Ru(II)–catalyzed [3+3] annulation of azomethine imines with iodonium ylides.
4. Iodonium Ylides Serve as Alkenylating Reagents
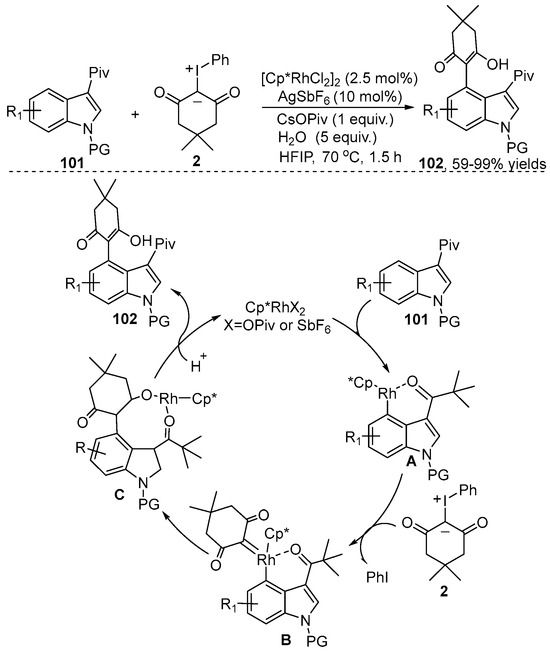
In contrast to the reactions mentioned above, iodonium ylides serve as an effective alkenylating reagent in C–H functionation reactions, which provides an alternative approach to selectively construct the corresponding alkenylating products. In this regard, the Zhang group [51] used pivaloyl as a directing group to accomplish [Cp*RhCl2]2-catalyzed C4-Selective C–H alkenylation of indole 101 with iodonium ylide 2 as the carbene precursor (Scheme 38). This alkenylation reaction gave product 102 in good yields and a high regioselectivity under redox neutral reaction conditions. A plausible mechanism is proposed (Scheme 38). First, a concerted metalation-deprotonation process of substrate 101 produced a rhodacyclic intermediate A. Then, the iodonium ylide 2 coordinated with species A to produce the high active rhodium-carbene species B, along with the elimination of PhI. Subsequent migratory insertion of the Rh-C bond into the carbene species B produced intermediate C. Finally, protonation of species C delivered the desired product 102 and released the active catalyst.
Scheme 38.
Rh(III)–catalyzed C(4)-selective C–H alkenylation of indoles with iodonium ylides, and the proposed reaction mechanism.
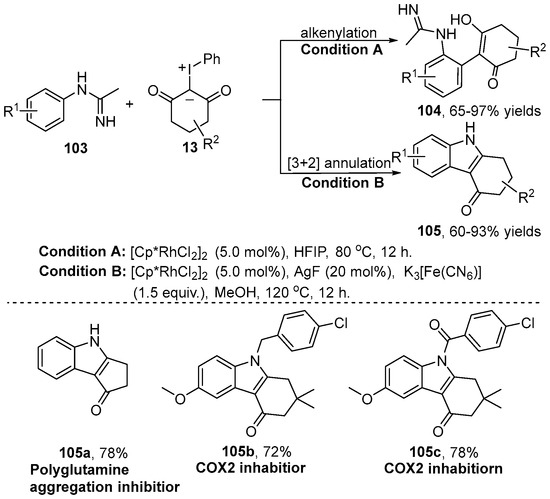
More recently, a condition-controlled reaction of N-aryl amidine 103 and iodonium ylide 13 via a Rh(III)-catalyzed C–H bond functionalization had been developed by the Wu group [52] (Scheme 39). This reaction was conducted in HFIP at 80 ℃ to give the alkenylating product 104. However, switching the solvent to MeOH in the presence of AgF/K3[Fe(CN)6] as the additive resulted in the formation of the [3+2] product carbazolone 105 through an intermolecular annulation process. It is worth mentioning that this protocol provided rapid and efficient access to synthesize the polyglutamine aggregation inhibitor 105a and the COX-2 inhibitor 105b–105c.
Scheme 39.
Rh(III)–catalyzed C–H alkenylation and cyclization of N-arylamidines with iodonium ylides.
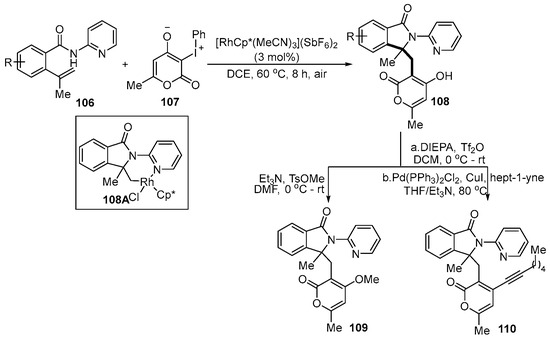
As we all know, the strategy of molecular hybridization has been widely applied to construct novel and highly-valued organic molecules. In this regard, Mai and co-workers [53] pioneered a Rh(III)-catalyzed tandem coupling reaction of iodonium ylide 107 with the C(sp3)-Rh species, produced by 5-exo-trig cyclization to accomplish molecular hybridization (Scheme 40). To demonstrate the potential application of this annulation reaction, product 108 was further derived to produce the corresponding products 109 and 2-pyrone 110 via methylation and Sonogashira coupling, respectively. Importantly, C(sp3)-Rh complex 108A was proven to be the key intermediate during the mechanism experiment study. The reaction mechanism was proposed to involve metal-mediated 5-exo-trig cyclization, coordination, carbene migration insertion and protonolysis process, in which two new chemical bonds and one stereogenic center were formed at the same time.
Scheme 40.
Rh(III)–catalyzed C–H annulation/coupling of isoindolin-1-ones with iodonium ylides.
Another C–H alkenylation reaction employing iodonium ylides based on carbene migratory insertion has been demonstrated by Liu and co-workers [54] (Scheme 41). They developed an inactivated methyl C–H alkenylation using iodonium ylide 112 as a carbene precursor to generate the coupling product 113. A wide range of substrates bearing various functional groups such as halogen, methoxy, trifluoromethyl and naphthyl rings were compatible with this reaction. To explore the reaction mechanism, the stable cyclometalated Rh(III) complex 113A was isolated, which could efficiently catalyze the reaction to generate the desired product.
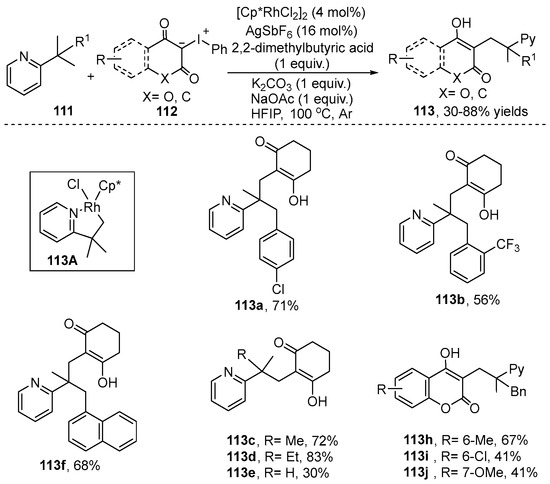
Scheme 41.
Rh(III)–catalyzed alkenylation of inactivated C(sp3)-H bond with iodonium ylides.
5. Conclusions
In summary, this paper briefly outlines the application of iodonium ylides in transition-metal-catalyzed C–H activation reactions, including [n+2] cyclization, [n+3] cyclization and alkenylation. Recent research progress indicates that iodonium ylides which serve as a new type of coupling reagent have shown tremendous potential in transition-metal-catalyzed C–H bond activation reactions and have been developed to effectively construct C–C or C–X bonds in complex organic molecules. Although great progress has been made in this field, there are still some limitations and challenges in the application of iodonium ylides. Previous reports have mainly focused on the reaction of iodonium ylides as a C2/C3 synthon and alkenylating reagent, and it is still necessary to expand the reaction model of iodonium ylides, such as potentially in free radical reactions. As for the transition-metal catalysts, Rh(III) and Ru(II) catalysts are the most widely used catalysts, and low-cost metal catalysts and highly active catalytic systems such as Ir, Pd and Co still need to be developed. In addition, the range of iodonium ylide substrates involved in such reactions are mainly confined to cyclic iodonium ylide compounds, while the metal-catalyzed C–H activation reactions with acyclic-derived iodonium ylides have rarely been reported. Therefore, the design and synthesis of iodonium ylides with more diverse structures to allow participation in C–H activation reactions remains a hot topic for future research. Above all, future research will further promote the application and development of iodonium ylides along these lines and bring more innovation and breakthroughs in the field of organic synthesis.
Author Contributions
J.L. and D.K. conceived the idea, discussed with all collaborators, and wrote the original draft; X.G. drew the molecular structures; R.Z., X.C. and S.W. revised and edited the paper. All authors have read and agreed to the published version of the manuscript.
Funding
Project supported by Hainan Provincial Natural Science Foundation of China (No. 222RC683), the Key R&D Projects of Hainan Province (No. ZDYF2024SHFZ065) and National Natural Science Foundation of China (No. 21901054).
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- De Frémont, P.; Marion, N.; Nolan, S.P. Carbenes: Synthesis, properties, and organometallic chemistry. Coord. Chem. Rev. 2009, 253, 862–892. [Google Scholar] [CrossRef]
- Doyle, M.P.; Duffy, R.; Ratnikov, M.; Zhou, L. Catalytic Carbene Insertion into C-H Bond. Chem. Rev. 2010, 110, 704–724. [Google Scholar] [CrossRef]
- Gillingham, D.; Fei, N. Catalytic X-H insertion reactions based on carbenoids. Chem. Soc. Rev. 2013, 42, 4918–4931. [Google Scholar] [CrossRef]
- Jia, M.; Ma, S. New Approaches to the Synthesis of Metal Carbenes. Angew. Chem. Int. Ed. Engl. 2016, 55, 9134–9166. [Google Scholar] [CrossRef] [PubMed]
- Nunewar, S.; Kumar, S.; Talakola, S.; Nanduri, S.; Kanchupalli, V. Co(III), Rh(III) & Ir(III)-Catalyzed Direct C-H Alkylation/Alkenylation/Arylation with Carbene Precursors. Chem. Asian J. 2021, 16, 443–459. [Google Scholar]
- Müller, P. Asymmetric Transfer of Carbenes with Phenyliodonium Ylides. Acc. Chem. Res. 2004, 37, 243–251. [Google Scholar] [CrossRef] [PubMed]
- Zhdankin, V.V.; Stang, P.J. Chemistry of Polyvalent Iodine. Chem. Rev. 2008, 108, 5299–5358. [Google Scholar] [CrossRef]
- Liu, T.X.; Yang, P.; Ma, N.; Li, X.; Wang, X.; Ma, J.; Zhang, G. Synthesis of Fullerene-Fused Allylbenzofurans via Palladium-Catalyzed Migration Reaction. Chin. J. Chem. 2023, 41, 1733–1739. [Google Scholar] [CrossRef]
- Zhdankin, V.V.; Yusubov, M.S.; Yoshimura, A. Iodonium ylides in organic synthesis. Arkivoc 2016, 1, 342–374. [Google Scholar]
- Ackermann, L. Carboxylate-assisted transition-metal-catalyzed C-H bond functionalizations: Mechanism and scope. Chem. Rev. 2011, 111, 1315–1345. [Google Scholar] [CrossRef]
- Sinha, S.K.; Guin, S.; Maiti, S.; Biswas, J.P.; Porey, S.; Maiti, D. Toolbox for Distal C-H Bond Functionalizations in Organic Molecules. Chem. Rev. 2022, 122, 5682–5841. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.Y.; Zhai, R.R.; Chen, X.; Wang, S.J. Recent Progress in C-H Bond Activation Reaction with Vinylene Carbonate. Chin. J. Org. Chem. 2023, 43, 3119–3134. [Google Scholar] [CrossRef]
- Zheng, C.; You, S.-L. Recent development of direct asymmetric functionalization of inert C–H bonds. RSC Adv. 2014, 4, 6173–6214. [Google Scholar] [CrossRef]
- Jiang, Y.; Li, P.; Zhao, J.; Liu, B.; Li, X. Iodonium Ylides as Carbene Precursors in Rh(III)-Catalyzed C-H Activation. Org. Lett. 2020, 22, 7475–7479. [Google Scholar] [CrossRef] [PubMed]
- Mayakrishnan, S.; Tamizmani, M.; Maheswari, N.U. Harnessing hypervalent iodonium ylides as carbene precursors: C-H activation of N-methoxybenzamides with a Rh(III)-catalyst. Chem. Commun. 2020, 56, 15462–15465. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.-P.; Xu, M.-M.; Zhang, R.-Y.; Xu, X.-P.; Ji, S.-J. Rh(III)-Catalyzed C(sp2)-H functionalization/cyclization cascade of N-carboxamide indole and iodonium reagents for access to indoloquinazolinone derivatives. Green Chem. 2021, 23, 6337–6340. [Google Scholar] [CrossRef]
- Sarkar, A.; Saha, M.; Das, A.R. Ru(II)-catalyzed chelation assisted C(sp2)-H bond functionalization along with concomitant (4+2) annulation. Org. Biomol. Chem. 2023, 21, 5567–5586. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Shi, S.; Ding, W.; He, Z.; Shen, Y.; Nian, Y.; Wu, X. Accessing 7,8-Dihydroquinoline-2,5-diones via Rh-Catalyzed Olefinic C-H Activation/[4+2] Cyclization. Org. Lett. 2024, 26, 5136–5140. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Zhou, Y.; Li, H.; Lei, J.; Bing, P.; He, B.; Li, Y. A Facile Route to Pyrazolo[1,2-a]cinnoline via Rhodium(III)-catalyzed Annulation of Pyrazolidinoes and Iodonium Ylides. Asian J. Org. Chem. 2022, 11, e202100656. [Google Scholar] [CrossRef]
- Li, R.; Hou, Y.-X.; Xu, J.-H.; Gao, Y.; Hu, X.-Q. Divergent synthesis of fused N-heterocycles via rhodium-catalysed [4+2] cyclization of pyrazolidinones with iodonium ylides. Org. Chem. Front. 2022, 9, 2181–2186. [Google Scholar] [CrossRef]
- Pan, C.; Yuan, C.; Chen, D.; Chen, Y.; Yu, J.T. Rh(III)-Catalyzed C-H Activation/Annulation of N-Methyl Arylhydrazines with Iodonium Ylides toward Ring-fused Cinnolines. Asian J. Org. Chem. 2022, 11, e202100809. [Google Scholar] [CrossRef]
- Li, H.; Gu, H.; Lu, Y.; Xu, N.; Han, N.; Li, J.; Liu, J.; Liu, J. Synthesis of Tetrahydrocarbazol-4-ones via Rh(III)-Catalyzed C-H Activation/Annulation of Arylhydrazines with Iodonium Ylides. J. Org. Chem. 2022, 87, 8142–8150. [Google Scholar] [CrossRef]
- Huang, G.; Shan, Y.; Yu, J.T.; Pan, C. Rhodium-catalyzed C-H activation/cyclization of aryl sulfoximines with iodonium ylides towards polycyclic 1,2-benzothiazines. Org. Biomol. Chem. 2021, 19, 10085–10089. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Liu, F.; Du, F.; Zeng, L.; Chen, Z. Cp∗Rh/Ag catalyzed C–H activation/cyclization sequences of NH-sulfoximines to fused aza-polyheterocycles under gentle conditions. Green Synth. Catal. 2023, 4, 160–168. [Google Scholar] [CrossRef]
- Liu, C.; Dai, Q.; Li, Y.; Huang, C.; Guo, L.; Yang, Z. Rh(III)-Catalyzed C-H Annulation of N-Nitrosoanilines with Iodonium Ylides for the Synthesis of N-Alkyl Indoles. J. Org. Chem. 2023, 88, 7281–7289. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Yang, Y.; Fang, F.; Liu, C.; Li, C.; Wang, D.; Liu, H. Rapid Access to Polysubstituted Tetrahydrocarbazol-4-ones via Sequential Selective C-H Functionalization from N-Nitrosoanilines. Chin. J. Chem. 2023, 41, 1957–1962. [Google Scholar] [CrossRef]
- Wang, K.; Song, X.; Xin, Y.; Zhang, X.; Fan, X. Condition-Controlled Selective Synthesis of Pyranone-Tethered Indazoles or Carbazoles through the Cascade Reactions of N-Nitrosoanilines with Iodonium Ylides. Org. Lett. 2023, 25, 4422–4428. [Google Scholar] [CrossRef] [PubMed]
- Jiao, L.; Zhang, B.; Qu, B.; Zhai, R.; Chen, X. Rh(III)-Catalyzed Direct C-H Cyclization of N-Nitrosoanilines with 1,3-Dicarbonyl Compounds: A Route to Tetrahydrocarbazol-4-ones. J. Org. Chem. 2023, 88, 10662–10669. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, M.; Liu, M.; Liu, Z.; Qiu, Y.; Zhang, Z.; Yu, F.; Huang, J. Rh(III)-catalyzed selective mono- and dual-functionalization/cyclization of 1-aryl-5-aminopyrazoles with iodonium ylides. Chem. Commun. 2023, 60, 432–435. [Google Scholar] [CrossRef]
- Zhai, R.; Jiao, L.; Qu, B.; Yang, X.; Chen, X. Rh(III)-Catalyzed C-H Cyclization of Primary Benzylamines with Iodonium Ylides toward Dihydrophenanthridin-1-ones. Asian J. Org. Chem. 2022, 11, e202200492. [Google Scholar] [CrossRef]
- Zhang, M.; Chen, L.; Sun, H.; Liu, Z.; Yan, S.J.; Yu, F. Rh(III)-Catalyzed [3+2] Annulation/Pinacol Rearrangement Reaction of Enaminones with Iodonium Ylides: Direct Synthesis of 2-Spirocyclo-pyrrol-3-ones. Org. Lett. 2023, 25, 7214–7219. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Chen, L.; Sun, H.; Liu, Z.; Huang, J.; Yu, F. Unprecedented chemoselective Ru(III)-catalyzed [3+2] annulation of enaminones with iodonium ylides for the synthesis of functionalized 3a,7a-dihydroxy hexahydro-4H-indol-4-ones. Org. Chem. Front. 2023, 10, 5660–5666. [Google Scholar] [CrossRef]
- Li, X.; Shen, Y.; Zhang, G.; Zheng, X.; Zhao, Q.; Song, Z. Ru(II)-Catalyzed Decarbonylative Alkylation and Annulations of Benzaldehydes with Iodonium Ylides under Chelation Assistance. Org. Lett. 2022, 24, 5281–5286. [Google Scholar] [CrossRef] [PubMed]
- Nunewar, S.; Kumar, S.; Priyanka, P.; Girase, P.; Kanchupalli, V. The solvent-controlled Rh(III)-catalyzed switchable [4+2] annulation of 2-arylIndoles with iodonium ylides. Chem. Commun. 2022, 58, 6140–6143. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Chang, J.; Shen, J.; Cui, X. One-pot access to indazole fused-phenanthridinones via Rh(III)-catalyzed [4+2] annulation. Green Synth. Catal. 2023, 2666–5549. [Google Scholar] [CrossRef]
- Nunewar, S.; Kumar, S.; Meshram, A.W.; Kanchupalli, V. Ru(II)-Catalyzed C-H Functionalization of 2-Arylbenzimidazoles with Iodonium Ylides: A Straightforward Access to Bridgehead Polycyclic N-Heterocycles. J. Org. Chem. 2022, 87, 13757–13762. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Lv, G.; Lin, Z.; Tang, J.; Chen, J.; Liu, J.; Hai, L.; Wu, Y. Mild construction of N-fused polycyclic compounds via Rh(III)/EosinY co-catalyze C-H activation. Green Synth. Catal. 2022, 5, 108–111. [Google Scholar] [CrossRef]
- Yan, K.; Liu, X.; Wen, J.; Li, Q.; Zhou, B.; Zhang, Y.; Wang, X. Synthesis of Substituted Pyrido[1,2-a]benzimidazoles by Ruthenium-Catalyzed C−H Bond Activation and Tandem Cyclization of 2-Arylimidazo[1,2-a]pyridines with Iodonium Ylides. Eur. J. Org. Chem. 2023, 26, e202300684. [Google Scholar] [CrossRef]
- Zhong, Z.; Liang, M.; Zhang, Z.; Cui, H.; Wang, N.; Mai, S.; Tao, H. Rh(III)-Catalyzed C-H Annulation of Alkenyl- or Arylimidazoles and (Hetero)cyclic 1,3-Dicarbonyl Compounds: A Rapid Access to Imidazo-Fused Polycyclic Compounds. Org. Lett. 2022, 24, 4850–4854. [Google Scholar] [CrossRef]
- Yang, Z.; Liu, J.; Li, Y.; Ding, J.; Zheng, L.; Liu, Z.Q. Three-Component Synthesis of Isoquinolone Derivatives via Rh(III)-Catalyzed C-H Activation and Tandem Annulation. J. Org. Chem. 2022, 87, 14809–14818. [Google Scholar] [CrossRef]
- Chen, W.L.; Song, J.L.; Fang, S.; Li, J.B.; Zhang, S.S.; Shu, B. Rh(III)-catalyzed C(sp2)-H functionalization/[4+2] annulation of oxadiazolones with iodonium ylides to access diverse fused-isoquinolines and fused-pyridines. Chem. Commun. 2024, 60, 6560–6563. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhou, B.; Yan, K.; Wen, J.; Li, Q.; Wang, X.; Wang, X. Application of Sulfoxonium Ylides or Iodonium Ylides in Rhodium-Catalyzed Synthesis of Phenanthrenes. Adv. Synth. Catal. 2024, 366, 1744–1750. [Google Scholar] [CrossRef]
- Nunewar, S.; Kumar, S.; Pandhare, H.; Nanduri, S.; Kanchupalli, V. Rh(III)-Catalyzed Chemodivergent Annulations between Indoles and Iodonium Carbenes: A Rapid Access to Tricyclic and Tetracyclic N-Heterocylces. Org. Lett. 2021, 23, 4233–4238. [Google Scholar] [CrossRef] [PubMed]
- Lv, G.; Zhang, Q.; Zhang, C.; Chen, Y.; Lin, Z.; Lai, R.; Yang, Z.; Wu, Y. The Pyridotriazole Works as a Traceless Directing Group: A C-H Activation/Annulation Cascade Reaction with Iodonium Ylides. Org. Lett. 2023, 25, 4022–4027. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Nunewar, S.; Kanchupalli, V. Rh(III)-Catalyzed Cross-Coupling/Annulation of Two Carbene Precursors: Construction of Dihydrobenzo[c]chromen-6-one Scaffolds and Application in the Total Synthesis of Cannabinol. Asian J. Org. Chem. 2021, 11, e202100689. [Google Scholar] [CrossRef]
- Yin, C.; Li, L.; Yu, C. Rh(III)-catalyzed C-H annulation of sulfoxonium ylides with iodonium ylides towards isocoumarins. Org. Biomol. Chem. 2022, 20, 1112–1116. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Chen, L.; Liu, D.; Liu, Z.; Huang, J.; Li, X.; Yu, F. Rh(III)-catalyzed cascade annulation reaction of N,N-dimethyl enaminones with iodonium ylides to give substituted isocoumarins. New J. Chem. 2023, 47, 12274–12278. [Google Scholar] [CrossRef]
- Wang, Q.; Li, Y.; Sun, J.; Chen, S.; Li, H.; Zhou, Y.; Li, J.; Liu, H. Rh-Catalyzed C-H Activation/Annulation of Enaminones and Cyclic 1,3-Dicarbonyl Compounds: An Access to Isocoumarins. J. Org. Chem. 2023, 88, 5348–5358. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Li, X.; Liu, B. Rh(III)-Catalyzed Efficient Synthesis of Isocoumarins from Cyclohexanediones. Chin. J. Org. Chem. 2021, 41, 4476–4483. [Google Scholar] [CrossRef]
- Ren, J.; Pi, C.; Cui, X.; Wu, Y. Transition- Metal-Controlled Divergent Annulations of Azomethine Imines with Iodonium Ylides via C-Centered [1,2]-Rearrangement. Org. Lett. 2023, 25, 2582–2587. [Google Scholar] [CrossRef]
- Wu, F.; Xiao, L.; Xie, H.; Chen, S.Y.; Song, J.L.; Zheng, Y.C.; Liu, Y.Z.; Zhang, S.S. Rhodium(III)-catalyzed regioselective C(sp(2))-H activation of indoles at the C4-position with iodonium ylides. Org. Biomol. Chem. 2022, 20, 5055–5059. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Yang, L.; Pi, C.; Cui, X.; Wu, Y. Rhodium(III)-Catalyzed Divergent C-H Functionalization of N-Aryl Amidines with Iodonium Ylides: Access to Carbazolones and Zwitterionic Salts. Adv. Synth. Catal. 2023, 365, 1817–1823. [Google Scholar] [CrossRef]
- Zhang, Z.; Su, B.; Gong, J.; Tao, H.; Mai, S. Rhodium-Catalyzed Difunctionalization of Alkenes Using Cyclic 1,3-Dicarbonyl-Derived Iodonium Ylides. Org. Lett. 2024, 26, 1886–1890. [Google Scholar] [CrossRef] [PubMed]
- Xie, P.; Gao, H.; Li, X.; Jiang, Y.; Liu, B. Rh(III)-Catalyzed C-C coupling of unactivated C(sp3)-H bonds with iodonium ylides for accessing all-carbon quaternary centers. Org. Chem. Front. 2022, 9, 3823–3827. [Google Scholar] [CrossRef]
- Kumar, S.; Borkar, V.; Mujahid, M.; Nunewar, S.; Kanchupalli, V. Iodonium Ylides: An emerging and alternative carbene precursor for C-H functionalizations. Org. Biomol. Chem. 2023, 21, 24–38. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).