Abstract
The results of this study showed that the compounds synthesized by the authors have significant potential due to their antibacterial and cytotoxic properties. The apparent antibacterial activity demonstrated by the compounds suggests that they are active antimicrobial agents against common microbial pathogens that cause various socially significant infectious diseases. Compound 6 showed pronounced antimicrobial activity against the Gram-positive test strain Staphylococcus aureus ATCC 6538, and compound 7 demonstrated pronounced antimicrobial activity against the Gram-negative test strain Escherichia coli ATCC 25922 (MIC = 6.3 µg/mL). This allowed us to consider these compounds to have great potential.
1. Introduction
Terpenoids are a class of compounds that make up a large group of secondary metabolites, which are the most important and structurally diverse phytochemicals. These compounds are synthesized by a wide variety of plants, fungi and bacteria [1,2,3,4]. Terpenoid metabolites are responsible for many important functions in plants. They are used by plants to perform various basic growth and development functions, but most terpenoids are used for specialized chemical interactions and protection in abiotic and biotic environments. On the other hand, in other organisms, such as bacteria and fungi, terpenoids perform functions that include electron transfer, the formation of cell walls and membranes, chemical protection from predators and the establishment of symbiotic relationships [5].
It is worth mentioning the most common advanced methods of green chemistry used to extract biologically active compounds from natural sources. In recent years, recovery methods involving plant extracts instead of chemical ones have been widely used [6,7,8,9]. Additionally, microbial production of terpenoids has become an alternative source of these compounds in a process that exploits cheap raw materials obtained from biomass [10].
Terpenoids are of high value in the pharmaceutical, perfumery and food industries due to their biological and pharmacological properties, and they are also used as biofuels [11,12,13,14,15].
Triterpenoids and their derivatives belonging to this class are the most widespread group in nature. These compounds demonstrate a wide range of biological activities, such as anti-inflammatory [16,17], antiviral [18,19], antibacterial [20,21], antituberculosis [22], antioxidant, antifungal, physical [23] and inhibitory activities [24,25]. Recent studies have shown triterpenoids to be promising agents in the treatment and inhibition of breast cancer through the introduction of several molecular mechanisms of action on breast cancer cells [26].
The authors of [27] studied the anticancer properties of triterpenoids and found that 2,3,22,23-tetrahydroxy-2,6,10,15,19,23-hexamethyl-6,10,14,18-tetracosatrene had selective biological activity in leukemia and breast cancer cells. It was established that some new triterpenoids with fragments of pyrazole, isoxazole and piran-4-on substituted in the molecules had the most pronounced cytotoxic activity against cancer cell lines (IC50 = 4.31–15.6; 8.33 µm) [28].
The pharmacological activities of many betulonic acid derivatives belonging to the class of pentacyclic triterpenoids of the lupane type were determined. Thus, the antitumor activity of new betulonic acid derivatives with O- and N-propyl was studied in relation to a group of 60 human cancer cells. The compounds showed antitumor activity against most of these cells. They were more active than doxorubicin against HCT-15 colon cancer cells and ovarian cancer CA/ADDRESS [29].
The introduction of secondary amines into betulonic acid amides led to the production of derivatives with pronounced antispasmodic activity not characteristic of the triterpene skeleton [30]. C-28 imidazolides containing fragments of 3-oxo-, 3-hydroxymino- and 2-cyano-2,3-seco-4(23)-en in the lupane A ring demonstrated antitumor activity in in vitro experiments studying their antitumor activity, significantly suppressing or inhibiting the growth of lung, colon and breast cancer cells and central nervous system diseases that caused death from ovarian, prostate and kidney cancer, as well as leukemia and melanoma [31].
Cytotoxic studies of nine different human tumor cells showed that N-methylpiperazinyl amide of betulonic acid inhibited the growth of leukemia cells (SR), non-small cell lung cancer (NCI-h460) and colon cancer (hct-116) [32].
In addition, it was shown that new betulonic acid amides with piperazine derivatives had different antibacterial activities. It was also found that betulonic acid amides with a hydroxyl radical had an anticholestatic effect on mice, i.e., when piperidine nitroxide was introduced into the lupane nucleus, its hepatoprotective activity increased [33,34].
Continuing research on the modification of triterpenoids in order to study the biological activity, we synthesized new derivatives of betulonic acid.
2. Results and Discussion
2.1. Chemistry
Betulonic acid is a lupane-type triterpenic acid with a wide range of biological activities. The presence of reaction centers, such as hydroxy, keto, carboxyl and double bonds, in the composition determines the possibilities of chemical modification of molecules of this triterpenoid. Its chemical modification can lead to the formation of compounds whose activity exceeds that of the natural precursor. For example, the introduction of a triazole fragment into the composition of a molecule led to derivatives with higher biological activity [35,36,37,38,39,40]. Compounds with a wide range of biological activities synthesized on the basis of betulonic acid are also known [41,42,43,44,45].
In order to expand a number of biologically active compounds while continuing work on the modification of triterpenoids, reactions based on betulonic acid were carried out.
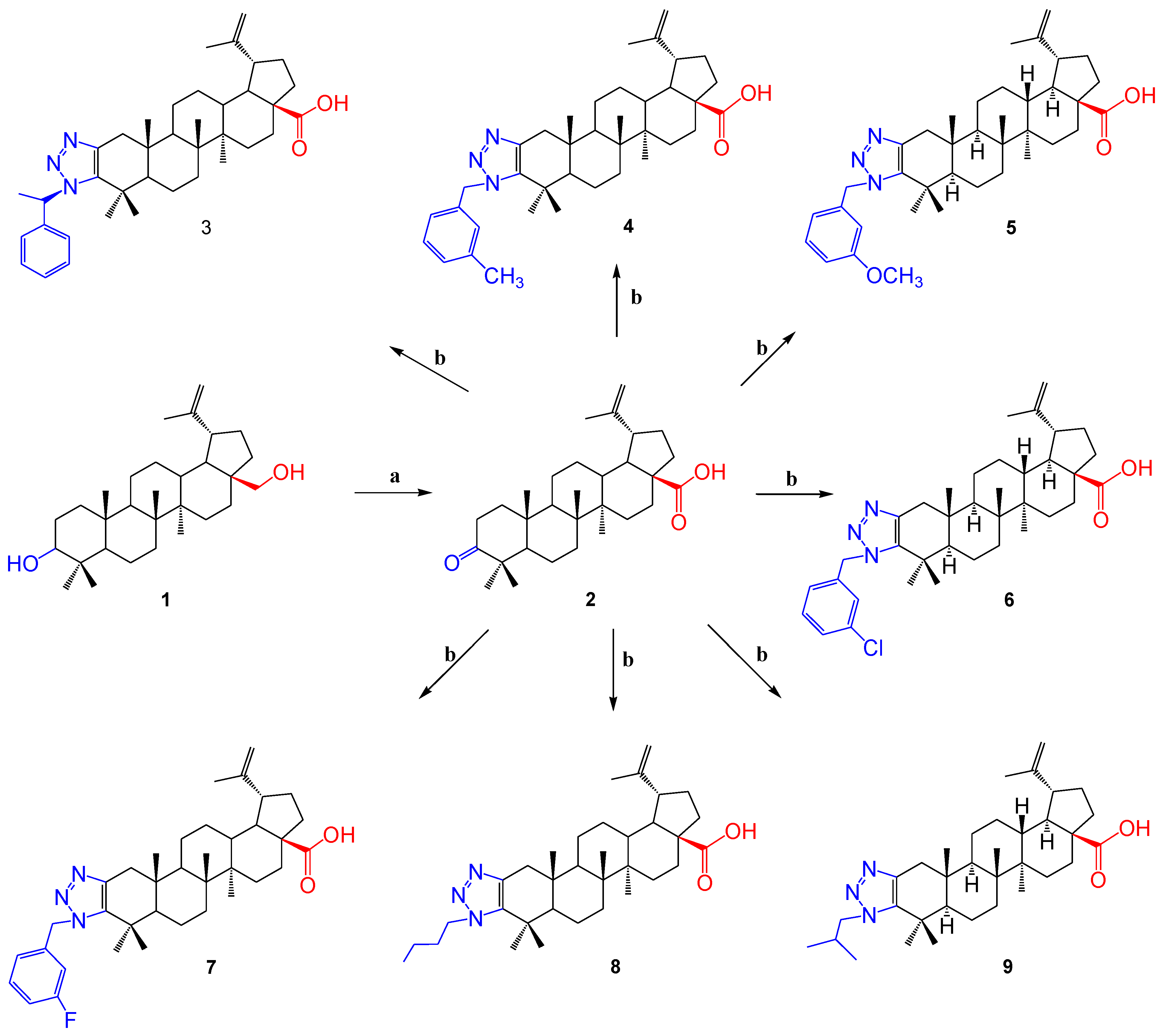
The first series of derivatives was synthesized in accordance with the sequence of reactions shown in Scheme 1.
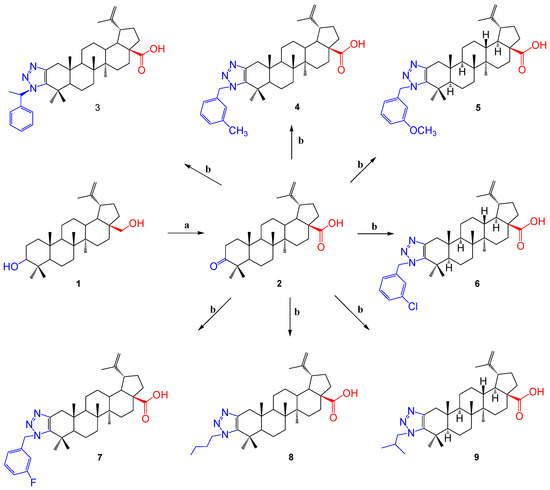
Scheme 1.
Reagents and conditions: (a) Jones reagent, acetone, RT; (b) primary amine, 4-phenylnitroazide, toluene, 24 h, 100 °C.
For triazolization, primary aromatic (containing chloro, fluoro, methyl and methoxy groups in the aromatic ring), linear (butylamine) and branched (isopropyl) amines were taken. The column chromatography method was used to isolate the formed products. The yield of the obtained products 3–9 was 60–68%.
The yield was calculated for the substance obtained after column chromatography. Each stage in the separation of the resulting product was carefully carried out, and no loss of product occurred. It was observed that a relatively high-yield product was formed by the interaction of betulonic acid 2 with 3-methylbenzylamine (68%). Compared with other products (chloro, fluoro, butyl and propyl), there were no sharp differences in yields, with the exception of methyoxy-group-containing compound 3 and methylphenylamine-containing product 5.
In previous work [46], the mechanisms of the triazole formation reaction were shown, indicating two alternative discoveries of the triazoline intermediate cycle. The role of 4-nitrophenylazide in this reaction is explained by the fact that it formally acts as a diazotransfer agent, with the formation of 4-nitroaniline as a by-product.
Spectroscopic (1H and 13C NMR) and mass spectrometric methods were used to establish the obtained compounds. In the 1H NMR spectra of the synthesized betulonic acid triazole derivatives 3–7, the signals of the triazole residue were shown in the area δ 7.33–5.61 p.m., and in the spectrum of the 8 and 9 compounds it was observed that additional signals were formed in comparison with the initial molecule; the signals were clearly shown in the area δ 2.91, 4.29 p.m. for 8 and δ 2.92, 4.09 p.m. for 9. Loss of the characteristic signal of the carbonyl group at the C-3 position of the initial molecule in the 13C NMR spectra of the resulting compounds provided evidence of a triazolation reaction. The molecular ion peak [M + H]+ was present in the mass spectra of the compounds.
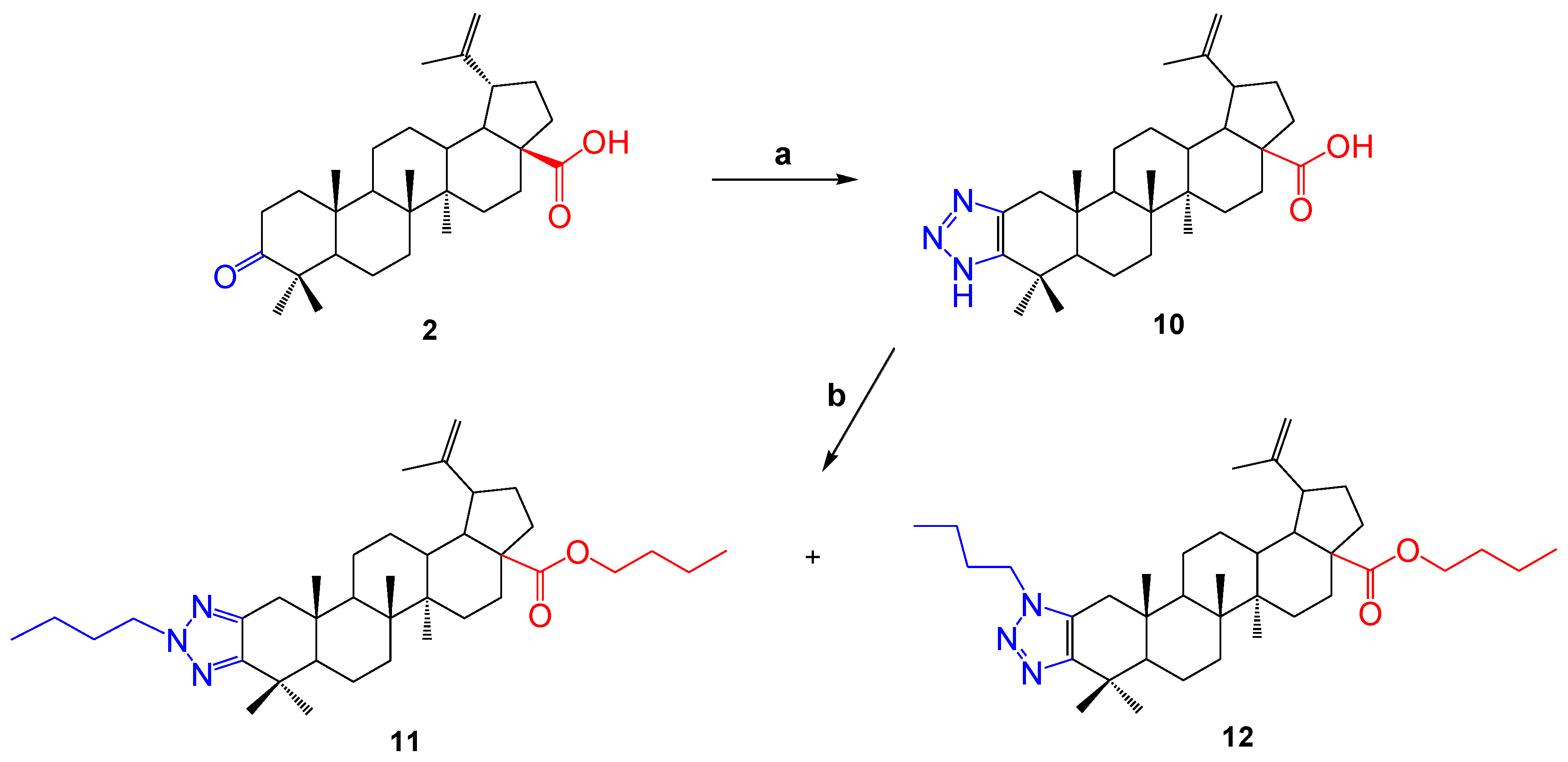
In order to continue obtaining new derivatives of betulonic acid, the triazolation reaction was carried out in the presence of ammonium acetate, as a result of which compound 10 was obtained. Then, the compound was dissolved in methanol and brombutane and potassium tret-butoxide (tButOK) were added to it. As a result, a mixture of two substances (11 and 12) was formed. In this case, it was observed that a carboxyl-group proton exchange situation occurred. The yield of the formed compounds was 46 and 20%, respectively.
The compounds 11 and 12 were separated using column chromatography. The structure of the synthesized compounds was determined by NMR spectroscopy and mass spectrometry. By analyzing the spectral data and taking into account the molecular ionic peak in the mass spectra of the compounds, it was proved that the molecules had the structures shown in Scheme 2. In the 1H NMR spectrum of compound 11, the signals of the protons of the exomethylene group at the C-20 position were present in the area δ 4.76 and 4.63 p.m., and in compound 12 they were present in the area δ 4.75 and 4.61 p.m. The presence of a butyl residue at C-28 was evidenced by the shift of the carbonyl group signal in the 13C NMR spectrum to a strong field compared to molecule 10.
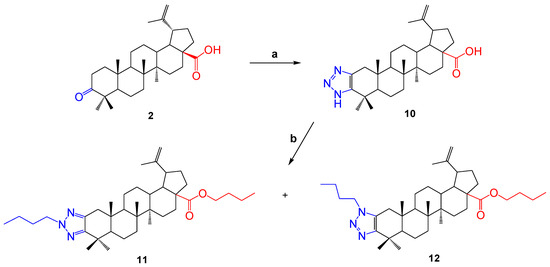
Scheme 2.
Reagents and conditions: (a) ammonium acetate, 4-nitrophenyl azide, DMF, 80 °C, 24 h; (b) tButOK, 1-bromobutane, methanol, 60 °C, 12 h.
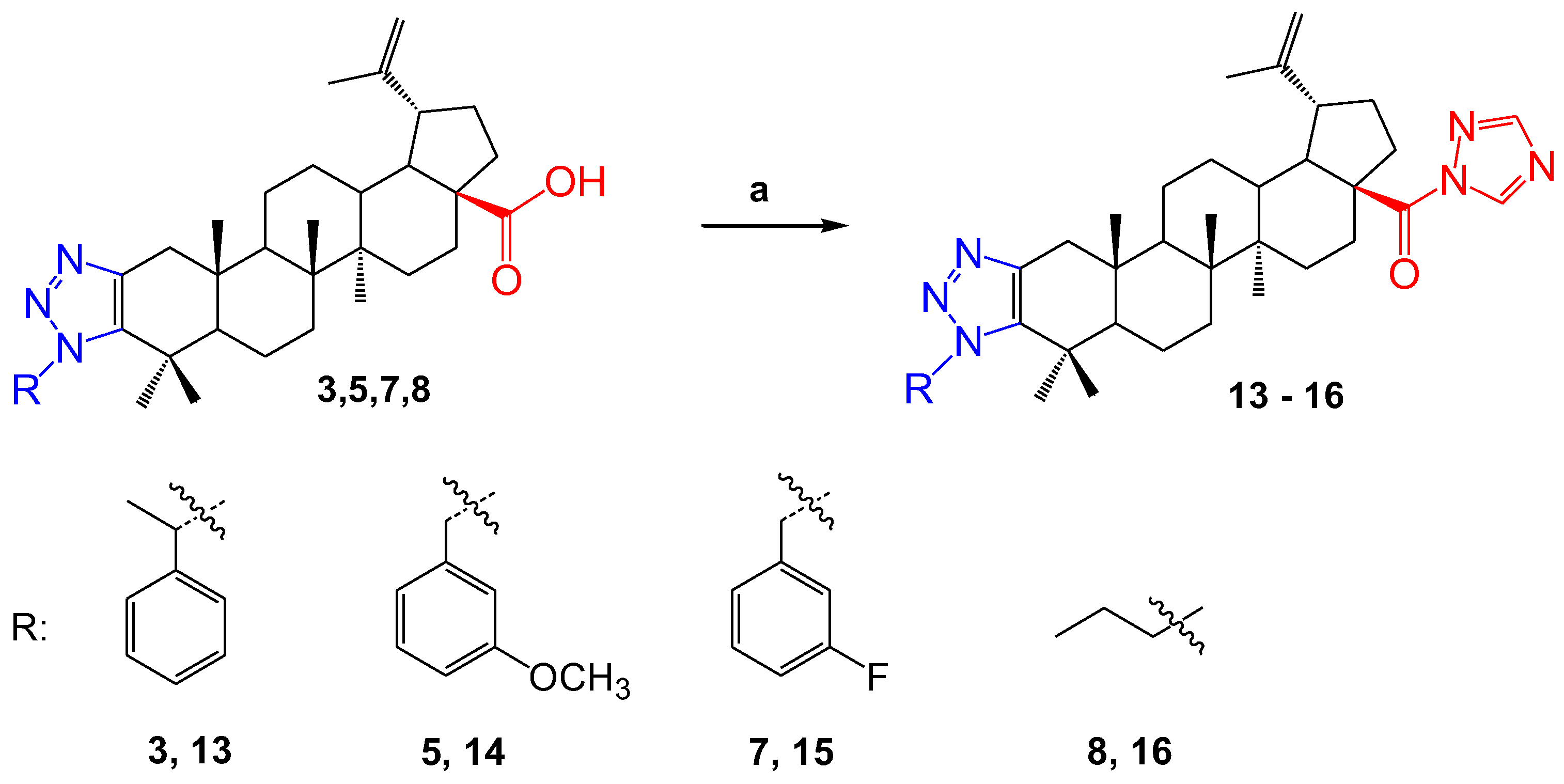
In order to study the biological activity, the betulonic acid derivatives 3, 5, 7, and 8 were chemically modified. The reactions were carried out by reacting 1,1’-carbonyldi-(1,2,4-triazole) in THF. As a result, colorless powdery substances 13–16 were obtained (Scheme 3). The yield of the products was very high, with the exception of compound 14, the yield of which was 52%.
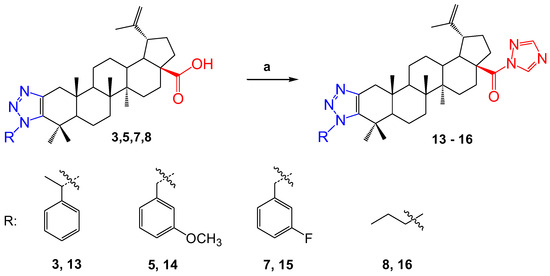
Scheme 3.
Reagents and conditions: (a) CDT, THF, 70 °C.
In the 1NMR spectra of the compounds, protons in the triazole ring at the C-28 position were observed as singlets at δ 8.93 and 7.99 p.m. for 13, at δ 8.92 and 7.99 p.m. for 14 and 15, and at δ 8.93 and 8.00 p.m. for 16. In the 13C NMR spectra, it was observed that, due to the formation of a triazole ring in place C-28, the signal of the carbonyl group shifted to a weak field, that is, it was manifested in regions δ 173.38, 173.39, 173.38 and 173.39 p.m., respectively.
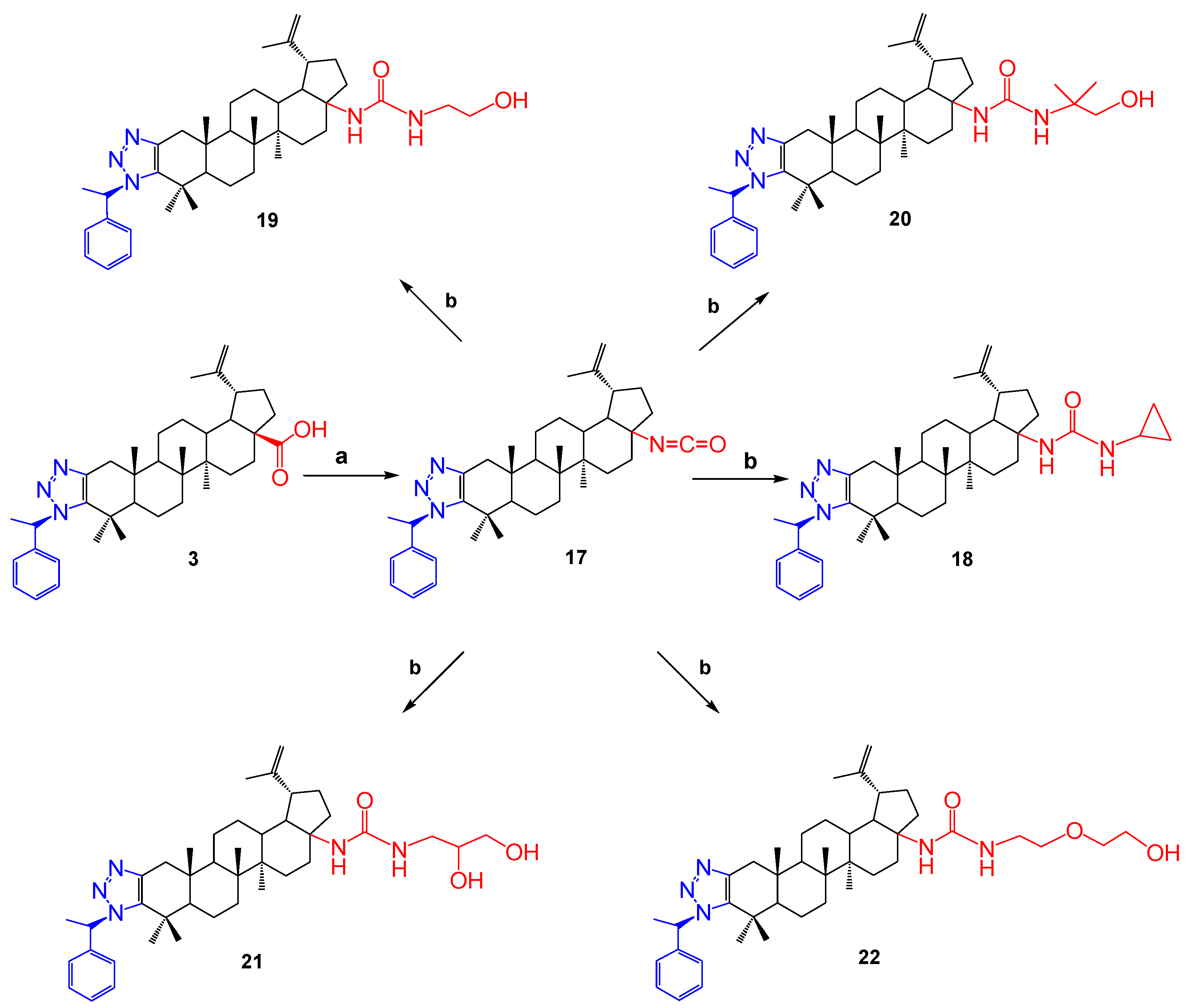
Continuing further modifications of the triazole derivatives of betulonic acid, compound 3 reacted with diphenylphosphoryl azide (DPPA) in toluene in the presence of triethylamine. The resulting carbamide derivative 17 was included in the further reaction. Then, the corresponding amines were added. As a result, the high-yield colorless powder substances 18–22 were obtained (Scheme 4).
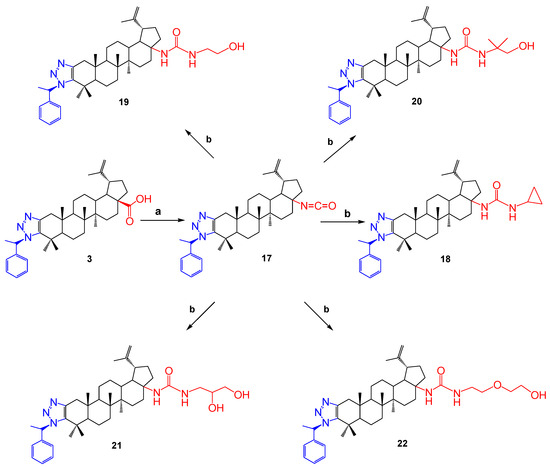
Scheme 4.
Reagents and conditions: (a) DPPA, toluene, Et3N; (b) amine, toluene, 110 °C.
The structure of the synthesized compounds was determined by spectroscopic and high-resolution mass spectrometry methods.
All synthesized compounds were tested for antimicrobial and cytotoxic activity.
2.2. Evaluation of the Biological Activity
2.2.1. Antimicrobial Activity
The antimicrobial activity of the samples was studied on recommended reference test microorganisms, facultative anaerobic Gram-positive cocci Staphylococcus aureus ATCC 6538, aerobic Gram-positive spore-forming Bacillus subtilis ATCC 6633, Gram-negative bacillus facultative anaerobes Escherichia coli ATCC 25922, aerobic Pseudomonas aeruginosa ATCC 27853 and k yeast fungus Candida albicans ATCC 10231, by serial dilution with determination of the minimum inhibitory concentration (MIC) [47,48]. The test strains of microorganisms used in the study were obtained from the American Type Culture Collection.
The results of the study of the antimicrobial activity of the samples by serial dilution are shown in Table 1.

Table 1.
Antimicrobial activity (MIC values in µg/mL) of test compounds in relation to reference test strains.
As a result of the antimicrobial study, it was found that the tested compounds exhibited antibacterial activity against opportunistic test strains to varying degrees. An analysis of the antimicrobial activity of the tested substances showed that its manifestation depends on the type of pathogen. The test strains of Staphylococcus aureus and Escherichia coli were most sensitive to all the compounds presented.
It was revealed that compound 6 showed pronounced antimicrobial activity against the Gram-positive Staphylococcus aureus test strain ATCC 6538 and that compound 7 showed pronounced antimicrobial activity against the Gram-negative Escherichia coli test strain ATCC 25922, their MICs being 6.3 µg/mL. At the same time, their antimicrobial activity was comparable to the activity of the comparison drug ceftriaxone, the MIC of which was also 6.3 µg/mL, and exceeded the activity of the comparison drug benzylpenicillin sodium salt against staphylococcus by two times and that against E. coli by three times.
Compounds 3 and 18 showed moderate antibacterial activity against both the Gram-positive test strain Staphylococcus aureus ATCC 6538 and the Gram-negative test microorganism Escherichia coli, their minimally inhibitory concentrations being 12.5 µg/mL in relation to the test strains.
The Gram-negative Pseudomonas aeruginosa test strain proved to be the most resistant to the action of these compounds. None of the tested compounds, except for compound 7, which had a weak MIC = 50 µg/kg, showed antibacterial properties against this microorganism.
Samples 4, 7, 8, 13, 15, 18, 19 and 21 showed moderate antibacterial activity against the Gram-positive test strain Staphylococcus aureus ATCC 65; their MICs were 12.5 and 25 µg/mL.
Compounds 2, 3, 5, 7, 19 and 20 showed a slight antifungal effect against the yeast-like fungus Candida albicans ATCC 10231 at concentrations of 25–50 µg/mL, which were lower than the activity of the comparison drug nystatin (MIC = 12.5 µg/mL).
Thus, among the new synthesized derivatives 3–10 and 13–22 were compounds with antibacterial activity comparable to the activity of the drug ceftriaxone and exceeding the activity of benzylpenicillin sodium.
Compound 6 turned out to be the most active against Staphylococcus aureus, while compound 7 was the most active against the Gram-negative test microorganism Escherichia coli. This allowed us to consider these compounds as very promising for the search for new antibacterial drugs, though further in-depth research is required.
2.2.2. Cytotoxic Activity
These new synthesized compounds were subjected to the Artemia salina (Leach) lethality test. Larvicidal activity based on the percentage of larval mortality was evaluated after 24 h exposure to the treatments.
According to Meyer et al. [49], who classified substances into toxic (LC50 value < 1000 mcg/mL) and nontoxic (LC50 value > 1000 mcg/mL), almost all the tested compounds showed good cytotoxic activity against artemia compared to the reference compound.
The cytotoxicity of the compounds was evaluated by means of the survival test of larvae of Artemia salina (Leach) crustaceans under in vitro cultivation conditions [49,50]. The results are shown in Table 2.

Table 2.
Cytotoxic activity of the compounds.
The study showed that samples of the tested compounds 2–4, 6–10, 13–15, 18–20 and 22 exhibited cytotoxic activity against larvae of the marine crustaceans Artemia salina (Leach).
Since the test for mortality of shrimp in brine has a strong correlation with tests for cytotoxicity against human cancer cells, it could be used to screen for antitumor potential. The aforementioned samples could be considered potential candidates for an antitumor compound of plant origin. It was found that these compounds can be sources of cytotoxic and antitumor compounds. Of course, it is necessary to conduct studies to assess their antitumor activity using human cancer cell lines in order to identify their true potential for therapeutic use.
The results obtained during this study showed that the synthesized compounds had significant potential due to their antibacterial and cytotoxic properties. Among the synthesized compounds, it was found that 6 and 7 had promising antibacterial properties and exhibited cytotoxicity.
The pronounced antibacterial activity demonstrated in this study for some compounds indicated them to be potential antimicrobial agents active against common microbial pathogens that cause various socially significant infectious diseases.
If we consider the relationship between the “structure” and the “biological activity” of the synthesized compounds, when triazole fragments were introduced into the structures of the molecules, the antimicrobial activities of the derivatives in relation to the Gram-positive and Gram-negative strains were relatively different. It was found that the activity of chlorine-containing triazole derivative 6 (MIC = 6.3 µg/mL) against the Staphylococcus aureus strain was significantly higher—about eight times that of the initial betulonic acid 2 (MIC = 50 µg/mL). And it was noticed that the activity of compounds 3, 4, 7 and 8 (MIC = 25 µg/mL) increased two times in relation to this strain compared to the original molecule. The fluorinated triazole derivative 7 (MIC = 6.3 µg/mL) showed very high antimicrobial activity in relation to the Escherichia coli strain, while the original betulonic acid 2 showed no activity against this strain. In addition, compounds 3–6 (MIC = 12.5–50 µg/mL), namely, phenyl-, methyl-, methoxy- and chlorine-containing triazole derivatives, also showed activity against this strain (Escherichia coli); the initial betulonic acid 2 was resistant to this strain, i.e., it did not show activity. Of the synthesized compounds shown in Scheme 1, only the fluorinated triazole derivative 7 was active against the Pseudomonas aeruginosa strain and the Candida albicans fungus. As for the fungus Candida albicans, the activity of compound 7 (MIC = 25 µg/mL) was twice as high as that of betulonic acid 2 (MIC = 50 µg/mL).
If we consider the relationship between the “structure” and the “biological activity” of the triazole derivatives of betulonic acid 13–16 obtained by further conversion (shown in Scheme 3), there was a decrease in biological activity in relation to all strains compared with the initial compounds 3, 5, 7 and 8. It was noted that the antimicrobial effect of the fluorinated triazole derivative 15 (MIC = 12.5 µg/mL) in relation to the Staphylococcus aureus strain was twice as high as that of the initial compound 7 (MIC = 50 µg/mL).
Considering the relationship between “structure” and “biological activity” in the N-substituted triazole derivatives 17–22 (shown in Scheme 4), it was noticed that antimicrobial activity in relation to Gram-positive and Gram-negative strains decreased or remained unchanged compared to the original molecule 3. Compound 19 (MIC = 25 µg/mL) was active in relation to the yeast-like fungus Candida albicans. It showed two times more activity compared to the original molecule 3 (MIC = 50 µg/mL).
When considering the relationship between “structure” and “biological activity” in relation to cytotoxicity, the following regularity was observed. For example, of the compounds shown in Scheme 1, the cytotoxicity of phenyltriazole derivative 3 (LD50 = 82.5 µg/mL) and chlorine-containing triazole derivative 6 (LD50 = 82.8 µg/mL) was slightly higher compared to that of the initial betulonic acid 2 (LD50 = 88.7 µg/mL), and the rest of the compounds, especially 4, 8 and 9 (LD50 = 95.6–107.2 µg/mL), showed a decrease in activity compared to the original molecule 2. It was observed that the cytotoxicity of the triazole derivative with methoxy group 5 was lost compared to the initial compound 2 (LD50 = 88.7 µg/mL).
When comparing the cytotoxicity of compounds 13–16 (LD50 = 85.1–105.9 µg/mL) with that of the starting compounds 3, 5, 7 and 8 (LD50 = 82.5–107.2 µg/mL), it was found that the cytotoxicity of compounds 14 and 16 (LD50 = 90.5 and 85.1 µg/mL) increased.
In addition, compounds 18–20 and 22 (LD50 = 63.5–74.1 µg/mL) were found to be cytotoxic compared to the original molecule 3 (LD50 = 82.5 µg/mL). And in compounds 17 and 21, on the contrary, activity was lost.
So, considering the relationship between “structure” and “biological activity”, the following conclusion can be made in relation to these synthesized derivatives. By introducing a triazole cycle with various substituents into a molecule, substances with antibacterial action can be obtained. In particular, halogen-containing triazole derivatives, i.e., chlorine and fluorine atoms, showed very high antimicrobial activity. It turned out that the presence of a fluorine atom and an ethanolamine fragment in the molecules of derivatives obtained with further conversion of triazole derivatives led to a doubling of activity against microbes and fungi. In addition, it was found that the synthesized derivatives had high antimicrobial activity due to the presence of phenyl, methyl and methoxy groups in the molecules. The introduction of the triazole cycle and substituted fragments into the synthesized compounds did not lead to a significant change in cytotoxicity compared with the initial molecules. However, in some compounds containing cyclopropane, ethylhydroxy and propylhydroxy fragments introduced into the molecules increased the cytotoxicity compared to that of the starting triazole derivatives.
3. Materials and Methods
3.1. General Chemistry Section
The 1H and 13C spectra of the compounds were measured on a Bruker (Billerica, MA, USA) AMX 400 MHz instrument. Tetramethylsilane and an NMR solvent were used in some spectra as internal standards. The melting points were determined using the Reichert (Carrollton, TX, USA) Thermovar. For column chromatography, 70−230 mesh silica 60 (E.M. Merck, Rahway, NJ, USA) was used as the stationary phase. Dichlorometane, petroleum ether and ethylacetate were used as eluents. The TLC (thin-layer chromatography) results were checked on silica gel 0.20 mm 60 with a mixture of ethanol and sulfuric acid (10 mL of sulfuric acid and 90 mL of ethanol), and UV was also used. Chemicals from commercial sources were used without further purification. Reaction dry solvents (toluene, THF, DMF and CH2Cl2) from commercial sources were used. UHPLC-ESI-Q-TOF-MS analyses of small molecules were performed using a Dionex (Sunnyvale, CA, USA) Ultimate (Arlington, VA, USA) 3000 UHPLC coupled to a Zorbax (Santa Clara, CA, USA) RRHP Eclipse Plus C18 column (2.1 × 100 mm, 1.8 µL) connected to a Bruker Impact II QTOF mass spectrometer. Mobile phases consisted of water (A) and acetonitrile (B), each supplemented with 0.1% formic acid. The following gradient was used at a flow rate of 0.200 mL/min: 0–2.5 min 5% B, 2.5–14 min 5–100% B, 14–19 min 100% B, 19–20.4 min 100–5% B, 20.4–25 min 5% B. The mass spectrometer was operated in positive-ion mode with a scan range of 50–3000 m/z. The source conditions were as follows: end plate offset at −500 V; capillary at −4500 V; nebulizer gas (N2) at 1.6 bar; dry gas (N2) at 8 L min−1; dry temperature at 180 °C. The ion transfer conditions were as follows: ion funnel RF at 200 Vpp; multiple RF at 200 Vpp; quadrupole low mass at 55 m/z; collision energy at 5.0 eV; collision RF at 600 Vpp; ion cooler RF at 50–350 Vpp; transfer time at 121 μs; pre-pulse storage time of 1 μs. Calibration was performed with 1 mM sodium formate through a loop injection of 20 μL at the start of each run. Also, mass spectra were acquired on a quadrupole orthogonal acceleration time-of-flight mass spectrometer (Synapt G2 HDMS, Waters, Milford, MA, USA). Samples were infused at 3 µL/min, and spectra were obtained in positive (or negative) ionization mode with a resolution of 15,000 (FWHM) using leucine enkephalin as the lock mass.
Betulonic acid 2 was prepared as previously reported [51]. The spectral data corresponded to the data reported in the literature [52,53]. The 1H, 13C NMR and mass spectra of compounds 2–22 can be found in the Supplementary Materials.
3.2. Experimental Section
- 3-Oxo-lup-20(29)-en-28-oic Acid (Betulonic Acid, 2). Betulin (5.0 g, 11.3 mmol) was placed in a 0.5 l flask filled with acetone (150 mL) and dissolved in an ultrasonic water bath. Freshly prepared Jones reagent (6.65 g Na2Cr2O7 and 6 mL H2SO4 in 50 mL water) was added drop by drop to the cooled (on an ice bath) solution. The color of the solution began to change. The reaction mixture was allowed to heat up to room temperature and continued to be stirred for 5 h. The course of the reaction was checked by TLC. Then, MeOH (100 mL) was added to the reaction mixture, followed by water (50 mL). The resulting precipitate was filtered and washed with water (50 mL). The crude product was dried, then dissolved in Et2O (60 mL) and washed with water (30 mL), 7.5% hydrochloric acid (20 mL), water (20 mL), saturated aqueous solution NaHCO3 (20 mL) and water (20 mL). The ether layer was driven off on a rotary evaporator, and the remaining residue was purified by column chromatography (silica gel); a mixture of heptane and ethyl acetate (80:20) was used as an eluent. Compound 2 was a colorless powder. The product yield was 1.55 g (30%).
- 1H NMR (400 MHz, CDCl3): δ 4.74 (d, J = 2.3 Hz, 1H), 4.66–4.58 (m, 1H), 3.01 (td, J = 10.7, 4.6 Hz, 1H), 2.56–2.35 (m, 2H), 2.33–2.17 (m, 2H), 2.07–1.83 (m, 2H), 1.70 (s, 4H), 1.64 (t, J = 11.4 Hz, 1H), 1.59–1.14 (m, 14H), 1.13–1.04 (m, 4H), 1.03–0.95 (m, 9H), 0.93 (s, 3H).
- 13C NMR (101 MHz, CDCl3): δ 218.23, 181.72, 150.32, 109.79, 56.37, 54.94, 49.85, 49.19, 47.34, 46.89, 42.49, 40.64, 39.61, 38.51, 37.04, 36.92, 34.13, 33.60, 32.10, 30.55, 29.68, 26.64, 25.49, 21.37, 21.00, 19.63, 19.37, 15.96, 15.82, 14.63.
- 1′-((S)-1-Phenylethyl)-1H′-lup-2-eno-[2,3-d]-[1,2,3]-triazole-28 oic Acid (3). Betulonic acid (1.0 g, 1 equiv., 2.2 mmol), 4-nitrophenyl azide (360 mg, 1.3 equiv., 2.86 mmol), (S)-(–)-α-methylbenzylamine (350 mg, 1.3 equiv., 2.86 mmol) and 4 Å molecular sieves (200 mg) were added to a dried reaction tube with a screw cap equipped with a magnetic stirrer. The mixture was dissolved in dry toluene (5 mL), and the reaction mixture was stirred at 100 °C for 24 h. A mixture of H2SO4 and ethanol (10:90) was used to visualize the TLC plates. The crude reaction mixture was purified directly using column chromatography (silica gel), first using dichloromethane to remove all 4-nitroaniline formed during the reaction, and then a mixture of petroleum ether and ethyl acetate was used as an eluent. Compound 3 was powdery with a yellowish tinge, m.p. 325–327 °C. The yield was 790 mg (61%). The spectroscopic data for compound 3 were consistent with previously reported data for this compound [41].
- 1H NMR (400 MHz, CDCl3): δ 7.33–7.25 (m, 3H), 7.25–7.19 (m, 2H), 5.72 (q, J = 7.0 Hz, 1H), 4.77 (d, J = 2.3 Hz, 1H), 4.64 (m, 1H), 3.03 (td, J = 10.6, 4.5 Hz, 1H), 2.96 (d, J = 15.3 Hz, 1H), 2.32–2.20 (m, 2H), 2.15 (d, J = 15.3 Hz, 1H), 2.05–1.95 (m, 5H), 1.81–1.60 (m, 5H), 1.61–1.32 (m, 11H), 1.26 (s, 5H), 1.10 (s, 3H), 0.99 (s, 6H), 0.81 (s, 3H).
- 13C NMR (101 MHz, CDCl3): δ 181.07, 150.22, 141.79, 141.05, 137.53, 128.64, 127.56, 126.24, 109.88, 59.28, 56.38, 54.78, 49.34, 49.21, 46.90, 42.45, 40.60, 38.91, 38.53, 38.32, 37.04, 33.80, 33.41, 32.08, 30.59, 29.80, 28.89, 25.50, 23.73, 21.36, 21.31, 19.42, 18.91, 16.25, 15.70, 14.65.
- 1′-(3-Methylbenzyl)-1H′-lup-2-eno-[2,3-d]-[1,2,3]-triazole-28-oic Acid (4). Betulonic acid 2 (100 mg, 1 equiv., 0.22 mmol), 4-nitrophenyl azide (72 mg, 2 equiv., 0.44 mmol), 3-methylbenzylamine (74.6 mg, 2.8 equiv., 0.616 mmol) and 4 Å molecular sieves (50 mg) were added to a dried reaction tube with a screw cap equipped with a magnetic stirrer. The mixture was dissolved in dry toluene (1 mL), and the reaction mixture was stirred at 100 °C for 24 h. A mixture of H2SO4 and ethanol (10:90) was used to visualize the TLC plates. The crude reaction mixture was purified directly using column chromatography (silica gel), first using dichloromethane to remove all 4-nitroaniline formed during the reaction, and then a mixture of petroleum ether and ethyl acetate (10:1) was used as an eluent. Compound 4 was a pale-yellow powder, m.p. 261–264 °C. The yield was 72 mg (68%).
- 1H NMR (400 MHz, CDCl3): δ 7.17 (t, J = 7.6 Hz, 1H), 7.06 (d, J = 7.6 Hz, 1H), 6.85 (s, 1H), 6.81 (d, J = 7.7 Hz, 1H), 5.62 (m, 2H), 4.76 (d, J = 2.3 Hz, 1H), 4.64 (m, 1H), 4.12 (q, J = 7.2 Hz, OH), 3.04 (td, J = 10.6, 4.5 Hz, 1H), 2.96 (d, J = 15.3 Hz, 1H), 2.25 (m, 6H), 1.99 (m, 2H), 1.77 (d, J = 12.8 Hz, 1H), 1.71 (s, 3H), 1.45 (m, 13H), 1.17 (s, 3H), 1.04 (s, 3H), 0.99 (d, J = 9.2 Hz, 6H), 0.90 (m, 1H), 0.78 (s, 3H).
- 13C NMR (101 MHz, CDCl3): δ 181.38, 150.25, 141.84, 138.49, 138.00, 136.38, 128.59, 128.52, 127.03, 123.46, 109.86, 56.40, 54.59, 52.81, 49.29, 49.19, 46.90, 42.44, 40.56, 38.97, 38.51, 38.36, 37.04, 33.71, 33.35, 32.08, 30.58, 29.79, 28.77, 25.50, 21.41, 21.37, 21.31, 19.42, 18.91, 16.08, 15.70, 14.65.
- HRMS (ESI+): m/z calculated for C38H54N3O2 [M + H]+: 584.42160, found: 584.4216.
- 1′-(3-Methoxybenzyl)-1H′-lup-2-eno-[2,3-d]-[1,2,3]-triazole-28-oic Acid (5). Betulonic acid (100 mg, 1 equiv., 0.22 mmol), 4-nitrophenyl azide (72 mg, 2 equiv., 0.44 mmol), 3-methoxybenzylamine (84.5 mg, 2.8 equiv., 0.616 mmol) and 4 Å molecular sieves (50 mg) were added to a dried reaction tube with a screw cap equipped with a magnetic stirrer. The mixture was dissolved in dry toluene (1 mL), and the reaction mixture was stirred at 100 °C for 18 h. A mixture of H2SO4 and ethanol (10:90) was used to visualize the TLC plates. The crude reaction mixture was purified directly using column chromatography (silica gel), first using dichloromethane to remove all 4-nitroaniline formed during the reaction, and then a mixture of petroleum ether and ethyl acetate was used as an eluent. Compound 5 was powdery with a yellowish tinge, m.p. 205–208 °C. The yield was 78 mg (60%).
- 1H NMR (400 MHz, CDCl3): δ 7.21 (td, J = 7.9, 3.3 Hz, 1H), 6.83–6.75 (m, 1H), 6.65–6.54 (m, 3H), 5.62 (d, J = 3.8 Hz, 3H), 4.76 (d, J = 2.3 Hz, 1H), 4.67–4.63 (m, 1H), 3.74 (d, J = 1.6 Hz, 4H), 3.09–2.92 (m, 2H), 2.26 (ddd, J = 15.5, 9.7, 3.5 Hz, 2H), 2.21–2.14 (m, 1H), 2.06–1.82 (m, 3H), 1.81–1.75 (m, 2H), 1.71 (s, 3H), 1.68 (s, 0H), 1.61–1.36 (m, 10H), 1.35–1.18 (m, 4H), 1.17 (d, J = 2.6 Hz, 3H), 1.04 (s, 3H), 1.02–0.96 (m, 8H), 0.81 (s, 1H), 0.77 (s, 3H).
- 13C NMR (101 MHz, CDCl3): δ 180.81, 159.97, 150.24, 141.89, 138.09, 138.04, 129.78, 118.65, 113.31, 111.96, 109.87, 56.36, 55.23, 54.79, 54.59, 52.74, 52.66, 49.30, 49.19, 46.88, 44.09, 42.45, 40.56, 40.36, 38.98, 38.48, 38.37, 37.03, 33.72, 33.35, 32.07, 30.57, 29.79, 28.73, 25.50, 21.38, 21.31, 19.42, 18.91, 16.08, 15.71, 15.19, 14.65.
- HRMS (ESI+): m/z calculated for C38H54N3O3 [M + H]+: 600.4160, found: 600.4194.
- 1′-(3-Chlorobenzyl)-1H′-lup-2-eno-[2,3-d]-[1,2,3]-triazole-28-oic Acid (6). Betulonic acid 2 (100 mg, 1 equiv., 0.22 mmol), 4-nitrophenyl azide (72 mg, 2 equiv., 0.44 mmol), 3-chlorobenzylamine (87 mg, 2.8 equiv., 0.616 mmol) and 4 Å molecular sieves (50 mg) were added to a dried reaction tube with a screw cap equipped with a magnetic stirrer. The mixture was dissolved in dry toluene (1 mL), and the reaction mixture was stirred at 100 °C for 24 h. A mixture of H2SO4 and ethanol (10:90) was used to visualize the TLC plates. The crude reaction mixture was purified directly using column chromatography (silica gel), first using dichloromethane to remove all 4-nitroaniline formed during the reaction, and then a mixture of petroleum ether and ethyl acetate (1:1) was used as an eluent. Compound 6 was a pale-yellow powder, m.p. 288–290 °C. The yield was 65 mg (62%).
- 1H NMR (400 MHz, CDCl3): δ 7.28–7.19 (m, 2H), 7.03 (d, J = 2.0 Hz, 1H), 6.89 (dt, J = 5.7, 2.1 Hz, 1H), 5.61 (d, J = 5.5 Hz, 2H), 4.76 (d, J = 2.3 Hz, 1H), 4.64 (t, J = 1.9 Hz, 1H), 3.04 (td, J = 10.6, 4.6 Hz, 1H), 2.96 (d, J = 15.4 Hz, 1H), 2.28 (ddd, J = 14.7, 9.0, 3.2 Hz, 2H), 2.23–2.15 (m, 1H), 2.00 (ddd, J = 17.8, 11.6, 5.5 Hz, 2H), 1.83–1.73 (m, 1H), 1.71 (s, 3H), 1.67–1.19 (m, 13H), 1.17 (s, 4H), 1.06–0.95 (m, 9H), 0.78 (s, 3H).
- 13C NMR (101 MHz, CDCl3): δ 181.63, 150.23, 142.05, 130.06, 128.07, 126.53, 124.55, 109.87, 56.41, 54.51, 52.16, 49.27, 49.20, 46.91, 42.44, 40.55, 38.98, 38.53, 38.29, 37.03, 33.69, 33.31, 32.07, 30.58, 29.80, 28.83, 25.47, 21.41, 21.37, 19.42, 18.88, 16.09, 15.73, 14.64.
- HRMS (ESI+): m/z calculated for C37H51ClN3O2 [M + H]+: 604.36698, found: 604.3661.
- 1′-(3-Fluorobenzyl)-1H′-lup-2-eno-[2,3-d]-[1,2,3]-triazole-28-oic Acid (7). Betulonic acid (100 mg, 1 equiv., 0.22 mmol), 4-nitrophenyl azide (72 mg, 2 equiv., 0.44 mmol), 3-fluorobenzylamine (77 mg, 2.8 equiv., 0.616 mmol) and 4 Å molecular sieves (50 mg) were added to a dried reaction tube with a screw cap equipped with a magnetic stirrer. The mixture was dissolved in dry toluene (1 mL), and the reaction mixture was stirred at 100 °C for 22 h. A mixture of H2SO4 and ethanol (10:90) was used to visualize the TLC plates. The crude reaction mixture was purified directly using column chromatography (silica gel), first using dichloromethane to remove all 4-nitroaniline formed during the reaction, and then a mixture of petroleum ether and ethyl acetate was used as an eluent. Compound 7 was powdery with a yellowish tinge, m.p. 302–305 °C. The yield was 82 mg (64%).
- 1H NMR (400 MHz, CDCl3): δ 7.27 (d, J = 5.5 Hz, 2H), 7.00–6.92 (m, 1H), 6.81 (ddd, JHF = 7.8 Hz, JHH =1.8, 0.9 Hz, 1H), 6.71 (dt, JHF = 9.6 Hz, JHH = 2.2 Hz, 1H), 5.68–5.58 (m, 2H), 4.76 (d, J = 2.3 Hz, 1H), 4.67–4.62 (m, 1H), 3.03 (td, J = 10.6, 4.6 Hz, 1H), 2.96 (d, J = 15.4 Hz, 1H), 2.27 (td, J = 12.7, 11.3, 3.3 Hz, 2H), 2.23–2.15 (m, 1H), 2.07–1.93 (m, 2H), 1.77 (dd, J = 11.4, 4.1 Hz, 1H), 1.71 (s, 3H), 1.66 (t, J = 11.4 Hz, 1H), 1.62–1.33 (m, 9H), 1.33–1.18 (m, 3H), 1.17 (s, 3H), 1.04 (s, 3H), 0.99 (d, J = 8.9 Hz, 6H), 0.78 (s, 3H).
- 13C NMR (101 MHz, CDCl3): δ 181.18, 161.82 (d, 1JC-F = 245.5 Hz), 150.22, 142.05, 139.03 (d, 3JC-F = 7.2 Hz), 138.10, 130.36 (d, 3JC-F = 8.2 Hz), 121.99 (d, 4JC-F = 2.9 Hz), 114.80 (d, 2JC-F = 22.5 Hz), 113.50 (d, 2JC-F = 21.1 Hz), 109.88, 56.38, 54.53, 52.24, 49.28, 49.20, 46.91, 42.45, 40.56, 38.98, 38.53, 38.31, 37.02, 33.69, 33.32, 32.07, 30.58, 29.80, 28.78, 25.48, 21.37, 19.42, 18.89, 16.10, 15.73, 14.65.
- HRMS (ESI+): m/z calculated for C37H51FN3O2 [M + H]+: 588.3960, found: 588.3966.
- 1′-Butyl-1H′-lup-2-eno-[2,3-d]-[1,2,3]-triazole-28-oic Acid (8). Betulonic acid (100 mg, 1 equiv., 0.22 mmol), 4-nitrophenyl azide (36 mg, 1 equiv., 0.22 mmol), n-butylamine (21 mg, 1.3 equiv., 0.286 mmol) and 4 Å molecular sieves (50 mg) were added to a dried reaction tube with a screw cap equipped with a magnetic stirrer. The mixture was dissolved in dry toluene (1 mL), and the reaction mixture was stirred at 100 °C for 24 h. A mixture of H2SO4 and ethanol (10:90) was used to visualize the TLC plates. The crude reaction mixture was purified directly using column chromatography (silica gel), first using dichloromethane to remove all 4-nitroaniline formed during the reaction, and then a mixture of petroleum ether and ethyl acetate was used as an eluent. Compound 8 was powdery with a yellowish tinge, m.p. 288–290 °C. The yield was 74 mg (63%).
- 1H NMR (400 MHz, CDCl3): δ 4.76 (d, J = 2.3 Hz, 1H), 4.67–4.61 (m, 1H), 4.29 (td, J = 7.1, 1.6 Hz, 2H), 3.04 (td, J = 10.5, 4.5 Hz, 1H), 2.91 (d, J = 15.3 Hz, 1H), 2.34–2.20 (m, 2H), 2.13 (d, J = 15.4 Hz, 1H), 2.06–1.93 (m, 4H), 1.80–1.73 (m, 1H), 1.71 (s, 3H), 1.65 (d, J = 11.5 Hz, 1H), 1.62–1.33 (m, 11H), 1.30 (s, 3H), 1.26 (s, 4H), 1.18 (s, 3H), 1.04–0.93 (m, 10H), 0.93–0.80 (m, 1H), 0.77 (s, 3H).
- 13C NMR (101 MHz, CDCl3): δ 181.21, 150.24, 141.03, 109.86, 56.39, 54.68, 49.37, 49.30, 49.21, 46.90, 42.46, 40.59, 38.98, 38.54, 38.27, 37.05, 33.66, 33.39, 32.85, 32.09, 30.60, 29.81, 29.71, 28.68, 25.50, 21.33, 20.18, 19.43, 18.98, 16.06, 15.72, 14.69, 13.70.
- HRMS (ESI+): m/z calculated for C34H54N3O2 [M + H]+: 536.4211, found: 536.4214.
- 1′-Isobutyl-1H′-lup-2-eno-[2,3-d]-[1,2,3]-triazole-28-oic Acid (9). Betulonic acid 2 (100 mg, 1 equiv., 0.22 mmol), 4-nitrophenyl azide (36 mg, 1 equiv., 0.22 mmol), isobutylamine (21 mg, 1.3 equiv., 0.286 mmol) and 4 Å molecular sieves (50 mg) were added to a dried reaction tube with a screw cap equipped with a magnetic stirrer. The mixture was dissolved in dry toluene (1 mL), and the reaction mixture was stirred at 100 °C for 24 h. A mixture of H2SO4 and ethanol (10:90) was used to visualize the TLC plates. The crude reaction mixture was purified directly using column chromatography (silica gel), first using dichloromethane to remove all 4-nitroaniline formed during the reaction, and then a mixture of petroleum ether and ethyl acetate (10:1) was used as an eluent. Compound 9 was a pale-yellow powder, m.p. 299–301 °C. The yield was 68 mg (65%).
- 1H NMR (400 MHz, CDCl3): δ 4.76 (d, J = 2.3 Hz, 1H), 4.64 (t, J = 1.9 Hz, 1H), 4.16–4.03 (m, 2H), 3.04 (td, J = 10.6, 4.6 Hz, 1H), 2.92 (d, J = 15.3 Hz, 1H), 2.54 (dq, J = 13.8, 6.9 Hz, 1H), 2.35–2.19 (m, 2H), 2.14 (d, J = 15.3 Hz, 1H), 2.08–1.94 (m, 2H), 1.81–1.73 (m, 1H), 1.71 (s, 3H), 1.69–1.32 (m, 10H), 1.30 (s, 3H), 1.24 (d, J = 16.4 Hz, 3H), 1.17 (s, 3H), 1.15–1.03 (m, 1H), 1.03–0.93 (m, 13H), 0.93–0.83 (m, 1H), 0.77 (s, 3H).
- 13C NMR (101 MHz, CDCl3): δ 181.59, 150.26, 140.94, 137.79, 109.83, 56.56, 56.42, 54.74, 49.30, 49.22, 46.91, 42.45, 40.58, 38.90, 38.55, 38.28, 37.05, 33.73, 33.38, 32.10, 30.61, 29.82, 29.38, 28.90, 25.50, 21.57, 21.36, 20.25, 20.21, 19.43, 19.00, 16.04, 15.74, 14.68.
- HRMS (ESI+): m/z calculated for C34H54N3O2 [M + H]+: 536.42160, found: 536.4209.
- 1H′-Lup-2-eno-[2,3-d]-[1,2,3]-triazole-28-oic Acid (10). Betulonic acid 2 (100 mg, 1 equiv., 0.22 mmol), ammonium acetate (84.8 mg, 5 equiv., 1.1 mmol) and 4-nitrophenyl azide (45.8 mg, 1.3 equiv., 0.28 mmol) were dissolved in dry DMF (1 mL). The reaction mixture was stirred at 80 °C for 24 h. A mixture of H2SO4 and ethanol (10:90) was used to visualize the TLC plates. The crude reaction mixture was purified directly using column chromatography (silica gel), first using dichloromethane to remove all 4-nitroaniline formed during the reaction, and then a mixture of petroleum ether and ethyl acetate (10:1) was used as an eluent. Compound 10 was a pale-yellow powder, m.p. 158 °C. The yield was 48 mg (46%). The spectroscopic data for compound 10 were consistent with previously reported data for this compound [41].
- 1H NMR (400 MHz, CDCl3): δ 4.77 (d, J = 2.5 Hz, 1H), 4.64 (d, J = 1.9 Hz, 1H), 3.05 (td, J = 10.7, 4.6 Hz, 1H), 2.90 (d, J = 15.5 Hz, 1H), 2.36–2.22 (m, 2H), 2.19–2.09 (m, 1H), 2.08–1.93 (m, 2H), 1.83–1.74 (m, 1H), 1.72 (s, 3H), 1.68–1.36 (m, 15H), 1.32 (s, 4H), 1.30–1.18 (m, 4H), 1.18–1.04 (m, 1H), 1.01 (d, J = 10.2 Hz, 6H), 0.97–0.80 (m, 6H), 0.77 (s, 3H).
- 13C NMR (101 MHz, CDCl3): δ 181.38, 150.35, 149.92, 140.40, 109.80, 56.40, 53.42, 49.22, 49.09, 46.95, 42.51, 41.35, 40.75, 39.04, 38.51, 37.32, 37.07, 33.72, 33.38, 33.30, 32.16, 31.01, 30.62, 29.80, 29.06, 27.67, 25.51, 23.76, 22.63, 21.41, 20.45, 19.42, 19.16, 16.27, 15.69, 14.68.
- Compound (11) and (12). Compound 10 (181 mg) was dissolved in dry methanol (1.5 mL), then potassium tret-butoxide (tButOK) (100.08 mg, 0.91 mmol) was added. The reaction mixture was stirred at room temperature for 3 h, after which 1-bromobutane was slowly added and stirred at 60 °C for 12 h. The solvent was distilled in a rotary evaporator. The crude reaction mixture was purified directly using column chromatography (silica gel), using a mixture of petroleum ether and ethyl acetate as an eluent. Compound 11 was a colorless substance. The yield was 103 mg (46%).
- 1H NMR (400 MHz, CDCl3): δ 4.76 (d, J = 2.3 Hz, 1H), 4.63 (t, J = 1.8 Hz, 1H), 4.32 (t, J = 7.3 Hz, 2H), 4.09 (qt, J = 10.8, 6.6 Hz, 2H), 3.04 (td, J = 10.8, 4.5 Hz, 1H), 2.83 (d, J = 15.4 Hz, 1H), 2.29 (td, J = 12.4, 11.7, 3.6 Hz, 2H), 2.07 (d, J = 15.4 Hz, 1H), 1.96–1.85 (m, 4H), 1.80–1.72 (m, 1H), 1.71 (s, 3H), 1.68–1.30 (m, 15H), 1.29 (d, J = 4.0 Hz, 4H), 1.21 (s, 2H), 1.17 (d, J = 5.7 Hz, 4H), 1.15–1.02 (m, 1H), 1.00 (s, 3H), 0.98 (d, J = 4.3 Hz, 4H), 0.96–0.81 (m, 11H), 0.79 (s, 3H).
- 13C NMR (101 MHz, CDCl3): δ 176.22, 150.89, 150.54, 141.49, 109.63, 63.72, 56.58, 54.41, 53.50, 49.35, 49.17, 47.01, 42.46, 40.76, 38.90, 38.35, 37.62, 37.05, 33.50, 33.39, 32.14, 32.05, 31.08, 30.81, 30.68, 29.72, 25.61, 23.82, 21.44, 19.84, 19.44, 19.32, 19.21, 16.26, 15.63, 14.67, 13.72, 13.59.
- HRMS (ESI+): m/z calculated for C38H62N3O2 [M + H]+: 592.48420, found: 592.4850.
- Compound 12 was a colorless substance. The yield was 45 mg (20%).
- 1H NMR (400 MHz, CDCl3) δ 4.75 (d, J = 2.4 Hz, 1H), 4.61 (m, 1H), 4.17 (m, 1H), 4.10 (m, 3H), 3.05 (td, J = 10.8, 4.4 Hz, 1H), 2.61 (d, J = 15.3 Hz, 1H), 2.37–2.24 (m, 2H), 2.05–1.98 (m, 1H), 1.97–1.85 (m, 2H), 1.84–1.73 (m, 3H), 1.70 (s, 3H), 1.68–1.37 (m, 13H), 1.33 (d, J = 10.5 Hz, 4H), 1.27–1.20 (m, 6H), 1.18–1.03 (m, 2H), 1.01 (s, 3H), 0.98 (d, J = 5.2 Hz, 3H), 0.95 (d, J = 6.8 Hz, 4H), 0.93–0.82 (m, 5H), 0.79 (s, 3H).
- 13C NMR (101 MHz, CDCl3) δ 176.17, 150.68, 150.15, 129.16, 109.61, 63.76, 56.52, 53.37, 49.34, 49.27, 47.36, 47.00, 42.49, 40.87, 39.24, 38.31, 37.03, 36.06, 33.65, 33.36, 32.16, 32.09, 30.81, 30.70, 30.59, 29.70, 25.58, 23.08, 21.59, 19.86, 19.38, 19.31, 19.00, 16.63, 15.63, 14.65, 13.72, 13.60.
- HRMS (ESI+): m/z calculated for C38H62N3O2 [M + H]+: 592.48420, found: 592.4844.
- 1′-((S)-1-Phenylethyl)-1H′-lup-2-eno-[2,3-d]-[1,2,3]-triazole-28-(1H-triazol-1-yl) (13). Compound 3 (100 mg, 1 equiv., 0.171 mmol) was dissolved in 2 mL of THF, and 1,1′-carbonyldi-(1,2,4-triazole) (112.1 mg, 4 equiv., 0.684 mmol) was added. The reaction mixture was stirred at 70 °C. The reaction was carried out for 5 h. The solvent was distilled in a rotary evaporator. The remainder was chromatographed with silica gel in a column. When the column was eluted with a mixture of petroleum ether and ethyl acetate (5:3), compound 13 was isolated. Compound 13 was a colorless powder, m.p. 232–235 °C. The yield was 116 mg (107%).
- 1H NMR (400 MHz, CDCl3): δ 8.93 (s, 1H), 7.99 (s, 1H), 7.34–7.26 (m, 3H), 7.24–7.18 (m, 3H), 5.72 (q, J = 7.0 Hz, 1H), 4.79 (d, J = 2.1 Hz, 1H), 4.68 (t, J = 1.8 Hz, 1H), 3.04–2.90 (m, 3H), 2.74–2.55 (m, 2H), 2.20–2.09 (m, 2H), 2.02 (d, J = 7.0 Hz, 3H), 1.90–1.75 (m, 3H), 1.73 (s, 3H), 1.72–1.57 (m, 2H), 1.57–1.30 (m, 6H), 1.30–1.13 (m, 9H), 1.09 (s, 3H), 0.99 (d, J = 10.7 Hz, 6H), 0.95–0.86 (m, 1H), 0.85 (s, 3H).
- 13C NMR (101 MHz, CDCl3): δ 152.19, 145.19, 141.02, 128.64, 127.55, 126.23, 110.20, 59.28, 58.45, 54.84, 50.92, 49.54, 45.65, 42.33, 40.62, 38.94, 38.41, 37.19, 36.26, 33.81, 33.38, 31.47, 30.54, 29.97, 28.89, 25.53, 23.74, 21.52, 21.32, 19.40, 18.90, 16.29, 15.67, 14.65.
- HRMS (ESI+): m/z calculated for C40H55N6O [M + H]+: 635.4432, found: 635.4393.
- 1′-(3-Methoxybenzyl)-1H′-lup-2-eno-[2,3-d]-[1,2,3]-triazole-28-(1H-triazol-1-yl) (14). Compound 5 (93 mg, 1 equiv., 0.155 mmol) was dissolved in 2 mL of THF, and 1,1′-carbonyldi-(1,2,4-triazole) (152.6 mg, 6 equiv., 0.93 mmol) was added. The reaction mixture was stirred at 70 °C. The reaction was carried out for 24 h. The solvent was distilled in a rotary evaporator. The remainder was chromatographed with silica gel in a column. When the column was eluted with a mixture of petroleum ether and ethyl acetate (4:1), compound 14 was isolated. Compound 14 was a colorless powder, m.p. 279–282 °C. The yield was 52 mg (52%).
- 1H NMR (400 MHz, CDCl3): δ 8.92 (s, 1H), 7.99 (s, 1H), 7.21 (t, J = 7.9 Hz, 1H), 6.82–6.77 (m, 1H), 6.63–6.58 (m, 1H), 6.56 (t, J = 2.1 Hz, 1H), 5.62 (s, 2H), 4.80 (d, J = 2.2 Hz, 1H), 4.71–4.64 (m, 1H), 3.74 (s, 3H), 3.05–2.90 (m, 3H), 2.68 (td, J = 12.4, 3.4 Hz, 1H), 2.60 (dd, J = 12.9, 7.3 Hz, 1H), 2.17 (s, 5H), 1.92–1.75 (m, 3H), 1.74 (s, 3H), 1.72–1.64 (m, 1H), 1.63 (d, J = 1.9 Hz, 2H), 1.60–1.34 (m, 5H), 1.35–1.19 (m, 2H), 1.18 (s, 3H), 1.06 (s, 3H), 1.03 (s, 3H), 0.97 (s, 4H), 0.80 (s, 3H).
- 13C NMR (101 MHz, CDCl3): δ 173.39, 159.97, 152.21, 149.83, 145.18, 141.87, 138.10, 138.00, 129.78, 118.65, 113.27, 112.00, 110.20, 58.44, 55.23, 54.66, 52.74, 50.91, 49.49, 45.65, 42.33, 40.60, 39.01, 38.48, 37.18, 36.27, 33.74, 33.32, 31.47, 30.54, 29.96, 28.73, 25.53, 21.54, 21.35, 19.41, 18.91, 16.13, 15.65, 14.66.
- HRMS (ESI+): m/z calculated for C40H55N6O2 [M + H]+: 651.4381, found: 651.4378.
- 1′-(3-Fluorobenzyl)-1H′-lup-2-eno-[2,3-d]-[1,2,3]-triazole-28-(1H-triazol-1-yl) (15). Compound 7 (50 mg, 1 equiv., 0.085 mmol) was dissolved in 2 mL of THF, and 1,1′-carbonyldi-(1,2,4-triazole) (55.7 mg, 4 equiv., 0.34 mmol) was added. The reaction mixture was stirred at 70 °C. The reaction was carried out for 2.5 h. The solvent was distilled in a rotary evaporator. The formed compound was washed three times with cold acetone. Compound 15 was a colorless powder, m.p. 307–310 °C. The yield was 61 mg (112%).
- 1H NMR (400 MHz, CDCl3): δ 8.92 (s, 1H), 7.99 (s, 1H), 7.31–7.23 (m, 2H), 7.00–6.91 (m, 1H), 6.81 (dt, JHF = 7.8 Hz, JHH = 1.3 Hz, 1H), 6.72 (dt, JHF = 9.6 Hz, JHH =2.1 Hz, 1H), 5.64 (d, J = 3.0 Hz, 2H), 4.80 (d, J = 2.2 Hz, 1H), 4.68 (t, J = 1.8 Hz, 1H), 3.75 (tdd, J = 5.8, 2.5, 1.6 Hz, 1H), 3.04–2.90 (m, 3H), 2.68 (td, J = 12.4, 3.4 Hz, 1H), 2.64–2.55 (m, 1H), 2.20 (d, J = 15.4 Hz, 1H), 1.91–1.75 (m, 4H), 1.74 (s, 3H), 1.70–1.34 (m, 8H), 1.25 (ddd, J = 25.0, 8.5, 3.2 Hz, 3H), 1.17 (s, 4H), 1.07 (s, 3H), 1.03 (s, 3H), 0.97 (s, 3H), 0.81 (s, 3H).
- 13C NMR (101 MHz, CDCl3): δ 13C NMR (101 MHz, CDCl3): δ 173.38, 161.83 (d, 1JC-F = 245.5 Hz), 152.21, 149.82, 145.18, 142.04, 139.01 (d, 3JC-F = 7.2 Hz), 138.07, 130.35 (d, 3JC-F = 8.3 Hz), 121.94 (d, 4JC-F = 2.9 Hz), 114.83 (d, 2JC-F = 20.9 Hz), 113.52 (d, 2JC-F = 22.6 Hz), 110.21, 67.98, 58.44, 54.61, 52.25, 50.91, 49.49, 45.65, 42.34, 40.60, 39.01, 38.42, 37.18, 36.27, 33.72, 33.30, 31.46, 30.53, 29.96, 28.79, 25.52, 21.54, 21.41, 19.40, 18.90, 16.16, 15.65, 14.66.
- HRMS (ESI+): m/z calculated for C39H52FN6O [M + H]+: 639.4181, found: 639.4180.
- 1′-Butyl-1H′-lup-2-eno-[2,3-d]-[1,2,3]-triazole-28-(1H-triazol-1-yl) (16). Compound 8 (50 mg, 1 equiv., 0.0934 mmol) was dissolved in 2 mL of THF, and 1,1′-carbonyldi-(1,2,4-triazole) (61.2 mg, 4 equiv., 0.3736 mmol) was added. The reaction mixture was stirred at 70 °C. The reaction was carried out for 2 h. The solvent was distilled in a rotary evaporator. The formed compound was washed three times with cold acetone. Compound 16 was a colorless powder, m.p. 281–283° C. The yield was 59 mg (108%).
- 1H NMR (400 MHz, CDCl3): δ 8.93 (s, 1H), 8.00 (s, 1H), 7.27 (s, 1H), 4.79 (d, J = 2.2 Hz, 1H), 4.71–4.63 (m, 1H), 4.29 (td, J = 7.1, 1.4 Hz, 2H), 3.05–2.89 (m, 3H), 2.68 (td, J = 12.4, 3.4 Hz, 1H), 2.63–2.56 (m, 1H), 2.19–2.10 (m, 2H), 1.97 (pd, J = 7.1, 1.4 Hz, 2H), 1.89–1.75 (m, 3H), 1.71 (d, J = 17.7 Hz, 5H), 1.66–1.34 (m, 7H), 1.30 (s, 3H), 1.27–1.18 (m, 4H), 1.12 (td, J = 13.0, 4.3 Hz, 1H), 1.03 (s, 3H), 1.01–0.94 (m, 6H), 0.80 (s, 3H).
- 13C NMR (101 MHz, CDCl3): δ 173.39, 152.21, 149.82, 145.19, 141.02, 137.26, 110.20, 58.45, 54.75, 50.92, 49.50, 49.36, 45.65, 42.34, 40.62, 39.01, 38.39, 37.19, 36.27, 33.68, 33.36, 32.86, 31.48, 30.54, 29.98, 28.68, 25.53, 21.52, 21.36, 20.18, 19.40, 18.97, 16.11, 15.67, 14.70, 13.70.
- HRMS (ESI+): m/z calculated for C36H55N6O [M + H]+: 587.4432, found: 587.4438.
- 1′-((S)-1-Phenylethyl)-1H′-lup-2-eno-[2,3-d]-[1,2,3]-triazole-isocyanate (17). Compound 3 (200 mg, 1 equiv., 0.34 mmol) was dissolved in toluene (6 mL) in an ultrasonic bath, then triethylamine (0.0464 mL, 1 equiv., 0.34 mmol) and diphenylphosphoryl azide (0.0772 mL, 1 equiv., 0.34 mmol) were added. The reaction was carried out at room temperature (21 °C) for 6 h. At the end of the reaction, the solvent was distilled in a rotary evaporator. The remainder was chromatographed with silica gel in a column. Compound 17 was a colorless powder, m.p. 197–200 °C. The yield was 120 mg (61%).
- 1H NMR (400 MHz, CDCl3): δ 7.29 (m, 3H), 7.22 (m, 2H), 5.73 (q, J = 7.0 Hz, 1H), 4.77 (d, J = 2.1 Hz, 1H), 4.67 (m, 1H), 2.97 (d, J = 15.3 Hz, 1H), 2.56 (td, J = 10.9, 5.8 Hz, 1H), 2.13 (m, 2H), 2.02 (d, J = 7.0 Hz, 3H), 1.84 (m, 5H), 1.70 (d, J = 1.2 Hz, 3H), 1.52 (m, 8H), 1.29 (s, 3H), 1.20 (m, 2H), 1.11 (d, J = 1.8 Hz, 6H), 0.94 (s, 3H), 0.84 (m, 3H).
- 13C NMR (101 MHz, CDCl3): δ 148.71, 141.83, 140.99, 137.51, 128.64, 127.55, 126.24, 121.63, 110.63, 71.59, 59.27, 54.80, 49.32, 49.18, 48.10, 42.06, 40.66, 39.27, 39.17, 38.91, 38.42, 33.81, 33.57, 33.46, 29.29, 28.91, 27.81, 24.91, 23.74, 21.36, 21.34, 19.52, 18.89, 16.29, 15.69, 14.44.
- HRMS (ESI+): m/z calculated for C38H53N4O [M + H]+: 581.4214, found: 581.4218.
- 1′-((S)-1-Phenylethyl)-1H′-lup-2-eno-[2,3-d]-[1,2,3]-triazole-28-oic Acid derivative-1. (18). Compound 3 (100 mg, 1 equiv., 0.17 mmol) was dissolved in toluene (3 mL) in an ultrasonic bath, then triethylamine (0.024 mL, 1 equiv., 0.17 mmol) and diphenylphosphoryl azide (0.039 mL, 1 equiv., 0.17 mmol) were added. The reaction was carried out at room temperature (20 °C) for 7 h, after which cyclopropylamine (0.177 mL, 10 equiv., 1.7 mmol) in 0.5 mL of toluene was added to the reaction mixture. Then, it was heated at 110 °C for 4 h. At the end of the reaction, the solvent was distilled in a rotary evaporator. The remainder was chromatographed with silica gel in a column. During elution of the column with ethyl acetate, a colorless substance (compound 18) with a melting point of 200–203 °C was isolated. The yield was 66 mg (61%).
- 1H NMR (400 MHz, CDCl3): δ 7.34–7.25 (m, 3H), 7.23–7.18 (m, 2H), 5.73 (q, J = 7.0 Hz, 1H), 4.95 (s, 1H), 4.75 (d, J = 2.1 Hz, 1H), 4.69–4.60 (m, 2H), 2.96 (d, J = 15.2 Hz, 1H), 2.67 (dt, J = 13.2, 3.3 Hz, 1H), 2.54 (dd, J = 12.5, 8.1 Hz, 1H), 2.44 (tq, J = 7.9, 4.6, 4.1 Hz, 2H), 2.16 (d, J = 15.2 Hz, 1H), 2.07–1.91 (m, 4H), 1.82–1.64 (m, 7H), 1.62–1.16 (m, 11H), 1.10 (d, J = 9.6 Hz, 6H), 0.99 (s, 3H), 0.95–0.75 (m, 6H), 0.69–0.56 (m, 2H).
- 13C NMR (101 MHz, CDCl3): δ 158.04, 149.40, 141.76, 140.94, 137.53, 128.66, 127.59, 126.23, 110.27, 63.72, 59.28, 54.74, 49.31, 49.23, 48.33, 42.07, 40.64, 38.89, 38.42, 38.37, 35.19, 33.83, 33.15, 29.86, 29.33, 28.89, 27.34, 25.11, 23.72, 22.70, 21.48, 21.35, 19.28, 18.90, 16.29, 15.58, 14.48, 7.82, 7.35.
- HRMS (ESI+): m/z calculated for C41H60N5O [M + H]+: 638.4792, found: 638.4750.
- 1′-((S)-1-Phenylethyl)-1H′-lup-2-eno-[2,3-d]-[1,2,3]-triazole-28-oic Acid derivative-2. (19). Compound 3 (100 mg, 1 equiv., 0.17 mmol) was dissolved in toluene (3 mL) in an ultrasonic bath, then triethylamine (0.024 mL, 1 equiv., 0.17 mmol) and diphenylphosphoryl azide (0.039 mL, 1 equiv., 0.17 mmol) were added. The reaction was carried out at room temperature (21 °C) for 2 h, after which ethanolamine (0.1026 mL, 10 equiv., 1.7 mmol) in 0.5 mL of toluene was added to the reaction mixture. Then, it was heated at 110 °C for 7 h. At the end of the reaction, the solvent was distilled in a rotary evaporator. The remainder was chromatographed with silica gel in a column. During elution of the column with ethyl acetate, a colorless substance (compound 19) with a melting point of 202–204 °C was isolated. The yield was 126 mg (116%).
- 1H NMR (400 MHz, CDCl3): δ 7.35–7.23 (m, 2H), 7.27–7.14 (m, 2H), 5.79 (s, 1H), 5.75 (q, J = 6.9 Hz, 1H), 5.18 (s, 1H), 4.69 (d, J = 2.3 Hz, 1H), 4.62 (t, J = 1.9 Hz, 1H), 4.12 (q, J = 7.2 Hz, 1H), 3.77–3.60 (m, 3H), 3.60–3.45 (m, 1H), 3.24 (s, 2H), 2.91 (d, J = 15.3 Hz, 1H), 2.68–2.60 (m, 1H), 2.55 (td, J = 11.0, 5.0 Hz, 1H), 2.46 (dd, J = 12.2, 8.1 Hz, 1H), 2.21–2.15 (m, 1H), 2.14 (d, J = 14.3 Hz, 1H), 2.08 (s, 1H), 2.03 (d, J = 12.8 Hz, 2H), 2.02–1.92 (m, 2H), 1.86–1.75 (m, 1H), 1.74 (s, 1H), 1.70 (s, 3H), 1.65 (d, J = 11.6 Hz, 1H), 1.62–1.54 (m, 1H), 1.57–1.47 (m, 4H), 1.47–1.35 (m, 1H), 1.38–1.28 (m, 1H), 1.28 (s, 4H), 1.26 (s, 9H), 1.30–1.19 (m, 1H), 1.13 (s, 3H), 1.19–1.03 (m, 2H), 1.02 (s, 3H), 0.96 (s, 3H), 0.96–0.85 (m, 2H), 0.89–0.81 (m, 2H), 0.79 (s, 3H).
- 13C NMR (101 MHz, CDCl3): δ 174.49, 159.57, 149.75, 141.44, 140.91, 137.89, 128.73, 127.74, 126.14, 110.03, 64.03, 63.73, 59.28, 54.73, 49.53, 49.31, 47.47, 43.51, 42.07, 40.65, 38.86, 38.31, 37.51, 35.61, 33.85, 33.23, 31.93, 29.79, 29.71, 29.66, 29.37, 28.88, 27.43, 25.02, 23.58, 22.70, 21.49, 21.35, 19.23, 18.90, 16.35, 15.70, 14.41, 14.13.
- HRMS (ESI+): m/z calculated for C40H60N5O2 [M + H]+: 642.4742, found: 642.470.
- 1′-((S)-1-Phenylethyl)-1H′-lup-2-eno-[2,3-d]-[1,2,3]-triazole-28-oic Acid derivative-3. (20). Compound 3 (100 mg, 1 equiv., 0.17 mmol) was dissolved in toluene (3 mL) in an ultrasonic bath, then triethylamine (0.024 mL, 1 equiv., 0.17 mmol) and diphenylphosphoryl azide (0.039 mL, 1 equiv., 0.17 mmol) were added. The reaction was carried out at room temperature (21 °C) for 3 h, after which 2-amino-2-methyl-1-propanol (0.162 mL, 10 equiv., 1.7 mmol) in 0.5 mL of toluene was added to the reaction mixture. Then, it was heated at 110 °C for 3.5 h. At the end of the reaction, the solvent was distilled in a rotary evaporator. The remainder was chromatographed with silica gel in a column. During elution of the column with ethyl acetate, a colorless substance (compound 20) with a melting point of 176–178 °C was isolated. The yield was 96 mg (84%).
- 1H NMR (400 MHz, CDCl3): δ 7.30 (dd, J = 8.2, 6.3 Hz, 3H), 7.23–7.16 (m, 2H), 6.45 (s, 1H), 5.75 (q, J = 7.0 Hz, 1H), 5.25 (s, 1H), 5.05 (s, 1H), 4.65 (d, J = 2.3 Hz, 1H), 4.60 (t, J = 1.9 Hz, 1H), 3.55 (s, 2H), 2.92 (d, J = 15.2 Hz, 1H), 2.67 (dt, J = 13.4, 3.4 Hz, 1H), 2.44 (ddd, J = 20.1, 14.0, 9.6 Hz, 2H), 2.17 (d, J = 15.2 Hz, 1H), 2.08 (s, 0H), 2.02 (d, J = 7.0 Hz, 3H), 1.99–1.90 (m, 1H), 1.75 (td, J = 14.7, 14.1, 8.5 Hz, 2H), 1.68 (s, 3H), 1.62 (d, J = 11.7 Hz, 1H), 1.62–1.40 (m, 5H), 1.39–1.24 (m, 7H), 1.23 (d, J = 1.9 Hz, 6H), 1.09 (d, J = 22.1 Hz, 8H), 0.96 (s, 3H), 0.93–0.78 (m, 7H).
- 13C NMR (101 MHz, CDCl3): δ 158.88, 149.68, 141.54, 140.91, 128.70, 127.69, 126.18, 109.99, 72.29, 63.89, 59.30, 54.78, 54.76, 49.51, 49.35, 47.37, 42.04, 40.62, 38.89, 38.36, 37.52, 35.61, 33.85, 33.24, 29.84, 29.55, 28.89, 27.40, 25.26, 25.22, 25.04, 23.60, 21.50, 21.35, 19.29, 18.89, 16.33, 15.74, 14.45.
- HRMS (ESI+): m/z calculated for C42H64N5O2 [M + H]+: 670.5055, found: 670.5093.
- 1′-((S)-1-Phenylethyl)-1H′-lup-2-eno-[2,3-d]-[1,2,3]-triazole-28- oic Acid derivative-4. (21). Compound 3 (100 mg, 1 equiv., 0.17 mmol) was dissolved in toluene (3 mL) in an ultrasonic bath, then triethylamine (0.024 mL, 1 equiv., 0.17 mmol) and diphenylphosphoryl azide (0.039 mL, 1 equiv., 0.17 mmol) were added. The reaction was carried out at room temperature (22 °C) for 3 h, after which 3-amino-1,2-propanediol (0.1317 mL, 10 equiv., 1.7 mmol) was added to the reaction mixture. Then, it was heated at 110 °C for 2 h. At the end of the reaction, the solvent was distilled in a rotary evaporator. The remainder was chromatographed with silica gel in a column. Elution of the column with a mixture of ethyl acetate and methanol (10:1) isolated a colorless substance (compound 21) with a melting point of 182–185 °C. The yield was 89 mg (78%).
- 1H NMR (400 MHz, CDCl3): δ 7.35–7.23 (m, 3H), 7.18 (td, J = 7.3, 1.9 Hz, 2H), 5.95 (s, 1H), 5.76 (q, J = 7.3 Hz, 1H), 5.35–5.18 (m, 1H), 4.68 (s, 1H), 4.64–4.57 (m, 1H), 4.12 (q, J = 7.2 Hz, 1H), 3.82–3.70 (m, 1H), 3.70–3.58 (m, 1H), 3.59–3.39 (m, 2H), 3.26 (dt, J = 16.1, 5.3 Hz, 2H), 2.92 (dd, J = 20.5, 15.2 Hz, 1H), 2.69–2.59 (m, 1H), 2.54 (ddq, J = 16.3, 11.8, 6.9, 5.2 Hz, 1H), 2.42 (dd, J = 12.0, 8.1 Hz, 1H), 2.24–2.10 (m, 1H), 2.06 (d, J = 10.6 Hz, 1H), 2.01 (d, J = 6.9 Hz, 3H), 1.97–1.71 (m, 1H), 1.69 (s, 3H), 1.67–1.30 (m, 5H), 1.30–1.19 (m, 7H), 1.12 (d, J = 8.0 Hz, 4H), 1.03–0.89 (m, 6H), 0.89–0.80 (m, 1H), 0.78 (s, 2H).
- 13C NMR (101 MHz, CDCl3): δ 159.68, 149.68, 141.37, 140.94, 138.02, 128.76, 128.68, 127.79, 126.18, 126.08, 110.09, 71.98, 63.85, 63.83, 63.30, 59.27, 54.71, 49.45, 49.31, 47.39, 42.50, 42.06, 40.63, 38.86, 38.29, 37.50, 35.72, 33.86, 33.22, 30.84, 29.71, 28.85, 27.41, 25.62, 25.01, 23.63, 23.52, 21.52, 21.34, 19.22, 18.89, 16.40, 15.68, 14.43, 14.20.
- HRMS (ESI+): m/z calculated for C41H62N5O3 [M + H]+: 672.4847, found: 672.4858.
- 1′-((S)-1-Phenylethyl)-1H′-lup-2-eno-[2,3-d]-[1,2,3]-triazole-28-oic Acid derivative-5. (22). Compound 3 (100 mg, 1 equiv., 0.17 mmol) was dissolved in toluene (3 mL) in an ultrasonic bath, then triethylamine (0.024 mL, 1 equiv., 0.17 mmol) and diphenylphosphoryl azide (0.039 mL, 1 equiv., 0.17 mmol) were added. The reaction was carried out at room temperature (22 °C) for 3 h, after which 2-(2-aminoethoxy)ethanol (0.1705 mL, 10 equiv., 1.7 mmol) in 0.5 mL of toluene was added to the reaction mixture. Then, it was heated at 110 °C for 2 h. At the end of the reaction, the solvent was distilled in a rotary evaporator. The remainder was chromatographed with silica gel in a column. Elution of the column with a mixture of ethyl acetate and methanol (20:1) isolated a colorless substance (compound 22) with a melting point of 165–167 °C. The yield was 76 mg (65%).
- 1H NMR (400 MHz, CDCl3): δ 7.37–7.23 (m, 3H), 7.22–7.16 (m, 2H), 5.74 (q, J = 7.0 Hz, 1H), 5.49 (s, 1H), 4.76–4.67 (m, 2H), 4.62 (t, J = 1.9 Hz, 1H), 3.83–3.68 (m, 3H), 3.68–3.60 (m, 3H), 3.60–3.51 (m, 4H), 3.36 (d, J = 5.1 Hz, 2H), 2.93 (d, J = 15.2 Hz, 1H), 2.71–2.61 (m, 1H), 2.49 (ddt, J = 20.7, 12.4, 6.5 Hz, 2H), 2.16 (d, J = 15.2 Hz, 1H), 2.06 (d, J = 8.7 Hz, 0H), 2.01 (d, J = 7.0 Hz, 3H), 1.76 (d, J = 11.2 Hz, 2H), 1.70 (s, 3H), 1.68–1.28 (m, 7H), 1.27 (s, 7H), 1.19–1.02 (m, 7H), 0.96 (s, 3H), 0.90–0.82 (m, 5H), 0.80 (s, 3H).
- 13C NMR (101 MHz, CDCl3): δ 158.08, 149.69, 141.63, 140.96, 137.71, 128.69, 127.65, 126.19, 110.06, 72.36, 70.81, 63.60, 61.66, 61.14, 59.30, 54.74, 49.50, 49.27, 47.52, 42.07, 41.35, 40.63, 40.18, 38.87, 38.32, 37.63, 35.71, 33.83, 33.25, 29.81, 29.77, 28.88, 27.39, 25.03, 23.67, 22.63, 21.46, 21.35, 19.26, 18.91, 16.32, 15.71, 14.42.
- HRMS (ESI+): m/z calculated for C42H64N5O3 [M + H]+: 686.5004, found: 686.4954.
3.3. In Vitro Biological Assays
3.3.1. Antimicrobial Activity
The antimicrobial activity of the samples was evaluated in relation to strains of the Gram-positive bacteria Staphylococcus aureus and Bacillus subtilis, the Gram-negative bacteria Escherichia coli and Pseudomonas aeruginosa, and the yeast fungus Candida albicans by serial dilution with determination of the minimum inhibitory concentration (MIC). The test strains of the microorganisms used in the study were obtained from the American Type Culture Collection. An antibacterial drug, ceftriaxone; benzylpenicillin sodium salt; and an antifungal drug, nystatin, were used as comparison drugs and served as positive controls.
MIC was determined by the method of serial dilution of ethanol solutions of the test samples in a nutrient broth. Suspensions of test strains at a concentration of 106 CFU/mL were used to carry out the method of serial dilutions. A suspension of test strains of the microorganisms was prepared from daily cultures grown on mown agar at a temperature of 37 °C for 24 h and at 30 °C for 48 h for the yeast fungus Candida albicans. The antimicrobial activity of the samples was studied at dilutions in the range of 1.56–50 µg/mL. A quantity of 0.1 mL of microbial suspension at a concentration of 106 CFU/mL was added to each tube with a working dilution of each test sample. The procedure was repeated for all cultures tested. A suspension of microbes with a nutrient medium without a sample was placed in control tubes to serve as a negative control. The mixture was incubated in a thermostat for 24–48 h, depending on the class of microorganism.
Then, the presence of turbidity in each of the tubes was visually determined, and the one that contained a transparent suspension and the lowest concentration of the antimicrobial agent was selected. This concentration corresponded to the MIC. The results were averaged according to the data of three experiments.
3.3.2. Cytotoxic Activity
The cytotoxicity of the synthesized compounds against Artemia salina (Leach) was evaluated in accordance with the methodology proposed by Meyer et al. [49].
The cytotoxicity of the samples was evaluated in the survival test of larvae of Artemia salina (Leach) crustaceans.
The experiments were carried out on larvae of 2 days of age under in vitro cultivation conditions. The larvae were grown by immersing the eggs of Artemia salina (Leach) crustaceans in artificial seawater and incubating them for 48 h at a temperature of 37 °C. The samples were dissolved in 2 mL of ethanol, then 500 µL (3 parallels), 50 µL (3 parallels) and 5 µL (3 parallels) were taken from this solution. After evaporation of ethanol, 5 mL of artificial seawater was added to each bottle.
Thus, if the initial weight of the sample was 2 mg, then the final concentrations of the sample were 100 µg/mL, 10 µg/mL and 1 µg/mL, respectively, with 3 repetitions for each concentration. Ten larvae of 2-day-old Artemia salina crustaceans were planted in each vial using a Pasteur pipette. After that, all the vials were left at room temperature in the light for 24 h.
After 24 h, the surviving and dead larvae were counted. Then, using the obtained data on the upper and lower toxic limits, the half-toxic dose of the sample was calculated. The control was DMSO in equal amounts.
The test was performed using ready-made samples as well as a comparison drug, dactinomycin (actinomycin D), which had antitumor (cytotoxic) activity (producer: Sigma Aldrich, St. Louis, MO, USA).
Lethal concentrations of these compounds, leading to 50% death of the shrimp (LC50), and 95% confidence intervals were determined based on 24 h calculations with probit analysis, and LC50 values were obtained with a 95% confidence interval [49].
4. Conclusions
Thus, in the synthesis of heterocyclic derivatives based on lupane-structured triterpenoid betulonic acid, 21 compounds were obtained, 18 of which were new. The physico-chemical properties of the synthesized compounds were proven by spectroscopic methods. As a result of the study of biological activity, it was found that most of the tested compounds exhibited antimicrobial and cytotoxic activity. Compound 6 showed pronounced antimicrobial activity against the Gram-positive Staphylococcus aureus test strain ATCC 6538, and compound 7 showed pronounced antimicrobial activity against the Gram-negative Escherichia coli test strain ATCC 25922.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules29133149/s1, NMR, HRMS spectra.
Author Contributions
Conceptualization, R.I.J. and W.D.; methodology, R.I.J. and W.D.; validation, R.I.J. and W.D.; investigation, R.I.J., A.S.A., G.K.M. and R.B.S.; resources, W.D., R.I.J., S.A. and G.K.M.; writing—original draft preparation, R.I.J., Y.M.S. and A.S.A.; writing—review and editing, R.I.J., W.D., G.K.M. and Z.Z.Z.; visualization, R.I.J. and W.D.; supervision, R.I.J. and W.D.; project administration, R.I.J. and W.D.; funding acquisition, R.I.J., G.K.M., A.S.A., Z.Z.Z., Y.M.S. and R.B.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the JSC “Center for International Programs” (www.bolashak.gov.kz), the Ministry of Science and Higher Education of the Republic of Kazakhstan (grant no. AP19674667).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
The authors thank the staff for technical support with the NMR spectroscopy and for technical assistance with the mass spectrometry.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Myagchilov, A.V. Triterpenoids of Inflorescences of Synurus deltoides (Asteraceae) from Primorskii Krai. Dokl. Biol. Sci. 2023, 512, 333–335. [Google Scholar] [CrossRef]
- Huang, Y.; Zhang, E.B.; Luo, J.H.; Gu, W.J.; Tao, M.; Geng, H. Terpenoids from the Petroleum Ether Extract of Artemisia argyi. Chem. Nat. Compd. 2024, 60, 68–71. [Google Scholar] [CrossRef]
- Babekov, A.U.; Yuldasheva, N.M.; Ismailova, A.M.; Komilov, B.D.; Turgunov, K.K.; Tashkhodzhaev, B.; Eshbakova, K.A. Terpenoids from the Plant Ferula ferganensis. Chem. Nat. Compd. 2022, 58, 967–969. [Google Scholar] [CrossRef]
- Fan, Y.Z.; Tian, C.; Tong, S.Y.; Liu, Q.; Xu, F.; Shi, B.-B.; Ai, H.-L.; Liu, J.-K. The antifungal properties of terpenoids from the endophytic fungus Bipolaris eleusines. Nat. Prod. Bioprospect. 2023, 13, 43. [Google Scholar] [CrossRef]
- Tholl, D. Biosynthesis and Biological Functions of Terpenoids in Plants. Advances in Biochemical Engineering. In Biotechnology of Isoprenoids. Advances in Biochemical Engineering/Biotechnology; Springer International: Cham, Switzerland, 2015; pp. 63–106. [Google Scholar] [CrossRef]
- Castañeta, G.; Cifuentes, N.; Sepulveda, B.; Bárcenas-Pérez, D.; Cheel, J.; Areche, C. Untargeted Metabolomics by Using UHPLC–ESI–MS/MS of an Extract Obtained with Ethyl Lactate Green Solvent from Salvia rosmarinus. Separations 2022, 9, 327. [Google Scholar] [CrossRef]
- Ivakhnov, A.D.; Selivanova, N.V.; Krasikova, A.A.; Stavrianidi, A.N.; Gusakova, M.A.; Bogolitsyn, K.G. Extraction of Terpenes of Common Juniper Greenery Under Sub- and Supercritical Conditions. Russ. J. Phys. Chem. B 2022, 16, 1354–1360. [Google Scholar] [CrossRef]
- Venkatesh, K.S.; Krishnamoorthi, S.R.; Palani, N.S.; Thirumal, V.; Jose, S.P.; Wang, F.-M.; Ilangovan, R. Facile one step synthesis of novel TiO2 nanocoral by sol–gel method using Aloe vera plant extract. Indian J. Phys. 2015, 89, 445–452. [Google Scholar] [CrossRef]
- Pirsaheb, M.; Gholami, T.; Seifi, H.; Dawi, E.A.; Said, E.A.; Hamoody, A.-H.M.; Altimari, U.S.; Salavati-Niasari, M. Green synthesis of nanomaterials by using plant extracts as reducing and capping agents. Environ. Sci. Pollut. Res. 2024, 31, 24768–24787. [Google Scholar] [CrossRef]
- Cao, Y.; Xian, M. Recent Advances in Microbial Production of Terpenoids from Biomass-derived Feedstocks. Chem. Res. Chin. Univ. 2024, 40, 20–28. [Google Scholar] [CrossRef]
- Liu, J.K. Natural products in cosmetics. Nat. Prod. Bioprospect. 2022, 12, 40. [Google Scholar] [CrossRef] [PubMed]
- Gammatantrawet, N.; Nguyễn, C.T.; Susawaengsup, C.; Ramli, A.N.M.; Tongkoom, K.; Chatsungnoen, T.; Dangtungee, R.; Bhuyar, P. Phytochemistry of Medicinal Herbs Belongs to Asclepiadaceae Family for Therapeutic Applications: A Critical Review. Mol. Biotechnol. 2024. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.G.; Zhao, T.T.; Lu, N.; Yang, Y.A.; Zhu, H.L. Research Progress of Glycyrrhizic Acid on Antiviral Activity. Mol. Biotechnol. 2019, 19, 826–832. [Google Scholar] [CrossRef] [PubMed]
- Fan, M.; Yuan, S.; Li, L.; Zheng, J.; Zhao, D.; Wang, C.; Wang, H.; Liu, X.; Liu, J. Application of Terpenoid Compounds in Food and Pharmaceutical Products. Fermentation 2023, 9, 119. [Google Scholar] [CrossRef]
- Caputi, L.; Aprea, E. Use of terpenoids as natural flavouring compounds in food industry. Recent Patents Food Nutr. Agric. 2011, 3, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Lipeeva, A.V.; Dolgikh, M.P.; Tolstikova, T.G.; Shults, E.E. A Study of Plant Coumarins. 18. Conjugates of Coumarins with Lupane Triterpenoids and 1,2,3-Triazoles: Synthesis and Anti-Inflammatory Activity. Russ. J. Bioorg. Chem. 2020, 46, 125–132. [Google Scholar] [CrossRef]
- Ticona, L.A.A.; Serban, A.M.; Madorrán, M.J.P.; Fernández-Grifol, M.; Sánchez, Á.R. Anti-Melanogenic and Anti-Inflammatory Activities of Triterpenoids from Jatropha macrantha. Rev. Bras. Farmacogn. 2021, 31, 40–50. [Google Scholar] [CrossRef]
- Khusnutdinova, E.F.; Petrova, A.V.; Kazakova, O.B. Antiviral potency of lupane and oleanane alkynyl-derivatives against human cytomegalovirus and papillomavirus. J. Antibiot. 2024, 77, 50–56. [Google Scholar] [CrossRef]
- Smirnova, I.; Petrova, A.; Lobov, A.; Minnibaeva, E.; Phoung, T.T.T.; Van, L.T.; Khine, M.M.; Esaulkova, I.; Slita, A.; Zarubaev, V.; et al. Azepanodipterocarpol is potential candidate for inhibits influenza H1N1 type among other lupane, oleanane, and dammarane A-ring amino-triterpenoids. J. Antibiot. 2022, 75, 258–267. [Google Scholar] [CrossRef] [PubMed]
- Ayodele, O.A.; Awotuya, I.O.; Taiwo, B.J.; Osungunna, O.M.; Vuyisa, M.; Kasim, S.L. Two New Triterpenoids from the Leaf of Ficus vogelii and Their Antibacterial Activities. Chem. Afr. 2024, 7, 63–70. [Google Scholar] [CrossRef]
- Salimova, E.V.; Magafurova, A.A.; Tretyakova, E.V.; Kukovinets, O.S.; Parfenova, L.V. Indole Derivatives of Fusidane Triterpenoids: Synthesis and the Antibacterial Activity. Chem. Heterocycl. Compd. 2020, 56, 800–804. [Google Scholar] [CrossRef]
- Chinthanom, P.; Vichai, V.; Dokladda, K.; Sappan, M.; Thongpanchang, C.; Isaka, M. Semisynthetic modifications of antitubercular lanostane triterpenoids from Ganoderma. J. Antibiot. 2021, 74, 435–442. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.; Sajid, A.R.; Javeed, A.; Aslam, M.; Ahsan, T.; Hussain, D.; Mateen, A.; Li, X.; Qin, P.; Ji, M. Antioxidant, antifungal, and aphicidal activity of the triterpenoids spinasterol and 22,23-dihydrospinasterol from leaves of Citrullus colocynthis L. Sci. Rep. 2022, 12, 4910. [Google Scholar] [CrossRef] [PubMed]
- Hang, T.X.H.; Jarupinthusophon, S.; Hairani, R.; Nguyen, V.-K.; Chavasiri, W. Cycloartane-type triterpenoids from the leaves of Sandoricum koetjape and their efficacy on α-glucosidase inhibition activity. J. Nat. Med. 2024, 78, 655–663. [Google Scholar] [CrossRef] [PubMed]
- Desmiaty, Y.; Hanafi, M.; Saputri, F.C.; Elya, B.; Rifai, E.A.; Syahdi, R.R. Two triterpenoids from Rubus fraxinifolius leaves and their tyrosinase and elastase inhibitory activities. Sci. Rep. 2021, 11, 20452. [Google Scholar] [CrossRef] [PubMed]
- Aly, S.H.; Elbadry, A.M.M.; Doghish, A.S.; El-Nashar, H.A.S. Unveiling the pharmacological potential of plant triterpenoids in breast cancer management: An updated review. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2024. [Google Scholar] [CrossRef]
- Irungu, B.N.; Nyangi, M.; Ndombera, F.T. Anticancer potential of four triterpenoids against NCI-60 human tumor cell lines. Beni-Suef Univ. J. Basic Appl. Sci. 2024, 13, 50. [Google Scholar] [CrossRef]
- Nazarov, M.A.; Tolmacheva, I.A.; Gagarskih, O.N.; Grishko, V.V. Synthesis and cytotoxic activity of triterpenoids with N,O-heterocyclic fragments based on 2-formyl-1(2)-ene derivative of methyldihydrobetulonate. Chem. Pap. 2023, 77, 2219–2227. [Google Scholar] [CrossRef]
- Giniyatullina, G.V. Synthesis and Antitumor Activity of O- and N-Propylamino-Derivatives of Betulin. Chem. Nat. Compd. 2022, 58, 684–692. [Google Scholar] [CrossRef]
- Govdi, A.I.; Sorokina, I.V.; Baev, D.S.; Bryzgalov, A.O.; Tolstikova, T.G.; Tolstikov, G.A.; Vasilevsky, S.F. Acetylenic derivatives of betulonic acid amide as a new type of compounds possessing spasmolytic activity. Russ. Chem. Bull. 2015, 64, 1327–1334. [Google Scholar] [CrossRef]
- Kazakova, O.B.; Medvedeva, N.I.; Lopatina, T.V.; Apryshko, G.N.; Pugacheva, R.B.; Yavorskaya, N.P.; Golubeva, I.S.; Tolstikov, G.A. Synthesis and the antineoplastic activity of imidazolides of betulonic acid. Russ. J. Bioorg. Chem. 2015, 41, 305–314. [Google Scholar] [CrossRef]
- Giniyatullina, G.V.; Kazakova, O.B. Synthesis and Cytotoxicity of Lupane Mono- and Bis-Piperazinylamides. Chem. Nat. Compd. 2021, 57, 698–705. [Google Scholar] [CrossRef]
- Chue, K.T.; Chang, M.S.; Ten, L.N. Synthesis and antibacterial activity of betulonic acid amides with piperazine derivatives. Chem. Nat. Compd. 2011, 47, 759–763. [Google Scholar] [CrossRef]
- Sorokina, I.V.; Baev, D.S.; Zhukova, N.A.; Tolstikova, T.G.; Antimonova, A.N.; Petrenko, N.I.; Shults, E.E.; Grigor’ev, I.A. Hepatoprotective activity of betulonic acid amides containing piperidine or pyrrolidine nitroxide moieties. Russ. J. Bioorg. Chem. 2013, 39, 668–670. [Google Scholar] [CrossRef]
- Sunitha, V.; Kumar, A.K.; Saikrishna, B. Synthesis of Novel Benzofuran Based 1,2,3-Triazoles, Their Antimicrobial and Cytotoxic Activities, and Molecular Docking Studies. Russ. J. Gen. Chem. 2022, 92, 1348–1359. [Google Scholar] [CrossRef]
- Prasad, C.; Nagesh, P.; Kishan, C.; Krishna, V.M.; Balaswamy, A.; Manga, V.; Prashanth, B.; Aparna, Y. Synthesis, Antimicrobial Evaluation, and In Silico Molecular Docking Studies of Chalcone-Based 1,2,3-Triazoles. Russ. J. Gen. Chem. 2023, 93, 1162–1170. [Google Scholar] [CrossRef]
- Kaushik, C.P.; Chahal, M. Synthesis and antibacterial activity of benzothiazole and benzoxazole-appended substituted 1,2,3-triazoles. J. Chem. Sci. 2020, 132, 142. [Google Scholar] [CrossRef]
- Ashram, M.; Habashneh, A.Y.; Bardaweel, S.; Taha, M.O. A Click Synthesis, Molecular Docking and Biological Evaluation of 1,2,3-triazoles-benzoxazepine hybrid as potential anticancer agents. Med. Chem. Res. 2023, 32, 271–287. [Google Scholar] [CrossRef]
- Seck, I.; Ciss, I.; Diédhiou, A.; Baldé, M.; Ka, S.; Ba, L.A.; Ndoye, F.S.; Figadère, B.; Seon-Meniel, B.; Gomez, G.; et al. 1,2,3-triazenes and 1,2,3-triazoles as antileishmanial, antitrypanosomal, and antiplasmodial agents. Med. Chem. Res. 2023, 32, 158–164. [Google Scholar] [CrossRef]
- Kaushik, C.P.; Chahal, M. Synthesis, antimalarial and antioxidant activity of coumarin appended 1,4-disubstituted 1,2,3-triazoles. Monatsh. Chem. 2021, 152, 1001–1012. [Google Scholar] [CrossRef]
- Denisov, M.S. Synthesis of 2-Heteroylidene Triterpenoids: Complex Formation with Palladium and in vitro Cytotoxic Activity. Russ. J. Gen. Chem. 2023, 93, 37–42. [Google Scholar] [CrossRef]
- Tsepaeva, O.V.; Nemtarev, A.V.; Salikhova, T.I.; Abdullin, T.I.; Eva, L.R.G.; Khozyainova, S.A.; Mironov, V.F. Synthesis, Anticancer, and Antibacterial Activity of Betulinic and Betulonic Acid C-28-Triphenylphosphonium Conjugates with Variable Alkyl Linker Length. Anticancer Agents Med. Chem. 2020, 20, 286–300. [Google Scholar] [CrossRef]
- Kazakova, O.B.; Giniyatullina, G.V.; Mustafin, A.G.; Babkov, D.A.; Sokolova, E.V.; Spasov, A.A. Evaluation of Cytotoxicity and α-Glucosidase Inhibitory Activity of Amide and Polyamino-Derivatives of Lupane Triterpenoids. Molecules 2020, 25, 4833. [Google Scholar] [CrossRef]
- Vasilevsky, S.F.; Govdi, A.I.; Shults, E.E.; Shakirov, M.M.; Sorokina, I.V.; Tolstikova, T.G.; Baev, D.S.; Tolstikov, G.A.; Alabugin, I.V. Efficient synthesis of the first betulonic acid-acetylene hybrids and their hepatoprotective and anti-inflammatory activity. Bioorg. Med. Chem. 2009, 17, 5164–5169. [Google Scholar] [CrossRef]
- Yang, S.-J.; Liu, M.-C.; Zhao, Q.; Hu, D.-Y.; Xue, W.; Yang, S. Synthesis and biological evaluation of betulonic acid derivatives as antitumor agents. Eur. J. Med. Chem. 2015, 96, 58–65. [Google Scholar] [CrossRef]
- Prakash, R.; Opsomer, T.; Dehaen, W. Triazolization of Enolizable Ketones with Primary Amines: A General Strategy toward Multifunctional 1,2,3-Triazoles. Chem. Rec. 2021, 21, 376–385. [Google Scholar] [CrossRef]
- Tulegenova, A.U. State Pharmacopoeia of the Republic of Kazakhstan; Publishing House “Zhibek Zholy”: Almaty, Kazakhstan, 2015; Volume I, 720p. [Google Scholar]
- Mironov, A.N. (Ed.) Guidelines for Conducting Preclinical Studies of Drugs—Part 1; GRIF-K: Moscow, Russia, 2012; p. 206. [Google Scholar]
- Meyer, B.N.; Ferrigni, N.R.; Putnam, J.E.; Jacobsen, L.B.; Nicholsand, D.E.; McLaughlin, J.L. Brine Shrimp: A Convenient General Bioassay for Active Plant Constituents. Planta Medica 1982, 45, 31–34. [Google Scholar] [CrossRef]
- McLaughlin, L. Crown Gall Tumors on Potato Discs and Brine Shrimp Lethality: Two Simple Bioassays for Higher Plant Screening and Fractionation. Methods Plant Boichem. 1991, 6, 1–32. [Google Scholar]
- Genet, C.; Strehle, A.; Schmidt, C.; Boudjelal, G.; Lobstein, A.; Schoonjans, K.; Souchet, M.; Auwerx, J.; Saladin, R.; Wagner, A. Structure-Activity Relationship Study of Betulinic Acid, A Novel and Selective TGR5 Agonist, and Its Synthetic Derivatives: Potential Impact in Diabetes. J. Med. Chem. 2010, 53, 178–190. [Google Scholar] [CrossRef] [PubMed]
- Samoshina, N.F.; Denisenko, M.V.; Denisenko, V.A.; Uvarova, N.I. Synthesis of glycosides of lupane-type triterpene acids. Chem. Nat. Compd. 2003, 39, 575–582. [Google Scholar] [CrossRef]
- Barthel, A.; Stark, S.; Csuk, R. Oxidative transformations of betulinol. Tetrahedron 2008, 64, 9225–9229. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).