Self-Assembled Carbon Metal–Organic Framework Oxides Derived from Two Calcination Temperatures as Anode Material for Lithium-Ion Batteries
Abstract
1. Introduction
2. Results and Discussion
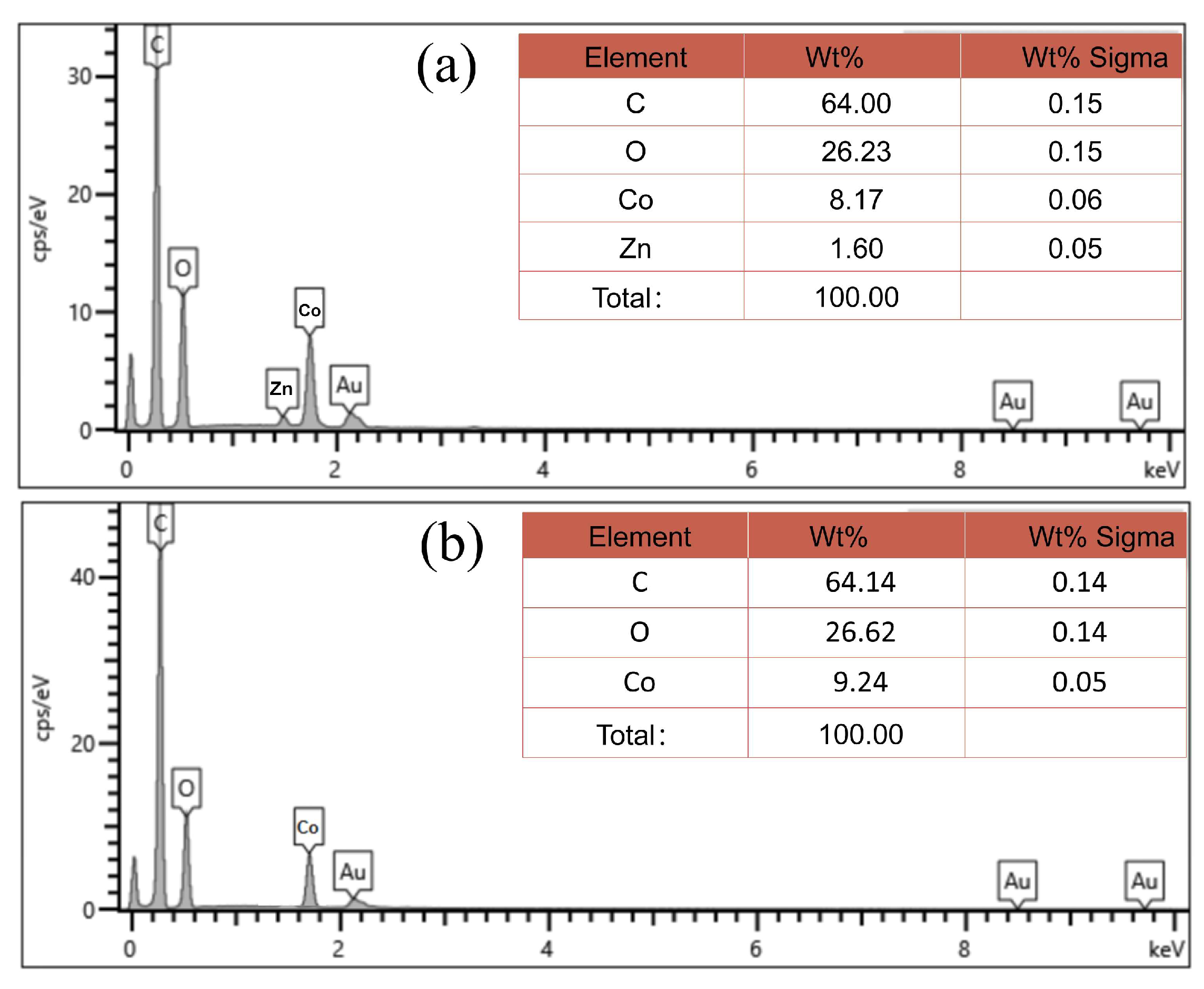
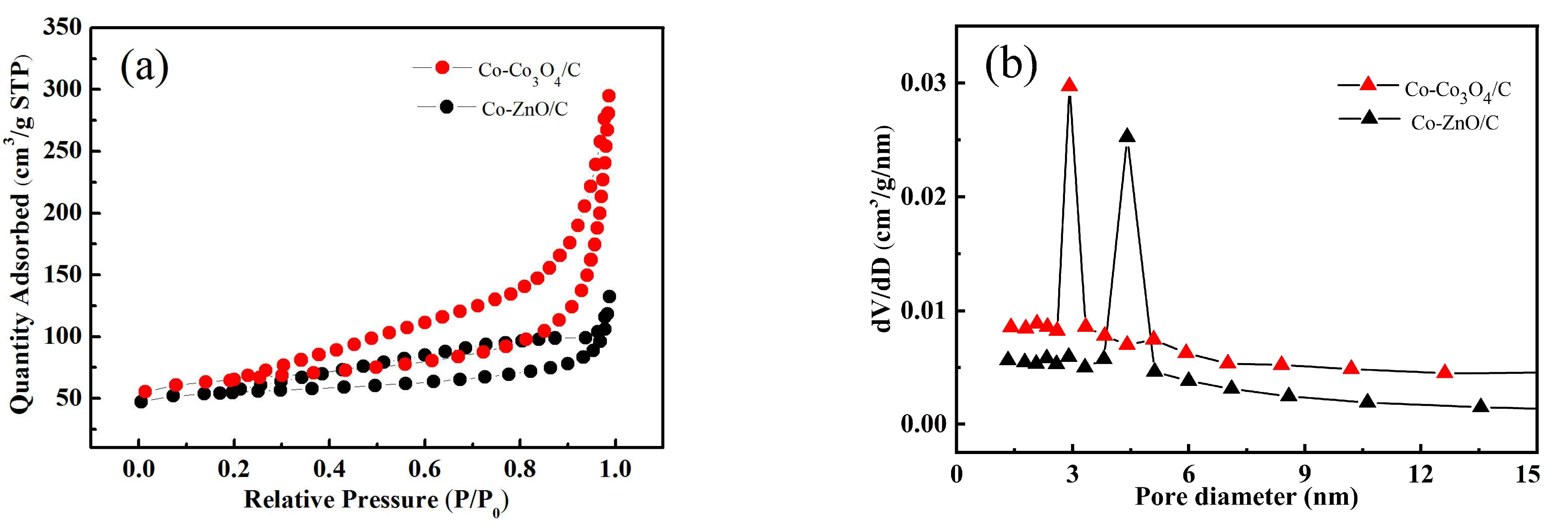
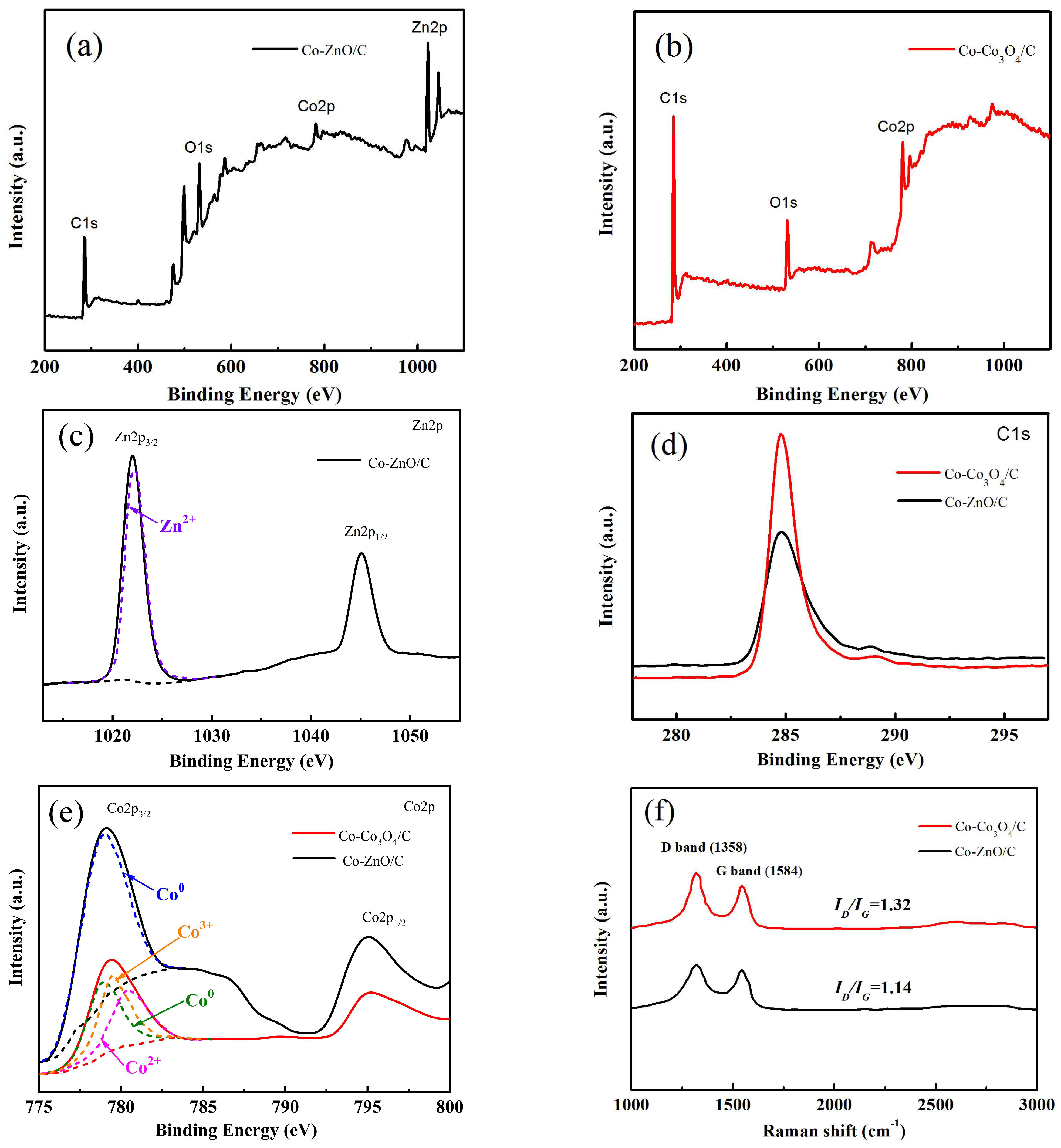
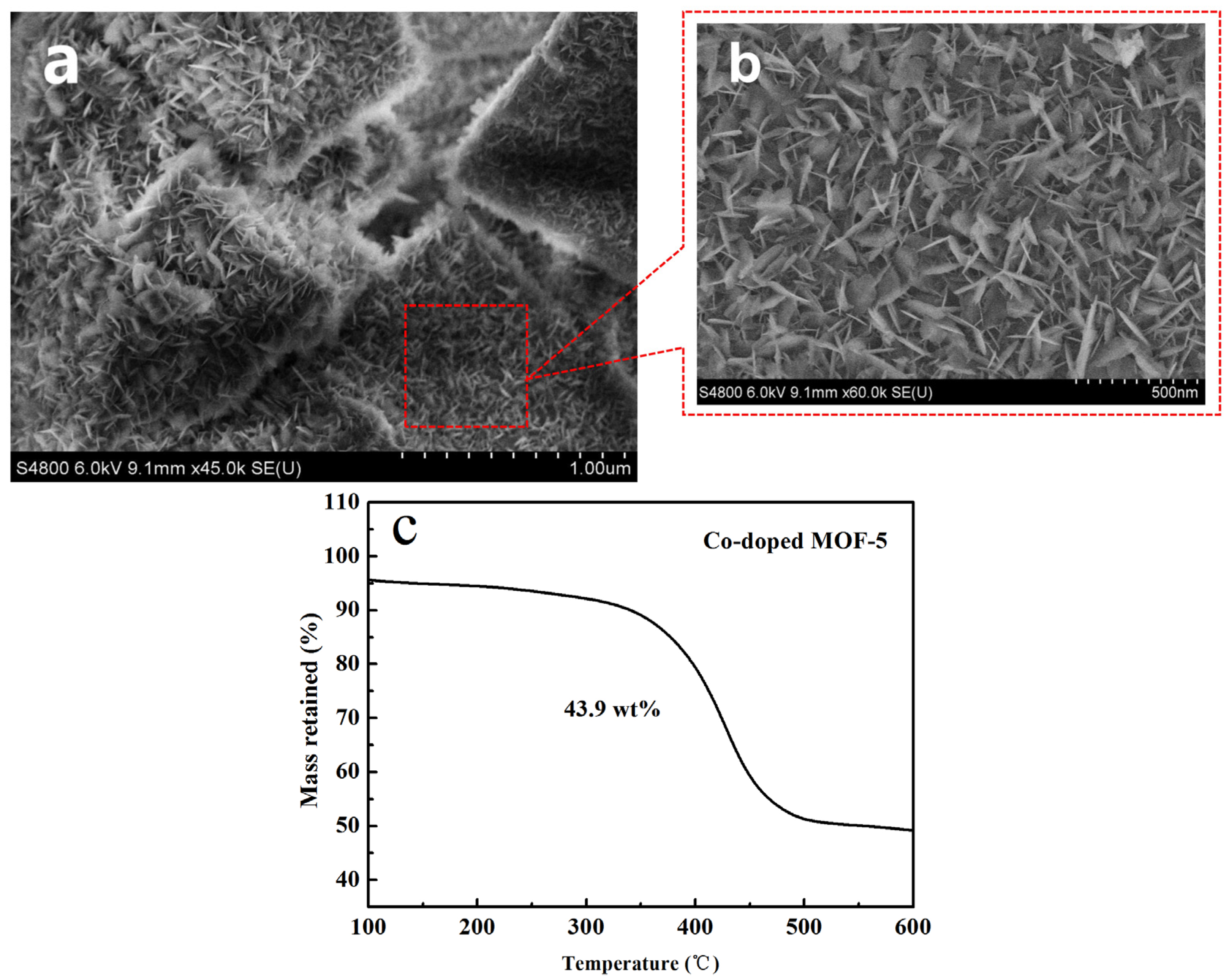
2.1. Structure and Morphology Characterization
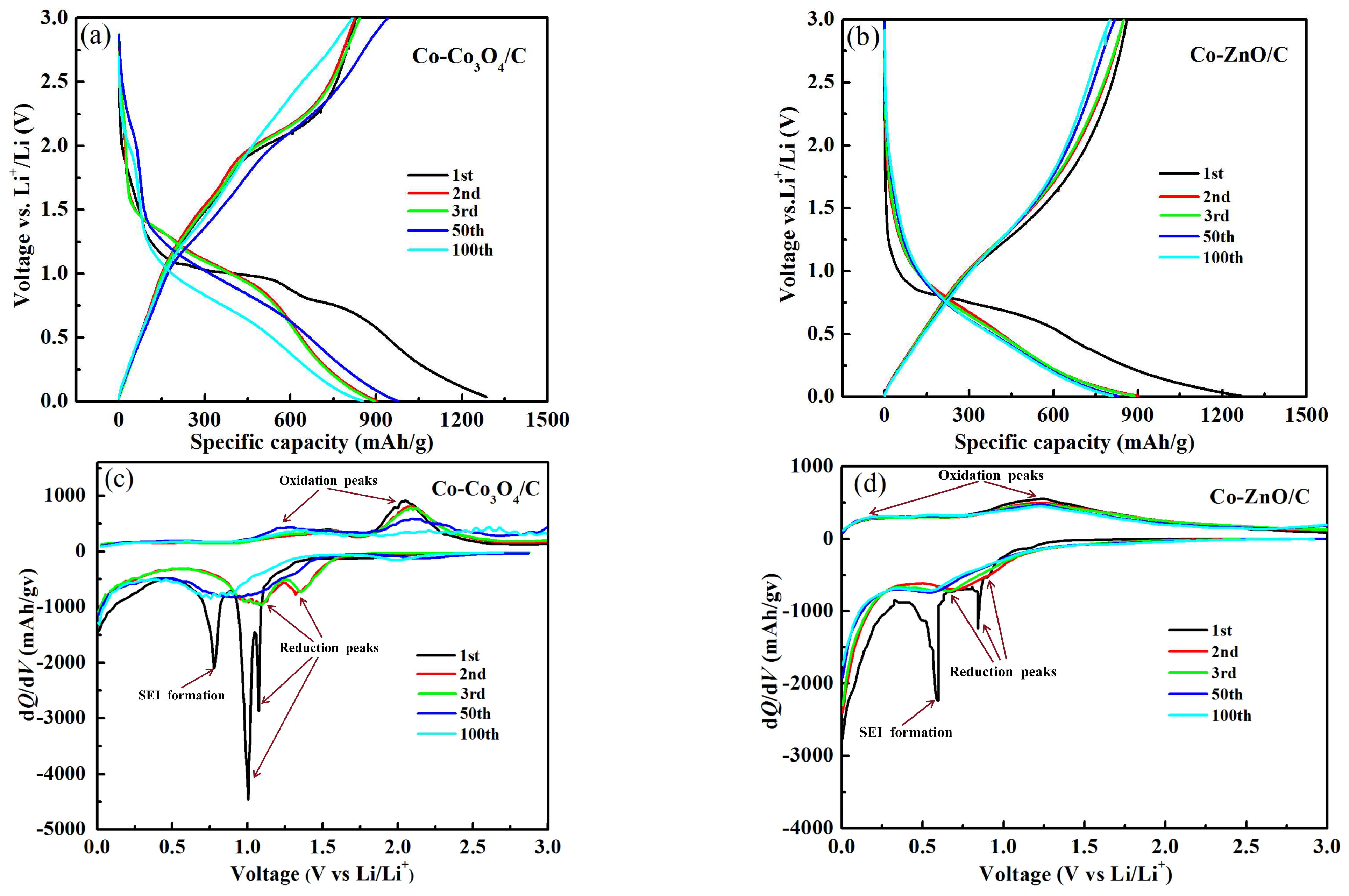
2.2. Electrochemical Analysis
3. Materials and Methods
3.1. Material Preparation
3.2. Material Characterization
3.3. Electrochemical Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sun, H.; Geng, Y.; Hu, L.; Shi, L.; Xu, T. Measuring China’s new energy vehicle patents: A social network analysis approach. Energy 2018, 153, 685–693. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, Y.; Lee, Y. Performance enhancement of spherical natural graphite by phenol resin in lithium ion batteries. J. Alloys Compd. 2006, 426, 218–222. [Google Scholar] [CrossRef]
- Cui, L.; Yang, Y.; Hsu, C.; Cui, Y. Carbon-silicon core-shell nanowires as high capacity electrode for lithium ion batteries. Nano Lett. 2009, 9, 3370–3374. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Yang, Z.; Cheng, J.; Zhong, X.; Gu, L.; Yu, Y. Germanium nanoparticles encapsulated in flexible carbon nanofibers as self-supported electrodes for high performance lithium-ion batteries. Nanoscale 2014, 6, 4532–4537. [Google Scholar] [CrossRef] [PubMed]
- Bodnarchuk, M.I.; Kravchyk, K.V.; Krumeich, F.; Wang, S.; Kovalenko, M.V. Colloidal tin-germanium nanorods and their Li-ion storage properties. ACS Nano 2014, 8, 2360–2368. [Google Scholar] [CrossRef] [PubMed]
- Shrivastav, V.; Sundriyal, S.; Goel, P.; Kaur, H.; Tuteja, S.K.; Vikrant, K.; Kim, K.; Tiwari, U.K.; Deep, A. Metal-organic frameworks (MOFs) and their composites as electrodes for lithium battery applications: Novel means for alternative energy storage. Coordin. Chem. Rev. 2019, 393, 48–78. [Google Scholar] [CrossRef]
- Xu, X.; Cao, R.; Jeong, S.; Cho, J. Spindle-like mesoporous α-Fe2O3 anode material prepared from MOF template for high-rate lithium batteries. Nano Lett. 2012, 12, 4988–4991. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.J.; Nam, S.; Kim, T.; Im, J.H.; Jung, H.; Kang, J.H.; Wi, S.; Park, B.; Park, C.R. Preparation and exceptional lithium anodic performance of porous carbon-coated ZnO quantum dots derived from a metal–organic framework. J. Am. Chem. Soc. 2013, 135, 7394–7397. [Google Scholar] [CrossRef]
- Zhang, G.; Hou, S.; Zhang, H.; Zeng, W.; Yan, F.; Li, C.; Duan, H. High-performance and ultra-stable lithium-ion batteries based on MOF-derived ZnO@ZnO quantum dots/C core-shell nanorod arrays on a carbon cloth anode. Adv. Mater. 2015, 27, 2400–2405. [Google Scholar] [CrossRef]
- Zou, F.; Hu, X.; Li, Z.; Qie, L.; Hu, C.; Zeng, R.; Jiang, Y.; Huang, Y. MOF-derived porous ZnO/ZnFe2O4/C octahedra with hollow interiors for high-rate lithium-ion batteries. Adv. Mater. 2014, 26, 6622–6628. [Google Scholar] [CrossRef]
- Wang, P.; Lang, J.; Liu, D.; Yan, X. TiO2 embedded in carbon submicron-tablets: Synthesis from a metal–organic framework precursor and application as a superior anode in lithium-ion batteries. Chem. Commun. 2015, 51, 11370–11373. [Google Scholar] [CrossRef]
- Shao, J.; Wan, Z.; Liu, H.; Zheng, H.; Gao, T.; Shen, M.; Qu, Q.; Zheng, H. Metal organic frameworks-derived Co3O4 hollow dodecahedrons with controllable interiors as outstanding anodes for Li storage. J. Mater. Chem. A 2014, 2, 12194–12200. [Google Scholar] [CrossRef]
- Wu, R.; Wang, D.P.; Rui, X.; Liu, B.; Zhou, K.; Law AW, K.; Yan, Q.; Wei, J.; Chen, Z. In-situ formation of hollow hybrids composed of cobalt sulfides embedded within porous carbon polyhedra/carbon nanotubes for high-performance lithium-ion batteries. Adv. Mater. 2015, 27, 3038–3044. [Google Scholar] [CrossRef]
- Yu, X.Y.; Yu, L.; Wu, H.B.; Lou, X.W. Formation of nickel sulfide nanoframes from metal-organic frameworks with enhanced pseudocapacitive and electrocatalytic properties. Angew. Chem. Int. Edit. 2015, 54, 5331–5335. [Google Scholar] [CrossRef]
- Liu, B.; Zhang, X.; Shioyama, H.; Mukai, T.; Sakai, T.; Xu, Q. Converting cobalt oxide subunits in cobalt metal-organic framework into agglomerated Co3O4 nanoparticles as an electrode material for lithium ion battery. J. Power Sources 2010, 195, 857–861. [Google Scholar] [CrossRef]
- Yang, S.J.; Kim, T.; Im, J.H.; Kim, Y.S.; Lee, K.; Jung, H.; Park, C.R. MOF-derived hierarchically porous carbon with exceptional porosity and hydrogen storage capacity. Chem. Mater. 2011, 24, 464–470. [Google Scholar] [CrossRef]
- Li, X. A modeling study of the pore size evolution in lithium-oxygen battery electrodes. J. Electrochem. Soc. 2015, 162, A1636–A1645. [Google Scholar] [CrossRef]
- Benramache, S.; Benhaoua, B.; Bentrah, H. Preparation of transparent, conductive ZnO:Co and ZnO:In thin films by ultrasonic spray method. J. Nanostruct. Chem. 2013, 1, 54. [Google Scholar] [CrossRef]
- Zhong, S.; Zhan, C.; Cao, D. Zeolitic imidazolate framework-derived nitrogen-doped porous carbons as high performance supercapacitor electrode materials. Carbon 2015, 85, 51–59. [Google Scholar] [CrossRef]
- Zou, F.; Chen, Y.; Liu, K.; Yu, Z.; Liang, W.; Bhaway, S.M.; Gao, M.; Zhu, Y. Metal organic frameworks derived hierarchical hollow NiO/Ni/graphene composites for lithium and sodium storage. ACS Nano 2015, 10, 377–386. [Google Scholar] [CrossRef]
- Lyu, S.; Wang, L.; Zhang, J.; Liu, C.; Sun, J.; Peng, B.; Wang, Y.; Rappé, K.G.; Zhang, Y.; Li, J.; et al. Role of active phase in fischer-tropsch synthesis: Experimental evidence of CO activation over single-phase cobalt catalysts. ACS Catal. 2018, 8, 7787–7798. [Google Scholar] [CrossRef]
- Winiarski, J.; Tylus, W.; Winiarska, K.; Szczygieł, I.; Szczygieł, B. XPS and FT-IR characterization of selected synthetic corrosion products of zinc expected in neutral environment containing chloride ions. J. Spectrosc. 2018, 2018, 1–14. [Google Scholar] [CrossRef]
- Hou, L.; Lian, L.; Zhang, L.; Pang, G.; Yuan, C.; Zhang, X. Self-sacrifice template fabrication of hierarchical mesoporous bi-component-active ZnO/ZnFe2O4 sub-microcubes as superior anode towards high-performance lithium-ion battery. Adv. Funct. Mater. 2015, 25, 238–246. [Google Scholar] [CrossRef]
- Deka, A.; Nanda, K.K. A comparison of ZnO films deposited on indium tin oxide and soda lime glass under identical conditions. AIP Adv. 2013, 3, 62104. [Google Scholar] [CrossRef]
- Wei, D.; Liu, Y.; Wang, Y.; Zhang, H.; Huang, L.; Yu, G. Synthesis of N-doped graphene by chemical vapor deposition and its electrical properties. Nano Lett. 2009, 9, 1752–1758. [Google Scholar] [CrossRef]
- Huang, J.; Wang, L.; Gu, C.; Zhai, M.; Liu, J. Preparation of hollow porous Co-doped SnO2 microcubes and their enhanced gas sensing property. CrystEngComm 2013, 15, 7515–7521. [Google Scholar] [CrossRef]
- Li, J.; Xiong, S.; Li, X.; Qian, Y. Spinel Mn1.5Co1.5O4 core-shell microspheres as Li-ion battery anode materials with a long cycle life and high capacity. J. Mater. Chem. 2012, 22, 23254–23259. [Google Scholar] [CrossRef]
- Zhou, K.; Lai, L.; Zhen, Y.; Hong, Z.; Guo, J.; Huang, Z. Rational design of Co3O4/Co/carbon nanocages composites from metal organic frameworks as an advanced lithium-ion battery anode. Chem. Eng. J. 2017, 316, 137–145. [Google Scholar] [CrossRef]
- Lukashuk, L.; Yigit, N.; Rameshan, R.; Kolar, E.; Teschner, D.; Hävecker, M.; Knop-Gericke, A.; Schlögl, R.; Föttinger, K.; Rupprechter, G. Operando insights into CO oxidation on cobalt oxide catalysts by NAP-XPS, FTIR, and XRD. ACS Catal. 2018, 8, 8630–8641. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, S.; Zang, Y.; Liu, R.; Liu, G.; Wang, G.; Zhang, Y.; Zhang, H.; Zhao, H. Co/Co9S8@S,N-doped porous graphene sheets derived from S, N dual organic ligands assembled Co-MOFs as superior electrocatalysts for full water splitting in alkaline media. Nano Energy 2016, 30, 93–102. [Google Scholar] [CrossRef]
- Boningari, T.; Pappas, D.K.; Smirniotis, P.G. Metal oxide-confined interweaved titania nanotubes M/TNT (M=Mn, Cu, Ce, Fe, V, Cr, and Co) for the selective catalytic reduction of NOx in the presence of excess oxygen. J. Catal. 2018, 365, 320–333. [Google Scholar] [CrossRef]
- Kong, B.; Zu, L.; Peng, C.; Zhang, Y.; Zhang, W.; Tang, J.; Selomulya, C.; Zhang, L.; Chen, H.; Wang, Y.; et al. Direct superassemblies of freestanding metal-carbon frameworks featuring reversible crystalline-phase transformation for electrochemical sodium storage. J. Am. Chem. Soc. 2016, 138, 16533–16541. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Wang, L.; Wang, A.; Zhang, B.; Pham, H.; Park, J.; He, X. Insight into uniform filming of LiF-rich interphase via synergistic adsorption for high-performance lithium metal anode. Exploration 2024, 4, 20230114. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Dong, W.; Riaz, M.S.; Ge, H.; Wang, X.; Liu, Z.; Huang, F. “Electron-sharing” mechanism promotes Co@Co3O4/CNTs composite as the high-capacity anode material of lithium-ion battery. ACS Appl. Mater. Inter. 2018, 10, 43641–43649. [Google Scholar] [CrossRef] [PubMed]
- Ge, X.; Li, Z.; Wang, C.; Yin, L. Metal–organic frameworks derived porous core/shell structured ZnO/ZnCo2O4/C hybrids as anodes for high-performance lithium-ion battery. ACS Appl. Mater. Inter. 2015, 7, 26633–26642. [Google Scholar] [CrossRef] [PubMed]
- Qiao, L.; Wang, X.; Qiao, L.; Sun, X.; Li, X.; Zheng, Y.; He, D. Single electrospun porous NiO-ZnO hybrid nanofibers as anode materials for advanced lithium-ion batteries. Nanoscale 2013, 5, 3037–3042. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.; Chen, B.; Qin, Y.; Yang, S.; Li, C.; Zuo, Y.; Liu, S.; Yang, J. Facile ultrasonic synthesis of CoO quantum dot/graphene nanosheet composites with high lithium storage capacity. ACS Nano 2012, 6, 1074–1081. [Google Scholar] [CrossRef]
- Wu, R.; Qian, X.; Rui, X.; Liu, H.; Yadian, B.; Zhou, K.; Wei, J.; Yan, Q.; Feng, X.; Long, Y.; et al. Zeolitic imidazolate framework 67-derived high symmetric porous Co3O4 hollow dodecahedra with highly enhanced lithium storage capability. Small 2014, 10, 1932–1938. [Google Scholar] [CrossRef]
- Xu, W.; Cui, X.; Xie, Z.; Dietrich, G.; Wang, Y. Integrated Co3O4/TiO2 composite hollow polyhedrons prepared via cation-exchange metal–organic framework for superior lithium-ion batteries. Electrochim. Acta 2016, 222, 1021–1028. [Google Scholar] [CrossRef]
- Cheng, H.; Xu, G.; Zhu, C.; Alhalili, Z.; Du, X.; Gao, G. Porous MOF derived TiO2/ZnO/C@ CNTs composites for enhancing lithium storage performance. Chem. Eng. J. 2023, 454, 140454. [Google Scholar] [CrossRef]
- Zhang, J.; Wan, J.; Wang, J.; Ren, H.; Yu, R.; Gu, L.; Liu, Y.; Feng, S.; Wang, D. Hollow multi-shelled structure with metal–organic–framework-derived coatings for enhanced lithium storage. Angew. Chem. 2019, 16, 5230–5235. [Google Scholar]
- Chen, Y.; Wang, Y.; Yang, H.; Hui, G.; Cai, X.; Guo, X.; Xu, B.; Lü, M.; Yuan, A. Facile synthesis of porous hollow Co3O4 microfibers derived-from metal–organic frameworks as an advanced anode for lithium ion batteries. Ceram. Int. 2017, 13, 9945–9950. [Google Scholar] [CrossRef]
- Huang, G.; Zhang, F.; Zhang, L.; Du, X.; Wang, J.; Wang, L. Hierarchical NiFe2O4/Fe2O3 nanotubes derived from metal organic frameworks for superior lithium ion battery anodes. J. Mater. Chem. A 2014, 21, 8048–8053. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, P.; Wang, B.; Wu, J.; Ning, S.; Xie, A.; Shen, Y. Hollow porous CuO/C nanorods as a high-performance anode for lithium ion batteries. J. Alloys Compd. 2018, 750, 77–84. [Google Scholar] [CrossRef]
- Zhao, X.; Xu, H.; Hui, Z.; Sun, Y.; Yu, C.; Xue, J.; Zhou, R.; Wang, L.; Dai, H.; Zhao, Y.; et al. Electrostatically assembling 2D nanosheets of MXene and MOF-derivatives into 3D hollow frameworks for enhanced lithium storage. Small 2019, 47, 1904255. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y.; Li, M.; Hu, X. Self-Assembled Carbon Metal–Organic Framework Oxides Derived from Two Calcination Temperatures as Anode Material for Lithium-Ion Batteries. Molecules 2024, 29, 3566. https://doi.org/10.3390/molecules29153566
Yang Y, Li M, Hu X. Self-Assembled Carbon Metal–Organic Framework Oxides Derived from Two Calcination Temperatures as Anode Material for Lithium-Ion Batteries. Molecules. 2024; 29(15):3566. https://doi.org/10.3390/molecules29153566
Chicago/Turabian StyleYang, Yang, Min Li, and Xiaoqin Hu. 2024. "Self-Assembled Carbon Metal–Organic Framework Oxides Derived from Two Calcination Temperatures as Anode Material for Lithium-Ion Batteries" Molecules 29, no. 15: 3566. https://doi.org/10.3390/molecules29153566
APA StyleYang, Y., Li, M., & Hu, X. (2024). Self-Assembled Carbon Metal–Organic Framework Oxides Derived from Two Calcination Temperatures as Anode Material for Lithium-Ion Batteries. Molecules, 29(15), 3566. https://doi.org/10.3390/molecules29153566






