Preliminary Study on Total Component Analysis and In Vitro Antitumor Activity of Eucalyptus Leaf Residues
Abstract
1. Introduction
2. Results and Discussion
2.1. Qualitative Analysis of Components in Eucalyptus Leaf Residue
2.1.1. Triterpenoids
2.1.2. Phloroglucinols
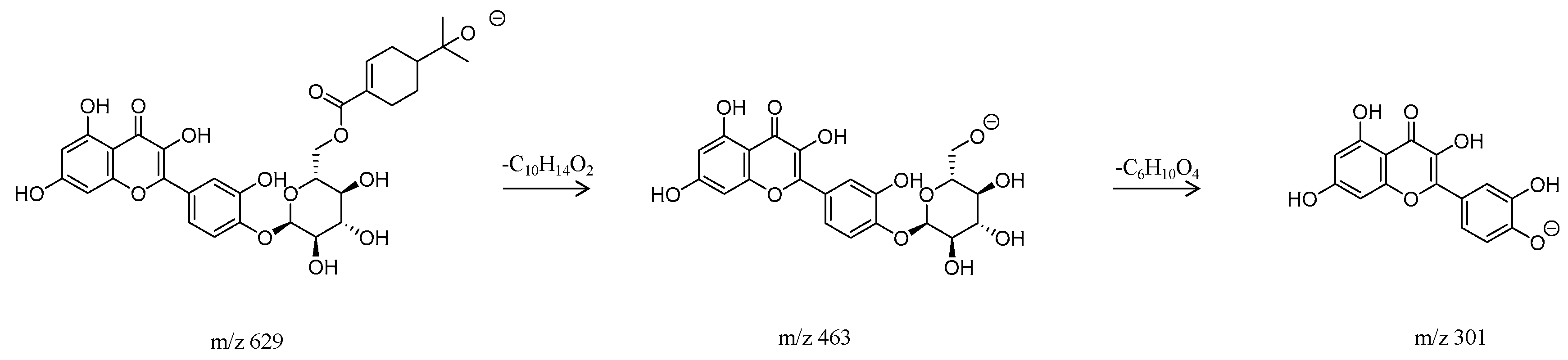
2.1.3. Flavonoids
2.2. Quantitative Analysis of Components in Eucalyptus Leaf Residue
2.3. In Vitro Antitumor Activity
3. Experimental Section
3.1. Instruments and Apparatus
3.2. Materials and Reagents
3.3. Sample Preparation and Enrichment
3.4. Experimental Methods
3.4.1. Chromatographic Conditions
3.4.2. Mass Spectrometry Conditions
3.4.3. Data Analysis
3.4.4. Quantitative Methods
3.4.5. Efficacy Experiments
In Vitro Antitumor Activity Experiments
Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fensham, R.J.; Laffineur, B.; Collingwood, T.D.; Beech, E.; Bell, S.; Hopper, S.D.; Phillips, G.; Rivers, M.C.; Walsh, N.; White, M. Rarity or decline: Key concepts for the Red List of Australian eucalypts. Biol. Conserv. 2020, 243, 108455. [Google Scholar] [CrossRef]
- Zhu, F.L.; Ren, S.X.; Qiu, B.L.; Huang, Z.; Peng, Z.Q. The abundance and population dynamics of Leptocybe invasa (Hymenoptera: Eulophidae) galls on Eucalyptus spp. in China. J. Integr. Agric. 2012, 11, 2116–2123. [Google Scholar] [CrossRef]
- Zhou, X.D.; Wingfield, M.J. Eucalypt diseases and their management in China: Keynote paper APPS 2011. Australas. Plant Pathol. 2011, 40, 339–345. [Google Scholar] [CrossRef]
- Salehi, B.; Sharifi-Rad, J.; Quispe, C.; Llaique, H.; Villalobos, M.; Smeriglio, A.; Martins, N. Insights into Eucalyptus genus chemical constituents, biological activities and health-promoting effects. Trends Food Sci. Tech. 2019, 91, 609–624. [Google Scholar] [CrossRef]
- Dhakad, A.K.; Pandey, V.V.; Beg, S.; Rawat, J.M.; Singh, A. Biological, medicinal and toxicological significance of Eucalyptus leaf essential oil: A review. J. Sci. Food Agric. 2018, 98, 833–848. [Google Scholar] [CrossRef] [PubMed]
- Heidari, A.; Khaki, E.; Younesi, H.; Lu, H.R. Evaluation of fast and slow pyrolysis methods for bio-oil and activated carbon production from eucalyptus wastes using a life cycle assessment approach. J. Clean. Prod. 2019, 241, 118394. [Google Scholar] [CrossRef]
- Okba, M.M.; El Gedaily, R.A.; Ashour, R.M. UPLC-PDA-ESI-qTOF-MS profiling and potent anti-HSV-II activity of Eucalyptus sideroxylon leaves. J. Chromatogr. B 2017, 1068, 335–342. [Google Scholar] [CrossRef]
- Ashour, R.M.S.; Okba, M.M.; Menze, E.T.; Gedaily, R.A.E. Eucalyptus sideroxylon bark anti-inflammatory potential, its UPLC-PDA-ESI-qTOF-MS profiling, and isolation of a new phloroglucinol. J. Chromatogr. Sci. 2019, 57, 565–574. [Google Scholar] [CrossRef]
- Tsiri, D.; Aligiannis, N.; Graikou, K.; Spyropoulos, C.; Chinou, I. Triterpenoids from Eucalyptus camaldulensis DEHNH. tissue cultures. Helv. Chim. Acta 2008, 91, 2110–2114. [Google Scholar] [CrossRef]
- Eyles, A.; Davies, N.W.; Mohammed, C. Novel detection of formylated phloroglucinol compounds (FPCs) in the wound wood of Eucalyptus globulus and E. nitens. J. Chem. Ecol. 2003, 29, 881–898. [Google Scholar] [CrossRef]
- Santos, B.M.; Zibrandtsen, J.F.S.; Gunbilig, D.; Sørensen, M.; Cozzi, F.; Boughton, B.A.; Heskes, A.M.; Neilson, E.H.J. Quantification and localization of formylated phloroglucinol compounds (FPCs) in Eucalyptus species. Front. Plant Sci. 2019, 10, 186. [Google Scholar] [CrossRef]
- Zhou, X.F.; Gao, Z.P. Plant origin and structural classification of phloroglucinols. J. Beijing Univ. Tradit. Chin. Med. 2012, 35, 399–405. [Google Scholar]
- Guo, Q.Y.; Huang, X.J.; Zhao, B.X.; Jian, Y.Q.; Luo, S.L.; Wang, Y.; Ye, W.C. Five new acylphloroglucinol glycosides from the leaves of Eucalyptus robusta. Nat. Prod. Commun. 2014, 9, 1934578X1400900218. [Google Scholar] [CrossRef]
- Amakura, Y.; Umino, Y.; Tsuji, S.; Ito, H.; Hatano, T.; Yoshida, T.; Tonogai, Y. Constituents and their antioxidative effects in eucalyptus leaf extract used as a natural food additive. Food Chem. 2002, 77, 47–56. [Google Scholar] [CrossRef]
- Marsh, K.J.; Saraf, I.; Hocart, C.H.; Youngento, K.; Singh, I.P.; Foleya, W.J. Occurrence and distribution of unsubstituted B-ring flavanones in Eucalyptus foliage. Phytochemistry 2019, 160, 31–39. [Google Scholar] [CrossRef]
- Saraf, I.; Marsh, K.J.; Vir, S.; Foley, W.J.; Singh, I.P. Quantitative analysis of various B-ring unsubstituted and substituted flavonoids in ten Australian species of Eucalyptus. Nat. Prod. Commun. 2017, 12, 1695–1699. [Google Scholar] [CrossRef]
- Chen, Z.; Huang, K.Y.; Ling, Y.; Goto, M.; Duan, H.Q.; Tong, X.H.; Liu, Y.L.; Cheng, Y.Y.; Natschke, S.L.M.; Yang, P.C.; et al. Discovery of an oleanolic acid/hederagenin-nitric oxide donor hybrid as an EGFR tyrosine kinase inhibitor for non-small-cell lung cancer. J. Nat. Prod. 2019, 82, 3065–3073. [Google Scholar] [CrossRef]
- Wang, H.; Zhong, W.; Zhao, J.; Zhang, H.; Zhang, Q.; Liang, Y.; Chen, S.; Liu, H.J.; Zong, S.M.; Tian, Y.X.; et al. Oleanolic acid inhibits epithelial-mesenchymal transition of hepatocellular carcinoma by promoting iNOS dimerization. Mol. Cancer Ther. 2019, 18, 62–74. [Google Scholar] [CrossRef]
- Kayouka, M.; Hamade, A.; Saliba, E.; Najjar, F.; Landy, D.; Gergesa, H.G. P-glycoprotein modulates oleanolic acid effects in hepatocytes cancer cells and zebrafish embryos. Chem.-Biol. Interact. 2020, 315, 108892. [Google Scholar] [CrossRef]
- Shopit, A.; Li, X.; Tang, Z.; Awsh, M.; Shobet, L.; Niu, M.Y.; Wang, H.Y.; Mousa, H.; Alshwmi, M.; Tesfaldet, T.; et al. MiR-421 up-regulation by the oleanolic acid derivative K73-03 regulates epigenetically SPINK1 transcription in pancreatic cancer cells leading to metabolic changes and enhanced apoptosis. Pharmacol. Res. 2020, 161, 105130. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Q.; Chen, Y.; Liang, C.L.; Liu, H.; Qiu, F.; Dai, Z. Antitumor effects of immunity-enhancing traditional Chinese medicine. Biomed. Pharmacother. 2020, 121, 109570. [Google Scholar] [CrossRef] [PubMed]
- Berillo, D.; Kozhahmetova, M.; Lebedeva, L. Overview of the biological activity of anthraquinons and flavanoids of the plant rumex species. Molecules 2022, 27, 1204. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Kaur, R.; Aggarwal, P.; Singh, G. Underutilized citrus species: An insight of their nutraceutical potential and importance for the development of functional food. Sci. Hortic. 2022, 296, 110909. [Google Scholar] [CrossRef]
- Wang, R.Q.; Guo, C.; Wang, Z.W.; Liu, D.L.; Niu, T.H.; Wu, G.T. Effects of Cremanthodium humile total terpenes on Th1/Th2 drift and Bax/Bcl-2 balance in H22 tumor-bearing mice. Immunol. J. 2018, 34, 101–108. [Google Scholar]
- Yang, Q.W.; Liang, H.P.; Chen, X.P.; Zhang, Z.F.; Liu, Y.Q. Effects of Celastrus orbiculatus extract on Eca-109 cell prolifeeration and invasion and metastasis of esophageal cancer. Heilongjiang Med. Pharmacy 2020, 43, 27–29. [Google Scholar]
- Liang, H.; He, J.; Zhang, S.C.; Dong, C.J.; Ma, A.G. Antitumor activities and its immunologic functions of Laurencia terpenoids. Chin. J. Mar. Drugs 2005, 24, 6–9. [Google Scholar]
- Vlčková, H.K.; Catapano, M.C.; Mitašík, L.; Kotland, O.; Nejmanová, I.; Pourová, J.; Nováková, L. Featuring ultimate sensitivity of high-resolution LC-MS analysis of phenolics in rat plasma. J. Sep. Sci. 2021, 44, 1893–1903. [Google Scholar] [CrossRef]
- Zandonadi, F.S.; Silva, A.A.R.; Melo, A.A.S.; Ignarro, R.S.; Matos, T.S.; Santos, E.A.; Sussulini, A. Understanding ayahuasca effects in major depressive disorder treatment through in vitro metabolomics and bioinformatics. Anal. Bioanal. Chem. 2023, 415, 4367–4384. [Google Scholar] [CrossRef]
- Zhu, H.; Lin, H.; Tan, J.; Wang, C.; Wang, H.; Wu, F.; Liu, J. UPLC-QTOF/MS-based nontargeted metabolomic analysis of mountain-and garden-cultivated Ginseng of different ages in Northeast China. Molecules 2018, 24, 33. [Google Scholar] [CrossRef]
- Wei, W.; Yu, Y.; Wang, X.; Yang, L.; Zhang, H.; Ji, H.; Guo, D. Simultaneous determination of bufalin and its nine metabolites in rat plasma for characterization of metabolic profiles and pharmacokinetic study by LC-MS/MS. Molecules 2019, 24, 1662. [Google Scholar] [CrossRef]
- Wei, L.; Gu, A.; Guo, Z.; Ding, J.; Jin, G.; Lei, Y. An integrated study on the fading mechanism of malachite green industrial dye for the marquisette curtain in the studio of cleansing fragrance, the palace museum (Beijing). Molecules 2022, 27, 4411. [Google Scholar] [CrossRef] [PubMed]
- GB/T 5009.10-1985; Determination of Crude Fiber in Vegetable Foods. Ministry of Health of the People Republic of China: Beijing, China, 1985.
- GB 5009.5-2016; Determination of Protein in Food of the People’s Republic of China. National Medical Products Administration: Beijing, China, 2016; p. 12.
- GB/T 23742-2009; Animal Feeding Stuffs-Determination of Ash Insoluble in Hydrochloric Acid. Chinese Academy of Agricultural Sciences: Beijing, China, 2009.
- GB 5009.124-2016; Determination of Amino Acid in Food. The National Standard of China: Beijing, China, 2016.
- Sun, F.; Liu, J.Y.; He, F.; Liu, Z.; Wang, D.M.; Wang, Y.F.; Yang, D.P. In-vitro antitumor activity evaluation of hyperforin derivatives. J. Asian Nat. Prod. Res. 2011, 13, 688–699. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Gao, G.; Gao, Y.L.; Xiong, L.; Li, X.; Guo, J.; Zhang, Y. Experimental research on the in vitro antitumor effects of Crataegus sanguinea. Cell Biochem. Biophys. 2013, 67, 207–213. [Google Scholar] [CrossRef] [PubMed]
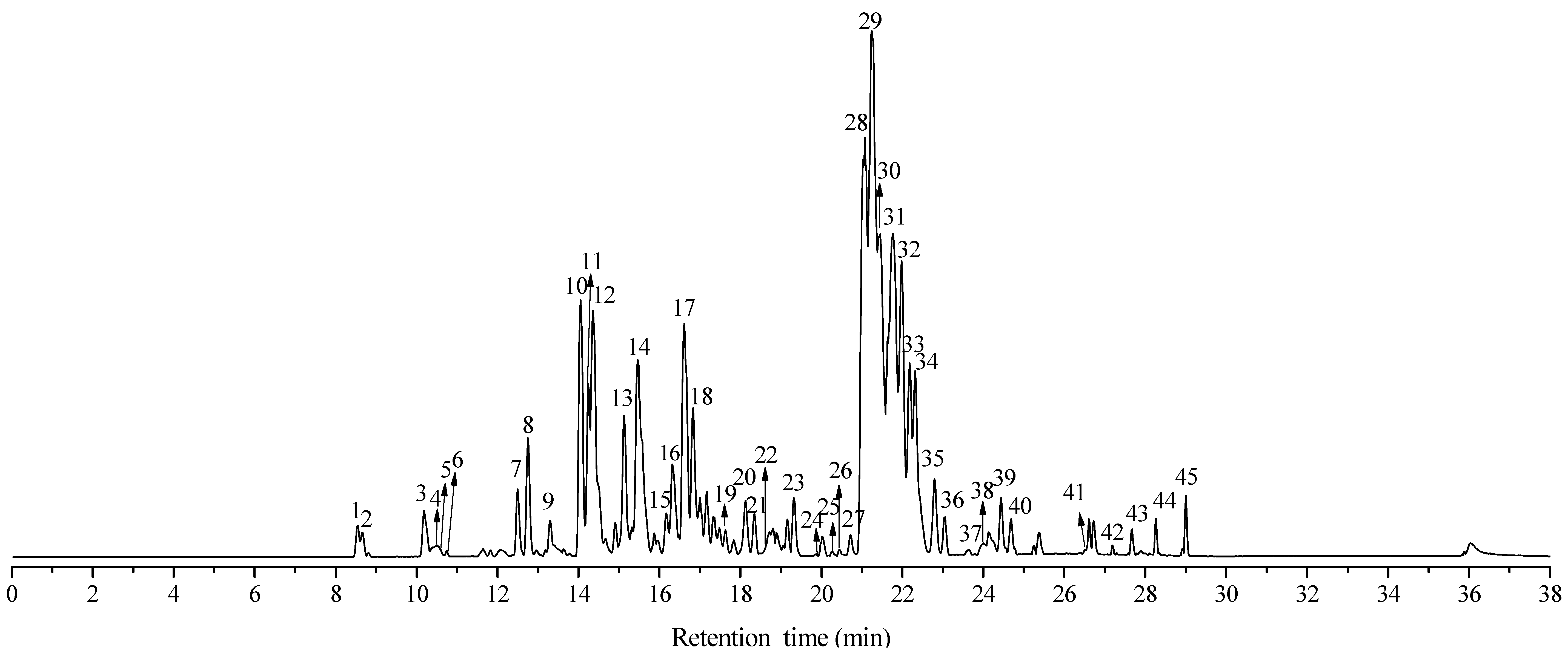
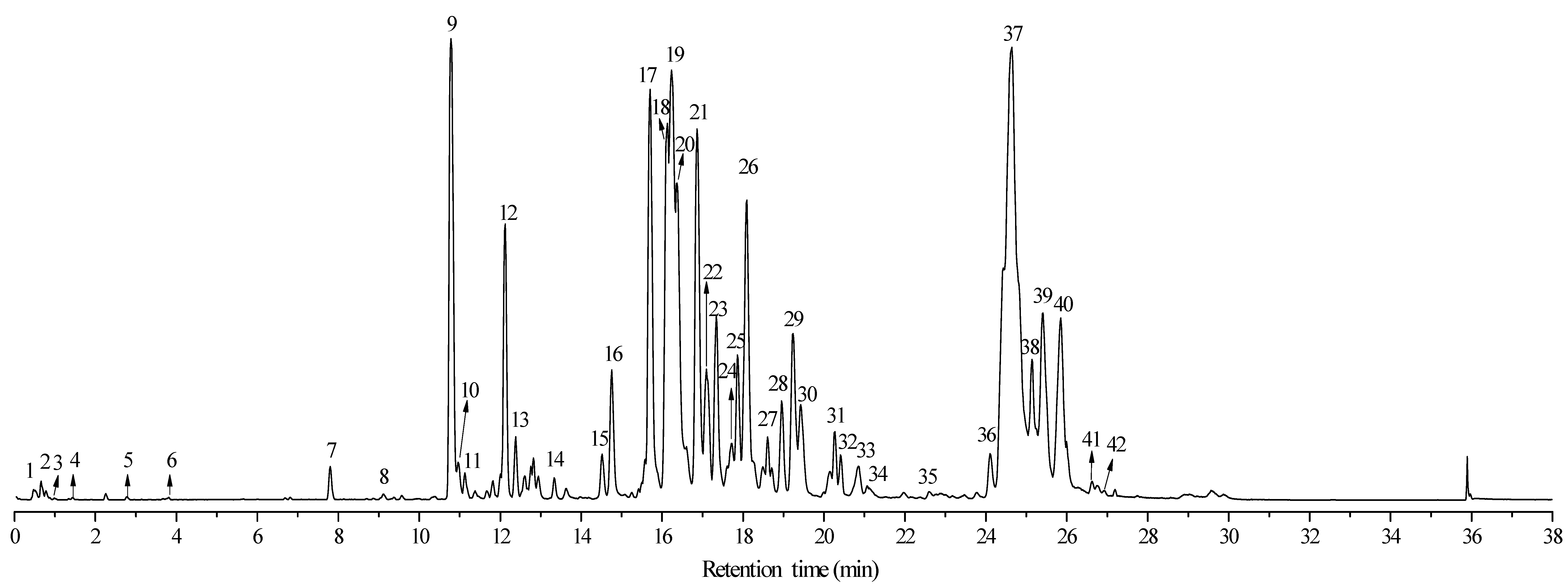

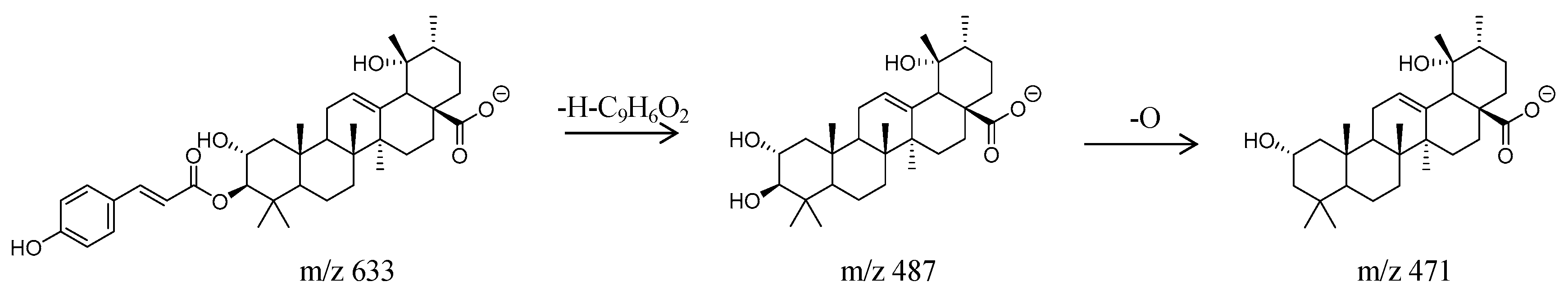
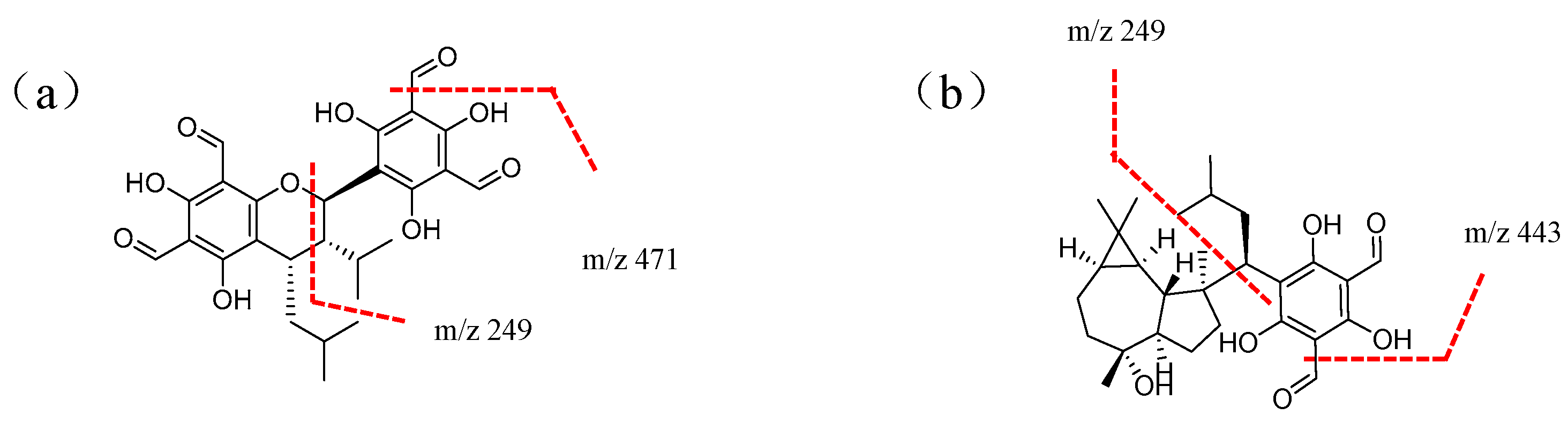
| No. | T (min) | [M − H]− (m/z) | Molecular Formula | Theoretical Molecular | Relative Error (ppm) | MSn (m/z) | Compounds | Classification |
|---|---|---|---|---|---|---|---|---|
| Weight | ||||||||
| 1 | 8.55 | 251.1372 | C14H20O4 | 251.1289 | 33 | MS2: 207.1435; 153.0010 | Tetradeca-trienedioic acid | Fatty acids |
| 2 | 8.66 | 251.1372 | C14H20O4 | 251.1289 | 33 | MS2: 207.1435; 153.0010 | Tetradeca-trienedioic acid isomer | Fatty acids |
| 3 | 10.18 | 251.1372 | C14H20O4 | 251.1289 | 33 | MS2: 207.1435; 153.0010 | Tetradeca-trienedioic acid isomer | Fatty acids |
| 4 | 10.39 | 325.1252 | C19H18O5 | 326.1154 | 30 | MS2: 325.1252, 310.0999, 309.0922, 295.0750, 282.1047, 267.0828, 239.0883, 177.0358, 150.0489, 133.0463 | Eucalyptol | Monoterpene |
| 5 | 10.52 | 265.1544 | C15H22O4 | 266.1517 | 10 | - | Leptospermone | Sesquiterpenketone |
| 6 | 10.74 | 489.3018 | C28H42O7 | 490.293 | 18 | MS2: 461.2899, 207.0286, 250.0839 | Macrocarpal J/I | Sesquiterpene |
| 7 | 12.49 | 485.2709 | C28H38O7 | 486.2617 | 19 | MS2: 207.0345, 183.0167 | Eucalyptone Isomer | Phloroglucinol sesquiterpenoids |
| 8 | 12.75 | 485.2709 | C28H38O7 | 486.2617 | 19 | MS2: 453.3354, 439.2457, 251.0918, 250.0839, 207.028 | Eucalyptone | Phloroglucinol sesquiterpenoids |
| 9 | 13.29 | 485.2709 | C28H38O7 | 486.2617 | 19 | MS1: 403.2620, 325.1956; MS2: 207.0345, 183.0167 | Eucalyptone Isomer | Phloroglucinol sesquiterpenoids |
| 10 | 13.46 | 455.3518 | C30H46O3 | 456.3603 | 19 | MS2: 455.3518, 407.3403, 363.3391, 248.9815 | Ursolic/Oleanolic/Betulinic Acid | Triterpene |
| 11 | 14.07 | 471.2937 | C28H40O6 | 472.2825 | 24 | MS1: 453.2439, 401.2091, 339.2095; MS2: 469.2737, 453.2439, 443.2924, 249.0845, 207.0374 | Macrocarpal A/B/D/E | Phloroglucinol sesquiterpenoids |
| 12 | 14.36 | 471.2937 | C28H40O6 | 472.2825 | 24 | MS2: 469.2737, 443.2924, 249.0845, 207.0374 | Macrocarpal A/B/D/E Isomer | Phloroglucinol sesquiterpenoids |
| 13 | 15.14 | 499.1778 | C26H28O10 | 500.1682 | 19 | MS1: 471.2937, 249.0845; MS2: 471.2937, 453.2814, 249.0845 | Sideroxylonal A/B/C | Phloroglucinol sesquiterpenoids |
| 14 | 15.46 | 471.2937 | C28H40O6 | 472.2825 | 24 | MS1: 339.2095; MS2: 207.0374 | Macrocarpal A/B/D/E Isomer | Phloroglucinol sesquiterpenoids |
| 15 | 15.85 | 487.343 | C30H48O5 | 488.3501 | 15 | - | Arjunolic/Asiatic Acid | Triterpene |
| 16 | 16.31 | 471.2937 | C28H40O6 | 472.2825 | 24 | MS2: 469.2737, 443.2924, 249.0845, 207.0374 | Macrocarpal A/B/D/E Isomer | Phloroglucinol sesquiterpenoids |
| 17 | 16.61 | 471.2937 | C28H40O6 | 472.2825 | 24 | MS2: 469.2737, 453.3529, 249.0845, 207.0374 | Macrocarpal A/B/D/E Isomer | Phloroglucinol sesquiterpenoids |
| 18 | 16.82 | 471.2937 | C28H40O6 | 472.2825 | 24 | MS2: 469.2737, 453.3529, 249.0845, 207.0374 | Macrocarpal A/B/D/E Isomer | Phloroglucinol sesquiterpenoids |
| 19 | 17.65 | 471.2892 | C28H40O6 | 472.2825 | 14 | MS1: 401.2091, 385.2156, 325.0000; MS2: 469.2737, 249.0845, 207.0345 | Macrocarpal A/B/D/E Isomer | Phloroglucinol sesquiterpenoids |
| 20 | 18.12 | 471.347 | C30H48O4 | 472.3552 | 17 | MS2: 469.2737, 249.0845, 207.0374 | Hydroxyursolic acid/Hederagenin | Triterpene |
| 21 | 18.33 | 385.2156 | C23H30O5 | 386.2093 | 16 | - | Euglobal | Phloroglucinol sesquiterpenoids |
| 22 | 18.56 | 497.3773 | C32H50O4 | 498.3709 | 13 | - | Oleanolic acid 3-acetate | Triterpene |
| 23 | 19.16 | 467.2581 | C28H36O6 | 468.2511 | 15 | MS2: 471.2892, 207.0374, 249.9943 | Unknown | Phloroglucinol sesquiterpenoids |
| 24 | 19.8 | 469.2737 | C28H36O6 | 470.2668 | 15 | MS2: 325.0000, 265.1544 (423.0046) | Withanolide A/Eucalrobusone O | Phloroglucinol sesquiterpenoids |
| 25 | 20.24 | 385.2156 | C23H30O5 | 386.2093 | 16 | - | Euglobal Isomer | Phloroglucinol sesquiterpenoids |
| 26 | 20.45 | 453.3011 | C29H42O4 | 454.3083 | 16 | MS1: 385.2156, 311.1812, 249.9896 | Unknown | - |
| 27 | 20.7 | 453.2788 | C28H38O5 | 454.2719 | 15 | MS2: 207.0374, 250.0936 | Macrocarpal C | Phloroglucinol sesquiterpenoids |
| 28 | 21.06 | 453.2788 | C28H38O5 | 454.2719 | 15 | MS2: 207.0374, 250.0936 | Macrocarpal C Isomer | Phloroglucinol sesquiterpenoids |
| 29 | 21.28 | 453.2788 | C28H38O5 | 454.2719 | 15 | MS2: 207.0374, 250.0936 | Macrocarpal C Isomer | Phloroglucinol sesquiterpenoids |
| 30 | 21.43 | 453.2788 | C28H38O5 | 454.2719 | 15 | MS2: 207.0374, 250.0936 | Macrocarpal C Isomer | Phloroglucinol sesquiterpenoids |
| 31 | 21.74 | 453.2788 | C28H38O5 | 454.2719 | 15 | MS2: 207.0374, 250.0936 | Macrocarpal C Isomer | Phloroglucinol sesquiterpenoids |
| 32 | 21.98 | 453.2788 | C28H38O=5 | 454.2719 | 15 | MS2: 207.0374, 250.0936 | Macrocarpal C Isomer | Phloroglucinol sesquiterpenoids |
| 33 | 22.19 | 453.2788 | C28H38O5 | 454.2719 | 15 | MS2: 207.0374, 250.0936 | Macrocarpal C Isomer | Phloroglucinol sesquiterpenoids |
| 34 | 22.32 | 453.2788 | C28H38O5 | 454.2719 | 15 | MS2: 207.0374, 250.0936 | Macrocarpal C Isomer | Phloroglucinol sesquiterpenoids |
| 35 | 22.81 | 453.2788 | C28H38O5 | 454.2719 | 15 | MS2: 207.0374, 250.0936 | Macrocarpal C Isomer | Phloroglucinol sesquiterpenoids |
| 36 | 23.03 | 453.2788 | C28H38O5 | 454.2719 | 15 | MS2: 207.0374, 250.0936 | Macrocarpal C Isomer | Phloroglucinol sesquiterpenoids |
| 37 | 23.62 | 633.3807 | C39H54O7 | 634.3869 | 10 | MS1: 568.3217, 525.3054, 485.2456, MS2: 471.2018, 248.9832 | 3-O-trans-p-Coumaroyltormentic acid | Triterpene |
| 38 | 24 | 703.3736 | C41H52O10 | 704.356 | 25 | MS2: 453.2788, 249.0845 | Unknown | Diterpene Phenol |
| 39 | 24.13 | 689.6077 | MS2: 393.326, 207.0345 | Unknown | Diterpene Phenol Sesquiterpene | |||
| 40 | 25.23 | 775.5295 | C51H66O10 | - | Unknown | Dimethylated Diterpene Olefin | ||
| 41 | 26.4 | 599.3742 | C39H52O5 | 600.3814 | 12 | - | Garcinialiptone | Triterpene |
| 42 | 27.21 | 281.2483 | C18H34O2 | 282.2558 | 27 | MS2: 249.2125, 181.2023 | Oleic Acid | Fatty Acid |
| 43 | 28.27 | 485.2536 | C28H38O7 | 486.2617 | 17 | MS2: 325.1920, 207.0374 | Eucalyptone Isomer | Formyl Sesquiterpene Phenol |
| 44 | 28.92 | 469.2584 | C28H38O6 | 470.2668 | 18 | MS2: 423.2351 | Withanolide A/Eucalrobusone O Isomer | Formyl Phloroglucinol Meroterpenoids |
| 45 | 29 | 741.5692 | C50H32O10 | - | Unknown | Phloroglucinol sesquiterpenoids |
| No. | RT (min) | [M − H]− (m/z) | Molecular Formula | Theoretical Molecular Weight | Relative Error (ppm) | MSn (m/z) | Compound | Classification |
|---|---|---|---|---|---|---|---|---|
| 1 | 0.51 | 353.1057 | C16H18O9 | 354.0950 | 30 | MS2: 165.0710, 203.0526, 233.0618, 259.0286, 275.1095, 335.0879 | Chlorogenic Acid | Polyphenol |
| 2 | 0.66 | 247.1153 | C14H16O4 | 248.1048 | 42 | MS2: 147.0990, 159.1346 | Isohistidine | Amino Acid |
| 3 | 0.98 | 497.1765 | C23H30O12 | 498.1737 | 6 | - | Eucaglobulin | Monoterpene |
| 4 | 1.43 | 629.2004 | C31H34O14 | 630.1948 | 9 | MS2: 301.0416, 300.0337, 463.0988 | Eucalmaidin D/Cypellogin A/B | Flavonoid |
| 5 | 2.78 | 561.2329 | C26H42O13 | 562.2625 | 53 | MS2: 285.0383, 257.0797, 183.1026 | 19-Hydroxycinnzeylanol 19-Glucoside | Diterpene Glycoside |
| 6 | 3.79 | 301.0025 | C14H6O8 | 302.0062 | 12 | 275.0965, 273.0799 | Ellagic acid | Polyphenol |
| 7 | 7.80 | 487.3430 | C30H48O5 | 488.3501 | 15 | - | Arjunolic/Asiatic Acid | Triterpene |
| 8 | 9.11 | 311.0909 | C18H16O5 | 312.0997 | 28 | - | Sideroxylin | Flavonoid |
| 9 | 10.79 | 489.2883 | C28H42O7 | 490.2930 | 10 | MS2: 461.2899, 207.0286, 250.0839 | Macrocarpal J/I | Phloroglucinol sesquiterpenoids |
| 10 | 10.95 | 489.2883 | C28H42O7 | 490.2930 | 10 | MS2: 461.2899, 207.0286, 250.0839 | Macrocarpal J/I Isomer | Phloroglucinol sesquiterpenoids |
| 11 | 11.13 | 471.3470 | C30H48O4 | 472.3552 | 17 | MS2: 453.3354, 249.0748, 207.0286 | Hydroxyursolic acid/Hederagenin | Triterpene |
| 12 | 12.12 | 489.2838 | C28H42O7 | 490.2930 | 19 | MS2: 461.2899, 457.2581, 443.2794, 250.0839, 207.0286 | Macrocarpal J/I Isomer | Phloroglucinol sesquiterpenoids |
| 13 | 12.37 | 471.3470 | C30H48O4 | 472.3552 | 17 | MS2: 453.3354, 249.0748, 207.0286 | Hydroxyursolic acid/Hederagenin Isomer | Triterpene |
| 14 | 13.34 | 489.2883 | C28H42O7 | 490.2930 | 10 | MS2: 461.2899, 207.0286, 250.0839 | Macrocarpal J/I Isomer | Phloroglucinol sesquiterpenoids |
| 15 | 14.53 | 485.2528 | C28H38O7 | 486.2617 | 18 | MS2: 453.3354, 439.2457, 251.0918, 250.0839, 207.028 | Eucalyptone | Phloroglucinol sesquiterpenoids |
| 16 | 14.75 | 485.2528 | C28H38O7 | 486.2617 | 18 | MS2: 453.3354, 439.2457, 251.0918, 250.0839, 207.028 | Eucalyptone Isomer | Phloroglucinol sesquiterpenoids |
| 17 | 15.71 | 617.3841 | C39H54O6 | 618.3920 | 13 | MS1: 499.1595, 485.2528, 471.2759, 455.2471, 325.1800, 161.9348; MS2: 497.3271, 451.2456 | O-p coumaroyl maslinic/alphitolic acid | Triterpene |
| 18 | 16.12 | 471.3470 | C30H48O4 | 472.3552 | 17 | MS2: 453.3354, 249.0748, 207.0286 | Hydroxyursolic acid/Hederagenin Isomer | Triterpene |
| 19 | 16.24 | 471.3470 | C30H48O4 | 472.3552 | 17 | MS2: 453.3354, 249.0748, 207.0286 | Hydroxyursolic acid/Hederagenin Isomer | Triterpene |
| 20 | 16.39 | 471.3470 | C30H48O4 | 472.3552 | 17 | MS2: 453.3354, 249.0748, 207.0286 | Hydroxyursolic acid/Hederagenin Isomer | Triterpene |
| 21 | 16.87 | 471.2759 | C28H40O6 | 472.2825 | 14 | MS2: 469.2737, 443.2924, 249.0845, 207.0374 | Macrocarpal A/B/D/E | Phloroglucinol sesquiterpenoids |
| 22 | 17.07 | 455.3514 | C30H48O3 | 456.3603 | 20 | MS1: 369.8584, 339.1982, 311.1667 | Ursolic/Oleanolic/Betulinic Acid | Triterpene |
| 23 | 17.31 | 499.1778 | C26H28O10 | 500.1682 | 19 | MS1: 471.2937, 249.0845; MS2: 471.2937, 453.2814, 249.0845 | Sideroxylonal A/B/C | Phloroglucinol sesquiterpenoids |
| 24 | 17.68 | 471.2937 | C28H40O6 | 472.2825 | 24 | MS1: 453.2439, 401.2091, 339.2095; MS2: 469.2737, 453.2439, 443.2924, 249.0845, 207.0374 | Macrocarpal A/B/D/E Isomer | Phloroglucinol sesquiterpenoids |
| 25 | 17.86 | 471.2937 | C28H40O6 | 472.2825 | 24 | MS1: 453.2439, 401.2091, 339.2095; MS2: 469.2737, 453.2439, 443.2924, 249.0845, 207.0374 | Isomer of Eucalyptal A/B/D/E | Phloroglucinol sesquiterpenoids |
| 26 | 18.07 | 471.2937 | C28H40O6 | 472.2825 | 24 | MS1: 453.2439, 401.2091, 339.2095; MS2: 469.2737, 453.2439, 443.2924, 249.0845, 207.0374 | Macrocarpal A/B/D/E Isomer | Phloroglucinol sesquiterpenoids |
| 27 | 18.6 | 453.3354 | C30H46O3 | 454.3446 | 20 | MS1: 325.1846 | Dehydroxyursolic Lactone | Triterpene |
| 28 | 18.94 | 471.2937 | C28H40O6 | 472.2825 | 24 | MS1: 453.2439, 401.2091, 339.2095; MS2: 469.2737, 453.2439, 443.2924, 249.0845, 207.0374 | Macrocarpal A/B/D/E Isomer | Phloroglucinol sesquiterpenoids |
| 29 | 19.42 | 471.2937 | C28H40O6 | 472.2825 | 24 | MS1: 453.2439, 401.2091, 339.2095; MS2: 469.2737, 453.2439, 443.2924, 249.0845, 207.0374 | Macrocarpal A/B/D/E Isomer | Phloroglucinol sesquiterpenoids |
| 30 | 20.12 | 499.1778 | C26H28O10 | 500.1682 | 19 | MS1: 471.2937, 249.0845; MS2: 471.2937, 453.2814, 249.0845 | Sideroxylonal A/B/C Isomer | Phloroglucinol sesquiterpenoids |
| 31 | 20.28 | 469.2560 | C28H38O6 | 470.2668 | 23 | MS1: 443.0080, 325.1809, 265.1477; MS2: 425.2687 | Withanolide A/Eucalrobusone O | Formyl Phloroglucinol Meroterpenoids |
| 32 | 20.4 | 599.3732 | C39H52O5 | 600.3814 | 14 | MS1: 455.2475 | Garcinialiptone | Triterpene |
| 33 | 20.78 | 629.2004 | C31H34O14 | 630.1948 | 9 | MS2: 301.0416, 300.0337, 463.0988 | Eucalmaidin D/Cypellogin A/B | Flavonoid |
| 34 | 21.06 | 453.2788 | C28H38O5 | 454.2719 | 15 | MS2: 207.0374, 250.0936 | Macrocarpal C | Phloroglucinol sesquiterpenoids |
| 35 | 22.57 | 453.2788 | C28H38O5 | 454.2719 | 15 | MS2: 207.0374, 250.0936 | Macrocarpal C Isomer | Phloroglucinol sesquiterpenoids |
| 36 | 24.11 | 453.2788 | C28H38O5 | 454.2719 | 15 | MS2: 207.0374, 250.0936 | Macrocarpal C Isomer | Phloroglucinol sesquiterpenoids |
| 37 | 24.6 | 453.2788 | C28H38O5 | 454.2719 | 15 | MS2: 207.0374, 250.0936 | Macrocarpal C Isomer | Phloroglucinol sesquiterpenoids |
| 38 | 25.16 | 453.2788 | C28H38O5 | 454.2719 | 15 | MS2: 207.0374, 250.0936 | Macrocarpal C Isomer | Phloroglucinol sesquiterpenoids |
| 39 | 25.42 | 453.2788 | C28H38O5 | 454.2719 | 15 | MS2: 207.0374, 250.0936 | Macrocarpal C Isomer | Phloroglucinol sesquiterpenoids |
| 40 | 25.83 | 453.2788 | C28H38O5 | 454.2719 | 15 | MS2: 207.0374, 250.0936 | Macrocarpal C Isomer | Phloroglucinol sesquiterpenoids |
| 41 | 26.69 | 607.3989 | C38H56O6 | 608.4077 | 14 | MS2: 249.0845, 207.0374 | Sesquiterpene Alcohol Ester | Sesquiterpene Phenol Alcohol |
| 42 | 26.95 | 453.2657 | C28H38O5 | 454.2719 | 14 | MS2: 207.0374, 250.0936 | Macrocarpal C Isomer | Phloroglucinol sesquiterpenoids |
| Composition | Content/% | Summation/% |
|---|---|---|
| Crude Fiber | 27.60 | 76.99 |
| Crude Protein | 5.64 | |
| Crude Fat | 4.85 | |
| Crude Ash | 4.10 | |
| Moisture | 18.40 | |
| Total Sugars | 6.86 | |
| Total Polyphenols | 4.77 | |
| Ursolic Acid | 0.586 | |
| Total Terpenes | 2.84 | |
| Phloroglucinols | 1.93 |
| Compound | Concentration /μg∙mL−1 | Inhibition Ratio/% | IC50/μg∙mL−1 | ||||
|---|---|---|---|---|---|---|---|
| MDA-MB- 231 | SGC- 7901 | Hela | MDA-MB- 231 | SGC- 7901 | Hela | ||
| Triterpenoids | 0.00 | 0.00 | 0.00 | 0.00 | 50.67 | 43.12 | 42.65 |
| 6.25 | 16.15 | 4.62 | 5.17 | ||||
| 12.50 | 32.01 | 4.24 | 9.83 | ||||
| 25.00 | 29.52 | 28.90 | 16.73 | ||||
| 50.00 | 46.63 | 62.87 | 57.97 | ||||
| 100.00 | 64.01 | 68.96 | 77.13 | ||||
| 5-FU | 0.00 | 0.00 | 0.00 | 0.00 | 2.36 | 12.61 | 22.81 |
| 6.25 | 60.95 | 35.05 | 17.47 | ||||
| 12.50 | 69.71 | 35.99 | 22.89 | ||||
| 25.00 | 73.26 | 38.72 | 34.07 | ||||
| 50.00 | 73.12 | 41.48 | 36.25 | ||||
| 100.00 | 73.04 | 50.26 | 40.07 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, J.; Wang, Z.; Cheng, X.; Lian, Y.; An, X.; Wu, D. Preliminary Study on Total Component Analysis and In Vitro Antitumor Activity of Eucalyptus Leaf Residues. Molecules 2024, 29, 280. https://doi.org/10.3390/molecules29020280
Wu J, Wang Z, Cheng X, Lian Y, An X, Wu D. Preliminary Study on Total Component Analysis and In Vitro Antitumor Activity of Eucalyptus Leaf Residues. Molecules. 2024; 29(2):280. https://doi.org/10.3390/molecules29020280
Chicago/Turabian StyleWu, Juanjuan, Zixuan Wang, Xinying Cheng, Yunhe Lian, Xiaodong An, and Di Wu. 2024. "Preliminary Study on Total Component Analysis and In Vitro Antitumor Activity of Eucalyptus Leaf Residues" Molecules 29, no. 2: 280. https://doi.org/10.3390/molecules29020280
APA StyleWu, J., Wang, Z., Cheng, X., Lian, Y., An, X., & Wu, D. (2024). Preliminary Study on Total Component Analysis and In Vitro Antitumor Activity of Eucalyptus Leaf Residues. Molecules, 29(2), 280. https://doi.org/10.3390/molecules29020280






