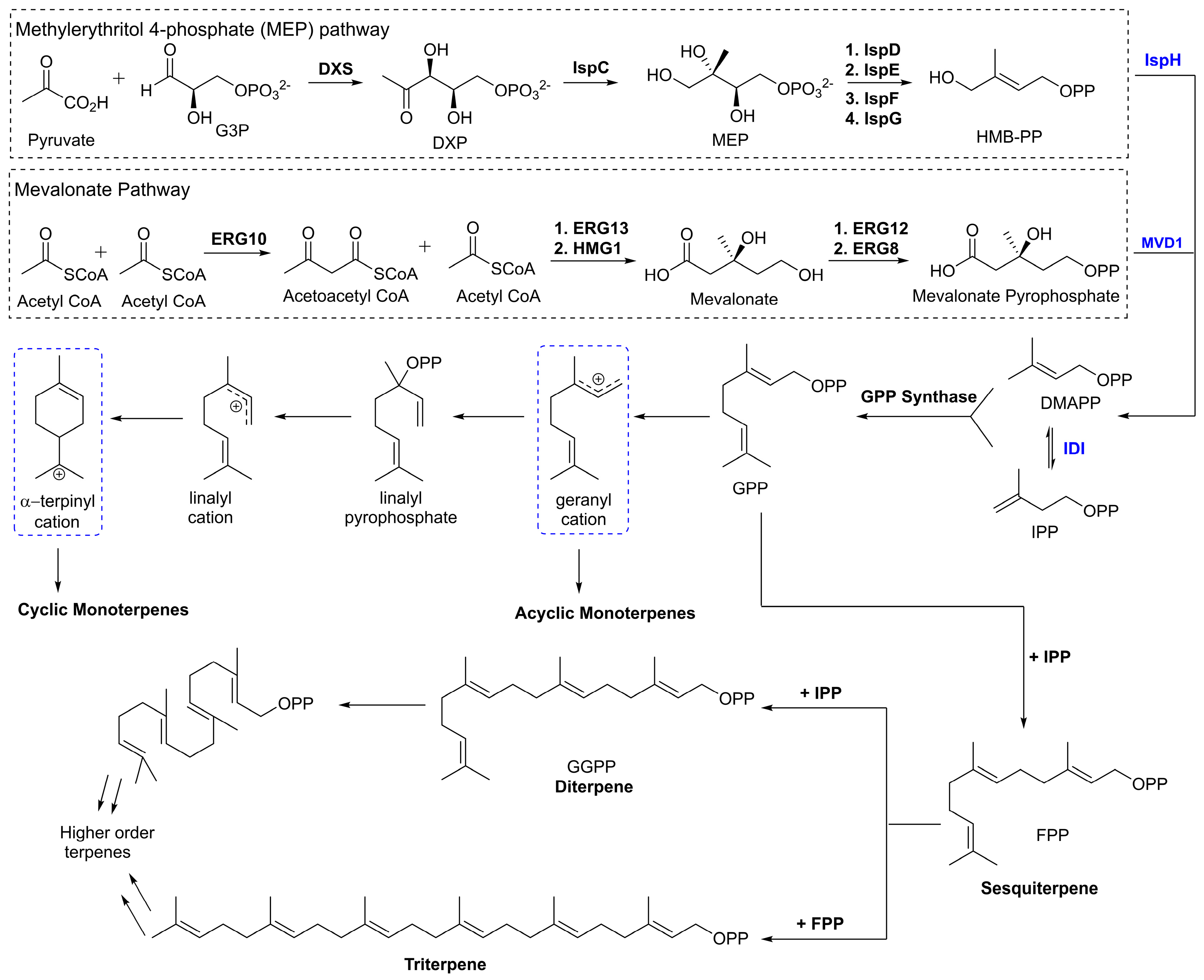
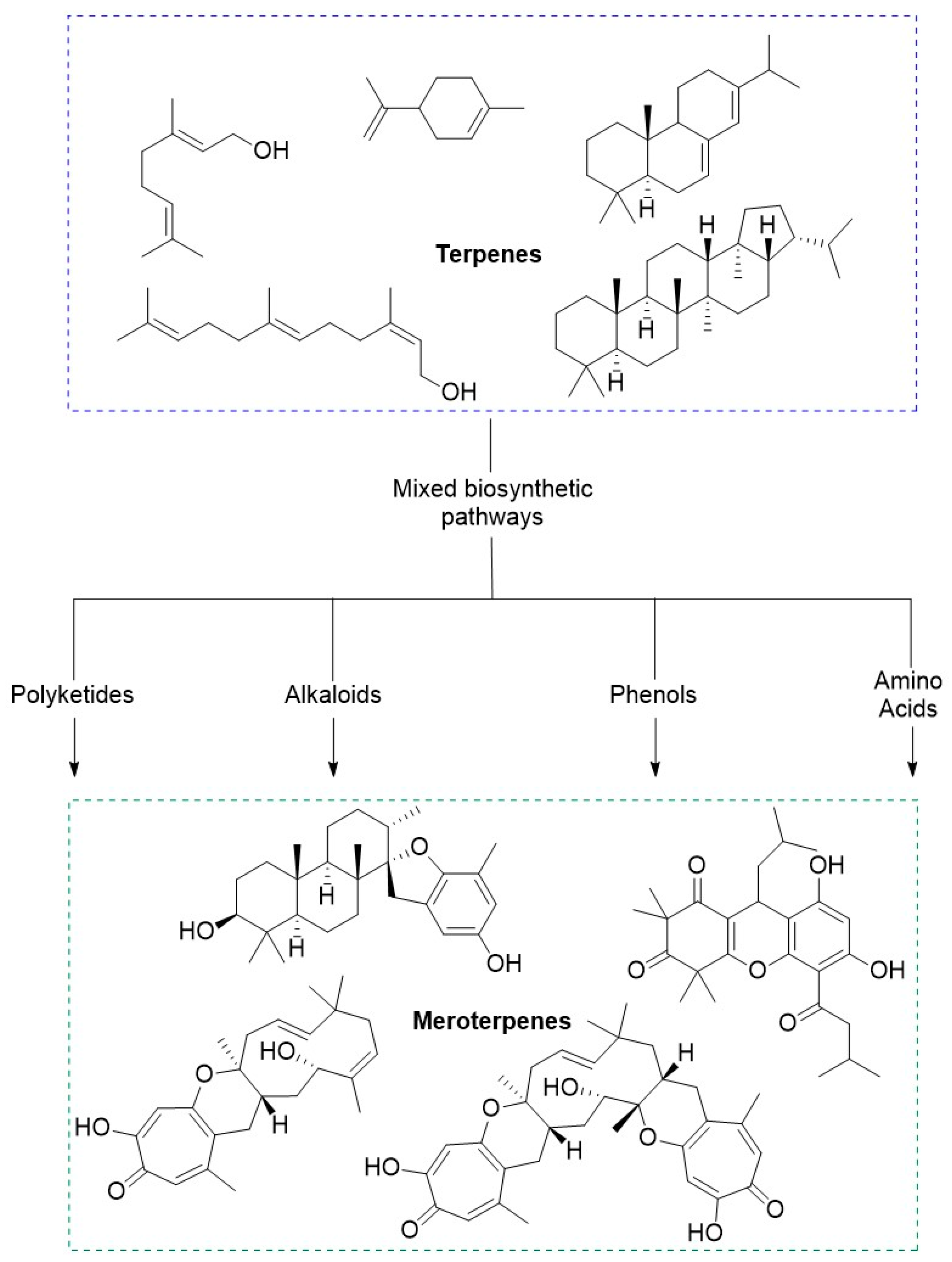
3. Chemistry and Biological Activities of Selected Meroterpenoids
Meroterpenoids are a large group of compounds characterized as being biosynthetically hybrid molecules, which typically involve the combination of a terpene with polyketides, alkaloids, phenols, or amino acids. The large potential for variations in the combination of synthons provides a class of molecules with a high degree of structural diversity. Recently, these natural products have gained attention from chemists and biologists alike as there are new chemical applications and synthetic strategies to access these complex molecular scaffolds. While many of these compounds have been isolated and identified there is a new need for larger quantities to pursue biological studies. Biological evaluations of these compounds have shown potential for therapeutic development, many of these intriguing meroterpene structures have already been reviewed [
64] and they are not included in this review. Specific synthetic lessons and innovative approaches are highlighted for the members of this meroterpene family, particularly natural products
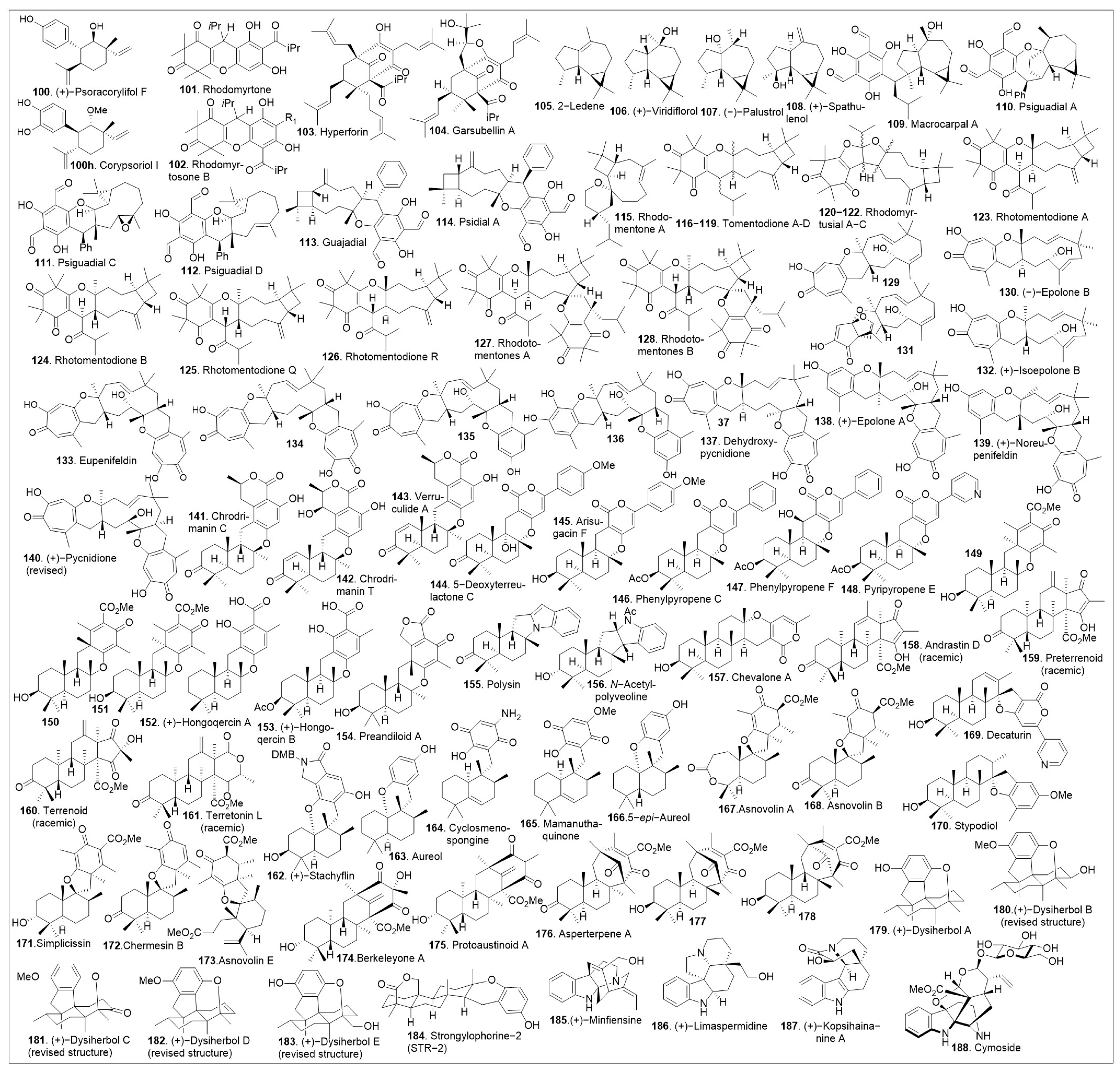
100–
188 (
Figure 5). This diagram highlights the complexity and diversity of these natural products containing 3 to 6 rings, which also includes an increase in ring size within this family of natural products.
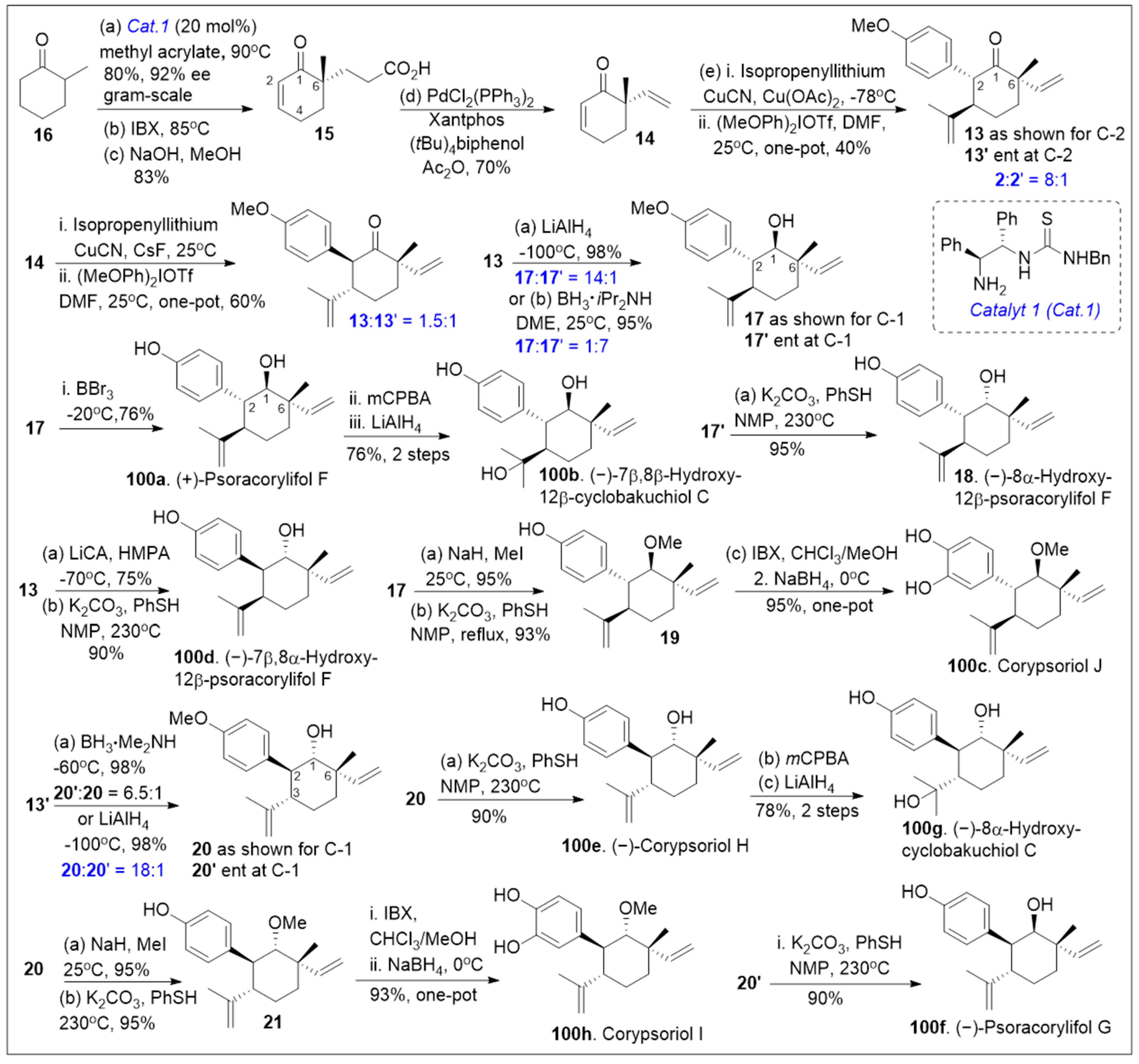
Total syntheses of small molecules can be challenging as insufficient functional groups are available to facilitate carbon-carbon bond formation and/or introduce chirality. Enantioselective divergent total syntheses for several meroterpenoids from
Psoralea corylifolia have been recently reported [
65]. The synthesis commenced with the readily available 2-methylcyclohexanone
16 in gram-scale, which was coupled with methyl acrylate mediated by thiourea catalyst (Cat. 1,
Scheme 1) followed by treatment with 2-iodoxybenzoic acid (IBX) to oxidatively generate the corresponding α,β- system and subsequent saponification furnished acid
15. The newly formed compound
15 was subjected to catalytic PdCl
2(PPh
3)
2 mediated intramolecular decarboxylative vinylation to establish the desired carbon quaternary center of enone
14. Compound
14 was then subjected to a 1,4-Michael addition reaction in the presence of copper and quenched with an aryl electrophile. This installs the α-arylation reaction sequence yielding diastereomers
13 and
13’, with compound
13 being predominant in 8:1 d.r. and can be separated by column chromatography. To improve the yield and favor a closer 1:1 ratio, Cesium fluoride was used instead of the copper acetate ligand which provided a 20% increase in yield. When applied properly, strategic conjugate addition reactions, when applied properly can expedite syntheses by forming carbon-carbon bonds efficiently, leading to improved overall yields. Compound
13 was subjected to reduction of the C-1 ketone with LiAlH
4 or amine borane reagent to enrich one diastereomer over the other one providing alcohols
17 and
17’ in either 14:1 or 1:7 respectively. Compound
17 can be deprotected by BBr
3 to yield the natural product (+)-psoracorylifol F (
100a) in good yield, which was subjected to regioselective
mCPBA epoxidation, and subsequent LiAlH
4 reductive opening of the epoxide provided (−)-7β,8β-hydroxy-12β-cyclobakuchiol C (
100b). Aromatic methoxy deprotection of compound
17’ by thermal treatment in the presence of the thio-anion led to the natural product (−)-8α-hydroxy-12β-psoracorylifol F (
18). Compound
13 was exposed to a three-step synthetic sequence strategy, α-epimerization, carbonyl reduction via NaBH
4 treatment, and heat-mediated thio-anion methyl deprotection of the phenol provided natural product (−)-7β,8α-hydroxy-12β-psoracorylifol F (
100d). Alternatively, compound
17 was treated with MeI to protect the secondary hydroxyl group, and the aromatic methoxy group was revealed under thio-anion reaction conditions to yield compound
19 in excellent yields. The resultant compound
19 was oxidized at the alpha phenol with IBX followed by reduction with NaBH
4 of the newly formed ortho-diketone to provide the natural product corypsoriol J (
100c). In parallel efforts, compound
13’ was subjected to carbonyl-reduction followed by LiAlH
4 or amine borane reagent to enhance the diastereoselectivity yielding alcohols
20 and
20’ in 6.5:1 or 18:1 ratio respectively. Deprotection of compound
14 mediated by thio-anion treatment under heat led to natural product (−)-corypsoriol H (
100e), which upon two-step sequence epoxidation mediated by
mCPBA and subsequent reductive opening of the epoxide with LiAlH
4 provided (−)-8α-hydroxy-cyclobakuchiol C (
100g). In a similar fashion, intermediate
20 was subjected to methyl-protecting group maneuver via intermediate
21, followed by one-pot oxidation mediated by IBX and subsequent reduction of the newly formed ortho-diketone. This, mediated by NaBH
4 yielded corypsoriol I (
100h). Finally, methyl deprotection of compound
20’ mediated by thermal treatment in the presence of thio-anion yielded (−)-psoracorylifol G (
100f). The short and efficient synthetic strategy enables access to a large number of natural products, which can be further investigated for their biological properties.
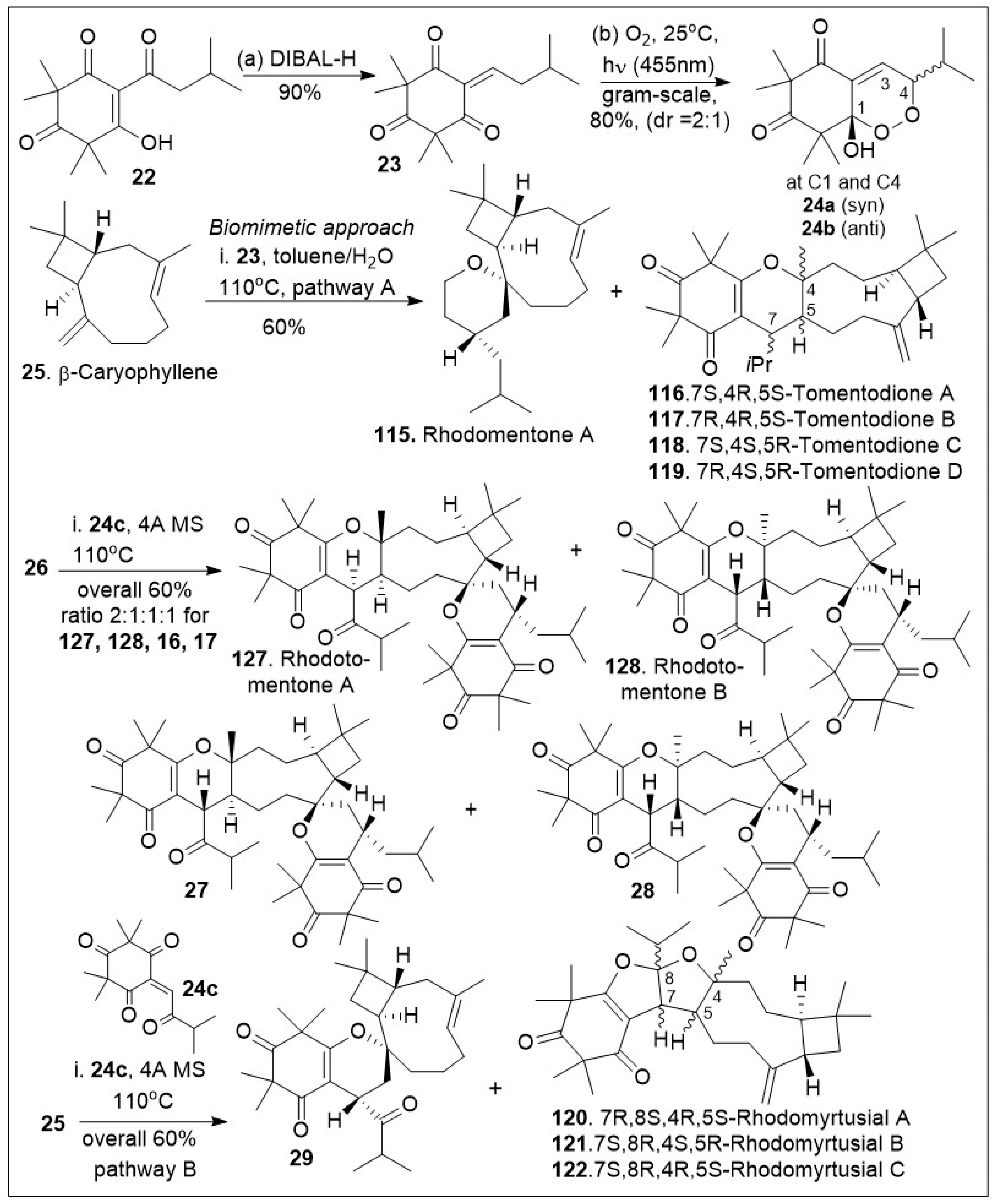
Rhodomyrtone belongs to a large and diverse family of tricyclic meroterpenoid systems isolated from
Rhodomyrtus tomentosa [
66,
67,
68,
69,
70]. This set of compounds has been shown to have strong antibacterial activity, acting primarily against gram-positive strains. In addition, the combined extracts of
Rhodomyrtus tomentosa have shown anti-cancer and anti-tumoral effects in various models [
66,
67]. The synthesis of the unusual caryophyllene-derived meroterpenoids (CDMTs) including rhodotomentone A (
127) and rhodotomentone B (
128) was reported via a biomimetic pathway [
68,
69,
70], (
Scheme 2). The synthesis began from the natural product leptospermone (
22), a commonly occurring acylphloroglucinol, which was subjected to dehydration mediated by DIBAL-H, yielding the dearomatized
ortho-quinone methide (
o-QM) intermediate
23 in gram-scale. This maneuver was followed by a [4+2] Diels-Alder photo-cycloaddition reaction with singlet oxygen, generated under blue LED (455 nm) radiation to provide endoperoxide intermediates
24a and
24b in 1:1 ratio. The formed
o-QM intermediate
23 was subsequently subjected to hetero-Diels-Alder (HDA) reaction with β-caryophyllene
25 to produce a mixture of rhodomentone A (
115), and the related natural products, tomentodione A, B, C and D (
116,
117,
118, and
119). Rhodomentone A (
115) can be treated with endoperoxide
24b to undergo the key Kornblum-DeLamare rearrangement and Diels-Alder cycloaddition reaction sequence under thermal reaction conditions. This produces rhodotomentone A(
127) and B(
128), along with two other rhodotomentone diastereoisomers, compounds
27 and
28. Additionally, endoperoxide
24b was reacted with β-caryophyllene (
25) to undergo the Kornblum-DeLamare rearrangement and Diels-Alder cycloaddition reaction sequence under thermal conditions to generate rhotomentodine A, B, Q and R (
123,
124,
125 and
126), along with rhodomyrtusial A, B and C (
120,
121 and
122) and compound
29 [70].
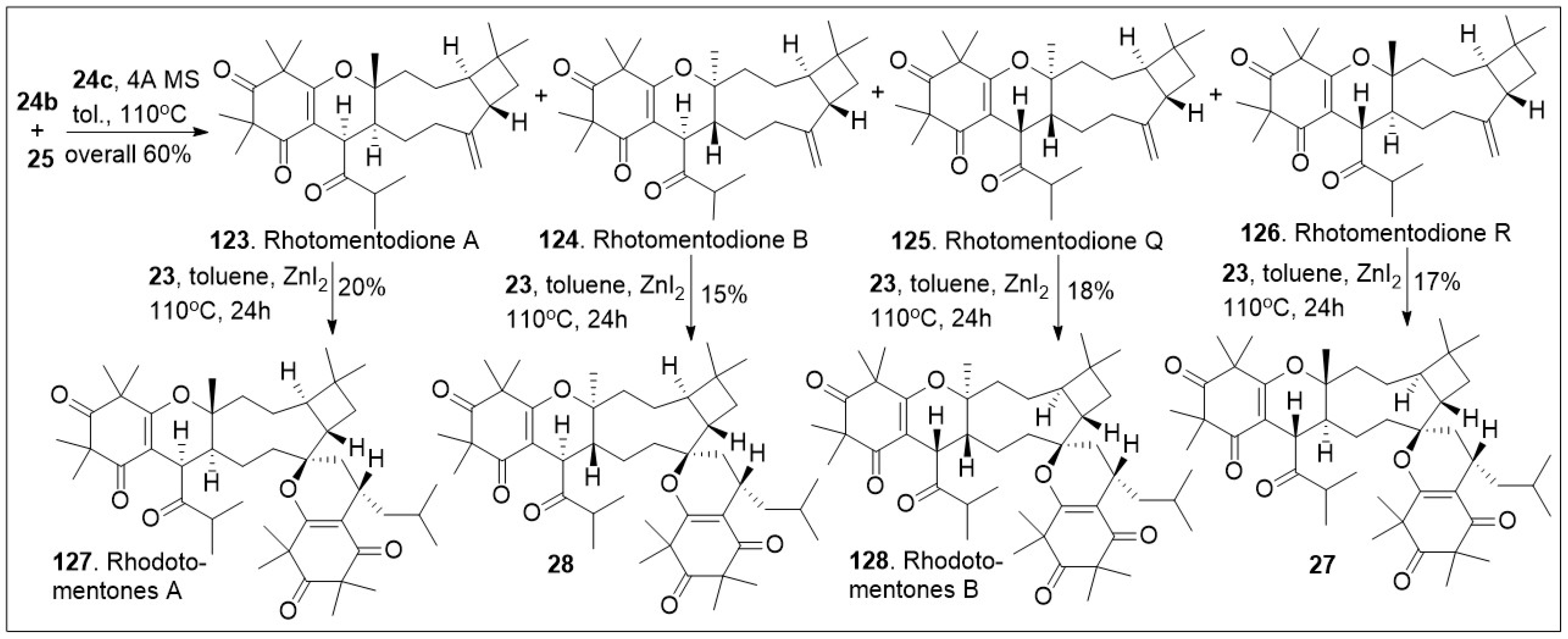
In an efficient approach (
Scheme 3), CDMTs
123,
124,
125 and
126 were easily converted to the corresponding natural products rhodomentone A (
127),
28, rhodomentone B (
128) and
27 respectively. This was carried out under hetero-Diels-Alder (HDA) reaction conditions, requiring Lewis acid, ZnI
2 and heat, in relatively modest yields. The synthetic approach offers rapid access to impressively complex structures [
70].
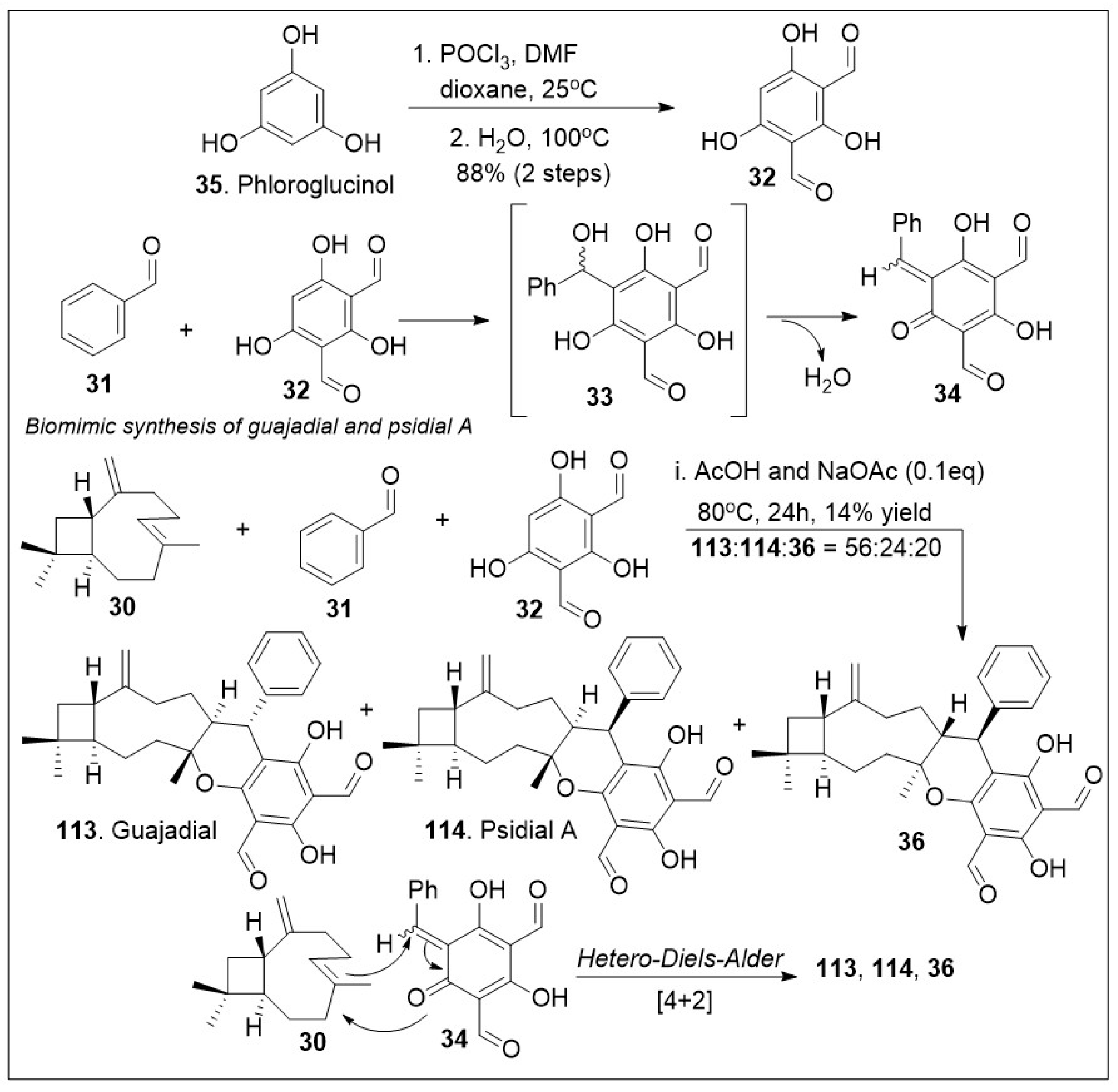
The meroterpenoids macrocarpals and psigudials isolated from
Psidium guajava a food crop widely abundant in tropical regions has been reported to display an array of medicinal properties [
71,
72]. These compounds have displayed significant biological properties as anti-HIV and anti-bacterial agents. To further evaluate their properties, access to sufficient quantities of these compounds is required. The application of a hetero-Diels-Alder (HDA) reaction in meroterpenoid synthesis is frequently utilized due to the likely probability that a similar pathway is taking place in nature to generate these natural products [
72]. A short biomimetic-inspired synthesis to guajadial
113 and compound
30 was recently reported (
Scheme 4). The synthesis commenced with a Knoevenagel condensation between benzaldehyde
31 and diformylphloroglucinol
32 providing compound
33 for in situ generated
o-quinone methide
34. The HDA cycloaddition reaction between caryophyllene
30 and
o-QM
34 led to guajadial
113, psidial A
114 and cycloadduct
36 [
72].
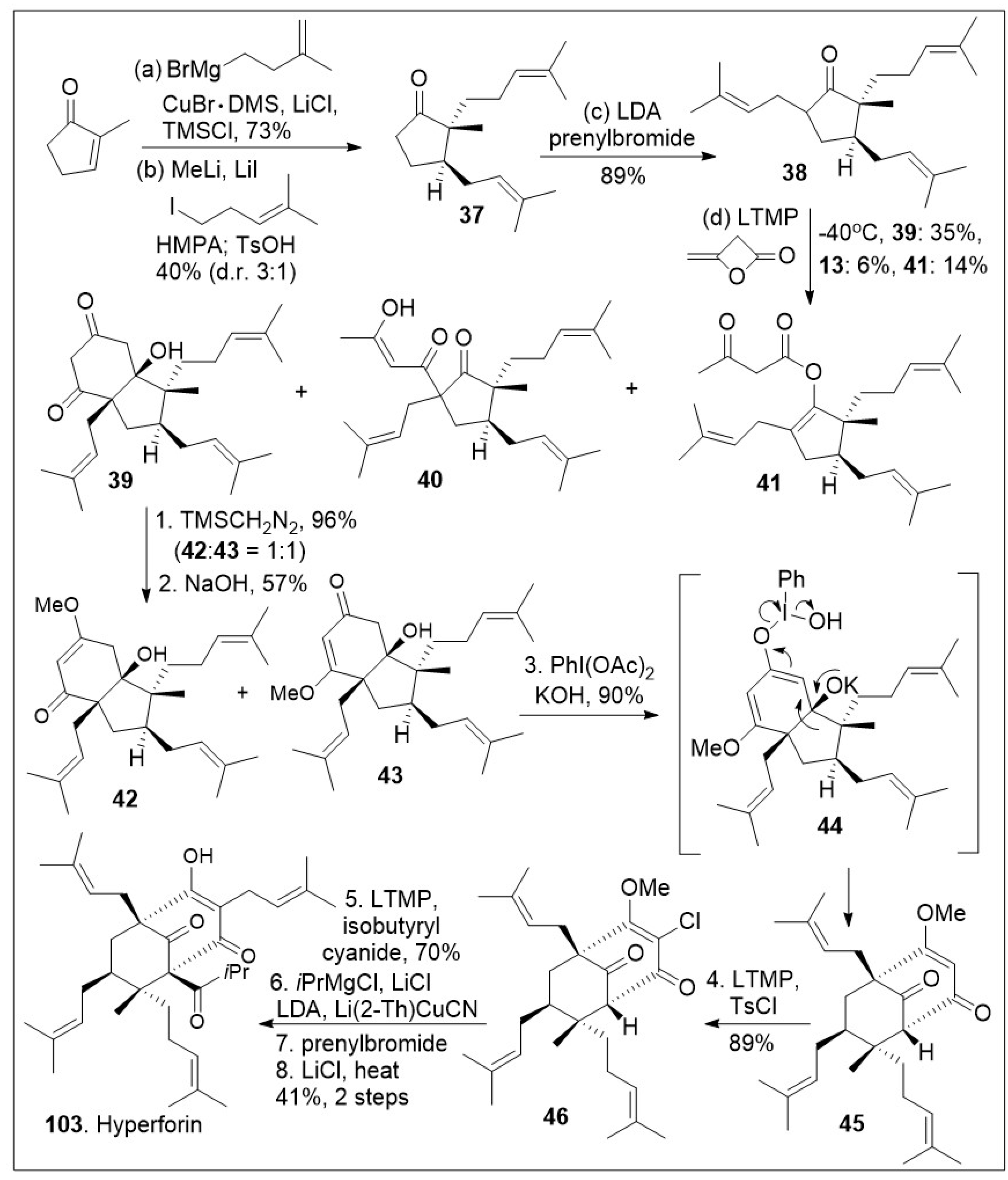
Hyperforin-like structures are a large family of meroterpenes that share a challenging molecular caged motif. A recent innovative synthetic strategy based on an annulative approach of polycyclic polyprenylated acylphloroglucinol (PPAP) meroterpenoids with 3,5-dimethylorsellinic (DMOA) was reported as shown in
Scheme 5 [
73]. The synthesis commenced with the readily available methylcyclopenenone synthon, which was subjected to sequential 1,4-copper-mediated conjugate addition reaction, namely formation of the lithium enolate followed by alkylative enolate trapping to generate the substituted cyclopetanone
37, which was subjected to further α-alkylated to furnish the highly substituted cyclopetanone
38. Next, cyclopetanone
38 was subjected to the key annulation reaction with β-lactone diketene, leading to diketone
39 as the major product, along with C-acylated product
40 and O-acylated product
41. Diketone
39 was further methylated with trimethylsilyldiazomethane to generate the vinylogous ester isomers
42 and
43. Compound
43 was subjected to oxidative rearrangement mediated by hypervalent iodine to provide product
45 with bicyclo[3.3.1]nonane ring system via intermediate
44. Compound
45 was subjected to a subsequent chlorination reaction to provide halogenated compound
46. Finally, a deprotonation/prenylation alkylation, acylation reaction sequence was used to introduce the last isoprenyl group generating the most challenging quaternary center. This was followed by deprotection of the methoxy group to yield hyperforin (
103) in modest yields. The short strategy is flexible enough to generate a potential diverse library of derivatives for future biological evaluations.
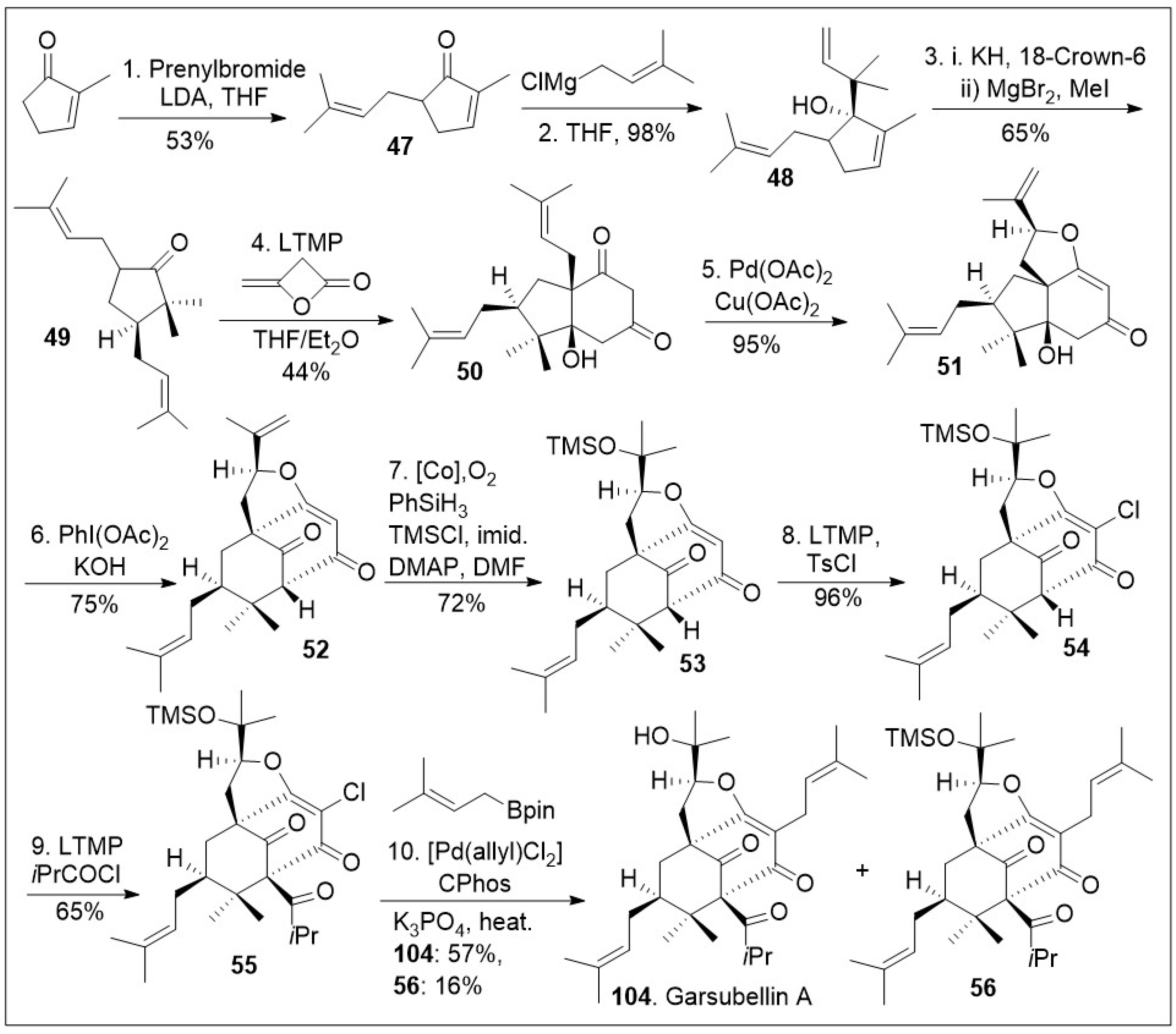
A similar approach has been applied to the caged meroterpenoid garsubellin A (
104, Scheme 6) [
73]. The total synthesis was initiated with methylcyclopenenone, which was α-prenylated to generate compound
47, followed by 1,2 Grignard reaction to access allylic alcohol
48. The resultant compound
48 was subjected to an anionic oxy-Cope rearrangement to provide bisprenylated cyclopentanone
49. Then, compound
49 underwent an annulation reaction with β-lactone diketene to produce diketone
50, followed by Pd(OAc)
2/Cu(OAc)
2 mediated unusual Wacker-type-intramolecular cyclization to provide tricycle system
51. Tricyclic system
51 was treated with PhI(OAc)
2 under basic condition for an oxidative ring expansion leading to compound
52, the needed bicyclo[3.3.1]nonane ring system that upon cobalt catalyzed Mukaiyama hydration reaction provided tricycle
53. At this point in the synthesis compound
53 was treated with lithium tetramethylpiperidide (LTMP), and TsCl to introduce the α-vinylogous chlorine to generate compound
54, followed by acylation to introduce the congested quaternary center leading to compound
55. Various types of alkylation reactions are feasible for such compound, but alkylation with borane prenyl system mediated by [Pd(allyl)Cl
2], and catalytic CPhos ligand under basic conditions introduce the prenyl group and remove the silyl protecting group to provide garsubellin A (
104), as well as compound
56, which could be treated with acid or base to yield garsubellin A (
104).
The polycyclic fused ring meroterpenes such as berkeleyone A have displayed anti-inflammatory activity [
74], while preandiloid A has shown antimicrobial properties. Asperterpene A was identified as a potent BACE-1 inhibitor with an IC
50 value of 78 nM against this enzyme, out preforming commercial BACE-1 inhibitors. Furthermore, in vivo animal model studies were congruent with the in vitro efficacy studies of this natural product [
74]. These findings indicate that these compounds require further studies and synthetic strategies to access them in large quantities.
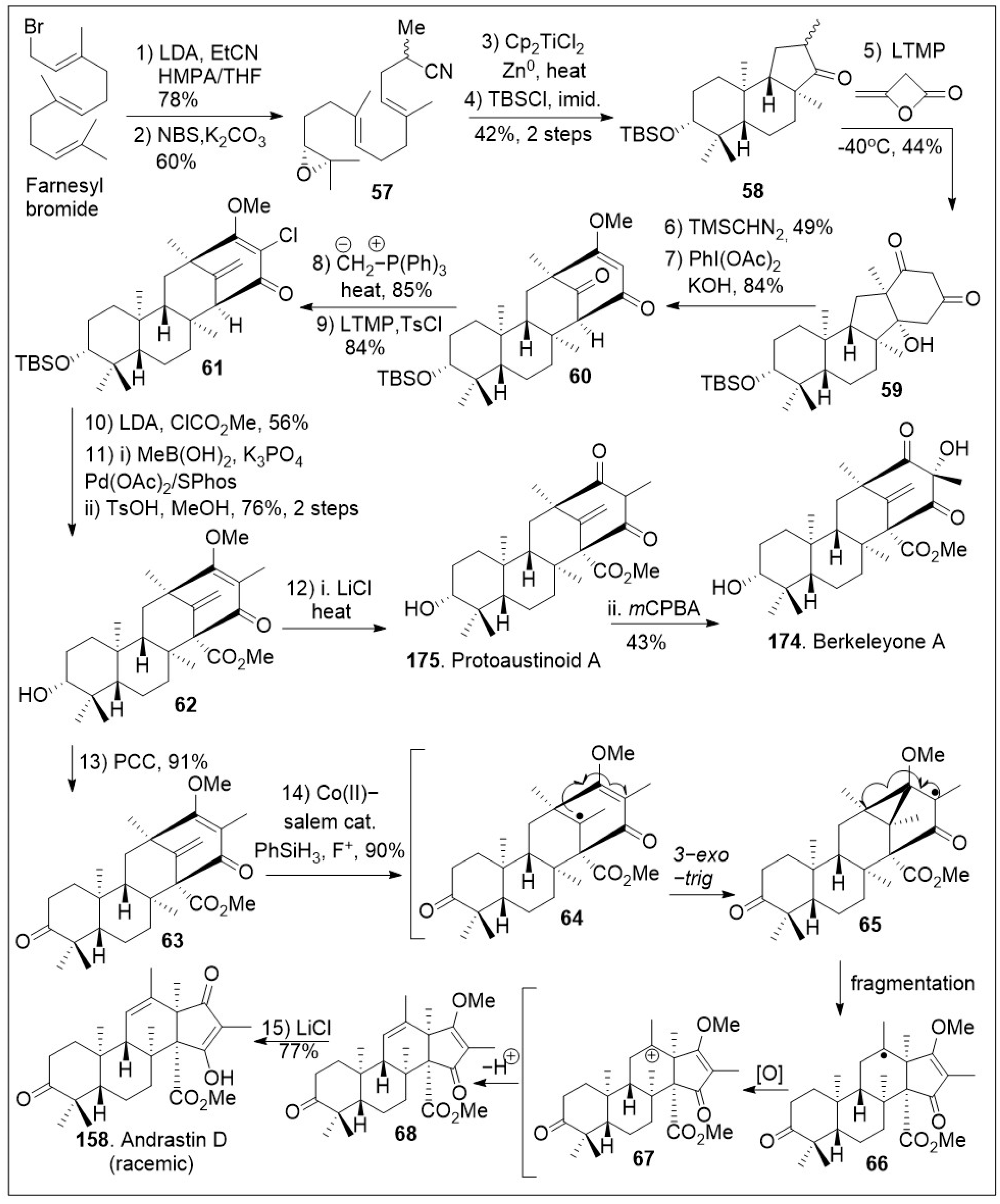
A successful approach to these natural products involves the use of a cyclization reaction to set up the tricyclic system in a single step as shown in
Scheme 7 [
73]. The synthesis began with farnesyl bromide being subjected to alkylation with the anion of propionitrile, followed by one-pot bromohydrin formation/cyclization reaction sequence to generate epoxide
57, which upon reductive epoxide-opening radical cyclization furnished tricyclic ketone
58. Then, ketone intermediate
58 was subjected to annulation reaction with β-lactone diketene to produce diketone
59, which was O-methylated, followed by oxidative ring expansion mediated by PhI(OAc)
2 under basic conditions to furnish bicyclo[3.3.1]nonane ring system
60. The newly formed tetracyclic
60 was exposed to Wittig olefination and subsequent chlorination reaction to yield halogenated polycyclic compound
61.
Upon further three-step α-alkylative methyl ester functionalization, methylation via Suzuki coupling reaction of the vinyl chloride with MeB(OH)
2, and acid mediated deprotection yielded advanced intermediate
62. Then, compound
62 was subjected to the final chloride-mediated demethylation, leading to the DMOA-derived metabolite, protoaustinoid A (
175). Compound
175 was further oxidized with
mCPBA to provide berkeleyone A (
174). In addition, advanced polycyclic compound
62 was treated with pyridinium chlorochromate (PCC) to generate compound
63, which was subjected to Shigehisa’s cobalt(II)-catalyzed hydroalkoxylation reaction leading to polycyclic intermediate
68. Finally, this compound was treated with LiCl to free the trapped enolate system, yielding the natural product andrastin D (
158) in racemic form. Furthermore, as shown in
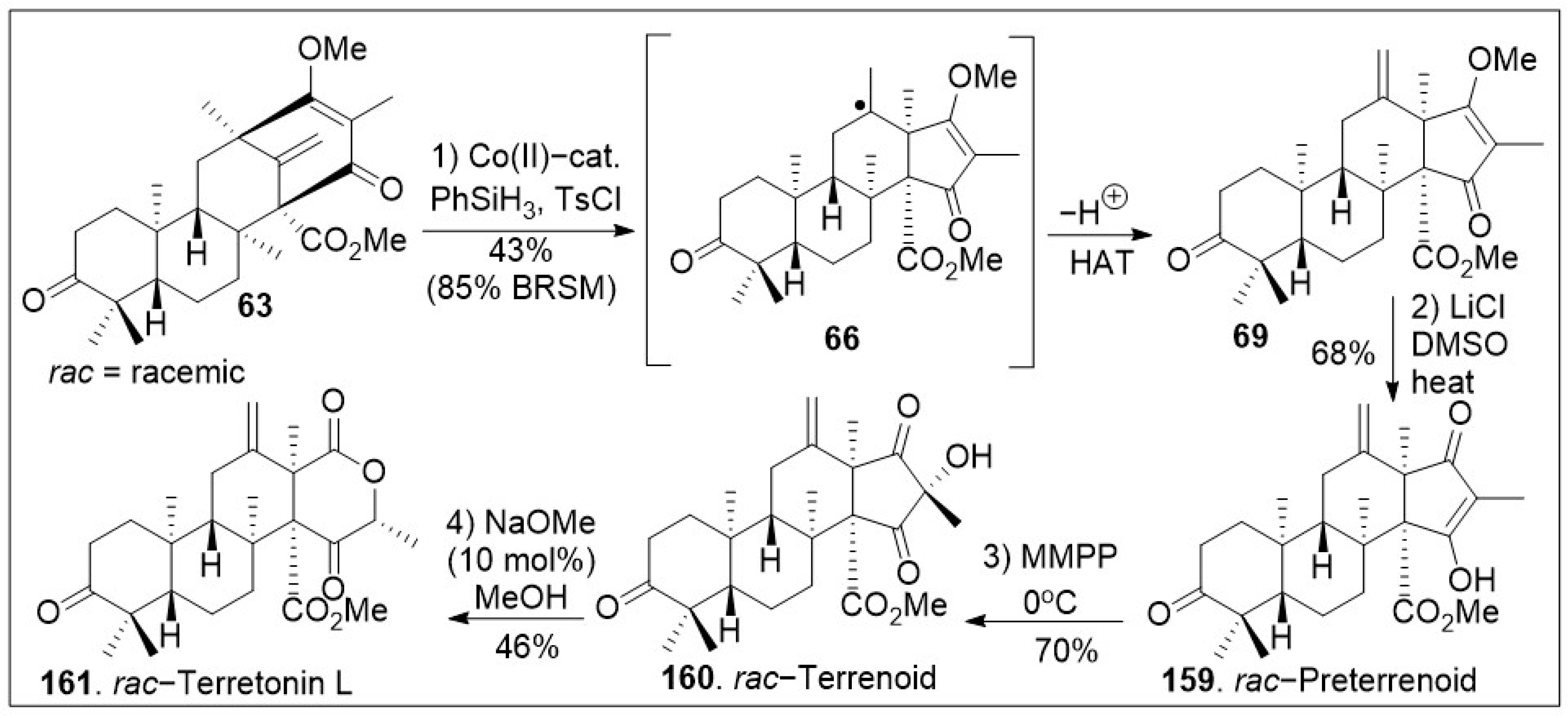
Scheme 8, the tetracyclic compound
63 was subjected to Carreira’s alkene hydrochlorination conditions to provide the polycyclic compound
69 via hydrogen-atom-transfer (HAT) process involving radical intermediate
66. Subsequent demethylation of polycyclic compound
69 mediated by LiCl yielded the natural product preterrenoid (
159), which underwent stereoselective α-oxidation mediated by magnesium monoperoxyphthalate (MMPP) to afford terrenoid (
160). Upon ring-expansion via retro-Claisen/esterification cascade reaction under basic conditions, compound
160 was converted to racemic terretonin L (
161). This synthetic strategy is efficient at enabling the generation of multiple members of this meroterpene family in good overall chemical yields [
73]. This approach is not asymmetric, however, there are multiple opportunities to introduce asymmetry.
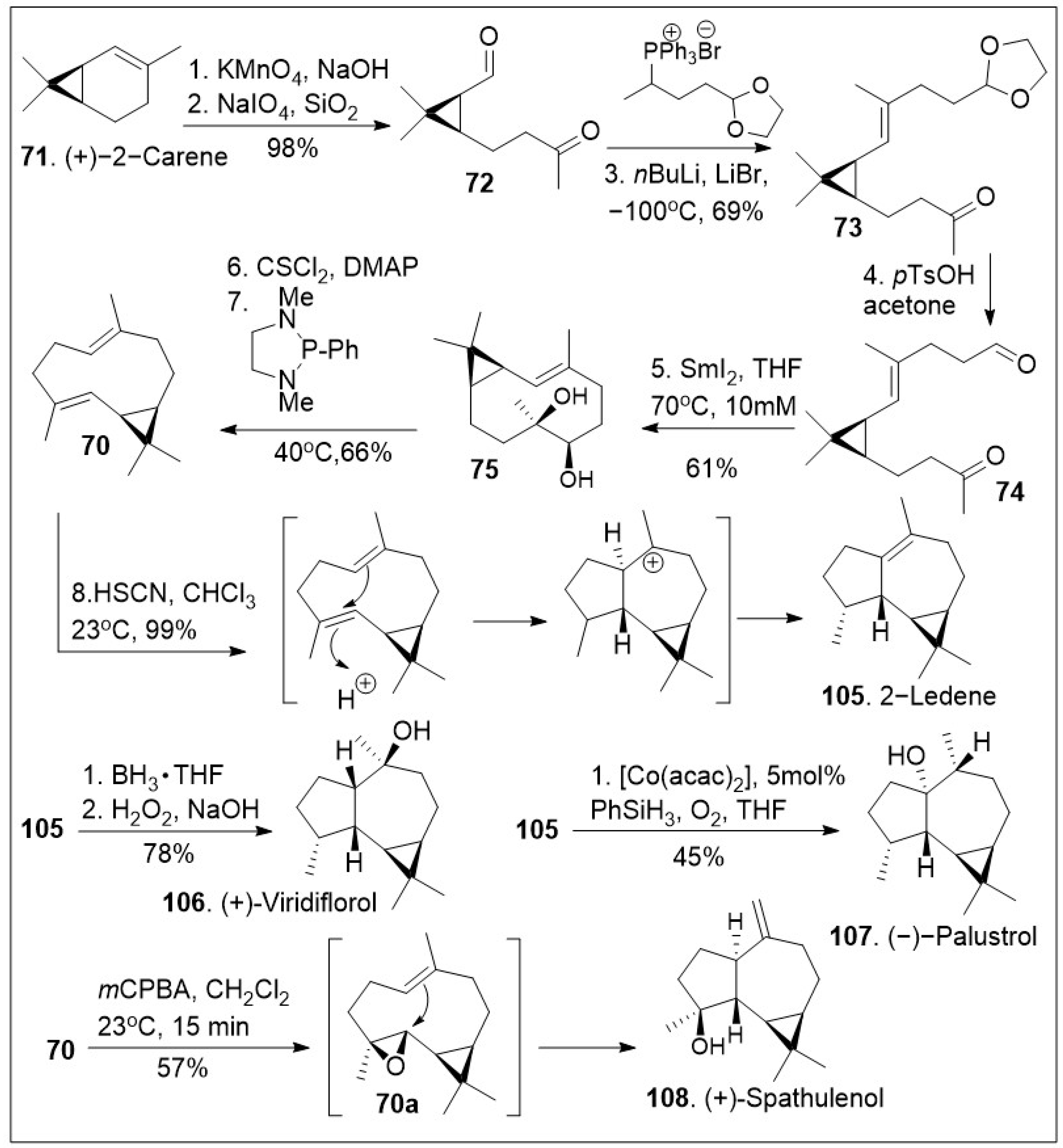
Forging complicated carbon-carbon bonds in complex natural products represents a challenge that requires groundbreaking synthetic endeavors or engaging in biomimetic synthesis, the process of imitating nature’s way to make molecules. Biomimetic synthesis of the psiguadial meroterpenoids featuring the key cationic cyclization reaction using terpene (+)-bicyclogermacrene
70 as the platform has been reported [
75] and it is depicted in
Scheme 9. The synthesis commenced with the generation of ketone-aldehyde
72, which was generated from (+)-2-carene (
71) upon treatment with KMnO
4 to mediate the dihydroxylation, and subsequent oxidative diol cleavage reaction. Compound
72 was further treated with Wittig reagent
A under basic reaction conditions to provide compound
73, which was deprotected, and cyclized under samarium mediation to provide the 10-membered cyclic system
75, which was then converted to (+)-bicyclogermacrene
70 with the required
E-olefin geometry using a modified Corey-Winter protocol. Compound
70 was then converted to 2-ledene (
105) via acid facilitated intramolecular cationic cyclization. The natural product
105 was transformed to (+)-viridiflorol (
106) upon treatment with hydroboration/hydroxylation conditions. Alternatively, compound
105 was subjected to Mukaiyama’s cobalt-catalyzed phenylsilane/O
2 hydration conditions to provide natural product (−)-palustrol
107. Finally, epoxidation of compound
70 was facilitated upon treatment with
mCPBA, followed by intramolecular cyclization provided (+)-spathulenol (
108).
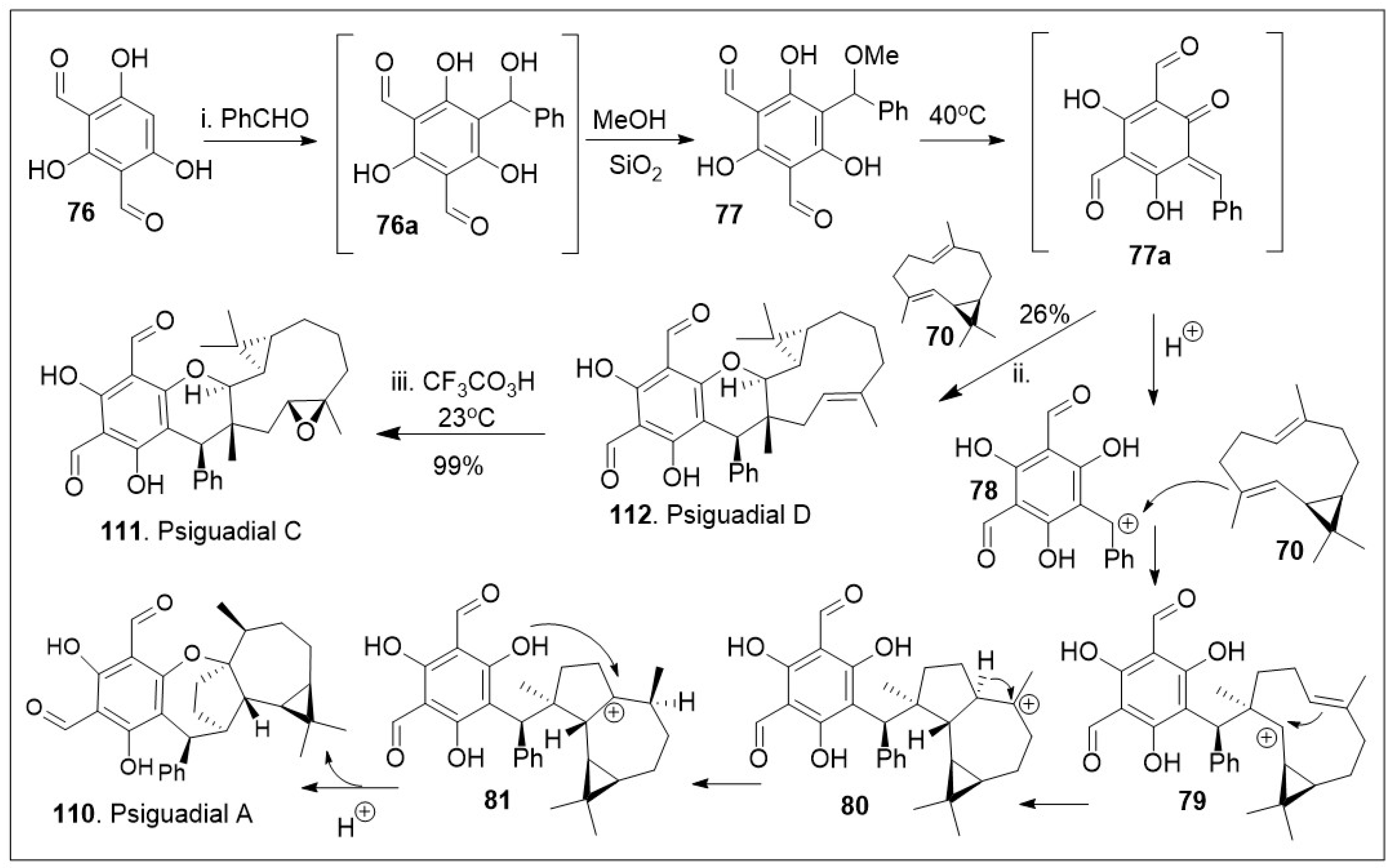
As highlighted in
Scheme 10, biomimetic approaches allow for flexible synthesis of various members of the same natural product family [
75]. Thus, compound
70 was treated with intermediate
o-QM
77 to enable the hetero-Diels-Alder (HDA) reaction, in order to yield the natural product psiguadial D (
112). The compound
o-QM
77 was synthesized in situ as the Knoevenagel-type condensation reaction product between bisformylated phloroglucinol
76 and phenyl aldehyde. Furthermore,
112 was converted to psigudial C (
111) via epoxidation reaction. Under the similar reaction conditions, the
o-QM
77 was unified with compound
4 via sequential cationic intermolecular coupling reaction followed by intramolecular cyclization cascade reactions. Briefly, intermediate
77 under acidic conditions generated the benzylic carbocation, which was able to react with compound
70 via addition reaction to yield intermediate
79. Compound
79 underwent an intramolecular cyclization, and a 1,2-hydride shift to establish the most stable cation
81. Finally, intramolecular cyclization from the phenolic system facilitated the installation of the last 7-member ring system of the natural product psiguadial A (
110).
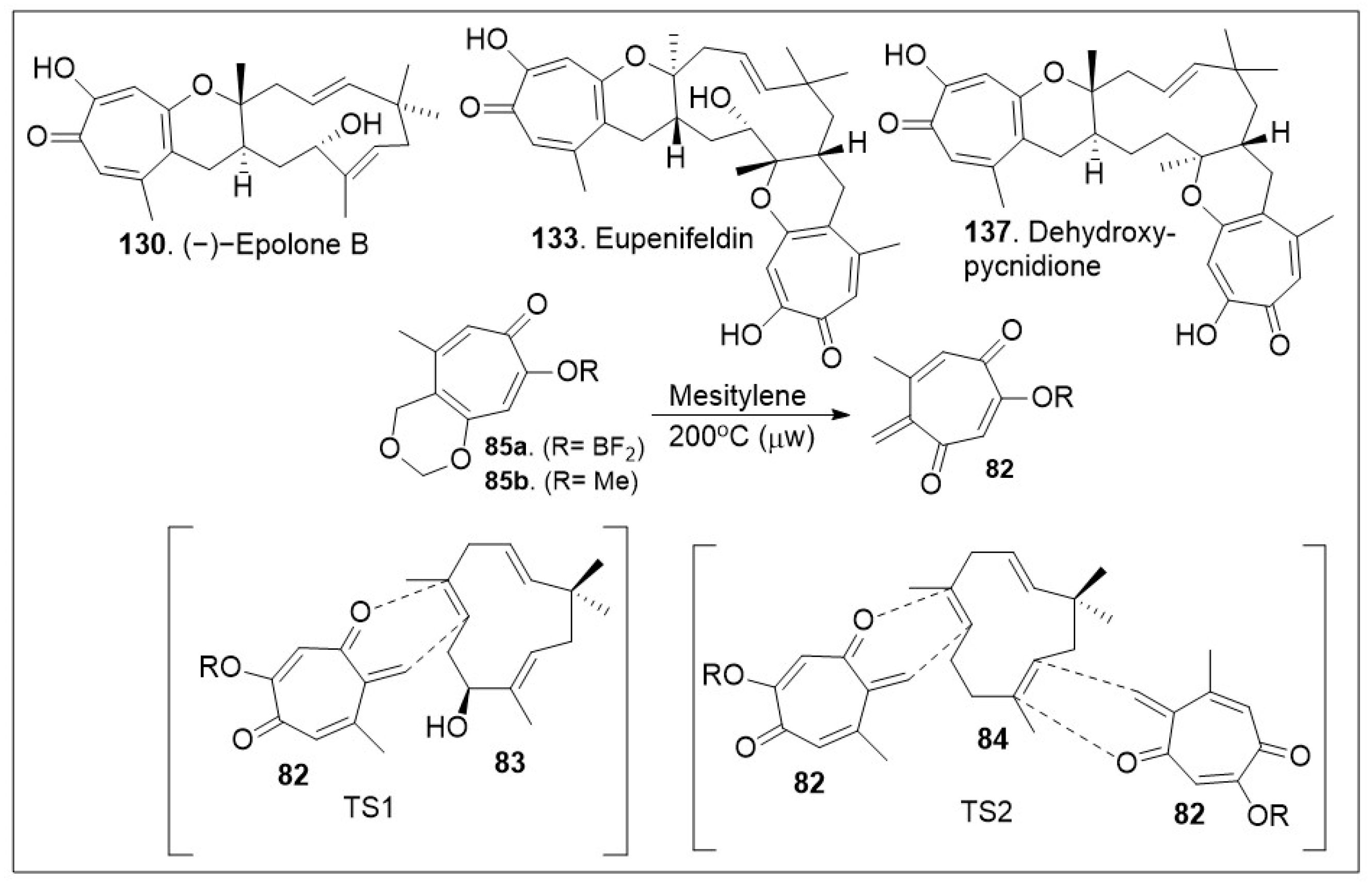
The sesquiterpene-tropolones belong to a class of structurally distinctive meroterpenoids characterized as sesquiterpene mono- and bistropolones, which includes pycnidione and eupenifeldin, featuring a unique polycyclic ring system with rich stereochemistry. These compounds are isolated from
Neosetophoma sp. a species of fungi. These compounds were tested against a series of cancer cell lines: human breast cancer (MDA-MB-231), two human ovarian cancer cell lines (OVCAR-3 and OVCAR-8), human mesothelioma (MSTO-211H), and human lung cancer (A549) to determine their IC
50 values; the most potent compound, eupenifeldin (
133,
Scheme 11) had IC
50 values of 2.83, 0.33, 0.02, 0.08, 1.33 μM for the above listed cell lines respectively [
76]. To further evaluate the cytotoxicity of these compounds towards healthy tissues a mitochondrial toxicity assay was conducted for compounds
129, 131 and
133 (
Figure 5). The results of this experiment showed no cytotoxicity up until a maximum dose of 12.5 μM, which was well above the majority of the IC
50 values for the compounds tested [
76]. Further study of this natural product was conducted by Maldonado et al. focusing on its effects on a panel of ovarian cancer cell lines. The antiproliferative effect (EC
50) of eupeninfeldin on three ovarian cancer cell lines (OVCAR-3, OVCAR-5, OVCAR-8) and nontumorigenic fallopian tube cells (FTT3-Tag) were determined to be 10, 11, 12, and 170 nM respectively [
77]. The results indicate the selectivity of the compound for cancer cell models over normal tissue with a greater than 10-fold difference. Additional mechanistic studies included cell migration assays, which provided favorable outcomes [
77]. To validate their in vitro findings, hollow fiber studies were conducted in murine models. OVCAR-3 and OVCAR-8 hollow fibers were implanted in mice, which were treated with eupeninfeldin or vehicle daily for 4 days. A significant reduction of OVCAR-3 cell model was observed while no significant inhibition of OVCAR-8 cell model was recorded suggesting the compound is working via a specific pathway. To further investigate the mechanism of action specific protein evaluation was conducted for apoptosis pathway (caspase 3/7 activation, PARP cleavage, autophagy assays) and global protein analysis using tandem mass tag (TMT), a chemical label technique that facilitates sample multiplexing in mass spectrometry (MS)-based quantification and identification of the proteome. Bafilomycin A1, a member of macrolide antibiotics and an autophagy inhibitor, was used as a co-treatment with eupenifeldin on their selected ovarian cancers cell lines (OVCAR-3, OVCAR-5, and OVCAR-8) to determine if this compound is mediating cell death through autophagy as a primary cell death modality. The resultant data indicated that indeed bafilomycin was able to significantly rescue the cells from death. Their combined findings indicate that this natural product displays selectivity. It is likely mediating cell death through autophagy [
77]. Pycnidione (
140,
Figure 5) is another example of bioactive sesquiterpene-bistropolone meroterpenoid natural products. First isolated from fungal species,
Phoma sp., this compound has been studied extensively for its biological activities. Kaneko studied the effects of pycnidione on leukemia cell models [
78]. This compound alone or in combination with bleomycin against a panel of human cancer cell lines had a significant anti-proliferative effect. Ongoing studies indicate that pycnidione inhibits of topoisomerase II at an IC
50 value of 19 μM and modulates the cell cycle at the G2 phase [
78]. These biological studies highlight the importance of having access to large quantities of these natural products for further derivatization to improve efficacy. The recent total synthesis of the structurally revised flagship members of the pycnidione family, namely (+)-pycnidione (
140), dehydroxypycnidione (
137) and (−)-epolone B (
130) was elegantly designed on a modular [4+2] inverse electron-demand hetero-Diels-Alder (HDA) reaction between the in situ generated
ortho-quinone methide (
o-QM)
82 and the corresponding dienophile, hydroxyhumulene
83 or humulene
84 (
Scheme 11). While the majority of [4+2] cycloaddition reactions require heat, microwave assistance can also promote the rection, and minimize decomposition of starting materials or intermediates. A unified synthetic endeavor was recently used to generate various members of these complex meroterpenes through the in situ generation of compound
82 (
Scheme 11). The resultant transition state of either dienophile
83 or
84 can proceed either as a single monocyclic formation step, TS1 or a bis-cyclization formation, TS2. They indicated that the reaction can be modified to favor one or various products. However, the diastereomers of eupenifeldin series have yet to be synthesized and remain a highly active area of research [
79].
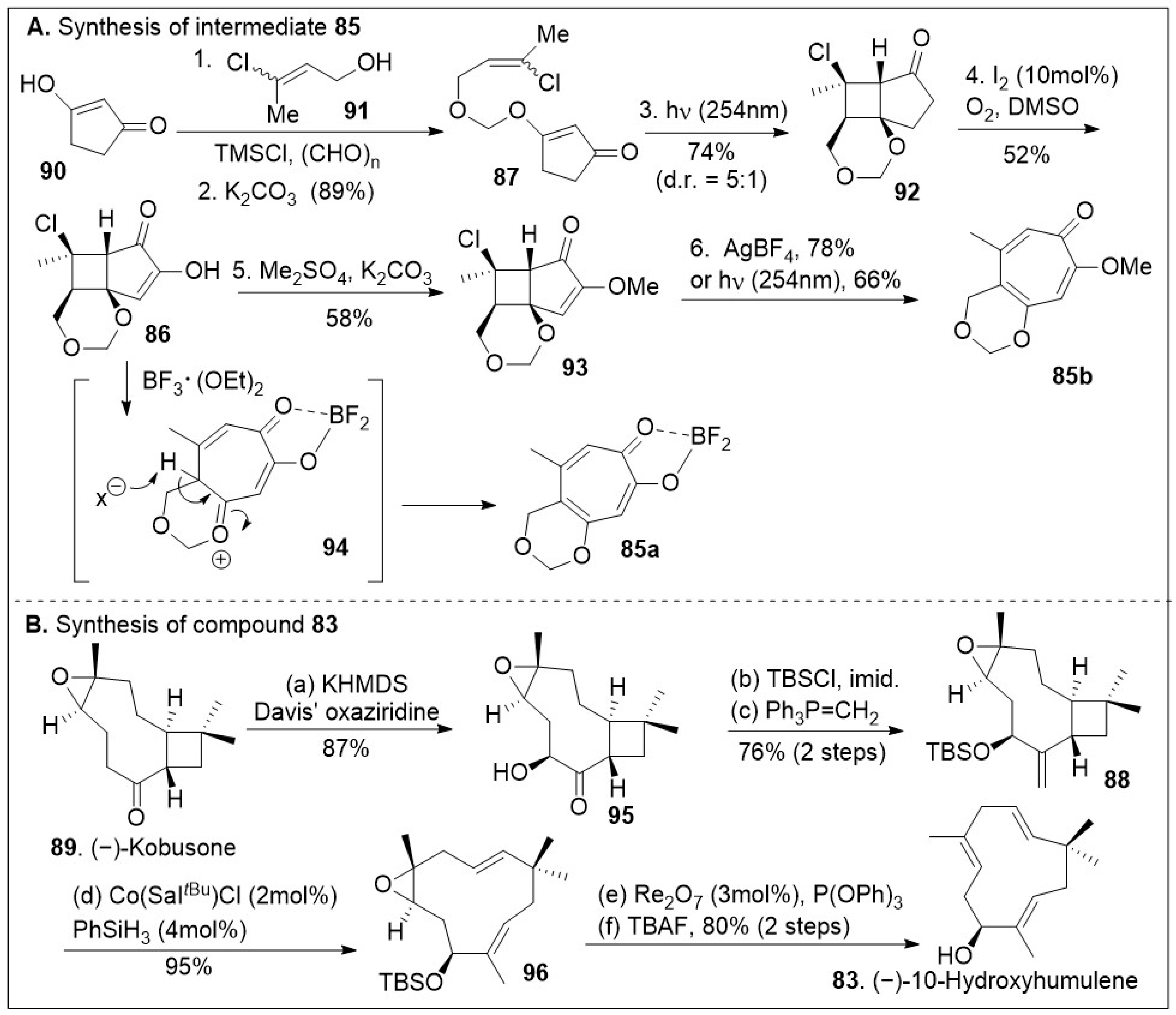
The synthesis capitalizes on the advantages to access building blocks
83,
o-quinone methide precursors
85a and
85b in sufficient quantities (
Scheme 12) [
79]. Their strategic synthesis began with 1,3-cyclopentanedione
90, followed by O-alkylation with
91, which is formed in situ with paraformaldehyde and chlorotrimethylsilane under basic conditions, providing the vinyl chloride
87 that can easily undergo a [2+2] intramolecular photocycloaddition furnishing tricycle
92. Light-driven [2+2] cycloaddition reactions are the most direct approach to build tetrasubstituted cyclobutanes that may lead to diverse structures. Upon one-pot iodination/Kornblum oxidation, the tricycle
92 was converted to hydroxy enone
86, followed by O-methylation provide intermediate
93. Use of either AgBF
4 or UV light (254 nm) facilitated the ring expansion via fragmentation of compound
93 to provide the methoxy-tropolone
85 in good yields (
Scheme 12A). Alternatively, treatment of compound
86 with Lewis acid, boron trifluoride diethyl etherate, resulted in tropolone
84. Thus, the use of simple transformations easily generates the large ring-containing molecule in sufficient quantities. (−)-Kobusone
89 was converted to the corresponding α-hydroxylated product
95 via Davis’ oxiziridine treatment as shown in
Scheme 12B. The resultant α-hydroxyl group was protected with a silyl group, followed by 1-carbon Wittig reaction yielding compound
88, setting the stage for the ring opening. This reaction previously discovered by the Shenvi group [
80] highlights the strategic use of Cobalt/Sal/silane catalyst systems to mediate a Hydrogen-Atom Transfer (HAT) retrocycloisomerization of compound
88 to afford compound
96 in excellent yields. Rhenium catalyzed deoxygenation and silyl group removal with TBAF provided the desired compound
83 as the starting material for the cycloaddition reaction.
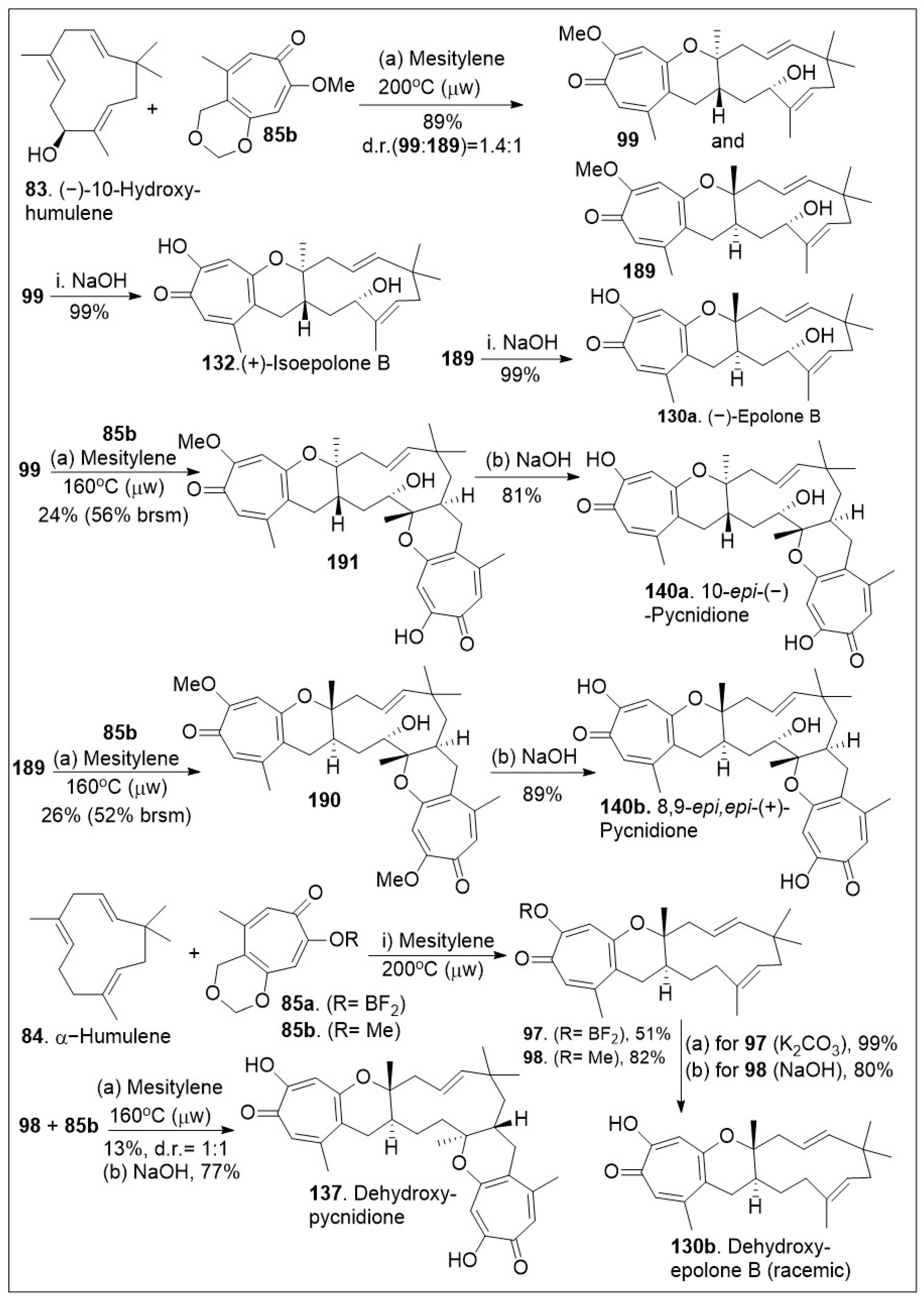
The application of this HDA reaction to various ene-systems is an efficient approach to generate natural products with high order architecture as shown in
Scheme 13. Furthermore, these types of cyclization reactions tend to be selective and can be applied to large scale settings, enabling carbon-carbon bond formations in an economical manner. With the in situ generated
o-QM
82 from tropolone
85a or
85b and dienophile
83, the platform was established for the [4+2] inverse electron demand HDA reaction [
79]. The thermal microwave assisted HDA reaction between (−)-hydroxyhumulene
83 and
o-QM (generated in situ from precursor
85) provided cycloadducts
99 and
189. Note that mesitylene was used rather than toluene to reach higher boiling points and provide the desired products in modest yields. Next deprotection of methoxy group of compounds
99 or
189 via base, led to the natural product epimers
132 or
130a respectively. Then, compound
99 was subjected to HDA reaction again with compound
85b to yield
191, which was deprotected under basic reaction conditions to afford the natural product epimer compound
140a. The same treatment could be provided to compound
189 to yield the natural product epimer
140b (8,9-
epi,
epi-(−)-pycnidione) in good yields. Finally, the HDA reaction of between humulene
84 (no chirality) and in situ generated
o-QM (from
85a or
85b) was conducted to produce compounds
97 or
98 respectively. Deprotection of these compounds led to the natural product
130b (dehydroxyepolone B) as a racemate in good yields. Compound
98 was further treated with dienophile for a second HDA reaction under the same reaction conditions to provide the adduct precursor of
137 in poor yields indicating the second HDA reaction is more difficult for
83 than for the allylic system
84. Perhaps, the presence of the hydroxyl group lowers the barrier slightly in favor of that second reaction. Deprotection of the methoxy group provided natural product
137 [
79].
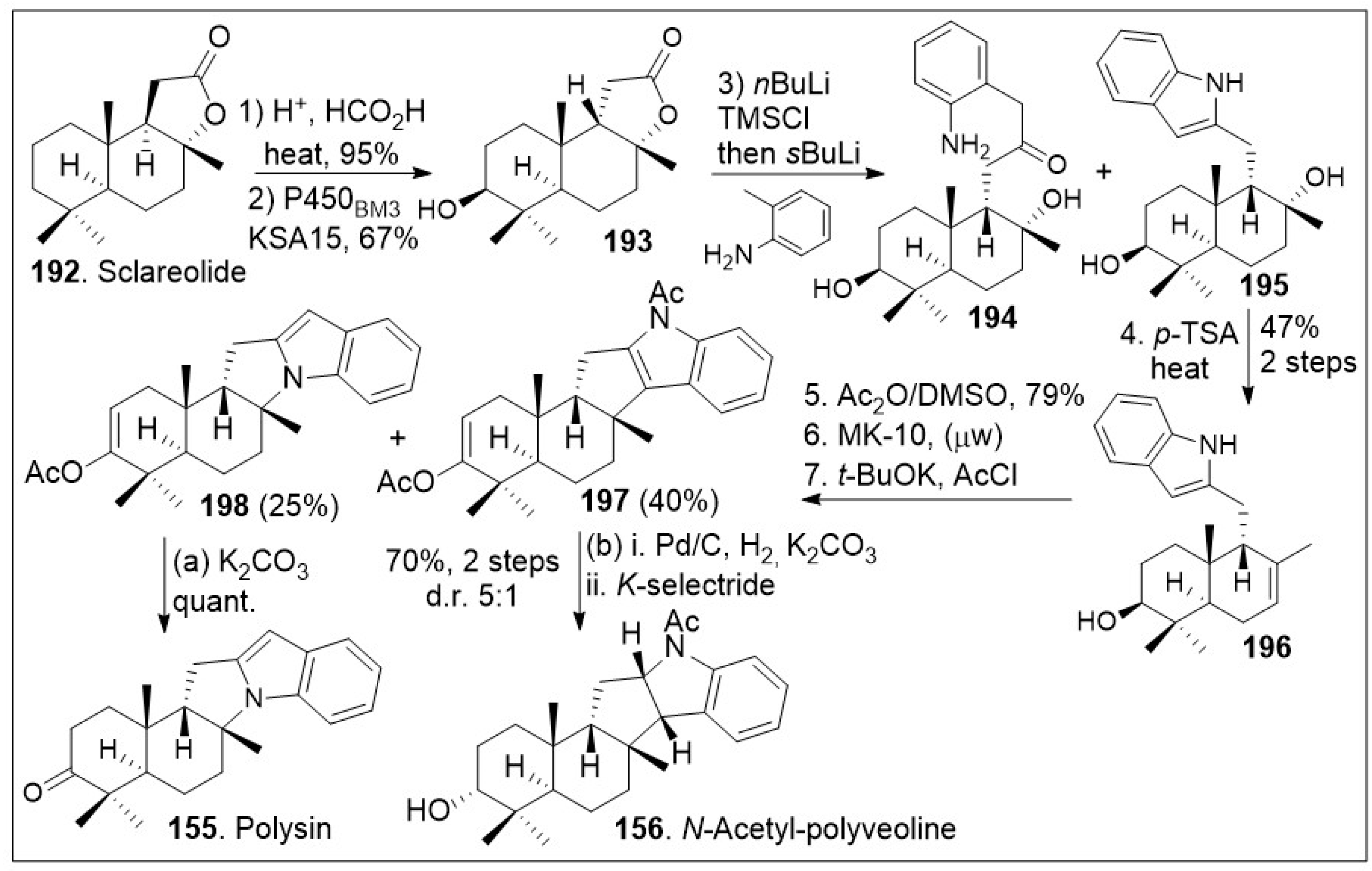
Recent synthetic approaches towards the
trans-
syn-fused drimane meroterpenoids have capitalized in advancements in chemoenzymatic reactions [
81] to facilitate carbon economic and diversity oriented synthetic strategies. The total synthesis application using a chiral-pool natural product is highlighted in
Scheme 14. The synthesis commenced with the commercially and readily available sclareolide
192, which was treated with acid to epimerize the β-hydrogen, and re-formation of the
syn-lactone (
199,
Scheme 15). Then, stereoselective enzymatic hydroxylation of the methylene group adjacent to the quaternary center using P450
BM3 variant KSA15 provided compound
193 in respectful yields in gram scale. Compound
193 was treated with
nBuLi, trapped with a silyl group, and coupled with 2-methyl aniline anion to provide compounds
194 and
195. This indole reaction known as Smith-modified Madelung indole synthesis protocol is usually high yielding and relatively compatible with other functional groups. This mixture was converted to
196 under acid treatment to facilitate the dehydration. System
196 was then exposed to Albright-Goldman oxidation to generate the corresponding ketone, which was further subjected to two-step microwave-promoted intramolecular Friedel-Crafts cyclization reaction and subsequent in situ trapping of the enolate with acetic anhydride, affording enol ether adducts
197 and
198. Next palladium catalyzed hydrogenation and subsequent K-selectride mediated diastereoselective reduction of enol ether adduct
197, provided
N-acetyl-polyveoline (
156). In parallel, compound
198 was treated with potassium carbonate to release the acetate group, resulting in the natural product polysin (
155) in good yield.
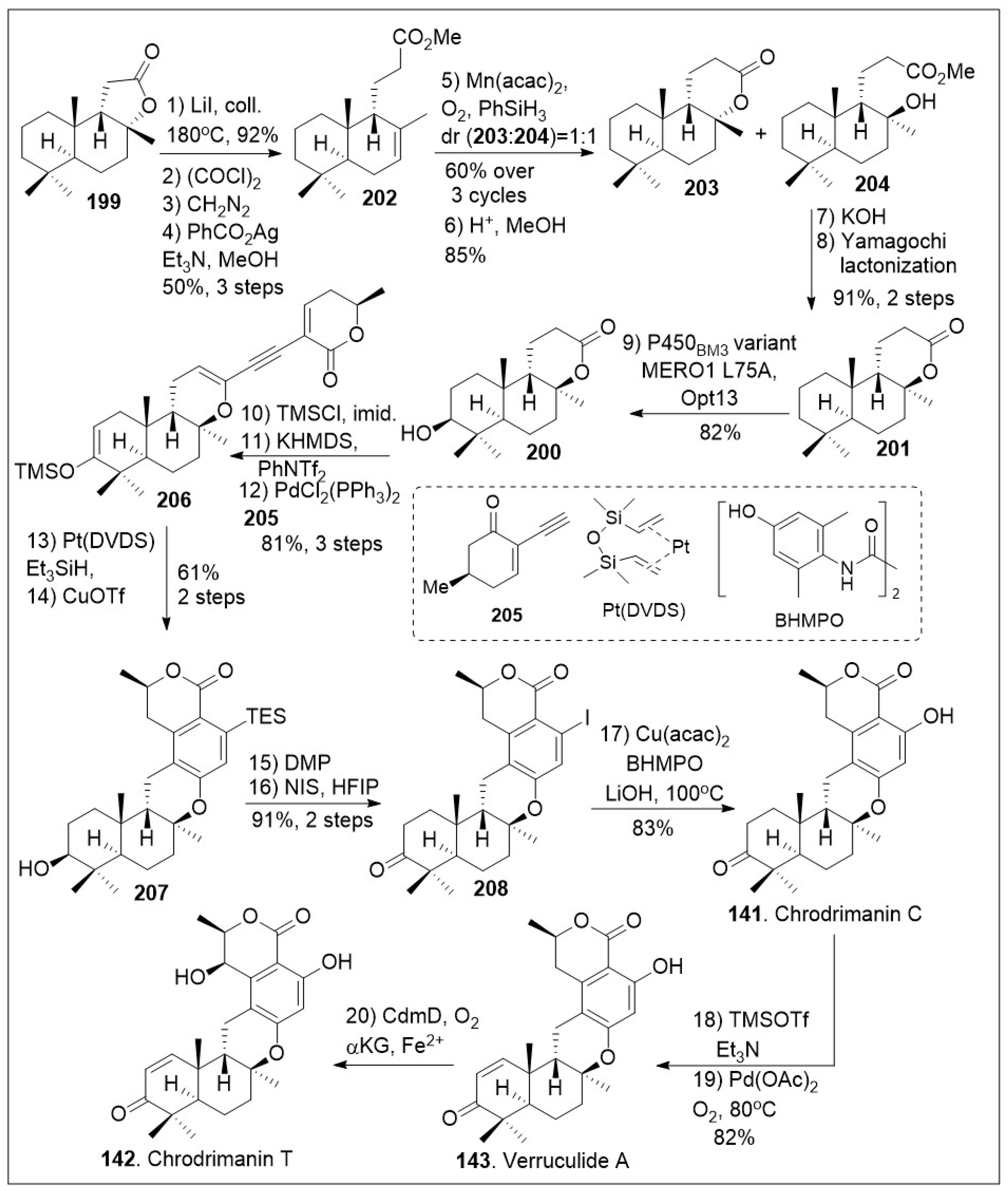
To generate other meroterpenoid family members (
Scheme 15), lactone
199 was converted to methyl ester
202 through a series of four linear steps [
81]. The lactone was first opened using heat, followed by three-step Arndt-Eistert homologation to furnish the corresponding methyl ester
202 in excellent yields. Then, compound
202 was treated with Mukaiyama hydration protocol which led to a mixture of lactone
203 and tertiary alcohol
204 in relatively good yields. In addition, lactone
203 was converted back to methyl ester
202 and recycled to increase the overall yield. Upon saponification and subsequent Yamaguchi lactonization of tertiary alcohol
204, the
trans-
syn-
trans-fused lactone
201 was generated. At this point, the application on enzymatic transformation at the methylene group adjacent to quaternary center was conducted. The enzymatic hydroxylation took place under P450
BM3 variant MERO1 L75A on a preparative scale to produce alcohol
200. Next vinyl triflate formation was conducted upon treatment of alcohol
200 with silyl group protection and trapping of the enolate to provide the corresponding triflate, which underwent Sonogashira coupling reaction with alkyne
205, to generate dienyne
206. Subsequent hydrosilylation of the newly formed dienyne
206 catalyzed by Platinum(0)-1,3-divinyl-1,1,3,3-tetramethyldisiloxane complex known as Pt(DVDS) in the presence of Et
3SiH, afforded the desired hydrosilylated triene intermediate, which could readily undergo 6π electrocyclization/aromatization reaction under copper(II) triflate to yield arene
207. The resultant compound was treated with Dess-Martin periodinane (DMP) to mediate the oxidation of the secondary hydroxyl group followed by desilylative iodinization under NIS mediation to provide iodo-arene
208. Compound
208 was subjected to copper-catalyzed hydroxylation of aryl halides under mild conditions, namely Cu(acac)
2 in the presence of
N,N′-bis(4-hydroxyl-2,6-dimethylphenyl)oxalamide (BHMPO) and base to yield chrodrimanin C (
141). The natural product
141 can be converted to verruculide A (
143) upon treatment with Saegusa 2-step protocol to install the α,β-unsaturated system. Furthermore, this natural product can be converted to chrodrimanin T (
142) under Fe(II)/α-ketoglutarate-dependent oxygenase treatment to introduce the hydroxyl group stereoselectively at the γ-position of the lactone ring. Overall, the Renata group provided an impressive use of enzymatic and modern organic chemistry reactions [
81].
Another effective synthetic approach using natural products as a chiral pool is the strategy towards the arisugacin natural products. These drimane meroterpenoids have been isolated from
Penicillium sp. and other sources. Their members have demonstrated acetylcholinesterase inhibitory activity in the nanomolar range [
82]. Access to both libraries and large quantities of these compounds is desirable to interrogate their mode of action. Stypodiol is a pentacyclic meroterpenoid isolated from the brown algae
S. flabelliforme, which displays a broad range of biological activity. Stypodiol has shown anti-proliferative effects against a number of cancer cell lines and also has antimicrobial capacity. This compound showed promising anti-proliferative activity in the low micromolar range against a human neuroblastoma cell line (SH-SY5Y) [
83]. While multiple cancer cell lines were tested most of them showed weak anti-proliferative effects, indicating the compound is likely to have selectivity towards a specific cancer subtype. Therefore, efficient synthetic approaches to this family of compounds are desirable. A recent rapid and modular approach using modern organic chemistry, such as radical-based reactions combined with chemoenzymatic reactions has been reported [
81]. As shown in
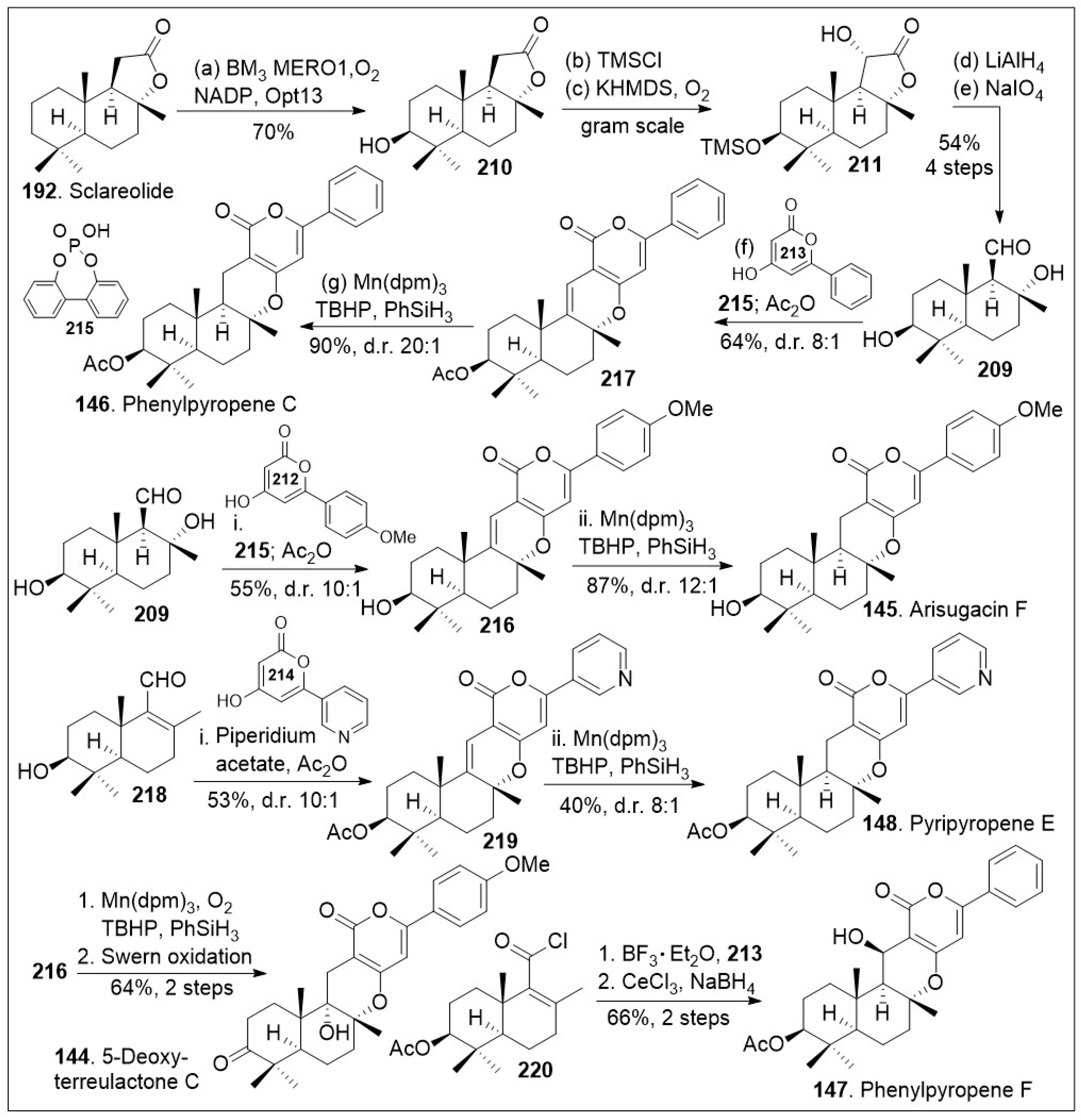
Scheme 16, the synthesis commenced with natural product sclareolide
192, which underwent enzymatic hydroxylation using P450
BM3 variant BM3 MERO1 in the presence of thermostabilized phosphate dehydrogenase (Opt13) at the methylene group adjacent to the quaternary center. The corresponding compound
210 was protected with a silyl group followed by stereoselective α-hydroxylation, conducted via trapping of the enolate with oxygen to efficiently generate compound
211. Next, lactone
211 was reduced to the triol system followed by oxidative cleavage of the diol moiety, rapidly furnishing the key aldehyde
209. Aldehyde
209 was coupled with pyrone
213 via a formal [3+3] cycloaddition/hydrogen atom transfer (HAT) reaction mediated by phosphoric acid
215 to generate cyclized product
217, which was acetylated in a one-pot procedure with acetic anhydride. Phosphoric acid catalyst promoted in situ alcohol dehydration and formal [3+3] cycloaddition to provide the shown stereocenter as the major product. Then, it was treated with Mn(dpm)
3 in the presence of TBHP (
tert-butyl hydrogen peroxide) and PhSiH
3 to facilitate a chemoselective HAT reduction reaction to provide the natural product phenylpyropene C (
146). This Mukaiyama reduction protocol has high stereospecificity as it favored the hydride addition from the bottom face, yielding the product in a 20 to 1 ratio with respect to its diastereoisomer.
Alternatively, aldehyde 209 was coupled with pyrone 212 via a formal [3+3] cycloaddition reaction mediated by phosphoric acid 215 to generate cyclized product 216, which was converted to arisugacin F (145) via chemoselective HAT reduction reaction mediated by Mn(dpm)3 in the presence of TBHP and PhSiH3. Compound 209 was dehydrated to provided enal 218, which was coupled with the shown pyrone via formal [3+3] cycloaddition mediated by piperidium acetate to generate compound 219. The corresponding compound can be then converted to the natural product pyripyropene E (148) via chemoselective HAT reduction reaction using their established reaction conditions for these systems in modest yields and good diastereomeric ratio. In addition, compound 216 was treated with Mukaiyama hydration chemoselective HAT reaction mediated by Mn(dpm)3 in the presence of oxygen gas and PhSiH3, followed by Swern oxidation to provide 5-deoxyterreulactone C (144) in good yields. Finally, the acyl chloride 220 that arises from aldehyde 209 was subjected to Fredel-Crafts acylation reaction with pyrone 213 mediated by BF3 etherate followed by cyclization reaction. Then, reduction of the ketone system under Luche reduction conditions provided the natural product phenylpyropene F (147). The total syntheses of various members of this drimane meroterpenoid family was achieved through the creative use of strategic enzymatic and chemoselective reactions to provide flexible, modular, and a generalized synthetic route that may facilitate biological studies of these natural products and their derivatives.
Another modular synthetic effort is highlighted in
Scheme 17 to access these natural products, but mainly to stypodiol [
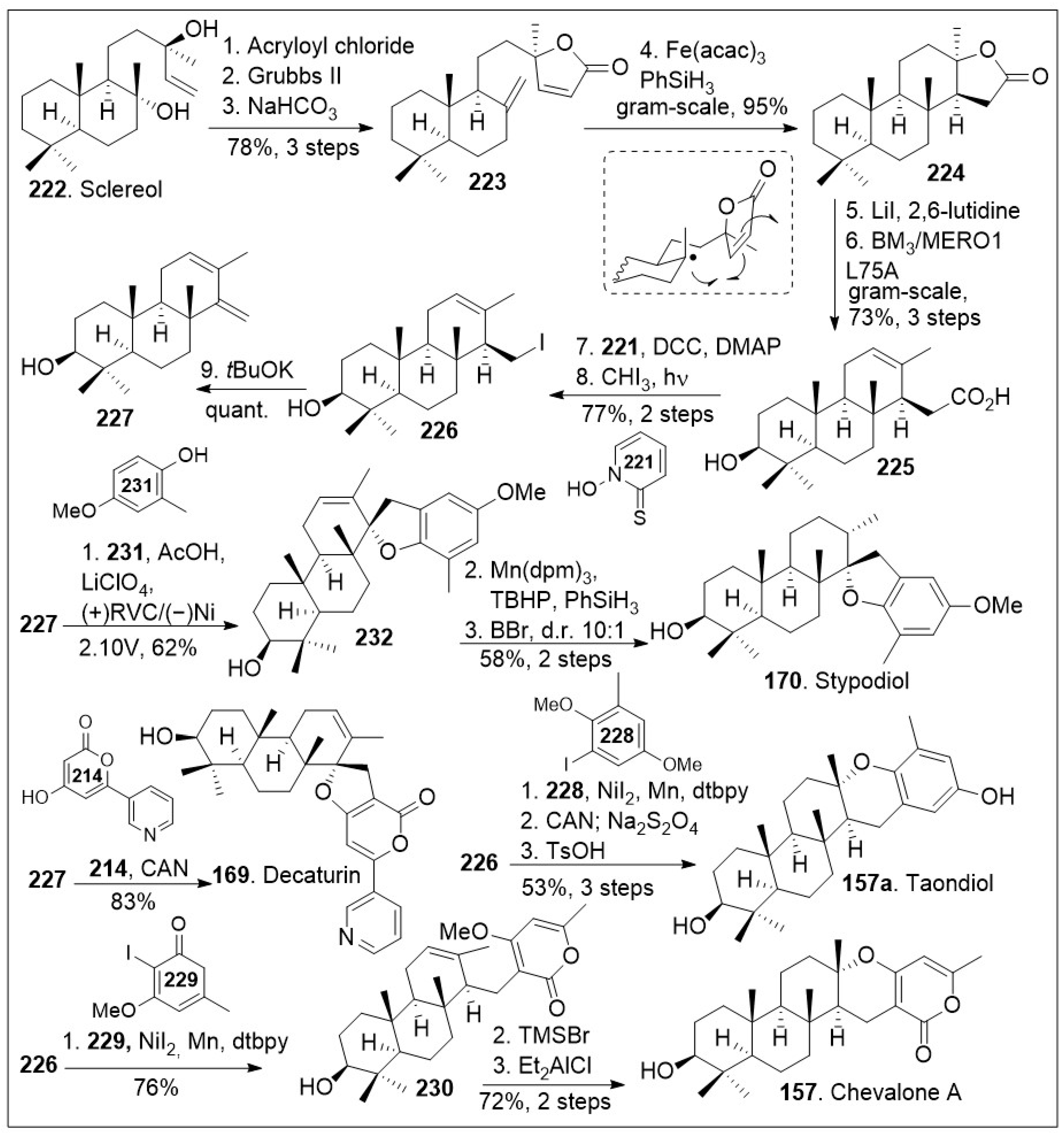
84]. The synthesis commenced with the available natural product sclareol
222, which was converted to intermediate
223 via a 3-step sequential protocol: acylation with acryloyl chloride, γ-lactone ring formation via intramolecular olefin metathesis mediated by Grubbs second generation catalyst, and finally dehydration reaction mediated by base treatment.
Next, gram-scale HAT-based intramolecular Giese type coupling reaction was conducted on compound
223 with Fe(acac)
3 in the presence of PhSiH
3, which goes through the radical transition state shown to preferentially provide the tetracyclic compound
224. Then, a two-step sequence mediated by lithium iodide resulted in the opening of the lactone ring and generation of the corresponding carboxylic acid. This was subjected to enzymatic hydroxylation using P450
BM3 variant BM3 L75A, leading to regioselective hydroxylated compound
225. The resultant compound could be treated with Barton reaction conditions to undergo decarboxylation, followed by photocatalyzed halogenation reaction to generate the corresponding iodide
226, which was then converted to diene
227 under basic conditions. Then, electrochemical SET-based [3+2] coupling reaction of diene
227 with phenol
231 via constant potential electrolysis (using reticulated vitreous carbon as cathode and Ni as the anode) generated advanced intermediate
232. Compound
232 was treated with Mn(dpm)
3 in the presence of TBHP and PhSiH
3 to induce diastereoselective HAT reduction followed by deprotection to generate the natural product stypodiol (
170) in good yield. Using a similar synthetic protocol, diene
227 was coupled with excess pyrone
214 via single-electron-transfer (SET) to promote a formal [3+2] cycloaddition mediated by cerium ammonium nitrate (CAN) to yield the natural product decaturin (
169). The iodide intermediate
226 was subjected to nickel-catalyzed cross-coupling with arene iodide
228, followed by the redox maneuver mediated by CAN and subsequent acid catalyzed intramolecular cyclization provided natural product taondiol (
157a). Furthermore, iodide intermediate
226 was subjected to nickel-catalyzed cross-coupling with pyrone iodide
229 to afford compound
230, which upon Lewis acid catalyzed intramolecular cyclization afforded the desired natural product chevalone A (
157) in good yields [
84]. This synthetic strategy offers the opportunity to access many derivatives of these meroterpene natural product derivatives by fine-tuning the coupling partner.
The hongoquercins are tetracyclic meroterpenoid natural products with the
trans-transoid decalin-dihydrobenzopyran ring system, which display a range of promising bioactivities. This subgroup of the meroterpenoids natural products incorporates a sesquiterpene unit to form the unique hongoquercins. These natural products have attracted attention because they pose a synthetic challenge with four continuous stereocenters and a highly substituted arene scaffold. Access to a modular synthesis can enable the establishment of a medicinal chemistry platform to investigate their exact mode of action. Over the past 25 years multiple synthetic endeavors have been reported towards meroterpenoid (+)-hongoquercin A (
152,
Scheme 18) and (+)-hongoquercin B (
153,
Scheme 19), but a recent simplified approach was disclosed by the Barrett group [
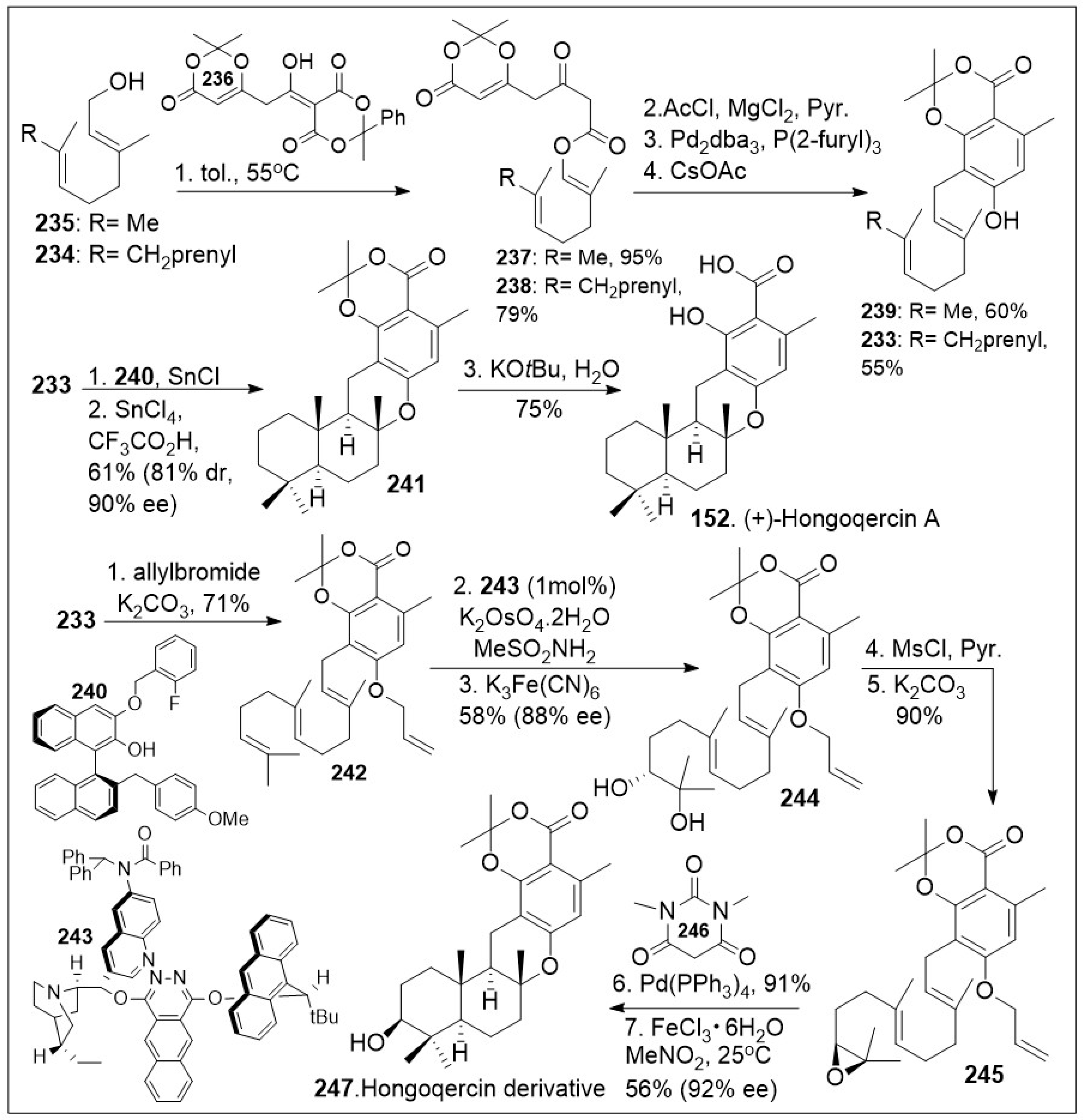
85], featuring a sequential polyketide aromatization and cationic polyene cyclization reaction. The synthesis commenced with thermolysis of dioxane-4,6-dione dioxanone
236 in the presence of
trans,
trans-farnesyl
234 or geraniol
235 to provide dioxinone β-keto ester
238 or
237 respectively, which upon C-acylation generated the corresponding ester systems. The resultant products underwent highly regioselective decarboxylative allylic rearrangement mediated by Pd
2dba
3, followed by in situ aromatization to provide farnesyl resorcylate
233 or the geranyl substituted analogue
239 respectively. Compound
233 was then treated with SnCl
4 complex with ligand
240 as a dual Bronsted and Lewis acid, in the presence of SnCl
4 and trifluoroacetic acid, which facilitated the enantioselective protonation of the substrate, followed by facile cationic intramolecular polyene cyclization reaction, leading to meroterpenoid
241. Compound
241 upon saponification provided the natural product (+)-hongoquercin A (
152). Alternatively, O-allylation of farnesyl resorcylate
233 with allylbromide under basic condition provided allyl ether
242, which upon asymmetric dihydroxylation generated diol
244. This step is followed by mesylating the secondary hydroxyl group, which enables the epoxide formation under a classic S
N2 reaction to provide epoxide
245. Compound
245 was then deprotected, followed by biomimetic cationic cyclization of epoxide mediated by Lewis acid, Iron (III) to generate the advanced meroterpenoid derivative
247 in good enantioselective excess.
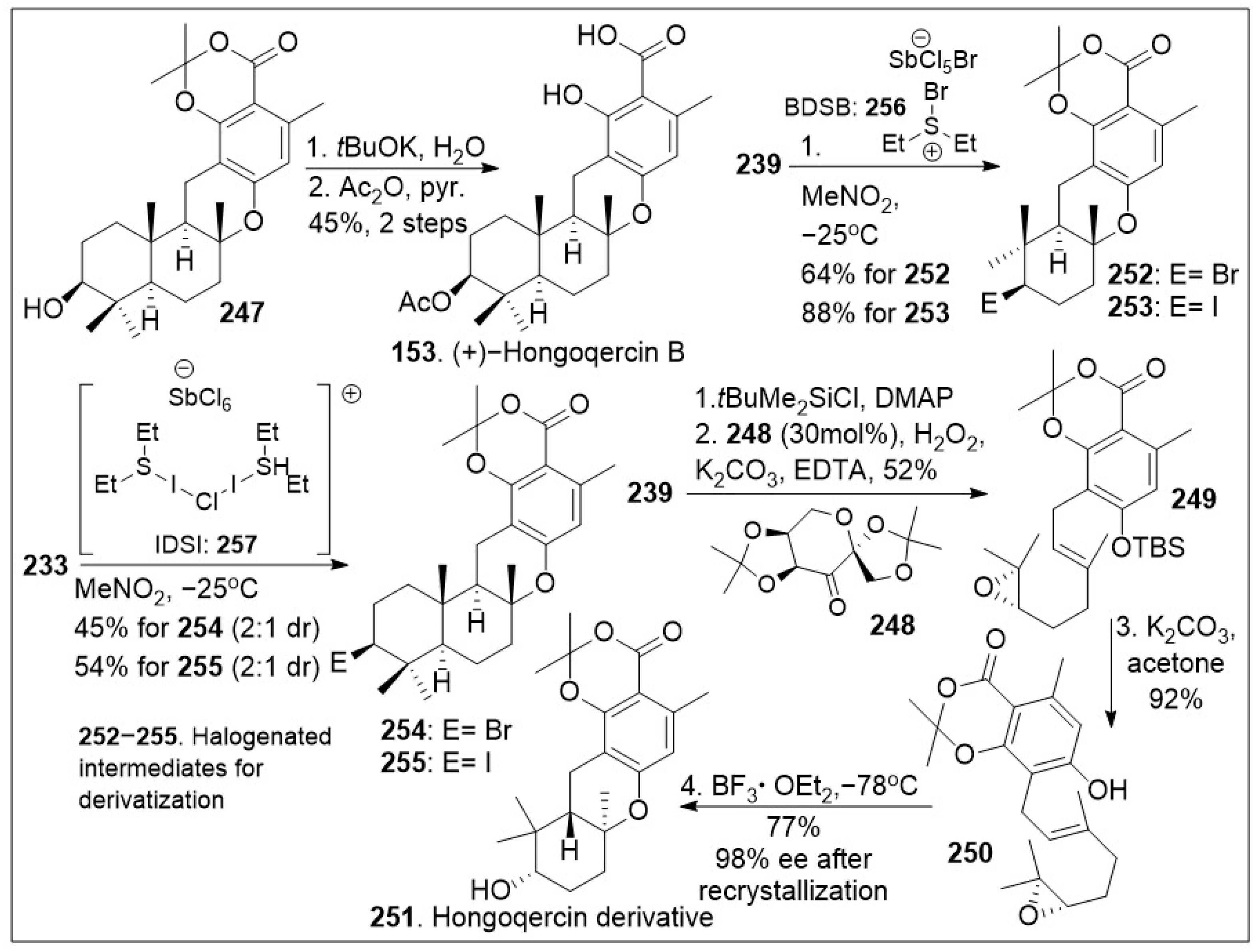
As shown in
Scheme 19, compound
247 was deprotected to yield the corresponding acid, followed by selective acetylation of the secondary hydroxyl group to provide natural product
153. Furthermore, halocyclization of farnesyl resorcylate
233 or geranyl-substituted analogue
239 (
Scheme 18) in the presence of halogenating reagents
256 (Bromodiethylsulfonium bromopentachloroantimonate V) or
257 (iodonium reagent), provided halogenated meroterpenoid
254/
255, or
252/
253 respectively in good yields [
85]. These important intermediates will allow the generation of several derivatives that can further provide insight into the biological mode of action of these natural products. An interesting meroterpenoid derivative of hongoquercin was generated geranyl-substituted resorcylate
239. This was protected with a silyl group followed by regioselective asymmetric epoxidation with Shi chiral ketone to generate epoxide
249. The resultant system was deprotected under basic conditions to yield epoxide
250, which underwent cationic polyene cyclization reaction mediated by boron trifluoride etherate to provide the advanced meroterpenoid derivative
251. This compound should serve as a chemical tool to interrogate the biological properties of these natural products.
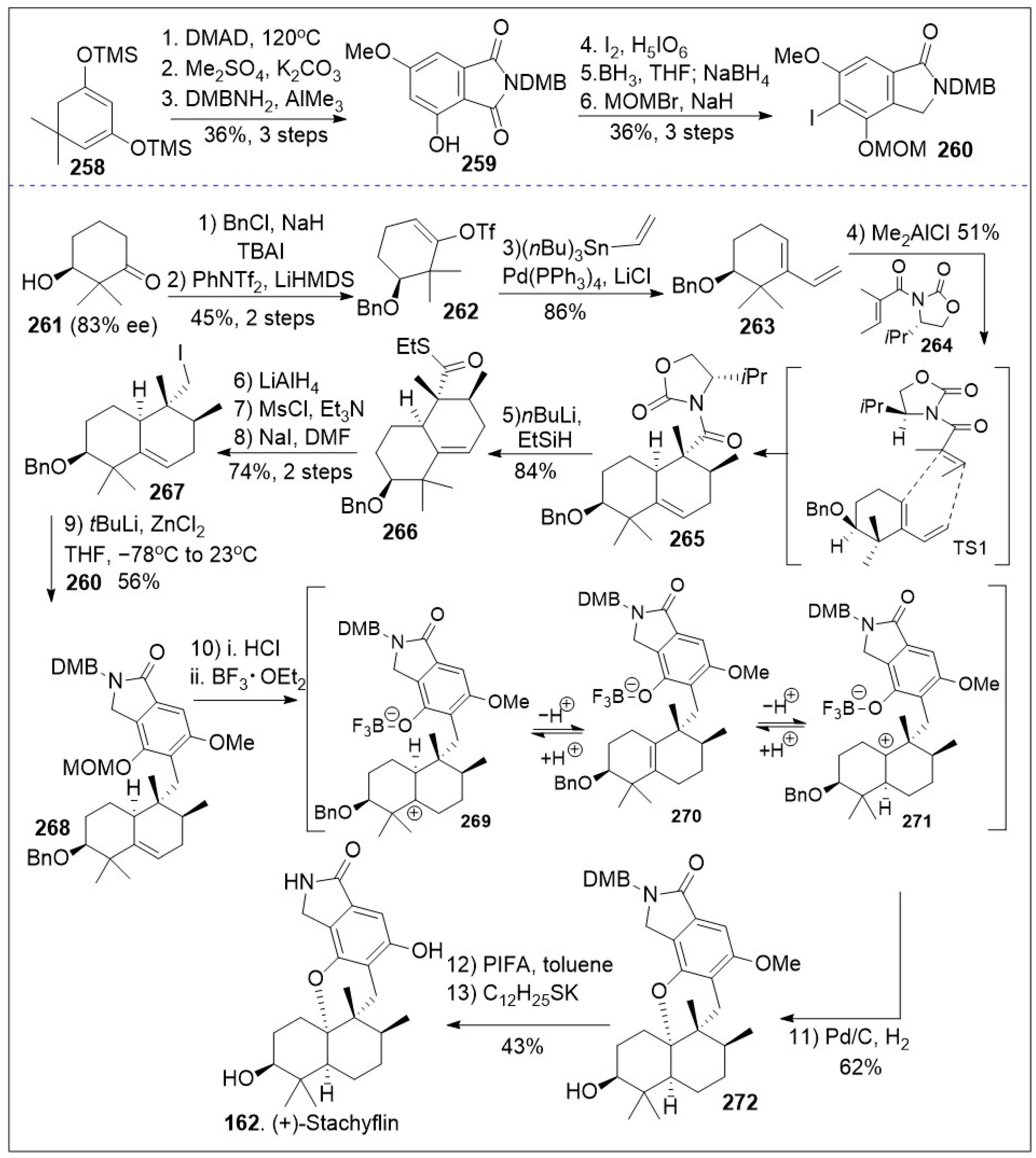
A modular synthetic approach to the tetracyclic meroterpenoids was recently reported [
86] and it is as illustrated in
Scheme 20. The synthesis featuring sterically demanding sp
2-sp
3 cross-coupling and acid mediated cyclization/isomerization for installation of the required
cis or
trans-decalin stereochemistry of the tetracyclic meroterpenoid scaffold is highlighted. The total synthesis commenced with a solvent-free Alder-Rickert reaction between the dimedone-derived bis-trimethylsilyl enol ether
258 and dimethyl 2-butynedioate (DMAD) provided resorcinol, followed by mono-methylation yielded phenol, which upon thermal treatment in the presence of 3,4-dimethoxybenzylamine (DMBNH
2) yielded imide
259. Regioselective halogenation of imide
259 generated intermediate
259a, which upon regioselective imide reduction provided key isoindolinone
260. Next diene
263 was generated from β-hydroxyketone
261 via three step protocol, involving the formation of benzyl-protected ketone, subsequent vinyltriflate formation, followed by Stille coupling with vinyl(
nBu
3)Sn mediated by Pd(PPh
3)
4. Then, a Lewis acid catalyzed Diels-Alder [4+2] cycloaddition reaction between diene
263 and dienophile
264 provided intermediate
265 as the major product due to the shown transition state. Compound
265, following a two-step sequential reduction reaction, generated alcohol via thioester intermediate
266, and it was converted to iodide
267 via two linear steps (mesylation of the alcohol followed by S
N2 reaction). Then, the key step was conducted, namely sp
2-sp
3 Negishi coupling of isoindolinone
260 with iodide
267 to provided 5,6-dehydrodecalin intermediate
268. Next compound
268 underwent deprotection, intramolecular cation trapping of the phenol group, followed by hydrogenation to yield the cyclized product
272. This established the required meroterpenoid scaffold. Lastly, global deprotection of intermediate
272 provided the natural product (+)-stachyflin (
162).
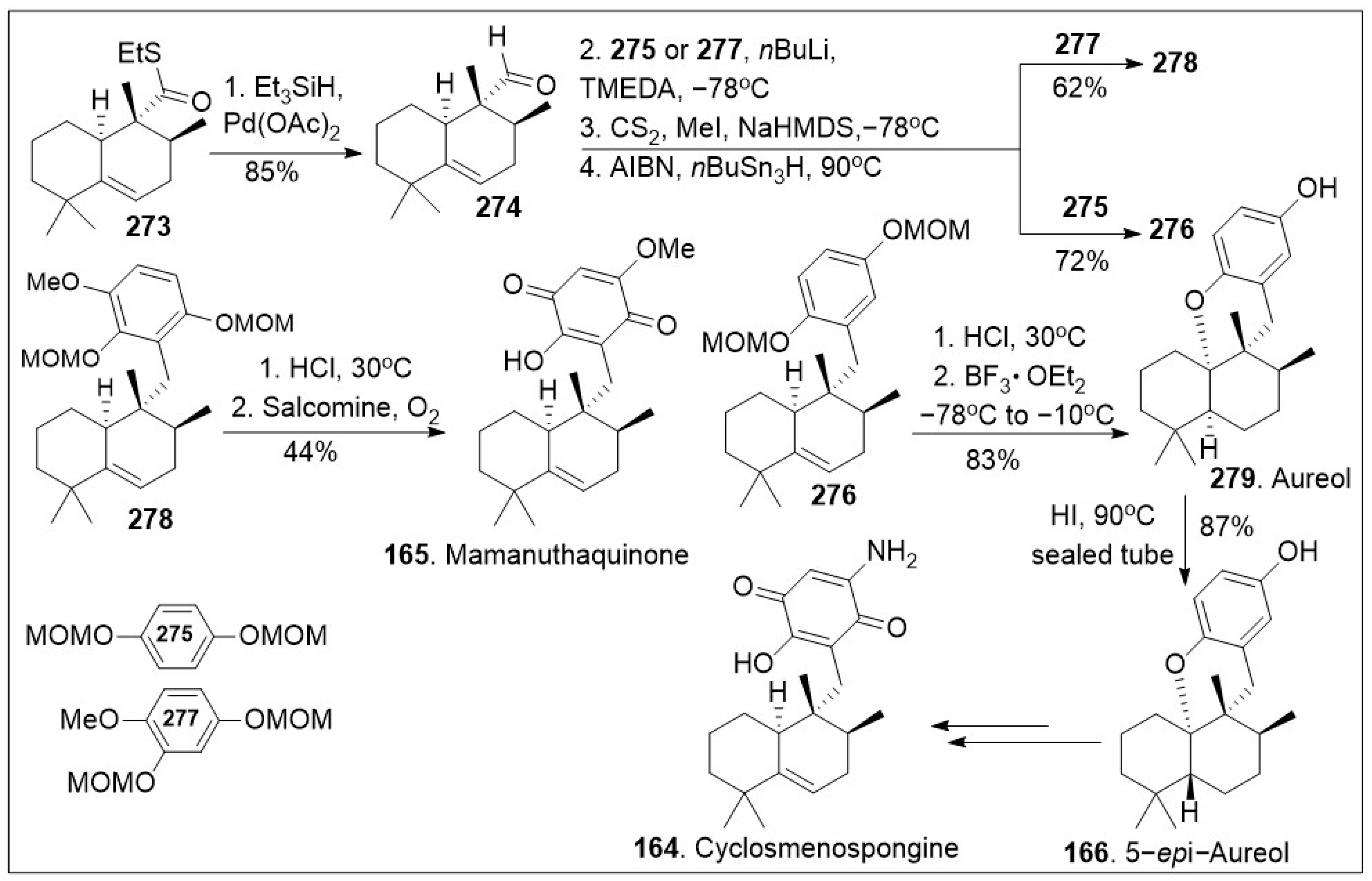
Next as shown in
Scheme 21, thioester
273 was reduced to the corresponding aldehyde
274 via Pd(II) in the presence of triethylsilane hydride in good yields. Then, aldehyde was coupled with the anion of arene
275 or
277, followed by 2-step Barton-McCombie deoxygenation to produce intermediate
276 or
278 respectively. Then, compound
278 was converted to mamanuthaquinone (
165) via global acid deprotection and salcomine (a coordination complex of salen ligand and cobalt) mediated oxidation reaction sequence in moderate yields. In addition, compound
276 was subjected to deprotection and intramolecular cation trapping with the free phenol group to provide natural product aureol (
279), which was subsequently converted to 5-
epi-aureol (
166) via epimerization of the decalin bridge carbon, using HI. Intermediate
166 is known to generate natural product cyclosmenospongine
164 [
86]. The efficient synthesis provides rapid access to various family members and the established platform will enable the generation of derivatives for further biological studies.
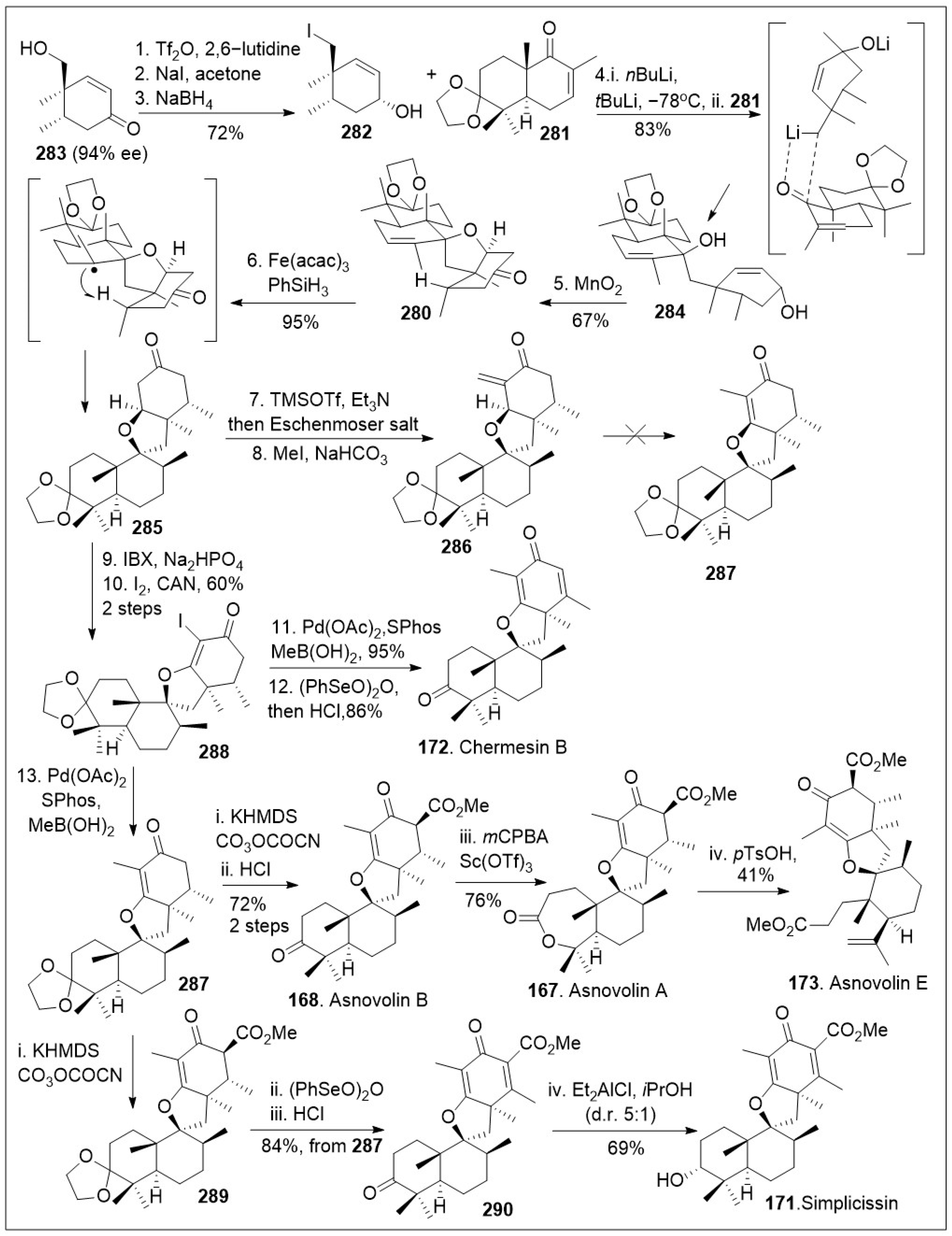
These unique spiromeroterpenoids share a common biosynthetic pathway that presumably arises from the terpenoid precursor farnesyldiphosphate and display promising biological properties [
87]. The total synthesis of asnovolins and related spiromeroterpenoids feature a key lithium mediated coupling reaction to establish the carbon-carbon bond formation that giving rise to the spirocenter, was recently reported by Porco [
87], shown in
Scheme 22. The synthesis began with known chiral hydroxy enone
283, which was converted to the corresponding neopentyl iodide via one-pot triflation, followed by an S
N2 reaction, which upon subsequent Luche reduction, provided allylic alcohol
282. Coupling reaction between the decalin system
281 and the lithium exchanged form of the iodide
282 via 1,2-addition reaction afforded compound
284. Next, treatment of the resultant product
284 with MnO
2 was converted to spirocycle
280 via tandem allylic oxidation, followed by oxa-Michael addition reaction sequence. Spirocycle
280 was then transformed to spirocyclic intermediate
285 via a metal hydride atom transfer (MHAT) process mediated by Fe(acac)
3 in the presence of hydride source PhSiH
3, establishing the desired stereochemistry. Enolization of spirocyclic intermediate
285 with TMSOTf followed by treatment with Eschenmoser’s salt, generated exocyclic enone
286. Under various reactions conditions compound
286 resisted isomerization to the endocyclic enone
287. Therefore, compound
285 was oxidized with 2-iodoxybenzoic acid (IBX) to the spirocyclic enone system, which was iodinated at the α-carbon to provide the halogenated spirocyclic intermediate
288. This system was then subjected to Suzuki coupling reaction to generate the desired endocyclic enone
287, followed by final desaturation, and protecting group removal to generate the spiromeroterpenoid chermesin B (
172). Compound
288 could be further functionalized via Pd mediated methylation to produce
287 again, which was treated with base to conduct an enolate acylation and deprotection to access the natural product asnovolin B (
168). The natural product
168 was then subjected to Baeyer-Villiger oxidation in the presence of Sc(OTf)
3 to yield asnovolin A (
167). The lactone ring opening of asnovolin A (
167) was conducted using methanolysis conditions to provide natural product asnovolin E (
173). In addition, compound
287 was acylated to generate compound
289, which was desaturated and deprotected to afford advanced spirocyclic intermediate
290. Finally, compound
290 was subjected to Meerwein-Ponndorf-Verley reduction conditions to produce simplicissin (
171) as the major product. The powerful linear synthesis enables access to many members of these natural products
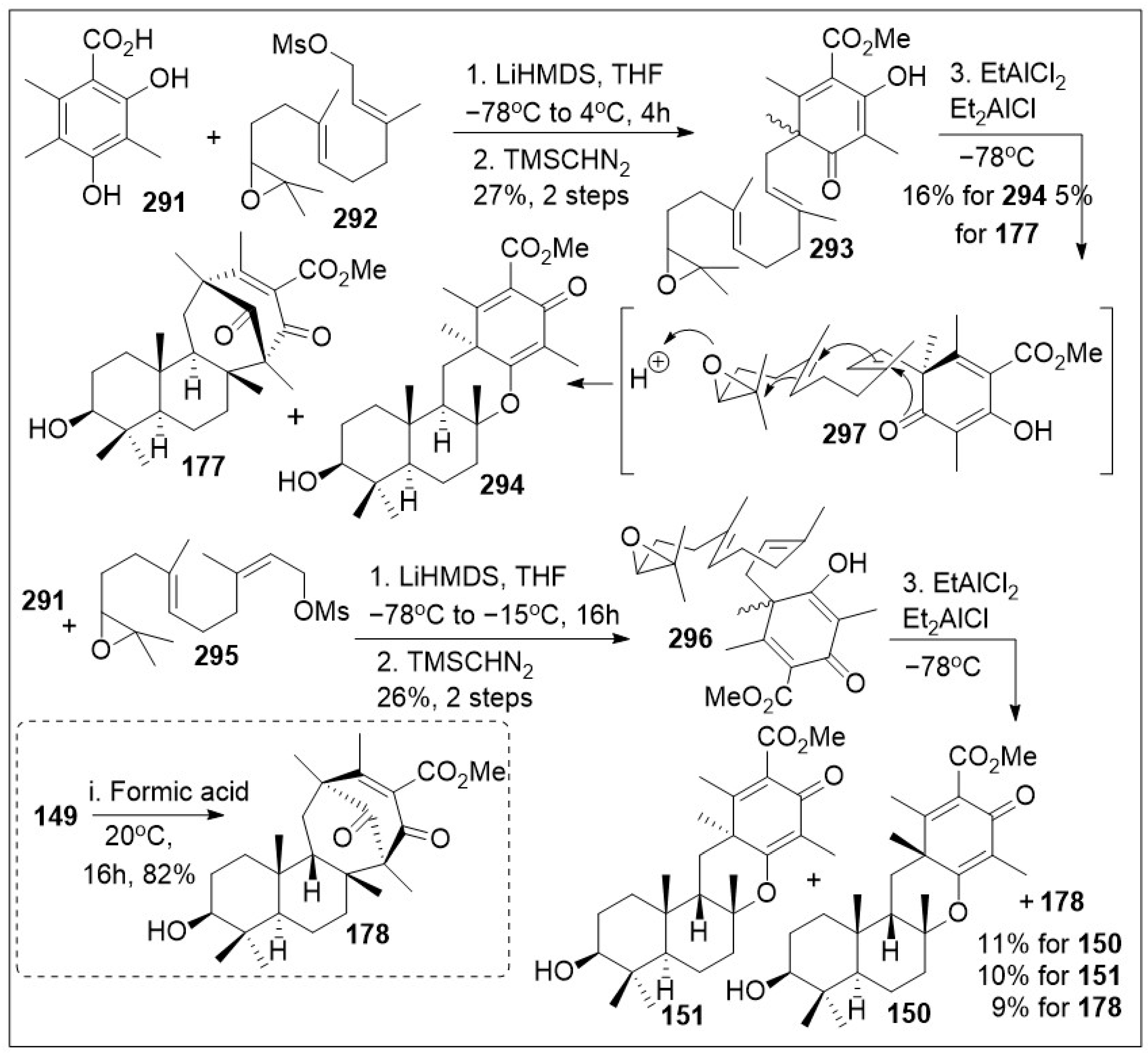
Asperterpene A and its related family are interesting structures with unique chemistry and biology. Asperterpene meroterpenoids displays an unprecedented 1,2,5-trimethyl-4,9-dioxobicyclo [3.3.1]non-2-ene-3-carboxylic acid moiety that is distinct from other similar meroterpenoids such as berkeleytrione and preaustinoid A. These natural products have exhibited promising BACE1 inhibitory activities in cell-based assays, with IC
50 values of 60–80 nM. This is more active than LY2811376 (IC
50 of 260 nM), a potent clinical BACE1 inhibitor produced by Eli Lilly. Additional in vivo studies of one of these natural products showed significant decrease of BACE1 activity and Ab42 levels in 3xTg AD mice, similar to LY2811376 [
74]. Therefore, the synthesis of these compounds has clinical relevance, and more potent derivatives could be generated once a streamlined synthetic endeavor has been established. An impressive biomimetic synthesis towards these natural products was recently reported as shown in
Scheme 23 [
88]. The synthesis began with the coupling reaction of 3,5-dimethylorsellinic acid
291 with (2
E,6
E)-10,11-epoxyfarnesyl mesylate
292 as the electrophile under basic condition, followed by in situ methyl esterification to generate (2
E,6
E)-10,11-epoxyfarnesyl-5-dimethylorsellinic acid methyl ester
293. Then, a dearomatization-driven polycyclization reaction was mediated by treatment with alkyl aluminum-pair as a Lewis acid system to promote the intramolecular polyene cyclization cascade reaction to afford asperterpene scaffold
177 and related meroterpenoid
149 via intermediate
297 in a 1 to 3 ratio favoring compound
294. Alternatively, compound
291 was coupled with electrophile (2
Z,6
E)-10,11-epoxyfarnesyl mesylate
295 (the stereoisomer of
292) using base treatment, followed by in situ methyl esterification to generate compound
296. The resultant product was treated with alkyl aluminum Lewis acid system to mediate the intramolecular polyene cyclization cascade reaction, which afforded meroterpenoid scaffolds
150,
151, and
178. In addition, it was found that compound
149 could be treated with formic acid to provide natural product
178 in good yields. While the chemical yields are modest, this synthetic approach is remarkable as several centers are established in a single step, generating high complexity rapidly.
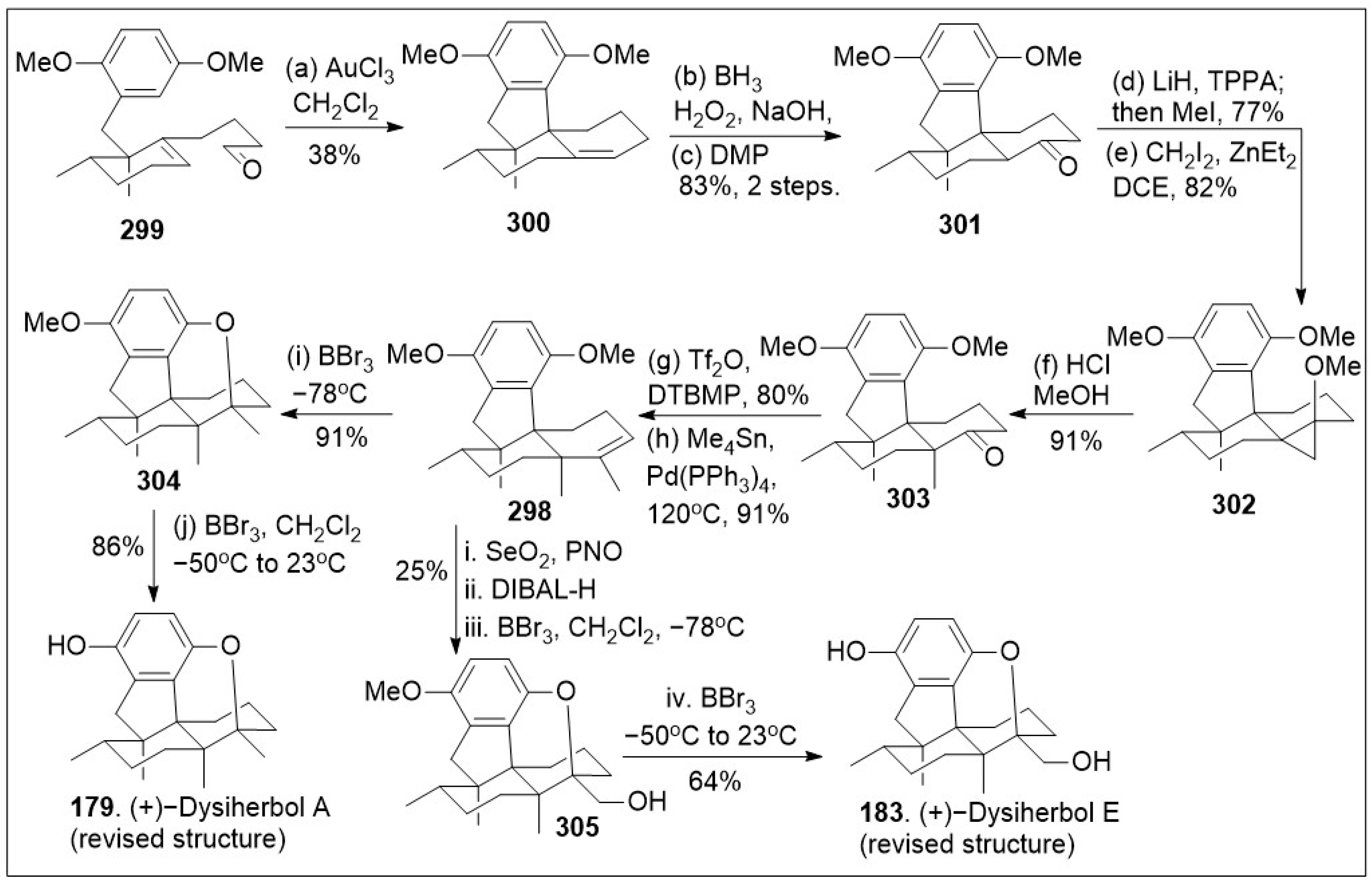
Dysiherbols A-C are a set of sesquiterpene hydroquinone meroterpenoids first isolated and characterized from
Dysidea sp., a species of marine sponge found in the South China Sea first isolated by Lin and co-workers [
89]. These natural products are structurally unified by a sterically compact 6/6/5/6/6 pentacyclic skeleton embracing an impressive oxabicyclo[3.3.1] ring system. There are 5−6 contiguous stereogenic centers in their molecular scaffold, four of which are quaternary centers. These natural products as extract mixtures were identified to inhibit NF-κB [
89]. Isolation and purification of these compounds demonstrated that the pure compounds had potent inhibitory values in the low micromolar range against NF-κB, finding IC
50 values of 0.49 to 6 μM for dysiherbols A, B, and C respectively. Additionally, these compounds were tested against a human myeloma cancer cell line, NCI H-929, and showed antiproliferation effects in the low micromolar range, with dysiherbols A being the most active with an EC
50 in the 500 nanomolar range. Recent divergent total syntheses of the revised structures of these sesquiterpene hydroquinone meroterpenoids (+)-dysiherbols A-E (
179–
183,
Figure 5) was reported as shown in
Scheme 24 and
Scheme 25 [
90]. The modular synthetic strategy began with compound
299 which had been reported to arise from the readily available dimethyl predysiherbol. Compound
299 was treated with gold to catalyze the tandem intramolecular cyclization reactions providing known Schmalz’s intermediate
300. Hydroboration/oxidative hydroxylation and subsequent Dess Martin Periodinane (DMP) oxidation reaction of compound
300 was converted to ketone
301. Then, compound
301 was followed by regioselective deprotonation/o-methylation under lithium hydride, α,α,α’-tris(4-hydroxyphenyl)-1-ethyl-4-isopropylbenzene (TPPA) followed by MeI, and subsequent Simmons-Smith cyclopropanation reaction sequence generated cyclopropane
302, which upon further proteolytic cleavage generated ketone
303. Then, enol triflate was formed to enable a Stille coupling reaction to afford compound
298. This compound was subjected to Lewis acid treatment at low temperatures to provide intermediate
304 through an intramolecular oxy-cyclization reaction, which upon increasing of the temperature under the same Lewis acid removed the additional methoxy group to yield the natural product (+)-dysiherbol A (
179). Alternatively, compound
298 was treated with a 2-step allylic oxidation and reduction reaction sequence, followed by Lewis acid mono deprotection and subsequent intramolecular oxy-cyclization to provide compound
305. Finally, compound
305 was deprotected to provide natural product (+)-dysherbol E (
183).
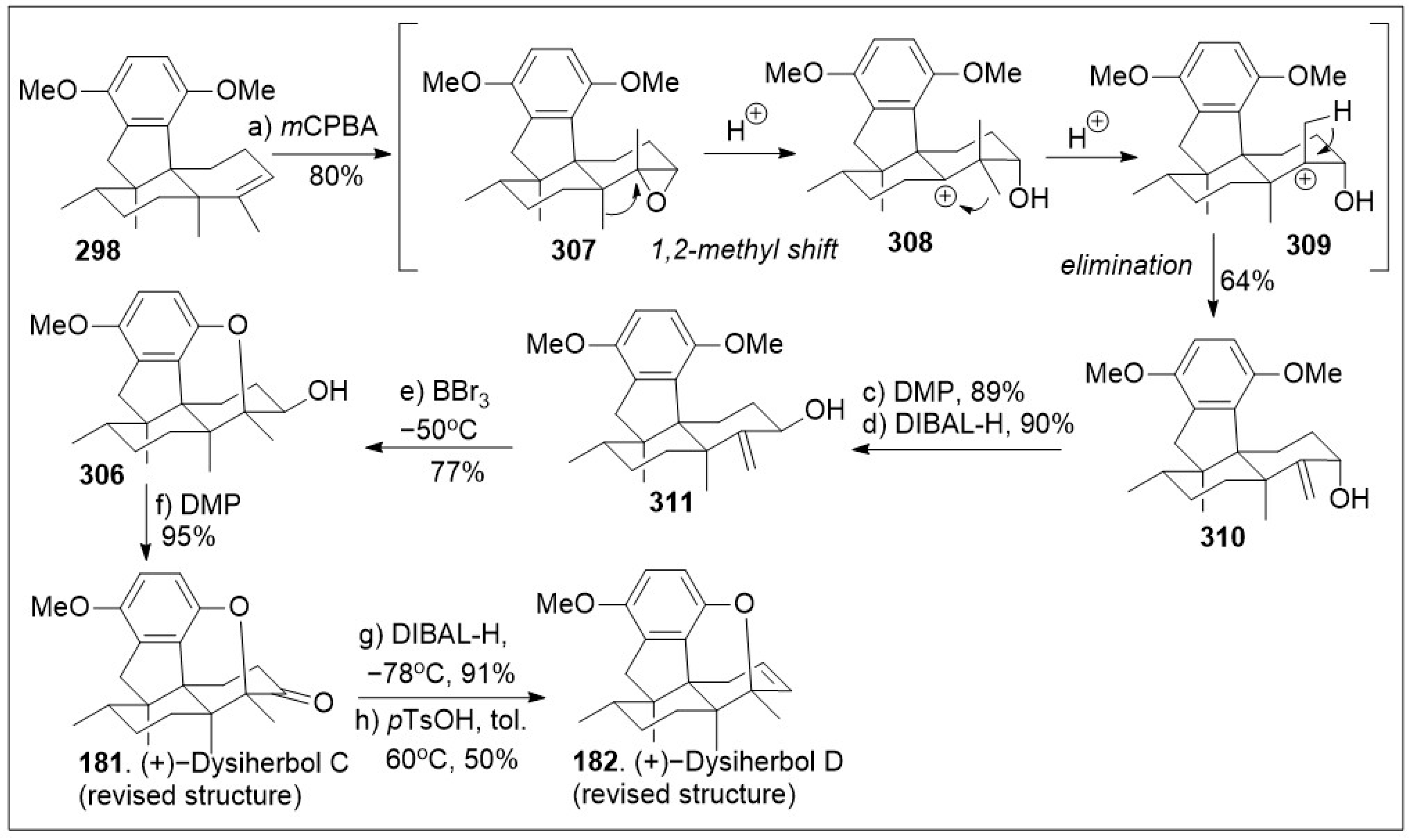
In parallel, as shown in
Scheme 25, compound
298 was treated with
mCPBA to undergo cationic 1,2-methyl shift and elimination reaction sequence to provide allylic alcohol
310. The hydroxyl group geometry in
310 was inverted via a 2 step-reaction transformation. Namely, oxidation of the hydroxyl group using DMP treatment, followed by DIBAL-H reduction, which provided allylic alcohol
311. Then, intramolecular oxy-cyclization of
311 mediated by BBr
3 generated cyclized ether
306, which was oxidized with DMP to yield (+)-dysiherbol C (
181). Reduction of this enone with DIBAL-H, followed by acid mediated dehydration reaction provided (+)-dysiherbol D (
182). In summary, this synthetic strategy of the tetracyclic core of dysiherbols through the use of critical acid mediated ring construction sequences [
90]. This unique synthetic strategy serves as the foundation to access the natural products and their derivatives.
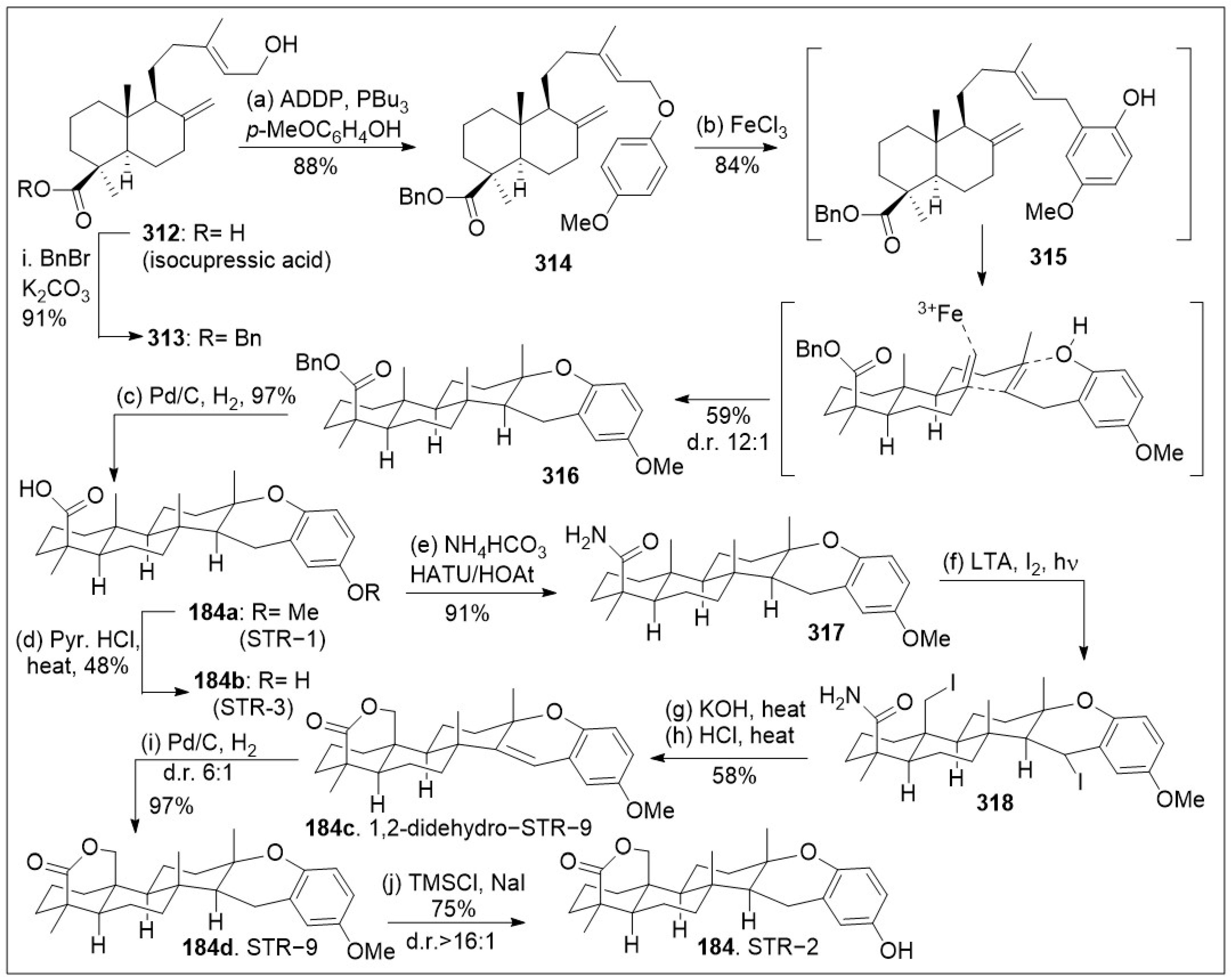
The strongylophorines meroditerpenoids have been isolated from various sources including the marine sponge
Petrosia corticata, and they have been demonstrated to be promising proteasome inhibitors [
91]. Access to these natural products to develop compound libraries is desirable. A recent streamlined total synthesis of strongylophorine-2 (STR-2,
184) is highlighted in
Scheme 26 [
92]. The synthesis capitalizes on a novel iron (III)-mediated rearrangement-cyclization cascade reaction. The synthesis commenced with the esterification of natural product isocuppressic acid
312, followed by treatment with 1,1'-(Azodicarbonyl)dipiperidine (ADDP) under Mitsunobu reaction conditions to etherify the allylic alcohol, generating compound
314. Next, compound
314 was exposed to Lewis acid Iron trichloride, which mediated the key acid catalyzed reaction that facilitated the C-O to C-C [
3,
3]-Claisen rearrangement, followed by ring closure to afford compound
316 as the major product as shown in
Scheme 26. Benzyl deprotection with Pd/C provided STR-1 (1
84a), and further deprotection of the methoxy group led to STR-3 (
184b). Alternatively,
184a could be subjected to amination conditions to yield compound
317, which was further functionalized with lead tetraacetate (LTA) and iodine-promoted hypoiodite reaction under photolysis to provide the di-iodo compound
318. Compound
318 was set for an intramolecular cyclization under basic conditions with elimination of the other iodo-group to provide 1,2-didehydro-STR-9 (
184c) as the unique intermediate, which was reduced under Pd/C to provide STR-9 (
184d). Next removal of the methoxy-protection group resulted in the natural product STR-2 (
184) in good yields.
Finally, a selected number of monoterpene indole alkaloids, which represent a large group of more than 3000 different compounds and are mainly present in three families:
Rubiaceae,
Apocynaceae, and
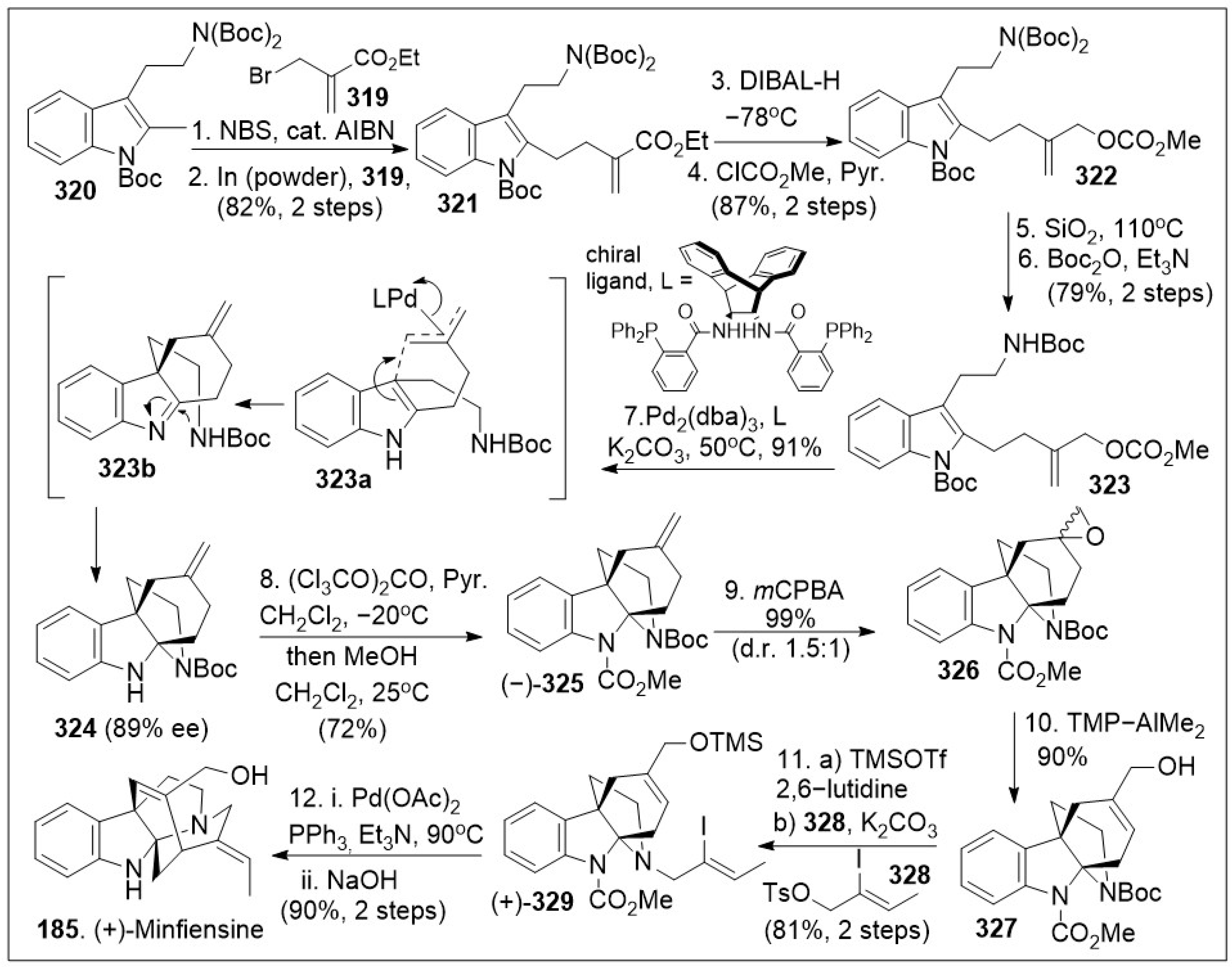
Loganiaceae will be included in this review. Numerous phytochemical studies have led to the discovery of potent bioactive molecules as exemplified by the anti-cancer natural product vinblastine. Minfiensine, a secoiridoid indole alkaloid-type, which was isolated from the African plant
Strychnos minfiensis by Massiot and co-workers in 1989 with no structural precedence at the time has since attracted various synthetic chemists. Extracts containing these meroterpene alkaloids are used throughout the world in the practice of traditional medicine, with some heightened interest in the medicinal potential of the pure natural products. A recent short total synthesis of monoterpene alkaloid (+)-minfiensine (
185) via an asymmetric palladium mediated cyclization reaction was reported [
93]. The total synthesis is depicted in
Scheme 27, and it commenced with the coupling reaction of readily available allylic halide
319 and methyltryptamine
320. The 2-step transformation took place via a
N-bromosuccinimide (NBS) mediated radical methyl-bromination of
320, followed by subsequent coupling with the allyl indium intermediate derived from the allylic halide
319 to provide compound
321, which upon DIBAL-H reduction and subsequent esterification produced allyl carbonate
322. Protecting group maneuver of compound
322 provided intermediate
323, which is subjected to an indole dearomative asymmetric allylic alkylation reaction mediated by Palladium (II) and the shown chiral ligand. The tetracyclic product
324 was generated in acceptable enantiomeric excess and good yields, and was
N-protected, followed by epoxidation with
mCPBA to afford a mixture of diastereomers of epoxide
326. Allylic alcohol
327 was subsequently generated from epoxide
326 via an epoxide ring-opening mediated by 2,2,6,6-Tetramethylpiperidine-dimethyl Aluminum complex (TMP-AlMe
2). Protection of the resultant alcohol with a silyl group followed by coupling with vinyl iodide
328 under basic condition provided
N-alkylated intermediate
329. Use of a Pd(OAc)
2-catalyzed intramolecular Heck-type reaction developed by Overman and Wrobleski for this molecule, followed by a spontaneous β-elimination reaction generated a polycyclic intermediate. This intermediate was deprotected under basic conditions provided (+)-minfiensine (
185). The synthesis is efficient at generating high complexity.
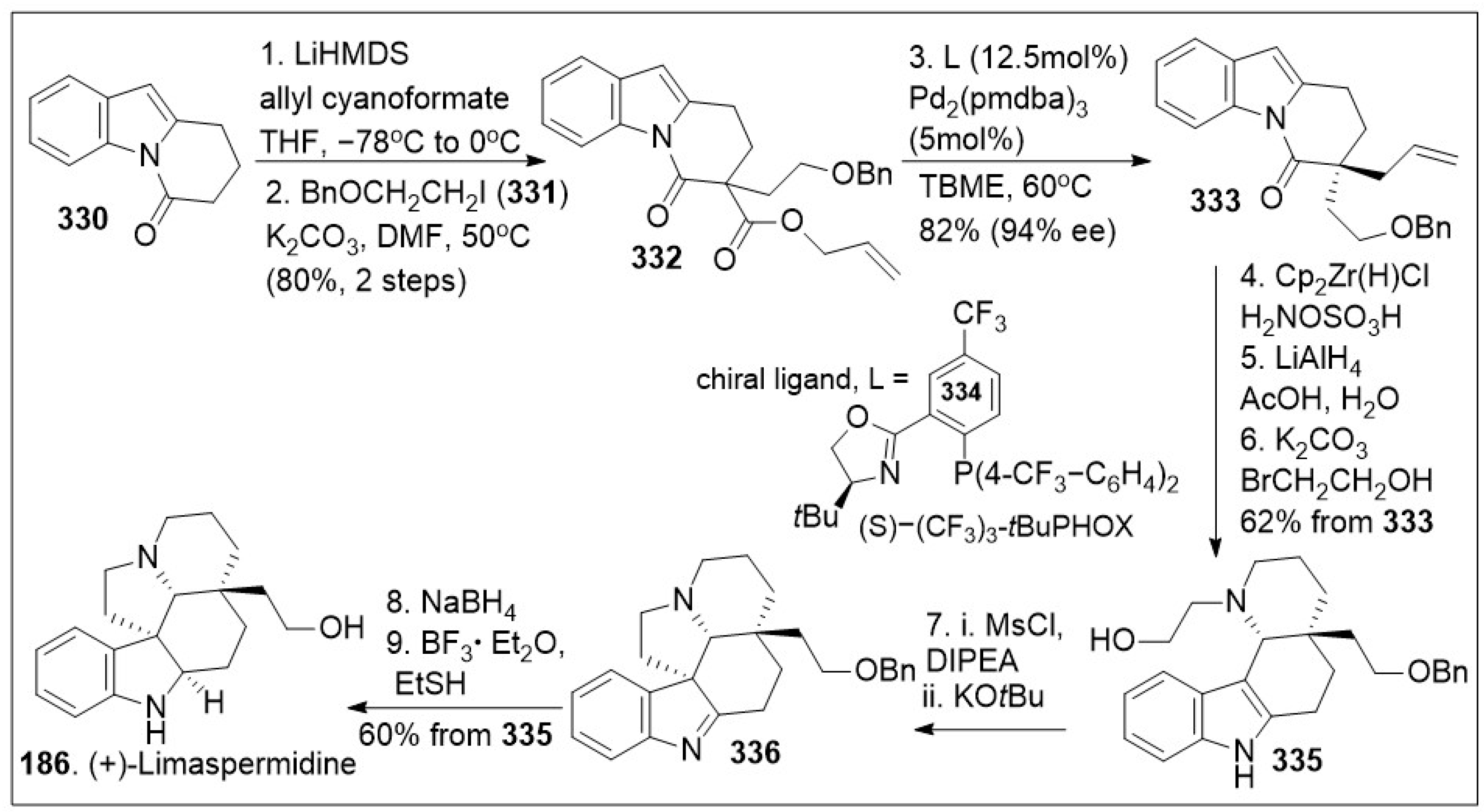
The total synthesis of meroterpene indole alkaloid (+)-limaspermidine (
186,
Scheme 28) was completed in a unified approach to demonstrate the power of palladium mediated cyclization reactions [
94].
Scheme 28 illustrates an efficient approach to this natural product from β-amidoester
332, which was generated from sequential double C-alkylation of dihydropyrido[1,2-a]indolone
330 first alkylation with allyl cyanoformate in the presence of LiHMDS, and second alkylation using (2-benzyloxy)ethyl iodide
331 under basic condition reactions. Then, decarboxylative asymmetric allylation of compound
332 was mediated by Pd
2(pmdba)
3 with a chiral ligand to provide compound
333 featuring the required α-quaternary all-carbon stereocenter in good enantioselective excess and chemical yields. Next, compound
333 was subjected to a formal anti-Markovnikov hydroamination reaction to generate the corresponding primary amine, which was directly treated with LiAlH
4 and quenched with aqueous acetic acid to promote intramolecular indole-iminium cyclization reaction. This furnished the
cis-fused tetracyclic system followed by chemoselective piperidine
N-alkylation to afford compound
335. Compound
335 was converted to polycyclic imine
336 via 2-step pyrrolidine annulation by generating the leaving group, followed by base treatment. Subsequent NaBH
4 mediated reduction of polycyclic imine
336 and benzyl deprotection provided the natural product (+)-limaspermidine
186 in overall good yields.
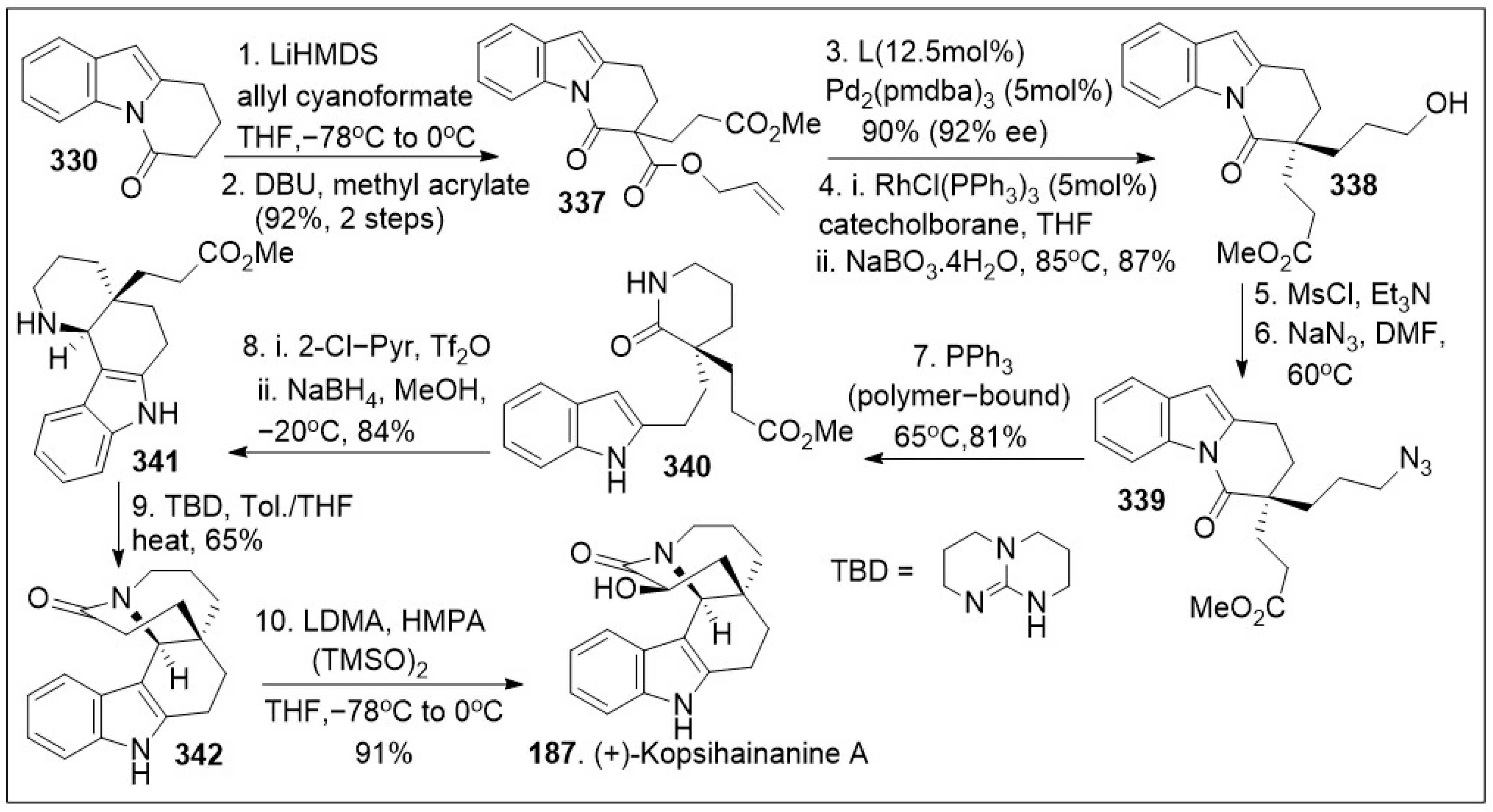
In parallel, other similar synthetic strategies to this family of meroterpenoids with alkaloid features have been reported [
94]. The total synthesis of (+)-kopsihainanine A (
187) is shown in
Scheme 29 and begins with β-amidoester
337, which was generated via sequential double α-C-alkylation of dihydropyrido[1,2-a]indolone
330, using allyl cyanoformate in the presence of LiHMDS. This was followed by treatment with methyl acrylate
331 under basic conditions. Further decarboxylative asymmetric allylation of compound
337 in the presence of Pd
2(pmdba)
3 with a chiral ligand provided an intermediate with the α-quaternary all-carbon stereocenter, which underwent Rh-catalyzed hydroboration to yield alcohol
338. Compound
338 was mesylated and displaced by the azide group to generate compound
339, followed by Staudinger reduction mediated by polymer-bond PPh
3 with concomitant translactamization, which provided
δ-lactam
340. Subsequent Bischler-Napieralski cyclization of
δ-lactam
340 furnished
trans-fused tetracycle
341. This was subjected to intramolecular lactamization between the piperidine nitrogen, and the pendant methyl ester group mediated by bicyclic guanidine base triazabicyclodecene (TBD) and generated pentacycle
342. Upon α-hydroxylation mediated by lithium dimethylamide (LDMA) in the presence of HMPA, using bis(trimethylsilyl) peroxide as the oxidant successfully afforded the natural product (+)-kopsihainanine A (
187). The overall synthetic maneuvers maximize the chemical yields, and a distinctive feature of this concise synthetic endeavors is the use of palladium-catalyzed reactions to form all the carbon-carbon bonds in the transformation of simple precursors to highly elaborate natural products with promising biological properties.
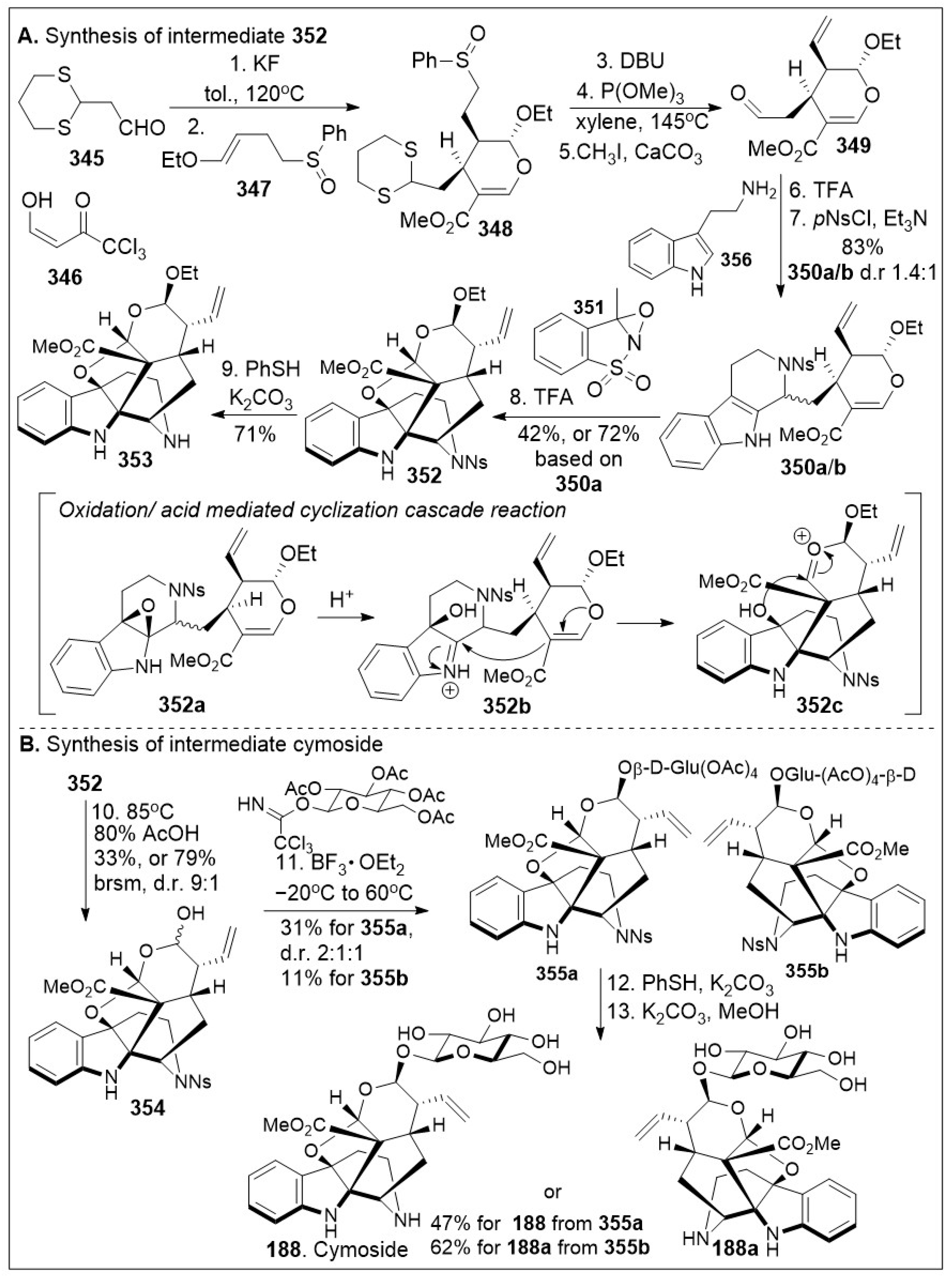
The total synthesis of cymoside, an oxidized derivative of strictosidine with an unprecedented hexacyclic skeleton which includes a double bridge linking the indole and the monoterpene moieties was isolated from the leaves of
Chimarrhis cymose and provides a fertile ground for organic chemists as shown in
Scheme 30. The total synthesis of cymoside (
188) via biomimetic oxidative cyclization was reported [
95]. The synthesis commenced with Knoevenagel condensation reaction between aldehyde
345 and 3-formyl-1,1,1-trichloroacetone
346 which generated enone intermediate, which upon inverse-demand hetero-Diels-Alder (HDA) reaction with enol ether
347 furnished cyclic intermediate
348 together with other diastereoisomers. Upon methanolysis and sulfoxide elimination, intermediate
348 was further converted to olefin intermediate, followed by deprotection furnished Tietze secologanin aglycon
349. Subsequent Pictet-Spengler reaction between the secologanin aglycon
349 and tryptamine
356, followed by protection of the secondary amine furnished the mixture of the protected strictosidine aglycon ethyl ether
350a and its epimer
350b. The biomimetic cascade reaction sequence of oxidation with oxaziridine
351 and acid catalyzed cyclization reaction of strictosidine aglycon ethyl ether
350a furnished the complete framework
352 of cymoside via intermediate
352a,
352b, and
352c, which was deprotected to generate ethyl ether aglycon
353 of cymoside. Hydrolysis of acetal
352 provided hemiacetal
354, which upon Schmit glycosylation with glycosyl trichloroacetimidate, generated glycosylated product
355a with its diastereoisomer, together with glycosylated product
355b. Global deprotection of intermediate
355a furnished the natural product cymoside
188, global deprotection of compound
355b provided product
188a as the diastereoisomer of cymoside
188.