Host–Guest Metal–Organic Frameworks-Based Long-Afterglow Luminescence Materials
Abstract
1. Introduction
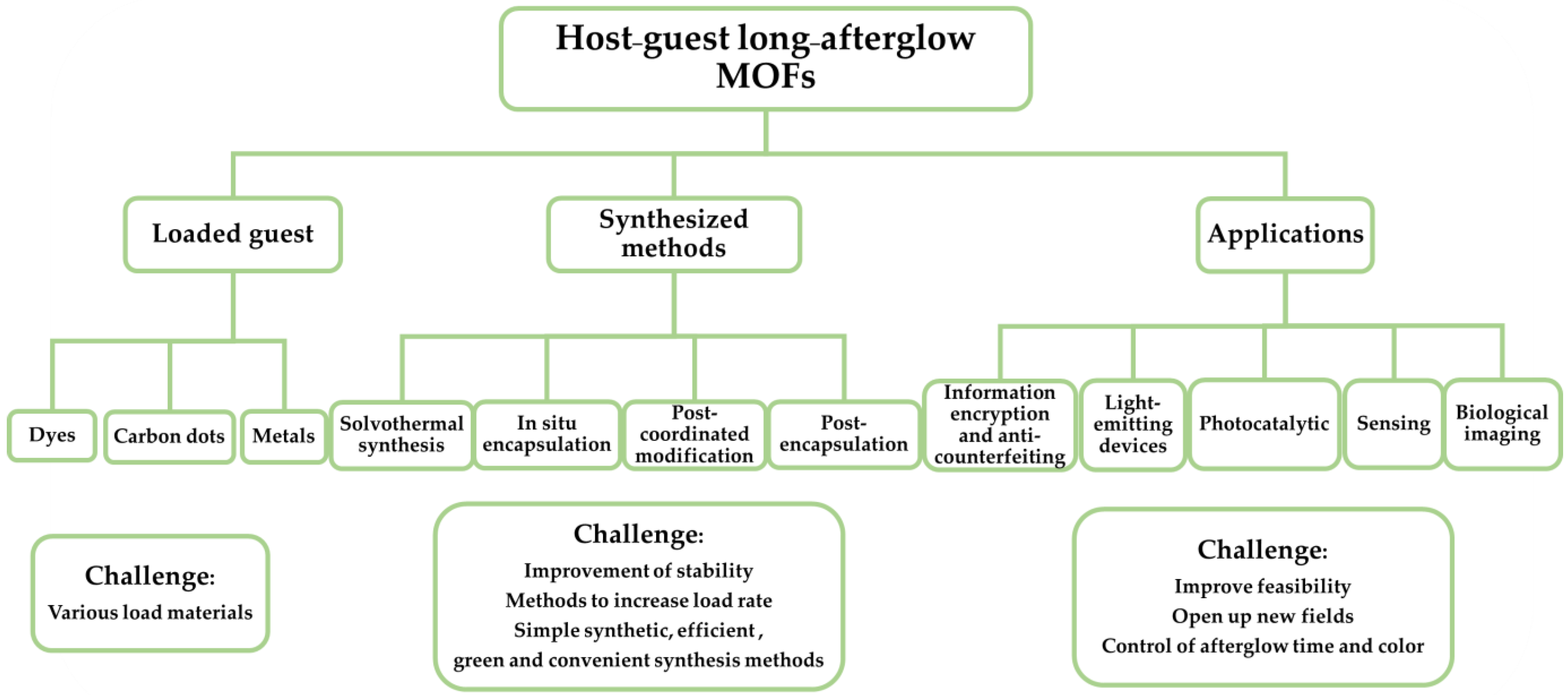
2. Synthesis and Classification of Afterglow Host-guest MOFs
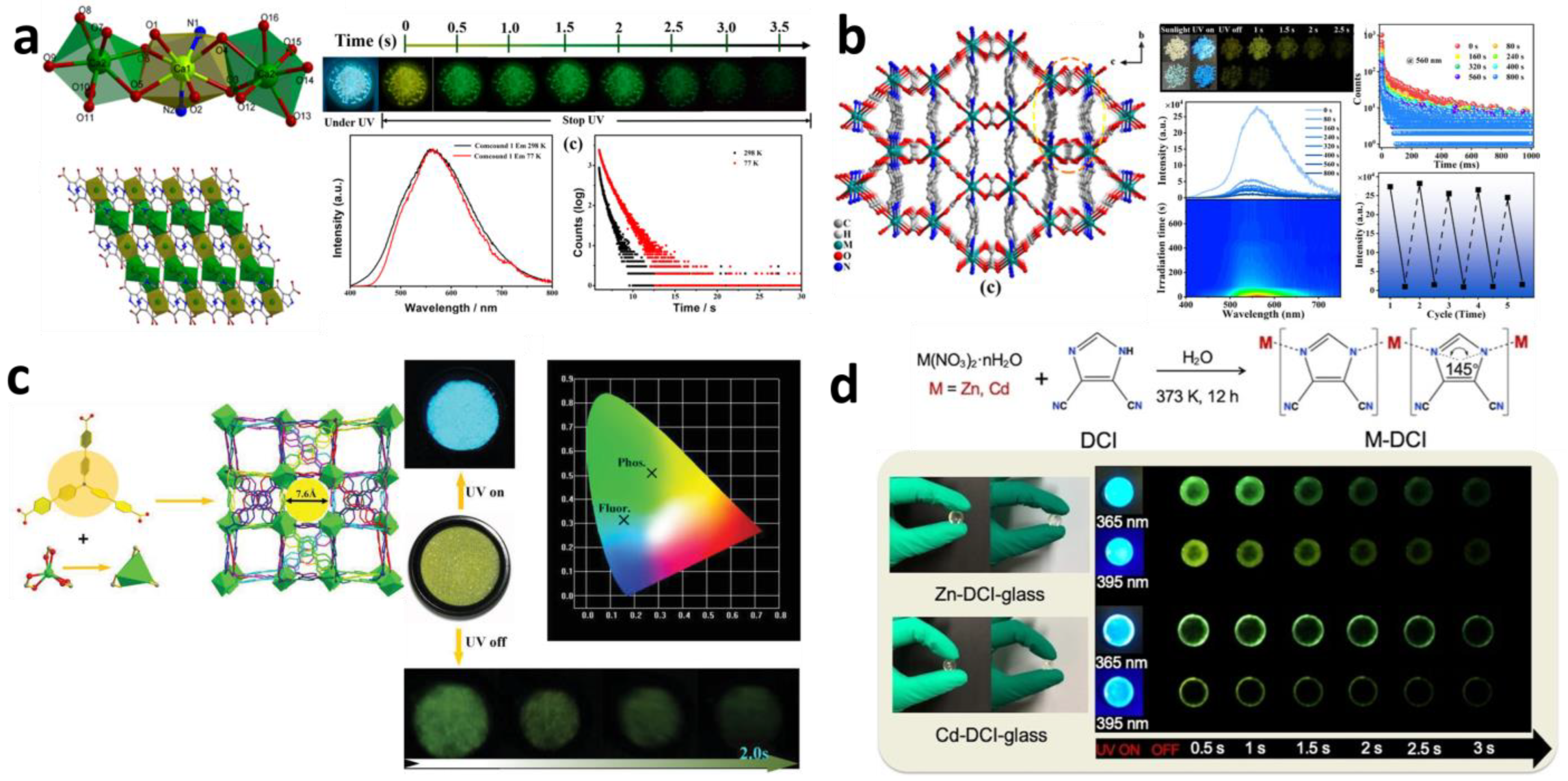
2.1. Synthesis Strategy for Mono-Component Afterglow PL MOFs
2.2. Synthesis Strategy for Host-guest MOF Afterglow Materials
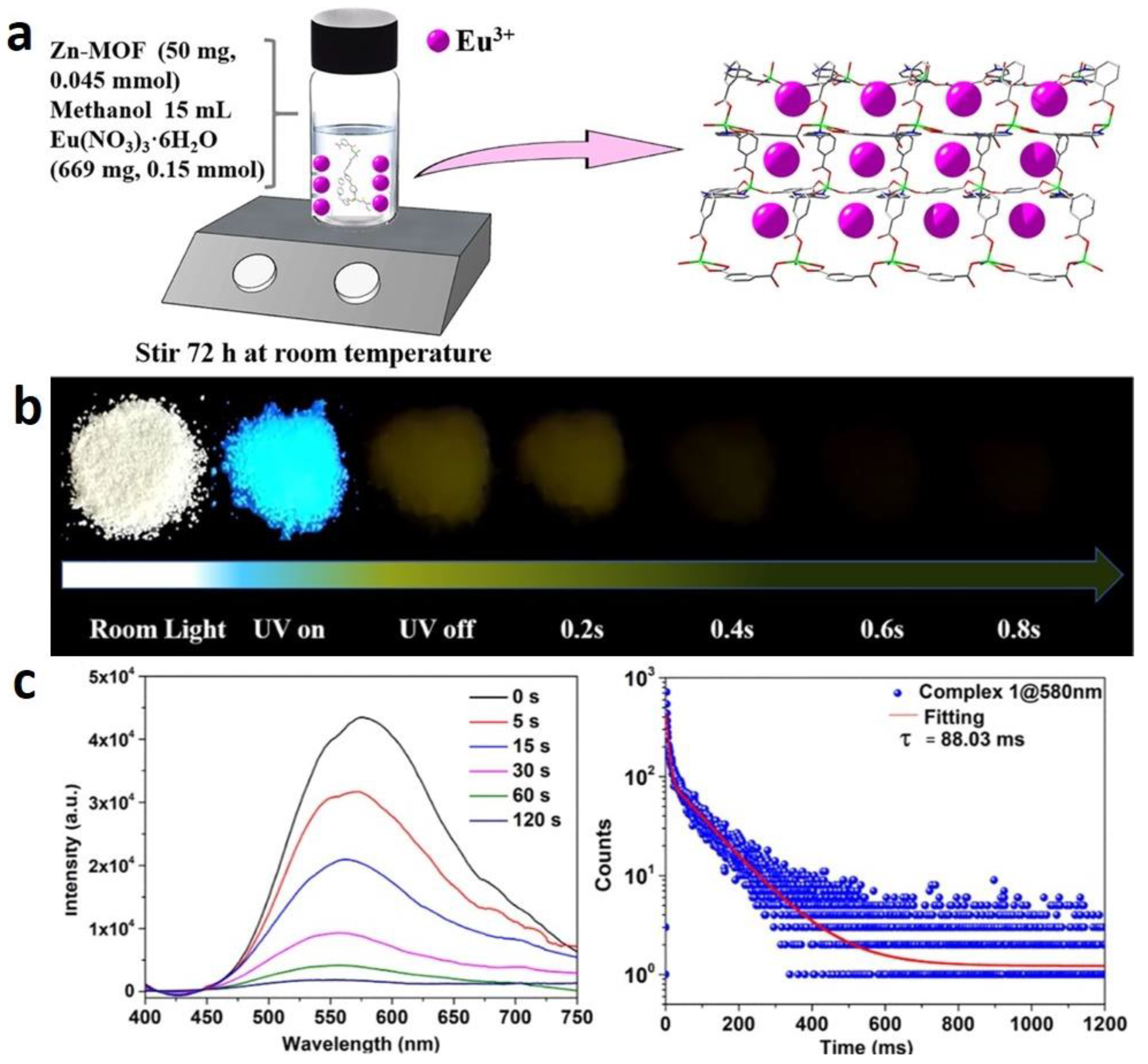
2.2.1. Solvothermal Synthesis
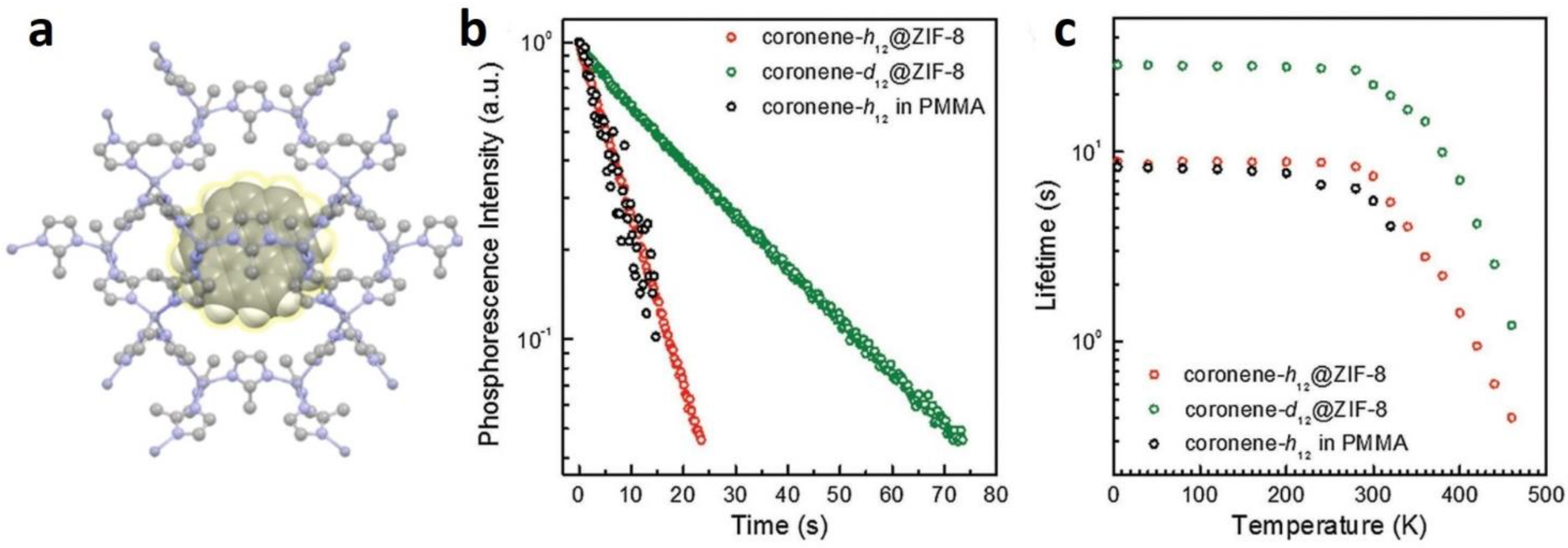
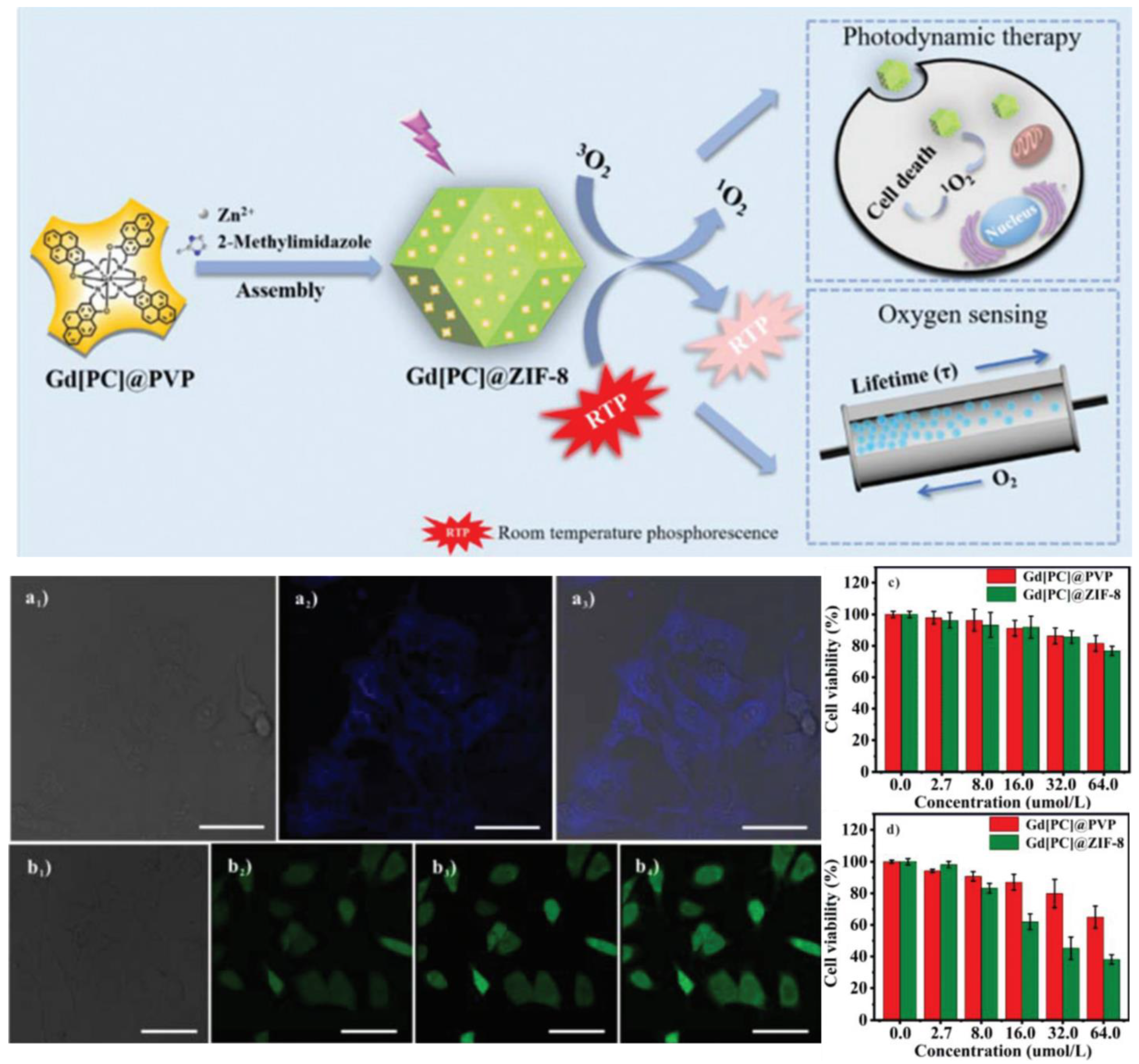
2.2.2. In Situ Encapsulation
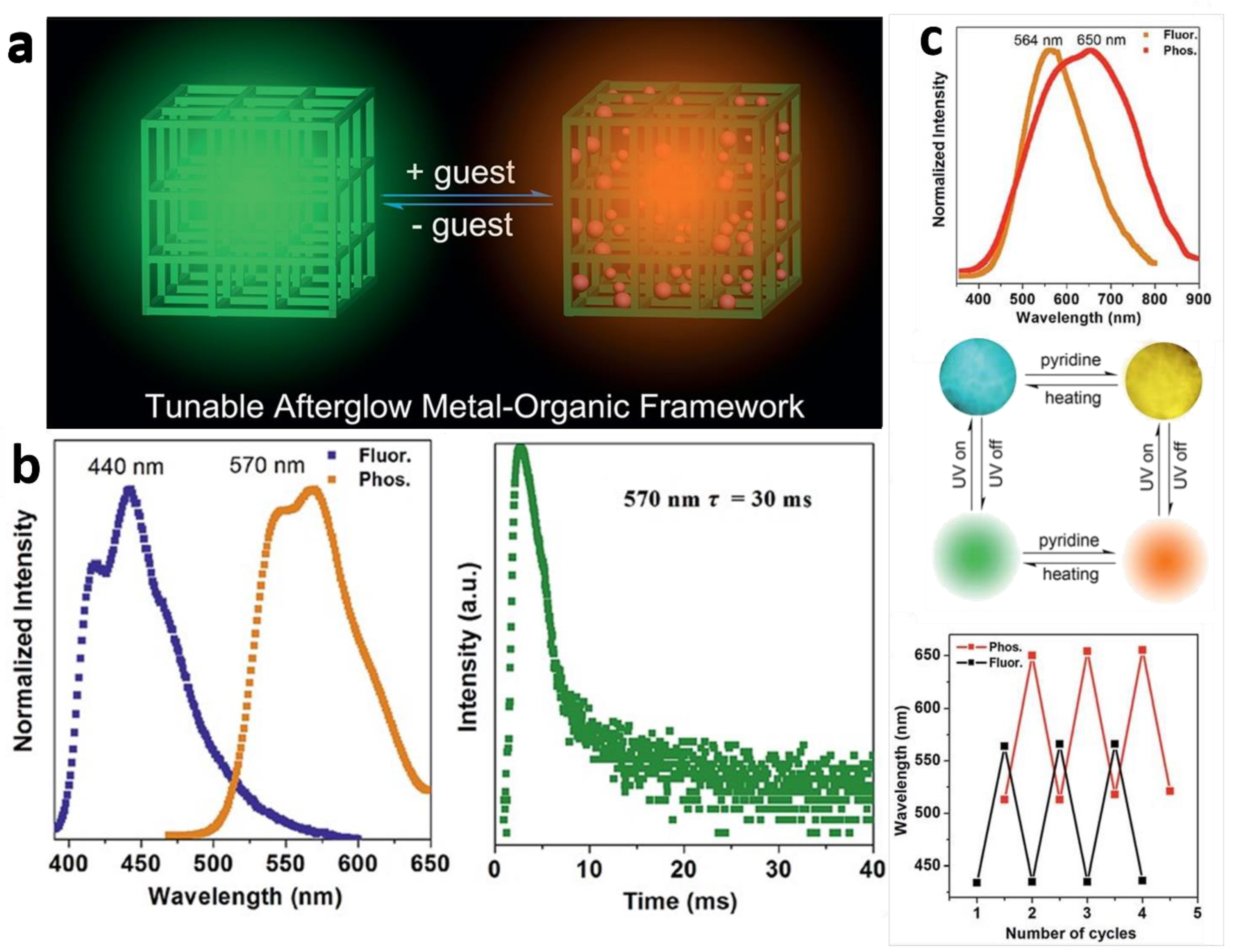
2.2.3. Post-Coordinated Modification
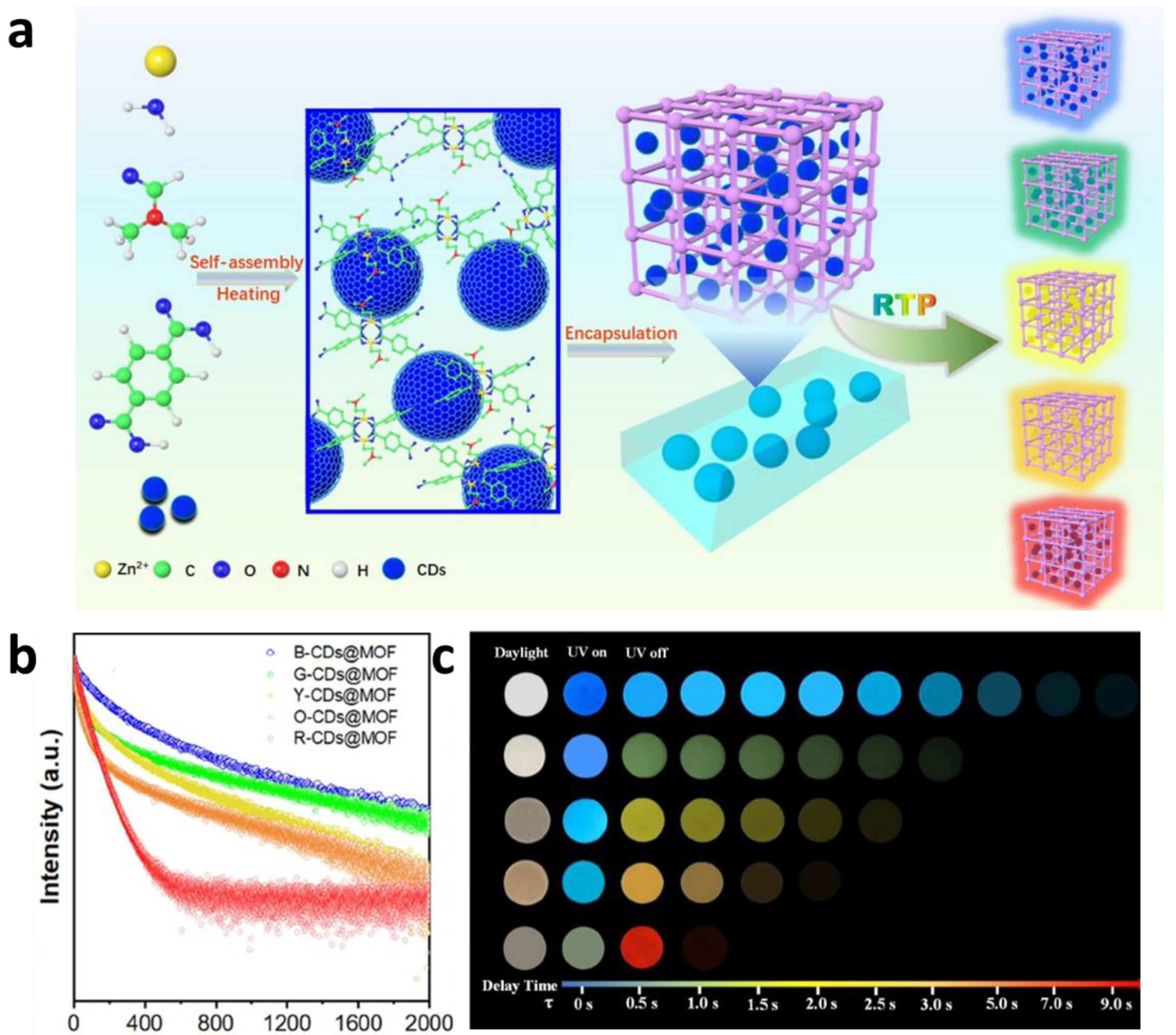
2.2.4. Post-Encapsulation
2.2.5. Other Synthesis Methods
| Code | Ligand | Coordinated Metal Ions | Guest | Phosphorescence Lifetime (ms) | Afterglow Color | T (K) | Ref. |
|---|---|---|---|---|---|---|---|
| 1 | 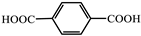 | Zn2+ | Pyridine | 470 | Green | RT | [45] |
| 2 | 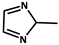 | Zn2+ | 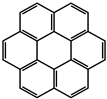 | 7400 22,400 | Green | 300 | [60] |
| 3 | 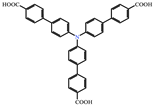 | Cd2+ | Coumarin 6, Acriflavine, Rhodamine B | 1180–672 | Green, yellow, and red in DMF solution | RT | [66] |
| 4 | 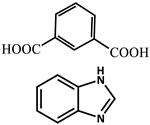 | Cd2+ | 4-Methylumbelliferone, Fluorescent Green B, Rhodamine 123, Rhodamine 6G, Rhodamine B | 293–765 | Green, yellow, orange, to red. | RT | [61] |
| 5 | 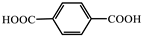 | Cd2+ | Eu3+ Tb3+ | 10.54 57.66 | Red | RT | [72] |
| 6 |  | Zn2+ | 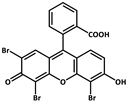 | 0.11, 3.57, 1.89 | Green to red | RT | [73] |
| 7 | 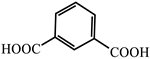 | Cd2+, Li+ | (Me2NH2)+ Mn2+ | 32 1.6–10.5 | Green Green to red | [74] | |
| 8 | 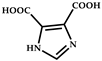 | Cd2+ | N,N-dimethylformamide | 187 196 | yellow to green | RT | [75] |
| 9 | 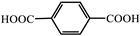 | Zn2+ | CDs | 85.67–1064.21 | Green | RT | [76] |
| 10 | 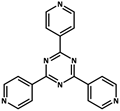 | Cd2+ | 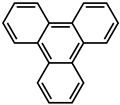 | 3230 | Green | 77 | [77] |
| 11 | 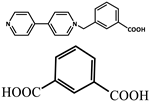 | Zn2+ | Eu3+ | 800 | Yellow | [78] | |
| 12 | 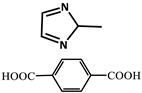 | Zn2+ | 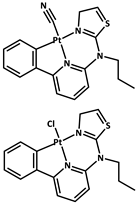 | 0.0184 0.0105 0.0111 | Green | 77 | [79] |
| 13 | 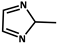 | Zn2+ | Gd[(Pyr)4cyclen] (Pyr = pyrenol) | 0.03695 | Green | 77 | [85] |
| 14 | 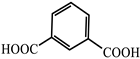 | Zn2+ | Rhodamine B | 926.56 | Red | RT | [80] |
| 15 | 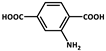 | Fe3+ | Zn3Ga2Ge2O10/Mn | 280,270 | Green | RT | [83] |
| 16 | 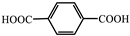 | Zn2+ | phosphors | 24.52–756.63 | Green to orange-red | RT | [84] |
3. Application of Afterglow MOFs
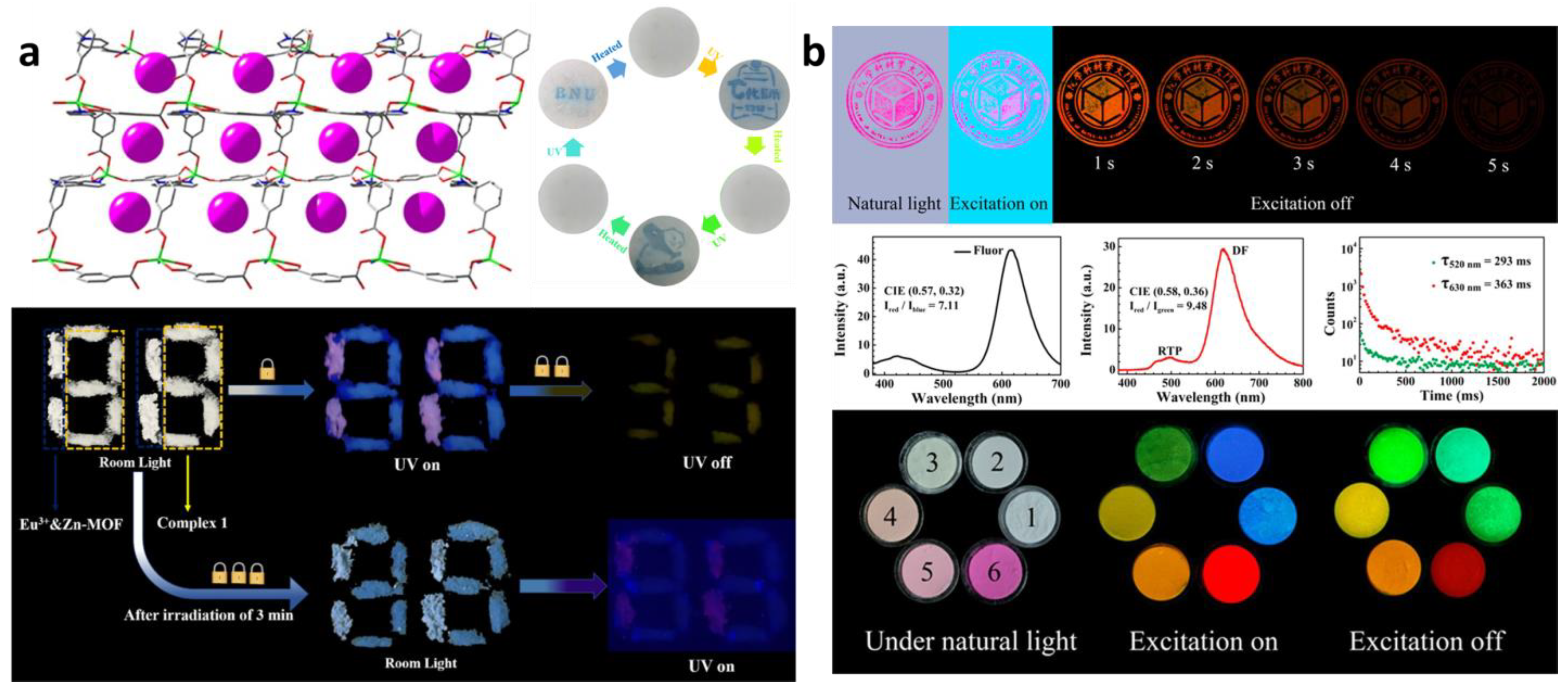
3.1. Information Encryption and Anti-Counterfeiting
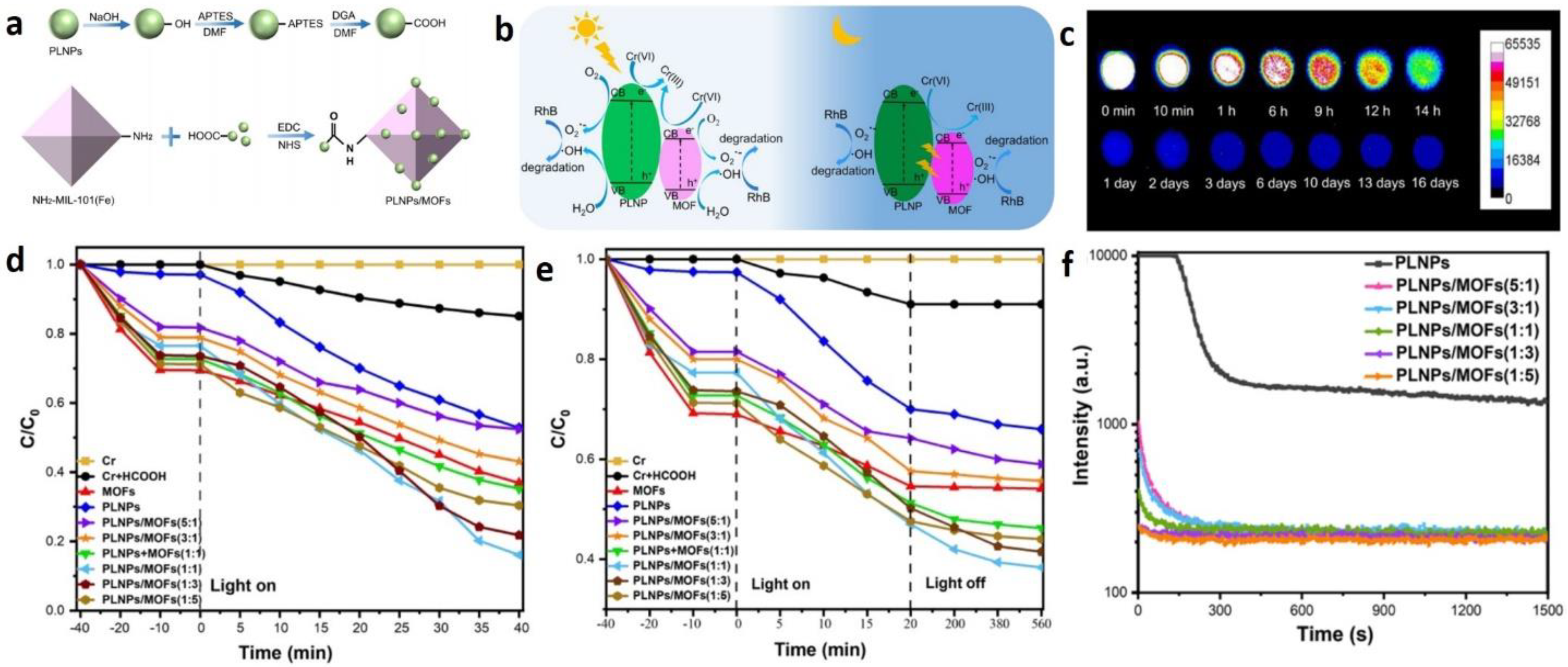
3.2. Photocatalysis
3.3. Sensing
3.4. Light-Emitting Devices
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Braslavsky, S.E. Glossary of Terms Used in Photochemistry, 3rd Edition (Iupac Recommendations 2006). Pure Appl. Chem. 2007, 79, 293–465. [Google Scholar] [CrossRef]
- Melhuish, W.H. Nomenclature, Symbols, Units and Their Usage in Spectrochemical Analysis-Part VI: Molecular Luminescence Spectroscopy. Pure Appl. Chem. 1984, 56, 231–245. [Google Scholar] [CrossRef]
- Matsuzawa, T.; Aoki, Y.; Takeuchi, N.; Murayama, Y. A New Long Phosphorescent Phosphor with High Brightness, SrAl2O4 : Eu2+, Dy3+. J. Electrochem. Soc. 1996, 143, 2670–2673. [Google Scholar] [CrossRef]
- Bian, L.; Ma, H.; Ye, W.; Lv, A.; Wang, H.; Jia, W.; Gu, L.; Shi, H.; An, Z.; Wei, H. Color-Tunable Ultralong Organic Phosphorescence Materials for Visual UV-Light Detection. Sci. China Chem. 2020, 63, 1443–1448. [Google Scholar] [CrossRef]
- Xu, S.; Chen, R.; Zheng, C.; Huang, W. Excited State Modulation for Organic Afterglow: Materials and Applications. Adv. Mater. 2016, 28, 9920–9940. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Tang, Z.; Luo, Y.; Yang, L.; He, M.; Ye, X.; Zheng, Z.; Cui, X.; Xia, C.; Wang, F. Visual Color Modulation and Luminescence Mechanism Studies on Mn/Eu Co-Doped Zn-Mg-Ge-O Long Afterglow System. Ceram. Int. 2020, 46, 14005–14018. [Google Scholar] [CrossRef]
- Lu, J.; Shen, L. Photoluminescence and Afterglow Luminescence Properties of a Green-Emitting Na2BeGeO4:Mn2+ Phosphor. Solid State Sci. 2018, 81, 66–70. [Google Scholar] [CrossRef]
- Park, I.J.; Roh, H.S.; Song, H.J.; Kim, D.H.; Kim, J.S.; Seong, W.M.; Kim, D.W.; Hong, K.S. Γ-Al2O3 Nanospheres-Directed Synthesis of Monodispersed BaAl2O4: Eu2+ Nanosphere Phosphors. CrystEngComm 2013, 15, 4797–4801. [Google Scholar] [CrossRef]
- Li, H.; Li, R.; Chang, C. Red-Shift and Improved Afterglow of Sr3Al2-XBxO5Cl2:Eu2+, Dy3+ Phosphors Via a Cation Substitution Strategy. Ceram. Int. 2022, 48, 1814–1819. [Google Scholar] [CrossRef]
- Xing, X.; Ling, D.; Tan, L. Microwave Synthesis of Casio3:(Eu2+, Dy3+) Nanorods and Verification on Luminescence Properties. J. Mater. Sci. Mater. Electron. 2014, 25, 4774–4778. [Google Scholar] [CrossRef]
- Cui, G.; Yang, X.; Zhang, Y.; Fan, Y.; Chen, P.; Cui, H.; Liu, Y.; Shi, X.; Shang, Q.; Tang, B. Round-the-Clock Photocatalytic Hydrogen Production with High Efficiency by a Lon-Afterglow Material. Angew. Chem. Int. Ed. 2018, 58, 1340–1344. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Pan, R.; Yuan, Z.; Hao, Q.; Niu, X.; Wang, R.; Ye, J.; Yang, H.Y.; Wu, Y. Functionalized Sr2MgSi2O7:(Eu,Dy)@Cds Heterojunction Photocatalyst for Round-the-Clock Hydrogen Production. Chem. Eng. J. 2024, 481, 148296. [Google Scholar] [CrossRef]
- Patel, D.K.; Sengupta, A.; Vishwanadh, B.; Sudarsan, V.; Vatsa, R.K.; Kadam, R.; Kulshreshtha, S.K. Local Environments around Eu3+ and Eu2+ Ions in Dual Light-Emitting BaSnO3:Eu Nanomaterials. Eur. J. Inorg. Chem. 2012, 2012, 1609–1619. [Google Scholar] [CrossRef]
- Li, C.; Song, Z.; Li, Y.; Lou, K.; Qiu, J.; Yang, Z.; Yin, Z.; Wang, X.; Wang, Q.; Wan, R. Enhanced Nir Downconversion Luminescence by Precipitating Nano Ca5(Po4)3f Crystals in Eu2+-Yb3+ Co-Doped Glass. Spectrochim. Acta A 2013, 114, 575–578. [Google Scholar] [CrossRef] [PubMed]
- Hasnat, M.; Lahti, V.; Byron, H.; Lastusaari, M.; Petit, L. Micro-Luminescence Measurement to Evidence Decomposition of Persistent Luminescent Particles During the Preparation of Novel Persistent Luminescent Tellurite Glasses. Scr. Mater. 2021, 199, 113864. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, P.; Xie, W.; Lin, Z.; Miao, X.; Li, S.; Wang, H.; Liu, W. Time-Dependent Multicolor Evolution of Photoluminescence and Afterglow in Lanthanide-Doped Gallate. Chem. Eng. J. 2023, 476, 146487. [Google Scholar] [CrossRef]
- Hu, T.; Cheng, H.; Yang, D.; Shao, K.; Teng, Y.; Pan, Z. In-Situ Formation of Oleic Acid Amide Ligand Boosts Persistent Luminescence of Zinc Gallate Nanoparticles toward Efficient Latent Fingermark Detection. Mater. Today Chem. 2023, 30, 101485. [Google Scholar] [CrossRef]
- Terraschke, H.; Wickleder, C. UV, Blue, Green, Yellow, Red, and Small: Newest Developments on Eu2+-Doped Nanophosphors. Chem. Rev. 2015, 115, 11352–11378. [Google Scholar] [CrossRef]
- Gupta, S.K.; Sudarshan, K.; Modak, B.; Gupta, R. Interstitial Zinc Boosted Light Tunability, Afterglow, and Ultrabright White Emission in Zinc Germanate (Zn2GeO4). ACS Appl. Electron. Mater. 2023, 5, 1286–1294. [Google Scholar] [CrossRef]
- Jiang, K.; Wang, Y.; Li, Z.; Lin, H. Afterglow of Carbon Dots: Mechanism, Strategy and Applications. Mater. Chem. Front. 2020, 4, 386–399. [Google Scholar] [CrossRef]
- Ran, Z.; Liu, J.; Zhuang, J.; Liu, Y.; Hu, C. Multicolor Afterglow from Carbon Dots: Preparation and Mechanism. Small Methods 2024, 8, 2301013. [Google Scholar] [CrossRef] [PubMed]
- Cobley, C.M.; Chen, J.; Cho, E.C.; Wang, L.V.; Xia, Y. Gold Nanostructures: A Class of Multifunctional Materials for Biomedical Applications. Chem. Soc. Rev. 2011, 40, 44–56. [Google Scholar] [CrossRef]
- Kuila, S.; George, S.J. Phosphorescence Energy Transfer: Ambient Afterglow Fluorescence from Water-Processable and Purely Organic Dyes Via Delayed Sensitization. Angew. Chem. Int. Ed. 2020, 59, 9393–9397. [Google Scholar] [CrossRef]
- Zhang, G.; Chen, J.; Payne, S.J.; Kooi, S.E.; Demas, J.N.; Fraser, C.L. Multi-Emissive Difluoroboron Dibenzoylmethane Polylactide Exhibiting Intense Fluorescence and Oxygen-Sensitive Room-Temperature Phosphorescence. J. Am. Chem. Soc. 2007, 129, 8942–8943. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Shi, Y.-J.; Chen, S.; Lin, Z.; Jiang, Z.; Liao, W.-M.; He, J. Dehydration-Triggered Afterglow Transition in a Mellitate-Based Coordination Polymer. Chem. Mater. 2023, 35, 3015–3023. [Google Scholar] [CrossRef]
- Chen, X.; Wang, G.; Wu, M.; Liu, J.; Liu, Z.; Wang, X.; Zou, Y.; Zhang, K. Merging Photoinitiated Bulk Polymerization and the Dopant-Matrix Design Strategy for Polymer-Based Organic Afterglow Materials. Polym. Chem. 2022, 13, 4641–4649. [Google Scholar] [CrossRef]
- Wei, H.; Zhang, Q.; Deng, Z.; Guan, G.; Dong, Z.; Cao, H.; Liang, P.; Lu, D.; Liu, S.; Yin, X.; et al. Lanthanide Inorganic Nanoparticles Enhance Semiconducting Polymer Nanoparticles Afterglow Luminescence for in Vivo Afterglow/Magnetic Resonance Imaging. Anal. Chem. 2024, 96, 7697–7705. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Du, L.; Zhao, W.; Zhao, Z.; Xiong, Y.; He, X.; Gao, P.F.; Alam, P.; Wang, C.; Li, Z. Ultralong Uv/Mechano-Excited Room Temperature Phosphorescence from Purely Organic Cluster Excitons. Nat. Commun. 2019, 10, 5161. [Google Scholar] [CrossRef]
- Zhu, C.Y.; Wang, Z.; Mo, J.T.; Fan, Y.N.; Pan, M. A Long Persistent Phosphorescent Metal-Organic Framework for Multi-Level Sensing of Oxygen. J. Mater. Chem. C 2020, 8, 9916–9922. [Google Scholar] [CrossRef]
- Faustini, M.; Nicole, L.; Ruiz-Hitzky, E.; Sanchez, C. History of Organic-Inorganic Hybrid Materials: Prehistory, Art, Science, and Advanced Applications. Adv. Funct. Mater. 2018, 28, 1704158. [Google Scholar] [CrossRef]
- Barbara, S.; Jerzy, C.; Mietek, J. Gas Adsorption Properties of Hybrid Graphene-MOF Materials. J. Colloid Interface Sci. 2018, 514, 801–813. [Google Scholar] [CrossRef]
- Houbertz, R.; Domann, G.; Cronauer, C.; Schmitt, A.; Martin, H.; Park, J.U.; FröHlich, L.; Buestrich, R.; Popall, M.; Streppel, U. Inorganic-Organic Hybrid Materials for Application in Optical Devices. Thin Solid Films 2014, 442, 194–200. [Google Scholar] [CrossRef]
- TranThi, T.H.; Dagnelie, R.; Crunaire, S.; Nicole, L. Optical Chemical Sensors Based on Hybrid Organic-Inorganic Sol-Gel Nanoreactors. Chem. Soc. Rev. 2011, 40, 621–639. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, L.; Xiao, F.S. Metal@Zeolite Hybrid Materials for Catalysis. ACS Cent. Sci. 2020, 6, 1685–1697. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Davis, C.E.; Groy, T.L.; Kelley, D.G.; Yaghi, O.M. Coordinatively Unsaturated Metal Centers in the Extended Porous Framework of Zn3(BDC)3·6CH3OH(BDC) 1,4-Benzenedicarboxylate). J. Am. Chem. Soc. 1998, 120, 2186–2187. [Google Scholar] [CrossRef]
- Yuan, J.; Dong, J.; Lei, S.; Hu, W. Long Afterglow MOFs: A Frontier Study on Synthesis and Applications. Mater. Chem. Front. 2021, 5, 6824–6849. [Google Scholar] [CrossRef]
- Fu, H.R.; Zhang, R.Y.; Ren, D.D.; Zhang, K.; Yuan, J.; Lu, X.Y.; Ding, Q.R. Chiral Coordination Assembly-Induced Phosphorescent Frameworks for Circularly Polarized Phosphorescence. Cryst. Growth Des. 2024, 24, 4819–4824. [Google Scholar] [CrossRef]
- Fu, H.R.; Ren, D.D.; Zhang, K.; Zhang, R.Y.; Lu, X.Y.; Wu, Y.P.; Aznarez, F.; Ding, Q.R.; Ma, L.F.; Li, D.S. Circularly Polarized Luminescence of Tetradentate-Ligand-Based Chiral MOFs: Structural Evidence of Chirality Inversion. ACS Materials Lett. 2024, 6, 2559–2568. [Google Scholar] [CrossRef]
- Zhou, J.; Liu, H.; Lin, Y.; Zhou, C.; Huang, A. Synthesis of Well-Shaped and High-Crystalline Ce-Based Metal Organic Framework for CO2/CH4 Separation. Microporous Mesoporous Mater. 2020, 302, 110224. [Google Scholar] [CrossRef]
- Qin, L.; Zheng, H. Structures and Applications of Metal-Organic Frameworks Featuring Metal Clusters. CrystEngComm 2017, 19, 745–757. [Google Scholar] [CrossRef]
- Ongari, D.; Tiana, D.; Stoneburner, S.J.; Gagliardi, L.; Smit, B. Origin of the Strong Interaction between Polar Molecules and Copper(Ii) Paddle-Wheels in Metal Organic Frameworks. J. Phys. Chem. C 2017, 121, 15135–15144. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Xia, W.; Cai, K.; Wu, Y.; Qiu, B.; Liang, Z.; Qu, C.; Zou, R. Metal-Organic Frameworks: Kinetic-Controlled Formation of Bimetallic Metal-Organic Framework Hybrid Structures (Small 41/2017). Small 2018, 13, 41. [Google Scholar] [CrossRef]
- Lu, D.F.; Wang, Z.W.; Wang, F.; Zhang, J. Phosphorescent Calcium-Based Metal-Organic Framework with Second-Scale Long Afterglow. Inorg. Chem. 2021, 60, 10075–10078. [Google Scholar] [CrossRef]
- Allendorf, M.D.; Bauer, C.A.; Bhakta, R.K.; Houk, R.J.T. Luminescent Metal-Organic Frameworks. Chem. Soc. Rev. 2009, 38, 1330–1352. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Yan, D. Long-Afterglow Metal-Organic Frameworks: Reversible Guest-Induced Phosphorescence Tunability. Chem. Sci. 2016, 7, 4519–4526. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.N.; Fan, T.T.; Wang, Q.S.; Han, H.L.; Li, X. Zn/Cd/Cu-Frameworks Constructed by 3,3′-Diphenyldicarboxylate and 1,4-Bis(1,2,4-Triazol-1-Yl)Butane: Syntheses, Structure, Luminescence and Luminescence Sensing for Metal Ion in Aqueous Medium. J. Solid State Chem. 2018, 258, 744–752. [Google Scholar] [CrossRef]
- Lo Presti, F.; Pellegrino, A.L.; Consoli, N.; Malandrino, G. Green Ultrasound-Assisted Synthesis of Rare-Earth-Based MOFs. Molecules 2023, 28, 6088. [Google Scholar] [CrossRef] [PubMed]
- Ajoyan, Z.; Mandl, G.A.; Donnarumma, P.R.; Quezada-Novoa, V.; Bicalho, H.A.; Titi, H.M.; Capobianco, J.A.; Howarth, A.J. Modulating Photo- and Radioluminescence in Tb(III) Cluster-Based Metal-Organic Frameworks. ACS Mater. Lett. 2022, 4, 1025–1031. [Google Scholar] [CrossRef]
- Liu, J.; Yang, L.Y.; Luo, F. A New Zn-Triazole MOF Showing Very Long-Lived Luminescence up to 3 S. J. Solid State Chem. 2021, 301, 122369. [Google Scholar] [CrossRef]
- Orfano, M.; Perego, J.; Cova, F.; Bezuidenhout, C.X.; Piva, S.; Dujardin, C.; Sabot, B.; Pierre, S.; Mai, P.; Daniel, C.; et al. Efficient Radioactive Gas Detection by Scintillating Porous Metal-Organic Frameworks. Nat. Photonics 2023, 17, 672–678. [Google Scholar] [CrossRef]
- Huang, M.; Liang, Z.; Huang, J.; Wen, Y.; Zhu, Q.-L.; Wu, X. Introduction of Multicomponent Dyes into 2d MOFs: A Strategy to Fabricate White Light-Emitting MOF Composite Nanosheets. ACS Appl. Mater. Interfaces 2023, 15, 11131–11140. [Google Scholar] [CrossRef]
- Li, H.; Li, Q.; Xu, Z. Lanthanide Cation Encapsulated in a Metal-Organic Framework as a White Led and Selective Naked-Eye Reversible Hcl Sensor. J. Mater. Chem. C 2019, 7, 2880–2885. [Google Scholar] [CrossRef]
- Zhang, L.; Li, H.; Yang, Y.; Yang, D.; Cui, Y.; Qian, G. Aligned Chromophores in a Host-Guest MOF Crystal for Switchable Polarized Nonlinear Optical Response. J. Mater. Chem. C 2022, 10, 14915–14920. [Google Scholar] [CrossRef]
- Zhai, R.; Zhu, Y.D.; Chang, l.M.; Gu, Z.G.; Zhang, J. Layer-by-Layer Grafting Dye on Enantiopure MOF Thin Films for Circularly Polarized Luminescence Amplification. Chinese J. struct. Chem. 2022, 41, 2209074–2209079. [Google Scholar] [CrossRef]
- Liu, J.; Fu, J.; Liu, T.; Shen, X.; Cheng, F. Encapsulating Electron-Rich Guest in a MOF Host through Donor-Acceptor Interaction for Highly Tunable Luminescence. Dyes Pigm. 2022, 205, 110542. [Google Scholar] [CrossRef]
- Falcaro, P.; Doonan, C. MOF Bio-Composites for Biocatalysis. Acta Crystallogr. Sect. A 2017, 73, C1030. [Google Scholar] [CrossRef]
- Tian, X.; Yi, Y.; Wu, Z.; Cheng, G.; Zheng, S.; Fang, B.; Wang, T.; Shchukin, D.G.; Hai, F.; Guo, J.; et al. Ionic Liquid Confined in MOF/Polymerized Ionic Network Core-Shell Host as a Solid Electrolyte for Lithium Batteries. Chem. Eng. Sci. 2023, 266, 118271. [Google Scholar] [CrossRef]
- Dou, J.; Chen, Q. MOFs in Emerging Solar Cells. Chin. J. Chem. 2023, 41, 695–709. [Google Scholar] [CrossRef]
- Zhang, C.; Yan, Z.P.; Dong, X.Y.; Han, Z.; Li, S.; Fu, T.; Zhu, Y.Y.; Zheng, Y.X.; Niu, Y.Y.; Zang, S.Q. Enantiomeric MOF Crystals Using Helical Channels as Palettes with Bright White Circularly Polarized Luminescence. Adv. Mater. 2020, 32, 2002914. [Google Scholar] [CrossRef] [PubMed]
- Mieno, H.; Kabe, R.; Notsuka, N.; Allendorf, M.D.; Adachi, C. Long-Lived Room-Temperature Phosphorescence of Coronene in Zeolitic Imidazolate Framework ZIF-8. Adv. Opt. Mater. 2016, 4, 1015–1021. [Google Scholar] [CrossRef]
- Liu, J.; Zhuang, Y.; Wang, L.; Zhou, T.; Hirosaki, N.; Xie, R.J. Achieving Multicolor Long-Lived Luminescence in Dye-Encapsulated Metal-Organic Frameworks and Its Application to Anticounterfeiting Stamps. ACS Appl. Mater. Interfaces 2018, 10, 1802–1809. [Google Scholar] [CrossRef] [PubMed]
- Cao, F.Y.; Liu, H.H.; Mu, Y.; Xue, Z.Z.; Li, J.H.; Wang, G. Enabling Dual Phosphorescence by Locating a Flexible Ligand in Zn-Based Hybrid Frameworks. J. Phys. Chem. Lett. 2022, 13, 6975–6980. [Google Scholar] [CrossRef]
- Alfonso Herrera, L.Á.; Beltrán, H.I. Infiltration as a Frontier Bandgap Engineering Strategy in MOFs: A Critical Review. Coord. Chem. Rev. 2024, 505, 215658. [Google Scholar] [CrossRef]
- Seco, J.M.; Pérez-Yáñez, S.; Briones, D.; García, J.Á.; Cepeda, J.; Rodríguez-Diéguez, A. Combining Polycarboxylate and Bipyridyl-Like Ligands in the Design of Luminescent Zinc and Cadmium Based Metal-Organic Frameworks. Cryst. Growth Des. 2017, 17, 3893–3906. [Google Scholar] [CrossRef]
- Ma, Y.; Fang, X.; Xiao, G.; Yan, D. Dynamic Manipulating Space-Resolved Persistent Luminescence in Core-Shell MOFs Heterostructures via Reversible Photochromism. Angew. Chem. Int. Ed. 2021, 61, e202114100. [Google Scholar] [CrossRef]
- Fu, H.; Wang, N.; Wu, X.X.; Li, F.F.; Zhao, Y.; Ma, L.; Du, M. Circularly Polarized Room-Temperature Phosphorescence and Encapsulation Engineering for MOF-Based Fluorescent/Phosphorescent White Light-Emitting Devices. Adv. Opt. Mater. 2020, 8, 2000330. [Google Scholar] [CrossRef]
- Zhou, B.; Qi, Z.; Yan, D. Highly Efficient and Direct Ultralong All-Phosphorescence from Metal-Organic Framework Photonic Glasses. Angew. Chem. Int. Ed. 2022, 61, e202208735. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Lin, Z.; Yan, D. Tuning Organic Room-Temperature Phosphorescence through the Confinement Effect of Inorganic Micro/Nanostructures. Small Struct. 2021, 2, 2100044. [Google Scholar] [CrossRef]
- Gu, Z.G.; Li, D.J.; Zheng, C.; Kang, Y.; Wöll, C.; Zhang, J. MOF-Templated Synthesis of Ultrasmall Photoluminescent Carbon-Nanodot Arrays for Optical Applications. Angew. Chem. Int. Ed. 2017, 56, 6853–6858. [Google Scholar] [CrossRef]
- Nadar, S.S.; Rathod, V.K. One Pot Synthesis of A-Amylase Metal Organic Framework (MOF)-Sponge Via Dip-Coating Technique. Int. J. Biol. Macromol. 2019, 138, 1035–1043. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, B.; Yu, X.; Li, J.; Shang, J.; Yu, J. Carbon Dots in Porous Materials: Host-Guest Synergy for Enhanced Performance. Angew. Chem. Int. Ed. 2020, 59, 19390–19402. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wang, K.Z.; Yan, D. Lanthanide Doped Coordination Polymers with Tunable Afterglow Based on Phosphorescence Energy Transfer. Chem. Commun. 2017, 53, 7752–7755. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.X.; Lu, X.M.; Chen, J.Y.; Yang, X.G.; Qin, W.J.; Ma, L.F. Long Afterglow of a Nonporous Coordination Polymer with Tunable Room-Temperature Phosphorescence by the Doping of Dye Molecules. Inorg. Chem. 2021, 60, 846–851. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.G.; Yan, D.P. Long-Lasting Phosphorescence with a Tunable Color in a Mn2+-Doped Anionic Metal-Organic Framework. J. Mater. Chem. C 2017, 5, 7898–7903. [Google Scholar] [CrossRef]
- Liu, S.; Lin, Y.; Yan, D. Dynamic Multi-Color Long-Afterglow and Cold-Warm White Light through Phosphorescence Resonance Energy Transfer in Host-Guest Metal-Organic Frameworks. Sci. China Chem. 2023, 66, 3532–3538. [Google Scholar] [CrossRef]
- Xu, B.; Wang, Z.; Shen, J.; Li, J.; Jia, Y.; Jiang, T.; Gao, Z.; Wang, X.; Meng, X. Metal-Organic Framework-Activated Full-Color Room-Temperature Phosphorescent Carbon Dots with a Wide Range of Tunable Lifetimes for 4D Coding Applications. J. Phys. Chem. C 2022, 126, 11701–11708. [Google Scholar] [CrossRef]
- Liu, X.T.; Hua, W.; Nie, H.-X.; Chen, M.; Chang, Z.; Bu, X.H. Manipulating Spatial Alignment of Donor and Acceptor in Host-Guest MOF for TADF. Nat. Sci. Rev. 2022, 9, nwab222. [Google Scholar] [CrossRef]
- Yang, D.D.; Zheng, H.W.; Fang, Y.H.; Liang, Q.F.; Han, Q.Z.; Shi, Y.S.; Zheng, X.J. Multistimuli-Responsive Materials Based on Zn(II)-Viologen Coordination Polymers and Their Applications in Inkless Print and Anticounterfeiting. Inorg. Chem. 2022, 61, 7513–7522. [Google Scholar] [CrossRef]
- Knedel, T.O.; Buss, S.; Maisuls, I.; Daniliuc, C.G.; Schlusener, C.; Brandt, P.; Weingart, O.; Vollrath, A.; Janiak, C.; Strassert, C.A. Encapsulation of Phosphorescent Pt(Ii) Complexes in Zn-Based Metal-Organic Frameworks toward Oxygen-Sensing Porous Materials. Inorg. Chem. 2020, 59, 7252–7264. [Google Scholar] [CrossRef]
- Yang, X.G.; Lu, X.M.; Zhai, Z.M.; Zhao, Y.; Liu, X.Y.; Ma, L.F.; Zang, S.Q. Facile Synthesis of a Micro-Scale MOF Host-Guest with Long-Lasting Phosphorescence and Enhanced Optoelectronic Performance. Chem. Commun. 2019, 55, 11099–11102. [Google Scholar] [CrossRef]
- Hou, Y.; Zhang, Z.; Lu, S.; Yuan, J.; Zhang, M. Highly Emissive Perylene Diimide-Based Metallacages and Their Host-Guest Chemistry for Information Encryption. J. Am. Chem. Soc. 2020, 142, 18763–18768. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, J.-S.; Liu, J.-Y.; Zhao, G.-J.; Liu, L.; Han, K.-L.; Cook, T.R.; Stang, P.J. Photophysical Properties of a Post-Self-Assembly Host/Guest Coordination Cage: Visible Light Driven Core-to-Cage Charge Transfer. J. Phys. Chem. Lett. 2015, 6, 1942–1947. [Google Scholar] [CrossRef]
- He, F.; Abulimiti, A.; Li, B.; Wu, Y.; Chen, Y.; Zhang, M.; Abdukayum, A. Persistent Luminescent Nanoparticle-Coated Metal-Organic Frameworks for Round-the-Clock Photocatalytic Degradation of Pollutants. ACS Appl. Nano Mater. 2023, 6, 12871–12881. [Google Scholar] [CrossRef]
- Ni, A.; Zhang, B.; Zhang, P.; Zhang, J.; Wang, H.; Feng, K.; Liu, S.; Ni, J.; Duan, C. Water Friendly Room Temperature Phosphorescence Doped Materials Prepared Via Metal Organic Framework Matrix Transformation. Dyes Pigm. 2023, 210, 110959. [Google Scholar] [CrossRef]
- Zhao, Z.; Ru, J.; Zhou, P.; Wang, Y.; Shan, C.; Yang, X.; Cao, J.; Liu, W.; Guo, H.; Tang, Y. A Smart Nanoprobe Based on a Gadolinium Complex Encapsulated by ZIF-8 with Enhanced Room Temperature Phosphorescence for Synchronous Oxygen Sensing and Photodynamic Therapy. Dalton Trans. 2019, 48, 16952–16960. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Guo, L.; Su, Q.; Su, X.; Wen, Y.; Wang, T.; Yang, P.; Xu, M.; Li, F. Afterglow Amplification for Fast and Sensitive Detection of Porphyria in Whole Blood. ACS Appl. Mater. Interfaces 2021, 13, 27991–27998. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Li, M.; Ge, L.; Liu, S.; Lv, W.; Yu, Y.; Tang, Y.; Han, C.; Li, M.; Tao, Y.; et al. Ultralong Red Room-Temperature Phosphorescence of 2D Organic-Inorganic Metal Halide Perovskites for Afterglow Red Leds and X-ray Scintillation Applications. Inorg. Chem. 2023, 62, 16538–16546. [Google Scholar] [CrossRef]
- Shi, R.H.; Long, Z.Q.; Wang, F.; Gong, L.Z.; Lin, X.Y.; Zhuang, G.L.; Lu, D.F. Two Calcium-Based Metal Organic Frameworks with Long Afterglow as Anticounterfeiting Materials. Chem. Eng. J. 2024, 479, 147851. [Google Scholar] [CrossRef]
- Luo, Z.; Liu, Y.; Liu, Y.; Li, C.; Li, Y.; Li, Q.; Wei, Y.; Zhang, L.; Xu, B.; Chang, X.; et al. Integrated Afterglow and Self-Trapped Exciton Emissions in Hybrid Metal Halides for Anti-Counterfeiting Applications. Adv. Mater. 2022, 34, 2200607. [Google Scholar] [CrossRef]
- Liu, D.; Huxford, R.C.; Lin, W. Phosphorescent Nanoscale Coordination Polymers as Contrast Agents for Optical Imaging. Angew. Chem. Int. Ed. 2011, 50, 3696–3700. [Google Scholar] [CrossRef]
- Ding, Y.; Ye, Y.; Wang, C.; Pei, L.; Mao, Q.; Liu, M.; Zheng, R.; Bokhari, A.; Han, N.; Zhong, J. “Light Battery” Role of Long Afterglow Phosphor for Round-the-Clock Environmental Photocatalysis. J. Clean. Prod. 2024, 450, 142041. [Google Scholar] [CrossRef]
- Wang, Z.; Zhu, C.Y.; Wei, Z.W.; Fan, Y.N.; Pan, M. Breathing-Ignited Long Persistent Luminescence in a Resilient Metal-Organic Framework. Chem. Mater. 2019, 32, 841–848. [Google Scholar] [CrossRef]
- Yu, X.; Ryadun, A.A.; Pavlov, D.I.; Guselnikova, T.Y.; Potapov, A.S.; Fedin, V.P. Ln-MOF-Based Hydrogel Films with Tunable Luminescence and Afterglow Behavior for Visual Detection of Ofloxacin and Anti-Counterfeiting Applications. Adv. Mater. 2024, 36, 2311939. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Mo, J.; Pan, J.; Pan, M. White Light and Color-Tuning Long Persistent Luminescence from Metal Halide Based Metal-Organic Frameworks. Adv. Funct. Mater. 2023, 33, 2300021. [Google Scholar] [CrossRef]
- Zheng, M.; Jin, Z.; Ma, Z.; Gu, Z.; Zhang, J. Photo-Curable 3D Printing of Circularly Polarized Afterglow Metal-Organic Framework Monoliths. Adv. Mater. 2024, 2313749, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Li, S.; Wen, L.; Xu, Z.; Li, H.; Ding, L.; Cheng, Y. Exploration of Double Z-Type Ternary Composite Long-Afterglow/Graphitic Carbon Nitride@Metal-Organic Framework for Photocatalytic Degradation of Methylene Blue. J. Colloid Interface Sci. 2023, 629, 409–421. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, X.Q.; Wang, D.; Zhou, Y.Q.; Li, M.Y.; Mo, J.-T. Reversible Acid-Base Long Persistent Luminescence Switch Based on Amino-Functionalized Metal-Organic Frameworks. Inorg. Chem. 2024, 63, 1188–1196. [Google Scholar] [CrossRef]
- Lv, Y.; Ding, D.; Zhuang, Y.; Feng, Y.; Shi, J.; Zhang, H.; Zhou, T.; Chen, H.; Xie, R. Chromium-Doped Zinc Gallogermanate@Zeolitic Imidazolate Framework-8: A Multifunctional Nanoplatform for Rechargeable in Vivo Persistent Luminescence Imaging and Ph-Responsive Drug Release. ACS Appl. Mater. Interfaces 2019, 11, 1907–1916. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.-C.; Gu, Z.-G.; Zhang, J. Host–Guest Metal–Organic Frameworks-Based Long-Afterglow Luminescence Materials. Molecules 2024, 29, 2989. https://doi.org/10.3390/molecules29132989
Zhang Z-C, Gu Z-G, Zhang J. Host–Guest Metal–Organic Frameworks-Based Long-Afterglow Luminescence Materials. Molecules. 2024; 29(13):2989. https://doi.org/10.3390/molecules29132989
Chicago/Turabian StyleZhang, Zhi-Chen, Zhi-Gang Gu, and Jian Zhang. 2024. "Host–Guest Metal–Organic Frameworks-Based Long-Afterglow Luminescence Materials" Molecules 29, no. 13: 2989. https://doi.org/10.3390/molecules29132989
APA StyleZhang, Z.-C., Gu, Z.-G., & Zhang, J. (2024). Host–Guest Metal–Organic Frameworks-Based Long-Afterglow Luminescence Materials. Molecules, 29(13), 2989. https://doi.org/10.3390/molecules29132989







