Abstract
In this study, facile construction engineering of Pr6O11@C with efficient photocatalytic activity was established. Taking advantage of the flocculation of Pr3+ in the base medium, acid red 14 (AR14) was flocculated together with Pr(OH)3 precipitate, in which Pr(OH)3 and AR14 mixed highly uniformly. Calcinated at high temperature in N2, a novel Pr6O11@C was successfully synthesized. The resulting materials were characterized by XRD, SEM, FT-IR, Raman, and XPS techniques. The results show that the cubic Pr6O11@C with Fm3m space group, similar to that of Pr6O11, was obtained. From the results of the photodegradation of AR14, it is found that the photocatalytic efficiency of Pr6O11@C is higher than that of pure Pr6O11 due to the formation of abundant carbon bonds and oxygen vacancies. Compared with pure Pr6O11 and other carbon-based composites, the acid resistance of Pr6O11@C is greatly improved due to the highly uniform dispersion of Pr6O11 and C, which lays a solid foundation for the practical application of Pr6O11@C. Moreover, the role of NH3·H2O and NaOH used as precipitants for the photocatalytic efficiency of Pr6O11 was investigated in detail.
1. Introduction
Due to the complete degradation of organic pollutants, photocatalytic technology has received extensive attention [1,2,3]. In general, TiO2, ZnO, CuS et al. were often chosen as photocatalysts to degrade organic pollutants [4,5,6]. However, many difficulties, such as the large band gap for TiO2 and ZnO [7,8] and the photocorrosion problem for sulfide [9], should be overcome when using these photocatalysts. Therefore, there is an urgent need to develop other photocatalysts with lower band gaps and stable performance.
Recently, rare earth oxides such as CeO2 have attracted considerable attention due to their potential photocatalytic activity and their stability during photocatalysis [10,11,12]. Unfortunately, pure CeO2 has a large band gap of about 3.2 eV, which greatly decreases its absorption of visible light and hence photocatalytic efficiency. Pr6O11, an n-type semiconductor with a band gap of around 1.77–3.3 eV [13], is very stable among the praseodymium oxide family at ambient temperature and pressure [14]. Consequently, Pr6O11 was chosen as a photocatalyst to degrade organic pollutants [15,16,17]. In order to reduce the band gap, carbon materials were introduced into Pr6O11 to prepare the Pr6O11@C composite. For example, Shende et al. synthesized a new Pr6O11/g-C3N4 heterostructure material by a single-step solvent-free solid-state method and found that the notable increase in the photocatalytic activity of the Pr6O11/g-C3N4 heterostructure was ascribed to the reduced band gap and hence the improved visible light absorption [13]. However, the synthetic process of g-C3N4 is relatively complex, and the interaction between g-C3N4 and Pr6O11 is weak. Can g-C3N4 be replaced by other carbon materials during the synthesis of Pr6O11@C with efficient photocatalytic activity?
Besides C3N4, activated carbon, carbon nanotube, grapheme, etc., are usually used as carbon sources to prepare carbon-based photocatalysts [18,19,20]. Nevertheless, the cost of carbon sources and the complex synthesis process of carbon-based catalysts hinder the practical application of these carbon materials. Moreover, these carbon-based Pr6O11 photocatalysts always react with H+ in an acid environment, which makes them unstable and limits their application in practice. Acid dyes containing rich carbon elements are always considered organic pollutants and removed by physical and/or chemical methods [21,22]. However, acid dyes are rarely used as carbon sources to synthesize carbon-based photocatalysts. Recently, it has been interesting to find that Pr3+ can flocculate acid red 14 (AR14) in the basic medium, which makes us consider the use of acid dyes such as AR14 as carbon sources for the preparation of Pr6O11@C. In the flocculation process, Pr3+ was precipitated into Pr(OH)3 by mixing with AR14 uniformly to form Pr(OH)3@AR14. Calcinated at high temperatures, Pr(OH)3 and AR14 can be changed into Pr6O11 and carbon, respectively. The synthetic process of Pr6O11@C based on this design may be illustrated in Scheme 1. To the best of our knowledge, there are no reports about the synthesis of Pr6O11@C via this facile construction engineering.
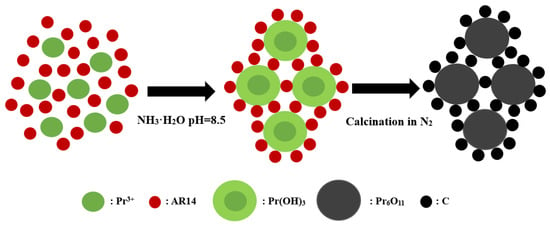
Scheme 1.
The scheme for the synthesis of Pr6O11@C.
Guided by the above idea, Pr6O11@C was synthesized using acid dye as a carbon source and Pr3+ as a flocculatant. The photocatalytic performance of Pr6O11@C was measured by the degradation of AR14, and the results show that the photocatalytic efficiency of Pr6O11@C is higher than that of pure Pr6O11 due to the uniform mixing of carbon with Pr6O11 and the efficient carbon bond formation rate. Accordingly, the acid resistance of Pr6O11 was greatly improved. Furthermore, the effect of different precipitants (NH3·H2O and NaOH) on the photocatalytic efficiency was also investigated.
2. Experimental
The experimental part is presented in the Supporting Materials.
3. Results and Discussion
Figure 1 shows the powder X-ray diffraction (XRD) patterns of Pr6O11 and Pr6O11@C prepared with different precipitants. The diffraction pattern of the synthesized samples can be ascribed to the cubic Pr6O11 with the Fm3m space group (JCPDS file No. 00-042-1121) [16]. It is clear from Figure 1 that all samples exhibit obvious peaks corresponding to the (111), (200), (220), (311), (222), (400), (331), (420), and (422) planes, indicating that both the precipitant and the introduction of carbon have no effect on the crystal structure of the prepared samples.
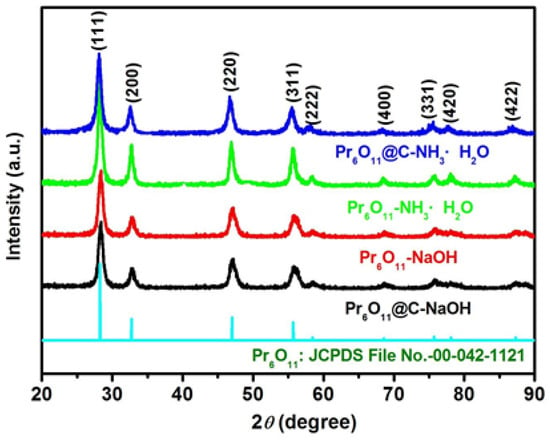
Figure 1.
XRD patterns of Pr6O11 and Pr6O11@C prepared with different precipitant.
The elemental mapping of O, Pr, and C from EDS is presented in Figure 2, and the results show that C was actually introduced into the resulting sample and that all the composited elements were evenly distributed in the sample. Similar phenomena can be found in other samples with different content of carbon, illustrating that AR14 can be changed into carbon in our experimental process.
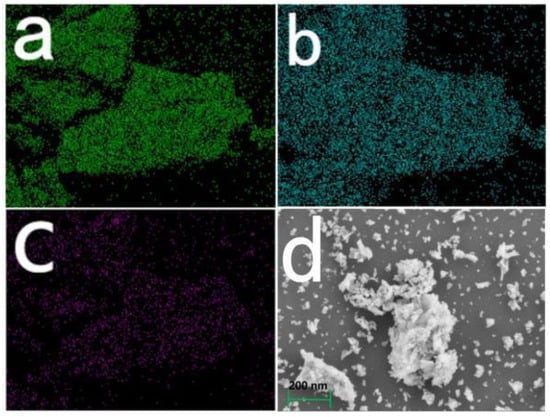
Figure 2.
Elemental mapping of (a) O, (b) Pr, (c) C and SEM image (d) of Pr6O11@C prepared using 100 mL of AR14 with a concentration of 0.075 mM as carbon source.
The photocatalytic efficiency of Pr6O11-NH3·H2O and Pr6O11-NaOH was evaluated by the degradation of AR14, which is presented in Figure 3. From Figure 3, it is easy to find that the photocatalytic efficiency of Pr6O11-NH3·H2O is much higher than that of Pr6O11-NaOH because the characteristic absorption peak intensity of AR14 at 516 nm is very low after degradation over Pr6O11-NH3·H2O. From the results of XRD, the structure of Pr6O11-NH3·H2O and Pr6O11-NaOH is similar. Why are the photocatalytic results so different?
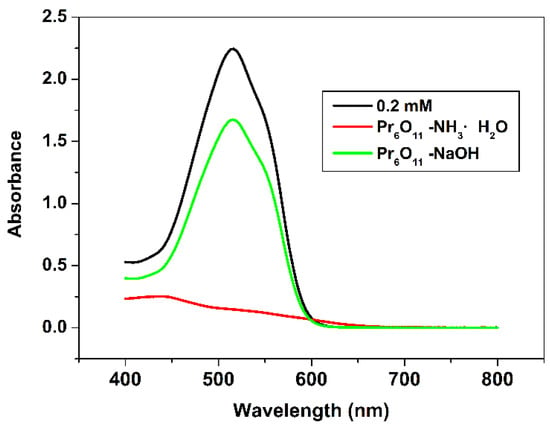
Figure 3.
UV-vis spectra of 0.2 mM AR14 after photodegradation over Pr6O11-NH3·H2O and Pr6O11-NaOH.
It is well known that the adsorption capability of organic pollutants over the catalyst plays an important role in photocatalytic efficiency [23]. In order to investigate the intrinsic reason for photocatalytic difference, the adsorption of 0.1 mmol/L (mM) AR14 on Pr6O11-NH3·H2O and Pr6O11-NaOH was carried out, and the results are shown in Figure 4. Compared with Figure 4a,b, it is obvious that the adsorption efficiency of Pr6O11-NH3·H2O is higher than that of Pr6O11-NaOH and that 30 min is enough for the adsorption equilibrium of AR14 over Pr6O11-NH3·H2O and Pr6O11-NaOH. From our previous report, it can be seen that the number of amino groups and hydroxyl groups in the sample prepared using NH3·H2O as the precipitant is higher than that of the sample synthesized using NaOH as the precipitant, which results in a higher adsorption efficiency [24].
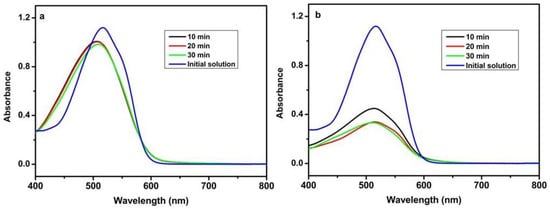
Figure 4.
UV-vis spectra of 0.1 mM AR14 after adsorption over Pr6O11-NaOH (a) and Pr6O11-NH3·H2O (b).
In order to investigate if there are amino groups and hydroxyl groups in Pr6O11-NaOH and Pr6O11-NH3·H2O, FT-IR was used, and the results are presented in Figure 5. It can be found from Figure 5 that amino groups and hydroxyl groups actually reside in Pr6O11-NH3·H2O because of the peaks at 1630 cm−1 ascribed to the O-H bending vibration [25] and 1467 cm−1 attributed to the N-H bending vibration mode [26]. The peak at 1630 cm−1 is also detected in Pr6O11-NaOH, but the intensity is lower than that of Pr6O11-NH3·H2O, implying that the hydroxyl content of Pr6O11-NH3·H2O is higher than that of Pr6O11-NaOH. According to the relevant literature [27,28,29], the peaks at 1489, 1436, and 1384 cm−1 are due to the stretching vibration of the redundant on the surface of the prepared samples. From Figure 5, it can be inferred that the number of in Pr6O11-NaOH is higher than that of Pr6O11-NH3·H2O because two peaks representing are detected on a curve, while only one characteristic peak is found on curve b. Consequently, the reasons for the higher adsorption efficiency of AR14 over Pr6O11-NH3·H2O can be presented as follows. On one hand, there are lots of hydroxyl and amino groups on the surface of Pr6O11-NH3·H2O, resulting in strong interaction between Pr6O11-NH3·H2O and AR14 due to the electrostatic attraction of sulfo groups (-) and - and in the acid system [24]. On the other hand, less content of in Pr6O11-NH3·H2O can decrease the repulsive force between and -. Moreover, the existence of on the surface of the photocatalyst can decrease the content of radicals, such as hydroxyl radicals, due to the capture of for radicals [30,31], which can also explain the lower photocatalytic efficiency of Pr6O11-NaOH.
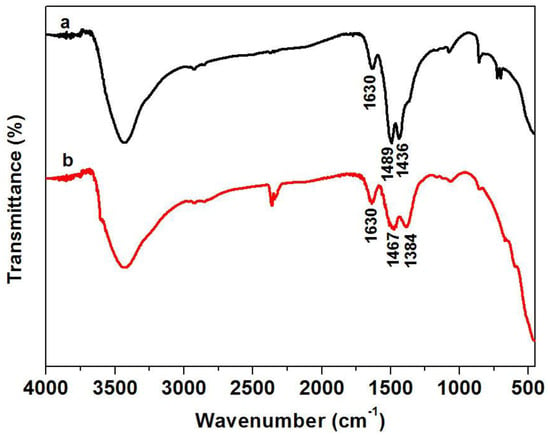
Figure 5.
FTIR spectra of Pr6O11-NaOH (a) and Pr6O11-NH3·H2O (b).
The light, especially for visible light, absorption efficiency of photocatalysts is another important factor affecting photocatalytic efficiency. From the diffuse reflectance UV-vis spectra of Pr6O11-NaOH and Pr6O11-NH3·H2O (Figure 6), it can be seen that the absorption efficiency of visible light over Pr6O11-NH3·H2O is much higher than that of Pr6O11-NaOH, implying that photogenerated electrons and holes can be efficiently produced and, hence, photocatalytic efficiency will be improved when Pr6O11-NH3·H2O is used as a photocatalyst.
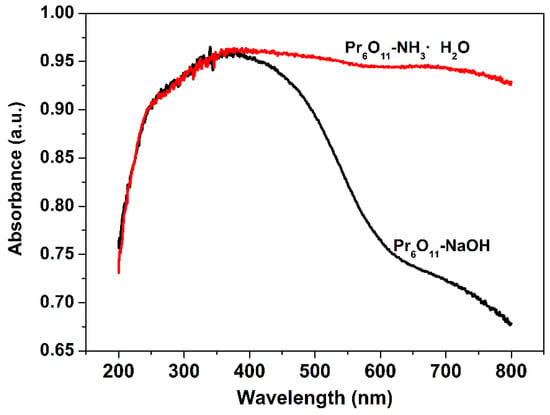
Figure 6.
Diffuse reflectance UV-vis spectra of Pr6O11-NaOH and Pr6O11-NH3·H2O.
As discussed above, Pr3+ and Pr4+ are always detected in Pr6O11, and the ratio of Pr4+ to Pr3+ can affect the photocatalytic efficiency of Pr6O11. The content of Pr4+ and Pr3+ in Pr6O11-NaOH and Pr6O11-NH3·H2O can be defined by the XPS spectra of Pr 3d, which is shown in Figure 7. The Pr 3d spectra in Figure 7a,b can be deconvoluted into five peaks centered at 933, 953, 948, 928, and 956 eV, respectively. The strong peaks at 933 and 953 eV correspond to Pr3+, and the other peaks are ascribed to Pr4+ [32,33]. According to the peak area of Pr4+ and Pr3+ in Figure 7, it can be concluded that the ratio of Pr4+ to Pr3+ in Pr6O11-NaOH (0.27) is higher than that of Pr6O11-NH3·H2O (0.25), implying less content of Pr4+ in Pr6O11-NH3·H2O. Therefore, we can conclude that more Pr4+ ions on the surface of Pr6O11-NH3·H2O were reduced to Pr3+, and thus more oxygen vacancies were formed. According to Gregson et al., trivalent lanthanide ions make it easier to form lanthanide carbon bonds than that of tetravalent ones [34]. Generally, the easy formation of carbon bonds in the photocatalyst is helpful for photocatalytic efficiency [35]. Moreover, oxygen vacancies can improve the separation of photogenerated electrons and holes, resulting in more efficient photocatalytic efficiency [36], which can be proved by the results of photocurrent over Pr6O11-NaOH and Pr6O11-NH3·H2O (Figure 11b). From the analysis of XPS spectra of Pr 3d, the results in Figure 3 can be further understood.
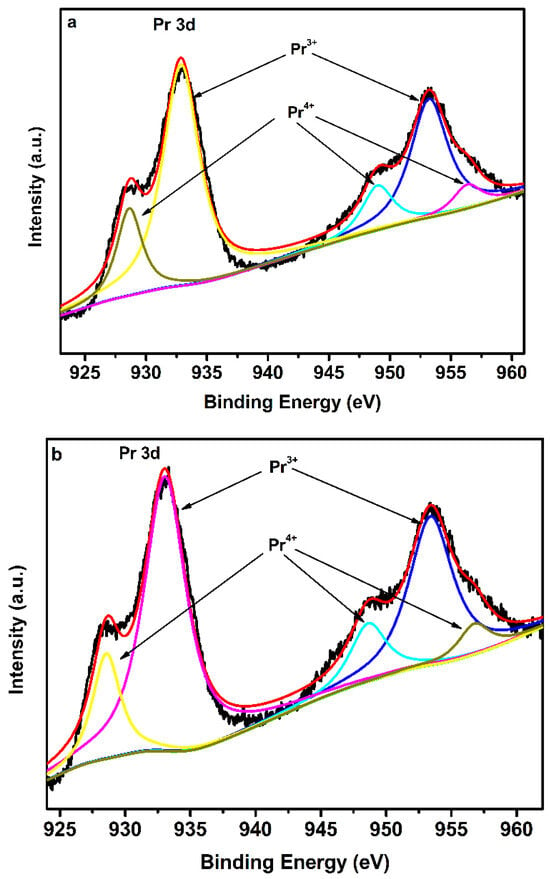
Figure 7.
XPS spectra of Pr 3d for Pr6O11-NaOH (a) and Pr6O11-NH3·H2O (b).
Besides the structural information given by XPS spectra of Pr 3d, XPS spectra of O 1s can also provide useful messages for the structure of Pr6O11. From XPS spectra of O 1s for Pr6O11-NaOH and Pr6O11-NH3·H2O (Figure 8), it can be inferred that the strength of the Pr-O bond in Pr6O11-NH3·H2O is higher than that in Pr6O11-NaOH because the peak area at about 528.30 and the characteristic binding energy of Pr-O [33] in Pr6O11-NH3·H2O are higher than those of Pr6O11-NaOH. A stronger Pr-O bond can make Pr6O11-NH3·H2O more stable, which is beneficial for its practical application. Moreover, more Pr-O bonds may be helpful for the transition of photogenerated electrons to the surface of the catalyst to combine the adsorbed oxygen and form a superoxide radical to degrade AR14. The peak at about 531 eV in Figure 8 can be attributed to oxygen species in the defects [37] and adsorbed oxygen [38]. Due to the high intensity, the peak at about 531 eV is mainly assigned to the adsorbed oxygen. It is clear from Figure 8 that the ratio of adsorbed oxygen to binding oxygen in Pr6O11-NaOH is higher than that in Pr6O11-NH3·H2O. It can be concluded from the literature [39] that the high content of adsorbed oxygen can result in poor activity of the catalyst, which can further prove the lower photocatalytic efficiency of Pr6O11-NaOH.
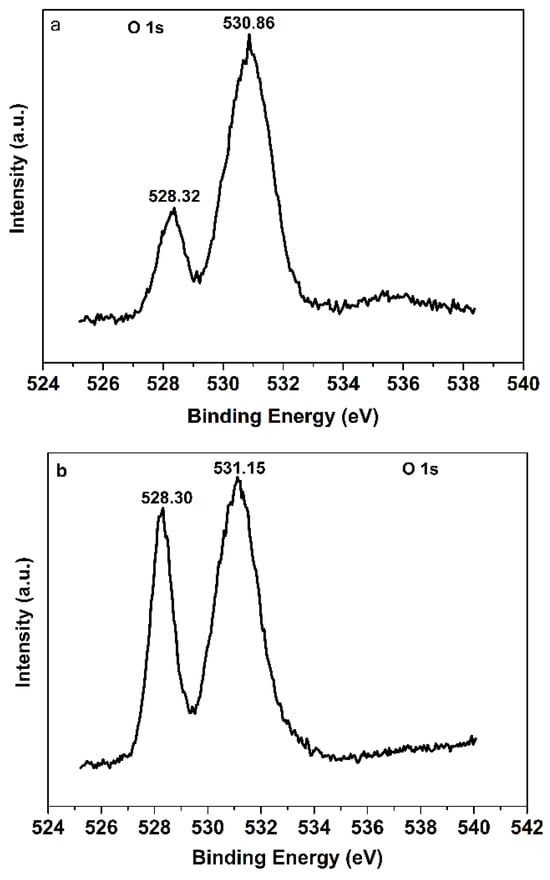
Figure 8.
XPS spectra of O 1s for Pr6O11-NaOH (a) and Pr6O11-NH3·H2O (b).
From the above discussion, it can be concluded that NH3·H2O is suitable for the preparation of Pr6O11 with enhanced photocatalytic efficiency. Therefore, NH3·H2O is designated as a precipitant to synthesize Pr6O11 and Pr6O11@C in the following context. The photodegradation of 0.3 mM AR14 over Pr6O11 and Pr6O11@C prepared via the route of Scheme 1 is illustrated in Figure 9. It is obvious that the introduction of carbon into Pr6O11 can improve the photocatalytic efficiency of Pr6O11 under the same experimental conditions. From the dark reaction, it is easy to find that the adsorption capacity of Pr6O11@C is higher than that of the pure composite, which is good for the enhancement of photocatalytic efficiency. Furthermore, it can be found from Figure 10a that, compared with Pr6O11, the UV-vis absorption of Pr6O11@C is more efficient, which is helpful for the improvement in photocatalytic activity. From Figure 10b, we can find that the intensity of peak at 543 nm, associated with Pr3+ and oxygen vacancy (positively charged) [40], of Pr6O11@C is higher than that of Pr6O11, implying more efficient photocatalysis of Pr6O11@C because the photogenerated electrons can be easily captured by the positively charged oxygen vacancies [34,36]. The existence of Pr3+ and the oxygen vacancy in Pr6O11@C is further demonstrated by the XPS spectra of Pr 3d for Pr6O11@C (Figure 10c) because the ratio of Pr4+ to Pr3+ is 0.22, which is lower than that of Pr6O11 (0.25). A possible reason for this is that the carbon produced by the pyrolysis of AR14 is active [35] and can reduce Pr4+ to Pr3+, resulting in the formation of oxygen vacancies via the replacement of Pr4+ by Pr3+.
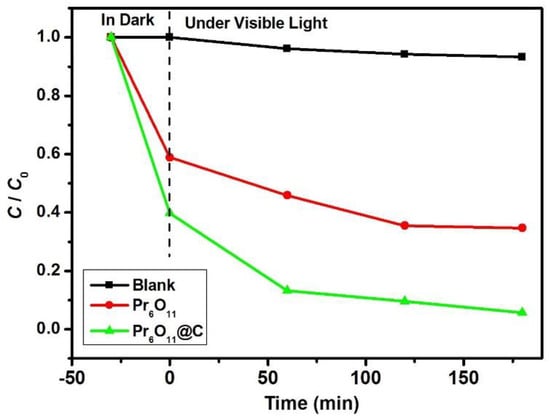
Figure 9.
The photodegradation of 0.3 mM AR14 in the presence of Pr6O11 and Pr6O11@C under visible light irradiation.
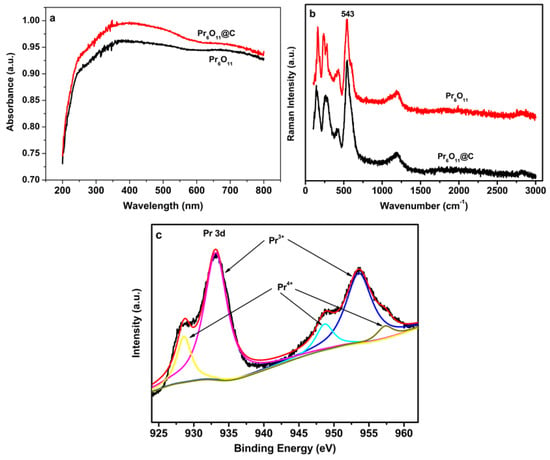
Figure 10.
Diffuse reflectance UV-vis spectra (a), Raman spectra of Pr6O11 and Pr6O11@C (b), and XPS spectra of Pr 3d for Pr6O11@C (c).
From our previous report, the formation of carbon bonds between carbon and photocatalyst can greatly improve photocatalytic efficiency [35]. The carbon bonding in Pr6O11@C can be investigated by XPS spectra of C 1s, which is presented in Figure 11a. The C1s spectra in Figure 11a can be deconvoluted into four peaks centered at 284.5, 285.9, 288.1, and 289.3 eV corresponding to C=C, C-OH, O=C and O-C=O bonds, respectively [41]. Consequently, it can be concluded that numerous carbon bonds were formed in Pr6O11@C, which can enhance the separation of photogenerated electrons and holes. In order to test this inference, a photocurrent experiment was carried out, and the results are shown in Figure 11b. It is obvious from Figure 11b that the photocurrent on Pr6O11@C is higher than that on Pr6O11, indicating more efficient photoelectron–hole separation efficiency for Pr6O11@C. It can be concluded from Figure S1 that AR14 was actually degraded over Pr6O11@C. From the results of Figure S2, it is obvious that 0.075 mM AR14 is the best concentration to synthesize Pr6O11@C with efficient photocatalytic efficiency. Moreover, Figure S3 proves that and OH• are the main oxidative species responsible for the photodegradation of AR14.
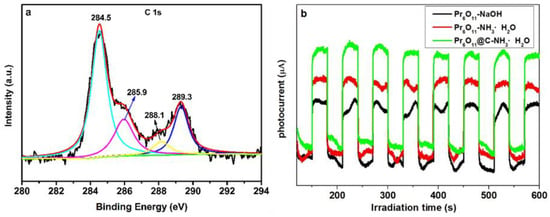
Figure 11.
XPS spectra of C 1s for Pr6O11@C (a), and photocurrent–time profiles of Pr6O11-NaOH, Pr6O11-NH3·H2O, and Pr6O11@C-NH3·H2O (b).
According to the above analysis, it can be concluded that lots of oxygen vacancies and carbon bonds were formed in the Pr6O11@C, and O2 can be adsorbed on the surface of Pr6O11@C. Therefore, under visible light irradiation, the electrons can be excited from VB to CB, intermediate energy levels resulting from oxygen vacancies, and transfer carbon bond and reach the surface of Pr6O11@C to combine with the adsorbed oxygen to form . On the other hand, the holes formed after the departure of excited electrons can interact with water to form OH• due to their strong oxidation. and OH• degrade AR14 together. Therefore, the possible photocatalytic mechanism of AR14 over Pr6O11@C can be illustrated by the schematic diagram of the energy band of Pr6O11@C, as shown in Figure 12.
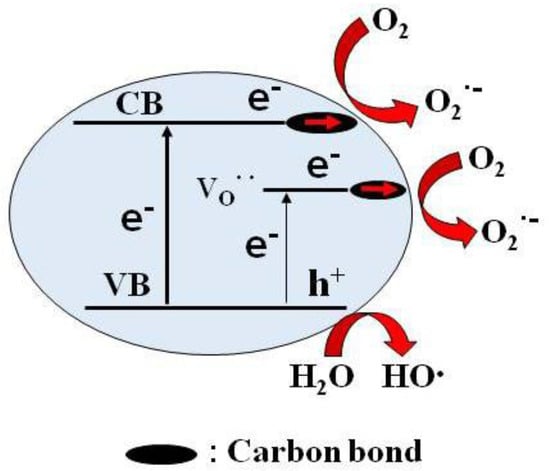
Figure 12.
Proposed photocatalytic mechanism over Pr6O11@C-NH3·H2O under the irradiation of visible light (: oxygen vacancy, h+: hole, VB: valence band, CB: conduction band).
From the viewpoint of practical application, a stable photocatalyst in a medium with different pH values is welcome. The effect of pH on the degradation of 0.3 mM AR14 over Pr6O11@C is shown in Figure 13. It is clear from Figure 13 that Pr6O11@C is stable in both acidic and alkaline media and that the photocatalytic efficiency of Pr6O11@C in an acid medium is higher than that of an alkaline medium. The reason for this may be that the adsorption ability of acid dyes over photocatalysts is increased in an acid medium [40]. It can be seen from Figure 13 that AR14 is almost degraded in the system with a pH of 5, and the degradation rate of AR14 is not obviously increased when the pH value is decreased to 3. From the experimental results, we found that pure Pr6O11 can be dissolved in the solution with a pH value of 4 because of an acid-base reaction. However, the prepared Pr6O11@C in this study is stable in the solution with a pH value of 3. In order to investigate the stability of other carbon-based Pr6O11 in acid solution, we synthesized Pr6O11@C using C3N4, carbon nanotube, and grapheme as carbon source. The preparation process is similar to Pr6O11@C prepared using AR14 as a carbon source. The AR 14 solution was replaced by a 100 mL solution containing 0.01 g C3N4 or carbon nanotube or grapheme. The results show that these carbon-based Pr6O11 materials are not stable in the solution with a pH value of 4, indicating that using AR14 as a carbon source is helpful for the stability of Pr6O11@C in the acid medium and that Pr6O11@C synthesized via Scheme 1 is a potential photocatalyst. A possible reason for the acid resistance of Pr6O11@C synthesized in this study is that carbon and Pr6O11 are mixed highly uniformly with each other, which can be seen from Scheme 1.
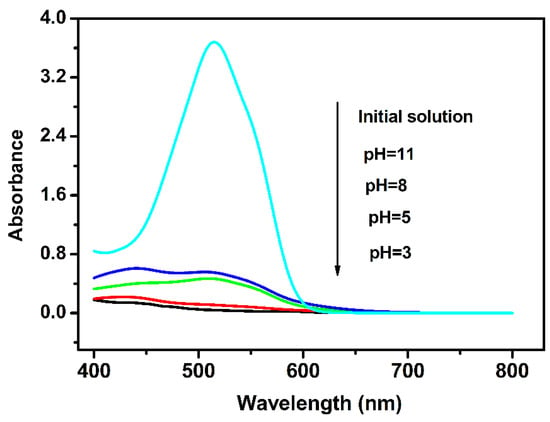
Figure 13.
Effect of pH on the degradation of 0.3 mM AR14 over Pr6O11@C for 180 min.
From the results of Figure 14a, it can be seen that Pr6O11@C demonstrates good photocatalytic stability because the sixth photocatalytic efficiency is similar to the first one. Moreover, the Pr3+ and Pr4+ are detected in Pr6O11@C after six cycles (Figure 14b), which is the same as that of the initial sample.
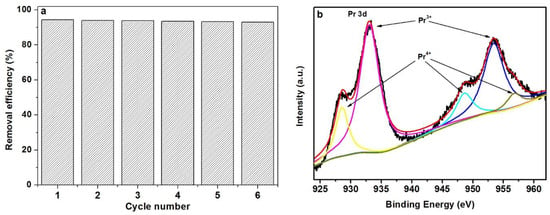
Figure 14.
Reuse performance (a) and XPS spectra of Pr 3d for Pr6O11@C after six photocatalytic cycles (b).
4. Conclusions
Pr6O11@C with efficient photocatalytic efficiency and acid resistance was prepared via facile construction engineering. As a precipitant, NH3·H2O is more suitable for the preparation of Pr6O11 with enhanced photocatalytic activity than that of NaOH because more amino groups and hydroxyl groups were detected in the sample synthesized using NH3·H2O as a precipitant. Moreover, the absorption intensity of visible light and the ratio of Pr3+ in Pr6O11-NH3·H2O are higher than those of Pr6O11-NaOH. Due to the formation of carbon bonds and oxygen vacancies, Pr6O11@C prepared using NH3·H2O as a precipitant has more efficient photocatalytic activity compared with that of the pure composite. The optimum one is 0.075 mM AR14 for synthesizing Pr6O11@C. and OH• are the main oxidative species responsible for the photodegradation of AR14. The use of AR14 as a carbon source is helpful for the stability of Pr6O11@C in the acid medium. Pr6O11@C had good photocatalytic stability, indicating that it has the potential application for the removal of organic pollutants.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules29153568/s1. Refs. [35,42] are cited in Supplementary Materials.
Author Contributions
Data curation, G.C., L.M., Y.T. and C.M.; Writing—original draft, S.H.; Writing—review & editing, P.A. All authors have read and agreed to the published version of the manuscript.
Funding
The Natural Science Foundation of China (21876158) and Key and general Projects of Jinhua Science and Technology Bureau (2022-1-077, 2022-4-042).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article and Supplementary Materials.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Liang, M.S.; Li, X.; Jiang, L.; Ran, P.; Wang, H.Z.; Chen, X.Z.; Xu, C.Y.; Tian, M.Y.; Wang, S.M.; Zhang, J.T.; et al. Femtosecond laser mediated fabrication of micro/nanostructured TiO2−x photoelectrodes: Hierarchical nanotubes array with oxygen vacancies and their photocatalysis properties. Appl. Catal. B-Environ. 2020, 119, 119231. [Google Scholar] [CrossRef]
- Zou, S.R.; Huang, C.; Liu, Y.; Zhou, J.W.; Zhou, T.F.; Hu, J.C. Emerging charge transfer in self-coupled polymorphs for promoting charge-carrier-involved photocatalysis. Chem. Eng. J. 2020, 396, 125213. [Google Scholar] [CrossRef]
- Camacho-Muñoz, D.; Lawton, L.A.; Edwards, C. Degradation of okadaic acid in seawater by UV/TiO2 photocatalysis—Proof of concept. Sci. Total Environ. 2020, 733, 139346. [Google Scholar] [CrossRef] [PubMed]
- Castellanos, R.M.; Bassin, J.P.; Dezotti, M.; Boaventura, R.A.R.; Vilar, V.J.P. Tube-in-tube membrane reactor for heterogeneous TiO2 photocatalysis with radial addition of H2O2. Chem. Eng. J. 2020, 395, 124998. [Google Scholar] [CrossRef]
- Lin, Y.; Hu, H.Y. Role of ZnO morphology in its reduction and photocatalysis. Appl. Surf. Sci. 2020, 502, 144202. [Google Scholar] [CrossRef]
- Hu, Y.; Gao, X.; Yu, L.; Wang, Y.; Ning, J.; Xu, S.; Lou, X.W. Carbon-coated CdS petalous nanostructures with enhanced photostability and photocatalytic activity. Angew. Chem. Int. Ed. 2013, 52, 5636–5639. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.; Khan, S.; Choi, J.; Dinh, D.T.T.; Lee, S.Y.; Paik, U.; Cho, S.H.; Kim, S. Synergetic control of band gap and structural transformation foroptimizing TiO2 photocatalysts. Appl. Catal. B-Environ. 2017, 210, 513–521. [Google Scholar] [CrossRef]
- Anandan, S.; Ohashi, N.; Miyauchi, M. ZnO-based visible-light photocatalyst: Band-gap engineering and multi-electron reduction by co-catalyst. Appl. Catal. B-Environ. 2010, 100, 502–509. [Google Scholar] [CrossRef]
- Ke, D.N.; Liu, S.L.; Dai, K.; Zhou, J.P.; Zhang, L.N.; Peng, T.Y. CdS/regenerated cellulose nanocomposite films for highly efficient photocatalytic H2 production under visible light irradiation. J. Phys. Chem. C 2009, 113, 16021–16026. [Google Scholar] [CrossRef]
- Tambat, S.; Umale, S.; Sontakke, S. Photocatalytic degradation of milling yellow dye using sol-gel synthesized CeO2. Mater. Res. Bull. 2016, 76, 466–472. [Google Scholar] [CrossRef]
- Ma, R.; Islam, M.J.; Reddy, D.A.; Kim, T.K. Transformation of CeO2 into a mixed phase CeO2/Ce2O3 nanohybrid by liquid phase pulsed laser ablation for enhanced photocatalytic activity through Z-scheme pattern. Ceram. Int. 2016, 42, 18495–18502. [Google Scholar] [CrossRef]
- Du, X.Q.; Zhang, Z.; Chen, H.; Liang, P. Preparation of CeO2 nanorods-reduced graphene oxide hybrid nanostructure with highly enhanced decolorization performance. Appl. Surf. Sci. 2020, 499, 143939. [Google Scholar] [CrossRef]
- Shende, A.G.; Ghugal, S.G.; Vidyasagar, D.; Kokane, S.B.; Jagannath; Umare, S.S.; Sasikala, R. Solvent free solid-state synthesis of Pr6O11/g-C3N4 visible light active photocatalyst for degradation of AV7 dye. Mater. Res. Bull. 2018, 107, 154–163. [Google Scholar] [CrossRef]
- Wang, X.; Yang, C.; Wang, T.M.; Liu, P. Praseodymium oxide/polypyrrole nanocomposites for electrochemical energy storage. Electrochim. Acta 2011, 58, 193–202. [Google Scholar] [CrossRef]
- Karunakaran, C.; Dhanalakshmi, R. Phenol degradationon Pr6O11 surface under UV-A light, synergistic photocatalysis by semiconductors. Radiat. Phys. Chem. 2009, 78, 8–12. [Google Scholar] [CrossRef]
- Zinatloo-Ajabshir, S.; Salavati-Niasari, M. Nanocrystalline Pr6O11: Synthesis, characterization, optical and photocatalytic properties. New J. Chem. 2015, 39, 3948–3955. [Google Scholar] [CrossRef]
- Karunakaran, C.; Dhanalakshmi, R.; Anilkumar, P. Photodegradation of carboxylic acids on Pr6O11 surface. Enhancement by semiconductors. Chem. Eng. J. 2009, 151, 46–50. [Google Scholar] [CrossRef]
- Jayakumar, G.; Irudayaraj, A.A.; Raj, A.D. Investigation on the synthesis and photocatalytic activity of activated carbon-cerium oxide (AC-CeO2) nanocomposite. Appl. Phys. A 2019, 125, 742. [Google Scholar] [CrossRef]
- Sapkota, K.P.; Lee, I.S.; Hanif, M.A.; Islam, M.A.; Akter, J.; Hahn, J.R. Enhanced visible-light photocatalysis of nanocomposites of copper oxide and single-walled carbon nanotubes for the degradation of methylene blue. Catalysts 2020, 10, 297. [Google Scholar] [CrossRef]
- Zhang, D.; Zhang, X.; Sun, Q.; Zheng, S.L.; Hao, J.Y.; Wang, Y. Continuous photocatalysis based on layer-by-layer assembly of separation-free TiO2/reduced graphene oxide film catalysts with increased charge transfer and active site. Eur. J. Inorg. Chem. 2019, 5, 721–729. [Google Scholar] [CrossRef]
- Budnyak, T.M.; Blachnio, M.; Slabon, A.; Jaworski, A.; Tertykh, V.A.; Derylo-Marczewska, A.; Marczewski, A.W. Chitosan deposited onto fumed silica surface as sustainable hybrid biosorbent for acid orange 8 dye capture: Effect of temperature in adsorption equilibrium and kinetics. J. Phys. Chem. C 2020, 124, 15312–15323. [Google Scholar] [CrossRef]
- Zhang, C.Y.; Yu, Y.G.; Wei, H.; Li, K.B. In situ growth of cube-like AgCl on montmorillonite as an efficient photocatalyst for dye (Acid Red 18) degradation. Appl. Surf. Sci. 2018, 456, 577–585. [Google Scholar] [CrossRef]
- Chen, F.H.; Li, S.S.; Chen, Q.T.; Zheng, X.J.; Liu, P.R.; Fang, S.M. 3D graphene aerogels-supported Ag and Ag@Ag3PO4 heterostructure for the efficient adsorption-photocatalysis capture of different dye pollutants in water. Mater. Res. Bull. 2018, 105, 334–341. [Google Scholar] [CrossRef]
- Wang, H.; Zhong, Y.C.; Yu, H.M.; Aprea, P.; Hao, S.Y. High-efficiency adsorption for acid dyes over CeO2 · xH2O synthesized by a facile method. J. Alloys Compd. 2019, 776, 96–104. [Google Scholar]
- Zhang, G.; He, Z.; Xu, W. A low-cost and high efficient zirconium-modified-Na-attapulgite adsorbent for fluoride removal from aqueous solutions. Chem. Eng. J. 2012, 183, 315–324. [Google Scholar] [CrossRef]
- Hao, S.Y.; Hou, J.; Aprea, P.; Deng, H.X. Amino-functionalized ceria with enhanced daylight photocatalytic efficiency. Ceram. Int. 2016, 42, 7440–7446. [Google Scholar] [CrossRef]
- Ren, H.M.; Cai, C.; Leng, C.B.; Pang, S.F.; Zhang, Y.H. Nucleation kinetics in mixed NaNO3/glycerol droplets investigated with the FTIR-ATR technique. J. Phy. Chem. B 2016, 120, 2913–2920. [Google Scholar] [CrossRef]
- Zhu, C.; Cheng, X.; Li, Y.; Tao, B.M. Influence of heat treatment on solidus temperature of NaNO3-KNO3 molten salt. Sol. Energy 2015, 118, 303–312. [Google Scholar]
- Castro, P.M.; Jagodzinski, P.W. FTIR and Raman spectra and structure of Cu(NO3)+ in aqueous solution and acetone. Spectrochim. Acta Part A 1991, 47, 1707–1720. [Google Scholar] [CrossRef]
- Kakavandi, B.; Bahari, N.; Kalantary, R.R.; Fard, E.D. Enhanced sono-photocatalysis of tetracycline antibiotic using TiO2 decorated on magnetic activated carbon (MAC@T) coupled with US and UV: A new hybrid system. Ultrason. Sonochem. 2019, 55, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Guo, Q.; Tang, G.; Peng, W.; He, D. Effects of inorganic ions on the photocatalytic degradation of carbamazepine. J. Water Reuse Desal. 2019, 9, 301–309. [Google Scholar] [CrossRef]
- Zhang, X.; Cheng, S.; Zhang, W.; Zhang, C.; Drewett, N.E.; Wang, X.Y.; Wang, D.; Yoo, S.J.; Kim, J.G.; Zheng, W.T. Mechanistic insight into nanoarchitected Ag/Pr6O11 catalysts for efficient CO oxidation. Ind. Eng. Chem. Res. 2017, 56, 11042–11048. [Google Scholar] [CrossRef]
- Jiang, N.; Zhou, X.; Jiang, Y.F.; Zhao, Z.W.; Ma, L.B.; Shen, C.C.; Liu, Y.N.; Yuan, C.Z.; Sahar, S.; Xu, A.W. Oxygen deficient Pr6O11 nanorod supported palladium nanoparticles: Highly active nanocatalysts for styrene and 4-nitrophenol hydrogenation reactions. RSC Adv. 2018, 8, 17504–17510. [Google Scholar] [CrossRef]
- Gregson, M.; Lu, E.; McMaster, J.; Lewis, W.; Blake, A.J.; Liddle, S.T. A Cerium(IV)-carbon multiple bond. Angew. Chem. Int. Ed. 2013, 52, 13016–13019. [Google Scholar] [CrossRef]
- Wang, H.; Shang, J.; Xiao, Z.L.; Aprea, P.; Hao, S.Y. Novel construction of carbon bonds in CeO2@C with efficiently photocatalytic activity. Dyes Pigments 2020, 182, 108669. [Google Scholar] [CrossRef]
- Hao, S.Y.; Hou, J.; Aprea, P.; Pepe, F. Mesoporous Ce-Pr-O solid solution with efficient photocatalytic activity under weak daylight irradiation. Appl. Catal B-Environ. 2014, 160–161, 566–573. [Google Scholar] [CrossRef]
- Fan, L.J.; Xi, K.; Zhou, Y.; Zhu, Q.L.; Chen, Y.F.; Lu, H.F. Design structure for CePr mixed oxide catalysts in soot combustion. RSC Adv. 2017, 7, 20309–20319. [Google Scholar] [CrossRef]
- Yu, L.; Li, G.; Zhang, X.; Ba, X.; Shi, G.; Li, Y.; Wong, P.K.; Yu, J.C.; Yu, Y. Enhanced activity and stability of carbon-decorated cuprous oxide mesoporous nanorods for CO2 reduction in artificial photosynthesis. ACS Catal. 2016, 6, 6444–6454. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, X.; Zhang, W.; Ge, X.; Wang, Y.; Zou, X.; Zhou, X.; Zheng, W. MXene-based quantum dots optimize hydrogen production via spontaneous evolution of Cl- to O- terminated surface groups. Energy Environ. Mater. 2022, 6, e12438. [Google Scholar] [CrossRef]
- Westermann, A.; Geantet, C.; Vernoux, P.; Rolidant, S. Defects band enhanced by resonance Raman effect in praseodymium doped CeO2. J. Raman Spectrosc. 2016, 47, 1276–1279. [Google Scholar] [CrossRef]
- Teng, C.C.; Ma, C.C.M.; Lu, C.H.; Yang, S.Y.; Lee, S.H.; Hsiao, M.C.; Yen, M.Y.; Chiou, K.C.; Lee, T.M. Thermal conductivity and structure of non-covalent functionalized graphene/epoxy composites. Carbon 2011, 49, 5107–5116. [Google Scholar] [CrossRef]
- Wang, X.T.; Zhou, J.Q.; Zhao, S.; Chen, X.; Yu, Y. Synergistic effect of adsorption and visible-light photocatalysis for organic pollutant removal over BiVO4/carbon sphere nanocomposites. Appl. Surf. Sci. 2018, 453, 394–404. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).