Ca-Doping Cobalt-Free Double Perovskite Oxide as a Cathode Material for Intermediate-Temperature Solid Oxide Fuel Cell
Abstract
1. Introduction
2. Results and Discussion
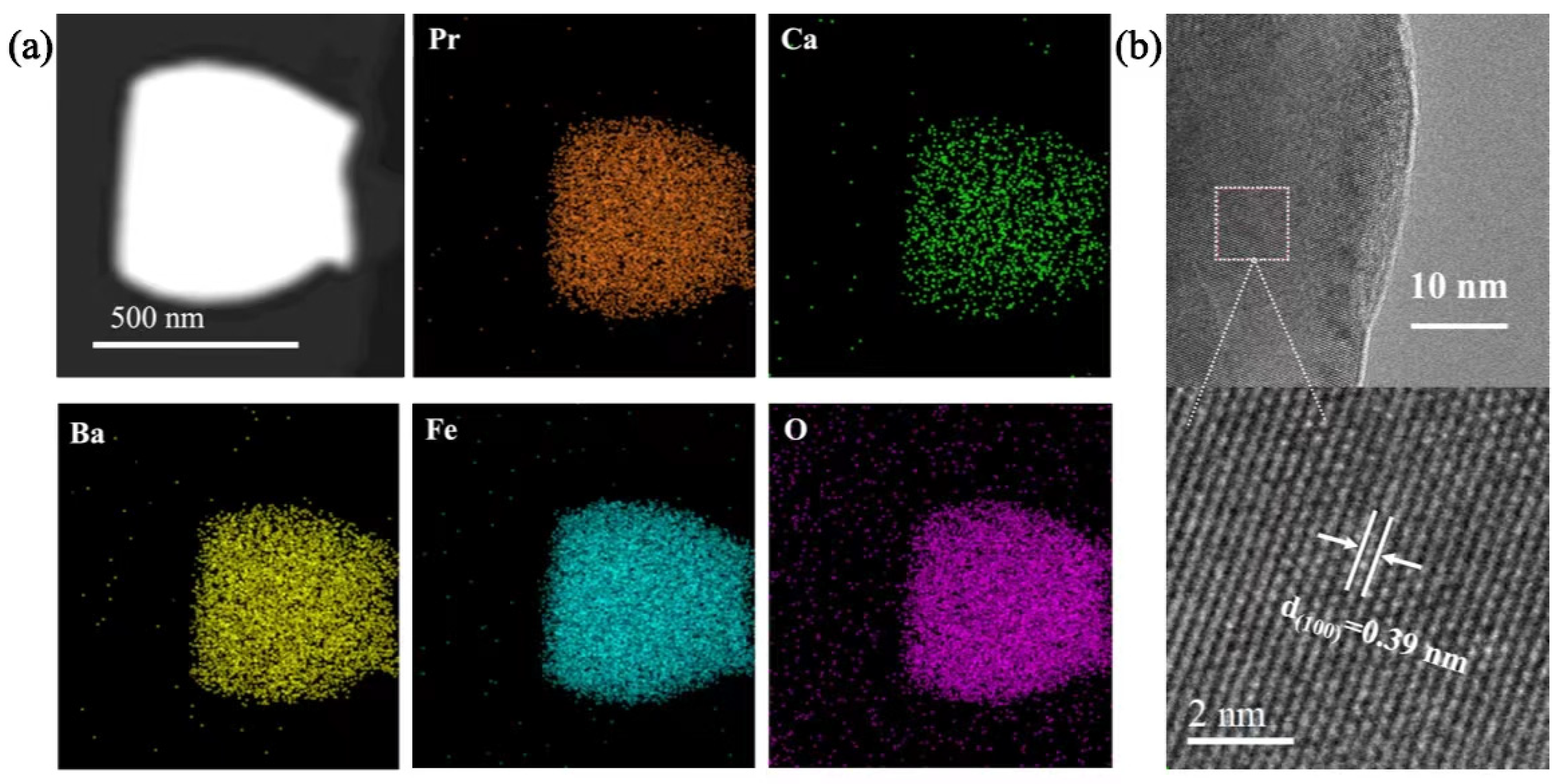
2.1. Elemental Composition and Crystal Structure
2.2. X-ray Photoelectron Spectroscopy Analysis
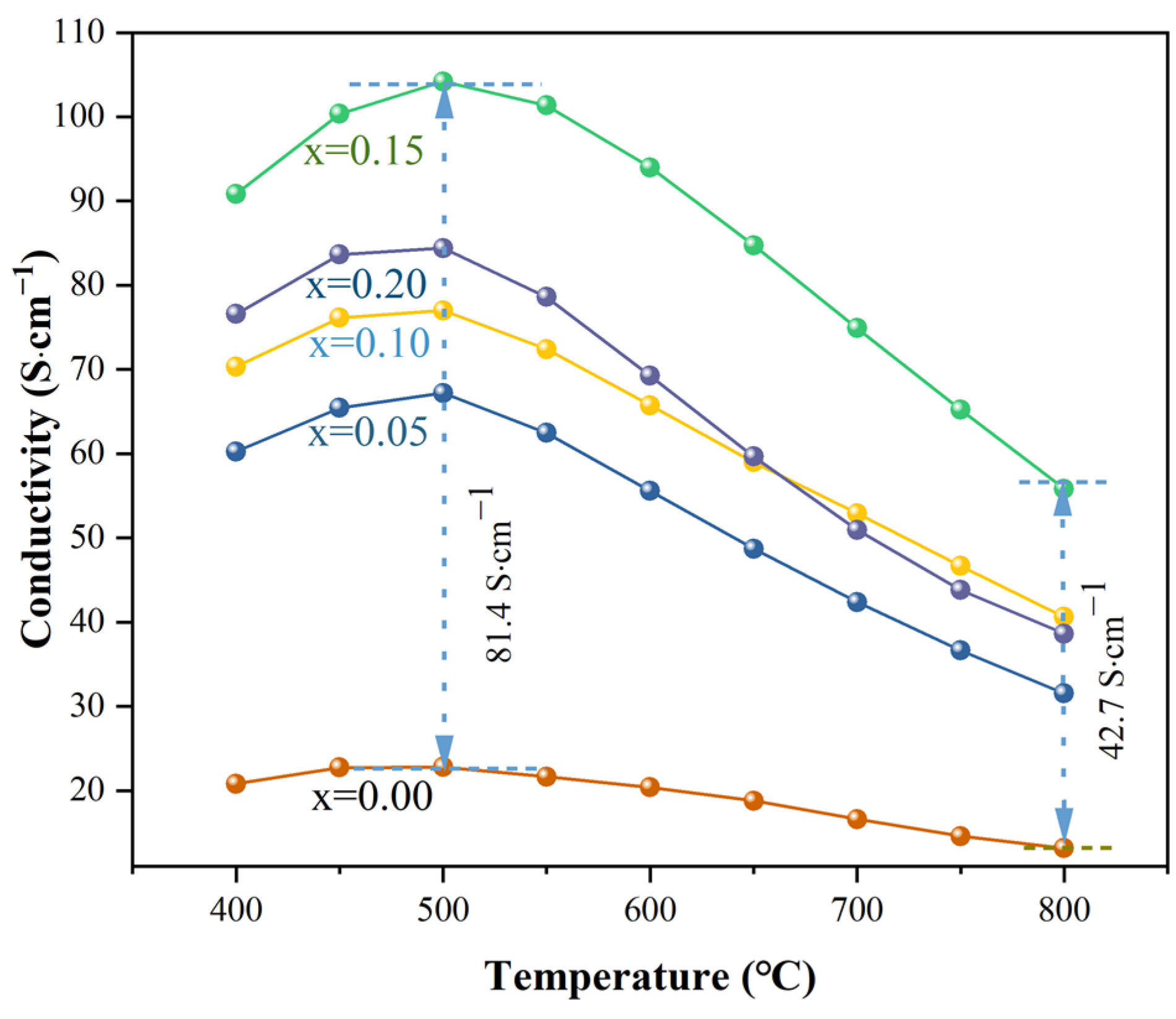
2.3. Electrical Conductivity
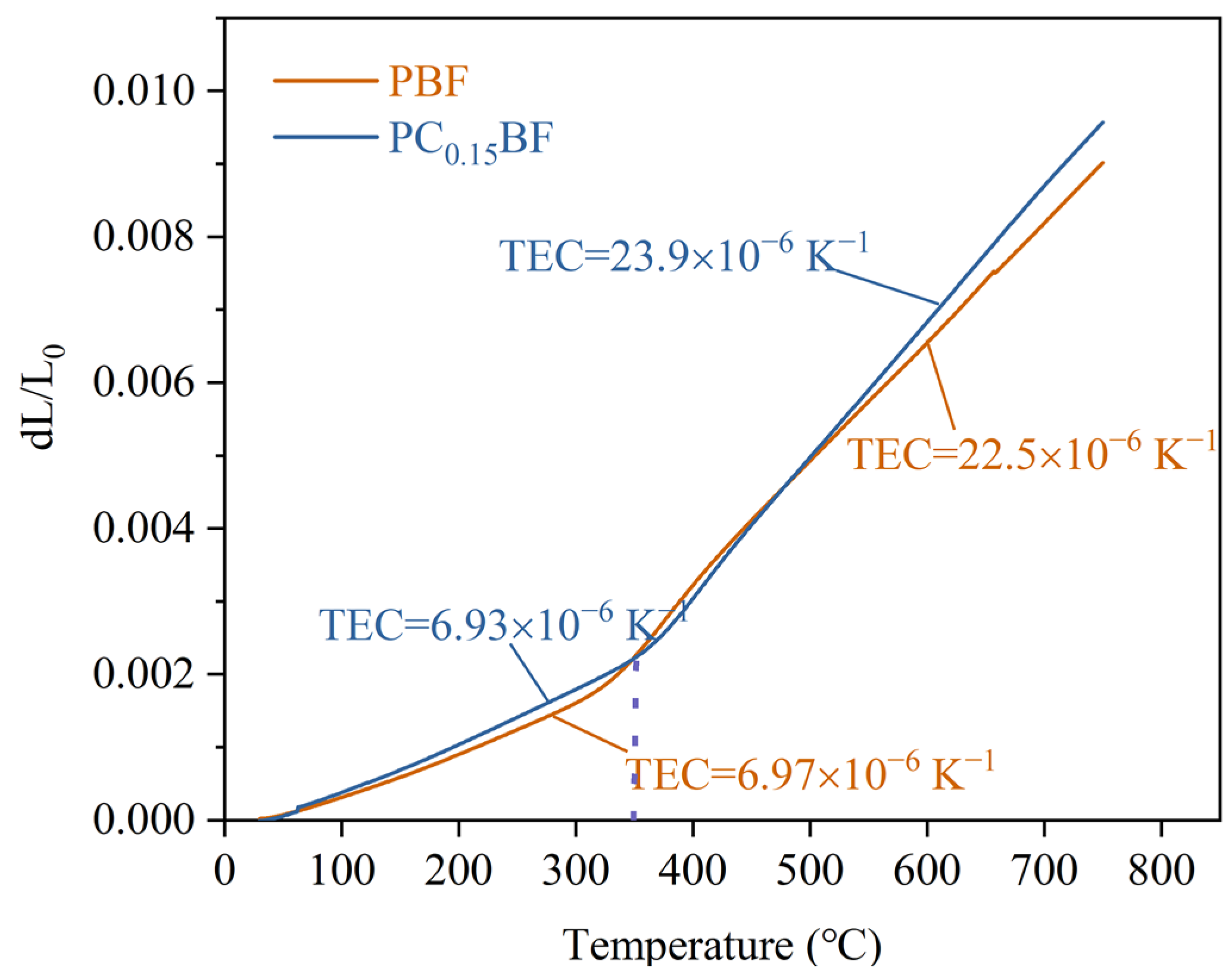
2.4. Thermal Expansion Behavior
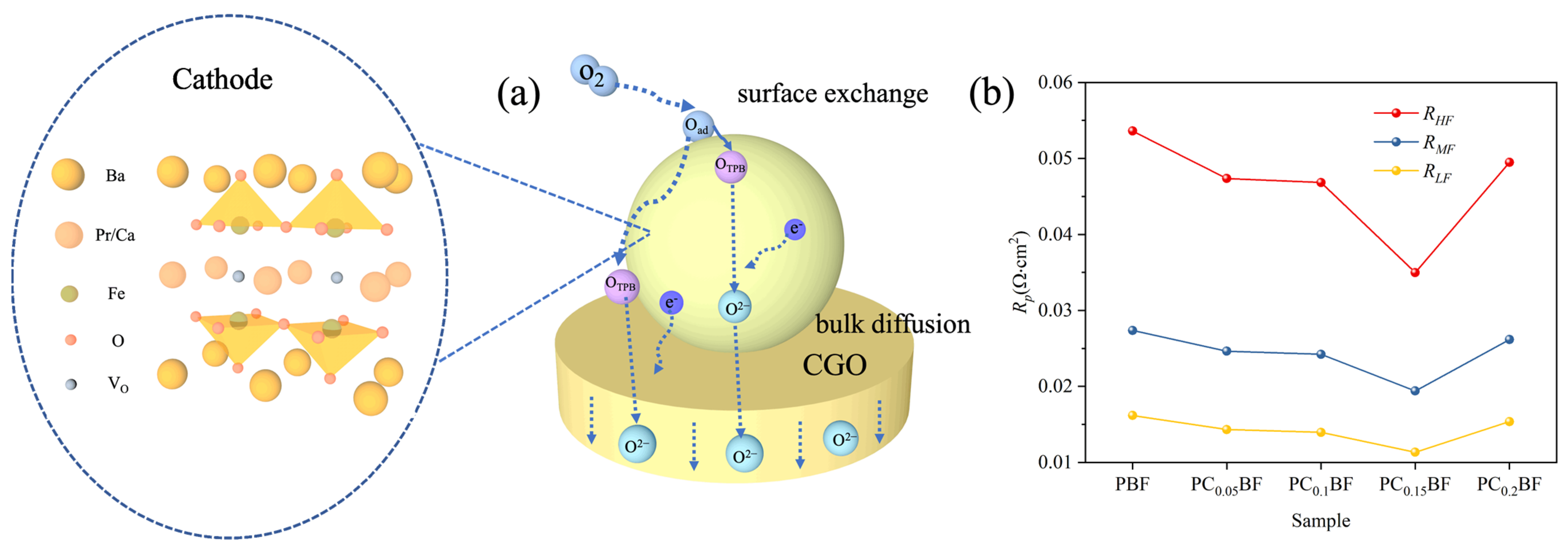
2.5. ORR Activity
| Sample | Electrode | Temperature (°C) | Rp (Ω·cm2) | Reference |
|---|---|---|---|---|
| La0.6Sr0.4Fe0.8Cu0.2O3-δ | SDC | 800 | 0.07 | [33] |
| Bi0.5Sr0.5Fe0.95P0.05O3−δ | GDC | 700 | 0.18 | [34] |
| La0.5Sr0.5Fe0.9Mo0.1O3–δ | SDC | 700 | 0.211 | [35] |
| Sr0.95Ti0.3Fe0.6Ni0.1O3−δ | GDC | 800 | 0.06 | [36] |
| Sm0.8La0.2BaFe2O6-δ | SDC | 800 | 0.12 | [37] |
| Pr1.85Ca0.15BaFe2O5+δ | CGO | 800 | 0.033 | This Work |
2.6. Single-Cell Performance Test
3. Materials and Methods
3.1. Powder Preparation
3.2. Physical and Chemical Properties
3.3. Preparation and Evaluation of Symmetric and Single Cells
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, S.; Wan, Y.; Xu, Z.; Xue, S.; Zhang, L.; Zhang, B.; Xia, C. La0.75Sr0.25Cr0.5Mn0.5O3−δ perovskite as a novel redox-stable efficient anode for solid oxide fuel cells. J. Mater. Chem. A 2020, 8, 11553–11563. [Google Scholar] [CrossRef]
- Dhongde, V.; Singh, A.; Kala, J.; Anjum, U.; Haider, M.A.; Basu, S. Radio-frequency magnetron sputtered thin-film La0.5Sr0.5Co0.95Nb0.05O3-δ perovskite electrodes for intermediate temperature symmetric solid oxide fuel cell (IT-SSOFC). Mater. Rep. Energy 2022, 2, 100095. [Google Scholar] [CrossRef]
- Fan, L.; Zhu, B.; Su, P.-C.; He, C. Nanomaterials and technologies for low temperature solid oxide fuel cells: Recent advances, challenges and opportunities. Nano Energy 2018, 45, 148–176. [Google Scholar] [CrossRef]
- Hanif, M.B.; Motola, M.; Qayyum, S.; Rauf, S.; Khalid, A.; Li, C.; Li, C. Recent advancements, doping strategies and the future perspective of perovskite-based solid oxide fuel cells for energy conversion. Chem. Eng. J. 2022, 428, 132603–132624. [Google Scholar] [CrossRef]
- Yu, X.; Wang, Z.; Ren, R.; Ma, M.; Xu, C.; Qiao, J.; Sun, W.; Sun, K.J. In Situ Self-Reconstructed Nanoheterostructure Catalysts for Promoting Oxygen Reduction Reaction. ACS Energy Lett. 2022, 7, 2961–2969. [Google Scholar] [CrossRef]
- Zhu, Z.; Shi, Y.; Aruta, C.; Yang, N. Improving Electronic Conductivity and Oxygen Reduction Activity in Sr-Doped Lanthanum Cobaltite Thin Films: Cobalt Valence State and Electronic Band Structure Effects. ACS App. Energy Mater. 2018, 1, 5308–5317. [Google Scholar] [CrossRef]
- Li, M.; Zhao, M.; Li, F.; Zhou, W.; Peterson, V.; Xu, X.; Shao, Z.; Gentle, I.; Zhu, Z. A niobium and tantalum co-doped perovskite cathode for solid oxide fuel cells operating below 500 °C. Nat. Commun. 2017, 8, 13990. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Zhong, Y.; Shao, Z. Double Perovskites in Catalysis, Electrocatalysis, and Photo(electro)catalysis. Trends Chem. 2019, 1, 410–424. [Google Scholar] [CrossRef]
- Zhang, W.; Shiraiwa, M.; Wang, N.; Ma, T.; Fujii, K.; Niwa, E.; Yashima, M.J. Pr/Ba cation-disordered perovskite Pr2/3Ba1/3CoO3−δ as a new bifunctional electrocatalyst for oxygen reduction and oxygen evolution reactions. Ceram. Soc. Jpn. 2018, 126, 814–819. [Google Scholar] [CrossRef]
- Kim, J.-H.; Manthiram, A. Layered LnBaCo2O5+δ perovskite cathodes for solid oxide fuel cells: An overview and perspective. J. Mater. Chem. A 2015, 3, 24195–24210. [Google Scholar] [CrossRef]
- Kim, J.-H.; Mogni, L.; Prado, F.; Caneiro, A.; Alonso, J.A.; Manthiram, A. High Temperature Crystal Chemistry and Oxygen Permeation Properties of the Mixed Ionic–Electronic Conductors LnBaCo2O5 + δ ( Ln = Lanthanide ). J. Electrochem. Soc. 2009, 156, 1376. [Google Scholar] [CrossRef]
- Wang, S.; Zan, J.; Qiu, W.; Zheng, D.; Li, F.; Chen, W.; Pei, Q.; Jiang, L. Evaluation of perovskite oxides LnBaCo2O5+δ (Ln = La, Pr, Nd and Sm) as cathode materials for IT-SOFC. J. Electroanal. Chem. 2021, 886, 115–144. [Google Scholar] [CrossRef]
- Kim, Y.N.; Kim, J.H.; Manthiram, A. Effect of Fe substitution on the structure and properties of LnBaCo2−xFexO5+δ (Ln = Nd and Gd) cathodes. J. Power Sources 2010, 195, 6411–6419. [Google Scholar] [CrossRef]
- Yoo, C.-Y.; Joo, J.H.; Lee, H.J.; Yu, J.H. The effects of Fe-substitution on the crystal structure and oxygen permeability of PrBaCo2O5+δ. Mater. Lett. 2013, 108, 65–68. [Google Scholar] [CrossRef]
- Wang, L.; Xie, P.; Bian, L.; Liu, X.; Chou, K. Performance of Ca-doped GdBa1-xCaxFe2O5+δ(x = 0, 0.1) as cathode materials for IT-SOFC application. Catal. Today 2018, 318, 132–136. [Google Scholar] [CrossRef]
- Jin, F.; Liu, X.; Chu, X.; Shen, Y.; Li, J. Effect of nonequivalent substitution of Pr3+/4+ with Ca2+ in PrBaCoFeO5+δ as cathodes for IT-SOFC. J. Mater. Sci. 2020, 56, 1147–1161. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, S.; Han, H.; Tang, K.; Xia, C. Cobalt-Free Double Perovskite Oxide as a Promising Cathode for Solid Oxide Fuel Cells. ACS Appl. Mater. Interfaces 2023, 15, 8253–8262. [Google Scholar] [CrossRef]
- Chen, Y.; Yoo, S.; Choi, Y.; Kim, J.; Ding, Y.; Pei, K.; Murphy, R.; Zhang, Y.; Zhao, B.; Zhang, W.; et al. A highly active, CO2-tolerant electrode for the oxygen reduction reaction. Energy Environ. Sci. 2018, 11, 2458–2466. [Google Scholar] [CrossRef]
- Li, G.; Gou, Y.; Cheng, X.; Bai, Z.; Ren, R.; Xu, C.; Qiao, J.; Sun, W.; Wang, Z.; Sun, K. Enhanced Electrochemical Performance of the Fe-Based Layered Perovskite Oxygen Electrode for Reversible Solid Oxide Cells. ACS Appl. Mater. Interfaces 2021, 13, 34282–34291. [Google Scholar] [CrossRef]
- Ren, R.; Wang, Z.; Meng, X.; Xu, C.; Qiao, J.; Sun, W.; Sun, K. Boosting the Electrochemical Performance of Fe-Based Layered Double Perovskite Cathodes by Zn2+ Doping for Solid Oxide Fuel Cells. ACS Appl. Mater. Interfaces 2020, 12, 23959–23967. [Google Scholar] [CrossRef]
- Jia, W.; Huang, Z.; Sun, W.; Wu, L.; Zheng, L.; Wang, Y.; Huang, J.; Yang, X.; Lv, M.; Ge, L. Flexible A-site doping La0.6-xMxSr0.4Co0.2Fe0.8O3 (M=Ca, Ba, Bi; x=0, 0.1, 0.2) as novel cathode material for intermediate-temperature solid oxide fuel cells: A first-principles study and experimental exploration. J. Power Sources 2021, 490, 22956–22964. [Google Scholar] [CrossRef]
- Jin, F.; Shen, Y.; Wang, R.; He, T. Double-perovskite PrBaCo2/3Fe2/3Cu2/3O5+δ as cathode material for intermediate-temperature solid-oxide fuel cells. J. Power Sources 2013, 234, 244–251. [Google Scholar] [CrossRef]
- Jin, F.; Xu, H.; Long, W.; Shen, Y.; He, T. Characterization and evaluation of double perovskites LnBaCoFeO5+δ (Ln = Pr and Nd) as intermediate-temperature solid oxide fuel cell cathodes. J. Power Sources 2013, 243, 10–18. [Google Scholar] [CrossRef]
- Xia, W.; Liu, X.; Jin, F.; Jia, X.; Shen, Y.; Li, J. Evaluation of calcium codoping in double perovskite PrBaCo2O5+δ as cathode material for IT-SOFCs. Electrochim. Acta 2020, 364, 1372–1374. [Google Scholar] [CrossRef]
- Lim, C.; Yang, Y.; Sin, Y.-W.; Choi, S.; Kim, G. Ca- and Ni-Doped Pr0.5Ba0.5FeO3−δ as a Highly Active and Robust Cathode for High-Temperature Solid Oxide Fuel Cell. Energy Fuels, 2020; 34, 11458–11463. [Google Scholar]
- Ghaffari, M.; Shannon, M.; Hui, H.; Tan, Q.; Irannejad, A. Preparation, surface state and band structure studies of SrTi(1 − x)Fe(x)O(3 − δ) (x = 0–1) perovskite-type nano structure by X-ray and ultraviolet photoelectron spectroscopy. Surf. Sci. 2012, 606, 670–677. [Google Scholar] [CrossRef]
- Li, X.; Jiang, X.; Xu, H.; Xu, Q.; Jiang, L.; Shi, Y.; Zhang, Q. Scandium-doped PrBaCo2−xScxO6−δ oxides as cathode material for intermediate-temperature solid oxide fuel cells. Int. J. Hydrogen Energy 2013, 38, 12035–12042. [Google Scholar] [CrossRef]
- Tahini, H.; Tan, X.; Schwingenschlögl, U.; Smith, S. Formation and Migration of Oxygen Vacancies in SrCoO3 and Their Effect on Oxygen Evolution Reactions. ACS Catal. 2016, 6, 5565–5570. [Google Scholar] [CrossRef]
- Hashim, S.; Liang, F.; Zhou, W.; Sunarso, J. Cobalt-Free Perovskite Cathodes for Solid Oxide Fuel Cells. ChemElectroChem 2019, 6, 3549–3569. [Google Scholar] [CrossRef]
- Zheng, Y.; Li, Q.; Chen, T.; Xu, C.; Wang, W. Quantitative contribution of resistance sources of components to stack performance for solid oxide electrolysis cells. J. Power Sources 2015, 274, 736–740. [Google Scholar] [CrossRef]
- Chen, T.; Pang, S.; Shen, X.; Jiang, X.; Wang, W. Evaluation of Ba-deficient PrBa1−xFe2O5+ oxides as cathode materials for intermediate-temperature solid oxide fuel cells. RSC Adv. 2016, 6, 13829–13836. [Google Scholar] [CrossRef]
- Lu, Y.; Zhao, H.; Chang, X.; Du, X.; Li, K.; Ma, Y.; Yi, S.; Du, Z.; Zheng, K.; Świerczek, K. Novel cobalt-free BaFe1−xGdxO3−δ perovskite membranes for oxygen separation. J. Mater. Chem. A 2016, 4, 10454–10466. [Google Scholar] [CrossRef]
- Zhou, Q.; Xu, L.; Guo, Y.; Jia, D.; Li, Y.; Wei, W. La0.6Sr0.4Fe0.8Cu0.2O3−δ perovskite oxide as cathode for IT-SOFC. Int. J. Hydrogen Energy 2012, 37, 11963–11968. [Google Scholar] [CrossRef]
- Gao, J.; Li, Q.; Zhang, Z.; Lü, Z.; Wei, B. A cobalt-free bismuth ferrite-based cathode for intermediate temperature solid oxide fuel cells. Electrochem. Commun. 2021, 125, 1069–1078. [Google Scholar] [CrossRef]
- Wu, M.; Cai, H.; Jin, F.; Sun, N.; Xu, J.; Zhang, L.; Han, X.; Wang, S.; Su, X.; Long, W.; et al. Assessment of cobalt–free ferrite–based perovskite Ln0.5Sr0.5Fe0.9Mo0.1O3–δ (Ln = lanthanide) as cathodes for IT-SOFCs. Eur. Ceram. Soc. 2021, 41, 2682–2690. [Google Scholar] [CrossRef]
- Ni, W.; Zhu, T.; Chen, X.; Zhong, Q.; Ma, W. Stable, efficient and cost-competitive Ni-substituted Sr(Ti,Fe)O3 cathode for solid oxide fuel cell: Effect of A-site deficiency. J. Power Sources 2020, 451, 22776–22782. [Google Scholar] [CrossRef]
- He, Z.; Xia, L.; Chen, Y.; Yu, J.; Huang, X.; Yu, Y. Layered perovskite Sm1−xLaxBaFe2O5+δ as cobalt-free cathodes for IT-SOFCs. RSC Adv. 2015, 5, 57592–57598. [Google Scholar] [CrossRef]
- Guan, T.; Sun, Y.; Yang, Z.; Jing, Y.; Guo, W. Anisotropic mechanical behavior of gadolinia-doped ceria solid electrolytes under electromechanical coupling field using atomistic simulations. Ceram Int. 2019, 45, 23355–23363. [Google Scholar] [CrossRef]
- Shi, H.; Su, C.; Ran, R.; Cao, J.; Shao, Z. Electrolyte materials for intermediate-temperature solid oxide fuel cells. Prog. Nat. Sci. Mater. Int. 2020, 30, 764–774. [Google Scholar] [CrossRef]

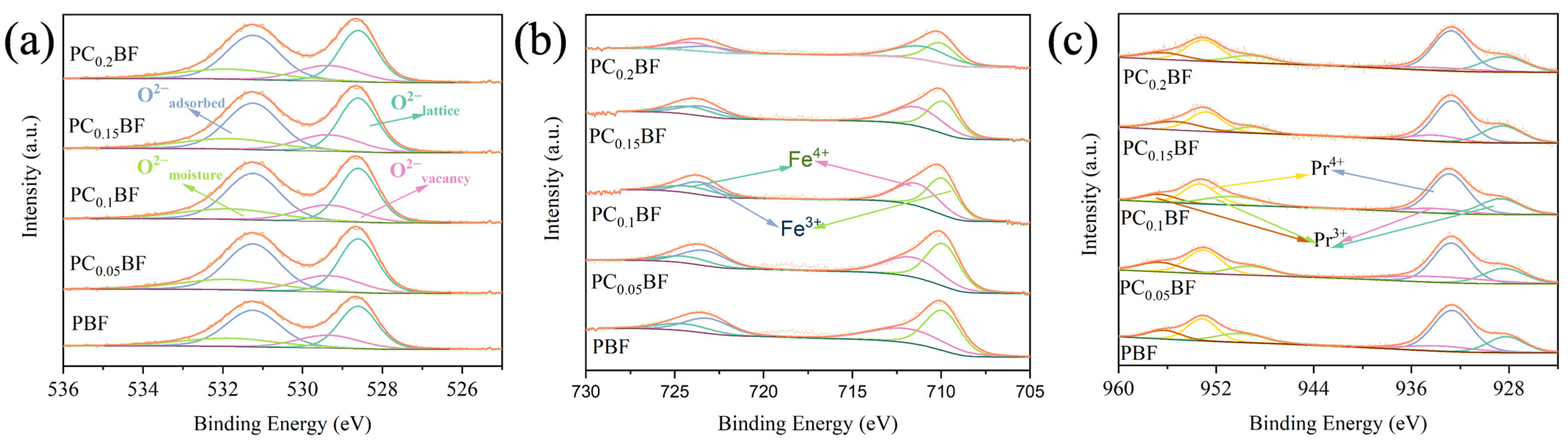


| Sample | Pr3+ (%) | Pr4+ (%) | Fe3+ (%) | Fe4+ (%) | (Oadsorbed + Ovacancy)/OLattice |
|---|---|---|---|---|---|
| x = 0.00 | 49.31 | 50.69 | 67.12 | 32.88 | 1.6894 |
| x = 0.05 | 44.66 | 55.34 | 64.36 | 35.64 | 1.7518 |
| x = 0.10 | 43.51 | 56.49 | 63.01 | 36.99 | 1.7945 |
| x = 0.15 | 41.81 | 58.19 | 60.13 | 39.87 | 1.8653 |
| x = 0.20 | 41.61 | 58.39 | 57.93 | 42.07 | 1.9422 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xue, L.; Li, S.; An, S.; Guo, Q.; Li, M.; Li, N. Ca-Doping Cobalt-Free Double Perovskite Oxide as a Cathode Material for Intermediate-Temperature Solid Oxide Fuel Cell. Molecules 2024, 29, 2991. https://doi.org/10.3390/molecules29132991
Xue L, Li S, An S, Guo Q, Li M, Li N. Ca-Doping Cobalt-Free Double Perovskite Oxide as a Cathode Material for Intermediate-Temperature Solid Oxide Fuel Cell. Molecules. 2024; 29(13):2991. https://doi.org/10.3390/molecules29132991
Chicago/Turabian StyleXue, Liangmei, Songbo Li, Shengli An, Qiming Guo, Mengxin Li, and Ning Li. 2024. "Ca-Doping Cobalt-Free Double Perovskite Oxide as a Cathode Material for Intermediate-Temperature Solid Oxide Fuel Cell" Molecules 29, no. 13: 2991. https://doi.org/10.3390/molecules29132991
APA StyleXue, L., Li, S., An, S., Guo, Q., Li, M., & Li, N. (2024). Ca-Doping Cobalt-Free Double Perovskite Oxide as a Cathode Material for Intermediate-Temperature Solid Oxide Fuel Cell. Molecules, 29(13), 2991. https://doi.org/10.3390/molecules29132991






